- 1Faculty of Medicine, New Vision University, Internship at Beni Suef University Hospital, Faiyum, Egypt
- 2Department of Family and Community medicine, College of Medicine, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 3Senior Nursing Specialist, General Administration of Health Programs and Chronic Diseases in Mother’s Health, Ministry of Health, Riyadh, Saudi Arabia
- 4Faculty of Medicine, Al-Azhar University, Damietta, Egypt
- 5Faculty of Medicine, New Vision University, Internship at Psychiatric hospital Beersheva, Beer Sheva, Palestine
- 6Faculty of Medicine, New Vision University, Nazareth, Georgia
- 7Faculty of Medicine, New Vision University, Chtoura, Lebanon
- 8Medical Research Center Department, Kateb University, Kabul, Afghanistan
- 9Department of Public Health and Community Medicine, Faculty of Medicine, Zagazig University, Zagazig, Egypt
- 10General Administration of Health Programs and Chronic Diseases in Mother’s Health, Ministry of Health, Riyadh, Saudi Arabia
Background: Autism Spectrum Disorder (ASD) is a complex lifelong neurodevelopmental disorder with a high and increasing global prevalence. Although the precise causes are unknown, both genetic and environmental factors, including maternal ones during pregnancy, significantly influence its development. Therefore, this study endeavors to explore the potential causes of autism, including maternal and paternal prenatal risk factors, as well as antenatal and natal maternal risk factors, and their associations with the severity of ASD in mothers of children with ASD, from February to May 2024.
Methods: At an autism center in Saudi Arabia, this cross-sectional study enrolled 168 mothers of children diagnosed with ASD. The web-based survey employs a structured questionnaire to gather comprehensive prenatal, natal, and demographic data. The collected data was coded and analyzed using suitable tests.
Results: The majority of the surveyed 168 mothers with autistic children reported having autism spectrum disorder (43.8%), moderate autism (31.9%), mild autism (15.6%), and severe autism (8.8%). Most autistic children had a history of one or both maternal and/or paternal antenatal exposures: 79.2% had soft drink consumption, 35.1% smoked, 24.4% had chronic physical diseases, and 20.8% had psychological disease. Regarding maternal antenatal conditions, 37% had a history of recurrent infection, 29.2% had anemia, 15.5% had a history of threatened abortion or bleeding, as well as exposure to air pollution, and 22 (13.1%) had a history of gestational diabetes. Significant (p <0.05) predictors of severe autism were gestational diabetes aOR 4.553 (95% CI: [1.518, 14.25], birth oxygen desaturation 4.142 (95% CI: [1.437, 12.45]. Furthermore, the likelihood of classifying a child’s ASD as severe increases by 7.1% with each year of age1.071 (95% CI: [1.002, 1.15].
Conclusion: ASD is a prevalent health condition that has many interrelationships with prenatal, maternal (medical, environmental, and psychosocial factors), and natal conditions. Prospective studies are essential for understanding and addressing these ASD risk factors.
1 Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by difficulties in social communication, interaction, and behavioral patterns that can affect everyone, manifest typically in early childhood, and have a significant impact on both individuals and society (1, 2). Researchers have identified approximately 1 in 68 children with some form of ASD. Despite the high global prevalence of ASD, which has increased over the past 50 years from 1:10000 live birth to 1:59 live birth, it remains incurable and, like any other childhood disorder, requires treatment and intervention. Although the precise causes are unknown, both genetic and environmental factors, including maternal factors during pregnancy, significantly influence its development (3, 4).
Many maternal factors can impact a child’s health in several ways. The uterine environment can be changed by a number of things, including polycystic ovary syndrome (PCOs), prenatal hyperandrogenism (male sex hormones), chronic anovulation, insulin resistance, metabolic syndrome, and chronic low-grade inflammation. These things may play a part in neurodevelopmental disorders like ASD, but the exact ways they cause these disorders are still not fully understood (5–7). Using some drugs (antidepressants, specifically selective serotonin reuptake inhibitors) during the second or third trimester of pregnancy may increase the risk of a child developing ASD (8).
Several studies have indicated that mothers with gestational diabetes mellitus (GDM)-associated altered glucose metabolism and insulin resistance conditions lead to neurodevelopmental disorders such as ASD. Moreover, pregnancy-associated inflammatory processes and oxidative stress may contribute to these adverse outcomes (9, 10). Additionally, tobacco smoke significantly impacts sperm DNA methylation and gene expression, potentially altering neuronal structure and leading to adverse birth outcomes such as low birth weight and limited growth in the fetus (11). Proper maternal nutrition during pregnancy plays a crucial role for optimal fetal development, growth, and health (12).
An autistic child prefers playing alone, avoids eye contact, has limited or repetitive patterns, and is at higher risk for injuries or abuse. This can sometimes be harmful. These deficits make it difficult for many with ASD to live independently. Therefore, family members and care providers deal with emotional, financial, and even physical stress and sexual health issues while raising a child with ASD, which appears to contribute to a general decrease in parental well-being and an increase in mental health concerns. The mother of an autistic child faces more stress, anxiety, depressive symptoms, social withdrawal, and fear compared to other mothers. Similar stages of grief, such as denial, anger, bargaining, depression, and acceptance, were experienced by others, along with a feeling of guilt as if they were responsible (13–16).
ASD research is scarce in developed nations like Saudi Arabia; hence, most clinical procedures are based on Western findings (17). Thus, the above-mentioned factor heightened interest in studying this topic, which is understandable. From February to May 2024, we conducted this cross-sectional study to investigate the severity level of ASD, the prenatal, maternal, and natal risk factors that might be associated with it during pregnancy, and their interrelationships among the mothers of ASD children.
2 Methods
2.1 Study design and setting
From February 2024 to May 2024, we conducted this web-based cross-sectional study in the Society of Autism Families in SA’s large cities, Riyadh, Jeddah, and Dammam. Despite SA’s vast size, most of its services are only available in its largest cities.
2.2 Participants
This cross-sectional study specifically targeted adult mothers who had received an ASD diagnosis for their children. Participants were eligible if their children were less than 18 years old, attended a pediatric outpatient clinic or an autism-specific center, and met the ASD diagnostic criteria. The American Psychiatric Association’s Diagnostic and Statistical Manual, Fifth Edition (DSM-5) provides information to help diagnose ASD. According to DSM-5 (16), a child must have persistent deficits in each of three areas of social communication and interaction, plus at least two of four types of restricted repetitive behaviors (15). Mothers with complex medical, psychological, or mental disorders were excluded as these disorders may affect recall, self-administration, and outcome accuracy.
2.3 Sample size and sampling techniques
We determined the sample size using the formula n = Z2P(1-P)/d2, where n stands for the sample size, Z for the desired level of confidence, P for the expected prevalence, and d for the precision or effect size. Despite a 2008 surge in local research, much remains unexplored. Saudi Arabia’s ASD prevalence is unknown. One 2002 survey found 42,500 autistic cases. Recent comprehensive reviews found that ASD prevalence in Arabian Gulf nations, including Saudi Arabia (SA), ranged from 1.4 to 29 per 10,000 (16). With a 95% confidence level and 80% power, the calculated sample size was determined to be 88 mothers of autistic children. To collect the sample, the official groups used an online Delphi panel.
2.4 Data collection
2.4.1 Data collection tool
A questionnaire was developed based on previous research (2, 4, 13–20). The questionnaire was initially drafted in English and then translated into Arabic. Two English-speaking translators performed the back translation, and they also consulted the original panel to address any issues or worries. To ensure content validity and reliability, the questionnaire underwent validation by three experts in public health, community medicine, and pediatrics. A survey was developed based on previous research (2, 4, 13–20). The questionnaire was initially drafted in English and then translated into Arabic. Two English-speaking translators performed the back translation, and they also consulted the original panel to address any issues or worries. To ensure content validity and reliability, the questionnaire underwent validation by three experts in public health, community medicine, and pediatrics. A pilot study was conducted on 10 participants who were not included in the main study to validate the questionnaire. We intentionally solicited feedback from participants, including inquiries about the clarity of the instructions, logistical, technical, and other concerns, as well as the difficulty of answering certain questions. After collecting and analyzing the pilot survey results, we addressed all the reported feedback, potentially correcting the questions or selecting the most appropriate types of queries. Cronbach’s alpha was calculated to estimate the questionnaire’s reliability, and a value of 0.78 was obtained.
The questionnaire is composed of five main sections, following the mothers’ written informed consent [found in Supplementary Material 1]. The first section includes the characteristics and demographic information of both the mother and father. 2) Prenatal risk factors for autism include health and pregnancy conditions, as well as adherence to required medications. 3) Antenatal risk factors include adherence to required follow-up, maternal factors such as environmental, psychosocial, and lifestyle-related dietary habits, and physical activity. We assessed psychosocial factors using the LIVES Daily Hassles score, a tool that evaluates the impact of frequent exposure to daily life stressors like family or marital conflict, income concerns, workload, time issues, concerns about future security, and environmental or housing conditions (20). 4) Natal-related risk factors. 5) The Autism Center uses the American Psychiatric Association’s DSM-5 classification, which provides information to the mothers regarding the diagnosis and severity of autism in their children (16).
2.4.2 The Data collection process
Data collection was carried out using a pre-tested, pre-coded, and well-structured questionnaire. We designed the questionnaire in Arabic using Google Forms and distributed it to all registered mothers of autistic children in all autism centers, leveraging popular and officially recognized social media platforms such as Facebook, Twitter, mother-registered emails, and WhatsApp groups within an autism-specific center. We sent frequent reminder messages and follow-up communications to increase the response rate until we reached the desired sample size.
2.5 Statistical analysis
The Jamovi software program, version 2.5.6, was used for data coding and analysis. The Shapiro-Wilk test was employed to assess the normal distribution of the data. Qualitative data was presented as frequencies and percentages, while quantitative data was displayed as mean and standard deviation (SD) if it demonstrated normal distribution or as median and interquartile range (IQR) if it did not.
For examining the relationship between autism severity and continuous variables, we employed one-way ANOVA (Kruskal-Wallis). The chi-square test (χ2) and Fisher’s exact test were used to analyze the distinction between autism severity and other categorical variables, with Fisher’s exact test for sample sizes less than five and chi-square for larger sample sizes. Variables that demonstrated a significant association underwent multivariate logistic regression to assess their predictive capability for ASD severity. We considered the results statistically significant when the probability was less than 0.05.
2.6 Ethical considerations
The institutional review board (IRP) at Princess Nourah bint Abdulrahman University registered this survey under the number 24-0363 and gave its approval on February 19, 2024. Before participating in the Google forum, we requested that all invited participants complete and check an informed consent box. Participation in the survey was voluntary and related to the autism center. Checking the consent box and completing the voluntary survey were considered evidence of providing valid, informed consent. The IRB application for this study, which received approval, described this consent process.
3 Results
3.1 Regarding the frequency or severity of autism
Regarding the frequency or severity of autism, a survey of 168 mothers with children with ASD revealed that 15.6% of these children had mild autism, 31.9% had moderate autism, 43.8% had autism spectrum disorder, and 8.8% had severe autism.
3.2 Demographics and prenatal history, and their correlation with the severity grades of ASD
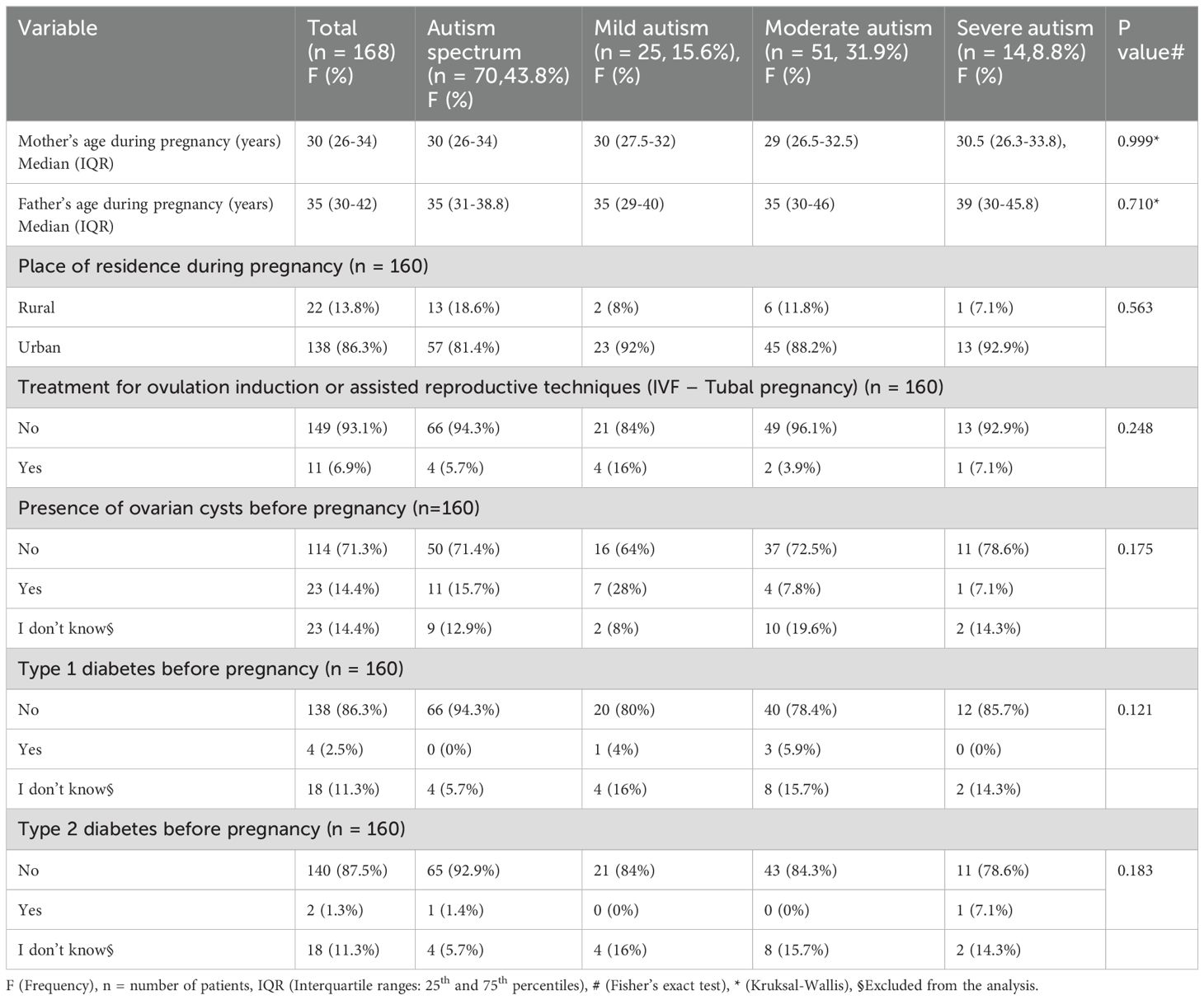
The median maternal age was 30 years, and the median paternal age was 35 years. Most participants resided in urban areas, 138 (86.3%), with slightly higher proportions in the mild and severe autism categories. In descending order regarding prenatal history or risk factors, 23 (14.4%) had ovarian cysts before pregnancy, 11 (6.9%) used assisted reproductive techniques (IVF-tubal pregnancy), 4 (2.5%) had type 1 diabetes, and 2 (1.3%) had type 2 diabetes before pregnancy. There was no statistically significant difference between the severity grades of ASD, demographics, and prenatal history (Table 1).
3.3 The relationship between the severity grades of ASD and the antenatal maternal and paternal exposure history or maternal antenatal conditions (medical and environmental factors)
Most autistic children had a history of one or both maternal and/or paternal antenatal exposures, as follows, in descending order: 77.7% had soft drink consumption, 30.6% smoked, 20.7% had chronic physical organic diseases (e.g., high blood pressure, asthma), 16.9% had psychological or neurological disease, and 13.3% had a history of diet product consumption (aspartame).
Regarding maternal antenatal conditions, the most common events, in descending order, were: 33.7% history of recurrent infection; 25.7% anemia during pregnancy; and 26 (16.3%) threatened abortion or bleeding during pregnancy. During pregnancy, there is a risk of exposure to air pollution, which includes elevated carbon levels, 22 (13.8%) cases of gestational diabetes, 14 (10.7%) individuals taking medications for chronic conditions such as insulin, thyroxine, corticosteroids, and hydroxychloroquine, and 8 (5%) individuals receiving the COVID-19 vaccine and antibiotics such as amoxicillin, cotrimoxazole, and penicillin.
The study found no statistically significant difference between the severity grades of ASD, antenatal maternal and paternal exposure history, or maternal antenatal conditions (p > 0.05), except for gestational diabetes during pregnancy (p = 0.047). The results showed that 4 (28.6%) of 14 cases had severe autism, 8 (15.7%) of 50 cases had moderate autism, and 0 (0%) cases had mild autism. As illustrated in detail in Table 2.
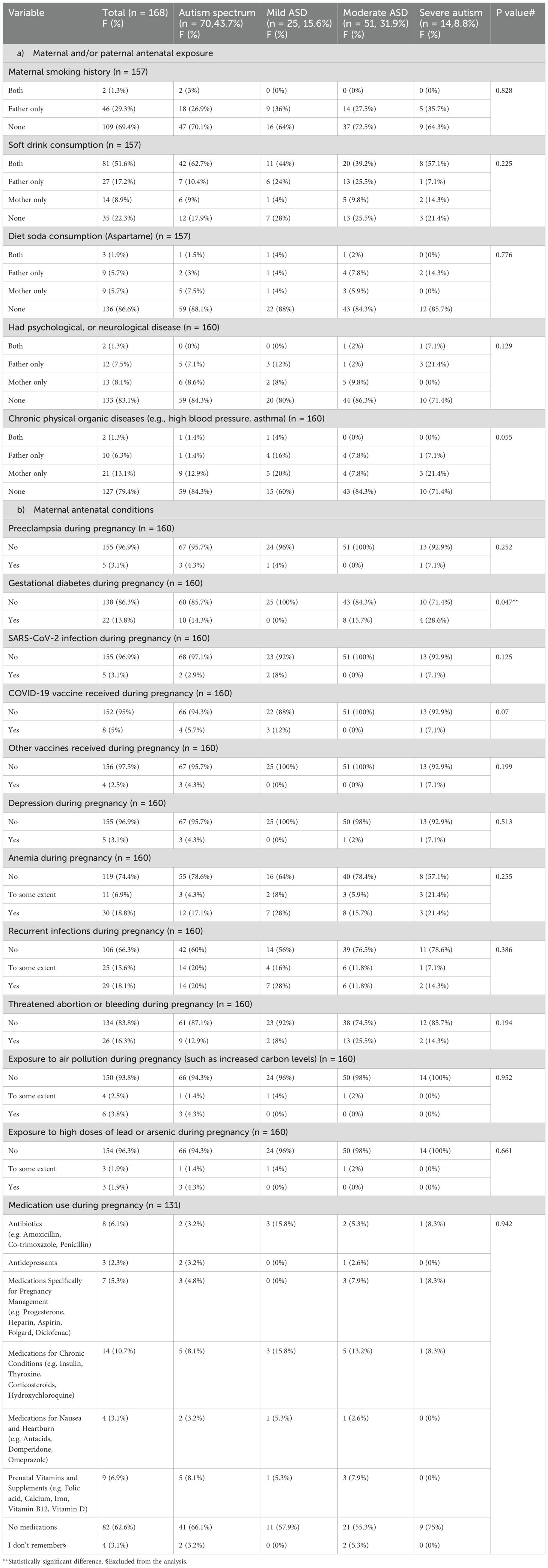
Table 2. Antenatal maternal and paternal history and their relationship with the severity grades of ASD.
3.4 Maternal lifestyle factors (including dietary and physical activity during pregnancy) and their relationship with the severity grades of ASD
Although approximately 45% of mothers didn’t remember their average daily intake, the majority reported less than the daily recommended diet of milk and dairy products. 67, 41.9%/94: vegetables 66, 41.3%/91: fruit 65, 65, 40.6%/93: water consumption 115, 71.9%): grains and bread 40, 25%/64; meat and legumes 43, 26.9%/85. The level of physical activity was 102 (63.7%). Routine household activities. The study found no significant association between the severity of ASD and the nutrition and lifestyle factors during pregnancy. As illustrated in detail in Table 3.
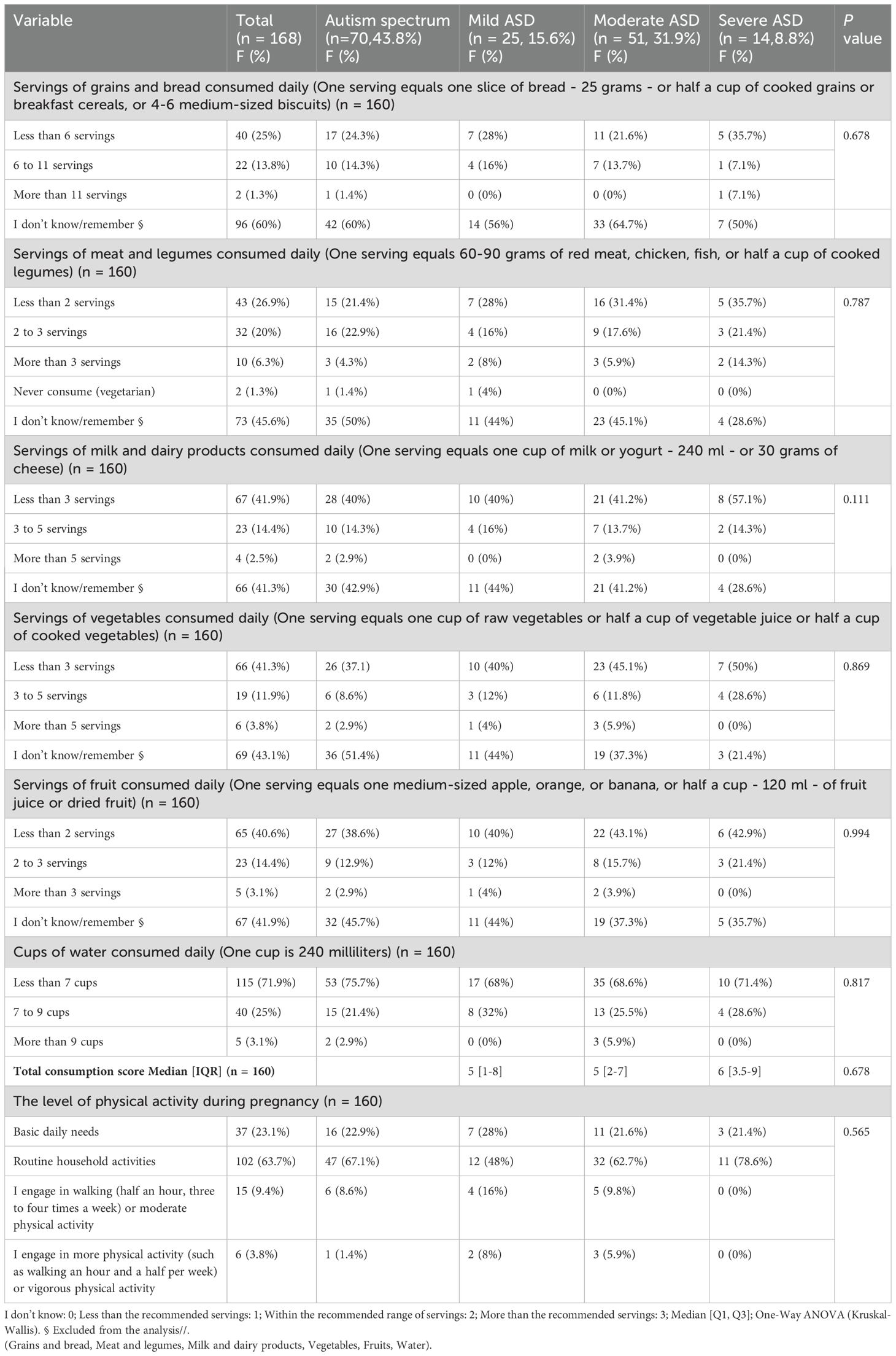
Table 3. Maternal lifestyle factors (including dietary and physical activity during pregnancy) and their relationship with the severity grades of ASD.
3.5 LIVES Daily Hassles Factors and Scale (psycho-social factors) during pregnancy and their relationship with the severity grades of ASD
In descending order, the majority reported that daily hassles, including household chores, spouse conflict, time pressure, financial concerns, internal fears, and work-related factors, had a negative impact on their health. The study found that there was no significant correlation between the severity of autism spectrum disorder (ASD) and the LIVES Daily Hassles Factors and Scale during pregnancy. However, the impact of household chores on health during pregnancy was significantly (P = 0.02) higher among individuals with severe autism (around 14.3%), compared to those with mild autism (12%) or moderate autism (5.9%). As illustrated in detail in Table 4.
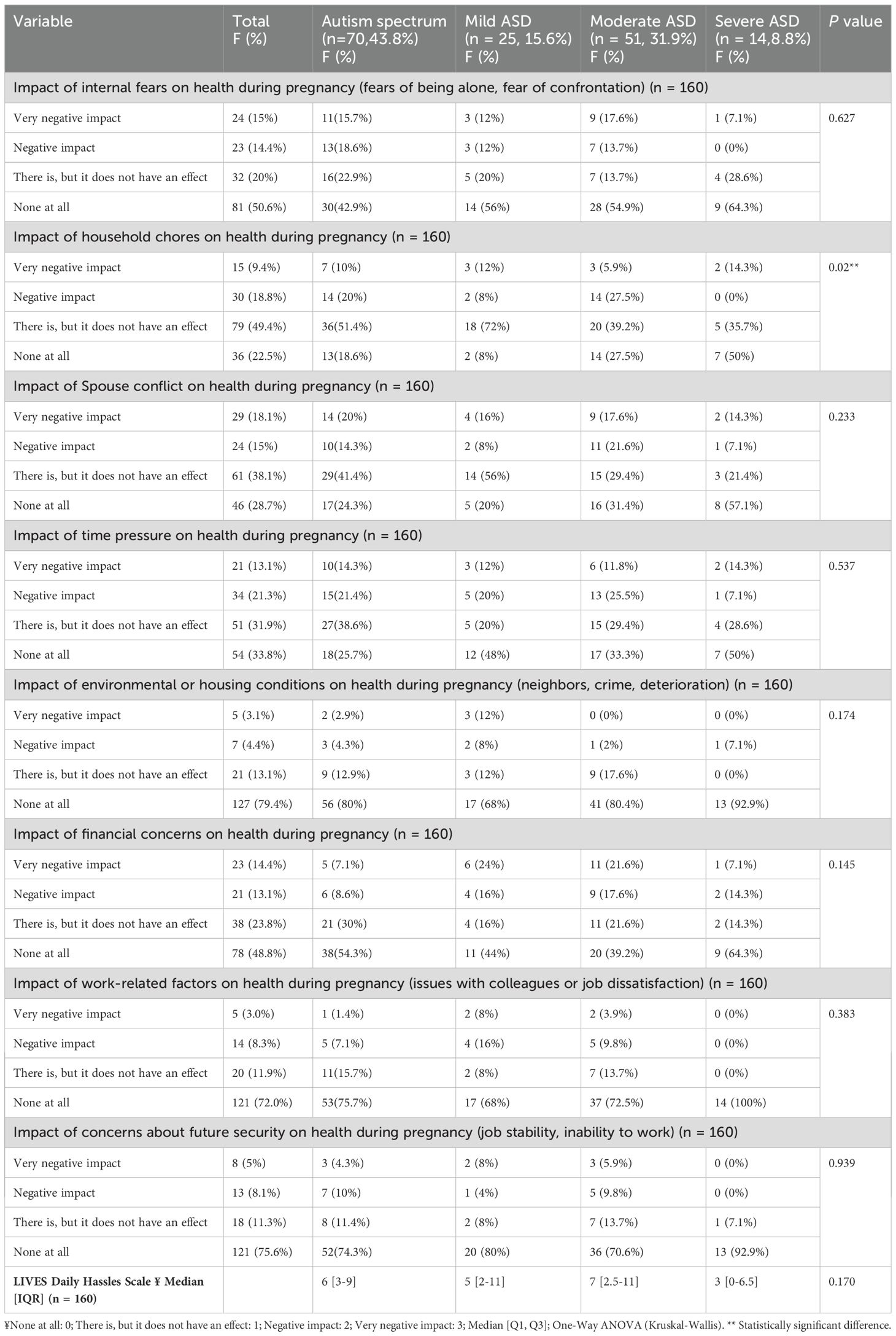
Table 4. LIVES daily hassles factors and scale during pregnancy and their relationship with the severity grades of ASD.
3.6 The natal history, ASD diagnosis history, and correlation with ASD severity grades
In terms of natal history and risk factors, there were 104 (65%) autistic children born in the government hospital. Out of these, 97 (60.6%) were born vaginally, 21.2% had an abnormal birth weight, 50 (31.3%) required admission to the neonatal intensive care unit, 23 (14.4%) had a history of oxygen desaturation, 11.9% had an abnormal birth length, and 16 (10%) had a history of any or recurrent infections. Only 88 (55%) cases with a history of autism received a diagnosis based on symptoms, with 82 (51.2%) families serving as the primary caregivers.
The study found no significant correlation between the severity of ASD and the natal history and ASD diagnosis. The study did, however, find a strong link (P = 0.021) between a child’s length at birth—which was higher at 3 (21.4%) in cases of severe autism compared to other grades less than 10%—and their oxygen desaturation—which was higher at 6 (42.9%) in cases of severe autism compared to other grades less than 14.3% (p = 0.017). In addition, the current median age of the child (years) is significantly (p = 0.035) higher in severe autism cases at 14 (12.25–18) compared to the median mild autism 7 (5.5–13), compared to the median moderate as well as autism spectrum. As illustrated in detail in Table 5.
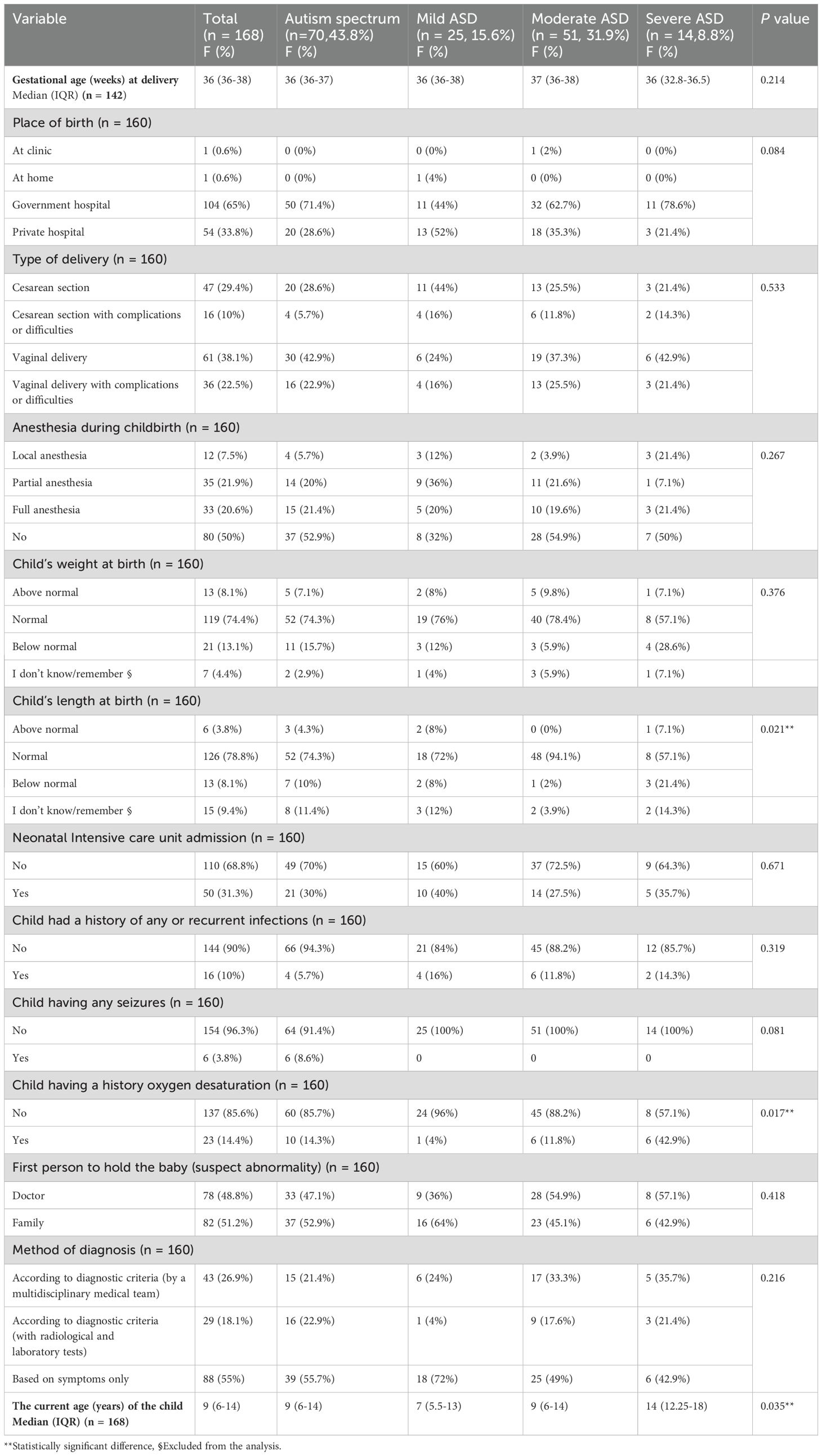
Table 5. The natal history and ASD diagnosis history and their relationship with the severity grades of ASD.
3.7 The predictors of severe ASD by ordinal logistic regression
3.7.1 In terms of antenatal history
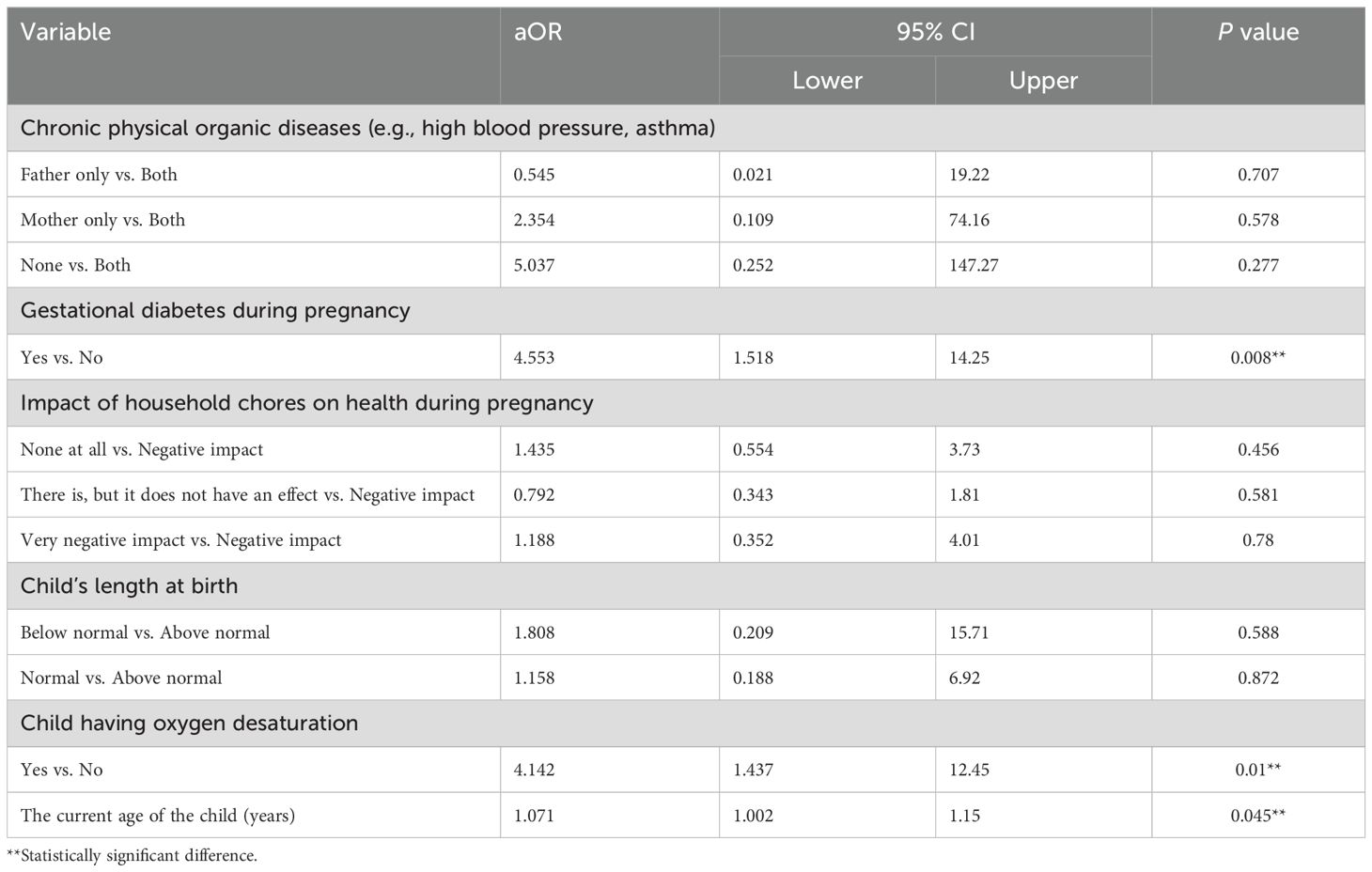
In terms of antenatal history, gestational diabetes during pregnancy is a strong predictor of severe autism. The aOR for the severity of ASD among women with gestational diabetes during pregnancy relative to those without gestational diabetes during pregnancy is 4.553 (95% CI: [1.518, 14.25], P = 0.008) (Table 6). This suggests that women with gestational diabetes during pregnancy are approximately 4.5 times more likely to have children with severe ASD compared to those without gestational diabetes during pregnancy.
3.7.2 Regarding natal history a child’s oxygen desaturation
Regarding natal history A child’s oxygen desaturation is a significant predictor of severe autism. The aOR for the severity of ASD among children with oxygen desaturation relative to those without oxygen desaturation is 4.142 (95% CI: [1.437, 12.45], P = 0.01). This suggests that children with oxygen desaturation are approximately 4.1 times more likely to develop severe ASD than those without.
3.7.3 In terms of autism history
In terms of autism history, a child’s current age in years is a significant predictor of severe autism. The aOR for the severity of ASD associated with each additional year of the child’s age is 1.071 (95% CI: [1.002, 1.15], P = 0.045). This indicates that for every one-year increase in the child’s age, the odds of being in a higher category of ASD severity relative to a lower category increase by approximately 7.1%.
4 Discussion
In SA, there is a significant gap between the demands of those diagnosed with ASD and the currently offered services (17). Early intervention focuses on language, play skills, social involvement, activities of daily living (ADLs), and disruptive behaviors. Genetic and epigenetic factors may contribute to ASD’s multifactorial origin (21). As a result, this local study from SA is critical to highlighting prenatal, antenatal, and natal-related factors, or associated ASD.
4.1 The frequency or severity of autism
In this study, the majority of the recruited 168 mothers with Autistic children reported having autism spectrum disorders (43.8%). Moderate autism accounts for 31.9%, mild autism for 15.6%, and severe autism (8.8%). According to the global classification of ASD severity, most mothers reported having ASD, a broader category that encompasses all levels of severity, including moderate, mild, and severe autism. Remember, diagnostic practices are crucial.
Rates of occurrence may be different depending on diagnostic criteria, cultural contexts, and research methods; without detailed sub-categorization, this isn’t standard practice (22). For example, a narrative review of UK clinical practice guidelines for ASD diagnosis shows that diagnostic rates vary depending on social and contextual factors (23). Furthermore, the level of maternal awareness and understanding were relatively low, with only 56.2% being able to identify and comprehend their child’s subcategorical classification.
4.2 Demographics and prenatal history and their relationship with the severity grades of ASD
4.2.1 Parental age and ASD
Although there was a non-significant association between parental age and ASD severity, those with severe autism had a relatively older median (IQR) parent age of 39 years (30–45.8), while other groups were 35 years. This may be due to the relatively small number of severe autism cases (8.8%). Previous research links older parental age to higher ASD risk in children, potentially due to genetic mutations in older gametes and exposure to various environmental factors over a longer period, some of which could impact the health and development of their offspring (5–24).
4.2.2 Polycystic ovarian syndrome
We found that 23 mothers (13.7%) of children with ASD had history of PCOS, with an average of 5% to 20% of PCOS among women of reproductive age and no significant correlation with the severity grades. In agreement with a large-scale study from Sweden, PCOS has identified a potential increased risk of birthing children with ASD. Although PCOs underlying pathogenic mechanisms have not been completely understood, prenatal hyperandrogenism (male sex hormones; chronic anovulation, insulin resistance, metabolic syndrome, chronic low-grade inflammation)—all these factors may affect the intrauterine environment and may play a role in neurodevelopmental disorders, including ASD (6–25).
4.3 Antenatal maternal and paternal exposure history or maternal antenatal conditions and their relationship with the severity grades of ASD
4.3.1 Soft drink consumption
Out of 168 mothers (8.3%), 81 (48.2%) reported that both fathers and mothers drank soft drinks, and 19% had a history of diet product consumption (aspartame). The FDA approved aspartame as a tabletop sweetener in 1981. By 1983, the FDA had reported many adverse events, e.g., depression, anxiety, headache complaints, and other neurological problems, including irritability, mood disorders, cognitive problems, and seizures (26, 27).
Moreover Aspartame-fed animals displayed cognitive problems, anxiety-related behaviors, and other noticeable neurophysiologic effects. This has led to the discovery of long-term health problems in humans and their offspring, as the availability of glutamine sulfhydryl (GSH) has greatly decreased. This is significant because GSH protects the developing brain by scavenging toxins, preventing oxidative stress, and supporting methylation. There are more free radicals, oxidative stress, lipid peroxidation, inflammation, mitochondrial dysfunction, excitotoxicity, neuronal apoptosis, serotonin, noradrenaline, and dopamine in the brain when aspartame and its byproducts are present (27–29).
4.3.2 Maternal smoking history
Maternal smoking history: 35.1% out of 168 mothers reported that fathers were smoking. The adjusted odds ratio (aOR) indicates the strength of this association, as well as a higher likelihood of having offspring with ASD (11). In agreement with other systematic reviews, tobacco smoke is the poisonous substance that most likely harms brain development. Nicotine affects nicotinic acetylcholine receptors in the offspring later in life, which mediate neural structural changes that can lead to adverse birth outcomes such as fetal growth restriction and low birth weight. Moreover, it significantly alters sperm DNA methylation and gene expression (11).
4.3.3 Medication use during pregnancy
Medication use during pregnancy We did not identify a notable association between medication use during pregnancy and ASD; however, other studies have suggested that the use of certain substances might be a risk factor for ASD. For instance, a study suggested that the use of antidepressants, specifically selective serotonin reuptake inhibitors, during the second and/or third trimesters increases the risk of ASD (8).
Among ASD mothers, only 9 (5.4%) consume prenatal vitamins and supplements (e.g., folic acid, calcium, iron, vitamin B12, and vitamin D). This indicates that consuming prenatal vitamins and supplements reduces the risk of ASD, and certain nutrients may have neuroprotective effects throughout development. This aligns with a systematic review that found that maternal prenatal vitamins, folic acid, and vitamin D reduce the risk of autism in offspring. Higher maternal intakes of folic acid, omega-6 fatty acids, and vitamin D during pregnancy reduced child autism risk, while women deficient in omega-3 and polyunsaturated fatty acids raised it (30, 31).
4.3.4 Infections and anemia during pregnancy
During pregnancy, 29 out of 168 mothers (17.3%) reported having recurrent infections. The nested case-control study found no overall significant association between any maternal infection during pregnancy and infections diagnosed during hospital admission, particularly bacterial infections (32). The lower level of immunity, along with the associated anemia, may pose a potential risk for ASD in 30 out of 168 mothers (17.9%). In alignment with other research, mothers diagnosed with anemia within the first 30 weeks of pregnancy had a higher prevalence of ASD, ADHD, and intellectual disabilities (33, 34).
4.3.5 The study examines the relationship between maternal lifestyle factors (such as dietary and physical activity during pregnancy) and severity grades
4.3.5.1 Nutrition during pregnancy
Although approximately 45% of mothers didn’t remember their average daily intake, the majority reported less than the daily recommended diet of milk and dairy products. 67, 78.7%/94: vegetables 66, 72.5%/91: fruit. 65, 69.9%/93: water, followed by grains and bread at 40, 60%/64, and meat and legumes at 45, 51.7%/87. This recall may indicate that the mothers did not follow an appropriate diet, which could potentially influence the risk of ASD. This pattern of dietary consumption is consistent with another two studies in SA (35, 36). This result aligns with the findings of another two United States cohorts, which suggested a positive association between the Western diet and autism-related traits (37).
Here are some key points highlighting the importance of nutrition during pregnancy: 1. Fetal Development: Adequate intake of nutrients such as folic acid, iron, calcium, and omega-3 fatty acids supports the development of the baby’s brain, bones, and overall body structure. 2. Birth Defect Prevention: Proper nutrition can help prevent birth defects. For instance, folic acid is known to reduce the risk of neural tube defects. 3. Healthy Birth Weight: A balanced diet helps to achieve a healthy birth weight, reducing the risk of complications during delivery and ensuring the baby has a favorable start in life. 4. Immune System Support: Nutrients like vitamins A, C, and E, along with zinc, support the development of the baby’s immune system, helping to protect against infections. 5. Long-term Health: Maternal nutrition can have long-term effects on the child’s health, influencing their risk of developing chronic conditions such as obesity, diabetes, and cardiovascular diseases later in life. 6. Cognitive Development: Essential fatty acids, particularly DHA, are critical for the development of the baby’s brain and eyes, potentially impacting cognitive function and vision (12).
4.3.5.2 The study focuses on the Daily Hassles Factors, their scale during pregnancy, and their relationship with the severity grades of ASD
In descending order, the majority reported that there was a negative impact on the health of the daily hassles: 78.5% of household chores, 72.6% of spouse conflict, 67.8% of time pressure, 53.6% of financial concerns, 51.8% of internal fears, and 27.9% of concerns about the future, as well as work-related factors. There was no significant correlation between the severity of ASD and the LIVES Daily Hassles Factors and Scale during pregnancy, according to the study. However, the impact of household chores on health during pregnancy was significantly (P = 0.02) higher among individuals with severe autism (around 14.3%), compared to those with mild autism (12%) or moderate autism (5.9%). Household chores and pregnancy stress: In our study, we identified a notable association between the stress from household chores during pregnancy and ASD development. This underscores the potential impact of prenatal stress on fetal development, possibly through hormonal changes or other stress responses. Our findings call for more support for expectant mothers to reduce stress and physical strain, which may lower ASD risk (38, 39).
4.4 The history of ASD diagnosis and its relationship to ASD severity grades
Two major domains of ASD diagnosis are social and communication interaction and restricted or repetitive conduct. In this study, only 88 (55%) cases received a diagnosis based on their symptoms, while 82 (51.3%) families handled the diagnosis. SA-related methodological issues, poor diagnostic competence, and lower parent awareness of ASD can all contribute to this gap, limiting the likelihood of recognizing symptoms and seeking therapy. Parents of autistic children face a lack of government and institutional support, as well as a lack of resources for autistic children, which may affect other siblings’ quality of life, their social and economic lives, family ties. In Saudi Arabia, 88.5% of parents of children with autism experience discomfort; out of 61 families with ASD, 37% felt ashamed for having autistic children, and 64% were concerned about others’ treatment. According to SA Khan et al.’s 2020 cross-sectional study, only 31% of children on the autistic spectrum could attend neighboring autism clinics, and 72% had no access to private ASD schools (40–42).
Radiological and laboratory tests diagnosed only 29 (18.1%) ASD cases, primarily diagnosing 16 (55%) cases of autism spectrum disorder. While SA recommends genetic testing for all ASD patients, chromosomal microarray analysis (CMA) is more effective when the cause of developmental delay is unknown, as 20-30% of individuals on the autism spectrum exhibit significant genetic variation and new mutations. Moreover, consanguinity in Saudi culture—57.7% of marriages—was considered in these recommendations (43, 44).
4.5 Factors associated with the severity of autism
4.5.1 Gestational diabetes mellitus
In this study, 22 out of 168 mothers (13.1%) reported having GDM, and there was a statistically significant association (p-value of 0.047) between GDM and the severity of ASD. Numerous mechanisms explain this association and GDM’s role. 1) GDM influences placental function, which in turn alters the delivery of nutrients and oxygen to the fetus, leading to hypoxia. Hypoxia (the developing brain doesn’t receive enough oxygen) can impair neurogenesis and lead to neuronal cell death, thereby contributing to neurodevelopmental impairments and potentially increasing the severity of ASD. Hypoxia can lead to high blood sugar levels, which can cause oxidative stress and inflammation in the developing fetal brain. 2) High blood sugar increases the production of reactive oxygen species, leading to oxidative stress, which can harm cellular structures such as lipids, proteins, and DNA, potentially disrupting normal brain development. 3) High blood sugar-induced inflammation can trigger the release of pro-inflammatory cytokines, potentially disrupting neuronal development and synaptic plasticity. These inflammatory processes can worsen the severity of ASD by further impairing neural connectivity and function (10, 45, 46). Moreover, this underscores the importance of closely monitoring and managing gestational diabetes during pregnancy.
4.5.2 Oxygen desaturation and ASD
Children exposed to oxygen desaturation at birth are more likely to develop severe ASD, according to a p-value of 0.017. This aligns with previous studies (45–49). This finding highlights the importance of early interventions for children with perinatal complications to potentially reduce ASD severity (47, 50). However, researchers are still exploring the exact pathological mechanisms that link oxygen desaturation to ASD. Hypoxia can cause inflammation, oxidative stress, and brain development disruptions, which may contribute to the development of ASD (4, 51).
4.5.3 Child’s age and ASD severity level
A 7.1% increase in the likelihood of classifying a child’s ASD as more severe with each year. This suggests that the challenges associated with ASD may become more pronounced as children age. However, it’s important to note that ASD symptoms can change over time, with some children showing a decrease in severity. This variability indicates that ASD is a dynamic condition, and interventions should be adaptable to each child’s changing needs (48).
4.6 Strengths and limitations
This study has many strengths, including the relatively large sample size, the use of a validated tool, the collection of paternal and maternal factors, and the comprehensiveness of prenatal, antenatal, and natal medical, environmental, dietary, physical, and psychosocial factors. Furthermore, we investigated the effect of frequent exposure to seven daily life stressors on health, which include family or marital conflict, income concerns, workload, time issues, concerns about future security, and environmental or housing conditions.
Although exploring the risk factors for autism with unknown causes is critical, the study has many limitations. For example, the study’s cross-sectional design introduces recall biases, as approximately 50% of mothers were unable to recall their dietary habits. Furthermore, the subjective report highlights the health effects of frequent exposure to seven daily life stressors during pregnancy. The web-based survey employs Delphi sampling techniques and excludes mothers with complex medical, psychological, or mental disorders, potentially influencing the results’ generalizability.
5 Conclusion
This study highlighted the maternal risk factors associated with autism spectrum disorders (ASD) in children. ASD is a diverse group of conditions characterized by some degree of difficulty with social interaction and communication.
Our study has shown a significant connection between gestational diabetes and autistic children, acknowledging the importance of interventions, screening tests, and lifestyle modifications during pregnancy. We also found a link between the severity of ASD and oxygen desaturation at birth. This highlights the importance of intervention in such cases to improve the child’s quality of life. Moreover, the mother’s stress and physical activity during household chores showed increased development of ASD in children, but a wider study is necessary to confirm the exact mechanism. Finally, the child’s age. The odds of having a more severe ASD increase with age.
6 Recommendation
After conducting this study, we concluded that understanding the relationship between the discussed factors and ASD development in children enables us to more effectively direct support services to meet the needs of these mothers and their children, enhancing their overall well-being.
● We recommend including social, psychological, work, and even financial support elements as essential components of the antenatal care program. Our findings reveal that over sixty percent of mothers with ASD faced daily challenges during pregnancy, which were related to the following: Factors include household chores, spouse conflict, time pressure, and financial concerns.
● Mass media health education campaigns aim to raise public awareness about the symptoms of ASD. In addition to educating mothers and fathers about sub categorical classification, management plans, prognosis, and ways to manage stigma-related issues and improve their life quality,
● To reduce the risk of ASD, the antenatal program should be comprehensive and include increased consumption of prenatal vitamins and supplements.
● These findings highlight the importance of monitoring and managing metabolic conditions, including gestational diabetes and infections, during pregnancy to mitigate potential risks to the child’s neurodevelopment.
● To strengthen the body of evidence, we recommend conducting similar studies on a random and representative sample in different settings, including mothers with complex medical, psychological, or mental disorders.
● Proper natal and postnatal care, including early and effective interventions for children with perinatal complications, has the potential to reduce ASD severity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Prince Nourah University institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AhA: Conceptualization, Writing – original draft, Writing – review & editing. FA: Conceptualization, Methodology, Validation, Writing – review & editing. AMA: Methodology, Data curation, Supervision, Writing – review & editing. AbA: Formal analysis, Data curation, Visualization, Writing – review & editing. ZA: Writing – original draft. TA: Writing – original draft. AAS: Writing – original draft. SA: Conceptualization, Methodology, Validation, Software, Supervision, Writing – original draft, Writing – review & editing. JS: Visualization, Software, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to express our special thanks to all the mothers recruited for this study. We extend our special thanks to the Society of Autism Families for their unwavering support, as well as to Eman Moraybed Alani, the Research and Development Manager at the Society of Autism Families, for her supportive and coordinated role in data collection and mother enrollment. Moreover, we would like to express our thanks to Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2024R290), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1467821/full#supplementary-material
Abbreviations
ASD, Autism spectrum disorder; DSM-5, Diagnostic and Statistical Manual, Fifth Edition; GDM, Gestational diabetes mellitus; GSH, Glutamine sulfhydryl; PCOS, Maternal polycystic ovary syndrome; SARS-CoV-2.
References
1. Muglia LJ, Benhalima K, Tong S, Ozanne S. Maternal factors during pregnancy influencing maternal, fetal, and childhood outcomes. BMC Med. (2022) 20(1):418. doi: 10.1186/s12916-022-02632-6
2. Zhong C, Tessing J, Lee BK, Lyall K. Maternal dietary factors and the risk of autism spectrum disorders: a systematic review of existing evidence. Autism Research. (2020) 13(10):1634–1658. doi: 10.1002/aur.2402.
3. Centers for Disease Control and Prevention Autism spectrum disorder (ASD) data & statistics (2016). Available online at: https://www.cdc.gov/ncbddd/autism/data.html (Accessed April 11, 2017).
4. Hyman SL, Levy SE, Myers SM. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. (2020) 145:e20193447. doi: 10.1542/peds.2019-3447
5. Gao Y, Yu Y, Xiao J, Luo J, Zhang Y, Tian Y, et al. Association of grandparental and parental age at childbirth with autism spectrum disorder in children. JAMA network Open. (2020) 3:e202868. doi: 10.1001/jamanetworkopen.2020.2868
6. Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C, et al. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry. (2016) 21:1441–8. doi: 10.1038/mp.2015.183
7. Ye W, Xie T, Song Y, Zhou L. The role of androgen and its related signals in PCOS. J Cell Mol Med. (2021) 25:1825–37. doi: 10.1111/jcmm.16205
8. Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. (2016) 170:117–24. doi: 10.1001/jamapediatrics.2015.3356
9. Xiang AH. Diabetes in pregnancy for mothers and offspring: reflection on 30 years of clinical and translational research: the 2022 Norbert Freinkel award lecture. Diabetes Care. (2023) 46:482–9. doi: 10.2337/dci22-0055
10. Xiang AH, Wang X, Martinez MP, Page K, Buchanan TA, Feldman RK. Maternal type 1 diabetes and risk of autism in offspring. JAMA. (2018) 320:89–91. doi: 10.1001/jama.2018.7614
11. Kim B, Ha M, Kim YS, Koh YJ, Dong S, Kwon HJ, et al. Prenatal exposure to paternal smoking and likelihood for autism spectrum disorder. Autism: Int J Res Pract. (2021) 25:1946–59. doi: 10.1177/13623613211007319
12. Qin Y, Xie L. Nutrition and supplements during pregnancy: A vital component in building the health and well-being of both the mother and the developing baby. Nutrients. (2023) 15:3395. doi: 10.3390/nu15153395
13. Autism Spectrum Disorder (ASD). Available online at: https://www.cdc.gov/ncbddd/autism/signs.html (accessed April 4, 2024).
14. Karst JS, Van Hecke AV. Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation. Clinical child and family psychology review. (2012) 15:247–277. doi: 10.1007/s10567-012-0119-6
15. Papadopoulos D. Mothers’ Experiences and challenges raising a child with autism spectrum disorder: A qualitative study. Brain sciences. (2021) 11(3):309. doi: 10.3390/brainsci11030309
16. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association (2013).
17. Alenezi S, Alyahya AS, AlKhalifah SM, Bakhsh HR, Alismail EH, Aldhalaan H, et al. Saudi expert consensus-based autism spectrum disorder statement: from screening to management. Children (Basel). (2022) 9:1269. doi: 10.3390/children9091269
18. Lu J, Wang Z, Liang Y, Yao P. Rethinking autism: the impact of maternal risk factors on autism development. American journal of translational research. (2022) 14(2):1136.
19. Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. (2014) 43:443–64. doi: 10.1093/ije/dyt282
20. Wright AGC, et al. Daily Stress and Hassles, in: The Oxford Handbook of Stress and Mental Health (2020). Oxford Library of Psychology (Accessed 22 June 2024).
21. Towle PO, Patrick PA, Ridgard T, Pham S, Marrus J. Is earlier better? The relationship between age when starting early intervention and outcomes for children with autism spectrum disorder: A selective review. Autism Res Treat. (2020) 2020:7605876. doi: 10.1155/2020/7605876
22. Wiggins LD, Reynolds A, Rice CE, Moody EJ, Bernal P, Blaskey L, et al. Using standardized diagnostic instruments to classify children with autism in the study to explore early development. J Autism Dev Disord. (2015) 45:1271–80. doi: 10.1007/s10803-014-2287-3
23. Tafla TL, Teixeira MCTV, Woodcock KA, Sowden-Carvalho S. Autism spectrum disorder diagnosis across cultures: Are diagnoses equivalent? Neurodiversity. (2024) 2. doi: 10.1177/27546330241226811
24. Sandin S, Schendel D, Magnusson P, Hultman C, Surén P, Susser E, et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry. (2016) 21:693–700. doi: 10.1038/mp.2015.70
25. Rudnicka E, Suchta K, Grymowicz M, Calik-Ksepka A, Smolarczyk K, Duszewska AM, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. (2021) 22:3789. doi: 10.3390/ijms22073789
26. Fowler SP, Gimeno Ruiz de Porras D, Swartz MD, Stigler Granados P, Heilbrun LP, Palmer RF. Daily early-life exposures to diet soda and aspartame are associated with autism in males: A case-control study. Nutrients. (2023) 15:3772. doi: 10.3390/nu15173772
27. Christian B, McConnaughey K, Bethea E, Brantley S, Coffey A, Hammond L, et al. Chronic aspartame affects T-maze performance, brain cholinergic receptors and Na+, K+-ATPase in rats. Pharmacol Biochem Behav. (2004) 78:121–12. doi: 10.1016/j.pbb.2004.02.017
28. Suez J, Cohen Y, Valdés-Mas R, Mor U, Dori-Bachash M, Federici S, et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell. (2022) 185:3307–3328.e19. doi: 10.1016/j.cell.2022.07.016
29. Gerasimidis K, Bryden K, Chen X, Papachristou E, Verney A, Roig M, et al. The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity. Eur J Nutr. (2019) 59:3213–30. doi: 10.1007/s00394-019-02161-8
30. Vecchione R, Wang S, Rando J, Chavarro JE, Croen LA, Fallin MD, et al. Maternal dietary patterns during pregnancy and child autism-related traits: results from two US cohorts. Nutrients. (2022) 14:2729. doi: 10.3390/nu14132729
31. Zhong C, Tessing J, Lee BK, Lyall K. Maternal dietary factors and the risk of autism spectrum disorders: A systematic review of existing evidence. Autism Res. (2020) 13:1634–58. doi: 10.1002/aur.v13.10
32. Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal infection during pregnancy and autism spectrum disorders. J Autism Dev Disord. (2015) 45:4015–25. doi: 10.1007/s10803-013-2016-3\
33. Hornig M, et al. Prenatal fever and autism risk. Mol Psychiatry. (2018) 23:759–66. doi: 10.1038/mp.2017.119
34. Wiegersma AM, Dalman C, Lee BK, Karlsson H, Gardner RM. Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA Psychiatry. (2019) 76:1294–304. doi: 10.1001/jamapsychiatry.2019.2309\
35. Sabur AM, Alsharief LA, Amer SA. Determinants of healthy food consumption and the effect of Saudi food related policies on the adult Saudi population, a national descriptive assessment 2019. Curr Res Nutr Food Sci. (2022) 10. doi: 10.12944/CRNFSJ.10.3.21
36. Amer SA, Alasmari SA, Amer MA, Aissa NA, Abd-Ellatif EE. Assessment of fast and junk food consumption and addiction among Saudi population. National descriptive study 2020. Curr Res Nutr Food Sci. (2022) 10. doi: 10.12944/CRNFSJ.10.1.02
37. Peretti S, Mariano M, Mazzocchetti C, Mazza M, Pino MC, Verrotti Di Pianella A, et al. Diet: The keystone of autism spectrum disorder? Nutr Neurosci. (2019) 22:825–39.
38. Abdelrazek MEG, Rice F. Maternal stress in pregnancy and child autism spectrum disorder: evaluating putative causal associations using a genetically informed design. BJPsych Open. (2021) 7, Suppl 1:S22. doi: 10.1192/bjo.2021.114
39. Herba CM, Glover V. The Developmental Effects of Prenatal Maternal Stress: Evolutionary Explanations. In: Wazana A, Székely E, Oberlander TF, editors. Prenatal Stress and Child Development. Springer, Cham (2021). doi: 10.1007/978-3-030-60159-1_3
40. Mostafa A. Addressing autism in the Arab world. Nat Middle East. (2011) 21:147. doi: 10.1038/nmiddleeast.2011.147
41. Al-Salehi SM, Al-Hifthy EH, Ghaziuddin M. Autism in Saudi Arabia: presentation, clinical correlates and comorbidity. Transcult Psychiatry. (2009) 46:340–7. doi: 10.1177/1363461509105823
42. Sulaimani M, Gut D. Autism in Saudi Arabia: present realities and future challenges. Rev Disabil Stud Int J. (2019) 15:2–8.
43. Kreiman BL, Boles RG. State of the art of genetic testing for patients with autism: A practical guide for clinicians. Semin Pediatr Neurol. (2020) 34:100804. doi: 10.1016/j.spen.2020.100804
44. El-Hazmi MA, al-Swailem AR, Warsy AS, al-Swailem AM, Sulaimani R, al-Meshari AA. Consanguinity among the Saudi Arabian population. J Med Genet. (1995) 32:623–6. doi: 10.1136/jmg.32.8.62
45. Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signaling. (2011) 15:3061–100. doi: 10.1089/ars.2010.3765
46. Aljumaiah MM, Alonazi MA, Al-Dbass AM, et al. Association of maternal diabetes and autism spectrum disorders in offspring: a study in a rodent model of autism. J Mol Neurosci. (2022) 72:349–58. doi: 10.1007/s12031-021-01912-9
47. Mandic-Maravic V, et al. autism spectrum disorders and Perinatal Complications-Is Oxidative Stress the Connection? Front Psychiatry. (2019) 10:675. doi: 10.3389/fpsyt.2019.00675
48. Waizbard-Bartov E, Ferrer E, Heath B, Rogers SJ, Nordahl CW, Solomon M, et al. Identifying autism symptom severity trajectories across childhood. Autism Res. (2022) 15:687–701. doi: 10.1002/aur.2674
49. Khan AS, AlGhadeer HA, Mohammed A, Al-Qassimi TMAJ, Al-Momen HH, Al-Nazzal MY. Autism in Saudi Arabia, a challenge to Saudi families: A cross-sectional study. Int J Med Dev Ctries. (2020) 4:1453–8. doi: 10.24911/IJMDC.51-1595277794
50. Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry J Ment Sci. (2009) 195:7–14. doi: 10.1192/bjp.bp.108.051672
Keywords: autism spectrum disorder, Saudi Arabia, antenatal, natal, prenatal, pregnancy, The LIVES Daily Hassles, maternal lifestyle factors
Citation: Abdelkader A, AlRadini F, Alosaimi A, Abbas A, Judeh Z, Emy Abu Esaid T, Saleh A, Shah J and Amer S (2024) Unveiling the influences of prenatal and maternal factors on the journey of an autistic child. Front. Psychiatry 15:1467821. doi: 10.3389/fpsyt.2024.1467821
Received: 20 July 2024; Accepted: 16 October 2024;
Published: 20 December 2024.
Edited by:
Rosa Calvo Escalona, Hospital Clinic of Barcelona, SpainReviewed by:
Antonio Narzisi, Stella Maris Foundation (IRCCS), ItalyAna Blázquez, Hospital Clinic of Barcelona, Spain
Copyright © 2024 Abdelkader, AlRadini, Alosaimi, Abbas, Judeh, Emy Abu Esaid, Saleh, Shah and Amer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samar Amer, ZHJfc2FtYXIxMUB5YWhvby5jb20=
†ORCID: Ahmed Abdelkader, orcid.org/0009-0006-2851-2835
Faten A. AlRadini, orcid.org/0000-0002-7835-8681
Ashwaq Ayidh M. Alosaimi, orcid.org/0000-0001-7009-5605
Samar Amer, orcid.org/0000-0002-9475-6372
 Ahmed Abdelkader
Ahmed Abdelkader Faten AlRadini2†
Faten AlRadini2† Tahneed Emy Abu Esaid
Tahneed Emy Abu Esaid Alaa Saleh
Alaa Saleh Samar Amer
Samar Amer