
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 26 February 2025
Sec. Psychological Therapy and Psychosomatics
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1519492
Psychotic symptoms are prevalent in individuals with various mental health disorders and frequently lead to adverse outcomes. In this study, we assessed the prevalence of psychotic symptoms and its associated conditions in a large sample of Chinese patients with somatic symptom disorder (SSD), which has not been examined systemically. We recruited 899 patients with SSD. We used the positive subscale of the Positive and Negative Syndrome Scale to assess psychotic symptoms in the participants. We evaluated the participants using the Hamilton Depression Rating Scale (HAMD), Hamilton Anxiety Rating Scale (HAMA) and Perceived Stress Scale (PSS).The prevalence of psychotic symptoms in participants was 10.2%. Compared with participants without psychotic symptoms, participants with psychotic symptoms had higher scores on the HAMD, HAMA and PSS scales and a shorter sleep duration. Based on the results of stepwise binary logistic regression analysis, the HAMA, HAMD and PSS were significantly associated with psychotic symptoms in the participants. Our findings suggest that psychotic symptoms are common in patients with SSD in the Chinese Han population. In addition, greater levels of anxiety, depression, and stress are potentially useful markers for predicting a greater risk of psychotic symptoms.
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), somatic symptom disorder (SSD) is the prototypical diagnosis for patients with somatic symptom and related disorders (1). Its major characteristics include somatic distress, catastrophizing cognitive behavior, health-related anxiety, and exaggerated reactions to somatic discomforts (2). SSD is a mental health condition reported in clinical settings, both in China and in other countries. This condition is widely reported in primary care in Western countries, with prevalence estimates ranging from 11.7% to 30.3% in studies conducted between 1999 and 2022 (3–6) In a 2020 survey of outpatients in China, the prevalence of DSM-IV SSD was found to be 33.8% (236/697) (7). This indicates how significant the issue is globally.
Even though the symptoms of SSD cannot be directly attributed to an organic pathology, patients with SSD are at a higher level of disability risk than patients with other conditions that are known to have greater disability risk than SSD (8). This not only restricts the social engagement of patients (9) but also profoundly affects their functional capabilities and overall quality of life (10). This causes physical and mental exhaustion in patients. Furthermore, because patients tend to use healthcare resources more frequently (8, 11), they incur significant health expenses, which increases the economic burden as well (9, 12). Thus, this condition also exerts considerable pressure on medical systems (13, 14). Reports indicate that, in the United States, the healthcare expenses for patients with SSD are six to fourteen times greater than the average healthcare expenses (15). Consequently, the effective clinical management of patients with SSD is a critical challenge in the mental healthcare system and requires urgent attention, given its inherent complexity and significance (16, 17).
Several studies have shown that the prevalence of a mental health condition along with other psychotic symptoms often correlates with more severe manifestations, elevated levels of suicidal ideation (18), anxiety (19), hypochondriasis (20), cognitive dysfunction (21), and A-type personality disorder (22), as well as poorer prognoses and higher rates of treatment resistance, among other adverse outcomes. Patients with SSD exhibit personality traits that predispose them to psychotic symptoms (23), characterized by heightened neuroticism (24) and signs of punishment sensitivity (25). Consequently, when patients with SSD display additional psychotic symptoms, they are likely to experience more severe repercussions than patients who have an isolated episode of SSD.
The increase in comorbidities of psychotic symptoms in SSD is associated with multifaceted mechanisms. Substantial evidence indicates that anxiety and depression may serve as potential etiological factors (26) and are common complications in patients with SSD. These negative emotional states heighten the risk of psychotic symptoms, frequently exacerbating both the severity and distress associated with such symptoms (27). Furthermore, SSD and psychotic symptoms have similar pathogenic mechanisms, with heightened life stress being a common causative factor (28, 29). The origins of this association remain unclear, but there may be potentially overlapping genetic risk factors that influence stress perception in patients with psychiatric disorders, with stress sensitivity being heritable (30). In previous studies, variables such as sex (31) and age (32, 33) have also been shown to influence the manifestation of psychotic symptoms, and therefore, they are potential risk factors that we need to consider.
Patients with both somatic symptom disorders and psychotic symptoms may experience more severe manifestations and additional adverse outcomes. However, the prevalence and risk factors associated with comorbid psychotic symptoms in patients with SSD has not been investigated sufficiently. Here, we examine the prevalence of psychotic symptoms in patients with SSD and the risk factors contributing to this prevalence.
We conducted a cross-sectional survey from January 2023 to April 2024 in The Third People’s Hospital of Ganzhou, Ganzhou People’s Hospital, and First Affiliated Hospital of Gannan Medical University, Jiangxi Province, China. The study protocol was approved by the Institutional Review Board of The Third People’s Hospital of Ganzhou. We obtained informed consent from all patients before requesting their participation in this study. The information provided by all respondents was confidential.
We recruited 899 participants from the outpatient departments of the psychiatry wards at The Third People’s Hospital of Ganzhou, Ganzhou People’s Hospital, and the First Affiliated Hospital of Gannan Medical University. The inclusion criteria were as follows: (1) Chinese Han nationality, based on self-reporting by the participants; (2) age of participants: 18-60 years; (3) diagnosis of SSD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria; (4) ability of participants to provide written informed consent. Before we assigned definitive diagnoses, we discussed them in our weekly team meeting, and a supervising senior physician with extensive clinical experience validated the diagnoses. Eight hundred and ninety-nine patients met the inclusion criteria. Ninety-eight patients were excluded for the following reasons: Ninety-eight patients were excluded for the following reasons: (1) they were pregnant or lactating (n=23); (2) they had substance use disorder (n=25), which was diagnosed according to DSM-5 criteria for substance use disorder; (3) they had severe personality disorder (n=13), as diagnosed using the DSM-5 criteria for severe personality disorders;(4) they had severe physical diseases (n=12); (5) they refused to participate in the study (n=20); (6) they were excluded for other unknown reasons (n=5).
Trained researchers systematically distributed questionnaires to each participant to gather comprehensive information, including their age, gender, educational background, marital status, body mass index (BMI), age of disease onset, and duration of disease. We meticulously reviewed the available medical records of participants. To address any missing data or ambiguous responses, we conducted supplementary interviews with relatives or attending physicians.
We used the positive subscale of the Positive and Negative Syndrome Scale (PANSS) to identify psychotic symptoms (34). It comprises seven items scored on a 7-point scale. The total score ranges from 7 (absent) to 49 (high) (extremely severe). Participants were considered to have psychotic symptoms if their score was greater than or equal to 15. Participants with a score less than or equal to 14 were considered to have no psychotic symptoms (34).
We used the 17-item Hamilton Depression Rating Scale (HAMD) (35) to evaluate the level of depression in patients. This scale comprises 17 items, including eight items rated on a five-point scale (0: not present, 4: severe) and nine items rated on a three-point scale (0: not present, 2: severe). We determined the presence and severity of depression based on the cumulative HAMD score. The Chinese version of this scale has been validated for its reliability and validity (35).
We used the 14-item Hamilton Anxiety Rating Scale (HAMA) (36) to assess the anxiety levels of participants. This scale comprises 14 items, measured using a five-point Likert scale (0: not present, 4: severe), with a maximum score of 56 points. We assessed the presence and severity of anxiety based on the total HAMA score. The Chinese version of this scale also has good reliability and validity (36).
The Perceived Stress Scale (PSS) (37) is used to measure stress levels in participants. The PSS has seven items, all of which are rated on a five-point Likert scale (0: not present, 4: severe). We instructed the participants to record their responses promptly based on their honest opinions about a particular event in the preceding month. We assessed the existence and degree of stress based on the total PSS score.
Two experienced psychiatrists with specialized training collected the above information. They had no prior knowledge of the clinical data of the participants. After repeated evaluation, the observer correlation coefficients of the HAMD total score and HAMA total score were found to be greater than 0.8.
For data analysis, we used the Kolmogorov Smirnov single-sample test to perform normality test on the data of each variable. The data were not normally distributed. When we compared the demographic and clinical variables between groups with and without symptoms of mental illness, we used the Mann-Whitney U test for continuous variables and the Chi-square test for categorical variables. We performed binary logistic regression analysis using factors closely related to psychotic symptoms, with gender and age included as covariates. We conducted statistical analyses using SPSS version 23.0.
Our study had 899 patients (579 females, 320 males). The median age of the patients was 33 years (range: 18 to 60 years). Of the patients, 597 had a bachelor’s degree or less advanced degree, and 302 patients had a bachelor’s degree or more advanced degree. The median age of illness onset was 32 years (range: 17 to 60 years). The median duration of illness was 11 years (range: 6.5 to 32 years). There were no significant differences in these demographic characteristics between the two groups of patients with and without psychotic symptoms.
Patients with somatic symptoms disorder had a 10.2% (92/899) prevalence of psychotic symptoms. Among patients, 9.59% (28/292) of males and 12.42% (64/515) of females exhibit psychotic symptoms; the difference was not significant (χ2 = 1.19, p = 0.275).We compared and presented the demographic and clinical characteristics of patients with and without psychotic symptoms in Table 1. We observed significant differences between the psychotic and non-psychotic patient groups with respect to the following variables: HAMD (Z = -11.374, p<0.001), HAMA (Z= -13.577, p<0.001), PSS total score (Z=-9.394, p<0.001). Patients with psychotic symptoms exhibited higher levels of anxiety and depression than patients without psychotic symptoms. In addition, patients with psychotic symptoms experienced more severe stress than patients without psychotic symptoms. Figures 1-3 display the significant differences in HAMD, HAMA, and PSS total scores, with the corresponding levels of significance marked.
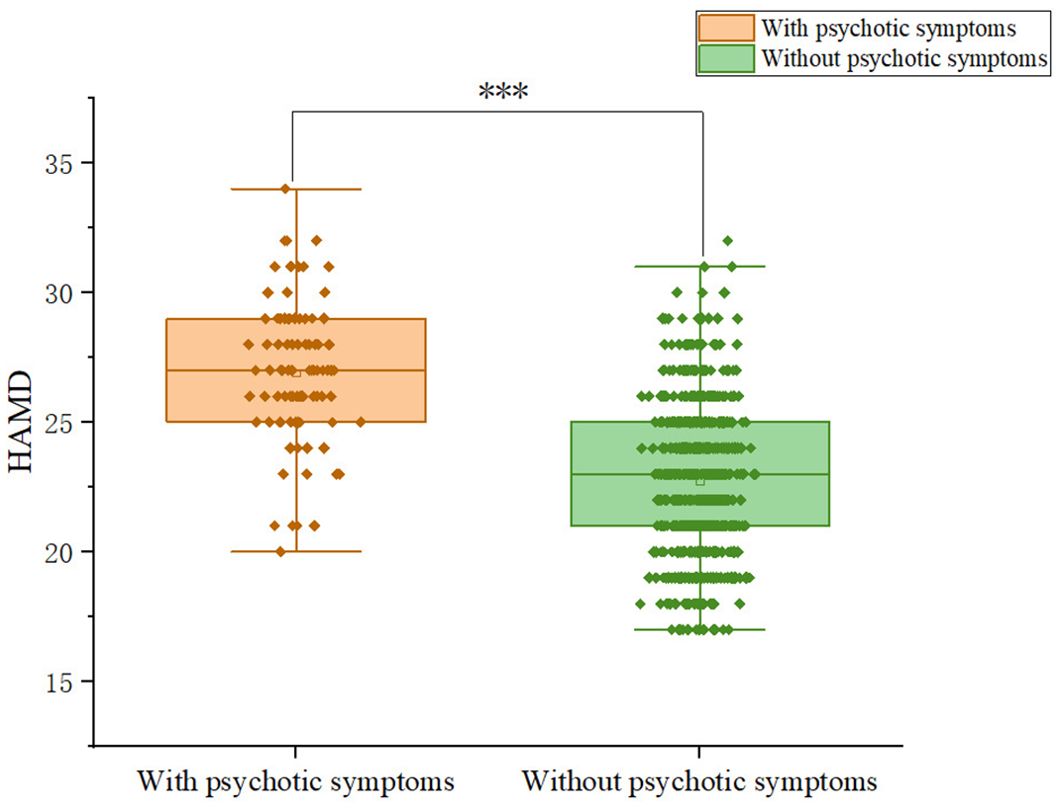
Figure 1. Differences in HAMD scores between participants with and without comorbid psychiatric symptoms.
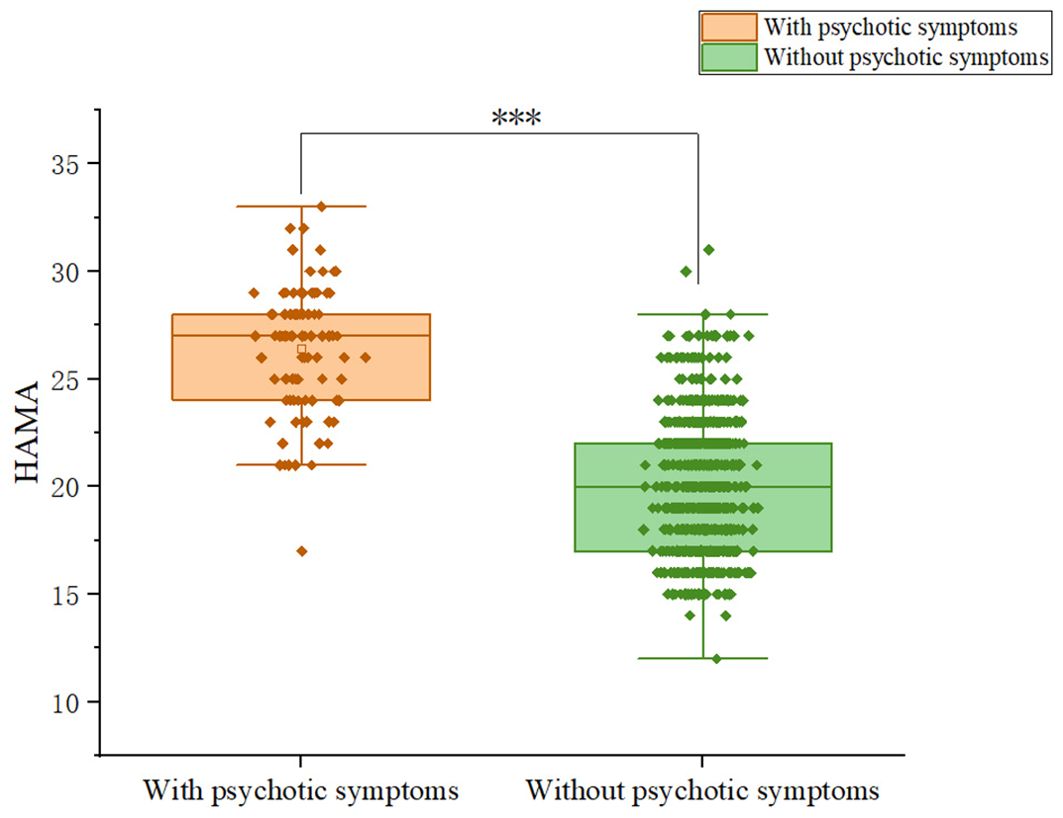
Figure 2. Differences in HAMA scores between participants with and without comorbid psychiatric symptoms.

Figure 3. Differences in PSS scores between participants with and without comorbid psychiatric symptoms.
We performed a stepwise forward binary logistic regression to identify the risk factors for psychotic symptoms. After controlling for the covariates of gender and age, the following variables reached statistical significance: The HAMA score (odds ratio = 1.175, 95% CI = 1.022—1.352, Wald χ2 = 71.927, p<0.001), HAMD score (odds ratio = 1.175, 95% CI = 1.022—1.352, Wald χ2 = 5.11 p = 0.024), PSS total score (odds ratio = 1.170, 95% CI = 1.044—1.312 Wald χ2 = 7.268, p = 0.007), were important predictors for psychotic symptoms in patients. The VIF values of the independent variables in the model are all less than 5, indicating that there is no significant multicollinearity issue among the predictors (35). Table 2 showed the results of the logistic regression.
To the best of our knowledge, this is the first study to identify the prevalence and risk factors of psychotic symptoms in Chinese Han patients with somatic symptoms disorder. We observed concomitant psychotic symptoms in 10.2% of patients with SSD. Additionally, some clinical characteristics, such as greater HAMD,HAMA and PSS scores were identified as risk factors for psychotic symptoms.
The prevalence rate of psychotic symptoms was higher in our sample than in some studies reporting the combined rate of other psychiatric disorders and psychotic symptoms (38). Compared to the prevalence of other comorbidities in patients with SSD, the prevalence of psychotic symptoms in patients with SSD surpasses the combined prevalence of SSD with depression (4.1%) and anxiety (5.5%) (39). This indicates that patients with SSD patients experience greater complications when they also experience psychotic symptoms; this warrants greater attention from researchers. To investigate the high co-incidence of psychotic symptoms in patients with SSD, we combined data from previous studies and arrived at the following plausible explanations. First, patients with SSD are more likely to be hypersensitive and react more severely to physical symptoms than patients with other mental health conditions (40). Some findings indicate that hallucinations are a response to hypervigilance (41, 42). Patients with SSD are prone to mental health symptoms, probably owing to their hypervigilance toward physical symptoms. Second, patients with SSD repeatedly seek medical treatment without being able to identify the cause (43) and repeatedly experience setbacks. Patients with SSD also exhibit a weak coping ability (44) and resilience (45). Also, because they do not have substantial coping ability and mental resilience to deal with such setbacks, they are more prone to psychotic symptoms (46).
Anxiety and depression are significantly correlated with psychotic symptoms (27, 47, 48)and represent the most prevalent complications among patients with SSD (49–51). Our research further elucidated that anxiety and depression are predictors of psychotic symptoms in patients with SSD. The predictive effects of anxiety and depression on psychotic symptoms have been extensively validated in previous studies (52–54). For instance, Machado et al. conducted a three-year longitudinal study demonstrating that symptoms of childhood anxiety in populations at a high risk for psychosis can serve as predictors of psychotic symptoms during adolescence (55). Smith et al. discovered that a greater severity of depression correlates with the intensity of auditory hallucinations and delusions of victimization (56). The mechanisms by which depression and anxiety influence psychotic symptoms have been reported in some studies. Morrison et al. (57) posited from a cognitive standpoint that anxiety and depression play a direct role in both the development and persistence of delusions and hallucinations. They stated that negative emotions may cause patients to misinterpret fundamentally regular experiences as threatening events, making them feel distressed and experience psychotic phenomena. This fosters a detrimental cycle of adverse emotions, physiological alterations, and safety-seeking behaviors in affected individuals. Bental et al. (58) reported an alternative explanation based on psychological defense mechanisms. They proposed that delusions—particularly those characterized by persecutory themes—are a defensive response to low self-esteem and depression. In summary, anxiety and depression not only represent prevalent mental health challenges for patients with SSD but also serve as significant predictors for psychotic symptoms. This finding highlights the importance of focusing on and effectively managing the emotional well-being of such patients during therapeutic interventions for SSD. Treatment strategies should include comprehensive psychological evaluations aimed at accurately identifying and assessing levels of anxiety and depression in such patients.
The experience of stress is frequently closely linked to the development of various mental health conditions (59–61). The findings from this study substantiate this notion and indicate that stress is a pivotal factor in forecasting psychotic symptoms in patients with SSD. Previous research findings have elucidated the underlying mechanisms through which stress influences psychotic symptoms across multiple dimensions, including psychological, physiological, and genetic factors. The stress-vulnerability model proposed by Zubin and Spring (62) underscores that experiences of stress play a crucial role in precipitating acute psychotic episodes. Specifically, inadequately managed stressful events, coupled with significant distress and anxiety, may exacerbate psychotic symptoms in individuals who are highly susceptible. Furthermore, stress can impact mental health via biological pathways. Psychosocial stressors can disrupt the functional equilibrium of the hypothalamic-pituitary-adrenal axis (63) and potentially influence neurotransmitter transmission (64). These biological alterations are integral to understanding how stress contributes to the pathological processes associated with mental disorders (65). From a genetic standpoint, evidence suggests the existence of familial predisposition of sensitivity to stress responses as a risk factor for psychotic symptoms (66). This sensitivity may be inherited through genetic mechanisms (30, 67). Given the multifaceted impact of stress on psychiatric manifestations spanning various domains, strengthening interdisciplinary collaboration among psychiatry, neurology, psychology, and other relevant fields to collectively deliver comprehensive medical services to affected patients is recommended.
In this study, we systematically investigated the prevalence of psychotic symptoms in patients with SSD, drawing on a large clinical data sample from China, and explored the potential predictive factors. This area of research has received relatively limited attention in research but holds significance in clinical practice. Our findings aim to enrich the investigations in this field by providing detailed and representative data. Additionally, these findings are closely linked to clinical practice and may offer more precise guidance for doctors in their daily work, aiding the identification and assessment of and interventions for psychotic symptoms in patients with SSD. This would help positively influence the rehabilitation process.
We observed several limitations of this study. First, the absence of healthy controls matched for age and gender with the participants is a significant limitation, which may have introduced bias when comparing our patient cohort with those from other studies. Second, due to the cross-sectional nature of our study, we were unable to establish causal relationships between clinical variables and psychotic symptoms in patients with somatic symptom disorder (SSD). Third, various genetic and environmental factors, such as genetic predisposition, levels of brain-derived neurotrophic factors, physical activity, and a family history of psychotic symptoms, may influence these symptoms. However, we did not collect such data in our investigation. Additionally, the sampling scope of this study was limited to Jiangxi Province, and future studies should aim to collect more representative data from a wider range of regions to enhance the generalizability of the findings. Furthermore, this study did not address the potential impact of socioeconomic status and healthcare accessibility on psychotic symptoms, which is an important factor for future investigations. In future research, large-scale controlled prospective studies should be conducted to better elucidate the relationship between these factors and psychotic symptoms.
In summary, the findings of this study revealed a 10.2% prevalence of psychotic symptoms in patients with SSD, suggesting that psychotic symptoms are common in patients with SSD in the Chinese Han population. Furthermore, our findings showed that greater levels of anxiety, depression and stress (indicated by high HAMA, HAMD, PSS) are risk factors for psychotic symptoms in patients with SSD. Psychotic symptoms result in increased anxiety, depression, and stress levels. Understanding the risk factors of psychotic symptoms can help identify their implications. This can help develop interventions and preventive methods and reduce the burden of psychotic symptoms in patients with SSD.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by The Institutional Review Board of the Third People’s Hospital of Ganzhou (ethics code: gzsyy2024044). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
JY: Formal analysis, Methodology, Writing – original draft. YZ: Data curation, Formal analysis, Investigation, Writing – review & editing. YBL: Data curation, Formal analysis, Investigation, Writing – review & editing. YPL: Data curation, Investigation, Writing – review & editing. HT: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Guha M. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Association. (2013).
2. Rief W, Martin A. How to use the new DSM-5 somatic symptom disorder diagnosis in research and practice: a critical evaluation and a proposal for modifications. Annu Rev Clin Psychol. (2014) 10:339–67. doi: 10.1146/annurev-clinpsy-032813-153745
3. Fink P, Sørensen L, Engberg M, Holm M, Munk-Jørgensen P. Somatization in primary care. Prevalence, health care utilization, and general practitioner recognition. Psychosomatics. (1999) 40:330–8. doi: 10.1016/S0033-3182(99)71228-4
4. Bener A, Al-Kazaz M, Ftouni D, Al-Harthy M, Dafeeah EE. Diagnostic overlap of depressive, anxiety, stress and somatoform disorders in primary care. Asia Pac Psychiatry. (2013) 5:E29–38. doi: 10.1111/j.1758-5872.2012.00215.x
5. Alosaimi FD, Abalhassan M, Alhaddad B, Alzain N, Fallata E, Alhabbad A, et al. Prevalence of metabolic syndrome and its components among patients with various psychiatric diagnoses and treatments: A cross-sectional study. Gen Hosp Psychiatry. (2017) 45:62–9. doi: 10.1016/j.genhosppsych.2016.12.007
6. Löwe B, Levenson J, Depping M, Hüsing P, Kohlmann S, Lehmann M, et al. Somatic symptom disorder: a scoping review on the empirical evidence of a new diagnosis. Psychol Med. (2022) 52:632–48. doi: 10.1017/S0033291721004177
7. Cao J, Wei J, Fritzsche K, Toussaint AC, Li T, Jiang Y, et al. Prevalence of DSM-5 somatic symptom disorder in Chinese outpatients from general hospital care. Gen Hosp Psychiatry. (2020) 62:63–71. doi: 10.1016/j.genhosppsych.2019.11.010
8. Harris AM, Orav EJ, Bates DW, Barsky AJ. Somatization increases disability independent of comorbidity. J Gen Intern Med. (2009) 24:155–61. doi: 10.1007/s11606-008-0845-0
9. Yang X, Luo J, Wang P, He Y, Wang C, Yang L, et al. Characteristics and economic burden of patients with somatoform disorders in Chinese general hospitals: a multicenter cross-sectional study. Ann Gen Psychiatry. (2023) 22:30. doi: 10.1186/s12991-023-00457-y
10. van Geelen SM, Rydelius PA, Hagquist C. Somatic symptoms and psychological concerns in a general adolescent population: Exploring the relevance of DSM-5 somatic symptom disorder. J Psychosom Res. (2015) 79:251–8. doi: 10.1016/j.jpsychores.2015.07.012
11. Kruse J, Heckrath C, Schmitz N, Alberti L, Tress W. Diagnosis and management of patients with psychogenic disorders in family practice. Results of a field study. Psychother Psychosom Med Psychol. (1999) 49:14–22.
12. Schumacher S, Rief W, Klaus K, Brähler E, Mewes R. Medium- and long-term prognostic validity of competing classification proposals for the former somatoform disorders. Psychol Med. (2017) 47(10):1719–32. doi: 10.1017/S0033291717000149
13. Smith GR Jr, Monson RA, Ray DC. Psychiatric consultation in somatization disorder. A randomized controlled study. N Engl J Med. (1986) 314:1407–13. doi: 10.1056/NEJM198605293142203
14. Barsky AJ, Orav EJ, Bates DW. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry. (2005) 62:903–10. doi: 10.1001/archpsyc.62.8.903
15. Smith GR Jr, Monson RA, Ray DC. Patients with multiple unexplained symptoms. Their characteristics, functional health, and health care utilization. Arch Intern Med. (1986) 146:69–72. doi: 10.1001/archinte.1986.00360130079012
16. Smith GR Jr, Rost K, Kashner TM. A trial of the effect of a standardized psychiatric consultation on health outcomes and costs in somatizing patients. Arch Gen Psychiatry. (1995) 52:238–43. doi: 10.1001/archpsyc.1995.03950150070012
17. Mayou EBR, Bass C, Sharpe AM. Treatment of functional somatic symptoms. New York: Oxford University Press (1995).
18. Thakur M, Hays J, Krishnanu KRR. Clinical, demographic and social characteristics of psychotic depression. Psychiatry Res. (1999) 86:99–106. doi: 10.1016/S0165-1781(99)00030-X
19. Charney DS, Nelson JC. Delusional and nondelusional unipolar depression: further evidence for distinct subtypes. Am J Psychiatry. (1981) 138:328–33. doi: 10.1176/ajp.138.3.328
20. Coryell W, Pfohl B, Zimmerman M. The clinical and neuroendocrine features of psychotic depression. J Nerv Ment Dis. (1984) 172:521–8. doi: 10.1097/00005053-198409000-00002
21. Gaudiano BA, Miller IW. Dysfunctional cognitions in hospitalized patients with psychotic versus nonpsychotic major depression. Compr Psychiatry. (2007) 48:357–65. doi: 10.1016/j.comppsych.2007.03.003
22. Serretti A, Lattuada E, Cusin C, Gasperini M, Smeraldi E. Clinical and demographic features of psychotic and nonpsychotic depression. Compr Psychiatry. (1999) 40:358–62. doi: 10.1016/S0010-440X(99)90141-4
23. Sevilla-Llewellyn-Jones J, Cano-Domínguez P, de-Luis-Matilla A, Peñuelas-Calvo I, Espina-Eizaguirre A, Moreno-Kustner B, et al. Personality traits and psychotic symptoms in recent onset of psychosis patients. Compr Psychiatry. (2017) 74:109–17. doi: 10.1016/j.comppsych.2017.01.006
24. Rosmalen JG, Neeleman J, Gans RO, de Jonge P. The association between neuroticism and self-reported common somatic symptoms in a population cohort. J Psychosom Res. (2007) 62:305–11. doi: 10.1016/j.jpsychores.2006.10.014
25. Russo J, Katon W, Sullivan M, Clark M, Buchwald D. Severity of somatization and its relationship to psychiatric disorders and personality. Psychosomatics. (1994) 35:546–56. doi: 10.1016/S0033-3182(94)71723-0
26. Lieb R, Meinlschmidt G, Araya R. Epidemiology of the association between somatoform disorders and anxiety and depressive disorders: an update. Psychosom Med. (2007) 69:860–3. doi: 10.1097/PSY.0b013e31815b0103
27. Hartley S, Barrowclough C, Haddock G. Anxiety and depression in psychosis: a systematic review of associations with positive psychotic symptoms. Acta Psychiatr Scand. (2013) 128:327–46. doi: 10.1111/acps.2013.128.issue-5
28. Chaumette B, Kebir O, Bourgin J, Godsil BP, Gaillard R, Jay TM, et al. Stress and psychotic transition: A literature review. L'encephale. (2016) 42(4):367–73. doi: 10.1016/j.encep.2015.10.001
29. von dem Knesebeck O, Lehmann M, Löwe B, Lüdecke D. Causal attributions for somatic symptom disorder. J Psychosom Res. (2020) 129:109910. doi: 10.1016/j.jpsychores.2019.109910
30. Wichers M, Myin-Germeys I, Jacobs N, Peeters F, Kenis G, Derom C, et al. Genetic risk of depression and stress-induced negative affect in daily life. Br J Psychiatry. (2007) 191(3):218–23. doi: 10.1192/bjp.bp.106.032201
31. Carter B, Wootten J, Archie S, Terry AL, Anderson KK. Sex and gender differences in symptoms of early psychosis: a systematic review and meta-analysis. Arch Womens Ment Health. (2022) 25:679–91. doi: 10.1007/s00737-022-01247-3
32. Köhler S, van Os J, de Graaf R, Vollebergh W, Verhey F, Krabbendam L. Psychosis risk as a function of age at onset: a comparison between early- and late-onset psychosis in a general population sample. Soc Psychiatry Psychiatr Epidemiol. (2007) 42:288–94. doi: 10.1007/s00127-007-0171-6
33. Schultze-Lutter F, Ruhrmann S, Michel C, Kindler J, Schimmelmann BG, Schmidt SJ. Age effects on basic symptoms in the community: A route to gain new insight into the neurodevelopment of psychosis. Eur Arch Psychiatry Clin Neurosci. (2020) 270:311–24. doi: 10.1007/s00406-018-0949-4
34. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
35. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
36. Thompson E. Hamilton Rating Scale for Anxiety (HAM-A). Occup Med (Lond). (2015) 65:601. doi: 10.1093/occmed/kqv054
38. Gaudiano BA, Dalrymple KL, Zimmerman M. Prevalence and clinical characteristics of psychotic versus nonpsychotic major depression in a general psychiatric outpatient clinic. Depression Anxiety. (2010) 26:54–64. doi: 10.1002/da.20470
39. de Waal MW, Arnold IA, Eekhof JA, van Hemert AM. Somatoform disorders in general practice: prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br J Psychiatry. (2004) 184:470–6. doi: 10.1192/bjp.184.6.470
40. Verkuil B, Brosschot JF, Thayer JF. A sensitive body or a sensitive mind? Associations among somatic sensitization, cognitive sensitization, health worry, and subjective health complaints. J Psychosom Res. (2007) 63:673–81. doi: 10.1016/j.jpsychores.2007.08.010
41. Kelleher I, Jenner JA, Cannon M. Psychotic symptoms in the general population - an evolutionary perspective. Br J Psychiatry. (2010) 197(3):167–9. doi: 10.1192/bjp.bp.109.076018
42. Wiersma D, Jenner JA, Nienhuis FJ, van de Willige G. Hallucination focused integrative treatment improves quality of life in schizophrenia patients. Acta Psychiatr Scand. (2004) 109:194–201. doi: 10.1046/j.0001-690X.2003.00237.x
43. Noeker M. Somatoforme Störungen - Einführung in denThemenschwerpunkt. KINDHEIT UND ENTWICKLUNG. (2002) 11:129–39. doi: 10.1026//0942-5403.11.3.129
44. Leonidou C, Panayiotou G, Bati A, Karekla M. Coping with psychosomatic symptoms: The buffering role of psychological flexibility and impact on quality of life. J Health Psychol. (2019) 24:175–87. doi: 10.1177/1359105316666657
45. Färber F, Rosendahl J. The association between resilience and mental health in the somatically ill. Dtsch Arztebl Int. (2018) 115:621–7. doi: 10.3238/arztebl.2018.0621
46. Campbell-Sills L, Cohan SL, Stein MB. Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behav Res Ther. (2006) 44:585–99. doi: 10.1016/j.brat.2005.05.001
47. Wigman JT, van Nierop M, Vollebergh WA, Lieb R, Beesdo-Baum K, Wittchen HU, et al. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity–implications for diagnosis and ultra-high risk research. Schizophr Bull. (2012) 38(2):247–57. doi: 10.1093/schbul/sbr196
48. Kanchanatawan B, Thika S, Sirivichayakul S, Carvalho AF, Geffard M, Maes M. In schizophrenia, depression, anxiety, and physiosomatic symptoms are strongly related to psychotic symptoms and excitation, impairments in episodic memory, and increased production of neurotoxic tryptophan catabolites: a multivariate and machine learning study. Neurotox Res. (2018) 33:641–55. doi: 10.1007/s12640-018-9868-4
49. Huang WL, Chang SS, Wu SC, Liao SC. Population-based prevalence of somatic symptom disorder and comorbid depression and anxiety in Taiwan. Asian J Psychiatr. (2023) 79:103382. doi: 10.1016/j.ajp.2022.103382
50. Haug TT, Mykletun A, Dahl AA. The association between anxiety, depression, and somatic symptoms in a large population: the HUNT-II study. Psychosom Med. (2004) 66:845–51. doi: 10.1097/01.psy.0000145823.85658.0c
51. Bekhuis E, Boschloo L, Rosmalen JG, Schoevers RA. Differential associations of specific depressive and anxiety disorders with somatic symptoms. J Psychosom Res. (2015) 78:116–22. doi: 10.1016/j.jpsychores.2014.11.007
52. Steinbrecher N, Hiller W. Course and prediction of somatoform disorder and medically unexplained symptoms in primary care. Gen Hosp Psychiatry. (2011) 33:318–26. doi: 10.1016/j.genhosppsych.2011.05.002
53. Creed FH, Tomenson B, Chew-Graham C, Macfarlane GJ, Davies I, Jackson J, et al. Multiple somatic symptoms predict impaired health status in functional somatic syndromes. Int J Behav Med. (2013) 20:194–205. doi: 10.1007/s12529-012-9257-y
54. Campo JV. Annual research review: functional somatic symptoms and associated anxiety and depression–developmental psychopathology in pediatric practice. J Child Psychol Psychiatry. (2012) 53:575–92. doi: 10.1111/j.1469-7610.2012.02535.x
55. MaChado V, Fonseca L, Barbosa MG, Bressan RA, Pan P, Rohde LA, et al. Childhood anxiety symptoms as a predictor of psychotic experiences in adolescence in a high-risk cohort for psychiatric disorders. Schizophr Bull Open. (2024) 5(1):sgae003. doi: 10.1093/schizbullopen/sgae003
56. Smith B, Fowler DG, Freeman D, Bebbington P, Bashforth H, Garety P, et al. Emotion and psychosis: links between depression, self-esteem, negative schematic beliefs and delusions and hallucinations. Schizophr Res. (2006) 86(1-3):181–8. doi: 10.1016/j.schres.2006.06.018
57. Morrison AP. The interpretation of intrusions in psychosis: an integrative cognitive approach to hallucinations and delusions. Behav Cogn Psychother. (2001) 29(3):257–76. doi: 10.1017/S1352465801003010
58. Bentall RP, Kinderman P, Kaney S. The self, attributional processes and abnormal beliefs: towards a model of persecutory delusions. Behav Res Ther. (1994) 32:331–41. doi: 10.1016/0005-7967(94)90131-7
59. Ottenweller JE, Natelson BH, Pitman DL, Drastal SD. Adrenocortical and behavioral responses to repeated stressors: toward an animal model of chronic stress and stress-related mental illness. Biol Psychiatry. (1989) 26:829–41. doi: 10.1016/0006-3223(89)90123-6
60. Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learn Mem. (2008) 15:551–64. doi: 10.1101/lm.921708
61. Bartolomucci A, Leopardi R. Stress and depression: preclinical research and clinical implications. PloS One. (2009) 4:e4265. doi: 10.1371/journal.pone.0004265
62. Zubin J, Spring B. Vulnerability–a new view of schizophrenia. J Abnorm Psychol. (1977) 86:103–26. doi: 10.1037/0021-843X.86.2.103
63. Pariante CM, Lapiz-Bluhm MD. The interface of stress and the HPA axis in behavioural phenotypes of mental illness. Curr Top Behav Neurosci. (2014) 18(Chapter 304):13–24. doi: 10.1007/7854_2014_304
64. Phillips LJ, Francey SM, Edwards J, McMurray N. Stress and psychosis: towards the development of new models of investigation. Clin Psychol Rev. (2007) 27:307–17. doi: 10.1016/j.cpr.2006.10.003
65. Strik W, Dierks T. Neurophysiological mechanisms of psychotic symptoms. Eur Arch Psychiatry Clin Neurosci. (2008) 258(Suppl 5):66–70. doi: 10.1007/s00406-008-5016-0
66. Lataster T, Collip D, Lardinois M, van Os J, Myin-Germeys I. Evidence for a familial correlation between increased reactivity to stress and positive psychotic symptoms. Acta Psychiatr Scand. (2010) 122:395–404. doi: 10.1111/j.1600-0447.2010.01566.x
Keywords: psychotic symptoms, somatic symptom disorder, risk factors, anxiety, depression, stress
Citation: Yuan J, Zhong Y, Li Y, Liao Y and Tang H (2025) Psychotic symptoms in Chinese patients with somatic symptom disorder: prevalence, risk factors, and associated conditions. Front. Psychiatry 16:1519492. doi: 10.3389/fpsyt.2025.1519492
Received: 30 October 2024; Accepted: 29 January 2025;
Published: 26 February 2025.
Edited by:
Yan-Min Xu, Wuhan Mental Health Center, ChinaReviewed by:
Shinsuke Hidese, Teikyo University, JapanCopyright © 2025 Yuan, Zhong, Li, Liao and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Tang, dGFuZ2hvbmdAZ211LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.