- 1Department of Child Health, Dalian Municipal Women and Children’s Medical Center (Group), Dalian, Liaoning, China
- 2Dalian Medical University, Dalian, Liaoning, China
- 3Department of Key Laboratory, Dalian Municipal Women and Children’s Medical Center (Group), Dalian, Liaoning, China
Introduction: Attention deficit hyperactivity disorder (ADHD) is a common neurodevelopmental disorders often co-occurring with sleep problems and other physical disorders. Vitamin D regulates sleep and supports normal brain function. Regrettably, no studies have looked at whether vitamin D insufficiency exacerbates sleep problems in ADHD children and further affects ADHD symptoms.
Objective: This study aimed to examine whether vitamin D insufficiency exacerbates sleep problems and ADHD symptoms in children aged 6–14 years.
Methods: This is a case-control study, 260 ADHD children (aged 6-14 years) were enrolled in, of whom 95 had vitamin D insufficiency and 165 had sufficiency. Collected all ADHD symptom severity and functional impairment scales, including Swanson, Nolan and Pelham (SNAP) scale, Integrated Visual and Auditory Continuous Performance Test (IVA-CPT), Conners parents symptom questionnaire (PSQ) and Weiss Functional Impairment Rating Scale-Parent Form (WFIRS-P). All guardians of children with ADHD complete the Children’s Sleep Habits Questionnaire (CSHQ).
Results: The CSHQ total scores of the ADHD children in both groups were significantly higher than 41, which means that ADHD children overall have sleep problems. Compared to ADHD children with vitamin D sufficiency group, we observed significantly higher sleep duration and sleep disordered breathing scores in ADHD children with vitamin D insufficiency group (all p< 0.05). However, there was no direct effect of vitamin D insufficiency on the type of ADHD, symptoms or functional impairment (all p> 0.05). Further analyses showed a correlation between the CSHQ and symptoms, functional impairment scores in children with ADHD.
Conclusion: Sleep problems are highly prevalent in children with ADHD. Vitamin D insufficiency has a significant impact on both sleep duration and sleep disordered breathing, but no notable direct effects on ADHD symptoms or functional impairment. Our findings underscore the importance of screening for vitamin D insufficiency in children with ADHD, particularly given its association with sleep disturbances, which may indirectly affect symptom severity.
1 Introduction
Attention deficit hyperactivity disorder (ADHD), prevalent in neurodevelopment, often emerges in childhood, characterized by persistent, impairing and developmentally inappropriate inattention/disorganization, with symptoms of hyperactivity, impulsivity, and inattention (1–3). Global estimates suggest ADHD prevalence of 5.29% in children and adolescents and more than 65% of ADHD children co-occurring with other developmental, psychiatric and physical disorders (4–6). The precise origin of ADHD remains unidentified, but the dopamine hypothesis gains support from the effectiveness of psychostimulants like methylphenidate, which inhibit neuronal dopamine transporters and prevent dopamine reuptake of from the synaptic cleft, as effective ADHD treatments (7).
A large body of experimental and clinical research indicates that vitamin D plays a role in regulating dopaminergic activity and may be associated with behavioral symptoms in ADHD patients (8, 9). The neuroactive steroid vitamin D has been shown to directly enhance the activity of tyrosine hydroxylase, the key enzyme in dopamine synthesis, by binding to a nuclear receptor known as vitamin D receptors (VDRs) (10). This has implications for the role of vitamin D in neurobiology and could provide insight into potential therapeutic applications. Additionally, ADHD is often accompanied by vitamin D deficiency (11). Studies indicate that vitamin D is essential for the proper functioning of the central nervous system, and may have a role in ADHD (12, 13). Vitamin D supplementation has been shown to positively impact cognitive function across multiple domains, including attention, opposition, hyperactivity, and impulsivity (14).
At the same time, vitamin D plays a role in sleep regulation (15). Specifically, vitamin D insufficiency can exacerbate sleep disorders and is causally linked to sleep difficulties and night awakenings in both children and adults (16–18). Several subjective and objective measures have shown that shorter sleep duration is associated with lower vitamin D levels (19, 20). Some studies have shown that restless leg syndrome is more common and severe in vitamin D insufficiency, suggesting that low vitamin D levels have a negative impact on sleep parameters (21).
Sleep problems are common in children with ADHD, with 25-50% experiencing difficulty sleeping, insomnia, night wakings and hypersomnia (22, 23). Adequate sleep is critical for early childhood neurodevelopment (24). Indeed, during early development, sleep plays a key role in healthy cognitive and psychosocial development (25). Numerous researches have highlighted the crucial role of sleep in regulation learning and memory functions (24, 26). Poor quality and reduced quantity of sleep negatively affect the development of cognitive skills and socio-emotional behavior (27).
Regrettably, few studies have investigated the effect of vitamin D insufficiency on ADHD symptoms and sleep. It is unclear whether vitamin D insufficiency accentuates sleep problems in children with ADHD, and whether sleep problems due to vitamin D insufficiency exacerbate ADHD symptoms and function impairments warrants further investigation. We hypothesized that vitamin D insufficiency would independently contribute to poorer sleep quality and increased functional impairment in children with ADHD. Therefore, we conducted this study to investigate the effect of vitamin D insufficiency on sleep and symptoms in children with ADHD.
2 Methods
2.1 Study design
This was a case-control study, which was conducted with ethics approval from the Ethics Committee of Dalian Municipal Women and Children’s Medical Center (Group) at the Dalian Medical University in Dalian, China (FEJT-KY-2024-106). The study enrolled subjects from the Department of Child Health at Dalian Municipal Women and Children’s Medical Center (Group) during the period of October 2023 to October 2024. All participants’ parents or guardians provided written agreement, and the research adhered to the ethical guidelines outlined in the Declaration of Helsinki.
2.2 Study population
The following is a description of the diagnostic and inclusion/exclusion standards for participants in this clinical study. Per the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), every participant fulfilled the clinical diagnostic criteria for ADHD (28). The formal diagnosis of ADHD was made by experienced child development and behavior specialist, collected 18-item Swanson, Nolan and Pelham (SNAP-IV) scale, Integrated Visual and Auditory Continuous Performance Test (IVA-CPT), Conners parents symptom questionnaire (PSQ) and Weiss Functional Impairment Rating Scale-Parent Form (WFIRS-P) to assess symptoms and function impairment. The IQ of every child was evaluated using the Wechsler Intelligence Scale for Children-Revised (29). All guardians of children with ADHD complete the Children’s Sleep Habits Questionnaire (CSHQ). All participants were included using consecutive sampling. In order to reduce perception bias, the guardians of ADHD children were strictly screened and required to accompany their children ≥5 days a week before they could fill the forms. Professionals will explain the requirements of filling out the questionnaire to the guardians before they fill it out, and will answer the guardians’ questions at any time during the filling process. This professional is a clinical staff member who is only responsible for the collection of clinical questionnaires and does not participate in the design of the study, compilation and analysis of data, etc.
Inclusion criteria for ADHD children with vitamin D insufficiency were: a) a formal diagnosis of ADHD; b) aged 6 to 14 years; c) 25(OH)D3 ≤ 50nmol/L; Inclusion criteria for vitamin D normal ADHD children were: a) a formal diagnosis of ADHD; b) aged 6 to 14 years; c) 25(OH)D3 > 50nmol/L. All children IQ >70.
Exclusion criteria for all participants were: a) aged <6 years or >14 years; b) having neurological conditions related to central function (e.g. narcolepsy, epilepsy, autism spectrum disorder, intellectual disability) or another significant mental health requiring hospitalization (e.g. depression, anxiety); c) any grave health condition, encompassing conditions like inflammatory bowel disease, cancer history, diabetes, liver or kidney disease; d) ongoing use of any medication, stimulants included; e) incomplete case information.
2.3 Study measures
2.3.1 SNAP-IV
Comprising 18 items, the SNAP rating scale features 9 questions about attention deficit disorder (ADD), 5 regarding hyperactivity, and 4 about impulsiveness (30). Hyperactivity disorder (HD) was the term used to describe the collective hyperactivity and impulsivity. Symptom evaluation employs a 4-level Likert scale, spanning from 0 (not at all) to 3 (very much). Scores on the SNAP subscale indicated the degree of ADHD (31).
2.3.2 IVA-CPT
IVA-CPT is a 20-minute examination featuring 500 1s and 2s trails in a pseudorandom order, was used to evaluate sustained selective attention (32). In this examination, the participant clicks the mouse solely upon seeing or hearing a 1, excluding a 2. Trained physicians conducted the tests on every participant, and responses were converted to standardizes scores based on age. This research utilized the full-scale attention quotient (FAQ) and the full-scale response control quotient (FRCQ) to assess the participants’ attention deficit and their ability to control responses, respectively (33).
2.3.3 PSQ
Conners developed this scale to compile an extensive checklist for parents to gather reports on the fundamental issues presented (34). The composition includes 48 elements and 6 subcategories, the composition encompasses indices for character problems index, psychosomatic disorders index, impulsivity index, learning problems index, anxiety index, and hyperactivity index (35). The assessment of the scale utilized a four-tier frequency scale ranging from “never” to “frequently”. An elevated score is associated with the intensity of the symptoms.
2.3.4 WFIRS-P
Comprising 50 questions, the WFIRS-P requires participants’ parents to evaluate their child’s functional disability in the previous month. The version 2 of the WFIRS-P was used in the research. WFIRS-P’s components are evaluated using a four-point Likert-type scale: 0 (never), 1 (sometimes), 2 (often) or 3 (very often), cumulatively yielding six distinct domain scores (Family, Life Skills, Social Activities, Child’s Self- Concept, Learning and School and Risky Activities). Elevated scores in every WFIRS- P domain index was indicative of greater functional impairment (36).
2.3.5 CSHQ
The CSHQ consists of a 35-item parent questionnaire. Parents are need to recall their children’s sleeping behaviors over a “typical” recent month. Items are assessed on a tripartite scale: “usually” indicates happening 5-7 times weekly; “sometimes” denotes 2-4 times weekly; and “rarely” represents 0-1 times weekly. Certain item scores will be inverted and require conversion for subsequent computations. Conceptually, the CSHQ is segmented into eight distinct subscales, each representing different sleep aspects: Bedtime Resistance, Sleep Anxiety, Sleep Onset Delay, Parasomnias, Sleep Duration, Sleep Disordered Breathing, Daytime Sleepiness, Night Wakings (37). The sum of the scores across the eight dimensions comprises the CSHQ total score, which reflects the overall quality of sleep. Higher scores indicate poorer sleep quality, CSHQ total score above 41 points was considered indicative of poor sleep quality.
2.3.6 Vitamin D testing
Serum 25(OH)D3 testing using AB SCIEX liquid chromatography- tandem mass spectrometry (HPLC MS TRIPLE QUAD 4500MD). Global Consensus Recommendations on Prevention and Management and Academy of Medicine, recommends the following classification of serum vitamin D status: deficiency (<30 nmol/L), insufficiency (30–50 nmol/L), sufficiency (>50 nmol/L) (38, 39). Besides, the Institute of Medicine issued a report stating that 25-hydroxyvitamin D concentrations of 50 nmol/L are adequate (40). Based on the above recommendations, in this study, we used 25(OH)D3≤ 50nmol/L to represent vitamin D insufficiency and 25(OH)D3> 50nmol/L to represent vitamin D sufficiency.
2.4 Statistical analyses
Analysis of the data was conducted with the SPSS software, version 26.0. The presentation of continuous variables was in the form of Mean ± SD. Student’s-t test for normally distributed, while the Mann-Whitney U test for the non-normally date. Categorical data are expressed in amount (%). The chi-square or corrected chi-Square test were used to analyzed the difference between categorical data. Spearman to analyze the clinical characteristics and CSHQ scores. p< 0.05 was deemed statistically significant.
3 Results
3.1 Characteristics of participants
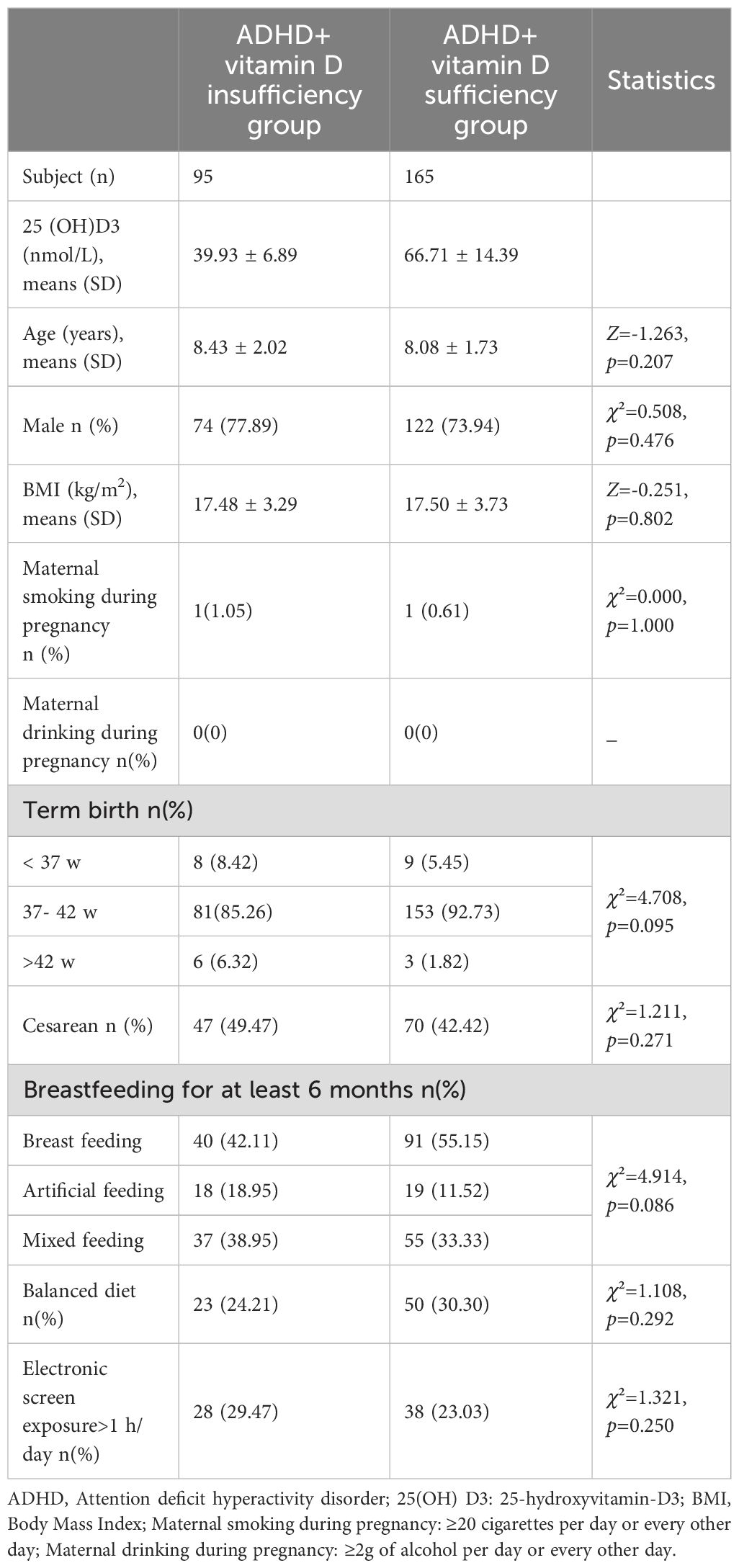
This study encompassed 260 children aged between 6 and 14 years diagnosed with ADHD, of whom 95 had vitamin D insufficiency (39.93± 6.89 nmol/L) and 165 had sufficiency (66.71 ± 14.39 nmol/L). The study’s clinical demographic characteristics are listed in Table 1. Basic characteristics like age, males, Body Mass Index (BMI), maternal smoking and drinking during pregnancy, birth history, feeding history and electronic screen exposure, exhibited no statistical difference between the groups (all p>0.05).
3.2 ADHD types, symptoms and functional scores between groups
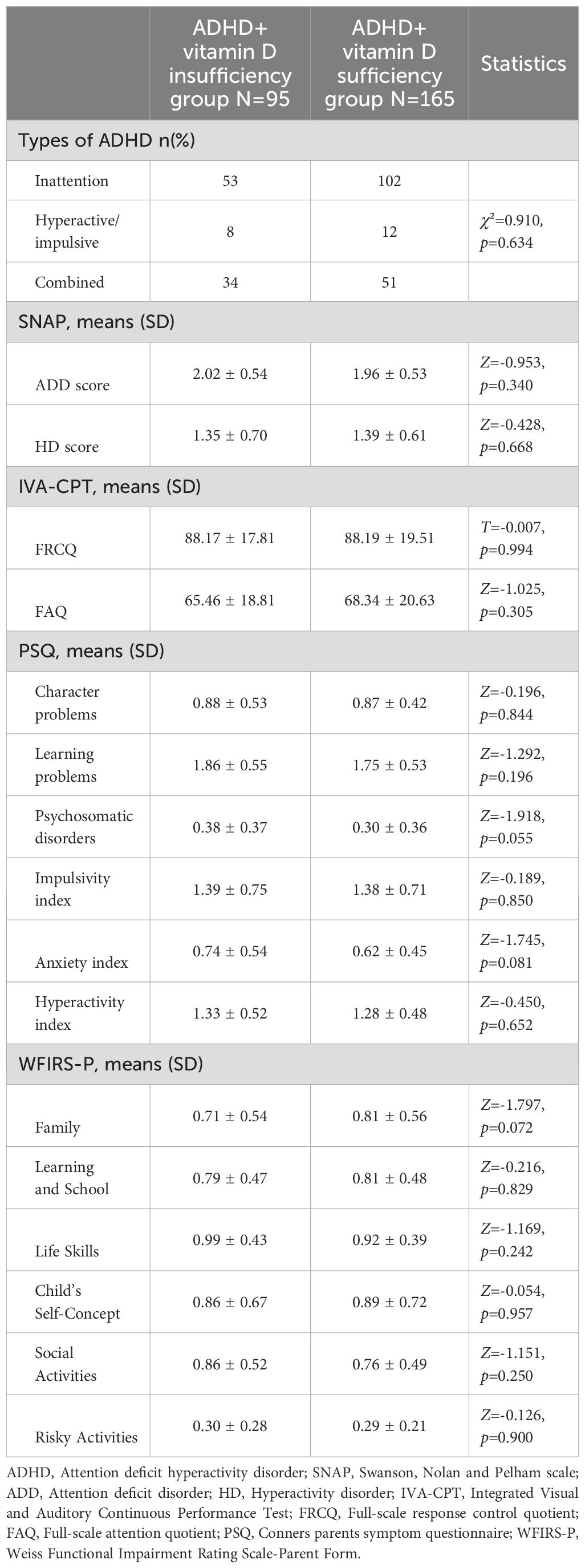
The results of the comparison of types, SNAP, IVA-CPT, PSQ and WFIRS-P between the ADHD combined vitamin D insufficiency and sufficiency groups were shown in Table 2. No statistical differences were seen between the two groups in the above indicators (all p> 0.05).
3.3 Sleep problems in ADHD with vitamin D insufficiency
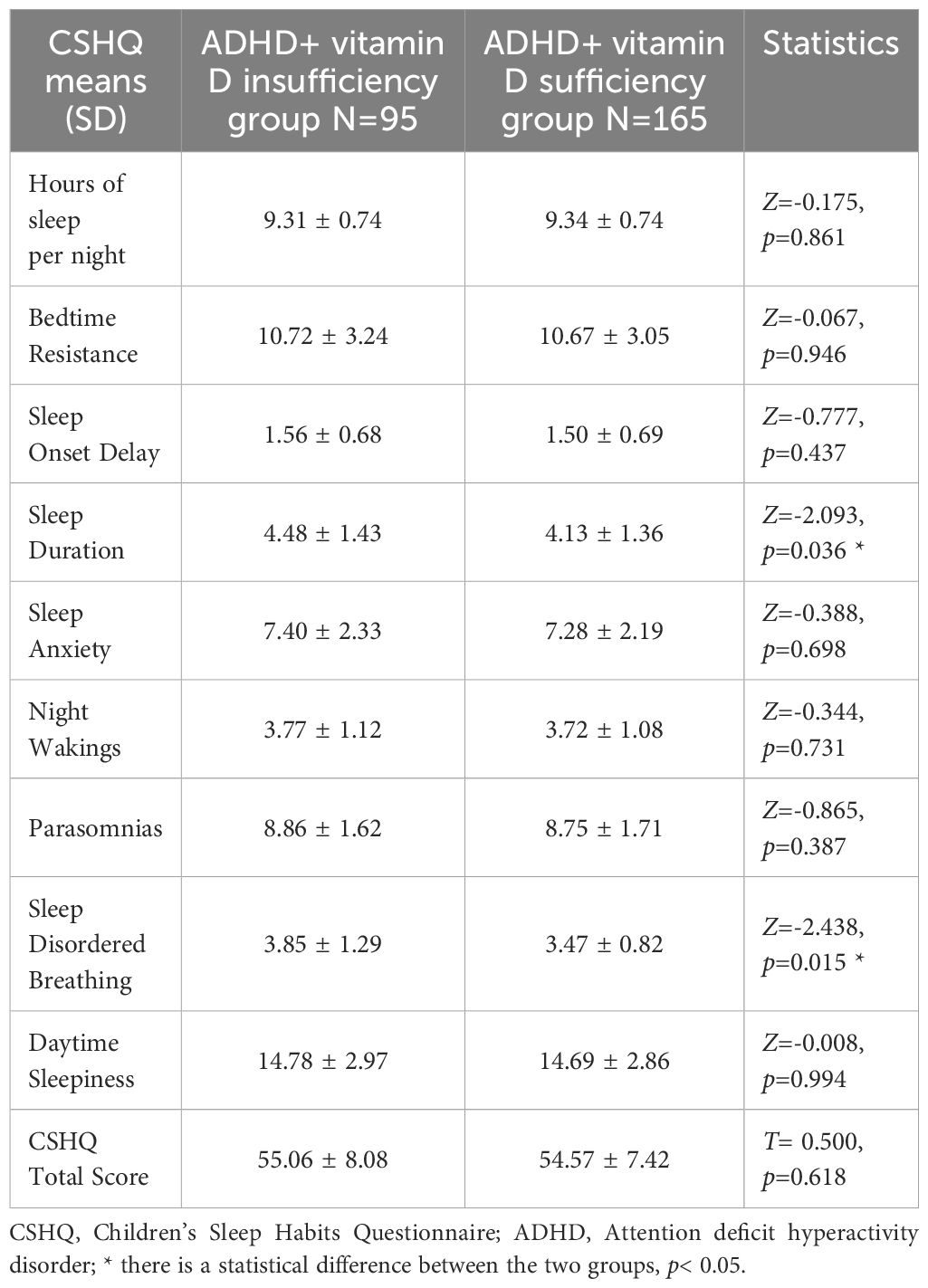
By analysis, it was found that the CSHQ total scores (54.75 ± 7.66) of the ADHD children in both groups were significantly higher than 41, which means that ADHD children overall have sleep problems. About the eight dimensions of CSHQ, the prominent statistically difference between groups were Sleep Duration and Sleep Disordered Breathing scores (all p< 0.05) (Figure 1), with no statistically significant differences in the remaining dimensions (all p> 0.05) (Table 3).
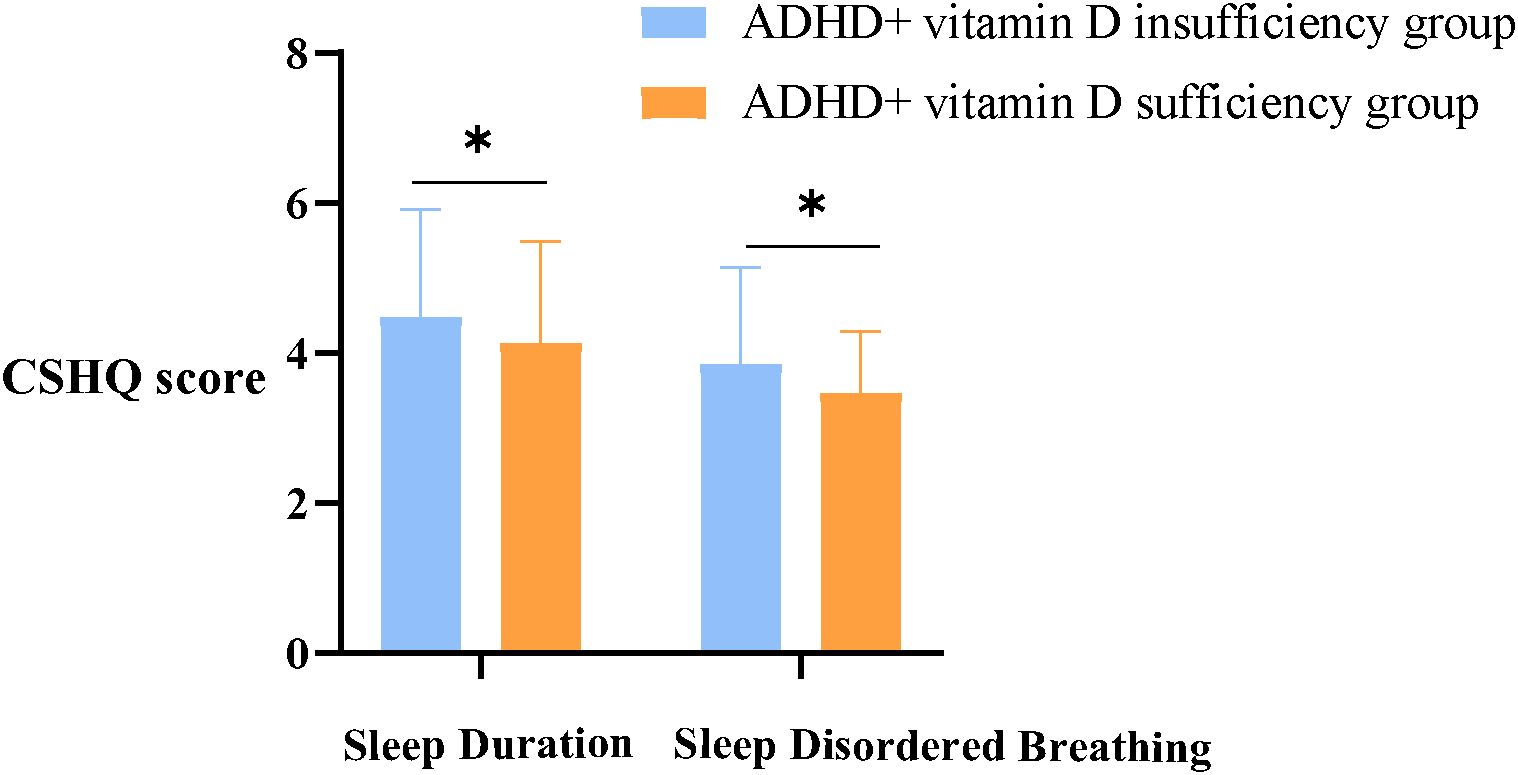
Figure 1. Sleep duration and sleep disordered breathing scores between groups. ADHD, Attention deficit hyperactivity disorder; CSHQ, Children’s Sleep Habits Questionnaire; *, there is a statistical difference between the two groups, p< 0.05.
3.4 ADHD symptoms and functional scores are associated with CSHQ
We identified that there was a correlation between the CSHQ score and ADHD symptoms, functional impairment scores (Figure 2). In particular, Sleep duration was positively correlated with Family and Life skills score in WFIRS-P (r= 0.179, r= 0.157, all p< 0.05); Sleep Disordered Breathing were positively correlated with learning problems, Anxiety index in PSQ (r= 0.135, r= 0.131, all p< 0.05) and Learning and School in WFIRS-P (r= 0.146, p< 0.05). In addition, Hyperactivity index in PSQ were positively correlated with Bedtime Resistance, Sleep Anxiety, Parasomnias and CSHQ total score (r= 0.159, r= 0.131, r= 0.142, r= 0.193, all p< 0.05); ADD and HD scores in SNAP were positively correlated with Parasomnias (r= 0.157, r= 0.239, all p< 0.05) (Supplementary Table 1).
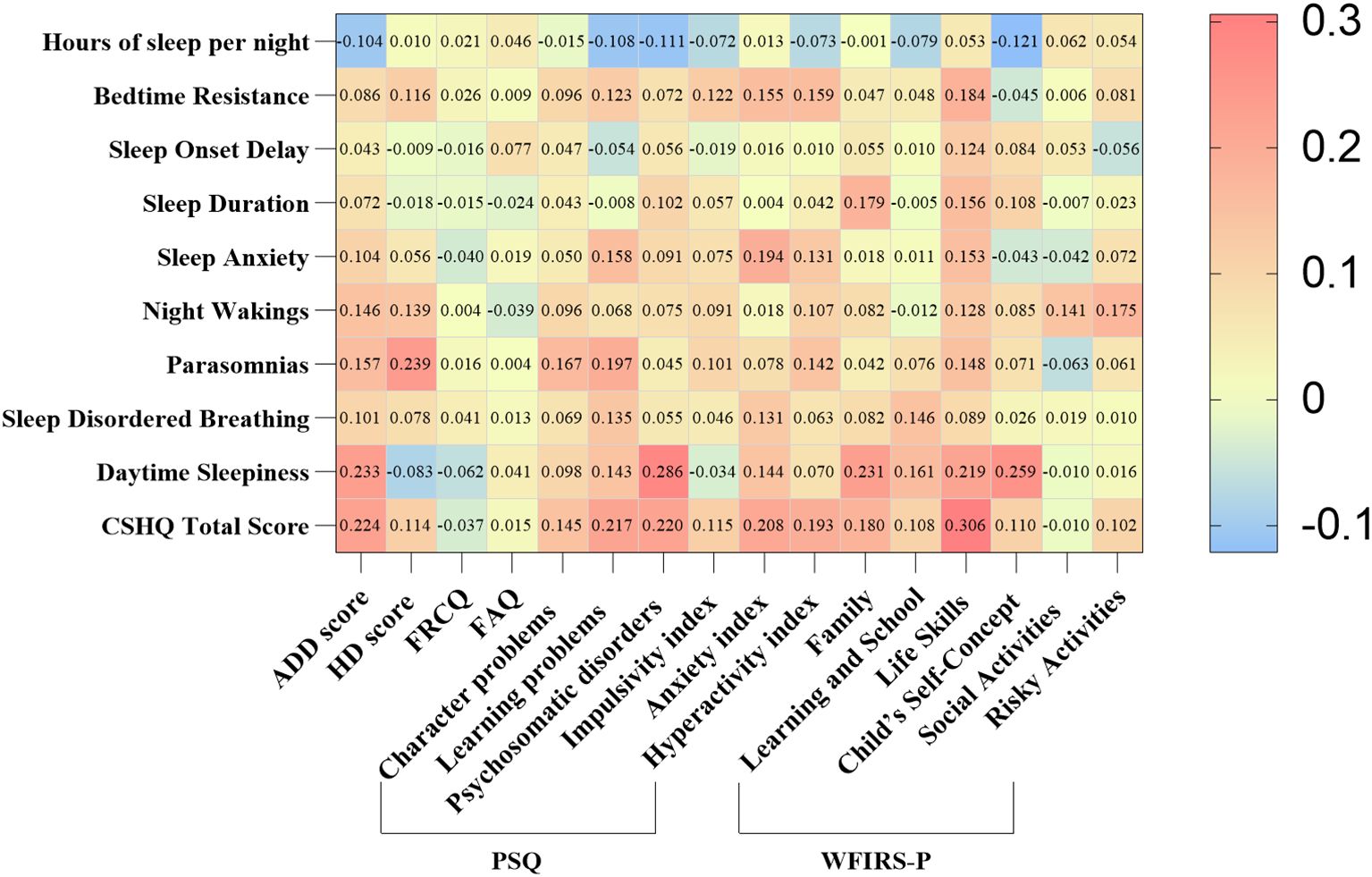
Figure 2. Correlation of symptoms and CSHQ scores in children with ADHD. ADHD, Attention deficit hyperactivity disorder; CSHQ, Children’s Sleep Habits Questionnaire; ADD, Attention deficit disorder; HD, Hyperactivity disorder; FRCQ, Full-scale response control quotient; FAQ, Full-scale attention quotient; PSQ, Conners parents symptom questionnaire; WFIRS-P, Weiss Functional Impairment Rating Scale-Parent Form. Using Spearman to analyze the relationship. Red color represents positive correlation, while blue color represents negative correlation.
4 Discussion
The objective of this study was to examine the impact of serum vitamin D levels on sleep problems, symptoms and functional scores in patients with ADHD. The primary finding was that sleep problems were highly prevalent in children with ADHD, and vitamin D insufficiency primarily influenced sleep duration and sleep disordered breathing, with no discernible direct effect on ADHD symptoms or functional impairment in ADHD children. This is the first study to simultaneously explore the effects of vitamin D insufficiency on sleep and clinical symptoms in children with ADHD.
Sleep problems pose a widespread health issue in children, and ADHD represents one of the significant diseases among the numerous co-morbid sleep disorders seen in this age group. Investigations have showed that the occurrence of ADHD in children alongside other sleep disorders is 74.6%, significantly higher than the previously reported range of 4.2%-6.5% in China (41, 42). Meanwhile, inattention and hyperactivity/impulsivity of ADHD were associated with sleep problem severity (43). This is consistent with our findings that CSHQ total scores in children with ADHD were generally higher than 41 and that CSHQ subscales correlated with ADHD symptom and functional impairment scores. Sleep problem may be a characteristic feature of ADHD or may interact with the disorder’s symptoms, either worsening or being worsened by them. Although it remains challenging to establish a causal relationship between ADHD and sleep problems, the clinical assessment of sleep in ADHD children is critically important.
While vitamin D’s main role is to maintain bone balance, its association with various other health issues like allergy, cardiovascular disease, infectious diseases, glucose metabolism/insulin resistance, etc (17, 44). Lately, an increasing body of research has revealed a connection between vitamin D and sleep problem. It has been observed that insufficient vitamin D levels correlate with reduced sleep duration, and sufficient vitamin D seems essential for sustaining sleep and minimizing night awakenings (15). Our study also found significant alterations in sleep duration in ADHD children with vitamin D insufficiency. Shortened sleep duration is a contributing factor to increased daytime sleepiness, inattention, and oppositional defiant disorder symptoms in children (45). While the precise process through which vitamin D influences sleep remains ambiguous, the crucial factor in this connection appears to be the manifestation of VDRs in brainstem regions implicated in sleep (16). Research has revealed the presence of VDRs in brain regions implicated in sleep control, including the prefrontal cortex, cingulate gyrus, hippocampal dentate gyrus, caudate nucleus, and the substantia nigra; indicating vitamin D’s involvement in sleep control (18, 46).
Our study found that vitamin D insufficiency had a significant effect on sleep disordered breathing in children with ADHD. Children with sleep disordered breathing often have difficulty paying attention, focusing, and being hyperactive during the day. Sleep disordered breathing can also impair memory and executive function, which are important for managing ADHD symptoms (51). Reduced levels of vitamin D in the serum are linked to a heightened risk of respiratory infections and a greater occurrence of allergic rhinitis (52, 53). Recurrent respiratory infections and immune system imbalance can result in tonsillar hypertrophy and chronic rhinitis. Both conditions increase the risk of sleep disordered breathing, particularly obstructive sleep apnea (OSA). OSA is a relatively common disorder in children, impact 3% to 4% of the total child population and is characterized as a disease with low inflammation. Two research showed a linear relationship between the levels of vitamin D and the likelihood of developing OSA (54). Furthermore, vitamin D could inhibit T cell growth, promote Foxp3+ Treg cells activation, and reduce Th17 cell differentiation and transcription, thus diminishing allergic inflammation (55, 56). This may be a possible mechanism for the occurrence of sleep disordered breathing in ADHD children with inadequate vitamin D levels.
In addition, serum vitamin D was negatively correlated with several biomarkers of systemic inflammation, such as high-sensitive C-reactive protein (hs-CRP), IL-6, and TNF-α levels (47). Studies have shown that increased levels of hs-CRP and IL-6 are associated with sleep disturbances, suggesting that inflammation can disrupt normal sleep patterns (48). And less than 5.5 hours sleep duration was significantly linked to increased hs-CRP levels (49). This means that the relationship between sleep and the immune system is bidirectional: lack of sleep can impair immune function, while an unbalanced immune response can disrupt sleep. Inflammatory mediators not only affect sleep, but also have an important impact on ADHD. It has been reported that IL-6 levels are significantly higher in patients with ADHD compared to their peers without ADHD (50). Elevated IL-6 may contribute to neuroinflammatory processes that affect brain function and behavior in ADHD patients. However, the effects of systemic inflammation caused by vitamin D insufficiency on sleep and ADHD need further exploration.
Despite the effects of vitamin D insufficiency on sleep in children with ADHD and may further contributary effect of sleep disturbances on ADHD symptoms, we did not identify any significant differences in clinical symptoms or functional impairment between children with ADHD who were vitamin D insufficient and those with normal vitamin D levels. Recent years have seen several studies investigate the relationship between vitamin D status and ADHD in children and adolescents; however, the results have been inconsistent (9, 13). One study found that improvement in attention was associated with an increase in 25(OH)D3 after one month of vitamin D supplementation in children with ADHD (57). A randomized controlled trial showed that while vitamin D and magnesium supplementation improved cognitive functions in children with ADHD, it did not significantly affect hyperactivity scores or conduct problems (58). A systematic review indicates that lower serum vitamin D levels are associated with ADHD, but the overall effect sizes are small (9). The contradictory outcomes described above may be related to the emergence of confounding factors or may be due to differences in sample sizes, demographic characteristics, and other features included in the study design. Our study showed that vitamin D insufficiency had no direct effect on ADHD symptoms. But given the direct effect of vitamin D insufficiency on sleep and the clear correlation between sleep and ADHD symptoms, we believe that vitamin D insufficiency has a potential effect on ADHD symptoms, which requires further exploration of the potential association between the two by expanding the sample size and using polysomnography.
Sleep plays an important role in neurodevelopment. Although this study found that vitamin D insufficiency did not have a direct effect on ADHD symptoms, its direct effect on sleep was definitive, and there is a correlation between sleep and ADHD symptoms. This alerts clinicians to the importance of assessing children’s sleep in the management of ADHD and to further explore possible causes of sleep problems in children, especially vitamin D levels. Further guide parents to improve the sleep of children with ADHD by supplementing vitamin D, lifestyle modifications, etc. At the same time, there is value in focusing on the effects of inadequate vitamin D levels early in life on sleep and neurodevelopment later in life. It is well known that vitamin D insufficiency in infancy and early childhood excites the sympathetic nerves, and children often exhibit irritability and sleep problems. However, few studies have explored the neurodevelopmental impact of sleep problems caused by vitamin D insufficiency in infancy and early childhood. And this has important implications for the future prevention and treatment of neurodevelopmental disorders in children, especially ADHD.
This study has some limitations. Firstly, sleep information was mainly obtained from subjective parental questionnaires. Subjective methods offer various advantages, including non-invasive collection and low cost. However, the use of subjective sleep measures is considered be affected by expectations, psychological influences, and responder bias. Polysomnography is widely recognized as the “gold standard” for measuring sleep, collecting comprehensive physiological data over full night, including brain activity, eye movement, heart rate, and blood oxygen levels (59). There were discrepancies between subjective report sleep and objective sleep measures. Therefore, there is a need to use a combination of subjective and objective to better understand sleep in children with ADHD. Secondly, the children with ADHD included in this study were all from northeastern China to ensure data stability and reduce the influence of latitude and longitude on the data, representing ADHD children in the north. However, the selected children are still geographically limited, and further expansion of the geographic area and sample size is needed to represent a wider range of children with ADHD. Thirdly, all participants in this study were ADHD children, and there was no investigation of vitamin D and sleep in non-ADHD children. In subsequent studies, we will further recruit non-ADHD children to observe whether there is a difference in the effect of vitamin D deficiency on sleep between the two groups of children. Lastly, longitudinal studies could better explore the effect of comorbid vitamin D insufficiency on ADHD symptoms and sleep, and in the next step we will conduct a prospective cohort study further explore the causal relationship.
5 Conclusion
Our research revealed that sleep problems are highly prevalent in children with ADHD and that vitamin D insufficiency further exacerbates sleep problems in children with ADHD, especially sleep duration and sleep disordered breathing. Nevertheless, vitamin D insufficiency does not appear to direct affect ADHD symptoms and functional impairment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Ethics Committee of Dalian Municipal Women and Children’s Medical Center (Group) at the Dalian Medical University in Dalian, China (FEJT-KY-2024-106). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
PZ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Writing – original draft. YL: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft. YM: Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing. TZ: Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Writing – review & editing. CZ: Formal analysis, Investigation, Resources, Software, Supervision, Validation, Writing – review & editing. HS: Funding acquisition, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Liaoning Provincial Natural Science Foundation (2023-BS-216) and Dalian Science and Technology Innovation Fund (2024JJ13PT055).
Acknowledgments
We acknowledge the contributions of key laboratory for early diagnosis and biotherapy of malignant tumors in children and women, Dalian women and children’s medical group that aided the efforts of the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1546692/full#supplementary-material
References
1. Faraone SV, Bellgrove MA, Brikell I, Cortese S, Hartman CA, Hollis C, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. (2024) 10(1):11. doi: 10.1038/s41572-024-00495-0
2. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th ed. Arlington, VA, US: American Psychiatric Publishing, Inc. (2013).
3. Leffa DT, Caye A, Rohde LA. ADHD in children and adults: diagnosis and prognosis. Curr topics Behav Neurosci. (2022) 57:1–18. doi: 10.1007/7854_2022_329
4. Antshel KM, Zhang-James Y, Wagner KE, Ledesma A, Faraone SV. An update on the comorbidity of ADHD and ASD: a focus on clinical management. Expert Rev Neurother. (2016) 16(3):279–93. doi: 10.1586/14737175.2016.1146591
5. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/S2215-0366(21)00395-3
6. May T, Birch E, Chaves K, Cranswick N, Culnane E, Delaney J, et al. The Australian evidence-based clinical practice guideline for attention deficit hyperactivity disorder. Aust New Z J Psychiatry. (2023) 57:1101–16. doi: 10.1177/00048674231166329
7. Macdonald HJ, Kleppe R, Szigetvari PD, Haavik J. The dopamine hypothesis for ADHD: An evaluation of evidence accumulated from human studies and animal models. Front Psychiatry. (2024) 15:1492126. doi: 10.3389/fpsyt.2024.1492126
8. Seyedi M, Gholami F, Samadi M, Djalali M, Effatpanah M, Yekaninejad MS, et al. The dopamine hypothesis for ADHD: An evaluation of evidence accumulated from human studies and animal models. Front Psychiatry. (2024) 15. doi: 10.3389/fpsyt.2024.1492126
9. Khoshbakht Y, Bidaki R, Salehi-Abargouei A. Vitamin D status and attention deficit hyperactivity disorder: A systematic review and meta-analysis of observational studies. Adv Nutr (Bethesda Md). (2018) 9:9–20. doi: 10.1093/advances/nmx002
10. Cui X, Pertile R, Liu P, Eyles DW. Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience. (2015) 304:90–100. doi: 10.1016/j.neuroscience.2015.07.048
11. Kotsi E, Kotsi E, Perrea DN. Vitamin D levels in children and adolescents with attention-deficit hyperactivity disorder (ADHD): a meta-analysis. Attention deficit hyperactivity Disord. (2019) 11:221–32. doi: 10.1007/s12402-018-0276-7
12. Bond M, Moll N, Rosello A, Bond R, Schnell J, Burger B, et al. Vitamin D levels in children and adolescents with chronic tic disorders: a multicentre study. Eur Child Adolesc Psychiatry. (2022) 31:1–12. doi: 10.1007/s00787-021-01757-y
13. Meyer T, Becker A, Sundermann J, Rothenberger A, Herrmann-Lingen C. Attention deficit-hyperactivity disorder is associated with reduced blood pressure and serum vitamin D levels: results from the nationwide German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Eur Child Adolesc Psychiatry. (2017) 26:165–75. doi: 10.1007/s00787-016-0852-3
14. Elshorbagy HH, Barseem NF, Abdelghani WE, Suliman HAI, Al-Shokary AH, Abdulsamea SE, et al. Impact of vitamin D supplementation on attention-deficit hyperactivity disorder in children. Ann pharmacotherapy. (2018) 52:623–31. doi: 10.1177/1060028018759471
15. Romano F, Muscogiuri G, Di Benedetto E, Zhukouskaya VV, Barrea L, Savastano S, et al. Vitamin D and sleep regulation: is there a role for vitamin D? Curr Pharm design. (2020) 26:2492–6. doi: 10.2174/1381612826666200310145935
16. Muscogiuri G, Barrea L, Scannapieco M, Di Somma C, Scacchi M, Aimaretti G, et al. The lullaby of the sun: the role of vitamin D in sleep disturbance. Sleep Med. (2019) 54:262–5. doi: 10.1016/j.sleep.2018.10.033
17. Al-Shawwa B, Ehsan Z, Ingram DG. Vitamin D and sleep in children. J Clin sleep medicine: JCSM: Off Publ Am Acad Sleep Med. (2020) 16:1119–23. doi: 10.5664/jcsm.8440
18. Prono F, Bernardi K, Ferri R, Bruni O. The role of vitamin D in sleep disorders of children and adolescents: A systematic review. Int J Mol Sci. (2022) 23(3):1430. doi: 10.3390/ijms23031430
19. Kim JH, Chang JH, Kim DY, Kang JW. Association between self-reported sleep duration and serum vitamin D level in elderly Korean adults. J Am Geriatrics Soc. (2014) 62(12):2327–32. doi: 10.1111/jgs.2014.62.issue-12
20. Al-Musharaf S. Changes in sleep patterns during pregnancy and predictive factors: A longitudinal study in saudi women. Nutrients. (2022) 14:2633. doi: 10.3390/nu14132633
21. Çakır T, Doğan G, Subaşı V, Filiz MB, Ülker N, Doğan ŞK, et al. An evaluation of sleep quality and the prevalence of restless leg syndrome in vitamin D deficiency. Acta neurologica Belgica. (2015) 115:623–7. doi: 10.1007/s13760-015-0474-4
22. Becker SP. ADHD and sleep: recent advances and future directions. Curr Opin Psychol. (2020) 34:50–6. doi: 10.1016/j.copsyc.2019.09.006
23. Spruyt K, Gozal D. Sleep disturbances in children with attention-deficit/hyperactivity disorder. Expert Rev Neurother. (2011) 11:565–77. doi: 10.1586/ern.11.7
24. Konrad C, Seehagen S. The effect of napping and nighttime sleep on memory in infants. Adv Child Dev Behav. (2021) 60:31–56. doi: 10.1016/bs.acdb.2020.08.003
25. Chaput JP, Gray CE, Poitras VJ, Carson V, Gruber R, Birken CS, et al. Systematic review of the relationships between sleep duration and health indicators in the early years (0-4 years). BMC Public Health. (2017) 17:855. doi: 10.1186/s12889-017-4850-2
26. Seehagen S, Charlton S, Starkey N, Fallaize A, Brown J, Jones K. The role of prior sleep for divergent thinking in infants. J Sleep Res. (2022) 31(2):e13457. doi: 10.1111/jsr.13457
27. Spruyt K. A review of developmental consequences of poor sleep in childhood. Sleep Med. (2019) 60:3–12. doi: 10.1016/j.sleep.2018.11.021
28. Wang LJ, Yang CY, Kuo HC, Chou WJ, Tsai CS, Lee SY. Effect of bifidobacterium bifidum on clinical characteristics and gut microbiota in attention-deficit/hyperactivity disorder. J Pers Med. (2022) 12(2):227. doi: 10.3390/jpm12020227
29. Zhuo L, Zhao X, Zhai Y, Zhao B, Tian L, Zhang Y, et al. Transcutaneous electrical acupoint stimulation for children with attention-deficit/hyperactivity disorder: a randomized clinical trial. Trans Psychiatry. (2022) 12:165. doi: 10.1038/s41398-022-01914-0
30. Khoshbakht Y, Moghtaderi F, Bidaki R, Hosseinzadeh M, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) diet on attention-deficit hyperactivity disorder (ADHD) symptoms: a randomized controlled clinical trial. Eur J Nutr. (2021) 60(7):3647–58. doi: 10.1007/s00394-021-02527-x
31. Gau SS, Shang CY, Liu SK, Lin CH, Swanson JM, Liu YC, et al. Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, version IV scale - parent form. Int J Methods Psychiatr Res. (2008) 17(1):35–44. doi: 10.1002/mpr.237
32. Kim J, Lee Y, Han D, Min K, Kim D, Lee C. The utility of quantitative electroencephalography and Integrated Visual and Auditory Continuous Performance Test as auxiliary tools for the Attention Deficit Hyperactivity Disorder diagnosis. Clin Neurophysiol. (2015) 126:532–40. doi: 10.1016/j.clinph.2014.06.034
33. Huang XX, Ou P, Qian QF, Huang Y. Long-term effectiveness of behavioural intervention in preschool children with attention deficit hyperactivity disorder in Southeast China - a randomized controlled trial. BMC Pediatr. (2021) 21(1):561. doi: 10.1186/s12887-021-03046-8
34. Conners CK. Symptom patterns in hyperkinetic, neurotic, and normal children. Child Dev. (1970) 41:667–82. doi: 10.2307/1127215
35. Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners Parent and Teacher Rating Scales. J Abnormal Child Psychol. (1978) 6:221–36. doi: 10.1007/BF00919127
36. Gajria K, Kosinski M, Sikirica V, Huss M, Livote E, Reilly K, et al. Psychometric validation of the Weiss Functional Impairment Rating Scale-Parent Report Form in children and adolescents with attention-deficit/hyperactivity disorder. Health Qual Life outcomes. (2015) 13. doi: 10.1186/s12955-015-0379-1
37. Owens JA, Spirito A, Mcguinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. (2000) 23:1043–51. doi: 10.1093/sleep/23.8.1d
38. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington (DC): National Academies Press (US) (2011).
39. Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. (2016) 101:394–415. doi: 10.1210/jc.2015-2175
40. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. (2011) 96:53–8. doi: 10.1210/jc.2010-2704
41. Yin H, Yang D, Yang L, Wu G. Relationship between sleep disorders and attention-deficit-hyperactivity disorder in children. Front Pediatr. (2022) 10:919572. doi: 10.3389/fped.2022.919572
42. Huang MM, Qian Z, Wang J, Vaughn MG, Lee YL, Dong GH. Validation of the sleep disturbance scale for children and prevalence of parent-reported sleep disorder symptoms in Chinese children. Sleep Med. (2014) 15(8):923–8. doi: 10.1016/j.sleep.2014.03.023
43. Evren B, Evren C, Dalbudak E, Topcu M, Kutlu N. The impact of depression, anxiety, neuroticism, and severity of Internet addiction symptoms on the relationship between probable ADHD and severity of insomnia among young adults. Psychiatry Res. (2019) 271:726–31. doi: 10.1016/j.psychres.2018.12.010
44. Kulie T, Groff A, Redmer J, Hounshell J, Schrager S. Vitamin D: an evidence-based review. J Am Board Family Med. (2009) 22(6):698–706. doi: 10.3122/jabfm.2009.06.090037
45. Becker SP, Epstein JN, Tamm L, Tilford AA, Tischner CM, Isaacson PA, et al. Shortened sleep duration causes sleepiness, inattention, and oppositionality in adolescents with attention-deficit/hyperactivity disorder: findings from a crossover sleep restriction/extension study. J Am Acad Child Adolesc Psychiatry. (2019) 58:433–42. doi: 10.1016/j.jaac.2018.09.439
46. Stumpf WE, O’brien LP. 1,25(OH)2 vitamin D3 sites of action in the brain. Histochemistry. (1987) 87:393–406. doi: 10.1007/BF00496810
47. Bellia A, Garcovich C, D’adamo M, Lombardo M, Tesauro M, Donadel G, et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Internal Emergency Med. (2013) 8:33–40. doi: 10.1007/s11739-011-0559-x
48. Dzierzewski JM, Donovan EK, Kay DB, Sannes TS, Bradbrook KE. Sleep inconsistency and markers of inflammation. Front Neurol. (2020) 11:1042. doi: 10.3389/fneur.2020.01042
49. Chiang JK. Short duration of sleep is associated with elevated high-sensitivity C-reactive protein level in Taiwanese adults: a cross-sectional study. J Clin sleep medicine: JCSM: Off Publ Am Acad Sleep Med. (2014) 10:743–9. doi: 10.5664/jcsm.3862
50. Chang JP, Mondelli V, Satyanarayanan SK, Chiang YJ, Chen HT, Su KP, et al. Cortisol, inflammatory biomarkers and neurotrophins in children and adolescents with attention deficit hyperactivity disorder (ADHD) in Taiwan. Brain behavior Immun. (2020) 88. doi: 10.1016/j.bbi.2020.05.017
51. Ivanov I, Miraglia B, Prodanova D, Newcorn JH. Sleep disordered breathing and risk for ADHD: review of supportive evidence and proposed underlying mechanisms. J Atten Disord. (2024) 28(5):686–98. doi: 10.1177/10870547241232313
52. Zhang P, Xu Q, Zhu R. Vitamin D and allergic diseases. Front Immunol. (2024) 15:1420883. doi: 10.3389/fimmu.2024.1420883
53. Marusca LM, Reddy G, Blaj M, Prathipati R, Rosca O, Bratosin F, et al. The effects of vitamin D supplementation on respiratory infections in children under 6 years old: A systematic review. Dis (Basel Switzerland). (2023) 11:104. doi: 10.3390/diseases11030104
54. Locci C, Ruiu A, Saderi L, Sotgiu G, Bassu S, Zaffanello M, et al. Relationships between 25-hydroxyvitamin D levels and obstructive sleep apnea severity in children: an observational study. J Clin Med. (2023) 12:1242. doi: 10.3390/jcm12031242
55. Tian HQ, Cheng L. The role of vitamin D in allergic rhinitis. Asia Pacific Allergy. (2017) 7:65–73. doi: 10.5415/apallergy.2017.7.2.65
56. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. (2020) 12:2097. doi: 10.3390/nu12072097
57. Miller MC, Pan X, Eugene Arnold L, Mulligan A, Connor S, Bergman R, et al. Vitamin D levels in children with attention deficit hyperactivity disorder: Association with seasonal and geographical variation, supplementation, inattention severity, and theta:beta ratio. Biol Psychol. (2021) 162. doi: 10.1016/j.biopsycho.2021.108099
58. Hemamy M, Pahlavani N, Amanollahi A, Islam SMS, McVicar J, Askari G, et al. The effect of vitamin D and magnesium supplementation on the mental health status of attention-deficit hyperactive children: a randomized controlled trial. BMC Pediatr. (2021) 21:178. doi: 10.1186/s12887-021-02631-1
Keywords: attention deficit hyperactivity disorder, Vitamin D, symptom, children’s sleep habits questionnaire, sleep disordered breathing
Citation: Zhang P, Liu Y, Ma Y, Zhao T, Zhang C and Sun H (2025) Vitamin D insufficiency and sleep disturbances in children with ADHD: a case-control study. Front. Psychiatry 16:1546692. doi: 10.3389/fpsyt.2025.1546692
Received: 17 December 2024; Accepted: 25 February 2025;
Published: 20 March 2025.
Edited by:
Jianda Kong, Qufu Normal University, ChinaReviewed by:
Veronika Maria Sidharta, Atma Jaya Catholic University of Indonesia, IndonesiaMohamed Abouzed, Al-Azhar University, Egypt
Intisar R. Sharba, University of Kufa, Iraq
Copyright © 2025 Zhang, Liu, Ma, Zhao, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Sun, MTM2MDQwOTMwODhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Panpan Zhang
Panpan Zhang Yang Liu1,2†
Yang Liu1,2† Hao Sun
Hao Sun