- 1Department of Psychosomatic Medicine and Psychotherapy, Riga Stradiņš University, Riga, Latvia
- 2Department of Depression and Crisis, National Centre of Mental Health, Riga, Latvia
- 3Faculty of Medicine, Riga Stradiņš University, Riga, Latvia
Severe acute respiratory sindrome - Coronavirus - 2 (SARS-CoV-2) (Coronavirus disease - 19 (COVID-19)) infection can result in long-term health consequences, such as long COVID. The clinical manifestations of long COVID include depression, anxiety, brain fog with cognitive dysfunction, memory issues, and fatigue. However, the links between vaccination and psychiatric disorders have been less studied. This article describes three patients who reported anxiety after receiving a complete course of the Pfizer-BioNTech BNT162b2 vaccine. It is important to explore the relationship between anxiety, other mental health disorders, and COVID-19 vaccination, as well as to investigate potential pathogenetic mechanisms.
1 Introduction
Currently, the persisting symptoms of SARS-CoV-2 (COVID-19) infection have received considerable clinical interest (1), with research focusing on both the early and later stages of the COVID-19, including long-lasting symptoms in some patients (2, 3). The inflammatory process, particularly cytokine storms, has also attracted significant attention, and researchers are currently investigating the persistence of symptoms in COVID-19 patients (4). The selective serotonin reuptake inhibitors (SSRIs), a group of antidepressant medications, have been reported to alleviate clinical symptoms observed during COVID-19 infection (5). However, in addition to examining the history and therapeutic approaches used to treat long COVID, the possibility of serotonin dysregulation (6) should be considered. An imbalance of the biogenic amine neurotransmitter serotonin is one of the primary hypotheses underlying the causes of anxiety and depression. However, the impact of vaccines on psychiatric symptoms remains unclear.
Various studies have shown that all approved COVID-19 vaccines are effective, safe, and immunogenic (7). Additionally, cohort studies examining the impact of COVID-19 vaccination on mental health have revealed increased risks of depression, anxiety, dissociative disorders, stress-related and somatoform disorders, as well as sleep disorders. Conversely, a reduction in the incidence and risk of schizophrenia and bipolar disorder has also been observed (8). However, psychiatric adverse effects associated with COVID-19 vaccination have not been sufficiently studied, and there is limited clarity regarding the impact of vaccines on psychiatric symptoms. In this report, we present a case series of three patients who reported experiencing anxiety after completing the vaccination schedule with the Pfizer-BioNTech BNT162b2 vaccine (9).
2 Case presentation
The inpatient unit for depression and crisis at the National Centre for Mental Health, which serves approximately 800,000 residents of Riga, the capital of Latvia, admitted three patients within a single month. These patients, all women aged 55 to 73, self-reported developing symptoms of anxiety and depression after receiving the Pfizer-BioNTech (BNT162b2) COVID-19 vaccine. The time between vaccination and the next hospitalization varied among the three patients (6, 16, and 33 months). Each patient had recovered from COVID-19 with no lasting consequences and had received both the Pfizer-BioNTech (BNT162b2) COVID-19 vaccine and a booster dose, after which anxiety and depression developed within 24 h. The number of episodes of depression and anxiety also varied between the cases.
3 Background history
Patient I is a 73-year-old woman of Latvian ethnicity, with primary education, from the Riga region. She reported experiencing episodic sleep disturbances and mild anxiety prior to the pandemic, which were diagnosed in 2018.
Patient II is a 67-year-old woman of Russian ethnicity, with secondary education, from the Riga region. She has undergone three hospitalizations. Prior to her postvaccination episode of anxiety and depression, she experienced two depressive episodes in 2012 and 2018, although inpatient therapy was not necessary for either of those previous episodes.
Patient III is a 55-year-old woman of Latvian ethnicity, with secondary education, from the Riga region. She reported a family history of psychiatric illness and a head injury at the age of 20. She has struggled with alcohol abuse and displayed a sluggish, deliberate manner. She began treatment for depression in 2009 after consulting a psychotherapist. In 2021, she experienced the first of four depressive episodes, all of which required hospitalization.
The anamnestic data for all cases are presented in Table 1.
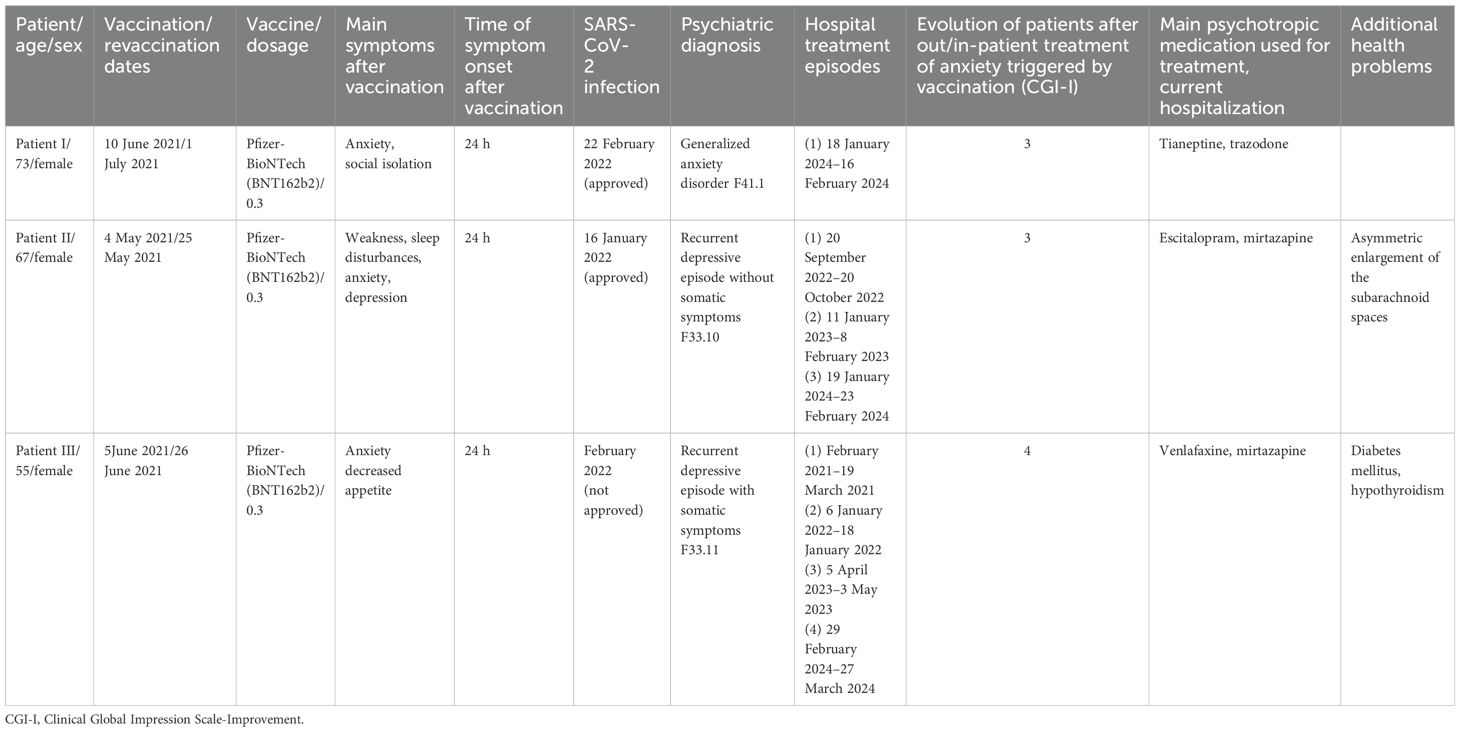
Table 1. Primary anamnestic information (vaccination, main symptoms, SARS-CoV-2 infection date, psychiatric diagnosis, hospital treatment episodes, medications given, and comorbidities) for the three patients.
4 Treatment episodes
4.1 Patient I
Patient I experienced an increase in anxiety within 24 h of receiving the vaccine on 10 June 2021 and a booster on 1 July 2021. The clinical manifestations included feeling anxious, constant worrying, difficulty relaxing, irritability, avoidance behaviors, chest pain, and elevated blood pressure.
The diagnosis of generalized anxiety disorder was made based on the diagnostic criteria of the International Statistical Classification of Diseases and Related Health Problems: Tenth Revision (ICD-10) (10) and the validated 7-Question Generalized Anxiety Disorder Scale (GAD-7) (11), as recommended by local clinical guidelines.
Patient I received outpatient treatment with medications (SSRI antidepressants, antihypertensives, and benzodiazepines), which led to minor clinical stabilization and a decrease in symptom intensity for about 27 months, followed by a worsening of symptoms in the last 6 months prior to hospitalization.
The patient was treated for 29 days at an inpatient unit with both pharmaceutical and nonpharmaceutical therapies (visual arts, music, theater, dance movement therapy, psychotherapy, and occupational therapy). Trazodone and tianeptine were administered to treat the patient’s anxiety, resulting in an improvement in her condition. Age-specific and nonsignificant changes were observed in subsequent examinations (computed tomography, electroencephalography, and transcranial and intracranial vessel duplex).
4.2 Patient II
The patient reported a significant decline in her health, along with anxiety and depression, following the administration of the Pfizer vaccine on 4 May 2021, and a booster on 25 May 2021.
After receiving the booster, the patient experienced another bout of anxiety and depression within 24 h, accompanied by persistent worrying, difficulty relaxing, restlessness, weakness, an inability to leave home due to anxiety, and loss of appetite, resulting in a weight loss of 10 kg.
The diagnosis of depressive episode without somatic symptoms was made based on applied diagnostic criteria of the ICD-10 and the validated Patient Health Questionnaire 9-Item Scale (PHQ-9) for depression and the GAD-7 for anxiety disorders, as recommended by local clinical guidelines.
The patient used antidepressants for a short period, experiencing some improvement.
In September 2022, 16 months after receiving the Pfizer vaccine, the patient was hospitalized for the first time due to increased anxiety. After 30 days of hospitalization, the patient’s condition improved with the daily administration of 20 mg of escitalopram.
The patient was re-hospitalized in 2023 due to tremors and extreme anxiety. After 28 days of hospitalization, her anxiety subsided with the daily administration of 30 mg of mirtazapine.
In 2024, the patient was hospitalized for a third time, and electroencephalography revealed the presence of encephalopathy.
4.3 Patient III
The patient attributed her second episode of depression and the onset of anxiety to receiving the vaccine on 5 June 2021, followed by the booster on 26 June 2021.
After receiving the COVID-19 vaccine (vaccine 2), the patient developed anxiety. The symptoms appeared within 24 h and included persistent worry, difficulty relaxing, irritability, restlessness, shaking of hands, and chest pain.
The diagnosis of recurrent depressive episode without somatic symptoms was made based on the diagnostic criteria from the ICD-10 and the validated PHQ-9 for depression and the GAD-7 for anxiety disorders, as recommended by local clinical guidelines.
The patient was hospitalized 6 months after vaccination in 2022 due to severe anxiety and depression. She was treated in a hospital ward for 22 days, with a daily dosage of 225 mg of venlafaxine and 15 mg of mirtazapine, resulting in improvement of her condition.
The patient was treated for a third time in 2023 in the hospital for 20 days due to depression and anxiety, with improvement in her condition. She has been diagnosed with diabetes mellitus.
In 2024, the patient was admitted to a psychiatric hospital for the fourth time for 27 days due to depression, low mood, and suicidal thoughts. The administration of vortioxetine (20 mg) showed promising results.
4.4 Current status
Following their last hospitalizations, all of the patients showed improvement and decreased anxiety levels. Currently, all the patients continue to take their medication and visit a psychiatrist on an outpatient basis.
5 Discussion
In general, COVID-19 vaccination is not associated with psychiatric adverse events, according to the meta-analysis (12).
Nevertheless, there is new evidence suggesting a risk that the COVID-19 vaccine administered to patients with pre-existing mental illness may contribute to their hospitalization (13), potentially as an anxiety response to the act of vaccination (14). Furthermore, a depressive episode occurring prior to receiving the Pfizer-BioNTech COVID-19 vaccine is a negative predictor of antibody titers (15).
Different mechanisms may play a role in the development of anxiety, including inflammatory processes, changes in psychotropic metabolism, autoimmune responses, and stress related to the vaccination itself (16).
In all three presented cases, the patients received a full course of the Pfizer-BioNTech (BNT162b2) vaccine between May and July of 2021 and subsequently developed anxiety.
Common aspects of all three cases include the same vaccine, pre-existing psychiatric symptoms, relatively severe symptoms of anxiety, and a prolonged recovery process.
Comorbidities and life events (e.g., head trauma, substance use such as alcohol and benzodiazepines) will be important aspects to consider in future surveys.
Moreover, each patient independently and actively reported their anxiety without prompting from a doctor. However, reports linking vaccination to the development of anxiety should be assessed with caution (17).
5.1 Limitations
The underlying cause of anxiety in these cases may not be connected to the Pfizer-BioNTech (BNT162b2) vaccination course, and no strong similarities were identified between the three cases. Patients may have attributed any health problems they experienced to either COVID-19 or the vaccination, without regard to evidence.
6 Conclusion
There may be a connection between the Pfizer-BioNTech (BNT162b2) COVID-19 vaccine and the development of anxiety symptoms in patients with pre-existing mental health problems. The potential underlying mechanism warrants further investigation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
No ethical committee decision was required for this case report. The studies were conducted in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MT: Conceptualization, Writing – original draft, Writing – review & editing. AL: Investigation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author has received financial benefits for participation in boards and as a speaker from the following pharmaceutical companies: Lundbeck, Janssen-Cilag, Gedeon Richter, Johnson & Johnson, Olainfarm, Grindex, and Medochemie.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nobile B, Durand M, Olie E, Gullaume S, Moles JP, Haffen E, et al. The anti-inflammatory effect of the tricyclic antidepressant clomipramine and its high penetration in the brain might be useful to prevent the psychiatric consequences of SARS-CoV-2 infection. Front Pharmacol. (2021) 12:615695. doi: 10.3389/fphar.2021.615695
2. D’Hondt S, Gisle L, De Pauw R, Van Cauteren D, Demarest S, Drieskens S, et al. Anxiety and depression in people with post-COVID condition: a Belgian population-based cohort study three months after SARS-CoV-2 infection. Soc Psychiatry Psychiatr Epidemiol. (2024) 59:2083–92. doi: 10.1007/s00127-024-02655-9
3. Taube M. Depression and brain fog as long-COVID mental health consequences: Difficult, complex and partially successful treatment of a 72-year-old patient-A case report. Front Psychiatry. (2023) 14:1153512. doi: 10.3389/fpsyt.2023.1153512
4. Shetty PA, Ayari L, Madry J, Betts C, Robinson DM, Kirmani BF. The relationship between COVID-19 and the development of depression: implications on mental health. Neurosci Insights. (2023) 18:26331055231191513. doi: 10.1177/26331055231191513
5. Gouda A, Mégarbane B. Molecular bases of serotonin reuptake inhibitor antidepressant-attributed effects in COVID-19: A new insight on the role of Bradykinins. J Pers Med. (2022) 12:1487. doi: 10.3390/jpm12091487
6. Wong A, Devason AS, Umana IC, Cox TO, Dohnalova L, Litichevskiy L, et al. Serotonin reduction in post-acute sequelae of viral infection. Cell. (2023) 186:4851–4867.e20. doi: 10.1016/j.cell.2023.09.013
7. Sharif N, Alzahrani KJ, Ahmed SN, Dey SK. Efficacy, immunogenicity and safety of COVID-19 vaccines: A systematic review and meta-analysis. Front Immunol. (2021) 12:714170. doi: 10.3389/fimmu.2021.714170
8. Kim HJ, Kim MH, Choi MG, Chun EM. Psychiatric adverse events following COVID-19 vaccination: a population-based cohort study in Seoul, South Korea. Mol Psychiatry. (2024) 29:3635–43. doi: 10.1038/s41380-024-02627-0
9. Teo SP. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J Pharm Pract. (2022) 35:947–51. doi: 10.1177/08971900211009650
10. World Health Organization. ICD-10: international statistical classification of diseases and related health problems: tenth revision. 2nd ed. Geneva, Switzerland: World Health Organization (2004). Available at: https://iris.who.int/handle/10665/42980.
11. Vrublevska J, Renemane L, Kivite-Urtane A, Rancans E. Validation of the generalized anxiety disorder scales (GAD-7 and GAD-2) in primary care settings in Latvia. Front Psychiatry. (2022) 13:972628. doi: 10.3389/fpsyt.2022.972628
12. Lee SE, Shim SR, Youn JH, Han HW. COVID-19 vaccination is not associated with psychiatric adverse events: A meta-analysis. Vaccines (Basel). (2023) 11:194. doi: 10.3390/vaccines11010194
13. Yiu HHE, Yan VKC, Wei Y, Ye X, Huang C, Castle DJ, et al. Risks of COVID-19-related hospitalisation and mortality among individuals with mental disorders following BNT162b2 and CoronaVac vaccinations: A case-control study. Psychiatry Res. (2023) 329:115515. doi: 10.1016/j.psychres.2023.115515
14. Parrino D, Frosolini A, Gallo C, De Siati RD, Spinato G, de Filippis C. Tinnitus following COVID-19 vaccination: report of three cases. Int J Audiol. (2022) 61:526–9. doi: 10.1080/14992027.2021.1931969
15. Kaneko H, Tsuboi H. Depressive symptoms predict antibody titers after a second dose of the SARS-CoV-2 BNT162b2 vaccine among hospital workers in Japan. Brain Behav Immun. (2023) 107:414–8. doi: 10.1016/j.bbi.2022.09.004
16. Mopuru R, Menon V. COVID-19 vaccine-related psychiatric adverse events: Mechanisms and considerations. Asian J Psychiatr. (2023) 79:103329. doi: 10.1016/j.ajp.2022.103329
Keywords: COVID-19 vaccines, SARS-CoV-2 vaccine, BioNTech162 vaccine, anxiety, case reports
Citation: Taube M and Lesiņa AA (2025) Case report: Development of anxiety symptoms after receiving the Pfizer-BioNTech (BNT162b2) COVID-19 vaccine: a case series. Front. Psychiatry 15:1514428. doi: 10.3389/fpsyt.2024.1514428
Received: 20 October 2024; Accepted: 26 December 2024;
Published: 22 January 2025.
Edited by:
Shinsuke Hidese, Teikyo University, JapanReviewed by:
Mario Di Fiorino, Psychiatry of Versilia Hospital, ItalyNelson Luis Cahuapaza-Gutierrez, Scientific University of the South, Peru
Copyright © 2025 Taube and Lesiņa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maris Taube, bWFyaXMudGF1YmVAcnN1Lmx2
 Maris Taube
Maris Taube Alise Alma Lesiņa3
Alise Alma Lesiņa3