- 1Department of Social Medicine and Public Health, Faculty of Public Health, Medical University of Plovdiv, Plovdiv, Bulgaria
- 2Institute for Rare Diseases, Plovdiv, Bulgaria
Objective: The aim of this study was to assess the attitudes and opinions on the potential use of whole-genome sequencing (WGS) in conjunction with the traditional newborn screening (NBS). We conducted an online survey among pediatricians and geneticists from Bulgaria. The study was based on the concept of non-selective WGS for all newborns and analysis of all genes.
Results/conclusion: In total, 120 out of 299 invited participants completed the survey, with an overall response rate of 40.1%. While half of the pediatricians surveyed supported population-based non-selective WGS in NBS, 65.2% of the geneticists expressed concerns. Most participants underlined that ethical issues were as important as medical ones and called for a stricter protection of affected individuals against any abuse of their personal data. Extensive genetic counseling and psychological support to families were mentioned as key elements in this potential activity. Nevertheless, both pediatricians and geneticists considered that NBS in Bulgaria could be further developed, with selective WGS being suggested as a potential option. While non-selective WGS for all newborns is not currently perceived as feasible, pediatricians and geneticists do believe that selective WGS could strengthen current NBS programs. Cross-border project collaborations may set the stage for generating experience and evidence on these complex issues.
Introduction
Newborn screening (NBS) is a public health service aimed at detecting apparently healthy infants with severe congenital disorders, for which there are available cost-effective identification and effective treatment. NBS is considered to be the longest running and most successful population screening activity worldwide (1). Many inborn conditions are potential candidates for NBS. World Health Organization’s Wilson–Jungner criteria are regarded as a benchmark that disorders need to meet in order to be included in an NBS panel. These indicators cover a range of issues, including knowledge on the disease, treatment, test validity, and costs (2).
Whole-genome sequencing (WGS) is viewed as a major vehicle for translating genetic and genomic advances into population health gains. The debate as to whether to include WGS in NBS is currently taking place in many jurisdictions (3–5). There are, however, considerable concerns among health-care practitioners, including limited benefit of WGS for most healthy people in the general population, lack of expertise among non-genetic health-care providers, potential negative implications for society and scarce economic resources (6–8). It is clear that there is a gap between what is technically possible and the clinical services available. The rate at which new disease genes are being identified is out-pacing the ability of medical professionals and health authorities to assess the potential risks and benefits of introducing WGS in NBS (9).
In Bulgaria, NBS started in 1979 and included testing for phenylketonuria and galactosemia. The latter was removed from the panel in 1989 due to lack of effectiveness. A pilot NBS for congenital hypothyroidism was carried out in 1993–1999 as a joint Swiss-Bulgarian research project. The current NBS panel in Bulgaria includes phenylketonuria, congenital hypothyroidism, and congenital adrenal hyperplasia, and it is mandated by law and publicly funded (10, 11).
Objective
The purpose of this study was to assess the attitudes and opinions of pediatricians and geneticists on the potential use of WGS for NBS in Bulgaria.
Materials and Methods
Definitions
This study is based on the concept of non-selective WGS for all newborns and analysis of all genes. This definition of WGS was presented to all study participants and included in the subsequent discussion of the results.
Study Design
Scope and format of the study were based on previous experiences by Ulm et al. and Joseph et al., as well as on the outcomes of an evaluation study of NBS for rare disorders in the Member States of the European Union (EU) in 2012 (5, 12, 13). The survey questionnaire consisted of 20 questions. Each question contained a free text field for providing additional information, if desired. Before starting the questionnaire, participants were given brief definitions of WGS and NBS. The questionnaire was piloted among a small group of medical professionals for input regarding clarity. The study was conducted through an online survey in March–April, 2017 in Bulgaria.
Ethical approval was not required for this study in accordance with the national and institutional guidelines. The survey was sociological from a methodological point of view, with no clinical research. No personal data were saved or analyzed.
Study Participants
Study participants included geneticists and pediatricians from Bulgaria. Selection of these target groups was based on the state-of-art of NBS in Bulgaria, as well as the perspectives of implementing WGS in the country. These medical professionals are currently involved in NBS and in the subsequent diagnostic confirmation, treatment, and follow-up of detected cases. Geneticists and pediatricians would be required to be actively engaged if WGS was incorporated into NBS in Bulgaria.
A convenient sample of participants was recruited from the membership of the Bulgarian Society of Human Genetics and Genomics and the Bulgarian Pediatric Association with publicly available email addresses. A total of 299 individuals were contacted by email to participate in the survey with an invitation letter describing the study. No incentives for participation were provided.
Data Analysis
Descriptive statistics were applied. Comparisons were made between different demographic variables to determine if they were associated with specific outcomes. Analyses specifically focused on the differences between pediatricians and geneticists. Chi-square and Fisher’s exact tests, as well as Mann–Whitney U-test were used to compare these two groups. Statistical significance was considered if the p-value was less than 0.05. Data were analyzed using SPSS (version 11.5; SPSS, Inc., Chicago, IL, USA).
Results
Profile of Survey Respondents
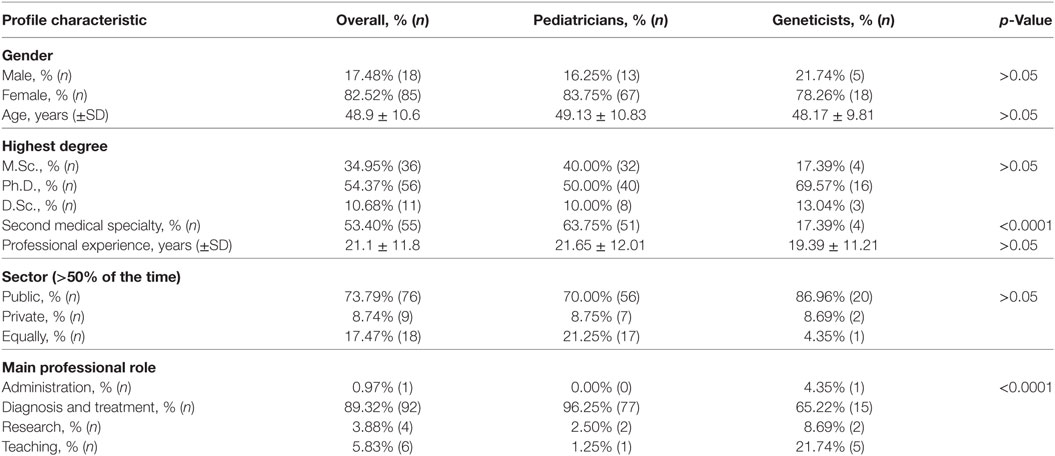
In total, 120 out of 299 invited participants completed the survey, with an overall response rate of 40.1%. Seventeen respondents indicated no medical specialty, so they were excluded from further analysis. Mean professional experience of the participants was 21 years with 54.4% of them having a Ph.D. degree (Table 1). The majority (89.3%) indicated a clinical role in a primary professional position, with 80 having a specialty of pediatrics (general pediatrics and/or profiled pediatrics) and 23 a specialty of genetics. Half of the respondents (53.4%) reported having a second specialty. Fifty-one pediatricians had a second, profiled specialty, as did four geneticists, who had a second specialty of pediatrics. Nevertheless, the latter were classified as geneticists for the purpose of cross-comparison. The groups of pediatricians and geneticists were found to be similar in terms of sex, age and professional experience (Table 1).
Knowledge and Awareness of NBS and WGS
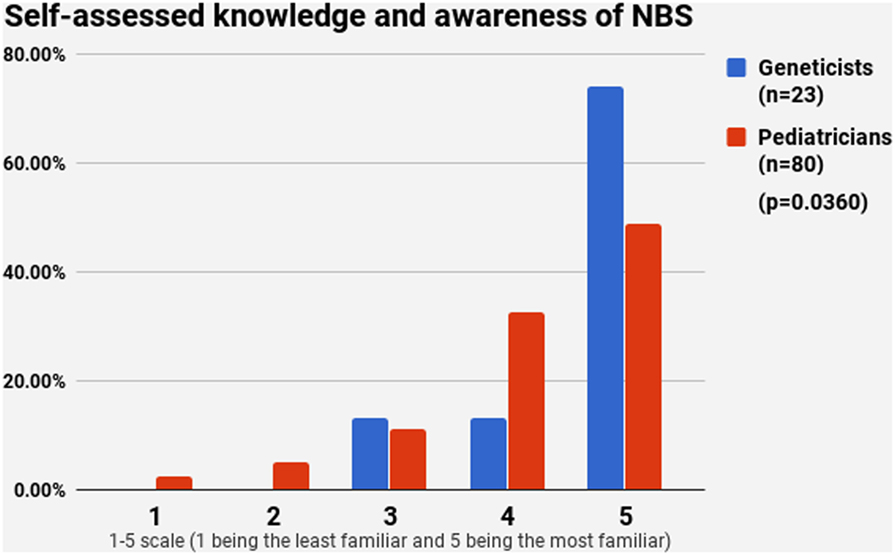
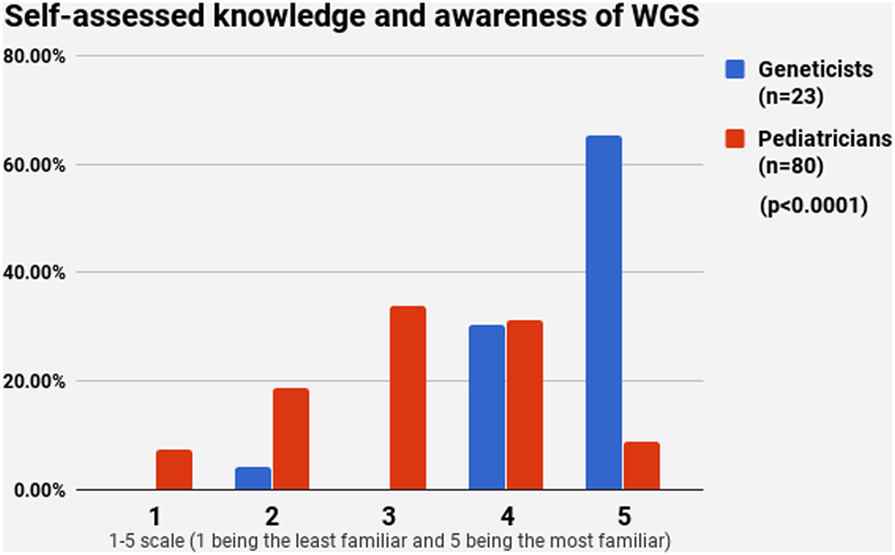
Half of the participants (54.4%) indicated a maximum level of knowledge on NBS on a self-assessment scale, while only 21.4% reported highest level of awareness on WGS. The difference between pediatricians and geneticists was statistically signficant in both cases (Figures 1 and 2). This was especially pronounced in regard to WGS, with 15 out of 23 geneticists declaring highest level of knowledge in comparison to only 7 out of 80 pediatricians. The split in self-assessed knowledge of NBS and WGS reflected on the question whether WGS could be implemented as an adjunct to NBS in Bulgaria (Figure 3). Whereas 25.0% (n = 20) of the pediatricians responded, they could not decide about this issue, all geneticists clearly indicated their opinion here. Only 34.8% (n = 8) of them supported the feasibility of WGS in NBS, while the rest opposed it (65.2%, n = 15). On the other side, pediatricians demonstrated enthusiasm, with 51.2% (n = 41) supporting the idea versus 23.8% (n = 19) being against. However, this disagreement was not statistically significant (p > 0.05).
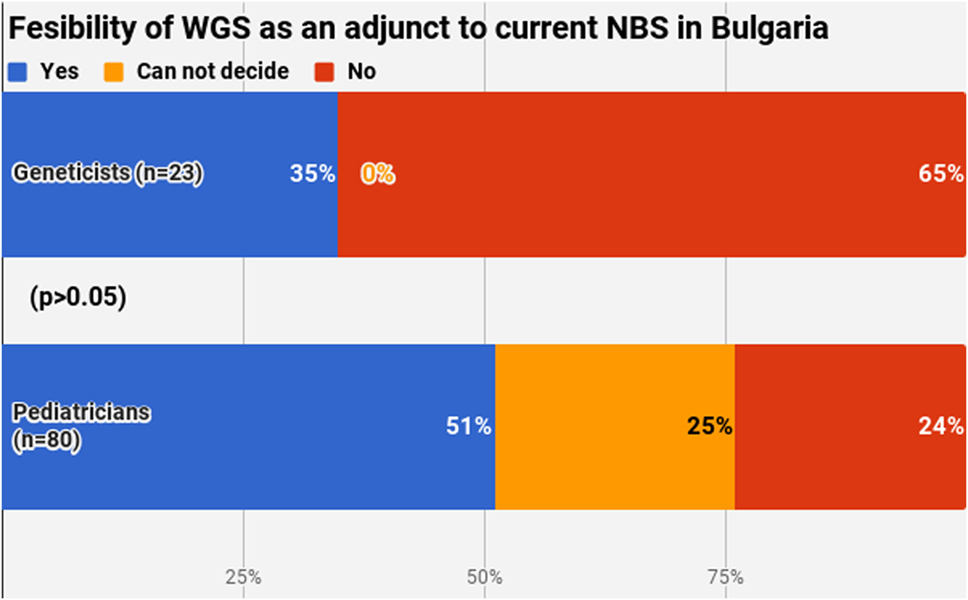
Figure 3. Fesibility of whole-genome sequencing as an adjunct to current newborn screening in Bulgaria.
Geneticists used the free text field to further point out their concerns (Table 2). The fact that most of the WGS data could not be directly linked to a specific role for the individual’s phenotype and development, thus generating lots of uncertainty and ambiguity, was emphasized. A large number of both groups raised the ethical issues that may occur, including the possibility of WGS data being used to the detriment of the individual. While high costs were rarely discussed, many participants mentioned that at this moment no country routinely used WGS as a part of NBS. Nevertheless, both pediatricians and geneticists considered that NBS in Bulgaria could be further developed, with selective WGS being suggested as a potential option by a large number of respondents.
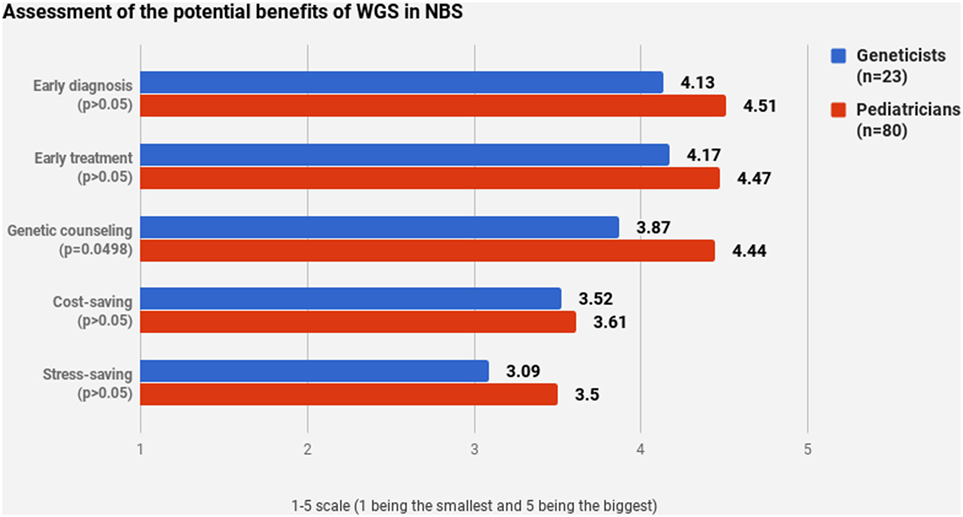
Pediatricians and geneticists largely agreed on their assessment of WGS’s potential benefits (Figures 4 and 5). WGS was seen as especially helpful for early diagnosis and treatment. Nevertheless, a stable scoring pattern was observed with pediatricians giving higher ratings than geneticists. The same outcome was observed when respondents were asked to evaluate WGS’s overall benefits in specific cases. The two groups agreed on the benefits of WGS in scenarios that are medically actionable, specifically for detection of treatable childhood-onset and treatable adult-onset conditions, as well as carriers and genetic markers. Statistically significant disagreement occurred in cases that are not medically actionable—childhood-onset non-treatable (p = 0.0427) and adult-onset non-treatable (p = 0.0038) conditions, as well as unknown phenotypes (p < 0.0001) (Figure 5).
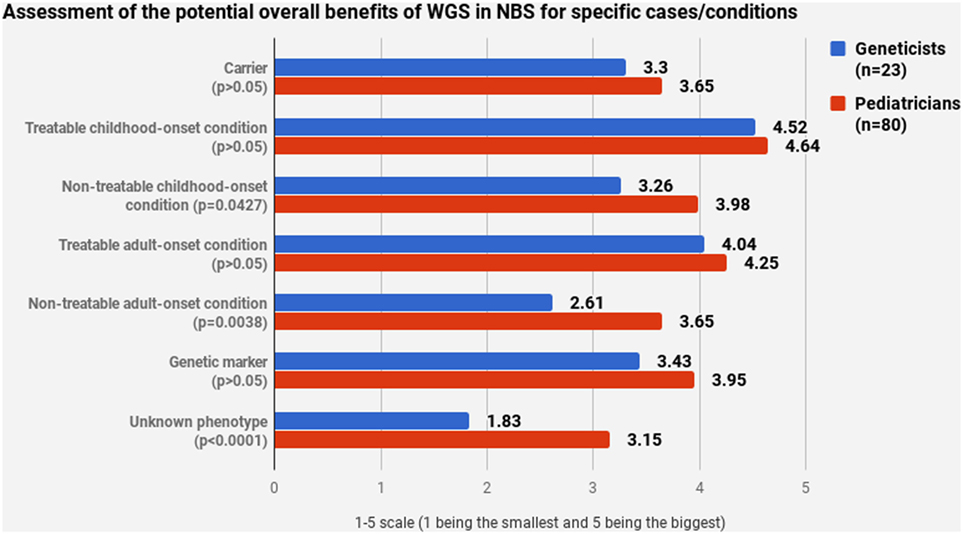
Figure 5. Assessment of the potential overall benefits of whole-genome sequencing in newborn screening for specific cases/conditions.
Regulatory Settings and Organizational Issues
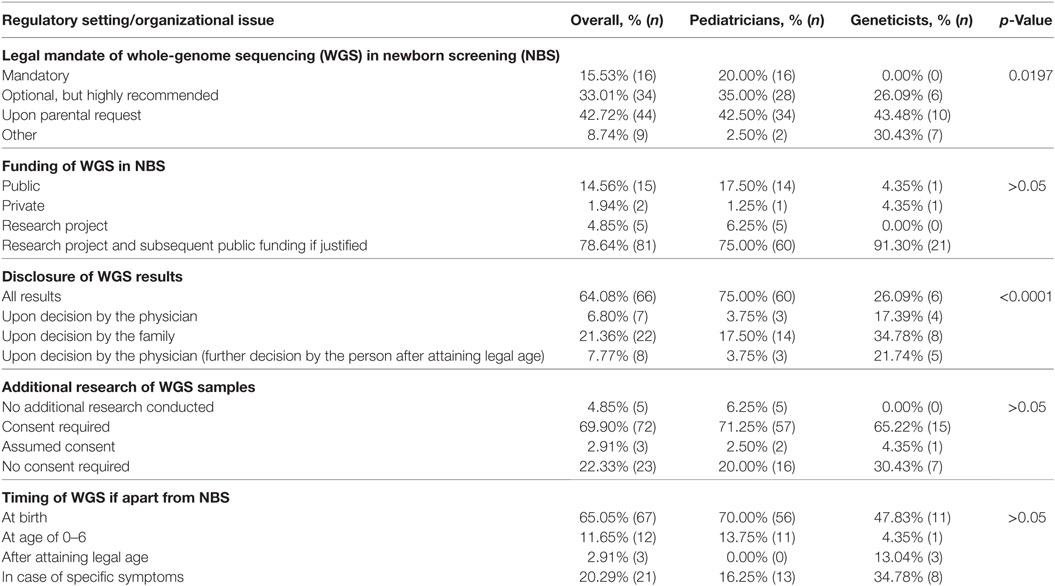
Study participants were asked about their opinion on regulatory settings and organizational issues if WGS is hypothetically introduced as a part of NBS in Bulgaria. Not a single geneticist supported the idea of making WGS mandatory, while 16 pediatricians expressed support (Table 3). Across both groups, 44 respondents (42.7%) believed that WGS should not be mandatory and must be available only upon parental request. While the majority of pediatricians indicated that WGS should be a mandatory or highly recommended service in this hypothetical case, most geneticists supported its use only upon request or in the presence of specific symptoms (p = 0.0197). This position was further reflected in the free text comments (Table 2). A geneticist pointed out that neither the projected high costs nor the inability to correctly interpret all WGS results are the main obstacle. It was the impact on the individual affected and his/her legitimate right not to know what genetic information he/she is carrying. The respondent stressed that WGS in NBS is effectively taking this power away from the person with no clear benefits in return.
A strong consensus was observed on the funding issues. Eighty-one respondents (78.6%) agreed that WGS should be initially funded through research projects and subsequently by public funds if substantial public health benefits are demonstrated (Table 3). There was no clear consensus among pediatricians and geneticists regarding the disclosure of results. While 75.5% (n = 60) of pediatricians believed that all WGS results should be disclosed, no option was supported by a majority of geneticists (p < 0.0001). Only 26.0% (n = 6) of them indicated that all results should be disclosed. Furthermore, many of the geneticists surveyed explained in the free text field that this process should be a result of extensive genetic counseling (Table 2). With regards to the use of WGS data for additional research purposes, a general consensus was reached, as 69.9% (n = 72) declared that additional research was only appropriate if informed consent was given. In case of WGS being implemented apart from NBS in Bulgaria, this activity was found to be most appropriate to conduct at birth, 65.0% (n = 67), and in case of specific symptoms, 20.4% (n = 21), with 70.0% of pediatricians and 47.8% of geneticists supporting the first option (p > 0.05).
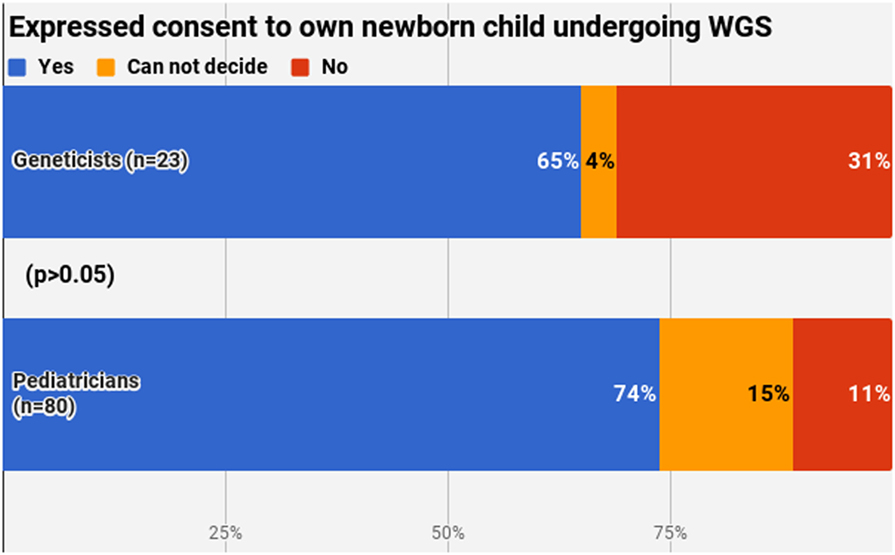
The final section of the questionnaire aimed to describe the personal attitude of the respondents. A clear majority (71.8%, n = 74) would consent to their own newborn undergoing WGS, with no significant difference between pediatricians and geneticists (p > 0.05) (Figure 6). Most common reasons for yes included the benefits of this procedure and WGS seen as the future of medicine. Most common reasons for no were the absence of a clear need for this service and the possible ethical issues arising.
Discussion
General Attitudes on WGS Implementation Alongside NBS
There is an ongoing debate regarding the role of WGS in NBS, but large-scale research activities, such as the USA’s Precision Medicine Initiative and the UK’s 100,000 genomes project, could lead to a reconsideration of WGS in all fields of medicine and health care. Respondents in our study admitted that WGS in NBS is hard to imagine in a small country with limited resources like Bulgaria, before this activity becomes commonly accepted and routinely applied in bigger jurisdictions. A geneticist commented that he/she does not see WGS in NBS being implemented in Bulgaria in the next 10 years. Nevertheless, all participants agreed that such a survey is needed in order to set up the stage for further research, as well as to dismiss uncertainty and speculations. As the issue of WGS in NBS is going to be raised more and more, the insights from our study could be highly beneficial at regional and European levels.
Previous studies extensively presented geneticists’, genetic counselors’, and parents’ perspectives, as well as general public views on WGS in NBS (5, 12, 14–17). To our best knowledge, our survey is the first to explore and cross-compare opinions of pediatricians and geneticists. This fills a substantial knowledge gap. There is no doubt that geneticists have a leading role in WGS. However, when evaluating the prospects of WGS in conjunction with NBS, pediatricians represent an important stakeholder whose views must be considered and taken into account. The opinion of pediatricians is crucial, given the fact that they would be exclusively involved in treatment and follow-up of all potential patients.
The demonstrated lack of clear consensus on whether WGS should be incorporated into NBS illustrates that there is a need for discussion and collaboration among all stakeholders before any major changes are being implemented. A recent study indicated that the vast majority of the American geneticists feel that WGS should not be currently used in NBS, and that if it to be used, it should not be mandatory (12). This attitude was greatly shared by geneticists from our sample. Pediatricians, however, had a rather different point of view. It is unclear if this difference is a result of the different level of expertise in WGS or it is because of the unique role of pediatricians in the treatment and follow-up of children. In our study, this specific group of respondents saw WGS benefits more positively in cases of non-treatable conditions than geneticists. So, a desire to manage the at-risk children might have been the basis for the discriminatory viewpoint.
Crucial Points for Consideration of WGS Implementation Alongside NBS
Ethical issues were the most commonly cited concerns by geneticists and pediatricians in our study. While participants greatly believed WGS and precise medicine are the future, a considerable number expressed disquiet about a number of potential risks, including discrimination, psychological stress, and invasion of privacy. One respondent stated his/her full support for the progress of medicine, but explicitly underlined that, as a person, he/she strongly favors patients’ right of autonomy and parents’ power not to consent even if WGS is made part of NBS. Bioethicists have been long exploring the friction between the new genetic and genomic technologies and the principle of respect for autonomy. As these services are becoming more and more routine, this demands a rethinking of traditional interpretations of the concept of informed consent (18). WGS implementation will trigger a more individualized and differentiated set-up for counseling and informed consent. Greater involvement of parents will require development of targeted educational strategies and outreach messages (17).
Potential discrimination was raised as a problem by our survey participants. One geneticist alerted to such dangers and the negative impact on societal acceptance and overall trust in health systems. A number of patient advocates have expressed concerns that clinical use of genetic technologies may reinforce and perpetuate stigmatization and discrimination in both medical and social contexts (19). A pediatrician from our research even pointed out stricter regulations as a prerequisite for a WGS application in NBS. In reality, ethical considerations are likely to dominate this debate, as WGS costs continue to reduce. Economic issues were barely mentioned as a crucial point for WGS implementation in NBS in Bulgaria.
Nevertheless, costs would be a major concern in any country intending to implement WGS use in NBS. Researchers recently evaluated the budget impact of WGS in Germany for all newborns and for diagnostic investigation of tumor patients in different oncologic indications. WGS in NBS was found to lead to costs of € 2.85 billion (an increase of total expenditure by 1.41%). Potential savings in terms of reduced costs for follow-up and improved cost-effectiveness of treatment were not quantified due to the fact that such estimations should be subject of indication-specific evaluations. Even so, this study concluded that WGS has the potential to generate a substantial number of deterministic findings for which treatment options are limited. The authors recommended the selective use of WGS, namely focusing on indications for which WGS has proven medical evidence, and thus saving public health resources (20).
Prospects for Selective WGS Screening
While there is significant number of objections about non-selective WGS for all newborns, selective WGS screening has been almost unanimously endorsed by previous studies. Cost analysis demonstrates that this procedure could be cost-effective and result in improvements in expectancy and quality of life for affected infants (12). Most recently, experts in genetics, pediatrics, public health, and health policy issued a joint statement, containing a set of recommendations to help inform and guide scientists and clinicians, as well as policy makers regarding the necessary considerations for the use of genome sequencing technologies in NBS. It was stressed that the main objective of NBS should be the targeted analysis and identification of gene variants conferring a high risk of preventable or treatable conditions, for which treatment has to start in the newborn period or in early childhood (21). Any change in the goals of NBS programs should be discussed carefully and represent the best interests of the child (3, 22). Opinions expressed by our study’s participants were consistent with those NBS policy benchmarks. Moreover, both geneticists and pediatricians supported expanding NBS with selective WGS for specific disorders, provided that broad societal consensus is reached and project funding is allocated.
Selective WGS embraces the same concept as NBS: to be predictable, preventive, and personalized. This activity will represent a step-increase in NBS, leading to opportunities for early detection, better management, and effective treatment throughout someone’s life, including late-onset disorders (23). Improved diagnostic capacity is of paramount importance in fields like rare diseases for example. Selective WGS in NBS; this could eliminate the need for an otherwise expensive diagnostic odyssey, by substantially decreasing extra referrals and psychological stress for patients and caregivers (12).
In our study, respondents suggested project funding and cross-border partnerships as optimal settings for launching selective WGS in NBS. European reference networks (ERNs) for rare diseases, which started in 2017, are a recent illustration of such collaborations (24, 25). ERNs are offering, in fact, a unique chance for advanced genetic and genomic approaches. These networks possess sufficient infrastructure and resources to initiate pilot projects to generate evidence on feasibility, efficiency, and cost-effectiveness of selective WGS in NBS. In turn, selective WGS screening could boost significantly rare disease diagnostic capacity at both EU and national levels. As congenital genetic disorders place a disproportionally high burden on families and health systems, selective WGS in NBS could have a major impact in medical practice and public health.
Conclusion
While non-selective WGS for all newborns is not currently perceived as feasible, pediatricians and geneticists do believe that selective WGS could strengthen current NBS programs. Cross-border project collaborations may set the stage for generating experience and evidence on these complex issues. Further research, including multi-stakeholder partnerships among pediatricians, geneticists, and patients should help guide the development of health policy and practice regarding this concept.
Ethics Statement
Approval by an Ethics committee was not required for this study. The survey was sociological from a methodological point of view, with no clinical research. No personal data were saved or analyzed.
Author Contributions
GI and SI contributed to the conception and design of the study, recruitment of survey respondents, analyzing the data, and writing the draft. SW contributed to the conception and design of the study, analyzing the data, and writing the draft. RS contributed to the conception and design of the study, analyzing the data and critical review of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The study was conducted as a research project within the Public Health Club of the Department of Social Medicine and Public Health at the Medical University of Plovdiv, Bulgaria. The Club was led by RS and GI and formed by the following medicine students (in alphabethical order): Asya Mihaylova, Ivan Atanasov, SI, Stefan Stefanov, SW, and Veselina Kanova. The authors would like to thank Prof. Jack Goldblatt for the critical review of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/article/10.3389/fpubh.2017.00308/full#supplementary-material.
References
1. Jansen ME, Lister KJ, van Kranen HJ, Cornel MC. Policy making in newborn screening needs a structured and transparent approach. Front Public Health (2017) 5:53. doi:10.3389/fpubh.2017.00053
2. Pourfarzam M, Zadhoush F. Newborn screening for inherited metabolic disorders; news and views. J Res Med Sci (2013) 18:801–8.
3. Friedman JM, Cornel MC, Goldenberg AJ, Lister KJ, Sénécal K, Vears DF, et al. Genomic newborn screening: public health policy considerations and recommendations. BMC Med Genomics (2017) 10:9. doi:10.1186/s12920-017-0247-4
4. De Laet C, Laeremans H, Ferster A, Gulbis B, Mansbach AL, Jonniaux E, et al. Newborn screening: the point of view of the pediatrician. Rev Med Brux (2015) 36:212–8.
5. Joseph G, Chen F, Harris-Wai J, Puck JM, Young C, Koenig BA. Parental views on expanded newborn screening using whole-genome sequencing. Pediatrics (2016) 137:S36–46. doi:10.1542/peds.2015-3731H
6. Janssens S, Chokoshvili D, Vears D, De Paepe A, Borry P. Attitudes of European geneticists regarding expanded carrier screening. J Obstet Gynecol Neonatal Nurs (2017) 46:63–71. doi:10.1016/j.jogn.2016.08.012
7. Botkin JR, Rothwell E. Whole genome sequencing and newborn screening. Curr Genet Med Rep (2016) 4:1–6. doi:10.1007/s40142-016-0084-3
8. Rego S. Newborn screening in the genomics era. J Law Biosci (2014) 1:369–77. doi:10.1093/jlb/lsu027
9. Andermann A, Blancquaert I, Beauchamp S, Déry V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bull World Health Organ (2008) 86:317–9. doi:10.2471/BLT.07.050112
10. Kremensky I. Genetic Screening and Diagnostics [DSc dissertation]. Sofia, Bulgarian: Medical University of Sofia (2006).
11. Stoeva I. Current status of endocrine screening programs in Bulgaria. Second National Conference for Rare Diseases and Orphan Drugs: Proceedings. Plovdiv, Bulgaria (2011). p. 16–21.
12. Ulm E, Feero WG, Dineen R, Charrow J, Wicklund C. Genetics professionals’ opinions of whole-genome sequencing in the newborn period. J Genet Couns (2015) 24:452–63. doi:10.1007/s10897-014-9779-3
13. Burgard P, Cornel M, Di Filippo F, Haege G, Hoffmann GF, Lindner M, et al. Report on the Practices of Newborn Screening for Rare Disorders Implemented in Member States of the European Union, Candidate, Potential Candidate and EFTA Countries. Luxembourg: European Commission (2012).
14. Kerruish N. Parents’ experiences 12 years after newborn screening for genetic susceptibility to type 1 diabetes and their attitudes to whole-genome sequencing in newborns. Genet Med (2016) 18:249–58. doi:10.1038/gim.2015.73
15. Nardini MD, Matthews AL, McCandless SE, Baumanis L, Goldenberg AJ. Genomic counseling in the newborn period: experiences and views of genetic counselors. J Genet Couns (2014) 23:506–15. doi:10.1007/s10897-014-9706-7
16. Bombard Y, Miller FA, Hayeems RZ, Barg C, Cressman C, Carroll JC, et al. Public views on participating in newborn screening using genome sequencing. Eur J Hum Genet (2014) 22:1248–54. doi:10.1038/ejhg.2014.22
17. Goldenberg AJ, Dodson DS, Davis MM, Tarini BA. Parents’ interest in whole-genome sequencing of newborns. Genet Med (2014) 16:78–84. doi:10.1038/gim.2013.76
18. Bunnik EM, de Jong A, Nijsingh N, de Wert GM. The new genetics and informed consent: differentiating choice to preserve autonomy. Bioethics (2013) 27:348–55. doi:10.1111/bioe.12030
19. Deem MJ. Whole-genome sequencing and disability in the NICU: exploring practical and ethical challenges. Pediatrics (2016) 137:S47–55. doi:10.1542/peds.2015-3731I
20. Plöthner M, Frank M, Graf von der Schulenburg JM. Whole-genome sequencing in German clinical practice: economic impacts of its use in selected areas of application. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz (2017) 60:143–50. doi:10.1007/s00103-016-2492-7
21. Howard HC, Knoppers BM, Cornel MC, Wright Clayton E, Sénécal K, Borry P, et al. Whole-genome sequencing in newborn screening? A statement on the continued importance of targeted approaches in newborn screening programs. Eur J Hum Genet (2015) 23:1593–600. doi:10.1038/ejhg.2014.289
22. Knoppers BM, Sénécal K, Borry P, Avard D. Whole-genome sequencing in newborn screening programs. Sci Transl Med (2014) 6:229cm2. doi:10.1126/scitranslmed.3008494
23. Francescatto L, Katsanis N. Newborn screening and the era of medical genomics. Semin Perinatol (2015) 39:617–22. doi:10.1053/j.semperi.2015.09.010
24. European Commission. Questions and Answers about European Reference Networks. (2017). Available from: http://europa.eu/rapid/press-release_MEMO-17-324_en.htm
Keywords: newborn screening, genetic screening, whole-genome sequencing, public health, rare diseases, European reference networks
Citation: Iskrov G, Ivanov S, Wrenn S and Stefanov R (2017) Whole-Genome Sequencing in Newborn Screening—Attitudes and Opinions of Bulgarian Pediatricians and Geneticists. Front. Public Health 5:308. doi: 10.3389/fpubh.2017.00308
Received: 06 August 2017; Accepted: 03 November 2017;
Published: 20 November 2017
Edited by:
Carmencita David Padilla, University of the Philippines Manila, PhilippinesReviewed by:
Meow-Keong Thong, University of Malaya, MalaysiaMichele Lloyd-Puryear, American College of Medical Genetics and Genomics, United States
Copyright: © 2017 Iskrov, Ivanov, Wrenn and Stefanov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgi Iskrov, aXNrcm92QHJhcmVkaXMub3Jn
 Georgi Iskrov
Georgi Iskrov Stefan Ivanov1
Stefan Ivanov1 Stephen Wrenn
Stephen Wrenn Rumen Stefanov
Rumen Stefanov