- 1Department of Orthopedics, The Second Hospital of Anhui Medical University, Hefei, China
- 2Department of Orthopedics, Shannan City People’s Hospital, Shannan, China
- 3Institute of Orthopedics, Research Center for Translational Medicine, The Second Hospital of Anhui Medical University, Hefei, China
Postoperative delirium (POD) represents a common neurological complication encountered predominantly among the elderly cohort undergoing surgical intervention for hip fractures. This phenomenon, particularly commonplace in geriatric populations with heightened preoperative risk profiles, pronounced comorbidities, and later stages of lifespan, poses complex clinical challenges. The impact of perioperative pharmacological interventions and anesthetic strategies on POD’s emergence cannot be understated, as it may profoundly affect the length of hospital stays, rehabilitation milestones, and the overall mortality hazard. The pharmacotherapeutic landscape for managing POD remains constrained, underscoring the imperative nature of preventive measures. Prudent preoperative risk stratification, meticulous intraoperative neuromonitoring guided by electroencephalographic studies, and a holistic postoperative patient welfare model are cornerstone recommendations in the quest to mitigate POD’s incidence. Nonetheless, an extensive exploration into the influence of anesthetic approaches and perioperative medications on the emergence of POD is yet to be satisfactorily charted. Our investigation endeavors to dissect the nexus between anesthetic modalities, perioperative pharmacological interventions, and POD incident rates among the elderly with hip fractures. This study spotlights pivotal determinants of POD in the wake of hip fracture surgery by evaluating and synthesizing data from peer-reviewed sources that adhere to rigorous inclusion criteria. Preliminary studies have revealed that certain anesthesia protocols and perioperative medications may increase the potential incidence of POD, such as higher depth of anesthesia or benzodiazepine use, and the incidence of POD in specific populations, such as patients with higher age, prior history of psychosis, and lower intraoperative oxygen saturation The findings from this study are instrumental in refining strategic perioperative plans tailored for the elderly recipients of hip fracture surgery, aimed at not only diminishing the incidence but also the gravity of POD. Despite these forward steps, the clinical uncertainty concerning the efficacy and safety of the specific drugs and surgical techniques in question remains. These lingering questions underscore the exigency for more extensive, empirically grounded research to consolidate the learnings of this investigation.
Highlights
● Most of the studies reviewed in this paper found no statistical difference in the effects of local and general anesthesia on postoperative delirium.
● There is a significant correlation between the use of benzodiazepines and postoperative delirium.
● The level of sedation affects the occurrence of postoperative delirium.
● Melatonin seems to be effective in preventing postoperative delirium.
● Based on the comparison of a large number of kinds of literature, it is concluded that the influence of perioperative drugs and anesthesia on postoperative delirium is not uniform.
1 Introduction
Postoperative delirium (POD) is a common clinical occurrence following hip fracture surgery, particularly among the elderly patient population (1, 2). Around one-quarter of these patients experience POD, which is a syndrome that often arises alongside other medical conditions and physical declines that are inherent to an aging demographic (3, 4). Delirium not only causes significant cognitive disturbances in attention, cognition, and orientation but also leads to prolonged hospital stays and increased short-term and long-term mortality rates for patients. This places additional physical, mental, and economic burdens on both patients and their families (5–7) Figure 1. Despite the fact that delirium after hip fracture is so common (8), the exact pathophysiological mechanisms remain to be conclusively elucidated (9), prevailing assumptions among researchers propose that the onset of delirium could be associated with factors such as neuroinflammation, dysfunction of the neuroendocrine system, augmented level of reactive oxidative stress (ROS), and aberrations in neurotransmitter functioning (10). A comprehensive understanding of its pathophysiology is crucial for the development of extensive preventative strategies and treatment approaches.
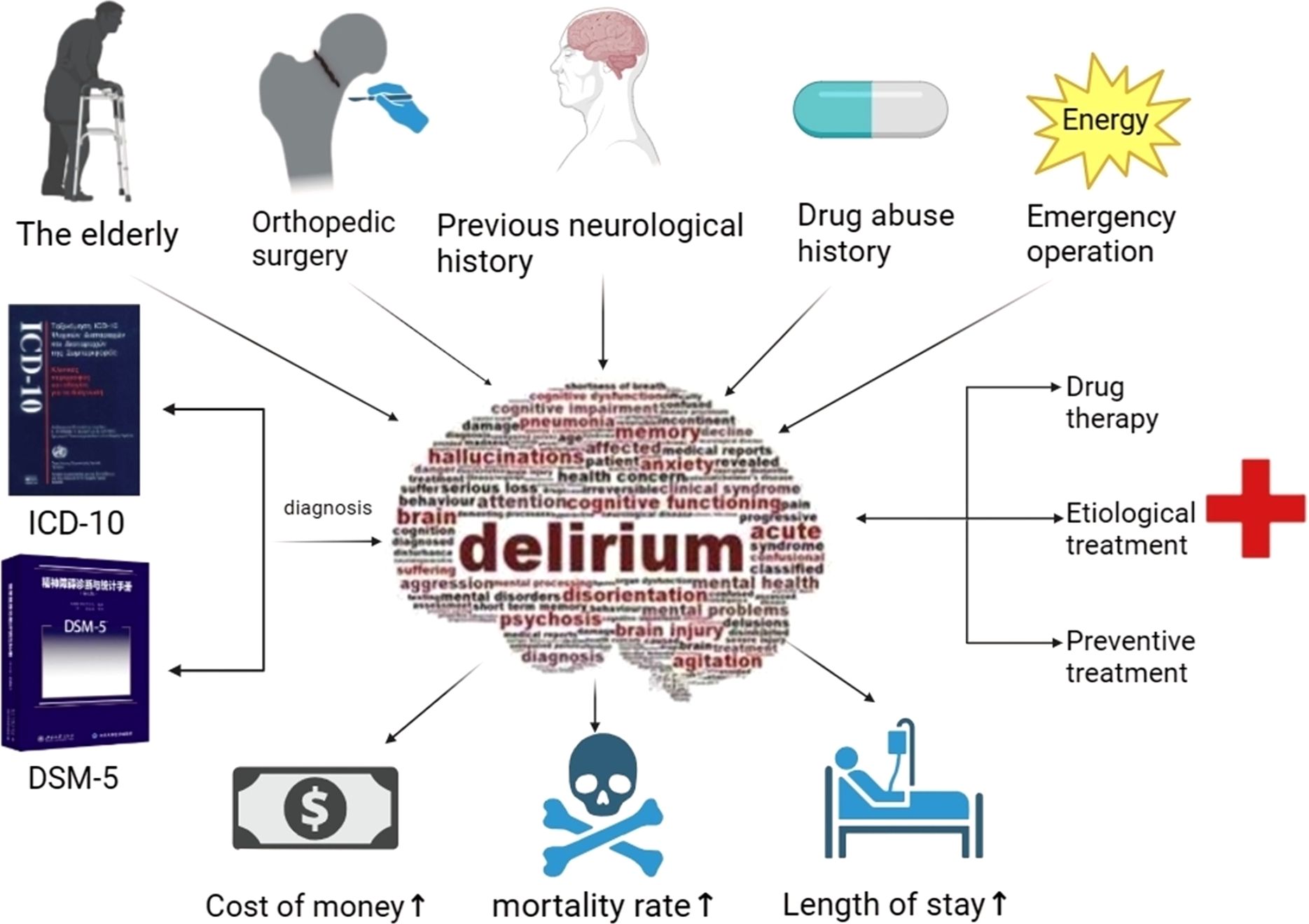
Figure 1. Provoking factors in delirium pathogenesis, the ultimate diagnostic benchmark, prognostic indices, and management strategies.
Non-pharmacological interventions have recently gained considerable attention in addressing POD (11). However, it is important not to dismiss the role of traditional pharmacological interventions and the significance of anesthetic drugs and perioperative drug support in the occurrence of delirium (12). In a study examining a population over 80 years of age hospitalized for hip fractures, multivariate logistic regression analysis demonstrated a correlation between the types and doses of drugs administered with the incidence of delirium (12). Given the instrumental role of anesthetic and perioperative medications in hip surgery, there is promise in mitigating this prevalent health issue through stringent oversight in medicinal administration. This emphasizes the importance of continuous evaluation of surgical procedures, anesthetic techniques, and pharmaceutical treatments in decreasing the prevalence of POD following hip fracture surgery.
This article reviews the effects of perioperative drug therapy, anesthesia methods, and anesthetic drugs on POD in elderly patients with hip fractures, to provide effective and feasible strategies for the prevention and treatment of POD in elderly patients with hip fractures.
2 Mechanism of delirium pathology
Currently, the intricate pathogenesis underlying POD following hip fracture in geriatric patients remains to be fully comprehended (9). Current research focuses on neuroinflammation, dysfunction of the neuroendocrine system, elevated levels of reactive oxidative stress (ROS), and abnormal neurotransmitter function (10). It appears improbable for a single proposed theory to comprehensively explicate either the etiological or the phenomenological elucidations of delirium. Instead, it is more plausible that the collaborative function of two or more theories accounts for the intricate cognitive and behavioral alterations that result in the ultimate biochemical aberrations, subsequently leading to delirium. To create a comprehensive understanding of intertwining these crucial theories, a novel theory termed “System Integration Failure” has been proposed (Figure 2). It delineates the varying contributions of each theory to present an intricate network, underscoring the intersections and reciprocalities. Additionally, it explicates how discrepancies in these contributions could contribute to the progression of delirium (13). Further exploration of the pathophysiological mechanism of delirium is expected to provide the scientific basis for formulating more effective prevention and management strategies. Future research will help uncover the deep causes of delirium development, thereby driving improvement and innovation in clinical practice.
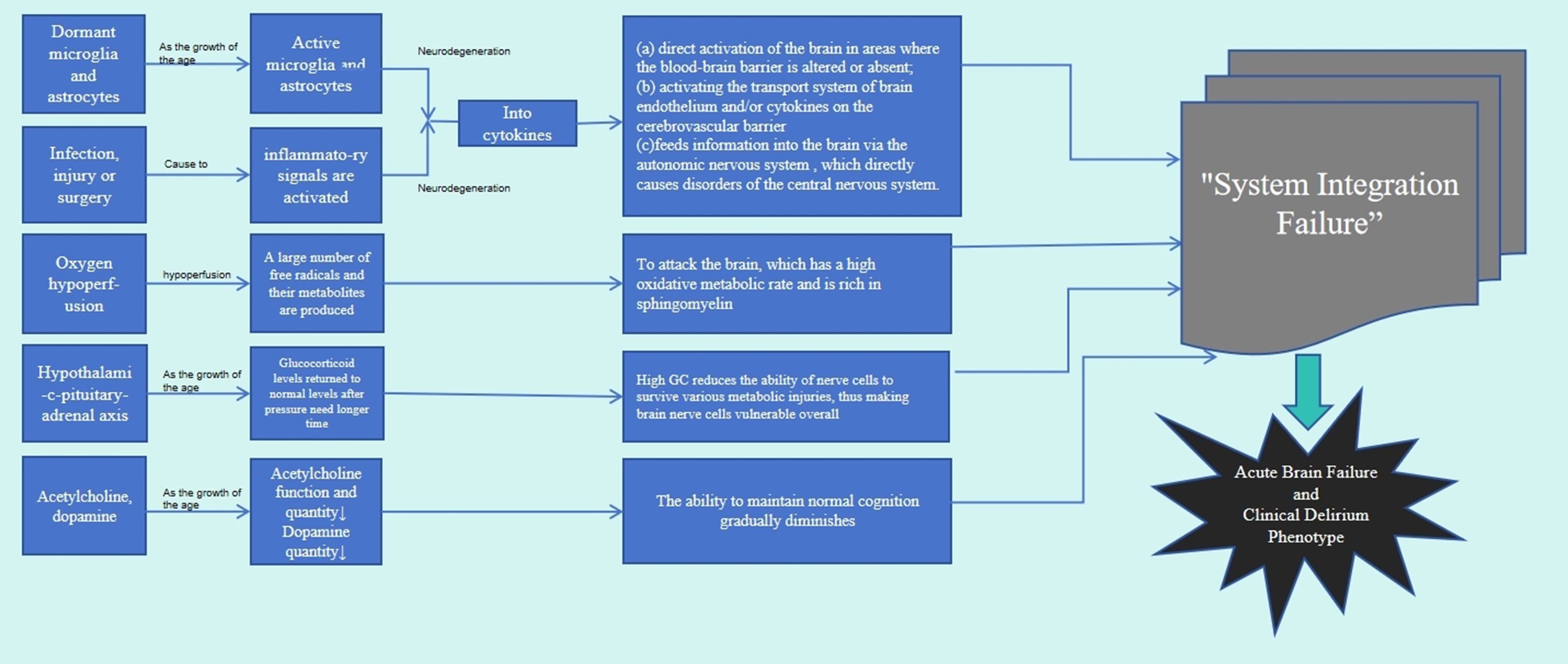
Figure 2. Four ways are down the nerve inflammation theory, theory of neuroendocrine dysfunction, reactive oxidative stress theory, and theory of neurotransmitter disorders. Through the interaction between the four approaches, the theory of System Integration Failure is finally obtained.
3 Epidemiology and Outcomes of POD
The incidence of postoperative hip fracture delirium exhibits considerable variability owing to a diversity of factors including patient demographics, surgical interventions, diagnostic methodologies, timeframes of assessment, and research designs. In the general adult population, the prevalence of POD falls within a range of 2.6% to 4.3% (14, 15). This incidence escalates markedly in older cohorts; individuals aged 60 and above experience a surge in POD rates to between 12.0% and 23.8% (16, 17). The prevalence among those over the age of 70 reaches 10.5% (15). Notably, the likelihood of delirium after hip fracture surgery amplifies drastically, with a noted increase in risk of 70-80%. Equally significant, delirium occurrence post-cardiovascular surgery is reported at 23.4% (18), while hip fracture surgery-related delirium stands at 16.9% (19). The incidence associated with emergency surgical procedures ranges from 22.7% to 26% (20, 21). Furthermore, the prevalence of delirium in patients admitted to the intensive care unit (ICU) postoperatively is documented at 24.4% (22). These data underscore the complexity of POD epidemiology and the need for multidimensional prevention and management strategies, giving contemporary clinicians new insight into the need to pay more attention to the occurrence of postoperative delirium, especially in older (> 70 years) patients with hip fractures, as well as in patients undergoing emergency surgery and IUC.
Delirium serves as an impactful prognostic indicator of adverse outcomes. In the immediate aftermath of surgery, delirium in patients has been correlated with extended hospitalizations, longer intervals to discharge, and an inflated rate of readmissions within 30 days, surpassing other major complications in its contributory role (23, 24). An emerging body of research suggests marked associations between the occurrence of delirium, amplified perioperative risk, and long-term mortality rates (3). Furthermore, delirium has been linked to a diminished health status and quality of life (25), and an elevated Alzheimer’s disease risk (23, 26). This underpins the salience of inhibitive measures against delirium, continual cognitive monitoring post-delirium, and ongoing surveillance of mortality rates in the wake of a delirium episode. Additional investigations within specific medical cohorts and Intensive Care Units are warranted. Future lines of inquiry ought to evaluate the relationship between the longevity, severity, and distinct types of delirium, and the impact on variables such as postoperative cognitive function, mortality, and dementia risk.
4 Risk factors for POD
Research has demonstrated that the incidence of POD in hip joint patients is associated with a multitude of factors, which can be classified into two categories: predisposing and precipitating risk factors (27) as delineated in Table 1. The predisposing factors encompass advanced age (28), gender (29), packed red blood cells implementation (30), occurrence of hearing loss (31), frailty (32, 33), antecedent history of mental disorders (34), renal failure (35), preoperative cognitive disability (29), lack of education (36, 37), level of serum albumin (35, 38), Charlson Comorbidity Index (CCI) (29), and high-risk assessment score (39). Precipitating factors comprise the depth of anesthesia level as monitored by brain function (40), instability of blood pressure levels (37), instance of alcohol withdrawal (39), degree of pain felt by the patient (41), medication administered perioperatively (42), the duration of mechanical ventilation (30, 34), cerebral oxygen saturation levels (43–45), the necessity for perioperative blood transfusion (46), elongated operation time (47), execution of emergency operation (48, 49), disturbances in fluid/electrolyte (34), atrial fibrillation (37), and duration of emergency room stay (34).
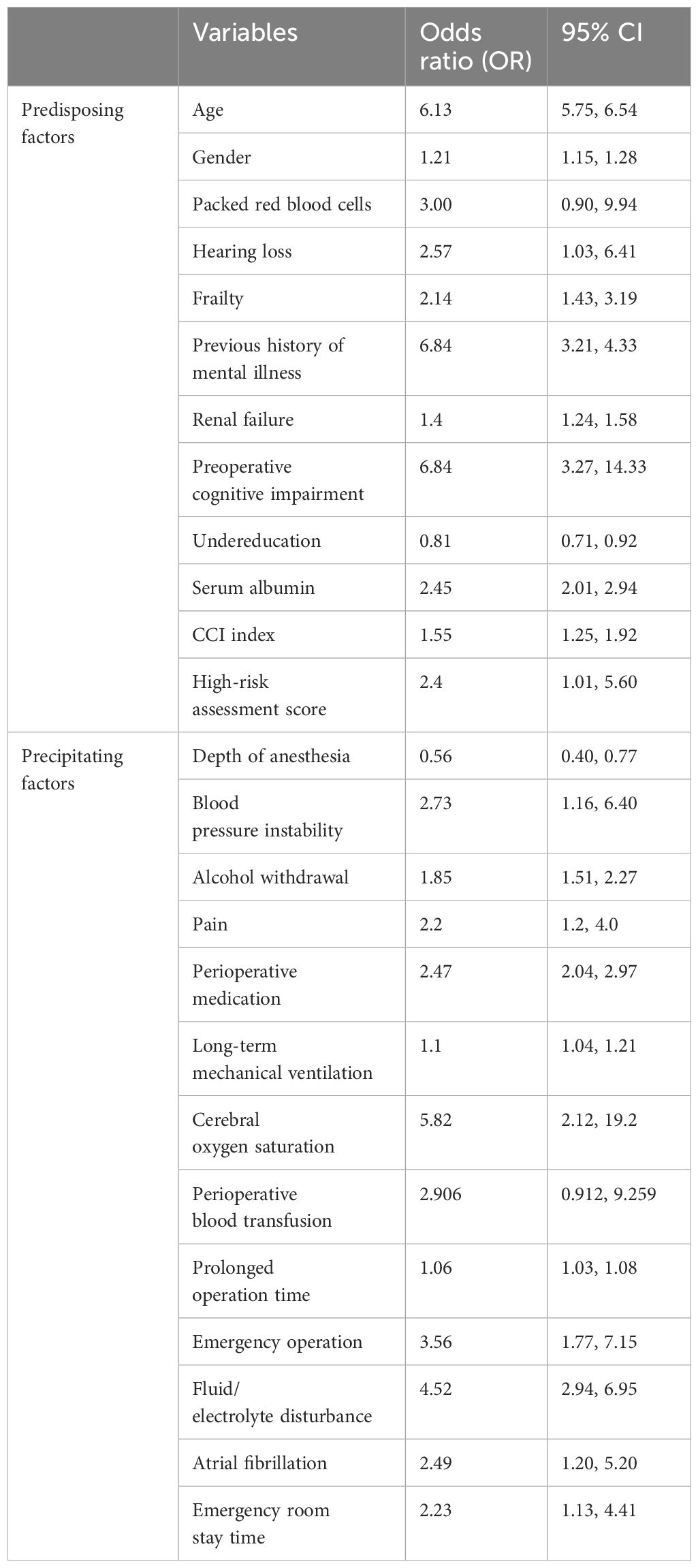
Table 1. Predisposing factors and Precipitating factors of POD and their odds ratio and 95% confidence interval (CI).
A plethora of literature accentuates the correlation between the onset of POD and advanced age (18, 28, 30), thereby corroborating that the age factor stands as a pivotal element in predicting the delirium risk index. This predilection might be ascribed to the prevalence of cerebrovascular diseases as well as heightened vascular fragility in older adults (50), sluggish blood flow, and neurotransmitter irregularities (51). Maldonado et al. have indicated that post the age of 50, the probability of succumbing to delirium elevates each successive year (52). Geriatric populations constitute the majority of maintenance drug users and are the most susceptible to adverse drug reactions, which can potentially account for the pronounced incidence of delirium in this demographic (53). The classification of the patient’s status by the American Society of Anesthesiologists and other co-existing diseases also influence the occurrence of POD. Furthermore, other co-morbidities can be appraised utilizing the improved Chalson Common Disease Index (CCI) (54). A pre-existing cognitive impairment and depressive dementia also determine the development of POD in most instances (55), which might be attributed to occurring alterations in the underlying neurotransmitter, and the predilection of hypoxia and anticholinergic drugs in these patients (43, 56), despite some diverging viewpoints concerning the influence of gender on the onset of POD (18, 57), which could be a result of sampling inaccuracies. Intraoperative pain which prompts a substantial stress response or necessitates the use of analgesic drugs (29, 41), aggravates the incidence of POD. Prolonged Intensive Care Unit (ICU) stays and hearing loss escalate patient anxiety (18, 31), thereby elevating the risk of POD in the backdrop of sub-optimal postoperative care.
This paper, in light of the preceding factors, endeavors to evaluate the association between the type of anesthesia and the routinely administered perioperative drugs, while leaving the discussion of other risk factors out of its ambit.
5 Diagnosis of POD
The severity of postoperative delirium is not in doubt, but its prevalence is often underestimated, leading to underdiagnosis. A meta-analysis published in 2021 noted that the DSM(Diagnostic and Statistical Manual of Mental Disorders)-5 diagnostic criteria for mental disorders are particularly sensitive to screening inpatients and patients with acute illness. However, this routine screening is time-consuming and requires significant training time (58). In addition, confusion assessment (CAM) is the most widely used screening tool with high sensitivity and specificity due to various modified and adapted versions, such as short CAM and Intensive Care Unit Confusion Assessment Method (CAM-ICU); However, specific training is needed to accurately implement this (59). In contrast, the Nurse Delirium Checklist (NUDESC), developed by Gaudreau et al. (60), is a quick and easy-to-complete tool for nurses, is easy to adapt, and has proven effective in multiple studies (15), diagnostic criteria are Table 2. However, this article has certain limitations, similar to other meta-analysis, it may be susceptible to publication bias, and the number of studies included in this review is limited due to clinical setting criteria. Therefore, for early detection and appropriate treatment to reduce the duration, severity, and negative consequences of postoperative delirium, clinicians may also subjectively detect delirium without the use of effective tools (61). Our literature review has clarified the five typical clinical presentation areas associated with delirium, which are (a) cognitive impairments, which manifest as distorted perception, compromised memory, deficient capacity for abstract thinking, and disorientation (62); (b) attentional deficits, evidenced by a diminished consciousness and a reduced ability to focus, sustain, or shift attention (63); (c) disturbances in circadian rhythms, indicative of erratic sleep-wake cycles (64); (d) affective instability, characterized by states of confusion, anxiety, irritability, and in some cases, explicit anger (65); (e) perturbations in the stream of mental activity which can resemble Schizophrenia or Affective Disorders (66). Taking these factors into account, we can provide better treatment for POD patients.
6 Anesthesia and POD
The impact of different anesthesia strategies and perioperative medications on the occurrence of POD in patients with hip fractures has been extensively studied (67–69). However, the existing research has not provided a clear evidence base from which definitive guidelines applicable to standard clinical practice can be derived (42). This manuscript will present a comprehensive data analysis, examining the influence of various anesthesia modalities and medications on POD. Due to the limited research on the incidence of POD after orthopedic surgery and anesthesia and perioperative drugs, the literature retrieved in this paper will also involve the incidence of POD after other types of surgery, so that more convincing research conclusions can be drawn.
6.1 Inhalational anesthesia vs. intravenous anesthesia
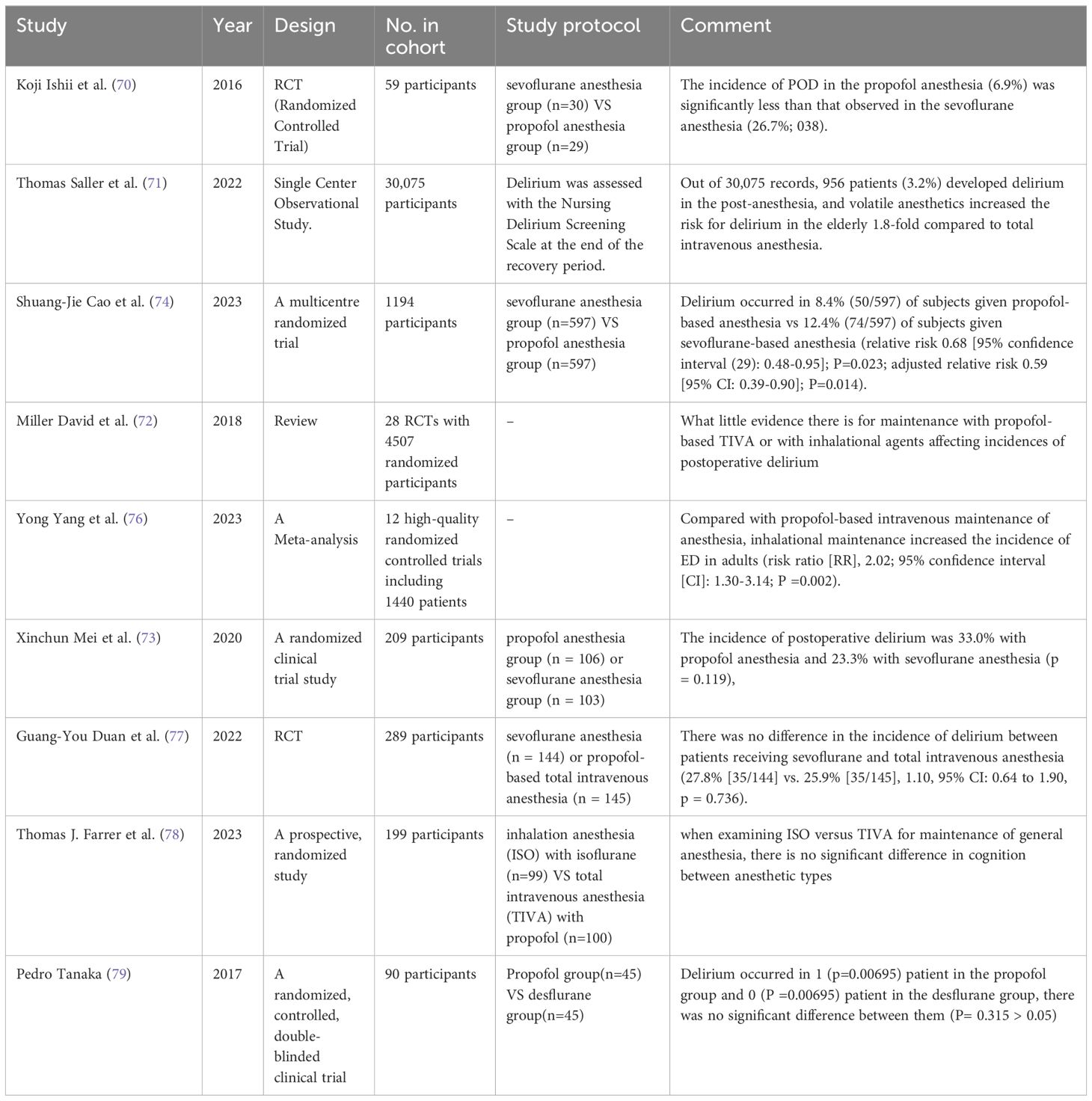
Through extensive literature research, scholars have presented divergent findings regarding the incidence of POD between inhalational anesthesia and intravenous anesthesia. There are two major viewpoints in this regard. Firstly, some researchers believe that there is a statistically significant difference between the incidence of POD under inhalation anesthesia and that under intravenous anesthesia (70–73). A single-center observational study involving 30,075 patients undergoing general anesthesia reported an increased risk of POD with volatile anesthetics. Another study conducted on elderly patients undergoing major cancer surgery compared propofol and sevoflurane anesthesia, and found that the incidence of POD was lower by one-third in the propofol anesthesia group (relative risk 0.68 [95% confidence interval: 0.48-0.95]; P=0.023; adjusted relative risk 0.59 [95% CI: 0.39-0.90]; P=0.014) (74). A retrospective study comparing patients aged 65 and above who received intravenous anesthesia or inhalational anesthesia in a Japanese hospital database indicated that total intravenous anesthesia (TIVA) was associated with a slightly lower incidence of POD compared to inhalational anesthesia (IA) (75). Furthermore, a meta-analysis of 12 high-quality randomized controlled trials involving 1,440 patients showed consistent results, with inhalational maintenance anesthesia associated with a higher incidence of POD compared to propofol-based intravenous maintenance anesthesia (risk ratio [RR]: 2.02; 95% confidence interval [CI]: 1.30-3.14; P = 0.002) (76).
On the contrary, the second viewpoint suggests that there is no statistically significant difference in the incidence of POD between inhalational anesthesia and intravenous anesthesia. A multicenter randomized controlled trial comparing sevoflurane with total intravenous anesthesia in patients undergoing valve replacement surgery showed no difference in the occurrence of POD between the two groups (27.8% [35/144] vs. 25.9% [35/145], 1.10, 95% CI: 0.64-1.90, p = 0.736) (77). Another randomized controlled study comparing isoflurane inhalation anesthesia (ISO) with propofol-based total intravenous anesthesia (TIVA) demonstrated no significant difference in cognition when ISO and TIVA were used for the maintenance of general anesthesia. The incidence of POD was also similar between the two anesthesia types (78). investigated the POD incidence in 100 elderly obese patients undergoing total knee replacement and reached a consistent conclusion that there was no difference in POD incidence between the desflurane and propofol groups (79).
The existing research literature supports two different views, although they are somewhat contradictory. These insights provide a strong evidence base for future prevention of POD through the selection of appropriate anesthesia methods. Further research should synthesize these different perspectives to reveal the exact association between anesthesia and POD and provide more precise guidance for clinical practice (Table 3).
6.2 General anesthesia and non-general anesthesia
Non-general anesthesia includes local anesthesia, spinal anesthesia, and nerve blocks. Spinal anesthesia, also known as half-body anesthesia, is further categorized into epidural anesthesia and subarachnoid anesthesia depending on the site of drug injection. Nerve blocks include common procedures such as brachial plexus block for upper limb surgery and cervical plexus block for neck surgery. Nowadays, ultrasound-guided nerve block techniques are widely used for adjunctive analgesia and reduction of anesthesia drug consumption. In this paper, we focused on comparing the incidence of POD after non-general anesthesia versus general anesthesia. Through an in-depth analysis of these data, we aim to provide conclusive evidence to support the selection of appropriate anesthesia methods in clinical practice. Our results will help guide medical decisions, ensure patient safety, and optimize surgical outcomes.
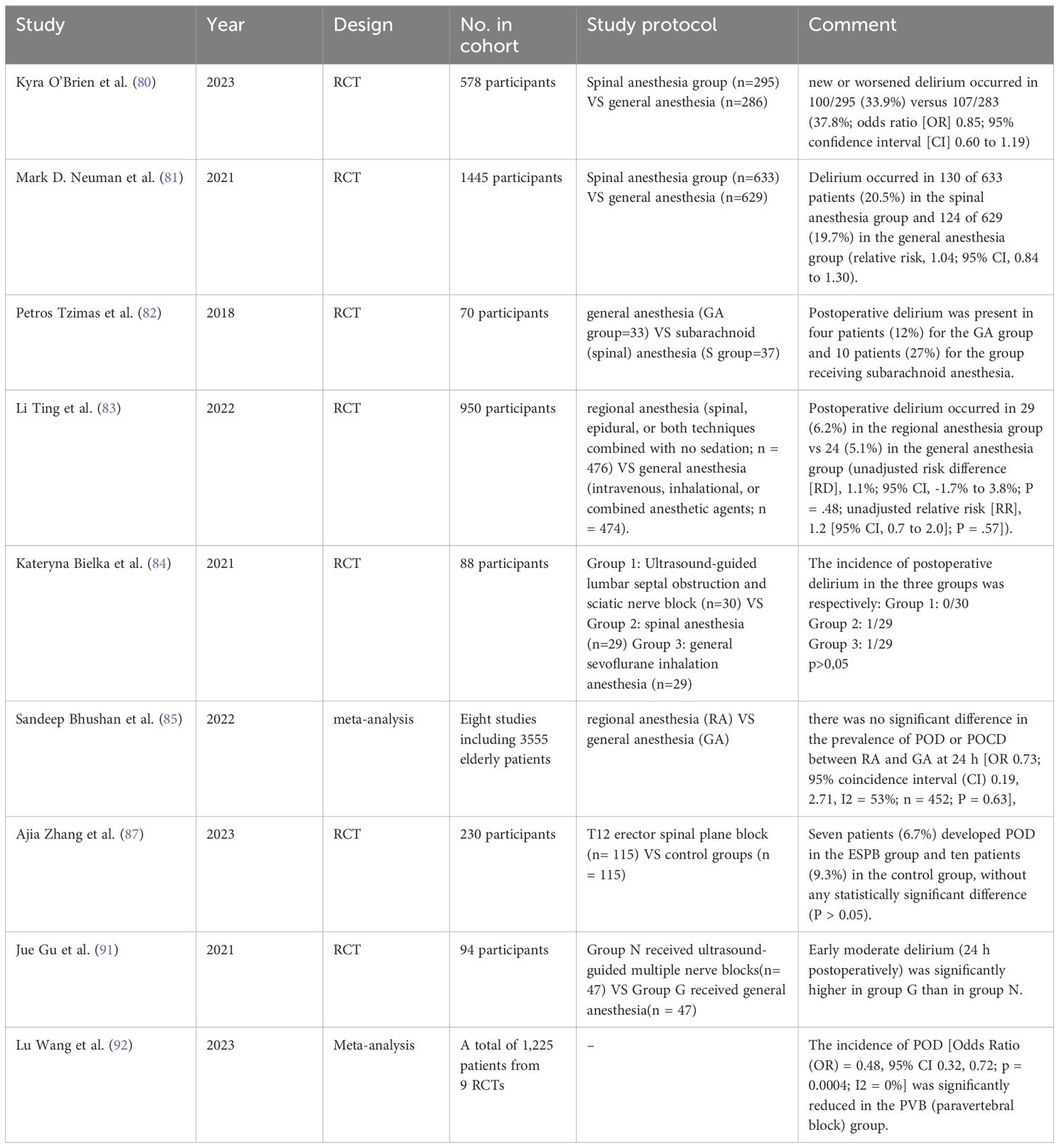
A randomized controlled trial analyzed the incidence of POD between subarachnoid anesthesia and general anesthesia in hip fracture surgery on patients aged 50 and above, the results showed no association between the type of anesthesia and the occurrence of POD (80). Another identical study made the same comparison and came to the same conclusion that among the 633 participants in the subarachnoid anesthesia group, 130 (20.5%) experienced delirium, while among the 629 patients in the general anesthesia group, 124 (19.7%) experienced delirium, with a relative risk of 1.04 (95% CI, 0.84 to 1.30), indicating no significant statistical difference in the occurrence of cognitive dysfunction between the two anesthesia methods (81). This finding is consistent with the conclusion of the study by Tzimas et al. (82). Additionally, a study comparing patients aged 65 and above who received hip fracture surgery found that compared to general anesthesia, subarachnoid anesthesia, epidural anesthesia, or a combination of the two did not significantly reduce the incidence of POD (83). Similarly, studies related to hip surgery have shown similar results (84–86). Furthermore, a study evaluated the effectiveness of T12 Erector Spinae Plane Block (ESPB) in elderly patients undergoing lumbar spine surgery. Although bilateral T12 ESPB reduced the Numerical Rating Scale (NRS) scores within 48 hours after lumbar spine surgery and decreased perioperative opioid consumption, it did not significantly reduce the incidence of POD. In the ESPB group, 7 cases (6.7%) had POD, while in the control group, 10 cases (9.3%) had POD, with no statistically significant difference (P > 0.05) (87).
In contrast to the above-mentioned block anesthesia methods, limited evidence suggests that only nerve blocks, among local anesthesia techniques, may reduce the incidence of delirium and are associated with shorter hospital stays, improved pain management, and decreased inflammation (88–90). In a study comparing ultrasound-guided multi-nerve blocks (myofascial block, lumbar plexus block, and femoral nerve block) with general anesthesia in elderly patients with hip fractures, satisfactory intraoperative pain management and reduced early postoperative cognitive impairment were achieved, making it a potential alternative anesthesia method for elderly hip fracture patients (91). Another meta-analysis of nine randomized controlled trials involving 1,225 patients found that perineural analgesia reduced postoperative pain scores and/or opioid consumption. In patients undergoing major surgery, perineural analgesia may prevent POD (92).
Although some studies have provided strong evidence to support the role of peripheral block anesthesia and peripheral analgesia in the prevention of POD, larger sample-size studies are needed to verify the universality and reliability of their effects. In addition, these studies should include longer-term follow-up to more accurately assess long-term cognitive function outcomes (Table 4).
6.3 Anesthesia depth
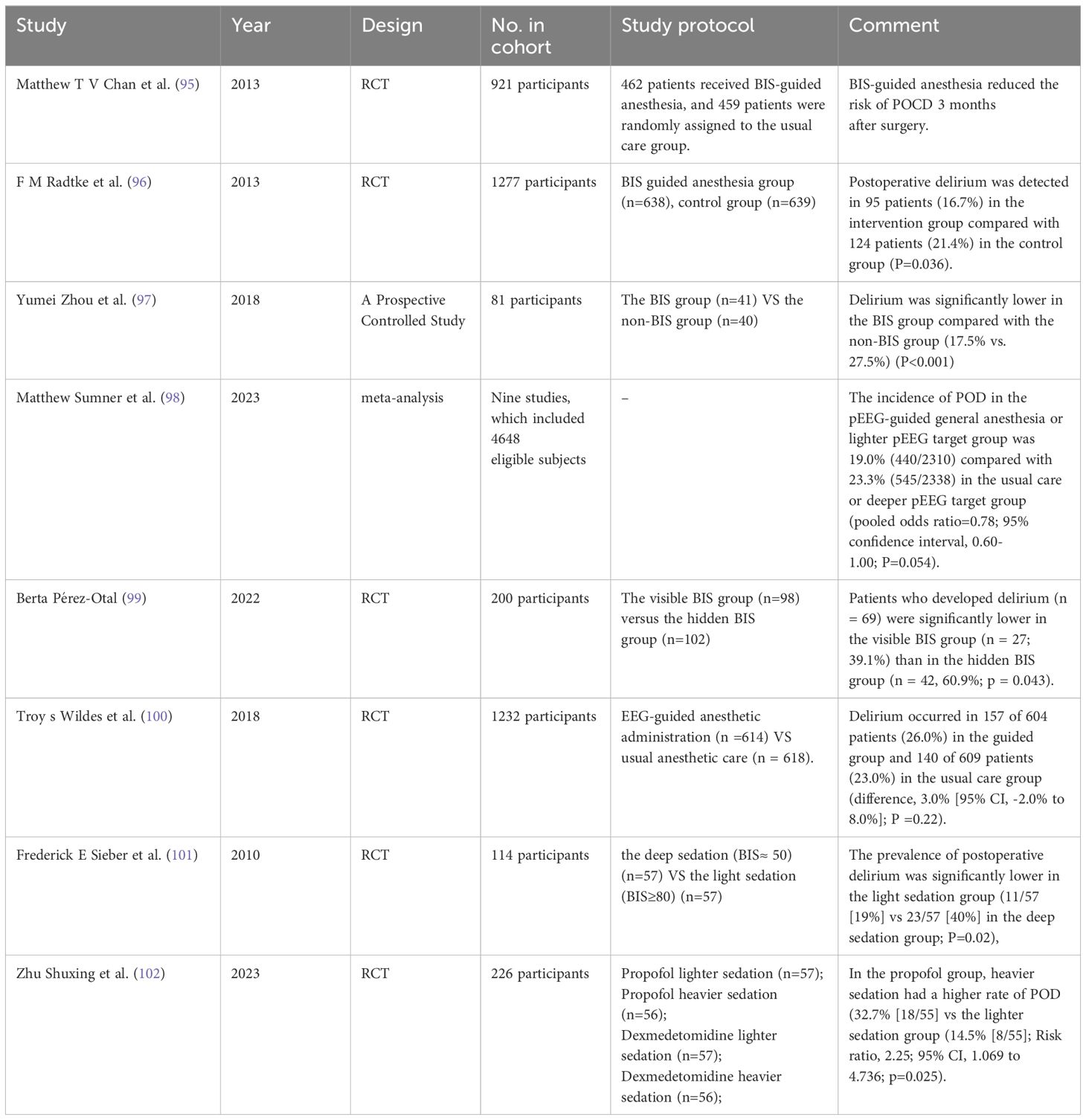
New evidence in the field of anesthesia indicates a significant impact of anesthesia depth on the occurrence of POD, which is a crucial determinant of patient outcomes (93). Anesthesia depth is reflected by the processed electroencephalogram (PEEG)-guided bispectral index (BIS) monitoring (94). According to the literature reviewed, most studies support a lower incidence of POD in anesthesia guided by BIS monitoring than under routine care (95–99). However, conflicting results have been reported in a randomized clinical trial where 1,232 patients were randomly assigned in a 1:1 ratio to receive anesthesia guided by PEEG (n=614) or routine anesthesia care (n=618). The findings showed no significant difference in the incidence of delirium between groups, with 157 patients (26.0%) experiencing POD in the guidance group of 604 patients, and 140 patients (23.0%) experiencing delirium in the routine care group of 609 patients (difference 3.0% [95% CI, -2.0% to 8.0%]; P = 0.22) (100). Considering the presence of non-objective factors, it can still be considered that intraoperative PEEG-guided monitoring anesthesia can prevent POD occurrence. Even more surprising, better control of anesthesia depth has been found to significantly prevent the occurrence of delirium, as many randomized clinical trials have categorized patients into light and deep anesthesia states to evaluate the incidence of POD, consistently showing a significant reduction in POD with lighter anesthesia (101, 102). The new strategies proposed in this study provide innovative insights for preventing POD occurrence, which not only enriches our understanding of the field but also provides a valuable reference for future research and practice (Table 5).
7 Perioperative drugs
A thorough examination of preventive measures for POD requires a comprehensive exploration of pharmacological interventions. However, the efficacy of pharmacological interventions for preventive pharmacotherapy of delirium remains uncertain due to inconclusive evidence currently available (103). The pathophysiological basis of delirium described earlier emphasizes the presumed efficacy of pharmacological agents. Potential therapeutic interventions may include reducing exposure to drugs with prominent anticholinergic properties (104), addressing and mitigating direct brain injury caused by infectious etiologies or drug toxicity, and alleviating physiological stress responses through the use of corticosteroids, benzodiazepines, dexmedetomidine, or melatonin. Looking ahead, this paper will focus on an in-depth analysis of drugs that have been extensively studied in contemporary research, as well as a comparative evaluation of these drugs.
7.1 Dexmedetomidine
Dexmedetomidine is a highly selective α-2 adrenoceptor agonist with a range of pharmacological properties, including sedation, anxiolysis, and mild analgesia, which contribute to its therapeutic efficacy in clinical settings (105). It has a favorable impact on cerebral hemodynamics characterized by a reduction in cerebral blood flow while maintaining coupling between cerebral blood flow and metabolism (106). Additionally, dexmedetomidine has been found to have opioid-sparing effects and enhance the analgesic effects of concomitant opioid medications (107).
In a prospective randomized controlled study, elderly patients 65 years or older undergoing total hip replacement under lumbar anesthesia combined with T12 paravertebral block were randomly assigned to receive supplemental propofol or dexmedetomidine sedation, the conclusion drawn was that intraoperative dexmedetomidine sedation may be associated with a lower incidence of POD (108). In another randomized clinical trial, 226 patients aged 65 or above undergoing hip joint surgery were randomly divided into four groups: propofol light sedation group, propofol deep sedation group, dexmedetomidine light sedation group, and dexmedetomidine deep sedation group. The occurrence of delirium was the primary outcome, and the same conclusion was reached: compared to propofol, dexmedetomidine had a lower incidence of delirium in elderly patients with hip joint fractures (102). In evaluating the relationship between POD and commonly used sedatives such as sevoflurane, desflurane, isoflurane, dexmedetomidine, propofol, midazolam, and ketamine, Cui et al. found that dexmedetomidine was associated with a lower incidence of POD, while midazolam was associated with more POD cases (44). Numerous studies have demonstrated the association between dexmedetomidine and a low incidence of POD (109–113) Although dexmedetomidine holds promising potential as a treatment for POD, further research is needed to establish its undeniable efficacy. Additionally, vigilance regarding adverse reactions is necessary, considering reports of cardiovascular events such as hypertension, tachycardia, myocardial ischemia, cerebrovascular accidents, and hypoxemia (114). Prospective studies should focus on the specific needs of elderly patients with hip fractures to elucidate an optimized treatment strategy that maximizes therapeutic benefits while minimizing risks (Table 6).
7.2 Benzodiazepines
As anxiolytics, sedatives, and hypnotics, benzodiazepines have significant efficacy and possess anticonvulsant and central muscle relaxant effects (115). The use of these compounds in the preoperative stage contributes to alleviating preoperative anxiety, enhancing anesthesia effects, reducing the required dose of anesthetic agents, improving perioperative safety, and promoting postoperative amnesia for intraoperative adverse stimuli (116). Monitoring studies in the United States have indicated that 80% of patients undergoing orthopedic surgery receive benzodiazepines during the perioperative period, despite the associated risks of POD and delayed neurocognitive recovery (115). It is important to recognize that the use of benzodiazepines often synergistically increases the consumption of opioids, with their influence extending to perioperative analgesia and indirectly impacting the manifestation of POD.
A retrospective cohort study by the American Geriatrics Society investigated the incidence of POD in patients with hip fractures and concluded that the use of long-acting benzodiazepines (OR 1.82, CI 1.74-1.89) and short-acting and long-acting benzodiazepines (OR 1.56, CI 1.48-1.63) was associated with an increased likelihood of POD (117). Similarly, an article from a national tertiary referral center in Thailand studying the relationship between cognitive dysfunction and selective major surgeries in elderly adults arrived at the same conclusion, considering benzodiazepines to be an independent predictive factor for the development of POD in elderly patients undergoing major surgeries (118). Cui et al. and Breschan et al. also hold a consistent view on this (44, 119). In terms of the use of benzodiazepines, a retrospective cohort study categorized patients receiving oral benzodiazepines into four groups: continued use, discontinued use, initiated use, and no use (never used). The outcome indicated that the abrupt cessation of perioperative benzodiazepines may be a risk factor for POD, thus suggesting a preventative approach to the clinical use of these drugs (120). Despite extensive research indicating an association between benzodiazepines and a higher incidence of POD, there still exist some conflicting views. Yang et al. randomly divided patients undergoing orthopedic surgery into two groups, one receiving remimazolam and the other receiving propofol anesthesia, and the results showed that, compared to propofol, remimazolam anesthesia was not associated with an increased incidence of POD in elderly orthopedic surgery patients (121). Another study investigating the impact of multimodal anxiety relief strategies, including oral melatonin or midazolam, on POD following sevoflurane anesthesia, found that the incidence of delirium in the midazolam group was similar to that of the placebo group (122).
The current literature shows that there are significant differences in the results of studies on the effect of benzodiazepines on POD incidence. Given this, caution is recommended against the use of this class of drugs in clinical practice, and further in-depth studies are carried out to clarify the mechanism of action and potential risks (Table 7).
7.3 Painkiller
Acute nociceptive stimulation is a key link between preoperative cognitive impairment and postoperative cognitive dysfunction, particularly delirium (123). The paradigm of perioperative pain management typically involves the combination of opioid analgesics and non-steroidal anti-inflammatory drugs (NSAIDs). It is important to recognize that while opioid therapy may potentially induce delirium (124), inadequate management of pain itself is a stronger independent risk factor for delirium (125), as demonstrated in clinical investigations of subjects undergoing interventions for hip fracture. The incidence of delirium increases when opioids are omitted or administered at subtherapeutic doses. Notably, patients receiving suboptimal pain management, compared to those receiving adequate analgesia, have a 9-fold increased risk of delirium development, even when cognitive function is preserved. Thus, inadequate analgesia and inappropriate pain management patterns have been described as risk factors for delirium in frail elderly populations (126).
Relying solely on opioid analgesics may not be sufficient to effectively alleviate pain, especially following orthopedic surgery. Therefore, NSAIDs are commonly used as adjunctive analgesics to opioids postoperatively (127). Ibuprofen and other related NSAIDs are widely used to alleviate pain and inflammation in various clinical conditions. In contrast, opioid-mediated analgesia does not alleviate the inflammatory component of pain perception and carries the potential for serious adverse effects (128).
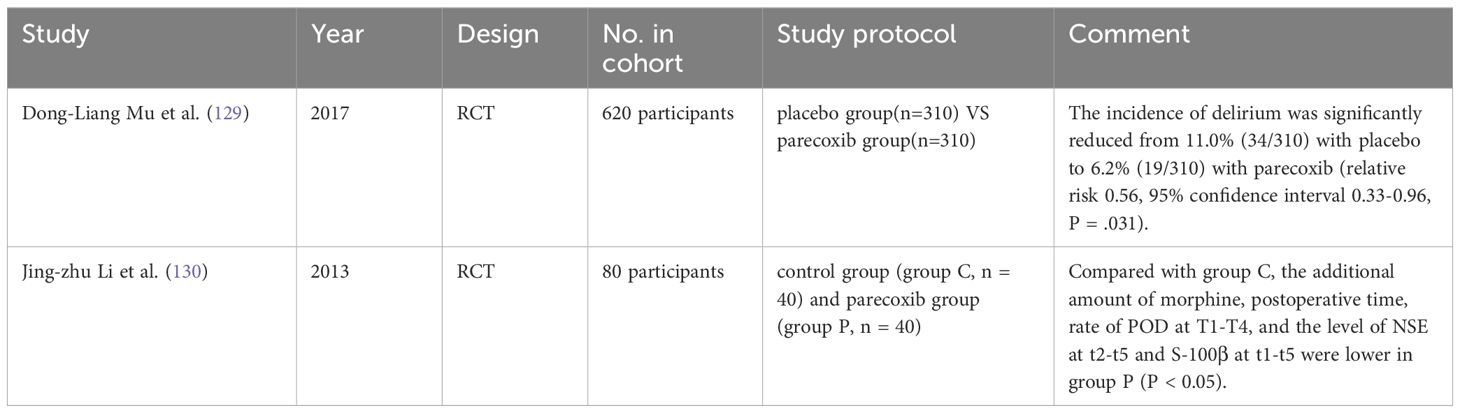
In a randomized, double-blind, multicenter trial, patients aged 60 or above undergoing elective total hip or knee arthroplasty were assigned to receive parecoxib or placebo in a 1:1 ratio, The results demonstrated that in a perioperative low-risk elderly population, the use of parecoxib as part of a multimodal analgesic regimen reduced the incidence of POD without increasing adverse outcomes when compared to intravenous morphine (129). Li et al. also concluded that parecoxib sodium analgesia reduced the incidence of POD and had a neuroprotective effect in elderly patients in a study of parecoxib sodium versus placebo-controlled hip replacement surgery (130). Regular intravenous administration of acetaminophen in combination with intravenous administration of propofol or dexmedetomidine is currently a high-quality strategy for reducing the frequency of POD (127). The study consistently concluded that multimodal analgesia strategies were effective in reducing the incidence of POD. This finding provides a clear guideline for the prevention of POD in clinical practice and has important practical significance (Table 8).
7.4 Steroid
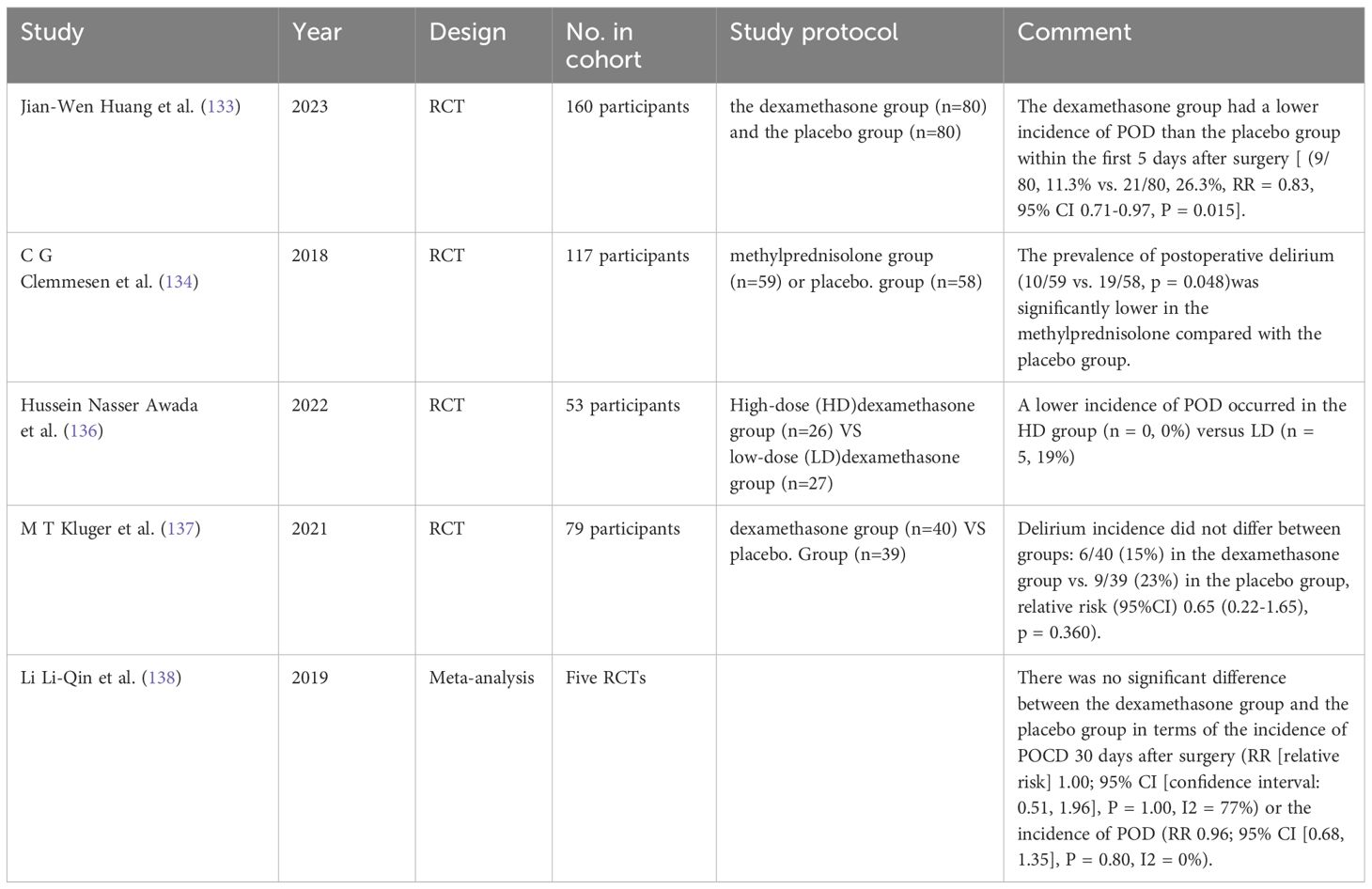
The immune-inflammatory pathway is associated with the pathogenesis of POD in patients with hip fractures (131), and corticosteroids have potent anti-inflammatory properties. The use of anti-inflammatory therapy in the prevention and treatment of delirium has been a strong research topic (132). Studies have shown that preoperative single-dose low-dose dexamethasone can reduce the incidence of POD in elderly orthopedic surgery patients (133, 134), with the greatest reduction observed in the absence of general anesthesia (135). Furthermore, compared to patients receiving low-dose glucocorticoids, those receiving high-dose glucocorticoids have a lower incidence of POD (136).
However, contradictory views to the above are still present. In patients with hip fractures, there was no significant difference in the severity of delirium scores between the dexamethasone and placebo groups, There was no difference in the incidence of delirium between the groups: 6/40 (15%) in the dexamethasone group and 9/39 (23%) in the placebo group (137). In addition, a meta-analysis indicated that prophylactic dexamethasone did not reduce the incidence of wound infection and POD (138). To advance research in this area, more experiments are urgently needed to explore alternative strategies for preventing postoperative cognitive impairment.
At the same time, it is crucial to delve deeper into the pathophysiological mechanisms behind these symptoms, which will help us understand POD more fully and develop more effective preventive measures (Table 9).
7.5 Antipsychotic drug
Antipsychotic treatment can generally be divided into two classes: classic first-generation antipsychotics (FGAs), also known as typical antipsychotics, mainly including chlorpromazine and haloperidol, and more modern second-generation antipsychotics (SGAs), commonly known as atypical antipsychotics, including quetiapine, risperidone, and olanzapine. Many researchers have studied the use of antipsychotic medications for the prevention of POD, and the results have been highly encouraging.
Haloperidol is a typical antipsychotic that primarily exerts its antipsychotic properties by selectively antagonizing dopamine/antipsychotic receptors in the brain, leading to alterations in dopamine turnover (139). Its clinical application extends to the treatment of schizophrenia and bipolar affective disorder (140), emphasizing its effective blockade of extrapyramidal dopamine and significant antiemetic effects (141). Fukata’s article is a good demonstration of this point of view (142). Nevertheless, in two randomized controlled clinical studies that compared haloperidol with placebo, prophylactic haloperidol use did not have a significantly better effect on POD incidence than placebo (143, 144). However, in elderly orthopedic patients undergoing knee or hip arthroplasty, preoperative oral administration of low-dose quetiapine one hour before surgery can reduce the occurrence of POD, improve postoperative sleep quality, and increase satisfaction with postoperative pain management at 24 hours (145).
In cardiac surgery, two studies have shown that the use of intraoperative risperidone is associated with a lower incidence of POD compared to placebo (11.1% vs. 31.7%, P=0.009, relative risk = 0.35, 95% confidence interval [CI] = 0.16-0.77) (146, 147). As an atypical antipsychotic, olanzapine significantly reduces the incidence of delirium when orally administered at 10 mg during the perioperative period. These findings suggest that olanzapine may be an effective strategy for preventing POD (148). However, despite the use of olanzapine for prophylaxis, a proportion of patients may still experience delirium. Risk reduction for delirium can be achieved through prophylactic administration of olanzapine, optimization of perfusion and oxygenation, and limiting intraoperative opioid use (149).
Taken together, we believe that these findings provide a new perspective for the future clinical use of antipsychotics and are expected to guide clinicians more effectively to prevent POD. To ensure the reliability of these conclusions, more research is recommended to verify the accuracy and clinical applicability of these findings (Table 10).
7.6 Melatonin
Melatonin has a regulatory effect on the circadian rhythm and, due to its role in synchronizing circadian rhythms, it can improve sleep onset and enhance sleep quality. This neurohormone exhibits flexibility in modulating excitatory processes in the central nervous system. Reduced melatonin secretion has been associated with increased susceptibility to delirium, particularly in the elderly and individuals with cognitive impairments. In terms of the pathophysiology of delirium, melatonin has shown efficacy in alleviating oxidative stress in various conditions (150). Its mechanisms include direct neutralization of reactive oxygen and nitrogen species, as well as indirect promotion of antioxidant enzymes and inhibition of pro-oxidant catalysts (151). Furthermore, melatonin is involved in sequestering transitional metals (152) and reducing the formation of harmful hydroxyl radicals, thereby mitigating oxidative stress (153). Its anti-free radical properties further extend to reducing the side effects of various medications and methamphetamine, providing the biological basis for the use of melatonin in preventing delirium (154, 155).
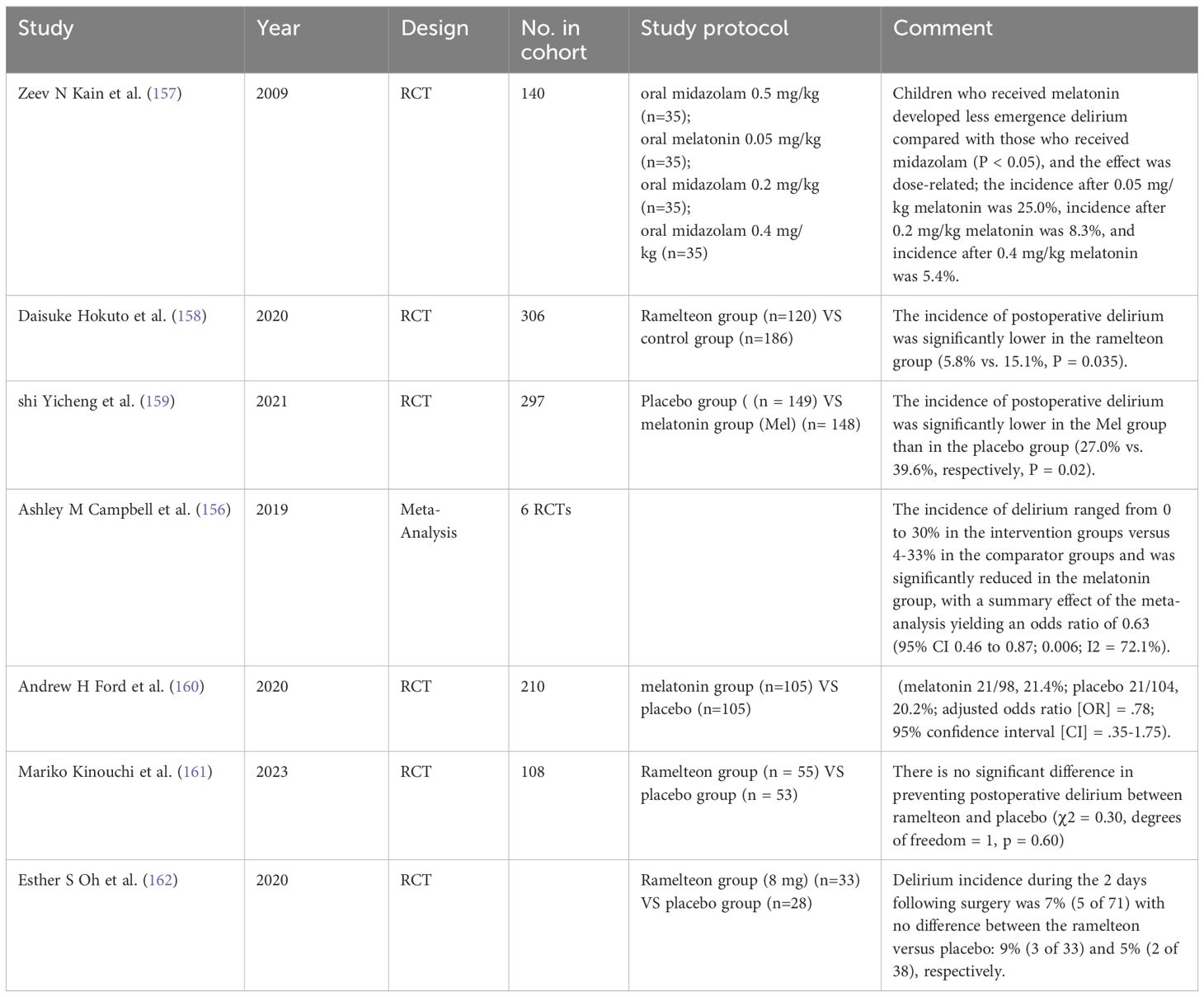
In a meta-analysis analyzing the incidence of POD in elderly surgical patients using melatonin or ramelteon as preventive measures compared to placebo, the occurrence of delirium in the placebo group ranged from 4% to 33% and was reduced to 0% to 30% in the melatonin group, with an odds ratio of 0.63 (95% CI, 0.46-0.87; P = 0.006) (156). Kain et al. believed that the incidence of delirium in the melatonin group was lower (P < 0.05), and the effect was dose-dependent.; the incidence rates after administration of melatonin at 0.05 mg/kg, 0.2 mg/kg, and 0.4 mg/kg were 25.0%, 8.3%, and 5.4%, respectively (157). A multimodal anti-anxiety approach, including oral melatonin, significantly reduced the emergence of delirium following sevoflurane anesthesia (122). While most studies suggest that melatonin may effectively reduce the incidence of POD (158, 159), in studies of cardiac surgery, the prophylactic use of melatonin did not show a statistically significant impact on POD (160). Furthermore, there is limited evidence that ramelteon is not effective in preventing POD (161, 162). Although the current findings diverge somewhat, they collectively highlight the importance of cross-disciplinary clinicians considering drug combination therapy in practice. This combined treatment strategy may help improve the effectiveness of prevention of POD and therefore deserves further exploration and validation in future clinical practice (Table 11).
7.7 Cholinergic agonists
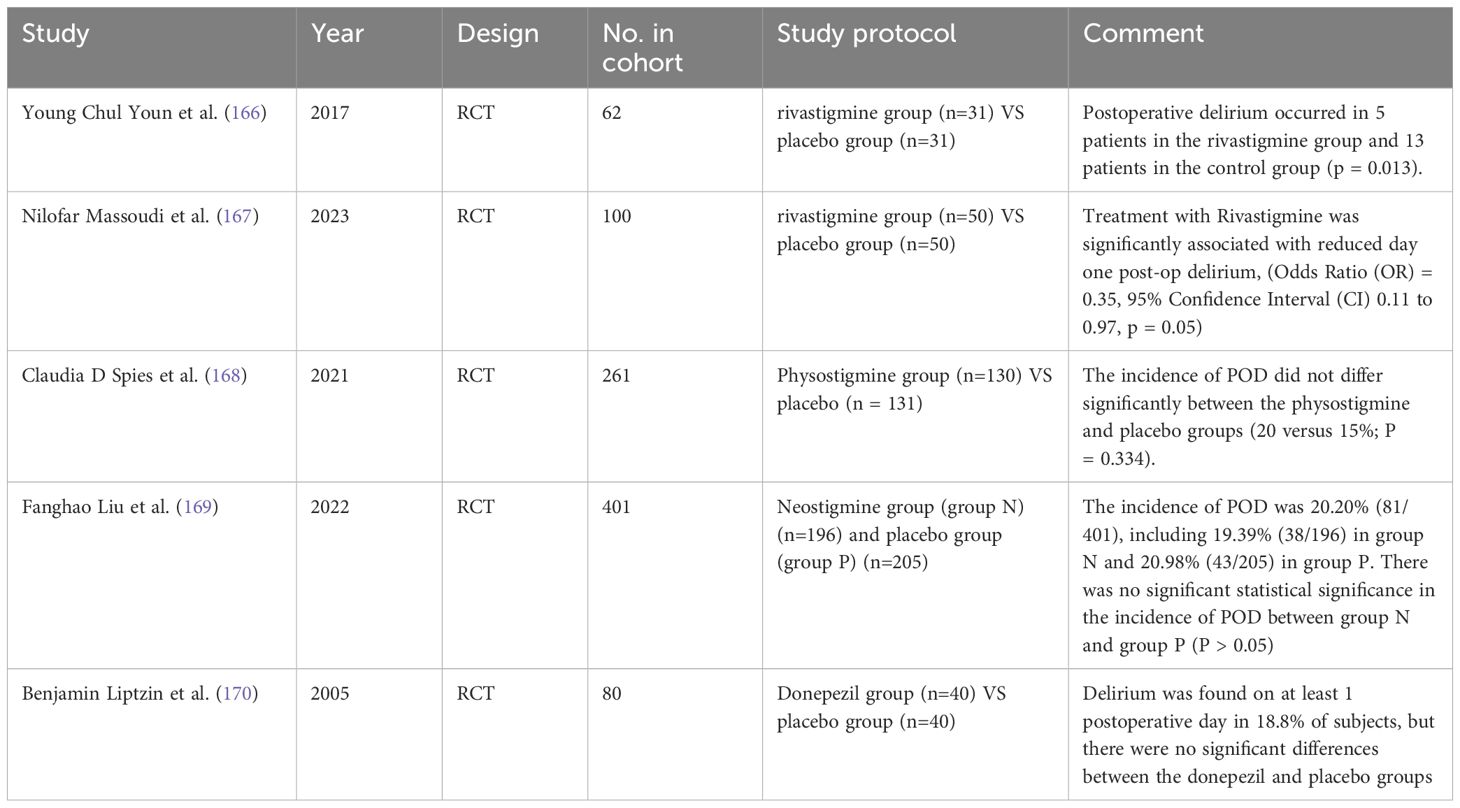
A large body of literature strongly indicates that alterations in cholinergic neurotransmission are associated with the occurrence of POD (163, 164). Genetic polymorphisms in cholinergic receptors, particularly the CHRM2 and CHRM4 genes (165), may support this relationship. While theoretically, interventions aimed at increasing acetylcholine (ACh) levels through the use of acetylcholinesterase inhibitors are considered potential interventions, existing controlled studies evaluating the efficacy of this treatment approach have yielded inconsistent results. Young et al. and Massoudi et al. supported this view, suggesting that sustained action of acetylcholine could significantly reduce the incidence of POD (166, 167). Other articles argue otherwise. A study found that administration of scopolamine within 24 hours after induction of anesthesia did not significantly change the incidence of POD (168). In two randomized controlled trials of acetylcholinesterase inhibitors, the authors randomly assigned patients to both the acetylcholinesterase inhibitor group and the control group, and they found surprisingly consistent results that there was no statistical difference in the incidence of POD between the control group and the acetylcholinesterase inhibitor (169, 170). This finding contradicts the idea that there is an association between cholinergic neurotransmission alterations and POD occurrence. To ensure the accuracy and reliability of the study, more studies are recommended to delve into the mechanism of action of the cholinergic system in POD occurrence and verify this paradoxical phenomenon.
Based on current research, analgesics are effective in preventing the development of POD. However, there is a discrepancy in the literature regarding the efficacy of alternative anesthetic techniques and perioperative medications in POD prevention. These conflicting findings present new avenues and challenges for future research in POD prevention and clinical pharmacology. Future studies should aim to clarify these differences and provide more definitive guidance for clinical practice (Table 12).
8 Prevention for POD
In 2015, the American Geriatrics Society disseminated an investigative synopsis addressing the incidence of POD amongst the geriatric demographic. The report delineated three pivotal non-pharmacologic directives: (i) standardization of educational initiatives within health-care institutions; (ii) implementation of multifaceted, non-pharmacological prophylaxes for individuals identified as high-risk, under the vigilant oversight of an interdisciplinary cohort; (iii) comprehensive interventions for patients with a confirmed diagnosis. Furthermore, the report underscored the dearth of robust evidence endorsing the utilization of technological apparatuses in management (171).
Preventive strategies, coupled with prompt recognition, are deemed fundamental in forestalling POD after hip fracture surgeries. Medical practitioners during the perioperative timeline should maintain heightened vigilance for POD onset, especially in patients presenting with both intrinsic and extrinsic risk factors. Contemporary strides have yielded POD risk assessment models (172, 173), instrumental in the stratification of delirium risk among this patient cohort, thereby guiding the formulation of preventative strategies.
Healthcare proxies often serve as initial observers of behavioral shifts in post-surgical patients; hence they must be adequately trained to recognize the indicators of POD at the earliest instance. Standard nursing interventions encompass the strategic placement of timekeeping devices and calendars to assist with temporal and spatial reorientation, fostering visitations by kin, establishing non-pharmacological routines to enhance sleep quality, delineating nocturnal rest from daytime activities, abating disruptive auditory stimuli, endorsing the utilization of sensory aids, assuring optimal hydration and nutritional status to mitigate malnutrition, and the vigorous management of pain (Figure 3). Furthermore, our analysis incorporated a comparison of geriatric orthopedic care methodologies. The geriatric orthopedic care paradigm integrates a multidisciplinary protocol, which encapsulates comprehensive assessments and optimization of the geriatric population post-orthopedic interventions. An abundance of research has verified that perioperative multimodal care substantially diminishes the incidence of POD (P <.00001) (174).

Figure 3. Strategies for the non-pharmacological mitigation of delirium and subsequent therapeutic interventions upon detection: a holistic approach to patient care.
9 Conclusion
While the utilization of surgical anesthesia and perioperative pharmacotherapy presents an elevated predisposition towards the advent of POD in hip surgery, judicious regulation of dosage and meticulous selection of pharmacological agents can mitigate such risk. It is incumbent upon anesthesiologists and surgical care teams to perform a comprehensive assessment of the patient’s chronological age, surgical category, and pathophysiological status among other criteria, to formulate an optimal anesthetic plan that encompasses the judicious choice of drugs and techniques. Additionally, family members and healthcare providers must possess cognizance of the potential for postoperative cognitive dysfunction, actively engaging in preventive strategies to attenuate the incidence.
Postoperative cognitive dysfunction, notably delirium, represents a prevalent post-surgical complication in geriatric cohorts after femoral fractures. The application of anesthesia and ancillary perioperative medications requires heightened scrutiny. Healthcare practitioners must exercise a strategic approach in the preoperative and intraoperative phases, inclusive of a thorough appraisal of the patient’s health profile, leading to the strategic administration of anesthetic agents and perioperative medications, with the express intent of minimizing the manifestation of POD.
Author contributions
SLa: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing. SLi: Conceptualization, Investigation, Project administration, Validation, Writing – review & editing. HW: Data curation, Funding acquisition, Project administration, Validation, Visualization, Writing – review & editing. SD: Conceptualization, Formal analysis, Methodology, Resources, Visualization, Writing – original draft. KS: Conceptualization, Data curation, Investigation, Project administration, Validation, Writing – original draft. CY: Data curation, Formal analysis, Funding acquisition, Resources, Validation, Writing – original draft, Writing – review & editing. LY: Conceptualization, Data curation, Project administration, Validation, Writing – original draft. LC: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing. JL: Conceptualization, Investigation, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the National first-class undergraduate professional construction project (clinical medicine) sub-project of Anhui Medical University, Natural Science Foundation of Hefei City (grant number: 2022041 to JL), Clinical Research Cultivation Program of the Second Affiliated Hospital of Anhui Medical University (grant number: 2020LCZD20 to JL), Basic and Clinical Cooperative Research Promotion Plan of Anhui Medical University (grant number: 2020xkjT040 to JL), Group Medical Aid Project of Natural Science Foundation of Tibet Autonomous Region (grant number: XZ2023ZR-ZY47(Z)), and Technology Plan Project of Shannan City (SNSBJKJJHXM2023015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rizk P, Morris W, Oladeji P, Huo M. Review of postoperative delirium in geriatric patients undergoing hip surgery. Geriatric Orthopaedic Surg Rehabil. (2016) 7:100–5. doi: 10.1177/2151458516641162
2. Fricchione GL, Nejad SH, Esses JA, Cummings TJ Jr, Querques J, Cassem NH, et al. Postoperative delirium. Am J Psychiatry. (2008) 165:803–12. doi: 10.1176/appi.ajp.2008.08020181
3. Bai J, Liang Y, Zhang P, Liang X, He J, Wang J, et al. Association between postoperative delirium and mortality in elderly patients undergoing hip fractures surgery: a meta-analysis. Osteoporosis Int. (2020) 31:317–26. doi: 10.1016/j.jclinane.2023.111221
4. Gao R, Yang Z-Z, Li M, Shi Z-C, Fu Q. Probable risk factors for postoperative delirium in patients undergoing spinal surgery. Eur Spine J. (2008) 17:1531–7. doi: 10.1007/s00586-008-0771-1
6. Park EA, Kim MY. Postoperative delirium is associated with negative outcomes and long-term mortality in elderly Koreans: a retrospective observational study. Medicina. (2019) 55:618. doi: 10.3390/medicina55100618
7. Shi Z, Mei X, Li C, Chen Y, Zheng H, Wu Y, et al. Postoperative delirium is associated with long-term decline in activities of daily living. Anesthesiology. (2019) 131:492–500. doi: 10.1097/ALN.0000000000002849
8. Marshall MC, Soucy MD. Delirium in the intensive care unit. Crit Care Nurs Q. (2003) 26:172–8. doi: 10.1097/00002727-200307000-00002
9. Lemstra AW, Kalisvaart KJ, Vreeswijk R, van Gool WA, Eikelenboom P. Pre-operative inflammatory markers and the risk of postoperative delirium in elderly patients. Int J Geriatric Psychiatry. (2008) 23:943–8. doi: 10.1002/gps.2015
10. Gamberale R, D’Orlando C, Brunelli S, Meneveri R, Mazzola P, Foti G, et al. Study protocol: understanding the pathophysiologic mechanisms underlying delirium in older people undergoing hip fracture surgery. BMC Geriatr. (2021) 21:633. doi: 10.1186/s12877-021-02584-1
11. Hughes CG, Boncyk CS, Culley DJ, Fleisher LA, Leung JM, McDonagh DL, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analgesia. (2020) 130:1572–90. doi: 10.1213/ANE.0000000000004641
12. Plaza-Carmona M, Requena-Hernández C, Jiménez-Mola S. Predictors of delirium in octogenarian patients hospitalized for a hip fracture. Int J Environ Res Public Health. (2020) 17:7467. doi: 10.3390/ijerph17207467
13. Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatric Psychiatry. (2018) 33:1428–57. doi: 10.1002/gps.4823
14. Cozowicz C, Memtsoudis SG, Poeran J. Risk factors for postoperative delirium in patients undergoing lower extremity joint arthroplasty: a retrospective population-based cohort study. Regional Anesth Pain Med. (2020) 46(1):94–95. doi: 10.1136/rapm-2020-101617
15. Winter A, Steurer MP, Dullenkopf A. Postoperative delirium assessed by post anesthesia care unit staff utilizing the Nursing Delirium Screening Scale: a prospective observational study of 1000 patients in a single Swiss institution. BMC Anesthesiol. (2015) 15:184. doi: 10.1186/s12871-015-0168-8
16. Silva AR, Regueira P, Albuquerque E, Baldeiras I, Cardoso AL, Santana I, et al. Estimates of geriatric delirium frequency in noncardiac surgeries and its evaluation across the years: a systematic review and meta-analysis. J Am Med Directors Assoc. (2021) 22:613–20.e9. doi: 10.1016/j.jamda.2020.08.017
17. Berian JR, Zhou L, Russell MM, Hornor MA, Cohen ME, Finlayson E, et al. Postoperative delirium as a target for surgical quality improvement. Ann Surg. (2018) 268:93–9. doi: 10.1097/SLA.0000000000002436
18. Oldroyd C, Scholz AF, Hinchliffe RJ, McCarthy K, Hewitt J, Quinn TJ. A systematic review and meta-analysis of factors for delirium in vascular surgical patients. J Vasc Surg. (2017) 66:1269–79.e9. doi: 10.1016/j.jvs.2017.04.077
19. Wu J, Yin Y, Jin M, Li B. The risk factors for postoperative delirium in adult patients after hip fracture surgery: a systematic review and meta-analysis. Int J Geriatric Psychiatry. (2021) 36:3–14. doi: 10.1002/gps.5408
20. Saravana-Bawan B, Warkentin LM, Rucker D, Carr F, Churchill TA, Khadaroo RG. Incidence and predictors of postoperative delirium in the older acute care surgery population: a prospective study. Can J Surg. (2019) 62:33. doi: 10.1503/cjs.016817
21. Saljuqi AT, Hanna K, Asmar S, Tang A, Zeeshan M, Gries L, et al. Prospective evaluation of delirium in geriatric patients undergoing emergency general surgery. J Am Coll Surgeons. (2020) 230:758–65. doi: 10.1016/j.jamcollsurg.2020.01.029
22. Chaiwat O, Chanidnuan M, Pancharoen W, Vijitmala K, Danpornprasert P, Toadithep P, et al. Postoperative delirium in critically ill surgical patients: incidence, risk factors, and predictive scores. BMC Anesthesiology. (2019) 19:1–10. doi: 10.1186/s12871-019-0694-x
23. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, Van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. (2010) 304:443–51. doi: 10.1001/jama.2010.1013
24. Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, et al. Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg. (2015) 150:1134–40. doi: 10.1001/jamasurg.2015.2606
25. Crocker E, Beggs T, Hassan A, Denault A, Lamarche Y, Bagshaw S, et al. Long-term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thorac Surg. (2016) 102:1391–9. doi: 10.1016/j.athoracsur.2016.04.071
26. Pereira JVB, Aung Thein MZ, Nitchingham A, Caplan GA. Delirium in older adults is associated with development of new dementia: a systematic review and meta-analysis. Int J Geriatric Psychiatry. (2021) 36:993–1003. doi: 10.1002/gps.5508
27. Wittmann M, Kirfel A, Jossen D, Mayr A, Menzenbach J. The impact of perioperative and predisposing risk factors on the development of postoperative delirium and a possible gender difference. Geriatrics. (2022) 7:65. doi: 10.3390/geriatrics7030065
28. Aitken SJ, Blyth FM, Naganathan V. Incidence, prognostic factors and impact of postoperative delirium after major vascular surgery: a meta-analysis and systematic review. Vasc Med. (2017) 22:387–97. doi: 10.1177/1358863X17721639
29. Yang Z, Wang X-F, Yang L-F, Fang C, Gu X-K, Guo H-W. Prevalence and risk factors for postoperative delirium in patients with colorectal carcinoma: a systematic review and meta-analysis. Int J Colorectal Dis. (2020) 35:547–57. doi: 10.1007/s00384-020-03505-1
30. Burkhart CS, Dell-Kuster S, Gamberini M, Moeckli A, Grapow M, Filipovic M, et al. Modifiable and nonmodifiable risk factors for postoperative delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothoracic Vasc Anesth. (2010) 24:555–9. doi: 10.1053/j.jvca.2010.01.003
31. E Silva LOJ, Berning MJ, Stanich JA, Gerberi DJ, Murad MH, Han JH, et al. Risk factors for delirium in older adults in the emergency department: a systematic review and meta-analysis. Ann Emergency Med. (2021) 78:549–65. doi: 10.1016/j.annemergmed.2021.03.005
32. Persico I, Cesari M, Morandi A, Haas J, Mazzola P, Zambon A, et al. Frailty and delirium in older adults: a systematic review and meta-analysis of the literature. J Am Geriatrics Soc. (2018) 66:2022–30. doi: 10.1111/jgs.15503
33. Gracie TJ, Caufield-Noll C, Wang N-Y, Sieber FE. The association of preoperative frailty and postoperative delirium: a meta-analysis. Anesthesia & Analgesia (2021) 133(2):314–23. doi: 10.1213/ANE.0000000000005609
34. Chen H, Mo L, Hu H, Ou Y, Luo J. Risk factors of postoperative delirium after cardiac surgery: a meta-analysis. J Cardiothoracic Surg. (2021) 16:1–11. doi: 10.1186/s13019-021-01496-w
35. Yang Q, Wang J, Huang X, Xu Y, Zhang Y. Incidence and risk factors associated with postoperative delirium following primary elective total hip arthroplasty: a retrospective nationwide inpatient sample database study. BMC Psychiatry. (2020) 20:1–9. doi: 10.1186/s12888-020-02742-6
36. Guan HL, Liu H, Hu XY, Abdul M, Dai MS, Gao X, et al. Urinary albumin creatinine ratio associated with postoperative delirium in elderly patients undergoing elective non-cardiac surgery: A prospective observational study. CNS Neurosci Ther. (2022) 28:521–30. doi: 10.1111/cns.13717
37. Oliveira FR, Oliveira VH, Oliveira ÍM, Lima JW, Calderaro D, Gualandro DM, et al. Hypertension, mitral valve disease, atrial fibrillation and low education level predict delirium and worst outcome after cardiac surgery in older adults. BMC Anesthesiology. (2018) 18:1–8. doi: 10.1186/s12871-018-0481-0
38. Jiang L, Lei G. Albumin/fibrinogen ratio, an independent risk factor for postoperative delirium after total joint arthroplasty. Geriatrics Gerontology Int. (2022) 22:412–7. doi: 10.1111/ggi.14381
39. Scholz A, Oldroyd C, McCarthy K, Quinn T, Hewitt J. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. J Br Surg. (2016) 103:e21–e8. doi: 10.1002/bjs.10062
40. Abbott TE, Pearse RM. Depth of anesthesia and postoperative delirium. JAMA. (2019) 321:459–60. doi: 10.1001/jama.2019.0164
41. Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analgesia. (2006) 102:1267–73. doi: 10.1213/01.ane.0000199156.59226.af
42. Weinstein S, Poultsides L, Baaklini L, Mörwald E, Cozowicz C, Saleh J, et al. Postoperative delirium in total knee and hip arthroplasty patients: a study of perioperative modifiable risk factors. Br J Anaesth. (2018) 120:999–1008. doi: 10.1016/j.bja.2017.12.046
43. Cui F, Zhao W, Mu D-L, Zhao X, Li X-Y, Wang D-X, et al. Association between cerebral desaturation and postoperative delirium in thoracotomy with one-lung ventilation: a prospective cohort study. Anesth Analgesia. (2021) 133:176–86. doi: 10.1213/ANE.0000000000005489
44. Cui Y, Li G, Cao R, Luan L, Kla KM. The effect of perioperative anesthetics for prevention of postoperative delirium on general anesthesia: a network meta-analysis. J Clin Anesth. (2020) 59:89–98. doi: 10.1016/j.jclinane.2019.06.028
45. Roberts ML, Lin H-M, Tinuoye E, Cohen E, Flores RM, Fischer GW, et al. The association of cerebral desaturation during one-lung ventilation and postoperative recovery: a prospective observational cohort study. J Cardiothoracic Vasc Anesth. (2021) 35:542–50. doi: 10.1053/j.jvca.2020.07.065
46. Chou MY, Wang YC, Peng LN, Liang CK, Chu CS, Liao MC, et al. Intraoperative blood transfusion predicts postoperative delirium among older patients undergoing elective orthopedic surgery: a prospective cohort study. Int J Geriatric Psychiatry. (2019) 34:881–8. doi: 10.1002/gps.5086
47. Ravi B, Pincus D, Choi S, Jenkinson R, Wasserstein DN, Redelmeier DA. Association of duration of surgery with postoperative delirium among patients receiving hip fracture repair. JAMA Network Open. (2019) 2:e190111–e. doi: 10.1001/jamanetworkopen.2019.0111
48. Yan E, Veitch M, Saripella A, Alhamdah Y, Butris N, Tang-Wai DF, et al. Association between postoperative delirium and adverse outcomes in older surgical patients: A systematic review and meta-analysis. J Clin Anesth. (2023) 90:111221. doi: 10.1016/j.jclinane.2023.111221
49. Zhang Y, He S-T, Nie B, Li X-Y, Wang D-X. Emergence delirium is associated with increased postoperative delirium in elderly: a prospective observational study. J Anesth. (2020) 34:675–87. doi: 10.1007/s00540-020-02805-8
50. Ohl ICB, Chavaglia SRR, Ohl RIB, Lopes MCBT, Campanharo CRV, Okuno MFP, et al. Evaluation of delirium in aged patients assisted at emergency hospital service. Rev Bras Enfermagem. (2019) 72:153–60. doi: 10.1590/0034-7167-2018-0386
51. Chen Y-L, Lin H-C, Lin K-H, Lin L-S, Hsieh C-E, Ko C-J, et al. Low hemoglobin level is associated with the development of delirium after hepatectomy for hepatocellular carcinoma patients. PloS One. (2015) 10:e0119199. doi: 10.1371/journal.pone.0119199
52. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clinics. (2017) 33:461–519. doi: 10.1016/j.ccc.2017.03.013
53. Larson EB, Kukull WA, Buchner D, Reifler BV. Adverse drug reactions associated with global cognitive impairment in elderly persons. Ann Internal Med. (1987) 107:169–73. doi: 10.7326/0003-4819-107-2-169
54. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
55. Tully PJ, Baker RA, Winefield HR, Turnbull DA. Depression, anxiety disorders and Type D personality as risk factors for delirium after cardiac surgery. Aust New Z J Psychiatry. (2010) 44:1005–11. doi: 10.3109/00048674.2010.495053
56. Egberts A, Moreno-Gonzalez R, Alan H, Ziere G, Mattace-Raso FU. Anticholinergic drug burden and delirium: a systematic review. J Am Med Directors Assoc. (2021) 22:65–73.e4. doi: 10.1016/j.jamda.2020.04.019
57. Hirsch J, DePalma G, Tsai T, Sands L, Leung J. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. Br J Anaesthesia. (2015) 115:418–26. doi: 10.1093/bja/aeu458
58. Chanques G, Ely EW, Garnier O, Perrigault F, Eloi A, Carr J, et al. The 2014 updated version of the Confusion Assessment Method for the Intensive Care Unit compared to the 5th version of the Diagnostic and Statistical Manual of Mental Disorders and other current methods used by intensivists. Ann Intensive Care. (2018) 8:33. doi: 10.1186/s13613-018-0377-7
59. De J, Wand AP. Delirium screening: A systematic review of delirium screening tools in hospitalized patients. Gerontologist. (2015) 55:1079–99. doi: 10.1093/geront/gnv100
60. Gaudreau JD, Gagnon P, Harel F, Tremblay A, Roy MA. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage. (2005) 29:368–75. doi: 10.1016/j.jpainsymman.2004.07.009
61. Bilotta F, Lauretta MP, Borozdina A, Mizikov VM, Rosa G. Postoperative delirium: risk factors, diagnosis and perioperative care. Minerva Anestesiol. (2013) 79:1066–76.
62. Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. (2004) 14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17
63. Morandi A, Davis D, Bellelli G, Arora RC, Caplan GA, Kamholz B, et al. The diagnosis of delirium superimposed on dementia: an emerging challenge. J Am Med Directors Assoc. (2017) 18:12–8. doi: 10.1016/j.jamda.2016.07.014
64. Daou M, Telias I, Younes M, Brochard L, Wilcox ME. Abnormal sleep, circadian rhythm disruption, and delirium in the ICU: are they related? Front Neurol. (2020) 11:549908. doi: 10.3389/fneur.2020.549908
65. Chevrolet J-C, Jolliet P. Clinical review: agitation and delirium in the critically ill–significance and management. Crit Care. (2007) 11:1–5. doi: 10.1186/cc5787
66. Trzepacz PT. The neuropathogenesis of delirium: a need to focus our research. Psychosomatics. (1994) 35:374–91. doi: 10.1016/S0033-3182(94)71759-X
67. Slor CJ, de Jonghe JF, Vreeswijk R, Groot E, Ploeg TV, Van Gool WA, et al. Anesthesia and postoperative delirium in older adults undergoing hip surgery. J Am Geriatr Soc. (2011) 59:1313–9. doi: 10.1111/j.1532-5415.2011.03452.x
68. Björkelund KB, Hommel A, Thorngren KG, Gustafson L, Larsson S, Lundberg DJAAS. Reducing delirium in elderly patients with hip fracture: a multi-factorial intervention study. Acta Anaesthesiol Scand. (2010) 54:678–88. doi: 10.1111/j.1399-6576.2010.02232.x
69. Genet B, Lamy T, Cohen-Bittan J, Glasman P, Verny M, Riou B, et al. Lack of association between perioperative medication and postoperative delirium in hip fracture patients in an orthogeriatric care pathway. J Am Med Dir Assoc. (2022) 23:623–30.e2. doi: 10.1016/j.jamda.2021.09.022
70. Ishii K, Makita T, Yamashita H, Matsunaga S, Akiyama D, Toba K, et al. Total intravenous anesthesia with propofol is associated with a lower rate of postoperative delirium in comparison with sevoflurane anesthesia in elderly patients. J Clin Anesth. (2016) 33:428–31. doi: 10.1016/j.jclinane.2016.04.043
71. Saller T, Hubig L, Seibold H, Schroeder Z, Wang B, Groene P, et al. Association between post-operative delirium and use of volatile anesthetics in the elderly: A real-world big data approach. J Clin Anesth. (2022) 83:110957. doi: 10.1016/j.jclinane.2022.110957
72. Miller D, Lewis SR, Pritchard MW, Schofield-Robinson OJ, Shelton CL, Alderson P, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev. (2018) 8:CD012317. doi: 10.1002/14651858.CD012317.pub2
73. Mei X, Zheng H-L, Li C, Ma X, Zheng H, Marcantonio E, et al. The effects of propofol and sevoflurane on postoperative delirium in older patients: a randomized clinical trial study. J Alzheimers Dis. (2020) 76:1627–36. doi: 10.3233/JAD-200322
74. Cao S-J, Zhang Y, Zhang Y-X, Zhao W, Pan L-H, Sun X-D, et al. Delirium in older patients given propofol or sevoflurane anaesthesia for major cancer surgery: a multicentre randomised trial. Br J Anaesth. (2023) 131:253–65. doi: 10.1016/j.bja.2023.04.024
75. Yoshimura M, Shiramoto H, Morimoto Y, Koga M. Comparison of total intravenous with inhalational anesthesia in terms of postoperative delirium and complications in older patients: a nationwide population-based study. J Anesth. (2022) 36:698–706. doi: 10.1007/s00540-022-03101-3
76. Yang Y, Feng L, Ji C, Lu K, Chen Y, Chen B. Inhalational versus propofol-based intravenous maintenance of anesthesia for emergence delirium in adults: a meta-analysis and trial sequential analysis. J Neurosurg Anesthesiol. (2023) 35:177–86. doi: 10.1097/ANA.0000000000000830
77. Duan GY, Duan ZX, Chen H, Chen F, Chen F, Du ZY, et al. Cognitive function and delirium following sevoflurane or propofol anesthesia for valve replacement surgery: A multicenter randomized controlled trial. Kaohsiung J Med Sci. (2023) 39:166–74. doi: 10.1002/kjm2.12618
78. Farrer TJ, Monk TG, McDonagh DL, Martin G, Pieper CF, Koltai D. A prospective randomized study examining the impact of intravenous versus inhalational anesthesia on postoperative cognitive decline and delirium. Appl Neuropsychol Adult. (2023), 1–7. doi: 10.1080/23279095.2023.2246612
79. Tanaka P, Goodman S, Sommer BR, Maloney W, Huddleston J, Lemmens HJ. The effect of desflurane versus propofol anesthesia on postoperative delirium in elderly obese patients undergoing total knee replacement: a randomized, controlled, double-blinded clinical trial. J Clin Anesth. (2017) 39:17–22. doi: 10.1016/j.jclinane.2017.03.015
80. O’Brien K, Feng R, Sieber F, Marcantonio ER, Tierney A, Magaziner J, et al. Outcomes with spinal versus general anesthesia for patients with and without preoperative cognitive impairment: Secondary analysis of a randomized clinical trial. Alzheimers Dement. (2023) 19:4008–19. doi: 10.1002/alz.13132
81. Neuman MD, Feng R, Carson JL, Gaskins LJ, Dillane D, Sessler DI, et al. Spinal anesthesia or general anesthesia for hip surgery in older adults. New Engl J Med. (2021) 385:2025–35. doi: 10.1056/NEJMoa2113514
82. Tzimas P, Samara E, Petrou A, Korompilias A, Chalkias A, Papadopoulos GJI. The influence of anesthetic techniques on postoperative cognitive function in elderly patients undergoing hip fracture surgery: general vs spinal anesthesia. Injury. (2018) 49:2221–6. doi: 10.1016/j.injury.2018.09.023
83. Li T, Li J, Yuan L, Wu J, Jiang C, Daniels J, et al. Effect of regional vs general anesthesia on incidence of postoperative delirium in older patients undergoing hip fracture surgery: the RAGA randomized trial. JAMA. (2022) 327:50–8. doi: 10.1001/jama.2021.22647
84. Bielka K, Kuchyn I, Tokar I, Artemenko V, Kashchii U. Psoas compartment block efficacy and safety for perioperative analgesia in the elderly with proximal femur fractures: a randomized controlled study. BMC Anesthesiol. (2021) 21:1–6. doi: 10.1186/s12871-021-01473-9
85. Bhushan S, Huang X, Duan Y, Xiao Z. The impact of regional versus general anesthesia on postoperative neurocognitive outcomes in elderly patients undergoing hip fracture surgery: A systematic review and meta-analysis. Int J Surg. (2022) 105:106854. doi: 10.1016/j.ijsu.2022.106854
86. Kamitani K, Higuchi A, Asahi T, Yoshida H. Postoperative delirium after general anesthesia vs. spinal anesthesia in geriatric patients. Masui. (2003) 52:972–5.
87. Zhang A, Chen J, Zhang X, Jiang T, Li D, Cai X, et al. Twelfth thoracic vertebra erector spinae plane block for postoperative analgesia and early recovery after lumbar spine surgery in elderly patients: a single-blind randomized controlled trial. BMC Anesthesiology. (2023) 23:402. doi: 10.1186/s12871-023-02351-2
88. Jin L, Yao R, Heng L, Pang B, Sun F-G, Shen Y, et al. Ultrasound-guided continuous thoracic paravertebral block alleviates postoperative delirium in elderly patients undergoing esophagectomy: a randomized controlled trial. Med (Baltimore). (2020) 99:e19896. doi: 10.1097/MD.0000000000019896
89. Dongjie Q, Longbiao Z, Peng L, Li J, Hongmeng X, Zhiyan C, et al. Effects of thoracic paravertebral nerve block on postoperative pain and postoperative delirium in elderly patients undergoing thoracoscopic lobectomy. Med (Baltimore). (2023) 102:e32907. doi: 10.1097/MD.0000000000032907
90. Lemke KA, Dawson SD. Local and regional anesthesia. Vet Clin North Am Small Anim Pract. (2000) 30:839–57. doi: 10.1016/S0195-5616(08)70010-X
91. Gu J, Wang E, Dai S, Dong R, Xu F, Shen Z, et al. Ultrasound-guided multiple nerve blocks: a safe and effective anesthetic modality in geriatric hip fracture patients. Clin J Pain. (2021) 37:881–6. doi: 10.1097/AJP.0000000000000988
92. Wang L, Wang F, Kang W, Gao G, Liu T, Chen B, et al. Impact of paravertebral block on perioperative neurocognitive disorder: a systematic review and meta-analysis of randomized controlled trials. Front Aging Neurosci. (2023) 15:1237001. doi: 10.3389/fnagi.2023.1237001
93. Luo C, Zou W. Cerebral monitoring of anaesthesia on reducing cognitive dysfunction and postoperative delirium: a systematic review. J Int Med Res. (2018) 46:4100–10. doi: 10.1177/0300060518786406
94. Johansen JW, Sebel PS. Development and clinical application of electroencephalographic bispectrum monitoring. Anesthesiology. (2000) 93:1336–44. doi: 10.1097/00000542-200011000-00029
95. Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesio. (2013) 25:33–42. doi: 10.1097/ANA.0b013e3182712fba
96. Radtke F, Franck M, Lendner J, Krüger S, Wernecke K, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. (2013) 110:i98–i105. doi: 10.1093/bja/aet055
97. Zhou Y, Li Y, Wang K. Bispectral index monitoring during anesthesia promotes early postoperative recovery of cognitive function and reduces acute delirium in elderly patients with colon carcinoma: a prospective controlled study using the attention network test. Med Sci Monit. (2018) 24:7785. doi: 10.12659/MSM.910124
98. Sumner M, Deng C, Evered L, Frampton C, Leslie K, Short T, et al. Processed electroencephalography-guided general anaesthesia to reduce postoperative delirium: a systematic review and meta-analysis. Br J Anaesth. (2023) 130:e243–e53. doi: 10.1016/j.bja.2022.01.006
99. Pérez-Otal B, Aragón-Benedí C, Pascual-Bellosta A, Ortega-Lucea S, Martínez-Ubieto J, Ramírez-Rodríguez JJSR. Neuromonitoring depth of anesthesia and its association with postoperative delirium. Sci Rep. (2022) 12:12703. doi: 10.1038/s41598-022-16466-y
100. Wildes TS, Mickle AM, Abdallah AB, Maybrier HR, Oberhaus J, Budelier TP, et al. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the ENGAGES randomized clinical trial. JAMA. (2019) 321:473–83. doi: 10.1001/jama.2018.22005
101. Sieber FE, Zakriya KJ, Gottschalk A, Blute M-R, Lee HB, Rosenberg PB, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clinic Proc. (2010) 85(1):18–26. doi: 10.4065/mcp.2009.0469
102. Zhu S, Liu Y, Wang X, Wang L, Li J, Xue X, et al. Different sedation strategies in older patients receiving spinal anesthesia for hip surgery on postoperative delirium: A randomized clinical trial. Drug Des Devel Ther. (2023) 17:3845–54. doi: 10.2147/DDDT.S439543
103. Reznik ME, Slooter AJC. Delirium management in the ICU. Curr Treat Options Neurol. (2019) 21:1–18. doi: 10.1007/s11940-019-0599-5
104. Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain–how well is it measured? Basic Clin Pharmacol Toxicol. (2014) 114:151–9. doi: 10.1111/bcpt.12140
105. Mo Y, Zimmermann AE. Role of dexmedetomidine for the prevention and treatment of delirium in intensive care unit patients. Ann Pharmacother. (2013) 47:869–76. doi: 10.1345/aph.1AR708
106. Lin N, Vutskits L, Bebawy JF, Gelb AW. Perspectives on dexmedetomidine use for neurosurgical patients. J Neurosurg Anesthesiol. (2019) 31:366–77. doi: 10.1097/ANA.0000000000000554
107. Weerink MA, Struys MM, Hannivoort LN, Barends CR, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. (2017) 56:893–913. doi: 10.1007/s40262-017-0507-7
108. Mei B, Meng G, Xu G, Cheng X, Chen S, Zhang Y, et al. Intraoperative sedation with dexmedetomidine is superior to propofol for elderly patients undergoing hip arthroplasty: a prospective randomized controlled study. Clin J Pain. (2018) 34:811–7. doi: 10.1097/AJP.0000000000000605
109. Aydogan MS, Korkmaz MF, Ozgül U, Erdogan MA, Yucel A, Karaman A, et al. Pain, fentanyl consumption, and delirium in adolescents after scoliosis surgery: dexmedetomidine vs midazolam. Paediatr Anaesth. (2013) 23:446–52. doi: 10.1111/pan.12128
110. Wang X, Wang J, Mu D, Wang DX. Dexmedetomidine combined with ropivacaine for continuous femoral nerve block improved postoperative sleep quality in elderly patients after total knee arthroplasty. Zhonghua Yi Xue Za Zhi. (2018) 98:728–32. doi: 10.3760/cma.j.issn.0376-2491.2018.10.003
111. Mei B, Xu G, Han W, Lu X, Liu R, Cheng X, et al. The benefit of dexmedetomidine on postoperative cognitive function is unrelated to the modulation on peripheral inflammation: a single-center, prospective, randomized study. Clin J Pain. (2020) 36:88–95. doi: 10.1097/AJP.0000000000000779
112. Shin H-J, Woo Nam S, Kim H, Yim S, Han S-H, Hwang J-W, et al. Postoperative delirium after dexmedetomidine versus propofol sedation in healthy older adults undergoing orthopedic lower limb surgery with spinal anesthesia: a randomized controlled trial. Anesthesiology. (2023) 138:164–71. doi: 10.1097/ALN.0000000000004488
113. Niu J-Y, Yang N, Tao Q-Y, He Y, Hou Y-B, Ning R-D, et al. Effect of different administration routes of dexmedetomidine on postoperative delirium in elderly patients undergoing elective spinal surgery: a prospective randomized double-blinded controlled trial. Anesth Analg. (2023) 136:1075–83. doi: 10.1213/ANE.0000000000006464
114. Zeng H, Li Z, He J, Fu W. Dexmedetomidine for the prevention of postoperative delirium in elderly patients undergoing noncardiac surgery: a meta-analysis of randomized controlled trials. PloS One. (2019) 14:e0218088. doi: 10.1371/journal.pone.0218088
115. Aucamp AK. Aspects of the pharmacokinetics and pharmacodynamics of benzodiazepines with particular reference to clobazam. Drug Development Research (1982) 2:117–26. doi: 10.1002/(ISSN)1098-2299
116. Wagner BK, Zavotsky KE, Sweeney JB, Palmeri BA, Hammond JS. Patient recall of therapeutic paralysis in a surgical critical care unit. Pharmacotherapy. (1998) 18:358–63. doi: 10.1002/j.1875-9114.1998.tb03862.x
117. Poeran J, Cozowicz C, Zubizarreta N, Weinstein SM, Deiner SG, Leipzig RM, et al. Modifiable factors associated with postoperative delirium after hip fracture repair: an age-stratified retrospective cohort study. Eur J Anaesthesiol. (2020) 37:649–58. doi: 10.1097/EJA.0000000000001197
118. Lertkovit S, Siriussawakul A, Suraarunsumrit P, Lertpipopmetha W, Manomaiwong N, Wivatdechakul W, et al. Polypharmacy in older adults undergoing major surgery: prevalence, association with postoperative cognitive dysfunction and potential associated anesthetic agents. Front Med (Lausanne). (2022) 9:811954. doi: 10.3389/fmed.2022.811954
119. Breschan C, Platzer M, Jost R, Stettner H, Likar RJPA. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatr Anaesth. (2007) 17:347–52. doi: 10.1111/j.1460-9592.2006.02101.x
120. Omichi C, Ayani N, Oya N, Matsumoto Y, Tanaka M, Morimoto T, et al. Association between discontinuation of benzodiazepine receptor agonists and post-operative delirium among inpatients with liaison intervention: A retrospective cohort study. Compr Psychiatry. (2021) 104:152216. doi: 10.2147/DDDT.S392569
121. Yang J-J, Lei L, Qiu D, Chen S, Xing L-K, Zhao J-W, et al. Effect of remimazolam on postoperative delirium in older adult patients undergoing orthopedic surgery: a prospective randomized controlled clinical trial. Drug Des Devel Ther. (2023) 17:143–53. doi: 10.2147/DDDT.S392569
122. Singla L, Mathew PJ, Jain A, Yaddanapudi S, Peters NJ. Oral melatonin as part of multimodal anxiolysis decreases emergence delirium in children whereas midazolam does not: A randomised, double-blind, placebo-controlled study. Eur J Anaesthesiology. (2021) 38:1130–7. doi: 10.1097/eja.0000000000001561
123. Ma JH, Liu YF, Hong H, Li CJ, Cui F, Mu DL, et al. Effect of acute pain on the association between preoperative cognitive impairment and postoperative delirium: a secondary analysis of three trials. Br J Anaesthesia. (2023) 130:e272–e80. doi: 10.1016/j.bja.2022.06.033
124. Duprey MS, Dijkstra-Kersten SMA, Zaal IJ, Briesacher BA, Saczynski JS, Griffith JL, et al. Opioid use increases the risk of delirium in critically ill adults independently of pain. Am J Respir Crit Care Med. (2021) 204:566–72. doi: 10.1164/rccm.202010-3794OC
125. Daoust R, Paquet J, Boucher V, Pelletier M, Gouin É, Émond M. Relationship between pain, opioid treatment, and delirium in older emergency department patients. Acad Emergency Med. (2020) 27:708–16. doi: 10.1111/acem.14033
126. Morrison RS, Magaziner J, Gilbert M, Koval KJ, McLaughlin MA, Orosz G, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. Journals Gerontology Ser A Biol Sci Med Sci. (2003) 58:76–81. doi: 10.1093/gerona/58.1.m76
127. Garimella V, Cellini C. Postoperative pain control. Clinics Colon And Rectal Surg. (2013) 26:191–6. doi: 10.1055/s-0033-1351138
128. Kroll PB, Meadows L, Rock A, Pavliv L. A multicenter, randomized, double-blind, placebo-controlled trial of intravenous ibuprofen (i.v.-ibuprofen) in the management of postoperative pain following abdominal hysterectomy. Pain Pract. (2011) 11:23–32. doi: 10.1111/j.1533-2500.2010.00402.x
129. Mu DL, Zhang DZ, Wang DX, Wang G, Li CJ, Meng ZT, et al. Parecoxib supplementation to morphine analgesia decreases incidence of delirium in elderly patients after hip or knee replacement surgery: A randomized controlled trial. Anesth Analgesia. (2017) 124:1992–2000. doi: 10.1213/ane.0000000000002095
130. Li JZ, Li XZ, Wang XM, Wang MS, Yu HF, Shi F, et al. Effects of parecoxib sodium analgesia on serum concentrations of neuron-specific enolase and S-100β and postoperative cognitive function of elderly patients undergoing acute replacement of femoral head. Zhonghua Yi Xue Za Zhi. (2013) 93:2152–4.
131. Clemmesen CG, Tavenier J, Andersen O, Palm H, Foss NB. Methylprednisolone and inflammatory stress response in older people undergoing surgery for hip fracture: a secondary analysis of a randomized controlled trial. Eur Geriatric Med. (2019) 10:913–21. doi: 10.1007/s41999-019-00231-y
132. Kenna HA, Poon AW, de los Angeles CP, Koran LM. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. (2011) 65:549–60. doi: 10.1111/j.1440-1819.2011.02260.x
133. Huang JW, Yang YF, Gao XS, Xu ZH. A single preoperative low-dose dexamethasone may reduce the incidence and severity of postoperative delirium in the geriatric intertrochanteric fracture patients with internal fixation surgery: an exploratory analysis of a randomized, placebo-controlled trial. J Orthopaedic Surg Res. (2023) 18:441. doi: 10.1186/s13018-023-03930-2
134. Clemmesen CG, Lunn TH, Kristensen MT, Palm H, Foss NB. Effect of a single pre-operative 125 mg dose of methylprednisolone on postoperative delirium in hip fracture patients; a randomised, double-blind, placebo-controlled trial. Anaesthesia. (2018) 73:1353–60. doi: 10.1111/anae.14406
135. Sakic L, Tonkovicc D, Hrgovic Z, Klasan A. Spinal dexamethasone effect on cognitive disorders after hip surgery. Med Arch. (2023) 77:18–23. doi: 10.5455/medarh.2023.77.18-23
136. Awada HN, Steinthorsdottir KJ, Schultz NA, Hillingsø JG, Larsen PN, Jans Ø, et al. High-dose preoperative glucocorticoid for prevention of emergence and postoperative delirium in liver resection: A double-blinded randomized clinical trial substudy. Acta Anaesthesiologica Scandinavica. (2022) 66:696–703. doi: 10.1111/aas.14057
137. Kluger MT, Skarin M, Collier J, Rice DA, McNair PJ, Seow MY, et al. Steroids to reduce the impact on delirium (STRIDE): a double-blind, randomised, placebo-controlled feasibility trial of pre-operative dexamethasone in people with hip fracture. Anaesthesia. (2021) 76:1031–41. doi: 10.1111/anae.15465
138. Li LQ, Wang C, Fang MD, Xu HY, Lu HL, Zhang HZ. Effects of dexamethasone on post-operative cognitive dysfunction and delirium in adults following general anaesthesia: a meta-analysis of randomised controlled trials. BMC Anesthesiology. (2019) 19:113. doi: 10.1186/s12871-019-0783-x
139. Muller P, Seeman P. Brain neurotransmitter receptors after long-term haloperidol: dopamine, acetylcholine, serotonin, alpha-noradrenergic and naloxone receptors. Life Sci. (1977) 21:1751–8. doi: 10.1016/0024-3205(77)90155-2
140. Puspitasari IM, Sinuraya RK, Rahayu C, Witriani W, Zannah U, Hafifah A, et al. Medication profile and treatment cost estimation among outpatients with schizophrenia, bipolar disorder, depression, and anxiety disorders in Indonesia. Neuropsychiatr Dis Treat. (2020) 16:815–28. doi: 10.2147/ndt.S240058
141. Prommer E. Role of haloperidol in palliative medicine: an update. Am J Hospice Palliative Care. (2012) 29:295–301. doi: 10.1177/1049909111423094
142. Fukata S, Kawabata Y, Fujishiro K, Kitagawa Y, Kuroiwa K, Akiyama H, et al. Haloperidol prophylaxis for preventing aggravation of postoperative delirium in elderly patients: a randomized, open-label prospective trial. Surg Today. (2017) 47:815–26. doi: 10.1007/s00595-016-1441-2
143. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, Vreeswijk R, Egberts TC, Burger BJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatrics Soc. (2005) 53:1658–66. doi: 10.1111/j.1532-5415.2005.53503.x
144. Khan BA, Perkins AJ, Campbell NL, Gao S, Khan SH, Wang S, et al. Preventing postoperative delirium after major noncardiac thoracic surgery-A randomized clinical trial. J Am Geriatrics Soc. (2018) 66:2289–97. doi: 10.1111/jgs.15640
145. Yang YP, Ding YY, Wang YY, Wang WW, Ye KP, Ye QG, et al. Effects of preoperative quetiapine on postoperative delirium and sleep quality in elderly orthopaedic patients. Zhonghua Yi Xue Za Zhi. (2023) 103:3252–7. doi: 10.3760/cma.j.cn112137-20230719-00029
146. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesthesia Intensive Care. (2007) 35:714–9. doi: 10.1177/0310057x0703500509
147. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. (2012) 116:987–97. doi: 10.1097/ALN.0b013e31825153cc
148. Larsen KA, Kelly SE, Stern TA, Bode RH Jr., Price LL, Hunter DJ, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. (2010) 51:409–18. doi: 10.1176/appi.psy.51.5.409
149. Jain FA, Brooks JO 3rd, Larsen KA, Kelly SE, Bode RH, Sweeney GA, et al. Individual risk profiles for postoperative delirium after joint replacement surgery. Psychosomatics. (2011) 52:410–6. doi: 10.1016/j.psym.2011.03.011
150. Tomás-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res. (2005) 39:99–104. doi: 10.1111/j.1600-079X.2005.00248.x
151. Reiter RJ, Tan DX, Pappolla MA. Melatonin relieves the neural oxidative burden that contributes to dementias. Ann New York Acad Sci. (2004) 1035:179–96. doi: 10.1196/annals.1332.012
152. Gulcin I, Buyukokuroglu ME, Kufrevioglu OI. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J Pineal Res. (2003) 34:278–81. doi: 10.1034/j.1600-079x.2003.00042.x
153. Reiter R, Tang L, Garcia JJ, Muñoz-Hoyos A. Pharmacological actions of melatonin in oxygen radical pathophysiology. Life Sci. (1997) 60:2255–71. doi: 10.1016/s0024-3205(97)00030-1
154. Kast RE. Agomelatine or ramelteon as treatment adjuncts in glioblastoma and other M1- or M2-expressing cancers. Contemp Oncol. (2015) 19:157–62. doi: 10.5114/wo.2015.51421
155. Jumnongprakhon P, Govitrapong P, Tocharus C, Tocharus J. Melatonin promotes blood-brain barrier integrity in methamphetamine-induced inflammation in primary rat brain microvascular endothelial cells. Brain Res. (2016) 1646:182–92. doi: 10.1016/j.brainres.2016.05.049
156. Campbell AM, Axon DR, Martin JR, Slack MK, Mollon L, Lee JK. Melatonin for the prevention of postoperative delirium in older adults: a systematic review and meta-analysis. BMC Geriatrics. (2019) 19:272. doi: 10.1186/s12877-019-1297-6
157. Kain ZN, MacLaren JE, Herrmann L, Mayes L, Rosenbaum A, Hata J, et al. Preoperative melatonin and its effects on induction and emergence in children undergoing anesthesia and surgery. Anesthesiology. (2009) 111:44–9. doi: 10.1097/ALN.0b013e3181a91870
158. Hokuto D, Nomi T, Yoshikawa T, Matsuo Y, Kamitani N, Sho M. Preventative effects of ramelteon against postoperative delirium after elective liver resection. PloS One. (2020) 15:e0241673. doi: 10.1371/journal.pone.0241673
159. Shi Y. Effects of melatonin on postoperative delirium after PCI in elderly patients: A randomized, single-center, double-blind, placebo-controlled trial. Heart Surg Forum. (2021) 24:E893–e7. doi: 10.1532/hsf.4049
160. Ford AH, Flicker L, Kelly R, Patel H, Passage J, Wibrow B, et al. The healthy heart-mind trial: randomized controlled trial of melatonin for prevention of delirium. J Am Geriatrics Soc. (2020) 68:112–9. doi: 10.1111/jgs.16162
161. Kinouchi M, Mihara T, Taguri M, Ogura M. The efficacy of ramelteon to prevent postoperative delirium after general anesthesia in the elderly: A double-blind, randomized, placebo-controlled trial. Am J Geriatric Psychiatry. (2023) 31:1178–89. doi: 10.1016/j.jagp.2023.07.011
162. Oh ES, Leoutsakos JM, Rosenberg PB, Pletnikova AM, Khanuja HS, Sterling RS, et al. Effects of ramelteon on the prevention of postoperative delirium in older patients undergoing orthopedic surgery: the RECOVER randomized controlled trial. Am J Geriatric Psychiatry. (2021) 29:90–100. doi: 10.1016/j.jagp.2020.05.006
163. Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. (1982) 217:408–14. doi: 10.1126/science.7046051
164. Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. Journals Gerontology Ser A Biol Sci Med Sci. (2008) 63:764–72. doi: 10.1093/gerona/63.7.764
165. Heinrich M, Sieg M, Kruppa J, Nürnberg P, Schreier PH, Heilmann-Heimbach S, et al. Association between genetic variants of the cholinergic system and postoperative delirium and cognitive dysfunction in elderly patients. BMC Med Genomics. (2021) 14:248. doi: 10.1186/s12920-021-01071-1
166. Youn YC, Shin HW, Choi BS, Kim S, Lee JY, Ha YC. Rivastigmine patch reduces the incidence of postoperative delirium in older patients with cognitive impairment. Int J Geriatric Psychiatry. (2017) 32:1079–84. doi: 10.1002/gps.4569
167. Massoudi N, Mohit B, Fathi M, Nooraei N, Hannani KK, ArianNik M. The impact of rivastigmine on post-surgical delirium and cognitive impairment; a randomized clinical trial. Int J Geriatric Psychiatry. (2023) 38:e5970. doi: 10.1002/gps.5970
168. Spies CD, Knaak C, Mertens M, Brockhaus WR, Shadenok A, Wiebach J, et al. Physostigmine for prevention of postoperative delirium and long-term cognitive dysfunction in liver surgery: A double-blinded randomised controlled trial. Eur J Anaesthesiology. (2021) 38:943–56. doi: 10.1097/eja.0000000000001456
169. Liu F, Lin X, Lin Y, Deng X, Guo Y, Wang B, et al. The effect of neostigmine on postoperative delirium after colon carcinoma surgery: a randomized, double-blind, controlled trial. BMC Anesthesiology. (2022) 22:267. doi: 10.1186/s12871-022-01804-4
170. Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post-surgical delirium. Am J Geriatric Psychiatry. (2005) 13:1100–6. doi: 10.1176/appi.ajgp.13.12.1100
171. Inouye SK, Robinson T, Blaum C, Busby-Whitehead J, Boustani M, Chalian A, et al. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surgeons. (2015) 220:136–48e1. doi: 10.1016/j.jamcollsurg.2014.10.019
172. Kim EM, Li G, Kim M. Development of a risk score to predict postoperative delirium in hip fracture patients. Anesth Analgesia. (2020) 130:79. doi: 10.1213/ANE.0000000000004386
173. Oberai T, Oosterhoff JH, Woodman R, Doornberg JN, Kerkhoffs G, Jaarsma R. Development of a postoperative delirium risk scoring tool using data from the Australian and New Zealand Hip Fracture Registry: an analysis of 6672 patients 2017-2018. Arch Gerontology Geriatrics. (2021) 94:104368. doi: 10.1016/j.archger.2021.104368
Keywords: delirium, perioperative medication, anesthesia, hip fractures, prevention, treatment
Citation: Lan S, Liang S, Wu H, Deng S, Sun K, Ye C, Yang L, Ciren L and Li J (2024) Strategies to prevent postoperative delirium: a comprehensive evaluation of anesthesia selection and drug intervention. Front. Psychiatry 15:1518460. doi: 10.3389/fpsyt.2024.1518460
Received: 28 October 2024; Accepted: 02 December 2024;
Published: 23 December 2024.
Edited by:
Cristiano Capurso, University of Foggia, ItalyReviewed by:
Antonio Leo, Istituto Santa Chiara, ItalyPallavi Joshi, Banner Alzheimer’s Institute, United States
Copyright © 2024 Lan, Liang, Wu, Deng, Sun, Ye, Yang, Ciren and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lunzhu Ciren, Q3JsdW56aHUyMDAwQDE2My5jb20=; Jun Li, YXlkbGlqdW5AYWhtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Shaoze Lan1†
Shaoze Lan1† Jun Li
Jun Li