
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 13 January 2025
Sec. Mood Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1506726
Objective: Studies have shown associations between Body Mass Index (BMI), High-Sensitivity C-reactive protein (HSCRP), and depressive symptoms(DP). However, the complex relationship between them remains uncertain. The objective of this research is to examine the correlation between them in a substantial sample that is representative of the national level.
Methods: Our analysis was based on the 2015-2016National Health and Nutrition Examination Survey (NHANES).DP was measured by the Patient Health Questionnaire-9 (PHQ-9). Using multivariable logistic regression analysis and stratified analysis, we examined the relationship between BMI, HSCRP, and DP. We applied generalized additive models to explore the non-linear relationships among variables.
Results: This study included a total of 4834 participants. The results revealed that BMI (P=0.002) and HSCRP (P=0.008) were risk factors for DP. The relationship between BMI and DP (P=0.035), BMI and HSCRP (P<0.001) were non-linear. The nonlinear association between HSCRP and DP (P=0.031), BMI and DP (P=9e-04) is significant in females when stratified by gender. No nonlinear association was found between BMI and DP (P =0.677) and between HSCRP and DP (P =0.439) in males. The results of the interaction test reveal a significant interaction between HSCRP and gender.
Conclusions: Research has found both BMI and HSCRP are risk factors for DP and the relationship between them was non-linear. The nonlinear associations between BMI and DP, as well as between HSCRP and DP, are gender-dependent.
Depression is a mental disorder that causes functional disabilities and a lack of interest in daily activities, affecting more than 260 million people around the world (1, 2). It is widely known that several factors contribute to depression’s etiology, with obesity playing a substantial role as a contributing factor. The number of obese individuals worldwide exceeds 650 million according to current data, as reported by the World Health Organization. Studies suggest that obesity is linked to depressive symptoms (DP) (3–5). BMI, as a commonly used indicator for assessing the level of obesity in individuals, has been widely employed in research examining the impact of obesity on health (6). Additionally, a study found that adults with high HSCRP were more likely to have depressive symptoms compared to those with low HSCRP (7).
The pathogenesis of DP may also be influenced by inflammation. Inflammation leads to the release of C-reactive protein (CRP) by the liver. Depression and CRP may be linked (8). High-sensitivity CRP (HSCRP) is a more precise biomarker for detecting low-grade systemic inflammation compared to C-reactive protein. The American Heart Association recognizes a threshold of 3 mg/L for low-grade inflammation (9). Research has demonstrated that persistent low-level peripheral inflammation can trigger sustained neuroinflammatory responses, potentially leading to changes in brain structure and function (10). Additionally, Chronic low-grade inflammation has also been proposed to contribute to the altered pathophysiology observed in depression (11, 12). A cohort study shows that serum HSCRP is an independent risk marker for new-onset major depressive disorder in women (13). Another study has found that around one-third of patients who have been diagnosed with depression exhibit HSCRP that is above a specific threshold (14). However, the available clinical evidence regarding these remains is not sufficient. The current research falls short in exploring gender differences. For instance, one study included only a female population and found that HSCRP is an independent prognostic marker for severe depression in women (13); another study did not fully utilize CRP as a continuous variable to deeply analyze its relationship with depression (14). The measurement of HSCRP may facilitate the identification of individuals at heightened risk for the development of depression, offering a rationale for early intervention. In addition, exploring the relationship between HSCRP and depression may provide a scientific basis for the use of anti-inflammatory drugs in depressed patients.
There are also associations between obesity and inflammation (15–19). In detail, these findings indicate that overweight markedly elevates the risk of clinically significant increases in HSCRP, particularly pronounced in individuals with obesity (BMI ≥30 kg/m²). The association between them varies across gender, ethnicity, and age. For instance, the Pearson correlation coefficients between BMI and the logarithmically transformed CRP are 0.360 and 0.370 for adults and children, respectively, with a 0.24 higher correlation for females than males, and a 0.15 higher correlation for North American/European individuals compared to Asians. Moreover, in individuals with obesity, HSCRP is significantly increased and elevated IL-6 is observed across all obesity categories. In summary, both BMI and HSCRP are associated with depression. Given the close relationship between obesity and inflammation, we hypothesized that BMI might mediate the association between HSCRP and DP. Therefore, we conducted a cross-sectional study using data from one NHANES survey cycle between 2015 and 2016. The objective of the research is to analyze the correlation between BMI, HSCRP, and DP and evaluate their impact across diverse populations.
Datasets were obtained from the NHANES, a survey that uses a probabilistic sampling design to ensure a representative sample. Research ethics review board approval was obtained and all participants provided informed consent. One survey cycle covering the period between 2015 and 2016 was analyzed. 9971 participants were initially enrolled in the cohort. After excluding 1215 participants due to missing BMI data, 1104 for the absence of HSCRP data, and 2818 individuals lacking PHQ-9 data, a total of 4834 subjects were included in the analysis, as depicted in Figure 1.
Figure 1. A flowchart of study population selection. NHANES, National Health and Nutrition Examination Survey; BMI, Body Mass Index; HSCRP, High-Sensitivity C-Reactive Protein; PHQ-9, Patient Health Questionnaire-9.
PHQ-9 is a validated tool for screening DP. The PHQ-9 score ranges from 0 to 27, with higher scores indicating more severe depressive symptoms (20). The PHQ-9 categorizes depression severity into four categories: mild (score of 5), moderate (score of 10), moderately severe (score of 15), and severe (score of 20) (21), each item on the PHQ-9 is scored from 0 (“not at all”) to 3 (“nearly every day”), assessing the frequency of depressive symptoms experienced during the last two weeks (22). Responses of “Refused” or “Do not know” were treated as missing data. If the PHQ-9 score is ≥10, then the subject is classified as having depression (21). The sensitivity and specificity of a PHQ-9 score ≥10 for severe depression are 88% and 88%, respectively (21). Participants were classified into depressed and non-depressed groups based on PHQ-9 scores.
BMI measurements were conducted by trained personnel. HSCRP was determined using a near-infrared particle immunoassay rate method.
We adopted the following criteria for selecting confounders: (a)These variables have previously been shown to be important factors in the association between BMI, HSCRP, and depression (23). Age was included based on this criterion; (b) These confounders were based on a change in the effect estimate exceeding 10% (24). Following this criterion, diabetes, HSCRP, gender, race, sleep disorders, and smoking were chosen as covariates. The physical activity questionnaire (PAQ), derived from the Global Physical Activity Questionnaire and administered at the Mobile Examination Center, was used to assess physical activity levels. These levels were defined by the WHO guidelines on PA (25): inactive (no engagement in strenuous or moderate intensity work activities), moderate (participation in sports without meeting the criteria for strenuous activity), and vigorous (engagement in ≥150 minutes moderate activity per week, ≥75 minutes vigorous exercise per week or strenuous work activities). Alcohol consumption was classified into non-drinkers (complete abstinence from alcohol), moderate drinkers (consumption of up to 2 alcoholic beverages per day for males and up to 1 alcoholic beverage per day for females), and heavy drinkers (consumption of more than 2 alcoholic beverages per day for males and more than 1 alcoholic beverage per day for females). Diabetes was categorized as no diabetes (not having been informed by a doctor of having diabetes) and having diabetes (having been informed by a doctor of having diabetes). Sleep disorders were categorized as having been told by a doctor of having trouble sleeping and not having been told of such difficulties. As for smoking, non-smokers were defined as individuals who have not consumed at least 100 cigarettes in their lifetime, whereas smokers (former smokers and current smokers) were those who have consumed at least 100 cigarettes throughout their lifetime.
The demographic characteristics of participants were analyzed by chi-square tests and the Kruskal-Wallis rank sum test for the non-DP subgroup and DP subgroup. For continuous variables, while for count variables with theoretical counts less than 10, Fisher’s exact probability test is applied. The association of DP, BMI, and HSCRP is based on multivariable logistic regression analysis. These models have DP as the dependent variable and BMI and HSCRP as independent variables. We used three models: Model 1 did not adjust for any covariates; Model 2 included sex, age, and race; Model 3 adjusted for gender, age, race, diabetes, sleep disorders and smoking. In addition, we performed gender-stratified analyses to investigate the sex-discrete relationships between DP, BMI, and HSCRP. Separate multivariable logistic regression analyses were employed for men and women, designating DP as the outcome variable, with BMI and HSCRP as predictors, while controlling for the previously discussed confounders. A generalized additive model was employed to examine the non-linear associations between BMI, HSCRP and DP. The model was adjusted for gender, age, race, diabetes, sleep disorders, and smoking to control for potential confounders. When analyzing the associations between BMI, HSCRP, and DP individually, DP, BMI, and HSCRP were included as additional covariates. The impact of BMI and HSCRP thresholds on DP was calculated separately using a two-segment linear regression model based on a smoothed curve. Interaction and stratified analyses were conducted according to five models (Crude: No factors have been adjusted; Model I: race, age; Model I*: race, age, and the interaction terms for the following variables: race; Model II: BMI, race, age, diabetes, sleep disorders and smoking; Model II*: BMI, race, age, diabetes, sleep disorders and smoking and the interaction terms for the following variables: BMI, race). Subgroup examinations were conducted with gender, smoking, hypertension, and sleep disorders as the stratification variables. We did mediation analysis using the product of coefficients method, and calculating the indirect effect of HSCRP on DP through BMI compared with the total effect of HSCRP on DP. The statistical analysis was carried out using R (version 4.2.0) and EmpowerStats (www.empowerstats.net, X&Y solutions, Inc. Boston, Massachusetts). Statistical significance was defined as less than 0.05 on a two-tailed basis.
Table 1 shows the characteristics of the baseline participants stratified by depressive symptoms. The average age was 48.400 ± 18.372 years, with males constituting 48.99% of the cohort. The subjects were categorized as follows: non-DP (PHQ-9 scores <10) and DP (PHQ-9 scores≥10) groups. Significant differences were observed in BMI and HSCRP between the two groups(BMI: [29.392 ± 6.986] vs. [31.160 ± 8.468], p<0.001; HSCRP: [3.942 ± 6.916] vs. [6.053 ± 12.818], p <0.001).
According to Table 2, An increment of 1Kg/m2 in BMI was associated with a respective increase in the risk of DP of 3.2%, 3.0%, and 2.2% across three models considered (Model 1: OR = 1.032, 95% CI: 1.019-1.045, P=<1e−5; Model 2: OR = 1.030, 95% CI: 1.017-1.044, P <1e−5; Model 3: OR = 1.022, 95% CI: 1.008-1.037, P =0.002); Similarly, An increment of 1mg/L in HSCRP was found to be associated with a respective elevation in the risk of developing DP by 2.3%, 2.2%, and 1.4% across three models (Model 1: OR=1.023, 95% CI: 1.013-1.033, P<1e−5; Model 2: OR=1.022, 95% CI: 1.012-1.032, P=2e−5; Model 3: OR=1.014, 95% CI: 1.004-1.024, P=0.008). These results suggest that BMI and HSCRP are risk factors for DP. In our gender-stratified analysis, it was revealed that both BMI (OR=1.036, 95% CI: 1.019-1.054, P=4e-5) and HSCRP (OR=1.027, 95% CI: 1.012-1.041, P=2.7e-4) are significant risk factors for the onset of DP exclusively in the female population in model3, the detailed information was provided in Supplementary Tables 1, 2.

Table 2. The association between BMI and HSCRP with the risk of DP using multivariable logistic regression.
Smooth curve fitting analyses provided insight into the nonlinear relationships between BMI, HSCRP, and DP (Figure 2). A consistent of confounders were adjusted for all analyses: sex, age, race, diabetes, sleep disorders, and smoking. In detail, the relationship between BMI and DP (P=0.035) was further modified for HSCRP; the correlation between HSCRP and DP (P =0.142) was additionally adjusted for BMI; and the association between BMI and HSCRP (P<0.001) was further refined by adjusting for DP (26, 27). After stratifying by gender there was no significant association between HSCRP and DP in males (P=0.439) as well as between BMI and DP in males (P=0.677). However, the nonlinear association between HSCRP and DP(P=0.031), BMI, and DP (P=9e-04) was significant in females (Figure 3).

Figure 2. Nonlinear associations between BMI, HSCRP, and DP. (A) BMI and probability of developing DP; (B) HSCRP and probability of developing DP; (C) BMI and HSCRP.The vertical scale ranges from 0.0 (no DP) to 1.0 (DP occurrence). Red lines indicate a smooth curve fit between variables, and blue bands indicate 95%CI. Gender, age, race, diabetes, sleep disorders, and smoking were adjusted. Adjusted model+HSCRP (A), Adjusted model+BMI (B), and Adjusted model+DP (C) were each included as confounders.
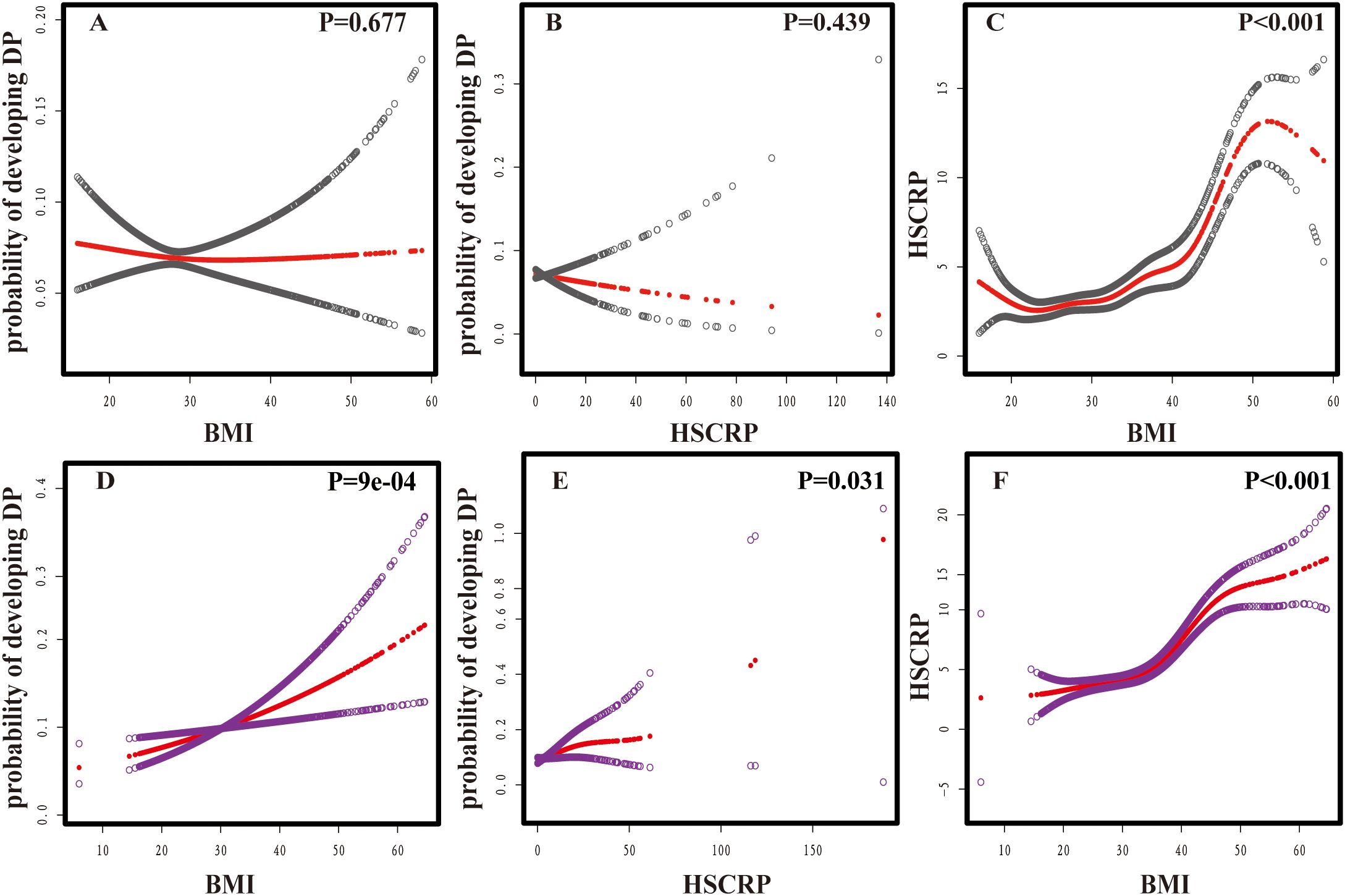
Figure 3. Nonlinear associations between BMI, HSCRP, and DP. (A) BMI and probability of developing DP in males; (B) HSCRP and probability of developing DP in males; (C) BMI and HSCRP in males. (D) BMI and probability of developing DP in females; (E) HSCRP and probability of developing DP in females; (F) BMI and HSCRP in females. The vertical scale ranges from 0.0 (no DP) to 1.0 (DP occurrence). Red lines indicate a smooth curve fit between variables; Grey bands indicate 95%CI in males; Purple bands indicate 95%CI in males. Gender, age, race, diabetes, sleep disorders, and smoking were adjusted. In the analyses of these non-linear associations, HSCRP (A, D), BMI (B, E), and DP (C, F) were each included as additional covariates.
In instances where the BMI was below 27.7, this effect is not statistically significant (P = 0.327). When BMI exceeded 27.7, the effect coefficient was 1.030, indicating that within this stratum, increments in BMI correlate with an elevated risk of DP, and this association was statistically significant (P = 0.002). The log-likelihood ratio test yields a P-value of 0.068; About HSCRP and DP, below 14.4 this threshold, the effect of HSCRP on DP was statistically significant, with an OR of 1.034 (95% CI: 1.004, 1.065; P = 0.028), above the threshold, the influence of HSCRP on DP was not statistically significant, with an OR of 1.003 (95% CI: 0.989, 1.017; P = 0.659). However, the difference in effect between the two segments (below and above the threshold) was not statistically significant, with an OR of 0.970 (95% CI: 0.935, 1.006; P = 0.106), therefore, the evidence for a significant change in the relationship between HSCRP and DP at this threshold was weak, as the difference in effects was not significant and the model fit is not improved. Further details are provided in the Supplementary Table 3.
Table 3 revealed that gender played an interactive role in the association between HSCRP and DP. The female had higher ORs between HSCRP and DP (OR =1.019; 95%CI, 1.004-1.034; P =0.011) than the male (OR =0.992; 95% CI, 0.970-1.014;P =0.487) in Model II*. In the other four models, similar results were obtained. In detail, among females, across all models, the OR for females was all greater than 1, with the 95% CI not encompassing 1, indicating that the association between heightened HSCRP and an elevated risk of DP is statistically significant; Among males, although a trend towards a decreased risk of depression with elevated HSCRP was observed, this observation did not achieve statistical significance. For model II and model II*, the OR for males was slightly below 1, with the 95% CI including 1, signifying that the association is not statistically significant. Similarly, we analyzed the interaction between gender and BMI to explore whether this interaction impacts the likelihood of DP. We found that in females, there was a positive correlation between BMI and the risk of DP (OR =1.026; 95% CI, 1.008, 1.044; P=0.005).In males, although there was a trend towards a reduced risk of DP with increasing BMI, this observation did not achieve statistical significance. (P interaction =0.810)in Model II* (Supplementary Table 4). In addition, we incorporated factors known to be associated with depression from the existing literature, such as hypertension, smoking, age, and sleep disorders (28, 29) to conduct analyses. The findings suggested a potential interaction between BMI and hypertension, and although its influence on DP was not statistically significant; No significant interaction was observed between smoking and BMI; Age might slightly modify the relationship between BMI and DP in some models, though the significance of this interaction was weak; Sleep disorders didn’t show a significant interaction with BMI about DP across most models; As for HSCRP and DP, no significant interaction between hypertension and HSCRP across all examined models; Upon adjustment for BMI, sex, age, and race, a significant positive correlation emerged between former smoking and DP. Furthermore, the inclusion of interaction terms for BMI, sex, and race indicated a significant moderating effect of smoking on the relationship between HSCRP and DP, which was also significant with the consideration of diabetes, sleep disorders, and other potential confounders and interaction terms; There was no significant difference between HSCRP and DP in different age groups; In the analysis stratified by the presence or absence of sleep disorders, no significant difference was detected in the relationship between HSCRP and DP between the two groups. Further details are provided in the Supplementary Tables 4-12.
Participants were stratified into two subgroups for analysis: a no-DP group (PHQ-9 < 10) and a DP group (PHQ-9 ≥10). We conducted an analysis to examine the mediating effect of HSCRP through BMI on DP. The results revealed a specific indirect effect of HSCRP through BMI on DP, with a coefficient estimate of OR=1.193e-3, and a 95% CI ranging from 1.160e-4 to 2.407e-3. The P-value was 0.032, indicating a statistically significant mediating role of BMI in the relationship between HSCRP and DP. This mediating effect accounted for 28.4% of the total effect(Supplementary Table 13). We further conducted separate mediation analyses for different gender subgroups. We found the association between HSCRP and the risk of DP may exhibit gender disparities, with BMI potentially acting as a mediator in females, whereas this mediating role is not apparent in males. Detailed results were presented in the Supplementary Tables 14, 15.
This study investigates the association between BMI, HSCRP, and DP. Multivariable logistic regression analysis provided strong evidence supporting the significant relationship of both BMI and HSCRP with DP. Furthermore, we identified non-linear correlations between BMI, DP, and HSCRP, with gender differences observed in these relationships. Subsequently, we investigated the interaction effect between BMI, HSCRP, and gender on the risk of DP. The results show a significant interaction between HSCRP and gender. Further stratified analysis revealed a significant relationship between HSCRP and DP in females, this may be related to fluctuations in ovarian hormones in women, for example, it has been found that fluctuations in ovarian hormones can regulate women’s susceptibility to stress and inflammation, resulting in DP (30); There is another reason, for example, that women may experience higher levels of stress, which is thought to increase inflammation, which can lead to depression (31). The mediation analysis revealed that HSCRP exerts a significant total effect on DP, with a portion of this effect being mediated through BMI. The mediation effect accounts for approximately 28.4% of the total effect. This is consistent with a previous study (32).
There is a nonlinear relationship between BMI and DP, consistent with results from a study in the Han Chinese population (33). From one perspective, increased BMI may lead to a state of chronic low-grade inflammation, which, by impacting brain function, elevates the risk of depression (34, 35). From another perspective, the intricate interplay between BMI and insulin resistance has been proposed as a potential biological pathway for depression (36, 37). Further analysis indicated the presence of a threshold effect between BMI and the risk of DP, such that when BMI is beyond 27.7, there is a marked increase in risk. Our findings suggest that clinicians can identify groups of patients who may benefit from early intervention by monitoring their BMI. Specifically, for individuals with BMI above a certain threshold, early intervention may be more effective. For example, we found that the risk of DP increased significantly when BMI exceeded 27.7. This finding has prompted clinicians to take more proactive preventive measures when these indicators exceed defined thresholds; The study also revealed that the impact of HSCRP on DP is significantly below a threshold of 14.4, but it becomes nonsignificant beyond this point. Despite indications of a threshold effect, the difference in the magnitude of the effect before and after the threshold was not pronounced, and it didn’t significantly enhance the model’s fit. Further research with larger sample sizes may be required to validate these findings.
Furthermore, our study also identified a nonlinear association between BMI and HSCRP. Although some studies did not explicitly explore this non-linear relationship, they did note a correlation between BMI and HSCRP (38–40), to some extent, this supports our conclusion. However, unhealthy eating patterns can lead to increased BMI and inflammation, which in turn are risk factors for depression. Instead, a healthy eating pattern can combat these risk factors, thereby reducing the risk of depression (41). Therefore, adopting a healthy eating pattern as a prevention strategy may not only help with weight loss but may also reduce the incidence of depression.
We are unique in our approach in that we explored the nonlinear association between BMI, HSCRP and DP. Fewer studies have explored the nonlinear nature of this relationship. In addition, our study revealed gender differences in the relationship between BMI and DP, and between HSCRP and DP. This may be related to the following factors, specifically, on one hand, females showed higher levels of inflammation (42) and obesity prevalence (43, 44); On the other hand, depressed women have higher leptin levels, which may be a key factor in the sex-specific association between BMI and DP (45); Similarly, the relationship between HSCRP and DP showed a gender difference. A possible explanation for this difference has to do with the influence of sex hormones, particularly periodic changes in ovarian hormones, which are known to regulate C-reactive protein levels, which in turn affect the state of depression (46, 47). However, this does not mean that inflammation is not a potential underlying factor for depression in males (48).
Nonetheless, this research does exhibit certain limitations. Firstly, due to NHANES’ use of a complex probability sampling design, which aims to represent the non-institutionalized civilian population of the United States, the homeless, military personnel, and residents in remote areas were not included. Despite some potential biases, after all, these groups constitute a small proportion of the overall population, therefore, their impact on the generalizability of the overall results is limited; Secondly, inflammation is not only associated with depression, but specifically treatment-resistant depression (49–51). The NHANES data includes PHQ-9 scores, which provide a measurement of the severity of DP. However, we must acknowledge that NHANES does not collect detailed information on the number of depressive episodes or the history of antidepressant medication use among participants. Consequently, we cannot directly distinguish between treatment-resistant depression and first-episode depression. Future research should consider these additional variables to gain a more comprehensive understanding of the relationship between depression and inflammation; In addition, the American Heart Association recognizes a threshold of 3mg/l for low-grade inflammation, yet in Table 1, the HSCRP in both non-DP (3.9) and DP (6.053) were above 3. This is the other limitation of our study. The high HSCRP in both groups indicates that our sample may not be representative of the general population with lower levels of inflammation. Therefore, our results may pertain more specifically to populations with higher inflammation levels and future research should examine the relationship across a broader range of HSCRP. Finally, it should be noted that this is a cross-sectional study, and future large-scale longitudinal research is needed to explore the causal relationships between them; Some data in the questionnaire, such as physical activity time, may be affected by recall bias, leading to inaccurate results; PHQ-9 is used for screening depression, but it is not the gold standard for diagnosing depression (12).
In conclusion, our results found that HSCRP and BMI are risk factors for the occurrence of DP. There was a nonlinear relationship between BMI and DP, BMI and HSCRP, respectively. After stratifying by gender, the nonlinear association between BMI and DP, as well as HSCRP and DP, no longer exists in males. Interaction and stratified analyses found that increased HSCRP are merely risk factors for DP in females. A larger prospective study with a larger sample size is needed to investigate the causal relationship between them in the future.
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2015.
Human subject research was granted ethical clearance by the NCHS Ethics Review Board (ERB). The research adhered to pertinent local statutes and organizational protocols. Documented consent for involvement in the study was obtained from the legal custodians or closest relatives of the participants.
YZ: Conceptualization, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. FZ: Investigation, Supervision, Validation, Writing – review & editing. YXZ: Supervision, Validation, Software, Writing – review & editing. CA: Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Thanks for the support of the Central Guided Local Science and Technology Development Funding Program (No.236Z7751G).
The authors extend their sincere appreciation to the participants of NHANES for their altruistic involvement, which has been instrumental to the advancement of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1506726/full#supplementary-material
1. Hammen C. Risk factors for depression: an autobiographical review. Annu Rev Clin Psychol. (2018) 14:1–28. doi: 10.1146/annurev-clinpsy-050817-084811
2. Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
3. Frank P, Jokela M, Batty GD, Lassale C, Steptoe A, Kivimäki M. Overweight, obesity, and individual symptoms of depression: A multicohort study with replication in UK Biobank. Brain Behav Immun. (2022) 105:192–200. doi: 10.1016/j.bbi.2022.07.009
4. He K, Pang T, Huang H. The relationship between depressive symptoms and BMI: 2005-2018 NHANES data. J Affect Disord. (2022) 313:151–7. doi: 10.1016/j.jad.2022.06.046
5. Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. (2019) 24:18–33. doi: 10.1038/s41380-018-0017-5
6. Must A, McKeown NM, Feingold KR, Anawalt B, Blackman MR, Boyce A, et al. The disease burden associated with overweight and obesity. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al, editors. Endotext. MDText.com, Inc.Copyright © 2000-2024, MDText.com, Inc, South Dartmouth (MA (2000).
7. Cho SH, Lim JE, Lee J, Lee JS, Jeong HG, Lee MS, et al. Association between high-sensitivity C-reactive protein levels and depression: Moderation by age, sex, obesity, and aerobic physical activity. J Affect Disord. (2021) 291:375–83. doi: 10.1016/j.jad.2021.05.040
8. Hickman RJ, Khambaty T, Stewart JC. C-reactive protein is elevated in atypical but not nonatypical depression: data from the National Health and Nutrition Examination survey (NHANES) 1999-2004. J Behav Med. (2014) 37:621–9. doi: 10.1007/s10865-013-9510-0
9. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. (2003) 107:363–9. doi: 10.1161/01.CIR.0000053730.47739.3C
10. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. (2016) 16:22–34. doi: 10.1038/nri.2015.5
11. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
12. McCarron RM, Shapiro B, Rawles J, Luo J. Depression. Ann Intern Med. (2021) 174:Itc65–itc80. doi: 10.7326/AITC202105180
13. Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. (2010) 197:372–7. doi: 10.1192/bjp.bp.109.076430
14. Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. (2019) 49:1958–70. doi: 10.1017/S0033291719001454
15. McLaughlin AP, Nikkheslat N, Hastings C, Nettis MA, Kose M, Worrell C, et al. The influence of comorbid depression and overweight status on peripheral inflammation and cortisol levels. psychol Med. (2022) 52:3289–96. doi: 10.1017/S0033291721000088
16. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. (1999) 282:2131–5. doi: 10.1001/jama.282.22.2131
17. Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. (2013) 14:232–44. doi: 10.1111/obr.2013.14.issue-3
18. Soriano-Guillén L, Hernández-García B, Pita J, Domínguez-Garrido N, Del Río-Camacho G, Rovira A. High-sensitivity C-reactive protein is a good marker of cardiovascular risk in obese children and adolescents. Eur J Endocrinol. (2008) 159:R1–4. doi: 10.1530/EJE-08-0212
19. Huet L, Delgado I, Dexpert S, Sauvant J, Aouizerate B, Beau C, et al. Relationship between body mass index and neuropsychiatric symptoms: Evidence and inflammatory correlates. Brain Behav Immun. (2021) 94:104–10. doi: 10.1016/j.bbi.2021.02.031
20. Lamers F, Jonkers CC, Bosma H, Penninx BW, Knottnerus JA, van Eijk JT. Summed score of the Patient Health Questionnaire-9 was a reliable and valid method for depression screening in chronically ill elderly patients. J Clin Epidemiol. (2008) 61:679–87. doi: 10.1016/j.jclinepi.2007.07.018
21. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
22. Shim RS, Baltrus P, Ye J, Rust G. Prevalence, treatment, and control of depressive symptoms in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2005-2008. J Am Board Fam Med. (2011) 24:33–8. doi: 10.3122/jabfm.2011.01.100121
23. Oh J, Chae JH, Kim TS. Age-specific association between body mass index and depression: The Korea National Health and Nutrition Examination Survey 2014. Int J Obes (Lond). (2018) 42:327–33. doi: 10.1038/ijo.2017.234
24. Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. (2014) 348:g14. doi: 10.1136/bmj.g14
25. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
26. Jung YE, Kang KY. Elevated hs-CRP level is associated with depression in younger adults: Results from the Korean National Health and Nutrition Examination Survey (KNHANES 2016). Psychoneuroendocrinology. (2019) 109:104397. doi: 10.1016/j.psyneuen.2019.104397
27. Kawamoto R, Kusunoki T, Abe M, Kohara K, Miki T. An association between body mass index and high-sensitivity C-reactive protein concentrations is influenced by age in community-dwelling persons. Ann Clin Biochem. (2013) 50:457–64. doi: 10.1177/0004563212473445
28. Cai Y, Chen M, Zhai W, Wang C. Interaction between trouble sleeping and depression on hypertension in the NHANES 2005-2018. BMC Public Health. (2022) 22:481. doi: 10.1186/s12889-022-12942-2
29. Fluharty M, Taylor AE, Grabski M, Munafò MR. The association of cigarette smoking with depression and anxiety: A systematic review. Nicotine Tob Res. (2017) 19:3–13. doi: 10.1093/ntr/ntw140
30. Slavich GM, Sacher J. Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacol (Berl). (2019) 236:3063–79. doi: 10.1007/s00213-019-05326-9
31. Lawn RB, Murchland AR, Kim Y, Chibnik LB, Tworoger SS, Rimm EB, et al. Trauma, psychological distress and markers of systemic inflammation among US women: A longitudinal study. Psychoneuroendocrinology. (2022) 145:105915. doi: 10.1016/j.psyneuen.2022.105915
32. Karageorgiou V, Casanova F, O’Loughlin J, Green H, McKinley TJ, Bowden J, et al. Body mass index and inflammation in depression and treatment-resistant depression: a Mendelian randomisation study. BMC Med. (2023) 21:355. doi: 10.1186/s12916-023-03001-7
33. Li C, Li X, Li Y, Niu X. The nonlinear relationship between body mass index (BMI) and perceived depression in the Chinese population. Psychol Res Behav Manage. (2023) 16:2103–24. doi: 10.2147/PRBM.S411112
34. Khanna D, Khanna S, Khanna P, Kahar P, Patel BM. Obesity: A chronic low-grade inflammation and its markers. Cureus. (2022) 14:e22711. doi: 10.7759/cureus.22711
35. Milano W, Ambrosio P, Carizzone F, De Biasio V, Di Munzio W, Foia MG, et al. Depression and obesity: analysis of common biomarkers. Diseases. (2020) 8:23. doi: 10.3390/diseases8020023
36. Zhang R, Dong SY, Wang F, Ma C, Zhao XL, Zeng Q, et al. Associations between body composition indices and metabolic disorders in Chinese adults: A cross-sectional observational study. Chin Med J (Engl). (2018) 131:379–88. doi: 10.4103/0366-6999.225059
37. Mehdi S, Wani SUD, Krishna KL, Kinattingal N, Roohi TF. A review on linking stress, depression, and insulin resistance via low-grade chronic inflammation. Biochem Biophys Rep. (2023) 36:101571. doi: 10.1016/j.bbrep.2023.101571
38. Chae WR, Nübel J, Baumert J, Gold SM, Otte C. Association of depression and obesity with C-reactive protein in Germany: A large nationally representative study. Brain Behav Immun. (2022) 103:223–31. doi: 10.1016/j.bbi.2022.04.024
39. Bulló M, García-Lorda P, Megias I, Salas-Salvadó J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. (2003) 11:525–31. doi: 10.1038/oby.2003.74
40. Festa A, D’Agostino R Jr., Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. (2001) 25:1407–15. doi: 10.1038/sj.ijo.0801792
41. Oddy WH, Allen KL, Trapp GSA, Ambrosini GL, Black LJ, Huang RC, et al. Dietary patterns, body mass index and inflammation: Pathways to depression and mental health problems in adolescents. Brain Behav Immun. (2018) 69:428–39. doi: 10.1016/j.bbi.2018.01.002
42. Cohen E, Margalit I, Shochat T, Goldberg E, Krause I. Markers of chronic inflammation in overweight and obese individuals and the role of gender: A cross-sectional study of a large cohort. J Inflammation Res. (2021) 14:567–73. doi: 10.2147/JIR.S294368
43. Garawi F, Devries K, Thorogood N, Uauy R. Global differences between women and men in the prevalence of obesity: is there an association with gender inequality? Eur J Clin Nutr. (2014) 68:1101–6. doi: 10.1038/ejcn.2014.86
44. Cooper AJ, Gupta SR, Moustafa AF, Chao AM. Sex/gender differences in obesity prevalence, comorbidities, and treatment. Curr Obes Rep. (2021) 10:458–66. doi: 10.1007/s13679-021-00453-x
45. Li L, Gower BA, Shelton RC, Wu X. Gender-specific relationship between obesity and major depression. Front Endocrinol (Lausanne). (2017) 8:292. doi: 10.3389/fendo.2017.00292
46. Jilma B, Dirnberger E, Löscher I, Rumplmayr A, Hildebrandt J, Eichler HG, et al. Menstrual cycle-associated changes in blood levels of interleukin-6, alpha1 acid glycoprotein, and C-reactive protein. J Lab Clin Med. (1997) 130:69–75. doi: 10.1016/S0022-2143(97)90060-3
47. Salkeld BD, MacAulay JC, Ball RW, Cannon JG. Modulation of body temperature, interleukin-6 and leptin by oral contraceptive use. Neuroimmunomodulation. (2001) 9:319–25. doi: 10.1159/000059389
48. Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence. (2014) 5:12–9. doi: 10.4161/viru.26982
49. Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. (2013) 70:31–41. doi: 10.1001/2013.jamapsychiatry.4
50. Treadway MT, Etuk SM, Cooper JA, Hossein S, Hahn E, Betters SA, et al. A randomized proof-of-mechanism trial of TNF antagonism for motivational deficits and related corticostriatal circuitry in depressed patients with high inflammation. Mol Psychiatry. (2024) 29:1–11. doi: 10.21203/rs.3.rs-3957252/v1
Keywords: BMI, PHQ-9, HSCRP, DP, inflammation
Citation: Zhang Y, Zhen F, Zhang Y and An C (2025) Associations between body mass index, high-sensitivity C-reactive protein, and depressive symptoms: NHANES 2015-2016. Front. Psychiatry 15:1506726. doi: 10.3389/fpsyt.2024.1506726
Received: 06 October 2024; Accepted: 16 December 2024;
Published: 13 January 2025.
Edited by:
Mark M. Rasenick, University of Illinois Chicago, United StatesReviewed by:
Qian Wu, The First Affiliated Hospital of Soochow University, ChinaCopyright © 2025 Zhang, Zhen, Zhang and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuixia An, YWN4c3VubnlAaGVibXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.