- 1Centre for Psychiatry and Psychotherapy, Justus-Liebig University Giessen, Giessen, Germany
- 2Centre for Mind, Brain and Behaviour (CMBB), University of Marburg and Justus-Liebig University Giessen, Marburg, Germany
- 3Department of Psychiatry and Psychotherapy, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany
Introduction: Transcranial alternating current stimulation (tACS) is a promising tool for modulating brain oscillations. This study investigated whether 5 Hz tACS could modulate neural oscillations in the prefrontal cortex and how this modulation impacts performance in working memory (WM) tasks.
Method: In two sessions, 28 healthy participants received 5 Hz tACS or sham stimulation over the left dorsolateral prefrontal cortex (DLPFC) while performing tasks with high and low WM loads. Resting-state EEG was recorded before and after stimulations for 5 minutes. EEG power was measured at electrodes surrounding the stimulation site.
Results: The results showed that tACS significantly improved reaction time (RT) compared to sham stimulation. This effect was task-specific, as tACS improved RT for hit responses only in high WM load trials, with no impact on low-load trials. Moreover, tACS significantly increased EEG power at 5 Hz and in the theta band compared to pre-stimulation levels.
Discussion: These findings demonstrate that tACS applied over left DLPFC modulates post-stimulation brain oscillations at the stimulation sites – known as tACS after-effects. Furthermore, the results suggest that 5 Hz tACS enhances response speed by elevating task-related activity in the prefrontal cortex to an optimal level for task performance.
Conclusion: In summary, the findings highlight the potential of tACS as a technique for modulating specific brain oscillations, with implications for research and therapeutic interventions.
1 Introduction
Working memory (WM) is a complex cognitive process that temporarily stores and manipulates information required for various cognitive functions including language comprehension, learning, problem-solving, and decision-making (1, 2). Deficits in WM leading to diminished cognitive activities have been observed in several neuropsychiatric and neurological disorders, such as depression (3, 4), schizophrenia (5–7), ADHD (8, 9), and Alzheimer’s disease (10, 11). Neuroimaging studies have identified brain regions, including the prefrontal cortex, superior and inferior parietal lobules, and the inferior temporal cortex, that underlie different components of WM (12–15). Moreover, electroencephalography (EEG) and magnetoencephalography (MEG) studies have demonstrated that neural oscillations within these brain regions play a major role in facilitating WM processing. WM is associated with enhanced oscillations and neural synchrony across multiple frequencies (16–19). However, there is evidence that specific frequency bands are particularly relevant to aspects of working memory. Notably, theta oscillations in the dorsolateral prefrontal cortex (DLPFC) have been found to be associated with WM performance (20–22). Alekseichuk et al. (16) found that synchronized theta and high gamma oscillations in the prefrontal cortex are essential for efficient spatial working memory function, particularly under higher cognitive demands. Theta appears to act as a coordinating rhythm and a potential organizer of working memory processing (16, 23).
The association between DLPFC theta oscillations and WM has been investigated by attempting to modulate oscillations within DLPFC using non-invasive brain stimulation techniques. Among brain stimulation techniques, transcranial alternating current stimulation (tACS) emerges as a promising tool in research and therapeutic endeavors due to its ability to modulate cortical excitability using low-intensity electrical currents (24). tACS delivers alternating electric current to specific brain regions through scalp electrodes affecting the neural oscillations at a particular frequency (25–28). tACS is believed to interact with ongoing rhythmic cortical activities and directly influence cognitive processes by entraining underlying neural oscillations at the frequency of stimulation (29, 30). The ability of tACS to modulate endogenous oscillations at stimulated frequency allows more direct and selective enhancements of processes underlying cognitions. Indeed, numerous studies reported that tACS enhanced performance in working memory (31–33), learning (34, 35), and decision-making (36, 37). Moreover, selective entrainment of specific neural oscillations through tACS has significant clinical implications as it could aid individuals with psychiatric and neurological disorders. Recent clinical applications of tACS have shown encouraging results in alleviating negative symptoms in several psychiatric conditions such as depression (38–40) and schizophrenia (28, 41).
Previous studies investigating the effects of tACS over DLPFC on working memory have produced mixed results (for details review Booth et al. (42); Senkowski et al. (43)). Several studies have reported that tACS applied simultaneously over frontal and parietal areas significantly improved WM performance in healthy individuals (18, 19, 30, 31, 44–47). However, studies examining the effects of tACS applied solely or separately over brain areas overlying the DLPFC are limited and the findings are inconsistent. For instance, Jaušovec et al. (45) observed positive effects of theta tACS on WM performance across different paradigms of WM when delivered over the frontal or parietal cortex. Jaušovec and Jaušovec (31) found that theta tACS over the left parietal brain area significantly increased WM capacity compared to sham tACS; however, no such effect was observed for tACS over the left frontal region. Our previous study found that 5 Hz tACS over the left DLPFC significantly improved performance in high-load WM tasks whereas tACS did not affect performance in low-load tasks (Rauh et al. (33)). These varying findings highlight the complexity of tACS effects on working memory and suggest that its impact may depend on multiple factors. Further research is needed to better understand the conditions under which tACS might influence WM performance and cognitive function.
Besides the effects of tACS on cognitive functions, a key area of research in tACS is its after-effects - lasting changes in brain activity and cognitive performance after the stimulation ends. Among the various frequencies used in tACS, theta and alpha frequencies have garnered particular interest due to their association with working memory, attention, and other cognitive functions. However, the existence and nature of tACS after-effects are debated, with studies reporting both positive and null results. For instance, Zaehle et al. (30) and Vossen et al. (29) found that alpha tACS improved cognitive performance and significantly increased alpha power compared to sham stimulation even 30 minutes after the stimulation ended. In contrast, Lafleur et al. (48) found no after-effects following 10 Hz and 20 Hz tACS applied over sensorimotor regions. Although many studies have explored tACS after-effects at alpha frequency (for a detailed review see De Koninck et al. (49)), research on tACS after-effects at theta frequency is scarce. D’Atri et al. (50) applied 5 Hz tACS to bilateral frontotemporal areas and observed significant increases in theta power in the parietooccipital area compared to the sham stimulation. Conversely, Pahor and Jaušovec (32) found a decrease in theta power measured 25 minutes after theta tACS stimulation. Briley et al. (51) reported increased task-related frontal theta power following theta tACS but observed no significant differences in resting theta power at 5 and 12 minutes post-stimulation compared to sham. These disparate findings highlight the need for further research to explore how to achieve sustained after-effects with theta tACS.
The present study investigates how targeted modulation of oscillations in the DLPFC influences task performance in healthy individuals. Building on our previous findings (Rauh et al. (33)), we applied 5 Hz (theta) tACS over the left DLPFC and examined its effects on performance in WM tasks. We hypothesized that theta tACS applied to the DLPFC would enhance performance in WM tasks compared to sham stimulation. Additionally, we explored whether theta tACS induces lasting changes in neural oscillations beyond the stimulation period - referred to as tACS after-effects. To this end, resting-state EEG was collected before and after the stimulations, and spectral power was measured from the EEG data. We anticipated an increase in post-stimulation theta power compared to pre-stimulation (baseline) levels. Furthermore, we investigated whether this enhancement in power was specific to the frequency of tACS.
2 Materials and methods
2.1 Participants
Twenty-eight healthy individuals participated in this study (Male = 10, Mean age = 32.5, SD = 13.22). Individuals with a history of neurological or psychiatric disease were excluded. All had normal or corrected-to-normal vision. They received compensation for their participation in the study. Each participant gave written informed consent to participate in the study. The Ethics Committee of the Justus-Liebig University Giessen reviewed and approved the study protocol.
2.2 Working memory tasks
The delayed match-to-sample task that examines load effects in visual WM was used in this study (33). The stimuli consisted of non-natural visual objects, specifically blurred outlines of random Tetris shapes (BORTs), presented under two conditions with varying WM loads. BORTs were chosen for their novelty and difficulty in verbalizing. The high-load condition featured four visual objects, while the low-load condition included two. To prevent recognition of previously viewed stimuli and subsequent ceiling effects, a large set of 504 BORTs was generated using a custom MATLAB script (448 for the experimental session and 56 for training). Within each session, no stimulus was repeated except for matching probe stimuli.
2.3 Procedure
The experiment was conducted in an electrically shielded and acoustically attenuated cabin. Participants were seated at a table, and the stimulus was presented using Presentation software (Neurobehavioral Systems, Berkeley, United States). They performed the delayed match-to-sample task described above. Each trial consisted of three phases: encoding, maintenance, and retrieval (Figure 1). During the encoding phase, either two (low-load condition) or four (high-load condition) different visual objects were shown sequentially for 600 ms each, resulting in an encoding phase duration of 1.2 to 2.4 s depending on the condition. Following the encoding phase, a fixation cross was displayed for 2 s, during which participants were instructed to memorize the displayed items (maintenance phase). In the retrieval phase, a probe stimulus was presented for 2 s, and participants were asked to indicate as quickly and accurately as possible whether this probe stimulus had been shown during the encoding phase. Responses were made via button press with the left index finger for a mismatch and the right index finger for a match. The intertrial interval was set to 3.5 s. The position of the target stimulus (first, second, third, or fourth in the encoding phase) was equally distributed and remained constant within each trial set. Each experimental session consisted of two blocks of 80 trials (40 trials per condition), presented in a randomized order. At the beginning of each experimental session, a training session of 16 trials was conducted to familiarize participants with the task. This study employed a cross-over design in which each subject participated in both sham and theta tACS - spaced 7 days apart. The same procedure was repeated for both sham and theta sessions. The order of the sham and tACS sessions was pseudo-randomized and balanced across participants. Each experimental session lasted approximately 50 minutes.
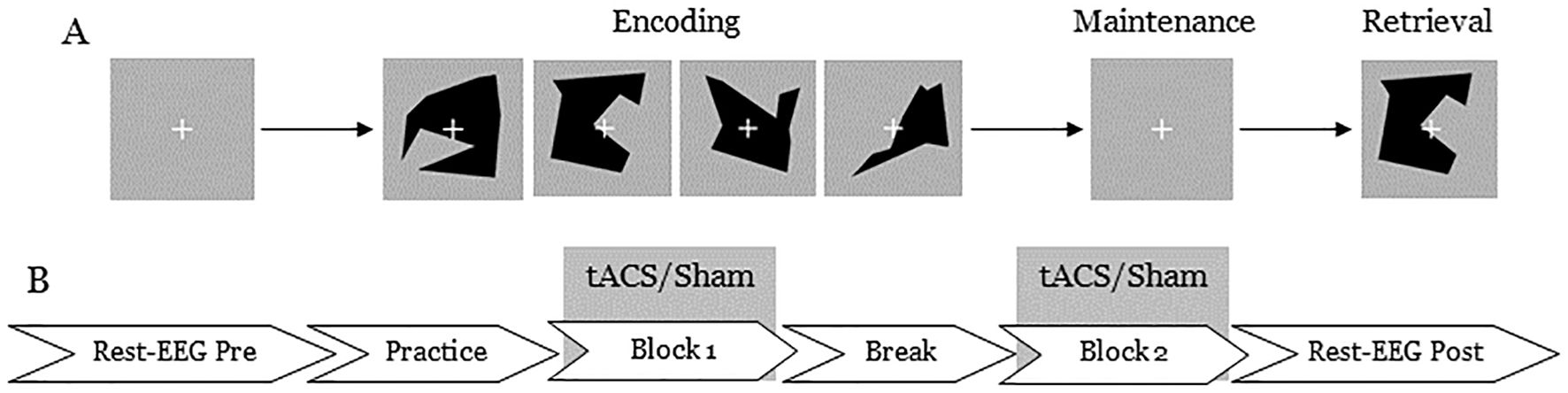
Figure 1. An illustration of (A) the delayed match-to-sample task used in the experiment and (B) stimulation and EEG data recording protocol. (A) Each trial began with a fixation cross for 2 seconds, followed by either 2 (low-load) or 4 (high-load) visual objects. Then, another fixation cross was presented for 2 seconds, followed by a probe stimulus. Participants responded by pressing a button to indicate whether they had seen the probe stimulus earlier. Each block lasted approximately 20 minutes. (B) Resting state EEG was collected for 5 minutes before and after the stimulation.
2.4 tACS parameters
A DC Stimulator MC (neuroConn GmbH, Ilmenau, Germany) was used for the electrical stimulation. The stimulation was delivered to the scalp area overlying the left DLPFC at 5 Hz. The 5 Hz stimulation frequency was chosen because, as discussed above, the effects of tACS at a slow theta frequency on brain rhythms have not been extensively studied. Moreover, 5 Hz oscillation has been used as an approximation of theta activity in previous studies employing the visual delayed match-to-sample paradigm, which is comparable to the task used in the present study (33, 52). We targeted the left DLPFC at coordinates (x, y, z = −40, 37, 24) in MNI space, identified in a previous study by Rauh et al. (33). Consequently, we deployed a “left frontal” high-definition model comprising three return electrodes positioned at F1, FC5 and AF3, and one stimulating electrode at F3. The anodal rubber electrode had a diameter of 2 cm and was surrounded by 3 “cathodal” Ag/AgCl electrodes. First, an EEG cap was placed on the participant’s head, and the target position for the rubber electrode was marked with a pen. The cap was then removed, and the rubber electrode was attached to the marked position using Ten20 paste (Weaver and Company, Aurora, USA). Subsequently, the EEG cap was refitted onto the head, with the remaining stimulation electrodes integrated directly into the cap. To minimize impedance, Signagel® Electrode Gel (Parker Laboratories, Fairfield, USA) was applied. To achieve a stimulation current of 1.5 mA (peak-to-peak for tACS) at F3, the stimulation currents were weighted -690 μA at F1 and -405 μA at both FC5 and AF3. The 5 Hz stimulation included a 10-second ramp-up and a 10-second ramp-down period. For the sham condition, the stimulation protocol included a 10-second ramp-up, 10 seconds of stimulation, and a 30-second ramp-down. In the sham stimulation, the current was turned off after the ramp-down period and remained off for the rest of the stimulation period. The stimulation session lasted 21 minutes.
2.5 EEG data recording and preprocessing
EEG data were recorded with 64 Ag/AgCl electrodes mounted on an elastic cap (ActiCaps, Brain Products, Munich, Germany), using the Brain Vision Recorder software version 1.21.0303 (Brain Products, Munich, Germany). The TP10 electrode was used as reference and the EEG data were sampled at 5000 Hz. Impedances were kept below 10 kΩ. EEG data were collected in three blocks: (1) baseline resting EEG for 5 minutes, (2) active or sham stimulation, (3) post-stimulation resting EEG for 5 minutes. For the resting EEG, the participants were instructed to close their eyes for 5 minutes. During the stimulation, they performed the task described above.
EEG data were preprocessed using the EEGLAB toolbox (53). EEG data were down-sampled to 500 Hz and bandpass filtered (0.5–100 Hz). The cleanLine plugin of EEGLAB was used to remove the 50 Hz electrical line noise. Channels containing excessive noise or artifacts were identified and removed using the EEGLAB plug-in FASTER (54). Across all participants, on average 2.10 (SD = 1.09, range 0–5) channels per subject were removed. Independent component analysis (ICA) was performed on continuous EEG data to remove ocular artifacts and generic noise. Artifactual independent components (ICs) were identified and removed from the data using the ICLabel plug‐in (55) of EEGLAB and through visual inspection of individual ICs. On average, 13.2 ICs (SD = 5.62, range 7-29) per subject were removed from the data. EEG data were then segmented into 1‐s epochs. Epochs containing artifacts were removed using a voltage threshold (± 100μV) rejection. This procedure removed on average 21.2 (SD = 40.3) epochs per subject. After artifact rejection, any missing channels were interpolated using spherical interpolation. The epoched data were then re-referenced to the average of all channels.
2.6 Spectral power analysis
The EEG epochs were processed using Welch’s method to estimate the PSD for each channel. Specifically, we applied a Hanning window of 0.5 seconds with a 0.25-second overlap to each epoch. The resulting PSD values (µV²/Hz) were averaged across all epochs for each channel and log-transformed (log10). To avoid negative values, 1 was added to the power values before the log transformation.
Power was extracted at 5 Hz and for the theta (4–6 Hz), delta (1–3 Hz), and alpha (7–12 Hz) bands. Finally, power was averaged across a cluster of electrodes (FC1, FC3, F5, Fz) surrounding the stimulation site (F3) in each frequency band of interest. Since the FC1, FC3, F5, and Fz electrodes surround the stimulation site (F3), analyzing power across this cluster allows for a more comprehensive assessment of the effects of stimulation on the targeted region. Although the primary frequency of interest was 5 Hz, we also analyzed power in the delta, theta, and alpha bands to assess whether the power modulation was specific to the stimulation frequency.
2.7 Statistical analyses procedure
We recorded response accuracy and reaction time for behavioral data analysis. We used d-prime values as outcome measures for response accuracy. D-prime, a discriminability index adapted from signal detection theory (56), measures the ability to correctly identify targets while minimizing false alarms and has been shown to have high sensitivity to detecting true signals or targets (57). Participants’ responses were categorized as either hits, misses, false alarms, or correct rejections. These response types were used to calculate d-prime metrics. Hit rate (H) was calculated as the proportion of correctly identified targets (i.e., H = Hits/Hits+Misses), and false alarm rate (FA) was the proportion of incorrect identifications (FA = False Alarms/False Alarms+Correct Rejections). To avoid issues with extreme values (e.g., hit rates of 1.0 or false alarm rates of 0), values were adjusted following standard practices: if H or FA equaled 1.0 or 0, they were adjusted by 1/2N, where N represented the number of trials. The d-prime index was then computed as the difference between the z-transformed hit rate and false alarm rate (d’ = Z(H)−Z(FA)). A repeated measures ANOVA was conducted with factors of Stimulation (Sham, tACS) and Task-load (Low, High) on d-prime scores. Reaction time (RT) was defined as the interval between the probe stimulus and the button press. RTs were also calculated for the 4 categories of responses. We performed a repeated measures ANOVA with factors of Stimulation (Sham, tACS), Task-load (Low, High), and Response (Hit, Miss, False Alarm, Correct Rejection). Baseline and post-stimulation EEG spectral power were compared using a repeated measures ANOVA with factors of Stimulation (Sham, tACS) and Time (Baseline, Post-stimulation). ANOVA was performed separately for each measured frequency. Follow-up analyses were performed to compare baseline and post-stimulation EEG spectral power using paired two-tailed t-tests. Power and behavioral data were normally distributed according to Shapiro-Wilk tests. Throughout the analyses, extreme scores (values exceeding ±3 SD from the mean) were excluded as outliers. Statistical analyses were performed using R.
3 Results
3.1 D-prime
Table 1 presents the means and standard deviations of the d-prime score. The ANOVA with factors Stimulation (Sham, Theta) and Task-load (Low, High) on d-prime scores revealed a main effect of Task-load (F(1, 26) = 131.66, p <.001, η2p = .835). Post hoc comparison with Bonferroni corrections showed that performance was significantly higher in low-load trials compared to high-load trials in both Sham (T(26) = 11.31, p <.001) and tACS (T(26) = 8.90, p <.001) conditions. Neither the main effect of Stimulation (F(1, 26) = .13, p = .725, η2p = .005) nor the Stimulation x Task-load interaction (F(1, 26) = .09, p = .769, η2p = .003) was significant. The results suggest that participants had higher accuracy in low-load trials and made more errors in high-load trials regardless of stimulation conditions (Figure 2A).
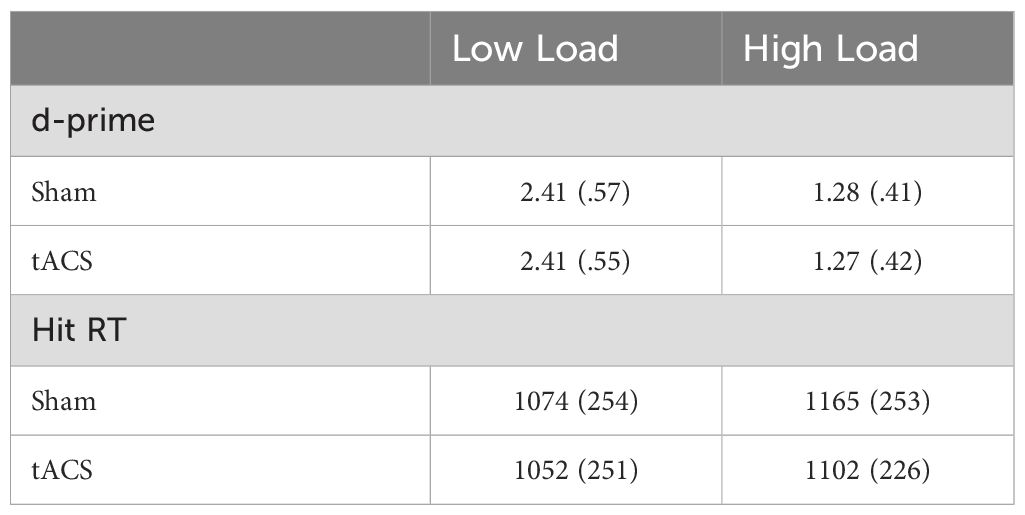
Table 1. Means and standard deviations (in parentheses) of d-prime scores and reaction times (ms) for correct responses in low- and high-load trials for Sham and tACS conditions.

Figure 2. Effects of Sham and tACS stimulations on working memory performance, measured by d-prime (A) and reaction time (RT) for hit responses (B). The violin plots display the distribution of individual data points shown as jittered dots. Red diamonds mark the mean and red error bars show the standard error. Significant differences between specific conditions are denoted by asterisks above the plots. * p <.05.
3.2 Reaction time
The three-way repeated-measures ANOVA of reaction time revealed the main effect of Task-load (F(1, 27) = 41.13, p <.001, η2p = .604), resulting from overall faster response for low load trials compared to the high load trials. The main effect of Response was also significant (F(3, 81) = 4.71, p = .004, η2p = .148). The main effect of Stimulation was not significant (F(1, 27) = 1.73, p = .199, η2p = .060). However, the Stimulation x Task-load x Response interaction was significant (F(3, 81) = 4.31, p = .007, η2p = .138). To explore the interaction, follow-up Stimulation x Task-load ANOVA was performed within each response category. The ANOVA for Hit response revealed a main effect of Stimulation (F(1, 27) = 4.93, p = .035, η2p = .154) and Task-load (F(1, 27) = 30.82, p <.001, η2p = .533). The Stimulation x Task-load interaction was also significant (F(1, 27) = 4.52, p = .043, η2p = .143). Post hoc comparisons after the Bonferroni correction showed that participants made significantly faster hit responses in high-load trials during active tACS than Sham stimulation (T(27) = 3.58, p = .008) (Figure 2B). In contrast, there was no significant difference in RTs for hit responses in low-load trials between Sham and active tACS stimulation conditions (T(27) = .86, p = .395) (Figure 2B). Table 1 presents a summary of RTs for hit responses. The ANOVA for Miss response revealed a main effect of Task-load (F(1, 27) = 11.64, p = .002, η2p = .301), resulting from faster overall RT in Low-load (M = 1027) compared to High-load (M = 1101) trials across stimulations. The main effect of Stimulation (F(1, 27) = .81, p = .375, η2p = .029) and Stimulation x Task-load interaction (F(1, 27) = .37, p = .55, η2p = .013) were not significant. The ANOVA for False alarms response revealed a main effect of Task-load (F(1, 27) = 10.26, p = .003, η2p = .277), resulting from higher overall RT in High-load (M = 1207) compared to Low-load (M = 937) trials across stimulations. The main effect of Stimulation (F(1, 27) = .77, p = .388, η2p = .028) and Stimulation x Task-load interaction (F(1, 27) = .003, p = .953, η2p = .00) were not significant. The ANOVA for Correct rejections response revealed a main effect of Task-load (F(1, 27) = 63.53, p <.001, η2p = .702), resulting from higher overall RT in High-load (M = 990) compared to Low-load (M = 878) trials across stimulations. The main effect of Stimulation (F(1, 27) = .44, p = .51, η2p = .016) and Stimulation x Task-load interaction (F(1, 27) = .69, p = .414, η2p = .025) were not significant. In summary, the RT analyses revealed that participants responded faster overall in low-load trials, and tACS stimulation specifically accelerated responses when participants correctly recognized stimuli presented during the encoding phase in the high-load trials. However, tACS did not affect error response times and the RTs related to the correct rejection of the stimuli. These behavioral results suggest that tACS may enhance the speed of accurate responses, particularly in high-load trials. However, the results should be interpreted cautiously rather than as conclusive evidence of a load-specific effect. Instead, they point to a promising trend that warrants further investigation, with additional data needed to confirm the reliability and replicability of the observed effects.
3.3 Spectral power
For power at the stimulation frequency of 5 Hz, the ANOVA revealed a main effect of Time (F(1, 27) = 7.15, p = .013, η2p = .210). Neither the main effect of Stimulation (F(1, 27) = .13, p = .723, η2p = .005) nor the Stimulation x Time interaction (F(1, 27) = .003, p = .952, η2p = .00) was significant. We further explored the main effect of Time by comparing baseline and post-stimulation power. Based on our a priori hypotheses, we conducted paired t-tests within each condition to investigate potential condition-specific effects. In the active tACS condition, post-stimulation power (M = .814, SD = .29) was significantly higher than baseline power (M = .778, SD = .28) at the stimulation frequency of 5 Hz (T(27) = 2.78, p = .01, Cohen’s d = .526). In contrast, no significant difference was observed between baseline (M = .805, SD = .30) and post-stimulation (M = .770, SD = .27) powers in the sham condition (T(27) = 1.71, p = .098, Cohen’s d = .324). The ANOVA comparing baseline and post-stimulation theta band power in sham and tACS stimulation conditions also revealed a main effect of Time (F(1, 27) = 5.17, p = .031, η2p = .160). The main effect of Stimulation (F(1, 27) = .28, p = .601, η2p = .01) and Stimulation x Time interaction (F(1, 27) = .306, p = .585, η2p = .011) were not significant. In the active tACS condition, post-stimulation (M = .847, SD = .24) theta power was significantly higher than baseline (M = .806, SD = .23) theta power (T(27) = 2.49, p = .019, Cohen’s d = .470). However, theta power did not differ between baseline (M = .802, SD = .22) and post-stimulation (M = .829, SD = .26) in the sham condition (T(27) = 1.26, p = .218, Cohen’s d = .239). Comparisons of baseline and post-stimulation power did not show significant differences in the delta and alpha bands for either the active tACS or sham conditions (all p >.05). Spectral power analyses demonstrated that 5 Hz tACS induced a significant increase in power and the power modulation was specific to the stimulation frequency (Figure 3).
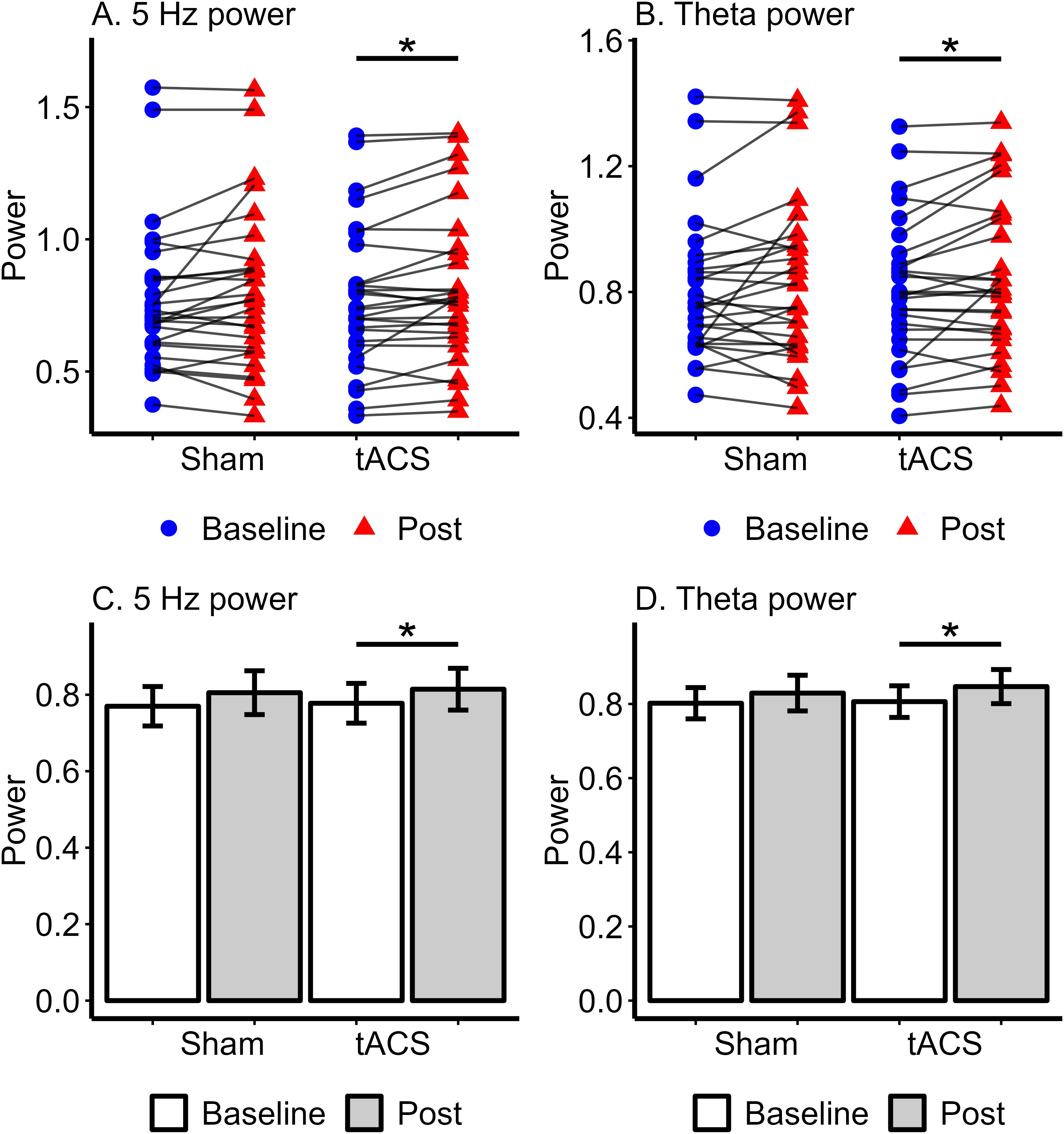
Figure 3. Spectral power at 5 Hz and theta band in Sham and tACS stimulation conditions. The upper (A, B) show individual power values with lines connecting baseline (pre-stimulation) and post-stimulation powers for each participant. (A) represents 5 Hz power, while (B) shows Theta power, with statistical significance indicated by an asterisk (*) between the baseline and post-stimulation powers in the tACS condition. The lower (C, D) present bar plots of mean power with standard error for each condition. (C) shows 5 Hz power, and (D) shows Theta power, with an asterisk (*) highlighting significant differences between baseline and post-tACS power. * p <.05.
4 Discussion
This study investigated how tACS at a slow theta frequency (5 Hz) modulated brain rhythms and task performance. Sham or 5 Hz tACS was applied to the left DLPFC while participants performed WM tasks with varying cognitive loads. RT and accuracy were measured during both tACS and sham conditions to assess task performance. Resting state EEG was collected before and after the stimulations, and spectral power was computed from EEG data. The results showed that tACS significantly enhanced task performance, particularly in high-load trials, as demonstrated by faster reaction times. Additionally, there was a significant increase in post-tACS spectral power at 5 Hz and in the theta band. This study builds on our previous work (33), in which we demonstrated that tACS at a slow theta frequency (5 Hz) over the left DLPFC improved working memory performance under high cognitive load conditions. In the present study, we found selective enhancement of RT in WM tasks. Additionally, we extend our earlier research by showing a significant increase in post-tACS spectral power, indicative of after-effects.
Our findings demonstrated that tACS improved RT in WM tasks selectively for cognitively demanding tasks. Specifically, response speed in high-load trials was significantly faster in the tACS condition compared to the sham condition. Notably, there were no effects of any stimulation on accuracy, suggesting that the improved response speed in high-load trials in the tACS condition did not involve a speed-accuracy trade-off. In a previous study, we found that 5 Hz tACS preferentially enhanced performance on high WM load tasks (33). Furthermore, our results are consistent with numerous previous studies reporting that tACS administered in the alpha (25, 36), theta (19, 33), and gamma (58, 59) ranges positively impacted cognitive functions. Hoy et al. (58) found that gamma-tACS improved WM performance only for stimuli that required greater cognitive load. The selective improvement of cognitive functions following tACS has also been reported in other domains (36, 59). The selective improvement in WM performance induced by tACS may result from the enhanced synchronization of neural oscillations. tACS is believed to entrain endogenous oscillations by enhancing coherence and synchronization of neuronal oscillations within and across brain regions (60, 61). The improved synchronization facilitates more efficient neural communication, which is particularly beneficial for cognitively demanding tasks in which the need for coordinated neural activity is higher. Furthermore, neural synchronization is considered pivotal in representing information in WM (62–64). The complex cognitive processes underlying WM are mediated by oscillatory activity across multiple frequency bands, involving both independent contributions and cross-frequency coupling (16–18, 21, 22). However, evidence suggests that specific frequency bands may play a more critical role in certain aspects of WM. Notably, frontal theta power is associated with memory load, with increased power correlating with the number of items maintained (65, 66). These findings highlight the facilitatory role of increased theta power in WM performance under higher cognitive load. Moreover, the absence of effects in the low-load condition is not surprising, given that low cognitive demand often leads to high performance and may cause ceiling effects. Therefore, the mechanism of tACS in enhancing neural synchrony likely accounts for the selective improvement of performance in WM tasks observed in this study. Our results illustrate the involvement of DLFPC in visual WM and the importance of theta oscillations in WM processes.
We found that 5 Hz tACS over the left DLPFC significantly increased spectral power post-stimulation compared to the pre-stimulation baseline level. The power enhancement, known as after-effects, was limited to the theta band and did not occur in the adjacent delta and alpha frequency bands. Our findings are consistent with the idea that tACS modulates brain oscillations in the frequency band corresponding to the stimulation frequency (25, 29, 30, 50). Although the exact neuronal mechanism of tACS is not fully understood, current research suggests that the after-effects may be primarily driven by neuronal entrainment or synaptic plasticity (26, 67, 68). The neuronal entrainment theory suggests that continuous stimulation with an oscillating current causes more individual neurons to synchronize their activity with the external rhythm, leading to a power increase across the entire network (68). It further suggests that neurons oscillating within the stimulation frequency range will become synchronized and entrained, while those with intrinsic frequencies outside this range will remain unaffected (68). Our findings may be explained by neuronal entrainment, as elevated power was observed specifically in the theta band while power in surrounding frequency bands did not change.
4.1 Limitations
Although this study was carefully designed and conducted, some limitations should be noted. The placement of the stimulation electrodes was based on previous studies, which may not be ideal. Given that tACS is more localized, individual differences in head anatomy could affect the results. Future studies using imaging techniques could address this issue. Additionally, we were unable to analyze the EEG data recorded during tACS due to artifacts introduced by the electrical stimulation. Currently, there is no established method to eliminate this noise effectively. Overcoming this challenge would provide crucial insights into the underlying processes of tACS and its effects on brain function. Our evaluation of working memory performance was based solely on reaction time (RT), accuracy and spectral power in the theta band. While these metrics are commonly used to assess task performance and neural activity, working memory is influenced by a range of factors, including cognitive strategies and individual differences in processing speed. Therefore, relying exclusively on RT and spectral power may not capture the full complexity of the cognitive processes involved in working memory tasks. Finally, we did not observe any effects of tACS on accuracy. While this outcome was not anticipated, previous research indicated that tACS may selectively improve task performance.
4.2 Conclusion
Our findings demonstrated that the application of tACS at a slow theta frequency (5 Hz) over left DLPFC improved performance in WM tasks preferentially in the higher cognitive load condition. Furthermore, the results showed a significant increase in post-tACS spectral power, known as after-effects. Our findings highlight the potential of tACS as a non-invasive brain stimulation method for modulating brain activity and enhancing cognitive function. Given that cognitive deficits are common in psychiatric and neurological conditions, these findings have significant implications for the clinical application of tACS in enhancing cognitive functions and overall wellbeing in clinical populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of Justus-Liebig University Giessen. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RD: Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Validation. OE: Data curation, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. JI: Data curation, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. JR: Conceptualization, Methodology, Supervision, Writing – review & editing. MS: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. EA: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. GL: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. CM: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by (1) the German Research Foundation through two projects: (a) the Collaborative Research Centre SFB936-Project C6-Third Funding Period to CM and GL, and (b) the Collaborative Research Centre SFB/TRR135-Project B7-Third Funding Period to CM, and (2) the DYNAMIC Centre, funded by the LOEWE program of the Hessian Ministry of Science and Arts (Grant Number: LOEWE 1/16/519/03/09.001 (0009)/98).
Acknowledgments
The authors would like to thank Christoph Ruprecht for his help in programming the experiment, and Ludmila Heck-Kucik and Daniel Erhard for their assistance in data collection.
Conflict of interest
The authors declare no conflicts of interest, financial interests, or personal relationships that could have influenced the findings reported in this paper.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
3. Gohier B, Ferracci L, Surguladze SA, Lawrence E, El Hage W, Kefi MZ, et al. Cognitive inhibition and working memory in unipolar depression. J Affect Disord. (2009) 116:100–5. doi: 10.1016/j.jad.2008.10.028
4. Nikolin S, Tan YY, Schwaab A, Moffa A, Loo CK, Martin D. An investigation of working memory deficits in depression using the n-back task: A systematic review and meta-analysis. J Affect Disord. (2021) 284:1–8. doi: 10.1016/j.jad.2021.01.084
5. Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. psychol Med 2008/10/23 Ed. (2009) 39:889–905. doi: 10.1017/S0033291708004558
6. Lee J, Park S. Working memory impairments in Schizophrenia: A meta-analysis. J Abnormal Psychol. (2005) 114:599–611. doi: 10.1037/0021-843X.114.4.599
7. Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. AJP. (2003) 160:1809–16. doi: 10.1176/appi.ajp.160.10.1809
8. Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS, Alderson RM. Working memory deficits and social problems in children with ADHD. J Abnormal Child Psychol. (2011) 39:805–17. doi: 10.1007/s10802-011-9492-8
9. Martinuseen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. (2005) 44:377–84. doi: 10.1097/01.chi.0000153228.72591.73
10. Belleville S, Chertkow H, Gauthier S. Working memory and control of attention in persons with Alzheimer’s disease and mild cognitive impairment. Neuropsychology. (2007) 21:458. doi: 10.1037/0894-4105.21.4.458
11. Kirova AM, Bays RB, Lagalwar S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. BioMed Res Int. (2015) 2015:748212. doi: 10.1155/2015/748212
12. Axmacher N, Schmitz DP, Wagner T, Elger CE, Fell J. Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory: A combined intracranial EEG and functional magnetic resonance imaging study. J Neurosci. (2008) 28:7304. doi: 10.1523/JNEUROSCI.1778-08.2008
13. Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. (2013) 49:1195–205. doi: 10.1016/j.cortex.2012.05.022
14. Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. (2003) 7:415–23. doi: 10.1016/S1364-6613(03)00197-9
15. Wang M, Yang P, Wan C, Jin Z, Zhang J, Li L. Evaluating the role of the dorsolateral prefrontal cortex and posterior parietal cortex in memory-guided attention with repetitive transcranial magnetic stimulation. Front Hum Neurosci. (2018) 12:236. doi: 10.3389/fnhum.2018.00236
16. Alekseichuk I, Turi Z, Amador de Lara G, Antal A, Paulus W. Spatial working memory in humans depends on theta and high gamma synchronization in the prefrontal cortex. Curr Biol. (2016) 26:1513–21. doi: 10.1016/j.cub.2016.04.035
17. Riddle J, Scimeca JM, Cellier D, Dhanani S, D’Esposito M. Causal evidence for a role of theta and alpha oscillations in the control of working memory. Curr Biol. (2020) 30:1748–54.e4. doi: 10.1016/j.cub.2020.02.065
18. Thompson L, Khuc J, Saccani MS, Zokaei N, Cappelletti M. Gamma oscillations modulate working memory recall precision. Exp Brain Res. (2021) 239:2711–24. doi: 10.1007/s00221-021-06051-6
19. Wolinski N, Cooper NR, Sauseng P, Romei V. The speed of parietal theta frequency drives visuospatial working memory capacity. PLoS Biol. (2018) 16:e2005348. doi: 10.1371/journal.pbio.2005348
20. Brzezicka A, Kamiński J, Reed CM, Chung JM, Mamelak AN, Rutishauser U. Working memory load-related theta power decreases in dorsolateral prefrontal cortex predict individual differences in performance. J Cogn Neurosci. (2019) 31:1290–307. doi: 10.1162/jocn_a_01417
21. Sauseng P, Klimesch W, Doppelmayr M, Hanslmayr S, Schabus M, Gruber WR. Theta coupling in the human electroencephalogram during a working memory task. Neurosci Letters. (2004) 354:123–6. doi: 10.1016/j.neulet.2003.10.002
22. Schicktanz N, Fastenrath M, Milnik A, Spalek K, Auschra B, Nyffeler T, et al. Continuous theta burst stimulation over the left dorsolateral prefrontal cortex decreases medium load working memory performance in healthy humans. PLoS One. (2015) 10:e0120640. doi: 10.1371/journal.pone.0120640
23. Diedrich L, Kolhoff HI, Bergmann C, Bähr M, Antal A. Boosting working memory in the elderly: driving prefrontal theta–gamma coupling via repeated neuromodulation. GeroScience. (2024) 1–16. doi: 10.1007/s11357-024-01272-3
24. Elyamany O, Leicht G, Herrmann CS, Mulert C. Transcranial alternating current stimulation (tACS): from basic mechanisms towards first applications in psychiatry. Eur Arch Psychiatry Clin Neurosci. (2021) 271:135–56. doi: 10.1007/s00406-020-01209-9
25. Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. (2014) 24:333–9. doi: 10.1016/j.cub.2013.12.041
26. Kasten FH, Dowsett J, Herrmann CS. Sustained Aftereffect of α-tACS Lasts Up to 70 min after Stimulation. Front Hum Neurosci. (2016) 10:245. doi: 10.3389/fnhum.2016.00245
27. Polanía R, Nitsche MA, Korman C, Batsikadze G, Paulus W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr Biol. (2012) 22:1314–8. doi: 10.1016/j.cub.2012.05.021
28. Yeh TC, Huang CC, Chung YA, Park SY, Im JJ, Lin YY, et al. Online left-hemispheric in-phase frontoparietal theta tACS modulates theta-band EEG source-based large-scale functional network connectivity in patients with schizophrenia: A randomized, double-blind, sham-controlled clinical trial. Biomedicines. (2023) 11:630. doi: 10.3390/biomedicines11020630
29. Vossen A, Gross J, Thut G. Alpha power increase after transcranial alternating current stimulation at alpha frequency (α-tACS) reflects plastic changes rather than entrainment. Brain Stimulation. (2015) 8:499–508. doi: 10.1016/j.brs.2014.12.004
30. Zaehle T, Rach S, Herrmann CS. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PloS One. (2010) 5:e13766. doi: 10.1371/journal.pone.0013766
31. Jaušovec N, Jaušovec K. Increasing working memory capacity with theta transcranial alternating current stimulation (tACS). Biol Psychol. (2014) 96:42–7. doi: 10.1016/j.biopsycho.2013.11.006
32. Pahor A, Jaušovec N. The effects of theta and gamma tACS on working memory and electrophysiology. Front Hum Neurosci. (2018) 11:651. doi: 10.3389/fnhum.2017.00651
33. Rauh J, Müller ASM, Nolte G, Haaf M, Mußmann M, Steinmann S, et al. Comparison of transcranial brain stimulation approaches: prefrontal theta alternating current stimulation enhances working memory performance. Front Psychiatry. (2023) 14:1140361. doi: 10.3389/fpsyt.2023.1140361
34. Wischnewski M, Zerr P, Schutter DJLG. Effects of theta transcranial alternating current stimulation over the frontal cortex on reversal learning. Brain Stimulation. (2016) 9:705–11. doi: 10.1016/j.brs.2016.04.011
35. Wischnewski M, Compen B. Effects of theta transcranial alternating current stimulation (tACS) on exploration and exploitation during uncertain decision-making. Behav Brain Res. (2022) 426:113840. doi: 10.1016/j.bbr.2022.113840
36. Moliadze V, Sierau L, Lyzhko E, Stenner T, Werchowski M, Siniatchkin M, et al. After-effects of 10 Hz tACS over the prefrontal cortex on phonological word decisions. Brain Stimulation. (2019) 12:1464–74. doi: 10.1016/j.brs.2019.06.021
37. Sela T, Kilim A, Lavidor M. Transcranial alternating current stimulation increases risk-taking behavior in the balloon analog risk task. Front Neurosci. (2012) 6:22. doi: 10.3389/fnins.2012.00022
38. Alexander ML, Alagapan S, Lugo CE, Mellin JM, Lustenberger C, Rubinow DR, et al. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Trans Psychiatry. (2019) 9:106. doi: 10.1038/s41398-019-0439-0
39. Riddle J, Rubinow DR, Frohlich F. A case study of weekly tACS for the treatment of major depressive disorder. Brain Stimulation: Basic Translational Clin Res Neuromodulation. (2020) 13:576–7. doi: 10.1016/j.brs.2019.12.016
40. Zhou J, Li D, Ye F, Liu R, Feng Y, Feng Z, et al. Effect of add-on transcranial alternating current stimulation (tACS) in major depressive disorder: a randomized controlled trial. Brain Stimulation. (2024) 17:760–8. doi: 10.1016/j.brs.2024.06.004
41. Kallel L, Mondino M, Brunelin J. Effects of theta-rhythm transcranial alternating current stimulation (4.5 Hz-tACS) in patients with clozapine-resistant negative symptoms of schizophrenia: a case series. J Neural Transmission. (2016) 123:1213–7. doi: 10.1007/s00702-016-1574-x
42. Booth SJ, Taylor JR, Brown LJE, Pobric G. The effects of transcranial alternating current stimulation on memory performance in healthy adults: A systematic review. Cortex. (2022) 147:112–39. doi: 10.1016/j.cortex.2021.12.001
43. Senkowski D, Sobirey R, Haslacher D, Soekadar SR. Boosting working memory: uncovering the differential effects of tDCS and tACS. Cereb Cortex Commun. (2022) 3:tgac018. doi: 10.1093/texcom/tgac018
44. Guo X, Li Z, Zhang L, Liu Q. Modulation of visual working memory performance via different theta frequency stimulations. Brain Sci. (2021) 11:1358. doi: 10.3390/brainsci11101358
45. Jaušovec N, Jaušovec K, Pahor A. The influence of theta transcranial alternating current stimulation (tACS) on working memory storage and processing functions. Acta Psychologica. (2014) 146:1–6. doi: 10.1016/j.actpsy.2013.11.011
46. Jones KT, Arciniega H, Berryhill ME. Replacing tDCS with theta tACS provides selective, but not general WM benefits. Brain Res. (2019) 1720:146324. doi: 10.1016/j.brainres.2019.146324
47. Sahu PP, Tseng P. Frontoparietal theta tACS nonselectively enhances encoding, maintenance, and retrieval stages in visuospatial working memory. Neurosci Res. (2021) 172:41–50. doi: 10.1016/j.neures.2021.05.005
48. Lafleur LP, Murray A, Desforges M, Pacheco-Barrios K, Fregni F, Tremblay S, et al. No aftereffects of high current density 10 Hz and 20 Hz tACS on sensorimotor alpha and beta oscillations. Sci Rep. (2021) 11:21416. doi: 10.1038/s41598-021-00850-1
49. De Koninck BP, Brazeau D, Guay S, Herrero Babiloni A, De Beaumont L. Transcranial alternating current stimulation to modulate alpha activity: A systematic review. Neuromodulation: Technol at Neural Interface. (2023) 26:1549–84. doi: 10.1016/j.neurom.2022.12.007
50. D’Atri A, Romano C, Gorgoni M, Scarpelli S, Alfonsi V, Ferrara M, et al. Bilateral 5 Hz transcranial alternating current stimulation on fronto-temporal areas modulates resting-state EEG. Sci Rep. (2017) 7:15672. doi: 10.1038/s41598-017-16003-2
51. Briley PM, Boutry C, Webster L, Veniero D, Harvey-Seutcheu C, Jung J, et al. Intermittent theta burst stimulation with synchronised transcranial alternating current stimulation leads to enhanced frontal theta oscillations and a positive shift in emotional bias. Imaging Neurosci. (2024) 2:1–14. doi: 10.1162/imag_a_00073
52. Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, et al. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci. (2009) 29:9481. doi: 10.1523/JNEUROSCI.1428-09.2009
53. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. (2004) 134:9–21. doi: 10.1016/j.jneumeth.2003.10.009
54. Nolan H, Whelan R, Reilly RB. FASTER: fully automated statistical thresholding for EEG artifact rejection. J Neurosci Methods. (2010) 192:152–62. doi: 10.1016/j.jneumeth.2010.07.015
55. Pion-Tonachini L, Kreutz-Delgado K, Makeig S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. NeuroImage. (2019) 198:181–97. doi: 10.1016/j.neuroimage.2019.05.026
56. Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp psychology: General. (1988) 117:34. doi: 10.1037/0096-3445.117.1.34
57. Haatveit BC, Sundet K, Hugdahl K, Ueland T, Melle I, Andreassen OA. The validity of d prime as a working memory index: Results from the “Bergen n-back task. J Clin Exp Neuropsychol. (2010) 32:871–80. doi: 10.1080/13803391003596421
58. Hoy KE, Bailey N, Arnold S, Windsor K, John J, Daskalakis ZJ, et al. The effect of γ-tACS on working memory performance in healthy controls. Brain Cognition. (2015) 101:51–6. doi: 10.1016/j.bandc.2015.11.002
59. Santarnecchi E, Polizzotto NR, Godone M, Giovannelli F, Feurra M, Matzen L, et al. Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr Biol. (2013) 23:1449–53. doi: 10.1016/j.cub.2013.06.022
60. Huang WA, Stitt IM, Negahbani E, Passey DJ, Ahn S, Davey M, et al. Transcranial alternating current stimulation entrains alpha oscillations by preferential phase synchronization of fast-spiking cortical neurons to stimulation waveform. Nat Commun. (2021) 12:3151. doi: 10.1038/s41467-021-23021-2
61. Violante IR, Li LM, Carmichael DW, Lorenz R, Leech R, Hampshire A, et al. Externally induced frontoparietal synchronization modulates network dynamics and enhances working memory performance. eLife. (2017) 6:e22001. Hamilton R, editor. doi: 10.7554/eLife.22001
62. Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. (2011) 12:105–18. doi: 10.1038/nrn2979
63. Mamashli F, Khan S, Hämäläinen M, Jas M, Raij T, Stufflebeam SM, et al. Synchronization patterns reveal neuronal coding of working memory content. Cell Rep. (2021) 36:109566. doi: 10.1016/j.celrep.2021.109566
64. Salazar RF, Dotson NM, Bressler SL, Gray CM. Content-specific fronto-parietal synchronization during visual working memory. Science. (2012) 338:1097–100. doi: 10.1126/science.1224000
65. Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. (2002) 15:1395–9. doi: 10.1046/j.1460-9568.2002.01975.x
66. Maurer U, Brem S, Liechti M, Maurizio S, Michels L, Brandeis D. Frontal midline theta reflects individual task performance in a working memory task. Brain Topography. (2015) 28:127–34. doi: 10.1007/s10548-014-0361-y
67. Helfrich RF, Schneider TR. Modulation of cortical network activity by transcranial alternating current stimulation. J Neurosci. (2013) 33:17551. doi: 10.1523/JNEUROSCI.3740-13.2013
Keywords: transcranial alternating current stimulation, EEG, working memory, theta power, dorsolateral prefrontal cortex
Citation: Debnath R, Elyamany O, Iffland JR, Rauh J, Siebert M, Andraes E, Leicht G and Mulert C (2025) Theta transcranial alternating current stimulation over the prefrontal cortex enhances theta power and working memory performance. Front. Psychiatry 15:1493675. doi: 10.3389/fpsyt.2024.1493675
Received: 09 September 2024; Accepted: 11 December 2024;
Published: 14 January 2025.
Edited by:
Yuanjun Xie, Fourth Military Medical University, ChinaReviewed by:
Stevan Nikolin, University of New South Wales, AustraliaXueli Chen, University of Science and Technology of China, China
Kai Guan, Information Science Academy of China Electronics Technology Group Corporation, China
Lukas Diedrich, University Medical Center Göttingen, Germany
Copyright © 2025 Debnath, Elyamany, Iffland, Rauh, Siebert, Andraes, Leicht and Mulert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranjan Debnath, cmFuamFuLmRlYm5hdGhAcHN5Y2hpYXQubWVkLnVuaS1naWVzc2VuLmRl
†These authors share first authorship
 Ranjan Debnath
Ranjan Debnath Osama Elyamany
Osama Elyamany Jona Ruben Iffland
Jona Ruben Iffland Jonas Rauh
Jonas Rauh Michael Siebert1
Michael Siebert1 Gregor Leicht
Gregor Leicht Christoph Mulert
Christoph Mulert