- 1International research center for Cognitive Applied Neuroscience (IrcCAN), Università Cattolica del Sacro Cuore, Milan, Italy
- 2Research Unit in Affective and Social Neuroscience, Department of Psychology, Università Cattolica del Sacro Cuore, Milan, Italy
1 EEG and executive functions across substance and behavioral addictions
Reward sensitivity and executive function (EF) impairment, including impaired decision-making and weak inhibitory control, are key characteristics observable at both behavioral and neural levels in addiction. These features are common to substance use disorder (SUD) and Gambling Disorder (GD), and to some extent, in Internet Gaming Disorder (IGD) (1). However, the research literature lacks consistency regarding these latter conditions and other behavioral disorders, necessitating further clarification.
Before, the Cortical Unbalance Model (2), which posits a left hemispheric imbalance in electrophysiological (EEG) delta, theta, and alpha cortical activity of the brain in addictive disorders, highlighted the significant role of both EEG markers and attitudes as potential precursors to this class of disorders (namely GD) with specific reference to reward sensitivity (3). On the other hand, several contributions stressed the role of Event Related Potentials (ERPs) as highly informative neurophysiological markers of neurocognitive impairment in addictive disorders, which can be useful starting from the diagnosis to cognitive rehabilitation and relapse prevention (4, 5). Such evidence showed how reward sensitivity and EFs impairment can be mapped with EEG markers in addiction. Understanding the neurocognitive impairment often accompanying addiction (6, 7) is crucial for developing effective assessment protocols and treatment strategies to enhance long-term therapeutic outcomes.
This opinion paper will discuss how these insights emphasize the need for an integrated addiction model that considers EEG correlates (both EEG frequency bands and ERPs) of impaired EFs, together with the evidence collected for the reward systems, and that guides neurocognitive screening procedures in SUD and behavioral addictions.
Indeed, it is argued that standardized procedures for the neurocognitive screening of EFs in patients with SUD and behavioral addiction could benefit from the inclusion of a set of computerized cognitive tasks with concomitant EEG data collection for determining the EEG correlates of EF impairment. Based on the EEG markers collected during the screening phase, and which can be continuously monitored even during daily life via wearable EEG devices, it will be possible to track neurocognitive impairment and its progress, as well as to propose personalized and targeted neurocognitive treatment approaches.
2 Concomitant collection of EEG markers during structured EF assessments
The recognition of cognitive impairments in addiction necessitates targeted assessment and intervention protocols. In a recent contribution, we have discussed how, despite the pivotal role of EFs dysfunction in influencing the clinical condition of SUD and behavioral addictions, a comprehensive understanding of the interrelationships and interdependencies among models of abuse, addiction-related neurofunctional changes, and specific patterns of neurocognitive/EF impairments remains a complex and largely unresolved matter (for an in-depth discussion on this subject, see (8). The identified contributing factors to these unresolved questions are:
- the absence of specialized assessment instruments designed to detect, qualify, and quantify the array of impaired higher cognitive functions in patients with addiction, considering its unique and frequently subtle manifestations.
- the lack of specific neurocognitive batteries with subtests dedicated to functions that are typically affected by addiction, such as inhibitory control.
- the peculiarity of the clinical subsamples with addiction, which are typically younger than reference clinical cohorts used to validate, screening tools designed for geriatric patients, and might present more subtle impairments, that require finer-grained evaluation (9, 10).
Given the need to reconceptualize the evaluation of addiction by proposing the assessment of EFs together with reward sensitivity in addiction, a new neurocognitive battery has been introduced (8). Indeed, recently, we developed a neurocognitive battery for the assessment of the executive functions in addiction (Battery for Executive Functions in Addiction – BFE-A, (8) for the Italian population, which encompasses five distinct neuropsychological tests and two tasks for the evaluation of the short- and long-term verbal memory, working memory, cognitive flexibility, focused attention, attention regulation and suppression of interference and inhibitory control (Figure 1). Specifically, the battery includes two contextualized tasks: a modified Stroop Task for Addiction (MST) and a Modified Go/No-go Task for Addiction (MGNT). The MST measures attention regulation and interference suppression, focusing on control of interference from addiction-related stimuli. The MGNT measures inhibitory control and attention bias suppression for addiction-related stimuli. Results in SUD populations suggest the BFE-A is a useful screening tool for addiction services, complementing the diagnostic process (12), though it lacks EEG marker integration.
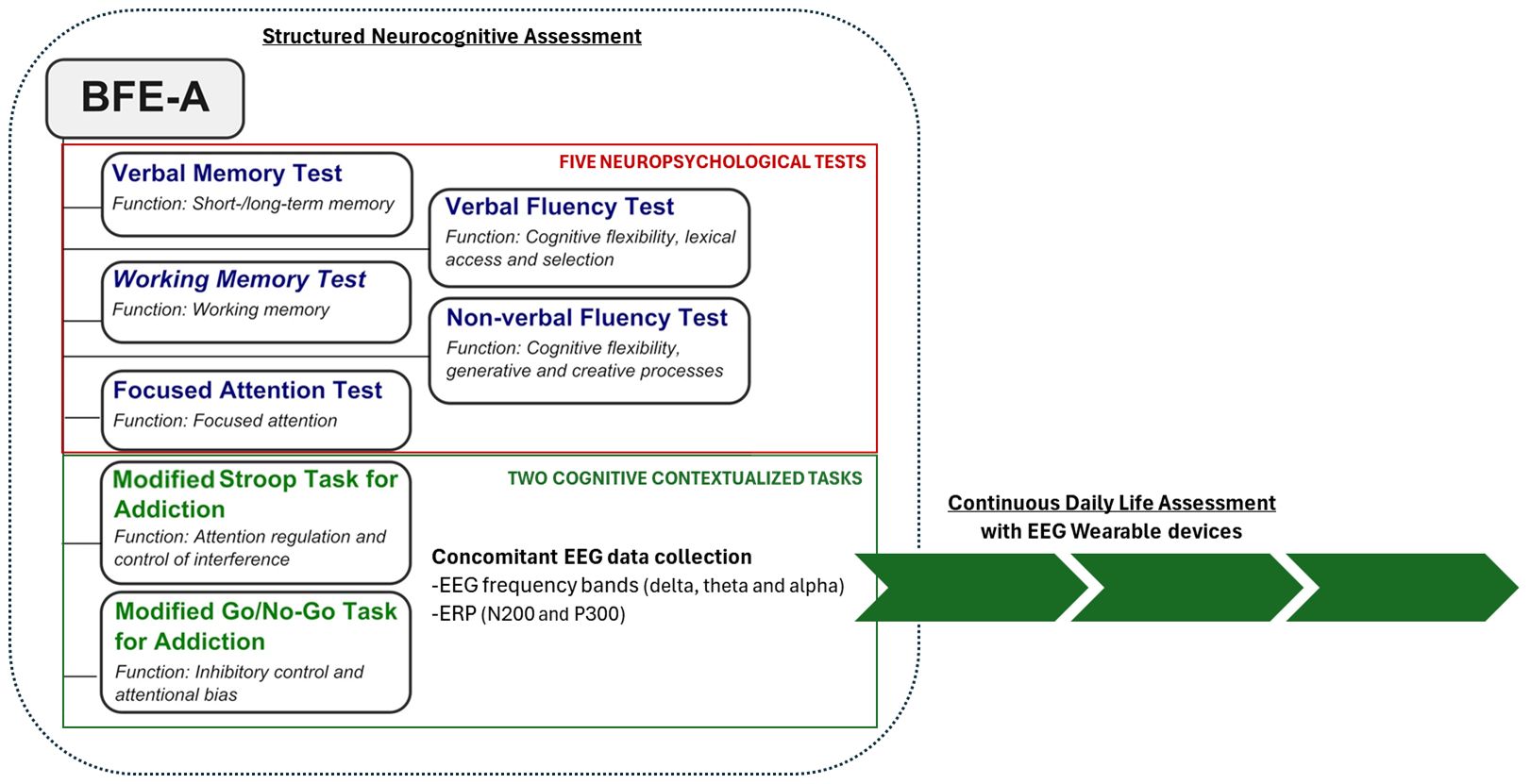
Figure 1. The figure illustrates the structure of BFE-A (retrieved and modified from 11) and outlines the proposal to collect EEG biomarkers during structured neurocognitive assessments, as well as to continuously monitor these biomarkers in daily life using wearable devices.
An extensive body of literature highlighted the value of exploiting the concurrent EEG recording during classical or contextualized cognitive tasks for measuring attention regulation and response inhibition in SUD (13) and various paradigms, such as oddball, Go/No-Go, Stroop, Cue-reactivity, to name a few, are employed to tackle the neuronal correlates of different cognitive and affective processes in the brain (14).
Distinct EEG markers (both in the time and frequency domain) have been found to be related to EF processes during Stroop and Go/No-Go task. During the Stroop task, the frontal theta amplitude was found to be larger in the incongruent condition, as well as the occipital alpha desynchronization persisted longer in the same condition. While the evoked delta amplitude is larger in the congruent condition (15). In the Go/No-Go task, theta and delta bands separately increased in relation to response inhibition and were uniquely related to the N200 and P300 ERP components (16).
Considering the executive impairment in addictions and the EEG frequency bands regarded as reliable markers of EF, lower delta, theta, and slow alpha power in alcoholics during Go and NoGo response at the Go/NoGo task (17).
Individuals with vulnerability to internet addiction showed a reduction of alpha band in the left prefrontal cortex during a contextualized Go/NoGo Taks for both Go and No-Go conditions (18) IGD patients have been reported to experience raised resting state gamma and reduced beta and delta activity (19).
With specific reference to the response inhibition impairment and ERP, the N200 and the P300 components have been identified as key markers for alcohol disorders (4). Domínguez-Centeno and colleagues (20) demonstrated how the increased amplitude of the P3 component collected during a classical letter Go/No-Go task may be considered a useful endophenotype and a vulnerability marker to develop addictive behavior. During a “contextual Go/No-Go task” reduced amplitude and latency on the N2 component and increased P3 latency were found in a group of polydrug users compared to controls (21).
In gambling and gaming studies alterations in the NoGo N2 are described consistently (22) with initial evidence also for the NoGo-P3. However, Simkute and colleagues (23) argued that a vast heterogeneity regarding the EEG experimental paradigms being used, and the lack of clear guidelines and standardized procedures prevent the identification of measures capable of reliably discriminating or characterizing the population susceptible to addictive behavior or being able to diagnose and monitor these disorders.
Regarding IGD, recent reviews on EEG markers of neurocognitive response (24, 25) reported how EEG generally revealed reduced beta waves and increased theta bands in gaming disorder. IGD with depression demonstrated increased theta and decreased alpha waves. Whereas increased P300 was frequently associated with the impaired excessive allocation of attentional resources of the internet addiction disorder towards addiction-specific cues. IGD had increased whole brain delta waves at baseline, which a showed significant reduction post-therapy.
The integration of the assessment with EEG biomarkers proved to be useful for several reasons such as the distinction between the quality and quantity of cognitive impairment, as well as between different phases of the progress of the clinical condition, both in terms of severity (26) that of clinical course and detoxification (27). Moreover, this behavioral and neurocognitive integrated approach can even be extended to study other emerging addictive behaviors, such as addiction to physical exercise or binge eating disorder (28).
3 Route to the interventions: extensive monitoring and continuous assessment of neurophysiological markers
In advocating a holistic approach to mental health care, particularly in addressing addiction, we propose that effective management and treatment necessitate thorough monitoring and assessment of patients’ behaviors and psycho- and neuro-physiological responses both during computerized tasks included in structured neuropsychological evaluations (such as the BFE-A) as well as during ecological conditions.
Wearables, such as smartwatches, fitness trackers, and biosensors, have emerged as promising tools for continuous and objective data collection in healthcare. These devices, equipped with various sensors, enable the collection of data on physical activity, sleep patterns and can even measure chemical biomarkers like alcohol levels or drug metabolites, making them valuable for addiction-related monitoring.
In recent experimental applications, wearables are primarily utilized for monitoring substance abuse and craving. For instance, wearable sensors have been tested to track transdermal alcohol levels in real-time, offering insights into alcohol consumption patterns (29). Additionally, these wearables can detect drug use through sweat analysis, contributing to the early identification of relapse risk (30). Equipped with physiological sensors, wearables can also record changes in electrophysiological signals such as heart rate, skin conductance, and other relevant physiological data associated with gambling cravings or high-risk situations. This information proves valuable in identifying vulnerable episodes and implementing harm reduction strategies (31).
Building upon a valuable yet limited evidence base, we suggest the next challenge in enhancing monitoring, personalized assessment, and therapy for addiction is to refine neurocognitive assessment protocols. This involves measuring neural resource expenditure and effort through non-invasive physiological recordings. The advent of wearable technology has opened new possibilities for real-world cognitive load measurement, extending neuroassessment beyond research labs (32, 33). Monitoring cognitive load can provide critical insights into neurological health, cognitive disorders, and rehabilitation progress. Traditional methods of cognitive load assessment often rely on laboratory-based tests, self-reports, or observer ratings. Wearable technology may offer an integrative set of markers to complement observed performance metrics and more deeply assess the efficiency of neurocognitive processes.
For instance, wearables with EEG sensors have demonstrated their capability in monitoring cognitive load fluctuations in Alzheimer’s patients (34). Additionally, the potential of wearables like smartwatches to assess cognitive load during stroke rehabilitation exercises has been tested, aiding in therapy plan customization and cognitive recovery tracking (35). Wearable EEG, especially, has proven to be both usable and beneficial in monitoring cognitive effort during neurorehabilitation exercises, tailoring therapy plans for individuals recovering from neurological disorders (36). Although current evidence in mental health is limited, these neighboring studies suggest novel opportunities that could and should be validated and implemented soon.
Moreover, based on the EEG markers collected during the screening phase, will be possible to propose personalized and targeted neurocognitive treatment approach, such as neuromodulation interventions with neurofeedback devices or neurostimulation interventions with transcranial direct current stimulation techniques, which could promote greater neural functioning in both SUD and behavioral addiction.
As a final note, while it must be acknowledged that basic and applied research and validation of actually feasible remote monitoring protocols using wearables – especially in care of psychopathology – is still at its beginning, a few notes concerning strong points and challenges of such digital-health applications can already be pointed-out for further and coming discussion.
Among the most reported advantages:
- Wearables offer continuous and objective data, reducing reliance on unreliable self-reporting in addiction treatment. This impartial stream of data helps healthcare providers make informed decisions based on concrete evidence, overcoming issues like denial, stigma, or memory lapses.
- Continuous data collection enables the assessment of trends and patterns over time, facilitating personalized treatment adjustments. This is particularly valuable in addiction treatment, allowing therapists to observe patient progress, identify triggers, and modify treatment plans accordingly.
- Wearables allow for remote monitoring, supporting patients regardless of their location, especially beneficial for those in outpatient or telehealth programs. This promotes a comprehensive and continuous care model.
- Many wearables include gamification elements and feedback mechanisms, motivating individuals in recovery to stay committed to their treatment plans through goal setting, earning rewards, or receiving real-time feedback. This contributes to better treatment outcomes.
- R&D in monitoring addiction with wearables increasingly involve tighter integration with machine learning and AI algorithms. Analyzing complex data patterns enhances predictive capabilities, enabling earlier detection of relapse risks and adaptive interventions.
4 Conclusive remarks
The integration of such integrated approach and wearable technologies into existing healthcare systems can be challenging, requiring significant infrastructure investments and training for healthcare professionals. There are potential risks of over-reliance on technology, which might lead to reduced patient engagement and a lack of personalized care. Moreover, it also must be acknowledged that a few issues contrast such strong points and still raise practical and ethical concerns regarding such applications. Among them:
- Collecting sensitive data raises privacy and ethical concerns. It is essential to implement robust data protection measures, ensure informed consent, and maintain transparency regarding data use and sharing. Adequate data storage systems must be established.
- The accuracy of wearable data varies between devices and individuals, necessitating validation studies for trustworthy and clinically relevant data.
- Cost can be a barrier to access for specific patient populations or services, depending on psycho-social and geographic contexts.
Cognitive impairments in addiction are increasingly recognized as significant contributors to the persistence and relapse of this complex disorder. Both clinical research and practice call for informative neurocognitive and neurofunctional markers that could complement standard psychodiagnostics process. Future efforts in digital health R&D should capitalize current methodological, scientific, and ethical debate to promote feasible, accessible, and valuable solutions.
Author contributions
MB: Writing – original draft, Writing – review & editing. LA: Writing – original draft, Writing – review & editing. DC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Fondazione Cariplo - Progetto “Empowering Emotions regulation and Executive functions in Behavioral Addictions (E3BA)”, ID. 2023-1612.
Conflict of interest
Two of the authors of this manuscript MB and DC are also the developer of the Battery for Executive Functions in Addiction Batteria per le Funzioni Esecutive nell’Addiction–BFE-A, an assessment tool that was born from the clinical and methodological need for population-specific tests of higher cognitive functioning in addiction.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Balconi M, Campanella S. Advances in Substance and behavioral addiction. The role of executive functions. 1st Edn. Balconi M, Campanella S, editors. Cham: Springer (2021). doi: 10.1007/978-3-030-82408-2
2. Balconi M, Finocchiaro R, Canavesio Y. Left hemispheric imbalance and reward mechanisms affect gambling behavior: The contribution of the metacognition and cortical brain oscillations. Clin EEG Neurosci. (2015) 46:197–207. doi: 10.1177/1550059413513261
3. Balconi M, Finocchiaro R, Canavesio Y. Reward-system effect (BAS rating), left hemispheric “unbalance” (alpha band oscillations) and decisional impairments in drug addiction. Addictive Behav. (2014) 39:1026–32. doi: 10.1016/j.addbeh.2014.02.007
4. Campanella S, Schroder E, Kajosch H, Noel X, Kornreich C. Why cognitive event-related potentials (ERPs) should have a role in the management of alcohol disorders. Neurosci Biobehav Rev. (2019) 106:234–44. doi: 10.1016/j.neubiorev.2018.06.016
5. Campanella S. Use of cognitive event-related potentials in the management of psychiatric disorders: Towards an individual follow-up and multi-component clinical approach. World J Psychiatry. (2021) 11:153–68. doi: 10.5498/wjp.v11.i5.153
6. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. (2016) 3:760–73. doi: 10.1016/S2215-0366(16)00104-8
7. Antons S, Brand M, Potenza MN. Neurobiology of cue-reactivity, craving, and inhibitory control in non-substance addictive behaviors. J Neurol Sci. (2020) 415:116952. doi: 10.1016/j.jns.2020.116952
8. Balconi M, Losasso D, Balena A, Crivelli D. Neurocognitive impairment in addiction: A digital tool for executive function assessment. Front Psychiatry. (2022) 13:955277. doi: 10.3389/fpsyt.2022.955277
9. Copersino ML, Fals-Stewart W, Fitzmaurice G, Schretlen DJ, Sokoloff J, Weiss RD. Rapid cognitive screening of patients with substance use disorders. Exp Clin Psychopharmacol. (2009) 17:337–44. doi: 10.1037/a0017260
10. Bruijnen CJWH, Jansen M, Dijkstra BAG, Walvoort SJW, Lugtmeijer S, Markus W, et al. The Montreal Cognitive Assessment (MoCA) as a cognitive screen in addiction health care: A validation study for clinical practice. J Subst Use. (2019) 24:47–54. doi: 10.1080/14659891.2018.1497102
11. Crivelli D, Balena A, Losasso D, Balconi M. Screening executive functions in substance-use disorder: first evidence from testing of the battery for executive functions in addiction (BFE-A). Int J Ment Health Addict. (2022) 22:1315–32. doi: 10.1007/s11469-022-00928-5
12. Balconi M, Losasso D, Balena A, Crivelli D. BFE-A - Batteria per le Funzioni Esecutive nell’Addiction [Battery for Exective Functions in Addiction]. Giunti Psychometrics (2022).
13. Houston RJ, Schlienz NJ. Event-related potentials as biomarkers of behavior change mechanisms in substance use disorder treatment. Biol Psychiatry Cognit Neurosci Neuroimaging. (2018) 3:30–40. doi: 10.1016/j.bpsc.2017.09.006
14. Habelt B, Arvaneh M, Bernhardt N, Minev I. Biomarkers and neuromodulation techniques in substance use disorders. Bioelectron Med. (2020) 6:1–17. doi: 10.1186/s42234-020-0040-0
15. Ergen M, Saban S, Kirmizi-Alsan E, Uslu A, Keskin-Ergen Y, Demiralp T. Time–frequency analysis of the event-related potentials associated with the Stroop test. Int J Psychophysiol. (2014) 94:463–72. doi: 10.1016/j.ijpsycho.2014.08.177
16. Harper J, Malone SM, Bernat EM. Theta and delta band activity explain N2 and P3 ERP component activity in a go/no-go task. Clin Neurophysiol. (2014) 125:124–32. doi: 10.1016/j.clinph.2013.06.025
17. Pandey AK, Kamarajan C, Manz N, Chorlian DB, Stimus A, Porjesz B. Delta, theta, and alpha event-related oscillations in alcoholics during Go/NoGo task: Neurocognitive deficits in execution, inhibition, and attention processing. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 65:158–71. doi: 10.1016/j.pnpbp.2015.10.002
18. Balconi M, Finocchiaro R. Deficit in rewarding mechanisms and prefrontal left/right cortical effect in vulnerability for internet addiction. Acta Neuropsychiatrica. (2016) 28:272–85. doi: 10.1017/neu.2016.9
19. Burleigh TL, Griffiths MD, Sumich A, Wang GY, Kuss DJ. Gaming disorder and internet addiction: A systematic review of resting-state EEG studies. Add Behav. (2020) 107:106429. doi: 10.1016/j.addbeh.2020.106429
20. Domínguez-Centeno I, Jurado-Barba R, Sion A, Martinez-Maldonado A, Castillo-Parra G, López-Muñoz F, et al. P3 component as a potential endophenotype for control inhibition in offspring of alcoholics. Alcohol Alcoholism. (2018) 53:699–706. doi: 10.1093/alcalc/agy051
21. Dousset C, Chenut C, Kajosch H, Kornreich C, Campanella S. Comparison of neural correlates of reactive inhibition in cocaine, heroin, and polydrug users through a contextual go/no-go task using event-related potentials. Biol (Basel). (2022) 11:1029. doi: 10.3390/biology11071029
22. Littel M, Van Den Berg I, Luijten M, Van Rooij AJ, Keemink L, Franken IHA. Error processing and response inhibition in excessive computer game players: An event-related potential study. Addict Biol. (2012) 17:934–47. doi: 10.1111/j.1369-1600.2012.00467.x
23. Simkute D, Dores AR, Barbosa F, Griskova-Bulanova I. Problematic gaming and gambling: A systematic review of task-specific EEG protocols. J Gambl Stud. (2024). doi: 10.1007/s10899-024-10332-4
24. Kashif S, Pandey S, Warriach ZI. Neurophysiological markers of internet gaming disorder: A literature review of electroencephalography studies. Cureus. (2021) 13:e17866. doi: 10.7759/cureus.17866
25. Sharifat H, Suppiah S. Electroencephalography-detected neurophysiology of internet addiction disorder and internet gaming disorder in adolescents - A review. Med J Malaysia. (2021) 76:401–13.
26. Sumiyoshi T, Campanella S, Giordano GM, Ishii R, Pogarell O. Understanding the pathophysiology of mental diseases and early diagnosis thanks to electrophysiological tools: some insights and empirical facts. Clin EEG Neurosci. (2024). doi: 10.1177/15500594241227485
27. Petit G, Cimochowska A, Kornreich C, Hanak C, Verbanck P, Campanella S. Neurophysiological correlates of response inhibition predict relapse in detoxified alcoholic patients: Some preliminary evidence from event-related potentials. Neuropsychiatr Dis Treat. (2014) 10:1025–37. doi: 10.2147/NDT.S61475
28. Yan WS, Liu MM, Liu SJ. A behavioral and event-related potentials study of food-related inhibitory control in probable binge eating disorder. Psychol Res Behav Manag. (2023) 16:4737–48. doi: 10.2147/PRBM.S441949
29. Campbell AS, Kim J, Wang J. Wearable electrochemical alcohol biosensors. Curr Opin Electrochem. (2018) 10:126–35. doi: 10.1016/j.coelec.2018.05.014
30. Oesterle TS, Karpyak VM, Coombes BJ, Athreya AP, Breitinger SA, Correa da Costa S, et al. Systematic review: Wearable remote monitoring to detect nonalcohol/nonnicotine-related substance use disorder symptoms. Am J Addict. (2022) 31:535–45. doi: 10.1111/ajad.13341
31. Ashrafioun L, Rosenberg H. Methods of assessing craving to gamble: A narrative review. Psychol Addictive Behav. (2012) 26:536–49. doi: 10.1037/a0026367
32. Crivelli D, Balconi M. Neuroassessment in sports: an integrative approach for performance and potential evaluation in athletes. Front Psychol. (2022) 13:747852. doi: 10.3389/fpsyg.2022.747852
33. Balconi M, Acconito C, Allegretta RA, Crivelli D. What is the relationship between metacognition and mental effort in executive functions? The contribution of neurophysiology. Behav Sci. (2023) 13:918. doi: 10.3390/bs13110918
34. Musaeus CS, Waldemar G, Andersen BB, Høgh P, Kidmose P, Hemmsen MC, et al. Long-term EEG monitoring in patients with alzheimer’s disease using ear-EEG: A feasibility study. J Alzheimer’s Dis. (2022) 90:1713–23. doi: 10.3233/JAD-220491
35. Chae SH, Kim Y, Lee KS, Park HS. Development and clinical evaluation of a web-based upper limb home rehabilitation system using a smartwatch and machine learning model for chronic stroke survivors: Prospective comparative study. JMIR Mhealth Uhealth. (2020) 8:e17216. doi: 10.2196/17216
Keywords: substance use disorders, behavioral addictions, executive functions, EEG, wearable devices
Citation: Balconi M, Angioletti L and Crivelli D (2024) Integrating EEG biomarkers in the neurocognitive screening of executive functions in substance and behavioral addiction. Front. Psychiatry 15:1472565. doi: 10.3389/fpsyt.2024.1472565
Received: 29 July 2024; Accepted: 05 September 2024;
Published: 19 September 2024.
Edited by:
Giorgio Di Lorenzo, University of Rome Tor Vergata, ItalyReviewed by:
Yong-guang Wang, Hangzhou Seventh Peoples Hospital, ChinaCopyright © 2024 Balconi, Angioletti and Crivelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Angioletti, bGF1cmEuYW5naW9sZXR0aTFAdW5pY2F0dC5pdA==
 Michela Balconi
Michela Balconi Laura Angioletti
Laura Angioletti Davide Crivelli
Davide Crivelli