- 1Peking University Sixth Hospital, Peking University Institute of Mental Health, NHC Key Laboratory of Mental Health (Peking University), National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Beijing, China
- 2The First Affiliated Hospital of Xinxiang Medical College, Xinxiang, Henan, China
- 3Beijing Key Laboratory of Mental Disorders, National Clinical Research Center for Mental Disorders and National Center for Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, China
- 4Ontario Institute for Studies in Education, University of Toronto, Toronto, ON, Canada
- 5Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China
Background: Increasing evidences suggests that depression is a heterogeneous clinical syndrome. Cognitive deficits in depression are associated with poor psychosocial functioning and worse response to conventional antidepressants. However, a consistent profile of neurocognitive abnormalities in depression remains unclear.
Objective: We used data-driven parsing of cognitive performance to reveal subgroups present across depressed individuals and then investigate the change pattern of cognitive subgroups across the course in follow-up.
Method: We assessed cognition in 163 patients with depression using The Chinese Brief Cognitive Test(C-BCT) and the scores were compared with those of 196 healthy controls (HCs). 58 patients were reassessed after 8 weeks. We used K-means cluster analysis to identify cognitive subgroups, and compared clinical variables among these subgroups. A linear mixed-effects model, incorporating time and group (with interaction term: time × group) as fixed effects, was used to assess cognitive changes over time. Stepwise logistic regression analysis was conducted to identify risk factors associated with these subgroups.
Results: Two distinct neurocognitive subgroups were identified: (1) a cognitive-impaired subgroup with global impairment across all domains assessed by the C-BCT, and (2) a cognitive-preserved subgroup, exhibited intact cognitive function, with performance well within the healthy range. The cognitive-impaired subgroup presented with more severe baseline symptoms, including depressed mood, guilt, suicidality, and poorer work performance. Significant group × time interactions were observed in the Trail Making Test Part A (TMT-A) and Continuous Performance Test (CPT), but not in Symbol Coding or Digit Span tests. Despite partial improvement in TMT-A and CPT tests, the cognitive-impaired subgroup's scores remained lower than those of the cognitive-preserved subgroup across all tests at the study endpoint. Multiple regression analysis indicated that longer illness duration, lower educational levels, and antipsychotic medication use may be risk factors for cognitive impairment.
Conclusion: This study identifies distinguishable cognitive subgroups in acute depression, thereby confirming the presence of cognitive heterogeneity. The cognitive-impaired subgroup exhibits distinct symptoms and persistent cognitive deficits even after treatment. Screening for cognitive dysfunction may facilitate more targeted interventions.
Clinical Trial Registration: https://www.chictr.org, identifier ChiCTR2400092796.
1 Introduction
Major Depressive Disorder (MDD) is a heterogeneous clinical syndrome (1, 2) that is diagnosed when a patient meets at least five of the nine symptoms listed in DSM-IV/DSM-5, accommodating multiple symptom combinations. Neuroimaging has greatly enhanced our understanding of mental disorders (3, 4). Previous studies have reported that data-driven analyses of biomarkers, such as brain connectivity, volume, and cortical thickness, can identify biologically distinct subgroups associated with specific cognitive performances (5) and predict clinical outcomes more accurately than traditional diagnostic categories (5–8). However, concerns regarding the cost and availability of MRI in practice (9), along with the test-retest reliability of measures derived from short-duration scans, may present substantial barriers to their clinical implementation (4).
Studies have shown that cognitive performance can be predicted by the brain’s functional connectivity patterns (10–13).Furthermore, cognitive impairment often predicts greater psychosocial dysfunction, including diminished quality of life, and social, occupational and global functioning (14–16). Thus, as a neurocognitive marker, cognitive performance can reflect an individual’s biological features to some extent and provide clinicians with valuable information about functionality. Cognitive assessments are relatively straightforward to administer, and their results are intuitively interpretable (17), making them a valuable tool for understanding the presentation of mental disorders, as conceptualized by the RDoC framework (18).
However, studies of cognitive impairment in MDD exhibit considerable heterogeneity. Most meta-analyses report cognitive deficits in executive function, memory, and attention in patients with MDD, with effect sizes ranging from small to moderate (19–23). Notably, a high degree of heterogeneity in results from meta-analyses is widely reported (19, 23, 24), accompanied with inconsistent findings. A meta-analysis found that working memory performance in patients does not significantly differ from that in healthy controls (24). Additionally, some previous studies have found that depressed adults do not exhibit impairments in any assessed cognitive functions (25, 26). Conflicting results are expected when significant neural heterogeneity exists within depressed patients but is overlooked in conventional group-based analyses (8, 27).
Several attempts have been made to specify more homogenous subgroups within MDD. Subtypes have been proposed based on specific combinations of symptoms, onset, course, or severity (28). Most traditional subtyping methods rely on pattern recognition and categorization derived from distinctions observed in clinical practice (29), yet cognitive function is often not a primary consideration in these schemes. More crucially, studies examining cognitive function across various subtypes of MDD have produced inconsistent findings. For instance, some studies suggest that patients with psychotic MDD have more severe cognitive impairments than those with non-psychotic MDD (30), while others report similar levels of impairment (31–34). This inconsistency suggests that traditional subtyping methods may have limited clinical utility in reflecting the cognitive profiles of individuals with MDD.
Additionally, to effectively leverage advances in neuroscience for understanding disease mechanisms, we need new approaches for patient stratification that recognize the complexity and continuous nature of psychiatric traits, and that are not constrained by current categorical approaches (35). The NIMH’s RDoC framework (36) promotes a research paradigm that begins with existing knowledge of behavior-brain relationships and connects these insights to clinical phenomena. Neurocognition, as an intermediary phenotype within behavior-brain interactions, holds significant potential for this purpose (37).Thus, exploring the heterogeneity of MDD through data-driven approach based on cognitive function may be a valuable avenue of investigation.
An early study supported the concept of subgroups and found that only a minority (<30%) of patients with MDD demonstrated measurable cognitive impairment and if this substantial minority was removed from the group statistical analyses, the significant effect sizes disappear (38). Several cross-sectional studies have reported three subgroups based on data-driven approaches. Pu et al. conducted hierarchical cluster analysis and identified three distinct neurocognitive subgroups: mild impairment, selective impairment, and global impairment (39). Similar findings were obtained through latent class analysis (40) and two-step clustering analysis (41). K-means cluster analysis can handle larger data sets than hierarchical clustering, and it uses random number seeds to ensure the stability of the initial central value (42). Limited longitudinal studies reported more on two subgroups identified by k-means clustering: a cognitively impaired group and a cognitively preserved group, characterizing different neurobiological profiles and allowing predictions of treatment response. Guo et al. discovered these two subtypes in the acute episode phase among MDD patients, and 80% of the patients remained in their original subgroup after six months of treatment (43). In a secondary analysis of a randomized clinical trial involving 1008 patients with MDD, 27% exhibited pre-treatment global cognitive impairment and significantly decreased brain response to a cognitive task, as well as poorer response to standard pharmacotherapy, thereby defining a cognitive biotype in MDD (44).
Consistent evidence indicates that cognition dysfunction may exist independently of depressive symptoms and persist during remission (45–49), contributing to and sustaining psychosocial impairment (50–52). Therefore, there is an urgent need for more longitudinal studies to validate the clinical value of these subtypes and to identify the cognitive trajectory of MDD from its initial onset. Additionally, although numerous standardized neurocognitive tests are available, employing generic neurocognitive tests could aid in considering cognition as a cross-diagnostic dimension (53, 54). Last but not least, the lack of readily administered objective cognitive tests impedes the routine screening of cognitive function in MDD by physicians in clinical practice (55).
Our primary research objectives are as follows: (a) We used a machine learning method, specifically cluster analysis, to identify cognitive subgroups within the broader MDD diagnosis, assessing whether they are distinguished by baseline patterns of clinical symptoms, reduced occupational function, and poorer response to antidepressant; (b) We also aimed to determine the cognitive trajectory of MDD in subgroups following eight weeks of acute phase treatment; (c) We used a digital assessment function, the Chinese Brief Cognitive Test (C-BCT), to assess cognitive function. The C-BCT is a cognitive test developed for schizophrenia, with the advantage of objectivity, rapid administration, and established norms within a healthy Chinese population. It has been used to assess cognitive function of MDD patients in several studies and one prospective cohort study (56, 57).
2 Materials and methods
2.1 Participants
We recruited 164 patients with depression from the outpatients at Peking University Sixth Hospital. The inclusion criteria included: (1) diagnosed with MDD and in a major depressive episode at the moment, (2) age 18 to 60 years, and (3) able to read and understand Mandarin. The healthy control group contains 196 community volunteers. The exclusion criteria included: (1) history of central nervous system trauma, neurological disorders, or comorbid psychiatric disorders (except for anxiety disorders) and (2) diagnosis of intellectual disabilities or pervasive developmental disorders (3) recent diagnosis of substance abuse or dependence (within the past three months), (4) physical illnesses affecting vision and hearing. Depressed participants with co-morbid anxiety were included to maximize the generalizability of the sample, provided that anxiety was not the primary focus of current treatment. Patients were diagnosed by trained psychiatrists using the Mini International Neuropsychological Interview (M.I.N.I.) (58), a structured psychiatric interview based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria.
All participants provided informed consent, and the study was conducted with approval from the local institutional Human Research Ethics Committee, adhering to the National Health and Medical Research Council guidelines for human research.
2.2 Procedures and research tools
2.2.1 Procedures
This is an eight-week observational study where all MDD patients received personalized antidepressant medication (SSRIs or SNRIs) prescribed by psychiatrist in an outpatient clinic. The researcher made no treatment-related recommendations and only recorded information about the medication. Demographic information, including sex, age, and education level, was collected for all participants. Patients underwent clinical and cognitive assessments at baseline and after eight weeks of follow-up. A total of 58 patients completed the follow-up; detailed reasons for loss to follow-up are provided in the Supplementary Materials (Supplementary Figure 1).
2.2.2 Measurements of cognitive function
The C-BCT was used to measure neurocognitive functioning (59, 60). C-BCT was initially developed for clinical trials targeting cognitive assessments in schizophrenia. Recent studies have demonstrated that similar instruments, such as the Brief Assessment of Cognition in Schizophrenia (BACS) (39, 61), can effectively in assess neurocognitive deficits in individuals with MDD.
The C-BCT comprises four tests that assess various cognitive domains: (1) Trail Making Test, Part A (TMT-A): the speed of information processing; (2) Symbol Coding: attention, the speed of information processing, and the executive function of transformation; (3) Continuous Performance Test (CPT): sustained and focused attention; (4) Digit Span: the ability of auditory verbal working memory. Patients with MDD underwent the C-BCT and raw subtest scores were standardized by creating age- and sex- corrected T-scores (59, 60), with higher scores reflecting better cognitive performance. We used single scores from cognitive item in the Hamilton Rating Scale for Anxiety (HAM-A) (62) to assess the level of subjective cognitive impairment, in contrast to objective cognitive performance.
2.2.3 Measurements of clinical features and occupational function
Current symptoms, age of onset, duration of illness, first episode of depression (FED) or recurrent major depression were collected using the M.I.N.I. interview. Evaluation of depression severity was conducted using the 17-item Hamilton Rating Scale for Depression (HRSD-17) (63), and anxiety levels were assessed using the HAM-A. The types and dose of patients’ medication were recorded in detail at baseline and at the end of the eight-week follow-up. When benzodiazepines were used more than 50% of the time in the previous week, the use was considered present and this variable was dichotomized into yes/no (64). Occupational function was assessed by asking patients if they had a break from work or study due to MDD.
2.3 Statistical analysis
We used R (Version 4.4.1) and Rstudio (Version 2024.04.2 + 764) to conduct cluster analyses. K-means cluster is one of the most commonly used unsupervised machine learning methods (65). We use the ‘cluster’ package to calculate the silhouette metric and the ‘factoextra’ package to plot the relationship between k and WSS (Total Within Sum of Squares). The optimal solution was selected by convergence across multiple criteria: (1) scree plot elbow method using WSS, (2) silhouette metric, and (3) clusters differ on a maximum number of inputs, while ensuring an adequate number of patients in each cluster.
Considering the autocorrelations among repetitive measurements of the same patient, we used a linear mixed effects model for continuous data. This analysis was also conducted in R (Version 4.4.1) using the ‘glmmTMB package’. The ‘ggplot2’ package was utilized to visualize the estimated mixed effects models. To measure the time effect, we entered the follow-up time (from baseline to the last follow-up appointment) as the fixed effect in the model. Different individuals may be prescribed different antidepressants; therefore, we converted the antidepressant doses to fluoxetine equivalents (mg) and included the ‘subject id, antidepressant’ as a random effect. To investigate group differences and group*time interactions, follow-up time and group (with interaction term: time*group) were entered as fixed effects.
Demographic, clinical, and functional variables were analyzed among resulting clusters within each patient group using one-way analysis of variance (ANOVA), Kruskal-Wallis test, or chi-square when appropriate, with effect-sizes also reported.
Stepwise logistic regression analysis was conducted to identify risk factors associated with each subgroup. Independent variables included age, sex, HRSD-17, HAM-A, Duration of illness, FMD, years of education, antidepressants (yes or no), benzodiazepines (yes or no), and antipsychotics(yes or no). Age of onset was excluded from the analysis due to the collinearity with age and age of onset. We use the ‘stepwise’ method to select variables for inclusion in the model.
3 Results
3.1 Demographic information
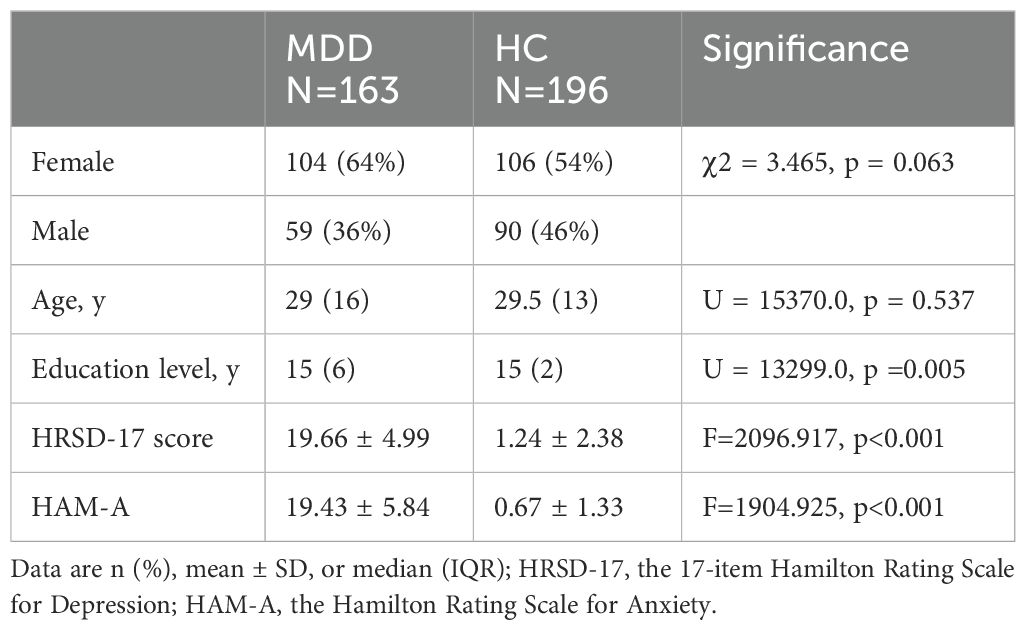
The 163 patients with MDD were 18 to 60 years old, with a median age of 29 years (IQR=16) and 64% were female. Of the 58 patients who participated in the follow-up study, 75% were female, with a median age of 29 years (IQR=15) (Supplementary Table 1). No significant differences were found in age (p = 0.537) or gender (p = 0.063) between patients with MDD and HCs. The MDD group had fewer years of education compared to HCs [15(6) vs. 15(2), p =0.005]. Patients with MDD exhibited moderate depressive symptoms (Table 1).
3.2 Deriving a cognitive subtype
The scree plot (Supplementary Figure 2) indicates an elbow at two clusters, after which the line flattens, indicating that additional clusters do not contribute to meaningfully separating the data and suggesting k=2 as the most optimal solution. Silhouette scores, which represent the mean silhouette coefficient across all instances of the dataset, range from -1 to1. Higher scores that closer to 1 indicate a model with more coherent clusters. Although the three-cluster solution yielded a silhouette score nearly identical to that of the two-cluster solution (0.32), it was not selected as it did not provide additional explanatory value (Supplementary Figure 2). Silhouette scores for k=4 to k=10 clusters were all lower than k=2. Scree plot and the silhouette metric indicated a two-cluster solution was optimal. The two-cluster solution showed significant differences across all cognitive test scores (all p < 0.001) (Figure 1).
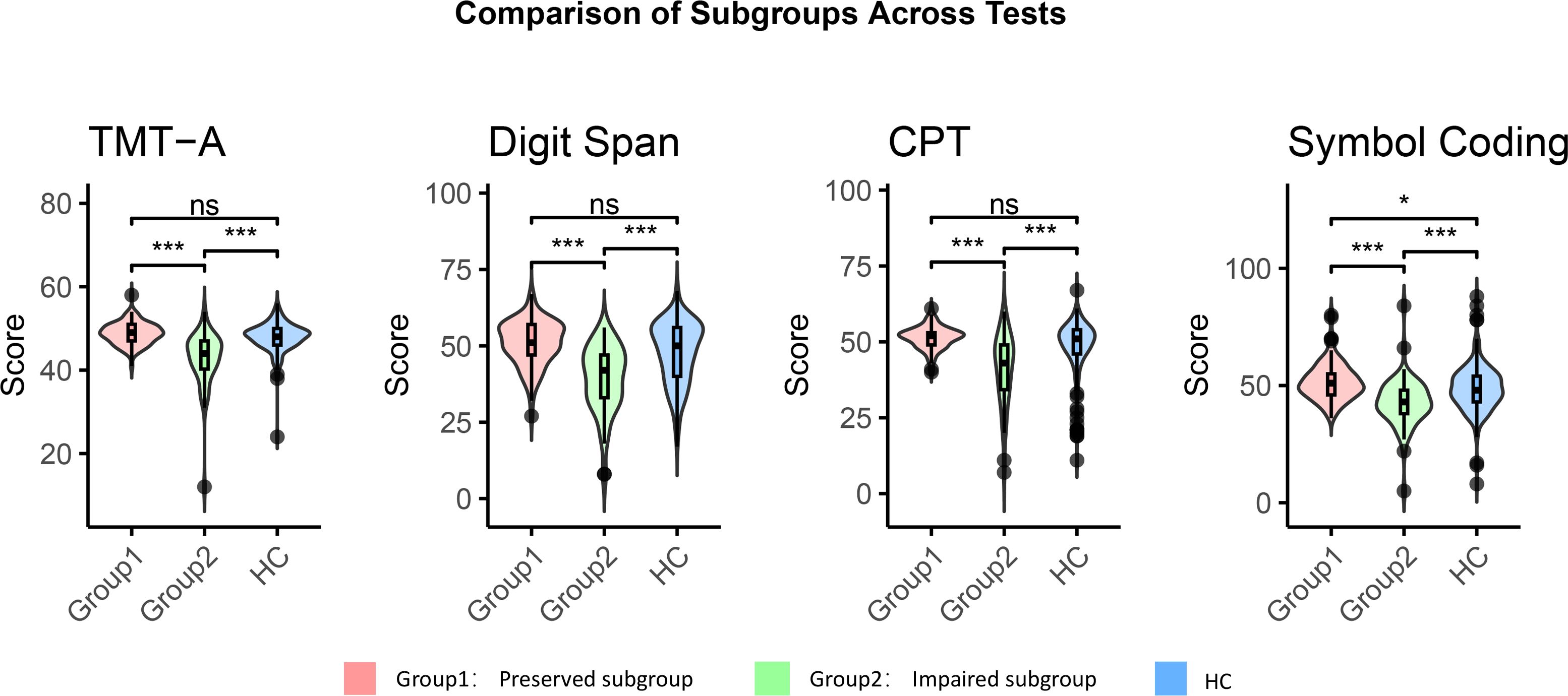
Figure 1. Cognitive function of the cognitive-impaired subgroup, the cognitive-preserved subgroup and HCs. Impaired group, cognitive-impaired subgroup; Preserved group, cognitive-preserved subgroup; HC, healthy control group; CPT, Continuous Performance Test; TMT-A, Trail Making Test, Part A. Comparative analysis was performed using the Mann-Whitney U test; all p values are adjusted for multiple comparisons with Bonferroni correction. * p<0.05. *** p<0.001. ns p>0.05.
The first cluster, referred to the cognitive-impaired subgroup, was characterized by significant impairments across all cognitive measures, and was present in 40% of individuals. Patients in cognitive-impaired subgroup demonstrated cognitive dysfunction across all tests when compared to HCs (all p < 0 .001, TMT-A: r=0.737; Digit Span: r=0.692; CPT: r=0.675; Symbol Coding: r=0.665) (Figure 1). The second cluster, referred to the cognitive-preserved subgroup, exhibited intact cognitive function, with performance well within the healthy range. Notably, this subgroup showed superior cognitive performance to the HCs on the Symbol Coding test (p = 0.011,r=0.414) (Figure 1). There was no significant difference between the scores of the two groups on the cognitive item in HAM-A, suggesting that both groups have similar levels of subjective cognitive function[2(1) vs. 2(2), p = 0.140)], despite differing significantly on all objective cognitive tests.
3.3 Baseline symptom profiles and occupation function
The severity of depressive symptoms, as measured by the HRSD-17, was significantly greater in the cognitive-impaired subgroup compared to the preserved subgroup (p < 0.001). Regarding individual symptoms, the cognitive-impaired subgroup has several profiles on HRSD-17, including more pronounced depressed mood (p =0.016), stronger feelings of guilty (p =0.048), higher frequency of suicidality(p =0.006), and poorer performance in work and activities (p =0.008). Additionally, a higher proportion of patients in the cognitive-impaired subgroup had stopped working due to MDD (Table 2).
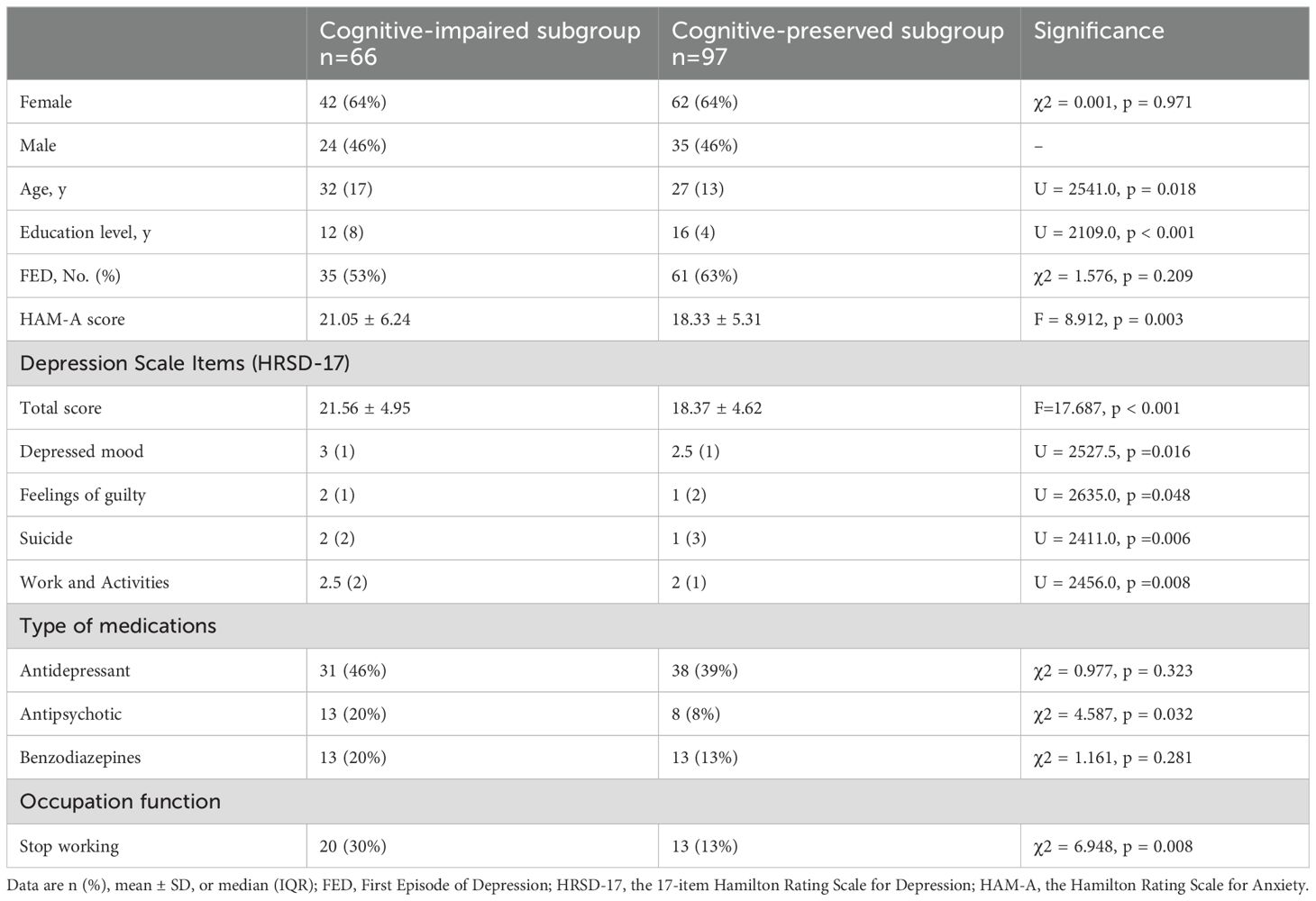
Table 2. Comparison between the two subgroups across demographics, clinical characteristics and occupation function.
3.4 Multivariate regression analysis to identify factors associated with cognition clusters
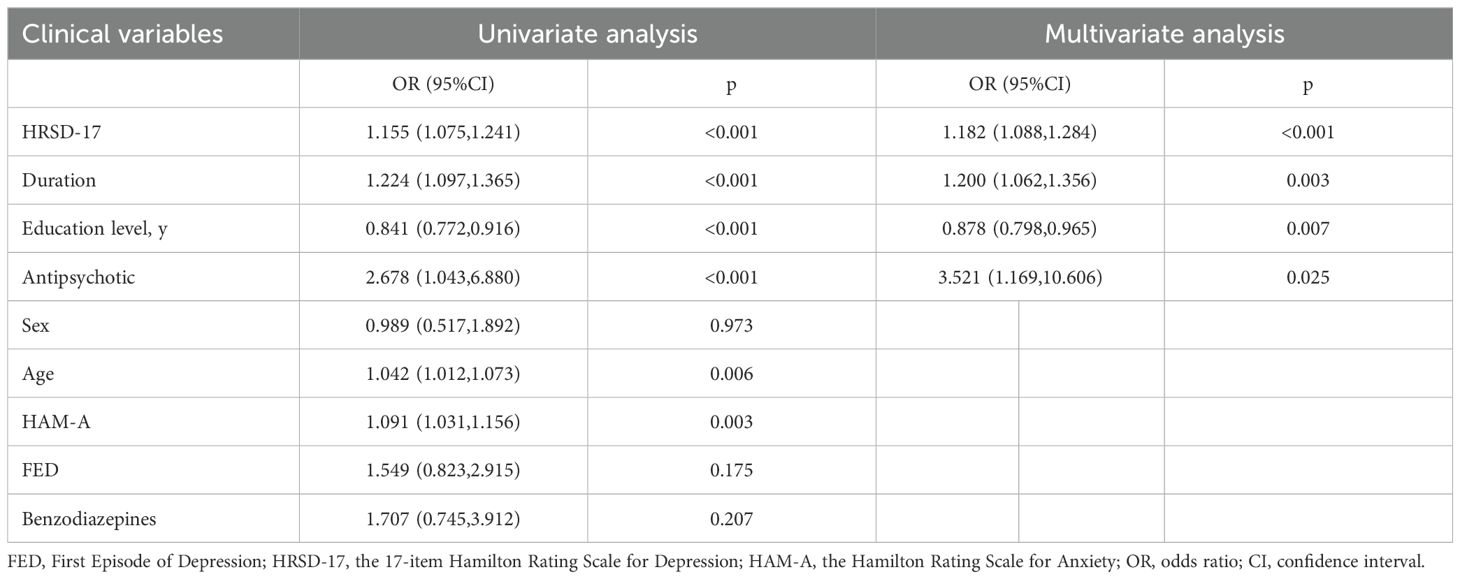
Binomial logistic regression analysis was used to identify the factors associated with cognition clusters. The following factors were included in the multivariate stepwise regression model: HRSD-17 (OR = 1.182, 95% CI [1.088–1.284], p < 0.001), duration (OR = 1.200, 95% CI [1.062–1.356], p = 0.003), years of education (OR = 0.878, 95% CI [0.798–0.965], p = 0.007), and antipsychotic use (OR = 3.521, 95% CI [1.169–10.606], p = 0.025) (Table 3), suggesting an association between these factors and the cognitive-impaired subgroup. However, the model’s fit requires further improvement, as the model containing four predictors had an R2 of 0.256, a rescaled R2 of 0.345, a sensitivity of 0.591, and a specificity of 0.845.
3.5 Cognitive subtype and treatment outcomes at eight weeks
No significant differences were found between the cognitive-impaired and cognitive-preserved subgroups at eight weeks in terms of HRSD-17 score (20.88 ± 4.01 vs. 18.26 ± 5.08, p = 0.491), treatment remission (50% vs. 64.7%, p = 0.263), and medication use [antidepressant dose (36.75 ± 20.26 vs. 39.19 ± 18.64 mg, p = 0.637), antipsychotic use (25.0% vs. 17.6%, p = 0.496), and benzodiazepine use (16.6% vs. 38.2%, p = 0.076)]. Nearly all patients were able to participate in work at the eight-week follow-up, and there was no significant difference between the two subgroups in the proportion of patients stopping work due to MDD (p = 0.230).
Significant group × time interactions were observed in TMT-A and CPT, but not in the Symbol Coding or Digit Span tests (Figure 2). These findings suggest that, after eight weeks of acute-phase treatment, the cognitive-impaired subgroup showed improvements in performance on the TMT-A and CPT, but no significant improvement on the Symbol Coding or Digit Span tests. Despite partial improvements in the TMT-A and CPT, the cognitive-impaired subgroup continued to score lower than the cognitive-preserved subgroup across all tests at the study endpoint (all p < 0.05) (Figure 2; Supplementary Table 1).
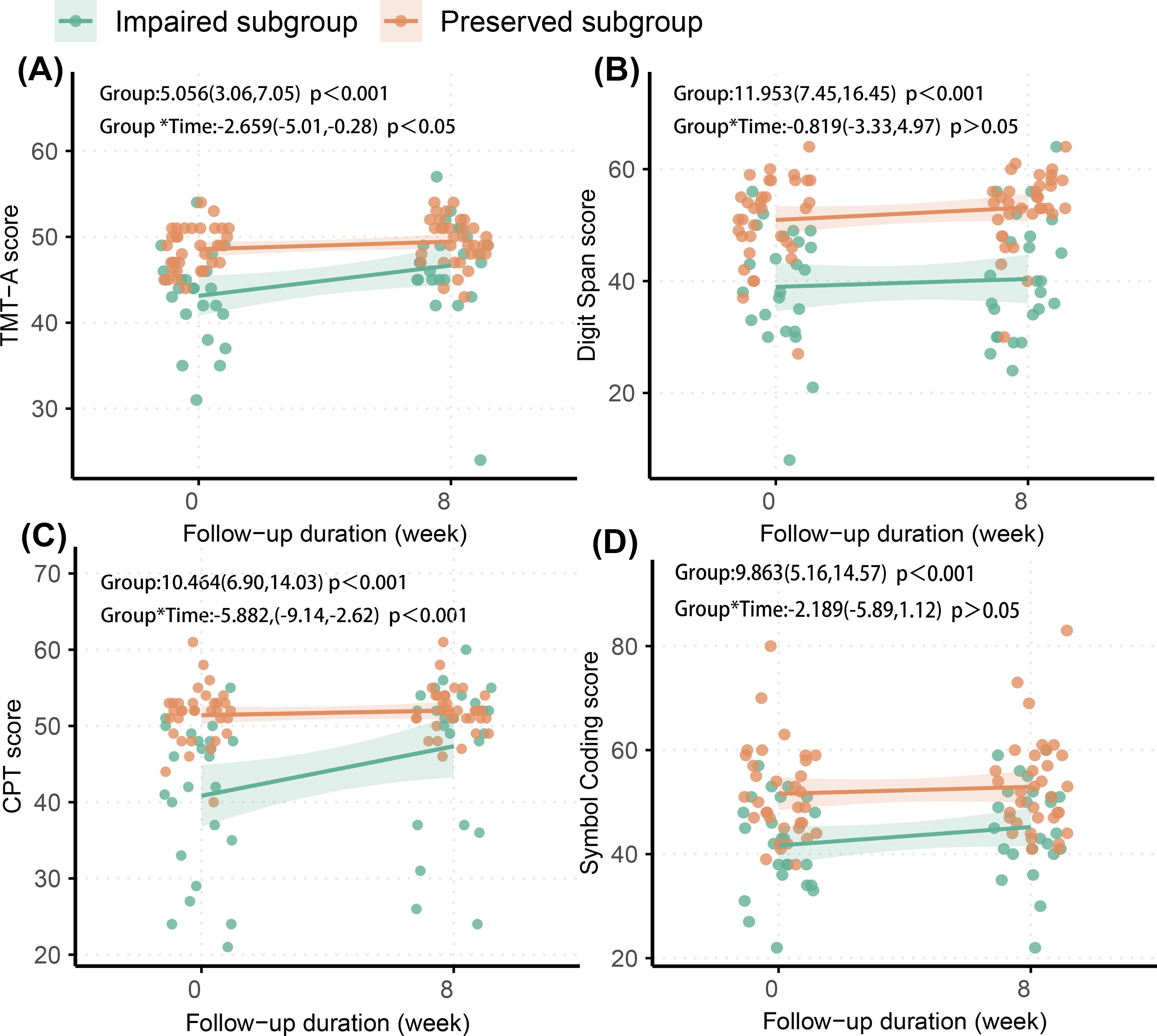
Figure 2. Longitudinal change of cognitive function in two subgroups. (A) TMT-A, Trail Making Test, Part A; (B) Digit Span; (C) CPT, Continuous Performance Test; (D) Symbol Coding. The effects (95% confidence interval) of group difference and interaction (group*time) in each figure were estimated in linear mixed effects mode.
4 Discussion
In this study, we identified a distinct cognitive-impaired subgroup in MDD using a machine learning clustering algorithm. This subgroup has greater severity in baseline symptoms including depressed mood, feelings of guilty, suicidality and poorer work performance. Despite improvement in depressive symptoms following acute-phase treatment, this subgroup still exhibited significant cognitive impairment.
The present study provides evidence for the existence of cognitive heterogeneity in patients with MDD during the acute episode. And after eight weeks of treatment, the cognitive-impaired subgroup remained preserved, performing worse than the cognitive-preserved group across all cognitive tests, which is consistent with previous longitudinal studies. Recent studies on functional connectivity have identified several fMRI-based biotypes in MDD, which characterize the neurobiological heterogeneity of the disorder and provide insights for personalized treatment. Notably, these fMRI-based biotypes have been shown to correlate with specific cognitive functions. Wen et al. applied a semi-supervised clustering method to regional grey matter (GM) brain volumes and identified two dimensions among patients with late-life depression. Patients in dimension 1 showed relatively preserved brain anatomy without white matter (WM) abnormalities. In contrast, patients in dimension 2 showed widespread brain atrophy and WM integrity deficits, along with cognitive impairment and higher depression severity (66). Similarly, another study identified a subtype in youth with internalizing symptoms, marked by elevated levels of psychopathology, impaired cognition, and multiple deficits apparent on multi-modal imaging (5). It is hypothesized that molecular alterations, along with concomitant changes in neuronal and glial morphometry and integrity, contribute to disruptions within and between brain circuits that are crucial for distinct cognitive domains (67–69). These studies suggest that classifying the cognitive heterogeneity associated with depression may provide a platform for better understanding the neurobiological underpinnings of the disease. Further research is needed to determine the neuroanatomical, neurophysiological, or neuroendocrine abnormalities specific to the cognitive-impaired subgroup.
The cognitive-impaired subgroup seems to have greater severity in negative thinking as assessed by HRSD-17, such as feelings of guilt and suicidality. Previous studies suggest that neuropsychological performance in depression may provide valuable information about risk for suicide. Deficits in interference processing, cognitive control, and memory performance have been found in past suicide attempters (70–72). These deficits are independent of clinical severity measures (70, 71), and residual cognitive deficits following symptomatic remission may contribute to suicide ideation in MDD (73). This highlights the need for greater attention to the safety of patients in the cognitive-impaired subgroup in clinical practice to prevent adverse events. Limited evidence suggests that shared neurobiological mechanisms underlying negative thinking and cognitive functioning may contribute to this relationship. Yang et al. identified a subgroup marked by poorer functioning across multiple cognition domains and increased brain activity in the anterior cingulate cortex and medial prefrontal cortex. This hyper-activation of the default mode could be linked to the Negative Cognition construct (74).
Another key finding of this study is that, even with significant symptom remission, the cognitive performance of the cognitive-impaired subgroup remained poorer than that of the cognitive-preserved subgroup across all tests. Several previous systematic reviews and meta-analyses have indicated that significant residual cognitive impairment persists during the remission phase of depression, including deficits in attention, learning and memory, working memory, and executive function (46, 47, 49). However, the effect sizes of these impairments appear to range from small to moderate, and the heterogeneity between studies should not be overlooked (45, 46). Differences between studies are not surprising when heterogeneity in cognition during remission persists and studies use diagnosis alone as inclusion criterion.
The improvement in CPT and TMT-A tests in the cognitive-impaired subgroup suggests partial recovery in processing speed and sustained visual attention following treatment. A meta-analysis involving 4,639 patients with MDD indicated a modest improvement in sustained visual attention and processing speed after treatment (45). Another meta-analysis comprising 33 studies found that antidepressants have a modest, positive effect on divided attention (75). However, the degree of improvement was insufficient to fully resolve these deficits. There was no improvement in Symbol Coding and Digit Span tests following pharmacological treatment, indicating that medication has limited impact on working memory and executive function related to transformation. Previous studies have suggested that antidepressants do not significant effect on working memory in patients with depression (75). Impaired working memory may contribute to rumination and difficulty breaking habitual thought patterns, thereby hindering effective reappraisal and problem-solving (76). Executive functioning was identified as the strongest independent predictor of functioning in remitted MDD patients (51). Residual cognitive deficits may contribute to ongoing occupational and social dysfunction (67, 77, 78). Furthermore, the persistence of cognitive impairment may interact with pre-existing emotional and social vulnerabilities, elevating the risk of recurrent depressive episodes (79, 80). Given the limited effectiveness of pharmacological treatment in improving cognitive function (81), combining other therapeutic approaches for patients in cognitive-impaired subgroup, such as cognitive rehabilitation training (82, 83) should be considered. Additionally, the use of vortioxetine may be a viable strategy, as it has demonstrated more definitive effects in improving cognitive function (84–87).
The results of multiple regression analysis identified longer duration of illness, lower educational attainment, and the use of antipsychotic medications as risk factors for cognitive impairment. Previous studies have similarly reported the negative effects of illness duration and education level on cognition (48, 88). While the effect of antipsychotic medication use is evident, we did not measure cognitive function before the initiation of the antipsychotics, leaving it unclear whether the medications use itself is merely indicative of underlying cognitive impairment risk or whether the medications contribute directly to cognitive dysfunction (e.g., through extrapyramidal side effects that affect cognition) (89). In this study, no significant impact of recurrence on cognitive function was found, despite previous research indicating that the number of depressive episodes is an important factor influencing cognitive function in depression patients (48, 49). This discrepancy may be due to our study simply categorization of patients as either first-episode or recurrent without accounting for the actual number of episodes. Additionally, the fit of the multiple regression models requires further improvement. Relying solely on these clinical risk factors may result in a relatively high false-negative rate, potentially leading to missed diagnoses of cognitive impairment. Therefore, it remains essential to conduct screening for cognitive function in patients with depression.
Our findings further support that subjective cognition may not accurately reflect objective cognitive function (73, 90). As a digital measurement tool, C-BCT offers a quick, easy-to-administer, and remotely accessible method for cognitive assessment in clinical practice. It covers various domains, including attention, working memory, information processing speed, and executive function. Its applicability across a range of diseases (56, 57, 60) also positions it a potential tool for cross-diagnostic cognitive assessments (53). As the C-BCT was originally designed to assess cognition in patients with schizophrenia, the use of a more comprehensive neurocognitive battery in patients with affective disorders (91, 92), including measures of affective processing, will be important in establishing and refining these cognitive profiles in future studies.
There are several limitations to our findings. First, the high dropout rate during the follow-up period may have resulted in an overrepresentation of patients in remission (the cognitive-impaired subgroup: 50%, the cognitive-preserved subgroup: 64.7%), which could affect our assessment of cognitive patterns during the remission phase. Second, although we considered the impact of medications, the relatively small sample size during follow-up prevented further analysis of medication dosage and type. Considering the abundant evidence of the deteriorating effect of anxiolytics on cognitive function (93–95), the effect of medication cannot be ignored. Third, our cognitive assessments did not include an evaluation of social cognition, and the assessments of occupational functioning and subjective cognitive impairment were relatively rudimentary. Finally, in our study, cognitive impairment was defined by comparison to a healthy control group. Future research may benefit from more comprehensive assessments of social cognitive function and subjective cognitive impairment, such as the Perceived Deficits Questionnaire for Depression (PDQ-D) (96). Future studies could further explore whether threshold-based criteria offer distinct advantages in reflecting functioning and predicting outcomes in MDD patients compared to data-driven approaches. Beyond clinical considerations, the subtype concept should be further validated in mechanistic studies, incorporating biological markers such as glucose, lipids, inflammatory indices, and neuroimaging. Additionally, further investigation into the cognitive effects of benzodiazepines is needed, supported by an expanded sample size. Longer follow-up periods are also needed to evaluate the stability of cognitive subtypes. It is important to recognize that cognitive symptoms should be considered a clinically significant treatment target, as improving affect alone is not sufficient for achieving functional or lasting recovery.
Data availability statement
The datasets presented in this article are not readily available due to the inclusion of patients’ personal health information. Requests to access the datasets should be directed to CX,eHVjaGVueWFuZ0BzdHUucGt1LmVkdS5jbg==.
Ethics statement
The studies involving humans were approved by Ethics Committee of Peking University Sixth Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CX: Conceptualization, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. YT: Investigation, Writing – review & editing. YL: Investigation, Writing – review & editing. JZ: Project administration, Writing – review & editing. ZL: Writing – review & editing. JL: Data curation, Investigation, Writing – review & editing. MW: Project administration, Writing – review & editing. TH: Methodology, Writing – review & editing. CS: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China Key International (Regional) Cooperative Research Programs (Grant No: 72110107003).
Acknowledgments
We would like to express our sincere gratitude to all the participants for their participation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1537331/full#supplementary-material
References
1. Ballard ED, Yarrington JS, Farmer CA, Lener MS, Kadriu B, Lally N, et al. Parsing the heterogeneity of depression: An exploratory factor analysis across commonly used depression rating scales. J Affect Disord. (2018) 231:51–7. doi: 10.1016/j.jad.2018.01.027
2. Fried EI, Nesse RM. Depression is not a consistent syndrome: An investigation of unique symptom patterns in the STAR*D study. J Affect Disord. (2015) 172:96–102. doi: 10.1016/j.jad.2014.10.010
3. Loosen AM, Kato A, Gu X. Revisiting the role of computational neuroimaging in the era of integrative neuroscience. Neuropsychopharmacology. (2024) 50:103–13. doi: 10.1038/s41386-024-01946-8
4. Nour MM, Liu Y, Dolan RJ. Functional neuroimaging in psychiatry and the case for failing better. Neuron. (2022) 110:2524–44. doi: 10.1016/j.neuron.2022.07.005
5. Kaczkurkin AN, Sotiras A, Baller EB, Barzilay R, Calkins ME, Chand GB, et al. Neurostructural heterogeneity in youths with internalizing symptoms. Biol Psychiatry. (2020) 87:473–82. doi: 10.1016/j.biopsych.2019.09.005
6. Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. (2017) 23:28–38. doi: 10.1038/nm.4246
7. Price RB, Lane S, Gates K, Kraynak TE, Horner MS, Thase ME, et al. Parsing heterogeneity in the brain connectivity of depressed and healthy adults during positive mood. Biol Psychiatry. (2017) 81:347–57. doi: 10.1016/j.biopsych.2016.06.023
8. Price RB, Gates K, Kraynak TE, Thase ME, Siegle GJ. Data-driven subgroups in depression derived from directed functional connectivity paths at rest. Neuropsychopharmacology. (2017) 42:2623–32. doi: 10.1038/npp.2017.97
9. Gabrieli JDE, Ghosh SS, Whitfield-Gabrieli S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron. (2015) 85:11–26. doi: 10.1016/j.neuron.2014.10.047
10. Park H-J, Friston K. Structural and functional brain networks: from connections to cognition. Science. (2013) 342:1238411. doi: 10.1126/science.1238411
11. Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. (2016) 19:165–71. doi: 10.1038/nn.4179
12. Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. (2017) 12:506–18. doi: 10.1038/nprot.2016.178
13. Thiebaut de Schotten M, Forkel SJ. The emergent properties of the connected brain. Science. (2022) 378:505–10. doi: 10.1126/science.abq2591
14. Cambridge OR, Knight MJ, Mills N, Baune BT. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: A systematic review. Psychiatry Res. (2018) 269:157–71. doi: 10.1016/j.psychres.2018.08.033
15. Sánchez-Torres AM, García de Jalón E, Gil-Berrozpe GJ, Peralta V, Cuesta MJ, PEPsNA Group. Cognitive intraindividual variability, cognitive impairment and psychosocial functioning in first-episode psychosis patients. Psychiatry Res. (2023) 328:115473. doi: 10.1016/j.psychres.2023.115473
16. Solé B, Jiménez E, Torrent C, Reinares M, Bonnin CDM, Torres I, et al. Cognitive impairment in bipolar disorder: treatment and prevention strategies. Int J Neuropsychopharmacol. (2017) 20:670–80. doi: 10.1093/ijnp/pyx032
17. Russo M, Mahon K, Burdick KE. Measuring cognitive function in MDD: emerging assessment tools. Depress Anxiety. (2015) 32:262–9. doi: 10.1002/da.22297
18. Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. (2014) 171:395–7. doi: 10.1176/appi.ajp.2014.14020138
19. Little B, Anwyll M, Norsworthy L, Corbett L, Schultz-Froggatt M, Gallagher P. Processing speed and sustained attention in bipolar disorder and major depressive disorder: A systematic review and meta-analysis. Bipolar Disord. (2024) 26:109–28. doi: 10.1111/bdi.13396
20. McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. (2009) 119:1–8. doi: 10.1016/j.jad.2009.04.022
21. Rhee TG, Shim SR, Manning KJ, Tennen HA, Kaster TS, d’Andrea G, et al. Neuropsychological assessments of cognitive impairment in major depressive disorder: A systematic review and meta-analysis with meta-regression. Psychother Psychosomatics. (2024) 93:8. doi: 10.1159/000535665
22. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. (2014) 44:2029–40. doi: 10.1017/S0033291713002535
23. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. (2013) 139:81–132. doi: 10.1037/a0028727
24. Lee RSC, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord. (2012) 140:113–24. doi: 10.1016/j.jad.2011.10.023
25. Castaneda AE, Suvisaari J, Marttunen M, Perälä J, Saarni SI, Aalto-Setälä T, et al. Cognitive functioning in a population-based sample of young adults with a history of non-psychotic unipolar depressive disorders without psychiatric comorbidity. J Affect Disord. (2008) 110:36–45. doi: 10.1016/j.jad.2007.12.239
26. Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: evidence of modest impairment. Biol Psychiatry. (2001) 50:35–43. doi: 10.1016/s0006-3223(00)01072-6
27. Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry. (2008) 69:1122–30. doi: 10.4088/jcp.v69n0712
28. Rush AJ. The varied clinical presentations of major depressive disorder. J Clin Psychiatry. (2007) 68:4–10.
29. van Loo HM, de Jonge P, Romeijn J-W, Kessler RC, Schoevers RA. Data-driven subtypes of major depressive disorder: a systematic review. BMC Med. (2012) 10:156. doi: 10.1186/1741-7015-10-156
30. Kim DK, Kim BL, Sohn SE, Lim SW, Na DG, Paik CH, et al. Candidate neuroanatomic substrates of psychosis in old-aged depression. Prog Neuropsychopharmacol Biol Psychiatry. (1999) 23:793–807. doi: 10.1016/s0278-5846(99)00041-x
31. Nelson EB, Sax KW, Strakowski SM. Attentional performance in patients with psychotic and nonpsychotic major depression and schizophrenia. Am J Psychiatry. (1998) 155:137–9. doi: 10.1176/ajp.155.1.137
32. Schatzberg AF, Posener JA, DeBattista C, Kalehzan BM, Rothschild AJ, Shear PK. Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. Am J Psychiatry. (2000) 157:1095–100. doi: 10.1176/appi.ajp.157.7.1095
33. Fleming SK, Blasey C, Schatzberg AF. Neuropsychological correlates of psychotic features in major depressive disorders: a review and meta-analysis. J Psychiatr Res. (2004) 38:27–35. doi: 10.1016/s0022-3956(03)00100-6
34. Politis A, Lykouras L, Mourtzouchou P, Christodoulou GN. Attentional disturbances in patients with unipolar psychotic depression: a selective and sustained attention study. Compr Psychiatry. (2004) 45:452–9. doi: 10.1016/j.comppsych.2004.07.007
35. Owen MJ. New approaches to psychiatric diagnostic classification. Neuron. (2014) 84:564–71. doi: 10.1016/j.neuron.2014.10.028
36. Doherty JL, Owen MJ. The Research Domain Criteria: moving the goalposts to change the game. Br J Psychiatry. (2014) 204:171–3. doi: 10.1192/bjp.bp.113.133330
37. Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. (2014) 13:28–35. doi: 10.1002/wps.20087
38. Iverson GL, Brooks BL, Langenecker SA, Young AH. Identifying a cognitive impairment subgroup in adults with mood disorders. J Affect Disord. (2011) 132:360–7. doi: 10.1016/j.jad.2011.03.001
39. Pu S, Noda T, Setoyama S, Nakagome K. Empirical evidence for discrete neurocognitive subgroups in patients with non-psychotic major depressive disorder: clinical implications. Psychol Med. (2018) 48:2717–29. doi: 10.1017/S003329171800034X
40. Martin DM, Wollny-Huttarsch D, Nikolin S, McClintock SM, Alonzo A, Lisanby SH, et al. Neurocognitive subgroups in major depressive disorder. Neuropsychology. (2020) 34:726–34. doi: 10.1037/neu0000626
41. Vicent-Gil M, Portella MJ, Serra-Blasco M, Navarra-Ventura G, Crivillés S, Aguilar E, et al. Dealing with heterogeneity of cognitive dysfunction in acute depression: a clustering approach. Psychol Med. (2021) 51:2886–94. doi: 10.1017/S0033291720001567
42. Singh T, Saxena N, Khurana M, Singh D, Abdalla M, Alshazly H. Data clustering using moth-flame optimization algorithm. Sensors (Basel). (2021) 21:4086. doi: 10.3390/s21124086
43. Guo W, Liu B, Wei X, Ju Y, Wang M, Dong Q, et al. The longitudinal change pattern of cognitive subtypes in medication-free patients with major depressive disorder: a cluster analysis. Psychiatry Res. (2023) 327:115413. doi: 10.1016/j.psychres.2023.115413
44. Hack LM, Tozzi L, Zenteno S, Olmsted AM, Hilton R, Jubeir J, et al. A cognitive biotype of depression linking symptoms, behavior measures, neural circuits, and differential treatment outcomes: A prespecified secondary analysis of a randomized clinical trial. JAMA Network Open. (2023) 6:e2318411. doi: 10.1001/jamanetworkopen.2023.18411
45. Ahern E, White J, Slattery E. Change in cognitive function over the course of major depressive disorder: A systematic review and meta-analysis. Neuropsychol Rev. (2024). doi: 10.1007/s11065-023-09629-9
46. Bernhardt M, Klauke S, Schröder A. Longitudinal course of cognitive function across treatment in patients with MDD: A meta-analysis. J Affect Disord. (2019) 249:52–62. doi: 10.1016/j.jad.2019.02.021
47. Kriesche D, Woll CFJ, Tschentscher N, Engel RR, Karch S. Neurocognitive deficits in depression: a systematic review of cognitive impairment in the acute and remitted state. Eur Arch Psychiatry Clin Neurosci. (2023) 273:1105–28. doi: 10.1007/s00406-022-01479-5
48. McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Kudlow P, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. (2013) 30:515–27. doi: 10.1002/da.22063
49. Semkovska M, Quinlivan L, O’Grady T, Johnson R, Collins A, O’Connor J, et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatry. (2019) 6:851–61. doi: 10.1016/S2215-0366(19)30291-3
50. Godard J, Baruch P, Grondin S, Lafleur MF. Psychosocial and neurocognitive functioning in unipolar and bipolar depression: a 12-month prospective study. Psychiatry Res. (2012) 196:145–53. doi: 10.1016/j.psychres.2011.09.013
51. Knight MJ, Air T, Baune BT. The role of cognitive impairment in psychosocial functioning in remitted depression. J Affect Disord. (2018) 235:129–34. doi: 10.1016/j.jad.2018.04.051
52. Woo YS, Rosenblat JD, Kakar R, Bahk W-M, McIntyre RS. Cognitive deficits as a mediator of poor occupational function in remitted major depressive disorder patients. Clin Psychopharmacol Neurosci. (2016) 14:1–16. doi: 10.9758/cpn.2016.14.1.1
53. Abramovitch A, Short T, Schweiger A. The C Factor: Cognitive dysfunction as a transdiagnostic dimension in psychopathology. Clin Psychol Rev. (2021) 86:102007. doi: 10.1016/j.cpr.2021.102007
54. Wenzel J, Badde L, Haas SS, Bonivento C, Van Rheenen TE, Antonucci LA, et al. Transdiagnostic subgroups of cognitive impairment in early affective and psychotic illness. Neuropsychopharmacology. (2024) 49:573–83. doi: 10.1038/s41386-023-01729-7
55. McAllister-Williams RH, Bones K, Goodwin GM, Harrison J, Katona C, Rasmussen J, et al. Analysing UK clinicians’ understanding of cognitive symptoms in major depression: A survey of primary care physicians and psychiatrists. J Affect Disord. (2017) 207:346–52. doi: 10.1016/j.jad.2016.09.036
56. Jia X, Wang T, Han H, Liu J, Wang L, Tian B, et al. Correlation of cognitive function and clinical characteristics in adolescent depressive disorder patients with self-injury behavior. Chin J Behav Med Brain Sci. (2023) 12:707–13. doi: 10.3760/cma.j.cn371468-20230127-00034
57. Zhou J, Xu J, Liu R, Qi H, Yang J, Guo T, et al. A prospective cohort study of depression (PROUD) in China: rationale and design. Curr Med (Cham Switzerland). (2023) 2:1. doi: 10.1007/s44194-022-00018-7
58. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59:22–33;quiz 34-57.
59. Ye S, Xie M, Yu X, Wu R, Liu D, Hu S, et al. The Chinese brief cognitive test: normative data stratified by gender, age and education. Front Psychiatry. (2022) 13:933642. doi: 10.3389/fpsyt.2022.933642
60. Zhu J, Li J, Zhou L, Xu L, Pu C, Huang B, et al. Eye movements as predictor of cognitive improvement after cognitive remediation therapy in patients with schizophrenia. Front Psychiatry. (2024) 15:1395198. doi: 10.3389/fpsyt.2024.1395198
61. Hidese S, Ota M, Wakabayashi C, Noda T, Ozawa H, Okubo T, et al. Effects of chronic l-theanine administration in patients with major depressive disorder: an open-label study. Acta Neuropsychiatr. (2017) 29:72–9. doi: 10.1017/neu.2016.33
62. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
63. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
64. Korten NCM, Penninx BWJH, Kok RM, Stek ML, Oude Voshaar RC, Deeg DJH, et al. Heterogeneity of late-life depression: relationship with cognitive functioning. Int Psychogeriatr. (2014) 26:953–63. doi: 10.1017/S1041610214000155
65. Coorey CP, Sharma A, Muller S, Yang JYH. Prediction modeling-part 2: using machine learning strategies to improve transplantation outcomes. Kidney Int. (2021) 99:817–23. doi: 10.1016/j.kint.2020.08.026
66. Wen J, Fu CHY, Tosun D, Veturi Y, Yang Z, Abdulkadir A, et al. Characterizing heterogeneity in neuroimaging, cognition, clinical symptoms, and genetics among patients with late-life depression. JAMA Psychiatry. (2022) 79:464–74. doi: 10.1001/jamapsychiatry.2022.0020
67. Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D. The role of cognitive impairment in general functioning in major depression. Psychiatry Res. (2010) 176:183–9. doi: 10.1016/j.psychres.2008.12.001
68. Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. (2010) 167:1305–20. doi: 10.1176/appi.ajp.2009.10030434
69. Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discovery. (2012) 11:141–68. doi: 10.1038/nrd3628
70. Keilp JG, Gorlyn M, Russell M, Oquendo MA, Burke AK, Harkavy-Friedman J, et al. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med. (2013) 43:539–51. doi: 10.1017/S0033291712001419
71. Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ. Attention deficit in depressed suicide attempters. Psychiatry Res. (2008) 159:7–17. doi: 10.1016/j.psychres.2007.08.020
72. Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. Am J Psychiatry. (2001) 158:735–41. doi: 10.1176/appi.ajp.158.5.735
73. Knight MJ, Baune BT. Cognitive dysfunction in major depressive disorder. Curr Opin Psychiatry. (2018) 31:26. doi: 10.1097/YCO.0000000000000378
74. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depression Anxiety. (2017) 34:9–24. doi: 10.1002/da.22556
75. Prado CE, Watt S, Crowe SF. A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and non-depressed samples. Neuropsychol Rev. (2018) 28:32–72. doi: 10.1007/s11065-018-9369-5
76. Joormann J. Cognitive inhibition and emotion regulation in depression. Curr Dir psychol Sci. (2010) 19:161–6. doi: 10.1177/0963721410370293
77. Clark M, DiBenedetti D, Perez V. Cognitive dysfunction and work productivity in major depressive disorder. Expert Rev Pharmacoeconomics Outcomes Res. (2016) 16(4):455–63. doi: 10.1080/14737167.2016.1195688
78. McIntyre RS, Lee Y. Cognition in major depressive disorder: a “Systemically Important Functional Index” (SIFI). Curr Opin Psychiatry. (2016) 29:48–55. doi: 10.1097/YCO.0000000000000221
79. Cha DS, Carmona NE, Subramaniapillai M, Mansur RB, Lee Y, Hon Lee J, et al. Cognitive impairment as measured by the THINC-integrated tool (THINC-it): Association with psychosocial function in major depressive disorder. J Affect Disord. (2017) 222:14–20. doi: 10.1016/j.jad.2017.06.036
80. Weightman MJ, Air TM, Baune BT. A review of the role of social cognition in major depressive disorder. Front Psychiatry. (2014) 5:179. doi: 10.3389/fpsyt.2014.00179
81. Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A. Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised longitudinal study. Lancet Psychiatry. (2016) 3:425–35. doi: 10.1016/S2215-0366(16)00012-2
82. Mokhtari S, Mokhtari A, Bakizadeh F, Moradi A, Shalbafan M. Cognitive rehabilitation for improving cognitive functions and reducing the severity of depressive symptoms in adult patients with Major Depressive Disorder: a systematic review and meta-analysis of randomized controlled clinical trials. BMC Psychiatry. (2023) 23:77. doi: 10.1186/s12888-023-04554-w
83. Motter JN, Pimontel MA, Rindskopf D, Devanand DP, Doraiswamy PM, Sneed JR. Computerized cognitive training and functional recovery in major depressive disorder: A meta-analysis. J Affect Disord. (2016) 189:184–91. doi: 10.1016/j.jad.2015.09.022
84. Baune BT, Brignone M, Larsen KG. A network meta-analysis comparing effects of various antidepressant classes on the digit symbol substitution test (DSST) as a measure of cognitive dysfunction in patients with major depressive disorder. Int J Neuropsychopharmacol. (2018) 21:97–107. doi: 10.1093/ijnp/pyx070
85. Harrison JE, Lophaven S, Olsen CK. Which cognitive domains are improved by treatment with vortioxetine? Int J Neuropsychopharmacol. (2016) 19:pyw054. doi: 10.1093/ijnp/pyw054
86. Rosenblat JD, Kakar R, McIntyre RS. The cognitive effects of antidepressants in major depressive disorder: A systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol. (2016) 19:pyv082. doi: 10.1093/ijnp/pyv082
87. Salagre E, Solé B, Tomioka Y, Fernandes BS, Hidalgo-Mazzei D, Garriga M, et al. Treatment of neurocognitive symptoms in unipolar depression: A systematic review and future perspectives. J Affect Disord. (2017) 221:205–21. doi: 10.1016/j.jad.2017.06.034
88. Hu Y, Li J, Zhao Y, Dong Z, Qiu P, Yang S, et al. Memory and processing speed impairments in first-episode drug-naïve patients with major depressive disorder. J Affect Disord. (2023) 322:99–107. doi: 10.1016/j.jad.2022.10.048
89. Fava GA, Rafanelli C. Iatrogenic factors in psychopathology. Psychother Psychosomatics. (2019) 88:129–40. doi: 10.1159/000500151
90. Naismith SL, Longley WA, Scott EM, Hickie IB. Disability in major depression related to self-rated and objectively-measured cognitive deficits: a preliminary study. BMC Psychiatry. (2007) 7:32. doi: 10.1186/1471-244X-7-32
91. Bauer IE, Keefe RSE, Sanches M, Suchting R, Green CE, Soares JC. Evaluation of cognitive function in bipolar disorder using the Brief Assessment of Cognition in Affective Disorders (BAC-A). J Psychiatr Res. (2015) 60:81–6. doi: 10.1016/j.jpsychires.2014.10.002
92. Lee C-Y, Wang L-J, Lee Y, Hung C-F, Huang Y-C, Lee M-I, et al. Differentiating bipolar disorders from unipolar depression by applying the Brief Assessment of Cognition in Affective Disorders. psychol Med. (2018) 48:929–38. doi: 10.1017/S003329171700229X
93. Griffin CE, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. (2013) 13:214–23.
94. Ra S, Tl S. A review of the neuropsychological effects of commonly used prescription medications(1998). Available online at (Accessed January 7, 2025).
95. Kishi T, Moriwaki M, Kawashima K, Okochi T, Fukuo Y, Kitajima T, et al. Investigation of clinical factors influencing cognitive function in Japanese schizophrenia. Neurosci Res. (2010) 66:340–4. doi: 10.1016/j.neures.2009.12.007
Keywords: depression, cognitive subtype, cluster analysis, heterogeneity, longitudinal study
Citation: Xu C, Tao Y, Lin Y, Zhu J, Li Z, Li J, Wang M, Huang T and Shi C (2025) Parsing the heterogeneity of depression: a data-driven subgroup derived from cognitive function. Front. Psychiatry 16:1537331. doi: 10.3389/fpsyt.2025.1537331
Received: 30 November 2024; Accepted: 13 January 2025;
Published: 30 January 2025.
Edited by:
Gábor Gazdag, Jahn Ferenc Dél-Pesti Kórház és Rendelőintézet, HungaryReviewed by:
Kai-Chun Yang, Taipei Veterans General Hospital, TaiwanNa Qu, Wuhan Mental Health Center, China
Copyright © 2025 Xu, Tao, Lin, Zhu, Li, Li, Wang, Huang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Shi, c2hpY2h1YW5AYmptdS5lZHUuY24=; Tao Huang, aHVhbmd0YW9AYmptdS5lZHUuY24=
 Chenyang Xu
Chenyang Xu Yanbao Tao2
Yanbao Tao2 Zhuoran Li
Zhuoran Li Mingqia Wang
Mingqia Wang Chuan Shi
Chuan Shi