- 1Department of Infectious Diseases, Chongqing Public Health Medical Center, Chongqing, China
- 2College of Public Health, Chongqing Medical University, Chongqing, China
Cumulative evidence indicates that compared to HIV negative individuals, people living with HIV (PLWH) have a higher likelihood of developing depression, anxiety, and cognitive disorders. Depression, which is known to be a persistent and overwhelming feeling of sadness accompanied by a loss of interest in usual activities, is one of the most common mental illnesses encountered during HIV infection. Experts believe that several factors such as neuroinflammation, life stressors, lack of sleep, poor nutritional state, opportunistic infections and comorbidities, and HIV medications are contributing factors favoring the development of depression in PLWH. However, the fundamental mechanisms which underlie the involvement of these factors in the emergence of depression in the context of HIV remain poorly explored. Past researches describing the role of one or two of the preceding factors do exist; however, very few articles tackle this important topic while considering the several different putative causative factors comprehensively in the particular context of HIV infection. Herein, we elaborate on the factors currently understood to be responsible for the development of depression, and discuss the particular fundamental mechanisms whereby each factor may result in the outcome of depression. We believe that the understanding of these factors and of their underlying mechanisms is essential for the development of future therapeutic interventions to alleviate the burden of depression commonly seen in PLWH, and therefore facilitate the development of strategies to improve their overall quality of life.
1 Introduction
Depression, also referred to as major depressive disorder or clinical depression, is a mood disorder that causes a persistent feeling of sadness and a loss of interest in usual activities. It is characterized by low mood, diminished self-worth, pessimistic thoughts, poor concentration, somatic symptoms (such as poor appetite, somnipathy, and fatigue), and increased withdrawal from social activities (1). Depression has the potential to lead to personal suffering, decreased productivity, increased health care costs, and a high risk of suicide. According to the World Health Organization (WHO), depression is the most prevalent psychiatric disorder in the world, with a global prevalence of 4% in 2017 (2). It is estimated that at least one in six people suffers from depression at some point in their lives (3), and according to the WHO, cases of anxiety and depression worldwide have increased by more than 25% as a direct consequence of the COVID-19 pandemic (4).
Depression is the most common comorbidity and neuropsychiatric complication in people living with HIV (PLWH) (5). This is due to the long-term effects of HIV infection itself (particularly the underlying chronic inflammatory state) (6) and the adverse effects of modern antiretroviral therapy (ART) on the central nervous system (CNS) (7), notwithstanding the fact that the widespread use of modern ART has significantly improved the survival and quality of life of PLWH (8). Past studies (9, 10) have shown that the prevalence of depression among PLWH exceeds that in the general population by a factor of three, thus further decreasing the already deteriorated overall quality of life of PLWH, and further augmenting the pre-existing psychological toll of the almost ubiquitous social rejection and stigmatization that this category of patient has had to endure. In one systematic review, Niu et al., analyzed 94 studies concerning the mental health of PLWH in China between 1998 and 2014, and observed that depression was highly prevalent in Chinese PLWH, with prevalence exceeding 60% in most included studies (11). In a more recent publication, the same team emphasized the need for improved screening of newly diagnosed HIV-positive individuals, as at least 10% of these individuals have persistent depression (12). It has been reported that the tandem of HIV-associated co-morbidity with depression often leads to longer-lasting depression and more severe depressive symptoms, such as a greater degree of mental stress and low self-esteem, decreased appetite, poor sleep quality, and a higher prevalence of Major Depressive Disorder (MDD) (13). In another systematic review, Lofgren et al., pooled 70 research articles from 16 African countries for analysis, and observed that the prevalence of MDD in Sub-Saharan PLWH was 15.3% (14). Notably, it was believed that the prevalence of depression in PLWH differs between high-income and low-income countries. Indeed, wealthy countries like Canada and the US have prevalence rates of 20-40% (15, 16). Differences in the observed prevalence of depression between wealthy countries and low-income countries (such as those in Sub-Saharan Africa) may be a consequence of their under-representation in the literature, poor access to care, lower awareness of depression, and the stigma associated with depression, amongst other factors (17–19). Otherwise, prevalence rates of between 20-40% have been reported in countries such as Cameroon (26.7%) (20) and Ethiopia (36.65%) (21).
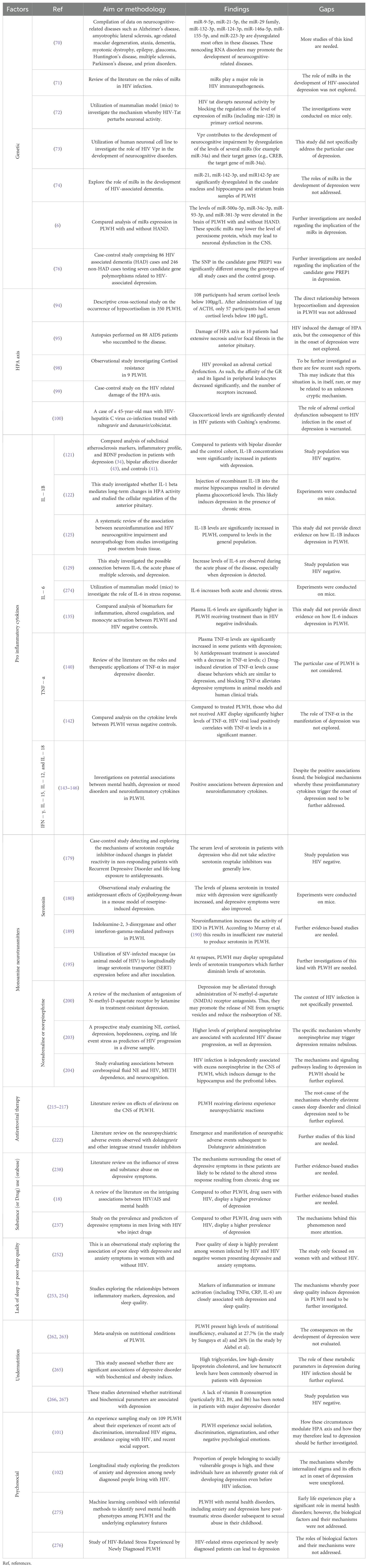
It is generally accepted that the etiology of depression is associated with many factors, and includes genetic factors, neuroendocrine factors, neuroimmune factors, and changes in concentrations of neurotransmitters (22). Up until now, several hypotheses, such as the hypothalamus-pituitary-adrenal (HPA) hypothesis (23), the cytokine hypothesis (24), and the monoamine neurotransmitter hypothesis (25) have all been proposed as the basis for the pathogenesis of depression. However, it is worth noting that the pathogenic mechanisms underlying the emergence of depression in the context of HIV infection remains sparsely researched, as this investigational realm is, and has been, challenging as a consequence of the diverse cultural considerations that need to be addressed in the assessment of depression, and also the diverse variety of investigational methods and tools used for its diagnosis (Table 1). These factors may certainly represent etiological clues; however, it is generally accepted that additional factors and the specific anatomical states induced by HIV infection, such as the persistent underlying chronic inflammation (particularly in the brain, and consequences on neuroendocrine factors, neuroimmune factors, cytokine and neurotransmitter expression, etc.), HIV medication, the lack of sleep, poor nutrition, etc., may be considered as further etiological elements as well. In this article, we provide an extensive review of factors favoring the emergence of depression (as reported in contemporary literature), and the manner in which the association of these factors with HIV infection materially increases the likelihood for the onset of depression in PLWH.
2 Genetic factors
2.1 Evidence
Although depression is not a hereditary disease, it does possess a genetic tendency. In 2000, one meta-analysis analyzed five twin family studies and observed that depression has a family aggregation, and a heritability of 37% (62). The quality of the analyzed studies was robust; however, the potential impact of the environment on individuals included in each study was not considered. In 2006, a twin study with a very large sample size was conducted in Sweden (63). Through personal interviews with 42,161 individual twins (including 15,493 complete pairs), the prevalence of depression was assessed by utilizing the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) diagnostic criteria. Their results indicated that the heritability of depression was 38%. Subsequently in 2014, a family study (64), which included 447 probands and 2082 of their relatives, reached a similar conclusion, i.e., that major depression exhibits a family aggregation (OR=2.26; 1.58-3.22; h2 = 0.20). Tansey et al. (65), estimated the contribution of common genetic variations to antidepressant response using Genome-Wide Complex Trait Analysis in a combined sample of 2799 antidepressant-treated subjects with major depressive disorder and genome-wide genotypic data. Their observation was that common genetic variants explain 42% of individual differences in antidepressant response (SE=0.180, p=0.009). The observations of the preceding studies thus confirm the familial aggregation of depression; however, from the perspective of HIV infection, this question remains to be further clarified. In order to obtain a clearer picture with respect to the specific case of PLWH with depression, we believe that a concise review of the contemporary literature related to genome-wide association studies (GWAS) and genome wide transcriptomic analysis is necessary.
2.2 Genome-wide transcriptomic analysis and GWAS in HIV-associated depression
It is known that HIV infection can be transmitted vertically from mother to child (66); however, this does not mean that HIV is heritable, nor that HIV is a genetic disease, as vertical transmission does not involve alterations to genetic material. Therefore, it is not logical or appropriate to search for relationships between HIV and depression on the basis of classical genetics. The emergence of the field of epigenetics thus provides us with a novel and exciting avenue from which to approach this subject. The preceding field of study, by definition, describes heritable changes in gene expression that are independent of changes in the regulated DNA sequence itself. Although DNA methylation and histone modification are two mechanisms that mediate epigenetic modification, noncoding microRNAs (miRs) are thought to be associated with epigenetic modification in two ways (67). Thus, miRs may induce DNA methylation either by interacting with the messenger RNA (mRNA) [miR-165 and miR-166 interact with PHABULOSA (PHB) mRNA to alter the chromatin of the template PHB gene (68)] or by targeting key DNA methylation enzymes (examples: DNMT1, 3a, and 3b) (69). In one systematic analysis by Juzwik et al., it was proposed that noncoding RNA disorders may promote the development of neurocognitive-related diseases, and especially that miRs affect transcription by stabilizing mRNA and preventing translation (70). In the particular case of HIV infection, our group has reported that miRs play a major role in HIV immunopathogenesis (by increasing or decreasing HIV replication) (71), and thus a closer look at the role of miRs in the development of HIV-associated depression is warranted.
Genome-wide transcriptomic analytic techniques, such as microarrays and RNA-seq, can identify changes in miRs and other noncoding RNAs. However, only a few studies have used human brain samples to study changes in miRs during HIV infection. The earliest research related to this can be traced back to 2008, when Eletto et al., using mice with HIV encephalopathy, identified a mechanism whereby HIV tat disrupts neuronal activity i.e., blocks the regulation of the level of expression of miRs (including mir-128) in primary cortical neurons (72). During this period, Mukerjee et al., using the Western blot technique and immunohistochemical analysis, observed that HIV-1 viral protein R (Vpr) plays an important role in neuronal dysfunction by regulating the level (upregulation or downregulation) of several miRs (for example miR-34a was found to be upregulated) and their specific target genes (e.g., CREB, the target gene of miR-34a), which may lead to the development of neurocognitive impairment (73). Yelamanchili et al., observed that miR-21, miR-142-3p, and miR142-5p are significantly dysregulated in the caudate nucleus and hippocampus and striatum brain samples of PLWH, and in SIV-infected rhesus monkeys (74). Subsequently, Xu et al., observed 17 significantly altered miRNAs in the brain tissues of patients with HIV-1 associated neurocognitive disorder (HAND), and compared them with miRNA samples from HIV positive individuals without HAND (6). Their results indicated the levels of miR-500a-5p, miR-34c-3p, miR-93-3p, and miR-381-3p were not only elevated in the brain of patients with HAND, but are also elevated in PLWH who do not have HAND (6). The expression of these specific miRNAs in PLWH will lower the level of peroxisome protein, which may lead to neuronal dysfunction in the CNS as demonstrated by a previous research team (75).
In addition to the study of epigenetics, candidate gene study and GWAS also point out another direction from which to study HIV-associated neurological diseases. This focuses mainly on the research of SNPs and genes related to the mutation of genes associated with the immune system. One case-control study comprising 86 HIV-associated depression cases and 246 non-HIV-associated depression cases tested seven candidate gene polymorphisms related to HIV-associated depression. Results from the study indicate that the CCR5 wt/Δ32 genotype is related to the duration of disease; however, the SNP in the candidate gene PREP1 was significantly different among the genotypes of all study cases and the control group (76). Interestingly, recent research shows that CCR5-Δ32 mutations may lead to the failure of HIV-1 gp120 to effectively bind to CCR5, thus preventing HIV from entering host cells (77). There are also a number of candidate gene studies (78) which focus on the genetic variations of TNFα and dopaminergic genes. However, it seems that these only play a role in HIV-associated neurocognitive disorders and HIV-associated dementia. Moreover, the sample populations in these studies were not specifically diagnosed with HIV-associated depression as a primary diagnosis. However, it is known that dopamine and TNFα levels also play important roles in the occurrence and development of depression. It is regrettable that only a small number of GWAS in HIV-related fields focus on HAND, and thus far GWAS related to HIV-associated depression have not, as yet, been published.
3 Neuroendocrine factors
3.1 The HPA axis and the HPA hypothesis for depression
Other than genetic factors, neuroendocrine factors should also be considered in the etiology of depression. Indeed, the HPA axis, which consists of the hypothalamus, the pituitary, and the adrenal glands [which regulates the production of glucocorticoids (GC)], has been implicated in the pathophysiology of anxiety and depression, as well as in the everyday processes of normal cognitive functioning (79). The HPA hypothesis suggests that depression is caused by hyperactivity of the HPA axis. When depression occurs in an individual, the sensitivity of the glucocorticoid receptor (GR) is impaired, which leads to an inhibition of the negative feedback mechanism regulating corticotropin-releasing hormone (CRH) secretion, which consequently leads to excessive central secretion of CRH, and thus increased circulating GC (80). It is worth mentioning that the sensitivity of the GR is basically regulated by the FKBP5 gene, encoding the FKBP51 gene, a co-chaperone of heat-shock protein 90 (hsp90). When FKBP51 binds to the GR, the affinity of glucocorticoid binding decreases, and the efficiency of GR transport to the nucleus decreases. FKBP5 mRNA and protein expression are induced by GR activation, and provide a negative feedback loop for GR sensitivity (81). At a cellular level, these genetic variations, combined with epigenetic changes, may explain structural and functional changes in several brain regions (82). When the body is in a state of excessive activation of the HPA axis, the likelihood of suffering from depression will also be substantially increased, because under normal conditions, lower GC levels interact with cortical and limbic structures (such as the prefrontal cortex and the hippocampus) to promote cognitive function and emotional processing. However, extensive research has shown that during the imbalance of homeostasis seen in chronic stress, a series of neurobiochemical changes (such as excessive GC production and hyperactivation of GR) result in reduced neurogenesis and neuroplasticity, especially in the hippocampus (83, 84). Thus, radiographic studies have shown that morphological changes occur in the hippocampus of patients with depression, and that the extent of hippocampal change positively correlates with the clinical course of depression (85).
3.2 The HPA axis in PLWH
HIV infection causes dysfunction in multiple somatic systems, including in the HPA axis. AIDS/HIV reduces host immune activity and alters cellular biological pathways through HIV encoded proteins, directly and indirectly affecting the HPA axis via the ‘stress’ represented by, amongst others, the ever-present opportunistic infections associated with the chronic immunodeficient state present in these patients, and due to the various adverse effects of the therapeutic compounds used to treat patients with HIV infection and AIDS (86).
From a pathophysiological perspective, the hypothalamus, the pituitary gland, and the adrenal gland all develop functional damage in PLWH. Firstly, the adrenal glands are often adversely affected in PLWH, and these patients often experience symptoms such as weakness, fatigue, and weight loss, which are symptoms that are similar to those seen in patients with chronic adrenal insufficiency (87). It is known that during early HIV infection, adrenal stimulation with adrenocorticotropic hormone (ACTH) induces an inadequate response in 14% of PLWH. The proportion with an inadequate GC response is further reduced in AIDS patients (54%) (87–89), suggesting progressive damage of the adrenal glands which is initiated at the early stage of HIV infection, and which further deteriorates during the AIDS stage. This is further corroborated at autopsy, where the adrenal glands are often found to be damaged in HIV/AIDS patients, mainly due to infections by opportunistic pathogens (e.g., Cytomegalovirus, Mycobacterium, Cryptococcus. Toxoplasmosis, and Pneumocystis) rather than by HIV itself, and which is facilitated by the profound immune deficiency ubiquitously present in PLWH at the AIDS stage of HIV infection (90). These opportunistic infections may be caused by the pathogens described, but may also encompass malignant tumors (such as non-Hodgkin’s lymphoma and Kaposi’s sarcoma) (91). In one study conducted in Nigeria in which 113 PLWH were recruited, the prevalence of adrenal insufficiency among PLWH was observed to be as high as 34% (92). During recent times, the incident rate of adrenal inflammation has declined significantly, as the inherent immunological functioning of PLWH has been observed to be more effectively preserved and the incidence of severe opportunistic infections has been observed to have decreased significantly, due to the widespread use of modern ART (93). With respect to the pituitary gland, it seems that the functional secretion of ACTH is generally retained in HIV/AIDS patients. As such, in one large study of 350 PLWH, 30.9% of the participants had serum cortisol levels below 100μg/L; however, after the administration of 1μg of ACTH, only 16.3% of subjects were observed to have serum cortisol levels below 180 μg/L (94). Then, in another study, autopsies were performed on 88 AIDS patients who succumbed to the disease, and only ten patients were observed to have extensive necrosis and/or focal fibrosis in the anterior pituitary (95).
Relative to the hypothalamus and pituitary gland components of the HPA axis, the adrenal glands sustain the most severe damage secondary to HIV infection. In chronic adrenocortical hypofunction/insufficiency, endocrine abnormalities are particularly serious, and may have a significant impact on the mental state of patients in the later stages of the disease, resulting in depression (96). When patients are diagnosed with adrenal insufficiency, most patients will choose long-term glucocorticoid treatment (97). As early as 1992, Norbiato et al., conducted a study that included nine PLWH with Cushing’s syndrome (98). In these patients, the affinity of the GR and its ligand in peripheral leukocytes decreased significantly, and the number of receptors increased, indicating that adrenal cortical dysfunction in these patients may have been caused by decreased sensitivity of peripheral tissues to glucocorticoids. Similar conclusions were also presented in another study (99). However, there are few recent such reports, and this may indicate that this situation is, in itself, rare, or may be related to an unknown cryptic mechanism. Nevertheless, it has been reliably demonstrated that glucocorticoid levels are significantly elevated in HIV patients with Cushing’s syndrome (100).
From a psychological perspective, PLWH are known to inherently be prone to stressful social circumstances, such as social isolation, discrimination, stigmatization, and other negative psychological emotions (101). Additionally, among PLWH (who, broadly speaking, tend to be economically vulnerable, tend to belong to ethnic minorities, tend to belong to sexual minorities, tend to be recreational drug users, and tend to be sex workers), the proportion of people belonging to socially vulnerable groups is high, and these individuals have an inherently greater risk of developing depression even prior to becoming infected with HIV (102). These ‘negative’ circumstances may activate the CNS to generate a series of stress responses, and this process is mainly mediated through the HPA axis (103). The HPA axis thus acts as a medium for the body to react to sources of stress. The end point of this endocrine pathway is the release of cortisol from the adrenal cortex. As a consequence, in these patients, the HPA axis may remain in an activated state for an extended period of time, and during this extended period of time, excessive glucocorticoids are produced and circulate within the body.
In PLWH, it is both the compensatory increase in production of glucocorticoids caused by adrenal insufficiency and the long-term and enduring negative life circumstances and events that lead to the overactivation of the HPA axis, and it is this overactivation that ultimately leads to the increased levels of circulating glucocorticoids. As mentioned above, glucocorticoids circulating in the body at an elevated level for an extended period of time will have a much greater impact on the CNS of the human body (especially in the hippocampus) (104), and may also predispose individuals to be more likely to develop depression. Future investigations will be necessary to clarify the preceding hypothesis and its fundamental mechanisms.
4 Neuroimmune factors
4.1 The cytokine hypothesis
Over recent years, researchers have observed that depression is associated with other chronic inflammatory and autoimmune diseases (105). As such, the cytokine hypothesis is an important theory that offers a further explanation for the pathogenesis of depression. The occurrence of depression is often accompanied by inflammatory responses, and the body’s baseline immunological functioning is thus altered by the presence of depression (106). Most studies concerning the mechanisms whereby an increase in cytokine levels causes depression focus on three likely explanations. Firstly, the release of proinflammatory cytokines may stimulate the HPA axis to secrete GCs (107). The proinflammatory effect of GCs is considered to be the basis of neurodegeneration (108). This is singularly important in the hippocampus, which is a brain region that controls cognitive function, and is an area of the brain which is particularly vulnerable to neuroinflammation and neurodegeneration (109). Secondly, on the one hand, cytokines may alter serotonin transporter (SERT) levels, and the consequent lower concentrations of serotonin induce the body to be more prone to anxiety and major depressive disorder (110). On the other hand, cytokines may activate indoleamine 2,3 dioxygenase (IDO) and increase 5-HT consumption through the kynurenine pathway, thus reducing 5-HT levels by negatively influencing tryptophan metabolism (111, 112). Thirdly, cytokines may materially affect neural plasticity by reducing the production of neurons, promoting the death of cortical nerves, and reducing the expression of brain-derived neurotrophic factor (BDNF) in the limbic system of the brain (113). Carlos et al., have utilized an in vitro approach in primary hippocampal neurons, and have observed that in the prolonged presence of IL-1β, BDNF is unable to efficiently deliver long-distance signaling via the retrograde transport of signaling endosomes (114). Thus, neural plasticity will be adversely affected.
4.2 Cytokines in HIV-associated depression
During the first few weeks of HIV infection, HIV may enter the CNS via a “Trojan horse” mechanism that permits infected immune cells to penetrate the blood-brain barrier (115). In conjunction with the trojan horse method, it is recognized that transcytosis, which is defined by the transport of HIV particles from the blood to the brain via epithelial cells, may result in CNS invasion by HIV (116). This allows HIV-activated monocytes, microglia, and other infected immune system cells to release viral proteins and infectious particles within the CNS, and subsequently promotes the inflammatory reaction, which substantially encourages the release of cytokines, and leads to the manifestation of a “sickness behavior” characterized by insomnia, loss of appetite, anhedonia, and difficulties with memory and concentration (117, 118). In order to counteract this, the body will activate the sympathetic adrenal medullary axis and the HPA axis (119). Thus, the activation of the HPA axis may be seen as an adaptive response to inflammation. For acute diseases, the “sickness behavior” subsides when the inflammation decreases and eventually dissipates. However, for chronic diseases such as HIV infection, the burden of HIV viral infection does not reduce. This leads to persistent clinical symptoms of chronic disease, and constitutes a significant obstacle to patient rehabilitation. Saloner et al. (120), recruited 143 PLWH to explore the relationship between depressive symptoms and inflammation, and observed that PLWH with depressive symptoms may be afflicted with an inflammatory subtype of depression, and may be particularly susceptible to neurocognitive changes.
4.2.1 Interleukin-1 beta
Elevated levels of IL-1B are associated with depressive symptoms. In a study by Uint et al., researchers analyzed the plasma cytokine concentrations of 34 patients with depression, 43 patients with bipolar affective disorder, and 41 controls (121). It was observed that, compared to those with bipolar disorder and the control cohort, IL-1B concentrations were significantly increased in patients with depression (p=0.005). The preceding authors believe that IL-1B may be thus used as an indicator for the early diagnosis of patients with depression (121). In another study, researchers injected recombinant IL-1B into the murine hippocampus, and this resulted in elevated plasma glucocorticoid levels, which is more likely to induce depression in the presence of chronic stress (122). Of note, IL-1B receptors are widely expressed in the hippocampus (123). According to Mussano et al., the reason for this is that IL-1B is neurotoxic, and promotes neuronal death (124). Studies have also shown that IL-1B levels are significantly increased in PLWH, compared to levels in the general population (125). IL-1B also induces viral replication in cells infected by HIV (126), and the concentration of IL-1B is elevated in the serum of PLWH (127). These findings may predispose PLWH to an increased susceptibility to the development of depression.
4.2.2 Interleukin 6
In addition to IL-1B, IL-6 is of particular importance in the study of depression. Evidence from animal and human clinical studies suggest that elevated levels of IL-6 may interfere with the normal physiological response to stress, and cause depression through activation of the hypothalamic-pituitary-adrenal axis (128). Notably, researchers have demonstrated that an increase in IL-6 levels may further promote depression particularly in individuals affected by comorbidities and/or diseases such as multiple sclerosis (129) and cancer (130). Additionally, studies have shown that IL-6 increases both acute and chronic stress (131). In the study by Gothoda et al., in which ten patients underwent liver resection (an example of acute stress), a significant and positive correlation between acute surgical stress and blood IL-6 levels has been observed (132). In one longitudinal community study assessing the relationship between chronic stress (caring for a spouse with dementia) and IL-6 production, it was observed that the average increase in IL-6 levels in caregivers was approximately four times higher than in noncarers (133). Human and animal clinical studies have also shown that stress may increase IL-6 levels. Elevated IL-6 levels may lead to HPA axis dysfunction, altered synaptic neurotransmission, and reduced neurotrophic factors, which may generate depression (134). Whether it is acute stress, chronic stress, or hyperactivity of the HPA axis, PLWH tend to be predisposed to them all. HIV infection has been demonstrated to induce monocytes and macrophages to express and secrete IL-6. Even with HIV virological suppression, plasma IL-6 levels are significantly higher in PLWH receiving treatment than in uninfected individuals (135). The acute and chronic stress, and the possible long-term neuroinflammation faced by PLWH may lead to higher circulating levels of IL-6, making these individuals much more susceptible to the development of HIV-associated depression.
4.2.3 Tumor necrosis factor-alpha
There is sufficient evidence in the literature to demonstrate that TNF-α is involved not only in the pathophysiology of cancer, but also in immunological functioning and the inflammatory process (136, 137). TNF-α is also an important cytokine involved in HIV-associated depression. TNF-α directly affects neuronal function and survival, regulates the production and secretion of neurotransmitters, and influences (either positively or negatively, depending on physiological or pathophysiological conditions) synaptic transmission (138, 139). TNF-α may also increase blood-brain barrier (BBB) permeability, which may be accompanied by depressive behaviors (138). The following perspectives were cited in a systematic review by Ma et al., viz., a) Plasma TNF-α levels are significantly increased in some patients with depression; b) Antidepressant treatment is associated with a decrease in TNF-α levels; c) Drug-induced elevation of TNF-α levels cause disease behaviors which are similar to depression, and blocking TNF-α alleviates depressive symptoms in animal models and human clinical trials (140). In HIV infection, TNF-α activates NFkB which in turn, upon activation, is translocated in the nucleus, where it binds near the HIV transcription initiation site to promote HIV expression and virion production (141). One study by Okay et al., observed that PLWH who did not receive antiretroviral therapy had higher TNF-α levels than those who received treatment, and both these cohorts had higher TNF-α levels than the normal healthy population (142). At the same time, HIV viral load positively correlates with TNF-α levels in a significant manner (142). Thus, during HIV infection, elevated TNF-α levels disrupts the integrity of the BBB and triggers neuroinflammation, which may substantially increase the likelihood of emergence of HIV-associated depression.
4.2.4 Other neuroinflammatory cytokines
In the HIV context, several studies have observed positive associations between depression and neuroinflammatory cytokines such as interferon gamma (IFN-γ), IL-15, 12, and 18 (143–146).
4.2.5 Chemokines
Chemokines are signaling proteins secreted by certain cells, and have the ability to induce directional chemotaxis in adjacent reactive cells. Over recent years, studies have demonstrated that the relationship between depression and chemokines is relatively close, and that some chemokines have a material impact on depression, e.g., the chemokines CXCL8 (147), CCL2 (148), and CCL3 (149). The relationship between HIV and the chemokine CXCL8 has already been suggested in studies of HIV infection, and most related studies have reported that CXCL8 levels are increased in the serum of PLWH (150). In a review by Mamik and Ghorpade, CXCL-8 was observed to correlate with neurological impairment and HIV disease severity (151). Specifically, the preceding authors have indicated that during HIV-associated neuroinflammation, the levels of CXCL8 are elevated in the brain and in cerebrospinal fluid. In this context, CXCL8 contributes to recruitment of neutrophils and monocytes within the brain. Mamik and Ghorpade believe that targeting CXCL8 and reducing levels of CXCL8 within the brain may reduce immune cell recruitment, and thus prevent certain neuro-inflammatory/degenerative diseases, e.g., Alzheimer’s disease, Parkinson’s disease, HIV-associated neurodegenerative disorder, and possibly depression (151). In a study by Lehmann et al., it was observed that Nef-induced CCL2 expression contributes to HIV brain invasion and neuronal dysfunction, which may also greatly increase the risk of depression (152). Furthermore, other than CXCl8 and CCL2, Schaller et al., have indicated that in the context of inflammation, CCL3 participates in the recruitment and activation of granulocytes by binding to receptors CCR1, CCR4, and CCR5 (153). At present, it is known that CCL3 may be associated with a delay of the progression of HIV disease; however, whether CCL3 is indeed related to HIV-associated depression warrants further study (154). Furthermore, other studies (146, 155, 156) have demonstrated a positive association between interferon-gamma-induced protein (IP)-10 and depressive symptoms. A broader presentation of the implications of cytokines in depression in PLWH has been extensively reviewed by Rakshasa-Loots (157).
5 Monoamine neurotransmitters
5.1 The monoamine hypothesis
The monoamine hypothesis is currently recognized as one of the fundamental etiological hypotheses for the development of depression (25). The theory argues that depression is caused by a decrease or a functional impairment of monoamine neurotransmitter concentrations within the synaptic spaces of the CNS (158). This theory asserts that depression is closely associated with low levels of monoamines such as DA, serotonin, and NE in the brain (158). These neurotransmitters have a wide range of biological activities, and are involved in many physiological responses of the CNS, such as emotional responses, mental activities, and sleep. When their activity or quantity decreases, the probability of developing depression increases substantially (159).
5.2 Monoamine neurotransmitters and HIV-associated depression
5.2.1 Dopamine
DA is the most abundant catecholamine neurotransmitter in the brain. As a neurotransmitter, DA regulates various physiological functions of the CNS. A decrease in DA levels will directly affect the patient’s mood, leading to a poor mental state (160). Over time, anxiety and/or depression may thus manifest (160). In 2004, one study suggested that depression may be associated with low levels of extracellular DA (161). A number of studies have since demonstrated that low levels of DA may be associated with depression. In one study in China, Zhang et al., observed that murine models with low DA levels are more prone to depressive and anxious behavior (162). A similar conclusion was reached in a study by Mallo et al., which observed that murine models of depression have lower levels of extracellular DA (163). Many similar studies exist in the literature, all of which point to the close relationship between low levels of DA and depression (160, 164, 165).
DA remains a factor that warrants discussion in the study of the pathogenesis of HIV-associated depression. Recent studies have demonstrated that HIV infection and psychoactive substances have the ability to affect the integrity of the basal ganglia (BG), which suggests that dopaminergic system dysfunction may be a potential mechanism for the development of HIV-associated depression (166). Saloner et al., used the Baker Depression Inventory-II (BDI-II) to assess the severity of current depression by recruiting 225 participants and measuring their DA levels in cerebrospinal fluid (167). These investigators observed that when DA levels in CSF are decreased, the incidence of depression in PLWH increased (167). In another study by Goulding et al., a significant reduction in tyrosine hydroxylase (TH) levels was observed in the brain of PLWH (168). TH is the rate-limiting enzyme for DA synthesis (168). The conclusions of both of the preceding studies suggest that HIV-associated depression may be closely associated with low levels of DA in the brain.
Furthermore, Kumar et al., have observed that HIV has a wide range of influence in dopaminergic areas of the brain, such as the frontocortical area, the basal ganglia, the caudate, the putamen, the globus pallidus, and importantly, in the substantia nigra, the main site of DA synthesis (169). In the early stages of HIV infection, infected monocytes migrate across the BBB, infiltrate the brain, and infect microglia (170), causing microglial dysfunction (171). After induction of the HIV-1 Tat protein, both the microglia and the functioning of the DA system are altered. Specifically, Tat simultaneously reduces the number of microglia and DA neurons in the substantia nigra pars compacta (172). Subsequently, HIV-1 viral proteins disrupt microglial proteins and receptors that regulate microglia-mediated phagocytosis of neurites, and pre- and post-synaptic phagocytosis (173). Finally, HIV-1 viral proteins may alter the bidirectional relationship between the dopaminergic system and synaptic structures (174). Collectively, evidence indicates that HIV infection leads to microglial dysfunction, which may have major consequences by inducing reduced DA levels and synaptic dysfunction, and this has meaningful ramifications for the development and progression of HIV-associated depression.
5.2.2 5-Hydroxytryptamine
5-hydroxytryptamine (also known as serotonin) was initially isolated in serum, and exists widely in mammalian tissues, especially in the cerebral cortex and in synapses (175). 5-HT is not only an inhibitory neurotransmitter but is also a messenger molecule that can produce pleasant emotions, and affects almost every aspect of brain activity, from regulation of emotions, the adjustment of energy levels, the influencing of memory, to shaping one’s outlook on life (176). As the relative therapeutic efficacy of the selective serotonin reuptake inhibitor (SSRI) class of drugs (such as fluoxetine and paroxetine) for the treatment of depression has become widely acknowledged, the relationship between serotonin levels and depression has received increasing scrutiny (177).
Many studies have confirmed that depression is related to low levels of serotonin (178). In a study by Aleksovski et al., the serum level of serotonin in patients with depression who did not take SSRIs was generally low (179). In an investigation by Park et al., the levels of plasma serotonin in treated mice with depression were significantly increased, and depressive symptoms were also improved (180). Many past studies have determined that abnormal levels of 5-HT and its metabolites are present in the CSF of patients with depression (181). The discovery of multiple serotonin receptor subtypes has further indicated that the serotonin system has an association with the pathogenesis of depression. As such, Bhatt’s study has indicated that in the CNS, 5-HT3 receptors (5-HT3R) are located in regions which have significance to the vomiting reflex, the perception of pain, the reward system, cognition, and depression and anxiety control (182). Recently, Zięba et al., have pointed out that 5-HT2A receptor promotion has an antidepressant effect (183).
In PLWH, the levels of 5-HT become significantly reduced (184). As an essential amino acid, tryptophan (TRP) participates in the kynurenine (KYN) pathway (185) through indoleamine-2,3-dioxidase (IDO) (186), in addition to participating in the production of new proteins and serotonin in vivo (187). The activity of IDO may be enhanced by inflammatory cytokines (188). Interestingly, when humans are infected by HIV, the induced neuroinflammation increases the activity of IDO (189), and an increased amount of tryptophan is metabolized to KYN (190), resulting in insufficient raw material to produce 5-HT. It is worth mentioning that many PLWH treated with ART continue to suffer from HIV-associated neurocognitive impairment, which may well be associated with an increase in IDO activity and KYN production, and a decrease in 5-HT levels (191). Other mechanisms, such as the depletion of “good” bacteria [butyrate-producing bacteria such as Akkermansia muciniphila (192, 193)] in the gut microbiome and the relative depletion of enteroendocrine cells in the gut [which has been reported during HIV infection (194)] may also be responsible for the low levels of serotonin observed in PLWH. Additionally, Shah et al., have demonstrated that at synapses, PLWH may display upregulated levels of serotonin transporters which further diminish levels of serotonin (195), suggesting that the relative lack of serotonin may be a possible mechanism explaining the onset of depression.
5.2.3 Noradrenaline or norepinephrine
NE is a common neurotransmitter. Adrenaline is converted to NE, is synthesized in presynaptic membrane vesicles, and is released into the synaptic junction (196). While this process requires calcium ions, the rate of NE synthesis is also regulated by calcium ions (197).
Researchers believe that depression occurs in an individual when norepinephrine levels are diminished, and the use of drugs which consume catecholamines may induce the development of depression (198). As a clinical example, it is known that the antihypertensive drug, reserpine, may consume norepinephrine in the synaptic cleft, and subsequently may cause depression (199). N-methyl-d-aspartate (NMDA) receptor antagonists may promote the release of NE from synaptic vesicles and reduce the reabsorption of NE, thus alleviating depression (200). It is also known that long-term treatment of patients with depression with the tricyclic antidepressant, imipramine, may cause blockage of the reabsorption of norepinephrine by the presynaptic membrane, thus promoting an increase in norepinephrine concentration within the synaptic cleft, and improving symptoms and signs of depression (201). Also, measurements of levels of 3-methoxy-4-hydroxyphenylglycol (MHPG), which is a metabolite of NE in the brain, may be used as a predictive marker to evaluate the occurrence and development of depression (202).
Among studies related to the pathogenesis of HIV-associated depression, there are a few studies in the literature concerning the association between the noradrenergic system and the pathogenesis of HIV-associated depression. Studies related to norepinephrine or serotonin and their association with HIV-associated depression are rare. Interestingly, one prospective study by Ironson et al., observed that higher levels of peripheral norepinephrine are associated with accelerated HIV disease progression, as manifested by an increased plasma viral load and decreased CD4+ T-cell counts (203). However, the specific mechanism whereby this may occur remains nebulous. Another study by Saloner et al., has indicated that HIV infection is independently associated with excess norepinephrine in the patient’s CNS, and that excess noradrenergic stimulation induces damage to the hippocampus and the prefrontal lobes, making it more likely to develop depression (204).
Increases in NE concentrations may exacerbate neuroinflammation during HIV infection, even among successfully ART-treated PLWH (204); however, further directed research is warranted in order to determine which specific cellular and cytokine components are involved in the consequent neuroinflammation induced by increases in NE concentrations in the brain.
5.2.4 Other neurotransmitter systems
It is now understood that glutamatergic dysfunction may also lead to the progression of depressive symptoms. This theory emerged from studies using animal models in which the authors demonstrated that HIV proteins (Tat and gp120 for example) may directly and indirectly enhance glutamatergic signaling (205, 206). Consequently, the increased glutamate release and reduced uptake may lead to neuronal injury, which may potentially explain the mechanisms whereby glutamate is associated with HAND and depressive symptoms (207). Lastly, the ability of the brain to adapt and change, which is referred to as neuroplasticity, may be a significant factor as well. Indeed, neuroplasticity may play a role in changes seen in neuroinflammatory factors as well as neurotrophic factors, including neurotransmitters, and therefore contribute to depression and cognitive impairment in PLWH (208, 209). HIV is known for its ability to induce neurological problems such as HAND (210). The development of HAND potentially results from the neuroplasticity that is subsequent to changes orchestrated by HIV infection, which ultimately may provoke the development of depressive symptoms.
6 Antiretroviral therapy
Up until the present time, there is no specific available drug or combination of drugs that can cure HIV infection or AIDS (211). In clinical practice, modern ART is usually employed to control and suppress HIV infection. In practical terms, this translates to the regular and lifelong consumption of several (usually three or four) different antiretroviral drugs daily. However, due to the complex modern ART drug combinations now utilized, ART administration tends to induce a series of adverse reactions which materially reduces the quality of life of ART-treated patients. Some antiviral drugs may cause serious neuropsychiatric complications, such as irritability, cognitive disorders, sleep disorders, and even depression (212).
Efavirenz, one of the most widely used non-nucleoside reverse transcriptase inhibitors (NNRTIs), is a common component of modern ART regimens, and is usually used in combination with two nucleoside reverse transcriptase inhibitors (NRTIs). However, the growing concern with respect to its adverse reactions (213) have sometimes led to the substitution of Efavirenz by other drugs in the initial treatment selection, or the conversion of the patients ART regimen to a non-Efavirenz regimen in ART-experienced patients (214). The reason for this is that the most common adverse manifestation experienced by patients receiving Efavirenz treatment are neuropsychiatric reactions (215, 216), which range from short-term effects, such as nightmares, dizziness, insomnia, tension, and attention deficit, to long-term serious symptoms, which include depression, suicidal ideation, and even overt psychosis (217). The sleep disorders and clinical depression caused by long-term use of Efavirenz is one of the most common reasons for patients to cease using this drug.
In 2007, Raltegravir was the first drug of the integrase strand transfer inhibitor (INSTI) class of antiretrovirals to be approved for use, with Elvitegravir and Dolutegravir subsequently being approved for use in 2012 and 2013, respectively (218, 219). These drugs inhibit retroviral integrases, which integrate viral DNA transcripts (via reverse transcription synthesis) into the host cell genome. It is speculated that their overall tolerance may be superior, compared to reverse transcriptase inhibitors (220). However, they may have an impact on the nervous system and have the capacity to induce depression, especially with treatment with Dolutegravir (221). In a recent cohort study involving more than 6,400 patients in different countries, about 3.5% (ranging from 1.4-7.2%) of patients receiving Dolutegravir treatment were observed to actively choose drug withdrawal as a direct consequence of the emergence and manifestation of neuropathic adverse events (222). This drug withdrawal rate was also observed to be higher than those of separate randomized clinical trials utilizing other INSTIs, such as Elvitegravir or Raltegravir (222).
The mechanisms underlying the effects of NNRTIs, INSTIs and other antiretroviral drugs on the CNS include: (i) provoke both oxidative stress and mitochondrial toxicity (223, 224); (ii) disrupt synaptic connections and alter neurotransmitter (acetylcholine) release (225); (iii) induce the release of proinflammatory cytokines (226–230); and (iv) inhibit the DNA polymerase gamma activity and cause mitochondrial toxicity and oxidative stress (231, 232). With the recent progressive implementation of long-acting ART, it is believed that the effects of these newer ART regimens on depression will be considerably reduced. Indeed, long-acting ARTs offer tremendous opportunities to (i) improve HIV treatment adherence, (ii) reduce the onset of opportunistic infections, (iii) reduce risks of drug–drug interactions (DDIs), (iv) reduce toxicity, and (v) reduce the stress and stigma related to compliance with the necessarily rigid daily ART treatment (233–236). Collectively, the preceding advantages may potentially help to mitigate the onset of HIV-associated depression subsequent to ART administration. However, only future evidence-based data will assist in appreciating the real effect that long-acting ART has on the depressive symptoms seen in PLWH.
7 Additional factors
7.1 Substance (or drug) use (or abuse)
Other than HIV mediation, it is worth noting that drug users with HIV, display a higher prevalence of depression (compared to other PLWH) (18, 237). The mechanisms surrounding the onset of depressive symptoms in these patients are likely to be related to the altered stress response resulting from chronic drug use (238). Indeed, recreational drug use and abuse induce alterations to the stress and reward pathways. Of note, acute administration of alcohol, marijuana, cocaine, and amphetamines activates (i) brain reward pathways (referred to as mesocorticolimbic dopaminergic systems), and (ii) brain stress pathways (including the corticotropin release factor (CRF)-HPA axis and autonomic nervous system pathways). Additionally, elevated ACTH and corticosterone levels have been reported in the plasma of recreational drug users (239, 240). Decreased levels of cortisol have been noted in cases of opiate consumption (241). Over time, regular and chronic consumption of recreational drugs induces addictive behavior, which may lead to the development of depressive symptoms and compulsive behaviors associated with drug seeking (242). In terms of mechanisms related to these outcomes, it is necessary to recall that recreational drugs are also stressors which increase the levels of GCs as well as the release of dopamine (243, 244). However, chronic release of GCs may inhibit dopamine synthesis, particularly when the HPA-axis is altered (245). Thus, to achieve a dopamine reward, addicted drug users tend to increase their drug consumption in an attempt to force the release of dopamine, which further deteriorates the HPA axis and increases their risk of development of depressive symptoms.
7.2 Lack of sleep or poor sleep quality
Gutierrez et al., and Wu et al., have reported in their systematic reviews that 30 to 90% of PLWH experience poor sleep quality (246, 247). Interestingly, people older than 40 years old are particularly affected (246). In other studies, sleep disturbances (poor sleep quality, insomnia, and shorter total sleep duration) have been reported among PLWH (248, 249). Thanks to recent investigations, sleep disturbances are becoming recognized as a potential cause of mental health disorders seen in PLWH (250, 251). In a recent study, Daubert et al., have further demonstrated that poor quality of sleep is highly prevalent among women living with HIV and HIV negative women presenting depressive and anxiety symptoms (252), suggesting that attention to sleep quality should be one of the priorities of the clinical management of chronic HIV.
The mechanisms whereby the lack of sleep or poor sleep quality may induce the development of depression during HIV infection are not fully understood. However, it is known that in the general population and in PLWH, markers of inflammation or immune activation (such as TNFα, CRP, IL-6) are closely associated to both depression (253) and sleep quality (254). As indicated previously, TRP participates in the KYN pathway (185) through IDO (186). Importantly, TRP is also a precursor of melatonin, which is an important factor in depression (255). Thus, in a context of HIV infection where inflammation increases the activity of IDO (189) resulting in significant diminution of TRP (256), melatonin synthesis may be affected leading to lack of sleep and an increased risk of depression. Evidence collated by Cho et al., indicates that only depressed patients display an association between poor sleep quality and kynurenine metabolism (257). Interestingly, they have also found that poor sleep quality is also associated with increased C-reactive protein levels in this category of participants, which reinforces the role played by inflammation in depression (257). HIV infection is well known for establishing a chronic state of inflammation and triggering the activity of IDO (258). Therefore, it is quite legitimate to consider the complex relationships between inflammation or immune activation, sleep quality, and depressive symptoms as one of the pathways which explains how poor quality of sleep may induce depression in PLWH.
7.3 Undernutrition
HIV infection is also associated with undernutrition (with commonest cases encountered in low- and middle-income countries) (259), and evidence suggests that these entities promote each other (260, 261). As indicated by Alebel et al., undernutrition represents one of the major contributors to HIV/AIDS-related deaths, and promotes the emergence of opportunistic infections (262). Investigations conducted in Tanzania (263) and Ethiopia (262) have reported that PLWH present high levels of nutritional insufficiency, evaluated at 27.7% and 26%, respectively. Recently, in their meta-analysis, Seid et al., have found that 23.74% of HIV-positive adults using ART in sub-Saharan Africa are undernourished (264). Undernutrition is associated with (i) anemia, (ii) loss of appetite, poor nutritional absorption, increased energy need, and (iii) loss of weight and a skinny appearance (259). Unfortunately, loss of weight and a skinny appearance may lead to self-stigmatization, social isolation, and depression.
Interestingly, it is also worth noting that some metabolic parameters and nutrients have been proposed as markers of depression. As such, high triglycerides, low high-density lipoprotein cholesterol (HDL-C), and low hematocrit levels have been commonly observed in patients with depression (265). Particular attention on these metabolic parameters in PLWH may be necessary for confirming their roles in depression during HIV infection. That said, a lack of vitamin B consumption (particularly B12, B9, and B6) (266, 267) has been noted in patients with major depressive disorder. A positive association between (i) low vitamin D serum levels and depression and (ii) poor outdoor exposure to sunshine and depression has also been observed by Anglin et al. (268), and Penckofer et al. (269),, respectively. Similarly, low circulating levels of omega 3 polyunsaturated fatty acid has been associated with major depressive disorder (270). In terms of minerals, depression has been noted in people [particularly women (271)] having an insufficient intake of (i) calcium, iron, magnesium, and zinc (272), as well as (ii) potassium, phosphorus, and copper (271). Interestingly, these nutrients are vital for monoamine synthesis, neuroinflammation control, neuroprotection, and for the synthesis of growth factors (273). Thus, undernutrition may be considered as an epigenetic factor influencing depression. In the context of HIV infection, it is therefore legitimate to assume that undernutrition may foster a fertile milieu which encourages the development of depression.
An overall representation of the factors involved in the pathogenesis of HIV-associated depression is shown in Figure 1. Likewise, Table 2 summarizes key studies, their methodologies, and findings used to discuss the influence of biological factors in the development of depression in PLWH.
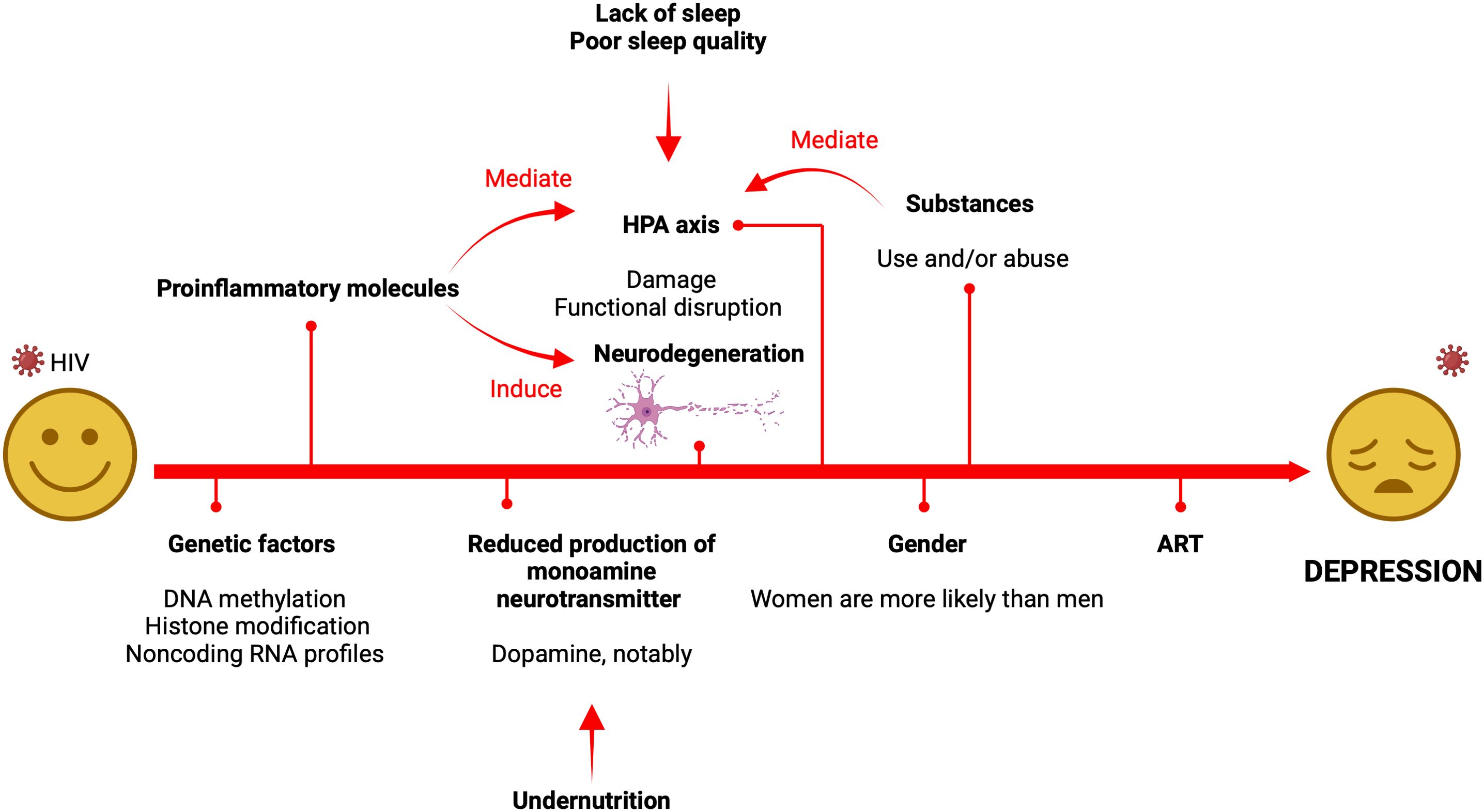
Figure 1. Summary of the factors and mechanisms involved in the pathogenesis of HIV-associated depression. It should be noted that the psychosocial framework of depression, which is not provided in this visual, also plays a significant role as it contributes to the alteration of the HPA axis. The main mechanisms responsible for depression include HPA-axis dysfunction, inflammation/neurodegeneration pathways, and neurotransmitter systems which are significantly perturbed.
7.4 Psychosocial factors
Lastly, it is worth nothing that in addition to the biological factors and mechanisms extensively expounded in this article, pre-existing psychosocial factors also play a major role in the development of depression in PLWH. In other words, the psychosocial milieu within which the individual exists significantly contributes to the development of mental health dysfunction, or more specifically depression, in PLWH. To illustrate this point, a recent publication by Rubin et al. (275), clearly presents some evidence. Indeed, they demonstrate that with respect to the psychosocial traits of PLWH, 4 clusters of mental health phenotypes in PLWH may be identified. Thus, clusters 1, 2, 3, and 4 designate patients with significant post-traumatic stress disorder (PTSD) symptoms, anxiety, mixed anxiety/depression, and no clinical mental health symptoms, respectively. Interestingly, cluster 1 (significant PTSD) individuals were observed to have an inherently higher rate of childhood sexual abuse compared to (i) cluster 4 (PLWH with no clinical mental health symptoms) and (ii) cluster 2 and 3 (PLWH with anxiety and/or depression who display minimal PTSD symptoms) individuals. Further comparative analysis revealed that compared to cluster 4, those with minimal PTSD symptoms were also observed to have had experienced higher rates of childhood sexual abuse. Although the cases of sexual abuse cannot be generalized to the entire population of PLWH suffering from depression, this study highlights the critical role that early life adversity plays in the onset of mental health disorders in PWLH in adulthood. Similarly, Huang et al. (276), have observed that HIV-related stress experienced by newly diagnosed patients may lead to depression. They have suggested that in order to mitigate the onset of anxiety and depression in these patients, interventions aimed at reducing stress among PLWH should be taken into consideration for the management of known stressors such as discrimination/stigma, fear of infecting others, and excessive attention to physical functions, amongst others. Furthermore, studies have also shown that PLWH (i) experience stressful social circumstances (social isolation, discrimination, stigmatization, and other negative psychological factors) (101) and (ii) tend to belong to socially vulnerable groups (sex workers, economically vulnerable, ethnic minorities, and other vulnerable groups) (102). Collectively, these ‘negative’ circumstances may modulate and facilitate the onset of depressive symptoms via a series of stress responses which potentially alters the homeostatic functioning of the HPA axis.
8 Conclusions and perspectives
Due to its high prevalence and severity, clinical depression in PLWH has evolved into a global public health problem. HIV-associated depression most likely results from enigmatically complex interactions amongst genetic, biological, and psychosocial factors. HIV infection is often accompanied by HPA axis damage and functional disruption, a large release of inflammatory cytokines, a reduction of monoamine neurotransmitters (especially DA), and increased neuronal death, all of which may increase the incident rate of HIV-associated depression. Additionally, some commonly used modern antiretroviral drugs (especially Efavirenz and Dolutegravir) may also have a negative impact on the CNS of PLWH, making patients more susceptible to the development of depression. The causes of HIV-related depression are, thus, complex and multifactorial, among which the release of cytokines, overactivation of the HPA axis, and changes in levels of monoamine neurotransmitters play key roles. Over the long term, treatment of depression in HIV/AIDS patients and the psychological monitoring of these patients should thus be optimized. With respect to ART treatment, it would be interesting to investigate the effects of long-acting antiretroviral therapy (which is a novel, efficacious, and much more robust therapeutic approach against HIV infection) on depression. In other words, does a switch to long-acting therapy favor or mitigate the onset of depression in PLWH? Only future studies will provide clearer answers to this question. Moreover, clinicians should be aware of the potential emergence of depression in PLWH, and at the same time anticipate and actively draft individualized patient treatment plans to improve the quality of life of all patients afflicted by HIV-related disease. Interventions should thus be directed towards those who are more likely to be affected by depression. As such, it is critical to indicate that there are differences between women and men with respect to the incidence of depression. Indeed, it seems that women infected by HIV are more likely to develop depression than men infected by HIV (277). Among the reasons for this, structural barriers, internalized stigma, and masculine coping styles are high on the list. Compared to men with HIV, women with HIV experiencing structural barriers seem to be more likely to develop depression through mental health impairment, as reported by Waldron et al. (278),. Besides, Celeste-Villalvir et al. (279), and Waldron et al. (278), have observed that women with HIV, compared to men with HIV, tend to experience internalized stigma, which makes it difficult for them to seek help and care. This context may also lead to depression. Lastly, according to Shi et al. (280),, men with HIV (as a consequence of their masculine coping style) are less likely to seek help when they have depression. Instead, men may express their depression through other symptoms, such as aggression and substance abuse (280). This results in fewer men being diagnosed with depression and consequently, this translates to a higher proportion of women being diagnosed with depression than men.
This narrative review (when compared to other recent related publications) aims to address and elucidate the fundamental mechanisms involved in the emergence of depression in the context of HIV infection. As such, factors favoring the emergence of depression are reported on, and the mechanisms potentially involved in the pathogenesis of depression in PLWH are presented as well. However, as a narrative review, this article does not provide original evidence-based data. Thus, the observations and the conclusive remarks remain subjective, and only further observational or evidence-based studies will provide stronger and more credible observations and conclusions to the topic at hand. Moreover, the selection of reference articles used in this study may be construed to be prejudicial, as article selection is inherently based on the subjective opinions and preconceptions of the specific authors involved in the study. Indeed, due to the very large number of publications concerning this topic present in the contemporary literature since the beginning of HIV epidemic, some may consider that the present review of the literature may not be robust enough, may be inappropriate, or is incomplete.
Notwithstanding these limitations, the present article provides a deeper appreciation of the roles of the HPA axis, inflammation, and monoamine neurotransmitters in the manifestation of depression in PLWH. Although there are several factors associated with depression in PLWH, most of these factors are closely related to HPA axis functionality, inflammation, and neurotransmitter release. As such, orienting future interventions which target restoration of the HPA axis, reduction (or mitigation) of the inflammatory syndrome, and regulation of the expression of dopamine (for instance) may be reasonable and viable approaches to treat depression in PLWH. The regulation of inflammation and/or the expression of neurotransmitters such as dopamine may be achieved pharmacologically. Regarding the restoration of the HPA, two strategies (used separately or in tandem) exist (281). Firstly, the restoration of the HPA axis may be achieved through lifestyle adjustments. For example, these adjustments can be oriented toward addressing (i) the underlying psychological problems, (ii) the nutritional habits, (iii) the quality of sleep, and associating (iv) regular physical activity to ultimately mitigate the effects of chronic stress on the HPA axis. Secondly, other than lifestyle adjustments, a pharmacological approach may be explored. This mainly relies on administration of corticosteroids followed by an assessment of HPA function. However, further work is necessary in the area of the epigenetics of depression in the context of HIV infection. It would be intellectually interesting, for instance, to study the levels of methylation of the genes that are involved in depression, and also the expression of miRs and their implications in the manifestation of depression in PLWH.
Contemporary literature indicates that considerable progress has been made in biomedical research concerning the pathogenesis of HIV-related depression. Nonetheless, it is necessary to redouble our efforts to fundamentally and accurately describe this specific subtype of clinical depression, and to clearly elucidate its etiology, pathophysiology, evaluation, treatment, and prognosis in a more robust and meaningful manner. Further directed research is therefore warranted in order to achieve these lofty but achievable objectives.
Author contributions
SZ: Conceptualization, Writing – original draft. WW: Writing – original draft. YC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Chongqing AIDS Medical Research Center Construction Program, the Chongqing Disease Prevention and Public Health Research Center Construction Program, and the Chongqing Key Public Health Disciplines Improvement Project. The funders had no role in study design, data collection, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Luxton R, Kyriakopoulos M. Depression in children and young people: identification and management NICE guidelines. Arch Dis Child Educ Pract Ed. (2022) 107:36–8. doi: 10.1136/archdischild-2020-320020
2. Edinoff AN, Akuly HA, Hanna TA, Ochoa CO, Patti SJ, Ghaffar YA, et al. Selective serotonin reuptake inhibitors and adverse effects: A narrative review. Neurol Int. (2021) 13:387–401. doi: 10.3390/neurolint13030038
3. Tol WA, Leku MR, Lakin DP, Carswell K, Augustinavicius J, Adaku A, et al. Guided self-help to reduce psychological distress in South Sudanese female refugees in Uganda: a cluster randomised trial. Lancet Glob Health. (2020) 8:e254–63. doi: 10.1016/s2214-109x(19)30504-2
4. World. COVID-19 pandemic triggers 25% increase in prevalence of anxiety and depression worldwide . Available online at: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide (Accessed April 08, 2025).
5. Gebru T, Ejara D, Yalew A, Deyessa N. Prevalence of depression and associated factors among HIV/AIDS patients attending antiretroviral therapy clinic at Adama Hospital Medical College, Adama, Central Ethiopia. Sci Rep. (2024) 14:1642. doi: 10.1038/s41598-024-52142-z
6. Xu Z, Asahchop EL, Branton WG, Gelman BB, Power C, Hobman TC. MicroRNAs upregulated during HIV infection target peroxisome biogenesis factors: Implications for virus biology, disease mechanisms and neuropathology. PLoS Pathog. (2017) 13:e1006360. doi: 10.1371/journal.ppat.1006360
7. Muche EA, Kiflu M, Ayalew MB. Patient reported central nervous system adverse events of efavirenz-based antiretroviral therapy in people living with HIV in northwest Ethiopia. HIV AIDS (Auckl). (2020) 12:601–9. doi: 10.2147/hiv.s276111
8. Sekine Y, Kawaguchi T, Kunimoto Y, Masuda J, Numata A, Hirano A, et al. Adherence to anti-retroviral therapy, decisional conflicts, and health-related quality of life among treatment-naïve individuals living with HIV: a DEARS-J observational study. J Pharm Health Care Sci. (2023) 9:9. doi: 10.1186/s40780-023-00277-y
9. Troncoso FT, de Oliveira Conterno L. Prevalence of neurocognitive disorders and depression in a Brazilian HIV population. Rev Soc Bras Med Trop. (2015) 48:390–8. doi: 10.1590/0037-8682-0034-2015
10. Burki T. Guidelines for visceral leishmaniasis and HIV co-infection. Lancet Infect Dis. (2022) 22:1124–5. doi: 10.1016/s1473-3099(22)00461-3
11. Niu L, Luo D, Liu Y, Silenzio VM, Xiao S. The mental health of people living with HIV in China, 1998-2014: A systematic review. PLoS One. (2016) 11:e0153489. doi: 10.1371/journal.pone.0153489
12. Niu L, Luo D, Chen X, Wang M, Zhou W, Zhang D, et al. Longitudinal trajectories of emotional problems and unmet mental health needs among people newly diagnosed with HIV in China. J Int AIDS Soc. (2019) 22:e25332. doi: 10.1002/jia2.25332
13. Jiang H, Chen S, Huang X, Huang R, Lin P, Cheng W, et al. Prevalence of and factors associated with major depressive disorder among HIV-positive individuals in Guangdong, China. J Affect Disord. (2019) 246:474–9. doi: 10.1016/j.jad.2018.12.130
14. Lofgren SM, Bond DJ, Nakasujja N, Boulware DR. Burden of depression in outpatient HIV-infected adults in sub-Saharan Africa; systematic review and meta-analysis. AIDS Behav. (2020) 24:1752–64. doi: 10.1007/s10461-019-02706-2
15. Choi SK, Boyle E, Cairney J, Collins EJ, Gardner S, Bacon J, et al. Prevalence, recurrence, and incidence of current depressive symptoms among people living with HIV in Ontario, Canada: results from the Ontario HIV treatment network cohort study. PLoS One. (2016) 11:e0165816. doi: 10.1371/journal.pone.0165816
16. Pence BW, Mills JC, Bengtson AM, Gaynes BN, Breger TL, Cook RL, et al. Association of increased chronicity of depression with HIV appointment attendance, treatment failure, and mortality among HIV-infected adults in the United States. JAMA Psychiatry. (2018) 75:379–85. doi: 10.1001/jamapsychiatry.2017.4726
17. Woods WA, Watson M, Ranaweera S, Tajuria G, Sumathipala A. Under-representation of low and middle income countries (LMIC) in the research literature: Ethical issues arising from a survey of five leading medical journals: have the trends changed? Glob Public Health. (2023) 18:2229890. doi: 10.1080/17441692.2023.2229890
18. Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: the need for an integrated response. Aids. (2019) 33:1411–20. doi: 10.1097/qad.0000000000002227
19. Chibanda D. Depression and HIV: integrated care towards 90-90-90. Int Health. (2017) 9:77–9. doi: 10.1093/inthealth/ihw058
20. Ngum PA, Fon PN, Ngu RC, Verla VS, Luma HN. Depression among HIV/AIDS patients on highly active antiretroviral therapy in the southwest regional hospitals of Cameroon: A cross-sectional study. Neurol Ther. (2017) 6:103–14. doi: 10.1007/s40120-017-0065-9
21. Amare T, Getinet W, Shumet S, Asrat B. Prevalence and associated factors of depression among PLHIV in Ethiopia: systematic review and meta-analysis, 2017. AIDS Res Treat. (2018) 2018:5462959. doi: 10.1155/2018/5462959
22. Sarno E, Moeser AJ, Robison AJ. Neuroimmunology of depression. Adv Pharmacol. (2021) 91:259–92. doi: 10.1016/bs.apha.2021.03.004
23. Juruena MF, Bocharova M, Agustini B, Young AH. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J Affect Disord. (2018) 233:45–67. doi: 10.1016/j.jad.2017.09.052
24. Bhatt S, Devadoss T, Jha NK, Baidya M, Gupta G, Chellappan DK, et al. Targeting inflammation: a potential approach for the treatment of depression. Metab Brain Dis. (2023) 38:45–59. doi: 10.1007/s11011-022-01095-1
25. Loula R, Monteiro LHA. Monoamine neurotransmitters and mood swings: a dynamical systems approach. Math Biosci Eng. (2022) 19:4075–83. doi: 10.3934/mbe.2022187
26. Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. psychol Corporation. (1996). doi: 10.1037/t00742-000
27. Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and validity of the beck depression inventory-II with adoslescent psychiatric inpatients. psychol Assess. (2004) 16:120–32. doi: 10.1037/1040-3590.16.2.120
28. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. (1961) 4:561–71. doi: 10.1001/archpsyc.1961.01710120031004
29. Radloff LS. The CES-D scale:A self-report depression scale for research in the general population. Appl psychol Measurement. (1977) 1:385–401. doi: 10.1177/014662167700100306
30. Saracino RM, Cham H, Rosenfeld B, Nelson CJ. Confirmatory factor analysis of the center for epidemiologic studies depression scale in oncology with examination of invariance between younger and Older patients. Eur J psychol Assess. (2020) 36:229–36. doi: 10.1027/1015-5759/a000510
31. Th. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy. (1990) 16:199–208. doi: 10.1016/0168-8510(90)90421-9
32. Brooks R. EuroQol: the current state of play. Health Policy. (1996) 37:53–72. doi: 10.1016/0168-8510(96)00822-6
33. Devlin NJ, Brooks R. EQ-5D and the EuroQol group: past, present and future. Appl Health Economics Health Policy. (2017) 15:127–37. doi: 10.1007/s40258-017-0310-5
34. Hamilton MA. Rating scale for depression. J Neurology Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
35. Trajković G, Starčević V, Latas M, Leštarević M, Ille T, Bukumirić Z, et al. Reliability of the Hamilton Rating Scale for Depression: A meta-analysis over a period of 49years. Psychiatry Res. (2011) 189:1–9. doi: 10.1016/j.psychres.2010.12.007
36. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. (2018) 134:382–9. doi: 10.1192/bjp.134.4.382
37. Davidson J, Turnbull CD, Strickland R, Miller R, Graves K. The Montgomery-Åsberg Depression Scale: reliability and validity. Acta Psychiatrica Scandinavica. (1986) 73:544–8. doi: 10.1111/j.1600-0447.1986.tb02723.x
38. D’Zurilla TJ, Nezu AM. Development and preliminary evaluation of the socialproblem-solving inventory. psychol Assess. (1990) 2:156–63.
39. D’Zurilla TJ, Nezu AM, Maydeu-Olivares A. Social problem-solving inventory-revised (SPSI-R). Multi-Health Syst. (2002).
40. Meranda PF. BASC: behavior assessment system for children. Measurement Eval Couns Dev. (1996) 28:229–32.
41. Reynolds CR, Kamphaus RW. Behavior assessment system for children (3rd ed.). Pearson Assessments. (2015).
42. Achenbach TM. The child behavior profile: an empirically based system for assessing children’s behavioral problems and competencies. Int J Ment Health. (1978) 7:24–42. doi: 10.1080/00207411.1978.11448806
43. Kariuki SM, Abubakar A, Murray E, Stein A, Newton CRJC. Evaluation of psychometric properties and factorial structure of the pre-school child behaviour checklist at the Kenyan Coast. Child Adolesc Psychiatry Ment Health. (2016) 10:1. doi: 10.1186/s13034-015-0089-9
44. Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica: Int J Child &Adolescent Psychiatry. (1981) 46:305–15.
45. Sun S, Wang S. The children’s depression inventory in worldwide child development research: A reliability generalization study. J Child Family Stud. (2015) 24:2352–63. doi: 10.1007/s10826-014-0038-x
46. Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. (1979) 64:442–50. doi: 10.1542/peds.64.4.442
47. Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ. Psychometric properties of the children’s depression rating scale–revised in adolescents. J Child Adolesc Psychopharmacol. (2010) 20:513–6. doi: 10.1089/cap.2010.0063
48. Neufeld E, O’Rourke N, Donnelly M. Enhanced measurement sensitivity of hopeless ideation among older adults at risk of self-harm: Reliability and validity of Likert-type responses to the Beck Hopelessness Scale. Aging Ment Health. (2010) 14:752–6. doi: 10.1080/13607860903421052
49. Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. (2003) 54:573–83. doi: 10.1016/S0006-3223(02)01866-8
50. Martin A, Rief W, Klaiberg A, Braehler E. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. (2006) 28:71–7. doi: 10.1016/j.genhosppsych.2005.07.003
51. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Internal Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
52. Robitaille A, Cappeliez P, Coulombe D, Webster JD. Factorial structure and psychometric properties of the reminiscence functions scale. Aging Ment Health. (2010) 14:184–92. doi: 10.1080/13607860903167820
53. Webster JD. Construction and validation of the reminiscence functions scale. J Gerontology. (1993) 48:P256–62. doi: 10.1093/geronj/48.5.P256
54. Ware JE Jr., Sherbourne CD. The MOS 36-ltem short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. (1992) 30:473–83. doi: 10.1097/00005650-199206000-00002
55. Gameroff MJ, Wickramaratne P, Weissman MM. Testing the short and screener versions of the social adjustment scale – self-report (SAS-SR). Int J Methods Psychiatr Res. (2012) 21:52–65. doi: 10.1002/mpr.358
56. Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Arch Gen Psychiatry. (1976) 33:1111–5. doi: 10.1001/archpsyc.1976.01770090101010
57. Tyrer P, Nur U, Crawford M, Karlsen S, MacLean C, Rao B, et al. The social functioning questionnaire: A rapid and robust measure of perceived functioning. Int J Soc Psychiatry. (2005) 51:265–75. doi: 10.1177/0020764005057391
58. Lopez MN, Quan NM, Carvajal PM. A psychometric study of the geriatric depression scale. Eur J psychol Assess. (2010) 26:55–60. doi: 10.1027/1015-5759/a000008
59. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. (1982) 17:37–49. doi: 10.1016/0022-3956(82)90033-4
60. Neugarten BL, Havighurst RJ, Tobin SS. The measurement of life satisfaction*. J Gerontology. (1961) 16:134–43. doi: 10.1093/geronj/16.2.134
61. Adams DL. Analysis of a life satisfaction index. J Gerontology. (1969) 24:470–4. doi: 10.1093/geronj/24.4.470
62. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. (2000) 157:1552–62. doi: 10.1176/appi.ajp.157.10.1552
63. Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. (2006) 163:109–14. doi: 10.1176/appi.ajp.163.1.109
64. Merikangas KR, Cui L, Heaton L, Nakamura E, Roca C, Ding J, et al. Independence of familial transmission of mania and depression: results of the NIMH family study of affective spectrum disorders. Mol Psychiatry. (2014) 19:214–9. doi: 10.1038/mp.2013.116
65. Tansey KE, Guipponi M, Hu X, Domenici E, Lewis G, Malafosse A, et al. Contribution of common genetic variants to antidepressant response. Biol Psychiatry. (2013) 73:679–82. doi: 10.1016/j.biopsych.2012.10.030
66. Cardenas MC, Farnan S, Hamel BL, Mejia Plazas MC, Sintim-Aboagye E, Littlefield DR, et al. Prevention of the vertical transmission of HIV; A recap of the journey so far. Viruses. (2023) 15:849. doi: 10.3390/v15040849
67. Yao Q, Chen Y, Zhou X. The roles of microRNAs in epigenetic regulation. Curr Opin Chem Biol. (2019) 51:11–7. doi: 10.1016/j.cbpa.2019.01.024
68. Bao N, Lye KW, Barton MK. MicroRNA binding sites in Arabidopsis class III HD-ZIP mRNAs are required for methylation of the template chromosome. Dev Cell. (2004) 7:653–62. doi: 10.1016/j.devcel.2004.10.003
69. Rajewsky N. microRNA target predictions in animals. Nat Genet. (2006) 38 Suppl:S8–13. doi: 10.1038/ng1798
70. Juźwik CA, D SS, Zhang Y, Paradis-Isler N, Sylvester A, Amar-Zifkin A, et al. microRNA dysregulation in neurodegenerative diseases: A systematic review. Prog Neurobiol. (2019) 182:101664. doi: 10.1016/j.pneurobio.2019.101664
71. Rashid F, Zaongo SD, Song F, Chen Y. The diverse roles of miRNAs in HIV pathogenesis: Current understanding and future perspectives. Front Immunol. (2022) 13:1091543. doi: 10.3389/fimmu.2022.1091543
72. Eletto D, Russo G, Passiatore G, Del Valle L, Giordano A, Khalili K, et al. Inhibition of SNAP25 expression by HIV-1 Tat involves the activity of mir-128a. J Cell Physiol. (2008) 216:764–70. doi: 10.1002/jcp.21452
73. Mukerjee R, Chang JR, Del Valle L, Bagashev A, Gayed MM, Lyde RB, et al. Deregulation of microRNAs by HIV-1 Vpr protein leads to the development of neurocognitive disorders. J Biol Chem. (2011) 286:34976–85. doi: 10.1074/jbc.M111.241547
74. Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS. MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell Death Dis. (2010) 1:e77. doi: 10.1038/cddis.2010.56
75. Ryan KM, Patterson I, McLoughlin DM. Peroxisome proliferator-activated receptor gamma co-activator-1 alpha in depression and the response to electroconvulsive therapy. Psychol Med. (2019) 49:1859–68. doi: 10.1017/s0033291718002556
76. Bol SM, Booiman T, van Manen D, Bunnik EM, van Sighem AI, Sieberer M, et al. Single nucleotide polymorphism in gene encoding transcription factor Prep1 is associated with HIV-1-associated dementia. PLoS One. (2012) 7:e30990. doi: 10.1371/journal.pone.0030990
77. Jasinska AJ, Pandrea I, Apetrei C. CCR5 as a coreceptor for human immunodeficiency virus and simian immunodeficiency viruses: A prototypic love-hate affair. Front Immunol. (2022) 13:835994. doi: 10.3389/fimmu.2022.835994
78. Sundermann EE, Bishop JR, Rubin LH, Little DM, Meyer VJ, Martin E, et al. Genetic predictor of working memory and prefrontal function in women with HIV. J Neurovirol. (2015) 21:81–91. doi: 10.1007/s13365-014-0305-z
79. Mikulska J, Juszczyk G, Gawrońska-Grzywacz M, Herbet M. HPA axis in the pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci. (2021) 11:1298. doi: 10.3390/brainsci11101298
80. Timmermans S, Souffriau J, Libert C. A general introduction to glucocorticoid biology. Front Immunol. (2019) 10:1545. doi: 10.3389/fimmu.2019.01545
81. Matosin N, Arloth J, Czamara D, Edmond KZ, Maitra M, Fröhlich AS, et al. Associations of psychiatric disease and ageing with FKBP5 expression converge on superficial layer neurons of the neocortex. Acta Neuropathol. (2023) 145:439–59. doi: 10.1007/s00401-023-02541-9
82. Tozzi L, Farrell C, Booij L, Doolin K, Nemoda Z, Szyf M, et al. Epigenetic changes of FKBP5 as a link connecting genetic and environmental risk factors with structural and functional brain changes in major depression. Neuropsychopharmacology. (2018) 43:1138–45. doi: 10.1038/npp.2017.290
83. Hartmann J, Bajaj T, Klengel C, Chatzinakos C, Ebert T, Dedic N, et al. Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. (2021) 35:109185. doi: 10.1016/j.celrep.2021.109185
84. Podgorny OV, Gulyaeva NV. Glucocorticoid-mediated mechanisms of hippocampal damage: Contribution of subgranular neurogenesis. J Neurochem. (2021) 157:370–92. doi: 10.1111/jnc.15265
85. Zacková L, Jáni M, Brázdil M, Nikolova YS, Marečková K. Cognitive impairment and depression: Meta-analysis of structural magnetic resonance imaging studies. NeuroImage Clin. (2021) 32:102830. doi: 10.1016/j.nicl.2021.102830
86. Lubitz S. Adrenal function in HIV infection. In: Davies TF, editor. A Case-Based Guide to Clinical Endocrinology. Springer International Publishing, Cham (2022). p. 543–52.
87. Mifsud S, Gauci Z, Gruppetta M, Mallia Azzopardi C, Fava S. Adrenal insufficiency in HIV/AIDS: a review. Expert Rev Endocrinol Metab. (2021) 16:351–62. doi: 10.1080/17446651.2021.1979393
88. Villette JM, Bourin P, Doinel C, Mansour I, Fiet J, Boudou P, et al. Circadian variations in plasma levels of hypophyseal, adrenocortical and testicular hormones in men infected with human immunodeficiency virus. J Clin Endocrinol Metab. (1990) 70:572–7. doi: 10.1210/jcem-70-3-572
89. Dobs AS, Dempsey MA, Ladenson PW, Polk BF. Endocrine disorders in men infected with human immunodeficiency virus. Am J Med. (1988) 84:611–6. doi: 10.1016/0002-9343(88)90144-1
90. Nassoro DD, Mkhoi ML, Sabi I, Meremo AJ, Lawala PS, Mwakyula IH. Adrenal insufficiency: A forgotten diagnosis in HIV/AIDS patients in developing countries. Int J Endocrinol. (2019) 2019:2342857. doi: 10.1155/2019/2342857
91. Mathavan A, Mathavan A, Mathavan M, Altshuler E. Management of adrenal insufficiency in the setting of chronic HIV and advanced extra-adrenal Hodgkin lymphoma. BMJ Case Rep. (2022) 15:e249269. doi: 10.1136/bcr-2022-249269
92. Odeniyi IA, Fasanmade OA, Ajala MO, Ohwovoriole AE. Adrenocortical function in Nigerians with human immunodeficiency virus infection. Ghana Med J. (2013) 47:171–7.
93. Lo J, Grinspoon SK. Adrenal function in HIV infection. Curr Opin Endocrinol Diabetes Obes. (2010) 17:205–9. doi: 10.1097/MED.0b013e3283394441
94. Akase IE, Habib AG, Bakari AG, Muhammad H, Gezawa I, Nashabaru I, et al. Occurrence of hypocortisolism in HIV patients: Is the picture changing? Ghana Med J. (2018) 52:147–52. doi: 10.4314/gmj.v52i3.7
95. Ferreiro J, Vinters HV. Pathology of the pituitary gland in patients with the acquired immune deficiency syndrome (AIDS). Pathology. (1988) 20:211–5. doi: 10.3109/00313028809059495
96. Bertorini TE, Perez A. Neurologic complications of disorders of the adrenal glands. Handb Clin Neurol. (2014) 120:749–71. doi: 10.1016/b978-0-7020-4087-0.00050-4
97. Almeida MQ, Mendonca BB. Adrenal insufficiency and glucocorticoid use during the COVID-19 pandemic. Clinics (Sao Paulo). (2020) 75:e2022. doi: 10.6061/clinics/2020/e2022
98. Norbiato G, Bevilacqua M, Vago T, Baldi G, Chebat E, Bertora P, et al. Cortisol resistance in acquired immunodeficiency syndrome. J Clin Endocrinol Metab. (1992) 74:608–13. doi: 10.1210/jcem.74.3.1740494
99. Vagnucci AH, Winkelstein A. Circadian rhythm of lymphocytes and their glucocorticoid receptors in HIV-infected homosexual men. J Acquir Immune Defic Syndr (1988). (1993) 6:1238–47.
100. Benido Silva V, Cardoso J, Esteves Brandão M, Mesquita I, Pereira MT. Iatrogenic Cushing’s syndrome: the result of cobicistat and glucocorticoid interaction in an HIV patient after bariatric surgery. Cureus. (2023) 15:e34367. doi: 10.7759/cureus.34367
101. Fazeli PL, Turan JM, Budhwani H, Smith W, Raper JL, Mugavero MJ, et al. Moment-to-moment within-person associations between acts of discrimination and internalized stigma in people living with HIV: An experience sampling study. Stigma Health. (2017) 2:216–28. doi: 10.1037/sah0000051
102. Garrido-Hernansaiz H, Alonso-Tapia J. Predictors of anxiety and depression among newly diagnosed people living with HIV: A longitudinal study. Scand J Psychol. (2020) 61:616–24. doi: 10.1111/sjop.12621
103. Juruena MF, Eror F, Cleare AJ, Young AH. The role of early life stress in HPA axis and anxiety. Adv Exp Med Biol. (2020) 1191:141–53. doi: 10.1007/978-981-32-9705-0_9
104. Naughton M, Dinan TG, Scott LV. Corticotropin-releasing hormone and the hypothalamic-pituitary-adrenal axis in psychiatric disease. Handb Clin Neurol. (2014) 124:69–91. doi: 10.1016/b978-0-444-59602-4.00005-8
105. Whitehouse CE, Fisk JD, Bernstein CN, Berrigan LI, Bolton JM, Graff LA, et al. Comorbid anxiety, depression, and cognition in MS and other immune-mediated disorders. Neurology. (2019) 92:e406–17. doi: 10.1212/wnl.0000000000006854
106. Gałecki P, Talarowska M. Inflammatory theory of depression. Psychiatr Pol. (2018) 52:437–47. doi: 10.12740/pp/76863
107. Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:760–8. doi: 10.1016/j.pnpbp.2010.06.020
108. Liu H, Kaur J, Dashtipour K, Kinyamu R, Ribak CE, Friedman LK. Suppression of hippocampal neurogenesis is associated with developmental stage, number of perinatal seizure episodes, and glucocorticosteroid level. Exp Neurol. (2003) 184:196–213. doi: 10.1016/s0014-4886(03)00207-3
109. Bolshakov AP, Tret’yakova LV, Kvichansky AA, Gulyaeva NV. Glucocorticoids: Dr. Jekyll and Mr. Hyde of hippocampal neuroinflammation. Biochem (Mosc). (2021) 86:156–67. doi: 10.1134/s0006297921020048
110. Yang KC, Liu MN, Liou YJ, Hu LY, Yang BH, Chou YH. Interleukin-1 family and serotonin transporter in first-episode, drug-naive major depressive disorder: A pilot study. J Psychiatr Res. (2021) 135:174–80. doi: 10.1016/j.jpsychires.2021.01.018
111. Zádor F, Joca S, Nagy-Grócz G, Dvorácskó S, Szűcs E, Tömböly C, et al. Pro-inflammatory cytokines: potential links between the endocannabinoid system and the kynurenine pathway in depression. Int J Mol Sci. (2021) 22:5903. doi: 10.3390/ijms22115903
112. Ramírez LA, Pérez-Padilla EA, García-Oscos F, Salgado H, Atzori M, Pineda JC. A new theory of depression based on the serotonin/kynurenine relationship and the hypothalamicpituitary- adrenal axis. Biomedica. (2018) 38:437–50. doi: 10.7705/biomedica.v38i3.3688
113. Zhang J, He H, Qiao Y, Zhou T, He H, Yi S, et al. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia. (2020) 68:2674–92. doi: 10.1002/glia.23878
114. Carlos AJ, Tong L, Prieto GA, Cotman CW. IL-1β impairs retrograde flow of BDNF signaling by attenuating endosome trafficking. J Neuroinflamm. (2017) 14:29. doi: 10.1186/s12974-017-0803-z
115. Ash MK, Al-Harthi L, Schneider JR. HIV in the brain: identifying viral reservoirs and addressing the challenges of an HIV cure. Vaccines (Basel). (2021) 9:867. doi: 10.3390/vaccines9080867
116. Rojas-Celis V, Valiente-Echeverría F, Soto-Rifo R, Toro-Ascuy D. New challenges of HIV-1 infection: how HIV-1 attacks and resides in the central nervous system. Cells. (2019) 8:1245. doi: 10.3390/cells8101245
117. Izquierdo-Useros N, Naranjo-Gómez M, Erkizia I, Puertas MC, Borràs FE, Blanco J, et al. HIV and mature dendritic cells: Trojan exosomes riding the Trojan horse? PloS Pathog. (2010) 6:e1000740. doi: 10.1371/journal.ppat.1000740
118. Maes M, Berk M, Goehler L, Song C, Anderson G, Gałecki P, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. (2012) 10:66. doi: 10.1186/1741-7015-10-66
119. Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. (2008) 455:894–902. doi: 10.1038/nature07455
120. Saloner R, Paolillo EW, Heaton RK, Grelotti DJ, Stein MB, Miller AH, et al. Chronically elevated depressive symptoms interact with acute increases in inflammation to predict worse neurocognition among people with HIV. J Neurovirol. (2021) 27:160–7. doi: 10.1007/s13365-020-00925-1
121. Uint L, Bastos GM, Thurow HS, Borges JB, Hirata TDC, França JID, et al. Increased levels of plasma IL-1b and BDNF can predict resistant depression patients. Rev Assoc Med Bras (1992). (2019) 65:361–9. doi: 10.1590/1806-9282.65.3.361
122. Parsadaniantz SM, Batsché E, Gegout-Pottie P, Terlain B, Gillet P, Netter P, et al. Effects of continuous infusion of interleukin 1 beta on corticotropin-releasing hormone (CRH), CRH receptors, proopiomelanocortin gene expression and secretion of corticotropin, beta-endorphin and corticosterone. Neuroendocrinology. (1997) 65:53–63. doi: 10.1159/000127164
123. Salvo-Romero E, Stokes P, Gareau MG. Microbiota-immune interactions: from gut to brain. LymphoSign J. (2020) 7:1–23. doi: 10.14785/lymphosign-2019-0018
124. Mussano F, Genova T, Munaron L, Petrillo S, Erovigni F, Carossa S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets. (2016) 27:467–71. doi: 10.3109/09537104.2016.1143922
125. Williams ME, Naudé PJW. The relationship between HIV-1 neuroinflammation, neurocognitive impairment and encephalitis pathology: A systematic review of studies investigating post-mortem brain tissue. Rev Med Virol. (2024) 34:e2519. doi: 10.1002/rmv.2519
126. Feria MG, Taborda NA, Hernandez JC, Rugeles MT. HIV replication is associated to inflammasomes activation, IL-1β, IL-18 and caspase-1 expression in GALT and peripheral blood. PLoS One. (2018) 13:e0192845. doi: 10.1371/journal.pone.0192845
127. Nabatanzi R, Ssekamatte P, Castelnuovo B, Kambugu A, Nakanjako D. Increased levels of caspase-1 and IL-1β Among adults with persistent immune activation after 12 years of suppressive antiretroviral therapy in the infectious diseases institute HIV treatment cohort. Open Forum Infect Dis. (2023) 10:ofad539. doi: 10.1093/ofid/ofad539
128. Ting EY, Yang AC, Tsai SJ. Role of interleukin-6 in depressive disorder. Int J Mol Sci. (2020) 21:2194. doi: 10.3390/ijms21062194
129. Koutsouraki E, Hatzifilipou E, Michmizos D, Cotsavasiloglou C, Costa V, Baloyannis S. Increase in interleukin-6 levels is related to depressive phenomena in the acute (relapsing) phase of multiple sclerosis. J Neuropsychiatry Clin Neurosci. (2011) 23:442–8. doi: 10.1176/jnp.23.4.jnp442
130. Jehn CF, Kühnhardt D, Bartholomae A, Pfeiffer S, Schmid P, Possinger K, et al. Association of IL-6, hypothalamus-pituitary-adrenal axis function, and depression in patients with cancer. Integr Cancer Ther. (2010) 9:270–5. doi: 10.1177/1534735410370036
131. Darcy J, Tseng YH. The link between stress and IL-6 is heating up. Cell Metab. (2020) 32:152–3. doi: 10.1016/j.cmet.2020.07.011
132. Gotohda N, Iwagaki H, Ozaki M, Kinoshita T, Konishi M, Nakagohri T, et al. Significant correlation between surgical stress of hepatectomy and changes in the serum levels of HGF, IL-6 and soluble Fas in patients with viral hepatitis. Hepatogastroenterology. (2008) 55:1400–3.
133. Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. (2003) 100:9090–5. doi: 10.1073/pnas.1531903100
134. Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun. (2017) 64:208–19. doi: 10.1016/j.bbi.2017.01.011
135. Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. (2012) 55:126–36. doi: 10.1093/cid/cis406
136. Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. (2016) 12:49–62. doi: 10.1038/nrrheum.2015.169
137. van Loo G, Bertrand MJM. Death by TNF: a road to inflammation. Nat Rev Immunol. (2023) 23:289–303. doi: 10.1038/s41577-022-00792-3
138. Probert L. TNF. and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience. (2015) 302:2–22. doi: 10.1016/j.neuroscience.2015.06.038
139. Habbas S, Santello M, Becker D, Stubbe H, Zappia G, Liaudet N, et al. Neuroinflammatory TNFα Impairs memory via astrocyte signaling. Cell. (2015) 163:1730–41. doi: 10.1016/j.cell.2015.11.023
140. Ma K, Zhang H, Baloch Z. Pathogenetic and therapeutic applications of tumor necrosis factor-α (TNF-α) in major depressive disorder: A systematic review. Int J Mol Sci. (2016) 17:733. doi: 10.3390/ijms17050733
141. Ownby RL, Kumar AM, Benny Fernandez J, Moleon-Borodowsky I, Gonzalez L, Eisdorfer S, et al. Tumor necrosis factor-alpha levels in HIV-1 seropositive injecting drug users. J Neuroimmune Pharmacol. (2009) 4:350–8. doi: 10.1007/s11481-009-9150-x
142. Okay G, Koc MM, Guler EM, Yabaci A, Kocyigit A, Akkoyunlu Y. The effect of antiretroviral therapy on IL-6, IL-1β, TNF-α, IFN-γ Levels and their relationship with HIV-RNA and CD4+ T cells in HIV patients. Curr HIV Res. (2020) 18:354–61. doi: 10.2174/1570162x18666200712174642
143. Derry HM, Johnston CD, Burchett CO, Brennan-Ing M, Karpiak S, Zhu YS, et al. Links between inflammation, mood, and physical function among older adults with HIV. J Gerontol B Psychol Sci Soc Sci. (2022) 77:50–60. doi: 10.1093/geronb/gbab027
144. Derry-Vick HM, Johnston CD, Brennan-Ing M, Burchett CO, Glesby N, Zhu YS, et al. Pain is associated with depressive symptoms, inflammation, and poorer physical function in older adults with HIV. Psychosom Med. (2022) 84:957–65. doi: 10.1097/psy.0000000000001119
145. Memiah P, Nkinda L, Majigo M, Humwa F, Haile ZT, Muthoka K, et al. Mental health symptoms and inflammatory markers among HIV infected patients in Tanzania. BMC Public Health. (2021) 21:1113. doi: 10.1186/s12889-021-11064-5
146. Rivera-Rivera Y, García Y, Toro V, Cappas N, López P, Yamamura Y, et al. Depression correlates with increased plasma levels of inflammatory cytokines and a dysregulated oxidant/antioxidant balance in HIV-1-infected subjects undergoing antiretroviral therapy. J Clin Cell Immunol. (2014) 5:1000276. doi: 10.4172/2155-9899.1000276
147. Milenkovic VM, Stanton EH, Nothdurfter C, Rupprecht R, Wetzel CH. The role of chemokines in the pathophysiology of major depressive disorder. Int J Mol Sci. (2019) 20:2283. doi: 10.3390/ijms20092283
148. Romero-Sanchiz P, Nogueira-Arjona R, Araos P, Serrano A, Barrios V, Argente J, et al. Variation in chemokines plasma concentrations in primary care depressed patients associated with Internet-based cognitive-behavioral therapy. Sci Rep. (2020) 10:1078. doi: 10.1038/s41598-020-57967-y
149. Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry. (2018) 23:48–58. doi: 10.1038/mp.2017.205
150. Lin WW, Nelson AN, Ryon JJ, Moss WJ, Griffin DE. Plasma cytokines and chemokines in Zambian children with measles: innate responses and association with HIV-1 coinfection and in-hospital mortality. J Infect Dis. (2017) 215:830–9. doi: 10.1093/infdis/jix012
151. Mamik MK, Ghorpade A. CXCL8 as a potential therapeutic target for HIV-associated neurocognitive disorders. Curr Drug Targets. (2016) 17:111–21. doi: 10.2174/1389450116666150626124544
152. Lehmann MH, Lehmann JM, Erfle V. Nef-induced CCL2 expression contributes to HIV/SIV brain invasion and neuronal dysfunction. Front Immunol. (2019) 10:2447. doi: 10.3389/fimmu.2019.02447
153. Schaller TH, Batich KA, Suryadevara CM, Desai R, Sampson JH. Chemokines as adjuvants for immunotherapy: implications for immune activation with CCL3. Expert Rev Clin Immunol. (2017) 13:1049–60. doi: 10.1080/1744666x.2017.1384313
154. Flórez-Álvarez L, Hernandez JC, Zapata W. NK cells in HIV-1 infection: from basic science to vaccine strategies. Front Immunol. (2018) 9:2290. doi: 10.3389/fimmu.2018.02290
155. Rubin LH, Langenecker SA, Phan KL, Keating SM, Neigh GN, Weber KM, et al. Remitted depression and cognition in HIV: The role of cortisol and inflammation. Psychoneuroendocrinology. (2020) 114:104609. doi: 10.1016/j.psyneuen.2020.104609
156. Lu H, Surkan PJ, Irwin MR, Treisman GJ, Breen EC, Sacktor N, et al. Inflammation and risk of depression in HIV: prospective findings from the multicenter AIDS cohort study. Am J Epidemiol. (2019) 188:1994–2003. doi: 10.1093/aje/kwz190
157. Mudra Rakshasa-Loots A. Depression and HIV: a scoping review in search of neuroimmune biomarkers. Brain Commun. (2023) 5:fcad231. doi: 10.1093/braincomms/fcad231
158. Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. (2008) 69 Suppl E1:4–7.
159. Perez-Caballero L, Torres-Sanchez S, Romero-López-Alberca C, González-Saiz F, Mico JA, Berrocoso E. Monoaminergic system and depression. Cell Tissue Res. (2019) 377:107–13. doi: 10.1007/s00441-018-2978-8
160. Belujon P, Grace AA. Dopamine system dysregulation in major depressive disorders. Int J Neuropsychopharmacol. (2017) 20:1036–46. doi: 10.1093/ijnp/pyx056
161. Dailly E, Chenu F, Renard CE, Bourin M. Dopamine, depression and antidepressants. Fundam Clin Pharmacol. (2004) 18:601–7. doi: 10.1111/j.1472-8206.2004.00287.x
162. Zhang M, Liu Y, Zhao M, Tang W, Wang X, Dong Z, et al. Depression and anxiety behaviour in a rat model of chronic migraine. J Headache Pain. (2017) 18:27. doi: 10.1186/s10194-017-0736-z
163. Mällo T, Alttoa A, Kõiv K, Tõnissaar M, Eller M, Harro J. Rats with persistently low or high exploratory activity: behaviour in tests of anxiety and depression, and extracellular levels of dopamine. Behav Brain Res. (2007) 177:269–81. doi: 10.1016/j.bbr.2006.11.022
164. Martin-Soelch C. Is depression associated with dysfunction of the central reward system? Biochem Soc Trans. (2009) 37:313–7. doi: 10.1042/bst0370313
165. Tamura T, Sugihara G, Okita K, Mukai Y, Matsuda H, Shiwaku H, et al. Dopamine dysfunction in depression: application of texture analysis to dopamine transporter single-photon emission computed tomography imaging. Transl Psychiatry. (2022) 12:309. doi: 10.1038/s41398-022-02080-z
166. Monick AJ, Joyce MR, Chugh N, Creighton JA, Morgan OP, Strain EC, et al. Characterization of basal ganglia volume changes in the context of HIV and polysubstance use. Sci Rep. (2022) 12:4357. doi: 10.1038/s41598-022-08364-0
167. Saloner R, Cherner M, Grelotti DJ, Paolillo EW, Moore DJ, Heaton RK, et al. Lower CSF homovanillic acid relates to higher burden of neuroinflammation and depression in people with HIV disease. Brain Behav Immun. (2020) 90:353–63. doi: 10.1016/j.bbi.2020.09.012
168. Goulding DR, Kraft A, Mouton PR, McPherson CA, Avdoshina V, Mocchetti I, et al. Age-related decrease in tyrosine hydroxylase immunoreactivity in the substantia nigra and region-specific changes in microglia morphology in HIV-1 Tg rats. Neurotox Res. (2019) 36:563–82. doi: 10.1007/s12640-019-00077-z
169. Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, et al. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol. (2009) 15:257–74. doi: 10.1080/13550280902973952
170. Ko A, Kang G, Hattler JB, Galadima HI, Zhang J, Li Q, et al. Macrophages but not Astrocytes Harbor HIV DNA in the Brains of HIV-1-Infected Aviremic Individuals on Suppressive Antiretroviral Therapy. J Neuroimmune Pharmacol. (2019) 14:110–9. doi: 10.1007/s11481-018-9809-2
171. Thangaraj A, Chivero ET, Tripathi A, Singh S, Niu F, Guo ML, et al. HIV TAT-mediated microglial senescence: Role of SIRT3-dependent mitochondrial oxidative stress. Redox Biol. (2021) 40:101843. doi: 10.1016/j.redox.2020.101843
172. Miller DR, Shaerzadeh F, Phan L, Sharif N, Gamble-George J, McLaughlin JP, et al. HIV-1 Tat regulation of dopamine transmission and microglial reactivity is brain region specific. Glia. (2018) 66:1915–28. doi: 10.1002/glia.23447
173. Mishra N, Mohata M, Aggarwal H, Chaudhary O, Das BK, Sinha S, et al. Expression of complement receptor 3 (CR3) and regulatory protein CD46 on dendritic cells of antiretroviral naïve and treated HIV-1 infected individuals: Correlation with immune activation status. Mol Immunol. (2018) 96:83–7. doi: 10.1016/j.molimm.2018.02.011
174. Roscoe RF Jr., Mactutus CF, Booze RM. HIV-1 transgenic female rat: synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol. (2014) 9:642–53. doi: 10.1007/s11481-014-9555-z
175. Bakshi A, Tadi P. Biochemistry, serotonin. In: StatPearls. StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC, Treasure Island (FL (2024).
176. De Deurwaerdère P, Di Giovanni G. Serotonin in health and disease. Int J Mol Sci. (2020) 21:3500. doi: 10.3390/ijms21103500
177. Micheli L, Ceccarelli M, D’Andrea G, Tirone F. Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise. Brain Res Bull. (2018) 143:181–93. doi: 10.1016/j.brainresbull.2018.09.002
178. Bamalan OA, Moore MJ, Al Khalili Y. Physiology, serotonin. In: StatPearls. StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC, Treasure Island (FL (2024).
179. Aleksovski B, Neceva V, Vujović V, Manusheva N, Rendevski V, Novotni A, et al. SSRI-reduced platelet reactivity in non-responding patients with life-long Recurrent Depressive Disorder: Detection and involved mechanisms. Thromb Res. (2018) 165:24–32. doi: 10.1016/j.thromres.2018.03.006
180. Park BK, Kim YR, Kim YH, Yang C, Seo CS, Jung IC, et al. Antidepressant-like effects of Gyejibokryeong-Hwan in a mouse model of reserpine-induced depression. BioMed Res Int. (2018) 2018:5845491. doi: 10.1155/2018/5845491
181. Liu Y, Zhao J, Guo W. Emotional roles of mono-aminergic neurotransmitters in major depressive disorder and anxiety disorders. Front Psychol. (2018) 9:2201. doi: 10.3389/fpsyg.2018.02201
182. Bhatt S, Devadoss T, Manjula SN, Rajangam J. 5-HT(3) receptor antagonism a potential therapeutic approach for the treatment of depression and other disorders. Curr Neuropharmacol. (2021) 19:1545–59. doi: 10.2174/1570159x18666201015155816
183. Zięba A, Stępnicki P, Matosiuk D, Kaczor AA. Overcoming depression with 5-HT(2A) receptor ligands. Int J Mol Sci. (2021) 23:10. doi: 10.3390/ijms23010010
184. Wang X, Mehra S, Kaushal D, Veazey RS, Xu H. Abnormal tryptophan metabolism in HIV and mycobacterium tuberculosis infection. Front Microbiol. (2021) 12:666227. doi: 10.3389/fmicb.2021.666227
185. Le Floc’h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. (2011) 41:1195–205. doi: 10.1007/s00726-010-0752-7
186. Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. (2017) 10:1178646917691938. doi: 10.1177/1178646917691938
187. Friedman M. Analysis, nutrition, and health benefits of tryptophan. Int J Tryptophan Res. (2018) 11:1178646918802282. doi: 10.1177/1178646918802282
188. Baumgartner R, Forteza MJ, Ketelhuth DFJ. The interplay between cytokines and the Kynurenine pathway in inflammation and atherosclerosis. Cytokine. (2019) 122:154148. doi: 10.1016/j.cyto.2017.09.004
189. Schroecksnadel K, Zangerle R, Bellmann-Weiler R, Garimorth K, Weiss G, Fuchs D. Indoleamine-2, 3-dioxygenase and other interferon-gamma-mediated pathways in patients with human immunodeficiency virus infection. Curr Drug Metab. (2007) 8:225–36. doi: 10.2174/138920007780362608
190. Murray MF. Tryptophan depletion and HIV infection: a metabolic link to pathogenesis. Lancet Infect Dis. (2003) 3:644–52. doi: 10.1016/s1473-3099(03)00773-4
191. Ferrell D, Giunta B. The impact of HIV-1 on neurogenesis: implications for HAND. Cell Mol Life Sci. (2014) 71:4387–92. doi: 10.1007/s00018-014-1702-4
192. Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. (2019) 4:623–32. doi: 10.1038/s41564-018-0337-x
193. Geng ST, Zhang ZY, Wang YX, Lu D, Yu J, Zhang JB, et al. Regulation of gut microbiota on immune reconstitution in patients with acquired immunodeficiency syndrome. Front Microbiol. (2020) 11:594820. doi: 10.3389/fmicb.2020.594820
194. van Marle G, Sharkey KA, Gill MJ, Church DL. Gastrointestinal viral load and enteroendocrine cell number are associated with altered survival in HIV-1 infected individuals. PLoS One. (2013) 8:e75967. doi: 10.1371/journal.pone.0075967
195. Shah S, Sinharay S, Matsuda K, Schreiber-Stainthorp W, Muthusamy S, Lee D, et al. Potential mechanism for HIV-associated depression: upregulation of serotonin transporters in SIV-infected macaques detected by 11C-DASB PET. Front Psychiatry. (2019) 10:362. doi: 10.3389/fpsyt.2019.00362
196. Hussain LS, Reddy V, Maani CV. Physiology, noradrenergic synapse. In: StatPearls. StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC, Treasure Island (FL (2024).
197. Fiore F, Alhalaseh K, Dereddi RR, Bodaleo Torres F, Çoban I, Harb A, et al. Norepinephrine regulates calcium signals and fate of oligodendrocyte precursor cells in the mouse cerebral cortex. Nat Commun. (2023) 14:8122. doi: 10.1038/s41467-023-43920-w
198. Maletic V, Eramo A, Gwin K, Offord SJ, Duffy RA. The role of norepinephrine and its α-adrenergic receptors in the pathophysiology and treatment of major depressive disorder and schizophrenia: A systematic review. Front Psychiatry. (2017) 8:42. doi: 10.3389/fpsyt.2017.00042
199. Khurana K, Bansal N. Lacidipine attenuates reserpine-induced depression-like behavior and oxido-nitrosative stress in mice. Naunyn Schmiedebergs Arch Pharmacol. (2019) 392:1265–75. doi: 10.1007/s00210-019-01667-6
200. Sattar Y, Wilson J, Khan AM, Adnan M, Azzopardi Larios D, Shrestha S, et al. A review of the mechanism of antagonism of N-methyl-D-aspartate receptor by ketamine in treatment-resistant depression. Cureus. (2018) 10:e2652. doi: 10.7759/cureus.2652
201. Fayez R, Gupta V. Imipramine. In: StatPearls. StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC, Treasure Island (FL (2024).
202. Nobis A, Zalewski D, Waszkiewicz N. Peripheral markers of depression. J Clin Med. (2020) 9:3793. doi: 10.3390/jcm9123793
203. Ironson G, O’Cleirigh C, Kumar M, Kaplan L, Balbin E, Kelsch CB, et al. Psychosocial and neurohormonal predictors of HIV disease progression (CD4 cells and viral load): A 4 year prospective study. AIDS Behav. (2015) 19:1388–97. doi: 10.1007/s10461-014-0877-x
204. Saloner R, Cherner M, Iudicello JE, Heaton RK, Letendre SL, Ellis RJ. Cerebrospinal fluid norepinephrine and neurocognition in HIV and methamphetamine dependence. J Acquir Immune Defic Syndr. (2020) 85:e12–22. doi: 10.1097/qai.0000000000002422
205. Potter MC, Figuera-Losada M, Rojas C, Slusher BS. Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. (2013) 8:594–607. doi: 10.1007/s11481-013-9442-z
206. Marino J, Maubert ME, Mele AR, Spector C, Wigdahl B, Nonnemacher MR. Functional impact of HIV-1 Tat on cells of the CNS and its role in HAND. Cell Mol Life Sci. (2020) 77:5079–99. doi: 10.1007/s00018-020-03561-4
207. Vázquez-Santiago FJ, Noel RJ Jr., Porter JT, Rivera-Amill V. Glutamate metabolism and HIV-associated neurocognitive disorders. J Neurovirol. (2014) 20:315–31. doi: 10.1007/s13365-014-0258-2
208. Mudra Rakshasa-Loots A, Whalley HC, Vera JH, Cox SR. Neuroinflammation in HIV-associated depression: evidence and future perspectives. Mol Psychiatry. (2022) 27:3619–32. doi: 10.1038/s41380-022-01619-2
209. Michael H, Mpofana T, Ramlall S, Oosthuizen F. The role of brain derived neurotrophic factor in HIV-associated neurocognitive disorder: from the bench-top to the bedside. Neuropsychiatr Dis Treat. (2020) 16:355–67. doi: 10.2147/ndt.s232836
210. Hu A, Zaongo SD, Harypursat V, Wang X, Ouyang J, Chen Y. HIV-associated neurocognitive disorder: key implications of the microbiota-gut-brain axis. Front Microbiol. (2024) 15:1428239. doi: 10.3389/fmicb.2024.1428239
211. World. HIV and AIDS (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (Accessed April 08, 2025).
212. Winias S, Radithia D, Savitri Ernawati D. Neuropathy complication of antiretroviral therapy in HIV/AIDS patients. Oral Dis. (2020) 26 Suppl 1:149–52. doi: 10.1111/odi.13398
213. Drury A, Gleadow-Ware S, Gilfillan S, Ahrens J. HIV and mental illness in Malawi and the neuropsychiatric sequelae of efavirenz. Malawi Med J. (2018) 30:40–5. doi: 10.4314/mmj.v30i1.9
214. Xia H, Huang XJ, Hu Y, Gao LY, Wu Y, Wu H, et al. Switching from efavirenz to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide reduces central nervous system symptoms in people living with HIV. Chin Med J (Engl). (2021) 134:2850–6. doi: 10.1097/cm9.0000000000001824
215. Zareifopoulos N, Lagadinou M, Karela A, Kyriakopoulou O, Velissaris D. Neuropsychiatric effects of antiviral drugs. Cureus. (2020) 12:e9536. doi: 10.7759/cureus.9536
216. Costa B, Vale N. Efavirenz: history, development and future. Biomolecules. (2022) 13:88. doi: 10.3390/biom13010088
217. Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, Esplugues JV. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother. (2015) 70:2693–708. doi: 10.1093/jac/dkv183
218. Cocohoba J, Dong BJ. Raltegravir: the first HIV integrase inhibitor. Clin Ther. (2008) 30:1747–65. doi: 10.1016/j.clinthera.2008.10.012
219. Fantauzzi A, Mezzaroma I. Dolutegravir: clinical efficacy and role in HIV therapy. Ther Adv Chronic Dis. (2014) 5:164–77. doi: 10.1177/2040622314530461
220. Cid-Silva P, Llibre JM, Fernández-Bargiela N, Margusino-Framiñán L, Balboa-Barreiro V, Pernas-Souto B, et al. Clinical experience with the integrase inhibitors dolutegravir and elvitegravir in HIV-infected patients: efficacy, safety and tolerance. Basic Clin Pharmacol Toxicol. (2017) 121:442–6. doi: 10.1111/bcpt.12828
221. Hoffmann C, Welz T, Sabranski M, Kolb M, Wolf E, Stellbrink HJ, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. (2017) 18:56–63. doi: 10.1111/hiv.12468
222. Hoffmann C, Llibre JM. Neuropsychiatric adverse events with dolutegravir and other integrase strand transfer inhibitors. AIDS Rev. (2019) 21:4–10. doi: 10.24875/AIDSRev.19000023
223. Stern AL, Lee RN, Panvelker N, Li J, Harowitz J, Jordan-Sciutto KL, et al. Differential effects of antiretroviral drugs on neurons in vitro: roles for oxidative stress and integrated stress response. J Neuroimmune Pharmacol. (2018) 13:64–76. doi: 10.1007/s11481-017-9761-6
224. Nooka S, Ghorpade A. Organellar stress intersects the astrocyte endoplasmic reticulum, mitochondria and nucleolus in HIV associated neurodegeneration. Cell Death Dis. (2018) 9:317. doi: 10.1038/s41419-018-0341-3
225. Ekins S, Mathews P, Saito EK, Diaz N, Naylor D, Chung J, et al. α7-Nicotinic acetylcholine receptor inhibition by indinavir: implications for cognitive dysfunction in treated HIV disease. Aids. (2017) 31:1083–9. doi: 10.1097/qad.0000000000001488
226. Wu T, Zhang J, Geng M, Tang SJ, Zhang W, Shu J. Nucleoside reverse transcriptase inhibitors (NRTIs) induce proinflammatory cytokines in the CNS via Wnt5a signaling. Sci Rep. (2017) 7:4117. doi: 10.1038/s41598-017-03446-w
227. Yu F, Li W, Wang L, Dai Y, Lu X, Wang Q, et al. Combining new non-nucleoside reverse transcriptase inhibitors (RTIs) with AZT results in strong synergism against multi-RTI-resistant HIV-1 strains. Molecules. (2018) 23:1599. doi: 10.3390/molecules23071599
228. Yang Y, Liu X, Wu T, Zhang W, Shu J, He Y, et al. Quercetin attenuates AZT-induced neuroinflammation in the CNS. Sci Rep. (2018) 8:6194. doi: 10.1038/s41598-018-24618-2
229. Sörstedt E, Carlander C, Flamholc L, Hejdeman B, Svedhem V, Sönnerborg A, et al. Effect of dolutegravir in combination with Nucleoside Reverse Transcriptase Inhibitors (NRTIs) on people living with HIV who have pre-existing NRTI mutations. Int J Antimicrob Agents. (2018) 51:733–8. doi: 10.1016/j.ijantimicag.2018.01.009
230. Ciavatta VT, Bichler EK, Speigel IA, Elder CC, Teng SL, Tyor WR, et al. In vitro and Ex vivo Neurotoxic Effects of Efavirenz are Greater than Those of Other Common Antiretrovirals. Neurochem Res. (2017) 42:3220–32. doi: 10.1007/s11064-017-2358-x
231. Bienstock RJ, Copeland WC. Molecular insights into NRTI inhibition and mitochondrial toxicity revealed from a structural model of the human mitochondrial DNA polymerase. Mitochondrion. (2004) 4:203–13. doi: 10.1016/j.mito.2004.05.018
232. Lanman T, Letendre S, Ma Q, Bang A, Ellis R. CNS neurotoxicity of antiretrovirals. J Neuroimmune Pharmacol. (2021) 16:130–43. doi: 10.1007/s11481-019-09886-7
233. Sciannameo S, Zalazar V, Spadaccini L, Duarte M, Cahn P, Aristegui I, et al. Preference for long-acting injectable for ART and PrEP among people with and without HIV: a cross-sectional study in Argentina. Ther Adv Infect Dis. (2024) 11:20499361241228341. doi: 10.1177/20499361241228341
234. Benning L, Mantsios A, Kerrigan D, Coleman JS, Golub E, Blackstock O, et al. Examining adherence barriers among women with HIV to tailor outreach for long-acting injectable antiretroviral therapy. BMC Womens Health. (2020) 20:152. doi: 10.1186/s12905-020-01011-8
235. Dandachi D, Dang BN, Lucari B, Swindells S, Giordano TP. Acceptability and preferences for long-acting antiretroviral formulations among people with HIV infection. AIDS Care. (2021) 33:801–9. doi: 10.1080/09540121.2020.1764906
236. Leonard A, Broussard J, Wilson NL, Dawson-Rose C. Cabotegravir and rilpivirine: A long-acting injectable antiretroviral treatment for human immunodeficiency virus. J Nurse Practitioners. (2021) 18:17–21. doi: 10.1016/j.nurpra.2021.11.022
237. Levintow SN, Pence BW, Ha TV, Minh NL, Sripaipan T, Latkin CA, et al. Prevalence and predictors of depressive symptoms among HIV-positive men who inject drugs in Vietnam. PLoS One. (2018) 13:e0191548. doi: 10.1371/journal.pone.0191548
238. Andersen SL. Stress, sensitive periods, and substance abuse. Neurobiol Stress. (2019) 10:100140. doi: 10.1016/j.ynstr.2018.100140
239. Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic-pituitary-adrenal responses to mild acute stress. Neuropsychopharmacology. (2008) 33:721–30. doi: 10.1038/sj.npp.1301466
240. Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rössler A, et al. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biol Psychiatry. (2014) 76:289–96. doi: 10.1016/j.biopsych.2013.10.019
241. Massaccesi C, Willeit M, Quednow BB, Nater UM, Lamm C, Müller D, et al. Opioid-blunted cortisol response to stress is associated with increased negative mood and wanting of social reward. Neuropsychopharmacology. (2022) 47:1798–807. doi: 10.1038/s41386-022-01283-8
242. Ciucă Anghel DM, Nițescu GV, Tiron AT, Guțu CM, Baconi DL. Understanding the mechanisms of action and effects of drugs of abuse. Molecules. (2023) 28:4969. doi: 10.3390/molecules28134969
243. Tomas-Roig J, Havemann-Reinecke U. Gene expression signature in brain regions exposed to long-term psychosocial stress following acute challenge with cannabinoid drugs. Psychoneuroendocrinology. (2019) 102:1–8. doi: 10.1016/j.psyneuen.2018.11.023
244. Takahashi H, Takada Y, Nagai N, Urano T, Takada A. Effects of nicotine and footshock stress on dopamine release in the striatum and nucleus accumbens. Brain Res Bull. (1998) 45:157–62. doi: 10.1016/s0361-9230(97)00332-8
245. Holloway AL, Schaid MD, Lerner TN. Chronically dysregulated corticosterone impairs dopaminergic transmission in the dorsomedial striatum by sex-divergent mechanisms. Neuropsychopharmacology. (2023) 48:1328–37. doi: 10.1038/s41386-023-01551-1
246. Gutierrez J, Tedaldi EM, Armon C, Patel V, Hart R, Buchacz K. Sleep disturbances in HIV-infected patients associated with depression and high risk of obstructive sleep apnea. SAGE Open Med. (2019) 7:2050312119842268. doi: 10.1177/2050312119842268
247. Wu J, Wu H, Lu C, Guo L, Li P. Self-reported sleep disturbances in HIV-infected people: a meta-analysis of prevalence and moderators. Sleep Med. (2015) 16:901–7. doi: 10.1016/j.sleep.2015.03.027
248. Borker PV, Macatangay BJ, Margolick JB, Punjabi NM, Rinaldo CR, Stosor V, et al. Shorter total sleep time is associated with lower CD4+/CD8+ T cell ratios in virally suppressed men with HIV. Sleep Adv. (2024) 5:zpae001. doi: 10.1093/sleepadvances/zpae001
249. Petrakis V, Steiropoulos P, Papanas N, Trypsianis G, Papazoglou D, Panagopoulos P. Quality of sleep in people living with HIV in the era of highly active antiretroviral treatment. Int J STD AIDS. (2023) 34:191–202. doi: 10.1177/09564624221146608
250. Irwin MR, Archer G, Olmstead R, Brown TT, Teplin LA, Patel SR, et al. Increased risk of depression in non-depressed HIV infected men with sleep disturbance: Prospective findings from the Multicenter AIDS Cohort Study. EBioMedicine. (2018) 36:454–60. doi: 10.1016/j.ebiom.2018.09.028
251. Rogers BG, Lee JS, Bainter SA, Bedoya CA, Pinkston M, Safren SA. A multilevel examination of sleep, depression, and quality of life in people living with HIV/AIDS. J Health Psychol. (2020) 25:1556–66. doi: 10.1177/1359105318765632
252. Daubert E, French AL, Burgess HJ, Sharma A, Gustafson D, Cribbs SK, et al. Association of poor sleep with depressive and anxiety symptoms by HIV disease status: women’s interagency HIV study. J Acquir Immune Defic Syndr. (2022) 89:222–30. doi: 10.1097/qai.0000000000002847
253. Liu H, Wu X, Wang Y, Liu X, Peng D, Wu Y, et al. TNF-α, IL-6 and hsCRP in patients with melancholic, atypical and anxious depression: an antibody array analysis related to somatic symptoms. Gen Psychiatr. (2022) 35:e100844. doi: 10.1136/gpsych-2022-100844
254. Huang Y, Jiang Y, Zhu M. The relationship between global sleep score and inflammatory markers in obese adults from the United States. Nat Sci Sleep. (2019) 11:317–24. doi: 10.2147/nss.s220436
255. Davis I, Liu A. What is the tryptophan kynurenine pathway and why is it important to neurotherapeutics? Expert Rev Neurother. (2015) 15:719–21. doi: 10.1586/14737175.2015.1049999
256. Dantzer R. Role of the kynurenine metabolism pathway in inflammation-induced depression: preclinical approaches. Curr Top Behav Neurosci. (2017) 31:117–38. doi: 10.1007/7854_2016_6
257. Cho HJ, Savitz J, Dantzer R, Teague TK, Drevets WC, Irwin MR. Sleep disturbance and kynurenine metabolism in depression. J Psychosom Res. (2017) 99:1–7. doi: 10.1016/j.jpsychores.2017.05.016
258. Sultana S, Elengickal A, Bensreti H, Belin de Chantemèle E, McGee-Lawrence ME, Hamrick MW. The kynurenine pathway in HIV, frailty and inflammaging. Front Immunol. (2023) 14:1244622. doi: 10.3389/fimmu.2023.1244622
259. Alum EU, Obeagu EI, Ugwu OPC, Samson AO, Adepoju AO, Amusa MO. Inclusion of nutritional counseling and mental health services in HIV/AIDS management: A paradigm shift. Med (Baltimore). (2023) 102:e35673. doi: 10.1097/md.0000000000035673
260. Gebru TH, Mekonen HH, Kiros KG. Undernutrition and associated factors among adult HIV/AIDS patients receiving antiretroviral therapy in eastern zone of Tigray, Northern Ethiopia: a cross-sectional study. Arch Public Health. (2020) 78:100. doi: 10.1186/s13690-020-00486-z
261. Shifera N, Yosef T, Matiyas R, Kassie A, Assefa A, Molla A. Undernutrition and Associated Risk Factors among Adult HIV/AIDS Patients Attending Antiretroviral Therapy at Public Hospitals of Bench Sheko Zone, Southwest Ethiopia. J Int Assoc Provid AIDS Care. (2022) 21:23259582221079154. doi: 10.1177/23259582221079154
262. Alebel A, Kibret GD, Petrucka P, Tesema C, Moges NA, Wagnew F, et al. Undernutrition among Ethiopian adults living with HIV: a meta-analysis. BMC Nutr. (2020) 6:10. doi: 10.1186/s40795-020-00334-x
263. Sunguya BF, Ulenga NK, Siril H, Puryear S, Aris E, Mtisi E, et al. High magnitude of under nutrition among HIV infected adults who have not started ART in Tanzania–a call to include nutrition care and treatment in the test and treat model. BMC Nutr. (2017) 3:58. doi: 10.1186/s40795-017-0180-0
264. Seid A, Seid O, Workineh Y, Dessie G, Bitew ZW. Prevalence of undernutrition and associated factors among adults taking antiretroviral therapy in sub-Saharan Africa: A systematic review and meta-analysis. PLoS One. (2023) 18:e0283502. doi: 10.1371/journal.pone.0283502
265. Lee BJ. Association of depressive disorder with biochemical and anthropometric indices in adult men and women. Sci Rep. (2021) 11:13596. doi: 10.1038/s41598-021-93103-0
266. Kaner G, Soylu M, Yüksel N, Inanç N, Ongan D, Başmısırlı E. Evaluation of nutritional status of patients with depression. BioMed Res Int. (2015) 2015:521481. doi: 10.1155/2015/521481
267. Gougeon L, Payette H, Morais JA, Gaudreau P, Shatenstein B, Gray-Donald K. Intakes of folate, vitamin B6 and B12 and risk of depression in community-dwelling older adults: the Quebec Longitudinal Study on Nutrition and Aging. Eur J Clin Nutr. (2016) 70:380–5. doi: 10.1038/ejcn.2015.202
268. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:100–7. doi: 10.1192/bjp.bp.111.106666
269. Penckofer S, Kouba J, Byrn M, Estwing Ferrans C. Vitamin D and depression: where is all the sunshine? Issues Ment Health Nurs. (2010) 31:385–93. doi: 10.3109/01612840903437657
270. Ciesielski TH, Williams SM. Low Omega-3 intake is associated with high rates of depression and preterm birth on the country level. Sci Rep. (2020) 10:19749. doi: 10.1038/s41598-020-76552-x
271. Nguyen TTT, Miyagi S, Tsujiguchi H, Kambayashi Y, Hara A, Nakamura H, et al. Association between Lower Intake of Minerals and Depressive Symptoms among Elderly Japanese Women but Not Men: Findings from Shika Study. Nutrients. (2019) 11:389. doi: 10.3390/nu11020389
272. Miki T, Kochi T, Eguchi M, Kuwahara K, Tsuruoka H, Kurotani K, et al. Dietary intake of minerals in relation to depressive symptoms in Japanese employees: the Furukawa Nutrition and Health Study. Nutrition. (2015) 31:686–90. doi: 10.1016/j.nut.2014.11.002
273. Aly J, Engmann O. The way to a human’s brain goes through their stomach: dietary factors in major depressive disorder. Front Neurosci. (2020) 14:582853. doi: 10.3389/fnins.2020.582853
274. Qing H, Desrouleaux R, Israni-Winger K, Mineur YS, Fogelman N, Zhang C, et al. Origin and function of stress-induced IL-6 in murine models. Cell. (2020) 182:372–87.e14. doi: 10.1016/j.cell.2020.05.054
275. Rubin LH, Cho K, Bolzenius J, Mannarino J, Easter RE, Dastgheyb RM, et al. Mental health phenotypes of well-controlled HIV in Uganda. Front Public Health. (2024) 12:1407413. doi: 10.3389/fpubh.2024.1407413
276. Huang Y, Luo D, Chen X, Zhang D, Huang Z, Xiao S. HIV-related stress experienced by newly diagnosed people living with HIV in China: A 1-year longitudinal study. Int J Environ Res Public Health. (2020) 17:2681. doi: 10.3390/ijerph17082681
277. Seth P, Kidder D, Pals S, Parent J, Mbatia R, Chesang K, et al. Psychosocial functioning and depressive symptoms among HIV-positive persons receiving care and treatment in Kenya, Namibia, and Tanzania. Prev Sci. (2014) 15:318–28. doi: 10.1007/s11121-013-0420-8
278. Waldron EM, Burnett-Zeigler I, Wee V, Ng YW, Koenig LJ, Pederson AB, et al. Mental health in women living with HIV: the unique and unmet needs. J Int Assoc Provid AIDS Care. (2021) 20:2325958220985665. doi: 10.1177/2325958220985665
279. Celeste-Villalvir A, Payan DD, Armenta G, Palar K, Then-Paulino A, Acevedo R, et al. Exploring gender differences in HIV-related stigma and social support in a low-resource setting: A qualitative study in the Dominican Republic. PLoS One. (2023) 18:e0290228. doi: 10.1371/journal.pone.0290228
280. Shi P, Yang A, Zhao Q, Chen Z, Ren X, Dai Q. A hypothesis of gender differences in self-reporting symptom of depression: implications to solve under-diagnosis and under-treatment of depression in males. Front Psychiatry. (2021) 12:589687. doi: 10.3389/fpsyt.2021.589687
Keywords: HIV, depression, factors, pathogenesis, mechanisms
Citation: Zaongo SD, Wu W and Chen Y (2025) Pathogenesis of HIV-associated depression: contributing factors and underlying mechanisms. Front. Psychiatry 16:1557816. doi: 10.3389/fpsyt.2025.1557816
Received: 10 January 2025; Accepted: 31 March 2025;
Published: 17 April 2025.
Edited by:
Yueheng Tang, Huazhong University of Science and Technology, ChinaReviewed by:
Addisu Girma Komba, Dr. Bogalech Gebre Memorial General Hospital, EthiopiaJiang Zhongsheng, Liuzhou People’s Hospital, China
Kim Madundo, Kilimanjaro Christian Medical University College, Tanzania
Copyright © 2025 Zaongo, Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaokai Chen, eWFva2FpY2hlbkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Silvere D. Zaongo
Silvere D. Zaongo Wenlin Wu1,2†
Wenlin Wu1,2† Yaokai Chen
Yaokai Chen