- 1Faculty of Residency, Riga Stradiņš University, Riga, Latvia
- 2Department of Depression and Crisis, National Center of Mental Health, Riga, Latvia
- 3Department of Psychosomatic Medicine and Psychotherapy, Riga Stradiņš University, Riga, Latvia
Lewy body dementia is associated with abnormal eosinophilic A-synuclein neural inclusions (Lewy bodies) in the brain. It is a neurodegenerative illness—and the second most common type of dementia after Alzheimer’s disease—that causes memory loss and severe problems in carrying out daily activities. In this report, we describe a case of Lewy body dementia that began with early psychotic symptoms with atypical features (transition from hallucinosis (hallucinatory insight) to true visual hallucinations) —without Parkinsonism. The patient exhibited sensitivity to first generation antipsychotic medication, which led to a worsening of her symptoms. Physicians should consider all possible diagnoses when confronted with atypical, early symptoms of visual hallucinosis or true hallucinations and dementia without Parkinsonism. Choosing antipsychotic medicines should be made with care given these patients’ possible sensitivity to antipsychotics. The selection of antipsychotics should be consider among first, second and third generation options.
1 Introduction
Dementia with Lewy bodies is the second most common form of neurodegenerative illness with an age of onset ranging between 50 and 80 years (1). This neurodegenerative illness is similar to Parkinson’s disease. Consensus diagnostic criteria for dementia with Lewy bodies are divided in to the Central features (progressive dementia severe enough to interfere with normal social function, deficits of attention, executive function, and visuospatial ability), Core features (fluctuating cognition, recurrent visual hallucinations, spontaneous parkinsonism), Suggestive features (rapid eye movement sleep behavior disorder, severe sensitivity to antipsychotics, low dopamine transporter uptake in the basal ganglia), Supportive features (repeated falls and syncope, transient unexplained loss of consciousness, severe autonomic dysfunction, non-visual hallucinations, systematized delusions, depression, relative preservation of medial temporal lobe structures, generalized low uptake on single photon emission computer tomography (CT) perfusion or positron emission tomography (PET) metabolism with reduced occipital activity, abnormal metaiodobenzylguanidine myocardial scintigraphy, prominent slow wave activity on electroencephalogram with temporal lobe transient shape waves (2, 3).
Dementia with Lewy bodies is often misdiagnosed as Alzheimer’s disease or Parkinson’s disease dementia (4). Visual hallucinations, parkinsonism, and fluctuating cognition are specific signs of mild to moderate dementia with Lewy bodies. These symptoms are typical of Alzheimer’s disease in later stages of dementia with Lewy bodies as well and complicate diagnostics (5).
Lewy body dementia has a poor prognosis, rapid cognitive impairment, and a significant negative impact on patient quality of life (6).
This particular case is interesting with early psychotic symptoms with atypical features (transition from hallucinosis (hallucinatory insight) to true visual hallucinations) with late and atypically long-time development of Parkinsonism.
From the perspective of the clinical diagnostic approach, it is crucial to consider misperceptions such as pareidolias and visual nonthreatening hallucinations, particularly in the early stages of the disease, even if parkinsonism is not present.
2 Case description
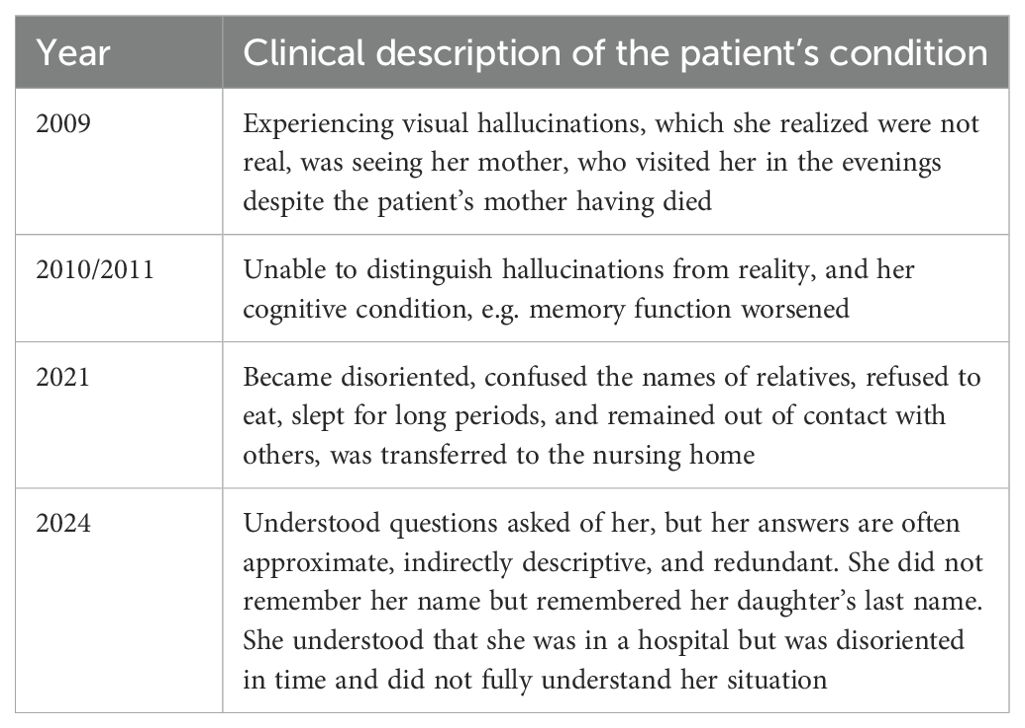
In 2024 a 91-year-old woman was hospitalized for a second time in his life after she was found at home lying on the floor, confused and speech impaired. Symptoms development during the years are described in the Table 1.
Medical history obtained from the patient’s son revealed that approximately 15 years ago, the patient began experiencing visual hallucinations, which she realized were not real. At the time, the patient told her son she was seeing her mother, who visited her in the evenings despite the patient’s mother having died. As the patient’s mental condition worsened, she was unable to distinguish hallucinations from reality, and her cognitive condition, e.g. memory function worsened. The patient’s first hospitalization occurred in 2021, when she became disoriented, confused the names of relatives, refused to eat, slept for long periods, and remained out of contact with others. During this period, the patient was transferred to a nursing home, where she gradually resumed eating and walking, and her consciousness cleared.
In 2024, the patient returned to the hospital with complaints similar to the ones she had in 2021. This time, her interactions with other patients in the hospital was satisfactory. She usually understood questions asked of her, but her answers were often approximate, indirectly descriptive, and redundant. She did not remember her name but remembered her daughter’s last name. She understood that she was in a hospital but was disoriented in time and did not fully understand her situation. On some days, she thought she was traveling by train, and on other days she believed it was Christmas. She willingly engaged in conversations and was polite and sincere. Her speech was rapid, sometimes poorly modulated, and difficult to understand. She often forgot words and spoke using descriptive phrases. When asked about berries she said she had picked that day, she might reply that she really picked grapes, then change her mind and say someone else picked them and brought them to her, and then change her mind again and declare that she ‘picked them in her sleep, in a dream’. In general, the patient was unconcerned about her perceptions and memory disorder. Her mood was slightly elevated. Her thought patterns were medium-paced, although sometimes she would exhibit obsessive, repetitive thinking, or she would lose her train of thought. At times she could also be illogical and contradictory.
3 Diagnostic assessment
A diagnosis of Lewy body dementia is made in this particular case based on consensus criteria that includes progressive dementia severe enough to interfere with social and occupational functioning, with attention and executive function damage (3). Recurrent visual hallucinations, cognitive fluctuations, and electroencephalography with temporal lobe transparent sharp waves suggested a diagnosis of Lewy body dementia in this patient. However, the late onset of Parkinsonism is atypical for this diagnosis (2).
From a neurological perspective, the patient demonstrated a positive bilateral palmomental reflex, which may indicate frontal lobe dysfunction. A positive Babinski’s reflex on the right side was also observed. On the left side of the body, there was slight stiffness in the arm and leg, and the cogwheel was observed in the left hand. No resting tremors were observed.
The patient was given 5 mg olanzapine tablets once daily and five drops of 0.2% haloperidol oral solution twice daily, mostly to address hallucinations.
However, she exhibited a pronounced sensitivity to antipsychotics and became rigid, drowsy, and withdrawn.
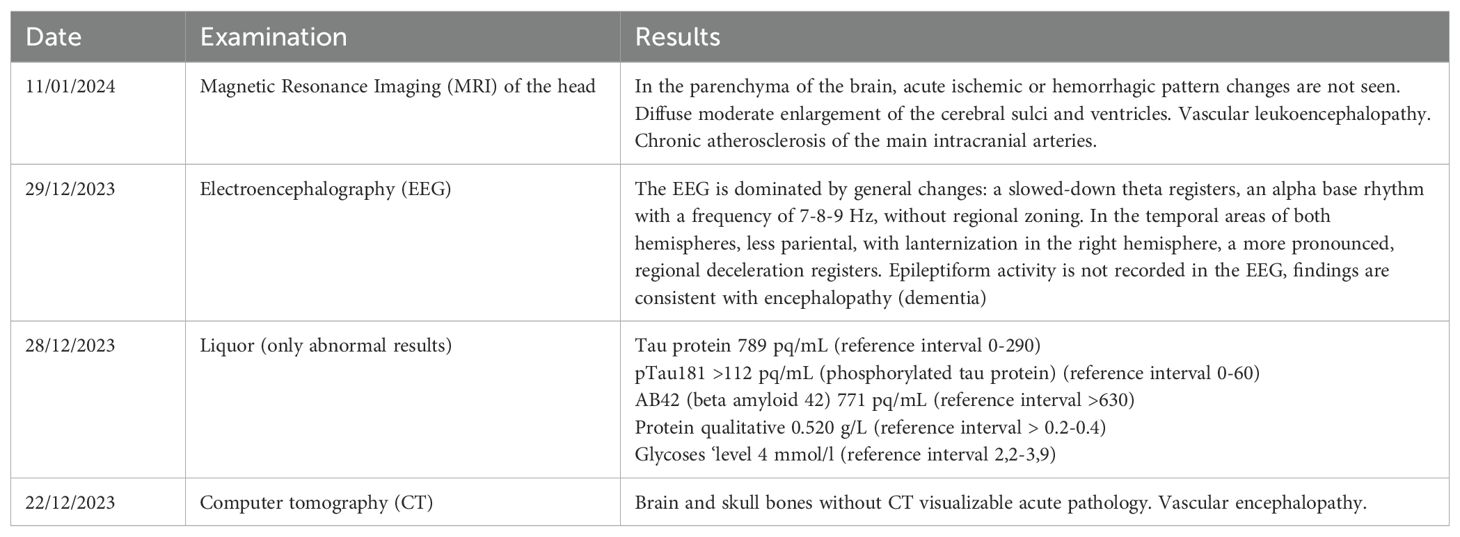
Due to these side effects, the patient underwent a computed tomography scan of the brain. There was no evidence of acute ischemia, although vascular encephalopathy was noted. To reduce the side effects, the haloperidol was removed from her treatment regime.
A lumbar puncture was performed on the patient. No cytosis was detected, but Tau protein levels were elevated, indicating neurodegeneration. The patient also underwent a computed tomography scan of the brain. There was no evidence of acute ischemia, although vascular encephalopathy was noted.
The results of examinations performed in an inpatient neurological setting are presented in Table 2.
In the hospital, the patient’s condition remained stable. Given that she has pronounced cognitive deficits and requires help caring for herself, she was transferred to a long-term care facility.
4 Discussion
Lewy body dementia is associated with abnormal eosinophilic A-synuclein neural inclusions (Lewy bodies) in the brain (7). An official diagnosis is made through an autopsy (6). New methods, like skin biopsy are introduced and will be performed in the future (8). We performed a lumbal puncture and found elevated TAU proteins suggestive of the disease. Lewy body dementia patients present with a fluctuating cognitive state, with neuropsychiatric, sleep, motor, and autonomic symptoms (9). In this case, the patient experienced hallucinations 15 years before present hospitalization in 2024 and severe dementia. Furthermore, the course of her disease fluctuated.
Visual hallucinations in the case of dementia with Lewy bodies are usually well formed and featured people, children, or animals (4). Visual hallucinations in early dementia are highly specific for a diagnosis of dementia with Lewy bodies (10). The hallucinations in dementia with Lewy bodies usually are nonthreatening misperceptions of ambiguous stimuli, termed pareidolias (11). In contrast in Alzheimer’s disease, hallucinations generally have a threatening quality (12). Diagnosis of dementia with Lewy bodies is less likely if parkinsonism does not develop until severe dementia (2).
In this patient, the initial dose of haloperidol may have been too high. Given the patient’s age, typical antipsychotics should have been introduced gradually via slow, daily up titration. Moreover, patients with Lewy body dementia may be sensitive to antipsychotic drugs, which can cause serious side effects, such as worsening extrapyramidal symptoms, aggression, and agitation (13, 14). For this patient 5 mg Olanzapine tablets taken orally once a day was sufficient. Risk of extrapyramidal symptoms are higher with typical antipsychotics and higher potency atypical antipsychotics, such as olanzapine and risperidone (15) Using typical antipsychotics, such as haloperidol, was an dangerous decision in this case. If nonpharmacological treatments fail to address aggressive behavior or related distress, haloperiodol will be used; however, safer alternatives, such as aripiprazole, will be taken into consideration (16). Cholinesterase inhibitor therapy is safe and effective treatment for psychotic symptoms in dementia with Lewy bodies (11, 17).
4.1 Limitations
The development of hallucinations in our patient may be associated with the development of other prevailing vascular processes. Moreover, the diagnosis of Lewi body dementia was clinical. The presence and severity of the symptoms was evaluated by a clinician based on their own experience and knowledge, and the diagnosis was not confirmed histologically. Various factors (vascular and metabolic) can interact during the development of dementia; therefore, patient reactions to medicines may be related to processes other than those related to Lewy body dementia.
5 Conclusion
In this patient, her disease presented with early psychotic symptoms— atypical visual hallucinations with specific development from hallucinosis till true visual hallucinations without Parkinsonism. Physicians should consider all possible diagnoses when confronted with atypical, early symptoms of dementia without Parkinsonism and with slow decrease of cognitive function in atypically long period of time. Choosing antipsychotic medicines should be made with care given these patients’ possible sensitivity to antipsychotics.
6 Patient perspective
Currently, the patient is receiving complete care in a social care institution. Her cognitive functions, based on the opinion of her relatives, have not improved, and she is experiencing movement difficulties.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LE: Writing – original draft. MT: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
MT has received financial benefits for participation in boards, and as a speaker from the following pharmaceutical companies: Lundbeck, Janssen-Cilag, Gedeon Richter, Johnson & Johnson, Olainfarm, Grindex, and Medochemie.
The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saint-Aubert L, Pariente J, Dumas H, Payoux P, Brandel J-P, Puel M, et al. Case report of Lewy body disease mimicking Creutzfeldt-Jakob disease in a 44-year-old man. BMC Neurol. (2016) 16:122. doi: 10.1186/s12883-016-0643-y
2. Taylor J-P, McKeith IG, Burn DJ, Boeve BF, Weintraub D, Bamford C, et al. New evidence on the management of Lewy body dementia. Lancet Neurol. (2020) 19:157–69. doi: 10.1016/S1474-4422(19)30153-X
3. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. (2017) 89:88–100. doi: 10.1212/WNL.0000000000004058
4. Walker Z, Possin KL, Boeve BF, Aarsland D. Lewy body dementias. Lancet. (2015) 386:1683–97. doi: 10.1016/S0140-6736(15)00462-6
5. Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. (2010) 257(3):359–66. doi: 10.1007/s00415-009-5324-y
6. Prasad S, Katta MR, Abhishek S, Sridhar R, Valisekka SS, Hameed M, et al. Recent advances in Lewy body dementia: A comprehensive review. Dis Mon. (2023) 69:101441. doi: 10.1016/j.disamonth.2022.101441
7. Sanford AM. Lewy body dementia. Clin Geriatr Med. (2018) 34:603–15. doi: 10.1016/j.cger.2018.06.007
8. Kawada T. Skin biopsy detection of phosphorylated α-synuclein to identify patients with synucleinopathies. J Neuropathology Exp Neurology. (2024) 83:988–9. doi: 10.1093/jnen/nlae089
9. Gomperts SN. Lewy body dementias: dementia with Lewy bodies and Parkinson disease dementia. Continuum (Minneap Minn). (2016) 22:435–63. doi: 10.1212/CON.0000000000000309
10. Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, et al. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun. (2013) 1:65. doi: 10.1186/2051-5960-1-65
11. Boot BP. Comprehensive treatment of dementia with Lewy bodies. Alzheimers Res Ther. (2015) 7:45. doi: 10.1186/s13195-015-0128-z
12. Gauthier S, Cummings J, Ballard C, Brodaty H, Grossberg G, Robert P, et al. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. (2010) 22(3):346–72. doi: 10.1017/S1041610209991505
13. Burgett RN, Tm F, Beireis LA. Acute treatment of psychotic symptoms in a newly diagnosed Lewy body dementia patient with an accelerated titration schedule of rivastigmine and de-escalation of antipsychotics. BMJ Case Rep. (2019) 12:e230193. doi: 10.1136/bcr-2019-230193
14. Kyle K, Bronstein JM. Treatment of psychosis in Parkinson’s disease and dementia with Lewy bodies: A review. Parkinsonism Relat Disord. (2020) 75:55–62. doi: 10.1016/j.parkreldis.2020.05.026
15. Armstrong MJ, Weintraub D. The Case for Antipsychotics in Dementia with Lewy Bodies. Mov Disord Clin Pract. (2016) 4:32–5. doi: 10.1002/mdc3.12383
16. Maddalena S, Magistri C, Mellini C, Sarli G. Aripiprazole for treating delirium: A systematic review-Is it a valid yet understudied treatment? J Psychopharmacol. (2024) 38:507–14. doi: 10.1177/02698811241249648
Keywords: dementia, Lewi bodies, psychosis, antipsychotics, sensitivity
Citation: Ercika L and Taube M (2025) Case Report: Lewy body dementia with unusual psychotic symptoms, atypically late parkinsonism, and patient sensitivity to first generation antipsychotics. Front. Psychiatry 16:1551581. doi: 10.3389/fpsyt.2025.1551581
Received: 25 December 2024; Accepted: 20 March 2025;
Published: 09 April 2025.
Edited by:
Chi Shen, Xi’an Jiaotong University, ChinaReviewed by:
Juan Moisés De La Serna, International University of La Rioja, SpainMario Di Fiorino, Psychiatry of Versilia Hospital, Italy
Carlo Magistri, Azienda Sanitaria Locale di Viterbo, Italy
Copyright © 2025 Ercika and Taube. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maris Taube, bWFyaXMudGF1YmVAcnN1Lmx2
 Lolita Ercika
Lolita Ercika Maris Taube
Maris Taube