- 1Department of Pharmacy, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, Jiangsu, China
- 2Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy & School of Pharmacy, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3Department of Pharmacy, The Suqian Clinical College of Xuzhou Medical University, Suqian, Jiangsu, China
- 4Department of Pharmacy, Wuxi Maternity and Child Health Care Hospital, Wuxi, Jiangsu, China
- 5School of Nursing, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 6Department of Pharmacy, Suzhou Research Center of Medical School, Suzhou Hospital, Affiliated Hospital of Medical School, Nanjing University, Suzhou, Jiangsu, China
- 7Department of Pharmacy, Shenzhen Hospital, Southern Medical University, Shenzhen, China
- 8Department of Pharmacy, Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University, Xuzhou, Jiangsu, China
Objective: Bipolar affective disorder (BAD) is a mood disorder with high morbidity and mortality. Quetiapine can be used in the treatment of patients with BAD; however, the precise administration regimen of quetiapine in these patients is still unknown. In this study, a population pharmacokinetic (PPK) model of quetiapine in patients with BAD was constructed based on model-informed precision dosing (MIPD) and real-world clinical data and an optimal initial dose of quetiapine in these patients was recommended.
Methods: A total of 99 patients with BAD treated with quetiapine were included. At the same time, the quetiapine concentrations, the physical and chemical indices of the patients, and the drug combination information were collected. A quetiapine PPK model for patients with BAD was then constructed and an initial dose based on Monte Carlo simulation was recommended.
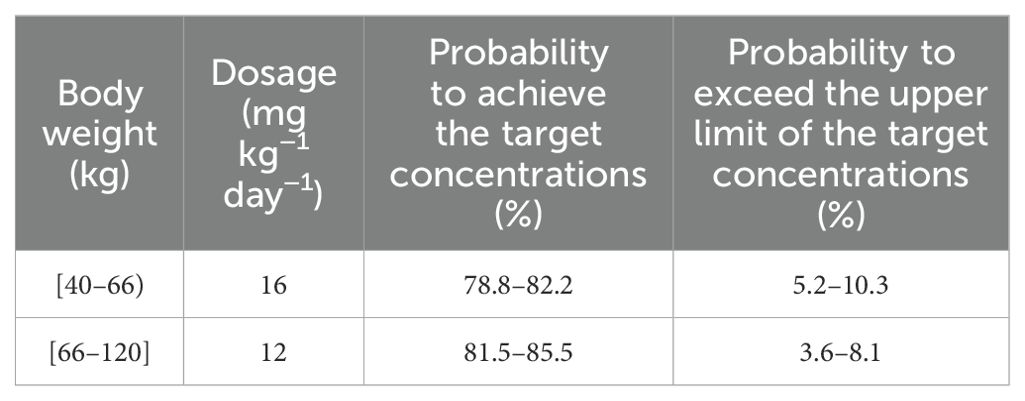
Results: In the final model of quetiapine for patients with BAD, the apparent oral clearance (CL/F) and the apparent volume of distribution (V/F) were 76.1 L/h and 530 L, respectively. For patients with BAD weighing 40–66 kg, the initial dose recommendation was 16 mg kg−1 day−1, the probability of reaching the therapeutic window was 78.8%–82.2%, and the probability of exceeding the upper limit of the therapeutic window was 5.2%–10.3%. For patients with BAD weighing 66–120 kg, the initial dose recommendation was 12 mg kg−1 day−1, the probability of reaching the therapeutic window was 81.5%–85.5%, and the probability of exceeding the upper limit of the therapeutic window was 3.6%–8.1%.
Conclusion: The present study, for the first time, recommended an initial dose of quetiapine in patients with BAD based on MIPD and real-world data, providing an individualized reference for the administration of quetiapine in these patients.
1 Introduction
Bipolar affective disorder (BAD) is a mood disorder that is clinically manifested as manic episodes or as both depressive and manic episodes (1–6). Studies found that anxiety disorders have become the first major category of mental diseases, followed by mood disorders, the second major category of mental diseases, with a prevalence rate of 0.8%–9.6%. The lifetime prevalence of BAD is 1.02% (7, 8). BAD has high disability and fatality rates, and its all-cause mortality rate is higher than that of the general population, approximately twice that of the general population. In addition, studies found that the suicide rates of patients with BAD are the highest among all mental diseases, with the suicide risk being 20–30 times higher than that of the general population (9–14). In short, the learning, work, and self-care abilities of patients with BAD are reduced and the quality of life is decreased, as well as even suicide in severe cases, causing serious trauma and economic burden to families and society.
Quetiapine is an atypical antipsychotic drug, which is an antagonist of various neurotransmitter receptors in the brain and can be applied for all types of schizophrenia (15–18). Furthermore, quetiapine can reduce the emotional symptoms associated with schizophrenia, such as depression, anxiety, and cognitive deficits, and can also be used in the treatment of BAD (19–22). However, the pharmacokinetic variation of quetiapine is large, and it is difficult to formulate the optimal drug administration regimen in clinical practice. In this study, a population pharmacokinetic (PPK) model of quetiapine in patients with BAD was built based on model-informed precision dosing (MIPD) and real-world clinical data and an optimal initial dose of quetiapine for these patients was recommended.
2 Methods
2.1 Data collection and patient information
Patients with BAD who were treated with quetiapine from Xuzhou Oriental Hospital affiliated with Xuzhou Medical University between July 2020 and August 2023 were included, retrospectively. The criteria for inclusion were as follows: i) diagnosed with BAD; ii) treated with quetiapine; and iii) therapeutic drug monitoring (TDM) for quetiapine. The research was approved by the Research Ethics Committee of Xuzhou Oriental Hospital affiliated with Xuzhou Medical University. The quetiapine concentrations in patients with BAD, along with the physiological and biochemical indicators, and the concomitant medications were collected from real-world data, including TDM and medical record system.
A total of 99 patients with BAD treated with quetiapine were included for analysis, which comprised 66 men and 33 women aged 17.05–69.47 years and weighing 43.00–119.00 kg. The demographic data for BAD and the drug combinations are shown in Tables 1 and 2, respectively.
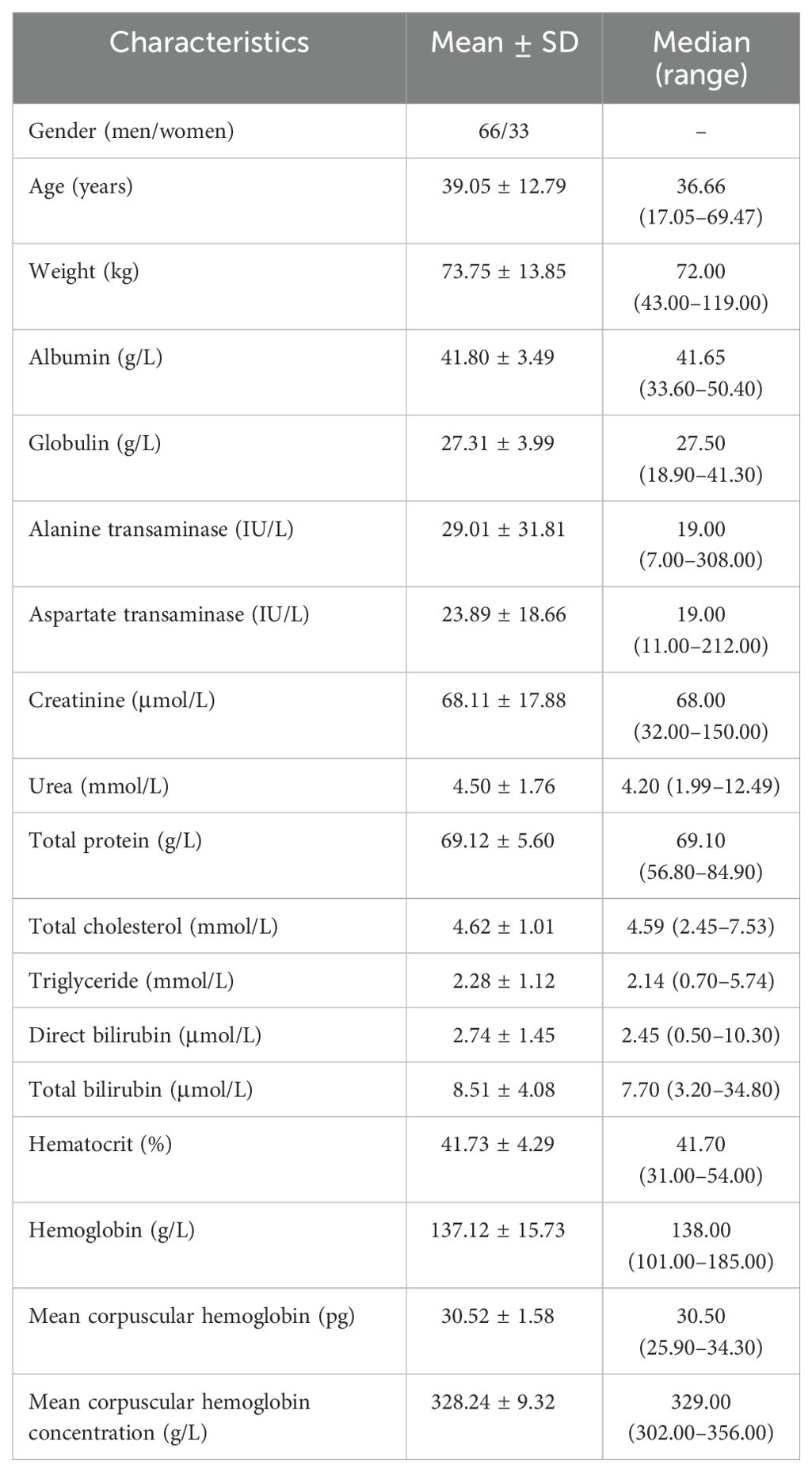
Table 1. Demographic data of patients with bipolar affective disorder treated with quetiapine (n = 99).
2.2 Modeling
A quetiapine PPK model for patients with BAD was built using non-linear mixed effect modeling (NONMEM), including apparent oral clearance (CL/F), apparent volume of distribution (V/F), and the absorption rate constant (Ka, fixed at 1.46/h) (23).
Equation 1 expresses the inter-individual variability:
where Ci is the individual parameter, TV(C) represents typical individual parameter (individual parameter with representative characteristics), and ηi denotes symmetrical distribution (in statistics, the distribution of index data is symmetrical; i.e., the distribution shape of the data on both sides of the mean is similar).
Equation 2 presents the random residual variability:
where Ei denotes the observed concentrations, Gi represents the individual predicted concentrations, and ϵn denotes symmetrical distribution.
Equation 3 displays the relationship of the pharmacokinetic parameters with weight:
where Li is the i-th individual parameter, Ni is the i-th individual weight, Nstd is the standard weight of 70 kg, and Lstd represents typical individual parameter. M is the allometric coefficient: 0.75 for CL/F and 1 for V/F (24).
Equations 4, 5 determine the pharmacokinetic parameters between the continuous covariates and the categorical covariates, respectively:
where Ri is the individual parameter, TV(R) represents typical individual parameter, Z denotes the parameter to be estimated, Si is the covariate of the i-th individual, and Sm is the population median for the covariate.
The stepwise method was used to evaluate the covariate, in which the objective function value (OFV) decreasing more than 3.84 (p < 0.05) and increasing more than 6.63 (p < 0.01) were the inclusion and exclusion standards, respectively (25, 26).
2.3 Model evaluation
The final quetiapine PPK model for patients with BAD was evaluated using visual graphics and bootstrap, where bootstrap sampling usually refers to reliable random sampling using the bootstrap framework. In fields such as data analytics and machine learning, random sampling is a common technique used to create subsets of a dataset in order to better understand and model the entire dataset.
2.4 Simulation
The quetiapine concentrations of patients with BAD were simulated using the Monte Carlo method, where the therapeutic window (valid therapeutic reference values) was 100–500 ng/ml (22). We simulated 1,000 virtual patients with BAD who weighed 40, 60, 80, 100, and 120 kg for 1, 4, 8, 12, 16, 20, 24, and 28 mg kg−1 day−1 quetiapine. The probability of reaching the therapeutic window was selected as the assessment index. At the same time, the present study evaluated the probability of exceeding the upper limit (maximum concentration, 500 ng/ml) of the therapeutic window, which was the safety index.
3 Results
3.1 Modeling
The final quetiapine PPK model for patients with BAD is shown as follows (Equation 6 and 7):
CL/F and V/F represent the apparent oral clearance and the apparent volume of distribution, respectively.
3.2 Evaluation
A visual representation of the quetiapine PPK model for patients with BAD is displayed in Figure 1, which showed that the quetiapine concentrations of these patients were well predicted by the final model. The individual plots are shown in Figure 2, which indicated that the quetiapine PPK model of the patients with BAD could accurately predict the quetiapine concentrations at the individual level. The results of bootstrap validation are presented in Table 3, which showed that the final quetiapine PPK model of the patients with BAD was accurate and reliable.
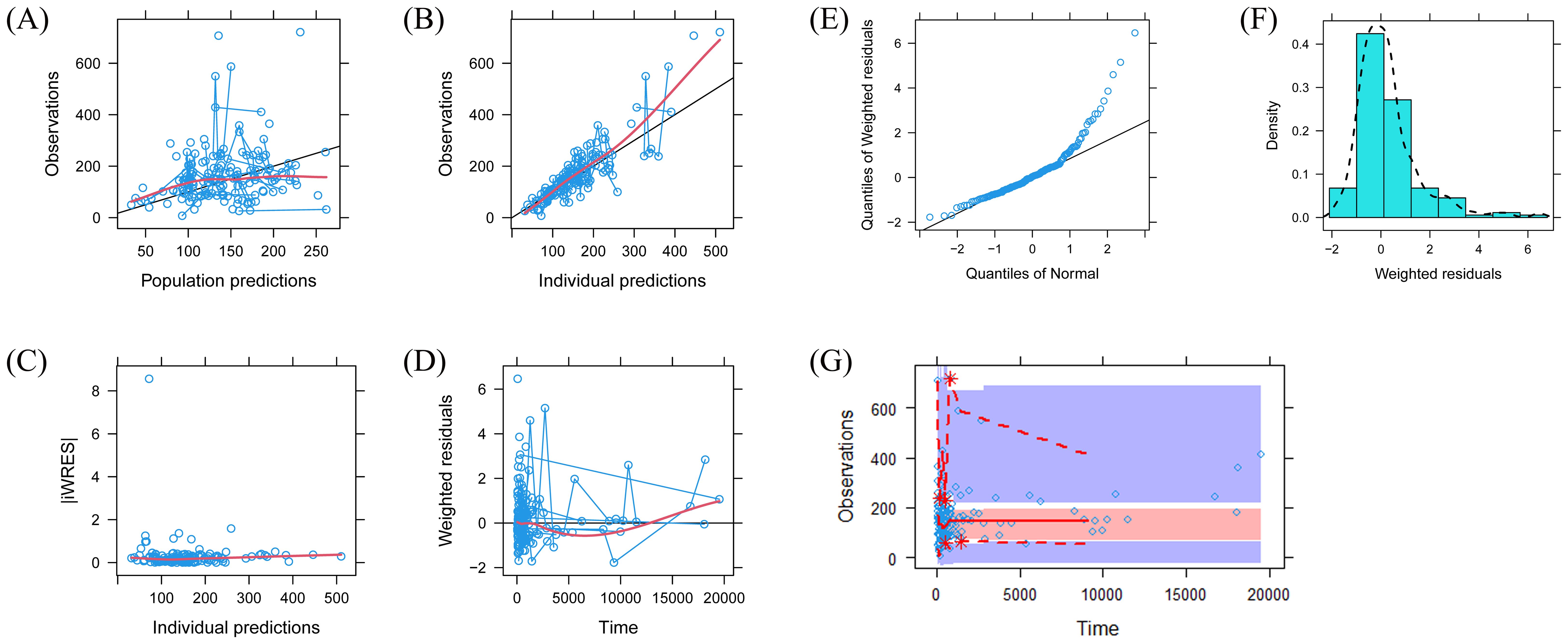
Figure 1. Model evaluation. (A) Observations vs. population predictions. (B) Observations vs. individual predictions. (C) Absolute value of the weighted residuals of individuals (│iWRES│) vs. individual predictions. (D) Weighted residuals vs. time. (E) Quantiles of the weighted residuals vs. quantiles of normal. (F) Density vs. weighted residuals. (G) Visual predictive check (VPC) of the model.
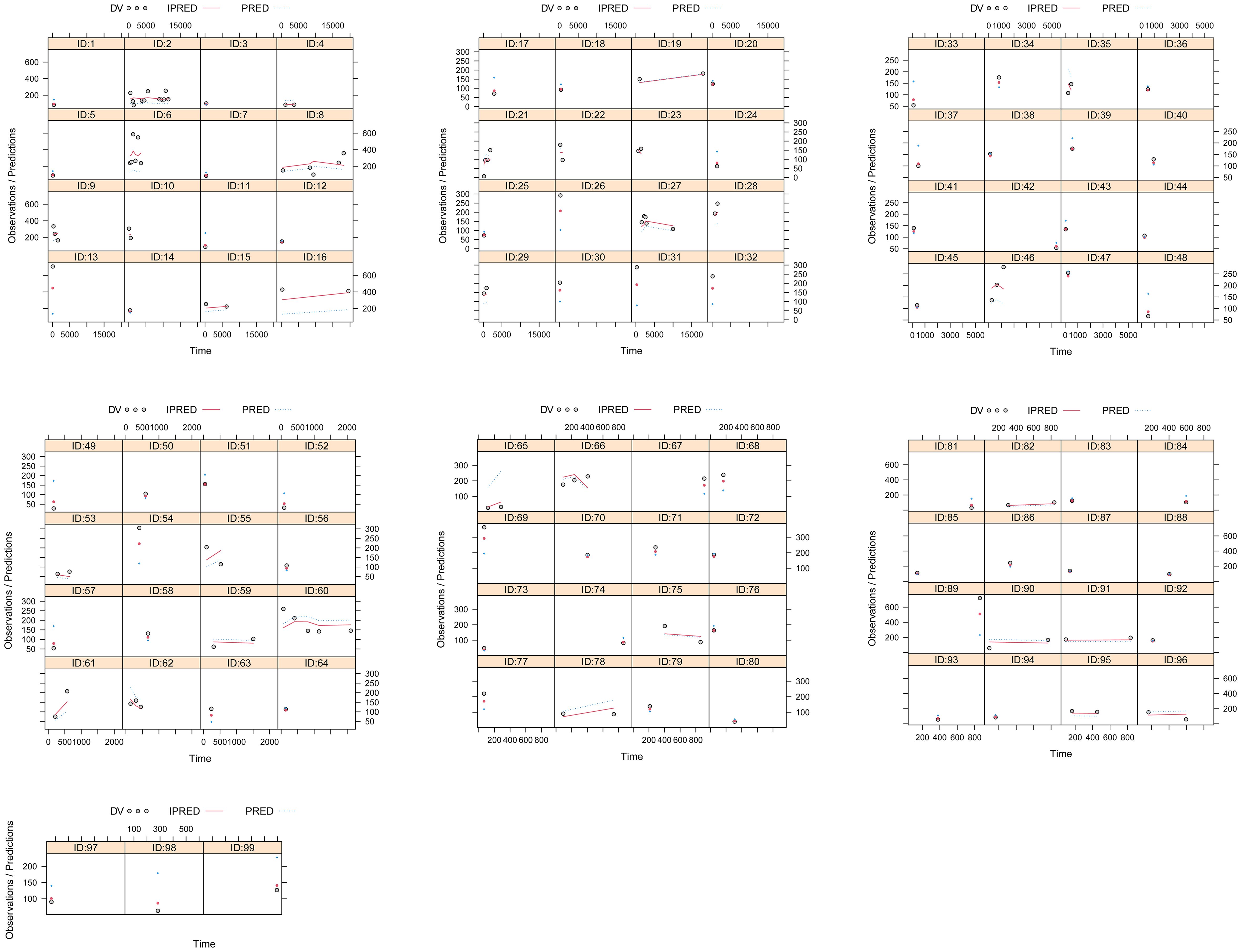
Figure 2. Individual plots. ID, patient ID number; DV, measured concentration value; IPRED, individual predictive value. PRED, population predictive value.
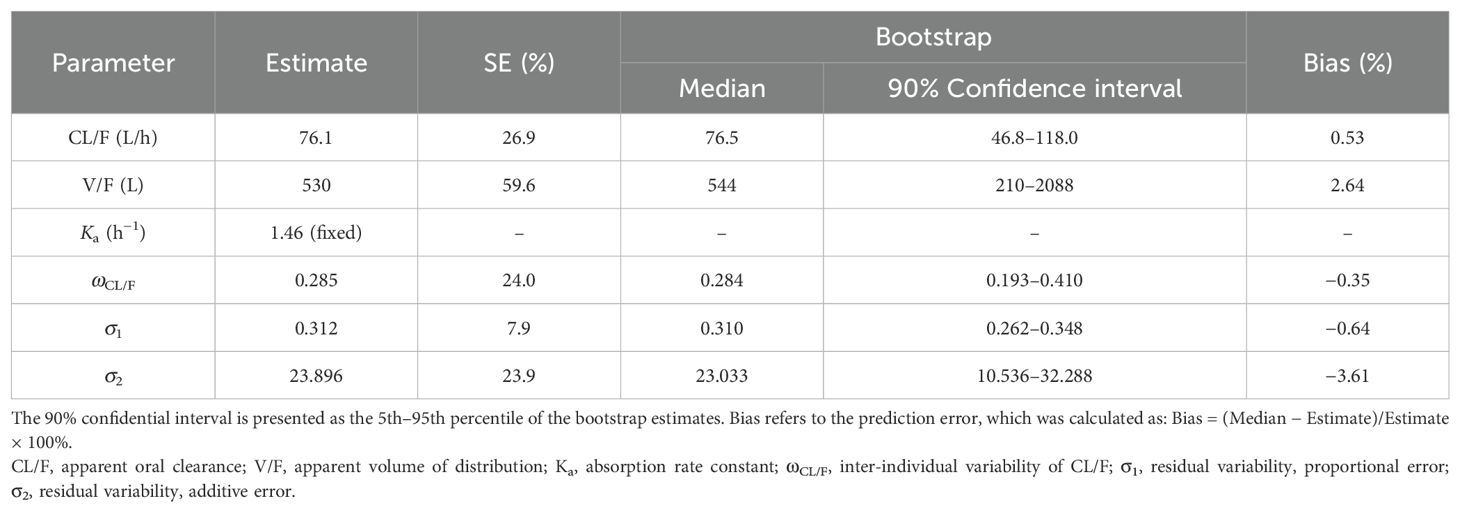
Table 3. Parameter estimates of the quetiapine final model and bootstrap validation in patients with bipolar affective disorder.
3.3 Recommended dosage
The simulated quetiapine concentrations in patients with BAD are shown in Figure 3. Figures 3A-E display the data for patients with BAD weighing 40, 60, 80, 100, and 120 kg, respectively. The probability of reaching the therapeutic window of quetiapine in patients with BAD is shown in Figure 4. For patients with BAD weighing 40–66 kg, the initial dose recommendation was 16 mg kg−1 day−1 and the probability of reaching the therapeutic window was 78.8%–82.2%. For patients with BAD weighing 66–120 kg, the initial dose recommendation was 12 mg kg−1 day−1 and the probability of reaching the therapeutic window was 81.5%–85.5%, as shown in Table 4.
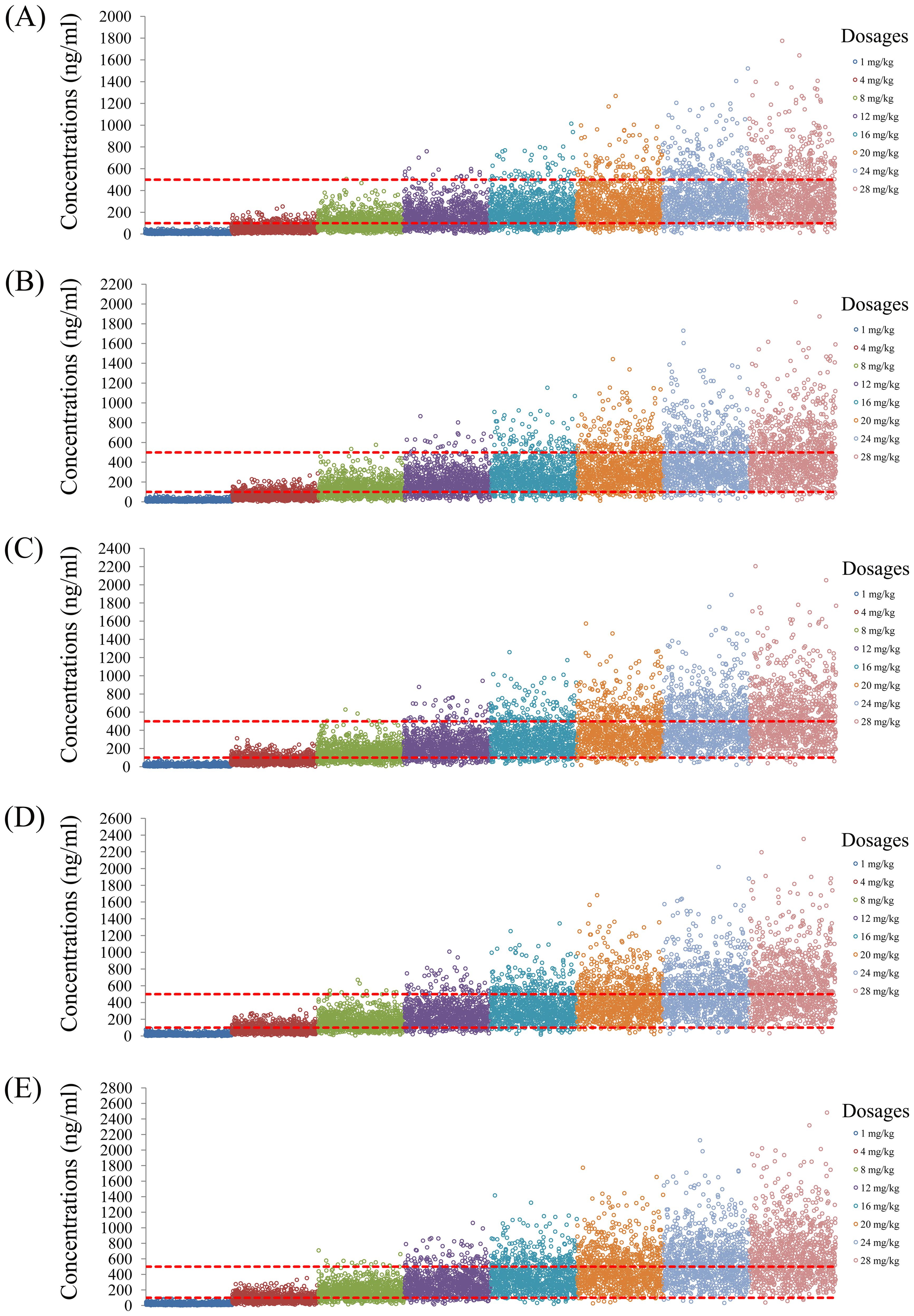
Figure 3. Simulated quetiapine concentrations in patients with bipolar affective disorder (BAD). (A–E) Patients with BAD weighing 40 kg (A), 60 kg (B), 80 kg (C), 100 kg (D), and 120 kg (E).
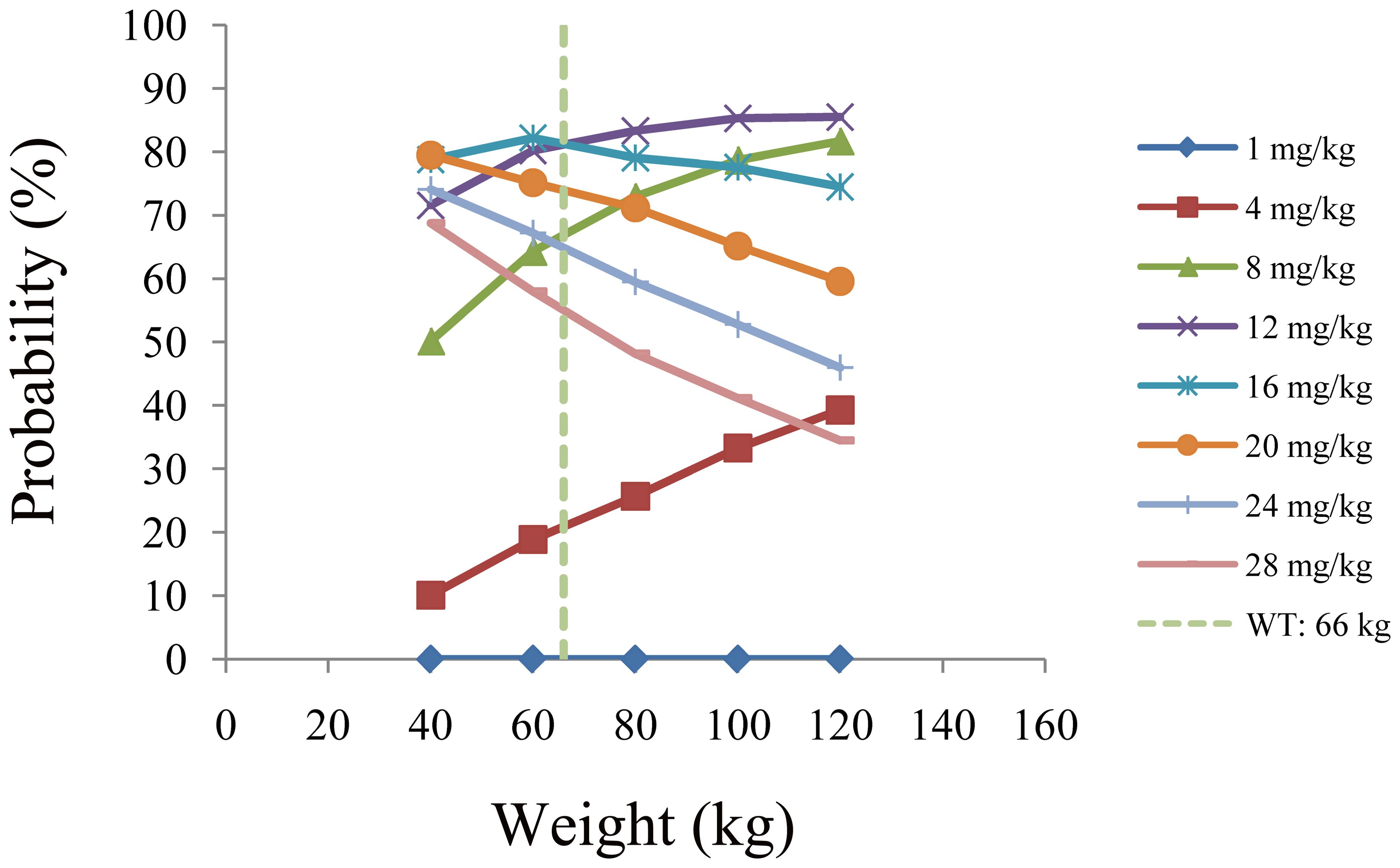
Figure 4. Probability of reaching the therapeutic window of quetiapine in patients with bipolar affective disorder.
3.4 Safety evaluation
The probability of exceeding the upper limit of the therapeutic window (500 ng/ml) at 1,000 simulated concentrations as the safety evaluation is shown in Figure 5. For patients with BAD weighing 40–66 kg, the initial dose recommendation was 16 mg kg−1 day−1 and the probability of exceeding the upper limit of the therapeutic window was 5.2%–10.3%. For those weighing 66–120 kg, the initial dose recommendation was 12 mg kg−1 day−1 and the probability of exceeding the upper limit of the therapeutic window was 3.6%–8.1%, as shown in Table 4.
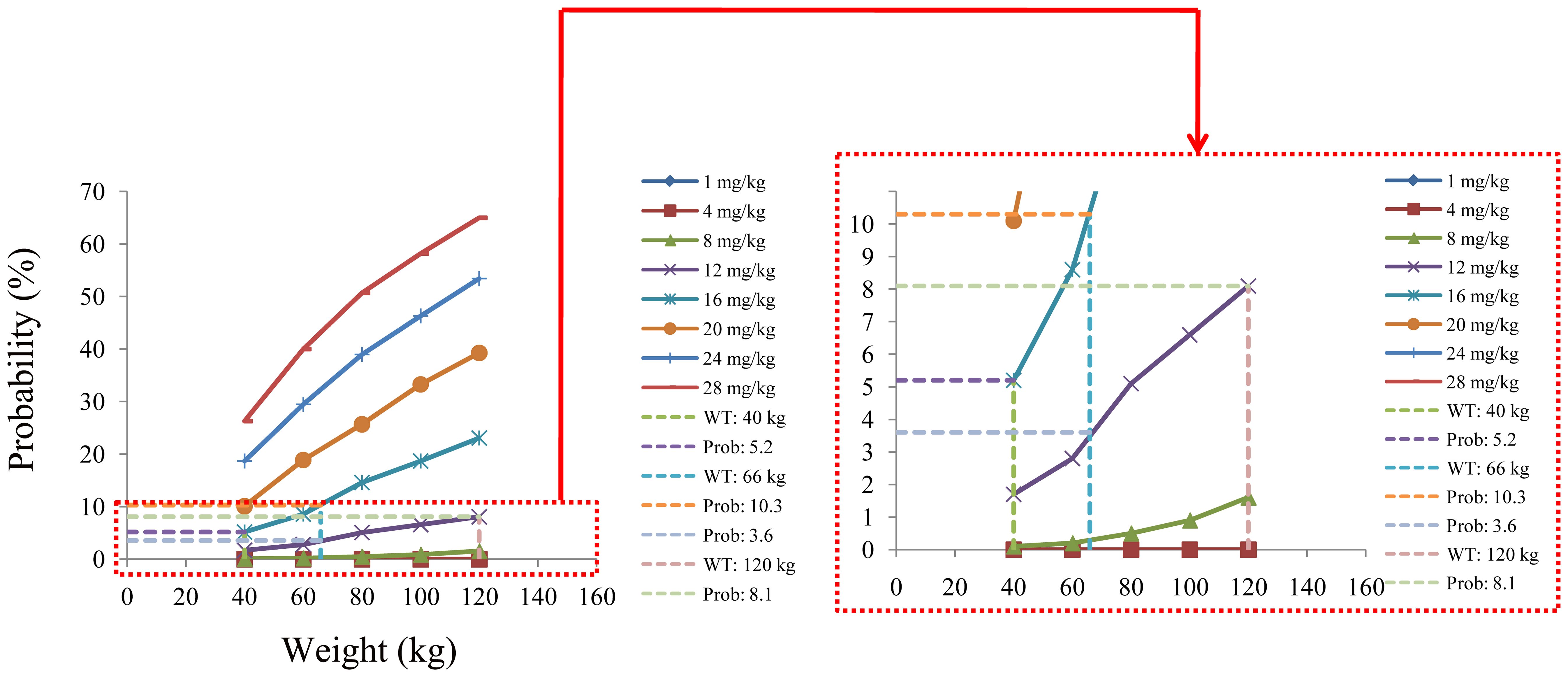
Figure 5. Probability of exceeding the upper limit of the therapeutic window of quetiapine in patients with bipolar affective disorder.
4 Discussion
Quetiapine is a dibenzodiazepine compound with a molecular structure similar to those of clozapine and perphenazine. It is not only effective in both positive and negative symptoms of schizophrenia but can also improve the cognitive function of patients. In addition, quetiapine has been used in the treatment of BAD (27). Quetiapine is rapidly absorbed by mouth, peaking at approximately 1.5 h and stabilizing within 48 h, with a half-life of approximately 7 h. The plasma protein binding rate is approximately 83%, while the bioavailability of oral tablets is close to 100%. In addition, quetiapine is completely metabolized in vivo, and its main metabolic pathway involves CYP3A4 and CYP2D6 (28). Therefore, the possible drug interactions caused by complex drug combinations in the course of clinical use deserve attention. Complex drug interactions, especially pharmacokinetic interactions, could affect the clearance rate of quetiapine and consequently affect its concentration, leading to differences in the dose requirements of different patients with BAD.
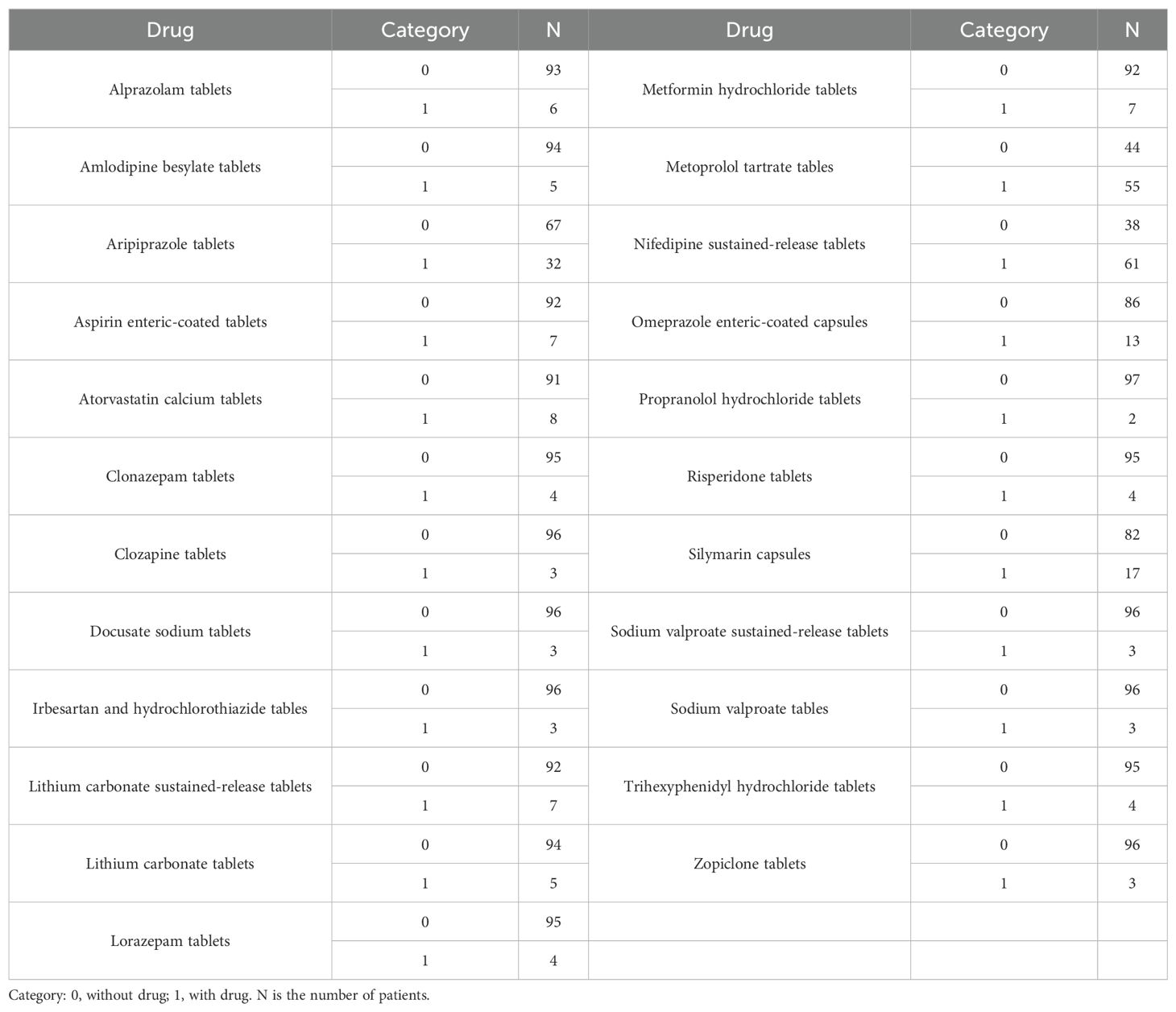
In this study, it was found that the co-medications (in tablet form, unless otherwise indicated) in patients with BAD included alprazolam, amlodipine besylate, aripiprazole, enteric-coated aspirin, atorvastatin calcium, clonazepam, clozapine, docusate sodium, irbesartan and hydrochlorothiazide, sustained-release lithium carbonate, lithium carbonate, lorazepam, metformin hydrochloride, metoprolol tartrate, sustained-release nifedipine, enteric-coated omeprazole capsules, propranolol hydrochloride, risperidone, silymarin capsules, sustained-release sodium valproate, sodium valproate, trihexyphenidyl hydrochloride, and zopiclone. To investigate whether these drug combinations and other factors affected quetiapine clearance in patients with BAD, MIPD was used.
MIPD integrated multifarious information including patients, drugs, and diseases via mathematical modeling and simulation technology, the obvious advantage of which was improving the safety, effectiveness, economy, and adherence to pharmacotherapy compared with empirical medication (29). MIPD has been widely used. For example, Zhou et al. reported on the optimization of oral isavuconazole for a population in a special physiological or pathological state using a physiologically based pharmacokinetic MIPD (35). Janssen Daalen et al. conducted a clinical trial on MIPD using machine learning for levothyroxine in general practice (30). Power-Hays et al. reported on the validation of HdxSim as a clinical decision support tool for MIPD of hydroxyurea in children with sickle cell anemia (31). Chen et al. conducted a real-world study on the initial dosage optimization of olanzapine in patients with BAD based on MIPD (32). Schatz et al. reported on the predictive performance of multi-model approaches for MIPD of piperacillin in critically ill patients (33). Villeneuve et al. reported on the rejection-free survival at 3 years of kidney transplant recipients with MIPD of mycophenolate mofetil (34). Thus, in this study, a PPK model of quetiapine was constructed for patients with BAD based on MIPD and real-world clinical data and an optimal initial dose of quetiapine for these patients was recommended.
In the final model of quetiapine in patients with BAD, the CL/F and V/F were 76.1 L/h and 530 L, respectively. The combinations of the drugs discussed above were not included into the final model affecting quetiapine clearance in patients with BAD: only body weight affected the clearance of quetiapine. Furthermore, based on Monte Carlo simulations, we predicted patients with BAD in the 40- to 120-kg weight range and found that, for those weighing 40–66 kg, the initial dose recommendation was 16 mg kg−1 day−1, the probability of reaching the therapeutic window was 78.8%–82.2%, and the probability of exceeding the upper limit of the therapeutic window was 5.2%–10.3%. For patients with BAD weighing 66–120 kg, the initial dose recommendation was 12 mg kg−1 day−1, the probability of reaching the therapeutic window was 81.5%–85.5%, and the probability of exceeding the upper limit of the therapeutic window was 3.6%–8.1%.
This study has some limitations. The influence of gene polymorphism was not considered. To the best of our knowledge, in the clinical use of quetiapine, its genotype identification is not a routine clinical test item, and the real-world data of quetiapine did not include genotype polymorphism. The MIPD model in this study was also based on real-world data to build a prediction model that can be used for real-world clinical drug use, which would more closely resemble real-world clinical use than models containing genes. In addition, the study focused on the initial dose recommendation; thus, the course of long-term treatment needs to be adjusted based on TDM in order to evaluate the efficacy and safety of quetiapine.
5 Conclusion
The present study, for the first time, recommended an initial dose of quetiapine in patients with BAD based on MIPD and real-world data, providing an individualized reference for the administration of quetiapine in patients with BAD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the data were retrospectively collected without patient identifiers.
Author contributions
Z-QZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft. Y-WJ: Conceptualization, Investigation, Methodology, Software, Validation, Writing – review & editing. DY: Writing – review & editing, Investigation, Methodology, Software, Supervision. XC: Software, Writing – original draft, Writing – review & editing. S-MH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. C-XL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. CZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. D-DW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The Xuzhou Special Fund for Promoting Scientific and Technological Innovation (No. KC23217, No. KC23254), The Medical Research Project of Jiangsu Provincial Health Commission (No. Z2023010), Jiangsu Province Education Science Planning Project (No. C/2022/01/36), Xuzhou Medical University Labor Education Special Project (No. X1d202209), Jiangsu Province Higher Education Informatization Research Topic (No. 2023JSETKT136), Xuzhou Medical University Research Topic of Higher Education Teaching Reform (No. Xjyzrd202304).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cattarinussi G, Heidari-Foroozan M, Jafary H, Mohammadi E, Sambataro F, Ferro A, et al. Resting-state functional magnetic resonance imaging alterations in first-degree relatives of individuals with bipolar disorder: A systematic review. J Affect Disord. (2024) 365:321–31. doi: 10.1016/j.jad.2024.08.040
2. Sun P, Feng S, Yu H, Wang X, Fang Y. Two hub genes of bipolar disorder, a bioinformatics study based on the GEO database. IBRO Neurosci Rep. (2024) 17:122–30. doi: 10.1016/j.ibneur.2024.07.006
3. Sun P, Wang X, Wang S, Jia X, Feng S, Chen J, et al. Bipolar disorder: Construction and analysis of a joint diagnostic model using random forest and feedforward neural networks. IBRO Neurosci Rep. (2024) 17:145–53. doi: 10.1016/j.ibneur.2024.07.007
4. Wang SM, Chang HH, Chang YH, Tsai TY, Chen PS, Lu RB, et al. Shortening of telomere length may be associated with inflammatory cytokine levels in patients with bipolar disorder. J Affect Disord. (2024) 365:155–61. doi: 10.1016/j.jad.2024.08.084
5. Zhao T, Zhang HL, Feng J, Cui L, Sun L, Li HJ, et al. Impact of UGT1A4 and UGT2B7 polymorphisms on lamotrigine plasma concentration in patients with bipolar disorder. Pharmacogenet Genomics. (2024) 34:261–7. doi: 10.1097/FPC.0000000000000543
6. Martinez B, Peplow PV. MicroRNAs as potential diagnostic biomarkers for bipolar disorder. Neural Regener Res. (2025) 20:1681–95. doi: 10.4103/NRR.NRR-D-23-01588
7. Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. Jama. (2004) 291:2581–90. doi: 10.1001/jama.291.21.2581
8. Moreira ALR, Van Meter A, Genzlinger J, Youngstrom EA. Review and meta-analysis of epidemiologic studies of adult bipolar disorder. J Clin Psychiatry. (2017) 78:e1259–69. doi: 10.4088/JCP.16r11165
9. Sharma R, Markar HR. Mortality in affective disorder. J Affect Disord. (1994) 31:91–6. doi: 10.1016/0165-0327(94)90112-0
10. Tondo L, Isacsson G, Baldessarini R. Suicidal behaviour in bipolar disorder: risk and prevention. CNS Drugs. (2003) 17:491–511. doi: 10.2165/00023210-200317070-00003
11. Baldessarini RJ, Pompili M, Tondo L. Suicide in bipolar disorder: Risks and management. CNS Spectr. (2006) 11:465–71. doi: 10.1017/s1092852900014681
12. Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. (2006) 8:625–39. doi: 10.1111/j.1399-5618.2006.00344.x
13. Pompili M, Gonda X, Serafini G, Innamorati M, Sher L, Amore M, et al. Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar Disord. (2013) 15:457–90. doi: 10.1111/bdi.12087
14. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. (2015) 72:334–41. doi: 10.1001/jamapsychiatry.2014.2502
15. Hao Y, Zhang J, Yang L, Zhou C, Yu Z, Gao F, et al. A machine learning model for predicting blood concentration of quetiapine in patients with schizophrenia and depression based on real-world data. Br J Clin Pharmacol. (2023) 89:2714–25. doi: 10.1111/bcp.15734
16. Terao I, Yokoi A, Fukushima H. Comparative efficacy of quetiapine by dose and formulation for psychosis in schizophrenia: A systematic review and dose-response model-based network meta-analysis. J Psychopharmacol. (2023) 37:953–9. doi: 10.1177/02698811231200020
17. Dias BB, Carreno F, Helfer VE, Olivo LB, Staudt KJ, Paese K, et al. Pharmacokinetic/pharmacodynamic modeling of cortical dopamine concentrations after quetiapine lipid core nanocapsules administration to schizophrenia phenotyped rats. CPT Pharmacometrics Syst Pharmacol. (2024) 13:638–48. doi: 10.1002/psp4.13107
18. Kim SH, Jung DU, Kim DH, Lee JS, Lee KU, Won S, et al. Efficacy and safety of lurasidone vs. Quetiapine XR in acutely psychotic patients with schizophrenia in korea: A randomized, double-blind, active-controlled trial. Psychiatry Investig. (2024) 21:762–71. doi: 10.30773/pi.2024.0052
19. Ferrari M, Godio M, Martini S, Callegari C, Cosentino M, Marino F. Inflammatory markers at baseline correlate with subsequent clinical response to quetiapine in patients with bipolar disorder. Hum Psychopharmacol. (2022) 37:e2854. doi: 10.1002/hup.2854
20. Sahraian A, Ghahremanpouri B, Mowla A. Is quetiapine effective for obsessive and compulsive symptoms in patients with bipolar disorder? A randomized, double-blind, placebo-controlled clinical trial. CNS Spectr. (2022) 27:634–8. doi: 10.1017/S1092852921000572
21. Qi L, Qiu Y, Li S, Yi N, Li C, Teng Z, et al. Single-cell immune profiling reveals broad anti-inflammation response in bipolar disorder patients with quetiapine and valproate treatment. iScience. (2023) 26:107057. doi: 10.1016/j.isci.2023.107057
22. Lin M, Zhang Y, Lv D, Xu N, Yang X, Liu X, et al. The impact of CYP3A5*3 on oral quetiapine: A population pharmacokinetic model in Chinese bipolar disorder patients. J Affect Disord. (2024) 351:309–13. doi: 10.1016/j.jad.2024.01.170
23. Zhou D, Bui KH, Li J, Al-Huniti N. Population pharmacokinetic modeling of quetiapine after administration of seroquel and seroquel XR formulations to Western and Chinese patients with schizophrenia, schizoaffective disorder, or bipolar disorder. J Clin Pharmacol. (2015) 55:1248–55. doi: 10.1002/jcph.544
24. Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. (2008) 48:303–32. doi: 10.1146/annurev.pharmtox.48.113006.094708
25. Chen X, Wang J, Lan J, Ge X, Xu H, Zhang Y, et al. Initial sirolimus dosage recommendations for pediatric patients with PIK3CD mutation-related immunodeficiency disease. Front Pharmacol. (2022) 13:919487. doi: 10.3389/fphar.2022.919487
26. Shen W, Hu K, Shi HZ, Jiang L, Zhang YJ, He SM, et al. Effects of sex differences and combined use of clozapine on initial dosage optimization of valproic acid in patients with bipolar disorder. Curr Pharm Des. (2024) 30:2290–302. doi: 10.2174/0113816128323367240704095109
27. Fauska C, Bastiampillai T, Adams RJ, Wittert G, Eckert DJ, Loffler KA. Effects of the antipsychotic quetiapine on sleep and breathing: a review of clinical findings and potential mechanisms. J Sleep Res. (2024) 33:e14051. doi: 10.1111/jsr.14051
28. DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. (2001) 40:509–22. doi: 10.2165/00003088-200140070-00003
29. Hu K, Fu M, Huang X, He S, Jiao Z, Wang D. Editorial: Model-informed drug development and precision dosing in clinical pharmacology practice. Front Pharmacol. (2023) 14:1224980. doi: 10.3389/fphar.2023.1224980
30. Janssen Daalen JM, Doesburg D, Hunik L, Kessel R, Herngreen T, Knol D, et al. Model-informed precision dosing using machine learning for levothyroxine in general practice: development, validation and clinical simulation trial. Clin Pharmacol Ther. (2024) 116:824–33. doi: 10.1002/cpt.3293
31. Power-Hays A, Dong M, Punt N, Mizuno T, Smart LR, Vinks AA, et al. Rationale, development, and validation of hdxSim, a clinical decision support tool for model-informed precision dosing of hydroxyurea for children with sickle cell anemia. Clin Pharmacol Ther. (2024) 116:670–7. doi: 10.1002/cpt.3119
32. Chen X, Hu K, Shi HZ, Chen L, Zhang YJ, He SM, et al. Initial dosage optimization of olanzapine in patients with bipolar disorder based on model-informed precision dosing: a study from the real world. Front Pharmacol. (2024) 15:1444169. doi: 10.3389/fphar.2024.1444169
33. Schatz LM, Greppmair S, Kunzelmann AK, Starp J, Brinkmann A, Roehr A, et al. Predictive performance of multi-model approaches for model-informed precision dosing of piperacillin in critically ill patients. Int J Antimicrob Agents. (2024) 64(4):107305. doi: 10.1016/j.ijantimicag.2024.107305
34. Villeneuve C, Humeau A, Monchaud C, Labriffe M, Rerolle JP, Couzi L, et al. Better rejection-free survival at three years in kidney transplant recipients with model-informed precision dosing of mycophenolate mofetil. Clin Pharmacol Ther. (2024) 116:351–62. doi: 10.1002/cpt.3206
35. Zhou J, Xu B, Zheng Y, Huang H, Wei Z, Chen S, et al. Optimization of oral isavuconazole dose for population in special physiological or pathological state: a physiologically based pharmacokinetics model-informed precision dosing. J Antimicrob Chemother. (2024) 79(9):2379–89. doi: 10.1093/jac/dkae240
Keywords: model-informed precision dosing, quetiapine, bipolar affective disorder, initial dose recommendation, real-world data
Citation: Zheng Z-Q, Jin Y-W, Yin D, Chen X, He S-M, Liu C-X, Zhang C and Wang D-D (2024) Model-informed precision dosing of quetiapine in bipolar affective disorder patients: initial dose recommendation. Front. Psychiatry 15:1497119. doi: 10.3389/fpsyt.2024.1497119
Received: 25 September 2024; Accepted: 11 November 2024;
Published: 04 December 2024.
Edited by:
Barbara Carpita, University of Pisa, ItalyReviewed by:
Rafal Roman Jaeschke, Jagiellonian University Medical College, PolandDu Lei, University of Cincinnati, United States
Copyright © 2024 Zheng, Jin, Yin, Chen, He, Liu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su-Mei He, aGVoZTgyMDRAMTYzLmNvbQ==; Chen-Xu Liu, MTgzNjEyMDcxNzlAMTYzLmNvbQ==; Cun Zhang, MTc1MTI2NTYzNjVAMTYzLmNvbQ==; Dong-Dong Wang, MTM4NTIwMjk1OTFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zi-Qiang Zheng1†
Zi-Qiang Zheng1† Xiao Chen
Xiao Chen Su-Mei He
Su-Mei He Cun Zhang
Cun Zhang Dong-Dong Wang
Dong-Dong Wang