
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 31 March 2025
Sec. Autism
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1536361
 Assia Riccioni1*†§
Assia Riccioni1*†§ Maria Pontillo2†
Maria Pontillo2† Lenardo Emberti Gialloreti3
Lenardo Emberti Gialloreti3 Mariagrazia Cicala3
Mariagrazia Cicala3 Michelangelo Vasta4,5
Michelangelo Vasta4,5 Mattia Gatto1,4
Mattia Gatto1,4 Lucrezia Arturi4
Lucrezia Arturi4 Martina Siracusano1,3
Martina Siracusano1,3 Michelangelo Di Luzio2
Michelangelo Di Luzio2 Stefano Vicari2,6‡
Stefano Vicari2,6‡ Luigi Mazzone1,4‡
Luigi Mazzone1,4‡Introduction: Despite evidence suggesting increased rates of psychosis in individuals with autism spectrum disorder (ASD), the detection of prodromal psychotic symptoms, including attenuated psychosis syndrome (APS), remains underexplored in this population.
Methods: The primary aim of the present study was to characterize the clinical phenotype of young individuals with ASD who also present with APS (ASD/APS; n = 48) in comparison with individuals with APS only (n = 93) and those with ASD only (n = 30) (age range 9–23 years). Assessments included standardized measures of autistic symptoms (Autism Diagnostic Observation Schedule–Second Edition; ADOS-2), pre-psychotic symptoms (Structured Interview for Psychosis-Risk Syndromes; SIPS), and cognitive and adaptive functioning.
Results: Overall, the ASD/APS group demonstrated significantly poorer general adaptive skills compared with the APS group (p = 0.006) and the ASD group (p = 0.005). Compared with the APS group, the ASD/APS group exhibited lower scores across all SIPS domains, with the exception of SIPS-P1 (unusual thought content/delusional ideas; p = 0.062; t = −1.882; F = 5.44) and SIPS-P3 (grandiosity; p = 0.156; t = −1.435; F = 22.6). In contrast, the ASD/APS group displayed significantly higher scores in the repetitive and restricted behavior domain compared with the ASD group (p < 0.001). Notably, there were no significant differences in the age of APS onset across groups (p = 0.601; t = 0.525; F = 0.253).
Discussion: These findings provide a more nuanced characterization of APS features in individuals with ASD and emphasize the importance of screening for APS in this population, particularly those considered at increased risk. Early detection and intervention could facilitate timely therapeutic support, potentially improving long-term outcomes for these individuals.
Adolescence (10–19 years) (1) is a critical developmental period for the emergence of numerous premorbid “red flags” associated with severe mental illnesses, including psychosis (2). Psychotic disorders are often preceded by atypical developmental trajectories (3, 4) and a prodromal phase referred to as Clinical High-Risk for Psychosis (CHR-P) (5). CHR-P describes a clinical condition characterized by subthreshold psychotic symptoms, associated with an increased risk of developing a full psychotic disorder within the subsequent 2–5 years (6–8).
The CHR-P framework encompasses three distinct clinical subgroups: Attenuated Psychotic Syndrome (APS), Brief (and Limited) Intermittent Psychotic Symptoms (BLIPS or BIPS), and Genetic Risk and Deterioration Syndrome (GRD) (9, 10). Among these, APS is the most prevalent (11) and is currently recognized as the strongest clinical predictor of conversion to psychosis in the general population (12). Introduced in the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5) Research Appendix (Section III) in 2013, APS is characterized by attenuated delusions, hallucinations, or disorganized speech that occur at least once per week over the past month but are not severe enough to meet the diagnostic threshold for a psychotic disorder (13).
Detection of APS plays a pivotal role in identifying CHR-P status and enabling timely preventive interventions, which can reduce the likelihood of progression to full-blown psychosis and improve long-term outcomes (5, 6). Early identification of APS is particularly important for vulnerable populations with preexisting conditions associated with an elevated risk of severe mental illness, such as autism spectrum disorder (ASD) (14–16).
ASD is a lifelong neurodevelopmental condition characterized by impairments in social-communicative skills and the presence of restricted and stereotyped patterns of behavior and interests (13). Individuals with ASD are known to be at an increased risk for developing one or more psychiatric disorders over their lifetime (17–19). Among these psychiatric co-occurring conditions, psychotic disorders are reported at varying rates, ranging from 0.6% (95% CI = 0.3–1.1) (20–22) to 9.4% (95% CI = 7.52–11.72) in adult populations (23). Moreover, research has shown that individuals with ASD are not only at increased risk for concomitant psychotic symptoms, but that individuals with psychotic disorders may also exhibit higher levels of autistic traits (24).
Thus, despite ASD and psychosis being distinct conditions, there is an emerging hypothesis suggesting a continuum between them (25, 26). This hypothesis is supported by evidence of shared neurodevelopmental origins and overlapping clinical features, particularly related to impairments in social and communicative skills (21, 27–29). Notably, core ASD symptoms—such as reduced eye contact, poor emotional and social reciprocity, and stereotyped language—can resemble psychotic symptoms, including social withdrawal, blunted affect, and disorganized speech (16, 27, 29). Consequently, identifying and characterizing psychotic symptoms in individuals with ASD remains challenging (4, 16, 27), further complicated by evidence that the presentation of psychotic symptoms in ASD may differ from that observed in the general population, often exhibiting a greater emphasis on affective symptoms, a more acute onset, and a transient course (15). As a result, the detection and characterization of psychotic symptoms—especially during the prodromal phase—remains a significant challenge in individuals with ASD (16, 29). Therefore, while available studies indicate an increased prevalence of attenuated psychotic symptoms (APS) in ASD (30–34), it remains unclear whether prodromal symptoms in ASD mirror those experienced by the general population (16, 30, 33, 35, 36). In response, research on the overlap and semiology of prodromal psychotic symptoms in ASD is rapidly expanding (26, 28, 30, 33–35).
In this context, we recently conducted a preliminary study (33) to examine autistic and psychotic symptoms, as well as cognitive and adaptive skill profiles, in a sample of young individuals with ASD (ages 10–23 years) with (n = 13) or without (n = 18) concomitant APS. Our findings revealed that individuals with ASD/APS exhibited more severe autistic symptoms, greater social skills impairments (in social awareness and social cognition), and worse general adaptive functioning compared with the ASD-only group. Furthermore, the primary differences in psychotic symptoms between the ASD/APS and ASD groups were observed in the domains of positive and disorganized symptoms, supporting the notion that the overlap between ASD and psychosis is more pronounced in negative symptoms rather than positive ones (4, 16, 37). However, the relatively small sample size (n = 31) and the lack of an APS-only control group limited our ability to fully explore and characterize the differences in social skills impairment and psychotic symptom profiles between individuals with ASD and those in the general population. Nevertheless, disseminating knowledge about the clinical characteristics of APS in ASD is crucial for informing clinical prognosis and therapeutic strategies for individuals with ASD.
It is worth noting that, to date, there is a paucity of observational empirical studies on this topic. Most existing data come from retrospective studies (29, 30, 36) or involve small sample sizes (29, 33), limiting the ability to draw definitive conclusions. Moreover, no previous studies have directly compared the clinical profiles of individuals with ASD/APS to those with APS (without ASD) and ASD alone.
Therefore, the primary aim of the present study was to explore and characterize the clinical profile of a sample of young individuals with ASD presenting concomitant APS, in comparison with individuals with APS alone (without ASD), aged 9–23 years. This was achieved through the administration of standardized, gold-standard assessments of both autistic and psychotic symptoms, as well as cognitive and adaptive functioning.
This is an observational cohort study conducted in the context of previous research projects (33). The study was approved by the Independent Ethical Committee of the University Hospital, Fondazione Policlinico Tor Vergata (Register number 126/18), and informed consent was obtained from all legal holders of custody.
Our sample was constituted by individuals (age range 9–23 years) recruited from the Child Psychiatry Unit of the University of Rome Tor Vergata Hospital and from the Child and Adolescent Neuropsychiatry Unit of the Bambino Gesù Children’s Hospital, between January 2019 and September 2023. Specifically, the participants included in the present study were assessed for their eligibility by a multidisciplinary team (child psychiatrists and psychologist). To minimize the risk of symptom misinterpretation or underestimation, the clinical assessments were conducted by expert clinicians with specialized knowledge in both autism and psychosis.
In order to be eligible, participants were required to have (1) a condition of Attenuated Psychosis Syndrome (APS), considered confirmed with a score of 3, 4, of 5 on the Structured Interview for Psychosis-Risk Syndromes (SIPS) (38) and/or (2) a diagnosis of Autism Spectrum Disorder (ASD) without language and/or cognitive impairment (Intelligence Quotient - IQ above 70), performed on the basis of the Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition (DSM–5) or the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5 TR) criteria (13), supported by the administration of the Autism Diagnostic Observation Schedule–Second Edition (ADOS–2) (39).
The adopted exclusion criteria were the presence of IQ equal or below 70, non-fluent speech, epilepsy, and other concurrent psychiatric or neurodevelopmental conditions (e.g., obsessive–compulsive disorder, attention deficit, and hyperactivity disorder).
A comprehensive clinical assessment of cognitive and adaptive skills, as well as of autistic and psychotic symptoms, was performed as described below.
Based on age and each individual’s ability to cooperate, all individuals included in our sample underwent a non-verbal or verbal cognitive evaluation to assess the IQ. Specifically, the Leiter International Performance Scale-Revised (Leiter-R) (40), which is not reliant on verbal skills, was chosen for children and adolescents with more severe communications impairments and limited levels of cooperation. Otherwise, the Wechsler Intelligence Scale for Children-fourth Edition (WISC-IV) (41) or the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (42) tests, including verbal language in the assessment of IQ, were used.
For all of these scales, raw scores were converted into composite scores, and a mean and standard deviation (SD) IQ value of 100 ± 15 was considered.
The Adaptive Behavior Assessment System, Second Edition (ABAS-II) (43), was administered to parents of all included individuals. In particular, the “5–21 years” ABAS-II form was used. Parents were asked to rate the child’s skills to complete an activity (from 0 = “not able to” to 3 = “able to do it and always performs it when needed”) in regard to 10 functioning areas (i.e., communication, use of the environment, preschool competences, domestic behavior, health and safety, play, self-care, self-control, social abilities, and motility). The questionnaire provides three main adaptive domains: conceptual (CAD), practical (PAD), social (SAD), and a comprehensive score, General Adaptive Composite (GAC). Each of these indexes is standardized with a mean of 100 and an SD of 15.
The Structured Interview for Psychosis-Risk Syndromes (SIPS) (38) was administrated by expert clinicians to all included individuals. The SIPS is a semi-structured interview, which rates along four major symptom dimensions on the Scale of Prodromal Symptoms (SOPS) among four symptom domains: positive symptoms (SIPS-P items P1–P5: P1 unusual thought content; P2 suspiciousness; P3 grandiosity; P4 perceptual abnormalities; and P5 disorganized communication), negative symptoms (SIPS-N items N1–N6: N1 social anhedonia; N2 avolition; N3 expression of emotion; N4 experience of emotions and self; N5 ideational richness; and N6 occupational functioning), disorganized symptoms (SIPS-D items D1–D4: D1 odd behavior or appearance; D2 bizarre thinking; D3 trouble with focus and attention; and D4 impaired personal hygiene), and general symptoms (SIPS-G items G1–G4: G1 sleep disturbance; G2 dysphoric mood; G3 motor disturbances; and G4 impaired tolerance to normal stress). Each item has a severity scale ranging from 0 (Absent) to 6 (Severe/Extreme).
Based on the SIPS/SOPS criteria, the presence of an APS condition is confirmed with a score of 3, 4, of 5 on the SIPS positive symptoms scale (SIPS-P) (12, 38, 44).
For the purpose of the present study, both the total and the single-item scores for each SIPS subscale (SIPS-P, SIPS-N, SIPS-D, SIPS-G, and SIPS total score) were analyzed.
The Autism Diagnostic Observation Schedule–Second Edition - ADOS-2 (39) was administered to all included individuals. The ADOS-2 is a semi-structured observational tool considered as the gold standard for the assessment of autistic symptoms. It includes five modules (Toddler, 1, 2, 3, and 4) selected on the basis of age and expressive language level. The ADOS-2 algorithm is organized in Social Affect (SA), Restricted and Repetitive Behaviors (RRB), and the total score (TOT). Modules 1, 2, and 3 provide the Calibrated Severity Score (CSS), ranging from 1 to 10, which indicates autism symptom severity. Module 4 is used with verbally fluent adults who are likely to demonstrate a wide range of abilities. For the present study, module 3 and module 4 were administered.
Even if module 4 does not provide a CSS, a revised algorithm is available in order to provide a calibrated score that can be compared with algorithms used for ADOS-2 Modules 1–3 (45).
A descriptive analysis was performed to report the number of subjects in each group, the male–female ratio, and the means ± SD of the subjects’ age. Differences in age, gender, IQ, adaptive functioning, autistic symptom levels, and psychotic symptoms between groups were assessed using independent samples t-tests, Mann–Whitney tests, independent samples Kruskal–Wallis tests, one-way ANOVA with post-hoc analysis adjusted for multiple comparisons (Tukey HSD), and Pearson chi-squared tests, where appropriate. Bivariate Spearman’s correlations were applied to estimate the relationships between SIPS subscale scores and the IQ values. Results are presented as number of observations and percentages or means ± SD. An alpha level of 0.05 was used for all statistical analyses. All statistical analyses were performed using SPSS v.26.0 (IBM Corp., Armonk, NY, USA).
A final sample of n=171 individuals was included. Specifically, we included a group of individuals with APS (n= 93; M:F= 47:46; age: 15.3 ± 2.4) in comparison with a group of ASD/APS (n= 48; M:F= 31:17; age: 15.2 ± 2.6). We further included a control group of individuals with ASD (n=30; M:F=25:5; age: 14.8 ± 2.9). Samples’ demographic and clinical data are summarized in Table 1.
No statistically significant differences emerged between groups (APS vs. ASD/APS vs. ASD) in terms of age (F=0.382, p=0.683), gender (χ2 = 3.205, p= 0.073), and IQ (F=1.793, p=0.170).
Nonetheless, the ASD/APS group showed lower scores in the ABAS-II GAC domain, reaching a statistically significant level when compared with both the APS (p= 0.006) and the ASD (p=0.005) groups. By contrast, no statistically significant differences came out in the ABAS-II GAC indexes between the ASD and APS groups (p=0.423), except for the ABAS-II SAD domain, which resulted more impaired in the ASD group (p=0.008).
In terms of psychotic symptoms, the APS group showed higher psychotic symptom level in all SIPS domains when compared with the ASD/APS individuals (SIPS-P: U=2933, p=0.002; SIPS-N: U=3182, p<0.001; SIPS-D: U=3169, p<0.001; SIPS-G: U=3184.5, p<0.001) (for SIPS/SOPS single-item scores, please refer to Table 1). Specifically, the APS individuals presented higher scores in all SIPS subitems when compared with the ASD/APS group, except for the SIPS-P P1 (APS vs. ASD/APS: 3.4 ± 1.1 vs. 3.1 ± 0.9; U=2671, p=0.03) and SIPS-P P3 items (APS vs. ASD/APS: 2.4 ± 1.4 vs. 1.9 ± 2.1; U=2690, p= 0.04). Being in line, the ASD/APS group reached a mean SIPS-P score ≥3 (thus confirming the presence of an APS condition) only in the P1 item (unusual thought content/delusion ideas; 3.1 ± 0.9) (Table 2). More in detail, in the ASD/APS group, a score within 3 and 5 (thus confirming an APS condition) was reached in 81.2% in P1, in 60.4% in P2, in 37.5% in P3, in 54.2% in P4, in 50% in P5 (Table 2). To note, within the APS and ASD/APS groups, no statistically significant correlation emerged between the SIPS subscales scores and the IQ value (SIPS-P: r=−0.004, p=0.959; SIPS-N: r=−0.114, p= 0.136; SIPS-D: r=0.022, p= 0.779; SIPS-G: r=0.001, p= 0.998) and between the SIPS subscales and the ABAS-II GAC indexes (SIPS-P: r=0.025, p=0.778; SIPS-N: r=0.047, p= 0.598; SIPS-D: r=0.075, p= 0.401; SIPS-G: r=0.148, p= 0.093).
Focusing on the autistic symptoms profile, the mean ADOS 2-CSS score was 6.2 ± 1.5 in the ASD group; 5.7 ± 2.1 in the ASD/APS group and 0.1 ± 0.2 in the APS individuals (Table 1). No statistically significant differences emerged in terms of ADOS-2 CSS score (p=0.234) and the ADOS-2_SA domain (p=0.591) between autistic individuals presenting or not presenting concomitant APS (ASD vs. ASD/APS). By contrast, the ASD/APS group showed greater scores in the ADOS-2_RRB domain when compared with the ASD (p<0.001) (Figure 1).
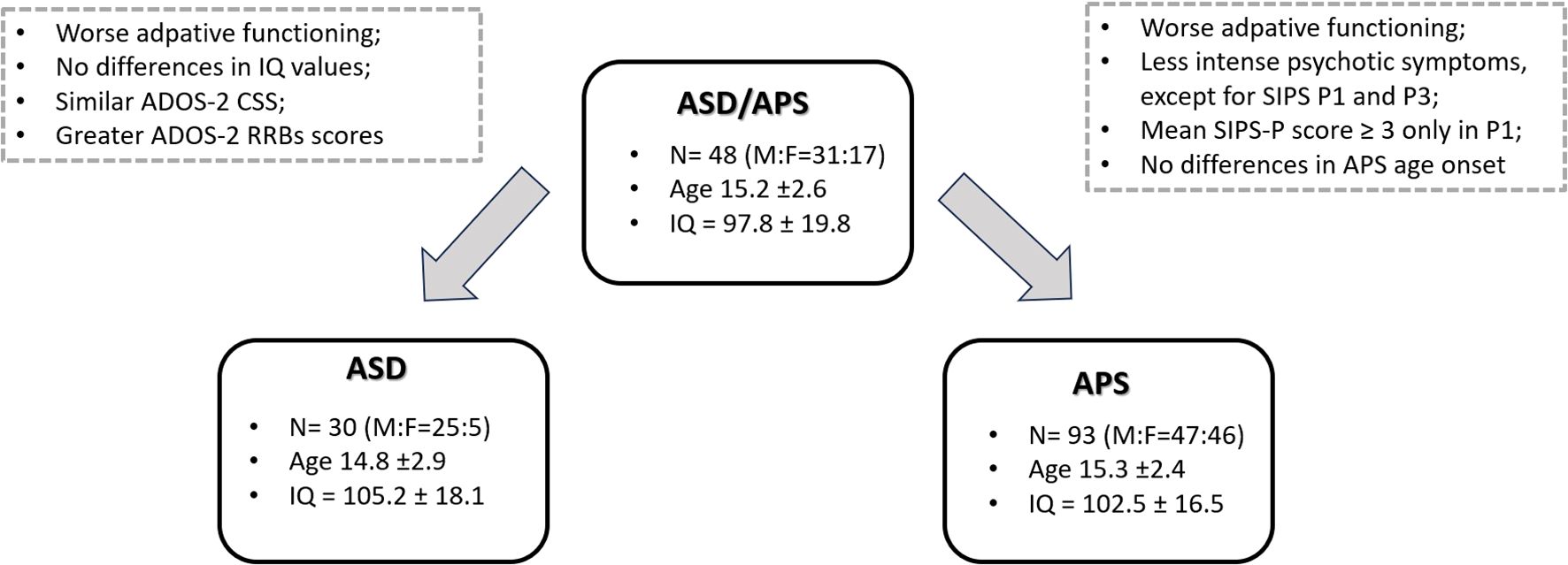
Figure 1. Mean clinical differences between the ASD/APS group and the ASD and APS groups, respectively.
Within all groups of participants, the post-hoc ANOVA model highlighted a negative association between the ADOS-2 CSS and the SIPS-P scores (t=3.006; p=0.004). By contrast, no statistically significant association was found between SIPS-P and IQ (t=1.251; p=0.215), gender (t=0.726; p=0.470), and ABAS-II GAC (t=1.974; p=0.053).
Finally, no statistically significant differences came out in terms of age onset for the APS condition between ASD/APS (13.5 ± 2.0) and the APS individuals (13.3 ± 2.2) (F=0.253; p=0.601).
To the best of our knowledge, this is the first study aimed at characterizing the clinical phenotype of autistic individuals with concomitant APS, in comparison with individuals with APS (without ASD), in terms of autistic and prodromal psychotic symptoms as well as cognitive and adaptive skills, through the administration of gold standard tools. Specifically, we aimed to explore whether individuals with ASD exhibit prodromal psychotic symptoms comparable with those experienced by the general population, with a focus on identifying potential clinical markers for APS in ASD.
Consistent with our previous study (33), our results demonstrated that individuals with ASD/APS exhibited greater impairment in general adaptive functioning skills (ABAS-GAC) compared with those with ASD alone or APS alone. This finding supports the notion that concomitant attenuated psychotic symptoms in autistic individuals may contribute to more significant impairments in adaptive functioning, which could, in turn, negatively affect quality of life and lead to poorer mental health outcomes (19, 46). Notably, data from our previous study (33) also highlighted that individuals with ASD/APS who later converted to full psychosis showed greater baseline impairments in adaptive skills (ABAS-II GAC) compared with those who did not convert. Thus, enhancing adaptive functioning skills in ASD individuals considered at increased risk for APS may play a crucial role in therapeutic strategies and long-term outcomes.
Focusing on the psychotic symptoms assessment, when compared with APS individuals the ASD/APS group overall presented lower scores in all the SIPS domains, except for the SIPS-P1 (unusual thought content/delusional ideas) and the SIPS-P3 (grandiosity) items. More in detail, in ASD/APS individuals, a mean SIPS-P score ≥3 (thus supporting the APS condition definition) was found only in the SIPS-P1 item. To note, unusual thought content, delusional ideas, and grandiosity are symptoms of disorganized thinking, which refers to disjointed and incoherent thought processes (13). The presence of delusional beliefs and ideation in individuals with ASD has been extensively documented, commonly characterized by “delusions of reference,” “delusions of thought insertion and withdrawal,” and “unusual ideas” (16, 47). Nonetheless, it is important to note that in ASD, individuals could be particularly challenging to distinguish between psychotic features—such as delusional beliefs and disorganized speech—and ASD core symptoms particularly referred to as RRBs, which include stereotyped languages or restricted interests (27). Along this, based on the autistic symptoms profile characterization, ASD/APS individuals included in our sample showed greater scores in the terms of RRBs in comparison with ASD. In this context, it is important to underline that in the present study the clinical assessment was performed by expert clinicians in both autism and psychosis in order to avoid possible symptoms’ under/mis-interpretation. Therefore, we could hypothesize that ASD individuals who presented greater symptoms severity in terms of RRBs could be the one at increased risk for APS, specifically detected by a higher score in the SIPS-P 1 item. As an element of proof that RRBs could play a crucial role, Jutla et al. (3) recently investigated whether previous neurodevelopmental symptoms could predict that an individual at clinical high risk (CHR) will convert to psychosis in a sample of n=151 CHR cohort, showing that Restricted and Repetitive Behaviors during childhood increase the risk of later conversion (OR 13.29, 95% CI 1.5–309.84). Consistent with a transdiagnostic perspective, available studies in the field have highlighted a neurodevelopmental-phenomenological bottom-up model, suggesting that a history of neurodevelopmental RRBs, particularly those related to sensory phenomena, may precede the emergence of obsessive-compulsive behaviors (48, 49), well recognized as a risk factor for psychosis (50–52).
Additionally, across all groups in our study, we found a negative association between the ADOS-2 CSS and SIPS-P scores. This suggests that individuals with more severe autistic symptoms exhibited less pronounced positive psychotic symptoms (independent of IQ, adaptive skills, and gender). This finding supports the hypothesis that positive psychotic symptoms, particularly those related to the SIPS-P1 item, may effectively define the APS condition, even in individuals with ASD.
Finally, it is important to note that no differences in terms of APS age onset came out between ASD/APS and the APS individuals. Despite this, while the APS population generally refers independently to clinicians due to symptoms distress arisen from a previous relatively well-being status, the perception of APS symptoms onset in ASD individuals could be cloudier. Indeed, the attention given to the autistic condition di per se could divert focus from possible concomitant conditions, particularly referred to as premorbid psychotic symptoms. As a consequence, clinicians and researchers are strongly invited not only to investigate and screen for APS in ASD individuals considered at increased risk (i.e., poorer adaptive skills, increased RRBs) but also to further explore clinical features of APS in ASD.
Despite our study highlighting several promising findings with possible important implications for daily clinical practice and future research perspectives, our data need to be supported by evidence coming from other studies specifically aimed to define the APS in ASD. Identifying potential clinical markers of APS in ASD could assist clinicians in developing timely and tailored therapeutic and educational interventions for this population.
The data that support the finding of this study are available on request from the corresponding author AR.
The studies involving humans were approved by Independent Ethical Committee of the University Hospital, Fondazione Policlinico Tor Vergata (Register number 126/18). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
AR: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. MP: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – review & editing. LG: Formal analysis, Writing – review & editing, Supervision. MC: Formal analysis, Writing – review & editing. MV: Data curation, Investigation, Writing – review & editing. MG: Data curation, Investigation, Writing – review & editing. LA: Data curation, Investigation, Writing – review & editing. MS: Data curation, Supervision, Writing – review & editing. MD: Data curation, Investigation, Writing – review & editing. SV: Conceptualization, Methodology, Supervision, Writing – review & editing. LM: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. (2018) 2(3):223–8. doi: 10.1016/S2352-4642(18)30022-1
2. Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. (2022) 27(1):281–95. doi: 10.1038/s41380-021-01161-7
3. Jutla A, Califano A, Dishy G, Hesson H, Kennedy L, Lundgren B, et al. Neurodevelopmental predictors of conversion to schizophrenia and other psychotic disorders in adolescence and young adulthood in clinical high risk individuals. Schizophr Res. (2020) 224:170–2. doi: 10.1016/j.schres.2020.10.008
4. Guerrera S, Pontillo M, Chieppa F, Passarini S, Di Vincenzo C, Casula L, et al. Autism Spectrum Disorder and Early Psychosis: a narrative review from a neurodevelopmental perspective. Front Psychiatry. (2024) 15:1362511. doi: 10.3389/fpsyt.2024.1362511
5. Raballo A, Poletti M, Preti A. Clinical High Risk for Psychosis (CHR-P) in children and adolescents: a roadmap to strengthen clinical utility through conceptual clarity. Eur Child Adolesc Psychiatry. (2024) 33(6):1997–9. doi: 10.1007/s00787-023-02139-2
6. Raballo A, Poletti M, Preti A, McGorry P. Clinical high risk for psychosis in children and adolescents: A meta-analysis of transition prevalences. Schizophr Res. (2022) 243:254–61. doi: 10.1016/j.schres.2020.03.063
7. Lång U, Yates K, Leacy FP, Clarke MC, McNicholas F, Cannon M, et al. Systematic review and meta-analysis: Psychosis risk in children and adolescents with an at-risk mental state. J Am Acad Child Adolesc Psychiatry. (2022) 61(5):615–25. doi: 10.1016/j.jaac.2021.07.593
8. Tor J, Dolz M, Sintes A, Muñoz D, Pardo M, de la Serna E, et al. Clinical high risk for psychosis in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. (2018) 27(6):683–700. doi: 10.1007/s00787-017-1046-3
9. Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. (2013) 70(1):107–20. doi: 10.1001/jamapsychiatry.2013.269
10. Fusar-Poli P. The clinical high-risk state for psychosis (CHR-P), version IISchizophr bull. (2017) 43(1):44–7. doi: 10.1093/schbul/sbw158
11. Fusar-Poli P, Cappucciati M, Borgwardt S, Woods SW, Addington J, Nelson B, et al. Heterogeneity of psychosis risk within individuals at clinical high risk: A meta-analytical stratification. JAMA Psychiatry. (2016) 73(2):113–20. doi: 10.1001/jamapsychiatry.2015.2324
12. Salazar de Pablo G, Catalan A, Fusar-Poli P. Clinical validity of DSM-5 attenuated psychosis syndrome: Advances in diagnosis, prognosis, and treatment. JAMA Psychiatry. (2020) 77(3):311–20. doi: 10.1001/jamapsychiatry.2019.3561
13. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition. Arlington: American Psychiatric Association (2013).
14. Schiffman J, Carpenter WT. Attenuated psychosis syndrome: benefits of explicit recognition. Shanghai Arch Psychiatry. (2015) 27(1):48–51. doi: 10.11919/j.issn.1002-0829.215015
15. Larson FV, Wagner AP, Jones PB, Tantam D, Lai MC, Baron-Cohen S, et al. Psychosis in autism: comparison of the features of both conditions in a dually affected cohort. Br J Psychiatry. (2017) 210(4):269–75. doi: 10.1192/bjp.bp.116.187682
16. Ribolsi M, Fiori Nastro F, Pelle M, Medici C, Sacchetto S, Lisi G, et al. Recognizing psychosis in autism spectrum disorder. Front Psychiatry. (2022) 13:768586. doi: 10.3389/fpsyt.2022.768586
17. Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. (2019) 6(10):819–29. doi: 10.1016/S2215-0366(19)30289-5
18. Hossain MM, Khan N, Sultana A, Ma P, McKyer ELJ, Ahmed HU, et al. Prevalence of comorbid psychiatric disorders among people with autism spectrum disorder: An umbrella review of systematic reviews and meta-analyses. Psychiatry Res. (2020) 287:112922. doi: 10.1016/j.psychres.2020.112922
19. Hollocks MJ, Leno VC, Chandler S, White P, Yorke I, Charman T, et al. Psychiatric conditions in autistic adolescents: longitudinal stability from childhood and associated risk factors. Eur Child Adolesc Psychiatry. (2023) 32(11):2197–208. doi: 10.1007/s00787-022-02065-9
20. Chisholm K, Lin A, Abu-Akel A, Wood SJ. The association between autism and schizophrenia spectrum disorders: A review of eight alternate models of co-occurrence. Neurosci Biobehav Rev. (2015) 55:173–83. doi: 10.1016/j.neubiorev.2015.04.012
21. Kincaid DL, Doris M, Shannon C, Mulholland C. What is the prevalence of autism spectrum disorder and ASD traits in psychosis? A systematic review. Psychiatry Res. (2017) 250:99–105. doi: 10.1016/j.psychres.2017.01.017
22. Mutluer T, Aslan Genç H, Özcan Morey A, Yapici Eser H, Ertinmaz B, Can M, et al. Population-based psychiatric comorbidity in children and adolescents with autism spectrum disorder: A meta-analysis. Front Psychiatry. (2022) 13:856208. doi: 10.3389/fpsyt.2022.856208
23. Varcin KJ, Herniman SE, Lin A, Chen Y, Perry Y, Pugh C, et al. Occurrence of psychosis and bipolar disorder in adults with autism: A systematic review and meta-analysis. Neurosci Biobehav Rev. (2022) 134:104543. doi: 10.1016/j.neubiorev.2022.104543
24. De Crescenzo F, Postorino V, Siracusano M, Riccioni A, Armando M, Curatolo P, et al. Autistic symptoms in schizophrenia spectrum disorders: A systematic review and meta-analysis. Front Psychiatry. (2019) 10:78. doi: 10.3389/fpsyt.2019.00078
25. Crespi B, Badcock C. Psychosis and autism as diametrical disorders of the social brain. Behav Brain Sci. (2008) 31(3):241–320. doi: 10.1017/S0140525X08004214
26. Vaquerizo-Serrano J, Salazar de Pablo G, Singh J, Santosh P. Autism spectrum disorder and clinical high risk for psychosis: A systematic review and meta-analysis. J Autism Dev Disord. (2022) 52(4):1568–86. doi: 10.1007/s10803-021-05046-0
27. Cochran DM, Dvir Y, Frazier JA. "Autism-plus" spectrum disorders: intersection with psychosis and the schizophrenia spectrum. Child Adolesc Psychiatr Clin N Am. (2013) 22(4):609–27. doi: 10.1016/j.chc.2013.04.005
28. Maat A, Therman S, Swaab H, Ziermans T. The attenuated psychosis syndrome and facial affect processing in adolescents with and without autism. Front Psychiatry. (2020) 11:759. doi: 10.3389/fpsyt.2020.00759
29. Jutla A, Foss-Feig J, Veenstra-VanderWeele J. Autism spectrum disorder and schizophrenia: An updated conceptual review. Autism Res. (2022) 15(3):384–412. doi: 10.1002/aur.2659
30. Foss-Feig JH, Velthorst E, Smith L, Reichenberg A, Addington J, Cadenhead KS, et al. Clinical profiles and conversion rates among young individuals with autism spectrum disorder who present to clinical high risk for psychosis services. J Am Acad Child Adolesc Psychiatry. (2019) 58(6):582–8. doi: 10.1016/j.jaac.2018.09.446
31. Gadow KD. Schizophrenia spectrum and attention-deficit/hyperactivity disorder symptoms in autism spectrum disorder and controls. J Am Acad Child Adolesc Psychiatry. (2012) 51(10):1076–84. doi: 10.1016/j.jaac.2012.08.001
32. Selten JP, Lundberg M, Rai D, Magnusson C. Risks for nonaffective psychotic disorder and bipolar disorder in young people with autism spectrum disorder: a population-based study. JAMA Psychiatry. (2015) 72(5):483–9. doi: 10.1001/jamapsychiatry.2014.3059
33. Riccioni A, Siracusano M, Vasta M, Ribolsi M, Nastro FF, Gialloreti LE, et al. Clinical profile and conversion rate to full psychosis in a prospective cohort study of youth affected by autism spectrum disorder and attenuated psychosis syndrome: A preliminary report. Front Psychiatry. (2022) 13:950888. doi: 10.3389/fpsyt.2022.950888
34. Bortoletto R, Bassani L, Garzitto M, Lamberti M, Simonati A, Darra F, et al. Risk of psychosis in autism spectrum disorder individuals exposed to psychosocial stressors: A 9-year chart review study. Autism Res. (2023) 16(11):2139–49. doi: 10.1002/aur.3042
35. Di Lorenzo G, Riccioni A, Ribolsi M, Siracusano M, Curatolo P, Mazzone L. Auditory mismatch negativity in youth affected by autism spectrum disorder with and without attenuated psychosis syndrome. Front Psychiatry. (2020) 11:555340. doi: 10.3389/fpsyt.2020.555340
36. Foss-Feig JH, Guillory SB, Roach BJ, Velthorst E, Hamilton H, Bachman P, et al. Abnormally large baseline P300 amplitude is associated with conversion to psychosis in clinical high risk individuals with a history of autism: A pilot study. Front Psychiatry. (2021) 12:591127. doi: 10.3389/fpsyt.2021.591127
37. Trevisan DA, Foss-Feig JH, Naples AJ, Srihari V, Anticevic A, McPartland JC. Autism spectrum disorder and schizophrenia are better differentiated by positive symptoms than negative symptoms. Front Psychiatry. (2020) 11:548. doi: 10.3389/fpsyt.2020.00548
38. Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. (2003) 29(4):703–15. doi: 10.1093/oxfordjournals.schbul.a007040
39. Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Services (2012).
40. Roid G, Miller L. Examiner’s Manual: Leiter International Performance Scale-Revised. Wood Dale, IL: Stoelting Co (1997).
41. Wechsler D. Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV) Technical and Interpretive Manual. San Antonio, TX: Psychological Corporation (2003). doi: 10.1037/t15174-000
43. Harrison PL, Oakland T. Adaptive Behavior Assessment SystemR Second Edition ABAS R-II. San Antonio: Harcourt (2003).
44. Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. (2002) 159(5):863–5. doi: 10.1176/appi.ajp.159.5.863
45. Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord. (2014) 44(8):1996–2012. doi: 10.1007/s10803-014-2080-3
46. Mandelli V, Landi I, Busuoli EM, Courchesne E, Pierce K, Lombardo MV. Prognostic early snapshot stratification of autism based on adaptive functioning. Nat Ment Health. (2023) 1:327–36. doi: 10.1038/s44220-023-00056-6
47. Abell F, Hare DJ. An experimental investigation of the phenomenology of delusional beliefs in people with asperger syndrome. Autism. (2005) 9(5):515–31. doi: 10.1177/1362361305057857
48. Poletti M, Gebhardt E, Raballo A. Along the fringes of Agency: neurodevelopmental account of the obsessive mind. CNS spectrums. (2022) 27(5):557–60. doi: 10.1017/S1092852921000560
49. Poletti M, Gebhardt E, Pelizza L, Preti A, Raballo A. Neurodevelopmental antecedents and sensory phenomena in obsessive compulsive disorder: A systematic review supporting a phenomenological-developmental model. Psychopathology. (2023) 56(4):295–305. doi: 10.1159/000526708
50. Niendam TA, Berzak J, Cannon TD, Bearden CE. Obsessive compulsive symptoms in the psychosis prodrome: correlates of clinical and functional outcome. Schizophr Res. (2009) 108(1-3):170–5. doi: 10.1016/j.schres.2008.11.023
51. Averna R, Pontillo M, Demaria F, Armando M, Santonastaso O, Pucciarini ML, et al. Prevalence and clinical significance of symptoms at ultra high risk for psychosis in children and adolescents with obsessive-Compulsive disorder: is there an association with global, role, and social functioning? Brain Sci. (2018) 8:181. doi: 10.3390/brainsci8100181
52. Borrelli DF, Ottoni R, Provettini A, Morabito C, Dell’Uva L, Marchesi C, et al. A clinical investigation of psychotic vulnerability in early-onset obsessive-Compulsive Disorder through Cognitive-Perceptive basic symptoms. Eur Arch Psychiatry Clin Neurosci. (2024) 274:195–205. doi: 10.1007/s00406-022-01543-0
Keywords: clinical high risk, prodrome, psychosis, neurodevelopment, schizophrenia, autism
Citation: Riccioni A, Pontillo M, Gialloreti LE, Cicala M, Vasta M, Gatto M, Arturi L, Siracusano M, Di Luzio M, Vicari S and Mazzone L (2025) Investigating the attenuated psychosis syndrome in youth with autism spectrum disorder: results from an observational study. Front. Psychiatry 16:1536361. doi: 10.3389/fpsyt.2025.1536361
Received: 28 November 2024; Accepted: 05 March 2025;
Published: 31 March 2025.
Edited by:
Soumitra Das, Western Health, AustraliaReviewed by:
Michele Poletti, IRCCS Local Health Authority of Reggio Emilia, ItalyCopyright © 2025 Riccioni, Pontillo, Gialloreti, Cicala, Vasta, Gatto, Arturi, Siracusano, Di Luzio, Vicari and Mazzone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Assia Riccioni, YXNzaWFyaWNjaW9uaUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
§ORCID: Assia Riccioni, orcid.org/0000-0001-6496-8756
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.