- Department of Psychiatry, Third People’s Hospital of Zhongshan City, Zhongshan, China
Objective: To investigate the correlation between BDNF gene polymorphism, BDNF levels, and susceptibility to mild cognitive impairment (MCI).
Methods: In this study, we investigated 107 elderly adults individuals from a community in Zhongshan, Guangdong Province, with an average age of 73.17 ± 7.081 years. The participants included 52 patients with Mild Cognitive Impairment due to Alzheimer’s Disease and 55 cognitively normal elderly adults control subjects. The two groups were matched based on gender, age, and education level. We assessed their cognitive functions and analyzed their genotypes and serum BDNF levels. Analysis of covariance (ANCOVA) was used to evaluate the differences in serum BDNF levels between the MCI group and the control group. Multivariate linear regression was utilized to analyze the association between BDNF levels and susceptibility to MCI, as well as cognitive functions. Multivariate logistic regression was employed to investigate the association between BDNF gene polymorphisms and the risk of developing MCI, along with their interactions.
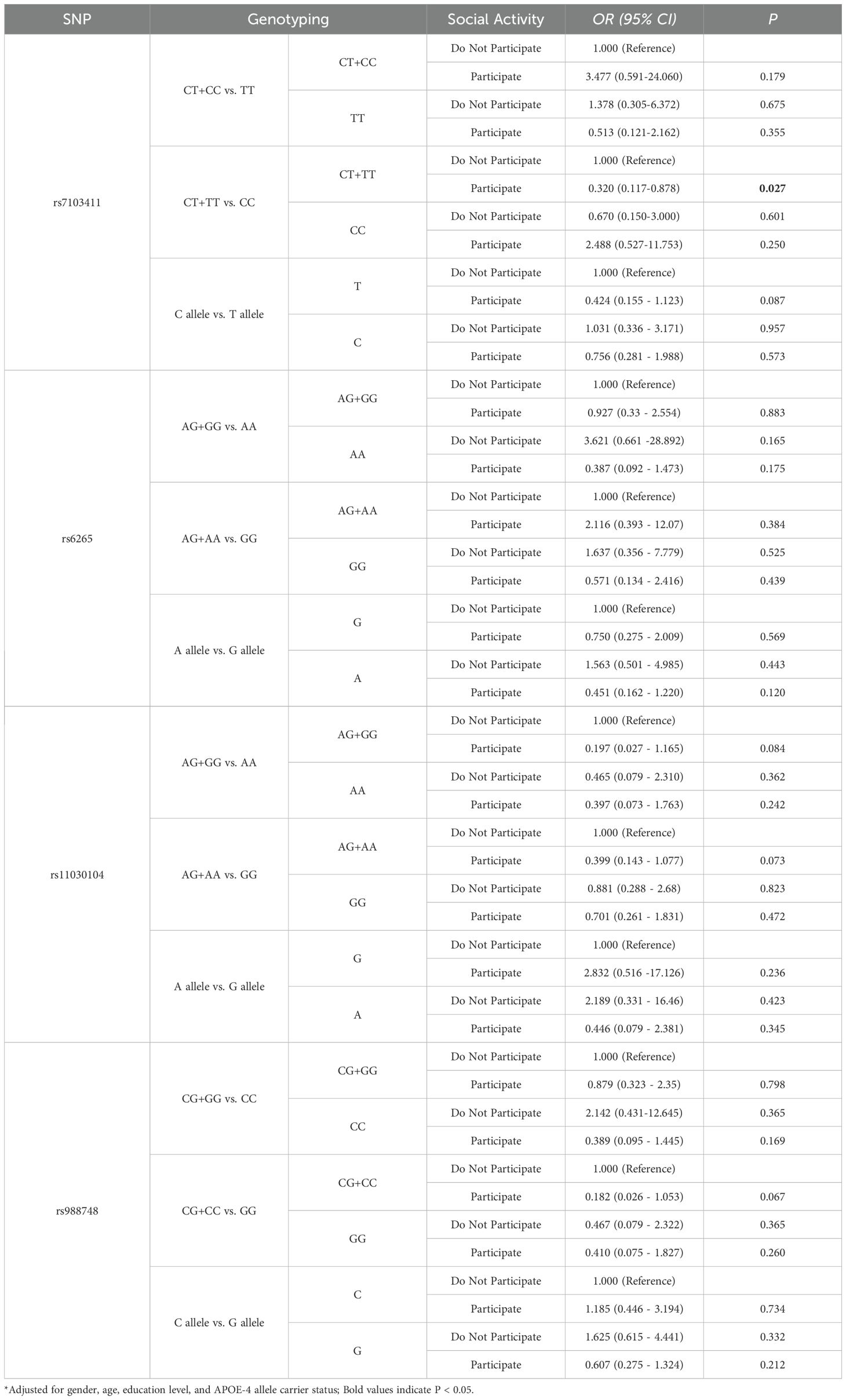
Results: The ANCOVA analysis indicated that there was no significant difference in serum BDNF levels between the MCI group and the control group (P > 0.05). Correlation analysis revealed a negative correlation between Mini-Mental Status Examination (MMSE) total scores and MCI (r = -0.461, P = 0.001), with significant correlations observed in orientation (r = -0.420, P = 0.002). Multiple linear regression analysis showed that specific polymorphisms, including rs7103411 (CT+TT vs. CC), rs6265 (CT and CT+TT vs. CC), rs11030104 (AG and AG+GG vs. AA), and rs988748 (CG+CC vs. GG), were significantly associated with decreased serum BDNF levels (P < 0.05). Multivariate logistic regression showed that rs7103411 polymorphism was associated with susceptibility to MCI; individuals with the CT or CC genotype had a 0.370 times lower risk of developing MCI compared to those with the TT genotype (OR = 0.370, 95% CI: 0.141-0.970, P = 0.043). A significant interaction was found between rs7103411 and social activity, which influenced the risk of developing MCI. Specifically, individuals with the CT or TT genotype of rs7103411 who engaged in social activities had a significantly lower risk of developing MCI (OR = 0.32, 95% CI: 0.117-0.878, P = 0.027).
Conclusion: This study indicates that BDNF rs7103411、rs6265、rs11030104 and rs988748 are associated with decreased serum BDNF levels in MCI patients. Individuals carrying the TT genotype in the BDNF rs7103411 gene are associated with an increased susceptibility to MCI. Individuals with the rs7103411 CT or TT genotype who participated in social activities showed a significantly reduced risk of developing MCI, suggesting that the interaction between the BDNF rs7103411 genotype and social activity can help reduce the risk of MCI.
1 Introduction
Mild Cognitive Impairment (MCI) is one of the common neurocognitive disorders and a significant public health issue, with its prevalence increasing with age. Its prevalence increases with age, the prevalence is 6.7% in individuals aged 60-64 years and 25.2% in those aged 80-84 years (1). MCI due to AD is considered to be associated with an increased risk of developing dementia, particularly Alzheimer’s dementia (1). Therefore, it is essential to identify and intervene early in cases of MCI (1). A nationwide cross-sectional study in China showed that the prevalence of mild cognitive impairment (MCI) is 15.5%, with approximately 38.77 million people affected by this condition (2). Early intervention can help delay the progression of the disease and ensure better outcomes.
Brain-Derived Neurotrophic Factor (BDNF) is a member of the neurotrophin family that plays an important role in promoting the growth, differentiation, and survival of neurons. It is crucial for neuroplasticity and cognitive function (3). Some studies suggest that BDNF levels are linked to the occurrence of neurodegenerative disorders such as Alzheimer’s Disease (AD), Frontotemporal Dementia (FTD), Lewy Body Dementia (LBD), and Vascular Dementia (VAD). However, studies on peripheral BDNF levels in patients with AD and MCI have yielded inconsistent results. On one hand, some studies have found significantly lower serum BDNF levels in AD and MCI patients compared to healthy controls (4–8). On the other hand, other studies have reported increased peripheral levels of BDNF in AD and MCI patients compared to healthy individuals (9–11). Furthermore, there are discrepancies in the trends of BDNF level changes: some studies show a decrease in peripheral BDNF levels during the MCI stage and an increase during the AD stage (12), while others indicate an increase during the MCI stage followed by a decrease during the AD stage (13). These conflicting results may be related to sample differences (population, medication use, etc.), methodological differences (cognitive assessment tools, sample handling, etc.), and environmental factors (lifestyle, etc.).
BDNF gene polymorphism is associated with neurodegenerative diseases, with the rs6265 (Val66Met) polymorphism linked to the risk of Alzheimer’s Disease (AD) (14, 15) and related to cognitive function (6, 16–21). Moreover, the secretion of BDNF is affected by BDNF rs6265 polymorphism (22, 23), indicating that BDNF gene polymorphism may impact the expression and activity of the protein, thereby influencing individual neuroplasticity and cognitive function. A meta-analysis indicated that individuals carrying the Met/Met genotype not only have poorer cognitive abilities but also lower serum BDNF levels (19). Studies show that BDNF gene polymorphism is significantly associated with memory function (24), while other studies find no significant link between gene polymorphism and cognitive function (25). These conflicting results may be due to the complex interactions between multiple genes affecting cognitive function and insufficient consideration of gene-environment interactions (26, 27). Although previous research has focused on the relationship between BDNF gene polymorphisms and cognitive functions, the majority of studies have concentrated on rs6265. There is less research on other polymorphic segments, particularly concerning interactions with environmental factors such as lifestyle.
The impact of genetics and environment on Alzheimer’s Disease (AD) is not independent; the interplay between immutable risk factors such as genetics and modifiable factors like environment and physical activity can influence the onset of AD (28). Besides the impact of BDNF gene polymorphism on cognition, social activity, as a significant lifestyle factor, has been shown to positively affect cognition in the elderly adults (29, 30). Active participation in social activities not only provides cognitive stimulation but may also indirectly affect BDNF levels and function by reducing stress and enhancing social support (31). Engaging more in social activities could help prevent or delay cognitive decline in elderly adults patients with Mild Cognitive Impairment (MCI) (32). A longitudinal study showed that in elderly individuals without cognitive impairment potentially modifiable factor such as social isolation may contribure to a condition of biopsychosocial frailty, which may increase the risk of dementia (33). Therefore, exploring the interactions between BDNF gene polymorphism, BDNF levels, and social engagement is crucial for understanding the complex etiology of MCI and its prevention strategies.
This study aims to explore the relationship between BDNF gene polymorphism and susceptibility to MCI, as well as the connection between BDNF levels and cognitive function in MCI patients. Additionally, the study will examine the impact of the interaction between social activities and BDNF polymorphism on the risk of MCI. Due to the lack of obvious symptoms, elderly individuals with mild cognitive impairment (MCI) may not receive timely diagnosis and medical treatment, leading them to often reside in the community rather than in hospitals. Therefore, early identification of MCI among community-dwelling elderly individuals is crucial. This study focuses on the elderly community population, aiming to provide a theoretical basis for the early diagnosis and personalized intervention of MCI.
2 Methods
2.1 Study sampling
In May 2023, a simple random sampling was employed to select one district in Zhongshan, Guangdong Province. A total of 500 community-dwelling elderly persons aged 60 and over were randomly selected to participate in the questionnaire survey. During the screening process, neuropsychological tests, including the Mini-Mental Status Examination (MMSE), were used to assess cognitive function. The inclusion criteria encompassed individuals aged 60 years and above who did not have any known major cerebrovascular diseases, dementia or other pathological cognitive impairments, acute functional mental disorders (including schizophrenia or bipolar disorder), stroke history, etc. Of the 91 people diagnosed with MCI, 52 agreed to participate in the study. A total of 55 participants were matched by sex, age, and education as a control group to the cognitively normal people in the same area. A total of 107 people were actively involved in the study by undergoing blood collection procedures.
2.2 Clinical assessment
Mini-Mental Status Examination(MMSE) is currently the most valuable screening tool for identifying the early stages of Alzheimer’s disease. It is widely used in community epidemiological surveys and has good reliability and validity. In this study, MMSE was used to screen for Mild Cognitive Impairment (MCI) by assessing various aspects of cognitive function, including orientation, memory, attention and calculation, recall ability, and language ability. The scale is composed of 30 items, including orientation, memory, attention and calculation, recall ability, and language ability in 5 dimensions; the correct answer gets 1 point, and the wrong answer gets 0 points, for a total of 30 points. Activities of daily living (ADL)(Wade and Collin, 1988) scale to measure the daily living ability of community-dwelling elders. This scale comprises ten items, including the ability to: eat, bathing, grooming, dressing, stool control, urination control, toileting, transfer, walking on level ground, and going up and downstairs. Responses to each of the ten items on the scale are scored as 0 (cannot perform or can partially perform) or 5 (can perform). The total score ranges from 0 (low-functioning, dependent) to 100 (high-functioning, independent). Collection of Demographic Characteristics: The demographic characteristics of participants were collected through a standardized questionnaire, including information such as age, gender, education level, and lifestyle. The questionnaire was completed during face-to-face interviews conducted by trained investigators to ensure data accuracy and consistency. Height and weight were measured on-site by medical personnel.
Diagnostic criteria for MCI due to AD (34): (1) Concern regarding a change in cognition; (2) Impairment in one or more cognitive domains; (3) Preservation of independence in functional abilities; (4) Not demented.The inclusion criteria encompassed individuals aged 60 years and above who did not have any known major cerebrovascular diseases, dementia or other pathological cognitive impairments, acute functional mental disorders (including schizophrenia or bipolar disorder), stroke history and traumatic brain injury. Patients with a history of alcohol abuse, drug addiction, and long-term use of medications that affect cognitive function, such as glucocorticoids, antipsychotic drugs, and sedative-hypnotic drugs, are excluded.
2.3 Brain-derived neurotrophic factor
Serum BDNF levels in MCI patients and control groups were measured using the enzyme-linked immunosorbent assay (ELISA) calibrated with an ELISA reader. The ELISA kits were purchased from BOSTER, and the experiment was conducted strictly following the instructions provided with the kits. The equipment used included a centrifuge (XinAo, GENIUS 6K-C model), a full-wavelength ELISA reader (ThermoFisher, Multiskan SkyHigh model), a constant temperature water bath (BIOBASE, BJPX-WB26 model), and a washer (Antu, IWO-960 model). Blood samples were collected after obtaining written informed consent from the participants, who voluntarily joined the study following a clear explanation of its purpose and procedures. The blood collection was conducted by certified medical professionals under aseptic conditions, ensuring the validity of the samples and the safety of the participants. Furthermore, all research procedures were approved by an ethics committee, adhering to international ethical standards.
2.4 Genotyping
Peripheral venous blood was collected into an ethylenediaminetetraacetic acid-containing vacuum anticoagulant tube and centrifuged at 3,300 rpm at room temperature. The leukocytes in the middle layer were collected and stored in a cryopreservation tube at −80°C for subsequent use. A TIANamp Genomic DNA Kit (centrifugal column type; DP304; Tiangen, Beijing, China) was used to extract genomic DNA, and the optical density was determined using a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). Genomic DNA extraction results were evaluated by gel electrophoresis. By analyzing the two key loci of the APOE gene, the 112th position (rs429358) and the 158th position (rs7412), the genotype is determined. The ϵ2, ϵ3, and ϵ4 alleles are defined by the base combinations at these loci: T at the 112th position and T at the 158th position indicates ϵ2; T at the 112th position and C at the 158th position indicates ϵ3; and C at both the 112th and 158th positions indicates ϵ4. If the genotype contains the ϵ4 allele (e.g., ϵ3/ϵ4 or ϵ4/ϵ4), the individual is classified as an APOE4 carrier. In this study, genetic polymorphisms related to cognitive function (22) (e.g., rs6265, rs7103411), BDNF expression (35) (rs11030100), Alzheimer’s disease (36) (rs11030104), and other psychiatric disorders (37) (rs988748) were selected to investigate their potential associations in patients with mild cognitive impairment (MCI).
The results of linkage disequilibrium analysis show that rs11030104 and rs6265 (R²=0.907, DP=0.961), rs11030104 and rs7103411 (R²=0.927, DP=1), rs11030104 and rs988748 (R²=0.908, DP=1), rs6265 and rs7103411 (R²=0.837, DP=0.960), rs6265 and rs988748 (R²=0.817, DP=0.960), and rs7103411 and rs988748 (R²=0.942, DP=0.981). Among these, the DP values for rs11030104, rs7103411, and rs988748 are equal to 1, indicating that these three SNPs are in complete linkage. Hardy-Weinberg analysis shows that all loci, except for rs11030100 (P <0.001), follow Hardy-Weinberg equilibrium.
2.5 Statistical analysis
Analysis was conducted using R software version 4.1.3. Normally distributed continuous variables were described as mean ± standard deviation, and non-normally distributed variables were described using the median (interquartile range). Categorical variables were expressed as percentages (%). The t-test and chi-square test were used to assess differences between the two groups. Analysis of covariance (ANCOVA) was used to compare differences in BDNF levels between the MCI group and the control group. Multiple linear regression was used to analyze the association between BDNF gene polymorphism and serum BDNF levels. Multivariate logistic regression was used to assess the association between BDNF polymorphism and susceptibility to MCI, as well as the interaction between the BDNF gene and environmental factors.
3 Results
3.1 Basic characteristics of the study subjects
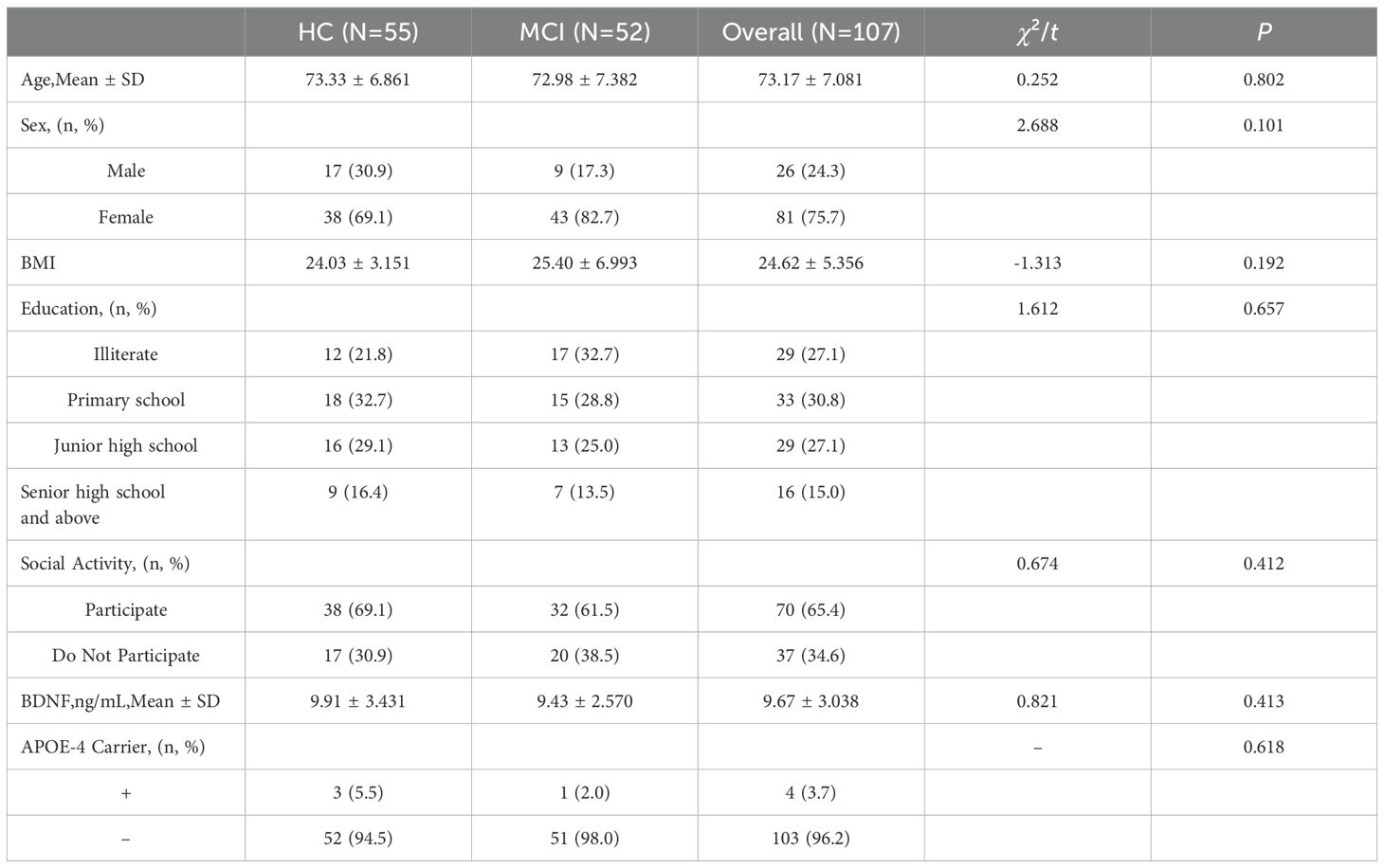
The study included 107 participants, consisting of 52 MCI patients and 55 cognitively normal elderly adults individuals. The basic characteristics of the subjects are presented in Table 1. There were no statistically significant differences between the MCI group and the healthy control (HC) group in terms of age, gender, BMI, education level, social activities, and serum BDNF concentrations (all P > 0.05).
3.2 Correlation between serum BDNF levels and cognitive function
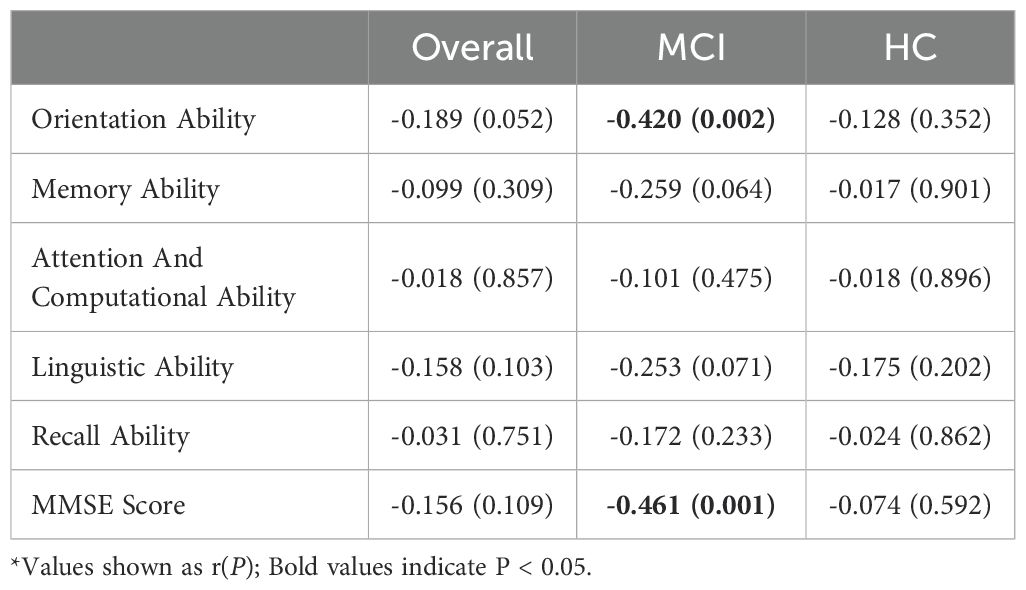
As shown in Table 2, in all participants, including both the MCI group and the healthy control group, BDNF levels were not significantly correlated with MMSE total scores or scores in various cognitive domains (all P > 0.05). In the normal control group, BDNF levels were also not significantly correlated with MMSE total scores or scores in any cognitive domains (all P > 0.05). However, for MCI patients, BDNF levels were significantly correlated with MMSE total scores (r = -0.461, P = 0.001) and orientation (r = -0.420, P = 0.002), but not with memory, attention and calculation, language abilities, or recall capabilities (all P > 0.05).
3.3 BDNF gene polymorphism and susceptibility to MCI
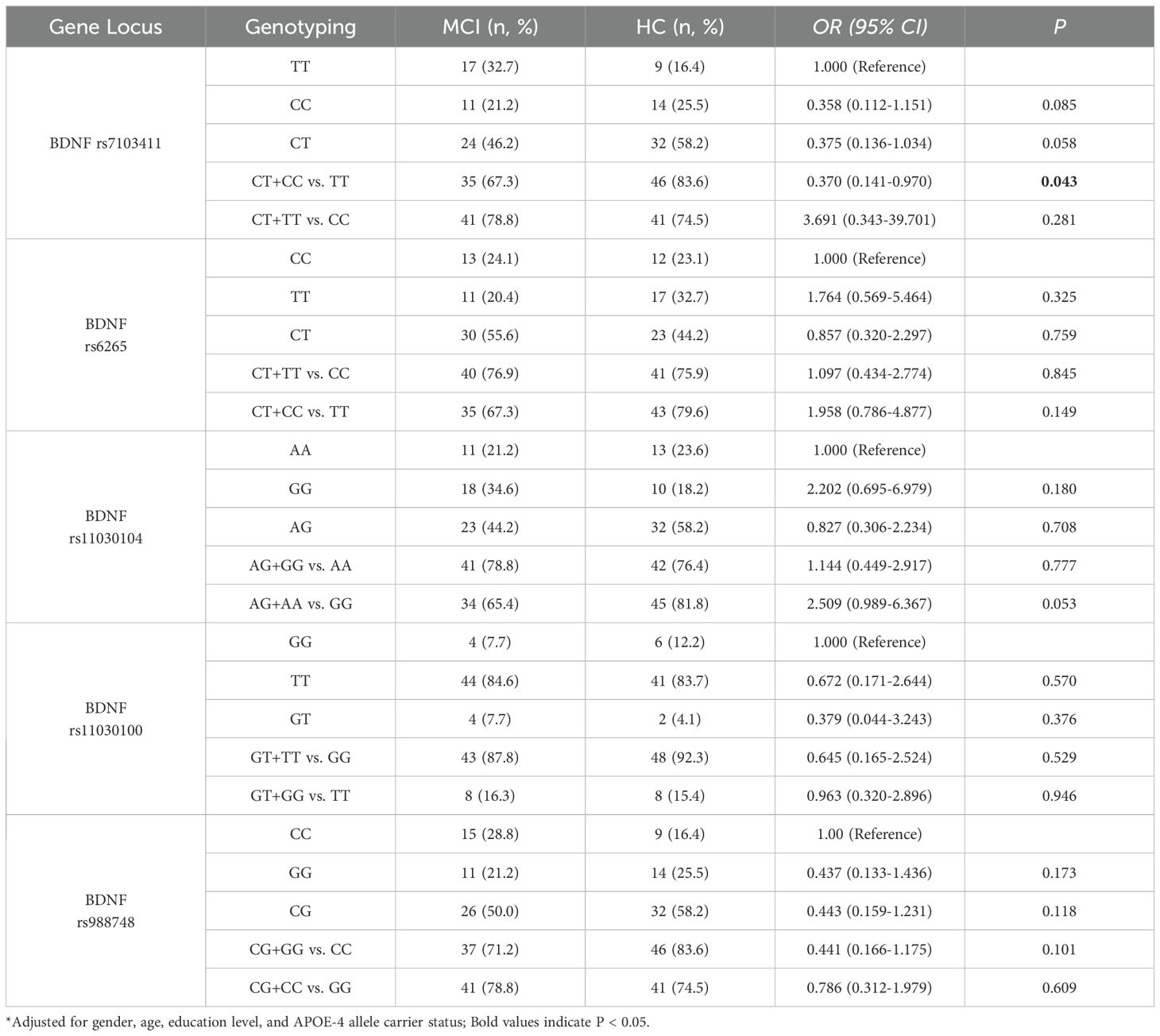
A comparison of genotype frequencies between MCI patients and the control group is presented in Table 3. After adjusting for age, gender, educational level, and APOE carrier status, the genetic model analysis of the BDNF rs7103411 locus between the MCI group and the HC group showed that compared to the frequency of CT plus CC genotypes, the frequency of the TT genotype was significantly higher in the MCI group compared to the HC group, indicating that the TT genotype may be associated with an increased risk of developing MCI, although the significance was modest (P = 0.043). No statistically significant differences were found at other genetic loci. (all P >0.05).
3.4 BDNF polymorphism and BDNF levels
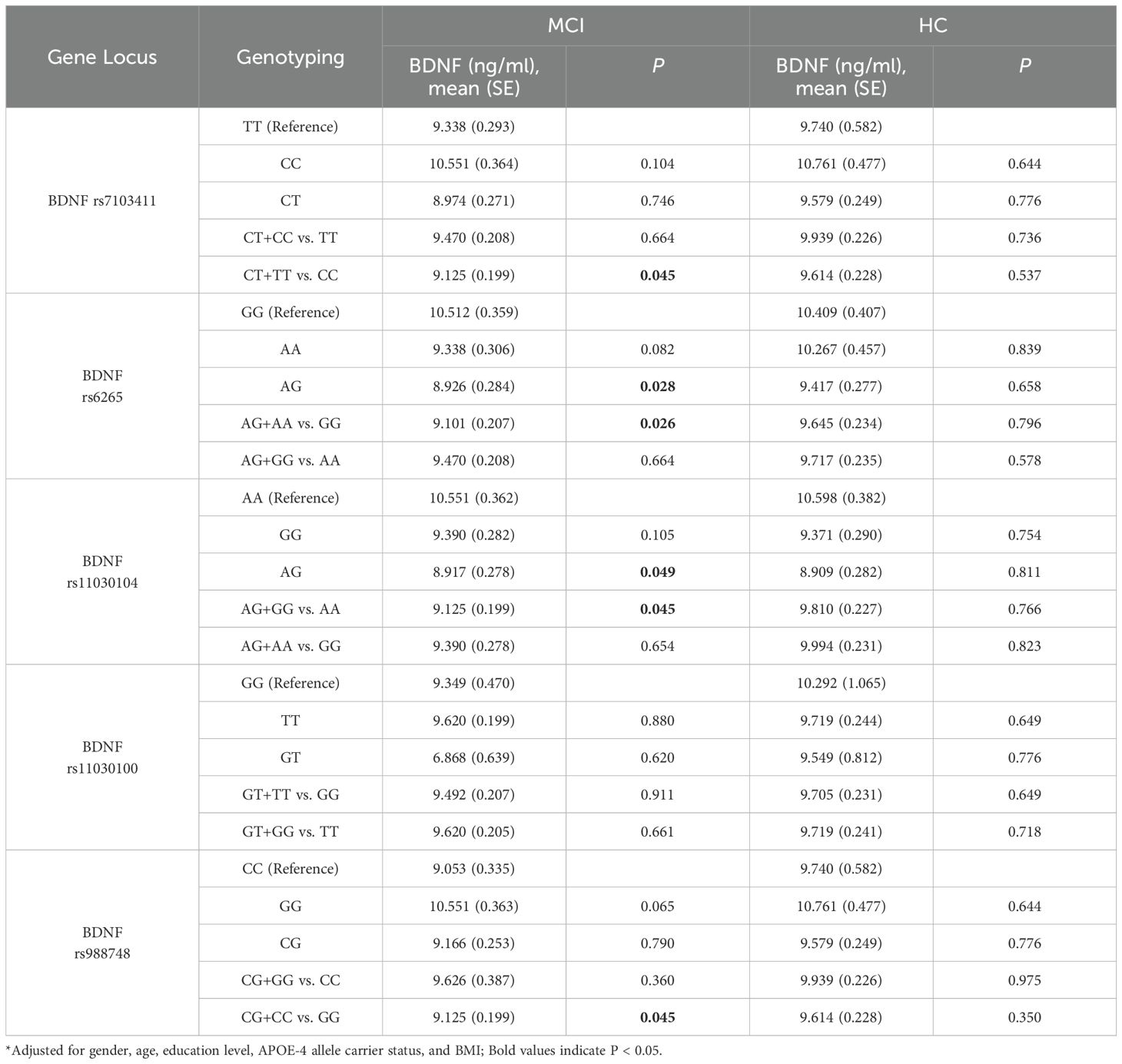
Covariance analysis was used to explore the serum BDNF levels of BDNF genotype polymorphisms in the elderly adults, as shown in Table 4. The results showed that in the healthy control group, there were no significant differences in BDNF levels among different genotypes at each locus. In the MCI group, carriers of the rs7103411 T allele had lower BDNF levels compared to CC homozygotes (CT+TT vs. CC) (P = 0.045). For rs6265, the AG genotype had significantly lower BDNF levels compared to the GG genotype (P = 0.028), and carriers of the G allele had significantly lower levels compared to A homozygotes (AG+AA vs. GG) (P = 0.026). For rs11030104, the AG genotype had significantly lower BDNF levels compared to the AA genotype (P = 0.049), and carriers of the G allele had significantly lower levels compared to A homozygotes (AG+GG vs. AA) (P = 0.045). For rs988748, carriers of the C allele had significantly lower BDNF levels compared to G homozygotes (CG+CC vs. GG) (P = 0.045).
3.5 BDNF rs7103411 polymorphism interaction on cognitive function
After adjusting for gender, age, educational level, and APOE-4 allele carriage, multivariate logistic regression analysis showed a significant interaction between BDNF rs7103411 and social activity (P = 0.033). Further analysis revealed that individuals with the CT or TT genotype in rs7103411 polymorphisms of BDNF gene who participated to social activities showed a significantly reduced risk for MCI, as compared to individuals who did not participate to social activities(OR = 0.320, 95% CI: 0.117-0.878, P = 0.027), as shown in Table 5. No statistically significant differences were found at other genetic loci. (all P >0.05).
4 Discussion
Gene-environment interactions play a crucial role in complex diseases such as neurodegenerative disorders (38). We found a significant interaction between BDNF rs7103411 polymorphism and social activity. Among carriers of the T allele, those who engaged in social activities had a significantly lower risk of developing MCI compared to those who did not participate. Social activities, as a key lifestyle factor, have profound impacts on cognitive function and psychological health in the elderly adults. Especially in elderly populations, promoting social engagement and reducing feelings of loneliness may help maintain and enhance cognitive functions (31, 39, 40). Cognitive reserve might be a potential mechanism by which social isolation affects cognitive function (40), where having extensive social connections and engaging in challenging, complex social interactions can provide mental stimulation and thus build cognitive reserve (41). Ward et al.’s study revealed that the Val66Met polymorphism of the BDNF gene might indirectly influence cognitive function by regulating an individual’s use of cognitive reserves, particularly in executive functions (42). Additionally, elderly adults with depression or anxiety might experience more negative social interactions, which could exacerbate feelings of loneliness and isolation. Evans et al. highlighted the relationship between social isolation and symptoms of depression and anxiety, as well as their interaction with cognitive function in the elderly adults (43). A study has been shown that patients with early-onset AD who carry the ApoE4 allele in relatively early stages might develop a greater impairment on episodic verbal memory task, as compared to ApoE4 non-carriers (44). Furthermore, studies have revealed the interaction between BDNF and feelings of loneliness impacting cognitive decline, particularly manifesting as more pronounced semantic memory deterioration and increased feelings of loneliness among participants with lower BDNF expression levels (45). This finding further supports the protective mechanism of BDNF on cognitive functions.These findings, together with our study, emphasize the complexity of interactions among social engagement, gene polymorphism, and cognitive reserve, providing insights into the etiology and prevention strategies for MCI.
BDNF gene polymorphisms are a focal point in research on Alzheimer’s disease (AD) and Mild Cognitive Impairment (MCI), revealing their significant role as prognostic markers in memory decline and hippocampal atrophy in MCI patients, and their close association with the progression of MCI (15). In healthy adults with high levels of Aβ accumulation in the brain, carriers of the Met allele exhibit greater declines in cognitive function and hippocampal volume (20), further underscoring the potential role of the BDNF gene in regulating cognitive processes. This study found that BDNF rs7103411 polymorphism is associated with MCI risk. In a study of an elderly adults German cohort, rs7103411 polymorphism was significantly related to memory performance, with carriers of the C allele showing an increased risk of cognitive decline (including memory and perceptual speed) (22). However, in our research, individuals carrying the C allele were found to have a reduced risk of MCI compared to TT individuals. This could be due to differences in genetic background, gender stratification, and differences in cognitive function tests. A study in an English community of elderly adults did not find an association between rs7103411 and cognitive function, which might primarily be due to the use of a haplotype analysis method (25). Additionally, we observed lower serum BDNF levels in rs7103411 T allele carriers compared to CC individuals among MCI patients, further suggesting that BDNF gene polymorphism might affect cognitive functions and their decline by modulating BDNF expression levels. Moreover, cognitive function and susceptibility to MCI may be influenced by complex interactions among multiple genes (26). The effects of a single gene polymorphism may be offset or enhanced by the actions of other genes, making it difficult to consistently observe the impacts of individual polymorphisms across all studies (46).
This study did not find an association between polymorphisms rs6265, rs11030104, rs11030100, and rs988748 with susceptibility to Mild Cognitive Impairment (MCI). Beyond the limitations of sample size and study type, the cohort selected from community-dwelling elderly adults with MCI had relatively short disease duration and milder disease severity, which may have hindered the observation of correlations between cognitive abilities and both BDNF levels and genetic polymorphisms. Similarly, previous research has shown that rs6265 polymorphism is not significantly associated with the age of onset, severity, or rate of cognitive decline in Alzheimer’s disease (AD) (47, 48). No significant association was found between rs11030104 and cognitive decline in AD patients either (49). Currently, the understanding of the specific roles of the BDNF gene in cognitive function and the development of MCI is still limited. The biological effects of polymorphisms may involve complex molecular pathways and networks that are not yet fully understood and should be further explored in future research. For example, genetic variants affecting amyloid precursor protein (APP), tau binding proteins, immunity, and lipid metabolism play a critical role in AD, as shown by a largely cited study (50), with underwent an Author correction (51). Differences in definitions of MCI or cognitive decline and variations in studies across different subtypes may lead to contradictory conclusions (1, 52). Additionally, due to the limitations of the survey content, the impact of gene-environment interactions may not have been fully considered, which can also contribute to divergent study results (27, 53, 54).
This study indicates that multiple single nucleotide polymorphisms (SNPs) within the BDNF gene, including rs6265, rs11030104, rs7103411, and rs988748, are associated with serum BDNF levels in patients with Mild Cognitive Impairment (MCI). Reduced BDNF levels and signaling are linked to neurodegenerative diseases such as MCI, Alzheimer’s Disease (AD), and Parkinson’s Disease (PD) (3). Notably, the rs6265 polymorphism, consistent with findings from numerous studies, has been shown to correlate with BDNF levels and is closely associated with key neurophysiological processes involved in cognition (23, 55, 56). Specifically, rs6265 is linked to changes in hippocampal volume, cortical thickness, and abnormalities in synaptic connections, which are critical indicators of cognitive function (55, 56). Further research has also highlighted the role of rs6265 in learning, memory retention, executive functions, and the decline in perceptual speed among the elderly adults (57, 58). The evidence from this study further supports the significant role of the rs6265 polymorphism in cognitive dysfunction. Polymorphisms rs11030104, rs7103411, and rs988748 show differences in BDNF expression levels within haplotypes (59). Higher BDNF expression levels may be associated with poorer cognitive performance, including overall cognition, episodic memory, executive functions, visuospatial abilities, and semantic processing (60). Although our study identified differences in serum BDNF levels across multiple BDNF SNPs, aside from rs7103411, no other polymorphisms were found to be associated with MCI. This analysis might be influenced by the study’s sample originating from community-dwelling elderly adults individuals selected without self-perceived symptoms, whose symptoms may not be readily noticeable. Secondly, changes in serum BDNF levels as early biomarkers for MCI or AD might occur before the observable progression of the disease.
Current research on the peripheral BDNF levels in patients with AD and MCI shows inconsistent results. Some studies suggest that BDNF levels are elevated in MCI patients, possibly reflecting a compensatory neurorestorative mechanism at the early stages of AD (11, 13, 61). However, in AD patients, extensive neuronal damage and depleted cognitive reserves lead to a significant decrease in BDNF levels (4, 13). In rat models, homozygous rats for rs6265 show a notable reduction in BDNF release compared to heterozygous rats, yet they exhibit significantly enhanced synaptic growth capabilities (62). Some researchers hypothesize that a decrease in serum BDNF levels is a late event in the progression of AD hence changes in BDNF levels during the MCI stage might not be pronounced (4). Concurrently, our study observed a negative correlation between BDNF levels and scores on MMSE and orientation tasks in MCI patients. These findings further support the hypothesis that BDNF serves as a compensatory and protective mechanism in patients with MCI or early-stage AD. The role of BDNF in neuroprotection and neuroregeneration has been extensively studied, and our results did not find a significant correlation between serum BDNF levels and MCI status. This may be due to the influence of various biological, methodological, and environmental factors on serum BDNF levels.
Our study has certain limitations, including a small sample size, a cross-sectional study design, and potential confounding factors. Future research should employ larger sample sizes and longitudinal study designs, and consider a wider range of genetic and environmental factors to delve deeper into the mechanisms of BDNF in the development of MCI. Additionally, exploring the interactions between BDNF and other biomarkers will be an important direction for future studies. Previous research has primarily focused on the Val66Met polymorphism, with the relatively limited investigation into other polymorphic sites within the BDNF gene. This finding suggests that polymorphic sites other than Val66Met may also play roles in cognitive decline and susceptibility to MCI, which warrants further verification in future studies. Considering BDNF’s critical role in neuroprotection and neuroplasticity, our findings underscore the need for future research to thoroughly explore the complex biological mechanisms between BDNF and MCI, as well as the potential long-term effects on cognitive function in the elderly adults.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the research ethics committee of the Third People’s Hospital of Zhongshan City (opinion number SSYLL20220401). This study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZT: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. PJ: Methodology, Writing – original draft, Writing – review & editing. YZ: Data curation, Investigation, Writing – original draft, Writing – review & editing. CK: Investigation, Writing – original draft, Writing – review & editing. JL: Investigation, Writing – original draft, Writing – review & editing. XL: Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Social Welfare Science and Technology Research Major Project in Zhongshan (grant number 2022B3017) and the Project of the Inheritance and Innovation of Traditional Chinese Medicine in Zhongshan City (grant number 2024B3025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: Mild cognitive impairment. Neurology. (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
2. Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. (2020) 5:e661–71. doi: 10.1016/S2468-2667(20)30185-7
3. Ventriglia M, Zanardini R, Bonomini C, Zanetti O, Volpe D, Pasqualetti P, et al. Serum brain-derived neurotrophic factor levels in different neurological diseases. BioMed Res Int. (2013) 2013:901082. doi: 10.1155/2013/901082
4. Ng TKS, Ho CSH, Tam WWS, Kua EH, Ho RCM. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with alzheimer’s disease (AD): A systematic review and meta-analysis. Int J Mol Sci. (2019) 20(2):257. doi: 10.3390/ijms20020257
5. Xie B, Zhou H, Liu W, Yu W, Liu Z, Jiang L, et al. Evaluation of the diagnostic value of peripheral BDNF levels for Alzheimer’s disease and mild cognitive impairment: results of a meta-analysis. Int J Neurosci. (2020) 130:218–30. doi: 10.1080/00207454.2019.1667794
6. Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, et al. Effect of BDNF val66Met on memory decline and hippocampal atrophy in prodromal alzheimer’s disease: A preliminary study. PloS One. (2014) 9:e86498. doi: 10.1371/journal.pone.0086498
7. Mori Y, Tsuji M, Oguchi T, Kasuga K, Kimura A, Futamura A, et al. Serum BDNF as a potential biomarker of alzheimer’s disease: verification through assessment of serum, cerebrospinal fluid, and medial temporal lobe atrophy. Front Neurol. (2021) 12:653267. doi: 10.3389/fneur.2021.653267
8. Qin XY, Cao C, Cawley NX, Liu TT, Yuan J, Loh YP, et al. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer’s disease: a meta-analysis study (N=7277). Mol Psychiatry. (2017) 22:312–20. doi: 10.1038/mp.2016.62
9. Angelucci F, Spalletta G, Iulio F di, Ciaramella FA, Salani F, Varsi AE, et al. Alzheimers disease (AD) and mild cognitive impairment (MCI) patients are characterized by increased BDNF serum levels. Curr Alzheimer Res. (2010) 7:15–20. doi: 10.2174/156720510790274473
10. Ng TKS, Coughlan C, Heyn PC, Tagawa A, Carollo JJ, Kua EH, et al. Increased plasma brain-derived neurotrophic factor (BDNF) as a potential biomarker for and compensatory mechanism in mild cognitive impairment: a case-control study. Aging. (2021) 13:22666–89. doi: 10.18632/aging.203598
11. Faria MC, Gonçalves GS, Rocha NP, Moraes EN, Bicalho MA, Gualberto Cintra MT, et al. Increased plasma levels of BDNF and inflammatory markers in Alzheimer’s disease. J Psychiatr Res. (2014) 53:166–72. doi: 10.1016/j.jpsychires.2014.01.019
12. Qian F, Liu J, Yang H, Zhu H, Wang Z, Wu Y, et al. Association of plasma brain-derived neurotrophic factor with Alzheimer’s disease and its influencing factors in Chinese elderly population. Front Aging Neurosci. (2022) 14:987244. doi: 10.3389/fnagi.2022.987244
13. Kim BY, Lee SH, Graham PL, Angelucci F, Lucia A, Pareja-Galeano H, et al. Peripheral brain-derived neurotrophic factor levels in alzheimer’s disease and mild cognitive impairment: a comprehensive systematic review and meta-analysis. Mol Neurobiol. (2017) 54:7297–311. doi: 10.1007/s12035-016-0192-9
14. Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. (2007) 39:17–23. doi: 10.1038/ng1934
15. Bessi V, Mazzeo S, Bagnoli S, Padiglioni S, Carraro M, Piaceri I, et al. The implication of BDNF Val66Met polymorphism in progression from subjective cognitive decline to mild cognitive impairment and Alzheimer’s disease: a 9-year follow-up study. Eur Arch Psychiatry Clin Neurosci. (2020) 270:471–82. doi: 10.1007/s00406-019-01069-y
16. Lee SY, Wang TY, Chen SL, Chang YH, Chen PS, Huang SY, et al. The correlation between plasma brain-derived neurotrophic factor and cognitive function in bipolar disorder is modulated by the BDNF Val66Met polymorphism. Sci Rep. (2016) 6:37950. doi: 10.1038/srep37950
17. Voineskos AN, Lerch JP, Felsky D, Shaikh S, Rajji TK, Miranda D, et al. The brain-derived neurotrophic factor Val66Met polymorphism and prediction of neural risk for Alzheimer disease. Arch Gen Psychiatry. (2011) 68:198–206. doi: 10.1001/archgenpsychiatry.2010.194
18. Andrews SJ, Das D, Cherbuin N, Anstey KJ, Easteal S. Association of genetic risk factors with cognitive decline: the PATH through life project. Neurobiol Aging. (2016) 41:150–8. doi: 10.1016/j.neurobiolaging.2016.02.016
19. Farcas A, Hindmarch C, Iftene F. BDNF gene Val66Met polymorphisms as a predictor for clinical presentation in schizophrenia – recent findings. Front Psychiatry. (2023) 14:1234220. doi: 10.3389/fpsyt.2023.1234220
20. Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, et al. BDNF Val66Met, Aβ amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol Aging. (2013) 34:2457–64. doi: 10.1016/j.neurobiolaging.2013.05.006
21. Abanmy N, Alsabhan J, Gard P, Scutt G. Association between the Val66Met polymorphism (rs6265/G196A) of the BDNF gene and cognitive performance with SSRI use in Arab Alzheimer’s disease patients. Saudi Pharm J SPJ. (2021) 29:1392–8. doi: 10.1016/j.jsps.2021.10.007
22. Laing KR, Mitchell D, Wersching H, Czira ME, Berger K, Baune BT. Brain-derived neurotrophic factor (BDNF) gene: a gender-specific role in cognitive function during normal cognitive aging of the MEMO-Study? Age. (2012) 34:1011–22. doi: 10.1007/s11357-011-9275-8
23. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. (2003) 112:257–69. doi: 10.1016/S0092-8674(03)00035-7
24. Notaras M, van den Buuse M. Brain-derived neurotrophic factor (BDNF): novel insights into regulation and genetic variation. Neuroscientist. (2019) 25:434–54. doi: 10.1177/1073858418810142
25. Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P, et al. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. (2008) 7:411–7. doi: 10.1111/j.1601-183X.2007.00363.x
26. Almkvist O, Graff C. The APOE ϵ4 allele affects cognitive functions differently in carriers of APP mutations compared to carriers of PSEN1 mutations in autosomal-dominant alzheimer’s disease. Genes. (2021) 12:1954. doi: 10.3390/genes12121954
27. Chiasseu M, Fesharaki-Zadeh A, Saito T, Saido TC, Strittmatter SM. Gene-environment interaction promotes Alzheimer’s risk as revealed by synergy of repeated mild traumatic brain injury and mouse App knock-in. Neurobiol Dis. (2020) 145:105059. doi: 10.1016/j.nbd.2020.105059
28. Finch CE, Kulminski AM. The AD exposome. Alzheimers Dement J Alzheimers Assoc. (2019) 15:1123–32. doi: 10.1016/j.jalz.2019.06.3914
29. Sommerlad A, Kivimäki M, Larson EB, Röhr S, Shirai K, Singh-Manoux A, et al. Social participation and risk of developing dementia. Nat Aging. (2023) 3:532–45. doi: 10.1038/s43587-023-00387-0
30. Niti M, Yap KB, Kua EH, Tan CH, Ng TP. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-ϵ4 genotype in Chinese older adults. Int Psychogeriatr. (2008) 20:237–51. doi: 10.1017/S1041610207006655
31. Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. (2004) 3:343–53. doi: 10.1016/S1474-4422(04)00767-7
32. Hughes TF, Flatt JD, Fu B, Chang CCH, Ganguli M. Engagement in social activities and progression from mild to severe cognitive impairment: the MYHAT study. Int Psychogeriatr. (2013) 25:587–95. doi: 10.1017/S1041610212002086
33. Solfrizzi V, Scafato E, Lozupone M, Seripa D, Schilardi A, Custodero C, et al. Biopsychosocial frailty and the risk of incident dementia: The Italian longitudinal study on aging. Alzheimers Dement J Alzheimers Assoc. (2019) 15:1019–28. doi: 10.1016/j.jalz.2019.04.013
34. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. (2011) 7:270–9. doi: 10.1016/j.jalz.2011.03.008
35. Donati F, Sian V, Biasini GM, de la Torre X, Folchitto F, Botrè F. Serum levels of brain-derived neurotrophic factor and other neurotrophins in elite athletes: potential markers of the use of transcranial direct current stimulation in sport. Front Sports Act Living. (2021) 3:619573. doi: 10.3389/fspor.2021.619573
36. Huang R, Huang J, Cathcart H, Smith S, Poduslo SE. Genetic variants in brain-derived neurotrophic factor associated with Alzheimer’s disease. J Med Genet. (2007) 44:e66. doi: 10.1136/jmg.2006.044883
37. Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, et al. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biol Psychiatry. (2005) 58:307–14. doi: 10.1016/j.biopsych.2005.04.006
38. Duman RS. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues Clin Neurosci. (2009) 11:239–55. doi: 10.31887/DCNS.2009.11.3/rsduman
39. Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cognit Sci. (2009) 13:447–54. doi: 10.1016/j.tics.2009.06.005
40. Evans IEM, Llewellyn DJ, Matthews FE, Woods RT, Brayne C, Clare L, et al. Social isolation, cognitive reserve, and cognition in healthy older people. PloS One. (2018) 13:e0201008. doi: 10.1371/journal.pone.0201008
41. Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. (2006) 5:406–12. doi: 10.1016/S1474-4422(06)70417-3
42. Ward DD, Summers MJ, Saunders NL, Ritchie K, Summers JJ, Vickers JC. The BDNF Val66Met polymorphism moderates the relationship between cognitive reserve and executive function. Transl Psychiatry. (2015) 5:e590–0. doi: 10.1038/tp.2015.82
43. Evans IEM, Llewellyn DJ, Matthews FE, Woods RT, Brayne C, Clare L. Social isolation, cognitive reserve, and cognition in older people with depression and anxiety. Aging Ment Health. (2019) 23:1691–700. doi: 10.1080/13607863.2018.1506742
44. Marra C, Bizzarro A, Daniele A, De Luca L, Ferraccioli M, Valenza A, et al. Apolipoprotein E epsilon4 allele differently affects the patterns of neuropsychological presentation in early- and late-onset Alzheimer’s disease patients. Dement Geriatr Cognit Disord. (2004) 18:125–31. doi: 10.1159/000079191
45. Dabiri S, Mwendwa DT, Campbell A. Psychological and neurobiological mechanisms involved in the relationship between loneliness and cognitive decline in older adults. Brain Behav Immun. (2024) 116:10–21. doi: 10.1016/j.bbi.2023.11.034
46. Tisato V, Zuliani G, Vigliano M, Longo G, Franchini E, Secchiero P, et al. Gene-gene interactions among coding genes of iron-homeostasis proteins and APOE-alleles in cognitive impairment diseases. PloS One. (2018) 13:e0193867. doi: 10.1371/journal.pone.0193867
47. Chuu JYJ, Taylor JL, Tinklenberg J, Noda A, Yesavage J, Murphy GM. The brain-derived neurotrophic factor Val66Met polymorphism and rate of decline in Alzheimer’s disease. J Alzheimers Dis JAD. (2006) 9:43–9. doi: 10.3233/jad-2006-9104
48. He X, Zhang Z, Zhang J, Zhou Y, Tang M, Wu C, et al. Lack of association between the BDNF gene val66Met polymorphism and alzheimer disease in a chinese han population. Neuropsychobiology. (2007) 55:151–5. doi: 10.1159/000106473
49. Honea RA, Cruchaga C, Perea RD, Saykin AJ, Burns JM, Weinberger DR, et al. Characterizing the role of brain derived neurotrophic factor genetic variation in alzheimer’s disease neurodegeneration. PloS One. (2013) 8:e76001. doi: 10.1371/journal.pone.0076001
50. Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. (2019) 51:414–30. doi: 10.1038/s41588-019-0358-2
51. Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Author Correction: Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. (2019) 51:1423–4. doi: 10.1038/s41588-019-0495-7
52. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. (2014) 275:214–28. doi: 10.1111/joim.12190
53. Mosca A, Sperduti S, Pop V, Ciavardelli D, Granzotto A, Punzi M, et al. Influence of APOE and RNF219 on behavioral and cognitive features of female patients affected by mild cognitive impairment or alzheimer’s disease. Front Aging Neurosci. (2018) 10:92. doi: 10.3389/fnagi.2018.00092
54. Tully PJ, Helmer C, Peters R, Tzourio C. Exploiting drug-apolipoprotein E gene interactions in hypertension to preserve cognitive function: the 3-city cohort study. J Am Med Dir Assoc. (2019) 20:188–194.e4. doi: 10.1016/j.jamda.2018.08.002
55. Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. (2006) 59:812–5. doi: 10.1016/j.biopsych.2005.09.022
56. Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic Variant BDNF (Val66Met) Polymorphism Alters Anxiety-Related Behavior. Science. (2006) 314:140–3. doi: 10.1126/science.1129663
57. Kennedy KM, Reese ED, Horn MM, Sizemore AN, Unni AK, Meerbrey ME, et al. BDNF val66met polymorphism affects aging of multiple types of memory. Brain Res. (2015) 1612:104–17. doi: 10.1016/j.brainres.2014.09.044
58. Goto T, Saligan LN, Li X, Xiang L, Kwiat C, Nguyen C, et al. Associations of brain-derived neurotrophic factor rs6265 polymorphism and cognitive function in breast cancer survivors from a cross-sectional study. Cancer Med. (2024) 13:e6975. doi: 10.1002/cam4.6975
59. Devlin P, Cao X, Stanfill AG. Genotype-expression interactions for BDNF across human brain regions. BMC Genomics. (2021) 22(1):207. doi: 10.1186/s12864-021-07525-1
60. Elahi FM, Casaletto KB, La Joie R, Walters SM, Harvey D, Wolf A, et al. Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. (2020) 16:681–95. doi: 10.1016/j.jalz.2019.09.004
61. Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, et al. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J Neural Transm. (2006) 113:1217–24. doi: 10.1007/s00702-005-0397-y
Keywords: gene polymorphism, social activities, MCI, BDNF, elderly adults
Citation: Tan Z, Ping J, Zhang Y, Kong C, Luo J and Liu X (2025) The impact of the interaction between BDNF rs7103411 gene polymorphism and social activities on mild cognitive impairment in community-dwelling elderly adults. Front. Psychiatry 15:1469671. doi: 10.3389/fpsyt.2024.1469671
Received: 25 July 2024; Accepted: 12 December 2024;
Published: 14 January 2025.
Edited by:
Olusegun Baiyewu, University of Ibadan, NigeriaReviewed by:
Antonio Daniele, Catholic University of the Sacred Heart, Rome, ItalyJian-Huan Chen, Jiangnan University, China
Copyright © 2025 Tan, Ping, Zhang, Kong, Luo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxia Liu, c3lsaXV4aW54aWFAMTYzLmNvbQ==
 Zhenkun Tan
Zhenkun Tan Junjiao Ping
Junjiao Ping Ying Zhang
Ying Zhang Chuijia Kong
Chuijia Kong Jiali Luo
Jiali Luo Xinxia Liu
Xinxia Liu