- Shenzhen Mental Health Centre, Shenzhen Kangning Hospital, Shenzhen, China
Schizophrenia is a serious psychiatric disorder of multifactorial triggers, with a lifetime prevalence of about 1% in all countries of the world, with a slightly higher prevalence in males than in females, and with a peak incidence between the ages of 15-35 years old, and a poor prognosis for most of the patients, which imposes a heavy burden on the society and the family. Early intervention is important for the prognosis and regression of patients. More and more studies have found that the imbalance of oxidation and antioxidant, and the persistent damage to the brain by oxidative stress play an important role in the occurrence and development of schizophrenia. Antioxidants, as additive therapy, play an important role in improving symptoms as well as preventing relapse in patients with schizophrenia. This paper intends to address the pathogenesis of oxidative stress injury and schizophrenia, and the significance of oxidative stress in the treatment of schizophrenia.
Introduction
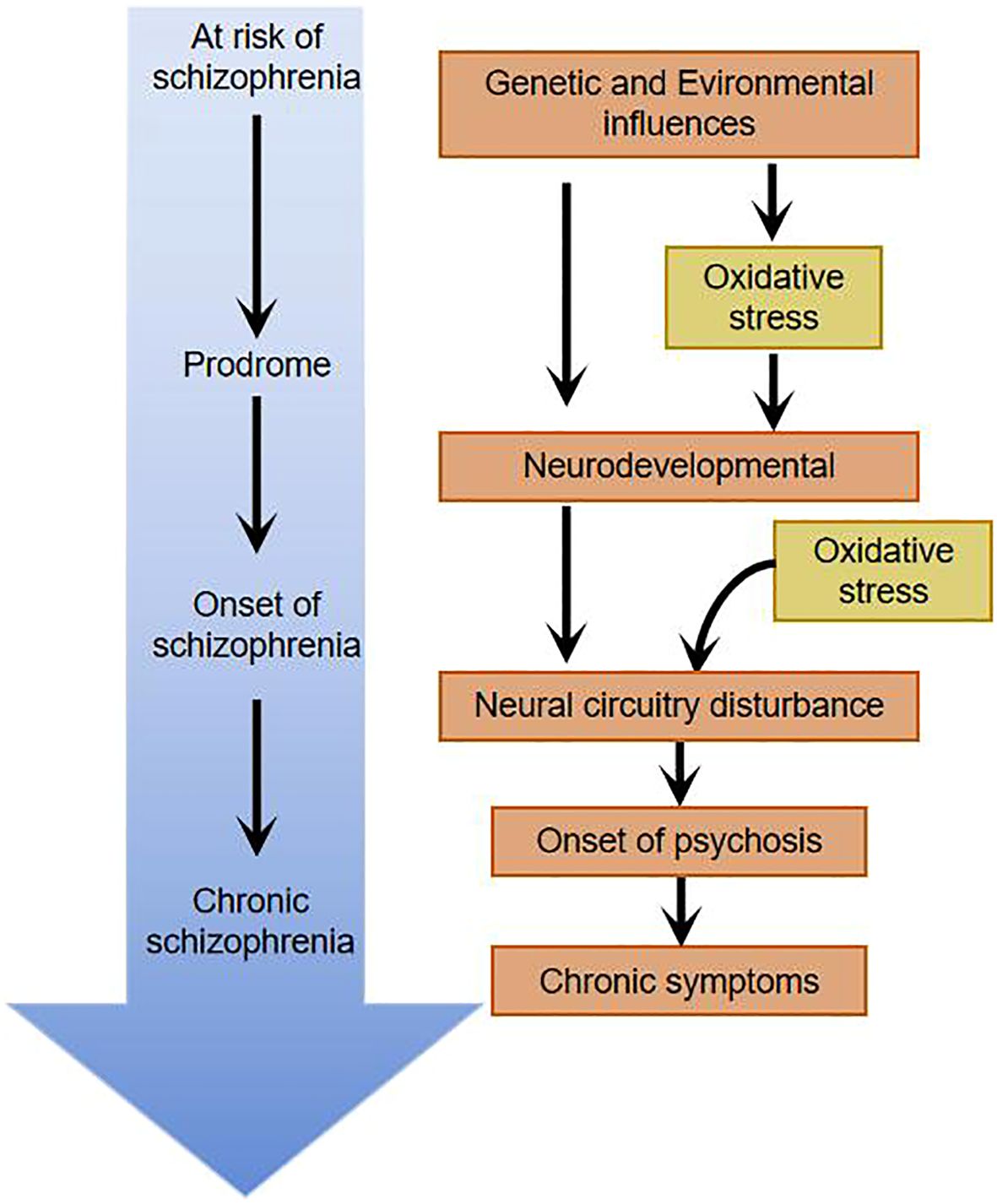
Schizophrenia is a chronic, severe, and psychiatric disorder of multifactorial onset that affects approximately 1% of the world’s population (1). It is characterized by positive symptoms (e.g., hallucinations and delusions), negative symptoms (e.g., depressed mood, laziness and social withdrawal) and cognitive impairment (2). The course of the disease is often prolonged, accounting for more than half of psychiatric inpatients, and about half of the patients eventually develop psychiatric disability, which places a heavy burden on society and families. Although most patients are diagnosed in adulthood, cognitive impairment and social deficits may appear as early as adolescence (3, 4). A growing number of studies have found that oxidative stress damage, which occurs since early childhood, sustains damage to the brain, affects neuronal growth and development, and influences neuronal structure and function, and may play an important role in the pathophysiological mechanisms of schizophrenia (5–7).
Oxidation and antioxidant
Under normal conditions, there is an oxidative system and an antioxidant defense system in the human body, both of which are in dynamic equilibrium. The oxidative system includes: superoxide anion, hydroxyl radicals, etc. The antioxidant defense system includes: superoxide dismutase, catalase and other enzymatic antioxidants as well as non-enzymatic antioxidants such as albumin, uric acid and glutathione. Oxidative stress occurs when the organism is subjected to a variety of internal and external environmental stimuli and there is an imbalance between oxidative and antioxidant processes. Appropriate oxidative stress is necessary for the normal physiological function of the organism, and excessive oxidative stress may have deleterious effects. The main targets of oxidative stress damage are DNA, lipids and proteins (8–10). Oxidative stress damage can lead to mutations in DNA by inducing nucleic acid strand breaks and purine oxidation, damage to cell membranes and organelle membranes through lipid peroxidation, and it can also lead to denaturation of proteins, which loses its original function and affects the structure and function of cells (11). The brain accounts for 2% of the body’s total weight but consumes about 20% of the body’s oxygen (Figure 1). Compared with other organs in the body, the brain consumes more oxygen, generates more free radicals, has a higher concentration of polyunsaturated fatty acids, and a lower level of antioxidants, and is therefore more susceptible to damage caused by oxidative stress (12).
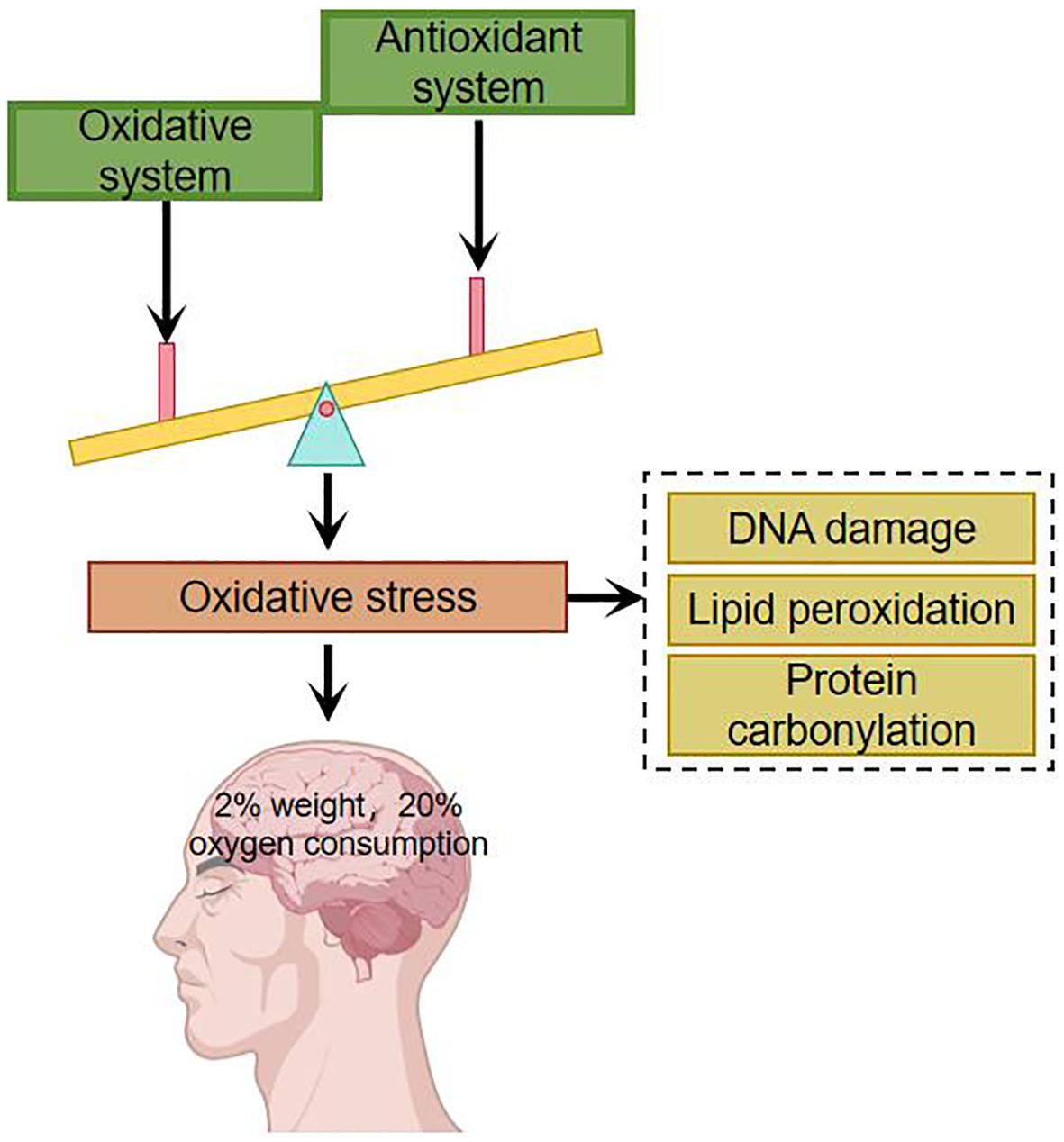
Figure 1. Imbalances in the oxidative and antioxidant systems have a particularly significant impact on the brain.
Oxidative stress damage may be linked to schizophrenia mechanisms
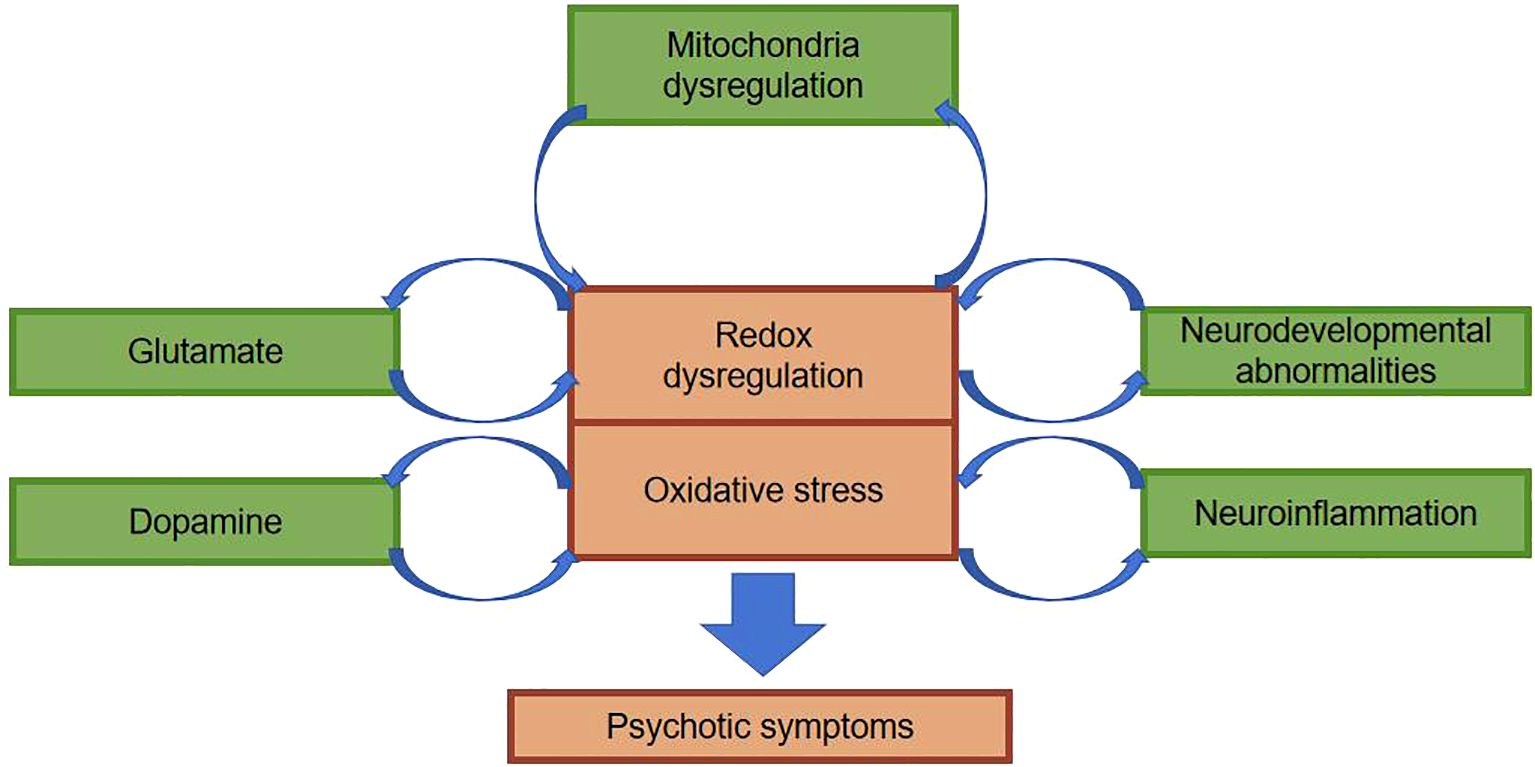
Social isolation, vitamin D deficiency, chronic stress during adolescence, perinatal infections, inflammation, etc.-all of which ultimately lead to oxidative stress (5, 13). Under normal conditions, the body’s oxidation and antioxidant activity are in dynamic equilibrium; under abnormal conditions, free radicals that are not scavenged from the body can react with normal tissue cells, damaging the cellular structure and causing cellular dysfunction. Measurement of the concentration of free radicals in the body is impractical because of their chemically reactive nature resulting in low levels and short half-lives in the body. In clinical studies, the level of oxidative stress is reflected indirectly by antioxidant enzymes, non-enzymatic antioxidants, and peroxidation products. 1954 Hoffer et al. suggested for the first time that oxidative stress damage plays an important role in the pathogenesis of schizophrenia by examining the levels of oxidative stressors in patients. Studies of oxidative stress damage in schizophrenic patients have been conducted mainly through blood (e.g., leukocytes, lymphocytes, albumin, uric acid, etc.), cerebrospinal fluid, and postmortem brain tissue of patients. Researchers have found that schizophrenic patients have higher peroxidation products detectable in peripheral blood and cerebrospinal fluid than the normal population, suggesting that schizophrenic patients have higher oxidative stress damage in their organisms than the normal population (14, 15). In order to exclude the influence of medication and disease duration on the study, some researchers studied patients with first-episode unmedicated schizophrenia and found that markers of oxidative stress damage were significantly elevated compared to the normal population (16) and that there was a significant correlation between the severity of psychiatric symptoms and markers of oxidative stress damage (5, 17). Researchers have found that serum superoxide dismutase (SOD) is significantly lower and catalase (CAT) and malondialdehyde (MDA) levels are significantly higher in patients with schizophrenia compared to the normal population (18). Studies of brain tissue from postmortem patients have clearly identified oxidative stress damage (19, 20), but confounding factors, such as the effect of other somatic diseases comorbid before death, cannot be excluded. Schizophrenia is a disorder that mostly develops in young adulthood, and testing the brains of elderly patients after death does not truly reflect the pathophysiological changes in the patients’ brains. A major advancement in research in recent years has been the use of MRS to study brain tissue from living subjects, where researchers have found that glutathione (GSH) levels in the medial prefrontal cortex of patients with first-episode schizophrenia who were not on medication were reduced by approximately 52% compared to the normal population (20). Do KQ et al. studied cerebrospinal fluid from first-episode unmedicated patients and found that GSH levels were reduced by 27% compared to the normal population (21), and Yao et al. measured the caudate nucleus of postmortem patients and found a 41% reduction in GSH levels (22). The brain, as 20% of the oxygen consumption in the organism, undergoes redox reactions all the time, generating a large number of free radicals. The active free radicals in the brain produce neurotoxic effects and sustain damage to nerve cells, causing peroxidation of cell membranes and organelle membranes, impaired membrane fluidity and stability, affecting nerve cell plasticity and neurotransmitter vesicle transmission, and affecting chemical reactions taking place in nerve cells, which may accelerate or lead to schizophrenia and a range of clinical manifestations (Figure 2).
The classical dopamine hypothesis of schizophrenia suggests that schizophrenics have central dopamine hyperfunction compared to normal individuals. Whereas the metabolism of dopamine generates large amounts of hydrogen peroxide, hydrogen peroxide generates reactive oxygen radicals via auto-oxidation of dopamine. These reactive oxygen species may subsequently interact with superoxide dismutase and glutathione, leading to reduced levels of these antioxidants, making the brain’s ability to cope with oxidative stress damage much reduced and further accelerating neuronal cell damage in the corresponding brain regions (23). Glutathione (GSH) redox system plays an important role in reducing the damage caused by oxidative stress in the organism. GSH is one of the major free radical scavengers in the brain, which can be converted to oxidized glutathione (GSSG) by glutathione peroxidase (GPx) to deplete reactive oxygen radicals, and GSSG can then be reduced to GSH by glutathione reductase (GR).Therefore, measurement of the body GSH, GSSG and their related enzyme responses are important for assessing oxidative stress injury. A study comparing GSH, GSSG, GPx and GR levels in the caudate region of the brain after death in schizophrenia patients and normal subjects showed that the schizophrenia group had significantly lower levels of GSH, GPx, and GR than the control group, which did not have any psychiatric disorders (24).The potential mechanism by which the reduced level of GSH affects the morphology of neurons may be through oxidative stress damage inducing lipid peroxidation, the protein denaturation, which further leads to cell membrane alterations and cytoskeletal changes, affecting the normal functioning of neuronal cells in the corresponding brain regions involved in schizophrenia (25).
The glutamate hypothesis of schizophrenia suggests that the function of N-methyl-D-aspartate (NMDA) receptors is impaired in this disorder. This hypothesis derives from the fact that drugs, including phenylcyclohexylpiperidine (PCP), cause schizophrenia-like psychotic symptoms in humans by blocking the function of the NMDA receptor, and that such psychotic symptoms are difficult to diagnostically distinguish from schizophrenia (26). Glutamate, as the main excitatory neurotransmitter in the central nervous system, also has a high level of excitotoxicity, and this excitotoxicity is mainly affected through oxidative stress damage, and its toxic effects on cells are mainly through two mechanisms: one is that the influx of calcium ions may trigger the infiltration of isotonic fluids, which can subject the cells to mechanical stress, which can in turn affect the function of the cells or even lead to cell death. The other is that the excitatory cycle induced by the entry of large amounts of calcium ions into the cell stimulates the further release of glutamate, the latter of which may lead to the further exacerbation of oxidative stress injury (27).
Previous studies on schizophrenia has predominantly concentrated on dopaminergic and glutamatergic neurotransmitters. Recently, the neuroinflammatory theory puts forth a viewpoint that dysfunction of the body’s immune system as a consequence of genetic and environmental factors may be a paramount pathologic mechanism in schizophrenia (28, 29). Interactions between neuroinflammation and oxidative stress affect the functional integrity of a neuron as well as the integrity of the meshwork surrounding that neuron. Aside from that, the association between chronic inflammation and oxidative stress facilitates the release of pro-inflammatory cytokines, the stimulation of astrocyte activity, as well as the dysregulation of dopaminergic and glutamatergic pathways, which ultimately gives rise to the onset of schizophrenic symptoms (Figure 3) (30). As already demonstrated by relevant imaging studies, during schizophrenic episodes, changes occur in brain structure, most commonly manifested as a reduction in hippocampal and cortical volume, accompanied by ventricular enlargement, which is independent of pharmacological treatment (31). In contrast to Alzheimer’s disease, the schizophrenia brain changes are not progressive neuronal degeneration and death, but rather changes in brain organization and in the size of neurons and other brain cells. Molecular studies have displayed altered expression of inflammatory markers in the prefrontal and temporal cortex and hippocampus of schizophrenic patients. This phenomenon suggests that the body may have oxidative stress as well as altered inflammatory responses. Clozapine, featured by its enduring immunosuppressive effects and anti-inflammatory properties that alleviate microglial activation, is associated with its adverse effect of agranulocytosis, which is also correlated with its anti-inflammatory action and abatement of oxidative stress damage. As a consequence, lessening inflammatory response and oxidative stress damage is expected to be a target goal for the future treatment of schizophrenia (32).
The central nervous system contains about 100 billion neurons. Astrocytes, oligodendrocytes, and microglia, as well as other cell types, collectively make up the adult brain and spinal cord. Oxidative stress damage early in life may disrupt the normal developmental stages of the brain (Figure 3). In particular, synapses form and connect, and these changes ultimately result in abnormal information processing and, ultimately, to schizophrenia (33). It’s particularly noteworthy that there are dramatic abnormalities in brain development and maturation in patients with schizophrenia spectrum disorders. First and foremost, a multitude of neuroblasts fail to reach the correct location during the 4-6 months of gestation, and autopsy studies reveal that they are buried deep in the white matter of the brain (34). On top of that, during childhood and adolescence, individuals in the pre-schizophrenic phase show excessive loss of neuronal and synaptic connectivity to the extent that, during psychotic episodes, about one-third to one-half of patients show marked atrophic changes and enlargement of the lateral ventricles (33, 35). The enlarged ventricles mirror the loss of brain tissue in schizophrenic patients: oxidative stress damage continues after psychotic episodes, the loss of brain volume continues to worsen, and appears to correlate with the duration of untreated psychosis and the number of exacerbations. Patients suffering from schizophrenia are in an exacerbation phase, where oxidative stress damage persists and keeps damaging the patient’s brain, which exerts a paramount impact on the patient’s prognosis. In addition, some scholars have reported that the patient’s prognosis worsens the longer the condition stays untreated (36, 37).
It has been found that micronutrient imbalances are present in patients with schizophrenia (38). Iron ions play an important role in the body, with nearly two-thirds of its total amount present in hemoglobin. Most of the remaining iron ions are located in the brain, liver, spleen and heart. Iron ions are adept at catalyzing redox reactions. Transferrin and ferritin are iron transport proteins, both with antioxidant properties. These two proteins act as antioxidants and reduce oxidative stress damage by lowering the concentration of free iron ions. It has been found that serum levels of iron, ferritin and transferrin are significantly lower in schizophrenic patients compared to normal controls, which also suggests that oxidative stress damage may play an important role in the development and progression of schizophrenic patients (39).
Impaired cognitive function is one of the important symptom clusters of schizophrenia, which mainly manifests impaired attention, memory, visual and verbal learning ability, etc. More and more studies have found that oxidative stress injury is involved in the pathological process of cognitive dysfunction in schizophrenia patients (16, 40). It has been found that the higher the severity of cognitive impairment in patients with schizophrenia, the lower the body’s antioxidant capacity, the increased protein oxidation in the brain, and the more severe the cognitive impairment (41, 42). Some researchers have studied first-degree relatives of schizophrenic patients and similarly found impaired cognitive functioning compared to the normal population (43). It is suggested that in clinical practice, reducing the level of oxidative stress in patients with early-onset schizophrenia may be beneficial in improving their cognitive function (44, 45).
Oxidative stress and schizophrenia treatment
Oxidative stress is amenable to early intervention and treatment, and there is a large therapeutic potential in this area. The effect of antipsychotic drugs on oxidative stress in the body is not yet clear, and more studies suggest that antipsychotic drugs play an important role in reducing oxidative stress damage in the body (46, 47). A prospective study conducted by Meng-Chang et al. at the Department of Psychiatry, Chang Gung Hospital, Taiwan, found a significant negative correlation between the scores of positive and negative symptom scales in the acute phase of schizophrenia and the level of antioxidants in the organism, and after a 4-week antipsychotic medication, the level of oxidative stress in the organism was significantly reduced compared to the previous one, suggesting that antipsychotic medication may have an effect on the organism’s oxidative stress levels (48). A study conducted by Cristiano et al. involving more than 100 patients treated with antipsychotic medication for 11 weeks found that risperidone was able to significantly reduce oxidative stress levels, and that there was no correlation between the decrease in oxidative stress levels and symptomatic improvement, whereas there was a correlation with the use of risperidone, which ruled out an effect of psychiatric symptomatic improvement on oxidative stress levels (49). oxidative stress levels (40).Parikh et al. found increased levels of lipid peroxidation in the brains of rats chronically treated with haloperidol, whereas no such changes were detected in animals chronically treated with risperidone (as well as olanzapine and clozapine) after 90 days, suggesting that the effects of different antipsychotic medications on oxidative stress may also vary (50). 2022 Goh et al. conducted a Meta-analysis study that included 71 papers involving 4588 patients with schizophrenia and 3983 healthy controls, and found that schizophrenia is associated with an impaired organismal antioxidant defense system, especially with regard to non-enzymatic antioxidants. In addition, antipsychotic drugs and their discontinuation may also alter enzymatic and non-enzymatic antioxidant defense systems. Different types of samples (erythrocytes, plasma, serum, and whole blood) and different measurement techniques may lead to differences in the results of antioxidant activity assays, and confounding factors such as the patient’s age, gender, disease duration, comorbidities with other diseases, and patient condition may have an effect on antioxidant activity (6, 51, 52).
Antioxidants are able to play a role in the development, progression and treatment of schizophrenia by scavenging reactive oxygen radicals and reducing the level of oxidative stress in the body. Antioxidants such as vitamin E, N-acetylcysteine (NAC), and melatonin have been investigated as adjunctive treatments for schizophrenia, where they can reduce oxidative stress damage and improve patients’ symptoms (53). In a previous study of an animal model of schizophrenia (Gclm knockout mice), NAC was given to counteract oxidative stress damage in the early stages of the disease, and the abnormal behavior of the animal model improved (54). A review of the literature on NAC for the treatment of schizophrenia has revealed that NAC is an effective adjunct to the treatment of schizophrenia, improving psychotic symptoms and cognitive functioning, and additionally this therapeutic effect appears to play a role in schizophrenia at all stages of the disease (55, 56). Studies on hesperidin, a naturally occurring antioxidant and neuroprotective flavonoid, have revealed that hesperidin reduces the level of oxidative stress in prefrontal cortex, striatum and hippocampal regions, and prevents and reverses ketamine-induced schizophrenia-like behavior in mice. It is suggested that hesperidin can be used as a nutritional supplement to prevent as well as assist in the treatment of disorders such as schizophrenia as an important agent in nutritional psychiatry (57). It has been found that appropriate supplementation of the diet with foods containing antioxidants can be helpful in the treatment of schizophrenic patients, and omega-3 polyunsaturated fatty acids, among others, are often used as antioxidant potentiators in the treatment of schizophrenia, which have been found to prevent neuronal cells from suffering from oxidative stress damage, mainly by enhancing the stability of lipid membranes (58–60). Similarly, combination therapy with ginkgo biloba extract and haloperidol has been reported to achieve better efficacy, enhancing the effectiveness of antipsychotics and reducing some extrapyramidal side effects (44). Digoxin, commonly isolated from various plants, is a citrus nutrient that has been shown to increase intracellular antioxidant capacity and alleviate symptoms associated with neurological disorders. One researcher found that digoxin applied to mice prevented schizophrenia-like behaviors and attenuated oxidative stress in ketamine schizophrenic mice (61). Oxidative stress is more prominent in the early stages of schizophrenia disease, and researchers hope to use oxidative stress indicators as an important reference for measuring the severity of the disease in the early stages as well as the efficacy of subsequent treatment (62). Advances in neuroimaging techniques, particularly the measurement of glutathione (GSH) levels in the brain, which are important for antioxidant defense, by magnetic resonance, have gradually made this idea a possibility. A growing number of studies have found that reducing the body’s level of oxidative stress, intervening early in the treatment of people at risk for schizophrenia, and reducing the duration of the untreated period can slow down the deterioration of cognitive functioning, improve psychotic symptoms, enhance patients’ recovery, and may even change the trajectory of the disease. Early cognitive-behavioral therapy can be administered to adjust the patient’s lifestyle, and if necessary, small-dose antipsychotic medication can be given to reduce the level of oxidative stress in combination with antioxidants and other agents to slow down neuronal cell damage.
Summary
The etiology of schizophrenia has not yet been clarified, and more and more studies have shown that schizophrenia is a progressive neurodevelopmental disorder, and multiple factors play a role in the onset and development of the disease. The level of oxidative stress in the brain of schizophrenic patients is significantly higher than that of the normal population, and the level of enzyme and non-enzymatic antioxidants is significantly decreased, especially in the acute phase, when the symptoms of hallucinatory delusions are obvious, and the organism’s oxidative stress damage is more severe relative to the smooth phase. However, the exact molecular mechanisms of the disease are unknown. The imbalance between oxidative and antioxidant causes the internal environment in which neurons live to be compromised, affecting substances such as lipids, proteins and nucleic acids in neuronal cells, and affecting neuronal structure as well as function. Early intervention therapy with antioxidant supplementation to reduce the level of oxidative stress and oxidative stress damage may provide a new breakthrough point in the treatment of schizophrenia.
Author contributions
KZ: Formal analysis, Investigation, Methodology, Writing – original draft. LZ: Conceptualization, Data curation, Writing – review & editing. YC: Conceptualization, Data curation, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare no financial support was received for the research, authorship, and/or publication of this article. Supported by Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (No.SZGSP013).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Moreno-Küstner B, Martín C, Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PloS One. (2018) 13:e0195687. doi: 10.1371/journal.pone.0195687
2. Chien YL, Hwu HG, Hwang TJ, Hsieh MH, Liu CC, Lin-Shiau SY, et al. Clinical implications of oxidative stress in schizophrenia: Acute relapse and chronic stable phase. Prog Neuropsychopharmacol Biol Psychiatry. (2020) 99:109868. doi: 10.1016/j.pnpbp.2020.109868
3. Cruz BF, de Campos-Carli SM, de Oliveira AM, de Brito CB, Garcia ZM, do Nascimento Arifa RD, et al. Investigating potential associations between neurocognition/social cognition and oxidative stress in schizophrenia. Psychiatry Res. (2021) 298:113832. doi: 10.1016/j.psychres.2021.113832
4. Lin CH, Lane HY. Early identification and intervention of schizophrenia: Insight from hypotheses of glutamate dysfunction and oxidative stress. Front Psychiatry. (2019) 10:93. doi: 10.3389/fpsyt.2019.00093
5. Dudzinska E, Szymona K, Bogucki J, Koch W, Cholewinska E, Sitarz R, et al. Increased markers of oxidative stress and positive correlation low-grade inflammation with positive symptoms in the first episode of schizophrenia in drug-naïve patients. J Clin Med. (2022) 11:2551. doi: 10.3390/jcm11092551
6. Solberg DK, Refsum H, Andreassen OA, Bentsen H. A five-year follow-up study of antioxidants, oxidative stress and polyunsaturated fatty acids in schizophrenia. Acta Neuropsychiatr. (2019) 31:202–12. doi: 10.1017/neu.2019.14
7. Okusaga OO. Accelerated aging in schizophrenia patients: the potential role of oxidative stress. Aging Dis. (2014) 5:256–62. doi: 10.14336/ad.2014.0500256
8. Martins SG, Zilhão R, Thorsteinsdóttir S, Carlos AR. Linking oxidative stress and DNA damage to changes in the expression of extracellular matrix components. Front Genet. (2021) 12:673002. doi: 10.3389/fgene.2021.673002
9. Sertan Copoglu U, Virit O, Hanifi Kokacya M, Orkmez M, Bulbul F, Binnur Erbagci A, et al. Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res. (2015) 229:200–5. doi: 10.1016/j.psychres.2015.07.036
10. Şimşek Ş, Gençoğlan S, Yüksel T, Kaplan İ, Alaca R, Aktaş H. Oxidative stress and DNA damage in untreated first-episode psychosis in adolescents. Neuropsychobiology. (2016) 73:92–7. doi: 10.1159/000444488
11. Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. (2023) 97:2499–574. doi: 10.1007/s00204-023-03562-9
12. Juchnowicz D, Dzikowski M, Rog J, Waszkiewicz N, Zalewska A, Maciejczyk M, et al. Oxidative stress biomarkers as a predictor of stage illness and clinical course of schizophrenia. Front Psychiatry. (2021) 12:728986. doi: 10.3389/fpsyt.2021.728986
13. Potanin SS, Morozova MA. [Oxidative stress in schizophrenia as a promising target for psychopharmacotherapy]. Zh Nevrol Psikhiatr Im S S Korsakova. (2021) 121:131–8. doi: 10.17116/jnevro2021121091131
14. Buosi P, Borghi FA, Lopes AM, Facincani IDS, Fernandes-Ferreira R, Oliveira-Brancati CIF, et al. Oxidative stress biomarkers in treatment-responsive and treatment-resistant schizophrenia patients. Trends Psychiatry Psychother. (2021) 43:278–85. doi: 10.47626/2237-6089-2020-0078
15. Bitanihirwe BK, Woo TU. Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev. (2011) 35:878–93. doi: 10.1016/j.neubiorev.2010.10.008
16. Zhu S, Zhao L, Fan Y, Lv Q, Wu K, Lang X, et al. Interaction between TNF-α and oxidative stress status in first-episode drug-naïve schizophrenia. Psychoneuroendocrinology. (2020) 114:104595. doi: 10.1016/j.psyneuen.2020.104595
17. Bošković M, Grabnar I, Terzič T, Kores Plesničar B, Vovk T. Oxidative stress in schizophrenia patients treated with long-acting haloperidol decanoate. Psychiatry Res. (2013) 210:761–8. doi: 10.1016/j.psychres.2013.08.035
18. Ma J, Yan L, Guo T, Yang S, Ni D, Liu Y, et al. A pilot study of biomarkers of oxidative stress in serum and schizophrenia. Psychiatry Res. (2020) 284:112757. doi: 10.1016/j.psychres.2020.112757
19. Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. (2011) 14:123–30. doi: 10.1017/s1461145710000805
20. Yao JK, Leonard S, Reddy RD. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr Bull. (2004) 30:923–34. doi: 10.1093/oxfordjournals.schbul.a007142
21. Do KQ, Trabesinger AH, Kirsten-Krüger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. (2000) 12:3721–8. doi: 10.1046/j.1460-9568.2000.00229.x
22. Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. (2006) 22:83–93. doi: 10.1155/2006/248387
23. Khan FZ, Sultana SP, Mullick MS, Akhter N. Oxidative Stress and Antioxidant Status in Schizophrenia Patients. J Armed Forces Med Coll. (2016) 12:40–3. doi: 10.3329/jafmc.v12i2.41087
24. Tsugawa S, Noda Y, Tarumi R, Mimura Y, Yoshida K, Iwata Y, et al. Glutathione levels and activities of glutathione metabolism enzymes in patients with schizophrenia: A systematic review and meta-analysis. J Psychopharmacol. (2019) 33:1199–214. doi: 10.1177/0269881119845820
25. Yang AC, Tsai SJ. New targets for schizophrenia treatment beyond the dopamine hypothesis. Int J Mol Sci. (2017) 18:1689. doi: 10.3390/ijms18081689
26. Zain MA, Rouhollahi E, Pandy V, Mani V, Majeed ABA, Wong WF, et al. Phencyclidine dose optimisation for induction of spatial learning and memory deficits related to schizophrenia in C57BL/6 mice. Exp Anim. (2018) 67:421–9. doi: 10.1538/expanim.18-0006
27. Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci. (2016) 17:125–34. doi: 10.1038/nrn.2015.19
28. Chauhan P, Kaur G, Prasad R, Singh H. Pharmacotherapy of schizophrenia: immunological aspects and potential role of immunotherapy. Expert Rev Neurother. (2021) 21:1441–53. doi: 10.1080/14737175.2021.1994857
29. Nguyen KD, Amerio A, Aguglia A, Magnani L, Parise A, Conio B, et al. Microglia and other cellular mediators of immunological dysfunction in schizophrenia: A narrative synthesis of clinical findings. Cells. (2023) 12:2099. doi: 10.3390/cells12162099
30. Momtazmanesh S, Zare-Shahabadi A, Rezaei N. Cytokine alterations in schizophrenia: An updated review. Front Psychiatry. (2019) 10:892. doi: 10.3389/fpsyt.2019.00892
31. Guo J, He C, Song H, Gao H, Yao S, Dong SS, et al. Unveiling promising neuroimaging biomarkers for schizophrenia through clinical and genetic perspectives. Neurosci Bull. (2024) 40:1333–52. doi: 10.1007/s12264-024-01214-1
32. Möller M, Du Preez JL, Viljoen FP, Berk M, Emsley R, Harvey BH. Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. Brain Behav Immun. (2013) 30:156–67. doi: 10.1016/j.bbi.2012.12.011
33. Cummings MA, Arias AW, Stahl SM. What is the neurobiology of schizophrenia? CNS Spectr. (2024) 30:1–6. doi: 10.1017/s1092852924000518
34. Khavari B, Mahmoudi E, Geaghan MP, Cairns MJ. Oxidative stress impact on the transcriptome of differentiating neuroblastoma cells: Implication for psychiatric disorders. Int J Mol Sci. (2020) 21:9182. doi: 10.3390/ijms21239182
35. Obi-Nagata K, Temma Y, Hayashi-Takagi A. Synaptic functions and their disruption in schizophrenia: From clinical evidence to synaptic optogenetics in an animal model. Proc Jpn Acad Ser B Phys Biol Sci. (2019) 95:179–97. doi: 10.2183/pjab.95.014
36. Wang MY, Ho NF, Sum MY, Collinson SL, Sim K. Impact of duration of untreated psychosis and premorbid intelligence on cognitive functioning in patients with first-episode schizophrenia. Schizophr Res. (2016) 175:97–102. doi: 10.1016/j.schres.2016.04.002
37. Zheng LR, Wang YX, Li M, Li LJ, Kang Z, Guo XF, et al. A study of cerebral cortex in untreated first-episode schizophrenics. Natl Med J China. (2013) 93:3252–5.
38. Uddin SMN, Sultana F, Uddin MG, Dewan SMR, Hossain MK, Islam MS. Effect of antioxidant, malondialdehyde, macro-mineral, and trace element serum concentrations in Bangladeshi patients with schizophrenia: A case-control study. Health Sci Rep. (2021) 4:e291. doi: 10.1002/hsr2.291
39. Chen P, Wang D, Xiu M, Chen D, Lackey B, Wu HE, et al. Polymorphism of transferrin gene impacts the mediating effects of psychotic symptoms on the relationship between oxidative stress and cognition in patients with chronic schizophrenia. Antioxidants (Basel). (2022) 11:125. doi: 10.3390/antiox11010125
40. Gonzalez-Liencres C, Tas C, Brown EC, Erdin S, Onur E, Cubukcoglu Z, et al. Oxidative stress in schizophrenia: A case-control study on the effects on social cognition and neurocognition. BMC Psychiatry. (2014) 14:268. doi: 10.1186/s12888-014-0268-x
41. Wiedlocha M, Zborowska N, Marcinowicz P, Debowska W, Debowska M, Zalewska A, et al. Oxidative stress biomarkers among schizophrenia inpatients. Brain Sci. (2023) 13:490. doi: 10.3390/brainsci13030490
42. Li H, Huang Y, Liang L, Li H, Li S, Feng Y, et al. The relationship between the gut microbiota and oxidative stress in the cognitive function of schizophrenia: A pilot study in China. Schizophr Res. (2024) 267:444–50. doi: 10.1016/j.schres.2024.03.053
43. Çilem Kızılpınar S, Çiğdem Aydemır M, Doğan Ö, Bahar Atak-Akkus F, Baran Z. Social cognition and oxidative stress in schizophrenia patients and first-degree relatives of patients. Psychiatr Danub. (2023) 35:523–34. doi: 10.24869/psyd.2023.523
44. Knable MB. Extract of Ginkgo biloba added to haloperidol was effective for positive symptoms in refractory schizophrenia. Evid Based Ment Health. (2002) 5:90. doi: 10.1136/ebmh.5.3.90
45. Yang M, Li J, Yang H, Yan L, Liu D, Zhu L, et al. Cognitive impairment and psychopathology are related to plasma oxidative stress in long term hospitalized patients with chronic schizophrenia. Front Psychiatry. (2022) 13:896694. doi: 10.3389/fpsyt.2022.896694
46. Wang K, Xiu M, Su X, Wu F, Zhang X. Association between changes in total antioxidant levels and clinical symptom improvement in patients with antipsychotic-naive first-episode schizophrenia after 3 months of risperidone monotherapy. Antioxidants (Basel). (2022) 11:646. doi: 10.3390/antiox11040646
47. Ansari Z, Pawar S, Seetharaman R. Neuroinflammation and oxidative stress in schizophrenia: are these opportunities for repurposing? Postgrad Med. (2022) 134:187–99. doi: 10.1080/00325481.2021.2006514
48. Tsai MC, Liou CW, Lin TK, Lin IM, Huang TL. Changes in oxidative stress markers in patients with schizophrenia: the effect of antipsychotic drugs. Psychiatry Res. (2013) 209:284–90. doi: 10.1016/j.psychres.2013.01.023
49. Noto C, Ota VK, Gadelha A, Noto MN, Barbosa DS, Bonifácio KL, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. (2015) 68:210–6. doi: 10.1016/j.jpsychires.2015.07.003
50. Parikh V, Khan MM, Mahadik SP. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. (2003) 37:43–51. doi: 10.1016/s0022-3956(02)00048-1
51. Goh XX, Tang PY, Tee SF. Effects of antipsychotics on antioxidant defence system in patients with schizophrenia: A meta-analysis. Psychiatry Res. (2022) 309:114429. doi: 10.1016/j.psychres.2022.114429
52. Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fatty Acids. (2003) 69:393–9. doi: 10.1016/j.plefa.2003.08.010
53. Patel S, Sharma D, Kalia K, Tiwari V. Crosstalk between endoplasmic reticulum stress and oxidative stress in schizophrenia: The dawn of new therapeutic approaches. Neurosci Biobehav Rev. (2017) 83:589–603. doi: 10.1016/j.neubiorev.2017.08.025
54. Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: Reversal by N-acetylcysteine. Biol Psychiatry. (2013) 73:574–82. doi: 10.1016/j.biopsych.2012.09.020
55. Yolland CO, Hanratty D, Neill E, Rossell SL, Berk M, Dean OM, et al. Meta-analysis of randomised controlled trials with N-acetylcysteine in the treatment of schizophrenia. Aust N Z J Psychiatry. (2020) 54:453–66. doi: 10.1177/0004867419893439
56. Minarini A, Ferrari S, Galletti M, Giambalvo N, Perrone D, Rioli G, et al. N-acetylcysteine in the treatment of psychiatric disorders: Current status and future prospects. Expert Opin Drug Metab Toxicol. (2017) 13:279–92. doi: 10.1080/17425255.2017.1251580
57. Ishola IO, Ben-Azu B, Adebayo OA, Ajayi AM, Omorodion IL, Edje KE, et al. Prevention and reversal of ketamine-induced experimental psychosis in mice by the neuroactive flavonoid, hesperidin: The role of oxidative and cholinergic mechanisms. Brain Res Bull. (2021) 177:239–51. doi: 10.1016/j.brainresbull.2021.10.007
58. Mitra S, Natarajan R, Ziedonis D, Fan X. Antioxidant and anti-inflammatory nutrient status, supplementation, and mechanisms in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2017) 78:1–11. doi: 10.1016/j.pnpbp.2017.05.005
59. Tharoor H, Mara S, Gopal S. Role of novel dietary supplement N-acetyl cysteine in treating negative symptoms in schizophrenia: A 6-month follow-up study. Indian J Psychol Med. (2018) 40:139–42. doi: 10.4103/ijpsym.Ijpsym_322_17
60. Dean OM, van den Buuse M, Bush AI, Copolov DL, Ng F, Dodd S, et al. A role for glutathione in the pathophysiology of bipolar disorder and schizophrenia? Animal models and relevance to clinical practice. Curr Med Chem. (2009) 16:2965–76. doi: 10.2174/092986709788803060
61. Okubo Eneni AE, Ben-Azu B, Mayowa Ajayi A, Oladele Aderibigbe A. Diosmin attenuates schizophrenia-like behavior, oxidative stress, and acetylcholinesterase activity in mice. Drug Metab Pers Ther. (2020) 35:20200119. doi: 10.1515/dmdi-2020-0119
Keywords: schizophrenia, oxidation and antioxidant, oxidative stress, treatment, prognosis
Citation: Zhang K, Zou L and Cai Y (2025) Progress in the study of oxidative stress damage in patients with schizophrenia: challenges and opportunities. Front. Psychiatry 16:1505397. doi: 10.3389/fpsyt.2025.1505397
Received: 02 October 2024; Accepted: 05 February 2025;
Published: 21 February 2025.
Edited by:
Sergei V. Fedorovich, Belarusian State University, BelarusReviewed by:
Aksana Hubich, Belarusian State University, BelarusAlexandrina Curpan, Alexandru Ioan Cuza University, Romania
Copyright © 2025 Zhang, Zou and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Cai, Y2FpeWk4MTZAMTYzLmNvbQ==
 Kaiguo Zhang
Kaiguo Zhang Lijing Zou
Lijing Zou