
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 11 April 2025
Sec. Schizophrenia
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1552451
This article is part of the Research TopicUnraveling the Nexus of Inflammatory Biomarkers and Cognitive Dysfunction in Schizophrenia: Implications for Treatment and Disease ProgressionView all 4 articles
Objective: The present study sought to evaluate the correlation between cognitive impairment (CI) and inflammatory indicators such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammatory index (SII) in schizophrenia patients.
Methods: This study included 331 schizophrenia inpatients. General data and laboratory findings (neutrophil, lymphocyte, platelet, etc.) were gathered, and then calculating NLR, PLR, and SII. A Chinese version of the Mini Mental State Examination (MMSE) was used for the assessment of cognitive function, and then the patients in the CI group were categorized into mild CI, moderate CI, and severe CI groups. Comparing the differences in NLR, PLR, and SII between the CN group and the CI group, as well as different CI groups, and analyzing the relationship between the NLR, PLR, and SII and the mechanism of CI in schizophrenia.
Results: There were 145 (43.8%) patients with cognitive impairment. Compared to the CN group, the CI group had higher NLR, PLR, and SII than the CN group, although their lymphocyte was lower. The NLR and SII were higher in the moderate CI group than in the mild CI group. NLR, PLR, and SII were significantly inversely correlated with the total score of cognitive function and scores across all aspects, whereas lymphocytes were considerably positively correlated. Higher NLR, PLR, and SII were substantially related to an increased risk of CI, but higher lymphocytes were associated with a decreased risk of CI.
Conclusion: NLR, PLR, and SII may be serum inflammatory markers of CI in schizophrenia, and lymphocytes may be protective variables for cognitive function in schizophrenia.
The cause of schizophrenia, a severe mental condition, remains unknown. It impairs patients’ social functioning by causing anomalies in perception, emotion, will, conduct, and thought. Positive and negative symptoms were previously believed to be the most common forms of symptoms related to schizophrenia. In recent years, psychiatrists have increasingly focused on cognitive impairment (CI) as an independent third core symptom of schizophrenia, based on an increasing number of clinicalobservations and research findings. CI refers to visuospatial orientation, language, calculation, memory, executive, and comprehension judgment of several degrees of impairment (1–3), reflecting the patient’s function. Studies have shown CI has more severe effects on patients’ social functioning. Schizophrenia patients usually have worse cognitive function compared to the general population (4, 5). Even though a patient’s psychiatric symptoms have subsided, CI still exists, causing decreased treatment adherence and increased risk of relapse of the illness, which is closely linked to unfavorable outcomes (6). Therefore, it is important to find effective cognitive interventions to improve the prognosis of patients with schizophrenia. However, there are presently no reliable medications to treat CI in schizophrenic patients. Antipsychotic drugs could improve psychiatric symptoms but do not improve cognitive function (7). We may find more effective ways to improve patients’ cognitive function if we have a deeper understanding of the biological mechanism of cognitive CI in schizophrenia patients. However, the pathophysiology of CI in schizophrenia is yet unknown.
Schizophrenia, a complex psychiatric disorder with unclear etiology, involves multi-system interactions in its pathophysiology. Immune dysregulation has been consistently observed in schizophrenia patients, with chronic inflammation implicated in disease mechanisms (8). Ballaz and Bourin et al. (9) emphasized the potential critical role of neuroinflammation in schizophrenia pathogenesis. They proposed that microglial activation, cytokine imbalance, hypothalamic-pituitary-adrenal (HPA) axis dysregulation, oxidative stress, and kynurenine pathway alterations might collectively disrupt neurotransmitter regulation and neuroplasticity, potentially manifesting as clinical symptoms. Specifically, cerebral inflammation in schizophrenia patients activates microglia to release pro-inflammatory factors, disrupting neuronal function and synaptic plasticity, which may manifest as clinical symptoms. Cytokine imbalance could influence schizophrenia pathology through its effects on dopamine and glutamate systems. Furthermore, the authors highlighted bidirectional interactions between neuroinflammation and chronic stress: sustained stress activates the HPA axis, increases cortisol release, and subsequently induces systemic inflammatory responses. Notably, current evidence remains inconclusive regarding whether the development of cognitive impairment (CI) in schizophrenia patients involves peripheral inflammation or neuroinflammatory alterations. In recent years, there has been a growing body of research exploring the relationship between cognitive function and inflammatory responses in patients with mental disorders. Increasing evidence suggests that inflammatory responses are closely related to cognitive impairment (CI) and may be an important mechanism underlying the occurrence of CI (10). Studies have found that the level of cognitive function in patients with bipolar disorder and depression disorder is related to inflammatory cytokines (11, 12). It has also been suggested that inflammation may be an important neuropathologic mechanism for cognitive decline and dementia in elderly patients (13). Studies on schizophrenia have also discovered a negative correlation between CRP (C-reactive protein, CRP) levels and cognitive function in individuals with schizophrenia (14, 15). In addition, when antipsychotic medications are combined with immunomodulators or anti-inflammatory treatments, cognitive improvement is more noticeable in people with schizophrenia (16). A review has concluded that CI in schizophrenia may be related to peripheral inflammation (17), when the body experiences inflammation, the central nervous system’s local inflammatory response overactivated the brain’s microglia, causing them to release high levels of inflammatory mediators like IL-6, TNF-α, IL-1β, and other cytokines, which impairs cognitive function (18–20). Therefore, the activation of inflammatory response and the dysregulation of the immune system may play an important role in schizophrenia CI, and peripheral inflammatory markers may be a simple and effective biological target for improving cognitive function in schizophrenia patients. However, Current research remains limited regarding the association between cognitive impairment and immune-inflammatory responses in schizophrenia, and traditional inflammatory cytokines often require complex techniques like ELISA require specialized equipment and trained personnel, posing significant technical and financial challenges. There is an urgent need to identify accessible and cost-effective inflammatory biomarkers for clinical studies. In recent years, Neutrophils and lymphocytes ratio (NLR), platelet and lymphocyte ratio (PLR), systemic immune inflammation index (systemic immune - inflammation index, SII index) [calculating formula for neutrophils (platelets lymphocyte ratio] such as a new type of periarteritis). These indicators are easy to evaluate, low cost, can be obtained from retrospective data, and can accurately represent the body’s inflammation, so they have been applied in more and more studies (21–23). Compared to traditional inflammation markers (e.g., IL-6, TNF-α, IL-1β, etc.), NLR, PLR, and SII are easier and cheaper to measure. These markers use simple blood test results (already done in most clinics) and don’t require extra costs or equipment. They are especially useful for large studies or areas with limited resources. Traditional markers like CRP mainly show short-term inflammation and can vary easily with external factors. In contrast, NLR, PLR, and SII track changes in common blood cells (neutrophils, lymphocytes, platelets) over time. These combined measures are more stable and give a fuller picture of immune system activity in mental disorders compared to single markers like CRP. Therefore, NLR, PLR and SII, as easily accessible, low-cost, more comprehensive and stable biomarkers, can be more easily integrated into routine clinical practice for early detection and monitoring of cognitive impairment in schizophrenic patients. This research investigates the relationship between NLR, PLR, SII index, and the risk of CI in schizophrenia patients through cross-sectional studies. Furthermore, it investigates the function of immunological inflammatory response in the etiology of CI in schizophrenia patients, providing theoretical evidence for future research. It provides a new idea and method for cognitive therapy of schizophrenic patients.
The subjects of the study were schizophrenia patients hospitalized in Minhang Mental Health Center from March 1, 2024, to October 31, 2024. The patients were enrolled according to admission criteria and divided into CI and CN (Cognitive normal) groups according to cognitive function level. The patients in the CI group were categorized into three groups: mild CI group, moderate CI group, and severe CI group.
Entry criteria: (1) Age 18-75 years old, regardless of gender; (2) It meets the diagnostic criteria of schizophrenia in ICD-10(International Classification of Disease-10, ICD-10); (3) Have sufficient audiovisual and understanding ability, no language communication barriers, and can complete the survey and cognitive function test (4); Obtaining informed consent.
Exclusion criteria: (1) patients with dementia, epilepsy, stroke, brain trauma, and other diseases that seriously affect cognitive function; (2) Long-term chronic infectious diseases, tumors, and other serious physical diseases, recent trauma, surgery, there are obvious infections; (3) Patients who have received immunosuppressants, non-steroidal anti-inflammatory drugs, antibiotics, glucocorticoids or MECT within the last 1 month; (4) Intellectual Disability, Down’s syndrome and dementia; (5) Women during pregnancy or breastfeeding; Tobacco, alcohol and other psychoactive substance abusers.
The study followed the principles of the Declaration of Helsinki, and is approved by the Ethics Committee of Shanghai Minhang Mental Health Center (LW202401).
The research group created the questionnaire, which included name, age, gender, marital status, education level, occupational status, length of hospital stay, prior history, family history, age of onset, course of disease, medication, etc. To analyze antipsychotic medication effects on inflammatory markers in schizophrenia, this study stratifies participants into three pharmacotherapy groups: Typical antipsychotic monotherapy (e.g., haloperidol), Atypical antipsychotic monotherapy (e.g., risperidone), Combination therapy (typical + atypical agents). Medication data were extracted from electronic medical records. All antipsychotic doses were standardized to chlorpromazine equivalents (CPZE) using validated conversion methods.
Trained psychiatrists used a brief mental state examination (MMSE) to assess cognitive function. MMSE is one of the most prevalent and widely used CI assessment tools in the world, and is particularly suitable for rapid assessment of hospitalized patients. The scale contains five dimensions: orientation, memory, attention, numeracy, and language, and recall. MMSE has been shown to have good reliability and validity in patients with schizophrenia, and its advantages such as simple operation and easy access to coordination have been widely used in the assessment of cognitive function in psychiatric patients. With an overall MMSE score of 30, the cut-off point for screening for cognitive impairment in patients with schizophrenia is 24, and patients with an MMSE score of 24 and below are considered to have cognitive impairment (CI) (24).
Blood samples (5 mL) were collected from fasting patients via antecubital venipuncture under standardized phlebotomy protocols between 06:00–07:00 AM using EDTA-K2 vacuum tubes. Samples were mixed gently for 5 minutes, briefly remixed, and transported to the Clinical Laboratory of Shanghai Minhang Mental Health Center within 2 hours for complete blood count (CBC) analysis on the XN-1000 SA-01 automated hematology analyzer. Record the neutrophil count, lymphocyte count, monocyte count, and platelet count, then calculate the values of NLR, PLR, and SII index by the formula. All patients were required to fast for more than 12 hours before blood was extracted, high-protein, greasy food, and alcohol were prohibited the day before blood was extracted.
Statistical analyses were conducted using SPSS 26.0. Continuous variables were first assessed for normality via the Shapiro-Wilk test. As all continuous variables exhibited non-normal distributions (skewed), data were reported as median (interquartile range) [M (P25, P75)]. Categorical data were compared via chi-square (χ²) tests, presented as frequencies (%). Between-group differences in continuous variables used nonparametric tests (Mann-Whitney U/Kruskal-Wallis), with Bonferroni corrected pairwise comparisons (P<0.0167 for 3+ groups). Spearman correlation analyzed variable relationships. Statistical significance was defined as P<0.05.
This study included 331 patients in total, 186 (56.2%) in the CN group and 145 (43.8%) in the CI group. Since all continuous variables are skewed, the median (interquartile range) [M (P25, P75)] is used to express them.
There was no significant difference between the CN and CI groups in terms of gender, history of alcohol and tobacco use prior to hospitalization, family history of mental illness, history of diabetes or hypertension, BMI, first -generation or second-generation use of antipsychotic drugs, use of combination drugs, and uric acid. The two groups differed significantly in terms of age, marriage, education, age of first onset, and equivalent dose of chlorpromazine (P < 0.05). Compared with the CN group, schizophrenia patients with CI had a higher age, a lower proportion of high school or above education, a higher proportion of divorced and widowed patients, and a lower equivalent dose of chlorpromazine (Table 1).
Significant differences were seen in NLR, PLR, SII, and lymphocytes levels between the CN and CI groups (P < 0.001). The NLR [2.00(1.62-2.70)], PLR [140(107.41-180.00)], and SII [498.75(363.61-640.79)] in the CI group were higher than the NLR [1.67 (1.24-2.22)], PLR 114.50 (91.13-147.56)], SII[397.73 (247.85-566.31)] in the CN group, whereas the lymphocytes 1.60 (1.30-2.15] in the CI group was lower than [the lymphocytes [2.10(1.70-2.50)] in the CN group. There was no significant difference in neutrophils and platelets counts between the two groups(P>0.05) (Figure 1).
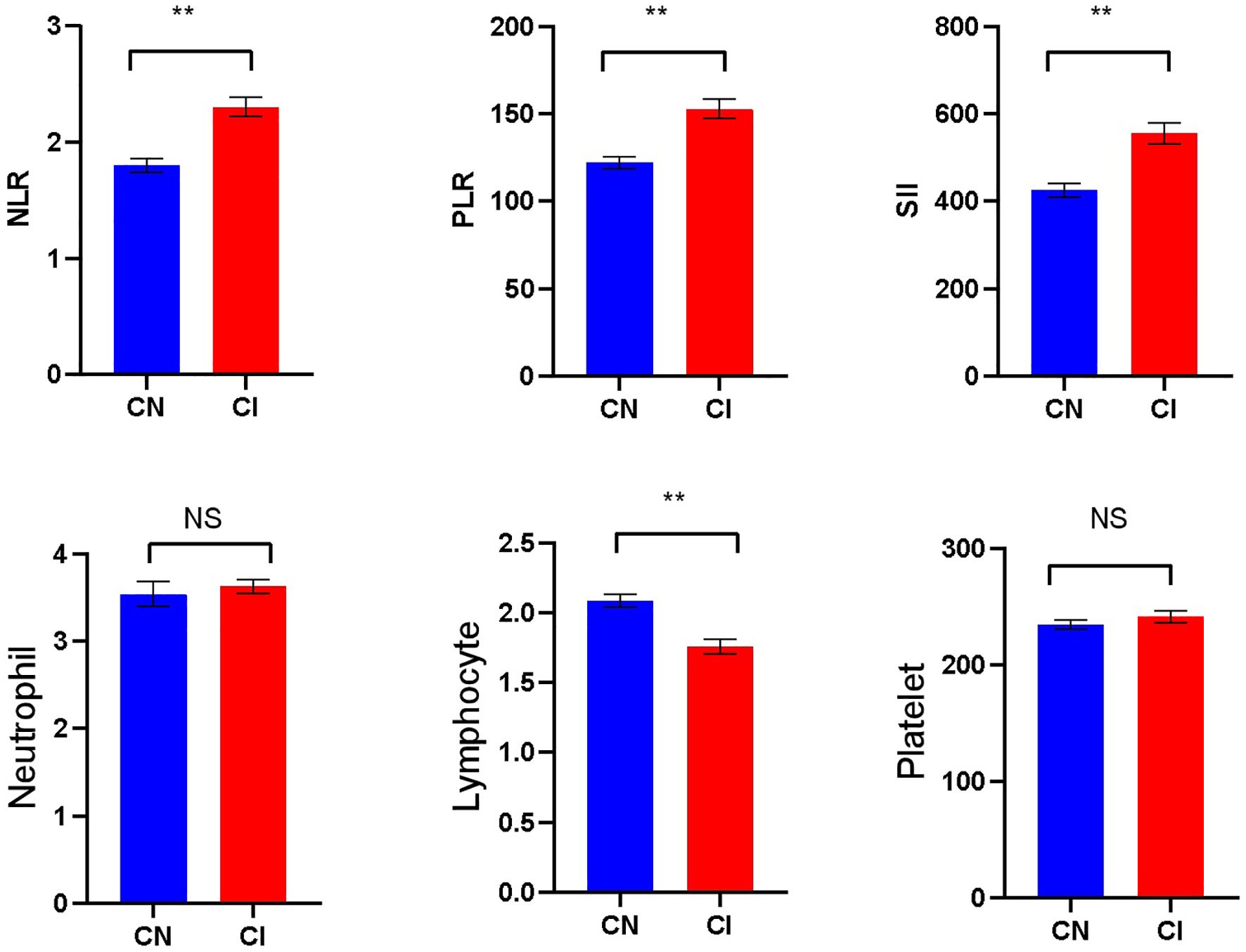
Figure 1. Comparison of laboratory indicators betweenthe CN and CI groups (** p<0.001; NS, No significant).
There were statistically significant differences in the levels of NLR and SII among schizophrenia patients in the mild, moderate, and severe CI groups (P<0.05), but no significant differences in the levels of PLR, neutrophils, lymphocytes, and platelets(P>0.05). Bonferroni correction was applied to three pairwise comparisons (Mann-Whitney U) across three groups, with a corrected significance threshold of α = 0.05/3 = 0.0167. Group differences were considered statistically significant at P < 0.0167. It was found that there was a significant difference in NLR (P=0.002) only between patients in the mild CI and moderate CI groups, and the NLR [2.23 (1.78-2.93)] in the moderate CI group was higher than the NLR [1.77 (1.46-2.27)] in the mild CI group. There was no statistically significant differences between the remaining groups (P > 0.0167) (Figure 2).
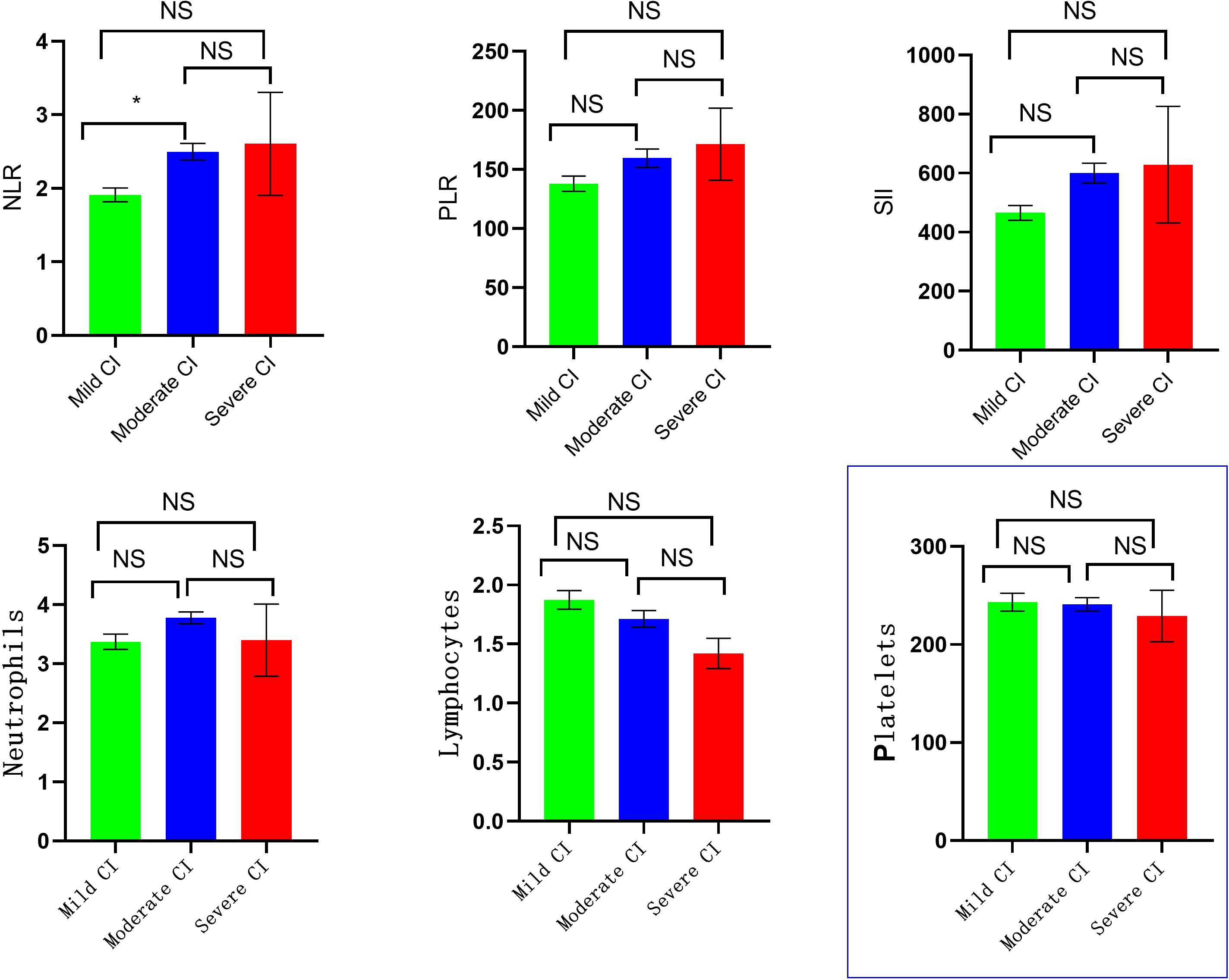
Figure 2. Comparison of laboratory indicators in different degrees of CI group. (* p < 0.0167, NS, No Significant).
The correlation between each research factor and cognitive function score was analyzed by Spearman correlation analysis. The findings demonstrated that lymphocytes had a significantly positive correlation with the overall score of cognitive function and scores of all dimensions, while NLR, PLR, and SII had a significantly negative correlation with these scores. Neutrophils were only negatively correlated with the total score of cognitive function, attention and computation scores. There was no significant correlation between platelets and the total score of cognitive function, as well as the scores of the various dimensions (Figure 3).
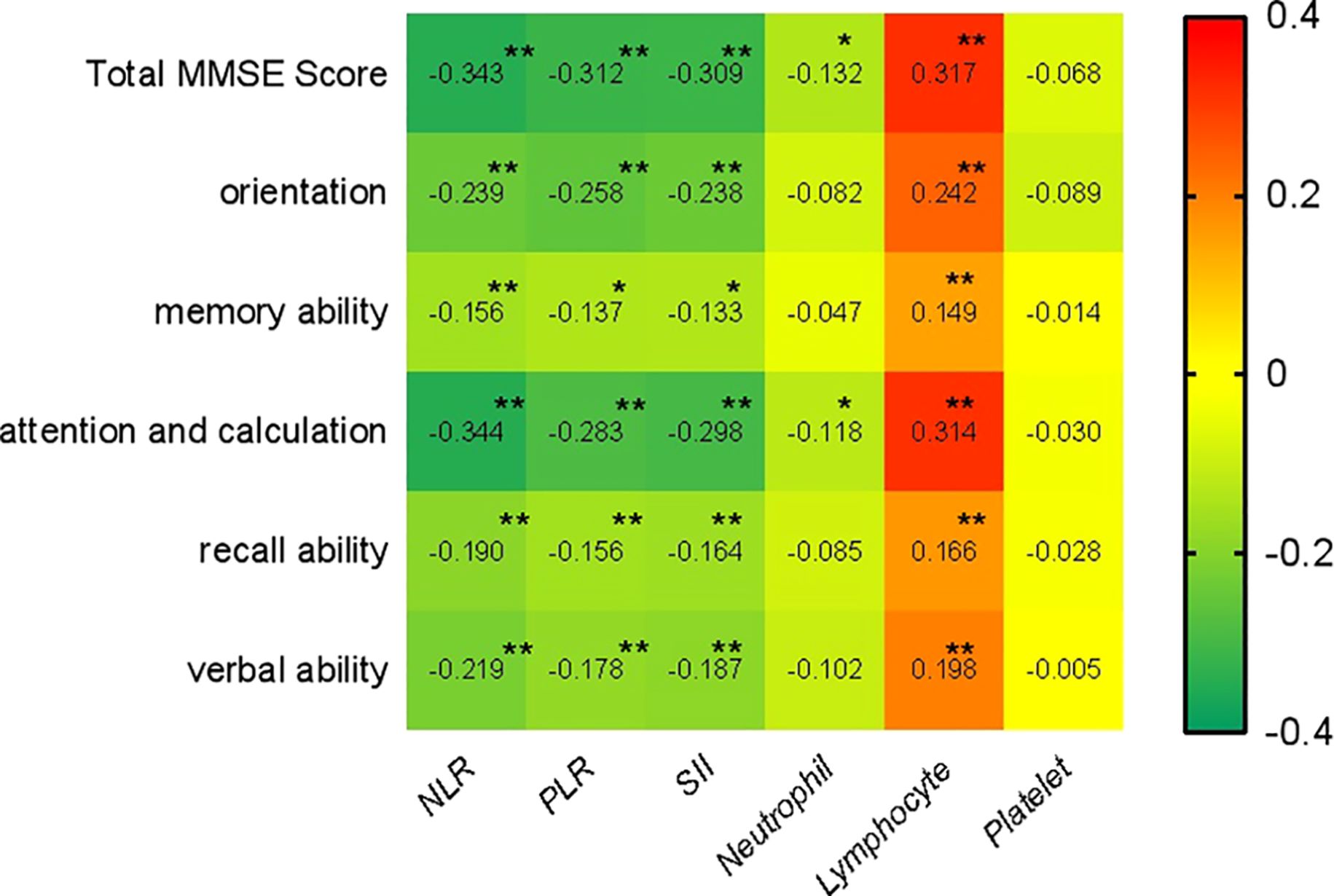
Figure 3. Spearman Spearman correlation analysis of NLR, PLR, and SII with cognitive function. (** p < 0.001, * p < 0.05).
The correlation between different inflammatory markers and the CI risk were analyzed by the logistic regression method. With cognitive impairment (CI) status (0 = no CI, 1 = CI) as the dependent variable, and NLR, PLR, SII, neutrophils, lymphocytes, and platelets were each tested as independent variables in separate logistic regression models, the analysis results showed that without adjusting any covariates, the higher NLR(OR: 1.894, 95%CI: 1.435-2.499), PLR (OR: 1.011,95%CI: 1.006-1.016), SII (OR: 1.002, 95%CI: 1.001-1.003) were significantly associated with a higher CI risk, and higher lymphocytes (OR: 0.413, 95%CI: 0.281-0.605) were associated with a lower CI risk. After accounting for confounding factors such as sex, age, education, marriage, alcohol use, smoking history, age of first onset, total duration of schizophrenia, family history of psychosis, hypertension, diabetes, antipsychotic drug use (Typical antipsychotic monotherapy, Atypical antipsychotic monotherapy or Combination therapy), chlorpromazine equivalent dose (CPZE), BMI, uric acid, the results still showed a higher NLR(OR:2.033, 95%CI: 1.507-2.743), PLR (OR: 1.012, 95%CI: 1.007-1.018), SII (OR: 1.003, 95%CI:1.001-1.004) were significantly associated with a higher CI risk, and higher lymphocytes(OR: 0.367, 95%CI: 0.235-0.574) were associated with a lower CI risk. There was no significant correlation between neutrophils and platelets and the CI risk (Table 2). Stratified analyses of key confounders (age, illness duration, chlorpromazine equivalents, medication regimens [first generation antipsychotics, second-generation antipsychotics, or combination]) demonstrated no significant interactions with NLR, PLR, or SII (P value for interaction>0.05).The inflammatory marker-cognition associations maintained directional consistency across strata, confirming robustness (Supplementary Tables 1–6).
Linear regression analyses were performed with MMSE score as the dependent variable, modeling NLR, PLR, SII, neutrophils, lymphocytes, and platelets each as the independent variable in separate models, the analysis showed that NLR, PLR, and SII were negatively correlated with the total cognitive function score and the scores of each dimension (β < 0, p < 0.001), and lymphocyte was positively correlated with the total cognitive function score and the scores of each dimension (β > 0, p < 0.001), when covariates were not adjusted. Neutrophils, platelets, and total cognitive function score and each dimension score were not significantly correlated. After accounting for confounding factors such as sex, age, education, marriage, alcohol use, smoking history, age of first onset, total duration of schizophrenia, family history of psychosis, hypertension, diabetes, antipsychotic drug use (Typical antipsychotic monotherapy, Atypical antipsychotic monotherapy or Combination therapy), chlorpromazine equivalent dose (CPZE), BMI, uric acid, the results still showed that NLR, PLR, and SII were negatively correlated with the total cognitive function score and each dimension (β < 0, p < 0.001), and lymphocytes were positively correlated with the total cognitive function score and each dimension (β > 0, p < 0.001). There was no significant correlation between neutrophils, platelets and total cognitive function score and each dimension score (Table 3). Variance inflation factor (VIF) analysis revealed values of 1.084 (NLR), 1.092 (PLR), 1.063 (SII), 1.019 (neutrophils), 1.163 (lymphocytes), and 1.068 (platelets), all below the threshold of 5, indicating no significant multicollinearity.
ROC curves evaluated NLR, PLR, SII, neutrophils, lymphocytes, and platelets as predictors of cognitive impairment in schizophrenia. Significant predictors (P<0.05) included NLR (AUC=0.66, 95%CI 0.60–0.72), PLR (AUC=0.65, 95%CI 0.59–0.71), SII (AUC=0.65, 95%CI 0.59–0.71), and lymphocytes (AUC=0.34, 95%CI 0.28–0.40). NLR, PLR, and SII showed moderate diagnostic efficacy (AUC>0.65), with optimal cutoffs at 1.73 (NLR), 131.5 (PLR), and 403 (SII). Lymphocytes demonstrated inverse prediction (AUC<0.5). Neutrophils (AUC=0.55, P=0.11) and platelets (AUC=0.53, P=0.40) showed no predictive value (Table 4).
NLR, PLR, and SII are blood biomarkers reflecting the inflammatory state of the body, which have attracted much attention in recent years, and they are new research hotspots in the field of cognitive disorders. Compared to conventional cytokine biomarkers (IL-6, IL-1β, TNF α), hematological indices (NLR, PLR, SII) provide cost-effective clinical utility through routine complete blood count (CBC) testing, less subject to transient fluctuations, and stably capture multidimensional features of chronic immune dysregulation, enabling direct integration into standardized diagnostic protocols for inflammatory monitoring. NLR, PLR, and SII are cheaper and easier to obtain than traditional inflammatory factors including interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α). Additionally, neutrophils, lymphocytes, and platelets play a crucial role in the inflammatory response. When inflammation occurs in the body, neutrophils increase and activate the adaptive immune response mediated by lymphocytes, resulting in lymphocyte apoptosis, and then increasing the levels of NLR. NLR thus represents a balance between two complementary immune pathways (intrinsic and adaptive immunity) (25), is less affected by various other states, and is more stable than counting white blood cell subsets alone (26), with more reliable and accurate results. Platelets are non-specific inflammatory indicators that are commonly associated with hemostasis and thrombosis. Recent research has revealed that platelets play an essential role in the body’s inflammatory response and immunological regulation (27, 28). Neutrophils, platelets, and lymphocytes participate in different inflammatory or immune pathways in the body, while NLR, PLR, and SII combine these three complementary cells, so they are more reliable and predictive than evaluating the effects of neutrophils, platelets, or lymphocytes alone, because they can objectively reflect the balance of systemic inflammation and immunological response, are less impacted by confounding factors, and can be used to evaluate chronic systemic inflammation (29). In recent years, NLR, PLR, and SII have been gradually applied to the study of various psychiatric disorders (30–32). Some scholars have used inflammatory markers such as NLR and PLR in studies on schizophrenia patients, and have obtained meaningful findings. Multiple studies have found that peripheral blood lymphocyte counts are reduced and neutrophil counts and NLR are increased (33–36) in schizophrenia patients compared to controls, and higher NLR may be a risk factor for schizophrenia patients (37). In addition, studies have found that NLR levels are associated with positive symptoms of schizophrenia, but not with negative symptoms (38). Studies have also shown that NLR levels is higher in the acute phase of schizophrenia than in the remission phase, and there may be some differences in NLR in different disease stages (39, 40). Additionally, Chinese researchers have discovered that the NLR and PLR of schizophrenia patients are higher than those of the healthy control group, as well as the NLR of schizophrenia patients and the healthy control group is different significantly (41). All of the researchers mentioned above have found that schizophrenia patients have abnormal markers such as NLR and PLR, which may be linked to various symptoms and the development of schizophrenia. However, few studies have investigated whether cognitive impairment in schizophrenia patients is linked to neutrophils, lymphocytes, NLR, PLR, SII, and so on.
Previous research has shown that higher NLR is associated with an increased risk of mild cognitive impairment in AD (42). The AKL study also discovered that PD patients’ NLR was considerably higher than that of healthy people (43). In research on elderly patients with ischemic stroke, NLR levels were discovered to be considerably greater in the cognitively impaired group than in the healthy control group, and NLR levels were associated with the severity of cognitive impairment (44). Some domestic scholars have evaluated the relationship between NLR level and cognitive function of elderly people in Chinese communities and found that the NLR level of elderly people with mild cognitive impairment is significantly increased (45, 46). There have been few investigations on the relationship between PLR, SII levels and cognitive function. Study on diabetes have found that PLR levels are significantly associated with cognitive decline in patients with type 2 diabetes (47). Another study monitored SII and cognitive function of patients before and after orthopedic surgery found that patients with higher SII before surgery were more likely to have lower cognitive function after surgery (48). These studies have shown that NLR, PLR, and SII are associated with impaired cognitive function in a variety of disorders and may have an important role in the development of cognitive impairment. This is consistent with our findings that in the present study, we found that schizophrenia patients in the group with cognitive impairment had higher NLR, PLR, and SII than patients in the cognitively normal group, and correlation analyses and logistic regression modeling also found that elevated NLR, PLR, and SII were associated with higher cognitive impairment. After incorporating confounding factors such as age, total duration of illness, and medication use, the above results were still presented, suggesting that cognitive dysfunction in schizophrenic patients may be somehow associated with alterations in NLR, PLR, and SII. NLR, PLR, and SII, as a composite of neutrophil, lymphocyte, and platelet counts, may reflect peripheral inflammation in the organism to some extent. In recent years, more and more evidence has shown that peripheral inflammation can also cross the blood-brain barrier to induce central inflammation and activate microglia, thus leading to the occurrence of cognitive impairment (49). The cerebral cortex and hippocampus contain high levels of pro-inflammatory cytokines (PICs) and their receptors, so the hippocampus is more vulnerable to inflammatory attack, which will affect the occurrence and transmission of nerves, destroy synaptic plasticity, and lead to atrophy of dendritic branches. Resulting in impaired cognitive function and brain atrophy (50). The brain tissue of schizophrenia patients is in a state of immunological inflammation, resulting in the release of a range of cytokines, which not only regulate cell growth and differentiation, but also participate in the body’s inflammatory damage process, while activating microglia of the central system, enhancing the synthesis of neurotoxic mediators (inflammatory factors and free radicals, etc.), and activating neurons’ inflammatory response. It leads to reduced expression of neuroplasticity protein, which is closely related to the pathogenesis of cognitive impairment (51–54). A growing body of research suggests that cognitive function is mediated by bidirectional interactions between the brain and the immune system (9, 55). Stress-induced activation of the HPA axis releases glucocorticoids, which modulate immune cell activity and inflammatory factor secretion. Reverse causality—cognitive impairment driving immune dysregulation—may also occur: neuroimmunoregulatory dysfunction in cognitively impaired patients may aberrantly activate peripheral immunity, elevating the neutrophil-to-lymphocyte ratio (NLR), platelet-to lymphocyte ratio (PLR), and systemic immune-inflammation index (SII). As across-sectional design, this study cannot establish temporal precedence between inflammation and cognitive decline. Longitudinal studies tracking marker-trajectory and cognitive changes are needed to disentangle causality. In addition, Previous studies have suggested that NLR, PLR, and SII may be associated with systemic oxidative injury through the following mechanisms: (i) neutrophil activation accompanied by ROS Release (56); (ii) platelet-derived ROS promote oxidative damage to the vascular endothelium (57); (iii) oxidative stress induced lymphocyte apoptosis may amplify NLR/PLR abnormalities (58). Oxidative stress markers were not directly measured in this study, It is difficult to fully distinguish whether these marker changes stem primarily from peripheral inflammation, systemic oxidative stress, or their combination. Future studies should employ multi-omics approaches to clarify pathways.
In this study, multiple dimensions of cognitive function were correlated, and it was found that NLR, PLR, and SII were negatively correlated with the patients’ verbal ability, memory ability, attention and calculation ability, and orientation scores. This suggests that NLR, PLR, and SII may be associated with impaired cognitive function across multiple dimensions. This is consistent with the study of Marsland et al. (59) who discovered that peripheral inflammation is related to a reduction in white matter, cerebral cortex, cortical surface area, and hippocampal volume in as well as declines in language, short-term memory, executive function, and spatial reasoning ability. Interestingly, our study found lymphocytes positively correlated with multidomain cognition, suggesting a protective role in schizophrenia. Lymphocytes—key immune components including helper T (Th) and regulatory T (Treg) cells—mediate dual functions: pro-inflammatory Th17 cells drive neuroinflammation, while Tregs suppress excessive immunity, protect neurons, and preserve cognition (60, 61). We hypothesize: In stable immunity (e.g., chronic phase), Tregs dominate, dampening inflammation and safeguarding cognitive domains. In immune imbalance (e.g., acute exacerbation), Th17 overactivation disrupts this balance, inducing cognitive decline. Notably, this protective potential reflects lymphocyte subtype dynamics—a nuance obscured in our study by measuring total lymphocytes (no Treg/Th17 distinction). Future studies should dissect subtype-specific roles to clarify protective mechanisms. Our study also found that neutrophils were only associated with total cognitive function scores, attention and numeracy, while platelets were not significantly associated with any of the cognitive dimensions. However, previous studies have suggested that platelets may also have an important role in the development of cognitive impairment. One study found that intracerebral white matter lesions and cognitive decline in patients with vascular dementia were associated with platelet activation (62). We speculate there are the following reasons: First, platelet activation may not necessarily exhibit quantitative changes. Second, PLR, NLR, SII—as composite indices—better reflect inflammatory-immune dysregulation by balancing pro-inflammatory (platelets/neutrophils) and anti-inflammatory (lymphocytes) components, minimizing confounding bias. Furthermore, differences in study population, sample size, and cross-sectional design (short observation time) may introduce data inaccuracies. Future studies should integrate longitudinal platelet function assays (e.g., mitochondrial ROS), expand sample size and average multiple measurements to reduce bias, and clarify causality.
In this study, NLR, PLR, SII were compared across CI severity: only NLR differed between mild vs moderate CI (Bonferroni-corrected p<0.0167), with no PLR, SII group differences. We hypothesized the following reasons: 1. Dynamic inflammation: inflammation peaks during the normal → mild CI transition (e.g., microglia hyperactivation, blood-brain barrier disruption), which results in a significant difference between normal and CI; in the moderate/severe phase, non-inflammatory mechanisms (neuronal loss, synaptic dysfunction) prevail due to neuroprotection/immunosuppression, thus blurring the mild-moderate-severe intergroup Differences. 2. Sample/statistical limitations: within groups, sample sizes may be small, reducing statistical power. 3. Lymphocyte subtype confounding: the total number of lymphocytes masks the opposing roles of Th17 (pro- inflammatory) and Treg (anti-inflammatory), which may affect the results. Future studies should increase each group sizes and longitudinally track NLR, PLR, SII alongside neuroinflammatory markers to dissect their stage specific trajectories in CI.
In this study, ROC analysis was conducted to predict cognitive impairment (CI) using NLR, PLR, SII. It was found that serum NLR/PLR/SII showed superior predictive efficacy for CI in schizophrenia, lymphocytes fair efficacy, and neutrophils/platelets poor efficacy. ROC curves yielded cutoffs of 1.730 (NLR), 131.5 (PLR), 403 SII), and 3.65 (lymphocytes). Regarding (the critical values for screening cognitive impairment, there are few relevant studies, and different studies report different critical values due to differences in sample characteristics, testing methods, etc. If NLR, PLR, and SII are validated as screening thresholds for cognitive impairment, If NLR, PLR and SII are recognized as screening thresholds for cognitive dysfunction, they will be valuable for:
1. Diagnostic aid: testing these markers at schizophrenia diagnosis can identify early-stage CI-risk patients (those with elevated NLR/PLR/SII should be prioritized for cognitive interventions).
2. Disease monitoring: regular testing assesses CI progression and guides treatment adjustment (persistently elevated markers may indicate worsening impairment requiring intensified interventions).
3. Treatment evaluation: post-treatment marker decreases may reflect cognitive improvement. Future research should conduct larger multicenter studies should validate screening thresholds in different populations, clinical situations and scenarios to optimize the use of markers in screening and early intervention for CI in schizophrenia.
This study has limitations: First, single-center/small-sample bias: All participants were recruited from one psychiatric hospital, limiting generalizability and necessitating future multicenter replication with expanded samples. Second, assessment bias: Cognitive scale administrations lacked rater blinding, potentially introducing observer bias despite standardized training. Third, cross-sectional design constraints: Baseline data preclude causal inference between inflammatory markers and CI—longitudinal studies tracking NLR, PLR, SII alongside repeated cognitive assessments are needed to establish temporal relationships. Fourth, mechanistic ambiguity: NLR, PLR, SII reflect both peripheral inflammation and systemic oxidative stress, which current methods cannot disentangle; future studies should employ multi-omics approaches to clarify pathways. Fifth, cognitive assessment limitations: While MMSE enabled rapid CI screening, it missed schizophrenia-specific deficits in executive function and social cognition—adopting the MCCB (MATRICS Consensus Cognitive Battery) in future studies will better capture multidomain impairments. However, this study offers preliminary evidence for future longitudinal investigations, wherein patients could be stratified by clinical characteristics to design targeted follow-ups.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Shanghai Minhang Mental Health Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
KC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. LW: Conceptualization, Data curation, Funding acquisition, Investigation, Resources, Writing – original draft. HN: Conceptualization, Data curation, Investigation, Writing – original draft. HP: Conceptualization, Data curation, Investigation, Writing – original draft. WZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Shanghai Municipal Health Commission (GWVI-11.2-XD25), the Health Committee of Minhang District in Shanghai (2025FM03), the Shanghai Municipal Commission of Science and Technology (24Y22800501 and 24Y22800503), and Shanghai Jiao Tong University (2023QN038, YG2024ZD24).
The authors are grateful to all participants for their contribution.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1552451/full#supplementary-material
1. Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. (2004) 56:301–7. doi: 10.1016/j.biopsych.2004.06.023
2. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia reliability, sensitivity and comparison with a standard neuro-cognitive battery. Schizophr Res. (2004) 68:283–97. doi: 10.1016/j.schres.2003.09.011
3. Dickinson D, Harvey PD. Systemic hypotheses for generalized cognitive deficits in schizophrenia a new take on an old problem. Schizophr Bull. (2009) 35:403–14. doi: 10.1093/schbul/sbn097
4. Bilder RM, Reiter G, Bates J, Lencz T, Szeszko P, Goldman RS, et al. Cognitive development in schizophrenia follow-back from the first episode. J Clin Exp Neuropsychol. (2006) 28:270–82. doi: 10.1080/1380339050036055
5. Hughes C, Kumari V, Soni W, Das M, Binneman B, Drozd S, et al. Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr Res. (2002) 59:137–46. doi: 10.1016/S0920-9964(01)00393-0
6. Zhang Z, Zhou FC, He F, Yang NB, Zhang L, Wang CY. Cognitive functioning in patients with first-episode schizophrenia and individuals at high-risk for psychosis. Chin Ment Health J. (2017) 31:345–9. doi: 10.3969/j.issn.1000-6729.2017.05.002
7. Geng XY, Yang GC, Zhang JJ, Zhai Y. Effects of clozapine and chlorpromazine on cognitive function and alexithymia in schizophrenia during maintenance therapy. J Int Psychiatry. (2020) 47:1142–1144 + 1147. doi: 10.13479/j.cnki.jip.2020.06.015
8. Birnbaum R, Weinberger DR. A genetics perspective on the role of the (neuro)immune system in schizophrenia. Schizophr Res. (2019) 217:105–13. doi: 10.1016/j.schres.2019.02.005
9. Ballaz S, Bourin M. Anti-inflammatory therapy as a promising target in neuropsychiatric disorders. Adv Exp Med Biol. (2023) 1411:459–86. doi: 10.1007/978-981-19-7376-5_20
10. Bradburn S, Murgatroyd C, Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. Ageing Res Rev. (2019) 50:1–8. doi: 10.1016/j.arr.2019.01.002
11. Barbosa IG, Rocha NP, Huguet RB, Ferreira RA, Salgado JV, Carvalho LA, et al. Executive dysfunction in euthymic bipolar disorder patients and its association with plasma biomarkers. J Affect Disord. (2012) 137:151–5. doi: 10.1016/j.jad.2011.12.034
12. Grassi-Oliveira R, Bauer ME, Pezzi JC, Teixeira AL, Brietzke E. Interleukin 6 and verbal memory in recurrent major depressive disorder. Neuroendocrinol Lett. (2011) 32:540–4. doi: 10.1016/j.amp.2011.03.009
13. BGorelick PB. Role of inflammation in cognitive impairment results of observational epidemiological studies and clinical trials. Ann N.Y AcadSci. (2010) 1207:155–62. doi: 10.1111/j.1749-6632.2010.05726.x
14. Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. (2007) 93:261–5. doi: 10.1016/j.schres.2007.03.022
15. Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, olken R. Additive effects of elevated C-reactive protein and exposure to Herpes Simplex Virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. (2012) 134:83–8. doi: 10.1016/j.schres.2011.10.003
16. Müller N, Riedel M, Schwarz MJ, Engel RR. Clinical effects of COX-2 inhibitors on cognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. (2005) 255:149–51. doi: 10.1007/s00406-004-0548-4
17. Misiak B, Stańczykiewicz B, Kotowicz K, Rybakowski JK, Samochowiec J, Frydecka D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: a systematic review. Schizophr Res. (2018) 192:16–29. doi: 10.1016/j.schres.2017.04.015
18. Shi YZ, Wang ZY, Lu L, Xv JQ, Yue LP, Du BG. Changes of IL-6in plasma and cerebrospinal fluid of schizophrenics with clozapine. J Clin Psychosomatic Disease. (2004) 10:153–5.
19. An HM, Tan YL, Shi J, Wang ZR, Soars JC, Wu JQ, et al. Altered IL-2, IL-6 and IL-8 serum levels in schizophrenia patients with tardive dyskinesia. Schizophr Res. (2015) 162:261–8. doi: 10.1016/j.schres.2014.12.037
20. Fan N, Luo Y, Xu K, Zhang M, Ke X, Huang X, et al. Relationship of serum levels of TNF-α, IL-6 and IL-18 and schizophrenia-like symptoms in chronic ketamine abusers. Schizophr Res. (2015) 169:10–5. doi: 10.1016/j.schres.2015.11.006
21. Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. (2001) 102:5–14.
22. Hu B, Yang X-R, Xu Y, Sun Y-F, Sun C, Guo W, et al. Systemic immuneInflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
23. Geng Y, Shao Y, Zhu D, Zheng X, Zhou Q, Zhou W, et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: A propensity score-matched analysis. Sci Rep. (2016) 6:39482. doi: 10.1038/srep39482
24. Ong HL, Subramaniam M, Abdin E, Wang P, Vaingankar JA, Lee S, et al. Performance of Mini-Mental State Examination (MMSE) in long-stay patients with schizophrenia or schizoaffective disorders in a psychiatric institute. Psychiatry Res. (2016) 241:256–62. doi: 10.1016/j.psychres.2016.04.116
25. Filippi MD. Neutrophil transendothelial migration: updates and new perspectives. Blood. (2019) 133:2149–58. doi: 10.1182/blood-2018-12-844605
26. Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long- term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. (2010) 106:470–6. doi: 10.1016/j.amjcard.2010.03.062
27. Taymez DG, Ucar E, Turkmen K, Ucar R, Afsar B, Gaipov A, et al. The predictive value of platelet/lymphocyte ratio in hemodialysis patients with erythropoietin resistance. Ther Apheresis Dialysis. (2016) 20:118–21. doi: 10.1111/1744-9987.12380
28. Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. (2018) 122:337–51. doi: 10.1161/CIRCRESAHA.117.310795
29. Mazza MG, Palladini M, de Lorenzo R, Magnaghi C, Poletti S, Furlan R, et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. (2021) 94:138–47. doi: 10.1016/j.bbi.2021.02.021
30. Zulfic Z, Weickert CS, Weickert TW, Liu D, Myles N, Galletly C, et al. Neutrophil-lymphocyte ratio - a simple, accessible measure of inflammation, morbidity and prognosis in psychiatric disorders. Australas Psychiatry. (2020) 28:454–8. doi: 10.1177/1039856220908172
31. Moody G, Miller BJ. Total and differential white blood cell counts and hemodynamics parameters in first- episode psychosis. Psychiatry Res. (2018) 260:307–12. doi: 10.1016/j.psychres.2017.11.086
32. Kalelioglu T, Akkus M, Karamustafalioglu N, Genc A, Genc ES, Cansiz A, et al. Neutrophil- lymphocyte and platelet-lymphocyte ratios as inflammation markers for bipolar disorder. Psychiatry Res. (2015) 228:925–7. doi: 10.1016/j.jad.2022.04.092
33. Semiz M, Yildirim O, Canan F, Demir S, Hasbek E, Tuman TC. Elevated neutrophil/lymphocyte ratio in patients with schizophrenia. Psychiatr Danub. (2014) 26:220–5.
34. Özdin S, Sarisoy G, Böke Ö. A comparison of the neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratios in schizophrenia and bipolar disorder patients - a retrospective file review. Nord J Psychiatry. (2017) 71:509–12. doi: 10.1080/08039488.2017.1340517
35. Jackson AJ, Miller BJ. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr Scand. (2020) 142:18 –26. doi: 10.1111/acps.v142.1
36. Karageorgiou V, Milas GP, Michopoulos I. Neutrophil-to-lymphocyte ratio in schizophrenia: a systematic review and meta-analysis. Schizophr Res. (2018) 2:4–12. doi: 10.1016/j.schres.2018.12.017
37. Yüksel RN, Ertek IE, Dikmen AU, Göka E. High neutrophil-lymphocyte ratio in schizophrenia independent of infectious and metabolic parameters. nord J Psychiatry. (2018) 72:336–40. doi: 10.1080/08039488.2018.1458899
38. Kovács MÁ, Tényi T, Kugyelka R, Prenek L, Hau L, Magyar ÉE, et al. Elevated osteopontin and interferon gamma serum levels and increased neutrophil-to-lymphocyte ratio are associated with the severity of symptoms in schizophrenia. Front Psychiatry. (2019) 10:996. doi: 10.3389/fpsyt.2019.00996
39. Bustan Y, Drapisz A, Ben Dor DH, Avrahami M, Schwartz-Lifshitz M, Weizman A, et al. Elevated neutrophil to lymphocyte ratio in non -affective psychotic adolescent inpatients: evidence for early association between inflammation and psychosis. Psychiatry Res. (2018) 262:149 –153. doi: 10.1016/j.psychres.2018.02.002
40. Özdin S, Böke Ö. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in different stages of schizophrenia. Psychiatry Res. (2019) 271:131–5. doi: 10.1016/j.psychres.2018.11.043
41. Zhu X, Zhou J, Zhu Y, Yan F, Han X, Tan Y, et al. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in schizophrenia. Australas Psychiatry. (2021) 30:95–9. doi: 10.1177/10398562211022753
42. Dong X, Nao J, Shi J, Zheng D. Predictive value of routine peripheral blood biomarkers in Alzheimer’s disease. Front Aging Neurosci. (2019) 11:332. doi: 10.3389/fnagi.2019.00332
43. Akil E, Bulut A, Kaplan İ, Özdemir HH, Arslan D, Aluçlu MU. The increase of carcinoembryonic antigen (CEA), high-sensitivity C-reactive protein, and neutrophil/lymphocyte ratio in Parkinson’s disease. Neurol Sci. (2015) 36:423–8. doi: 10.1007/s10072-014-1976-1
44. Liu ZL, Li W, Li HL, Feng P, Zhao HQ. A clinical study of blood inflammatory markers in patients with vascular cognitive impairment no dementia. Chin J Clin Neurosciences. (2013) 21:32–6. doi: 10.3969/j.issn.1008-0678.2013.01.005
45. Liu JH, Zhang YJ, Ma QH, Sun HP, Xu Y, Pan CW. Elevated blood neutrophil to lymphocyte ratio in older adults with cognitive impairment. Arch Gerontol Geriatr. (2020) 88:104041. doi: 10.1016/j.archger.2020.104041
46. An P, Zhou X, Du Y, Zhao J, Song A, Liu H, et al. Association of neutrophil-lymphocyte ratio with mild cognitive impairment in elderly Chinese adults: a case-control study. Curr Alzheimer Res. (2019) 16:1309–15. doi: 10.2174/1567205017666200103110521
47. Du L, Hu X, Zhang B, Miao X, Wang J, Shen J, et al. The relationship of platelet-to-lymphocyte ratio with cognitive decline in T2DM. Diabetol Metab Syndr. (2021) 13:151. doi: 10.1186/s13098-021-00772-y
48. Lu W, Zhang K, Chang X, Yu X, Bian J. The association between systemic immune-inflammation index and postoperative cognitive decline in elderly patients. Clin Interv Aging. (2022) 17:699–705. doi: 10.2147/CIA.S357319
49. Joshi N, Singh S. Updates on immunity and inflammation in Parkinson disease pathology. J Neurosci Res. (2018) 96:379–90. doi: 10.1002/jnr.24185
50. Chesnokova V, Pechnick RN, Wawrowsky K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav Immun. (2016) 58:1–8. doi: 10.1016/j.bbi.2016.01.017
51. Ren JN, Meng XX, Zhang JZ. Study on the changes of HMGB1, TNF-α, lL-1β, lL-6 and their interrelationship in schizophrenia. Med testing clinic. (2020) 31:59–61. doi: 10.3969/j.issn.1673-5013.2020.03.014
52. Zhang GR, Zhou GL. Study on the correlation between inflammatory factor levels and cognitive dysfunction in schizophrenia. Med Lab Sci Clinics. (2015) 18:50–2. doi: CNKI:SUN:HNSJ.0.2015-13-028
53. Xu SS, Jin XH, Lv SL, Wang YW, Zhao WW. Correlation of early cognitive dysfunction with metabolic indicators and inflammatory factors in Alzheimer’s disease. Neural Injury Funct Reconstruction. (2019) 14:647–9. doi: 10.16780/j.cnki.sjssgncj.2019.12.016
54. Kindler J, Lim CK, Weickert CS, Boerrigter D, Galletly C, Liu D, et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry. (2020) 25:2860–72. doi: 10.1038/s41380-019-0401-9
55. Zhao F, Li B, Yang W, Ge T, Cui R. Brain-immune interaction mechanisms: Implications for cognitive dysfunction in psychiatric disorders. Cell Prolif. (2022) 55:e13295. doi: 10.1111/cpr.v55.10
56. Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. (2006) 52:601–23. doi: 10.1373/clinchem.2005.061408
57. Violi F, Pignatelli P. Platelet oxidative stress and thrombosis. Thromb Res. (2012) 129:378–81. doi: 10.1016/j.thromres.2011.12.002
58. Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. (2013) 38:225–36. doi: 10.1016/j.immuni.2012.10.020
59. Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. (2015) 48:195–204. doi: 10.1016/j.bbi.2015.03.015
60. McQuillan K, Lynch MA, Mills KH. Activation of mixed glia by Abeta-specific Th1 and Th17 cells and its regulation by Th2 cells. Brain Behav Immun. (2010) 24:598–607. doi: 10.1016/j.bbi.2010.01.003
61. Moore TC, Hasenkrug KJ. B-Cell Control of Regulatory T Cells in friend virus infection. J Mol Biol. (2021) 433:166583. doi: 10.1016/j.jmb.2020.06.022
Keywords: cognitive impairment, neutrophil-to-lymphocyte ratio, platelet-tolymphocyte ratio, systemic immune-inflammatory index, schizophrenia
Citation: Chen K, Wang L, Ning H, Pan H and Zhang W (2025) Neutrophil-to-lymphocyte ratio; platelet-to-lymphocyte ratio; systemic immune-inflammatory Index: inflammatory indicators of cognitive impairment in schizophrenia patients. Front. Psychiatry 16:1552451. doi: 10.3389/fpsyt.2025.1552451
Received: 28 December 2024; Accepted: 24 March 2025;
Published: 11 April 2025.
Edited by:
Ya Wang, Capital Normal University, ChinaReviewed by:
Santiago J. Ballaz, Yachay Tech University, EcuadorCopyright © 2025 Chen, Wang, Ning, Pan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Chen, MTg4OTU2ODU2MzdAMTYzLmNvbQ==; Weibo Zhang, Wmhhbmd3ZWlibzYwMEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.