- 1Department of Magnetic Resonance Imaging (MRI), The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Psychiatry, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background: As a crucial node of the cortico-striato-thalamo-cortical (CSTC) loop, the striatum has long been considered to be involved in the pathophysiology of obsessive-compulsive disorder (OCD). Numerous neuroimaging studies have reported functional abnormalities of the striatum in OCD. However, altered dynamic functional connectivity (DFC) patterns of striatal subregions were rarely reported in patients with OCD.
Methods: We collected resting-state functional MRI data from 97 first episode and drug-naïve OCD patients and 106 HCs matched for gender and age. Seed-based whole-brain resting-state functional connectivity (RSFC) and DFC analysis were performed for 12 striatal subregions. Between-group differences of the mean RSFC and DFC were determined using a two-sample t-test. In addition, we performed a Spearman’s correlation analysis to examine the relationship between altered RSFC and DFC and the clinical characteristics of OCD.
Results: Patients with OCD exhibited increased RSFC between the superior ventral striatum (VSs) and the calcarine (CAL), lingual gyrus (LING), cuneus (CUN), supplementary motor area (SMA), precuneus (PCUN), paracentral lobule (PCL) and superior parietal gyrus (SPG). Increased RSFC between the left dorsal caudal putamen (DCP) and LING and inferior occipital gyrus (IOG) and increased RSFC between left ventral rostral putamen (VRP) and fusiform gyrus (FFG) were also found. in OCD group. The left dorsal caudate (DC) showed increased RSFC with CAL. In addition, OCD patients shows increased RSFC between multiple striatal seeds and cerebellum. The left VSs showed decreased DFC in the OCD patients with the PCUN, SPG and superior occipital gyrus (SOG). The right DC showed decreased DFC with the medial frontal gyrus orbital part (ORBmed), superior frontal gyrus orbital part (ORBsup) and gyrus rectus (REC). OCD severity was associated with DFC values between the right DC and ORBmed (r = 0.209, p = 0.044).
Conclusion: Our study reveals disrupted RSFC and DFC between the striatal subregions and widespread brain regions in OCD patients. The findings highlight the role of the striatum in the neuropathology of OCD at a refined anatomical level and support the CSTC model in OCD.
1 Introduction
Obsessive compulsive disorder (OCD) is a common and disabling mental disorder characterized by obsessions and/or compulsions (1). Obsessions refer to intrusive and unwanted repetitive thoughts, impulses, images or urges. Compulsions are repetitive mental activities or behaviors that an individual feels driven to perform. These behaviors are typically stereotyped and ritualistic, such as repeatedly washing hands, checking locks, or counting objects. OCD typically causes significant anxiety or distress, affecting the individual’s daily life, social interactions, work, and studies. The course of OCD is typically chronic, spanning over years or decades, and is characterized by recurrent episodes (2, 3). OCD has a lifetime prevalence of 2–3% (4), causing significant negative impact on public health (5). However, the pathophysiology of OCD remains incompletely elucidated. Resting-state functional magnetic resonance imaging (R-fMRI) is a technique using the blood oxygenation level dependent (BOLD) signal to measure metabolic activity in brain region (6). Previous R-fMRI studies have reported abnormalities in functional connectivity (FC) in multiple brain regions in OCD, particularly in the striatum (7, 8).
As a crucial node of the cortico-striato-thalamo-cortical (CSTC) loop, the striatum has long been considered to be involved in the pathophysiology of obsessive-compulsive disorder. The striatum consists of the caudate nucleus and the putamen which receives synaptic input from entire cerebral cortex and integrates affective, motor, and cognitive information (9–11). Neuroimaging studies have suggested the striatum is involved in various motor-related function, executive/cognitive control and reward related/motivational processes (12–15). The striatum is a complex composed of structurally and functionally heterogeneous subregions. Nucleus accumbens, dorsal caudate and putamen separately constitute the affective circuit, dorsal cognitive circuit and ventral cognitive circuit (16). Previous studies have confirmed the functional segregation between the ventral and dorsal striatum in reward system, planning and executive functions, and learning processes (17–21). Di Martino et al. have subdivided striatum by defining six seed regions and using these six seeds to explore the whole-brain FC (22). Specifically, inferior ventral striatum (VSi) was functionally connected with regions involved in emotional processing while superior ventral striatum (VSs) was connected with regions involved in executive function, decision making, and motor planning. Dorsal caudate (DC) was primarily associated with the regions involved in cognitive control such as dorsolateral prefrontal cortex, ventral lateral prefrontal cortex, anterior cingulate cortex. Thus, the segmentation of striatum into subregions and using them as seed will significantly enhance the accuracy of whole-brain FC analysis, offering profound understanding into the intricate functional networks of the brain.
Previous research on resting-state fMRI in obsessive-compulsive disorder were under the premise that the brain remained static, failed to fully utilize the rich temporal dynamics inherent in spontaneous BOLD FC. In contrast to the static FC model, DFC assesses the time-varying covariance of neural signals across brain regions, enabling to explore the temporal characteristics of FC (23). The sliding window analysis method is the most common strategy for examining dynamic FC (24–26). DFC has been extensively applied to investigate the neuropathology of mental disorders including major depressive disorders (27–29), generalized anxiety disorders (30, 31), schizophrenia (32, 33) and epilepsy (34, 35). Ding et al. have found that the DFC between the left superior temporal gyrus and the left cerebellum Crus I and left thalamus, and between the right supplementary motor area and right dorsolateral prefrontal cortex and left precuneus was decreased in OCD (36). Teng et al.’s research reported that OCD exhibited significantly decreased DFC variability between the left thalamus seed and the left cuneus and right lingual gyrus as well as decreased DFC variability between the bilateral cuneus seed and bilateral postcentral gyrus (37). However, the DFC of the striatal subregions in patients with OCD remains unclear. In this research, we applied whole-brain voxel-based RSFC and DFC methods to explore the static and dynamic function alterations at rest in patients with OCD. Furthermore, we examined the association between RSFC and DFC alterations and the severity of OCD. In addition, previous studies have indicated that pharmacological treatment can altered the brain functional connectivity patterns in patients with OCD (38). We included first-onset OCD patients who had not previously received any medication.
2 Materials and method
2.1 Participants
Patients with OCD were recruited from out-patient/inpatient services of Department of Psychiatry, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China. Patients were diagnosed by a chief physician and a well-trained psychiatrist, following the guidelines outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) for OCD. OCD patients were drug-naïve and should not have previous episodes. All patients were Han Chinese and right handedness. The severity of OCD was quantified using the Yale–Brown Obsessive Compulsive Scale (Y-BOCS) (39). The Self Rating Depression Scale (SDS) and the Self Rating Anxiety Scale (SAS) were used to evaluate depressive and anxiety symptoms. Furthermore, individuals who meet any of the following exclusion criteria will be excluded from participation in the current study: (1) Having comorbidity of other mental/psychotic disorders; (2) Taking drugs such as anesthesia and analgesia in the past 1 month; (3) History of alcohol or psychoactive substance abuse; (4) History of organic brain lesions, or other organic body disease; (5) Suffering from disorders of the nervous system, endocrine system, cardiovascular system, and respiratory system; (6) Contraindications for MRI scanning including implanted cardiac pacemakers, artificial heart valves, fixed dentures, metal braces, or other magnetizable foreign bodies in the body. Ninety-seven patients were included in the case group after meeting the above inclusion criteria. We recruited 106 HCs matched for gender and age from the general public through poster advertisement. The inclusion criteria for the HC group are: (1) Han Chinese and right-handed; (2) No obvious abnormalities were found in the head MRI; (3) No mental disorders or neurological diseases; In addition, exclusion criteria for HC group include the following: (1) Suffering from severe systemic diseases; (2) Alcoholism or substance abuse; (3) family history of hereditary neurological disorders; (3) Claustrophobia or having any metallic objects in the body.
All participants signed written informed consent forms before experiment. The study received approval from the research ethical committee of the First Affiliated Hospital of Zhengzhou University.
2.2 Data acquisition
The R-fMRI data of participants were acquired using on 3-Tesla GE Discovery MR750 scanner (General Electric, Fairfield Connecticut, USA). During R-fMRI scanning, all subjects were instructed to keep their eyes closed but stay awake, and avoid consciously engaging in thoughts or memories. First, each subject underwent conventional imaging sequences including T1-weighted, T2-weighted, and T2-weighted fluid-attenuated inversion recovery (FLAIR) to exclude organic lesions or other abnormalities. The scanning parameters employed in the resting-state functional scans were as follows: repetition time = 2000ms; echo time = 30ms; number of slices = 32; thickness = 4 mm; resolution matrix = 64 × 64; flip angle = 90°; field of view= 220 × 220 mm2; and slice gap = 0.5mm.
2.3 Data processing
The Data Processing & Analysis for Brain Imaging toolbox (DPABI, version 4.3; http://rfmri.org/dpabi) (40) was used for the analysis of R-fMRI data within a pipeline framework. The main steps were as follows: (1) removing the first 10 time points; (2) slice-timing correction and realignment; (3) correction of head motion (excluding data with translation over 3 mm or rotation over 3 degree); (4) spatial normalization into the standard Montreal Neurological Institute template (resampling voxel size, 3 × 3 × 3 mm3); (5) spatial smoothing with a Gaussian kernel of full-width at half-maximum of 6 mm; (6) detrending of BOLD signals for reducing low-frequency drift; (7) temporal band pass (0.01 and 0.1). Four subjects in the OCD group and two subjects in the HC group were excluded because head motion exceeded the previously set threshold. The final sample included 93 OCD patients and 104 HCs.
2.4 Dynamic and static FC analyses
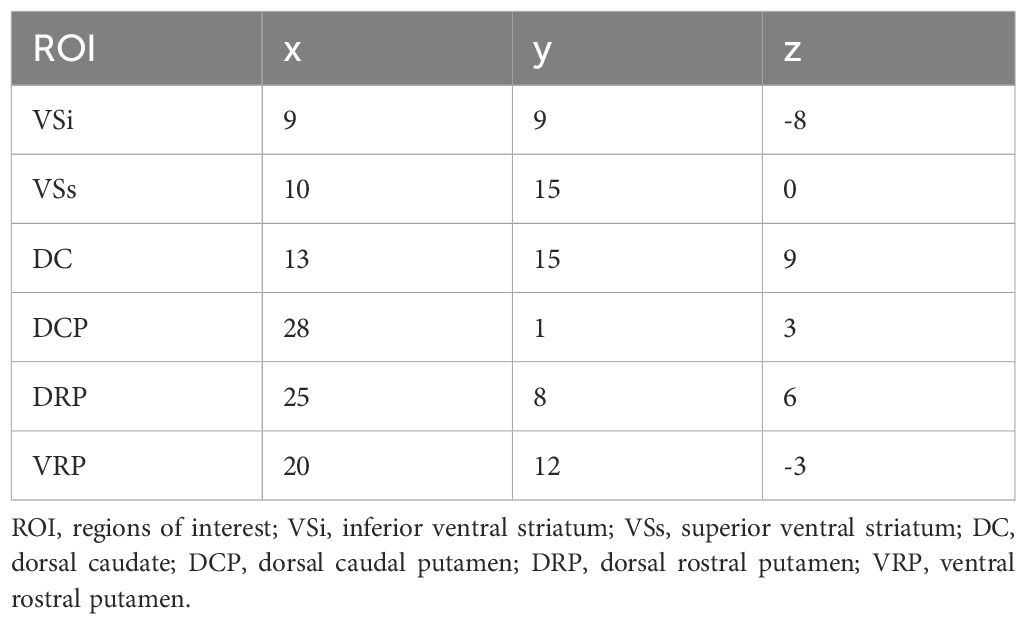
Based on the research by Di Martino and his colleagues, we used 12 seeds of striatum for whole-brain RSFC and DFC. For each hemisphere, six MNI coordinates were designated and seeds were defined as spherical mask with a 4mm radius centered around each coordinate. (22) (see Table 1). DPARSF was used for RSFC analysis. The mean time series of activity within this seed region was computed. Then, Pearson correlation analysis was used to calculate the temporal correlation between the mean time series of seed region and the time series of each voxel across the whole brain. The correlation coefficients were then transformed into Z-scores using Fisher’s r-to-z transformation to improve the normality of the data. Dynamic FC analysis was performed using Dynamic Brain Connectome (DynamicBC, version 2.0; http://restfmri.net/forum/DynamicBC) analysis toolbox (41). Sliding time-window analysis was adopted to characterize FC temporal dynamics. The window length is a crucial parameter, but there is currently no consensus on its selection. Based on previous research, the minimum window length should be at least 1/fmin to prevent the introduction of spurious fluctuations caused by shorter intervals. Here,fmin is the minimum frequency of time series (42). Simultaneously, the window length should not be excessively long to prevent disruption of the temporal variability dynamics of FC. (43). We set the length of the window to 50TR (1TR=2s) with an overlap of 0.8 which means the step size is 10TR. Within each window, we applied seed-based DFC analysis to compute the temporal correlation coefficients between the averaged time course of each ROI and all other voxels to build a FC map. Then, a Fisher’s r-toz transformation was applied for the resulting FC matrices in order to improve the normality of correlation distribution. For each subject, the standard deviation of zFC values was calculated to obtain the DFC values of all voxels within windows.
2.5 Statistical analyses
The statistical analysis was conducted using the statistical SPSS software (IBM SPSS Statistics for Windows, version 26.0. Armonk, NY: IBM Corp). Demographic and clinical data were analyzed using the chi-squared test for gender and independent two-sample t-tests for the other demographic characteristics between the two groups. The RSFC and DFC values were compared between the OCD group and HCs using voxel-wise two-sample t-tests in SPM12 toolbox, with age, gender, education level, and head motion included as covariates. The results were thresholded using Gaussian random field (GRF) correction, carried out via the DPABI toolbox, with a voxel-level threshold of p < 0.005 and a cluster-level threshold of p < 0.05. To examine the association of RSFC and DFC values with disease severity, we extracted the mean RSFC and DFC values from brain areas showing significant between-group differences and performed a correlation analysis with Y-BOCS total scores, SDS and SAS scores. Spearman’s correlation was analyzed through SPSS with Bonferroni correction.
2.6 Validation analyses
To verify the reliability of our findings on DFC, we used two additional window lengths of 60TR and 80TR both shifted by 10 TR to eliminate the impact of parameter selection and confirm the stability of DFC.
3 Results
3.1 Demographic and clinical data of patients
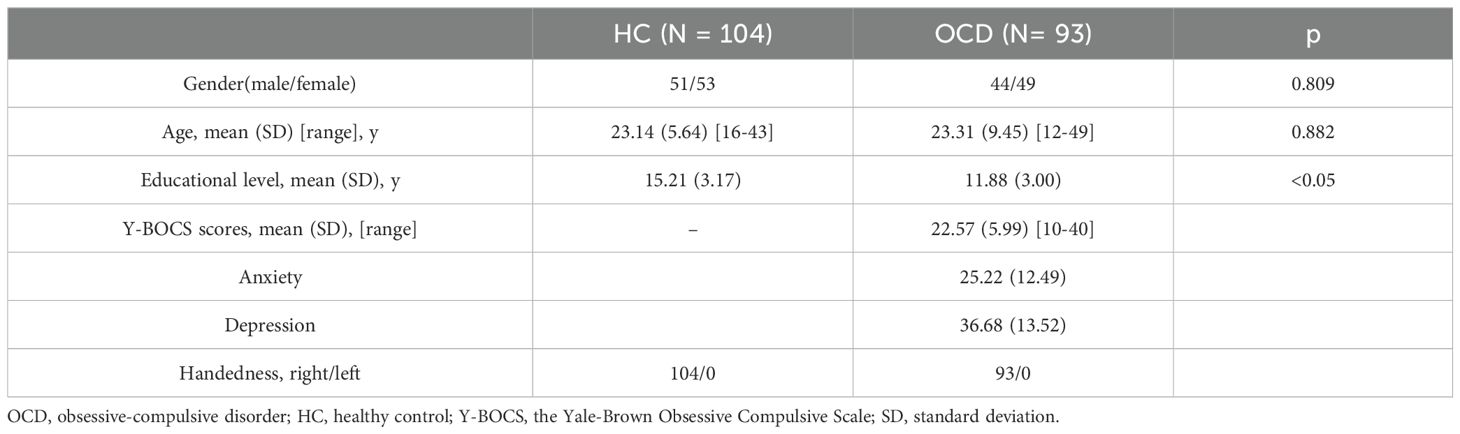
The clinical demographics were presented in Table 2. 93 OCD patients and 104 HCs were included in RSFC and DFC analyses. No significant group differences appeared in terms of sex composition, and age (p > 0.05). The HC group had significantly higher educational levels than the OCD group (p<0.05). The Y-BOCS scores of the OCD patients were higher than those of the HC group.
3.2 The resting-state functional connectivity analyses
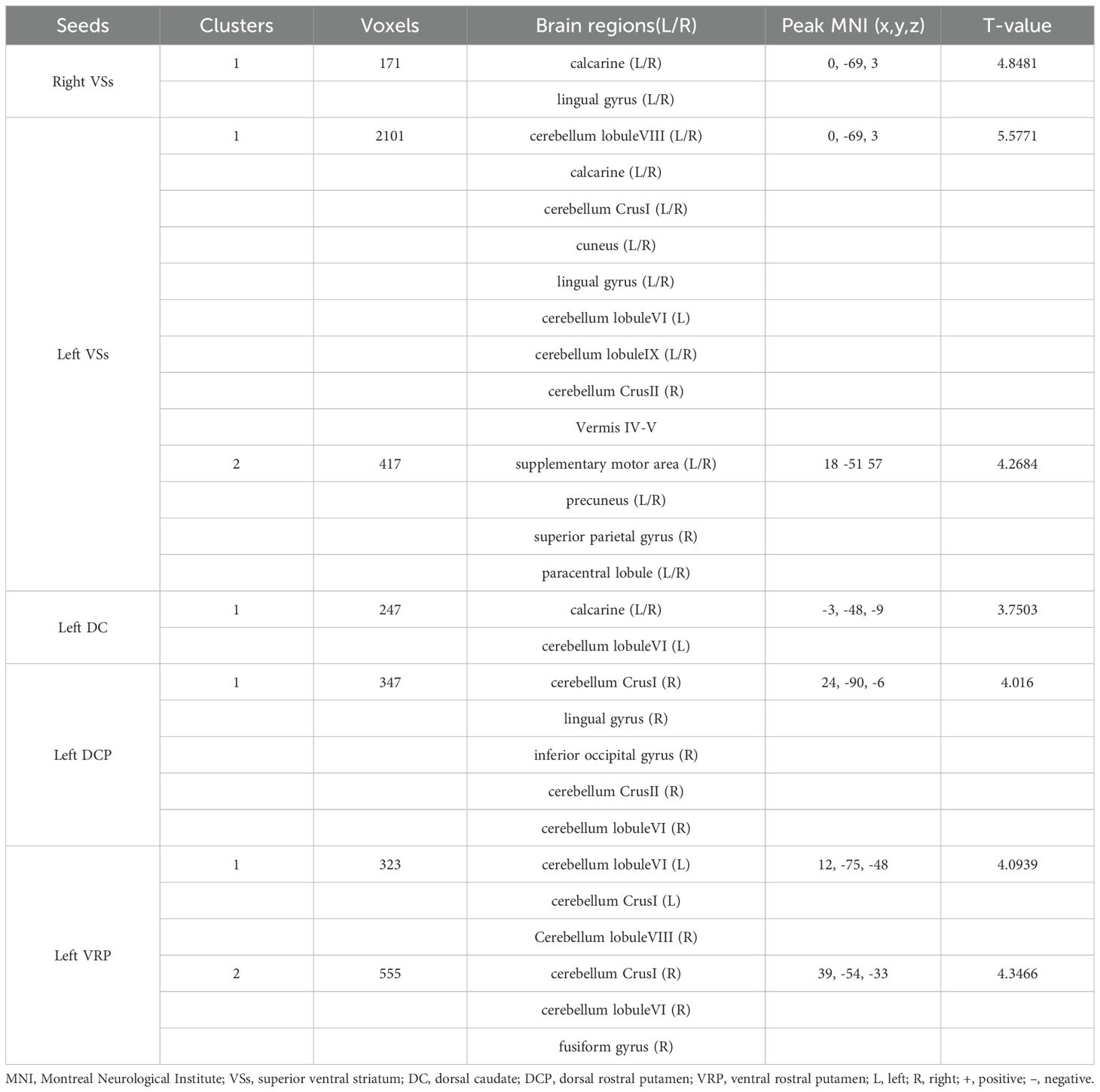
Patients with OCD exhibited increased RSFC between the right VSs and bilateral CAL and LING and increased RSFC between the left VSs and bilateral cerebellum, CAL, CUN, LING, SMA, PCUN, PCL and right SPG. Increased RSFC between the left DCP and right cerebellum, right LING and right IOG and increased RSFC between the left VRP and bilateral cerebellum and right FFG in OCD group were also found. Besides, the left DC showed increased RSFC with bilateral CAL and left cerebellum (Figures 1–3; Table 3).
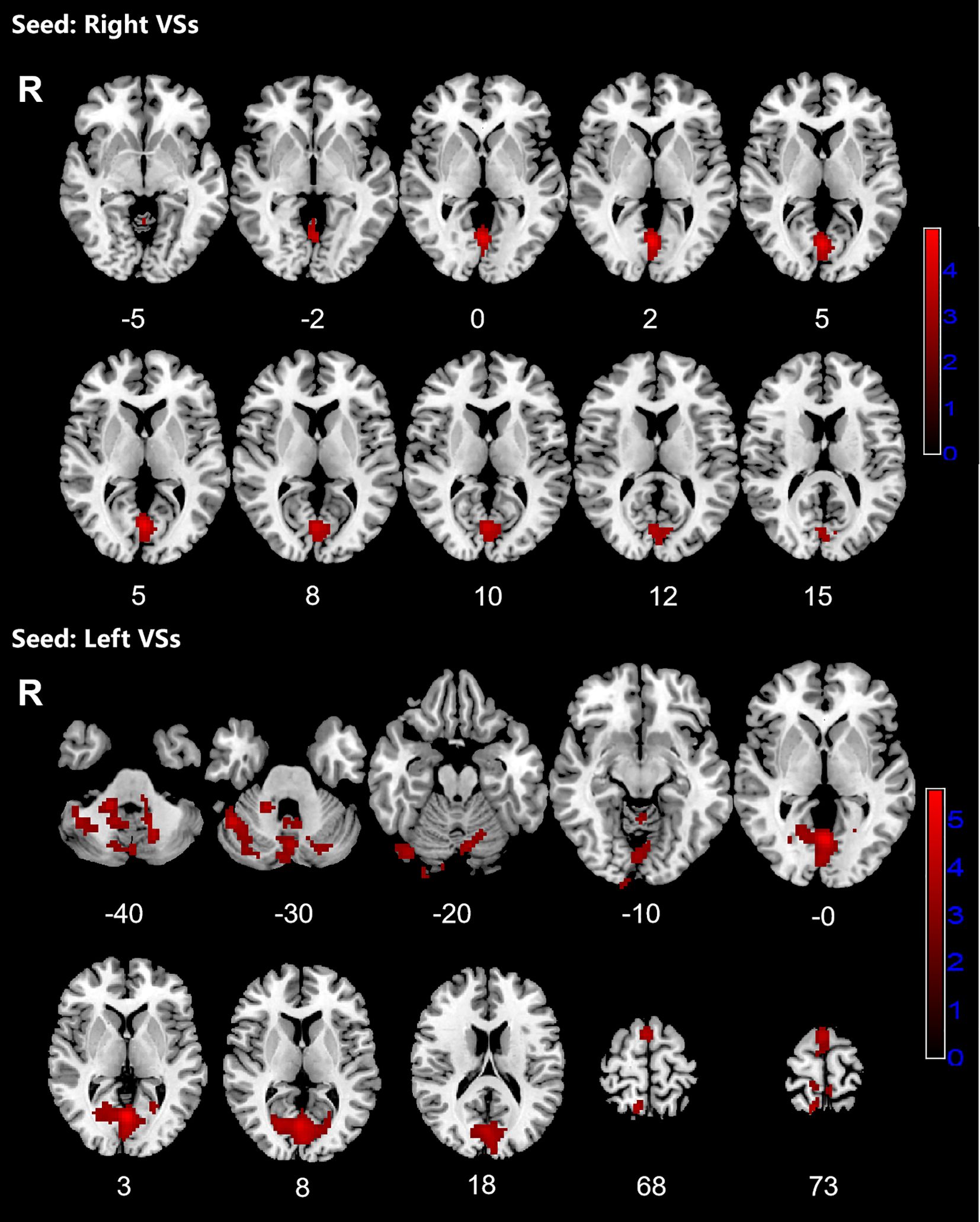
Figure 1. Significant increased resting-state functional connectivity (RSFC) of the bilateral superior ventral striatum (VSs) in OCD patients, compared with HC. The red regions in the brain slices present the location of difference (GRF corrected).
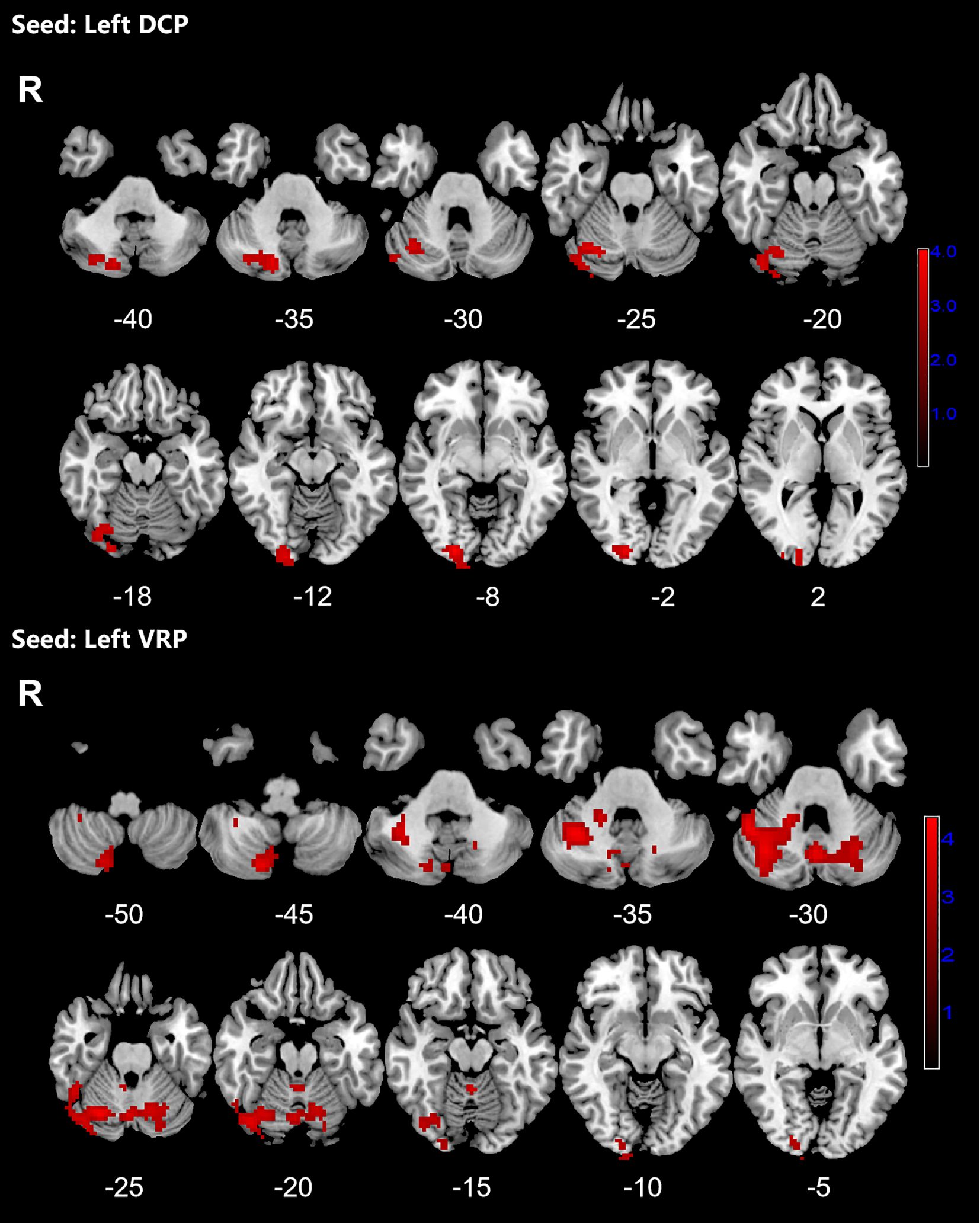
Figure 2. Significant increased resting-state functional connectivity (RSFC) of the left dorsal caudal putamen (DCP) and ventral rostral putamen (VRP) in OCD patients, compared with HC. The red regions in the brain slices present the location of difference (GRF corrected).

Figure 3. Significant increased resting-state functional connectivity (RSFC) of the left dorsal caudate (DC) and in OCD patients, compared with HC. The red regions in the brain slices present the location of difference (GRF corrected).
3.3 Dynamic functional connectivity analyses
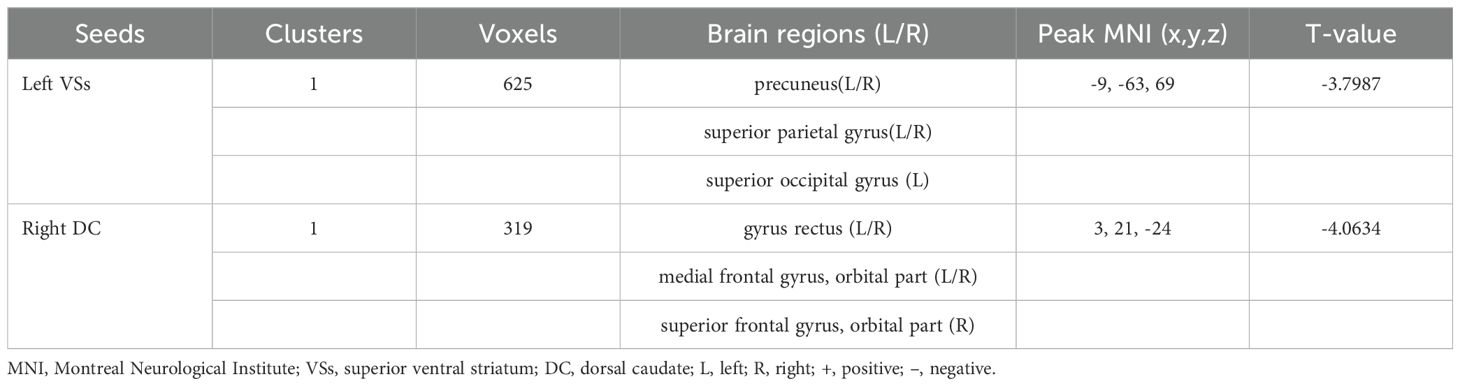
OCD group displayed significantly decreased DFC between the left VSs and bilateral PCUN, SPG and left SOG and decreased DFC between the right DC and bilateral ORBmed, REC and right ORBsup (Figure 4; Table 4).
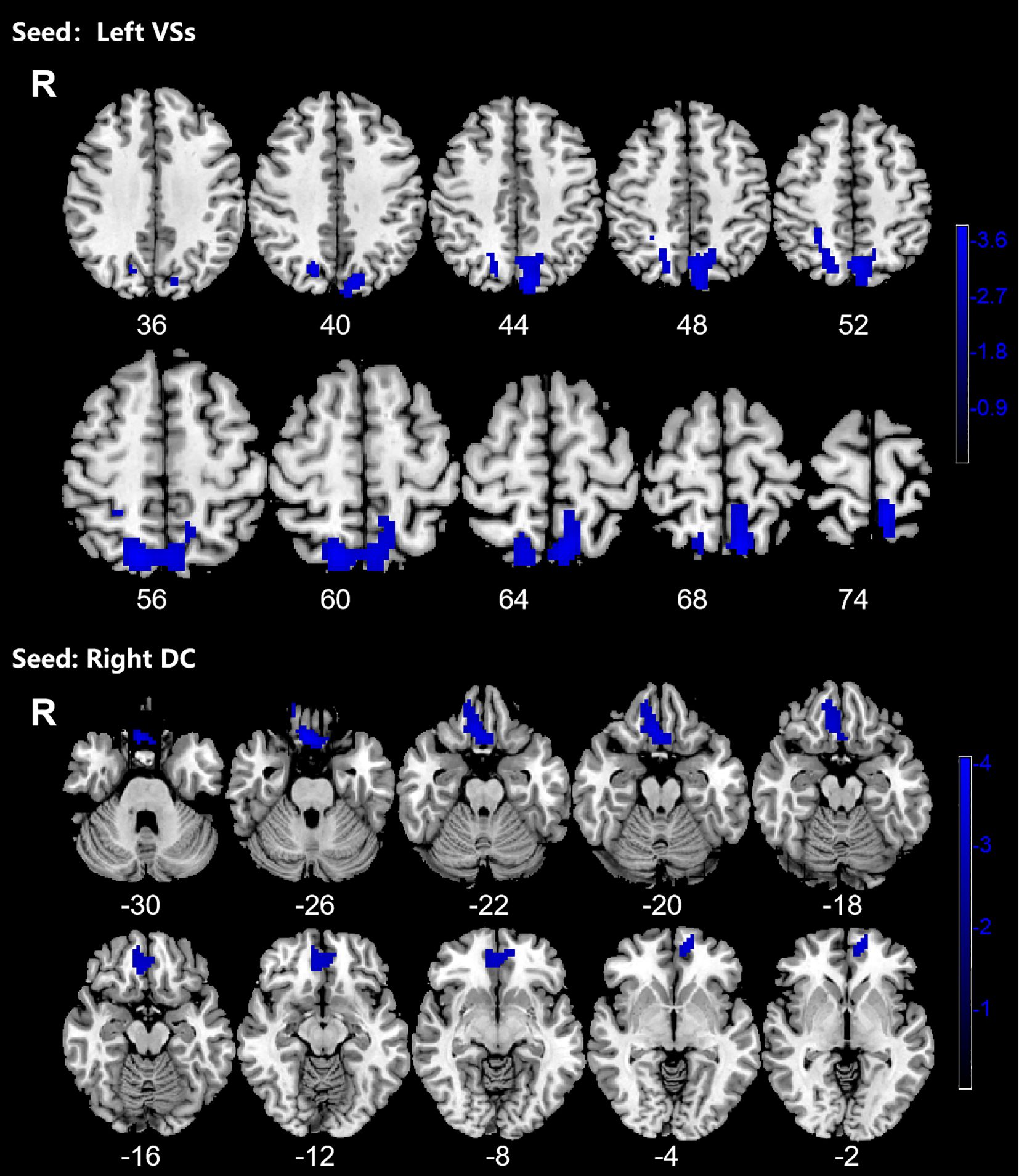
Figure 4. Significant decreased dynamic functional connectivity (DFC) of the left superior ventral striatum (VSs) and dorsal caudate (DC) in OCD patients, compared with HC. The blue regions in the brain slices present the location of difference (GRF corrected).
3.4 Spearman correlation analyses
Since the data for correlation analyses does not follow a normal distribution, we conduct a Spearman correlation analysis. The Spearman correlation analysis showed that decreased DFC of the left ORBmed was significantly related to Y-BOCS scores in OCD (Figure 5). However, the significance did not survive the Bonferroni correction (p < 0.05/10 = 0.005). The correlation analysis of brain regions with Y-BOCS scores are provided in Supplementary Table S3 in the Supplementary Materials. We did not find the correlation between the increased RSFC and Y-BOCS total scores. In addition, we did not find the correlation between the altered RSFC and DFC and anxiety and depression symptoms.
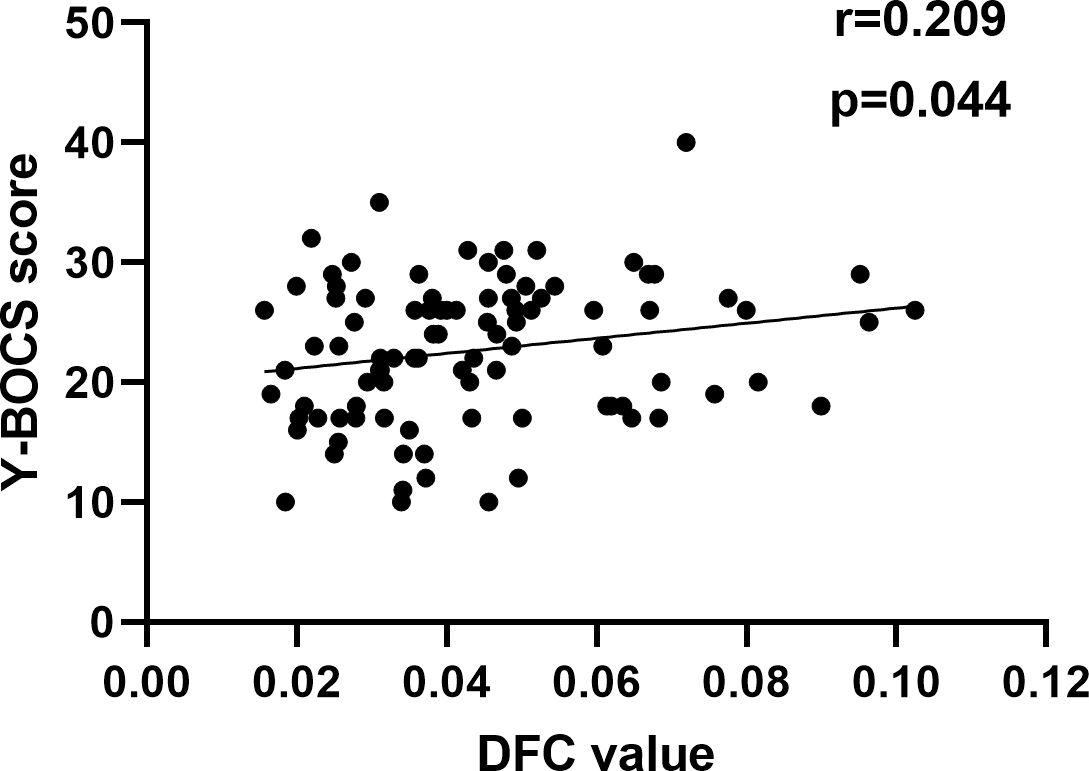
Figure 5. Correlation between altered DFC and Y-BOCS. A positive correlation between the altered DFC of the left ORBmed and Y-BOCS scores.
3.4 Validation results
The results of DFC between two groups using sliding window lengths of 60TR and 80TR were similar to those of DFC with sliding window length = 50 TR. The results of DFC with a window length of 60TR were showed in Supplementary Figure S1, and Supplementary Table S1 and the results of DFC with a window length of 80TR were showed in Supplementary Figure S2, and Supplementary Table S2 (see in the Supplementary Material).
4 Discussion
The present study examined the whole-brain RSFC and DFC using the subregions of the striatum as seeds in first-episode, drug-naïve OCD patients compared to healthy controls. We found that in OCD patients, the increased RSFC were mainly located within the cerebellum, SMA, parietal and occipital regions including CAL/LING, CUN, PCUN, PCL SPG, IOG. The results showed decreased DFC values between the left VSs and PCUN, SPG and SOG and decreased DFC values between the right DC and the ORBmed, ORBsup and REC. The correlation analysis showed that decreased DFC values of the ORBmed was significantly related to the severity of OCD.
In this study, we observed multiple striatal seeds such as bilateral VSs, left DC, left DCP showed increased RSFC with CAL and LING in patients with OCD. The CAL and LING are located in the occipital lobe and are parts of the visual cortex. The CAL plays an important role in integrating “visuopsychic” and “visuosensory” processing (44). The lingual gyrus is involved in the processing of visual information (45–47). Evidence from previous study suggests that abnormalities of visual perception involve in the neuromechanisms of OCD (48). A previous fMRI study showed that the lingual gyrus was activated when viewing neutral faces relative to scrambled images (49). The increased RSFC between the striatum and CAL and LING and may lead to overreaction to certain visual stimuli, triggering intense, involuntary and repetitive thoughts and behaviors.
We found the left VSs showed increased RSFC with the SMA, SPG and PCL. The SMA is located on the medial surface of the frontal lobe and plays an important role in movement planning and execution, as well as cognitive control (50–52). The SMA is part of the ‘sensorimotor’ CSTC of OCD, involving sensorimotor processes (1). A previous study foud that OCD patients exhibited excessive activation in the SMA during error processing (53). Another study showed increased RSFC between the right pre-SMA and the inferior frontal gyrus (IFG), and the increased FC was associated with impaired response inhibition in OCD patients (54). The SMA is also a key target for repetitive transcranial magnetic stimulation (rTMS) in the treatment of OCD. Multiple studies have shown that rTMS targeted at the SMA can effectively alleviate OCD symptoms (55–57). The PCL and SPG are also considered an important motor area, possibly related to the mental representation of movement (58). The increased functional connectivity between VSs and SMA, SPG and PCL may reflect deficits in cognitive and motor control.
Our results showed increased RSFC between multiple striatal seeds and the cerebellum. The cerebellum is traditionally thought to be involved in motor coordination and balance. Increasing evidence suggests that the cerebellum plays an important role in cognitive and emotional processes (59–61). The involvement of cerebellum in the pathophysiology of OCD has been confirmed. Previous studies have reported that OCD patient showed altered amplitude of low frequency fluctuations (fALFF) in cerebellum (62–64). Nabeyama et al. found that after behavioral therapy, OCD patients showed increased activation of the cerebellum during cognitive tasks (65). Abnormal functional connectivity between the cerebellum and widespread brain networks has been reported (66, 67). One previous study found increased global brain connectivity (GBC) in the putamen and cerebellar cortex of OCD patients (68). Zhang et al. found weakened functional connectivity between the cerebellar regions and the striatum (63). Differing from their findings, we observed increased RSFC between multiple striatal subregions and cerebellar regions. This could be attributed to functional heterogeneity of the striatum. Researches indicates that the basal ganglia and cerebellum are interconnected via dense disynaptic pathways, influencing cortical input and output (69–71). The deep cerebellar nuclei (DCN) neurons have a significant impact on striatal neuron activity through their output pathways, thereby modulating behavior related to reward processing (72). The enhanced FC between the striatum and cerebellum may be associated with impairments in executive control and the reward system.
It is worth noting that we found increased RSFC and decreased DFC between the left VSs and the precuneus in OCD patients compared with the HCs. Precuneus is part of the default mode network, involving in self-consciousness and self-referential processing (73–75). It plays a crucial role in a diverse array of cognitive functions, including visuo-spatial imagery, episodic memory retrieval and self-processing operations (76). It has been found that patients with OCD exhibit excessive activation of the precuneus when performing higher-order cognitive tasks, such as repetitive visual stimuli, working memory-related visuospatial task, or obsessive–compulsive symptom provoking tasks (77–81). Neuroimaging studies of resting-state functional imaging have reported abnormal functional connectivity between precuneus and widespread brain areas including parietal lobe, sensorimotor area, visual cortex, cerebellum, angular gyrus, and middle frontal gyrus (82, 83). A previous study reported OCD patients showed decreased functional connectivity between the caudate nucleus and precuneus during psychological distress, which was positively correlated with the severity of compulsive symptoms (84). The enhanced RSFC between the VSs and the precuneus may reflect an over-synchronization of these two regions in cognitive control and emotional regulation. As higher DFC indicates superior information processing capability and enables higher cognitive and behavioral flexibility (85). The reduced DFC between the left VSs may account for impairment of cognitive functions, self-referential processing in patients with OCD.
We also found the decreased DFC value between the right DC and the bilateral ORBmed, REC and right ORBsup. The orbitofronto-striatal circuit has consistently been thought to be associated with the neuropathology of OCD (86). Evidence from previous research indicates that the orbital frontal cortex (OFC) plays an important role in emotional regulation, reward processing, and decision-making (87–92). Functional brain imaging studies have demonstrated significantly increased OFC activity at rest in OCD (93, 94). A series of paradigm studies have reported abnormal activation of the OFC in OCD patients such as symptom provocation task, reversal learning and go/no-go task (95–98). Previous study reported increased functional connectivity between the striatal subregions and the OFC in OCD patients. (7, 99). The OFC plays an important integrative role between the internal state (regulated by the hypothalamus) and the external state (regulated by the striatum) (100). It is reported DC is more specifically associated with working memory and executive function. (16). The decreased DFC between DC and OFC may indicate reduced efficiency in information transfer between these brain regions, leading to the dysfunction of reward and executive system. In addition, we observed the DFC values of OFC correlate positively with YBOCS scores, suggesting that dysfunctions in OFC play a substantial role in OCD symptomatology. Nakao et al. found decreased activation in the OFC related to symptom provocation after symptom improvement (96). Researches have shown that OFC activity decreased with treatment response but increased with symptom provocation. Additionally, some studies reported that after treatment, there is a decrease in caudate glucose metabolism, while the caudate nucleus is activated during symptom provocation (101). As the severity of OCD increases, the enhanced DFC may reflect compensation for the excessive activity of the OFC, aiming to strengthen regulatory capacity by increasing interactions with other brain regions.
There remain a few possible limitations to consider in this study. First, there is no agreement on the selection of window length for the sliding-window method and different window lengths may lead to varied results. A shorter window may lead to higher noise and variability, while a longer window may fail to capture rapid dynamic changes (42, 43). We choose a window length of 50TR based on a comprehensive consideration of the data characteristics and experimental design. We conducted validation analysis that confirmed the stability of our results. Second, the correlation analysis did not show statistical significance after applying the Bonferroni correction. The lack of significance in the results may be attributed to factors such as a small sample size, weak correlations between variables, and overly stringent Bonferroni correction. It is important to note that even in the absence of statistical significance, the observed trends are still clinically significant and provide insights for future studies. In the future, more patients need to be included or other statistical correction methods can be used. Third, we did not find abnormal DFC using the putamen seed, contrary to many previous studies that have reported aberrant function of putamen in OCD. Therefore, it is important to emphasize that this is an exploratory study before drawing any conclusions.
5 Conclusion
This study explored the RSFC and DFC between striatal subregions and whole-brain areas in patients with OCD. We found increased RSFC and decreased DFC between the striatal subregions and widespread brain regions. Our findings expand on the existing literature and support the critical role of the striatum in the neuropathology of OCD.
Data availability statement
The datasets presented in this article are not readily available because the dataset is for internal use only by the Magnetic Resonance Imaging Department of the First Affiliated Hospital of Zhengzhou University. Requests to access the datasets should be directed to JC.
Ethics statement
The studies involving humans were approved by The Research Ethical Committee of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WS: Writing – original draft, Writing – review & editing. YT: Writing – original draft. HY: Investigation, Writing – original draft. HG: Writing – review & editing. BW: Writing – review & editing. ZL: Writing – original draft. YZ: Writing – review & editing. SH: Writing – review & editing. JC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research study was supported by the Natural Science Foundation of China (Nos.82471962, 62476252), Medical Science and Technology Research Project of Henan Province (SBGJ202302068 and 24A320069).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1529983/full#supplementary-material.
References
1. Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive–compulsive disorder. Nat Rev Dis Primers. (2019) 5:52. doi: 10.1038/s41572-019-0102-3
2. Sharma E, Math S. Course and outcome of obsessive–compulsive disorder. Indian J Psychiatry. (2019) 61:43. doi: 10.4103/psychiatry.IndianJPsychiatry_521_18
3. Van Dis EAM, Van Veen SC, Hagenaars MA, Batelaan NM, Bockting CLH, Van den Heuvel RM, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders: A systematic review and meta-analysis. JAMA Psychiatry. (2020) 77:265. doi: 10.1001/jamapsychiatry.2019.3986
4. Fontenelle LF, Mendlowicz MV, Versiani M. The descriptive epidemiology of obsessive–compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2006) 30(3):327–37. doi: 10.1016/j.pnpbp.2005.11.001
5. Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. (2010) 15:53–63. doi: 10.1038/mp.2008.94
6. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. (2007) 8:700–11. doi: 10.1038/nrn2201
7. Naze S, Hearne LJ, Roberts JA, Sanz-Leon P, Burgher B, Hall C, et al. Mechanisms of imbalanced frontostriatal functional connectivity in obsessive-compulsive disorder. Brain. (2022) 146(4):1322–7. doi: 10.1093/brain/awac425
8. Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. (2009) 66(11). doi: 10.1001/archgenpsychiatry.2009.152
9. Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. (2006) 16:1508–21. doi: 10.1093/cercor/bhj088
10. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. (1986) 9:357–81. doi: 10.1146/annurev.ne.09.030186.002041
11. Van Den Heuvel OA, Van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol. (2016) 26:810–27. doi: 10.1016/j.euroneuro.2015.12.005
12. Goulet-Kennedy J, Labbe S, Fecteau S. The involvement of the striatum in decision making. Dialogues Clin Neurosci. (2016) 18:55–63. doi: 10.31887/DCNS.2016.18.1/sfecteau
13. Delgado MR. Reward-related responses in the human striatum. Ann New York Acad Sci. (2007) 1104:70–88. doi: 10.1196/annals.1390.002
14. Hiebert NM, Lawrence MR, Ganjavi H, Watling M, Owen AM, Seergobin KN, et al. Striatum-mediated deficits in stimulus-response learning and decision-making in OCD. Front Psychiatry. (2020) 11:13. doi: 10.3389/fpsyt.2020.00013
15. Graybiel AM, Raucht SL. Toward a neurobiology review of obsessive-compulsive disorder. In: Hyman S, editor. The science of mental health, 1st ed. Routledge, New York (2022). p. 119–23. doi: 10.4324/9780203822937-13
16. Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. (2012) 16:43–51. doi: 10.1016/j.tics.2011.11.003
17. Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. (2006) 59:257–64. doi: 10.1002/ana.20742
18. Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. (2005) 18:411–7. doi: 10.1097/01.wco.0000173463.24758.f6
19. Vo K, Rutledge RB, Chatterjee A, Kable JW. Dorsal striatum is necessary for stimulus-value but not action-value learning in humans. Brain. (2014) 137:3129–35. doi: 10.1093/brain/awu277
20. Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making: figure 1. J Neurosci. (2007) 27:8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007
21. Balleine BW. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. (2010) 35(1):48–69. doi: 10.1038/npp.2009.131
22. Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: A resting state fMRI study. Cereb Cortex. (2008) 18:2735–47. doi: 10.1093/cercor/bhn041
23. Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage. (2013) 80:360–78. doi: 10.1016/j.neuroimage.2013.05.079
24. Jones DT, Vemuri P, Murphy MC, Gunter JL, Senjem ML, Machulda MM, et al. Non-stationarity in the “Resting brain’s” Modular architecture. PloS One. (2012) 7:e39731. doi: 10.1371/journal.pone.0039731
25. Chang C, Glover GH. Time–frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage. (2010) 50:81–98. doi: 10.1016/j.neuroimage.2009.12.011
26. Handwerker DA, Roopchansingh V, Gonzalez-Castillo J, et al. Periodic changes in fMRI connectivity. NeuroImage. (2012) 63:1712–9. doi: 10.1016/j.neuroimage.2012.06.078
27. Zhou B, Chen Y, Zheng R, Jiang Y, Li S, Wei Y, et al. Alterations of static and dynamic functional connectivity of the nucleus accumbens in patients with major depressive disorder. Front Psychiatry. (2022) 13:877417. doi: 10.3389/fpsyt.2022.877417
28. Zheng Y, Wu Y, Liu Y, Li D, Liang X, Chen Y, et al. Abnormal dynamic functional connectivity of thalamic subregions in patients with first-episode, drug-naïve major depressive disorder. Front Psychiatry. (2023) 14:1152332. doi: 10.3389/fpsyt.2023.1152332
29. Zheng R, Chen Y, Jiang Y, Zhou B, Li S, Wei Y, et al. Abnormal dynamic functional connectivity in first-episode, drug-naïve adolescents with major depressive disorder. J Neurosci Res. (2022) 100:1463–75. doi: 10.1002/jnr.25047
30. Li C, Xia L, Ma J, Li S, Liang S, Ma X, et al. Dynamic functional abnormalities in generalized anxiety disorders and their increased network segregation of a hyperarousal brain state modulated by insomnia. J Affect Disord. (2019) 246:338–45. doi: 10.1016/j.jad.2018.12.079
31. Yao Z, Liao M, Hu T, Zhang Z, Zhao Y, Zheng F, et al. An effective method to identify adolescent generalized anxiety disorder by temporal features of dynamic functional connectivity. Front Hum Neurosci. (2017) 11:492. doi: 10.3389/fnhum.2017.00492
32. Xue K, Chen J, Wei Y, Chen Y, Han S, Wang C, et al. Altered dynamic functional connectivity of auditory cortex and medial geniculate nucleus in first-episode, drug-naïve schizophrenia patients with and without auditory verbal hallucinations. Front Psychiatry. (2022) 13:963634. doi: 10.3389/fpsyt.2022.963634
33. Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage: Clin. (2014) 5:298–308. doi: 10.1016/j.nicl.2014.07.003
34. Liu F, Wang Y, Li M, Wang W, Li R, Zhang Z, et al. Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure: Dynamic FNC in IGE-GTCS. Hum Brain Mapp. (2017) 38:957–73. doi: 10.1002/hbm.23430
35. Li R, Liao W, Yu Y, Chen H, Guo X, Tang Y, et al. Differential patterns of dynamic functional connectivity variability of striato–cortical circuitry in children with benign epilepsy with centrotemporal spikes. Hum Brain Mapp. (2018) 39:1207–17. doi: 10.1002/hbm.23910
36. Ding Z, Ding Z, Chen Y, Lv D, Li T, Shang T, et al. Decreased gray matter volume and dynamic functional alterations in medicine-free obsessive-compulsive disorder. BMC Psychiatry. (2023) 23:289. doi: 10.1186/s12888-023-04740-w
37. Teng C, Zhang W, Zhang D, Shi X, Wu X, Qiao H, et al. Association between clinical features and decreased degree centrality and variability in dynamic functional connectivity in the obsessive–compulsive disorder. NeuroImage: Clin. (2024) 44:103665. doi: 10.1016/j.nicl.2024.103665
38. Bernstein GA, Cullen KR, Harris EC, Conelea CA, Zagoloff AD, Carstedt PA, et al. Sertraline effects on striatal resting-state functional connectivity in youth with obsessive-compulsive disorder: A pilot study. J Am Acad Child Adolesc Psychiatry. (2019) 58:486–95. doi: 10.1016/j.jaac.2018.07.897
39. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The yale-brown obsessive compulsive scale. Arch Gen Psychiatry. (1989) 46(11):1006–11. doi: 10.1001/archpsyc.1989.01810110048007
40. Yan C-G, Wang X-D, Zuo X-N, Zhang Y-F, Liu G. DPABI: data processing & Analysis for (Resting-state) brain imaging. Neuroinformatics. (2016) 14:339–51. doi: 10.1007/s12021-016-9299-4
41. Liao W, Wu G-R, Xu Q, Ji G-J, Zhnag Z, Zhang Y-F, et al. DynamicBC : A MATLAB toolbox for dynamic brain connectome analysis. Brain Connectivity. (2014) 4:780–90. doi: 10.1089/brain.2014.0253
42. Leonardi N, Van De Ville D. On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage. (2015) 104:430–6. doi: 10.1016/j.neuroimage.2014.09.007
43. Li J, Duan X, Cui Q, Chen H, Liao W. More than just statics: temporal dynamics of intrinsic brain activity predicts the suicidal ideation in depressed patients. psychol Med. (2019) 49:852–60. doi: 10.1017/S0033291718001502
44. Ffytche DH, Catani M. Beyond localization: from hodology to function. Philos Trans R Soc B: Biol Sci. (2005) 360:767–79. doi: 10.1098/rstb.2005.1621
45. Kitada R, Johnsrude IS, Kochiyama T, Lederman SJ. Brain networks involved in haptic and visual identification of facial expressions of emotion: An fMRI study. NeuroImage. (2010) 49:1677–89. doi: 10.1016/j.neuroimage.2009.09.014
46. De Gelder B, Tamietto M, Pegna AJ, Van Den Stock J. Visual imagery influences brain responses to visual stimulation in bilateral cortical blindness. Cortex. (2015) 72:15–26. doi: 10.1016/j.cortex.2014.11.009
47. Stern ER, Muratore AF, Taylor SF, Abelson JL, Hof PR, Goodman WK. Switching between internally and externally focused attention in obsessive-compulsive disorder: Abnormal visual cortex activation and connectivity. Psychiatry Research: Neuroimaging. (2017) 265:87–97. doi: 10.1016/j.pscychresns.2016.08.006
48. Gonçalves ÓF, Marques TR, Lori NF, Sampaio A, Branco MC. Obsessive–compulsive disorder as a visual processing impairment. Med Hypotheses. (2010) 74:107–9. doi: 10.1016/j.mehy.2009.07.048
49. Kesler/West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ. Neural substrates of facial emotion processing using fMRI. Cogn Brain Res. (2001) 11:213–26. doi: 10.1016/S0926-6410(00)00073-2
50. Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. (2008) 9:856–69. doi: 10.1038/nrn2478
51. Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. (2001) 11:663–72. doi: 10.1016/S0959-4388(01)00266-5
52. Nguyen VT, Breakspear M, Cunnington R. Reciprocal interactions of the SMA and cingulate cortex sustain premovement activity for voluntary actions. J Neurosci. (2014) 34:16397–407. doi: 10.1523/JNEUROSCI.2571-14.2014
53. Norman LJ, Taylor SF, Liu Y, Radua J, Chye Y, De Wit SJ, et al. Error processing and inhibitory control in obsessive-compulsive disorder: A meta-analysis using statistical parametric maps. Biol Psychiatry. (2019) 85:713–25. doi: 10.1016/j.biopsych.2018.11.010
54. Tomiyama H, Murayama K, Nemoto K, Tomita M, Hasuzawa S, Mizobe T, et al. Increased functional connectivity between presupplementary motor area and inferior frontal gyrus associated with the ability of motor response inhibition in obsessive–compulsive disorder. Hum Brain Mapp. (2022) 43:974–84. doi: 10.1002/hbm.25699
55. Tadayonnejad R, Corlier J, Valles TE, Citrenbaum C, Matthews C, Einstein E, et al. Safety and efficacy of targeting the supplementary motor area with double-cone deep transcranial magnetic stimulation vs figure-eight coil in treatment of obsessive-compulsive disorder with comorbid major depressive disorder. J Psychiatr Res. (2024) 179:295–9. doi: 10.1016/j.jpsychires.2024.09.026
56. Berlim MT, Neufeld NH, Van Den Eynde F. Repetitive transcranial magnetic stimulation (rTMS) for obsessive–compulsive disorder (OCD): An exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res. (2013) 47:999–1006. doi: 10.1016/j.jpsychires.2013.03.022
57. Rostami R, Kazemi R, Jabbari A, Madani AS, Rostami H, Taherpour MA, et al. Efficacy and clinical predictors of response to rTMS treatment in pharmacoresistant obsessive-compulsive disorder (OCD): a retrospective study. BMC Psychiatry. (2020) 20:372. doi: 10.1186/s12888-020-02769-9
58. Péran P, Démonet J-F, Cherubini A, Carbebat D, Caltagirone C, Sabatini UM. Mental representations of action: The neural correlates of the verbal and motor components. Brain Res. (2010) 1328:89–103. doi: 10.1016/j.brainres.2010.02.082
59. Schutter DJLG, Van Honk J. The cerebellum on the rise in human emotion. Cerebellum. (2005) 4:290–4. doi: 10.1080/14734220500348584
60. Schmahmann JD. The cerebellum and cognition. Neurosci Lett. (2019) 688:62–75. doi: 10.1016/j.neulet.2018.07.005
61. Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. (2012) 11:352–65. doi: 10.1007/s12311-011-0260-7
62. Qiu L, Fu X, Wang S, Tang Q, Chen X, Cheng L, et al. Abnormal regional spontaneous neuronal activity associated with symptom severity in treatment-naive patients with obsessive-compulsive disorder revealed by resting-state functional MRI. Neurosci Lett. (2017) 640:99–104. doi: 10.1016/j.neulet.2017.01.024
63. Zhang H, Wang B, Li K, Wang X, Li X, Zhu J, et al. Altered functional connectivity between the cerebellum and the cortico-striato-thalamo-cortical circuit in obsessive-compulsive disorder. Front Psychiatry. (2019) 10:522. doi: 10.3389/fpsyt.2019.00522
64. Meng Z, Zhang Z, Fan Q, Li Y. (2018). Altered fractional amplitude of low frequency fluctuations in unmedicated female patients with obsessive-compulsive disorder, in: 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI: IEEE. pp. 1144–7. doi: 10.1109/EMBC.2018.8512490
65. Nabeyama M, Nakagawa A, Yoshiura T, Nakao E, Togao O, Yoshizato C, et al. Functional MRI study of brain activation alterations in patients with obsessive–compulsive disorder after symptom improvement. Psychiatry Research: Neuroimaging. (2008) 163:236–47. doi: 10.1016/j.pscychresns.2007.11.001
66. Lv D, Ou Y, Chen Y, Yang R, Zhong Z, Jia C, et al. Increased cerebellar–default-mode network connectivity at rest in obsessive–compulsive disorder. Eur Arch Psychiatry Clin Neurosci. (2020) 270:1015–24. doi: 10.1007/s00406-019-01070-5
67. Xu T, Zhao Q, Wang P, Fan Q, Chen J, Zhang H, et al. Altered resting-state cerebellar-cerebral functional connectivity in obsessive-compulsive disorder. psychol Med. (2019) 49:1156–65. doi: 10.1017/S0033291718001915
68. Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. (2014) 75:595–605. doi: 10.1016/j.biopsych.2013.10.021
69. Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci. (2018) 19:338–50. doi: 10.1038/s41583-018-0002-7
70. Chen CH, Fremont R, Arteaga-Bracho EE, Khodakhah K. Short latency cerebellar modulation of the basal ganglia. Nat Neurosci. (2014) 17:1767–75. doi: 10.1038/nn.3868
71. Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci. (2010) 107:8452–6. doi: 10.1073/pnas.1000496107
72. Xiao L, Bornmann C, Hatstatt-Burklé L, Scheiffele P. Regulation of striatal cells and goal-directed behavior by cerebellar outputs. Nat Commun. (2018) 9:3133. doi: 10.1038/s41467-018-05565-y
73. Northoff G, Heinzel A, De Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage. (2006) 31:440–57. doi: 10.1016/j.neuroimage.2005.12.002
74. Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. (2012) 16:584–92. doi: 10.1016/j.tics.2012.10.008
75. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann New York Acad Sci. (2008) 1124:1–38. doi: 10.1196/annals.1440.011
76. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. (2006) 129:564–83. doi: 10.1093/brain/awl004
77. Thorsen AL, Hagland P, Radua J, Mataix-Cols D, Kvale G, Hansen B, et al. Emotional processing in obsessive-compulsive disorder: A systematic review and meta-analysis of 25 functional neuroimaging studies. Biol Psychiatry: Cogn Neurosci Neuroimaging. (2018) 3:563–71. doi: 10.1016/j.bpsc.2018.01.009
78. Rotge J-Y, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, et al. Provocation of obsessive–compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J Psychiatry Neurosci. (2008) 33(5):405–12.
79. Viard A, Flament MF, Artiges E, Dehaene S, Naccache L, Cohen D, et al. Cognitive control in childhood-onset obsessive–compulsive disorder: a functional MRI study. Psychol Med. (2005) 35:1007–17. doi: 10.1017/S0033291704004295
80. Viol K, Aas B, Kastinger A, Kronbichler M, Schöller H, Reiter EM, et al. Individual OCD-provoking stimuli activate disorder-related and self-related neuronal networks in fMRI. Psychiatry Research: Neuroimaging. (2019) 283:135–44. doi: 10.1016/j.pscychresns.2018.12.008
81. De Vries FE, De Wit SJ, Cath DC, Van Der Werf YD, Van Der Borden V, Van Rossum TB, et al. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. (2014) 76:878–87. doi: 10.1016/j.biopsych.2013.11.021
82. Fajnerova I, Gregus D, Francova A, Noskova E, Koprivova J, Stopkova P, et al. Functional connectivity changes in obsessive–compulsive disorder correspond to interference control and obsessions severity. Front Neurol. (2020) 11:568. doi: 10.3389/fneur.2020.00568
83. Ye Q, Zhang Z, Sun W, Fan Q, Li Y. Disrupted functional connectivity of precuneus subregions in obsessive-compulsive disorder. NeuroImage: Clin. (2021) 31:102720. doi: 10.1016/j.nicl.2021.102720
84. Van Der Straten A, Van Leeuwen W, Denys D, Van Marle H, Van Wingen G. The effect of distress on the balance between goal-directed and habit networks in obsessive-compulsive disorder. Trans Psychiatry. (2020) 10:73. doi: 10.1038/s41398-020-0744-7
85. Marusak HA, Calhoun VD, Brown S, Crespo LM, Sala-Hamrick K, Gotlib IH, et al. Dynamic functional connectivity of neurocognitive networks in children. Hum Brain Mapp. (2017) 38:97–108. doi: 10.1002/hbm.23346
86. Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited. Neurosci Biobehav Rev. (2008) 32:525–49. doi: 10.1016/j.neubiorev.2007.09.005
87. Klein-Flügge MC, Bongioanni A, Rushworth MFS. Medial and orbital frontal cortex in decision-making and flexible behavior. Neuron. (2022) 110:2743–70. doi: 10.1016/j.neuron.2022.05.022
88. Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Prog Neurobiol. (2008) 86:216–44. doi: 10.1016/j.pneurobio.2008.09.001
89. Lopatina N, McDannald MA, Styer CV, Peterson JF, Sadacca BF, Cheer JF, et al. Medial orbitofrontal neurons preferentially signal cues predicting changes in reward during unblocking. J Neurosci. (2016) 36:8416–24. doi: 10.1523/JNEUROSCI.1101-16.2016
90. Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. (1999) 398:704–8. doi: 10.1038/19525
91. Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. (2005) 6:691–702. doi: 10.1038/nrn1747
92. Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: Decision-making and reversal learning. Brain Cogn. (2004) 55:41–53. doi: 10.1016/S0278-2626(03)00284-7
93. Xia J, Fan J, Du H, Liu W, Li S, Zhu J, et al. Abnormal spontaneous neural activity in the medial prefrontal cortex and right superior temporal gyrus correlates with anhedonia severity in obsessive-compulsive disorder. J Affect Disord. (2019) 259:47–55. doi: 10.1016/j.jad.2019.08.019
94. Ping L, Su-Fang L, Hai-Ying H, Zhang-Ye D, Jia L, Zhi-Hua G, et al. Abnormal spontaneous neural activity in obsessive-compulsive disorder: A resting-state functional magnetic resonance imaging study. PloS One. (2013) 8:e67262. doi: 10.1371/journal.pone.0067262
95. Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol Psychiatry. (2007) 62:901–9. doi: 10.1016/j.biopsych.2006.12.007
96. Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: A functional magnetic resonance imaging study. Biol Psychiatry. (2005) 57:901–10. doi: 10.1016/j.biopsych.2004.12.039
97. Remijnse PL, Nielen MMA, Van Balkom AJLM, Cath DC, Van Oppen P, Uylings HBM, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. (2006) 63:1225. doi: 10.1001/archpsyc.63.11.1225
98. Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, Del Campo N, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. (2008) 321:421–2. doi: 10.1126/science.1154433
99. Abe Y, Sakai Y, Nishida S, Nakamae T, Yamada K, Fukui K, et al. Hyper-influence of the orbitofrontal cortex over the ventral striatum in obsessive-compulsive disorder. Eur Neuropsychopharmacol. (2015) 25:1898–905. doi: 10.1016/j.euroneuro.2015.08.017
100. Kopell BH, Greenberg BD. Anatomy and physiology of the basal ganglia: Implications for DBS in psychiatry. Neurosci Biobehav Rev. (2008) 32:408–22. doi: 10.1016/j.neubiorev.2007.07.004
Keywords: obsessive-compulsive disorder, striatum, caudate, functional magnetic resonance imaging, dynamic functional connectivity
Citation: Shi W, Tian Y, Yang H, Guo H, Wen B, Liu Z, Zhang Y, Han S and Cheng J (2025) Abnormal static and dynamic functional connectivity of striatal subregions in patients with obsessive-compulsive disorder. Front. Psychiatry 16:1529983. doi: 10.3389/fpsyt.2025.1529983
Received: 18 November 2024; Accepted: 30 January 2025;
Published: 21 February 2025.
Edited by:
Yoshiyuki Hirano, Chiba University, JapanReviewed by:
Debo Dong, Southwest University, ChinaJie Yang, Central South University, China
Xiaochen Zhang, Shanghai Jiao Tong University, China
Na Liu, Nanjing Brain Hospital Affiliated to Nanjing Medical University, China
Zhitao Gao, Chinese PLA General Hospital, China
Zibin Yang, Guangdong Second Provincial General Hospital, China
Copyright © 2025 Shi, Tian, Yang, Guo, Wen, Liu, Zhang, Han and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoqiang Han, c2hhb3FpYW5naGFuQDE2My5jb20=; Jingliang Cheng, ZmNjY2hlbmdqbEB6enUuZWR1LmNu
 Wenqing Shi1
Wenqing Shi1 Huirong Guo
Huirong Guo Baohong Wen
Baohong Wen Zijun Liu
Zijun Liu Yong Zhang
Yong Zhang Shaoqiang Han
Shaoqiang Han Jingliang Cheng
Jingliang Cheng