
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 21 February 2025
Sec. Neuroimaging
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1473913
Background: Aberrant interoceptive processing has been hypothesized to contribute to the pathophysiology of functional neurological disorder, although findings have been inconsistent. Here, we utilized functional magnetic resonance imaging (fMRI) to examine neural correlates of interoceptive attention – the conscious focus and awareness of bodily sensations – in functional movement disorder (FMD).
Methods: We used voxelwise analyses to compare blood oxygenation level-dependent responses between 13 adults with hyperkinetic FMD and 13 healthy controls (HCs) during a task requiring attention to different bodily sensations and to an exteroceptive stimulus. Additionally, we examined between-group differences in self-reported measures of interoception and evaluated their relationship with neural activity.
Results: Interoceptive conditions (heartbeat, stomach and ‘body’, indicating sensations from the body part or limb affected in FMD participants) activated a network involving the precuneus, the posterior cingulate cortex (PCC) and caudate nucleus (CN) bilaterally, and the right anterior insula (aINS) (p <0.05, corrected). Group differences in brain activity were mainly driven by processing of disease-related interoceptive signals, which in the FMD group was associated with a broader neural activation than monitoring gastric interoception, while no group differences were detected during cardiac interoception. Differences based on interoceptive focus (body vs heartbeat and stomach) between FMD subjects and HCs were found in PCC, CN, angular gyrus, thalamus, and in the mid-insula (p <0.05, corrected).
Conclusions: This is, to our knowledge, the first study showing that FMD is associated with abnormal interoceptive processing in regions involved in monitoring body state, attentional focus, and homeostatic inference.
Interoception refers to sensing, interpreting, and integrating a wide range of internal bodily signals (1). This complex process encompasses different dimensions, including interoceptive accuracy, attention, and sensibility (2, 3), which contribute to the homeostatic regulation of the body as well as to cognition, attention, and emotion processing (4, 5). Furthermore, interoceptive signals are critical in building body representations (6–8) and for the generation of subjective motor-related feelings states (3, 9, 10). At the neurocircuitry level, interoceptive processing has been consistently associated with activity in the dorsal mid-insular cortex, as well as in sensorimotor, temporal, and prefrontal cortex regions (11–19).
Alterations in interoception, and its underlying neurocircuitry, have been increasingly recognized as an important transdiagnostic component of different psychiatric disorders, including anxiety and mood disorders, eating disorders, addictive disorders, and somatic symptom disorders [for a review see (3)]. In recent years, interoceptive deficits have also been proposed to contribute to the generation of functional neurological symptoms (20). To test this hypothesis, several studies have examined behavioral correlates of interoceptive accuracy (the ability to accurately detect internal bodily sensations) in patients with Functional Neurological Disorders (FND), with a focus on heartbeat perception accuracy (21–28). Few studies have also examined interoceptive sensibility - the subjective perception and beliefs about one’s interoceptive accuracy and attention (26, 28, 29) - and its correlation with white matter integrity indices in FND patients) vs healthy controls (30).
Findings from these studies have been inconsistent, showing either reduced interoceptive accuracy in individuals with functional movement disorder (FMD) and functional seizures (FS) (21, 22, 25, 26, 29) or lack of group-level differences between patients and controls (23, 24, 28, 30). These discrepancies are likely due to several factors, including potential heterogeneity in interoceptive abilities among patients with different FND subtypes. Furthermore, behavioral measures of cardiac interoception accuracy do not capture impairment in other dimensions of interoception that may be implicated in FND (31).
In particular, a growing body of evidence suggests that individuals with FND selectively monitor disease-related somatic information and exhibit abnormal body-centered attention. For instance, FND patients have been shown to over-report somatic symptoms, compared to clinical assessment (21) or objective measures (32) of symptom frequency. These observations suggest that alterations in interoceptive attention (IA) - the conscious focus and awareness of bodily sensations (31) – may also contribute to the pathophysiology and symptomatology of FND. However, to date no published study has directly probed IA processing, and its neural correlates, in individuals with FND. Furthermore, research is needed to understand whether IA abilities vary according to the interoceptive signals processed, the increased weight attributed to disease-related interoceptive signals in FND may hijack IA allocated to other bodily signals.
To begin addressing this set of questions, in the current study we investigated neural mechanisms of IA in patients with FMD and healthy controls (HCs) during performance of an interoception attention task comprising different interoceptive conditions and an exteroceptive condition. Furthermore, we examined the relationship between neural activity during IA and individual differences in self‐reported interoception, as measured by the Multidimensional Scale of Interoceptive Awareness (MAIA) (33). We hypothesized that patients with FMD compared to controls would show increased activity in insula during the interoceptive conditions compared to exteroception. We also predicted that the magnitude of the hemodynamic response in this region during IA would correlate with self-reported measures of interoception in patients with FMD.
Participants in this study were recruited from the Human Motor Control Clinic at the National Institutes of Health (NIH) between April 2018 and April 2022 and belonged to a larger ongoing study investigating the clinical and neurobiological correlates of FMD. Study subjects partially overlap with those reported in previous articles (34, 35) and included 13 patients with diagnosis of FMD and 13 age- and sex-matched HCs. Exclusion criteria for FMD patients included movement symptoms affecting the head or neck; comorbid neurologic diseases; psychosis, bipolar disorder, or current substance abuse; current suicidality; disease severity requiring hospitalization; use of tricyclic antidepressants or antiepileptic medications; and abnormal clinical MRI brain. HCs were excluded for use of antidepressant medications within the last 6 months. All participants provided written informed consent. The NIH Institutional Review Board approved the study.
During the study, all participants underwent a physical and neurological exam. Diagnosis of ‘clinically definite’ FMD was made by at least 2 movement disorders specialists utilizing Fahn and Williams criteria. Participants also completed the Simplified-Functional Movement Disorders Rating Scale (S-FMDRS) (36), which was used to identify the limb or body part most affected, based on symptom severity and frequency. Anxious and depressive symptomatology were evaluated using the Hamilton Anxiety Rating Scale (HAM-A) (37) and the Hamilton Rating Scale for Depression (HAM-D) (38). We also administered the Multidimensional Assessment of Interoceptive Awareness (MAIA) to evaluate different self-reported components of interoception (33, 39). This 32-item questionnaire includes eight scales (i.e., Noticing, Not-Distracting, Not-Worrying, Attention Regulation, Emotional Awareness, Self-Regulation, Body Listening, Trusting) and has been extensively employed in both healthy and clinical populations. A major strength of the MAIA is the ability to differentiate between maladaptive and beneficial attention styles towards the body (39).
As part of the larger study in which they were enrolled, study subjects were also screened for psychiatric diagnoses using the Structured Clinical Interview for DSM-IV-TR, Patient Edition (SCID) (40).
Imaging was acquired during each visit with a 3-T MR750 GE scanner, using a 32-channel head coil. Each fMRI scan included five consecutive runs in the following order: anatomical scan (~5 min); resting state (~6 min), task (3 runs, ~9 min each), and resting state (~6 min). A single-shot, multi-echo, echo-planar imaging (EPI) sequence with Sensitivity Encoding (SENSE) was employed for blood oxygenation level-dependent (BOLD) fMRI scans. fMRI acquisition parameters were: repetition time (TR) = 2500 ms, number of echoes = 3, echo times (TEs) = 14.5, 32.3, 50.1 ms, flip angle (FA) = 75°, field of view (FOV) = 216 x 216 mm2, matrix size= 72x72, slice thickness: 3.0 mm, slices = 36, nominal voxel size: 3.0 x 3.0 x 3.0 mm3, repetitions = 144. The three echoes are used to differentiate BOLD vs non-BOLD signals in the fMRI data based on the TE-dependence of the signal of interest (e.i. related to neuronal activity). We used Tedana method within the afni_proc.py script to denoise data without conventional noise modeling (41). This method is based on performing Independent Component Analysis (ICA) and then compute the TE dependance of the signal in each. Those with a good fit are considered signal (fMRI) and those with poor fitting are considered noise. For an anatomical reference for the fMRI analyses, a T1-weighted MRI scan with magnetization-prepared rapid gradient echo (MPRAGE) sequence with SENSE was obtained (TR = 7.7ms, TE = 3.436ms, FA = 7°, FOV = 256 x 256 mm2, nominal voxel size: 1.0 x 1.0 x 1.0 mm3, slices = 176).
This task was modified from prior fMRI studies of interoception where subjects were asked to attend to their body sensations (11, 14, 15, 17, 18, 42). In brief, during each of the 3 runs of the task, participants alternated between two experimental conditions, the interoceptive attention and the exteroceptive attention condition. During the interoception condition, the word “HEART”, “STOMACH”, or “BODY” was presented for 10 seconds in the center of a screen, in black font against a white background. During this time, subjects were instructed to focus their attention on their heartbeat (H) or stomach distension (S). When patients with FMD saw the word “BODY” on the screen, they were asked to monitor sensations coming from the affected limb/body part (e.g., left leg; right arm), as identified by the S-FMDRS. If patients reported functional motor symptoms in different limbs/body parts, they were instructed to focus on the most severely affected. During these blocks, healthy controls focused on sensations from the limb/body part indicated by the matching FMD patient.
In this version of the task, the interoceptive conditions included monitoring both visceral and somatic signals, in line with the conceptualization of interoception as the sensing of all physiological tissues that relay a signal to the central nervous system about the current state of the body (42–45). Another fMRI study also employed a similar approach, with subjects attending to heartbeat as well as to skin temperature during the task (42). Furthermore, numerous imaging studies investigating interoceptive processing across a variety of psychiatric disorders employed tasks presenting disease-related stimuli [for a review see (46)].
The interoceptive attention task also involved an exteroceptive attention control condition, during which the word “TARGET” was presented in the center of the screen and randomly switched color from black to a lighter shade of gray, for 500 ms durations. Participants were instructed to focus their attention on the intensity of these color changes and to count the number of times they occurred during the 10-second exteroceptive trial.
One-half of the trials of the interoceptive and exteroceptive conditions were immediately followed by a response period during which subjects rated the intensity of interoceptive sensations (with “1” indicating no sensation, and “7” indicating an extremely strong sensation), or the number of color changes perceived in the exteroceptive trial, via an MRI-compatible button-box. After each rating, there was an intertrial interval (ITI) consisting of a fixation crosshair before the next block begun. The task was performed using E-Prime® 3.0 software (Psychology Software Tools, Pittsburgh, PA, USA).
After receiving verbal instructions about how to perform the task, all subjects underwent a practice session during which they were monitored while making stimulus intensity responses and were asked to indicate whether they had any remaining questions about the task demands.
Data processing of fMRI data was performed using AFNI [v16.2.16 (40); http://afni.nimh.nih.gov/afni]. The three runs of the task were processed together. Pre-processing steps are detailed in Supplementary Material [eAppendix (preprocess_taskFMRI_02b_TT27.sh].
Maps (βs) for each interoception conditions (body, heart, and stomach) against the exteroception condition (target) were created for each subject. A multivariate modeling using the AFNI program 3dMVM was set for the analysis. We evaluated the main effects of group (FMD or HCs) and interoceptive modality (heart, stomach, or body) and their interaction. For the IA conditions, all subject-level βs represent the signal change from the exteroceptive baseline condition. We performed comparisons across conditions within the same model (see script in supplements MVM_target_command.txt). Imaging results are reported at a voxel-wise threshold of p<0.005 and cluster size of 20, bi-sided, for a whole brain corrected significance of p <0.05.
To examine the relationship between brain activation during disease-related somatic (body) vs visceral interoception (stomach+ heart) and self-report measures of interoception as well as clinical measures of FMD, we correlated the individual β values derived from the contrast of somatic versus visceral interoception with the following questionnaire scores: MAIA, HAM-A, HAM-D, and FMDRS, using GraphPad Prism software version 8.0. Correlations were Bonferroni corrected to control for family-wise error for a significance level of p < 0.05.
Twenty-six participants, consisting of 13 patients with FMD and 13 age- and sex-matched HCs, were included in the analysis. Groups did not differ in terms of demographic data and exposure to childhood trauma (Table 1). Compared to HCs, patients reported greater anxiety symptom severity in the seven days prior to the study, which was in the mild range (HAM-A ratings= 10.3 ± 5.1), whereas both patients and HCs did not report depressive symptomatology (score < 10), as assessed by the HAM-D scale, Five patients had a lifetime diagnosis of comorbid psychiatric disorders (depressive disorders n=2, generalized anxiety disorder n=3, PTSD=1).
Clinically, patients reported an average FMD duration of 10.7 years (± 7), with a baseline S-FMDRS score of 14.5 (± 6.8). The abnormal movements included tremor (n = 7; seven upper extremities and three lower extremities), dystonia (n = 3 upper limb/shoulder), positive myoclonus (n = 2; shoulder/lower limb); mixed tremor/dystonia (n = 1; upper limb).
Whole brain analysis revealed a significant interaction between group (FMD > HCs) and stimulus (interoception > exteroception) [p< 0.05, corrected] in a large cluster encompassing the left and right posterior cingulate cortex (PCC) and the precuneus, as well as in the left and right caudate nucleus (CN), and in the right anterior insula (aINS) (Figure 1, Table 2). Specifically, compared to controls, patients with FMD exhibited increased hemodynamic response in these regions across interoceptive modalities. In separate comparisons of each interoception condition (heart [H], stomach [S], body [B]) to the exteroception condition (target [T]), we found significant group differences during somatic interoception compared to the exteroceptive condition (B > T), such that FMD patients had greater average activity in several regions part of the default mode network [DMN] (i.e., posterior cingulate, precuneus, angular gyrus, and medial prefrontal cortex), in the right cerebellum as well as in the bilateral CN [p< 0.05, corrected] (Figure 2A, Table 2). Precuneus activity was greater in patients with FMD compared to controls during visceral interoception [p< 0.05, corrected] (S > T), whereas no group differences in the hemodynamic response to heartbeat attention were found (Figure 2B, Table 2).
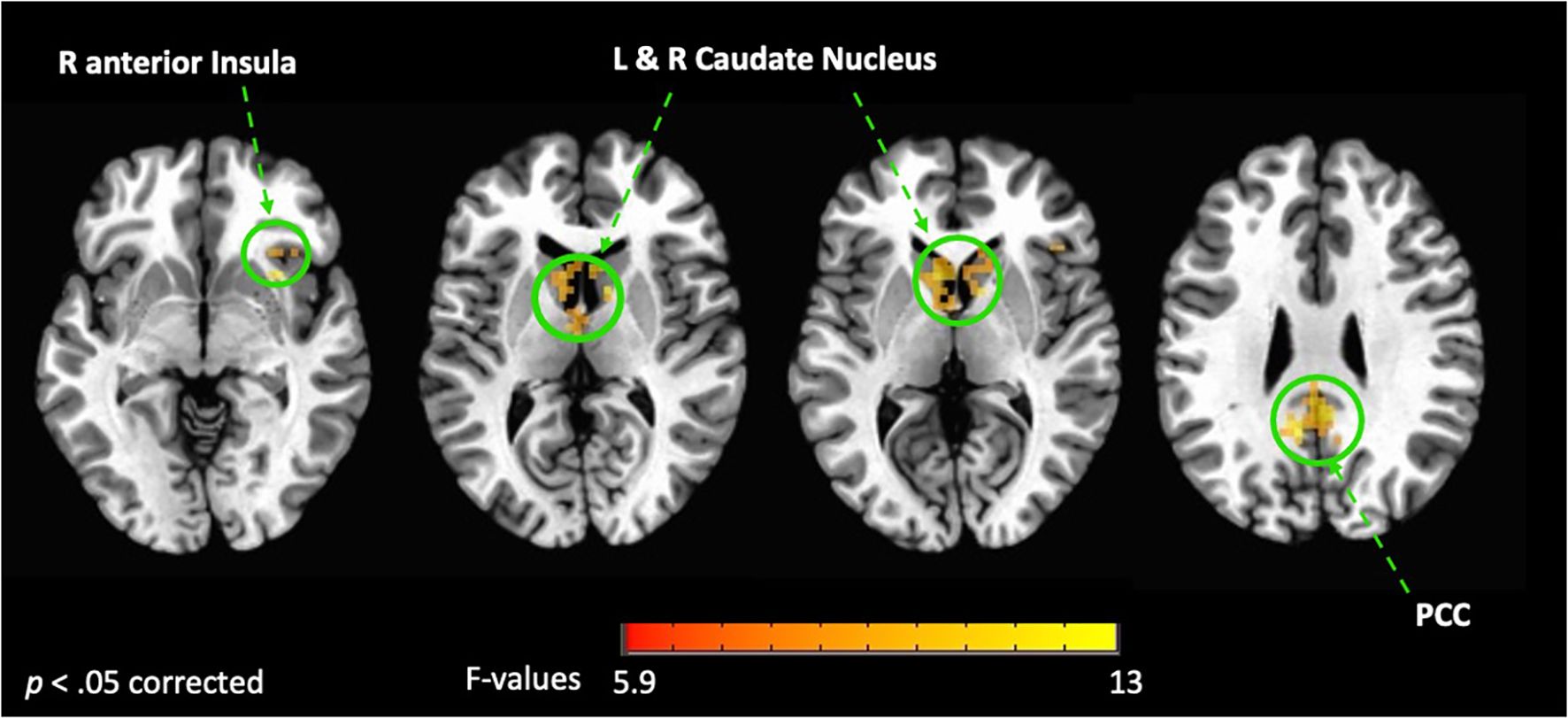
Figure 1. Interaction between groups (FMD vs HCs) and condition (interoception vs exteroception). This figure shows the brain regions activated during interoceptive conditions vs exteroception in patients with FMD compared to healthy controls (HCs). In these regions, hemodynamic activity was increased across interoceptive conditions in the FMD group compared to the HC group. All results shown were corrected for multiple comparisons (p < 0.05). FMD, functional movement disorder; PCC, posterior cingulate cortex; R, right; L, left.
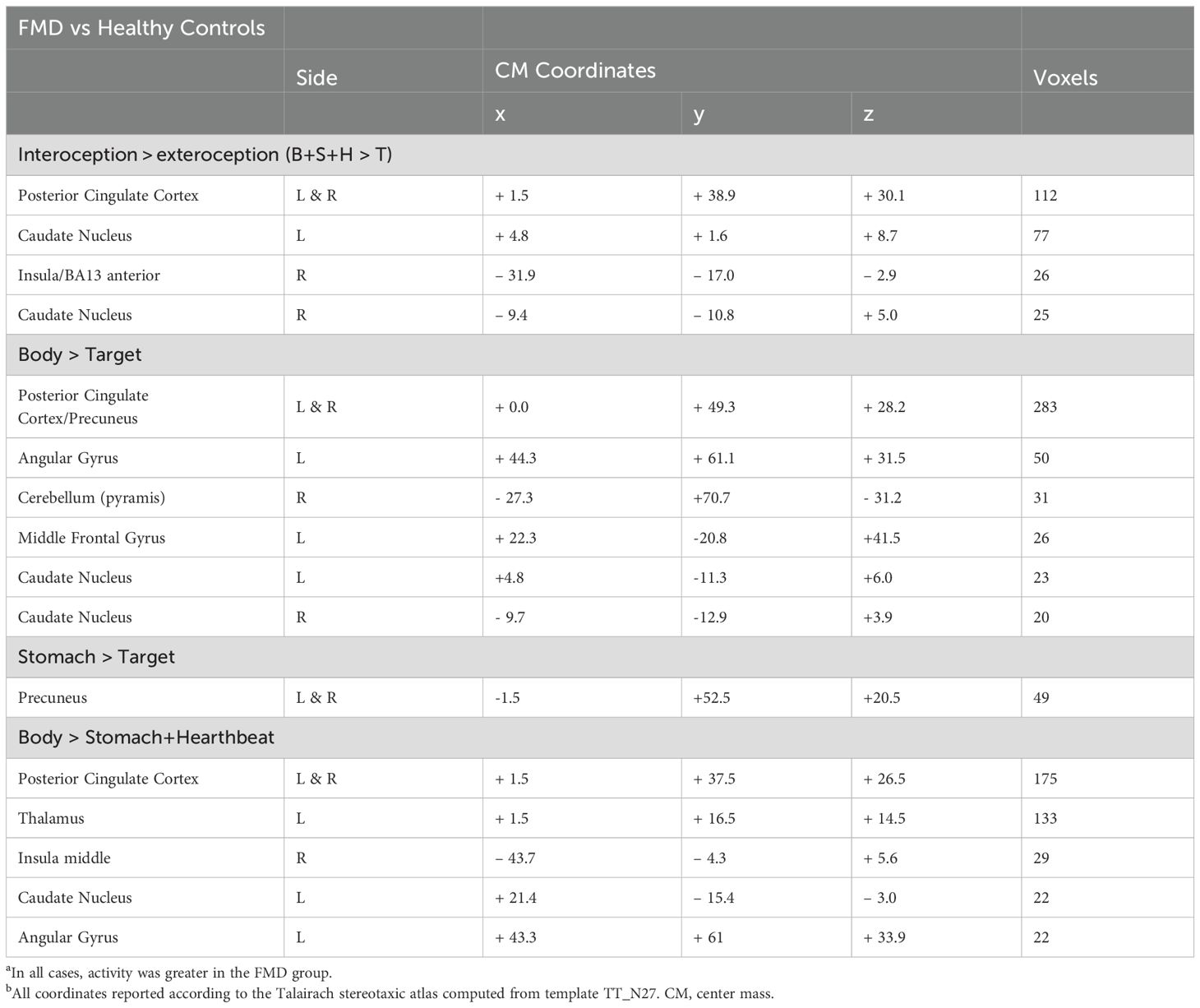
Table 2. Brain regions exhibiting group-differences in the hemodynamic response to interoception conditions versus exteroceptive attention.

Figure 2. Group differences in somatic and gastric interoception. In the FMD group, attending to disease-related interoceptive signals (A) elicited activity in several regions part of the default mode network, including the bilateral posterior cingulate/precuneus, left angular gyrus, right cerebellum as well as in the bilateral CN. Precuneus activity was also greater during gastric interoception (B) in patients with FMD compared to the HC group. All results shown were corrected for multiple comparisons (p < 0.05). FMD, functional movement disorder.
Next, we examined differences in BOLD activity during somatic interoception compared to the two visceral interoception conditions (B > S + H) between groups. In FMD patients, IA to bodily sensations was associated with increased BOLD response in several nodes of the DMN (i.e., left angular gyrus, left thalamus, and PCC bilaterally), in the right mid-insula and in the CN [p< 0.05, corrected] (Figure 3A, Table 2).
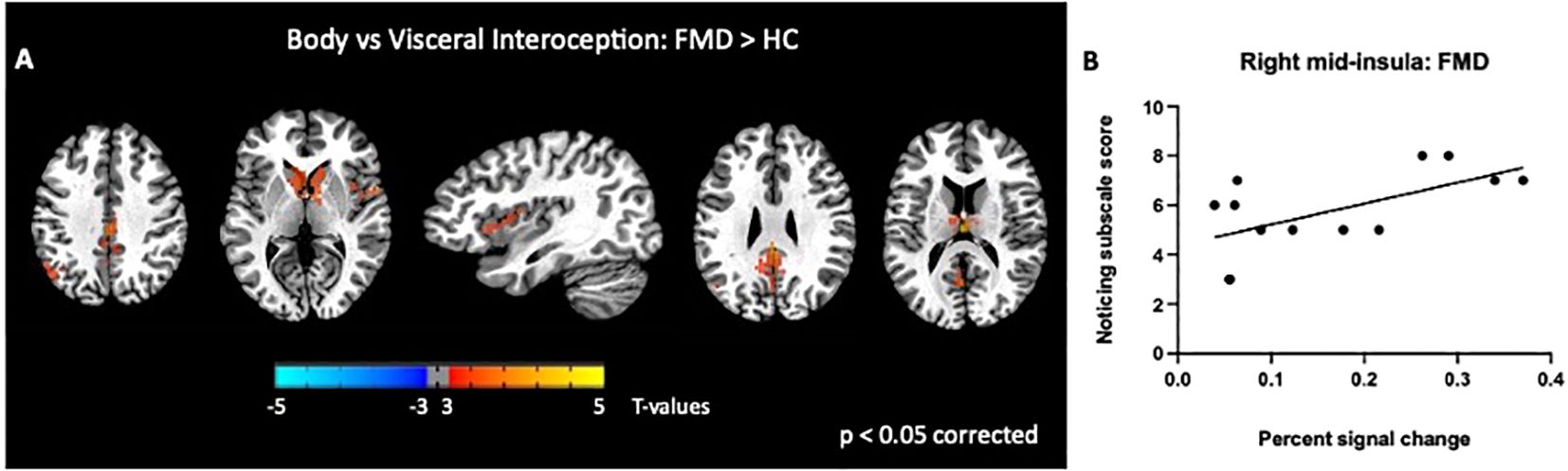
Figure 3. Group differences in somatic, disease-related interoception vs visceral interoception. In the FMD group compared to HCs, attending to disease-related interoceptive signals compared to other interoceptive signals (stomach + heartbeat) (A) elicited activity in several nodes of the DMN (i.e., left angular gyrus, left thalamus, and PCC bilaterally), in the right mid-insula and in the bilateral caudate nucleus (B). In subjects with FMD, activity in the right mid-insula was positively correlated with the MAIA Noticing subscale, which measures body awareness. All results shown were corrected for multiple comparisons (p <.05). FMD, functional movement disorder.
Scores for each of the eight MAIA subscales did not differ among patients and controls, in line with previous research (Supplementary Table 1). In the FMD group, we next assessed the relationship between MAIA subscale scores and BOLD responses in brain regions showing altered response during somatic vs visceral interoception (B > S + H). We chose to focus on this comparison, given our hypothesis that disease-related interoceptive signals would hijack interoceptive processing compared to other interoceptive signals.
We found a significant correlation between BOLD activity in the right mid-insula and the MAIA Noticing subscale score (r = 0.6; p=0.02; Figure 3B). We also examined the relationship between regional BOLD activity and several FMD-related clinical measures and found no significant correlations with S-FMDRS, HAM-D, and HAM-A total scores (data not shown).
To our knowledge, this study is the first to investigate the neural correlates of IA in individuals with FMD compared to healthy controls and to examine group-differences between hemodynamic responses to disease-related interoceptive signals vs other bodily signals. Our findings provide experimental evidence that FMD is associated with abnormal interoceptive activity in several regions, including the right insula, the bilateral posterior cingulate cortex, and the right caudate nucleus. Group-differences in brain activity were mainly driven by processing of disease-related interoceptive signals, which in patients with FMD was associated with a broader neural activation than monitoring other bodily sensations. Importantly, in this group, activity in the right mid-insula during disease-related vs visceral IA was positively correlated with the Noticing MAIA subscale scores, which measures individual’s body awareness.
In the comparison of interoception vs exteroception, we identified brain regions that have been consistently implicated in interoceptive processing, thus confirming the validity of the imaging paradigm used in our study. In line with our hypothesis, patients with FMD exhibited abnormal insular hemodynamic activity across interoceptive conditions relative to HCs, particularly within the right anterior subregion. The insula is a primary interoceptive cortex involved in salience, prediction, cognition, homeostasis, and self/emotional awareness (44, 47). Accumulating evidence suggests different roles for subregions of the insular cortex during interoception, such that bodily signals are mainly projected to the posterior insula and then transmitted forward along the rostrocaudal axis, integrated with other sensory inputs in the mid- insula, and finally re-represented in the aINS to be consciously available (44, 48, 49). As such, the right aINS contributes to the conscious interoceptive experience and has been identified as the main neural substrate of IA (50). Thus, our finding of greater activity in this subregion, together with evidence of abnormal body-centered attention (18, 21), may suggest that patients with FMD may be hypersensitive to, and perhaps constantly monitoring, interoceptive sensations. In support of this hypothesis, we observed that along with right aINS, interoceptive conditions vs exteroception also elicited greater activity within the PCC in FMD participants compared to controls. The PCC is a key node in the DMN (51, 52) and activity in this structure has been associated with decision-making, memory, body ownership, interoceptive and emotion processing, and modulation of arousal state (50, 53, 54). Notably, neuroimaging studies have consistently implicated the PCC in controlling the focus (internal vs external) and breadth (broad vs narrow) of attention, with studies reporting increased PCC activation when individuals direct attention internally (55, 56). Specifically, it has been proposed that the right aINS and the PCC form a system regulating the balance between internally and externally focused attention (53, 57). Thus, the coactivation of PCC and aINS in the FMD group during interoceptive processing vs exteroception further suggest that subjects with this disorder may have an impairment in shifting the attentional focus away from interoceptive signals.
Separate comparison of each interoceptive condition vs exteroception revealed no group-differences during cardiac interoception, supporting prior reports of normal heartbeat perception accuracy in individuals with FMD (23, 24, 28, 30). Conversely, increased activity in the precuneus – another key node of the DMN (58) - was observed during gastric interoception in FMD participants compared to controls, confirming that this network may play an important role in abnormal IA processes in FMD. Furthermore, the precuneus is also implicated in the sense of self and agency (59), which is impaired in FMD patients (60), and functional and structural alterations in this region have been reported in association with FMD (for a review see (61)). We further observed marked group-differences during processing of disease-related interoceptive signals vs exteroception, which in FMD patients elicited a broad activation across the DMN, including the angular gyrus, the mid- frontal gyrus, the PCC and the precuneus. Interestingly, the cerebellum also showed heightened activity in FMD patients compared to controls. This finding is in line with evidence of cerebellar contribution to both attentional (62, 63) and interoceptive processes (64, 65), and can also be explained in the context of prior imaging studies showing increased cerebellar volume and activity in FMD patients compared to either controls or patients with other movement disorders (for a review see (66)). Accumulating evidence also suggests that the cerebellum is part of the DMN (67, 68), thus its activation may reflect the widespread engagement of this network observed in our study, particularly in response to disease-related interoceptive signals. Previous studies have found that the DMN is characterized by overactivation and neurometabolic dysfunctions in children and adolescents with FND (69, 70): we expand on these findings by showing that these alterations may represent the neurobiological correlate of abnormal IA in subjects with FMD.
The current study also investigated for the first time group-differences in brain activation during disease-related somatic interoception compared to visceral interoception. As expected, individuals with FMD showed greater BOLD response to disease-related vs other bodily sensations in several brain regions. This pattern of activation suggests that ‘disease-centered’ attention allocation may happen not only at the expenses of external stimuli but also of other interoceptive signals. A potential explanation is that these signals are perceived as less salient compared to disease-related sensations, although we did not specifically tested for this hypothesis. However, it is worth mentioning that salience attribution is encoded by the aINS, while our results indicate increased activity in the mid-insula, in line with a prior study in healthy subjects showing that differences based on interoceptive focus (heartbeat vs skin temperature) were found in the mid-insula (14). According to active inference framework of interoceptive processing, this segment of the insula represents the key neural substrate of interoceptive prediction error, which occurs following a mismatch between interoceptive prediction signals issued by the anterior insula and incoming interoceptive signals arriving via the thalamus (71). Specifically, the middle and posterior insula compute the difference between the predicted interoceptive signal and the actual interoceptive signal, generating an error signal. Thus, our finding of increased activation in the segment of the insula during disease-related somatic interoception compared to visceral interoception may indicate a greater magnitude of disease-related interoceptive prediction errors, which may induce individuals with FMD to constantly monitor disease-related sensations in the attempt to match them with expected signals. In support of this hypothesis, we did found a positive, although marginally significant, correlation between BOLD response in the mid-insula and scores on the Noticing MAIA subscale, which assesses the spontaneous tendency to sense or notice bodily sensations.
A predictive coding account of our results is further suggested by evidence of greater activation in several nodes of the DMN, which, together with the insula and other cortical and subcortical regions, are part of unified, intrinsic large-scale brain network, the allostatic-interoceptive network, that modulates visceromotor and interoceptive processes with the goal to maintain or restore allostasis, while also supporting a wide range of psychological functions (emotions, memory, decision-making, pain), which rely on allostasis (64). Interestingly, this network also includes the CN, which showed greater activation in FMD subjects during disease-related somatic interoception compared to visceral interoception, as well as in the comparison between disease-relate bodily signals and exteroceptive condition. The CN has been associated with perceptual prediction errors (72), and a study evaluating the neural correlates of breach of expectations during the execution of a sequence of whole-body movements found that prediction-violating movements elicited CN activation (73). Taken together, our findings suggest that IA, particularly toward disease-related bodily signals, and interoceptive predictive error are closely linked in FMD, with abnormal IA resulting in higher weighting of prediction errors, which in turn may further contribute to maintain the attentional focus on disease-related bodily signals.
No study is without limitations. First, our sample size was modest, although it was in line with previous studies investigating group-differences in behavioral measures of interoception (23, 28) and included cases and controls closely matched for age and sex. Second, concerns about false positive rates in fMRI studies (74) might be raised given the use of a voxel-wise threshold of p<0.005 and cluster size correction based on random field theory. While recent evidence suggests that these concerns may be overstated (75), we also believed that the occurrence of false positive rates is unlikely given the extensive amount of neuroimaging studies implicating the insula and nodes of the DMN in interoception. Further, as the current study is the first to investigate the neural correlates of IA in FMD, we decided to adopt a voxel-wise approach and less stringent criteria, to investigate interoceptive relationships across the entire brain. However, it is crucial to replicate our findings using large datasets to assess the consistency and reliability of the results, reinforcing the significance and validity of our study’s outcomes. In interpreting our findings is also important to consider that we chose to use a version of the interoceptive task that included monitoring both visceral and somatic signals, in line with a broader conceptualization of interoception as the sensing of signals from the entire body, including skin, muscles, joints, in addition to viscera (42–45, 76). This conceptualization is supported by research showing that visceral and somatic afferents converge at several levels on their way to the brain (77). Furthermore, a study in healthy controls showed that both somatic and visceral interoception activated several brain regions, including the insula, supporting the validity of our study design (14). However, our characterization of the “heartbeat” and “stomach” conditions as involving visceral interoception and the “body” condition as involving “somatic” interoception involves assumptions that were not directly tested in the current study. As such, interpretation of the differences between interoceptive conditions should take into account the somatic and visceral processing may have been simultaneously engaged during each interoceptive condition. Another potential limitation is that FMD participants demonstrated higher levels of anxiety symptoms, which were in the moderate range, and greater psychiatric comorbidities (depressive and anxiety disorders, PTSD) compared to the HC group (see Table 1). We could not control for the potential effects of psychiatric comorbidities on brain activity, given that they were found exclusively in the FMD group and not in the control group, thus precluding their inclusion as covariates in the analyses. Thus, we cannot exclude the influence of psychiatric symptoms or comorbidities on our results, although prior studies examining IA in individuals with major depressive and anxiety disorders reported insula activation responses that were opposite to those observed in our study (78, 79). Future studies should enroll psychiatric controls to better uncover IA-related brain activation patterns uniquely associated with FMD. Lastly, we did not employ an accelerometer to record movements during the fMRI scanning, given that previous research suggests that external processes, such as cutaneous sensations, could affect interoceptive processing (45). However, we acknowledge that these data could have allowed examining the interaction between brain activation and movements evoked by interoceptive attention.
Despite these limitations, this study is the first to unravel the neural correlates of interoception attention in individuals with FMD, highlighting a critical role for regions involved in monitoring body state and in regulating attentional focus and homeostatic inference. Further studies are required to replicate our findings in larger samples and across distinct FND subtypes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the NIH Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
PAS: Conceptualization, Writing – original draft, Writing – review & editing. JP: Investigation, Methodology, Writing – review & editing, Formal analysis. MH: Conceptualization, Funding acquisition, Writing – review & editing. SH: Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work has been supported by the NINDS Intramural Research Program and the Mary Ann Tynan Fellowship Program/Brigham and Women’s Hospital.
We also acknowledge the support of the clinical research staff in the NINDS intramural program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1473913/full#supplementary-material
1. Chen WG, Schloesser D, Arensdorf AM, Simmons JM, Cui C, Valentino R, et al. The emerging science of interoception: sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci. (2021) 44(1):3–16. doi: 10.1016/j.tins.2020.10.007
2. Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol. (2015) 104:65–74. doi: 10.1016/j.biopsycho.2014.11.004
3. Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, et al. Interoception and mental health: A roadmap. Biol Psychiatry Cognit Neurosci Neuroimaging. (2018) 3(6):501–13. doi: 10.1016/j.bpsc.2017.12.004
4. Tan Y, Wang X, Blain SD, Jia L, Qiu J. Interoceptive attention facilitates emotion regulation strategy use. Int J Clin Health Psychol IJCHP. (2023) 23:100336. doi: 10.1016/j.ijchp.2022.100336
5. Garfinkel SN, Critchley HD. Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary: Soc Cognit Affect Neurosci. (2013) 8:231–4. doi: 10.1093/scan/nss140
6. Raimo S, Boccia M, Di Vita A, Cropano M, Guariglia C, Grossi D, et al. The body across adulthood: on the relation between interoception and body representations. Front Neurosci. (2021) 15:586684. doi: 10.3389/fnins.2021.586684
7. Raimo S, Di Vita A, Boccia M, Iona T, Cropano M, Gaita M, et al. The body across the lifespan: on the relation between interoceptive sensibility and high-order body representations. Brain Sci. (2021) 11(4):493. doi: 10.3390/brainsci11040493
8. Tsakiris M, Jiménez AT, Costantini M. Just a heartbeat away from one’s body: interoceptive sensitivity predicts malleability of body-representations. Proc R Soc B Biol Sci. (2011) 278:2470–6. doi: 10.1098/rspb.2010.2547
9. Price CJ, Hooven C. Interoceptive awareness skills for emotion regulation: theory and approach of mindful awareness in body-oriented therapy (MABT). Front Psychol. (2018) 9:798. doi: 10.3389/fpsyg.2018.00798
10. Quigley KS, Kanoski S, Grill WM, Barrett LF, Tsakiris M. Functions of interoception: from energy regulation to experience of the self. Trends Neurosci. (2021) 44:29–38. doi: 10.1016/j.tins.2020.09.008
11. Avery JA, Kerr KL, Ingeholm JE, Burrows K, Bodurka J, Simmons WK. A common gustatory and interoceptive representation in the human mid-insula: Gustatory-Interoceptive Overlap. Hum Brain Mapp. (2015) 36:2996–3006. doi: 10.1002/hbm.22823
12. Caseras X, Murphy K, Mataix-Cols D, López-Solà M, Soriano-Mas C, Ortriz H, et al. Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Hum Brain Mapp. (2013) 34(5):1220–9. doi: 10.1002/hbm.21503
13. Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. (2004) 7:189–95. doi: 10.1038/nn1176
14. Ernst J, Northoff G, Böker H, Seifritz E, Grimm S. Interoceptive awareness enhances neural activity during empathy. Hum Brain Mapp. (2013) 34:1615–24. doi: 10.1002/hbm.22014
15. Farb NAS, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb Cortex. (2013) 23:114–26. doi: 10.1093/cercor/bhr385
16. Pollatos O, Traut-Mattausch E, Schroeder H, Schandry R. Interoceptive awareness mediates the relationship between anxiety and the intensity of unpleasant feelings. J Anxiety Disord. (2007) 21:931–43. doi: 10.1016/j.janxdis.2006.12.004
17. Simmons WK, Rapuano KM, Kallman SJ, Ingeholm JE, Miller B, Gotts SJ, et al. Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nat Neurosci. (2013) 16(11):1551–2. doi: 10.1038/nn.3535
18. Wiebking C, Northoff G. Neural activity during interoceptive awareness and its associations with alexithymia—An fMRI study in major depressive disorder and non-psychiatric controls. Front Psychol. (2015) 6:589. doi: 10.3389/fpsyg.2015.00589
19. Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. (2012) 62:493–9. doi: 10.1016/j.neuroimage.2012.05.012
20. Van Den Bergh O, Witthöft M, Petersen S, Brown RJ. Symptoms and the body: Taking the inferential leap. Neurosci Biobehav Rev. (2017) 74:185–203. doi: 10.1016/j.neubiorev.2017.01.015
21. Ricciardi L, Demartini B, Crucianelli L, Krahé C, Edwards MJ, Fotopoulou A. Interoceptive awareness in patients with functional neurological symptoms. Biol Psychol. (2016) 113:68–74. doi: 10.1016/j.biopsycho.2015.10.009
22. Demartini B, Volpe R, Mattavelli G, Goeta D, D’Agostino A, Gambini O. The neuromodulatory effect of tDCS in patients affected by functional motor symptoms: an exploratory study. Neurol Sci. (2019) 40:1821–7. doi: 10.1007/s10072-019-03912-5
23. Jungilligens J, Wellmer J, Schlegel U, Kessler H, Axmacher N, Popkirov S. Impaired emotional and behavioural awareness and control in patients with dissociative seizures. Psychol Med. (2020) 50:2731–9. doi: 10.1017/S0033291719002861
24. Pick S, Rojas-Aguiluz M, Butler M, Mulrenan H, Nicholson TR, Goldstein LH. Dissociation and interoception in functional neurological disorder. Cognit Neuropsychiatry. (2020) 25:294–311. doi: 10.1080/13546805.2020.1791061
25. Williams IA, Reuber M, Levita L. Interoception and stress in patients with Functional Neurological Symptom Disorder. Cognit Neuropsychiatry. (2021) 26:75–94. doi: 10.1080/13546805.2020.1865895
26. Koreki A, Garfkinel SN, Critchley H, Cope S, Agrawal N, Edwards M, et al. Impaired cardiac modulation in patients with functional seizures: Results from a face intensity judgment task. Epilepsia. (2023) 64(11):3073–81. doi: 10.1111/epi.17761
27. Koreki A, Garfinkel S, Mula M, Agrawal N, Cope S, Eilon T, et al. Trait and state interoceptive abnormalities are associated with dissociation and seizure frequency in patients with functional seizures. Epilepsia. (2020) 61(6):1156–65. doi: 10.1111/epi.16532
28. Millman LSM, Short E, Stanton B, Winston JS, Nicholson TR, Mehta MA, et al. Interoception in functional motor symptoms and functional seizures: Preliminary evidence of intact accuracy alongside reduced insight and altered sensibility. Behav Res Ther. (2023) 168:104379. doi: 10.1016/j.brat.2023.104379
29. Ricciardi L, Nisticò V, Andrenelli E, Cunha JM, Demartini B, Kirsch LP, et al. Exploring three levels of interoception in people with functional motor disorders. Parkinsonism Relat Disord. (2021) 86:15–8. doi: 10.1016/j.parkreldis.2021.03.029
30. Sojka P, Diez I, Bareš M, Perez DL. Individual differences in interoceptive accuracy and prediction error in motor functional neurological disorders: A DTI study. Hum Brain Mapp. (2021) 42:1434–45. doi: 10.1002/hbm.25304
31. Murphy J, Catmur C, Bird G. Classifying individual differences in interoception: Implications for the measurement of interoceptive awareness. Psychon Bull Rev. (2019) 26:1467–71. doi: 10.3758/s13423-019-01632-7
32. Parees I, Saifee TA, Kassavetis P, Kojovic M, Rubio-Agusti I, Rothwell JC, et al. Believing is perceiving: mismatch between self-report and actigraphy in psychogenic tremor. Brain. (2012) 135(1):117–23. doi: 10.1093/brain/awr292
33. Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. The multidimensional assessment of interoceptive awareness (MAIA). PloS One. (2012) 7:e48230. doi: 10.1371/journal.pone.0048230
34. Spagnolo PA, Garvey M, Hallett M. A dimensional approach to functional movement disorders: Heresy or opportunity. Neurosci Biobehav Rev. (2021) 127:25–36. doi: 10.1016/j.neubiorev.2021.04.005
35. Spagnolo PA, Johnson K, Hodgkinson C, Goldman D, Hallett M. Methylome changes associated with functional movement/conversion disorder: Influence of biological sex and childhood abuse exposure. Prog Neuropsychopharmacol Biol Psychiatry. (2023) 125:110756. doi: 10.1016/j.pnpbp.2023.110756
36. Nielsen G, Ricciardi L, Meppelink AM, Holt K, Teodoro T, Edwards M. A simplified version of the psychogenic movement disorders rating scale: the simplified functional movement disorders rating scale (S-FMDRS). Mov Disord Clin Pract. (2017) 4:710–6. doi: 10.1002/mdc3.12475
37. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
38. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
39. Mehling W. Differentiating attention styles and regulatory aspects of self-reported interoceptive sensibility. Philos Trans R Soc Lond B Biol Sci. (2016) 371:20160013. doi: 10.1098/rstb.2016.0013
40. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute (2002).
41. Kundu P, Inati SJ, Evans JW, Luh WM, Bandettini PA. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage. (2012) 60:1759–70. doi: 10.1016/j.neuroimage.2011.12.028
42. Stern ER, Grimaldi SJ, Muratore A, Murrough J, Leibu E, Fleysher L, et al. Neural correlates of interoception: Effects of interoceptive focus and relationship to dimensional measures of body awareness. Hum Brain Mapp. (2017) 38(12):6068–82. doi: 10.1002/hbm.23811
43. Cameron OG. Interoception: the inside story—A model for psychosomatic processes. Psychosom Med. (2001) 63:697–710. doi: 10.1097/00006842-200109000-00001
44. Craig A. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. (2003) 13:500–5. doi: 10.1016/S0959-4388(03)00090-4. (Bud).
45. Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat Neurosci. (2009) 12:1494–6. doi: 10.1038/nn.2411
46. Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. (2007) 164:1476–88. doi: 10.1176/appi.ajp.2007.07030504
47. Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. (2006) 60:383–7. doi: 10.1016/j.biopsych.2006.03.042
48. Frot M, Faillenot I, Mauguière F. Processing of nociceptive input from posterior to anterior insula in humans. Hum Brain Mapp. (2014) 35:5486–99. doi: 10.1002/hbm.22565
49. Hassanpour MS, Simmons WK, Feinstein JS, Luo Q, Lapidus RC, Bodurka J, et al. The insular cortex dynamically maps changes in cardiorespiratory interoception. Neuropsychopharmacology. (2018) 43(2):426–34. doi: 10.1038/npp.2017.154
50. Wang X, Wu Q, Egan L, Gu X, Liu P, Gu H, et al. Anterior insular cortex plays a critical role in interoceptive attention. eLife. (2019) 8:e42265. doi: 10.7554/eLife.42265
51. Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex N Y N 1991. (2009) 19:72–8. doi: 10.1093/cercor/bhn059
52. Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci Off J Soc Neurosci. (2011) 31:3217–24. doi: 10.1523/JNEUROSCI.5626-10.2011
53. Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain J Neurol. (2014) 137:12–32. doi: 10.1093/brain/awt162
54. Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. (2005) 150:205–17. doi: 10.1016/S0079-6123(05)50015-3
55. Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. (2004) 24:10084–92. doi: 10.1523/JNEUROSCI.2625-04.2004
56. Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci. (2007) 104:642–7. doi: 10.1073/pnas.0610082104
57. Pearson JM, Hayden BY, Raghavachari S, Platt ML. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Curr Biol CB. (2009) 19:1532–7. doi: 10.1016/j.cub.2009.07.048
58. Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. (2014) 34:932–40. doi: 10.1523/JNEUROSCI.4227-13.2014
59. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. (2006) 129:564–83. doi: 10.1093/brain/awl004
60. Nahab FB, Kundu P, Maurer C, Shen Q, Hallett M. Impaired sense of agency in functional movement disorders: An fMRI study. PloS One. (2017) 12:e0172502. doi: 10.1371/journal.pone.0172502
61. Roelofs JJ, Teodoro T, Edwards MJ. Neuroimaging in functional movement disorders. Curr Neurol Neurosci Rep. (2019) 19:12. doi: 10.1007/s11910-019-0926-y
62. Gottwald B, Mihajlovic Z, Wilde B, Mehdorn HM. Does the cerebellum contribute to specific aspects of attention? Neuropsychologia. (2003) 41:1452–60. doi: 10.1016/s0028-3932(03)00090-3
63. Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. (2013) 80:807–15. doi: 10.1016/j.neuron.2013.10.044
64. Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, et al. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat Hum Behav. (2017) 1:0069. doi: 10.1038/s41562-017-0069
65. Smith SD, Nadeau C, Sorokopud-Jones M, Kornelsen J. The relationship between functional connectivity and interoceptive sensibility. Brain Connect. (2022) 12:417–31. doi: 10.1089/brain.2020.0777
66. Sasikumar S, Strafella AP. The neuroimaging evidence of brain abnormalities in functional movement disorders. Brain J Neurol. (2021) 144:2278–83. doi: 10.1093/brain/awab131
67. Balsters JH, Laird AR, Fox PT, Eickhoff SB. Bridging the gap between functional and anatomical features of cortico-cerebellar circuits using meta-analytic connectivity modeling. Hum Brain Mapp. (2014) 35:3152–69. doi: 10.1002/hbm.22392
68. Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci Off J Soc Neurosci. (2009) 29(26):8586–94. doi: 10.1523/JNEUROSCI.1868-09.2009
69. Charney M, Foster S, Shukla V, Zhao W, Jiang SH, Kozlowska K, et al. Neurometabolic alterations in children and adolescents with functional neurological disorder. NeuroImage Clin. (2024) 41:103557. doi: 10.1016/j.nicl.2023.103557
70. Kozlowska K, Chung J, Cruickshank B, McLean L, Scher S, Dale RC, et al. Blood CRP levels are elevated in children and adolescents with functional neurological symptom disorder. Eur Child Adolesc Psychiatry. (2019) 28(4):Article 4. doi: 10.1007/s00787-018-1212-2
71. Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. (2015) 16:419–29. doi: 10.1038/nrn3950
72. Schiffer AM, Ahlheim C, Wurm MF, Schubotz RI. Surprised at all the entropy: hippocampal, caudate and midbrain contributions to learning from prediction errors. PloS One. (2012) 7:e36445. doi: 10.1371/journal.pone.0036445
73. Schiffer AM, Schubotz RI. Caudate nucleus signals for breaches of expectation in a movement observation paradigm. Front Hum Neurosci. (2011) 5:38. doi: 10.3389/fnhum.2011.00038
74. Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. (2016) 113:7900–5. doi: 10.1073/pnas.1602413113
75. Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. (2017) 7:152–71. doi: 10.1089/brain.2016.0475
76. Crucianelli L, Ehrsson HH. The role of the skin in interoception: A neglected organ? Perspect Psychol Sci. (2023) 18:224–38. doi: 10.1177/17456916221094509
77. Van Oudenhove L, Kragel PA, Dupont P, Ly HG, Pazmany E, Enzlin P, et al. Common and distinct neural representations of aversive somatic and visceral stimulation in healthy individuals. Nat Commun. (2020) 11(1):5939. doi: 10.1038/s41467-020-19688-8
78. Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. (2014) 76:258–66. doi: 10.1016/j.biopsych.2013.11.027
Keywords: functional movement disorder, interoception, prediction error, insula, default mode network
Citation: Spagnolo PA, Parker JA, Hallett M and Horovitz SG (2025) Functional movement disorder is associated with abnormal interoceptive brain activity: a task-based functional MRI study. Front. Psychiatry 16:1473913. doi: 10.3389/fpsyt.2025.1473913
Received: 31 July 2024; Accepted: 27 January 2025;
Published: 21 February 2025.
Edited by:
Vaibhav A. Diwadkar, Wayne State University, United StatesReviewed by:
Sofya N. Morozova (kulikova), Research Center of Neurology (Russia), RussiaCopyright © 2025 Spagnolo, Parker, Hallett and Horovitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Primavera A. Spagnolo, cHNwYWdub2xvQGJ3aC5oYXJ2YXJkLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.