
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 01 November 2019
Sec. Children and Health
Volume 7 - 2019 | https://doi.org/10.3389/fpubh.2019.00311
This article is part of the Research TopicCannabis: A Public Health PerspectiveView all 4 articles
 Skyler Shollenbarger1
Skyler Shollenbarger1 Alicia M. Thomas1
Alicia M. Thomas1 Natasha E. Wade2
Natasha E. Wade2 Staci A. Gruber3,4
Staci A. Gruber3,4 Susan F. Tapert2
Susan F. Tapert2 Francesca M. Filbey5
Francesca M. Filbey5 Krista M. Lisdahl1*
Krista M. Lisdahl1*Objective: The endocannbinoid system and cannabis exposure has been implicated in emotional processing. The current study examined whether regular cannabis users demonstrated abnormal intrinsic (a.k.a. resting state) frontolimbic connectivity compared to non-users. A secondary aim examined the relationship between cannabis group connectivity differences and self-reported mood and affect symptoms.
Method: Participants included 79 cannabis-using and 80 non-using control emerging adults (ages of 18–30), balanced for gender, reading ability, and age. Standard multiple regressions were used to predict if cannabis group status was associated with frontolimbic connectivity after controlling for site, past month alcohol and nicotine use, and days of abstinence from cannabis.
Results: After controlling for research site, past month alcohol and nicotine use, and days of abstinence from cannabis, cannabis users demonstrated significantly greater connectivity between left rACC and the following: right rACC (p = 0.001; corrected p = 0.05; f2 = 0.55), left amygdala (p = 0.03; corrected p = 0.47; f 2 = 0.17), and left insula (p = 0.03; corrected p = 0.47; f 2 = 0.16). Among cannabis users, greater bilateral rACC connectivity was significantly associated with greater subthreshold depressive symptoms (p = 0.02).
Conclusions: Cannabis using young adults demonstrated greater connectivity within frontolimbic regions compared to controls. In cannabis users, greater bilateral rACC intrinsic connectivity was associated with greater levels of subthreshold depression symptoms. Current findings suggest that regular cannabis use during adolescence is associated with abnormal frontolimbic connectivity, especially in cognitive control and emotion regulation regions.
Cannabis remains one of the most popular used substances worldwide (1). Approximately, 35% of high school seniors and young adults ages 19–28 reported using cannabis in the past year (2). Cannabis use during youth has been a recent focus in public health research, as it may influence one's risk for reporting symptoms of anxiety and depression (3–14). A potential mechanism underlying cannabis' influence on mood and affective symptoms may involve frontolimbic functioning [see (15, 16)]. Understanding differences in frontolimbic connectivity among young adults with frequent cannabis use may provide insight into the etiology of associated mood or affective risk.
Cannabinoids in cannabis, such as Δ9-tetrahydrocanabidiol (or THC) and cannabidiol (CBD), are chemicals that mimic endogenous neurotransmitters anandamide and 2AG by binding to endocannabinoid (eCB) receptors CB1 and CB2 (17–20). THC is the main psychoactive component of cannabis and is responsible for the subjective “high” individuals experience [see (20–22)]. CB1 activity modulates the release of the neurotransmitters GABA and glutatmate (GLUT) [see (23)]. The eCB system modulates several functions related to physical (e.g., sleep, pain, and inflammation) and mental health, including regulation of emotional and stress responses [see (24–29)].
More specifically, the eCB system plays a role in mood and affect (28, 30–35), integrating reward feedback (36), and threat related signals (37–39). Brain regions primarily involved in the affective processing system include several interacting cortical and subcortical regions (e.g., amygdala, anterior cingulate gyrus or ACC, medial and inferior orbito-frontal, ventromedial or vmPFC, dorsomedial prefrontal cortex, ventral striatum, and insula) (40–44). This system is highly innervated with CB1 receptors (45–49) and animal models demonstrate developmental changes in CB1 expression within the mPFC, ACC and insula (50), suggesting the system demonstrates plasticity during adolescence. Therefore, repeated THC exposure during development may impact naturally occurring changes in eCB functioning within mesocorticolimbic regions (16). Indeed, daily cannabis users have shown decreased CB1 receptor density within frontolimbic regions (prefrontal cortex (PFC), ACC, and insula) compared to non-users which recovered after a month of abstinence (51). Further, acute THC administration has resulted in abnormal performance on behavioral measures of emotional processing (52–54), amygdala reactivity (38), and altered functional connectivity and signaling in PFC regions (15, 16, 53, 55–58). However, additional research is needed to confirm the influence of repeated THC exposure on affective outcomes in adolescents and young adults.
Due to the neuromodulatory role of the eCB system, examining brain functional connectivity is an important outcome to study in regular cannabis users. These relationships can be examined during tasks and also at rest, when individuals are not actively engaging in any specific cognitive tasks, called resting state, or intrinsic functional connectivity (ifcMRI) (59). Connectivity patterns in frontolimbic regions continue to develop into late adolescence and emerging adulthood; prefrontal maturation purports enhanced emotion regulation and behavior inhibition capabilities [see (60–68)], giving rise to a functional coupling between frontal and limbic regions (i.e., the frontolimbic network) (69). Collectively, the developmental changes in frontolimbic connectivity are thought to enhance socioemotional regulation [see (70–72)], specifically via functioning within the amygdala, medial PFC, vmPFC, ACC, insula, and inferior frontal gyrus (43, 73). A particular region within the PFC, the ACC, also undergoes significant age-related changes in intrinsic functional connectivity, particularly in rostral ACC (rACC) subregions involved in social cognition and emotion regulation (74). Therefore, this system may be particularly vulnerable to repeated THC exposure during development.
Thus far, studies have found slower response times in users when identifying emotional faces and more liberal criterion for selecting sadness (75), poorer facial recognition and emotion matching (76), and emotion identification and discrimination impairments (77) compared to non-users; though accuracy in emotion identification may not display a dose-dependent relationship (78). fMRI studies have found aberrant amygdala and ACC activity in young cannabis users during affective processing tasks, including blunted ACC and amygdala activation during sub-conscious facial viewing (79), blunted amygdala response among youth with comorbid cannabis dependence and depression (80), and greater amygdala reactivity to angry faces in young adolescents (81).
However, to date very few studies have examined intrinsic functional connectivity (ifcMRI) in adolescents and emerging adults (82–86). Studies to date in adolescent and young adult cannabis users (primarily male) have demonstrated increased intrinsic connectivity in frontal (superior, inferior frontal gyrus)-temporal gyrus-cerebellar regions (83), frontal-parietal-cerebellar network (84), increased middle-frontal and cingulate gyrus connectivity (85), and increased frontal gyrus activity along with reduced middle temporal activity (82). Increased connectivity patterns were linked with increased symptoms of cannabis dependence (83) and recent cannabis use frequency (84). In young adult males, cannabis use was linked with increased connectivity in insula and decreased connectivity in the anterior cingulate and midbrain, even after a month of abstinence (86). Thus, overall, young cannabis users appear to demonstrate increased intrinsic connectivity patterns, especially in frontal-limbic regions. Still, these studies were primarily in men (83, 84, 86), thus findings may not generalize to female users (87–90). Further, two studies did not control for comorbid alcohol use (83, 86) and despite the aforementioned link between cannabis use and affective processing, no studies to date have specifically examined affective processing networks in cannabis users. Therefore, additional research is needed to examine intrinsic connectivity in affective processing networks in larger samples that include both males and females, controlling for comorbid alcohol use.
The purpose of the current study was to explore whether regular cannabis use in adolescents and young adults was associated with aberrant ifcMRI frontolimbic connectivity at rest. We employed a priori region of interest analysis focusing on regions with reported cortical differences between young cannabis users and controls, including: vmPFC (91, 92), ACC (81, 93, 94), insula (95), and amygdala (88, 96, 97). This project utilized ifcMRI data from three collection sites from the Imaging Data in Emerging Adults with Addiction (IDEAA) Consortium (University of Wisconsin-Milwaukee or UWM; McLean Hospital/Harvard University or McLean; University of Texas—Dallas or UTD). The strength of utilizing multi-site data sets include excellent reliability and validity when combining multi-site ifcMRI data (98–107), increased generalizability of more heterogenous groups (i.e., improving sex, ethnicity, and geographic diversity), and larger sample sizes. It was hypothesized that cannabis users would demonstrate increased intrinsic connectivity patterns in regions subserving emotional expression [amygdala, insula, and caudal (cACC) and rostral ACC (rACC)]. Lastly, in order to interpret the findings, a secondary aim examined if group differences in connectivity were associated with cannabis users' self-reported anxiety and depressive symptoms.
Participants included 79 cannabis users (42 men and 37 women) and 80 (45 men and 35 women) controls aged 18–30 year old young adults devoid of major medical, psychiatric or neurologic comorbidities. This age restriction is to reduce potential differences in developmental stage since adolescents and emerging adults may have greater PFC and limbic development compared to adult participants (60–64, 68, 108). Study participants were selected from the IDEAA consortium subject pool (PIs: Krista Lisdahl, Ph. D., UWM; Staci Gruber, Ph. D., McLean Hospital/Havard, Susan Tapert, Ph.D., University of California-San Diego, and Francesca Filbey Ph.D., UTD; data from Dr. Tapert's lab did not include resting-state fMRI collection and therefore was not used in the current study).
Inclusion criteria included: right-handedness; had usable intrinsic ifcMRI data; fluency in English; and fit one of two groups: cannabis users (at least weekly cannabis use within the past 3 months, duration of use >1 year) and controls (never had a history of regular (>monthly) use; no recent past month use; no history of cannabis use disorder). Exclusion criteria included history of neurological illness or loss of consciousness >2 min; MRI contraindications (pregnancy, claustrophobia, weight over 250 lbs., ferromagnetic implants of any kind, pacemakers, or other devices in body); current use of psychoactive medication; current DSM-IV-TR (109) independent Axis I disorders (aside from substance use disorders); regular other illicit drug use (>20 times); and inability to remain abstinent from all drugs and alcohol for at least 12 h (ranged from 12 h to 21 days monitored abstinence across sites).
The Institutional Review Board for each site approved all aspects of data collection. Participants underwent site-specific IRB-approved consenting procedures, and completed screening sessions to ensure inclusion/exclusion criteria. Following study inclusion, the participants completed psychological questionnaires, underwent substance toxicology screening, and received an MRI at the individual collection sites. The ifcMRI data was collected before any fMRI task for each site.
Drug use prior to study participation was recorded by interview using temporal memory cues from a modified version of the Time-Line Follow-Back at each study site (110). Drug categories included quantity-standardized collection of: nicotine cigarettes (total number), alcohol (total standardized drinks), cannabis (total number of grams of dried flower1), and other illicit drugs (days used). Time-period covered for substance use assessment was 1 month [original data collected from each site ranged from 2 weeks (McLean), past 30 days (UTD), to past year (UWM); thus, total past month substance use was averaged for each participant collected from McLean, though all McLean users reported consistent daily patterns of use during this time].
The Beck Depression Inventory—second edition (BDI-II) (collected from all sites) measured self-reported symptoms of past 2-week depressive symptoms with a possible range of 0–63 total scores (111, 112). Low scores on the BDI-II are interpreted as ≤ 16 and elevated ≥17.
The Wechsler Abbreviated Scale of Intelligence (WASI)—Vocabulary subtest (113) (collected from McLean and UTD) and the Wide Range Achievement Test−4th edition (WRAT-IV). Reading subtest (114) (collected from UWM) measured verbal intelligence (115) and quality of education [see (116)]. Standardized (age-corrected) T-scores for each participant were used in the analyses.
Image processing followed standardized recommendations for fMRI processing (117, 118). ifcMRI scans were combined from three research sites; de-identified raw DICOM files were uploaded to the McLean Hospital server. UWM: Structural MRI (sMRI) scans were collected using a 3T GE MR750 scanner and SPGR sequence with the following parameters: TR/TE/TI = 8.2/3.4/450 ms, flip angle = 12°, FOV = 240, matrix size: 256 × 256 mm, slice thickness = 1 mm (along left-right direction), voxel size = 1 × 1 × 1 mm, 150 slices, total scan time = 8 min. ifcMRI scans were collected using a gradient echo, echoplanar sequence with ramp sampling correction using the intercomissural line (AC-PC) as a reference (TR: 2,000 ms, TE: 25 ms, FOV: 240, flip angle = 77°, matrix size: 64 × 64, 40 slices, reps: 240, thickness 3.7 mm). McLean: sMRI scans were collected using a 3T Siemens Magnetom TrioTim sngo MR B17 and MPRAGE sequence with the following parameters: TR/TE/TI = 2,000/2.15/1,100 ms, flip angle = 12°, FOV = 256 × 256 mm, slice thickness = 1.33 mm (along left-right direction), voxel size = 1.5 × 1.0 × 1.3 mm, total scan time = 9 min. ifcMRI scans were collected using a gradient echo, echo-planar sequence (TR: 2,500 ms, TE: 30 ms, flip angle: 82° degrees, matrix size: mm, 41 slices, voxel size: 3.5 × 3.5 × 2.5 mm3). UT Dallas: sMRI images were collected using a 3T Philips whole body scanner equipped with Quasar gradient subsystem (40 mT/m amplitude, a slew rate of 220 mT/m/ms). A 32-channel receive head phased array coil combined with body coil transmission to achieve greater sensitivity in cortical areas. sMRI scans utylized an MPRAGE sequence with the following parameters: TR/TE/TI = 2,100/3.70/1,100 ms, flip angle = 12°, FOV = 256 × 256 mm, slab thickness = 160 mm (along left-right direction), voxel size = 1 × 1 × 1 mm, total scan time = 3 min 57 s. fMRI scans were collected using a gradient echo, echo-planar sequence with the intercomissural line (AC-PC) as a reference (TR: 2.0 s, TE: 29 ms, flip angle: 75 degrees, matrix size: 64 × 64, 39 slices, voxel size: 3.44 × 3.44 × 3.5 mm3).
All images were preprocessed utilizing an identical pipeline, computing system, and software versions (no updates were conducted during data analysis) at UWM. Anatomical preprocessing utilized the CPAC analysis software for large multisite datasets (see: https://fcp-indi.github.io/), which utilized pre-existing imaging software, including AFNI (119), FSL (120), and ANTS (http://stnava.github.io/ANTs/). Data were deobliqued to align with X, Y, and Z coordinates; resampled to FSL friendly RPI anatomical convention; skull stripped; anatomical segmentation; and binarized threshold masks were created utilizing FSL's FAST; functional images were linearly registered to anatomical native space using FSL's FLIRT; anatomical images underwent non-linear transformation to MNI152 (voxel size = 2 mm3) standard brain template using ANTS. fMRI was also preprocessed using the CPAC software using the following steps: removal of the initial 5 time points to allow T1 stabilization; deoblique; resampling to RPI space; skull stripping; data was “scrubbed” using Framewise Displacement (121) with a maximum TR displacement set to 4 mm; image intensity normalization; linear and quadratic detrending to remove residual drift due to scanner heating and/or slower head movement; nuisance regression (white matter and cerebrospinal fluid) using 6 displacement and motion correction parameters using CompCor (applied prior to smoothing); spatial smoothing (Gaussian Kernal = 4 mm FWHM; Sigma = 2.54); and temporal filtering (Band Pass filter = 0.1–0.01 Hz). Frontolimbic ROI's. Cortical and subcortical ROI's were created using FreeSurfer's (122) cortical parcellation atlas [DKT40 atlas; (123)] and subcortical segmentation (124). ROI's included the bilateral rostral anterior cingulate (rACC), caudal anterior cingulate (cACC), ventral medial PFC (vmPFC), insula, and amygdala.
For each subject, the average time series was extracted for all aforementioned ROI's using the CPAC software. Next, the correlation coefficients for the time series were created using MATLAB (Version 8.0.0.783 64-bit maci64, 2012). Lastly, a series of standard multiple regressions were run to predict correlation coefficients between each set of ROIs; the primary predictor variable (cannabis group status), and covariates (past month nicotine use, past month alcohol use, MRI collection site, and duration of abstinence from cannabis prior to scan) were entered utilizing standard least squares multiple regression in SPSS (version 24). Specifically, the first block included all covariates (past month nicotine use, past month alcohol use, behavioral/MRI collection site, and duration of abstinence) and the second block included cannabis group status. False Discovery Rate correction [FDR; (125)] was implemented to correct for multiple comparisons. All correlation coefficients between ROIs were visually inspected for normality in distribution. Skewed distributions were transformed using a log10 transformation and used in the regression in place of the skewed correlation coefficients. There was no evidence of multicollinearity or homoscedasticity following inspection of the standardized residual for the variables of interest. Interpretations of statistical significance were made if p < 0.05. For ease of interpretation, regions with connectivity differences after correction for multiple comparisons were also displayed on an average template brain provided by BrainNet Viewer software [(126); see Figure 2 below].
Pearson r correlations were run between connectivity coefficients and total depressive symptoms among cannabis users (in regions predicted by cannabis use).
ANOVAs and χ2's tests examined whether cannabis users and controls differed in demographic variables (see Table 1). Cannabis users and controls did not differ in age [F(1, 157) = 1.1, p = 0.3], ethnicity group [64.6% Caucasian for cannabis users and 52.5% for controls, χ2 (1)2.4, p = 0.12], gender [46.8% female for cannabis users and 43.8% for controls, χ2 (1)0.15, p = 0.7], and premorbid intelligence [F(1, 156) = 0.46, p = 0.5].
As expected, cannabis users differed from controls in past month total grams [F(1, 157) = 91.1, p < 0.01], past month total days of cannabis use [F(1, 85) = 9,208.4, p < 0.01], past month total standard alcohol drinks [F(1, 157) = 20, p < 0.01], and past month total cigarettes [F(1, 157) = 7.3, p = 0.01]. The cannabis users were abstinent from cannabis for 12–24 h (27.8%); 2–3 days (39.2%); 4–7 days (5.1%); or 8 days or greater (27.8%).
Cannabis users reported significantly greater total BDI-II [F(1, 124) = 5.7, p = 0.02] scores compared to controls, although both groups' total BDI-II scores remained in the subclinical range.
After controlling for MRI collection site, past month alcohol and cigarette use (in standard units), and days abstinent from cannabis, cannabis users demonstrated significantly increased connectivity between left rACC and the following: right rACC [t(80) = 3.3, beta = 0.59, p = 0.001; FDR corrected p = 0.05; Cohen's f2 = 0.55], left amygdala [t(80) = 2.2, beta = 0.45, p = 0.03; FDR corrected p = 0.47; Cohen's f 2 = 0.17], left insula [t(80) = 2.2, beta = 0.45, p = 0.03; FDR corrected p = 0.47; Cohen's f 2 = 0.16]. There were no group differences where cannabis users demonstrated significant decreases in connectivity compared to controls (see Figure 1 for an image of the bilateral rACC).
Among cannabis users, greater bilateral rACC connectivity was significantly associated with greater total depressive symptoms [r = 0.29, n = 66, p = 0.02] (see Figure 2).
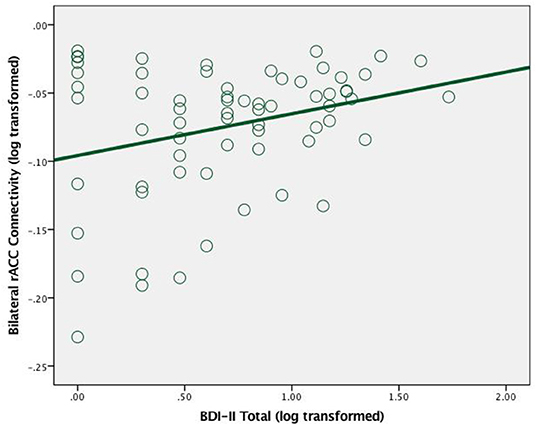
Figure 2. Scatterplot between total depression symptoms and bilateral rAcc connectivity (log transformed) in cannabis users.
The current study examined whether cannabis use was associated with frontolimbic intrinsic connectivity using a cross-sectional design in a sample devoid of independent Axis I anxiety or mood disorders. After controlling for MRI collection site, recent alcohol, and nicotine use, and abstinence from cannabis use, cannabis users demonstrated increased intrinsic connectivity between the left rACC and the following: left insula, left amygdala, and right rACC in comparison to controls, though only group differences between bilateral rACC survived after correcting for multiple comparisons. Further, we found that increased bilateral rACC connectivity was associated with greater subclinical depressive symptoms in cannabis users.
Current findings parallel previous intrinsic functional studies indicating frequent cannabis use among youth is associated with greater connectivity between frontal and temporal regions (83), and increased ACC connectivity in males (85). Resting state connectivity increases in comparison to controls was also reported within the medial frontal gyrus among a high-risk mostly male adolescent group (82). The present study adds to existing literature by including more females, controlling for other substance use and cannabis abstinence period, and relating the observed connectivity differences to mood-related symptoms. Task-based studies also report altered medial PFC activity associated with cannabis use among emerging adults (79, 127–134), suggesting chronic cannabis use is associated with region-specific changes in brain activity and connectivity among regions implicated in emotion regulation, identification, and modulation.
The current findings of abnormal functional connectivity in the rACC and limbic regions, which is consistent with our previous structural findings. Our team recently reported that greater cannabis use was related to reduced left rACC volume among young cannabis users, and smaller rACC volumes were also significantly associated with lower performance in an emotional discrimination task (94). Further, we also found reduced right ACC cortical thickness in a sample of young cannabis users, including a subset of cannabis users with a history of childhood attention deficit hyperactivity disorder, compared to non-using controls (93). The ACC undergoes significant developmental shifts in functional connectivity during young adulthood (74), has been implicated in ones' ability to detect and monitor self-produced errors (135, 136) whether one is conscious/aware of the error or not (137, 138). The ACC may be less engaged in cannabis users compared to controls during tasks requiring inhibitory control and error monitoring (131). The rostral subdivision of the ACC is functionally connected with the amygdala (139), forming a network for processing affective facets of behavior (140, 141). In concert with the insula, the ACC also serves to incorporate perceptual information with autonomic and emotional information (142). More specifically, the rACC has been posited to have top-down control influence, serving as a gatekeeper, between regions processing negative affective information and those integrating environmental stimuli [see (143, 144)], and demonstrates protracted development during young adulthood (74). The rACC is involved in implicit or automatic emotion regulation that occurs at a subconscious level (42). Indeed, lesions in the rACC are posited to impair ones' sensitivity to adjustments in personal performance during a cognitive control task (145). For example, cannabis users have demonstrated reduced P300 (attention to emotion) during implicit and empathic emotional processing paradigms, particularly for the highest using cannabis users that also demonstrated deficits in explicit processing of negative emotions (146). Thus, abnormalities in rACC structure and function may impact various behavioral aspects, including cognitive control and emotional regulation.
The current study suggests that chronic cannabis use may increase intrinsic connectivity between emotion regulation regions, which was opposite of our original hypothesis. A potential interpretation may include the inefficiency of prefrontal top-down regulation, as hypothesized by Behan et al. (84), suggesting reduced intrinsic amygdala responsiveness. Further, Pujol et al. (86) found reduced ACC and insula connectivity; however, the study did not examine subcomponents of the ACC and used seed-based rather than region of interest approaches. Thus, disruptions in rACC function may lead to challenges in modulating ones' mood, consistent with the current study findings, or adjusting to emotionally salient internal and external information. Indeed, we also found that increased depressive symptoms among cannabis users were associated with greater connectivity between the bilateral rACC. Alterations in rACC structure (147–150) and function [see (151–153)] have been previously linked with depressive and affective symptoms and antidepressant resonse (154). Though the current sample did not meet criteria for an Axis I mood or anxiety disorder, cannabis use may impact regions implicated in symptom manifestation. Although cannabis users reported significantly greater subclinical levels of depression, we are unable to determine whether the endorsed symptoms predated the initiation of cannabis use or whether the endorsed symptoms occurred during the course of regular cannabis use among users. Indeed, cross-sectional (8, 11–13) and longitudinal (5, 6, 13, 99, 155) studies among cannabis-using youth have found increased risk of mood and affective symptoms. Even casual cannabis using young adults report greater depressive symptomatology (156). Thus, structural and functional abnormalities within the rACC observed in cannabis users may result in mood dysregulation. Alternatively, subtle mood dysregulation may be a risk-factor for riskier cannabis use consumption.
Proposed theories accounting for these functional and behavioral differences in cannabis users may have multiple underlying etiologies. Chronic young adult cannabis users demonstrate abnormal CB1 receptor density in the ACC (51); thus, frequent cannabis use may influence continued white matter myelination and gray matter pruning within this region, impacting structural integrity (81, 91, 93, 157). Further, altering CB1 availability and eCB signaling may impact GABA and GLUT signaling, which is observed in the ACC of adolescents with chronic cannabis use (158, 159), suggesting continued cannabis use may impact healthy ACC functioning. Indeed, rACC glutamate levels have been associated with interactions between task-positive (supragenual ACC) and task-negative (perigenual ACC) subregions (160), suggesting excitatory activity at rest may alter one's ability to engage networks involved in environmental interaction. Thus, altered inhibitory eCB activity may account for changes in intrinsic ACC connectivity among users. It is also possible that abnormalities in rACC and increased symptoms of depression place adolescents and young adults at increased risk for regular cannabis use. Prospective longitudinal studies are needed to address causality.
In terms of youth treatment, there are potential interventions that may target ACC functioning to improve emotional regulation and mood in cannabis users. For example, activation within the ACC was associated with positive treatment outcomes following change talk among a diverse group of cannabis-using youth (161). Mindfulness-based mediation and a combination of mindfulness with aerobic exercise have also been associated with ACC specific changes [see (162)].
Findings from the current study should be considered in light of potential limitations. Although comorbid use of nicotine products was measured, some participants may have smoked cannabis with nicotine mixed in (e.g., blunts); this was not measured in the current study. Given the cross-sectional nature of the current study, potential differences in frontolimbic connectivity and subclinical mood symptoms may exist prior to the onset of frequent cannabis use and serve as a risk factor for regular cannabis use during adolescence (163, 164). The relationships between such factors and substance use patterns among youth have previously been investigated [see (165–175)]. Therefore, prospective, longitudinal studies are necessary to determine timing and causality.
In conclusion, the present multisite imaging study found that among otherwise healthy young adults devoid of independent mood or affective disorders, regular cannabis users had greater intrinsic connectivity between left and right rACC. The current study also found that greater intrinsic bilateral rACC connectivity was associated with greater subthreshold depressive symptoms among cannabis users. Results coincide and expand upon prior intrinsic and task-based imaging projects among young adults with chronic cannabis use, suggesting altered connectivity between regions with high cannabinoid receptor density that are imperative for emotional inhibition, recognition, and regulation. As THC content continues to rise (176–178), today's users may be at increased risk for elevated mood or anxiety symptoms (179–181). Considering these findings, it is recommended that youth delay regular use of cannabis until after peak brain maturation is achieved [see (182)]. In light of the current paper, cannabis interventions for youth may target improving anterior cingulate functioning, including aerobic exercise and mindfulness-based approaches [see (162, 183, 184)].
This study was carried out in accordance with the recommendations by the Institutional Review Board at each institution (UWM, McLean, and UTD). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
SS developed the aims and hypotheses, assisted with the design, pre-processed the fMRI scans, and conducted the analyses. AT assisted with the MRI preprocessing and data analyses. NW assisted with the IDEAA data management and merging, MRI pre-processing, and edited the manuscript. SG, ST, and FF were an IDEAA site PI, assisted with the design and data analysis, and edited the manuscript. KL was an IDEAA site PI, assisted with development of the aims and hypotheses, and supervised all MRI pre-processing, design, data analyses, and edited all versions of the manuscript.
This IDEAA consortium was supported by NIDA (R01 DA032646, PI: Gruber; R01 DA030354, PI: KL). ST's work was supported by R01 AA03419 and R01 DA021182. FF's work was supported by R01 DA042490, R01 DA030344, and R01 AA023658, and the Bert Moore Chair in BrainHealth.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. ^Cannabis concentrate usage was very low in the sample (n = 3); cannabis concentrates were converted into estimated equivalent grams of flower.
1. Kuddus M, Ginawi IM, Al-Hazimi A. Cannabis sativa: an ancient wild edible plant of India. Emirates J Food Agric. (2013) 25:736–45. doi: 10.9755/ejfa.v25i10.16400
2. Miech RA, Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use 1975-2016: Volume I, Secondary School Students. Ann Arbor, MI: Institute for Social Research, The University of Michigan (2017).
3. McGee R, Williams S, Poulton R, Moffitt T. A longitudinal study of cannabis use and mental health from adolescence to early adulthood. Addiction. (2000) 95:491–503. doi: 10.1046/j.1360-0443.2000.9544912.x
4. Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use, depression and anxiety among Australian adults: findings from the National Survey of Mental Health and Well-Being. Soc Psychiatry Psychiatr Epidemiol. (2001) 36:219–27. doi: 10.1007/s001270170052
5. Brook DW, Brook JS, Zhang C, Cohen P, Whiteman M. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch Gen Psychiatry. (2002) 59:1039–44. doi: 10.1001/archpsyc.59.11.1039
6. Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use andpsychosocial adjustment in adolescence and young adulthood. Addiction. (2002) 97:1123–35. doi: 10.1046/j.1360-0443.2002.00103.x
7. Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. (2002) 325:1195–8. doi: 10.1136/bmj.325.7374.1195
8. Rey JM, Sawyer MG, Raphael B, Patton GC, Lynskey M. Mentalhealth of teenagers who use cannabis: results of an Australian survey. Br J Psychiatry. (2002) 180:216–21. doi: 10.1192/bjp.180.3.216
9. Georgiades K, Boyle MH. Adolescent tobacco and cannabis use: young adult outcomes from the Ontario Child Health Study. J Child Psychol Psychiatry. (2007) 48:724–31. doi: 10.1111/j.1469-7610.2007.01740.x
10. Hayatbakhsh MR, Najman JM, Jamrozik K, Mamun AA, Alati R, Bor W. Cannabis and anxiety and depression in young adults: a large prospective study. J Am Acad Child Adolesc Psychiatry. (2007) 46:408–17. doi: 10.1097/chi.0b013e31802dc54d
11. Wittchen H, Fröhlich C, Behrendt S, Günther A, Rehm J, Zimmermann P, et al. Cannabis use and cannabis use disorders and their relationship to mental disorders: a 10-year prospective-longitudinal community study in adolescents. Drug Alcohol Dependence. (2007) 88:S60–70. doi: 10.1016/j.drugalcdep.2006.12.013
12. Fleming CB, Mason WA, Mazza JJ, Abbott RD, Catalano RF. Latent growth modeling of the relationship between depressive symptoms and substance use during adolescence. Psych Addict Behav. (2008) 22:186–97. doi: 10.1037/0893-164X.22.2.186
13. de Graaf R, Radovanovic M, van Laar M, Fairman B, Degenhardt L, Aguilar-Gaxiola S, et al. Early cannabis use and estimated risk of later onset depression spells: epidemiologic evidence from the population-based world health organization world mental health survey initiative. Am J Epidemiol. (2010) 172:149–59. doi: 10.1093/aje/kwq096
14. Degenhardt L, Coffey C, Romaniuk H, Swift W, Carlin JB, Hall WD, et al. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. (2013) 108:124–33. doi: 10.1111/j.1360-0443.2012.04015.x
15. Egerton A, Allison C, Brett RR, Pratt JA. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev. (2006) 30:680–95. doi: 10.1016/j.neubiorev.2005.12.002
16. Ellgren MM, Artmann AA, Tkalych OO, Gupta AA, Hansen HS, Hansen SH, et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. (2008) 18:826–34. doi: 10.1016/j.euroneuro.2008.06.009
17. Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. (2002) 54:161–202. doi: 10.1124/pr.54.2.161
18. Pacher P, Bátkai S, Kunos G. The endocannabinoids system as an emerging target of pharmacotherapy. Pharamcol Rev. (2006) 58:389–462. doi: 10.1124/pr.58.3.2
19. Mechoulam R, Peters M, Murillo-Rodriguez E, Hanuš L. Cannabidiol – recent advances. Chem Biodivers. (2007) 4:1678–92. doi: 10.1002/cbdv.200790147
20. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Ann Rev Psychol. (2013) 64:21–47. doi: 10.1146/annurev-psych-113011-143739
21. Ashton C H. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. (2001) 178:101–6. doi: 10.1192/bjp.178.2.101
22. Fišar Z. Phytocannabinoids and endocannabinoids. Curr Drug Abuse Rev. (2009) 2:51–75. doi: 10.2174/1874473710902010051
23. Howlett AC. The cannabinoid receptors. Prostagl Other Lipid Mediat. (2002) 68:619–31. doi: 10.1016/S0090-6980(02)00060-6
24. Moreira FA, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict Biol. (2008) 13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x
25. Solinas M, Goldberg SR, Piomeli D. The endocannabinoids system in brain reward processes. J Pharmacol. (2008) 154:369–83. doi: 10.1038/bjp.2008.130
26. Covey DP, Wenzel JM, Cheer JF. Cannabinoid modulation of drug reward and the implications of marijuana legalization. Brain Res. (2014) 1628(Pt A):233–43. doi: 10.1016/j.brainres.2014.11.034
27. Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. (2017) 43:155–72. doi: 10.1038/npp.2017.130
28. Witkin J, Tzavara E, Nomikos G. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. (2005) 16:315–31. doi: 10.1097/00008877-200509000-00005
29. McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev. (2014) 42:116–31. doi: 10.1016/j.neubiorev.2014.02.006
30. Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. (2002) 159:379–87. doi: 10.1007/s00213-001-0946-5
31. Ashton CH, Moore PB, Gallagher P, Young AH. Cannabinoids in bipolar affective disorder: a review and discussion of their therapeutic potential. J Psychopharmacol. (2005) 19:293–300. doi: 10.1177/0269881105051541
32. Hill M, Gorzalka B. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. (2005) 15:593–9. doi: 10.1016/j.euroneuro.2005.03.003
33. Adamczyk P, Golda A, McCreary A, Filip M, Przegalinski E. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J Physiol Pharmacol. (2008) 59:217–28. Available online at: http://www.jpp.krakow.pl/journal/archive/06_08/pdf/217_06_08_article.pdf
34. Hillard C, Weinlander K, Stuhr K. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. (2012) 204:207–29. doi: 10.1016/j.neuroscience.2011.11.020
35. Marco EM, Laviola G. The endocannabinoid system in the regulation of emotions throughout lifespan: a discussion on therapeutic perspectives. J Psychopharmacol. (2012) 26:150–63. doi: 10.1177/0269881111408459
36. Hell H, Jager G, Bossong M, Brouwer A, Jansma J, Zuurman L, et al. Involvement of the endocannabinoid system in reward processing in the human brain. Psychopharmacology. (2012) 219:981–90. doi: 10.1007/s00213-011-2428-8
37. Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat-and reward-related brain function. Biol Psychiatry. (2009) 66:9–16. doi: 10.1016/j.biopsych.2008.10.047
38. Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Witt H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. (2008) 28:2313–9. doi: 10.1523/JNEUROSCI.5603-07.2008
39. Pietrzak RH, Huang Y, Corsi-Travali S, Zheng M, Lin S, Henry S, et al. Cannabinoid type 1 receptor availability in the amygdala mediates threat processing in trauma survivors. Neuropsychopharmacology. (2014) 39:2519–28. doi: 10.1038/npp.2014.110
40. Patterson DW, Schmidt LA. Neuroanatomy of the human affective system. Brain Cognit. (2003) 52:24–6. doi: 10.1016/S0278-2626(03)00005-8
41. Pessoa L. A network model of the emotional brain. Trends Cogn. Sci. (2017) 21, 357–371. doi: 10.1016/j.tics.2017.03.002
42. Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. (2015) 16:693–700. doi: 10.1038/nrn4044
43. Frith CD, Frith U. Social cognition in humans. Curr Biol. (2007) 17:724–32. doi: 10.1016/j.cub.2007.05.068
44. Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain of emotion: a meta-analytic review. Behav Brain Sci. (2012) 35:121–42. doi: 10.1017/S0140525X11000446
45. Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. (1997) 77:299–318. doi: 10.1016/S0306-4522(96)00428-9
46. Mackie K. Distribution of cannabinoid receptors in the central and peripheralnervous system. Handbook Exp Pharmacol. (2005) 168:299–325. doi: 10.1007/3-540-26573-2_10
47. Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous 80 system structure—a short review. Pharmacol Biochem Behav. (2008) 90:501–11. doi: 10.1016/j.pbb.2008.05.010
48. Terry GE, Liow J, Zoghbi SS, Hirvonen J, Farris AG, Lerner A, et al. Quantitation of cannabinoid CB1 receptors in healthy human brain using positron emission tomography and an inverse agonist radioligand. Neuroimage. (2009) 48:362–70. doi: 10.1016/j.neuroimage.2009.06.059
49. Terry GE, Hirvonen J, Liow J-S, Zoghbi SS, Gladding R, Tauscher JT, et al. Imaging and quantitation of cannabinoid CB1 receptors in human and monkey brains using (18)F-labeled inverse agonist radioligands. J Nucl Med. (2010) 51:112–20. doi: 10.2967/jnumed.109.067074
50. Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. (2011) 65:278–86. doi: 10.1002/syn.20844
51. Hirvonen J, Goodwin RS, Li C, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. (2012) 17:642–9. doi: 10.1038/mp.2011.82
52. Ballard ME, Bedi G, de Wit H. Effects of delta-9-tetrahydrocannabinol on evaluation of emotional images. J Psychopharmacol. (2012) 26:1289–98. doi: 10.1177/0269881112446530
53. Bossong MG, van Hell HH, Jager G, Kahn RS, Ramsey NF, Jansma JM. The endocannabinoid system and emotional processing: a pharmacological fMRI study with Δ9-tetrahydrocannabinol. Eur Neuropsychopharmacol. (2013) 23:1687–97. doi: 10.1016/j.euroneuro.2013.06.009
54. Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ, et al. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomized, double-blind, 66 placebo-controlled study in cannabis users. Eur Neuropsychopharmacol. (2015) 25:325–34. doi: 10.1016/j.euroneuro.2014.11.014
55. Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, et al. Distinct effects of {delta}9-tetrahydrocannabinol and 62 cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. (2009) 66:95–105. doi: 10.1001/archgenpsychiatry.2008.519
56. Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, et al. Modulation of effective connectivity during emotional processing by delta 9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. (2010) 13:421–32. doi: 10.1017/S1461145709990617
57. Bhattacharyya S, Falkenberg I, Martin-Santos R, Atakan Z, Crippa JA, Giampietro V, et al. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology. (2015) 40:1343–52. doi: 10.1038/npp.2014.258
58. Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. Opposite effects of delta-9- tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. (2010) 35:764–74. doi: 10.1038/npp.2009.184
59. Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. (1997) 10:165–70. doi: 10.1002/(SICI)1099-1492(199706/08)10:4/5<165::AID-NBM454>3.0.CO;2-7
60. Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J Cogn Neurosci. (1997) 9:835–47. doi: 10.1162/jocn.1997.9.6.835
61. Joseph R. Environmental influences on neural plasticity, the limbic system, emotional development and attachment: a review. Child Psychiatry Hum Dev. (1999) 29:189–208. doi: 10.1023/A:1022660923605
62. Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. (2003) 20:420–8. doi: 10.1016/S1053-8119(03)00355-0
63. Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. (2005) 15:239–44. doi: 10.1016/j.conb.2005.03.012
64. Casey B, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. (2005) 9:104–10. doi: 10.1016/j.tics.2005.01.011
65. Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. (2006) 16:553–60. doi: 10.1093/cercor/bhj003
66. Choudhury S, Blakemore S, Charman T. Social cognitive development during adolescence. Soc Cogn Affect Neurosci. (2006) 1:165–74. doi: 10.1093/scan/nsl024
67. Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. (2007) 17:251–7. doi: 10.1016/j.conb.2007.03.009
68. Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. (2008) 28:62–77. doi: 10.1016/j.dr.2007.08.003
69. Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS. Functional differences in emotion processing during adolescence and early adulthood. Neuroimage. (2014) 91:70–6. doi: 10.1016/j.neuroimage.2014.01.035
70. Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. (2008) 9:267–77. doi: 10.1038/nrn2353
71. Braun K. The prefrontal-limbic system: development, neuroanatomy, function, and implications for socioemotional development. Clin Perinatol. (2011) 38:685–702. doi: 10.1016/j.clp.2011.08.013
72. Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. (2000) 11:43–8. doi: 10.1097/00001756-200001170-00009
73. Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Develop Neurosci. (2006) 1:1–8. doi: 10.1111/j.1467-7687.2005.00454.x
74. Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. (2008) 19:640–57. doi: 10.1093/cercor/bhn117
75. Platt B, Kamboj S, Morgan CJ, Curran HV. Processing dynamic facialaffect in frequent cannabis users: evidence of deficits in the speed of identifying emotional expressions. Drug Alcohol Depend. (2010) 112:27–32. doi: 10.1016/j.drugalcdep.2010.05.004
76. Huijbregts SC, Griffith-Lendering MF, Vollebergh WA, Swaab H. Neurocognitive moderation of associations between cannabis use and psychoneuroticism. J Clin Exp Neuropsychol. (2014) 36:794–805. doi: 10.1080/13803395.2014.943694
77. Bayrakci A, Sert E, Zorlu N, Erol A, Saricicek A, Mete L. Facial emotion recognition deficits in abstinent cannabis dependent patients. Compr Psychiatry. (2015) 58:160–4. doi: 10.1016/j.comppsych.2014.11.008
78. Hindocha C, Wollenberg O, Carter Leno V, Alvarez BO, Curran HV, Freeman TP. Emotional processing deficits in chronic cannabis use: a replication and extension. J Psychopharmacol. (2014) 28:466–71. doi: 10.1177/0269881114527359
79. Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective responsein marijuana smokers: An FMRI study. Drug Alcohol Depend. (2009) 105:139–53. doi: 10.1016/j.drugalcdep.2009.06.019
80. Cornelius JR, Aizenstein HJ, Hariri AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav. (2010) 35:644–6. doi: 10.1016/j.addbeh.2010.02.004
81. Spechler PA, Orr CA, Chaarani B, Kan KJ, Mackey S, et al. Cannabis use in early adolescence: evidence of amygdala hypersensitivity to signals of threat. Develop Cogn Neurosci. (2015) 16:63–70. doi: 10.1016/j.dcn.2015.08.007
82. Houck JM, Bryan AD, Ewing SF. Functional connectivity and cannabis use in high-risk adolescents. Am J Drug Alcohol Abuse. (2013) 39:414–23. doi: 10.3109/00952990.2013.837914
83. Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, et al. Altered resting-state connectivity in adolescent cannabis users. Am J Drug Alcohol Abuse. (2013) 39:372–81. doi: 10.3109/00952990.2013.848213
84. Behan B, Connolly CG, Datwani S, Doucet M, Ivanovic J, Morioka R, et al. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology. (2014) 84:131–7. doi: 10.1016/j.neuropharm.2013.05.027
85. Cheng H, Skosnik PD, Pruce BJ, Brumbaugh MS, Vollmer JM, Fridberg DJ, et al. Resting state functional magnetic resonance imaging reveals distinct brain activity in heavy cannabis users–a multi-voxel pattern analysis. J Psychopharmacol. (2014) 28:1030–40. doi: 10.1177/0269881114550354
86. Pujol J, Blanco-Hinoio L, Batalla A, López-Solà M, Harrison BJ, Soriano-Mas C, et al. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. (2014) 51:68–78. doi: 10.1016/j.jpsychires.2013.12.008
87. Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang T, Tapert SF. Prefrontal morphometry in abstinent adolescent marijuana users: Subtle gender effects. Addict Biol. (2009) 14:457–68. doi: 10.1111/j.1369-1600.2009.00166.x
88. McQueeny TM, Padula C, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. (2011) 224:128–34. doi: 10.1016/j.bbr.2011.05.031
89. Schepis TS, Desai RA, Cavallo DA, Smith AE, Mcfetridge A, Liss TB, et al. Gender differences in adolescent marijuana use and associated psychosocial characteristics. J Addict Med. (2011) 5:65–73. doi: 10.1097/ADM.0b013e3181d8dc62
90. Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc. (2012) 18:678–88. doi: 10.1017/S1355617712000276
91. Shollenbarger SG, Price J, Wieser J, Lisdahl KM. Impact of cannabis use on prefrontal and parietal cortex gyrification and surface area in adolescents and emerging adults. Develop Cogn Neurosci. (2015) 16:46–53. doi: 10.1016/j.dcn.2015.07.004
92. Price JS, McQueeny T, Shollenbarger SG, Browning EL, Wieser J, Lisdahl KM. Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacology. (2015) 232:2939–50. doi: 10.1007/s00213-015-3931-0
93. Lisdahl KM, Tamm L, Epstein JN, Jernigan T, Molina BSG, Hinshaw SP, et al. The impact of adhd persistence, recent cannabis use, and age of regular cannabis use onset on subcortical volume and cortical thickness in young adults. Drug Alcohol Depend. (2016) 161:135–46. doi: 10.1016/j.drugalcdep.2016.01.032
94. Maple KE, Thomas AM, Kangiser MM, Lisdahl KM. Anterior cingulate volume reductions in abstinent adolescent and young adult cannabis users: association with affective processing deficits. Psychiatry Res. (2019) 288:51–9. doi: 10.1016/j.pscychresns.2019.04.011
95. Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, et al. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res. (2011) 220:164–72. doi: 10.1016/j.bbr.2011.02.001
96. Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J. Neurosci. (2014) 34:5529–39. doi: 10.1523/JNEUROSCI.4745-13.2014
97. Schacht JP, Hutchison KE, Filbey FM. Associations between can- nabinoid receptor-1 (CNR1) variation and hippocampus and amyg- dala volumes in heavy cannabis users. Neuropsychopharmacology. (2012) 37:2368–76. doi: 10.1038/npp.2012.92
98. Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. (2010) 107:4734–9. doi: 10.1073/pnas.0911855107
99. Di X, Biswal BB. Dynamic brain functional connectivity modulated by resting-state networks. Brain Struct Funct. (2015) 220:37–46. doi: 10.1007/s00429-013-0634-3
100. Fox MD, Snyder AZ, Zacks JM, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. (2005) 102:9673–8. doi: 10.1073/pnas.0504136102
101. Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. (2005) 26:15–29. doi: 10.1002/hbm.20113
102. Marcus DS, Harms MP, Snyder AZ, Jenkinson M, Wilson JA, Glasser MF, et al. Human Connectome Project informatics: quality control, database services, and data visualization. Neuroimage. (2013) 80:202–19. doi: 10.1016/j.neuroimage.2013.05.077
103. Tomasi D, Volkow ND. Functional connectivity density mapping. Proc Natl Acad Sci USA. (2010) 107:9885–90. doi: 10.1073/pnas.1001414107
104. Yan C, Liu D, He Y, Zou Q, Zhu C, Zuo X, et al. Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS ONE. (2009) 4:5743. doi: 10.1371/journal.pone.0005743
105. Patriat R, Molloy EK, Meier TB, Kirk GR, Nair VA, Meyerand ME, et al. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed and fixated. Neuroimage. (2013) 78:463–73. doi: 10.1016/j.neuroimage.2013.04.013
106. Stonnington CM, Tan G, Kloppel S, Chu C, Draganski B, Jack CRJr, et al. Interpreting scan data acquired from multiple scanners: a study with Alzheimer's disease. Neuroimage. (2008) 39:1180–5. doi: 10.1016/j.neuroimage.2007.09.066
107. Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, et al. Network centrality in the human functional connectome. Cereb Cortex. (2012) 22:1862–75. doi: 10.1093/cercor/bhr269
108. Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through earlyadult- hood. Proc Natl Acad Sci USA. (2004) 101:8174–9. doi: 10.1073/pnas.0402680101
109. American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association.
110. Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behav Res Ther. (1979) 17:157–160. doi: 10.1016/0005-7967(79)90025-1
111. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation (1996). doi: 10.1037/t00742-000
112. Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the beck depression inventory-second edition in a sample of college students. Depress Anxiety. (2004) 19:187–9. doi: 10.1002/da.20002
113. Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psych Corp (1999).
114. Wilkinson G. Wide Range Achievement Test, (WRAT-4) Manual. 4th ed. Wilmington, DE: Wide Range, Inc. (2006). doi: 10.1037/t27160-000
115. Willshire DK, Kinsella G, Prior M. Estimating WAIS-R IQ from the national adult reading test: a cross-validation. J Clin Exp Neuropsychol. (1991) 13:204–16. doi: 10.1080/01688639108401038
116. Manly JJ, Jacobs DM, Touradji P. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J Int Neuropsychol Soc. (2002) 8:341–8. doi: 10.1017/S1355617702813157
117. Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. (2015) 105:536–51. doi: 10.1016/j.neuroimage.2014.10.044
118. Shirer WR, Jiang H, Price CM, Ng B, Greicius MD. Optimization of rs-fMRI Pre-processing for enhanced signal-noise separation, test-retest reliability, and group discrimination. Neuroimage. (2015) 117:67–79. doi: 10.1016/j.neuroimage.2015.05.015
119. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comp Biomed Res. (1996) 29:162–73. doi: 10.1006/cbmr.1996.0014
120. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. (2004) 23:208–19. doi: 10.1016/j.neuroimage.2004.07.051
121. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. (2012) 59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018
122. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. (1999) 9:179–94. doi: 10.1006/nimg.1998.0395
123. Klein A, Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front Neursci. (2012) 6:171. doi: 10.3389/fnins.2012.00171
124. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. (2002) 33:341–55. doi: 10.,1016/S0896-6273(02)00569-X
125. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
126. Xia M, Wang J, He Y. BraiNet viewer: a network visualization tool for human brain connectomics. PLoS ONE. (2013) 8:e68910. doi: 10.1371/journal.pone.0068910
127. Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. (2004) 23:914–20. doi: 10.1016/j.neuroimage.2004.07.032
128. Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorder. Drug Alcohol Depend. (2005) 79:201–10. doi: 10.1016/j.drugalcdep.2005.01.009
129. Cheng L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. (2006) 129:1096–112. doi: 10.1093/brain/awl064
130. Tapert S, Schweinsburg A, Drummond S, Paulus M, Brown S, Yang T, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. (2007) 194:173–83. doi: 10.1007/s00213-007-0823-y
131. Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. (2009) 34:2450–8. doi: 10.1038/npp.2009.67
132. Schweinsburg AD, Schweinsburg BC, Lisdahl Medina K, McQueeny T, Brown SA, Tapert SF. The influenct of recency of use on fMRI reponse during spatial working memory in adolesecent marijuana users. J Psychoactive Drugs. (2010) 42:401–12. doi: 10.1080/02791072.2010.10400703
133. Wesley MJ, Hanlon CA, Porrino LJ. Poor decition-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. (2011) 191:51–9. doi: 10.1016/j.pscychresns.2010.10.002
134. Sneider JT, Gruber SA, Rogowska J, Silveri MM, Yurgelun-Todd DA. A preliminary study of functional brain activation among marijuana users during performance of a virtual water maze task. J. Addict. (2013) 2013:461029. doi: 10.1155/2013/461029
135. Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. (2001) 38:752–60. doi: 10.1111/1469-8986.3850752
136. Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. Neuroimage. (2007) 34:1774–81. doi: 10.1016/j.neuroimage.2006.11.014
137. Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. (2005) 27:602–8. doi: 10.1016/j.neuroimage.2005.04.035
138. O'Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, et al. The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. Eur J Neurosci. (2007) 25:2571–9. doi: 10.1111/j.1460-9568.2007.05477.x
139. Beckman M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. (2009) 29:1175–90. doi: 10.1523/JNEUROSCI.3328-08.2009
140. Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. (1995) 118(Pt 1): 279–306. doi: 10.1093/brain/118.1.279
141. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. (2000) 4:215–22. doi: 10.1016/S1364-6613(00)01483-2
142. Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. (2007) 27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007
143. Cooney RE, Joormann J, Atlas LY, Eugène F, Gotlib IH. Remembering the good times: neural correlates of affect regulation. Neuroreport. (2007) 18:1771–4. doi: 10.1097/WNR.0b013e3282f16db4
144. Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. (2011) 15:85–93. doi: 10.1016/j.tics.2010.11.004
145. Di Pellegrino G, Ciaramelli E, Làdavas E. The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J Cogn Neurosci. (2007) 19:275–86. doi: 10.1162/jocn.2007.19.2.275
146. Troup LJ, Bastidas S, Nguyen MT, Andrzejewski JA, Bowers M, Nomi JS. An event-related potential study on the effects of cannabis on emotion processing. PLoS ONE. (2016) 11:e0149764.
147. Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. (2002) 51:342–4. doi: 10.1016/S0006-3223(01)01280-X
148. Hajek T, Kozeny J, Kopecek M, Alda M, Höschl C. Reduced subgenual cingulate volumes in mood disorders: a meta-analysis. J Psychiatry Neurosci. (2008) 33:91–9.
149. Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. (2007) 62:429–37. doi: 10.1016/j.biopsych.2006.09.020
150. Mayberg H, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. (2005) 45:651–60. doi: 10.1016/j.neuron.2005.02.014
151. Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. (2009) 13:663–81. doi: 10.1017/S1092852900013754
152. Strikwerda-Brown C, Davey CG, Whittle S, Allen NB, Byrne ML, Schwartz OS, et al. Mapping the relationship between subgenual cingulate cortex functional connectivity and depressive symptoms across adolescence. Soc Cogn Affect Neurosci. (2015) 10:961–8. doi: 10.1093/scan/nsu143
153. Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. (2007) 164:1476–88. doi: 10.1176/appi.ajp.2007.07030504
154. Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. (2001) 158:405–15. doi: 10.1176/appi.ajp.158.3.405
155. Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. (2007) 370:319–28. doi: 10.1016/S0140-6736(07)61162-3
156. Troup LJ, Andrzejewski JA, Braunwalder JT, Torrence RD. The relationship between cannabis use and measures of anxiety and depression in a sample of college campus cannabis users and non-users post state legalization in Colorado. PeerJ. (2016) 4:e2782. doi: 10.7717/peerj.2782
157. Shollenbarger SG, Price J, Wieser J, Lisdahl KM. Poorer frontolimbic white matter integrity is associated with chronic cannabis use, FAAH genotype, and increased depressive and apathy symptoms in adolescents and young adults. Neuroimage Clin. (2015) 8:117–25. doi: 10.1016/j.nicl.2015.03.024
158. Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. Neuroimage.(2011) 57:69–75 doi: 10.1016/j.neuroimage.2011.02.044
159. Prescot AP, Renshaw PF, Yurgelun-Todd DA. γ-amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug Alcohol Depend. (2013) 129:232–9. doi: 10.1016/j.drugalcdep.2013.02.028
160. Duncan NW, Enzi B, Wiebking C, Northoff G. Involvement of glutamate in rest-stimulus interaction between perigenual and supragenual anterior cingulate cortex: a combined fMRI-MRS study. Hum Brain Mapp. (2011) 32:2172–82. doi: 10.1002/hbm.21179
161. Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: evaluating adolescetns' response to a cannabis intervention. Psychol Addict Behav. (2013) 27:510–25. doi: 10.1037/a0029767
162. Paulus MP, Stewart JL, Haase L. Treatment approaches for interoceptive dysfunctions in drug addiction. Front Psychiatry. (2013) 4:137. doi: 10.3389/fpsyt.2013.00137
163. Boys A, Marsden J, Strang J. Understanding reasons for drug use amongst young people: a functional perspective. Health Educ Res. (2001) 16:457–69. doi: 10.1093/her/16.4.457
164. McCarty CA, Rhew IC, Murowchick E, McCauley E, Stoep AV. Emotional health predictors of substance use initiation during middle school. Psychol Add Behav. (2012) 26:351–7. doi: 10.1037/a0025630
165. Lopez B, Wang W, Schwartz SJ, Prado G, Huang S, Brown CH, et al. School, family, and peer factors and their association with substance use in hispanic adolescents. J Prim Prevent. (2009) 30:622–41. doi: 10.1007/s10935-009-0197-5
166. Prado G, Huang S, Schwartz SJ, Maldonado-Molina MM, Bandiera FC, de la Rosa M, et al. What accounts for differences in substance use among U.S.-born and immigrant hispanic adolescents?: results from a longitudinal prospective cohort study. J Adolesc Health. (2009) 45:118–25. doi: 10.1016/j.jadohealth.2008.12.011
167. Connell CM, Gilreath TD, Aklin WM, Brex RA. Social-ecological influences on patterns of substance use among non-metropolitan high school students. Am J Commun Psychol. (2010) 45:36–48. doi: 10.1007/s10464-009-9289-x
168. Kiesner J, Poulin F, Dishion TJ. Adolescent substance use with friends: moderating and mediating effects of parental monitoring and peer activity contexts. Merrill Palmer Q. (2010) 56:529–56. doi: 10.1353/mpq.2010.0002
169. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Substance Use. (2011) 16:150–60. doi: 10.3109/14659891.2010.519421
170. Karriker-Jaffe KJ. Areas of disadvantage: a systematic review of effects of area-level socioeconomic status on substance use outcomes. Drug Alcohol Rev. (2011) 30:84–95. doi: 10.1111/j.1465-3362.2010.00191.x
171. Van Ryzin MJ, Fosco GM, Dishion TJ. Family and peer predictors of substance use from early adolescence to early adulthood: an 11-year prospective analysis. Addict Behav. (2012) 37:1314–24. doi: 10.1016/j.addbeh.2012.06.020
172. Eisenberg ME, Toumbourou JW, Catalano RF, Hemphill SA. Social norms in the development of adolescent substance use: a longitudinal analysis of the international youth development study. J Youth Adolesc. (2014) 43:1486–97. doi: 10.1007/s10964-014-0111-1
173. Sitnick S, Shaw DS, Hyde L. Precursors of adolescent substance use from early childhood and early adolescence: testing a developmental cascade model. Develop Psychopathol. (2014) 26:125–40. doi: 10.1017/S0954579413000539
174. Unger JB. Cultural influences on substance use among hispanic adolescents and young adults: findings from project RED. Child Develop Perspect. (2014) 8:48–53. doi: 10.1111/cdep.12060
175. Bacio GA, Estrada Y, Huang S, Martínez M, Sardinas K, Prado G. Ecodevelopmental predictors of early initiation of alcohol, tobacco, and drug use among hispanic adolescents. J Sch Psychol. (2015) 53:195–208. doi: 10.1016/j.jsp.2015.02.001
176. Burgdorf JR, Kilmer B, Pacula RL. Heterogeneity in the composition of marijuana seized in California. Drug Alcohol Depend. (2011) 117:59–61. doi: 10.1016/j.drugalcdep.2010.11.031
177. ElSohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BFIII. Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980-1997. J Forensic Sci. (2000) 45:24–30. doi: 10.1520/JFS14636J
178. Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. (2010) 55:1209–17. doi: 10.1111/j.1556-4029.2010.01441.x
179. Gage S, Hickman M, Heron J, Munafò MR, Lewis G, Macleod J, et al. Associations of cannabis and cigarette use with depression and anxiety at age 18: findings from the Avon Longitudinal study of parents and children. PLoS ONE. (2015) 10:e0122896. doi: 10.1371/journal.pone.0122896
180. Kedzior KK, Laeber LT. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population- a meta-analysis of 31 studies. BMC Psychiatry. (2014) 14:1–39. doi: 10.1186/1471-244X-14-136
181. Chadwick B, Miller ML, Hurd YL. Cannabis use during adolescent development: suspectibility to psychiatric illness. Front Psychiatry. (2013) 4:129. doi: 10.3389/fpsyt.2013.00129
182. Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger SG. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. (2013) 4:53. doi: 10.3389/fpsyt.2013.00053
183. Lisdahl KM, Wright NE, Medina-Kirchner C, Maple KE, Shollenbarger S. Considering cannabis: the effects of regular cannabis use on neurocognition in adolescents and young adults. Curr Addict Rep. (2014) 1:144–56. doi: 10.1007/s40429-014-0019-6
Keywords: cannabis, resting state fMRI, young adults, adolescents, affective symptoms, depressive symptoms, connectivity analysis
Citation: Shollenbarger S, Thomas AM, Wade NE, Gruber SA, Tapert SF, Filbey FM and Lisdahl KM (2019) Intrinsic Frontolimbic Connectivity and Mood Symptoms in Young Adult Cannabis Users. Front. Public Health 7:311. doi: 10.3389/fpubh.2019.00311
Received: 16 April 2018; Accepted: 14 October 2019;
Published: 01 November 2019.
Edited by:
Blair Henry, Sunnybrook Health Science Centre, CanadaReviewed by:
Susan Elizabeth Esposito, Life University, United StatesCopyright © 2019 Shollenbarger, Thomas, Wade, Gruber, Tapert, Filbey and Lisdahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krista M. Lisdahl, a3Jpc3RhLm1lZGluYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.