
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 25 March 2025
Sec. Personality Disorders
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1539611
Introduction: Borderline personality disorder (BPD) patients exhibit high rates of co-occurring mental disorders. Though literature reports varying prevalence of substance use disorders (SUD) in BPD, they are frequent with prevalence rates of approximately 45%. This study examines the 12-month prevalence of SUDs in a German sample of BPD patients by semi-structured interviews and compared to medical records.
Methods: N=126 BPD patients were included. Twelve-month SUD prevalence was obtained by semi-structured clinical interview (SCID) and compared to reported prevalence in the general population and to diagnoses from medical records.
Results: Mean age of the sample was 37.5 (SD ± 11.5) years and N=61 (48%) patients were female. Compared to the general population, increased 12-month prevalence based on SCID was found for alcohol abuse (22.2%, +7.9-times), alcohol dependence (17.5%, +5.6-times), cannabis abuse (15.1%, +30.2-times), cannabis dependence (19.0%, +31.7-times), sedative abuse (3.2%, +4.6-times) and sedative dependence (3.2% +4.6-times). N=43 (34.1%) patients presented at least one diagnosis of substance abuse and N=43 (34.1%) presented at least one diagnosis of substance dependence. N=51 (40.1%) patients were diagnosed with at least one substance use or dependence disorder. On average, prevalence based on SCID exceeded prevalence obtained from medical records. Particularly, alcohol abuse (3.5-times), cannabis abuse (2.4-times) and dependence (2.2-times), and sedative- and stimulant abuse (both no diagnosis in medical record vs. 3.2% in SCID) were underrecognized. Furthermore, concordance rates between diagnoses based on medical record and SCID were below 30% for all substances.
Conclusion: Our data confirm high prevalence of SUDs in BPD patients. Of note, medical records underrecognized alcohol abuse and cannabis abuse and dependence. Substance abuse and dependence are primary risk factors of suicidal behaviors and completed suicide. SUDs have been shown to be amenable to psychotherapeutic interventions. Therefore, careful diagnosis of SUD by e.g. expert structured interviews and integration of SUDs in a multimodal treatment plan is recommended.
Consumption of psychoactive substances is common in the general population and their use and abuse is associated with a significant burden of disease worldwide (1). Current literature reports the highest number of disability-adjusted life years (DALY) and death for tobacco use with alcohol consumption and illegal drug use following in second and third place, respectively (2). Compared to the general population, use of psychoactive substances is more frequent in clinical samples. Particularly in the context of psychiatric disorders, it constitutes a common problem and substance use disorders (SUD) are frequent (3). In particular, alcohol use disorder is the most prevalent SUD in every category of psychiatric disorder, including personality disorders (3). Dual disorder patients, i.e. patients that are characterized by the co-occurrence of two syndromes, have a higher risk of all-cause mortality (4) and a less favorable prognosis due to a reduced effectiveness of pharmacological as well as psychosocial and psychotherapeutic interventions (5, 6). Patients with severe mental illness and SUD have higher rates of hospitalization and an increased number of contacts with psychiatric emergency departments compared to patients without SUD (7).
Among the personality disorders that commonly co-occur with SUD, borderline personality disorder (BPD) has received particular attention because of its complex clinical presentation. The symptoms of borderline personality disorder (BPD) can be grouped in three principles domains: emotional dysregulation, inconsistent identity and disturbed interpersonal functioning (8). Epidemiological studies suggest prevalence rates between 0.7-2.7% in non-clinical samples (9, 10) In clinical samples, BPD constitutes the most frequent personality disorder that affects up to 10% of psychiatric outpatients and up to 25% of inpatients (11). BPD is frequently associated with the co-occurrence of additional psychiatric disorders, with mood and anxiety disorders, non-borderline personality disorders, and SUD being the most prevalent (12). Vice versa, personality disorders are frequently diagnosed in patients that are treated for an SUD and a prevalence for BPD between 5-22% has been reported in this patient population (13, 14). This is in line with findings from a dedicated review article reporting a prevalence of 22.1% for current BPD among cases with a current SUD across studies (15). Similarly, high prevalence for any SUD has been described in patients with BPD, with dedicated cross sectional studies reporting rates between 19-87% for any SUD and 24-66% for an alcohol-related SUD (16). While diagnostic criteria for BPD and SUDs intersect, the overlap does not account for the pronounced observed co-occurrence of BPD and SUDs and BPD rates in SUD patients remain high even when substance related characteristics are not included in the diagnosis (17, 18).
Compelling evidence suggests an adverse impact of the co-occurrence of BPD in patients with SUDs (19). By comparison these patients are younger, more frequently female, and less frequently employed compared to SUD patients without BPD. Additionally, they display high rates of mood disorders and low levels of functioning (20, 21). Conversely, studies regarding the impact of a co-occurring SUD on BPD symptomatology and outcome are less frequent and yielded inconsistent results. Prior studies suggest that patients with co-occurring BPD and past or current SUD report higher impulsivity than those without SUD (22), although some found no significant differences in BPD symptoms (23, 24). However, suicide and self-harm thoughts are more common in BPD patients with SUD (25, 26), and their remission rates are lower. SUD is also a significantly worse prognostic factor than other co-occurring disorders, such as PTSD, anxiety, or mood disorders (27).
Previous studies have addressed prevalence rates of SUDs in BPD patients. However, most of these studies only distinguished between alcohol abuse and dependence (DSM-IV, ICD-10), while other substances have been commonly grouped as drug abuse and dependence (again DSM-IV, ICD-10).
In the present manuscript we assessed 12-month prevalence rates of substance abuse and dependence (by ICD-10 as we assessed a German population), and substance use disorder as defined by DSM-5 of individual substances in a sample of BPD patients by semi-structured clinical interview (SCID DSM-5). We compared obtained prevalence to data from the general population and to diagnoses obtained from medical records. To our knowledge a comparison of SCID diagnoses and medical records, evaluating potential over- or underdiagnosis of SUD in BPD has not been performed before.
The present study followed the principals of the Declaration of Helsinki and was approved by the local ethics committee at Hannover Medical School. All participants gave their consent before study inclusion. Structured clinical interviews and follow-up data were acquired between September 2021 and April 2023 (in one telephone interview session).
Patients with a prior diagnosis of borderline personality disorder that were previously treated at the ward for dialectical behavior therapy of the Department of Psychiatry, Social psychiatry, and Psychotherapy at Hannover Medical School (but not in a structured follow up) were contacted by telephone. Inclusion criteria were as follows: a diagnosis of BPD, an age of 18 years or older, treatment for BPD in MHH within the past year, and sufficient German langue skills to understand the consent form and to participate in the SCID. Severe cognitive disorders (known IQ <70; previous clinical diagnosis; severe intoxication) were considered an exclusion criterion. Off 324 patients contacted, N=126 patients with BPD agreed to partake in and were included in the study.
To assess the 12-month prevalence of mental and behavioral disorders due to psychoactive substance use (ICD-10-CM codes F10-F19) the German version of the semi-structured clinical interview (SCID) for Diagnostic and Statistical Manual-5 (DSM-5) was performed (28, 29). In particular, module E (Substance Use Disorders) with items E1-10 (Alcohol Use Disorders in the past 12 month) and E11-E36 (Nonalcohol Substance Use Disorders in the past 12 month) of the SCID-5 was applied by telephone interview by a trained research assistant under supervision (J.N.; supervision N.S. and K.G.K). Additionally, data regarding all prior psychiatric diagnoses, including substance abuse and dependence disorders were obtained from patient’s medical records by J.N.
We obtained ICD-10 SCID-validated diagnoses by matching ICD-10 criteria to the SCID-5-CV items. For harmful use that included the consumption with loss of control, as well as at least one item for social, psychological or physical harm (items E6-10 for alcohol and E28-32 for nonalcohol substances). For dependence we required 3 or more symptoms correlating with craving (items E3 for alcohol; E25 for nonalcohol substances), loss of control over consumption (E2-3 and E24-25), continued use despite harm (E6-7, E9-10; E28-29, E30-31), disregard of other activities in favor of substance use (E4, E8; E26, E30), development of tolerance (E11; E33), and withdrawal symptoms (E12, E34).
The mean age of BPD patients in the present sample was 37.5 (SD ± 11.5) years and N=61 (48%) were female. Based on medical record, prevalence of psychiatric comorbidity was high, with N=117 (93%) patients having at least one other F-diagnosis than BPD and F10 diagnoses. Prevalence of F-diagnoses based on medical record of the total sample as well as grouped by sex are summarized in Table 1.
Statistical analyses were performed using SPSS 28 (IBM, Armonk, NY, USA) and R 4.4.3 (Trophy Case; R Core TeamR Foundation for Statistical Computing, Vienna, AUSTRIA). Point prevalence rates based on SCID and medical record were calculated and 95% confidence intervals (CI) were computed using the Clopper-Pearson method. Shapiro-Wilk test was used to test for normal distribution of continuous data. Group comparisons of categorical data were carried out by use of Chi-Square test and Mann-Whitney U test was performed for comparison of continuous data. Two-tailed p-values are depicted and p≤.050 was considered statistically significant. Concordance statistics were calculated using Kendall’s tau.
Comparison of point prevalence rates of the three most frequently used substances in BPD patients to literature data from the German general population are depicted in Table 2 (30). The point prevalence of SUDs in the study’s BPD sample was significantly higher than reported data in the general population. This applied to both male and female patients.
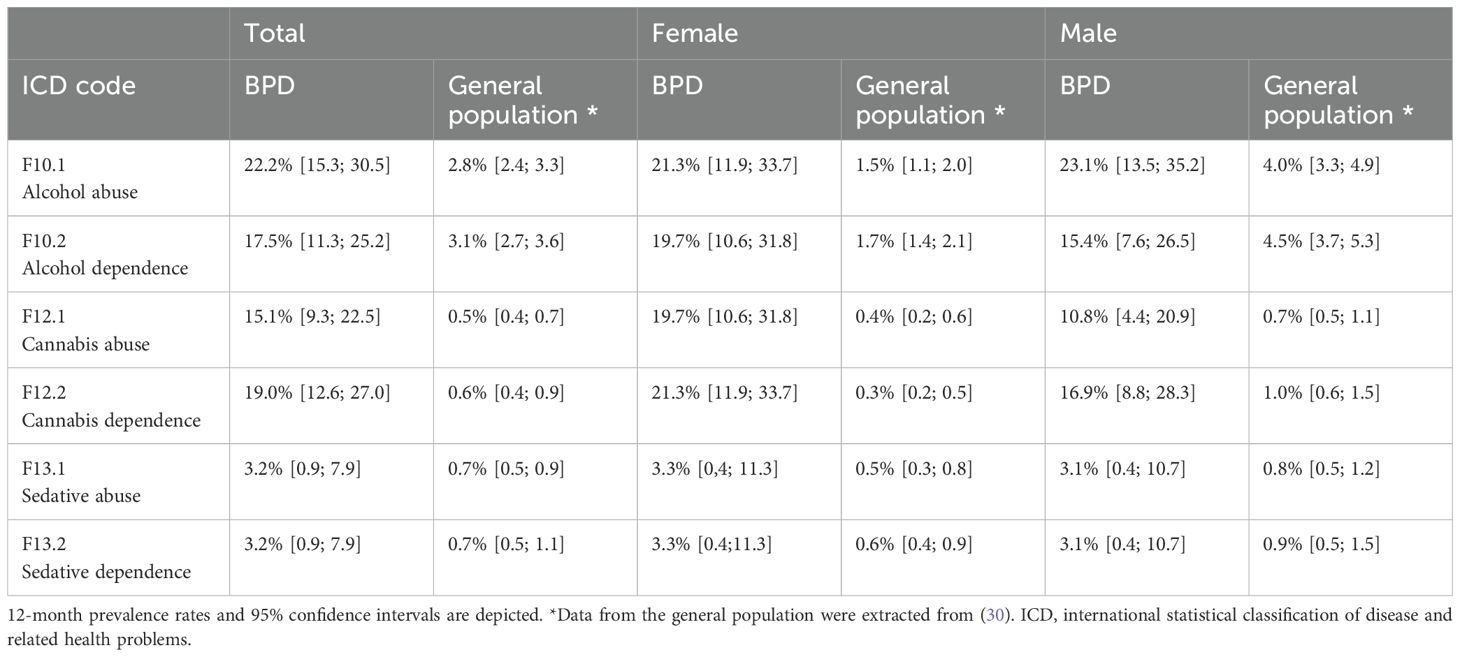
Table 2. Comparison of substance abuse and dependence in BPD patients with data from the general population.
Alcohol related disorders are the second most common SUD (or, for that matter, dependence and harmful use by ICD-10 criteria) in the general population. In this study’s BPD sample, we found a 7.9-times (female: 14.2-times, male: 5.8-times) higher prevalence of alcohol abuse while prevalence of alcohol dependence showed a 5.6-times increase (female: 11.6-times, male: 3.4-times) when compared to the general German population. Overall, N=50 (39.7%) BPD patients presented with an alcohol use disorder based on SCID.
Prevalence for cannabis related disorders was less than one percent in the general population (30). The present data indicate significantly higher point prevalence in BPD patients, with cannabis abuse exceeding data from the general population by 30.2-times (female: 49.3-times, male: 15.4-times) and cannabis dependence by 31.7-times (female: 71.0-times, male:16.9-times). Indeed, cannabis use disorders were the nearly as prevalent as alcohol use disorders in the present BPD sample, with N=43 (34.1%) displaying cannabis use or dependence disorders.
Concurrent with cannabis use disorders, prevalence of sedative abuse and dependence in the general population were less than one percent (30). In contrast, in this study’s sample point prevalence for sedative abuse fell above 3% for the total as well as the male and female subgroups. Sedative abuse was 4.6-times higher in the complete sample (female: 6.6-times, male: 3.9-times) than in the general population. Further, sedative dependence disorder was more frequent (4.6-times) in BPD patients compared to the general population (female: 5.2-times, male: 3.4-times). The overall 12-month prevalence rate for a sedative use disorder was 6.3% (N=8) in this study’s sample.
Based on data obtained from SCID, N=58 (46.0%) of BPD patients in the present sample had at least mild SUD (at least 2 DSM-5 criteria), while at least one diagnosis of moderate to severe SUD (four or more DSM-5 criteria) was found in N=41 (32.5%) of patients.
Male and female BPD patients in the present sample did not differ significantly with regard to mean age (female: 36.2 ± 11.1 years, male: 38.5 ± 11.9 years, U=1750.5, Z=-1.133, p=.257) or psychiatric comorbidities (Table 1).
No significant differences regarding prevalence rates of specific SUD determined by SCID were found in female compared to male BPD patients (Table 3). Additionally, female and male BPD patients did not differ significantly regarding frequencies of at least one diagnosis of any dependence or harmful use (Table 3).
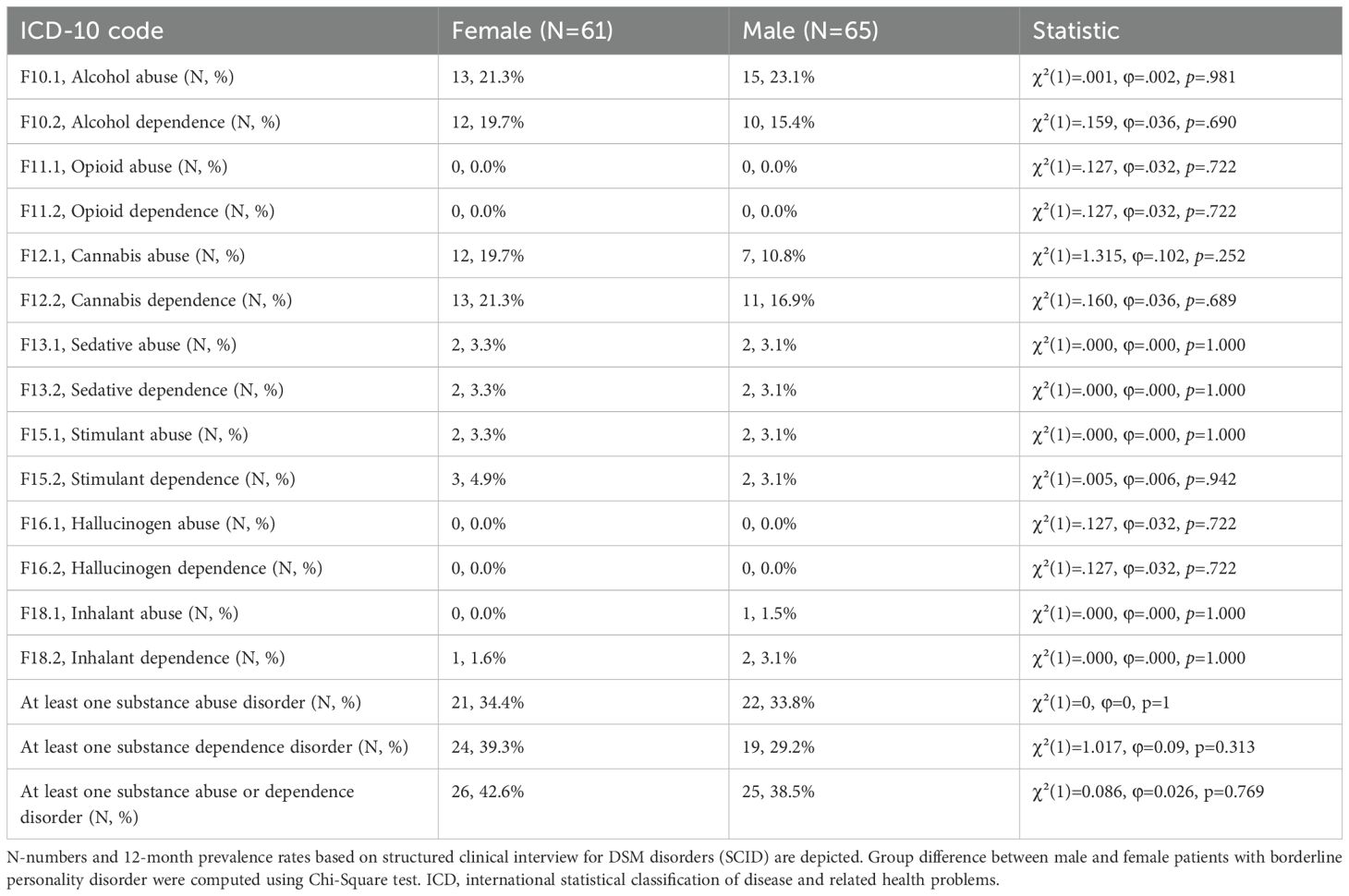
Table 3. Similar prevalence rates of substance abuse and dependence disorders in female and male BPD patients based on SCID.
Figures 1A, B as well as Table 4 compare numbers of substance abuse and substance dependence cases based on SCID diagnoses to case numbers obtained from medical records. Alcohol abuse and dependence and cannabis abuse and dependence were the most frequent ICD-10 diagnoses in medical records and SCID. However, present data suggest an underdiagnosis of SUD in patients with BPD. On average, prevalence rates based on SCID exceeded prevalence rates obtained from medical record. Particularly, alcohol abuse (3.5-times) and dependence (1.5-times), cannabis abuse (2.4-times) and dependence (2.2-times), sedative abuse (no diagnosis in medical record vs. 3.2% in SCID), and stimulant abuse (2.0-times) were underrecognized in medical records. Conversely, frequencies of opioid abuse and dependence and sedative dependence were low based on medical record as well as based on SCID.
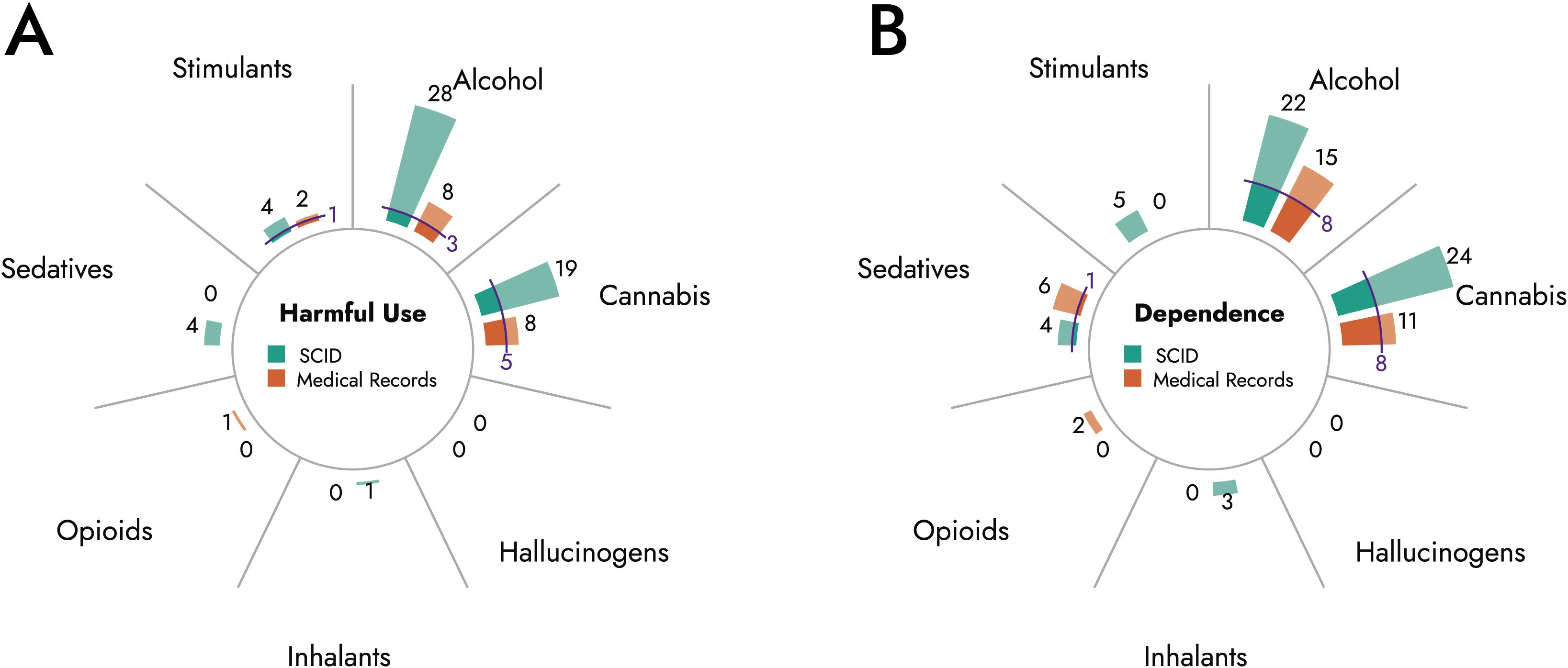
Figure 1. Comparison of substance abuse and dependence diagnoses based on SCID and medical record. Diagrams depict total number of cases diagnosed with harmful use (A) or dependence (B) of indicated substances based on SCID (green) or medical records (orange). Blue lines indicate cases with matching diagnoses in medical record and SCID.
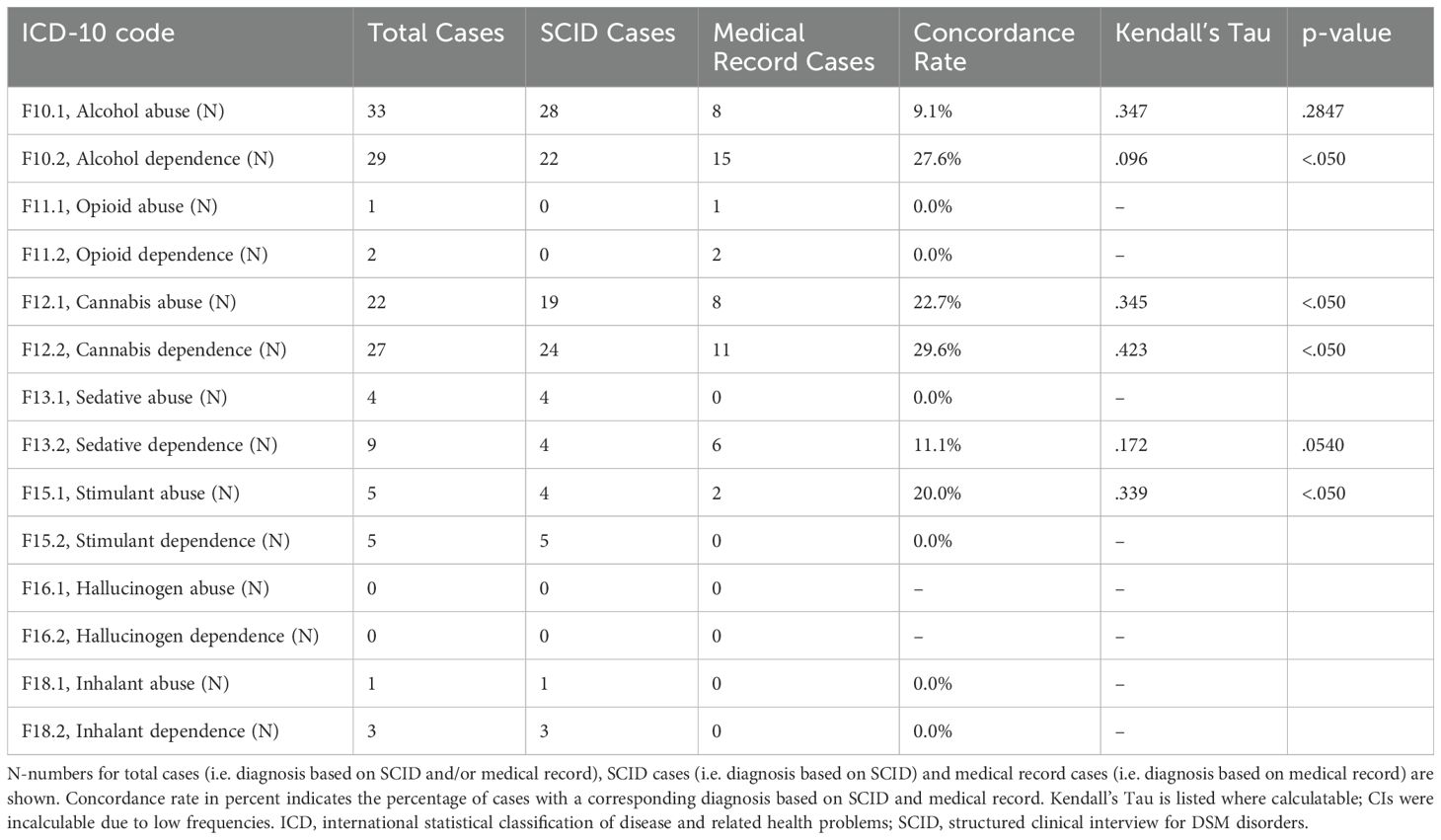
Table 4. Discrepancies between substance abuse and substance dependence diagnoses based on SCID and medical record.
Furthermore, concordance rates between diagnoses based on medical records and SCID were low (Table 4, Figures 1A, B). The highest concordance rates were observed for cannabis dependence (29.6%) and alcohol dependence (27.6%), while diagnoses for harmful use of sedatives or stimulants showed no overlap between medical records and SCID.
In the present study we describe increased 12-month prevalence rates of SUDs in patients with BPD compared to the general population. Our data are in line with previous studies describing an elevated prevalence for SUDs in BPD patients compared to the general population. In contrast to studies by others, we did not observe sex-specific effects on SUD prevalence in the present sample. Additionally, we report an underrepresentation of SUD diagnoses in medical records of BPD patients compared to data based on SCID-5. While DSM-5 criteria for substance use disorders that underly SCID differ from ICD-10 criteria that underly German medical records, and are more prone to diagnose a patient with SUD as even fulfilling two of eleven symptoms classifies as mild SUD, the differences in the current sample are too large to be explained by this effect.
The present study highlights a significantly higher 12-month prevalence for SUDs, particularly mild SUDs, in BPD patients compared to the general public. In this regard, our findings are in line with previous works that consistently report high co-occurrence rates for 12-month and lifetime prevalence for SUDs in BPD. A meta-analysis by Trull and colleagues that only included studies utilizing structured interviews for SUD diagnosis, found an average co-occurrence rate of approximately 45% for at least one current SUD in BPD patients across settings (15), which is slightly lower than the observed prevalence of 46% for any SUD in the present sample. In the general population alcohol is the most frequently used substance, excluding nicotine, by some margin (30). Prior studies in the context of BPD yielded heterogenous results concerning prevalence of alcohol use disorders. Trull and colleagues report high prevalence rates of approximately 46% for current alcohol use disorder in BPD patients across settings (15), while a second systemic review article reported a 12-month prevalence for alcohol use disorders between 13% and 31%, based on 16 studies that used structured (N=15) or unstructured (N=1) interviews for SUD diagnosis (31). Our data indicate a 12-month prevalence of 25% for alcohol use disorder based on SCID.
Contrarily to data from the general population and to prior studies in BPD patients, cannabis use disorders were more prevalent than alcohol-related disorders in the present sample (15, 30). There are fewer studies that assess cannabis use disorders in BPD patients than those targeted at comorbidity of BPD and alcohol-related disorders. This can be attributed to the fact that the majority of respective do not distinguish between different substances, but rather assessed drug use disorders in aggregate. The underlying rational for the combined analysis of nonalcoholic substances as one category might relate to the relatively low prevalence and subsequent limited sample sizes. However, one publication based on data from the National Institute on Alcohol Abuse and Alcoholism’s (NIAAA) NESARC study and that analyzed lifetime prevalence of substance use and dependence in BPD patients in relation to specific substances, reported a lifetime prevalence for cannabis use disorder of 31% in a sample of 1,030 BPD patients (32). This aligns with the 12-month prevalence of cannabis use disorder in this sample according to medical records whereas cannabis use disorders identified through SCID show a slight under representation in our sample (25.2%). While we can only speculate about the underlying reasons, several factors might be considered. Cannabis use has been increasing in the general population in recent years, owing to the progressive legalization and/or decriminalization in several countries, including Germany (33–35). Additionally, research suggests that coping motives as well as conformity motives are strongly associated with borderline features for both alcohol and cannabis (36). Therefore, particularly conformity motives may contribute to increased cannabis use in BPD patients given the increasing popularity of the substance in the general population. Further, studies have shown that cannabis use may exert anxiolytic effects, reduce impulsivity and self-harming behavior, and mitigate stress reactivity—features frequently observed in BPD (37–39).
Sedative use disorder was reported to have a lifetime prevalence of 8.4% in BPD patients (32). To the best of our knowledge, data regarding 12-month prevalence rates for sedative use disorders in this patient population are lacking to date. In the current sample, 12-month prevalence rates were with 4.8% markedly higher than in the general population.
Overall, our data are in line with previous studies reporting significantly increased rates of substance abuse and dependence in BPD patients compared to the general public.
In the general population, SUDs occur with a higher frequency in men compared to women (30). Similarly, studies assessing potential differences regarding frequency of SUDs in male and female patients with BPD have indicated higher prevalence rates for alcohol use disorders and substance use disorders in men compared to women (40–42). Contrarily, in the present sample no sex-specific differences in 12-month prevalence rates were observed for any substance, and additionally male and female patients did not differ significantly regarding the frequency of at least one substance use or dependence disorder. Given the overall higher rates of SUDs, particularly of alcohol-related disorders, in men in the general population, most SUDs appear to be stronger overrepresented in female BPD patients compared to males. Considering the overall higher risk of BPD-individuals for SUDs reported in literature (43).
While previous research regarding the impact of a co-occurring SUD on BPD symptomology and trajectory yielded inconsistent results, there is some evidence that indicates higher levels of suicidality and self-harm and less frequent remission from BPD in the context of any SUD (44, 45). Additionally, a co-occurring SUD was found to be associated with an increased likelihood of high-risk sexual behavior reflected by higher rates of sexual transmitted diseases in BPD patients (46).
Further, patients with co-occurring BPD and SUD might show decreased adherence to therapy when only one disorder is taken into account, which is reflected by increased dropout rates in dual patients (47). Likely due to the incompability of ongoing substance use and effective psychotherapy or physical inability to attend psychotherapy due to intoxication. Also, craving for substances can significantly impact treatment adherence in more intensive settings like day-hospitals or inpatient-treatments. Due to lack of evidence, this does remain speculative, however.
Given the described adverse effects of SUDs surprising little literature exists that covers treatment approaches for co-occurring BPD and SUD (48). Although psychotherapy is considered the first-line treatment option for BPD and also plays part in SUD treatment, studies assessing the efficacy of psychotherapy in dual disorder patients are scarce and limited by small sample sizes and variable outcomes. However, there is some evidence that structured integrative care, particularly dialectic behavior therapy for SUD (DBT-SUD) and to a lesser extent for dynamic deconstructive psychotherapy (DDP) are associated with beneficial outcomes (48). Pharmacologically there is little evidence for BPD but a co-occurring SUD might need substitution or can benefit from medication (e.g. bupropione for nicotine use or acamprosate for alcohol use disorders or naltrexone to reduce craving). Therefore, it is of importance to adequately diagnose SUDs in BPD patients to enable them to receive optimized treatment. However, data from this study suggests that in clinical practice the co-occurrence of both disorders is often overlooked – particularly in the case of cannabis use disorders.
An underdiagnosis of SUDs appears to be common, which is reflected in our data showing significantly lower prevalence rates for most SUDs based on data obtained from medical records compared to SCID.
In this regard, particularly substance abuse was more often not recognized in medical records compared to substance dependence. This may be due to the ICD-10 criteria requiring negative physical health consequences for the diagnosis of substance abuse whereas the adapted SCID-5 criteria, based on DSM-5, do not require this criterion for the definition of mild SUD. In clinical practice that may lead to underestimating the impact of a less severe SUD on the overall treatment as the substance abuse does not appear in diagnoses of discharge summaries and medical histories but may get lost in texts describing the patient’s behaviors. Considering the relevant negative impact of substance abuse on both physical and mental health, taking special care to test for even mild forms of substance abuse or applying DSM-5 criteria for the actual treatment, could improve overall outcomes. In addition, concordance rates between diagnoses based on medical records and SCID were low, suggesting that either at the time of medical record’s creation patients exhibited markedly different substance use behaviors or diagnostic criteria applied in clinical setting differed from the gold-standard criteria used in SCID. Another possible explanation was “carry-on” of old diagnoses in medical records that patients had had in previous treatments.
We are unaware of studies that focused on the accuracy of medical records regarding SUD diagnoses in BPD patients and similarly, we could not identify research that investigated potential reasons for the under-recognition of SUDs in this patient population. However, literature relating to dual patients with schizophrenia and SUDs suggest that under-reporting amongst patients is common (49). This is in line with a meta-analysis including studies that compared substance use based on self-report to laboratory tests in a general adult mental health setting (50). This study reported that laboratory test yielded positive results in 27% of patients that denied use of any substance with (50). Further, the authors also proposed that using structured interviews regarding substance use might yield more accurate results than other methods of clinical assessment. From a clinical perspective, factors such as shame about the use or abuse of psychoactive substances as well as a fear of being required to be abstinent from a substance they have found to be helpful in mitigating fear- and anxiety-related symptoms. They may, in that context, not even consider the use of the substance as “abuse” or “dependence”. Lastly, reporting regular use of substances and a diagnosis of a SUD limit access to psychotherapeutic treatments – abstinence may be a prerequisite for therapy or the insurance company may refuse reimbursement for patients consuming psychoactive substances. Patients, therefore, have many motives beyond the dysfunctional or stereotype to avoid mentioning problematic substance use.
The present study has several limitations that should be taken into account when interpreting the results. First, we did not record reasons for non-participation. Therefore, we cannot exclude the possibility of some bias regarding the present study sample, i.e. patients with problematic substance use might be less likely to agree to participate. Second, we did not perform a SCID for other psychiatric disorders other than SUDs. Therefore, we were unable to assess if the observed underdiagnosis of SUDs in medical records extended to other mental disorders or whether the observed discrepancy was specific for SUDs. Third, while studies suggest that SCID is a more reliable method for diagnosis of SUDs in patients with severe mental illnesses than other interview- or self-report instrument, SCID does rely on the accuracy of the information given by the patient. Therefore, we cannot exclude some inaccuracies regarding the reported 12-month prevalence rates.
Our study confirms high rates of SUDs in patients with BPD compared to the general population. Of note, particularly cannabis use and dependence was high in the present sample, exceeding prevalence rates for alcohol-related disorders. In contrast to prior studies, prevalence rates for any SUD did not significantly differ between sexes. Based on comparison with data retrieved from medical records, SUDs were often not adequately diagnosed in this patient population. While we can only speculate about the underlying reasons for this observed discrepancy, it is important to note the potential resulting adverse consequences for the individual patient but also for the health care system (51, 52). Given the unfavorable outcomes of BPD patients with co-occurring SUDs (44, 45, 53), adequate diagnostic evaluation and consequent referral to indicated therapies is of paramount importance to not only ensure short-term adherence but a favorable long-term outcome. Therefore, clinical professionals treating patients with BPD should encourage patients to be open about their substance use and not hesitate to diagnose and educate about SUDs to reduce stereotypes, encourage multi-disorder treatment and help their patients avoid long-term adverse health events.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee at Hannover Medical School. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
JN: Investigation, Writing – original draft. SB: Formal analysis, Visualization, Writing – original draft. BS: Formal Analysis, Visualization, Writing – original draft. NS: Writing – review & editing. KK: Conceptualization, Formal Analysis, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
KK received speaker honoraria and travel grants from EliLilly, Janssen, Takeda, Medice, Servier, Dr. Schwabe, and Idorsia. All other authors declare no conflict of interest.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rauschert C, Mockl J, Seitz NN, Wilms N, Olderbak S, Kraus L. The use of psychoactive substances in Germany. Dtsch Arztebl Int. (2022) 119:527–34. doi: 10.3238/arztebl.m2022.0244
2. Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. (2018) 113:1905–26. doi: 10.1111/add.14234
3. Toftdahl NG, Nordentoft M, Hjorthoj C. Prevalence of substance use disorders in psychiatric patients: A nationwide danish population-based study. Soc Psychiatry Psychiatr Epidemiol. (2016) 51:129–40. doi: 10.1007/s00127-015-1104-4
4. Hjorthoj C, Ostergaard ML, Benros ME, Toftdahl NG, Erlangsen A, Andersen JT, et al. Association between alcohol and substance use disorders and all-cause and cause-specific mortality in schizophrenia, bipolar disorder, and unipolar depression: A nationwide, prospective, register-based study. Lancet Psychiatry. (2015) 2:801–8. doi: 10.1016/S2215-0366(15)00207-2
5. Wobrock T, Falkai P, Schneider-Axmann T, Hasan A, Galderisi S, Davidson M, et al. Comorbid substance abuse in first-episode schizophrenia: effects on cognition and psychopathology in the eufest study. Schizophr Res. (2013) 147:132–9. doi: 10.1016/j.schres.2013.03.001
6. Large M, Mullin K, Gupta P, Harris A, Nielssen O. Systematic meta-analysis of outcomes associated with psychosis and co-morbid substance use. Aust N Z J Psychiatry. (2014) 48:418–32. doi: 10.1177/0004867414525838
7. Jorgensen KB, Nordentoft M, Hjorthoj C. Association between alcohol and substance use disorders and psychiatric service use in patients with severe mental illness: A nationwide danish register-based cohort study. Psychol Med. (2018) 48:2592–600. doi: 10.1017/S0033291718000223
8. Bohus M, Stoffers-Winterling J, Sharp C, Krause-Utz A, Schmahl C, Lieb K. Borderline personality disorder. Lancet. (2021) 398:1528–40. doi: 10.1016/S0140-6736(21)00476-1
9. Ellison WD, Rosenstein LK, Morgan TA, Zimmerman M. Community and clinical epidemiology of borderline personality disorder. Psychiatr Clinics North America. (2018) 41:561–73. doi: 10.1016/j.psc.2018.07.008
10. Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. (2018) 21:28–32. doi: 10.1016/j.copsyc.2017.09.001
11. Leichsenring F, Leibing E, Kruse J, New AS, Leweke F. Borderline personality disorder. Lancet. (2011) 377:74–84. doi: 10.1016/S0140-6736(10)61422-5
12. Shah R, Zanarini MC. Comorbidity of borderline personality disorder: current status and future directions. Psychiatr Clin North Am. (2018) 41:583–93. doi: 10.1016/j.psc.2018.07.009
13. Verheul R. Co-morbidity of personality disorders in individuals with substance use disorders. Eur Psychiatry. (2001) 16:274–82. doi: 10.1016/s0924-9338(01)00578-8
14. Howe LK, Fisher LR, Atkinson EA, Finn PR. Symptoms of anxiety, depression, and borderline personality in alcohol use disorder with and without comorbid substance use disorder. Alcohol. (2021) 90:19–25. doi: 10.1016/j.alcohol.2020.11.002
15. Trull TJ, Freeman LK, Vebares TJ, Choate AM, Helle AC, Wycoff AM. Borderline personality disorder and substance use disorders: an updated review. Borderline Pers Disord Emot Dysregul. (2018) 5:15. doi: 10.1186/s40479-018-0093-9
16. Zanarini MC, Frankenburg FR, Weingeroff JL, Reich DB, Fitzmaurice GM, Weiss RD. The course of substance use disorders in patients with borderline personality disorder and axis ii comparison subjects: A 10-year follow-up study. Addiction. (2011) 106:342–8. doi: 10.1111/j.1360-0443.2010.03176.x
17. Dulit RA, Fyer MR, Haas GL, Sullivan T, Frances AJ. Substance use in borderline personality disorder. Am J Psychiatry. (1990) 147:1002–7. doi: 10.1176/ajp.147.8.1002
18. Rounsaville BJ, Kranzler HR, Ball S, Tennen H, Poling J, Triffleman E. Personality disorders in substance abusers: relation to substance use. J Nerv Ment Dis. (1998) 186:87–95. doi: 10.1097/00005053-199802000-00004
19. Blay M, Verne M, Durpoix A, Benmakhlouf I, Labaume L. Clinical specificities of patients with substance use disorder and comorbid borderline personality disorder compared to patients with substance use disorder only: A retrospective study. J Addict Dis. (2024), 1–7. doi: 10.1080/10550887.2024.2363038
20. Barber JP, Frank A, Weiss RD, Blaine J, Siqueland L, Moras K, et al. Prevalence and correlates of personality disorder diagnoses among cocaine dependent outpatients. J Pers Disord. (1996) 10:297–311. doi: 10.1521/pedi.1996.10.4.297
21. Langas AM, Malt UF, Opjordsmoen S. In-depth study of personality disorders in first-admission patients with substance use disorders. BMC Psychiatry. (2012) 12:180. doi: 10.1186/1471-244X-12-180
22. Coffey SF, Schumacher JA, Baschnagel JS, Hawk LW, Holloman G. Impulsivity and risk-taking in borderline personality disorder with and without substance use disorders. Pers Disord. (2011) 2:128–41. doi: 10.1037/a0020574
23. Boog M, Dugonjic H, Arntz A, Goudriaan AE, Wetering B, Franken IHA. Borderline personality disorder with versus without alcohol use disorder: comparing impulsivity and schema modes. J Pers Disord. (2022) 36:1–18. doi: 10.1521/pedi_2021_35_521
24. Lee HJ, Bagge CL, Schumacher JA, Coffey SF. Does comorbid substance use disorder exacerbate borderline personality features? A comparison of borderline personality disorder individuals with vs. Without Current Substance Dependence. Pers Disord. (2010) 1:239–49. doi: 10.1037/a0017647
25. Links PS, Heslegrave RJ, Mitton JE, van Reekum R, Patrick J. Borderline personality disorder and substance abuse: consequences of comorbidity. Can J Psychiatry. (1995) 40:9–14. doi: 10.1177/070674379504000105
26. Stone MH. The Fate of Borderline Patients: Successful Outcome and Psychiatric Practice. New York: Guilford Publications (1990).
27. Zanarini MC, Frankenburg FR, Hennen J, Reich DB, Silk KR. Axis I comorbidity in patients with borderline personality disorder: 6-year follow-up and prediction of time to remission. Am J Psychiatry. (2004) 161:2108–14. doi: 10.1176/appi.ajp.161.11.2108
28. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Dsm-5. Washington, DC: American psychiatric association (2013).
29. First MB, Beesdo-Baum K, Zaudig M, Wittchen HU. Scid-5-Cv Strukturiertes Klinisches Interview Für Dsm-5-Störungen – Klinische Version: Deutsche Bearbeitung Des Structured Clinical Interview for Dsm-5 Disorders – Clinician Version. First VMB, Williams JBW, Karg RS, Spitzer RL, editors. Göttingen: Manual: Hogrefe (2019).
30. Atzendorf J, Rauschert C, Seitz NN, Lochbuhler K, Kraus L. The use of alcohol, tobacco, illegal drugs and medicines: an estimate of consumption and substance-Related disorders in Germany. Dtsch Arztebl Int. (2019) 116:577–84. doi: 10.3238/arztebl.2019.0577
31. Guy N, Newton-Howes G, Ford H, Williman J, Foulds J. The prevalence of comorbid alcohol use disorder in the presence of personality disorder: systematic review and explanatory modelling. Pers Ment Health. (2018) 12:216–28. doi: 10.1002/pmh.1415
32. Carpenter RW, Wood PK, Trull TJ. Comorbidity of borderline personality disorder and lifetime substance use disorders in a nationally representative sample. J Pers Disord. (2016) 30:336–50. doi: 10.1521/pedi_2015_29_197
33. Manthey J, Freeman TP, Kilian C, Lopez-Pelayo H, Rehm J. Public health monitoring of cannabis use in europe: prevalence of use, cannabis potency, and treatment rates. Lancet Reg Health Eur. (2021) 10:100227. doi: 10.1016/j.lanepe.2021.100227
34. Seitz NN, Lochbuhler K, Atzendorf J, Rauschert C, Pfeiffer-Gerschel T, Kraus L. Trends in substance use and related disorders: analysis of the epidemiological survey of substance abuse 1995 to 2018. Dtsch Arztebl Int. (2019) 116:585–91. doi: 10.3238/arztebl.2019.0585
35. Kotz D, Kastaun S, Manthey J, Hoch E, Klosterhalfen S. Cannabis use in Germany. Dtsch Arztebl Int. (2024) 121:52–7. doi: 10.3238/arztebl.m2023.0237
36. Vest NA, Murphy KT, Tragesser SL. Borderline personality disorder features and drinking, cannabis, and prescription opioid motives: differential associations across substance and sex. Addictive Behav. (2018) 87:46–54. doi: 10.1016/j.addbeh.2018.06.015
37. Childs E, Lutz JA, de Wit H. Dose-related effects of delta-9-thc on emotional responses to acute psychosocial stress. Drug Alcohol Depend. (2017) 177:136–44. doi: 10.1016/j.drugalcdep.2017.03.030
38. Grant JE, Odlaug BL, Chamberlain SR, Kim SW. Dronabinol, a cannabinoid agonist, reduces hair pulling in trichotillomania: A pilot study. Psychopharmacol (Berl). (2011) 218:493–502. doi: 10.1007/s00213-011-2347-8
39. Fabre LF, McLendon D. The efficacy and safety of nabilone (a synthetic cannabinoid) in the treatment of anxiety. J Clin Pharmacol. (1981) 21:377S–82S. doi: 10.1002/j.1552-4604.1981.tb02617.x
40. Tadic A, Wagner S, Hoch J, Baskaya O, von Cube R, Skaletz C, et al. Gender differences in axis I and axis ii comorbidity in patients with borderline personality disorder. Psychopathology. (2009) 42:257–63. doi: 10.1159/000224149
41. Sher L, Rutter SB, New AS, Siever LJ, Hazlett EA. Gender differences and similarities in aggression, suicidal behaviour, and psychiatric comorbidity in borderline personality disorder. Acta Psychiatr Scand. (2019) 139:145–53. doi: 10.1111/acps.12981
42. Johnson DM, Shea MT, Yen S, Battle CL, Zlotnick C, Sanislow CA, et al. Gender differences in borderline personality disorder: findings from the collaborative longitudinal personality disorders study. Compr Psychiatry. (2003) 44:284–92. doi: 10.1016/S0010-440X(03)00090-7
43. Tomko RL, Trull TJ, Wood PK, Sher KJ. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord. (2014) 28:734–50. doi: 10.1521/pedi_2012_26_093
44. Yen S, Shea MT, Pagano M, Sanislow CA, Grilo CM, McGlashan TH, et al. Axis I and axis ii disorders as predictors of prospective suicide attempts: findings from the collaborative longitudinal personality disorders study. J Abnorm Psychol. (2003) 112:375–81. doi: 10.1037/0021-843x.112.3.375
45. Darke S, Williamson A, Ross J, Teesson M, Lynskey M. Borderline personality disorder, antisocial personality disorder and risk-taking among heroin users: findings from the Australian treatment outcome study (Atos). Drug Alcohol Depend. (2004) 74:77–83. doi: 10.1016/j.drugalcdep.2003.12.002
46. Chen EY, Brown MZ, Lo TT, Linehan MM. Sexually transmitted disease rates and high-risk sexual behaviors in borderline personality disorder versus borderline personality disorder with substance use disorder. J Nerv Ment Dis. (2007) 195:125–9. doi: 10.1097/01.nmd.0000254745.35582.f6
47. Webb D, McMurran M. A comparison of women who continue and discontinue treatment for borderline personality disorder. Pers Ment Health. (2009) 3:142–9. doi: 10.1002/pmh.69
48. Lahaie F-S, Fraser R. Diagnosis and treatment of comorbid borderline personality disorder and substance use disorder. Can J Addict. (2018) 9:30–5. doi: 10.1097/CXA.0000000000000027
49. Bahorik AL, Newhill CE, Queen CC, Eack SM. Under-reporting of drug use among individuals with schizophrenia: prevalence and predictors. Psychol Med. (2014) 44:61–9. doi: 10.1017/S0033291713000548
50. Large MM, Smith G, Sara G, Paton MB, Kedzior KK, Nielssen OB. Meta-analysis of self-reported substance use compared with laboratory substance assay in general adult mental health settings. Int J Methods Psychiatr Res. (2012) 21:134–48. doi: 10.1002/mpr.1350
51. Heath LM, Laporte L, Paris J, Hamdullahpur K, Gill KJ. Substance misuse is associated with increased psychiatric severity among treatment-seeking individuals with borderline personality disorder. J Pers Disord. (2018) 32:694–708. doi: 10.1521/pedi_2017_31_307
52. Timko C, Chen S, Sempel J, Barnett P. Dual diagnosis patients in community or hospital care: one-year outcomes and health care utilization and costs. J Ment Health. (2006) 15:163–77. doi: 10.1080/09638230600559631
53. Zanarini MC, Frankenburg FR, Glass IV, Fitzmaurice GM. The 24-year course of symptomatic disorders in patients with borderline personality disorder and personality-disordered comparison subjects: description and prediction of recovery from bpd. J Clin Psychiatry. (2024) 85:24m15370. doi: 10.4088/JCP.24m15370
Keywords: borderline personality disorder, substance use disorder, alcohol use disorder, cannabis use disorder, prevalence analysis, medical record, SCID
Citation: Nabel J, Bertele S, Stapel B, Scharn N and Kahl KG (2025) Unseen dualities: underdiagnosis of substance use disorders in borderline personality disorder. Front. Psychiatry 16:1539611. doi: 10.3389/fpsyt.2025.1539611
Received: 04 December 2024; Accepted: 10 March 2025;
Published: 25 March 2025.
Edited by:
Lionel Cailhol, University Institute in Mental Health of Montreal, CanadaReviewed by:
Amaury Durpoix, Hôpitaux Universitaires de Strasbourg, FranceCopyright © 2025 Nabel, Bertele, Stapel, Scharn and Kahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai G. Kahl, a2FobC5rYWlAbWgtaGFubm92ZXIuZGU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.