- 1Institute for Drug and Alcohol Studies, Virginia Commonwealth University, Richmond, VA, United States
- 2Department of Cognitive Psychology, Institute for Cognitive Science Studies (ICSS), Tehran, Iran
- 3Department of Cognitive Science and Psychology, New Bulgarian University, Sofia, Bulgaria
- 4Metacognium LLC, Austin, TX, United States
- 5Department of Psychiatry, University of Minnesota, Minneapolis, MN, United States
- 6Department of Psychiatry, Virginia Commonwealth University, Richmond, VA, United States
Over the past few decades, our understanding of substance use disorders (SUD) has been reshaped by evidence from neuroscience, which suggests that SUD are characterized by specific neuromarkers that transcend traditional diagnostic boundaries and act as pre-diagnostic markers that could be targeted through preventive attempts. Connectivity-based neuromarkers or brain networks have emerged as a promising framework, providing new insights into the neurocognitive mechanisms of SUD. Utilizing this data-driven framework assists prevention and intervention developers in offering a non-judgmental insight for adolescents regarding the potential vulnerability of neurocognitive systems to continued substance use. Given the importance of such awareness, this paper proposes a neural network-informed approach based on research domain criteria (RDoC) to characterize the content of neuroscience-informed psychoeducation designed for SUD. Furthermore, we argue that various features related to content and structure need to be considered when developing such interventions delivered through digital platforms (e.g., apps and websites). Finally, we introduce a theory-driven app called “NIPA”, developed with the aim of increasing adolescents’ awareness and resilience to the effects of drugs and other emotional triggers on brain and cognitive functions.
1 Introduction
Psychoeducation has been widely applied for different health related conditions (mental and medical disorders) in both clinical and community settings. Broadly speaking, psychoeducation is a process of teaching clients specific and general information about their illness and treatment (1). Specific information provides more detailed illness-related information, e.g., diagnosis and therapeutic trajectories, while general information includes more translational and general content, such as problem-solving skills and healthy life-style (1). Since the 1980s, when Anderson et al. introduced the term psychoeducation (2), translational research has reshaped the psychoeducational regimens and transferred findings from basic science to more practical level. By growing the knowledge of neuroscience in the context of mental illness, our understanding of risk factors, precursors, neural correlates, and therapeutic approaches has been much improved and the field of neuroscience-informed psychoeducation has emerged. “Neuroeducation”, “internal education”, “neuroscience literacy”, “neuroliteracy” and “Brain talk” are other relevant terms used to describe a class of interventions intended to educate individuals and reframe their perceptions of mental illness using neurobiological knowledge (3–5).
The application of psychoeducation in the treatment of substance use disorders (SUD) can be traced back to the 1940s, when alcoholism was acknowledged as an independent public-health problem and “the mantle of stigma covered the subject” (6). Early interventions targeted primarily treatment-seeking individuals who were at later stages of the addiction cycle characterizing severe SUD, rather than earlier stages such as substance experimentation or problem use (7, 8). Years later, psychoeducational interventions advanced by going beyond therapeutic interventions and getting applied as a tool for addiction prevention, particularly for children and adolescents. The first flashes of a psychoeducational program for children appeared in a commercial cartoon produced by the Partnership for a Drug-Free America (PDFA) in response to a cartoon that tricked kids to smoke. The PDFA’s cartoon featured the effects of drugs on the brain, depicting them as an egg dropping into a frying pan (9). Another example of such “Scare tactics” was the “Just Say No” campaign, known as Drug Abuse Resistance Education (D.A.R.E.), which aimed to teach students about the dangers of substance use by sending uniformed cops into the schools (10). These zero tolerance campaigns prevalent between the 1970s and 1980s were unsuccessful in reducing substance use behaviors.
Given the failure of such fear-based deterrence attempts, a new wave of educational approaches has emerged, characterized by non-judgmental and informative content that encourages students to make informed decisions about substance use (10). These educational approaches largely focused on knowledge and skill development in prevention, early intervention, and harm reduction formats. The knowledge includes information regarding the harmful effects of substance use and corrects normative expectations, while the skills training builds personal and social competencies, as well as establishes refusal skills (11). Accordingly, several successful educational interventions have been developed and tested in school settings; such as Red Frogs (12), Just Say Know Prevention Program (10), Unplugged (13), Life Skills Training (11), Project Towards No Drug Abuse (14), Reconnecting Youth (RY) (15), School Health and Alcohol Harm Reduction Project (SHAHRP) (16), Project ALERT (17) and ALERT Plus (18), Reasoning and Rehabilitation V2 (R&R2) program (19).
Over the past few years, interventions for adolescent have evolved from their initial focus on classic drug-related knowledge (e.g., the biological impact, including short- and long-term consequences of substance use, substance use standards, and prevalence) and skills training to leveraging adolescents’ curiosity about the brain’s involvement in substance use (20). The “Seductive Allure of Neuroscience” (SANE) (20) for adolescents has revealed a unique opportunity to integrate neuroscience knowledge with substance use prevention. The SANE proposes that psychological phenomena are more appealing and health messages more persuasive for adolescents when they are accompanied with brain-related information. For example, a recent harm reduction program, the ‘Respect Your Brain’, developed by Debenham and colleagues (2022), aimed to leverage neuroscience literacy of young people using a series of short animations (21). They found that watching brain-related animations could effectively engage people with the topics and positively change their attitudes towards the brain and substance use. Another program (22), which applied neuroscience to improve drug-based knowledge and teach practical coping skills is the ‘Illicit Project’. This program covered different aspects of the neuroscience of SUD in three modules including Alcohol and the Developing Brain; MDMA, Cannabis Use, Harm Reduction; and Mental Health and Wellbeing. Evidence from the pilot study indicates lasting effects (6 months) of the program on reducing drug and alcohol use compared to the control group who received health education. Therefore, it seems that knowledge about the role of the brain in the etiology and maintenance of SUD could lead to meaningful change in thoughts and behaviors (23). Moreover, given the high potential for learning and flexibility during adolescence, young people can benefit from preventive interventions to reduce the probability of SUD later in life (24, 25).
On the other hand, the rapid development of digital health technologies (e.g., mobile applications, websites), has introduced tremendous changes in the field of designing behavioral interventions and provides numerous opportunities for personalizing interventions and increasing their feasibility and accessibility. Digital platforms offer therapists and educators the chance to improve the quality of therapeutic and training services by incorporating unique features such as multimedia content, personalized feedback, and interactive elements. These platforms also allow services to be delivered affordably, regardless of time and location, which greatly enhances their scalability. Using digital health interventions also warrants the replicability and fidelity of the intervention and removes the barriers of differences in therapists’ skills and knowledge. Due to the popularity of digital health technologies, substantial progress has been made towards developing e-health preventive interventions (delivered via internet, computers, tablets, mobile technology, or tele-health) in the recent years (26–31). These interventions that typically offer a set of normative education and life skills training provide good empirical evidence and promising results about the efficacy of digital health in preventing or mitigating substance use in adolescents. Moreover, digital preventive interventions provide an opportunity for young users who prefer to remain anonymous during the training course and so reduce the stigma and embarrassment that they may perceive due to their needs for seeking help. This issue is complicated even further in adolescents, as they are highly impacted by stigma, and stigma can in turn create a major barrier to service seeking (32).
Acknowledging previous works, the present study aims to describe a conceptual framework for substance use prevention grounded in neuroscience knowledge and brain networks. Then, we propose key recommendations for developing a digital neuroscience-informed psychoeducation aimed at substance use prevention and introduce a sample educational app, termed ‘NIPA,’ as one of the novel tools developed to improve adolescents’ metacognitive awareness and enhance their resilience against SUD. Additionally, we provide preliminary feasibility and acceptability data regarding the app in a sample of college students and delineate the next steps for future studies.
2 Adolescents’ resilience against addiction: neural networks perspective
A large body of research has implicated the mechanisms involved in substance use vulnerability in adolescents. Family history, genetic and environmental factors, and neurodevelopmental adaptations are risk factors known to be associated with increased vulnerability in young people (33). Moreover, SUD is more probable in the presence of cognitive immaturities, particularly in memory and learning, goal-directed behaviors, decision making and impulse control which are common during adolescence (34, 35). According to the Dual Systems Model and the Maturational Imbalance Model, adolescent risk-taking results from a temporary imbalance between two neurobiological systems: the subcortical socioemotional system, which is responsive to emotion, reward, and novelty, and the prefrontal cognitive control system, which guides controlled action, planning, and decision-making (36). A key assumption of both models is that the socioemotional system (System 1) develops faster and earlier in adolescence than the cognitive control system (System 2). As a result, adolescents are presumed to be particularly vulnerable to high-risk behaviors and tend to pursue quicker rewards (e.g., instant pleasure, peer acceptance) (37, 38). Consequently, during adolescence neural networks may respond differently to emotionally salient stimuli, such as substances. For example, in a hypothetical scenario, a young person who is new to high school and unable to find close friends may be more vulnerable to experiment with drugs, in the hope of finding friends and being accepted by a group of peers. Initial recreational and exploratory substance use leads to neurobiological changes that contribute to repeated and problematic use. As a result of these alterations and subsequent neuroplasticity, brain networks show abnormal functional reorganizations, leading to disrupted cognitive functions. However, a balanced functioning of these two systems could underpin a trait termed Resilience.
In general, resilient people are characterized by their ability to recover quickly after experiencing environmental risk or adversity and by adaptively coping with negative events and emotions. Resilience can be inferred from resistance to maladaptive behaviors when individuals face tragic or life-threatening events (39). A growing body of neuroscientific research aims to elucidate the brain networks underlying resilience and connect them with related cognitive processes (24, 40). However, while relatively few studies have focused on the specific brain networks associated with resilience or vulnerability against SUD, growing evidence highlights the importance of specific neural networks in the progression of SUD (25, 35). These networks include the Attention Network, Default Mode Network, Salience Network, and the Executive Control Network. Each of these networks is involved in specific cognitive functions and their balanced interactions could enhance one’s ability to overcome emotional adversity, and resolve the barriers in favor of positive outcomes. One of the conceptual models that we have suggested to explain the underlying neurocognitive mechanisms underlying resilience is the “EASICoRe” model (41). This conceptual model defines the major cognitive target processes involved in the dynamic response to drug-related cues. According to this model, once vulnerable individuals are exposed to an Environmental trigger (both internal and external cues), their Attentional resources are selectively allocated to process different aspects of the trigger (e.g., emotional, physical), while their Memory is biased toward recollecting relevant drug-related memories. By integrating the information from attention and memory, the evaluation system starts processing the Salience of incoming information and comparing them with subjective goals/values based on available appraisal schemas. At this moment, various somatic signals originate from within the body transferring information related to bodily experiences (e.g., heart rate, respiration rates). These Interoceptive signals contribute to emotional/appetitive experience and affect decision-making particularly under risk and uncertainty. Followed by evaluation, Inhibitory Control may be activated to control impulsive desires and habits and direct behavior Response toward more goal-oriented action. From the perspective of neuroscience, each of these cognitive functions is correlated with an activation of a distinct brain network. (Figure 1). In the following section, we describe these four networks and their dynamic interactive roles in building resilience against addiction.
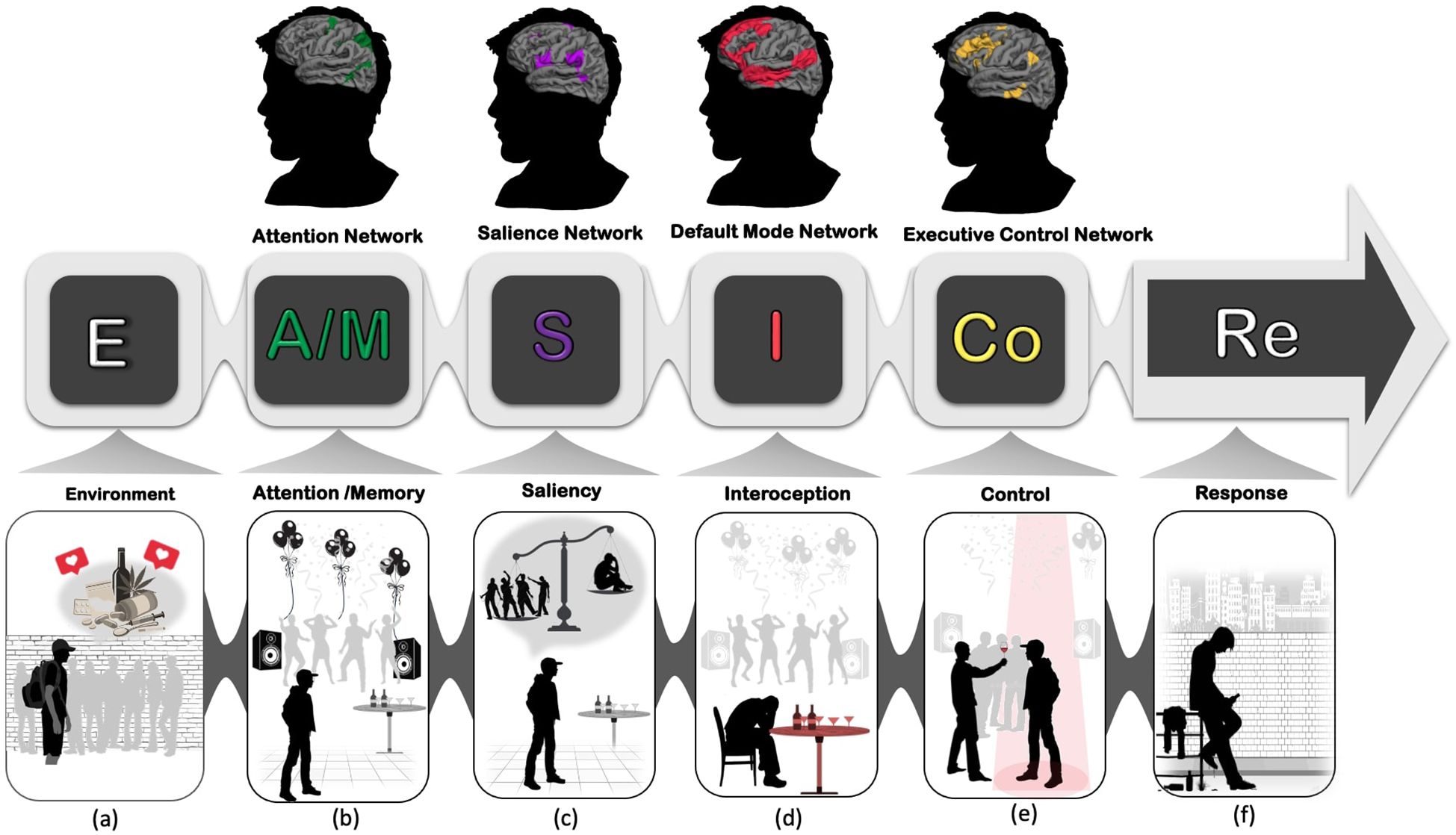
Figure 1. EASICoRe model as a neuroscience-informed conceptual framework, indicates different neurocognitive mechanisms involved in substance use vulnerability in adolescents. (A) Environment: Adolescents enter high school, experiencing increased peer pressure and exposure to environmental cues such as alcohol and drugs, along with a desire for social approval and being accepted into a group; (B) Attention/Memory: Gradually, adolescents’ attentional systems become biased towards drug/alcohol-related cues, and hedonic drug-related memories are consolidated in their memory; (C) Salience: Repeated exposure to these cues, leads the brain to overweight using drugs and alcohol as a prerequisite for remaining in the peer group over the fear of rejection and loneliness; (D) Interoception: Interoceptive signals such as heart rate and skin temperature are misinterpreted as a sign of urge to use drugs/alcohol; (E) Control: Executive control over inhibiting impulsive signals toward drug/alcohol use becomes increasingly challenging; (F) Response: Drug-taking behavior is reinforced by the hedonic pleasure experienced each time individuals use drugs/alcohol.
2.1 Attention Network (AN)
Attention regulation refers to the process by which individuals control how their attention is allocated toward specific stimuli while ignoring irrelevant ones. This regulatory process is crucial for human survival, as our attentional resources are capacity-limited, requiring us to be selective when faced with competing information (42). Individuals can regulate their attention through two distinct but intertwined systems: the dorsal attention network (DAN) and the ventral attention network (VAN) (43). DAN, which involves the frontal eye fields (FEF), the superior parietal lobules (SPL), and the inferior parietal sulci (IPS), is responsible for goal-directed processing and top-down, voluntary attentional allocation. In contrast, VAN, thought to comprise the right temporoparietal junction (TPJ) and ventral frontal cortex (VFC), is involved in detecting unexpected, unattended, or salient stimuli. This latter network is responsible for bottom-up allocation and is known to be involuntary (44, 45). Poor attention regulation, exhibited by changes in the functional architecture of DAN and VAN, is prevalent among substance users (46), who often show reduced ability to deliberately switch their attention away from drug-related stimuli and to ignore the thoughts and emotions that tempt them to focus on drug use.
2.2 Default Mode Network (DMN)
Addiction is a pathological learning disorder in which many individuals with SUD are unable to successfully retrieve the experienced negative consequences of their past actions, reflect on them, and plan for a drug-free future (47). Findings from resting-state functional connectivity neuroimaging studies have identified the role of the Default Mode Network (DMN) in mental time travel, where individuals move back and forth between the past, present, and future to reflect on their autobiographical experiences and predict possible future situations. The DMN is also involved in self-awareness and interoceptive processes which allow individuals to accurately perceive signals received from internal organs (i.e., heart, skin, muscle, stomach) and interpret them as a sign of specific emotional state (i.e., anxiety, craving, fatigue) (48–50). This network involves three main components: a midline subsystem (including the medial prefrontal cortex, posterior cingulate cortex, and precuneus), a medial temporal subsystem (including the medial temporal lobe, medial parietal cortex, inferior parietal lobe, and ventromedial prefrontal cortex), and a dorsal medial prefrontal subsystem (including the dorsal medial prefrontal cortex, temporoparietal junction, and lateral temporal cortex) (51). Impaired internally-oriented cognition, including memory retrieval, mental imagery, and prospective thinking, may lead to increased vulnerability to continued substance use in adolescents. When vulnerable individuals are exposed to drug-related cues, they might fail to mentally imagine the consequences of their drug use based on previous memories and, as a result, may be unable to plan protective actions. Moreover, disruption of the DMN contributes to poor self-awareness and inaccurate interoception, which could lead to maladaptive responses, such as drug use, to regain homeostatic balance.
2.3 Salience Network (SN)
SUD is characterized by heightened salience attributed to any form of drug-related cues across various sensory modalities (e.g., the sight of a lighter, the smell of a cigarette, the sound of rolling) and by an expectation of greater reward from obtaining them (52). A set of brain regions including the amygdala, the anterior insula and the dorsolateral cingulate cortex are involved in detecting salient competing stimuli, processing reward, interoception, motivation, and emotion (53, 54). The other critical role of the SN is in risky decision making, in which individuals prefer smaller immediate rewards rather than larger but delayed ones (55). Disrupted activity of the SN is associated with increased impulsivity and emotional reactivity. Lack of premeditation before using drugs is an example of impulsivity related to the activity of the SN.
2.4 Executive Control Network (ECN)
Compromised executive functions such as response inhibition, attention, working memory, planning, problem solving and flexibility, are known to be key cognitive factors involved in substance use initiation and maintenance (34). Impairments in these functions are associated with the ECN, a task-oriented brain network underlying various cognitive processes involved in decision-making and self-regulation (48). The ECN includes the dorsolateral prefrontal cortices, dorsomedial prefrontal cortex, inferior parietal lobule, anterior–superior posteromedial cortices, and medial temporal gyrus (56). One of the key functions of the ECN that is often impaired in substance users is cognitive flexibility. In clinical and experimental studies, poor flexibility is manifested by perseveration, characterized by the failure to change strategies in accordance with feedback (57).
According to the EASICoRe model, vulnerable adolescents become hypersensitive to drug-related cues (SN), fail to control their attentional resources in favor of ignoring these cues (DAN, VAN), exhibit weakened inhibition to regulate their behavior against substance use (ECN) and, ultimately, are less sensitive and less able to recall the experienced negative consequences of using drugs (DMN). In addition to their individual effects, these networks can interact with each other, yielding more complex phenotypes. For example, impaired decision-making in substance users could be due to many different reasons associated with aberrant functioning in distinct neural networks. It could be related to attentional and memory difficulties in remembering previous choices or consequences of actions (DAN, VAN). It could also be related to motivational mechanisms, which bias choice behavior such as hypersensitivity or hyposensitivity to reward and punishment (SN), or to cognitive control and/or cognitive flexibility difficulties (ECN), evidenced by perseveration on substance use despite potentially fatal negative consequences. These complex phenotypes may portend different biotypes of SUD and other psychiatric disorders which, in turn, may be targeted by more personalized interventions (58–61).
Therefore, neuroscience can inform adolescents about how continued substance use may alter brain functions and structures through a simplified and comprehensive educational format. To make such interventions more engaging and interactive, digital platforms could offer various opportunities to support this translation. In the following section, we discuss this novel approach in the field of prevention.
3 Digital neuroscience-informed psychoeducation for addiction prevention
Providing educational tools about substance use and addiction to adolescents is challenging, as they tend to be more influenced by their peers’ beliefs and may find it difficult to trust educational content, which traditionally tends to be stigmatizing and fear-based. The growth of the field of addiction neuroscience is a novel and promising path that helps prevention scientists translate the complex science of the brain into comprehensible materials. This brain-derived education that capitalizes on the SANE (20) phenomenon particularly when delivered via a digital platform, captivates younger audiences and facilitates dialogue between them and scientists who view addiction as a brain disorder rather than a moral or criminal issue (62). To facilitate the development of such digital educational intervention, we summarize the most important considerations in the following section in terms of content and structure.
3.1 Content
3.1.1 Science-delivered education
Adolescents trust more educational content which is evidence-based, non-stigmatizing and simple to comprehend. Using brain-based education that focuses on brain structure and function seems to be non-judgmental and engaging for young people. Within therapeutic settings, growing evidence suggests that providing patients with biological explanations of their disorders can reduce self-stigmatizing attitudes and potentially remove the mental barriers to recovery (23).
3.1.2 Familiarity
To persuade adolescents about the potential harm of drug use on the brain, symptoms should be explained in terms of concrete and tangible examples (e.g. vignettes), rather than abstract concepts, and clarified with illustrations of how they might interfere with daily functioning. For instance, how attention instability could affect productivity in studying.
3.1.3 Gamified cognitive exercises
Besides the educational facts and conceptual information regarding cognitive functions, offering cognitive games to increase individuals’ insight into the mechanisms behind these functions is particularly useful. Once individuals play these games, they can clearly understand how specific cognitive functions may be engaged both in performing the game and in activities of daily living.
3.1.4 Applied cognitive strategies
The feasibility of the provided brain strategies is evaluated through their applicability in the context of real life. Convenient, available, and simple strategies may be perceived as meaningful and relevant, encouraging individuals to adopt and maintain them in their daily lives.
3.2 Structure
3.2.1 Length of training session
A key factor in developing educational content, particularly for digital platforms, is the length of the training sessions. Due to our limited attention span, educational sessions that exceed 20-25 minutes might not contribute to efficient learning (63). Therefore, it is suggested to break down the length of the educational sessions into shorter episodes, separated by breaks or entertaining activities.
3.2.2 Multimedia content
Integrating various content modules, including animations, cartoons, text, games, videos and music, could enhance the learning experience and user engagement. For example, using a game-based approach to design brain exercises may be more suitable for adolescents and young adults, who are generally active game players and more familiar with such contents (64). Using cartoons could also strengthen the effectiveness of key messages as cartoons grab attention, facilitate memory, amplify self-awareness, boost self-affirmation, and provide useful decision-making heuristics and means for emotion regulation and mental time travel (8).
3.2.3 Self-assessment
A key phase in the learning process is self-evaluation. Educational interventions aimed at enhancing individual knowledge may have more lasting effects by allowing users to assess their own knowledge and learning (65).
3.2.4 Language
Using concise, digestible language and simple metaphors of well-known concepts such as “spotlight” for “attention”, could help bridge the gap between science and practice for adolescents. This language can facilitate engagement with the content while introducing scientific terminology, and allow young people to discuss addiction without any resistance. They can also use this shared language to talk with their counselor or therapist to have more effective communication (23).
4 NIPA example: neuroscience-informed psychoeducation for addiction
Based on the theoretical background on the role of neural networks in substance use vulnerability among adolescents, a mobile application referred to as the ‘Neuroscience-Informed Psychoeducation for Addiction (NIPA)1 was developed as a metacognitive awareness program to promote resilience in the face of emotional triggers, particularly drugs and alcohol. The program delivers psychoeducational content in four 20-minute-long sessions, including neuroscience-based education and cognitive games and training. Once individuals install the application on their mobile devices, the first session is unlocked, granting access to the full content. All sessions follow a similar structure, which includes the following sections:
4.1 Introduction (Knowledge)
Each session begins with an animation depicting a specific cognitive problem (attention and concentration, memory, flexibility and inhibition, impulsivity and decision making). Subsequently, individuals are asked about their personal experience with the cognitive problem(s).
4.2 Games (Practice)
Following the introduction and exploration of specific cognitive functions and difficulties, individuals play the first two levels of a game (levels 1-2), which engages the specific cognitive processes reviewed in that session. The games are designed to raise individuals’ awareness of how they use specific cognitive functions to solve game-based scenarios. For instance, after watching an animation illustrating how attention difficulties can interfere with daily tasks, individuals play a hidden objects game, applying their attention to find target images in crowded backgrounds. After the neuroscience-informed educational section (described below), individuals repeat the game with an increased level of difficulty (levels 3-4), this time with greater awareness of the specific cognitive processes involved.
4.3 Neuroscience-informed psychoeducation (Knowledge)
This animated section aligns with the previous ones and explains specific cognitive functions implicated in SUD and their underlying brain networks (DAN/VAN, DMN, SN, ECN). For example, different types of attention, including sustained, selective, divided, and flexible attention, as well as voluntary and involuntary attention, are described in the first session, and the dorsal and ventral attention networks are introduced as their underlying neural networks. Moreover, immediately after playing the second round of games, individuals are provided with further scientific evidence on how different brain regions within a network are activated to invoke a cognitive function (Table 1). In this section, individuals also learn about specific threats to brain functions as a result of using drugs, alcohol, and other emotional triggers. For example, in the first session, attentional bias is introduced as a result of the disruption of the normal functioning of voluntary and involuntary attention. This section is presented through engaging cartoons and animations.
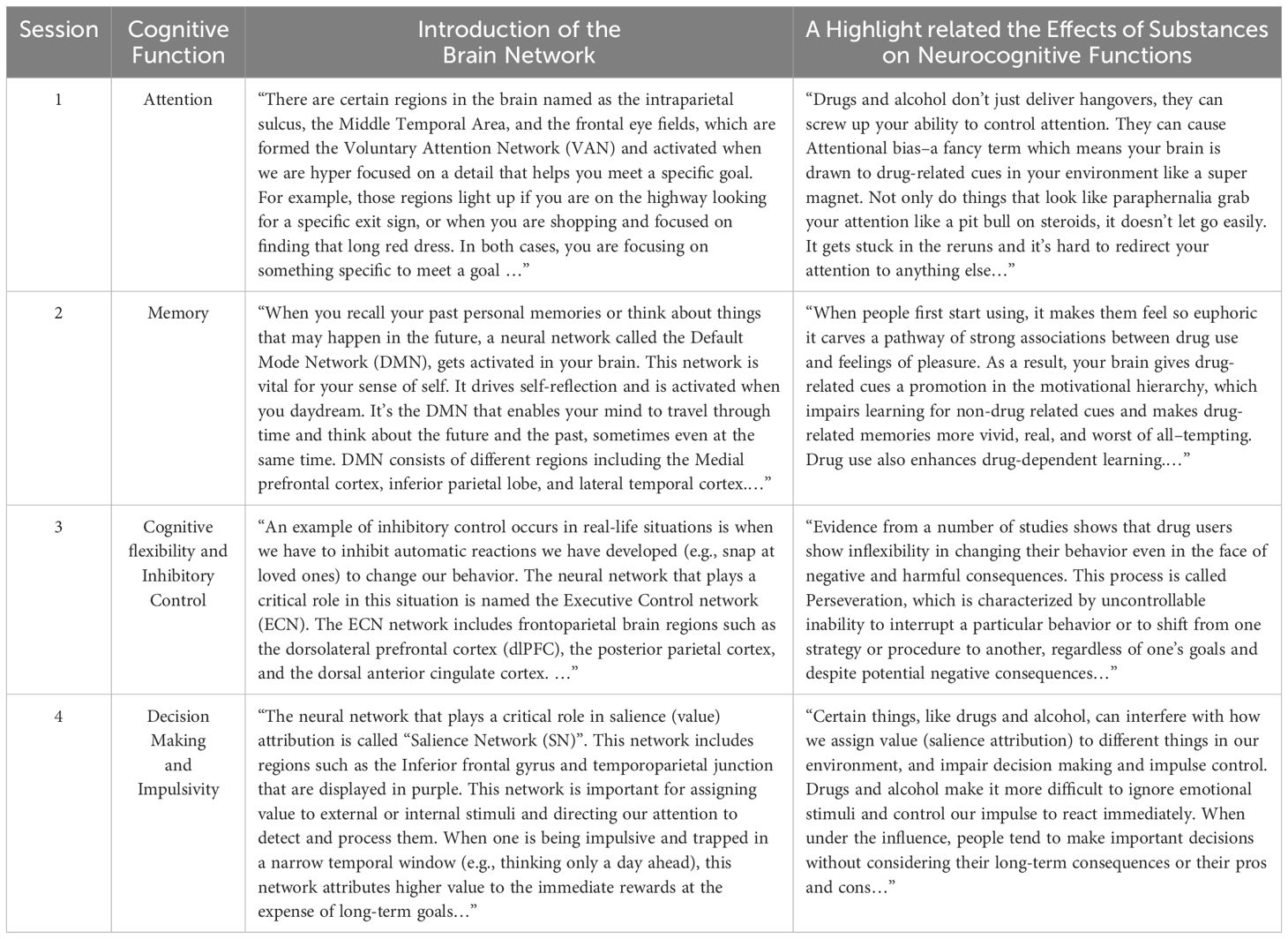
Table 1. Samples of narrations describing specific cognitive functions implicated in addiction and their underlying brain networks in the NIPA program.
4.4 Brain training Strategies (Skills)
The final section of each session is dedicated to providing four specific cognitive training strategies to boost the specific cognitive functions reviewed in the session, aiming to improve individuals’ resilience when exposed to drugs and other emotional triggers. Each strategy is accompanied by an exercise where individuals are required to apply the strategy they have learned. For example, in the first session, mindfulness, deep breathing, and focused reading and listening skills are learned and practiced through exercises.
4.5 Wrap-up (Practice)
Once individuals complete each session, they are provided with session highlights as a conclusion and are then directed to a multiple-choice exam. This section is designed to improve the learning experience and consists of 4-5 questions. Immediately after completing the quiz, they receive feedback and scores.
Throughout all the sessions, we used different comic characters, including ‘Mr. Brain,’ to narrate complex brain-based concepts and add a sense of humor to make the content more engaging.
5 Pilot study to test the feasibility and acceptability of NIPA
To investigate the feasibility and acceptability of NIPA, we conducted a pilot study with a sample of 85 undergraduate students (Mean age =19.09 years; Female=85%). Participants for the current study were included from an ongoing longitudinal cohort study of college students at a large, urban, mid-Atlantic public university. This study was approved by the university’s review board (HM20018784) and all participants provided informed consent. For a detailed review of study methods see (66). Participants were invited by email and screened for eligibility. Inclusion criteria included (1) being an undergraduate student age 18 or older (2); previous experience/use of alcohol, and/or tobacco, and/or cannabis, and/or other drugs; and (3) being willing and able to download the app and complete the program. Eligible participants were asked to complete a set of self-report assessments (e.g., Barkley Deficits in Executive Functioning Scale, Monetary Choice Questionnaires). Study data were collected and managed using REDCap electronic data capture tools (67, 68).
Once participants completed the pre-intervention baseline assessments, they started the NIPA program and after the final session of the intervention, they completed post-intervention assessment, which included feasibility and acceptability measures to evaluate the utility of NIPA as an educational program (Figure 2). The present paper reports the feasibility results, whereas the outcomes of the pre- and post-assessments are reported in another study.
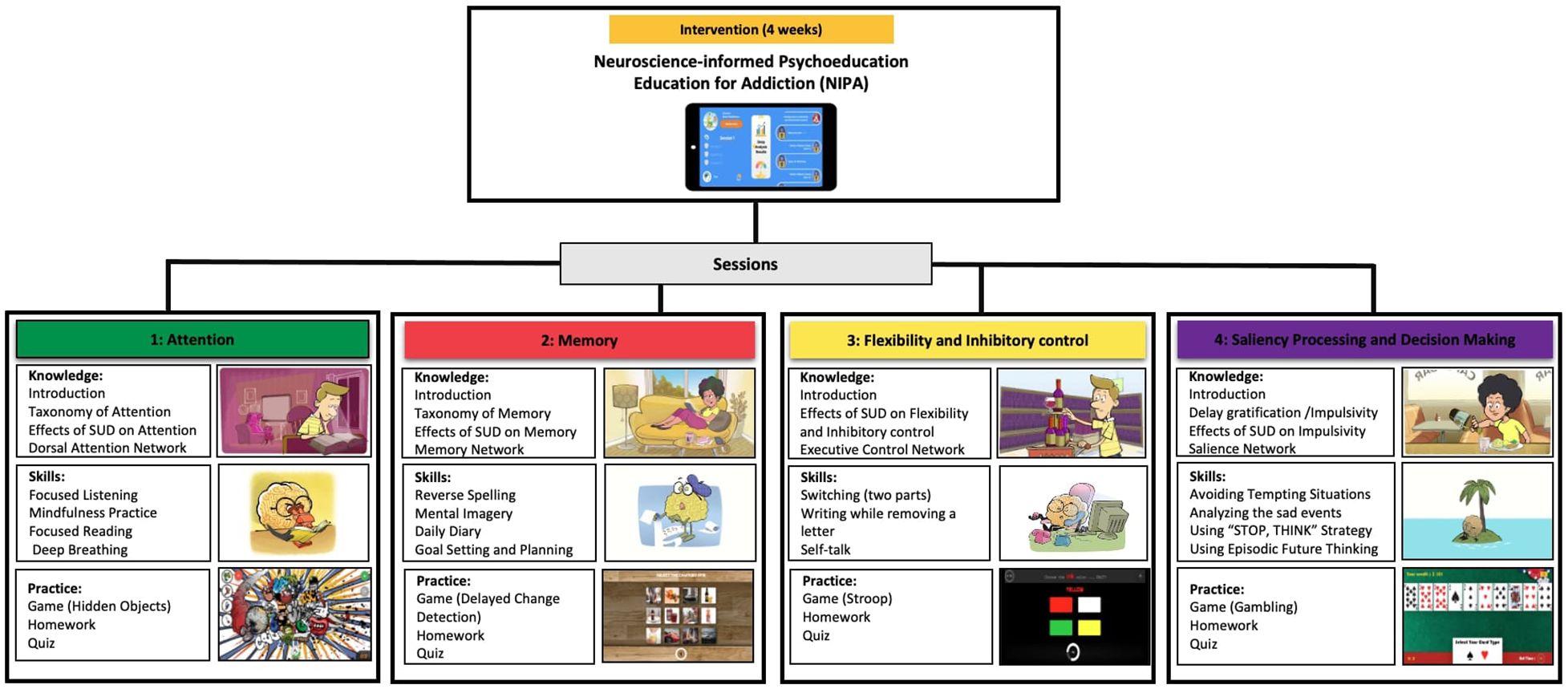
Figure 2. The architecture of the NIPA intervention, including cognitive domains (Attention, Memory, Flexibility, Decision Making), knowledge (Neuroscience-informed psychoeducation), skills (Brain training strategies), and practice (Games and quiz) designed in 4 sessions.
5.1 Attendance and completion of training sessions
We invited 100 students enrolled in the cohort project to participate in the study. Eighty-five participants expressed interest to participate and completed the pre-intervention assessment. Of these, 72 completed one session, 71 - two sessions, 69 - three sessions, and 68 - all four sessions, all of whom gave post-intervention feedback.
5.2 Acceptability measures
The acceptability questionnaire included 10 items rated on a 7-point Likert scale. The questionnaire covered eight areas including (1) perceived enjoyment [How much did you enjoy using the app]? (2), convenience [How easy did you find to install and use the app]? (3), perceived informativeness [How informative did you find the information provided about the brain and addiction]? (4), applicability [How much do you think the brain training strategies could be applicable to your daily routine]? (5), perceived effectiveness [How effective do you think the app might be]? (6), continued use [Would you like to continue using the app]? (7), recommendation to peers [Would you like to recommend the app to your friend]?. The last three questions were related to the overall program [How satisfied are you with the number of sessions? How satisfied are you with the session length? Overall, how satisfied are you with the program]?. Participants were also asked to identify their most and least likable part of using the app as well as the biggest barriers they encountered for completing the sessions. Table 2 shows the results from students’ perceived acceptability measures of the NIPA program.
According to participants’ rating, the games were the most (70.8%) and the brain training strategies were the least (33.8%) likable sections of using the app. The participants also identified the brain training strategies section as the biggest barrier to continuing using the app.
6 Discussion
As the age of drug use initiation decreases, interventions targeting the delay or reduction of problematic substance use among young people have expanded significantly. Preliminary forms of such interventions focused on reducing supply, reducing or delaying drug demand, and limiting the health and social harmful effects of substance use by criminalizing it (69). Although these classical approaches showed promise in reducing substance use by providing general knowledge about its negative consequences and teaching certain resistance skills, they appear to be ineffective in raising individuals’ awareness of the critical role of the brain in preventing or maintaining substance misuse. This kind of neuroscientific approach to reshaping the understanding of addiction as a brain disorder rather than a criminal justice issue, is an emerging field that seems to be more persuasive and acceptable among young people.
This new approach simplifies complex concepts about how different substances affect the brain, how neurocognitive mechanisms underlie the development of addiction, and how the brain can build resilience against SUD. Additionally, it could help adolescents better understand their own cognitive health. This is particularly important for adolescents with undiagnosed psychiatric disorders, such as ADHD. Parents and teachers of these youth might report behaviors such as leaving tasks incomplete, struggling to enjoy activities, and making impulsive decisions. Unaware of these symptoms and associated cognitive dysfunctions, adolescents become vulnerable to more serious challenges in daily life, such as a lack of persistence in pursuing long-term goals and being easily distracted by impulsive thoughts or risky behaviors such as substance use). Therefore, it seems necessary to provide adolescents with applied neuroscientific knowledge that can increase their understanding and awareness of cognitive functions, the long-term consequences of substance use on the brain, and evidence-based strategies to build resilience and improve brain health.
Moreover, framing drug-related education with brain-based language provides a safe learning environment for adolescents, where they don’t feel labeled or stigmatized for their attitudes toward substance use. The efficacy of such an intervention could increase even further when delivered through a digital platform, which is a salient communication tool for young people. Digital platforms have a number of benefits that can increase the efficacy of prevention and intervention efforts, such as: (1) higher anonymity compared to in-person sessions; (2) highly reproducible manner of intervention delivery; (3) by being available 24/7, they can provide ‘on demand’ therapeutic services when individuals need them most; (4) reduce stigma by diminishing the potential for public exposure; and (5) high scalability – increased access at low cost. Given the importance of expanding digital neuroscience-informed psychoeducation interventions for substance use prevention, we developed NIPA as a novel mobile app designed to inform adolescents about the neurocognitive mechanisms of addiction. It is noteworthy that NIPA uses a network perspective to discuss these neurocognitive processes affected by substance use, as brain networks provide a more accurate understanding of cognitive functions compared to other levels of analysis. This is supported by recent neuroimaging studies that focus on brain network analysis to represent the complex functional interactions between different regions underlying specific cognitive processes (70, 71).
Results from this pilot study support the feasibility and acceptability of NIPA among college students. We found that the brain games were the section liked the most by our participants, while the brain training strategies were rated lower compared to other sections. The mean scores for different measures of acceptability, including enjoyment, perceived convenience, informativeness, applicability, effectiveness, continuity, recommendation to peers, and satisfaction, were relatively good. Moreover, we received some qualitative feedback on various aspects of the program, which will be used to revise and modify subsequent versions of the app. These changes may help ensure that the content is more persuasive and acceptable for adolescents and emerging adults, enabling it to influence their awareness of the harmful effects of drugs on the brain and their intention to use or continue using them.
We wish to acknowledge a few limitations of our study. First, the small sample size and the lack of a control group reduce the generalizability and comparability of the results. Second, our sample was comprised predominantly of females with only a few male participants, which restricted our ability to examine gender differences in the analysis. Third, to improve learning and facilitate effective consolidation of the content, we are currently developing homework assignments to be completed between the sessions. Despite these limitations, our study introduces a novel psychoeducational tool in the field of addiction prevention for adolescents. Our findings support the feasibility of such neuroscience-informed interventions among adolescents in a college setting. Moreover, this app program has the potential to be used as a preventive tool for high school students and as a harm reduction intervention for adolescents who recreationally use drugs and alcohol. Additionally, that, due to its simple and comprehensible content, the app could be integrated into the therapeutic course of SUD treatment to raise patients’ awareness about the importance of seeking cognitive training interventions. However, further research is needed to determine whether using the app can change individuals’ attitudes and intentions toward drug use, as well as improve cognitive outcomes such as decision-making and impulsivity in each of these contexts.
Data availability statement
Data from this study are available via dbGaP (phs001754.v4.p2) or via c3BpdDRzY2llbmNlQHZjdS5lZHU= to qualified researchers who provide the appropriate signed data use agreement.
Ethics statement
The study involving humans was approved by Institutional review board (IRB) of Virginia Commonwealth University (protocol number HM20018784). The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TR: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. KaM: Formal analysis, Project administration, Writing – review & editing. EP: Writing - review & editing, Validation. KhM: Software, Writing – review & editing. HE: Conceptualization, Investigation, Supervision, Writing – review & editing. JV: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research reported in this publication was supported by the Fogarty International Center (FIC) and the National Institute on Drug Abuse (NIDA) under award number R01DA021421 (JV), by NIDA INVEST fellowship award (TR), and by the National Center for Advancing Translational Sciences (NCATS) under award number UM1TR004360. Spit for Science has been supported by Virginia Commonwealth University, P20AA017828, R37AA011408, K02AA018755, P50AA022537, and K01AA024152 from the National Institute on Alcohol Abuse and Alcoholism, UL1RR031990 from the National Center for Research Resources and National Institutes of Health Roadmap for Medical Research, as well as support by the Center for the Study of Tobacco Products at VCU. REDCap support provided by CTSA award UM1TR004360 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the views of the respective funding agencies.
Acknowledgments
We would like to thank the Spit for Science participants for making this study a success, as well as the many university faculty, students, and staff who contributed to the design and implementation of the project. We are also thankful to Anne Morris and Emily Lilley for their assistance with recruiting study participants. The Spit for Science Working Group: Director: Karen Chartier. Co-Director: Ananda Amstadter. Past Founding Director: Danielle M. Dick (2011-2022). Registry management: Emily Lilley, Renolda Gelzinis, Anne Morris. Data cleaning and management: Katie Bountress, Amy E. Adkins, Nathaniel Thomas, Zoe Neale, Kimberly Pedersen, Thomas Bannard & Seung B. Cho. Data collection: Kimberly Pedersen, Amy E. Adkins, Peter Barr, Holly Byers, Erin C. Berenz, Erin Caraway, Seung B. Cho, James S. Clifford, Megan Cooke, Elizabeth Do, Alexis C. Edwards, Neeru Goyal, Laura M. Hack, Lisa J. Halberstadt, Sage Hawn, Sally Kuo, Emily Lasko, Jennifer Lent, Mackenzie Lind, Elizabeth Long, Alexandra Martelli, Jacquelyn L. Meyers, Kerry Mitchell, Ashlee Moore, Arden Moscati, Aashir Nasim, Zoe Neale, Jill Opalesky, Cassie Overstreet, A. Christian Pais, Tarah Raldiris, Jessica Salvatore, Jeanne Savage, Rebecca Smith, David Sosnowski, Jinni Su, Nathaniel Thomas, Chloe Walker, Marcie Walsh, Teresa Willoughby, Madison Woodroof & Jia Yan. Genotypic data processing and cleaning: Cuie Sun, Brandon Wormley, Brien Riley, Fazil Aliev, Roseann E. Peterson & Bradley T. Webb.
Conflict of interest
KhM has ownership interests in Metacognium LLC, the company that developed the mobile application.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ This application is available under the name of Metacognium in App Store and Google Play.
References
1. Motlova LB, Balon R, Beresin EV, Brenner AM, Coverdale JH, Guerrero APS, et al. Psychoeducation as an opportunity for patients, psychiatrists, and psychiatric educators: why do we ignore it? Acad Psychiatry. (2017) 41:447–51. doi: 10.1007/s40596-017-0728-y
2. Sarkhel S, Singh O, Arora M. Clinical practice guidelines for psychoeducation in psychiatric disorders general principles of psychoeducation. Indian J Psychiatry. (2020) 62:319. doi: 10.4103/psychiatry.IndianJPsychiatry_780_19
3. Miller R. Neuroeducation: integrating brain-based psychoeducation into clinical practice. J Ment Health Counseling. (2016) 38:103–15. doi: 10.17744/mehc.38.2.02
4. Jolles J, Jolles DD. On neuroeducation: why and how to improve neuroscientific literacy in educational professionals. Front Psychol. (2021) 12:752151. doi: 10.3389/fpsyg.2021.752151
5. Han D, Chen S. Reducing the stigma of depression through neurobiology-based psychoeducation: A randomized controlled trial. Psychiatry Clin Neurosci. (2014) 68:666–73. doi: 10.1111/pcn.2014.68.issue-9
7. Rezapour T, Hatami J, Farhoudian A, Sofuoglu M, Noroozi A, Daneshmand R, et al. NEuro COgnitive REhabilitation for disease of addiction (NECOREDA) program: from development to trial. Basic Clin Neurosci. (2015) 6:291–8.
8. Rezapour T, Barzegari M, Sharifi E, Malmir N, Ghiasvand H, Salehi M, et al. Neuroscience-informed psychoeducation for recovery: A program to promote metacognition in people with substance use disorders. Basic Clin Neurosci. (2021) 12:597–606. doi: 10.32598/bcn.2021.809.3
9. Moreau J. I learned it by watching YOU !” The partnership for a drug-free america and the attack on “Responsible use” Education in the 1980s. J Soc History. (2016) 49:710–37. doi: 10.1093/jsh/shv062
10. Meredith LR, Maralit AM, Thomas SE, Rivers SL, Salazar CA, Anton RF, et al. Piloting of the Just Say Know prevention program: a psychoeducational approach to translating the neuroscience of addiction to youth. Am J Drug Alcohol Abuse. (2021) 47:16–25. doi: 10.1080/00952990.2020.1770777
11. Botvin GJ, Griffin KW. School-based programmes to prevent alcohol, tobacco and other drug use. Int Rev Psychiatry. (2007) 19:607–15. doi: 10.1080/09540260701797753
12. Quinn CA, Hides L, De Andrade D, Pocuca N, Wilson M, Kavanagh DJ. Impact of a brief psychoeducational intervention for reducing alcohol use and related harm in school leavers. Drug Alcohol Rev. (2019) 38:339–48. doi: 10.1111/dar.2019.38.issue-4
13. Gabrhelik R, Duncan A, Miovsky M, Furr-Holden CDM, Stastna L, Jurystova L. Unplugged”: A school-based randomized control trial to prevent and reduce adolescent substance use in the Czech Republic. Drug Alcohol Depend. (2012) 124:79–87. doi: 10.1016/j.drugalcdep.2011.12.010
14. Sussman S, Dent CW, Stacy AW. Project towards no drug abuse: A review of the findings and future directions. Am J Health Behav. (2002) 26:354–65. doi: 10.5993/AJHB.26.5.4
15. Cho H, Hallfors DD, Sánchez V. Evaluation of a high school peer group intervention for at-risk youth. J Abnorm Child Psychol. (2005) 33:363–74. doi: 10.1007/s10802-005-3574-4
16. McBride N, Farringdon F, Midford R, Meuleners L, Phillips M. Harm minimization in school drug education: final results of the School Health and Alcohol Harm Reduction Project (SHAHRP). Addiction. (2004) 99:278–91. doi: 10.1111/j.1360-0443.2003.00620.x
17. Orlando M, Ellickson PL, McCaffrey DF, Longshore DL. Mediation analysis of a school-based drug prevention program: effects of project ALERT. Prev Sci. (2005) 6:35–46. doi: 10.1007/s11121-005-1251-z
18. Longshore D, Ellickson PL, McCaffrey DF, Clair P. School-based drug prevention among at-risk adolescents: effects of ALERT plus. Health Educ Behav. (2007) 34:651–68. doi: 10.1177/1090198106294895
19. Alarcó-Rosales R, Sánchez-SanSegundo M, Ferrer-Cascales R, Albaladejo-Blazquez N, Lordan O, Zaragoza-Martí A. Effects of a School-Based Intervention for Preventing Substance Use among Adolescents at Risk of Academic Failure: A Pilot Study of the Reasoning and Rehabilitation V2 Program. Healthcare. (2021) 9:1488. doi: 10.3390/healthcare9111488
20. Weisberg DS, Keil FC, Goodstein J, Rawson E, Gray JR. The seductive allure of neuroscience explanations. J Cogn Neurosci. (2008) 20:470–7. doi: 10.1162/jocn.2008.20040
21. Debenham J, Birrell L, Champion K, Askovic M, Newton N. A pilot study of a neuroscience-based, harm minimisation programme in schools and youth centres in Australia. BMJ Open. (2020) 10:e033337. doi: 10.1136/bmjopen-2019-033337
22. Debenham J, Champion K, Birrell L, Newton N. Effectiveness of a neuroscience-based, harm reduction program for older adolescents: A cluster randomised controlled trial of the Illicit Project. Prev Med Rep. (2022) 26:101706. doi: 10.1016/j.pmedr.2022.101706
23. Kryza-Lacombe M, Richards E, Hansen N, Goldin P. Integrating neuroeducation into psychotherapy practice: Why and how to talk to patients about the brain. Behav Therapist. (2021) 44:361–70.
24. Fischer AS, Camacho MC, Ho TC, Whitfield-Gabrieli S, Gotlib IH. Neural markers of resilience in adolescent females at familial risk for major depressive disorder. JAMA Psychiatry. (2018) 75:493. doi: 10.1001/jamapsychiatry.2017.4516
25. Yip SW, Lichenstein SD, Liang Q, Chaarani B, Dager A, Pearlson G, et al. Brain networks and adolescent alcohol use. JAMA Psychiatry. (2023) 80:1131. doi: 10.1001/jamapsychiatry.2023.2949
26. Schinke S, Schwinn T. Gender-specific computer-based intervention for preventing drug abuse among girls. Am J Drug Alcohol Abuse. (2005) 31:609–16. doi: 10.1081/ADA-200068415
27. Lord SE, D’Amante D. 4: Efficacy of online alcohol and other drug prevention for early adolescents. J Adolesc Health. (2007) 40:S4. doi: 10.1016/j.jadohealth.2006.11.016
28. Griffin KW, Williams C, Botvin CM, Sousa S, Botvin GJ. Effectiveness of a hybrid digital substance abuse prevention approach combining e-Learning and in-person class sessions. Front Digit Health. (2022) 4:931276. doi: 10.3389/fdgth.2022.931276
29. Paz Castro R, Haug S, Wenger A, Schaub MP. Longer-term efficacy of a digital life-skills training for substance use prevention. Am J Prev Med. (2022) 63:944–53. doi: 10.1016/j.amepre.2022.06.017
30. Birrell L, Furneaux-Bate A, Chapman C, Newton NC. A mobile peer intervention for preventing mental health and substance use problems in adolescents: protocol for a randomized controlled trial (The mind your mate study). JMIR Res Protoc. (2021) 10:e26796. doi: 10.2196/26796
31. Newton NC, Chapman C, Slade T, Birrell L, Healy A, Mather M, et al. A national effectiveness trial of an eHealth program to prevent alcohol and cannabis misuse: responding to the replication crisis. Psychol Med. (2022) 52:274–82. doi: 10.1017/S0033291720001919
32. Sheikhan NY, Henderson JL, Halsall T, Daley M, Brownell S, Shah J, et al. Stigma as a barrier to early intervention among youth seeking mental health services in Ontario, Canada: a qualitative study. BMC Health Serv Res. (2023) 23:86. doi: 10.1186/s12913-023-09075-6
33. Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann New York Acad Sci. (2008) 1141:105–30. doi: 10.1196/nyas.2008.1141.issue-1
34. Squeglia LM, Cservenka A. Adolescence and drug use vulnerability: findings from neuroimaging. Curr Opin Behav Sci. (2017) 13:164–70. doi: 10.1016/j.cobeha.2016.12.005
35. Hernández-Álvarez DM, Pacheco L, Velasco-Segura R, Pérez de la Mora M, Tejeda-Romero C, González-García N. Default mode network efficiency is correlated with deficits in inhibition in adolescents with inhalant use disorder. Front Psychiatry. (2020) 11:209. doi: 10.3389/fpsyt.2020.00209
36. Meeus W, Vollebergh W, Branje S, Crocetti E, Ormel J, Van De Schoot R, et al. On imbalance of impulse control and sensation seeking and adolescent risk: an intra-individual developmental test of the dual systems and maturational imbalance models. J Youth Adolescence. (2021) 50:827–40. doi: 10.1007/s10964-021-01419-x
37. Peeters M, Oldehinkel T, Vollebergh W. Behavioral control and reward sensitivity in adolescents’ Risk taking behavior: A longitudinal TRAILS study. Front Psychol. (2017) 8:231. doi: 10.3389/fpsyg.2017.00231
38. Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. (2013) 9:449–61. doi: 10.2147/NDT.S39776
39. Ersche KD, Meng C, Ziauddeen H, Stochl J, Williams GB, Bullmore ET, et al. Brain networks underlying vulnerability and resilience to drug addiction. Proc Natl Acad Sci USA. (2020) 117:15253–61. doi: 10.1073/pnas.2002509117
40. Ota M, Nemoto K, Ishida I, Sato S, Asada T, Arai T, et al. Structural brain network correlated with the resilience to traumatic events in the healthy participants: An MRI study on healthy people in a stricken area of the Great East Japan Earthquake. psychol Trauma: Theory Research Practice Policy. (2022) 14:1035–9. doi: 10.1037/tra0000517
41. Rezapour T, Assari S, Kirlic N, Vassileva J, Ekhtiari H. Enhancing cognitive resilience in adolescence and young adults: A multidimensional approach. In: Croff JM, Beaman J, editors. Family Resilience and Recovery from Opioids and Other Addictions. Springer International Publishing, Cham (2021). p. 45–64. doi: 10.1007/978-3-030-56958-7_3
42. Lanssens A, Pizzamiglio G, Mantini D, Gillebert CR. Role of the dorsal attention network in distracter suppression based on features. Cogn Neurosci. (2020) 11:37–46. doi: 10.1080/17588928.2019.1683525
43. Alves PN, Forkel SJ, Corbetta M, Thiebaut De Schotten M. The subcortical and neurochemical organization of the ventral and dorsal attention networks. Commun Biol. (2022) 5:1343. doi: 10.1038/s42003-022-04281-0
44. Farrant K, Uddin LQ. Asymmetric development of dorsal and ventral attention networks in the human brain. Dev Cogn Neurosci. (2015) 12:165–74. doi: 10.1016/j.dcn.2015.02.001
45. Solís-Vivanco R, Jensen O, Bonnefond M. New insights on the ventral attention network: Active suppression and involuntary recruitment during a bimodal task. Hum Brain Mapping. (2021) 42:1699–713. doi: 10.1002/hbm.25322
46. Lee D, Lee J, Namkoong K, Jung YC. Altered functional connectivity of the dorsal attention network among problematic social network users. Addictive Behaviors. (2021) 116:106823. doi: 10.1016/j.addbeh.2021.106823
47. Hyman SE. Addiction: A disease of learning and memory. AJP. (2005) 162:1414–22. doi: 10.1176/appi.ajp.162.8.1414
48. Volkow ND, Blanco C. Substance use disorders: a comprehensive update of classification, epidemiology, neurobiology, clinical aspects, treatment and prevention. World Psychiatry. (2023) 22:203–29. doi: 10.1002/wps.21073
49. Ma N, Liu Y, Fu XM, Li N, Wang CX, Zhang H, et al. Abnormal brain default-mode network functional connectivity in drug addicts. PloS One. (2011) 6:e16560. doi: 10.1371/journal.pone.0016560
50. Gong M, Shen Y, Liang W, Zhang Z, He C, Lou M, et al. Impairments in the default mode and executive networks in methamphetamine users during short-term abstinence. IJGM. (2022) 15:6073–84. doi: 10.2147/IJGM.S369571
51. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. NeuroImage. (2019) 200:313–31. doi: 10.1016/j.neuroimage.2019.06.036
52. Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. (2011) 12:652–69. doi: 10.1038/nrn3119
53. Schimmelpfennig J, Topczewski J, Zajkowski W, Jankowiak-Siuda K. The role of the salience network in cognitive and affective deficits. Front Hum Neurosci. (2023) 17:1133367. doi: 10.3389/fnhum.2023.1133367
54. Padula CB, Tenekedjieva LT, McCalley DM, Al-Dasouqi H, Hanlon CA, Williams LM, et al. Targeting the salience network: A mini-review on a novel neuromodulation approach for treating alcohol use disorder. Front Psychiatry. (2022) 13:893833. doi: 10.3389/fpsyt.2022.893833
55. Grodin EN, Cortes CR, Spagnolo PA, Momenan R. Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug Alcohol Depend. (2017) 179:100–8. doi: 10.1016/j.drugalcdep.2017.06.014
56. Gotlieb R, Yang XF, Immordino-Yang MH. Default and executive networks’ roles in diverse adolescents’ emotionally engaged construals of complex social issues. Soc Cogn Affect Neurosci. (2022) 17:421–9. doi: 10.1093/scan/nsab108
57. McHugh MJ, Gu H, Yang Y, Adinoff B, Stein EA. Executive control network connectivity strength protects against relapse to cocaine use. Addict Biol. (2017) 22:1790–801. doi: 10.1111/adb.2017.22.issue-6
58. Ahn WY, Vasilev G, Lee SH, Busemeyer JR, Kruschke JK, Bechara A, et al. Decision-making in stimulant and opiate addicts in protracted abstinence: evidence from computational modeling with pure users. Front Psychol. (2014) 5:849/abstract. doi: 10.3389/fpsyg.2014.00849/abstract
59. Waltmann M, Herzog N, Reiter AMF, Villringer A, Horstmann A, Deserno L. Diminished reinforcement sensitivity in adolescence is associated with enhanced response switching and reduced coding of choice probability in the medial frontal pole. Dev Cogn Neurosci. (2023) 60:101226. doi: 10.1016/j.dcn.2023.101226
60. Tozzi L, Zhang X, Pines A, Olmsted AM, Zhai ES, Anene ET, et al. Personalized brain circuit scores identify clinically distinct biotypes in depression and anxiety. Nat Med. (2024) 30:2076–87. doi: 10.1038/s41591-024-03057-9
61. Vassileva J, Lee JH, Psederska E, Ahn WY. Utility of computational approaches for precision psychiatry: applications to substance use disorders. In: Stoyanov D, Draganski B, Brambilla P, Lamm C, editors. Computational Neuroscience, vol. 199 . Springer US, New York, NY (2023). p. 211–31. doi: 10.1007/978-1-0716-3230-7_14
62. MaChado Do Vale TC, Da Silva Chagas L, De Souza Pereira H, Giestal-de-Araujo E, Arévalo A, Oliveira-Silva-Bomfim P. Neuroscience outside the box: from the laboratory to discussing drug abuse at schools. Front Hum Neurosci. (2022) 16:782205. doi: 10.3389/fnhum.2022.782205
63. Norman MK. Twelve tips for reducing production time and increasing long-term usability of instructional video. Med Teacher. (2017) 39:808–12. doi: 10.1080/0142159X.2017.1322190
64. Lukka L, Palva JM. The development of game-based digital mental health interventions: bridging the paradigms of health care and entertainment. JMIR Serious Games. (2023) 11:e42173. doi: 10.2196/42173
65. Sharma R, Jain A, Gupta N, Garg S, Batta M, Dhir S. Impact of self-assessment by students on their learning. Int J App Basic Med Res. (2016) 6:226. doi: 10.4103/2229-516X.186961
66. Dick DM, Nasim A, Edwards AC, Salvatore JE, Cho SB, Adkins A, et al. Spit for Science: launching a longitudinal study of genetic and environmental influences on substance use and emotional health at a large US university. Front Genet. (2014) 5:47/abstract. doi: 10.3389/fgene.2014.00047/abstract
67. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Informatics. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
68. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Informatics. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
69. Sánchez-Puertas R, Vaca-Gallegos S, López-Núñez C, Ruisoto P. Prevention of alcohol consumption programs for children and youth: A narrative and critical review of recent publications. Front Psychol. (2022) 13:821867. doi: 10.3389/fpsyg.2022.821867
70. Medaglia JD, Lynall ME, Bassett DS. Cognitive network neuroscience. J Cogn Neurosci. (2015) 27:1471–91. doi: 10.1162/jocn_a_00810
Keywords: neuroscience-informed psychoeducation, addiction, adolescents, neurocognitive, research domain criteria (RDoC), metacogntive
Citation: Rezapour T, McLean KL, Psederska E, Maleki KN, Ekhtiari H and Vassileva J (2025) Neuroscience-informed psychoeducation for addiction: a conceptual and feasibility study. Front. Psychiatry 16:1527828. doi: 10.3389/fpsyt.2025.1527828
Received: 13 November 2024; Accepted: 20 January 2025;
Published: 12 February 2025.
Edited by:
Catalina Lopez-Quintero, University of Florida, United StatesReviewed by:
Ji-An Li, University of California, San Diego, United StatesDiana Fishbein, University of North Carolina at Chapel Hill, United States
Copyright © 2025 Rezapour, McLean, Psederska, Maleki, Ekhtiari and Vassileva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jasmin Vassileva, amx2YXNzaWxldmFAdmN1LmVkdQ==
 Tara Rezapour
Tara Rezapour Kayla L. McLean
Kayla L. McLean Elena Psederska
Elena Psederska Khashayar Niki Maleki
Khashayar Niki Maleki Hamed Ekhtiari
Hamed Ekhtiari Jasmin Vassileva
Jasmin Vassileva