
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 04 April 2025
Sec. Mood Disorders
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1525091
Background: It is thought that inflammation significantly contributes to the development of bipolar disorder (BD), and recent findings indicate a connection between cystatin C and immune-related inflammation. In this study, we investigated serum cystatin C levels in patients with BD and explored the relationship between cystatin C and inflammatory markers.
Methods: The study involved 3,647 individuals diagnosed with BD, comprising 2,431 with BD-manic (BD-M) and 1,216 with BD-depression (BD-D), alongside 3,500 healthy controls. The analysis covered cystatin C levels and inflammatory biomarkers obtained from complete blood counts across the various groups. The Spearman correlation test was used to examine the relationship between cystatin C and inflammatory markers. Logistic regression and ROC curve analyses assessed the predictive value of these markers for disease occurrence.
Results: Serum cystatin C levels were significantly elevated in BD patients, particularly those in manic episodes, compared to the healthy control group, with distinct correlation patterns with inflammatory biomarkers observed among the groups. Serum Cystatin C levels independently and positively indicated disease occurrence, showing improved diagnostic effectiveness when combined with inflammatory ratios.
Conclusion: Our research indicates that cystatin C could be involved in the pathophysiological mechanisms of BD by affecting pro-inflammatory processes. Additionally, it should be emphasized that cystatin C showed considerable predictive capacity in diagnosing BD, especially when used alongside various inflammatory markers.
Limitations: The cross-sectional study is limited to demonstrating associations rather than establishing causality. A thorough examination of sociodemographic factors and the severity of the disease could not be conducted.
Bipolar disorder (BD) represents a widespread, recurring, and incapacitating psychiatric illness distinguished by fluctuating episodes of depression accompanied by either mania or hypomania, impacting about 40 million people globally (1). Individuals with BD, a significant contributor to global disability, often face increased risks of suicidal behavior, cardiovascular diseases, aggression, and legal issues, potentially impairing social functioning and reducing quality of life (2, 3). Genetic predispositions and environmental conditions contribute to BD development, but the underlying mechanisms are not fully understood (4). In clinical practice, the diagnosis of bipolar disorder (BD) relies predominantly on subjective clinical symptoms and lacks objective biomarkers. Hence, it is crucial to delve deeper into the pathophysiological processes associated with BD and to pinpoint potential biomarkers for its identification, facilitating early diagnosis and prompt treatment.
Prior studies have demonstrated that immune dysfunction and inflammation are key factors in the development of BD (5, 6). These findings underscore the critical role of comprehending immune response mechanisms and inflammatory processes in the pathogenesis of BD. Furthermore, a rising number of inflammation-related biomarkers identified through hematological assessments have been found to correlate with the onset of BD (7–9), reinforcing the connection between immune response, inflammation, and the disease. Cystatin C, a prominent cysteine protease inhibitor predominantly located in the brain, has emerged as a factor intricately linked to immune inflammation (10). Originally identified by Jorgen Clausen as a protein present in cerebrospinal fluid, cystatin C has since been recognized as a vital component in various bodily fluids (11). The CST3 gene encodes human cystatin C, found in all mammalian tissues and fluids such as cerebrospinal fluid, blood plasma, urine, semen, and saliva (12). Cystatin C is predominantly produced by astrocytes in the central nervous system, with its cerebrospinal fluid concentration being quintuple that of the bloodstream (13, 14).
While cystatin C is mainly recognized as a marker for glomerular filtration rate (GFR), its biological functions go well beyond this designated role. This protein demonstrates both antibacterial and antiviral characteristics, affects tumor metastasis, aids in bone resorption, influences the immune system, and encourages cellular proliferation and growth (15). Growing evidence indicates that cystatin C plays a direct role in various immunological disorders, with its encoding gene being regulated by inflammatory mediators like cytokines in situations of inflammation or infection (16). Cystatin C influences inflammation and immune responses by altering cysteine protease activity or through mechanisms independent of its inhibitory functions (17). Studies on serum cystatin C levels have provided significant diagnostic insights into various inflammatory diseases (18). Recent scholarly interest has increased in the relationship between serum cystatin C levels and central nervous system disorders, including Alzheimer’s, Parkinson’s, and depression (19–21). Research reveals that patients experiencing depression exhibit considerably higher cystatin C levels compared to healthy individuals, which may be linked to immunological processes (22). Another study suggested that inflammatory depression is associated with selective glomerular hypofiltration (SGHS), which are characterized by a reduced estimated glomerular filtration rate (eGFR) based on cystatin C relative to eGFR based on creatinine (23). Furthermore, the pro-inflammatory cytokine IL-6 partially mediated the relationship between SGHS and depression. Similar to depression, BD is also characterized by chronic low-grade systemic inflammation (24). Whether cystatin C levels exhibit alterations in BD is equally worth investigating. Nonetheless, previous research has not assessed serum cystatin C levels in BD patients, especially during varying episodes such as manic (BD-M) and depressive (BD-D) phases.
Prior research has shown that the neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and system inflammation response index (SIRI) are effective inflammation biomarkers, readily derived from complete blood counts (7, 8). It has also been noted that these parameters are significantly elevated in individuals with BD and exhibit strong predictive capabilities regarding the disease’s occurrence. Considering the specific association of cystatin C and the derived ratios from complete blood counts with inflammation, further investigation into their relationship is warranted. Taking into account the predictive significance of such inflammatory biomarkers, the integration of cystatin C with these ratios could yield additional insights for predicting BD.
In this study, we leveraged comprehensive clinical data to investigate cystatin C concentration differences between BD patients (primarily those in manic or depressive episodes) and healthy controls, as well as between BD-M and BD-D subgroups, while also exploring associations between cystatin C and inflammatory markers to elucidate its potential role in BD pathophysiology. We further compared the diagnostic performance of cystatin C with established inflammatory indices and evaluated their combined utility, hypothesizing that cystatin C levels would be elevated in BD patients (with differential expression across mood states), correlate with inflammatory markers, and demonstrate diagnostic accuracy comparable to conventional inflammatory biomarkers.
This retrospective cross-sectional study was conducted in a real-world setting at Beijing Hui-Long-Guan Hospital from January 2015 to January 2021. We examined sociodemographic and hematological data of participants obtained from the hospital’s Electronic Medical Record System (EMRS). The study focused on hospitalized patients in the acute phase of BD. Our sample comprised 3,647 BD patients, which included 2,431 BD-M patients and 1,216 BD-D patients. The diagnosis of BD as well as disease episodes were based on the criteria of International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Participants were selected if they met these criteria: (a) a verified ICD-10 diagnosis of BD by two independent senior psychiatrists (BD-M: F31.0, F31.1, F31.2; BD-D: F31.3, F31.4, F31.5): (b) aged 18 to 65 years; and (c) availability of hematological data for Cystatin C, platelets, neutrophils, lymphocytes, and monocytes. The exclusion criteria encompassed: (a) the presence of comorbid psychiatric disorders;(b) significant infections, autoimmune disorders, malignant tumors, renal illnesses, liver diseases, diabetes, obesity, or pregnancy; and (c) prior use of nonsteroidal anti-inflammatory medications, steroids, or antibiotics within a month. In a parallel effort to establish a control group, we included 3,500 healthy individuals who were retrospectively selected from the EMRS of the Health Examination Center at Beijing Tong-Ren Hospital during the same study period. The healthy control group was composed of individuals who went to the hospital for routine health examinations. They had no current or past diagnoses of any mental disorders based on ICD-10 criteria, were between 18 and 65 years old, and met the same exclusion criteria as the patients.
The study involved the collection of both fundamental demographic characteristics, such as age and sex, as well as various hematological parameters. These parameters included levels of Cystatin C, uric acid, creatinine, urea, and the counts of platelets, neutrophils, lymphocytes, and monocytes. Data was sourced from EMRS, ensuring accuracy and reliability in the demographic and hematological information gathered. Fasting venous blood samples were collected from participants between 7 and 9 a.m. These samples were then subjected to analysis by professional laboratory technicians, who conducted routine blood tests alongside a series of biochemical assessments to generate comprehensive results. In analyzing the inflammatory responses in participants, particular calculations for various inflammatory ratios were employed: NLR = neutrophil/lymphocyte; MLR = monocyte/lymphocyte; PLR = platelet/lymphocyte; SII = platelet × neutrophil/lymphocyte; SIRI = monocyte × neutrophil/lymphocyte.
All statistical analyses were performed using SPSS version 25.0. Continuous variables are expressed as mean ± standard deviation, and categorical variables are represented as counts (percentages). The chi-square (χ²) test was utilized for the analysis of categorical data. The Kolmogorov-Smirnov test was used to assess the normal distribution of the study variables. The Q-Q plots and histograms suggest that the variables exhibit an approximate normal distribution. Given the large sample size in this study, continuous variables were compared using an independent t-test for two groups or a one-way ANOVA for three groups. An analysis of covariance (ANCOVA) within the General Linear Model framework was conducted to assess differences in biochemical test results and complete blood count across various categories. For each laboratory measurement, ANCOVA was performed with the laboratory data serving as the dependent variable, fixed factors comprised of the diagnostic groups, and covariates including age and sex. Post-hoc analysis, utilizing the Bonferroni correction, was carried out to examine inter-group differences. Spearman correlation analysis was used to examine the relationships between cystatin C, age, and inflammatory biomarkers. A logistic regression analysis was performed to evaluate the predictive value of cystatin C and inflammatory ratios in relation to the occurrence of BD, encompassing both manic and depressive episodes. ROC curves were utilized to assess the predictive accuracy of individual biomarkers and combined cystatin C models with various ratios for detecting BD across different episodes. Statistical significance was defined as P < 0.05.
There were no significant differences of age or sex between the BD and HC groups (P > 0.05). Importantly, individuals with BD showed significantly elevated cystatin C levels when compared to the HCs (P <0.001). The study found elevated creatinine levels and decreased uric acid and urea levels among the other variables analyzed (P < 0.001 for all). Besides, BD patients exhibited significantly elevated levels of platelets, neutrophils, monocytes, NLR, MLR, PLR, SII, and SIRI (P < 0.001 for all). The results were shown in Table 1 and Figure 1A.
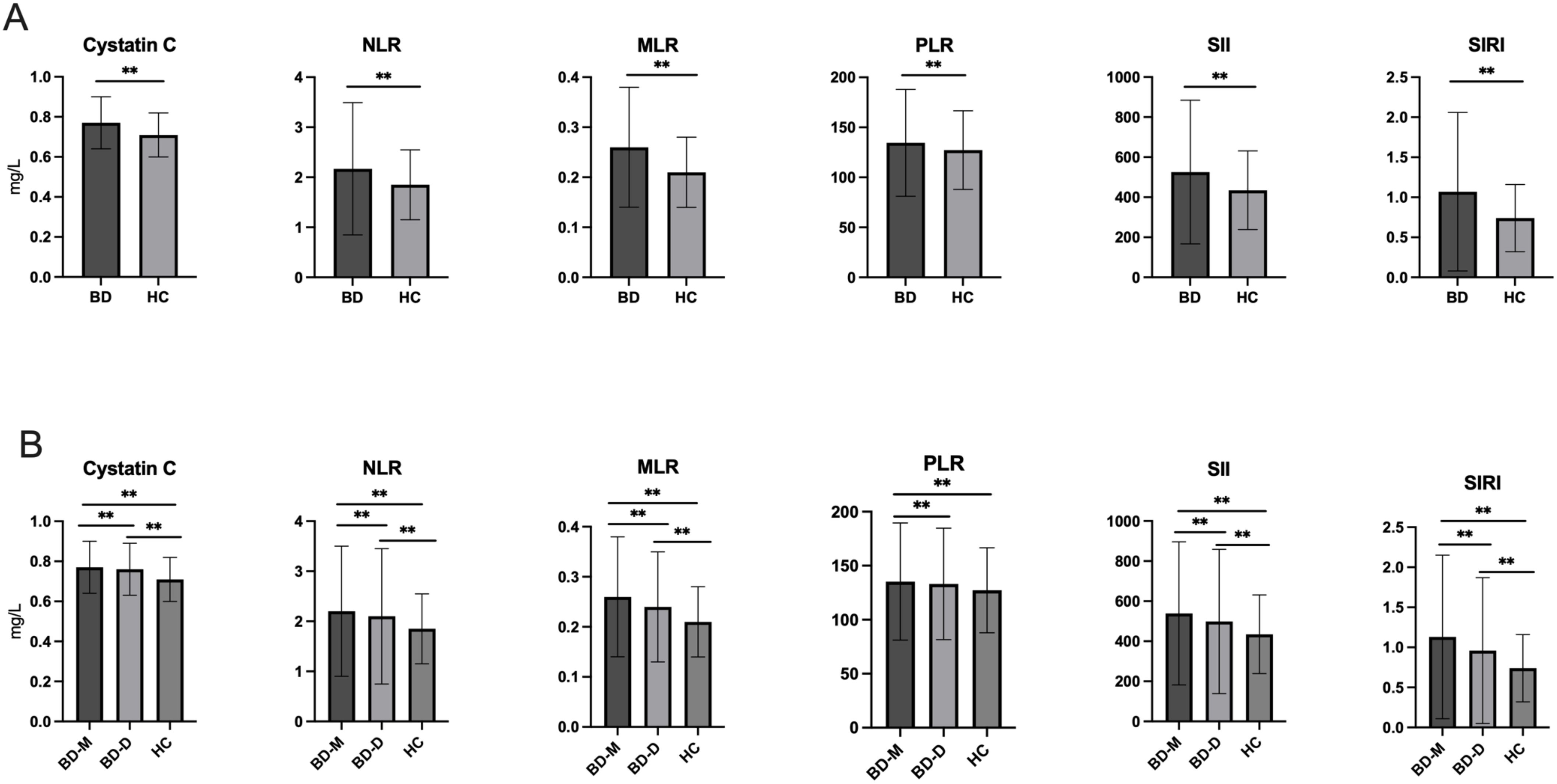
Figure 1. (A) Comparison of cystatin C, NLR, MLR, PLR, SII and SIRI between BD and HC; (B) Comparison of cystatin C NLR, MLR, PLR, SII and SIRI among BD-M, BD-D and HC groups. BD, Bipolar disorder; BD-M, bipolar disorder in manic episode; BD-D, bipolar disorder in depressive episode; HC, healthy controls; NLR, neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; SIRI, system inflammation response index. **P < 0.001.
Table 2 categorizes participants in the BD group into two subgroups: BD-M and BD-D. Chi-square analysis indicated no significant sex differences among the BD-D, BD-M, and HC groups (P > 0.05). ANOVA and post hoc tests revealed that the BD-M group (39.57 ± 12.49) was significantly younger than the BD-D group (40.62 ± 12.75) (P = 0.036). After adjusting for age and sex, significant differences were observed in cystatin C, uric acid, creatinine, urea, platelets, neutrophils, monocytes, NLR, MLR, PLR, SII, and SIRI levels among the three groups (P < 0.001 for all).The BD-M group showed significantly higher levels of cystatin C, creatinine, platelets, neutrophils, monocytes, NLR, MLR, PLR, SII, and SIRI (P < 0.001) compared to the HC group, along with reduced levels of uric acid and urea. The BD-D group exhibited higher levels of cystatin C, creatinine, neutrophils, monocytes, NLR, MLR, PLR, SII, and SIRI, and lower levels of uric acid and urea compared to the HC group (P < 0.001). Comparisons revealed that the BD-M group exhibited elevated levels of cystatin C, uric acid, platelets, neutrophils, monocytes, NLR, MLR, PLR, SII, and SIRI, but a reduced level of creatinine compared to the BD-D group (P < 0.001). Figure 1B illustrates the differences in cystatin C, NLR, MLR, PLR, SII, and SIRI across all groups.
We examined cystatin C levels in different groups, considering different clinical presentations and sex. According to ICD-10, we categorized patients into two groups based on the presence of psychotic symptoms: P (psychotic, ICD-10: F31.2, F31.5), or NP (non-psychotic, ICD-10: F31.0, F31.1, F31.3, F31.4). Table 3 and Figure 2 indicate no significant differences in cystatin C levels between patients with and without psychotic symptoms in the BD, BD-M, and BD-D groups (P > 0.05 for all). Nonetheless, both the BD cohort and the healthy control group displayed notably elevated cystatin C levels in male participants compared to their female counterparts (P <0.001 for all).
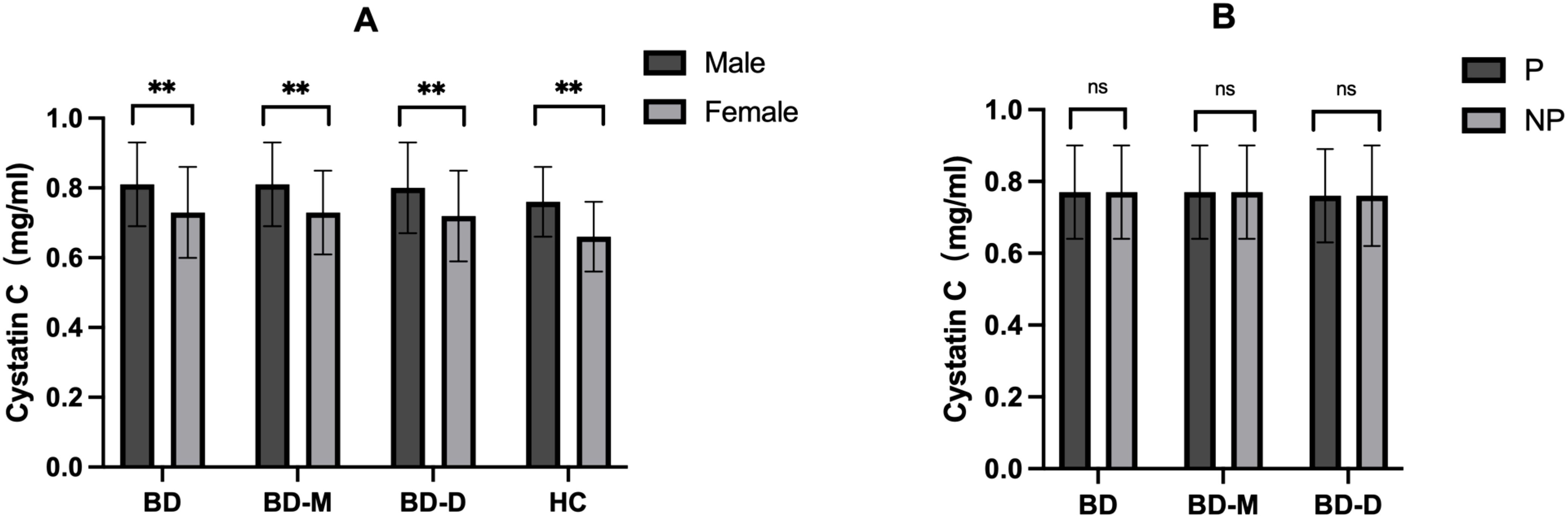
Figure 2. (A) Comparison of cystatin C among groups with different sex; (B) Comparison of cystatin C among groups with or without psychotic symptoms. BD, Bipolar disorder; BD-M, bipolar disorder in manic episode; BD-D, bipolar disorder in depressive episode; HC, healthy controls; P, patients with psychotic symptoms; NP, patients without psychotic symptoms. **P < 0.001; ns no significance.
Table 4 demonstrates a positive correlation between age and Cystatin C levels across all study groups (P < 0.001). In the HC group, cystatin C levels positively correlated with neutrophil, lymphocyte, and monocyte counts, as well as NLR, MLR, SII, and SIRI (P < 0.05). In contrast, a negative correlation was found between cystatin C levels and PLR (P <0.001). In the BD and BD-M groups, cystatin C levels showed positive correlations with neutrophils, lymphocytes, monocytes, MLR, and SIRI, while displaying negative correlations with platelets, PLR, and SII. In the BD-D group, cystatin C showed a positive correlation with lymphocyte and monocyte counts, and a negative correlation with platelet count, PLR, and SII.
Table 5 demonstrates that the binary logistic regression model for BD included age, sex, cystatin C, NLR, MLR, PLR, and SIRI, each independently linked to BD development. Moreover, sex, cystatin C, MLR, PLR, and SIRI exhibited positive correlations with BD, whereas age and NLR displayed negative associations. Aligning with the BD model, sex, cystatin C, MLR, PLR, and SIRI emerged as positive indicators for BD-M, while age and NLR were again inversely related to BD-M. In the BD-D subgroup, sex, cystatin C, MLR, and SIRI showed positive correlations with BD-D, whereas age was negatively associated. ROC curves were utilized to assess the diagnostic value of individual indicators like cystatin C, NLR, MLR, PLR, SII, and SIRI, along with combined models from previous logistic regression analyses. The findings are summarized in Tables 6–9 and Figure 3. In classifying BD, cystatin C had higher AUC than any other indicators, however, only the combined model achieved an AUC above 0.7. The results for the BD-M group were similar to those of the BD group. In the BD-D category, even the combined model showed the AUC value that did not exceed 0.7. We conducted further ROC analysis to differentiate between BD-M and BD-D; however, the AUC values for all indicators remained below 0.6.
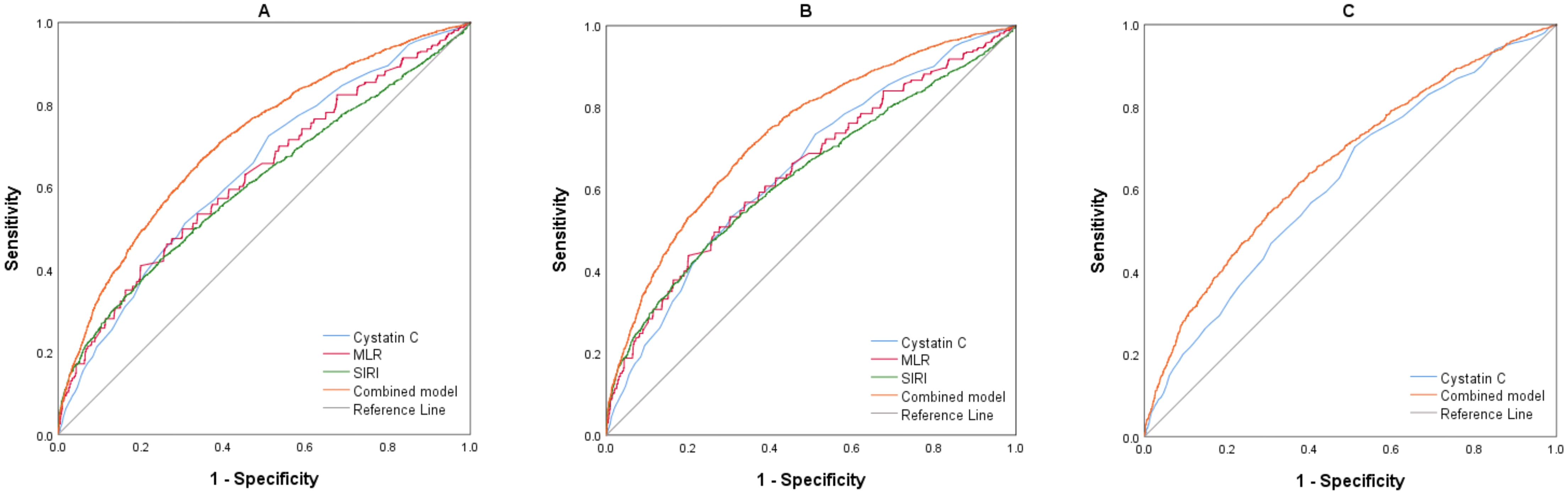
Figure 3. (A) ROC curves of the parameters for BD (BD vs. HC). (B) ROC curves of the parameters for BD-M (BD-M vs. HC). (C) ROC curves of the parameters for BD-D (BD-D vs. HC). BD, Bipolar disorder; BD-M, bipolar disorder in manic episode; BD-D, bipolar disorder in depressive episode; MLR, monocyte/lymphocyte ratio; SIRI, system inflammation response index.
This retrospective analysis found significantly elevated serum cystatin C levels in individuals with BD compared to healthy controls. While such findings might suggest renal dysfunction within the BD cohort, the uric acid, urea, and creatinine results provide contradictory evidence. Specifically, the BD group displayed notably elevated creatinine levels paired with reduced uric acid and urea levels, hinting at the potential influence of other mechanisms on cystatin C levels. Recent studies have explored the link between high serum cystatin C levels and psychiatric disorders, particularly depression. A cohort study involving 1,440 Chinese seniors over 60 identified a detrimental association between elevated serum cystatin C levels and heightened depression risk (25). An online cross-sectional study of 159 major depressive disorder (MDD) patients revealed a significant correlation between serum cystatin C levels and depressive symptom severity (22). Cystatin C has various biological functions potentially influencing depression development. Although BD and MDD are categorized as distinct clinical entities, they share etiological connections and clinical characteristics, including depressive symptoms (26), implying that the mechanisms through which cystatin C affects physiological and pathological states in MDD might also manifest in BD. BD, like MDD, is associated with systemic low-grade inflammation (27), marked by increased levels of inflammatory cytokines including IL-6, TNF-α, IL-1RA, and sIL-2R (28). Cystatin C, commonly used as a marker for glomerular filtration rate in clinical settings, is increasingly recognized for its role in immune responses to external and internal antigens in pathological conditions. This underscores cystatin C’s wider clinical significance, extending beyond mere kidney function assessment. The elevated serum levels of cystatin C observed in BD in our study may be attributed to its association with immune inflammation and modulation by cytokines, similar to findings in MDD reported in previous research.
This study found higher cystatin C levels in BD-M patients compared to those with BD-D. The clinical manifestations of the two episodes are distinct: the manic episode typically features elevated mood and increased energy or activity, while the depressive episode is marked by sadness and lack of pleasure (29). A study encompassing 8,332 BD patients revealed that elevated levels of CRP during manic episodes persisted even after adjustments for confounding variables, including age, sex, BMI, psychotic symptoms, and age at onset (30). This finding suggests an independent association between inflammatory changes and mood episodes. In comparison to depressive episodes, manic episodes might correlate with a more pronounced inflammatory response, which could influence cystatin C levels.
BD patients with psychotic symptoms have poorer outcomes than non-psychotic individuals, as indicated by higher hospitalization rates and longer stays (31). A study of 665 inpatients analyzed biochemical variables in BD patients with and without psychotic features. Findings revealed that patients with psychotic symptoms exhibited a higher NLR and lower total cholesterol and triglyceride levels, suggesting increased inflammation and reduced metabolic alterations (32). A recent study investigated associations between peripheral blood biomarkers (including cystatin C) and symptom severity across MDD, BD, and schizophrenia. Notably, cognitive-related symptoms such as auditory hallucinations were significantly correlated with elevated cystatin C levels (33). However, our study found no significant differences in cystatin C levels between patients with and without psychotic symptoms in both BD-M and BD-D groups. Since our study was cross-sectional, further research is needed to evaluate the possible link between psychotic symptoms and cystatin C levels. This study investigated the effects of sex and age on cystatin C levels in the BD, BD-M, BD-D, and HC groups. Our study found that males had higher cystatin C levels than females, and cystatin C levels positively correlated with age across all groups. This suggests that cystatin C concentrations may rise as age increases. These findings align with several prospective cross-sectional cohort studies in healthy populations (34, 35) and our study further corroborates these results in patients with BD.
Recently, inflammation ratios derived from complete blood counts have gained attention as superior biomarkers for indicating inflammation states. NLR, MLR, PLR, SII, and SIRI are derived from various combinations of neutrophils, lymphocytes, monocytes, and platelets. Neutrophils, lymphocytes, and monocytes are distinct white blood cell types that play unique roles in the innate and adaptive immune systems, contributing to defense against infections and immune-related diseases (36). Platelets, produced in the bone marrow, are crucial for hemostasis, wound healing, and angiogenesis; moreover, their role in inflammation across various diseases is supported by a substantial body of evidence (37). NLR, MLR, PLR, SII, and SIRI have been extensively studied in immune-inflammatory conditions like infectious inflammation and tumors (38–41), as well as in mental health disorders such as affective disorders and schizophrenia (7, 9, 42, 43). Our study revealed that all five ratios were elevated in BD patients, irrespective of manic or depressive episodes, compared to healthy controls. Furthermore, NLR, MLR, SII, and SIRI were significantly higher during manic episodes than during depressive episodes, highlighting the increased level of inflammation in manic episode, as demonstrated in previous research. The relationship between cystatin C and inflammatory markers differed among diagnostic groups and healthy controls. Cystatin C exhibited a weak yet significant correlation with all inflammatory ratios; however, this relationship may be influenced by different disease stages. A significant correlation between cystatin C and the NLR was observed exclusively in the HC group. Moreover, the HC group displayed higher correlation coefficients for MLR and SIRI in comparison to the diagnostic groups. This difference might be due to a more robust correlation involving cystatin C with neutrophils, lymphocytes, and monocytes within the HC group, along with the notable negative correlation between cystatin C and platelets that was found only in the disease groups. We propose that different stages of BD may involve distinct interactions affecting the relationship between cystatin C and immune cells such as neutrophils, lymphocytes, monocytes, and platelets. Nonetheless, as this study is cross-sectional in nature, additional research is necessary to investigate the pathophysiological connections linking cystatin C with these inflammatory biomarkers in BD.
In clinical practice, the diagnosis of BD largely depends on clinical features such as affective temperaments and negative clinical outcomes (44), which may not be sufficiently effective for the early detection of the disease due to its varied and confusing symptoms. Consequently, efforts have been directed toward identifying objective and reliable biological predictors to facilitate timely and efficient diagnosis (45). In recent years, a substantial body of research has investigated inflammation and oxidative stress-related peripheral blood biomarkers derived from complete blood counts or biochemical test data in BD (46, 47). Research indicates that NLR, MLR, PLR, SII, and SIRI are effective predictors of BD occurrence. However, the efficacy of these indicators is not entirely satisfactory, indicating that the search for more useful biomarkers remains worthwhile in the future. Meanwhile, cystatin C has been noted in some psychiatric disorders, and elevated serum cystatin C levels suggest potential diagnostic capabilities in these diseases (19, 21). In our research, we observed increased concentrations of cystatin C in patients with BD when compared to healthy individuals, highlighting its considerable potential in forecasting various stages of BD. Table 5’s binary logistic regression analysis identified serum cystatin C levels as independent and positive predictors of disease occurrence, particularly in the BD-M group. We performed receiver operating characteristic curve analyses to further investigate the diagnostic effectiveness of specific indicators, including cystatin C, NLR, MLR, PLR, SII, and SIRI, as well as combined models derived from previous logistic regression results. Cystatin C demonstrated the highest diagnostic effectiveness among the parameters evaluated, indicating its strong diagnostic potential in diagnosing BD. The logistic regression combined models demonstrated superior diagnostic effectiveness. The results indicate that cystatin C may act as a significant biomarker for diagnosing BD, especially when combined with other inflammatory markers. Moving forward, our efforts will focus on identifying more effective biomarkers and improving the integration of these indicators.
Our current study has various strengths and limitations. This study provides valuable real-world insights, enhancing knowledge in the field and underscoring its clinical importance. To the best of our understanding, this is the first large-scale study to analyze cystatin C levels across different episodes of BD and evaluate its diagnostic potential, both alone and in combination with other inflammatory ratios. This study represents the most comprehensive analysis of serum cystatin C levels and their association with peripheral inflammation ratios in BD to date. Nonetheless, it is crucial to recognize several limitations. Primarily, since this is a retrospective analysis, the severity of symptoms, illness duration, and frequency of episodes could not be assessed through structured psychiatric evaluations. Consequently, this study did not explore the relationship between cystatin C levels and disease severity. We did not perform a more detailed grouping based on ICD-10 criteria, and therefore cannot investigate changes in indicators across additional disease stages of BD. Due to feasibility constraints, we were unable to collect additional sociodemographic variables, including smoking habits, nutritional practices, body mass index, and social status. These variables may affect cystatin C levels and inflammation ratios. Our study did not consider treatment effects of medications, which may influence the levels of these indicators. Lastly, given that this is a cross-sectional analysis, it can only illustrate associations rather than establish causality. In the future, we intend to carry out more comprehensive research to explore the relationships between cystatin C and inflammatory biomarkers in more detailed BD subgroups over an extended period.
In this research, we noted increased serum concentrations of cystatin C among patients with BD, particularly during manic episodes. Our results further revealed a correlation between cystatin C levels and various immune cells, such as neutrophils, lymphocytes, monocytes, and platelets, along with derived biomarkers like the NLR, MLR, PLR, SII, and SIRI, indicating a relationship between cystatin C and inflammation. Additionally, our findings suggested that cystatin C might act as an independent risk factor for BD, including both depressive and manic phases, in comparison to healthy controls. We discovered that cystatin C exhibited superior diagnostic efficacy relative to other inflammatory indices, and an integrated model incorporating these factors displayed enhanced diagnostic capacity. In summary, our findings highlight the importance of cystatin C in BD, proposing its potential role in facilitating the diagnosis of this disorder in the future. We advocate for more research on cystatin C, which could yield deeper insights into its inflammatory roles and contribute to our comprehension of the pathophysiology of BD and various psychiatric conditions.
The data are not publicly available due to their containing information that could compromise the privacy of research participants. Requests to access these datasets should be directed to JC, Y2hlbmp4MTExMEAxNjMuY29t.
The studies involving humans were approved by Ethics Committee of Beijing Huilongguan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it is a retrospective and low-risk study.
CZ: Conceptualization, Formal Analysis, Writing – original draft. JM: Conceptualization, Formal Analysis, Writing – original draft. HG: Data curation, Writing – review & editing. YL: Data curation, Writing – review & editing. YW: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. JC: Conceptualization, Supervision, Writing – review & editing. JF: Data curation, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the Capital’s Funds for Health Improvement and Research (2022-4-2134), Beijing Hospitals Authority Youth Programme (QML20232002), Beijing Municipal Administration of Hospitals Incubating Program (PX2022079).
The authors thank all of the participants for their commitment to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nierenberg AA, Agustini B, Köhler-Forsberg O, Cusin C, Katz D, Sylvia LG, et al. Diagnosis and treatment of bipolar disorder: A review. JAMA. (2023) 330:1370. doi: 10.1001/jama.2023.18588
2. Krahn GL. WHO world report on disability: a review. Disabil Health J. (2011) 4:141–2. doi: 10.1016/j.dhjo.2011.05.001
3. Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Primers. (2018) 4:18008. doi: 10.1038/nrdp.2018.8
4. Freund N, Juckel G. Bipolar disorder: its etiology and how to model in rodents. Methods Mol Biol. (2019) 2011:61–77. doi: 10.1007/978-1-4939-9554-7_4
5. Hamdani N, Tamouza R, Leboyer M. Immuno- inflammatory markers of bipolar disorder: a review of evidence. Front Biosci (Elite Ed). (2012) 4:2170–82. doi: 10.2741/e534
6. Muneer A. Bipolar disorder: role of inflammation and the development of disease biomarkers. Psychiatry Investig. (2016) 13:18–33. doi: 10.4306/pi.2016.13.1.18
7. Dadouli K, Janho MB, Hatziefthimiou A, Voulgaridi I, Piaha K, Anagnostopoulos L, et al. Neutrophil-to-lymphocyte, monocyte-to-lymphocyte, platelet-to-lymphocyte ratio and systemic immune-inflammatory index in different states of bipolar disorder. Brain Sci. (2022) 12:1034. doi: 10.3390/brainsci12081034
8. Marazziti D, Torrigiani S, Carbone MG, Mucci F, Flamini W, Ivaldi T, et al. Neutrophil/lymphocyte, platelet/lymphocyte, and monocyte/lymphocyte ratios in mood disorders. Curr Med Chem. (2022) 29:5758–81. doi: 10.2174/0929867328666210922160116
9. Wei Y, Wang T, Li G, Feng J, Deng LB, Xu H, et al. Investigation of systemic immune-inflammation index, neutrophil/high-density lipoprotein ratio, lymphocyte/high-density lipoprotein ratio, and monocyte/high-density lipoprotein ratio as indicators of inflammation in patients with schizophrenia and bipolar disorder. Front Psychiatry. (2022) 13:941728. doi: 10.3389/fpsyt.2022.941728
10. Warfel AH, Zucker-Franklin D, Frangione B, Ghiso J. Constitutive secretion of cystatin C (gamma-trace) by monocytes and macrophages and its downregulation after stimulation. J Exp Med. (1987) 166:1912–7. doi: 10.1084/jem.166.6.1912
11. Mussap M, Plebani M. Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci. (2004) 41:467–550. doi: 10.1080/10408360490504934
12. Stańczykiewicz B, Łuc M, Banach M, Zabłocka A. Cystatins: unravelling the biological implications for neuroprotection. Arch Med Sci. (2024) 20:157–66. doi: 10.5114/aoms/171706
13. Nagai A, Terashima M, Sheikh AM, Notsu Y, Shimode K, Yamaguchi S, et al. Involvement of cystatin C in pathophysiology of CNS diseases. Front Biosci. (2008) 13:3470–9. doi: 10.2741/2941
14. Sundelöf J, Sundström J, Hansson O, Eriksdotter-Jönhagen M, Giedraitis VG, Larsson A, et al. Cystatin C levels are positively correlated with both Abeta42 and tau levels in cerebrospinal fluid in persons with Alzheimer’s disease, mild cognitive impairment, and healthy controls. J Alzheimers Dis. (2010) 21:471–8. doi: 10.3233/JAD-2010-091594
15. Gauthier S, Kaur G, Mi W, Tizon B, Levy E. Protective mechanisms by cystatin C in neurodegenerative diseases. Front Biosci (Schol Ed). (2011) 3:541–54. doi: 10.2741/s170
16. Xu N, Zhang YY, Lin Y, Bao B, Zheng L, Shi GP, et al. Increased levels of lysosomal cysteinyl cathepsins in human varicose veins: a histology study. Thromb Haemost. (2014) 111:333–44. doi: 10.1160/TH13-04-0309
17. Zi M, Xu Y. Involvement of cystatin C in immunity and apoptosis. Immunol Lett. (2018) 196:80–90. doi: 10.1016/j.imlet.2018.01.006
18. Werle B, Sauckel K, Nathanson CM, Cystatins C. E/M and F in human pleural fluids of patients with neoplastic and inflammatory lung disorders. Biol Chem. (2003) 384:281–7. doi: 10.1515/BC.2003.031
19. Chen WW, Cheng X, Zhang X, Zhang QS, Sun HQ, Huang WJ, et al. The expression features of serum Cystatin C and homocysteine of Parkinson’s disease with mild cognitive dysfunction. Eur Rev Med Pharmacol Sci. (2015) 19:2957–63.
20. Mathews PM, Levy E. Cystatin C in aging and in Alzheimer’s disease. Ageing Res Rev. (2016) 32:38–50. doi: 10.1016/j.arr.2016.06.003
21. Zhu L, Yu C, Chang Y, Sun S, Sun Z. Serum cystatin C is associated with depression after intracerebral hemorrhage. Neuropsychiatr Dis Treat. (2023) 19:1117–26. doi: 10.2147/NDT.S409421
22. Sun T, Chen Q, Li Y. Associations of serum cystatin C with depressive symptoms and suicidal ideation in major depressive disorder. BMC Psychiatry. (2021) 21:576. doi: 10.1186/s12888-021-03509-3
23. Söderberg Veibäck G, Malmgren L, Asp M, Ventorp F, Suneson K, Grudet C, et al. Inflammatory depression is associated with selective glomerular hypofiltration. J Affect Disord. (2024) 356:80–7. doi: 10.1016/j.jad.2024.04.007
24. Jones BDM, Daskalakis ZJ, Carvalho AF, Strawbridge R, Young AH, Mulsant BH, et al. Inflammation as a treatment target in mood disorders: review. BJPsych Open. (2020) 6:e60. doi: 10.1192/bjo.2020.43
25. Wu L, Yan Z, Jiang H, Xing H, Li H, Qiu C. Serum cystatin C, impaired kidney function, and geriatric depressive symptoms among older people living in a rural area: a population-based study. BMC Geriatr. (2018) 18:265. doi: 10.1186/s12877-018-0957-2
26. Hashimoto K. Metabolomics of major depressive disorder and bipolar disorder: overview and future perspective. Adv Clin Chem. (2018) 84:81–99. doi: 10.1016/bs.acc.2017.12.005
27. Osimo EF, Cardinal RN, Jones PB, Khandaker GM. Prevalence and correlates of low-grade systemic inflammation in adult psychiatric inpatients: An electronic health record-based study. Psychoneuroendocrinology. (2018) 91:226–34. doi: 10.1016/j.psyneuen.2018.02.031
28. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. (2016) 21:1696–709. doi: 10.1038/mp.2016.3
29. Yang R, Zhao Y, Tan Z, Lai J, Chen JS, Zhang XF, et al. Differentiation between bipolar disorder and major depressive disorder in adolescents: from clinical to biological biomarkers. Front Hum Neurosci. (2023) 17:1192544. doi: 10.3389/fnhum.2023.1192544
30. Lyu N, Zhao Q, Fu B, Li J, Wang H, Yang F, et al. Hormonal and inflammatory signatures of different mood episodes in bipolar disorder: a large-scale clinical study. BMC Psychiatry. (2023) 23:449. doi: 10.1186/s12888-023-04846-1
31. Chakrabarti S, Singh N. Psychotic symptoms in bipolar disorder and their impact on the illness: A systematic review. World J Psychiatry. (2022) 12:1204–32. doi: 10.5498/wjp.v12.i9.1204
32. Esposito CM, Barkin JL, Ceresa A, Nosari G, Paolo Di M, Legnani F, et al. Are there any differences in clinical and biochemical variables between bipolar patients with or without lifetime psychotic symptoms? J Clin Med. (2023) 12:5902. doi: 10.3390/jcm12185902
33. Qiu J, Yu C, Kuang Y, Hu Y, Zhu T, Qin K, et al. Association between psychiatric symptoms with multiple peripheral blood sample test: a 10-year retrospective study. Front Psychiatry. (2024) 15:1481006. doi: 10.3389/fpsyt.2024.1481006
34. Al Musaimi O, Abu-Nawwas AH, Al Shaer D, Khaleel NY, Fawzi M. Influence of age, gender, smoking, diabetes, thyroid and cardiac dysfunctions on cystatin C biomarker. Semergen. (2019) 45:44–51. doi: 10.1016/j.semerg.2018.07.005
35. Odden MC, Tager IB, Gansevoort RT, Bakker SJL, Katz R, Fried LF, et al. Age and cystatin C in healthy adults: a collaborative study. Nephrol Dial Transplant. (2010) 25:463–9. doi: 10.1093/ndt/gfp474
36. Saez A, Herrero-Fernandez B, Gomez-Bris R, Sánchez-Martinez H, Gonzalez-Granado JM. Pathophysiology of inflammatory bowel disease: innate immune system. Int J Mol Sci. (2023) 24:1526. doi: 10.3390/ijms24021526
37. Pogorzelska K, Krętowska A, Krawczuk-Rybak M, Sawicka-Żukowska M. Characteristics of platelet indices and their prognostic significance in selected medical condition - a systematic review. Adv Med Sci. (2020) 65:310–5. doi: 10.1016/j.advms.2020.05.002
38. Chen H, Wu X, Wen Z, Zhu Y, Liao L, Yang J. The clinicopathological and prognostic value of NLR, PLR and MLR in non-muscular invasive bladder cancer. Arch Esp Urol. (2022) 75:467–71. doi: 10.56434/j.arch.esp.urol.20227505.68
39. Ng WWS, Lam SM, Yan WW, Shum HP. NLR, MLR, PLR and RDW to predict outcome and differentiate between viral and bacterial pneumonia in the intensive care unit. Sci Rep. (2022) 12:15974. doi: 10.1038/s41598-022-20385-3
40. Wang RH, Wen WX, Jiang ZP, Du ZP, Ma ZH, Lu AL, et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. (2023) 14:1115031. doi: 10.3389/fimmu.2023.1115031
41. Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 US adults. J Clin Med. (2023) 12:1128. doi: 10.3390/jcm12031128
42. Özdin S, Böke Ö. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in different stages of schizophrenia. Psychiatry Res. (2019) 271:131–5. doi: 10.1016/j.psychres.2018.11.043
43. Wei Y, Feng J, Ma J, Chen D, Chen J. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in patients with affective disorders. J Affect Disord. (2022) 309:221–8. doi: 10.1016/j.jad.2022.04.092
44. Baldessarini RJ, Innamorati M, Erbuto D, Serafini GL, Fiorillo A, Amore M, et al. Differential associations of affective temperaments and diagnosis of major affective disorders with suicidal behavior. J Affect Disord. (2017) 210:19–21. doi: 10.1016/j.jad.2016.12.003
45. Hu X, Yu C, Dong T, Yang Z, Fang Y, Jiang Z. Biomarkers and detection methods of bipolar disorder. Biosens Bioelectron. (2023) 220:114842. doi: 10.1016/j.bios.2022.114842
46. Xu H, Li R, Wang L, Wang TT, Luo YH, Wei YY, et al. Non-enzymatic antioxidants, macro-minerals and monocyte/high-density lipoprotein cholesterol ratio among patients with bipolar disorder. J Affect Disord. (2023) 322:76–83. doi: 10.1016/j.jad.2022.11.017
Keywords: bipolar disorder, cystatin C, inflammation, biomarker, serum
Citation: Zhang C, Ma J, Gao H, Luo Y, Feng J, Wei Y and Chen J (2025) Investigation of serum cystatin C levels and their diagnostic value in combination with inflammatory ratios in patients with bipolar disorder. Front. Psychiatry 16:1525091. doi: 10.3389/fpsyt.2025.1525091
Received: 12 November 2024; Accepted: 17 March 2025;
Published: 04 April 2025.
Edited by:
Marcin Siwek, Jagiellonian University, PolandReviewed by:
Magdalena Sowa-Kućma, University of Rzeszow, PolandCopyright © 2025 Zhang, Ma, Gao, Luo, Feng, Wei and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingxu Chen, Y2hlbmp4MTExMEAxNjMuY29t; Yanyan Wei, d2VpZGF5YW42NkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.