
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 14 February 2025
Sec. Molecular Psychiatry
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1507064
This article is part of the Research TopicThe Individual and Joint Contributions of Molecular and Environmental Factors on Gene Expression and Psychopathology DevelopmentView all 6 articles
Introduction: The current study assessed the impact of self-reported stress measures on microRNA (miRNA) profiles in saliva exosomes. Saliva is one of the most accessible and non-invasive bodily fluids and exosomal miRNAs in saliva could be useful in (1) measuring stress states and (2) distinguishing between individuals suffering from high levels of chronic stress vs. adverse childhood experiences (ACEs). miRNAs are small, noncoding RNAs that act as gene regulators. Several studies have shown differential expressions of certain miRNA in neurological diseases and in stress, post-traumatic stress syndrome (PTSD) and anxiety. Detailed analyses of miRNA expressions and profiling of miRNAs among populations with various exposures to traumatic and life stressors have not been carried out. The goal of our study was to discover miRNAs associated with high chronic stress or childhood trauma.
Method: This study sought to explore miRNA expression in African American young women from a small, southern Historically Black College and University (HBCU). Twelve participants completed the social readjustment rating scale (SRRS), ACEs scale, and saliva collection and were divided into three groups based on ACE and chronic stress score: Low Chronic Stress (LCS; n = 4); High Chronic Stress (HCS; n = 4); High Chronic Stress + High ACEs (HCS+HA; n=4). A custom-made miRNA Taqman-Array tested for fold change in four miRNAs (i.e., miR-19b, miR-187, miR-34a and miR-135-3p).
Results: There was a significant downregulation of miR-19b (χ 2(2, N=12) = 7.42, p < 0.01, η²= 0.915), miR-187 (χ 2 (2, N = 12) = 7.36, p < 0.05, η²= 0.598), and miR-34a (χ 2(2, N = 12) = 7.42, p < 0.05, η²= 0.60). in both the HCS and the HCS+HA groups vs. LCS. Interestingly, miR-135-3p (χ 2(2, N = 12) = 8.00, p < 0.05, η²= 0.67. was upregulated in the HCS group vs. LCS and HCS+LA. Expression for miR-135-3p was not significantly different between LCS + HCS+HA.
Conclusion: Our analyses shows that miRNA extracted from salivary exosomes can be a reliable biomarker for stress and miR-135a-3p appears to be the most upregulated between LCS and HCS individuals and a potential candidate to corroborate self-reports on self-assessments and predict negative health outcomes. Given that HCS+HA did not show an upregulation of miR-135-3p but had similar expression in the other three miRs compared to HCS group may indicate an adaptive stress response following early life adversity. Further, downregulation in miR-135-3p in individuals with high levels of chronic stress could point to unknown childhood trauma exposure (e.g. closed adoptions, dissociative amnesia, abuse). A major limitation in this study is the small sample size and future directions include determining the predictive validity of these miRNAs in predicting onset of physical and mental health outcomes for early interventions in larger studies.
MicroRNAs (miR) have emerged as the critical regulators of gene expression at the post-transcriptional levels and are dynamically regulated during fear conditioning (1), stress adaptations (2, 3) and anxiety disorders (4). MiRNAs are small (18-22 nucleotides), non-coding RNAs that can regulate gene expression at transcriptional and post-transcriptional levels. Emerging data suggests human saliva may be a viable, non-invasive source to examine change in miRNA expression due to stress (5, 6) as well as detection and diagnosis of health outcomes (3, 7–13). Therefore, it would be logical to explore the role of chronic stress and stress associated with adverse childhood experiences (ACE) in regulating the expression of miRNAs to develop future non-invasive screens that predict negative health outcomes because of stress. ACEs include forms of abuse, neglect, household dysfunction, and other traumatic stressors experienced from birth to the age of 18. Kalmakis & Chandler (2015) (14) offer a more formal definition of adverse childhood experiences as “childhood events, varying in severity and often chronic, occurring in a child’s family or social environment that cause harm or distress, thereby disrupting the child’s physical or psychological health and development.” These are commonly measured using a self-report measure that gives each an ACE score based on their reported experiences.
Numerous studies have found a correlation between ACE scores and physical and mental health outcomes in adulthood. ACEs can evolve into abnormal fear processing and may result in deficiency in fear-inhibitory mechanisms and impairment in the ability to discriminate between safety and danger cues (15). Previous research on ACE indicates relationships between high ACE scores and adult alcohol problems (16), prescription drug use (17), migraines and vascular biomarkers (18), and decreased oxytocin levels (19). Higher ACE scores were correlated with anxiety disorder, PTSD, and bipolar disorder in a sample of low-income women (20). Salinas-Miranda et al., also found ACE to be linked to adult quality of life, particularly stress and sleep disorder (2015) (13). Numerous epidemiological studies suggest neurobiological impacts, particularly to the stress-response systems, from high level of ACEs (21). Early research on ACEs was conducted primarily among White, middle class populations. More contemporary research has expanded to include greater racial, ethnic, and socioeconomic diversity to explore how ACE impacts health outcomes. Studies of African Americans have found that high ACE scores are associated with greater risk of future cardiometabolic disorders and shortened leukocyte telomere length (22), and higher rates of substance use (23). Together, these studies provide evidence that early life adversity causes a wide range of negative outcomes in adulthood highlighting the need to identify biomarkers to screen for in early adulthood to lessen severity of negative outcomes later in adulthood.
Similarly, stressful life events have been found to correlate with both physical and mental health issues suggesting a greater need for understanding the role of stress interactions in onset of health issues (24, 25). Stressful life events are reported as more frequent among racial and ethnic minority groups (26) leading to a greater need for stress epigenetic research among these groups. Health-related stress events have also been linked to higher mortality rate (27) indicating the need for identifying non-invasive and high-throughput biomarker screens utilizing miRNAs found in salivary exosomes.
Several biomarkers of stress induced pathology have been evaluated, such as cortisol, salivary alpha-amylase, and inflammatory cytokines, but may be more unreliable in predicting outcomes due to modulations by circadian rhythm and other co-factors (28). miRNAs can be in various bodily fluids including blood, serum, plasma, and saliva (29). However, except for saliva, other means of exploring miRNAs are invasive. In saliva, miRNAs are well protected in exosomes and are considered a non-invasive source of biomarkers of clinically relevant consequences of stress (30). Even though miRNAs play an important role in responding to stress and other changes in a person’s environment, few miRNA studies have been conducted to understand alterations in miRNA in populations under stress (31–33) or to identify specific miRNA expressions in minority populations (34). We hypothesized that high levels of chronic stress would lead to changes in salivary microRNA expression that were distinct from low levels of chronic stress and that high levels of early life adversity, measured by the adverse childhood experience (ACE) scale would also show distinct miRNA profiles. Finally, studies focusing on African American populations, and specifically African American emerging adults are lacking in the current literature. Thus, this paper serves as a first to identify dynamic regulation of candidate miRNAs in a mostly African-American population characterized by high stress or low stress conditions that could serve as a clinically relevant biomarker screen for stress-related illnesses.
For this study we recruited female African Americans from within the university community located in a rural town (See Table 1 for Participant Demographics). The university is a Historically Black college (HBCU) with a 95% African American student population. The majority of students are from rural areas of the Southeast United States and 85% of the student population are Pell Grant eligible, indicating families fall below the Federal Poverty Line. The student population represents young adult African Americans who are likely to have been exposed to childhood or chronic stressors related to financial hardship. Twenty-six participants completed the consent process and survey packets. Out of 26 initial participants a total of 12 participants completed the survey packets and saliva collection (morning, afternoon, and evening) to allow for miRNA analysis for this pilot study.
Participants who responded to recruitment efforts on campus and met the study criteria came to the university campus, completed a consent form, and completed three questionnaires. After determining their stress using the ACE scale and the Social Readjustment Rating Scale (SRRS) they were divided into three groups: Low Chronic Stress (LCS), High Chronic Stress (HCS), High Chronic Stress + High ACEs (HCS+HE). Saliva collection took place over one week where participants signed up for a day to complete saliva collection in the morning, afternoon, and evening. Individuals who successfully submitted for saliva collection three times in one day and whose samples were viable were then analyzed.
The self-administered questionnaires consisted of 1) A demographic survey including age, gender, annual income, and health behavior survey measuring alcohol and tobacco use. The second self-administered questionnaire was the Adverse Childhood Experiences survey. This scale measures the number of Adverse Childhood Experiences before the age of 18. It consists of ten items. The lowest score a participant can earn is 0 and the highest score is 10. For the purpose of this study, scores of 3 or above were considered high scores, indicating childhood exposure to some level of abuse, neglect, or household dysfunction. The third scale was the Holmes and Rahe Social Readjustment Rating scale, which measures stressful life experience in the past year. The Holmes and Rahe Social Readjustment Rating Scale (SRSS) is a scale measuring stressful life events that have occurred in the past year (35). Each item on the scale is assigned a life change unit. Death of a spouse, partner, or parent, for example, is the life event with the highest number of life change units at 100. Minor violation of the law is a life event with the lowest number of life change units at 10. The 43 items list contains both positive and negative life events that are common stressors. The scale can be used to measure the number of life events experienced each year or by adding up the total number of life change units. The SRSS was originally developed to measure stressful life events and susceptibility to illness. Individuals scoring about 300 were found to be at greater risk of illness or health change in the coming year (35). For the purpose of this study, scores of 300 or more were therefore considered the cut off for high chronic stress scores. Studies using the SRSS have consistently found a relationship between psychological distress and subsequent occurrence of illness, despite criticisms of the life change unit assignment per life event.
Each participant’s survey packet and saliva samples were coded with a unique identifier to allow for matching a person’s miRNA profile analysis to their scores on the ACE and SRRS.
To evaluate the differentially expressed miRNAs we collected saliva samples from each participant at three different occasions during a single day. The exosomal miRNAs from each of the individual samples were isolated by utilizing total saliva exosome miRNA kit (Invitrogen Cat # 4478454) and characterized miRNA profile by utilizing custom made human miRNA proofing kit (ThermoFisher) Differentially expressed miRNAs were identified and statistically significant changes in miRNA expression were correlated with ACE and SRRS scores (Table 2).
Saliva samples were collected three times from each of the individuals: first, in the morning (7:30 AM to 10:00 AM). In the afternoon (12:00 PM to 2:30 PM), and in the evening 5 PM to 7 PM). These times of collections were chosen to normalize the biological changes that occur during the circadian rhythm. During the collection period, participants were seated straight up and were instructed to refrain from speaking or swallowing. They allowed the saliva to accumulate in the floor of the mouth, and then spit it through a funnel into a pre-cooled saliva tube. Saliva was collected using the Saliva RNA Collection and Preservation Device (Norgen, Cat#RU53810). At least 1 mL of whole saliva was collected. Salivary flow rate was calculated by dividing the volume of collected saliva by the duration of collection time. After collection of saliva, the specimens were stored at 4°C for up to 6 h, after which it was stored at −80°C until use. The miRNAs were isolated the same day of collection by utilizing exosome mRNA collection kit (Invitrogen).
Exosomes were isolated from saliva samples (0.5–1.0 mL) using total exosome isolation reagent (Invitrogen, Carlsbad, CA, USA), in accordance with the manufacturer’s protocol and using polyethylene glycol (PEG) based reagents for exosome precipitation (36). Briefly, whole saliva samples were centrifuged at 2000× g (gravity) for 10 min at room temperature to remove cells and debris. Supernatant were incubated with total exosome isolation reagent (Invitrogen Cat# 4484453) at 8°C for 1 h. After incubation, samples were centrifuged at 10,000× g for 1 h at 8°C. Residual supernatants were then discarded, and the exosome pellets were collected. Following exosome isolation, the pellet was treated with Rnase A to degrade any residual cellular RNAs in order to ensure that all detected RNA was exosomal in origin. The pellets were then re-suspended in a 200 μL volume (calculated by salivary supernatant volume) of exosome re-suspension buffer (provided by the manufacturer-Invitrogen).
In order to isolate and purify the miRs, we utilized TaqMan miRNA ABC purification kit (Applied Biosystems Cat #4473087). We isolated miRNAS according to the manufacturer’s protocol. Briefly, the total exosomal miRNAs isolated from each of the individual were transferred to tubes containing miRNA-beads, then the miRNAs were hybridized to the beads. The beads were isolated by magnetic rack, washed and miRNAs were eluted using 70°C Thermomixer at 1200 rpm.
We utilized Applied Biosystem “Custom RT and Preamplification (pools on Custom TaqMan Array microRNA Cards (Life Technologies, protocol #4478705) to analyze the differential expression of nine miRNAs. The cDNA synthesis was performed using customized miRNA Reverse Transcriptase (RT) pool without amplification. The comparative RT-PCR was performed with internal control U6 for each sample, according to the manufacturers’ protocol. The RT primer pool designed to analyze saliva miRNAs consisted of 4 (miR-19b, miR-187, miR-34a and miR-135-3p) miRNA specific primers sets, respectively. The following custom primers were used:
hsa-miR-19b-3p MIMAT0000074 UGUGCAAAUCCAUGCAAAACUGA;
hsa-miR-187-3p MIMAT0000262 UCGUGUCUUGUGUUGCAGCCGG;
hsa-miR-34a-3p MIMAT0004557 CAAUCAGCAAGUAUACUGCCCU;
hsa-miR-135a-3p MIMAT0004595 UAUAGGGAUUGGAGCCGUGGCG
A positive control reverse transcription reaction with the small nucleolar RNA U6 was performed using specific primers. These miRNAs were selected based on their relevance and expression levels in human saliva and plasma with respect to stress and anxiety.
After cDNA synthesis of samples, utilizing internal control U6, comparative real time PCR was carried out. Taqman® Universal Master Mix II (cat# 4440040) supplied by applied biosystem was used. Custom design plates with microRNA specific primers, Taqman® probe NGF-MGB, Taqman® assay 20x and passive reference ROX was used. 10 µl of cDNA sample and Taqman® master mix was added to bring the total volume of 20 µl. The cycle condition consisted of polymerase activation at 95°C for 10 minutes, then 40 cycle denaturation at 95°C for 15 seconds, followed by annealing/extension at 60°C for 1 minute. The experiment was repeated three times.
For internal control U6 Taqman® microRNA assay (Cat# 4427975) supplied by applied biosystem was used to perform RT-PCR. 1 µl Taqman® microRNA assay 20x, 5 µl of cDNA, 10 µl of PCR master mix, 4 µl of nucleases free water was mixed to make final volume of 20 µl. Delta cycle threshold was utilized to calculate the Fold Change for each microRNA sample.
Threshold cycle (CT) values were obtained from the qPCR analysis for both the target gene and U6 (37). ΔCT was calculated for each sample by subtracting the CT value of U6 (internal control) from the CT value of the target gene:
To calculate the relative expression of the target gene normalized to U6, the following formula was applied:
This equation derives from the assumption that a decrease of one CT corresponds to a doubling of the gene product. Therefore, 2−ΔCT provides a measure of how much more or less the target gene is expressed relative to the U6 control. A smaller ΔCT value leads to a larger 2−ΔCT indicating higher gene expression, while a larger ΔCT indicates lower expression. All reactions were performed in triplicate, and mean 2−ΔCT values were calculated for each sample. Data were expressed as mean ± standard deviation.
To evaluate the discrimination power of candidate miRNA biomarker for Stress in a limited sample size, we carried out a non-parametric Kruskal-Wallis test with Dunn’s post-hoc comparisons of four miRNAs expressions in three group of individuals: i) with low chronic stress, ii) High chronic stress and iii) high chronic stress with high ACE. Pearson correlations were run between candidate miRNA, SRSS, and ACEs. Significance was determined by p < 0.05. Statistical analysis was performed using JASP.
In the present study, we aim to identify miRs associated with high self-reported stress over the last year without ACE and with ACE as compared to controls with low stress over the last year in expression of salivary exosomal miRNAs. The major aim of the study was to identify certain miRNAs as a phenotypical marker of high stress conditions by characterizing miRs that are either significantly upregulated or downregulated as a function of both recent stress and ACEs.
Results from a Kruskal-Wallis test were significant (χ2(2, N=12) = 7.42, p < 0.01, η²= 0.915). Dunn’s post-hoc tests show LCS was significantly different compared to HCS (z=2.45, p <0.05) and HCS + HA (z=2.25, p <.05). HCS and HCS + HA were not significantly different (Figure 1A).
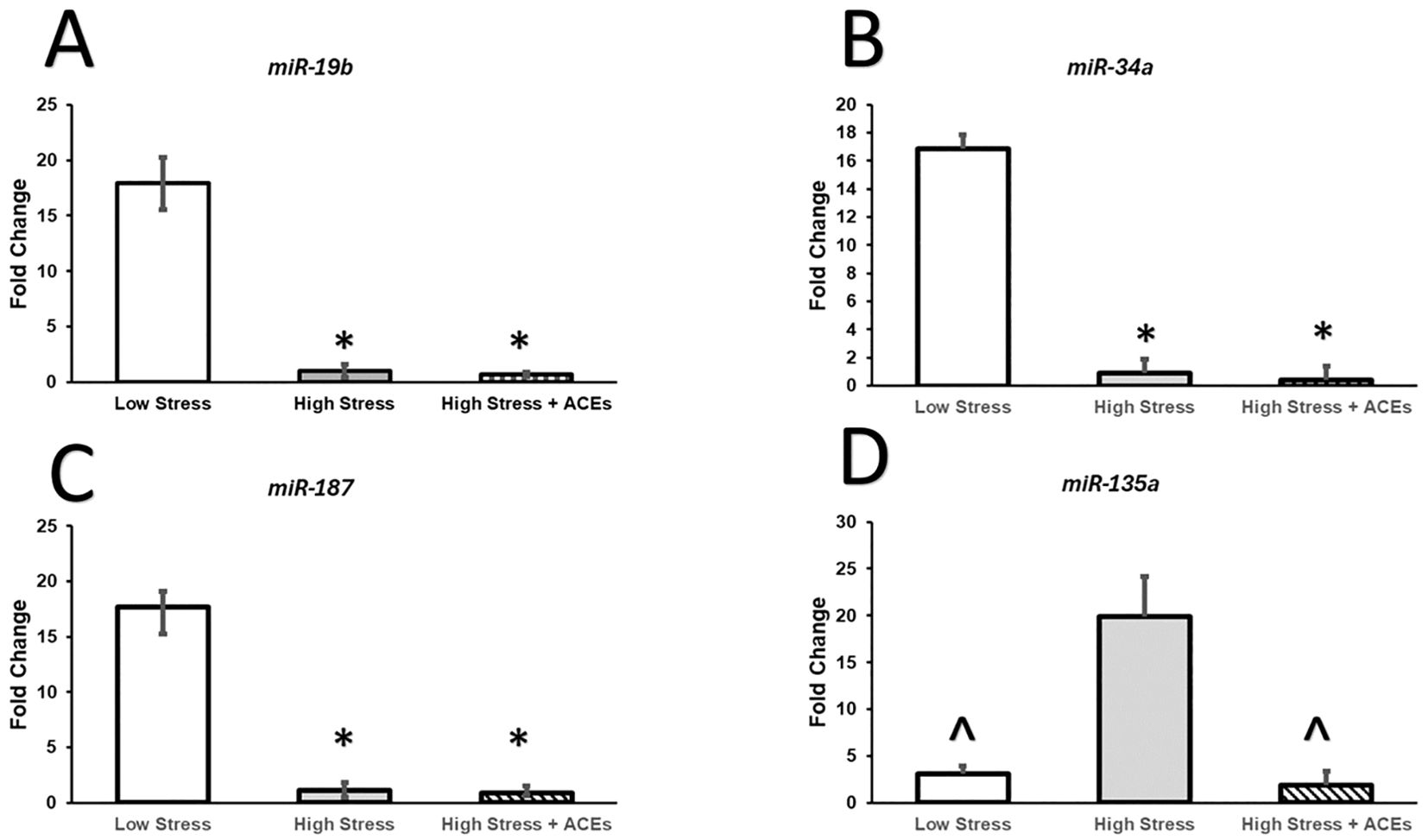
Figure 1. Relative expression of miRNAs is altered by High Chronic Stress and Adverse Childhood Experiences. (A–C) Relative expression of miR-19b, miR-34a, and miR-187 is decreased in individuals with High Chronic Stress (HCS) and High Chronic Stress coupled with High ACEs (HCS+HA). (D) Levels of High Chronic Stress increase expression of miR-135a compared to Low Chronic Stress (LCS) and High Chronic Stress coupled with High ACEs (HCS+HA). The decreased expression of miR-135a in High Stress with High ACEs could reflect an adaptive stress response due to the early insult to the stress response system. This profile could also help identify women with unknown childhood trauma and improve health intervention efficacy as early life stress manifests neurobiological alterations differently than chronic stress in adulthood. Bars represent group means and SEM is plotted by error bars. * depicts significant Bonferroni post hoc compared to LCS, ^ depicts significant Bonferroni post hoc compared to HCS.
Results from a Kruskal-Wallis test were significant (χ2 (2, N = 12) = 7.36, p < 0.05, η²= 0.598. Dunn’s post-hoc tests show miR-187 in the LCS condition was significantly higher compared to HCS (z=2.35, p < 0.05) and HCS + HA (z = 2.35, p < 0.05). HCS and HCS + HA were not significantly different (Figure 1B).
Results from a a Kruskal-Wallis test were significant (χ2(2, N = 12) = 7.42, p < 0.05, η²= 0.60. Post-hoc tests show miR-34a in the LCS condition was significantly higher compared to HCS (z = 2.45, p < 0.05) and HCS + HA (z = 2.26, p < 0.05). HCS and HCS + HA were not significantly different (Figure 1C).
Results from a Kruskal-Wallis test were significant (χ2 (2, N = 12) = 8.00, p < 0.05, η²= 0.67. Post-hoc tests show miR-135a-3p in the LCS condition was significantly lower compared to HCS (z=-1.96, p < 0.05) but was not different compared to HCS + HA. miR-135a-3- was significantly higher in the HCS compared to the HCS + HA condition (z = 2.75, p < 0.05) Figure 1D.
There was a significant main effect between groups on number of ACEs reported (χ2 (2, N = 12) = 10.24, p < 0.01). LCS had significantly lower reported ACEs compared to HCS + HA (t(11) = -8.38, p < 0.001) (Figure 2A). There were no significant differences between LCS and HCS.
There was a significant difference between groups on self-reported stressful events rated by the SRSS (χ2(2, N = 12) 7.54, p < 0.05, η²=.62 LCS had significantly lower SRSS scores compared to HCS (z = -2.16, p < 0.05) and HCS + HA (z = -2.55, p < 0.01). There was not a significant difference between HCS and HCS + HA Figure 2B. Further, there was a significant positive correlation between ACEs and SRSS (r = .82, p < 0.001).
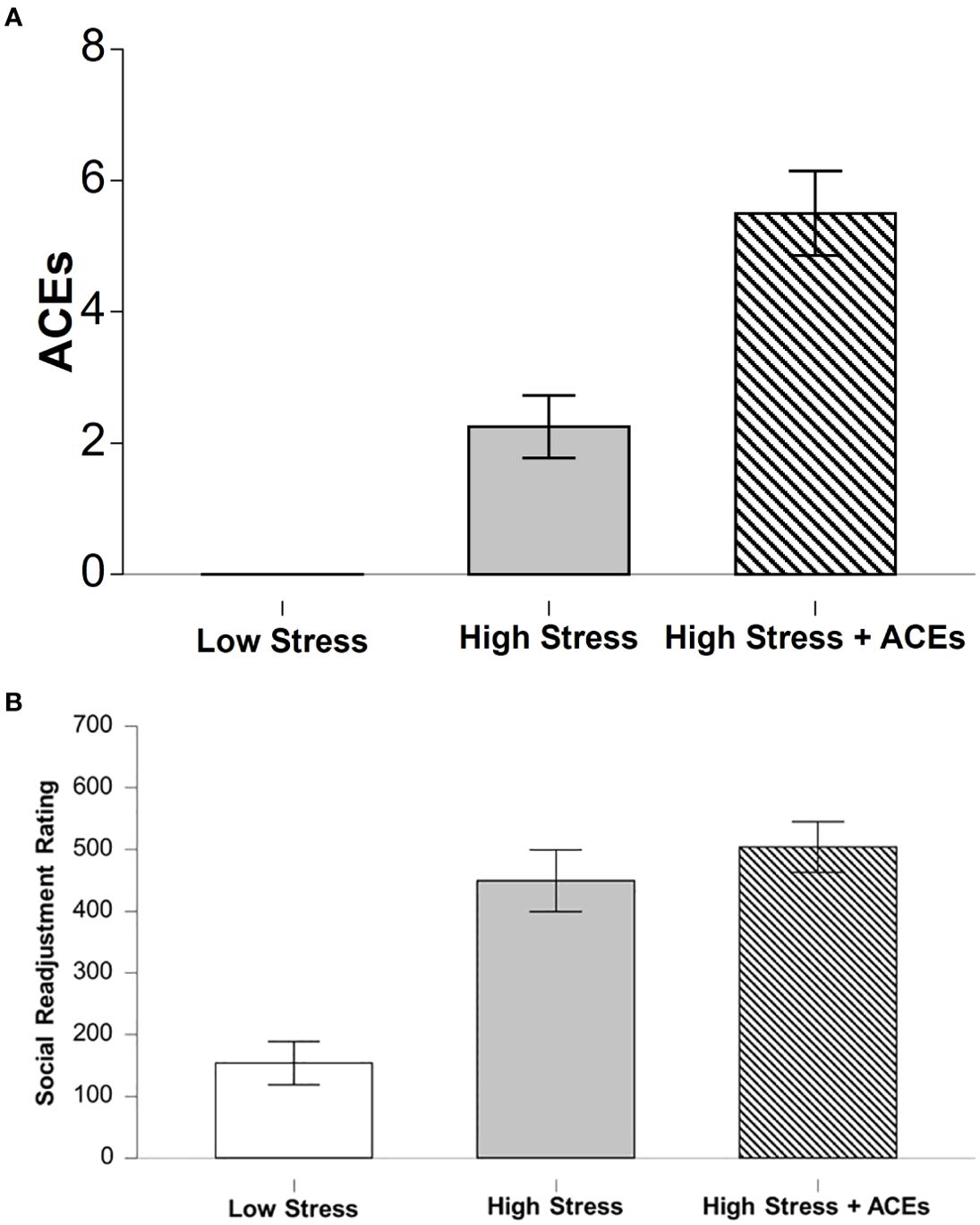
Figure 2. Self-reports of early life adversity and current chronic levels of stress. (A) The low stress group (LCS) had fewer reported ACEs compared to both stress groups and the highest incident was found in the High Stress + ACEs (HCS+HA) group. (B) The High Stress (HCS) and the High Stress + ACEs (HCS+HA) groups report comparable levels of high stress incidents over the last year. Bar graphs depict means and error bars reflect SEM. * depicts significant Bonferroni post hoc compared to LCS, ^ depicts significant Bonferroni post hoc compared to HCS+HA.
It is well documented that childhood adversity and chronic stress increase risk factors for many ailments and diseases including cardiovascular disease, autoimmune disorders, cancer and increased risk for addiction, culminating in premature death. Thus, it is vital to develop reliable, objective, non-invasive screens to monitor and prevent these consequences. Exosomal miRNAs extracted from saliva are a great candidate due to the non-invasive and relative ease of collection methods. miRNAs have become the focus of various studies given their role in the regulation of the central nervous system (CNS) through post-transcriptional gene silencing (38, 39). miRNAs are known to be important mediators of the brain genomic response to stress, including the hypothalamic-pituitary-adrenal axis and the autonomic nervous system (40, 41). Results from the current study demonstrate downregulation of candidate miRNAs as well as demonstrating a possible phenotype for stress adaptation due to cumulative effects of early life adversity coupled with chronic stress. These results also provide evidence that the subjectiveness of stressful experiences correlate with objective changes in epigenetic markers of stress.
Our results support existing literature demonstrating the causal roles of miR-19b, miR-187, miR-34a and miR-135-3p in regulating stress response. For example, decreased expression of miR-19b following trauma is positively associated with onset of PTSD in women but not men (42). Results from our current exploratory study corroborate this finding and is especially relevant given the participants were exclusively African American females, similar to the characteristics of study participants from Linnstaedt et al. Cortisol exposure also downregulated miR-19b expression in rat hippocampal progenitor cells (43) and future studies aim to examine how decreased miR-19b expression impacts learning and memory or increase risk for psychological disorders.
Both the HCS and the HCS + HA displayed decreased expression of miR-187. This is alarming given the role miR-187 in suppressing tumor growth (44, 45), suggesting a possible mechanism for chronic stress increasing cancer risk. In addition to increased cancer risk and poorer disease progression prognosis associated with decreased miR-187 expression, Zhang et al. (2018) (41) found lower expression of miR-187 induces retinal cell apoptosis. Further, patients with coronary artery disease also present with a significant decrease in miR-187 expression and experimental overexpression through knockdown of its downstream target, DYRK2, improved cardiomyocyte apoptosis in a hypoxia/reoxygenation model of myocardial infarction (46). Together with the results of this study findings indicate screens targeting miR-187 in early adulthood could aid in mitigating the risk of cancer, progressive vision loss, and cardiovascular disease in at-risk demographics, such as individuals exposed to early life adversity and high levels of chronic stress. Future work should examine if health interventions can increase the expression of miR-187 to improve health outcomes.
Similar to mi-187, both HCS and HCS + HA displayed decreased expression in salivary exosomes and downregulation of mir-34a is found in breast cancer, colon cancer, prostate cancer, and vascular disease (47). Others have noted appropriate levels of miR-134a act as a critical controller of tumor suppression such that overexpression of miR-134a is currently being investigated in clinical trials for cancer treatment (48). Recent studies show patients diagnosed with major depression disorder (MDD) demonstrate downregulation of plasma miR-134a and can be used to discriminate between controls, bipolar disorder, and schizophrenia (49). Further, early life stress and chronic levels of stress increase the risk for MDD (50, 51). Taken together with our results showing both high chronic stress and high rates of ACEs downregulate saliva miR-187 expression supports the idea of using miR-187 extracted from salivary exosomes as a non-invasive screen for predicting negative health outcomes.
High chronic stress levels increased expression of miR-135-3p compared to low chronic stress group and high chronic stress plus high ACEs group. An intriguing finding that supports others’ findings early life stress programs a maladaptive stress response stress experiences in adulthood (52, 53). For example, chronic stress exposure during adulthood increases both basal cortisol levels and cortisol responses to acute stressors (54, 55), but early life adversity blunts the cortisol response to acute stressors (56). Low levels of cortisol are observed in patients diagnosed with PTSD resulting from adverse early life abuse (57). Future work should further investigate disparate health outcomes in individuals with chronic stress exposure in adulthood vs. early life stress as well as the cumulative effects. The decrease in miR-135-3p expression observed in high chronic stress + HA compared to high levels of chronic stress could serve as a useful tool to parse out individuals with undisclosed or unknown early life stress, such as in cases of adoption or parental estrangement, to better screen for associated diseases and psychological disorders in these individuals and positively benefit public health initiatives.
Stress and stress related disorders are among the most prevalent psychiatric disorders and are the leading cause of global morbidities. In our preliminary pilot study we have identified dysregulations in four miRNAs that have a potential to be important biomarkers in salivary exosomes and be used to target interventions that increase stress resiliency and reduce the risk and severity of psychiatric disorders. Despite our small sample size in this exploratory pilot study, we were able to achieve large effect sizes in our statistical tests. However, the homogeneity and small sample of study participants limits generalizability. Additionally, we did not have enough participants who fell into a low stress/high ACE category to analyze this group. Next steps include replicating in a larger, heterogenous sample. In our current study we have not addressed the genes or mRNAs that are associated with downregulations or upregulations of human miRNAs as this was outside the scope of this feasibility study. There are 30-40 downstream targets of any one miRNAs and future preclinical and clinical work can identify downstream targets that could be screened for simultaneously to increase predictive validity.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Claflin University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AB: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. EH: Formal analysis, Writing – original draft, Writing – review & editing. OB: Investigation, Methodology, Supervision, Writing – original draft. PP: Formal analysis, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Li D, Liu J, Li S, Yang J, Sun H, Wang A. Fear conditioning downregulates miR-138 expression in the hippocampus to facilitate the formation of fear memory. NeuroReport. (2018) 29:1418. doi: 10.1097/WNR.0000000000001129
2. Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, et al. microRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J Neurosci. (2011) 31:14191–203. doi: 10.1523/JNEUROSCI.1673-11.2011
3. Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. (2012) 148:1172–87. doi: 10.1016/j.cell.2012.02.005
4. Chen S-D, Sun X-Y, Niu W, Kong L-M, He M-J, Fan H-M, et al. Correlation between the level of microRNA expression in peripheral blood mononuclear cells and symptomatology in patients with generalized anxiety disorder. Compr Psychiatry. (2016) 69:216–24. doi: 10.1016/j.comppsych.2016.05.006
5. Wiegand C, Heusser P, Klinger C, Cysarz D, Büssing A, Ostermann T, et al. Stress-associated changes in salivary microRNAs can be detected in response to the Trier Social Stress Test: An exploratory study. Sci Rep. (2018) 8:7112. doi: 10.1038/s41598-018-25554-x
6. Nagarajan MB, Tentori AM, Zhang WC, Slack FJ, Doyle PS. Spatially resolved and multiplexed MicroRNA quantification from tissue using nanoliter well arrays. Microsystems Nanoengineering. (2020) 6:1–9. doi: 10.1038/s41378-020-0169-8
7. Safari Z, Firouzi A, Rezaeikalantari N, Mohammadi S, Ranjbar N, Shahpori H, et al. The salivary exosomal microRNA as a potential biomarker in patients with periodontitis and oral cancers. Chem Biol Drug Design. (2023) 101:1204–15. doi: 10.1111/cbdd.14159
8. Bahbah E, Noehammer C, Pulverer W, Jung M, Weinhäusel A. Salivary biomarkers in cardiovascular disease: An insight into the current evidence. FEBS J (2021) 288. doi: 10.1111/febs.15689
9. Ubhale R, Dahake S, Madhu PP, Chhabra KG, Reche A, Bankar A, et al. Salivary biomarkers in COVID-19 patients. J Family Med Primary Care. (2022) 11:6778. doi: 10.4103/jfmpc.jfmpc_1569_21
10. Kułak-Bejda A, Waszkiewicz N, Bejda G, Zalewska A, Maciejczyk M. Diagnostic value of salivary markers in neuropsychiatric disorders. Dis Markers. (2019) 2019:4360612. doi: 10.1155/2019/4360612
11. Chen W, Cui Y, Wang J, Yuan Y, Sun X, Zhang L, et al. Effects of downregulated expression of microRNA-187 in gastric cancer. Exp Ther Med. (2018) 16:1061–70. doi: 10.3892/etm.2018.6318
12. Safari R, Jacques J-R, Brostaux Y, Willems L. Ablation of non-coding RNAs affects bovine leukemia virus B lymphocyte proliferation and abrogates oncogenesis. PloS Pathog. (2020) 16:e1008502. doi: 10.1371/journal.ppat.1008502
13. Salinas-Miranda AA, Salemi JL, King LM, Baldwin JA, Berry EL, Austin DA, et al. Adverse childhood experiences and health-related quality of life in adulthood: Revelations from a community needs assessment. Health Qual Life Outcomes. (2015) 13:123. doi: 10.1186/s12955-015-0323-4
14. Kalmakis KA, Chandler GE. Health consequences of adverse childhood experiences: A systematic review. J Am Assoc Nurse Practitioners. (2015) 27:457–65. doi: 10.1002/2327-6924.12215
15. Schellhaas S, Schmahl C, Bublatzky F. Social threat and safety learning in individuals with adverse childhood experiences: Electrocortical evidence on face processing, recognition, and working memory. Eur J Psychotraumatol. (2022) 13:2135195. doi: 10.1080/20008066.2022.2135195
16. Strine TW, Dube SR, Edwards VJ, Prehn AW, Rasmussen S, Wagenfeld M, et al. Associations between adverse childhood experiences, psychological distress, and adult alcohol problems. Am J Health Behav. (2012) 36(3):408–23. doi: 10.5993/AJHB.36.3.11
17. Anda RF, Brown DW, Felitti VJ, Dube SR, Giles WH. Adverse childhood experiences and prescription drug use in a cohort study of adult HMO patients. BMC Public Health. (2008) 8:198. doi: 10.1186/1471-2458-8-198
18. Tietjen GE, Khubchandani J, Herial NA, Shah K. Adverse childhood experiences are associated with migraine and vascular biomarkers. Headache. (2012) 52(6):920–9. doi: 10.1111/j.1526-4610.2012.02165.x
19. Opacka-Juffry J, Mohiyeddini C. Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress. (2012) 15(1):1–10. doi: 10.3109/10253890.2011.560309
20. Cambron C, Gringeri C, Vogel-Ferguson MB. Physical and mental health correlates of adverse childhood experiences among low-income women. Health Soc Work. (2014) 39(4):221–9. doi: 10.1093/hsw/hlu029
21. Anda R, Felitti V, Bremner J, Walker J, Whitfield C, Perry B, et al. The enduring effects of abuse and related adverse experiences in childhood: A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci (2006) 256:174–86. doi: 10.1007/s00406-005-0624-4
22. Kliewer W, Robins JL. Adverse childhood experiences are associated with cardiometabolic risk indicators and telomere length in low-income african-american adolescents. Int J Behav Med. (2022) 29:131–5. doi: 10.1007/s12529-021-09978-w
23. Forster M, Rogers CJ, Benjamin SM, Grigsby T, Lust K, Eisenberg ME. Adverse childhood experiences, ethnicity, and substance use among college students: findings from a two-state sample. Subst Use Misuse. (2019) 54:2368–79. doi: 10.1080/10826084.2019.1650772
24. Gili M, Toro MG, Armengol S, García-Campayo J, Castro A, Roca M. Functional impairment in patients with major depressive disorder and comorbid anxiety disorder. Can J Psychiatry. (2013) 58:679–86. doi: 10.1177/070674371305801205
25. Pagano ME, Skodol AE, Stout RL, Shea MT, Yen S, Grilo CM, et al. Stressful life events as predictors of functioning: Findings from the Collaborative Longitudinal Personality Disorders Study. Acta Psychiatrica Scandinavica. (2004) 110:421–9. doi: 10.1111/j.1600-0447.2004.00398.x
26. Hatch SL, Dohrenwend BP. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: A review of the research. Am J Community Psychol (2007) 40(3-4):313–32. doi: 10.1007/s10464-007-9134-z
27. Phillips D, Carroll. Stressful life-events exposure is associated with 17-year mortality, but it is health-related events that prove predictive. Br J Health Psychol (2008) 13:647–57.
28. Nater UM, Skoluda N, Strahler J. Biomarkers of stress in behavioural medicine. Curr Opin Psychiatry. (2013) 26:440. doi: 10.1097/YCO.0b013e328363b4ed
29. Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. (2007) 8:166. doi: 10.1186/1471-2164-8-166
30. Michael A, Bajracharya SD, Yuen PST, Zhou H, Star RA, Illei GG, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. (2010) 16:34–8. doi: 10.1111/j.1601-0825.2009.01604.x
31. Murphy CP, Singewald N. Role of microRNAs in anxiety and anxiety-related disorders. Curr Topics Behav Neurosci. (2019) 42:185–219. doi: 10.1007/7854_2019_109
32. Honda M, Kuwano Y, Katsuura-Kamano S, Kamezaki Y, Fujita K, Akaike Y, et al. Chronic academic stress increases a group of microRNAs in peripheral blood. PloS One. (2013) 8:e75960. doi: 10.1371/journal.pone.0075960
33. Leung AKL, Sharp PA. MicroRNA functions in stress responses. Mol Cell. (2010) 40:205–15. doi: 10.1016/j.molcel.2010.09.027
34. Varghese Rency S, Barefoot Megan E, Jain S, Chen Y, Zhang Y, Alley A, et al. Integrative analysis of DNA methylation and microRNA expression reveals mechanisms of racial heterogeneity in hepatocellular carcinoma. Front Genet (2021) 12:708326. doi: 10.3389/fgene.2021.708326
35. Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosomatic Res. (1967) 11:213–8. doi: 10.1016/0022-3999(67)90010-4
36. Rider M, Hurwitz S, Meckes D. ExtraPEG: A polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep (2016) 6:23978. doi: 10.1038/srep23978
37. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. (2008) 3:1101–8. doi: 10.1038/nprot.2008.73
38. Shu S, Jiang M, Deng X, Yue W, Cao X, Zhang K, et al. Heterochromatic silencing of immune-related genes in glia is required for BBB integrity and normal lifespan in drosophila. Aging Cell (2023) 22:e13947. doi: 10.1111/acel.13947
39. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet. (2008) 9:102–14. doi: 10.1038/nrg2290
40. Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. (2013) 33:9003–12. doi: 10.1523/JNEUROSCI.0914-13.2013
41. Zhang QL, Wang W, Alatantuya, Dongmei, Lu ZJ, Li LL, et al. Down-regulated miR-187 promotes oxidative stress-induced retinal cell apoptosis through P2X7 receptor. Int J Biol Macromolecules. (2018) 120:801–10. doi: 10.1016/j.ijbiomac.2018.08.166
42. Linnstaedt SD, Rueckeis CA, Riker KD, Pan Y, Wu A, Yu S, et al. microRNA-19b predicts widespread pain and posttraumatic stress symptom risk in a sex-dependent manner following trauma exposure. Pain. (2020) 161:47–60. doi: 10.1097/j.pain.0000000000001709
43. Mazzelli M, Maj C, Mariani N, Mora C, Begni V, Pariante CM, et al. The long-term effects of early life stress on the modulation of miR-19 levels. Front Psychiatry. (2020) 11:389. doi: 10.3389/fpsyt.2020.00389
44. Ng L, Wan TM, Iyer DN, Huang Z, Sin RW, Man AT, et al. High Levels of Tumor miR-187-3p-A Potential Tumor-Suppressor microRNA-Are Correlated with Poor Prognosis in Colorectal Cancer. Cells. (2022) 11(15):2421. doi: 10.3390/cells11152421
45. Chen X, Song QL, Ji R, Wang JY, Li ZH, Guo D, et al. MiR-187 regulates the proliferation, migration and invasion of human trophoblast cells by repressing BCL6-mediated activation of PI3K/AKT signaling. Placenta. (2022) 118:20–31. doi: 10.1016/j.placenta.2022.01.001
46. Zhu F, Yu Z, Li D. miR-187 modulates cardiomyocyte apoptosis and oxidative stress in myocardial infarction mice via negatively regulating DYRK2. Signa Vitae. (2021) 17(5):142–50.
47. Kalfert D, Ludvikova M, Pesta M, Ludvik J, Dostalova L, Kholová I. Multifunctional roles of miR-34a in cancer: A review with the emphasis on head and neck squamous cell carcinoma and thyroid cancer with clinical implications. Diagnostics. (2020) 10:563. doi: 10.3390/diagnostics10080563
48. Hong DS, Kang Y-K, Borad M, Sachdev J, Ejadi S, Lim HY, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. (2020) 122:1630–7. doi: 10.1038/s41416-020-0802-1
49. Zhang H, Liu X, Chen J, Cheng K, Bai S-J, Zheng P, et al. Circulating microRNA 134 sheds light on the diagnosis of major depressive disorder. Trans Psychiatry. (2020) 10:95. doi: 10.1038/s41398-020-0773-2
50. Almulla AF, Algon AAA, Maes M. Adverse childhood experiences and recent negative events are associated with activated immune and growth factor pathways, the phenome of first episode major depression and suicidal behaviors. Psychiatry Res. (2024) 334:115812. doi: 10.1016/j.psychres.2024.115812
51. Lo Iacono L, Ielpo D, Accoto A, Di Segni M, Babicola L, D’Addario SL, et al. MicroRNA-34a regulates the depression-like behavior in mice by modulating the expression of target genes in the dorsal raphè. Mol Neurobiol. (2020) 57:823–36. doi: 10.1007/s12035-019-01750-2
52. Dempster KS, O’Leary DD, MacNeil AJ, Hodges GJ, Wade TJ. Linking the hemodynamic consequences of adverse childhood experiences to an altered HPA axis and acute stress response. Brain Behavior Immun. (2021) 93:254–63. doi: 10.1016/j.bbi.2020.12.018
53. Roché S, Kearns H, Brindle RC. Testing adverse childhood experiences (ACEs) as a potential moderator of the association between current chronic stress and cardiovascular reactivity. Int J Psychophysiology: Off J Int Organ Psychophysiol. (2023) 193:112245. doi: 10.1016/j.ijpsycho.2023.112245
54. Cay M, Ucar C, Senol D, Cevirgen F, Ozbag D, Altay Z, et al. Effect of increase in cortisol level due to stress in healthy young individuals on dynamic and static balance scores. Northern Clinics Istanbul. (2018) 5:295–301. doi: 10.14744/nci.2017.42103
55. Belda X, Fuentes S, Daviu N, Nadal R, Armario A. Stress-induced sensitization: The hypothalamic–pituitary–adrenal axis and beyond. Stress. (2015) 18:269–79. doi: 10.3109/10253890.2015.1067678
56. Brindle RC, Pearson A, Ginty AT. Adverse childhood experiences (ACEs) relate to blunted cardiovascular and cortisol reactivity to acute laboratory stress: A systematic review and meta-analysis. Neurosci Biobehav Rev. (2022) 134:104530. doi: 10.1016/j.neubiorev.2022.104530
Keywords: microRNA, adverse childhood experiences, social readjustment rating scale, women, miR-19b, miR-187, miR-34a and miR-135-3p
Citation: Holliday E, Bagasra A, Bagasra O and Pandey P (2025) Assessing the feasibility of using salivary microRNAs as biomarkers to distinguish between chronic stress and childhood trauma in African American young women in an exploratory pilot study. Front. Psychiatry 16:1507064. doi: 10.3389/fpsyt.2025.1507064
Received: 07 October 2024; Accepted: 08 January 2025;
Published: 14 February 2025.
Edited by:
Diego Luiz Rovaris, University of São Paulo, BrazilReviewed by:
Jakub Tomasik, University of Cambridge, United KingdomCopyright © 2025 Holliday, Bagasra, Bagasra and Pandey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erica Holliday, ZWhvbGxpZDFAa2VubmVzYXcuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.