
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Psychiatry , 07 January 2025
Sec. Molecular Psychiatry
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1524555
This article is part of the Research Topic Neuroinflammation and Mood Disorders: Molecular Mechanisms and Therapeutic Implications View all 4 articles
Obesity has emerged as a significant health concern, particularly affecting young people worldwide. Its prevalence extends beyond Westernized countries and has been projected to rise from 107.7 million obese children and adolescents in 2015 to 254 million by 2030 (1). This metabolic disorder poses severe consequences for healthcare systems globally, as childhood obesity often persists into adulthood (2). Unlike other diseases, obesity is a pathological condition that renders individuals more susceptible to various disorders, including metabolic syndrome, cardiovascular disease, non-alcoholic fatty liver disease, and cancer (3).
Metabolic dysfunctions disrupt the structural and functional systems of humans, including the central nervous system (CNS) (4). The hypothalamus, a pivotal region situated between the CNS and periphery, serves as the central control center for energy homeostasis, body temperature, food intake, and other essential functions (5). Consuming a diet high in calories, particularly carbohydrates and lipids, triggers a vicious cycle of hyperactivation of central immune cells and neuroinflammatory mediators within the hypothalamus, resulting in widespread effects (6). Consequently, alterations in this key brain region led to impairments in all related neuronal circuits of other brain areas, including the mesolimbic dopamine (DA) system, hippocampus, nucleus accumbens, striatum, and cortex, which are primarily associated with functions such as cognition and mood regulation.
The gut microbiota plays a pivotal role in the pathophysiology of neuropsychiatric and neurodegenerative disorders, particularly in the context of obesity. The gut-brain axis mediates this relationship (7). Obesity-induced peripheral alterations influence brain function by enhancing neuroinflammation, altering neurotransmitter synthesis, and impairing insulin signaling (7). These mechanisms have been associated with elevated risks of depression, anxiety, cognitive decline, and neurodegenerative diseases in obese individuals (8).
Specifically, Proteobacteria and Cyanobacteria are overrepresented in the gut microbiota of obese patients (9, 10). The dysregulation of metabolites associated with these bacteria may contribute to systemic inflammation and oxidative damage, indirectly affecting neuroinflammatory pathways relevant to neuropsychiatric and neurodegenerative diseases (11, 12).
Obesity and mood disorders are intricately interconnected, and their pathological mechanisms share numerous characteristics (13). At the CNS level, obesity-related detrimental factors compromised the integrity of the blood-brain barrier (BBB), a pivotal barrier that prevents the entry of detrimental substances into the brain (14). The BBB is a sophisticated and highly specialized biological construct, characterized by its selective permeability and protective role within the CNS. Obesity leads to structural and functional alterations of the BBB, including increased permeability, altered transport mechanisms, and inflammatory responses (14). Consistently, our findings indicate that the disruption of the blood-brain barrier (BBB), induced by a high-fat diet (HFD) and evidenced by albumin extravasation in the hippocampus of obese mice, represents a critical mechanism that contribute to the pathogenesis of mood disorders (15). In this condition, peripheral signals via the gut-microbiota-brain axis can impact CNS activity, both negatively and positively (7). Obesity-induced metainflammation, characterized by increased levels of pro-inflammatory mediators, activates astrogliosis and microgliosis, leading to neuroinflammation (16). The role of microglia, the resident immune cells in the CNS, is particularly relevant in the context of neuroinflammation and mood disorders (17). Under normal conditions, microglia maintain homeostasis by regulating synaptic pruning and clearing cellular debris. However, in response to chronic stress or obesity-related inflammation, microglia can become activated and release inflammatory mediators that lead to neurotoxicity (18). The hyperactivation of central immune cells blocks the pivotal machinery responsible for neurogenesis, the process of neuronal renewal (19). Indeed, activated microglia reduce neurogenesis by suppressing neuronal stem cell proliferation, increasing apoptosis of neuronal progenitor cells, and decreasing survival of newly developing neurons and their integration into existing neuronal circuits (20). Furthermore, long-term consumption of a Western-style HFD, characterized by low fiber content, may lead to a substantial reduction in short-chain fatty acids, endogenous molecules with notable anti-inflammatory and neurogenesis-promoting properties (21). Our studies demonstrated that HFD feeding induced depressive- and anxiety-like behavior associated with intestinal dysbiosis, with the proliferation of inflammatory-related microbes, and alteration of gut tryptophan metabolite pathway (22). The production of toxic metabolites of tryptophan, such as quinolinic and kynurenic acid, may severally impact CNS homeostasis (23). The neurobiological alterations induced by a HFD encompass impairments in reward circuitry, diminished serotonin (5-HT) and DA levels, and augmented hypothalamic-pituitary-adrenal axis (HPA) activity, a critical component of the body’s stress response system (24). In particular, neuroinflammation is known to disrupt the balance of neurotransmitters essential for mood regulation, particularly 5-HT and gamma-aminobutyric acid (GABA). Elevated levels of interleukin (IL)-6 and tumor necrosis factor (TNF)-α have been associated with dysregulation of serotonergic and GABAergic systems, which may exacerbate mood disorders symptoms in individuals with obesity (25). These conditions can serve as the etiological basis for depressive and anxiety phenotypes.
Given the growing and intricate relationship between obesity and neuropsychiatric disorders, recent evidence also highlights shared mechanistic targets between the two conditions. For instance, peroxisome proliferator-activated receptor (PPAR)-α, whose role in lipid metabolism of various tissues is well-established in clinical therapy, has recently been recognized as an endogenous tranquilizer of mood disorders caused by dysmetabolism (26). PPAR-α is widely distributed across the amygdala, prefrontal cortex, thalamic nuclei, nucleus accumbens, ventral tegmental area (VTA), and basal ganglia (27). Moreover, research by Jiang et al. (28) demonstrated that the PPAR-α agonist WY14643 ameliorated depressive-like behaviors in mice, an effect attributed to the activation of the Brain-Derived Neurotrophic Factor signaling pathway.
Neurodegenerative diseases and obesity are increasingly recognized to have a complex, bidirectional relationship (29). Obesity, particularly in midlife, has been associated with an elevated risk of developing several neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and other forms of cognitive decline (30). This correlation is mediated by a combination of metabolic, inflammatory, and hormonal factors that affect brain health (31). These factors share biological mechanisms, including chronic inflammation, insulin resistance, hormonal changes (leptin and adipokines), oxidative stress and related mitochondrial dysfunction, and altered gut microbiota (32). Metainflammation, a key feature in many neurodegenerative diseases induced by dysmetabolism, impairs the BBB and triggers neuroinflammation (15). Neuroinflammation, in turn, accelerates neuronal damage and contributes to disease progression. Consistently, obesity increases the production of free radicals and decreases the antioxidant defenses, leading to oxidative stress (33). This process, coupled with impaired mitochondrial function, damages cellular structures, including lipids, proteins, and DNA, accelerating the degeneration of neurons and other brain cells (34).
Emerging research underscores the role of metabolic disorders, particularly obesity, in the etiology of AD, even if aging is the primary risk factor for AD (35). Therefore, obesity significantly correlates with cognitive dysfunction and is a substantial risk factor for developing AD (36). In this context, the excessive body weight can lead to inflammatory processes and metabolic derangements that may increase the risk of amyloid β (Aβ) accumulation in the brain (37). The connection between obesity and AD extends beyond Aβ deposition; both conditions are associated with a chronic low-grade inflammatory state, or metainflammation, characterized by oxidative stress and increased production of reactive oxygen species (38). These factors contribute to neuronal damage and cognitive decline (39). Furthermore, HFD-linked obesity has been observed to induce mitochondrial dysfunction and impair antioxidant defenses, impacting brain health (40).
Notably, the DA system, particularly the VTA, is involved in both obesity and cognitive function. Different types of HFDs alter this system, potentially affecting reward perceptions related to food intake (41).
Insulin signaling influences DA transporter activity, establishing a connection between metabolic health and cognitive outcomes (42). This connection appears to involve increased expression of the dopamine-degrading enzymes MAO-A and MAO-B, which reduces dopamine (DA) signaling (43). Furthermore, DA has been demonstrated to reduce microglial inflammation, potentially impacting the progression of neurodegenerative processes in AD (44). This intricate interplay implies that obesity not only increases the risk of developing AD but also contributes to its pathophysiology through mechanisms involving inflammation and metabolic dysfunction primarily affecting adipose tissue.
While PD is traditionally associated with genetic factors and environmental toxins, emerging research indicates that obesity may also contribute to both the development and progression of PD (29). Specifically, several studies have suggested that obesity, particularly in midlife, may increase the risk of developing PD later in life. For instance, individuals with a body mass index in the obese range at midlife exhibited a higher likelihood of developing PD in their later years (45).
Obesity has been linked to the worsening of both motor and non-motor symptoms of PD (46). It can impact the motor system by causing stiffness, decreasing flexibility, and hindering physical activity, which is already a significant challenge for individuals with PD. Additionally, obesity, particularly in older adults, has been linked to an increased risk of cognitive decline (29). The relationship between obesity and cognitive dysfunction in PD may be attributed to factors such as chronic inflammation, vascular health, and metabolic dysfunction, which can all contribute to worsen brain health in individuals with PD (47). Obesity leads to an increased systemic inflammation, during which adipocytes release pro-inflammatory cytokines such as TNF-α, IL-6, and C-reactive protein (48). Lipid overnutrition and subsequent metainflammation can have a direct impact on the brain, exacerbating neuroinflammation, which is a key player in the progression of PD. As it contributes to the degeneration of dopaminergic neurons, neuroinflammation plays a crucial role in the development of PD. In the brain, microglia are activated during neuroinflammation as a key mechanism in PD (49). Since microglia release neurotoxic substances that promote neuronal damage, obesity-induced inflammation may further exacerbate this microglial activation, accelerating the progression of PD (50).
At the molecular level, obesity has been demonstrated to influence brain circuits that regulate DA, potentially contributing to both the onset and progression of PD (51). Notably, leptin, primarily produced by adipocytes, plays a role in the regulation of the brain’s DA system (52). Research suggests that leptin resistance, as a hallmark of obesity, can impair DA release and signal transduction, potentially exacerbating the symptoms of PD (53).
The purpose of this opinion is to draw attention to the obesity-driven neuropsychiatric and neurodegenerative disorders, investigating how to address the “pandemic of wellness.” Identifying causal factors and their interrelationships in the development of obesity and its comorbidities, although a complex and intricate task, is crucial to prevent or counteract neuropsychiatric and neurodegenerative disorders caused by gluco-lipid dysmetabolism. The scientific community has made significant progress in understanding the pathogenic mechanisms involved in obesity-induced CNS disorders (Figure 1), but there is still much to accomplish. Furthermore, contemplating and possibly targeting the metabolic derangements due to obesity could be critical in developing innovative therapeutic strategies to counteract obesity-related central disorders, emphasizing the necessity for a comprehensive approach to brain health which takes into account the impact of metabolic disorders.
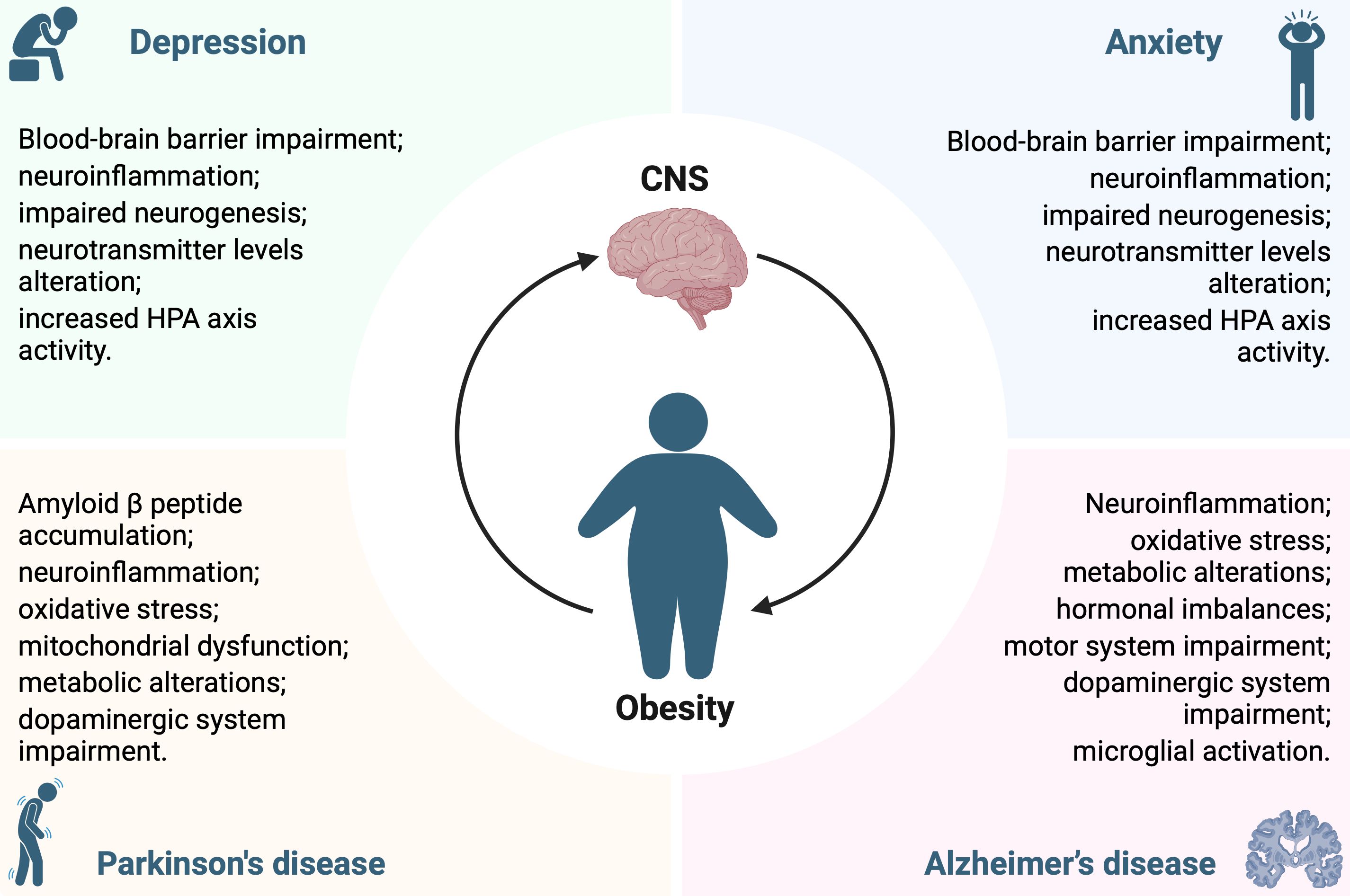
Figure 1. Pathological mechanisms underlying the interconnection between obesity and central nervous system disorders.
CP: Writing – original draft, Writing – review & editing. NO: Writing – original draft. FD: Writing – original draft. SM: Writing – original draft. AL: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AD, Alzheimer’s disease; Aβ, amyloid β; BBB, blood-brain barrier; CNS, central nervous system; DA, dopamine; GABA, gamma-aminobutyric acid; HFD, high-fat diet; HPA, hypothalamic-pituitary-adrenal axis; IL, interleukin; PD, Parkinson’s disease; PPAR, peroxisome proliferator-activated receptor; 5-HT, serotonin; TNF, tumor necrosis factor; VTA, ventral tegmental area.
1. Zhang X, Liu J, Ni Y, Yi C, Fang Y, Ning Q, et al. Global prevalence of overweight and obesity in children and adolescents: A systematic review and meta-analysis. JAMA Pediatr. (2024) 178:800–13. doi: 10.1001/jamapediatrics.2024.1576
2. Palacios-Marin I, Serra D, Jimenez-Chillaron JC, Herrero L, Todorcevic M. Childhood obesity: implications on adipose tissue dynamics and metabolic health. Obes Rev. (2023) 24:e13627. doi: 10.1111/obr.13627
3. Zhang X, Ha S, Lau HC, Yu J. Excess body weight: novel insights into its roles in obesity comorbidities. Semin Cancer Biol. (2023) 92:16–27. doi: 10.1016/j.semcancer.2023.03.008
4. Rojas M, Chavez-Castillo M, Pirela D, Parra H, Nava M, Chacin M, et al. Metabolic syndrome: is it time to add the central nervous system? Nutrients. (2021) 13. doi: 10.3390/nu13072254
5. Seong J, Kang JY, Sun JS, Kim KW. Hypothalamic inflammation and obesity: A mechanistic review. Arch Pharm Res. (2019) 42:383–92. doi: 10.1007/s12272-019-01138-9
6. Mukherjee S, Skrede S, Haugstoyl M, Lopez M, Ferno J. Peripheral and central macrophages in obesity. Front Endocrinol (Lausanne). (2023) 14:1232171. doi: 10.3389/fendo.2023.1232171
7. Asadi A, Shadab Mehr N, Mohamadi MH, Shokri F, Heidary M, Sadeghifard N, et al. Obesity and gut-microbiota-brain axis: A narrative review. J Clin Lab Anal. (2022) 36:e24420. doi: 10.1002/jcla.24420
8. Goralczyk-Binkowska A, Szmajda-Krygier D, Kozlowska E. The microbiota-gut-brain axis in psychiatric disorders. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms231911245
9. Machate DJ, Figueiredo PS, Marcelino G, Guimaraes RCA, Hiane PA, Bogo D, et al. Fatty acid diets: regulation of gut microbiota composition and obesity and its related metabolic dysbiosis. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21114093
10. Juarez-Fernandez M, Porras D, Petrov P, Roman-Saguillo S, Garcia-Mediavilla MV, Soluyanova P, et al. The synbiotic combination of akkermansia muciniphila and quercetin ameliorates early obesity and nafld through gut microbiota reshaping and bile acid metabolism modulation. Antioxidants (Basel). (2021) 10. doi: 10.3390/antiox10122001
11. Sini P, Dang TBC, Fais M, Galioto M, Padedda BM, Luglie A, et al. Cyanobacteria, cyanotoxins, and neurodegenerative diseases: dangerous liaisons. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22168726
12. Skrzypczak-Wiercioch A, Salat K. Lipopolysaccharide-induced model of neuroinflammation: mechanisms of action, research application and future directions for its use. Molecules. (2022) 27. doi: 10.3390/molecules27175481
13. Dionysopoulou S, Charmandari E, Bargiota A, Vlahos N, Mastorakos G, Valsamakis G. The role of hypothalamic inflammation in diet-induced obesity and its association with cognitive and mood disorders. Nutrients. (2021) 13. doi: 10.3390/nu13020498
14. Feng Z, Fang C, Ma Y, Chang J. Obesity-induced blood-brain barrier dysfunction: phenotypes and mechanisms. J Neuroinflamm. (2024) 21:110. doi: 10.1186/s12974-024-03104-9
15. Lama A, Pirozzi C, Severi I, Morgese MG, Senzacqua M, Annunziata C, et al. Palmitoylethanolamide dampens neuroinflammation and anxiety-like behavior in obese mice. Brain Behav Immun. (2022) 102:110–23. doi: 10.1016/j.bbi.2022.02.008
16. Rorato R, Ferreira NL, Oliveira FP, Fideles HJ, Camilo TA, Antunes-Rodrigues J, et al. Prolonged activation of brain cb2 signaling modulates hypothalamic microgliosis and astrogliosis in high fat diet-fed mice. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23105527
17. Lin MH, Cheng PC, Hsiao PJ, Chen SC, Hung CH, Kuo CH, et al. The glp-1 receptor agonist exenatide ameliorates neuroinflammation, locomotor activity, and anxiety-like behavior in mice with diet-induced obesity through the modulation of microglial M2 polarization and downregulation of sr-A4. Int Immunopharmacol. (2023) 115:109653. doi: 10.1016/j.intimp.2022.109653
18. Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacol (Berl). (2016) 233:1637–50. doi: 10.1007/s00213-016-4218-9
19. Nikolopoulos D, Manolakou T, Polissidis A, Filia A, Bertsias G, Koutmani Y, et al. Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via il-6 and il-18 mediate early diffuse neuropsychiatric lupus. Ann Rheum Dis. (2023) 82:646–57. doi: 10.1136/ard-2022-223506
20. Guo D, Xu Y, Liu Z, Wang Y, Xu X, Li C, et al. Igf2 inhibits hippocampal over-activated microglia and alleviates depression-like behavior in lps- treated male mice. Brain Res Bull. (2023) 194:1–12. doi: 10.1016/j.brainresbull.2023.01.001
21. den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a ppargamma-dependent switch from lipogenesis to fat oxidation. Diabetes. (2015) 64:2398–408. doi: 10.2337/db14-1213
22. Pirozzi C, Coretti L, Opallo N, Bove M, Annunziata C, Comella F, et al. Palmitoylethanolamide counteracts high-fat diet-induced gut dysfunction by reprogramming microbiota composition and affecting tryptophan metabolism. Front Nutr. (2023) 10:1143004. doi: 10.3389/fnut.2023.1143004
23. Zheng H, Teague TK, Yeh FC, Burrows K, Figueroa-Hall LK, Aupperle RL, et al. C-reactive protein and the kynurenic acid to quinolinic acid ratio are independently associated with white matter integrity in major depressive disorder. Brain Behav Immun. (2022) 105:180–9. doi: 10.1016/j.bbi.2022.07.011
24. Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from hpa axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. (2012) 1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x
25. Labban RSM, Alfawaz H, Almnaizel AT, Hassan WM, Bhat RS, Moubayed NM, et al. High-fat diet-induced obesity and impairment of brain neurotransmitter pool. Transl Neurosci. (2020) 11:147–60. doi: 10.1515/tnsci-2020-0099
26. Pinna G. Role of ppar-allopregnanolone signaling in behavioral and inflammatory gut-brain axis communications. Biol Psychiatry. (2023) 94:609–18. doi: 10.1016/j.biopsych.2023.04.025
27. Tufano M, Pinna G. Is there a future for ppars in the treatment of neuropsychiatric disorders? Molecules. (2020) 25. doi: 10.3390/molecules25051062
28. Jiang B, Huang C, Zhu Q, Tong LJ, Zhang W. Wy14643 produces anti-depressant-like effects in mice via the bdnf signaling pathway. Psychopharmacol (Berl). (2015) 232:1629–42. doi: 10.1007/s00213-014-3802-0
29. Neto A, Fernandes A, Barateiro A. The complex relationship between obesity and neurodegenerative diseases: an updated review. Front Cell Neurosci. (2023) 17:1294420. doi: 10.3389/fncel.2023.1294420
30. Procaccini C, Santopaolo M, Faicchia D, Colamatteo A, Formisano L, de Candia P, et al. Role of metabolism in neurodegenerative disorders. Metabolism. (2016) 65:1376–90. doi: 10.1016/j.metabol.2016.05.018
31. Ghosh-Swaby OR, Reichelt AC, Sheppard PAS, Davies J, Bussey TJ, Saksida LM. Metabolic hormones mediate cognition. Front Neuroendocrinol. (2022) 66:101009. doi: 10.1016/j.yfrne.2022.101009
32. Bittencourt A, Brum PO, Ribeiro CT, Gasparotto J, Bortolin RC, de Vargas AR, et al. High fat diet-induced obesity causes a reduction in brain tyrosine hydroxylase levels and non-motor features in rats through metabolic dysfunction, neuroinflammation and oxidative stress. Nutr Neurosci. (2022) 25:1026–40. doi: 10.1080/1028415X.2020.1831261
33. Cojocaru KA, Luchian I, Goriuc A, Antoci LM, Ciobanu CG, Popescu R, et al. Mitochondrial dysfunction, oxidative stress, and therapeutic strategies in diabetes, obesity, and cardiovascular disease. Antioxidants (Basel). (2023) 12. doi: 10.3390/antiox12030658
34. Ghosh P, Fontanella RA, Scisciola L, Taktaz F, Pesapane A, Basilicata MG, et al. Obesity-induced neuronal senescence: unraveling the pathophysiological links. Ageing Res Rev. (2024) 101:102533. doi: 10.1016/j.arr.2024.102533
35. Forny-Germano L, De Felice FG, Vieira M. The role of leptin and adiponectin in obesity-associated cognitive decline and alzheimer’s disease. Front Neurosci. (2018) 12:1027. doi: 10.3389/fnins.2018.01027
36. Cho SH, Jang M, Ju H, Kang MJ, Yun JM, Yun JW. Association of late-life body mass index with the risk of alzheimer disease: A 10-year nationwide population-based cohort study. Sci Rep. (2022) 12:15298. doi: 10.1038/s41598-022-19696-2
37. Vinuesa A, Pomilio C, Gregosa A, Bentivegna M, Presa J, Bellotto M, et al. Inflammation and insulin resistance as risk factors and potential therapeutic targets for alzheimer’s disease. Front Neurosci. (2021) 15:653651. doi: 10.3389/fnins.2021.653651
38. Picone P, Di Carlo M, Nuzzo D. Obesity and alzheimer’s disease: molecular bases. Eur J Neurosci. (2020) 52:3944–50. doi: 10.1111/ejn.14758
39. Naomi R, Teoh SH, Embong H, Balan SS, Othman F, Bahari H, et al. The role of oxidative stress and inflammation in obesity and its impact on cognitive impairments-a narrative review. Antioxidants (Basel). (2023) 12. doi: 10.3390/antiox12051071
40. Schmitt LO, Gaspar JM. Obesity-induced brain neuroinflammatory and mitochondrial changes. Metabolites. (2023) 13. doi: 10.3390/metabo13010086
41. Leite F, Ribeiro L. Dopaminergic pathways in obesity-associated inflammation. J Neuroimmune Pharmacol. (2020) 15:93–113. doi: 10.1007/s11481-019-09863-0
42. Fiory F, Perruolo G, Cimmino I, Cabaro S, Pignalosa FC, Miele C, et al. The relevance of insulin action in the dopaminergic system. Front Neurosci. (2019) 13:868. doi: 10.3389/fnins.2019.00868
43. Kleinridders A, Cai W, Cappellucci L, Ghazarian A, Collins WR, Vienberg SG, et al. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci U.S.A. (2015) 112:3463–8. doi: 10.1073/pnas.1500877112
44. Pike AF, Longhena F, Faustini G, van Eik JM, Gombert I, Herrebout MAC, et al. Dopamine signaling modulates microglial nlrp3 inflammasome activation: implications for parkinson’s disease. J Neuroinflamm. (2022) 19:50. doi: 10.1186/s12974-022-02410-4
45. Domenighetti C, Sugier PE, Ashok Kumar A Sreelatha, Schulte C, Grover S, Portugal B, et al. Association of body mass index and parkinson disease: A bidirectional mendelian randomization study. Neurology. (2024) 103:e209620. doi: 10.1212/WNL.0000000000209620
46. Vikdahl M, Carlsson M, Linder J, Forsgren L, Haglin L. Weight gain and increased central obesity in the early phase of parkinson’s disease. Clin Nutr. (2014) 33:1132–9. doi: 10.1016/j.clnu.2013.12.012
47. Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. (2014) 8:375. doi: 10.3389/fnins.2014.00375
48. Martins LB, Monteze NM, Calarge C, Ferreira AVM, Teixeira AL. Pathways linking obesity to neuropsychiatric disorders. Nutrition. (2019) 66:16–21. doi: 10.1016/j.nut.2019.03.017
49. Morris HR, Spillantini MG, Sue CM, Williams-Gray CH. The pathogenesis of parkinson’s disease. Lancet. (2024) 403:293–304. doi: 10.1016/S0140-6736(23)01478-2
50. Russo C, Valle MS, Russo A, Malaguarnera L. The interplay between ghrelin and microglia in neuroinflammation: implications for obesity and neurodegenerative diseases. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms232113432
51. Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. (2001) 357:354–7. doi: 10.1016/s0140-6736(00)03643-6
52. Caron A, Lee S, Elmquist JK, Gautron L. Leptin and brain-adipose crosstalks. Nat Rev Neurosci. (2018) 19:153–65. doi: 10.1038/nrn.2018.7
Keywords: Alzheimer's disease, Parkinson's disease, mood disorders, gut-brain axis, metabolic dysfunction, gut microbiota
Citation: Pirozzi C, Opallo N, Del Piano F, Melini S and Lama A (2025) Body and mind: how obesity triggers neuropsychiatric and neurodegenerative disorders. Front. Psychiatry 15:1524555. doi: 10.3389/fpsyt.2024.1524555
Received: 07 November 2024; Accepted: 19 December 2024;
Published: 07 January 2025.
Edited by:
Florian Freudenberg, University Hospital Frankfurt, GermanyReviewed by:
Marcel Pérez-Morales, Autonomous Metropolitan University, MexicoCopyright © 2025 Pirozzi, Opallo, Del Piano, Melini and Lama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudio Pirozzi, Y2xhdWRpby5waXJvenppQHVuaW5hLml0; Adriano Lama, YWRyaWFuby5sYW1hQHVuaW5hLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.