- 1School of Public Policy and Management, China University of Mining and Technology, Xuzhou, China
- 2School of Education Science, Jiangsu Normal University, Xuzhou, China
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by significant impairments in social interaction, often manifested in facial recognition deficits. These deficits hinder individuals with ASD from recognizing facial identities and interpreting emotions, further complicating social communication. This review explores the neural mechanisms underlying these deficits, focusing on both functional anomalies and anatomical differences in key brain regions such as the fusiform gyrus (FG), amygdala, superior temporal sulcus (STS), and prefrontal cortex (PFC). It has been found that the reduced activation in the FG and atypical activation of the amygdala and STS contribute to difficulties in processing facial cues, while increased reliance on the PFC for facial recognition tasks imposes a cognitive load. Additionally, disrupted functional and structural connectivity between these regions further exacerbates facial recognition challenges. Future research should emphasize longitudinal, multimodal neuroimaging approaches to better understand developmental trajectories and design personalized interventions, leveraging AI and machine learning to optimize therapeutic outcomes for individuals with ASD.
1 Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that frequently becomes apparent during the formative years of childhood, characterized by substantial impairments in social interaction. These deficits are most commonly expressed through difficulties in communication, circumscribed interests, and the exhibition of repetitive or stereotypical behaviors (1). The World Health Organization has estimated that ASD impacts approximately one child in every hundred worldwide (2). The most recent data provided by the Centers for Disease Control and Prevention (CDC) indicate that the prevalence of ASD in the United States is as high as 2.76% and about 1 in 38 children aged 8 years has been diagnosed with ASD (3).
Research indicates that individuals with ASD often exhibit impairments in facial recognition and employ atypical strategies for facial recognition (4). Behavioral studies have shown that children with ASD are less likely to make eye contact and show reduced interest in faces compared to typically developing children (5–7). These deficits extend to difficulties in recognizing facial identities, interpreting facial expressions, and understanding social cues conveyed through facial movements (8). Recently, a review of the last four decades of empirical research on facial recognition in ASD has revealed that individuals with ASD perform, on average, one standard deviation below their neurotypical peers on tasks involving facial identity and discrimination (9). In addition, research has also highlighted atypical strategies used by individuals with ASD for facial recognition. For example, they may focus on non-facial features or use alternative cognitive processes to recognize faces, which can further hinder their social interactions (10). These strategies might involve concentrating on individual facial features rather than integrating them into a holistic representation, leading to less accurate and slower face recognition (11).
Given the importance of facial recognition in social interactions, these deficits may contribute significantly to the social challenges faced by individuals with ASD. For example, failing to recognize a partner’s distress or joy can lead to responses that seem insensitive or out of place, undermining social bonds (12). Additionally, atypical gaze behavior, such as focusing more on the mouth than the eyes during social interactions, has been found in individuals with ASD and is associated with decreased social competence (6). This altered gaze pattern suggests a reduced salience of the eyes, a critical component of non-verbal communication, which may contribute to difficulties in interpreting social cues and hinder effective social interactions. While previous studies have examined the neural mechanisms behind facial recognition deficits in individuals with ASD, a critical gap remains in understanding how key brain regions dynamically interact during real-time social interactions. Much of the existing research focuses on isolated regions such as the fusiform gyrus (FG) and amygdala, often neglecting how these regions communicate within larger networks, especially under different cognitive loads and social contexts. This review goes beyond individual activation levels to investigate both structural and functional connectivity between critical regions, such as the superior temporal sulcus and prefrontal cortex. By exploring these network dynamics and the compensatory mechanisms individuals with ASD may rely on, this review offers a more holistic model that better captures the complexity of social cognition in real-world scenarios.
In this review, we conducted a comprehensive search of articles on facial recognition deficits in individuals with ASD using multiple academic databases and platforms. The databases included PubMed, ScienceDirect, Web of Science, SpringerLink, Elsevier, as well as psycINFO, Scopus, and Google Scholar etc. We used a combination of search terms such as “autism,” “abnormal” “face recognition,” “dysfunctional face processing,” “autism face processing,” “autism face recognition,” “brain regions,” “neural network,” “brain connectivity,” “anatomical anomalies,” “autism face processing fMRI/MEG/fNIRS,” “AI,” and “machine learning.” We applied inclusion criteria to select studies that specifically investigated the neural mechanisms underlying facial recognition deficits in ASD, focusing on structural and functional brain anomalies, connectivity, and neural networks. Studies were excluded if they lacked a direct examination of facial recognition processes or did not involve neuroimaging or brain-based analyses. After applying the inclusion and exclusion criteria, we ultimately identified 70 relevant articles. Our aim was to synthesize these findings to construct a more systematic theoretical model that better explains the pathological mechanisms of facial recognition deficits in ASD.
2 Neural anomalies in brain regions of facial recognition in ASD
The brain structure of the core facial recognition network primarily encompasses the inferior occipital gyri (IOG), FG, superior temporal sulcus (STS), amygdala, and prefrontal cortex (PFC). These structures are tasked with the processing of both variable and invariant facial features (13). Based on the comprehensive literature review, we found that the facial recognition impairments observed in individuals with ASD are associated with underlying anatomical irregularities within this core facial network, including reduced activation in the FG, atypical amygdala activation, hypoactivity in the STS, and increased activation of the PFC.
2.1 Reduced activation in the FG
Research has consistently identified the FG as a key region associated with the recognition of human faces (14). This area is selectively activated during the processing of facial stimuli, leading to its popular designation as the “fusiform face area” (FFA) (15, 16). A comprehensive meta-analysis of 100 functional magnetic resonance imaging (fMRI) studies involving facial emotion recognition in typically developing individuals has underscored the pivotal role of the FG in facial recognition processes (17). Three discrete facial selective regions are localized within the middle fusiform sulci, the posterior fusiform gyrus, and the inferior occipital gyrus (18).
Some studies have revealed that individuals with ASD exhibit reduced activation in the FG during facial recognition tasks. Nickl-Jockschat et al. (19) conducted a meta-analysis on face processing in ASD, finding reduced activation in the FG. Functional connectivity analyses revealed that this region is connected to the temporo-occipital cortex, inferior frontal and parietal cortices, thalamus, and amygdala. These results suggest that disrupted face processing in ASD is part of a broader network related to face, affective, and language processing, potentially contributing to the observed impairments and reduced interest in faces. A study by Ibrahim (20) using advanced imaging techniques found persistent reductions in FG activation in children with ASD, reinforcing the link between structural anomalies and face processing deficits.
Furthermore, research has indicated that individuals with ASD may have a thinner cortex in the left FG compared to typically developing individuals, with age showing a significant negative correlation with cortical thickness in those with ASD, a relationship that is not observed in typically developing individuals (21). A stereotactic-based study offers a more nuanced neuroanatomical explanation for the structural changes within the fusiform gyrus, reporting a decreased neuronal density in layer III, a reduced total number of neurons across layers III, V, and VI, and a diminished mean perinuclear volume of neurons in layers V and VI of individuals with ASD (22).
2.2 Atypical amygdala activation
Research indicates that the amygdala is capable of processing significant amounts of sophisticated sensory information. Each amygdala neuron can respond to somatosensory, visual, auditory, and all types of visceral inputs. The afferent nerve carrying this information reaches the amygdala in the opposite direction along the path followed by the efferent nerve of the amygdala (23). A neuroimaging study has demonstrated that the human amygdala becomes active during the interpretation of social signals, including gaze, facial expression recognition, and body language (24). Nickl-Jockschat and colleagues (19) discovered that the amygdala is associated with emotional domains such as fear, disgust, happiness, and sadness through functional decoding.
Studies have revealed that individuals with ASD exhibit atypical amygdala activation across a range of tasks involving social cognition, including inferring mental states from pictures of eyes and evaluating facial expressions (25, 26). A fMRI study conducted by Baron-Cohen and his team (24) found that individuals with ASD had a markedly reduced activation of the amygdala during mentalizing tasks—such as inferring intentions from eyes or facial expressions—compared to typically developing individuals. In this study, researchers compared the activation status of functional systems between individuals with ASD (ASD group) and typically developing individuals (control group) through a series of tasks designed to assess the theory of mind. It was observed that the ASD group failed to engage the amygdala and exhibited less partial activation in the frontal lobe compared to the control group. Additionally, the control group demonstrated significantly enhanced response capabilities in the left amygdala, the right insula, and the left inferior FG, whereas the ASD group showed a significantly stronger response in the bilateral superior temporal gyrus. This suggests that rather than using the amygdala for information-processing tasks, individuals with ASD may shift the processing load to temporal lobe structures that are specialized for labeling complex visual stimuli, which could be a compensatory mechanism for the atypical functioning of the amygdala (24). Furthermore, Nordahl et al. (20) supports these findings, indicating that atypical amygdala activation patterns in ASD may begin early in development and persist into adolescence, and the altered amygdala activation in response to different social traits, such as trustworthiness and dominance, in individuals with ASD.
However, it is crucial to note that hypersensitive responses of the amygdala to facial stimuli in ASD have also been reported. Lassalle et al. (27) demonstrated that when gaze is controlled and constrained to the eyes, individuals with ASD show increased activation in social brain regions, including the amygdala, particularly in response to low-intensity fearful faces. This heightened sensitivity is coupled with a lack of functional correlation between the amygdala and the ventromedial prefrontal cortex, indicating a potential excitatory/inhibitory imbalance in socio-affective processing. These findings highlight the context-dependent nature of amygdala responses in ASD, suggesting that both hypoactivation and hypersensitivity may contribute to the atypical processing of social stimuli.
2.3 Hypoactivity in the STS
The STS is a critical region involved in social perception, particularly in processing eye gaze and interpreting the intentions and emotions conveyed through facial movements. Eye gaze serves as a critical social cue, and anomalies in gaze behavior are a notable characteristic among individuals with ASD. Research has consistently shown that individuals with ASD exhibit deficits in visual reciprocity and social gaze behavior, which are key indicators of facial recognition deficits in this population (7, 8). Direct gaze, where one individual looks directly into the eyes of another, has a task-relevant perceptual advantage over avoidance gaze (where gaze is directed away). Direct gaze is more conducive to categorization, recognition, and memory of facial information (28). In terms of neural activity, direct gaze requires more activation of the STS than avoidance gaze. This is because the STS is closely related to the perception and regulation of gaze behavior and is involved in decoding communicative intentions behind eye movements and processing the social meaning of movement cues (29, 30).
Several neuroimaging studies using dynamic facial stimulation have demonstrated the critical role of the STS in processing gaze direction. For example, research by Pelphrey et al. (7) showed that the STS responds selectively to direct versus averted gaze, suggesting that it plays a crucial role in understanding where others are looking and inferring their intentions. However, in individuals with ASD, these studies have failed to demonstrate the same level of STS activation and modulation by gaze direction (31–33). This finding aligns with results from Castelli et al. (34), which revealed hypoactivity in the STS and reduced functional connectivity between the STS and a portion of the inferior occipital gyrus (visual area V3) during tasks involving the attribution of intention to moving geometric shapes. These hypoactivity in the STS may contribute to the significant impairment in social gaze observed in individuals with ASD. The inability to effectively process and respond to direct gaze can hinder social interactions, as gaze behavior is integral to understanding and engaging in social communication.
Moreover, the STS is involved in the perception of biological motion and the integration of visual and auditory information to comprehend social actions and intentions (35). Fry et al. (31) identified diminished face-selective network connectivity and reduced STS selectivity in individuals with ASD. In individuals with ASD, the STS’s difficulty in extracting social cues from visual facial information may stem from underactivation of the core nodes within the somatosensory cortex network, which processes visual facial information. This underactivation results in reduced inputs to the mirror neuron system (MNS), further complicating the decoding of social signals (36). Research has shown that the STS’s role in social cognition extends beyond gaze processing. It is also implicated in understanding and predicting others’ actions and intentions, a process that requires integrating various social cues (29, 37). Studies using tasks that involve interpreting social interactions or attributing mental states to others have consistently found reduced STS activation in individuals with ASD, highlighting the broad impact of STS hypoactivity on social cognition (34).
2.4 The increased activation of PFC
The PFC is a crucial brain region involved in higher-order cognitive processes, including decision-making, social behavior, and emotional regulation. It plays a significant role in supporting complex cognitive functions such as planning, problem-solving, and regulating emotions (38). In individuals with ASD, atypical activation patterns in the PFC have been observed during tasks involving social cognition (39). Another study has provided evidence that individuals with ASD may recruit the PFC as a compensatory mechanism for deficits in the primary facial recognition network. Typically, face recognition relies heavily on the FFA, amygdala, and STS. However, due to anomalies in these regions, individuals with ASD might rely more on cognitive strategies mediated by the PFC to recognize faces and interpret social cues (40). To support this, Kim et al. (41) demonstrated that verbalization tasks improved facial recognition in adolescents with ASD by engaging the PFC. This compensatory strategy may help mitigate deficits in the fusiform gyrus and amygdala, which are commonly underactivated in individuals with ASD.
The prefrontal cortex is known for its role in executive functions, which include managing attention, regulating behavior, and controlling impulses (38). These functions are essential for adaptive social behavior and emotional regulation. In neurotypical individuals, the automatic processing of social information is primarily mediated by the FFA, amygdala, and STS. However, the reliance on the PFC suggests that individuals with ASD may engage more in conscious, effortful processing of social information (42). For example, a study by Gilbert et al. (39) found that during tasks requiring social cognition, individuals with ASD showed greater activation in the medial prefrontal cortex (mPFC) compared to neurotypical controls. This increased activation was interpreted as evidence of compensatory recruitment of the PFC to support social processing. Similarly, Lombardo et al. (40) observed that individuals with ASD exhibited atypical patterns of activation in the PFC when interpreting social cues, suggesting that they might be compensating for deficits in more intuitive social processing networks.
This compensatory strategy, while potentially helpful in addressing some deficits, can be cognitively demanding. The PFC is engaged in effortful, controlled processing, which can lead to increased cognitive load and fatigue (43). This greater reliance on the PFC may not fully substitute for the more automatic and efficient processing of social information typically mediated by the FFA, amygdala, and STS. Consequently, while individuals with ASD may develop strategies to compensate for their face recognition deficits, these strategies might be less efficient and more taxing, potentially impacting their overall cognitive and emotional well-being (42).
3 Neural connectivity and structural anomalies of facial recognition in ASD
The section will explore the functional and structural brain connectivity in relation to facial recognition deficits in individuals with ASD. First, we will focus on how impaired functional connectivity between key regions, such as the FG, amygdala, PFC, and STS, contributes to social cognitive challenges. This section will highlight the impact of these disrupted neural connections on emotional processing and facial perception. Following this, the anatomical differences in these same regions will be examined, with emphasis on how structural anomalies in white matter tracts further impair communication between brain regions. By integrating both functional and structural perspectives, this review provides a comprehensive understanding of the neural mechanisms underlying facial recognition deficits in ASD.
3.1 Functional brain connectivity in ASD
In individuals with ASD, impaired functional connectivity between key brain regions involved in facial recognition contributes to social cognitive deficits. Vuilleumier et al. (44) demonstrated that amygdala damage correlates with underactivation of the FFA during facial recognition, while Anderson and Phelps (45) found that atypical amygdala activity affects the FFA’s ability to perceive facial emotions. Haxby et al. (30) further showed diminished connectivity between the FFA and other facial recognition regions in individuals with ASD, particularly in those with greater social deficits. Kleinhans et al. (46) similarly reported weaker connections between the FFA and amygdala, highlighting the importance of interaction between these regions for effective facial recognition.
Disrupted connectivity also involves the PFC and STS. Kana et al. (47) found reduced functional connectivity between the medial prefrontal cortex (mPFC) and amygdala during emotion processing, complicating compensatory mechanisms in ASD. This impaired connectivity persists into adulthood, as noted by Ammons et al. (48), further affecting social functioning. The PFC, while sometimes compensatory, cannot fully replace the automatic facial recognition processes typically handled by regions like the fusiform gyrus and STS. The importance of the STS in social cognition is further emphasized by studies examining its connectivity with other brain regions. For instance, Lombardo et al. (40) found that atypical STS connectivity with the mPFC and the amygdala in individuals with ASD correlated with difficulties in understanding social hierarchies and emotions. A study by Saitovitch et al. (49) also found that STS hypoactivity and reduced functional connectivity with other social brain regions were consistent markers of social cognition impairments in ASD. These findings suggest that STS hypoactivity contributes to a broader network dysfunction that underlies social cognitive impairments in ASD.
Additionally, resting-state fMRI studies further illustrate widespread connectivity issues. Schipul et al. (50) reported decreased connectivity between the amygdala and PFC at rest in individuals with ASD, which may hinder emotional processing and higher-order decision-making. Similarly, von dem Hagen et al. (51) identified atypical connectivity between the FG and the posterior superior temporal sulcus (pSTS), suggesting that the impaired coordination between facial perception and gaze processing regions contributes to the facial recognition deficits seen in ASD. These findings emphasize the importance of examining both structural and functional connectivity to fully understand the neural underpinnings of social cognitive impairments in ASD.
3.2 Anatomical differences and neural connectivity in ASD
In addition to functional anomalies in facial recognition networks, individuals with ASD exhibit structural differences that further contribute to their social cognitive impairments. Voxel-based morphometry (VBM) studies have consistently revealed volumetric alterations in key brain areas involved in social cognition. Ecker et al. (52) conducted a comprehensive VBM analysis, finding increased gray matter volume in the fusiform gyrus (FG) and reduced volume in the amygdala in individuals with ASD. These volumetric changes in regions responsible for processing facial identity and emotions suggest that anatomical anomalies may underlie the functional deficits observed in ASD. Similarly, McAlonan et al. (53) reported decreased gray matter in the superior temporal sulcus (STS), a region critical for interpreting dynamic facial features such as gaze direction and emotional expressions. Such structural variations may be linked to the challenges individuals with ASD face in social interactions, including difficulties with facial recognition and understanding social cues.
Neural connectivity studies provide further insights into how these anatomical differences disrupt communication between brain regions. Diffusion tensor imaging (DTI) has revealed altered white matter connectivity in ASD, particularly in pathways essential for social cognition. Lange et al. (54) found reduced fractional anisotropy (FA) in the inferior longitudinal fasciculus (ILF) and the uncinate fasciculus (UF)—fiber tracts that connect the FG to the amygdala and PFC. These pathways are vital for integrating facial identity and emotional information, and disruptions in their structural connectivity may contribute to the impairments in facial processing seen in ASD. Other studies utilizing advanced neuroimaging techniques, such as tractography, have reinforced these findings, showing that anomalies in these white matter tracts are associated with the severity of social and emotional difficulties (55, 56).
Functional brain connectivity analyses, particularly using resting-state fMRI, have also highlighted reduced communication between key facial recognition regions. Kleinhans et al. (46) demonstrated weakened connectivity between the FFA and the amygdala in individuals with ASD. This diminished functional integration of identity and affective processing supports the hypothesis that disruptions in the amygdaloid-fusiform network are central to the facial recognition deficits observed in ASD. More recently, Tseng et al. (57) extended these findings by showing that anomalies of functional connectivity between the FFA and other regions of the social brain network, such as the PFC and STS, may contribute to the broader social impairments seen in ASD. These studies emphasize the importance of investigating both structural and functional connectivity to understand the full extent of neural disruptions in ASD.
4 Implication and future directions
4.1 Potential implications for individuals with ASD
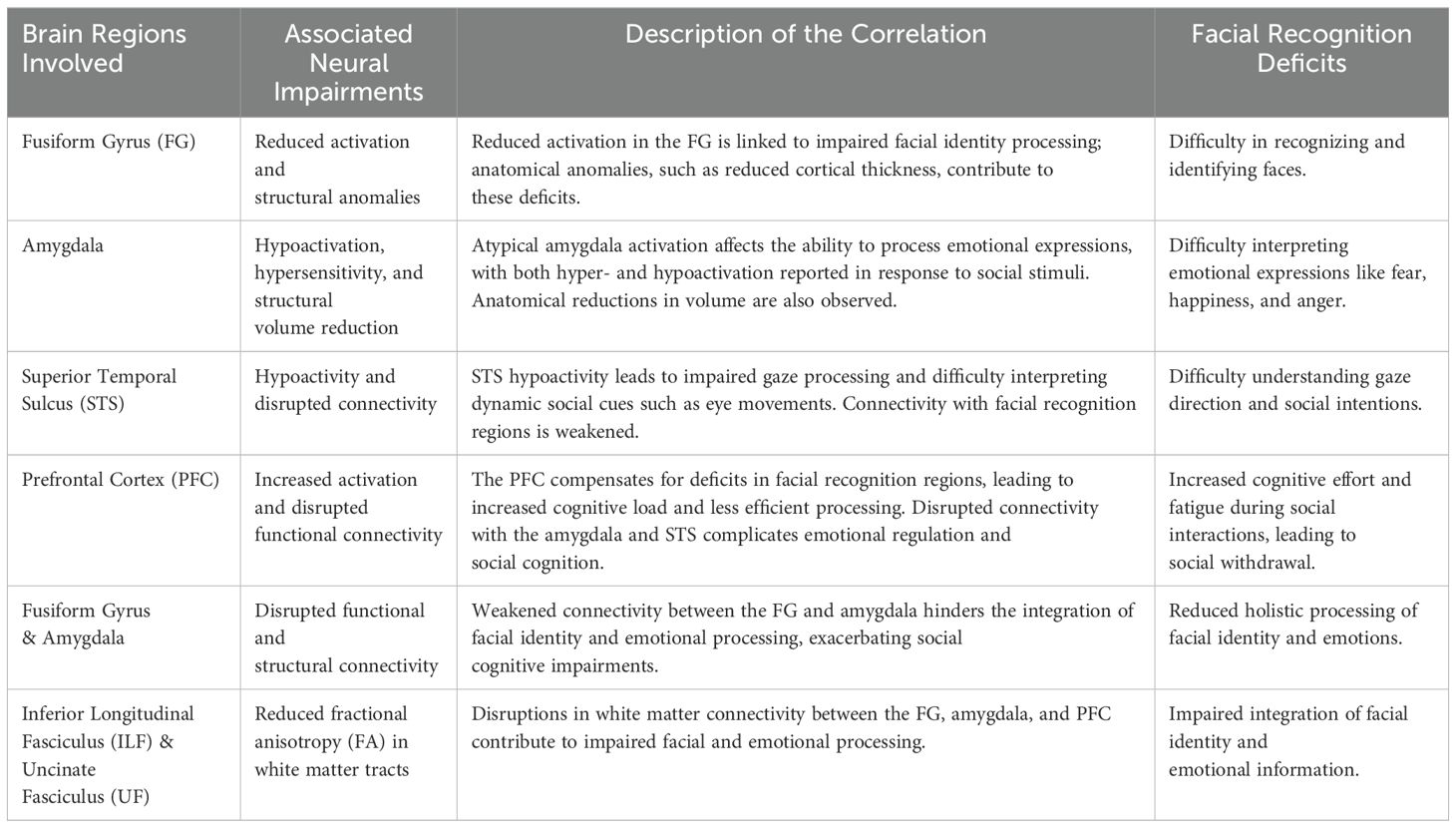
The neural anomalies found in key regions responsible for facial recognition, such as the FG, amygdala, STS, and PFC, have significant implications for the daily lives and functioning of individuals with ASD (Table 1). Reducing in FG activation, for example, can lead to difficulties in recognizing familiar faces, which are crucial for social interaction and relationship-building. This can result in increased social anxiety, avoidance behaviors, and challenges in forming personal or professional relationships. Atypical amygdala activation, which is critical for interpreting emotions, often results in difficulties for individuals with ASD to accurately read emotional expressions like fear, happiness, or anger. These deficits could lead to inappropriate or delayed responses in social settings, further complicating their ability to engage meaningfully in conversations or social activities. Additionally, hypoactivity in the STS hinders the processing of social cues such as eye gaze, making it harder for individuals with ASD to gauge the intentions or emotions of others during face-to-face interactions. Compensatory activation in the PFC, while helpful in some cases, imposes a heavy cognitive load on individuals with ASD. Social interactions that should be automatic become cognitively demanding, leading to fatigue, frustration, and eventual social withdrawal. This reliance on conscious effort in processing social information can make social engagements less enjoyable and more stressful, potentially exacerbating feelings of isolation. In sum, these neural anomalies contribute to the overall social challenges individuals with ASD face in daily life, including difficulty forming and maintaining relationships, understanding social contexts, and successfully navigating social environments. Addressing these challenges through early interventions aimed at mitigating neural dysfunction could have profound effects on improving social outcomes for individuals with ASD.
4.2 The future directions
4.2.1 longitudinal and multimodal studies on neural development with emphasis on neural differences
Future research should integrate longitudinal tracking of neural development in individuals with ASD from early childhood to adulthood, employing advanced neuroimaging techniques like fNIRS, fMRI, MEG, and EEG (58, 59). These multimodal approaches will help identify how brain structures and functions related to face processing change over time, highlighting both differences and strengths rather than just deficits. Longitudinal studies should aim to understand the natural developmental trajectory and stability of face processing abilities, identifying individual variations and neural patterns in a neutral sense. This approach will provide a balanced view of ASD brain development, including how critical periods for interventions might emerge to enhance social cognition, without solely focusing on therapeutic outcomes. Modalities like fNIRS offer naturalistic and portable measurements, while DTI and functional connectivity MRI (fcMRI) explore structural and functional networks, allowing for a comprehensive understanding of neural diversity in ASD.
4.2.2 Exploring individual differences and personalized approaches through AI and neuroimaging
Considering the heterogeneity in ASD, future research should explore individual neural mechanisms underlying face recognition deficits using neuroimaging biomarkers and advanced analysis methods (60–63). Identifying distinct neural and genetic markers will pave the way for personalized treatment approaches tailored to each individual’s unique neural and behavioral profile. Techniques like fMRI, EEG, and fNIRS can profile neural characteristics, while machine learning (ML) and computer vision can enhance predictive models for personalized interventions. For instance, ML models trained on both behavioral and neural data could aid in the prediction of treatment outcomes, while robot-assisted interventions (64, 65) provide a more holistic and individualized therapy strategy, considering both neurodiversity and unique developmental trajectories.
4.2.3 Integration of neuroimaging techniques with advanced analytical approaches and neurodiversity consideration
The fusion of neuroimaging techniques, including functional connectivity analyses and fNIRS, with computational modeling and AI holds significant promise for understanding the complex and diverse nature of ASD. Specifically, fNIRS offers the benefits of portability and naturalistic assessment (58), and can be used alongside EEG, MEG, and MRI to explore both task-specific activation and resting-state functional connectivity in ASD. Machine learning algorithms, such as convolutional neural networks (CNNs), Vision Transformer (ViT) models, and hybrid approaches (e.g., CNN with XGBoost or Random Forest), can be applied to neuroimaging data to uncover patterns related to face recognition deficits. Incorporating these technologies enables a nuanced understanding of the neural underpinnings of ASD (41, 66), focusing on individual differences and neural diversity, and aids in developing targeted interventions and diagnostic tools informed by data from diverse populations.
4.2.4 Leveraging AI for holistic understanding and support in ASD
Machine learning and big data analytics present new opportunities for refining ASD diagnosis and support strategies (58). Automated analysis of facial recognition patterns, eye movements, and neural data allows for the creation of sophisticated predictive models. Integrating neural indicators, such as brain activation and connectivity patterns, can improve the precision of ASD classification beyond traditional behavioral assessments. fNIRS and MEG offer promising data sources for these AI models, allowing for real-time analysis and potentially earlier detection of ASD symptoms (59). As AI models advance, they may facilitate more effective, targeted supports that adapt to the changing needs of individuals with ASD over time, emphasizing personalized growth and optimizing therapy effectiveness in alignment with the neurodiversity model.
5 Conclusion
This review highlights the complex neural basis of facial recognition deficits in individuals with ASD, emphasizing anatomical and functional anomalies across multiple brain regions. The FG, essential for facial identity recognition, shows reduced activation, with structural anomalies contributing to deficits. The amygdala, responsible for emotional expression processing, exhibits both hypoactivation and hypersensitivity, affecting facial emotion interpretation. The STS, involved in gaze processing and social cognition, shows hypoactivity, worsening social cue comprehension. The PFC, often compensatory, adds cognitive load, reducing facial recognition efficiency. Furthermore, the disruptions in structural and functional connectivity, such as between the FFA and amygdala, impair ASD’s facial identity and emotion integration. Neuroimaging studies (e.g., VBM, DTI) underscore the role of anatomical differences in these functional impairments, with disrupted connection between the PFC, amygdala, and STS suggesting inefficiency in compensatory mechanisms. Future research should use longitudinal, multimodal approaches to identify critical intervention periods. Personalized therapies, informed by neuroimaging and AI, hold promise for improving social cognition and quality of life in individuals with ASD through targeted interventions.
Author contributions
JL: Conceptualization, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. HC: Conceptualization, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. HW: Conceptualization, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing, Validation. ZW: Conceptualization, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing (2013).
2. Zeidan J, Fombonne E, Scorah J, Ibrahim A, Durkin MS, Saxena S, et al. Global prevalence of autism: A systematic review update. Autism Res. (2022) 15:778–90. doi: 10.1002/aur.v15.5
3. Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States (2023). Available online at: https://www.cdc.gov/mmwr/volumes/72/ss/ss7202a1.htm (Accessed March 24, 2023).
4. Minio-Paluello I, Porciello G, Pascual-Leone A, Baron-Cohen S. Face individual identity recognition: a potential endophenotype in autism. Mol Autism. (2020) 11:1–16. doi: 10.1186/s13229-020-00371-0
5. Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. (2013) 504:427–31. doi: 10.1038/nature12715
6. Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. (2002) 59:809–16. doi: 10.1001/archpsyc.59.9.809
7. Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. (2005) 128:1038–48. doi: 10.1093/brain/awh404
8. Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol. (2005) 27:403–24. doi: 10.1207/s15326942dn2703_6
9. Griffin JW, Bauer R, Scherf KS. A quantitative meta-analysis of face recognition deficits in autism: 40 years of research. psychol Bull. (2021) 147:268–92. doi: 10.1037/bul0000310
10. Behrmann M, Thomas C, Humphreys K. Seeing it differently: visual processing in autism. Trends Cogn Sci. (2006) 10:258–64. doi: 10.1016/j.tics.2006.05.001
11. Joseph RM, Tanaka J. Holistic and part-based face recognition in children with autism. J Child Psychol Psychiatry. (2003) 44:529–42. doi: 10.1111/jcpp.2003.44.issue-4
12. Yirmiya N, Kasari C, Sigman M, Mundy P. Empathy and cognition in high-functioning children with autism. Child Dev. (1992) 63:150–60. doi: 10.2307/1130909
13. Nomi JS, Uddin LQ. Face processing in autism spectrum disorders: From brain regions to brain networks. Neuropsychologia. (2015) 71:201–16. doi: 10.1016/j.neuropsychologia.2015.03.029
14. McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. J Cogn Neurosci. (1997) 9:605–10. doi: 10.1162/jocn.1997.9.5.605
15. Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. (1997) 17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997
16. Wardle SG, Taubert J, Teichmann L, Baker CI. Rapid and dynamic processing of face pareidolia in the human brain. Nat Commun. (2020) 11:4518. doi: 10.1038/s41467-020-18325-8
17. Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, et al. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. (2011) 54:2524–33. doi: 10.1016/j.neuroimage.2010.10.011
18. Weiner KS, Grill-Spector K. Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. Neuroimage. (2010) 52:1559–73. doi: 10.1016/j.neuroimage.2010.04.262
19. Nickl-Jockschat T, Rottschy C, Thommes J, Schneider F, Laird AR, Fox PT, et al. Neural networks related to dysfunctional face processing in autism spectrum disorder. Brain Structure Funct. (2015) 220:2355–71. doi: 10.1007/s00429-014-0791-z
20. Ibrahim K, Eilbott JA, Ventola, P, He G, Pelphrey KA, McCarthy G, et al. Reduced amygdala–prefrontal functional connectivity in children with autism spectrum disorder and co-occurring disruptive behavior. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. (2019) 4:1031–41. doi: 10.1016/j.bpsc.2019.01.009
21. Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. (2010) 133:3745–54. doi: 10.1093/brain/awq279
22. van Kooten IA, Palmen SJ, von Cappeln P, von Cappeln T, Korr H, Schmitz C. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. (2008) 131:987–99. doi: 10.1093/brain/awn033
23. Wang S, Li X. A revisit of the amygdala theory of autism: Twenty years after. Neuropsychologia. (2023) 183:108519. doi: 10.1016/j.neuropsychologia.2023.108519
24. Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. (1999) 11:1891–8. doi: 10.1046/j.1460-9568.1999.00621.x
25. Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. (2005) 23:125–41. doi: 10.1016/j.ijdevneu.2004.12.012
26. Sato W, Uono S, Kochiyama T. Neurocognitive mechanisms underlying social atypicalities in autism: weak amygdala’s emotional modulation hypothesis. Front Psychiatry. (2020) 11:552852. doi: 10.3389/fpsyt.2020.00864
27. Lassalle A, Åsberg Johnels J, Zürcher NR, Hippolyte L, Billstedt E, Ward N, et al. Hypersensitivity to low intensity fearful faces in autism when fixation is constrained to the eyes. Hum Brain Mapp. (2017) 38:5943–57. doi: 10.1002/hbm.v38.12
28. Senju A, Johnson MH. The eye contact effect: mechanisms and development. Trends Cogn Sci. (2009) 13:127–34. doi: 10.1016/j.tics.2008.11.009
29. Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. (2000) 4:267–78. doi: 10.1016/S1364-6613(00)01501-1
30. Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. (2000) 4:223–33. doi: 10.1016/S1364-6613(00)01482-0
31. Fry R, Lewis ME, Baker M. Investigating the influence of autism spectrum traits on face processing mechanisms in developmental prosopagnosia. J Autism Dev Disord. (2023) 53:193–207. doi: 10.1007/s10803-022-05705-w
32. Vaidya CJ, Foss-Feig J, Shook D, Kaplan L, Kenworthy L, Gaillard W. Controlling attention to gaze and arrows in childhood: an fMRI study of typical development and Autism Spectrum Disorders. Developmental Science. (2011) 14:911–24. doi: 10.1111/j.1467-7687.2011.01041.x
33. Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. Autism, the superior temporal sulcus and social perception. Trends Neurosci. (2006) 29:359–66. doi: 10.1016/j.tins.2006.06.004
34. Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. (2002) 125:1839–49. doi: 10.1093/brain/awf189
35. Grossman ED, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, et al. Brain areas involved in perception of biological motion. J Cogn Neurosci. (2000) 12:711–20. doi: 10.1162/089892900562417
36. Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. (2006) 9:28–30. doi: 10.1038/nn1611
37. Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. (2005) 43:1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013
38. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. (2001) 24:167–202. doi: 10.1146/annurev.neuro.24.1.167
39. Gilbert SJ, Bird G, Brindley R, Frith CD, Burgess PW. Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: an fMRI study. Neuropsychologia. (2008) 46:2281–91. doi: 10.1016/j.neuropsychologia.2008.03.025
40. Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, et al. Atypical neural self-representation in autism. Brain. (2011) 134:611–24. doi: 10.1093/brain/awp306
41. Kim M, Dooley K, Lu Q. Exploring intervention strategies to enhance face recognition abilities in adolescents with autism spectrum disorder: Assessing the impact of verbalization and Navon tasks. Adv Autism. (2023) 9:133–45. doi: 10.1108/AIA-03-2022-0011
42. Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. (2012) 36:1292–313. doi: 10.1016/j.neubiorev.2012.02.007
43. Shalom DB. The medial prefrontal cortex and integration in autism. Neuroscientist. (2009) 15:589–98. doi: 10.1177/1073858409336371
44. Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. (2004) 7:1271–8. doi: 10.1038/nn1341
45. Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. (2001) 411:305–9. doi: 10.1038/35077083
46. Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. (2008) 131:1000–12. doi: 10.1093/brain/awm334
47. Kana RK, Patriquin MA, Black BS, Channell MM, Wicker B. Altered medial frontal and superior temporal response to implicit processing of emotions in autism. Autism Res. (2016) 9:55–66. doi: 10.1002/aur.1496
48. Ammons CJ, Winslett ME, Bice J, Patel P, May KE, Kana RK. The Mid-Fusiform Sulcus in Autism Spectrum Disorder: Establishing a Novel Anatomical Landmark Related to Face Processing. Autism Research. (2021) 14:53–64. doi: 10.1002/aur.2425
49. Saitovitch A, Bargiacchi A, Chabane N, Brunelle F, Samson Y, Boddaert N, et al. Social cognition and the superior temporal sulcus: implications in autism. Revue Neurologique. (2012) 168:762–70. doi: 10.1016/j.neurol.2012.07.017
50. Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Front Syst Neurosci. (2011) 5:10. doi: 10.3389/fnsys.2011.00010
51. von dem Hagen EA, Nummenmaa L, Yu R, Engell AD, Ewbank MP, Calder AJ. Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cereb Cortex. (2013) 23:664–73. doi: 10.1093/cercor/bhq062
52. Ecker C, Ginestet C, Feng Y, Johnston P, Lombardo MV, Baron-Cohen S. Brain surface anatomy in adults with autism: The relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiatry. (2013) 70:59–70. doi: 10.1001/jamapsychiatry.2013.265
53. McAlonan GM, Cheung C, Cheung V, Wong N, Suckling J, Murphy DG. Mapping brain structure in autism: A voxel-based MRI study of volumetric differences and intercorrelations in a case-control sample. J Autism Dev Disord. (2005) 35:709–17. doi: 10.1093/brain/awh332
54. Lange N, Dubray MB, Lee JE, Froimowitz MP, Froehlich A, Alexander AL. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. (2010) 3:350–8. doi: 10.1002/aur.v3.6
55. Abbott AE, Nair A, Keown CL, Datko M, Jahedi A, Fishman I, et al. Patterns of atypical functional connectivity and behavioral links in autism differ between default, salience, and executive networks. Cereb Cortex. (2016) 26:4034–45. doi: 10.1093/cercor/bhv191
56. Ryan NP, Genc S, Beauchamp MH, Yeates KO, Hearps S, Silk TJ. White matter microstructure predicts longitudinal social cognitive outcomes after pediatric traumatic brain injury: A diffusion tensor imaging study. psychol Med. (2018) 48:679–91. doi: 10.1017/S0033291717002057
57. Tseng A, Camchong J, Francis SM, Mueller BA, Lim KO, Conelea CA, et al. Differential extrinsic brain network connectivity and social cognitive task-specific demands in autism spectrum disorder (ASD). J Psychiatr Res. (2022) 148:230–9. doi: 10.1016/j.jpsychires.2022.01.066
58. Zhang F, Roeyers H. Exploring brain functions in autism spectrum disorder: A systematic review on functional near-infrared spectroscopy (fNIRS) studies. Int J Psychophysiol. (2019) 137:41–53. doi: 10.1016/j.ijpsycho.2019.01.003
59. O’Reilly C, Lewis JD, Elsabbagh M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PloS One. (2017) 12:e0175870. doi: 10.1371/journal.pone.0175870
60. Almufareh MF, Kausar N, Khurshid K, Abdalla AN. Facial classification for autism spectrum disorder. J Disabil Res. (2024) 18:104–16. doi: 10.57197/JDR-2024-0025
61. Awaji B, Senan EM, Olayah F, Alshari EA, Alsulami M, Abosaq HA, et al. Hybrid techniques of facial feature image analysis for early detection of autism spectrum disorder based on combined CNN features. Diagnostics. (2023) 13:2948. doi: 10.3390/diagnostics13182948
62. Liu W, Li M, Yi L. Identifying children with autism spectrum disorder based on their face processing abnormality: A machine learning framework. Autism Res. (2016) 9:888–98. doi: 10.1002/aur.1615
63. Wu Q, Xiao X, Liu Y, Billinghurst M, Nanayakkara S. “Early autism screening in children using facial recognition”. In Extended Abstracts of the CHI Conference on Human Factors in Computing Systems. (2024) pp. 1–7. doi: 10.1145/3613905.3651045
64. Del Coco M, Leo D, Carcagnì D, Fama D, Spadaro D, Ruta D, et al. Study of mechanisms of social interaction stimulation in autism spectrum disorder by assisted humanoid robot. IEEE Trans Cogn Dev Syst. (2017) 10:993–1004. doi: 10.1109/TCDS.2017.2783684
65. Lecciso F, Levante. A, Fabio RA, Caprì T, Leo M, Carcagnì P, et al. Emotional expression in children with ASD: A pre-study on a two-group pre-post-test design comparing robot-based and computer-based training. Front Psychol. (2021) 12:678052. doi: 10.3389/fpsyg.2021.678052
Keywords: ASD, facial recognition deficits, FG, amygdala, STS, PFC, social cognition
Citation: Liu J, Chen H, Wang H and Wang Z (2025) Neural correlates of facial recognition deficits in autism spectrum disorder: a comprehensive review. Front. Psychiatry 15:1464142. doi: 10.3389/fpsyt.2024.1464142
Received: 13 July 2024; Accepted: 22 October 2024;
Published: 06 January 2025.
Edited by:
Margaret Lang Bauman, Boston University, United StatesReviewed by:
Marco Leo, National Research Council (CNR), ItalyMakoto Wada, National Rehabilitation Center for Persons with Disabilities, Japan
Copyright © 2025 Liu, Chen, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhidan Wang, endhbmcxOUBqc251LmVkdS5jbg==
 Jianmei Liu1,2
Jianmei Liu1,2 Huihui Chen
Huihui Chen Zhidan Wang
Zhidan Wang