- 1Unit of Psychiatry, Department of Medicine (DMED), University of Udine, Udine, Italy
- 2Institute of Biomolecular Chemistry, National Research Council (CNR), Pozzuoli, Italy
- 3Department of Medicine (DMED), University of Udine, Udine, Italy
- 4Institute of Clinical Pathology, Friuli Centrale Health University Authority (ASUFC), Udine, Italy
- 5Department of Chemical and Pharmaceutical Sciences, University of Trieste, Trieste, Italy
- 6Unit of Psychiatry, Friuli Centrale Health University Authority (ASUFC), Udine, Italy
- 7Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom
Despite promise of its supplementation as both monotherapy and add-on treatment in autism spectrum disorder (ASD), the biobehavioral effects of Palmitoylethanolamide (PEA) in autistic adults have never been explored so far. We discussed the cases of two autistic adults with different degrees of severity (level 1 and level 2) presenting with symptoms of psychic distress, who were treated with ultramicronized-PEA (um-PEA) 600 mg/day monotherapy for a sustained period of 4 months. The level 1 autistic patient showed improved depressive symptoms and social engagement at a 12-week follow-up, in parallel to a tendency toward reduced inflammatory response and enhanced endocannabinoid (eCB) signaling, partially relapsing after um-PEA discontinuation at four months. Opposedly, the level 2 autistic patient exhibited a generally stable psychosocial functioning for the initial 12 weeks, consistent with basically unchanged immune and eCBs levels, abruptly deteriorating and leading to antipsychotic initiation afterwards. No significant side effects were reported in both cases during the observation period. The two cases suggest that um-PEA could be an effective option for the treatment of psychic distress in level 1 autistic adults, warranting further investigation of its age- and level-specificity and of the biological underpinnings of its therapeutic effect in ASD.
1 Introduction
Autism Spectrum Disorder (ASD) is a complex multifactorial neurodevelopmental condition entailing difficulties in social interaction and restricted/repetitive behaviors and interests, presenting with varying degrees of severity (1–3). Autistic individuals often encounter numerous psychosocial barriers and challenges starting from early childhood (4–6), leading to limited social integration, poor job prospects, and a high incidence of comorbid psychic distress during adulthood (7–11). Detecting and intervening early on symptoms of psychic distress in autistic adults is therefore crucial to maintaining their quality of life, although mixed evidence regarding whether they may benefit from conventional psychotropic medications (12, 13) warrants the need to research into novel therapeutical targets. Despite being largely debated (14), a comprehensive understanding of the neurophysiological mechanisms underlying ASD is still hard to define. In recent years, growing evidence has converged toward immune dysregulation as a key contributor to the emergence and persistence of ASD traits, either systemically (15–19), or within the central nervous system (CNS), where it primarily occurs as glutamate excitotoxicity (20–23). Concurrently, over the past decades the endocannabinoid (eCB) system has drawn increasing attention for its ability to modulate neuroinflammation and glutamatergic signaling to dopaminergic neurons via the cannabinoid receptors (i.e., CB1 and CB2 receptors) interaction with both exogenous (i.e., phytocannabinoids) and endogenous (i.e., eCBs) ligands (24–26), with growing but sparse evidence of potential biobehavioral and therapeutic implications for ASD (27–29). Along with the major eCBs anandamide (AEA) and 2-arachidoylgylcerol (2-AG), several other bioactive mediators have been discovered, including the N-acylethanolamines (NAEs) palmitoylethanolamide (PEA) and oleoylethanolamide (OEA), monoacylglycerols, and other N-acylaminoacids/neurotransmitters, all of which are part of the expanded eCB system, known as the endocannabinoidome (eCBome) (30). In particular, PEA has been increasingly studied for its immunomodulatory properties, acting through an endocannabinoid-like mechanism both in the peripheral nervous system and in the CNS (31, 32), with biobehavioral correlates in several neurological and mental health diseases, including epilepsy (33), multiple sclerosis (34), cognitive decline (35), major depressive disorder (36), and psychosis (37). PEA presents a multi-faceted mechanism of action. First it acts as a direct agonist of the Peroxisome Proliferator Activated Receptor-α (PPAR-α) and of the G Protein-coupled Receptor 55 (GPR55), second as an allosteric modulator of the Transient Receptor Potential Vanilloid 1 (TRPV1), and third as an inhibitor of the Fatty-Acid Amide Hydrolase (FAAH) and a stimulator of the Diacylglycerol Lipase (DAGL), thus increasing the endogenous availability of AEA and 2-AG (31, 32). In addition to being naturally produced by several human cells and tissues in response to actual or potential damage, PEA can also be supplemented exogenously. In its ultra-micronized form (um-PEA), it shows enhanced bioavailability by more effectively penetrating the CNS (38).
The biobehavioral role of PEA in ASD has been recently interrogated, particularly for its therapeutic potential to target the immune-glutamatergic pathway via the eCB system modulation (39). While evidence has been reported on the beneficial effects of PEA supplementation, either as monotherapy or as add-on to treatment as usual with a safe and tolerable profile, on the core symptoms of autistic children and adolescents (40–42), to the best of our knowledge it has not yet been investigated among autistic adults.
Here, we present the cases of two autistic adults treated with um-PEA as monotherapy for symptoms of psychic distress over a sustained period of 4 months. To better account for heterogeneous effects of um-PEA across the autism spectrum (1), we describe the cases of patients differing in their levels of support needs and presence of comorbid intellectual disability.
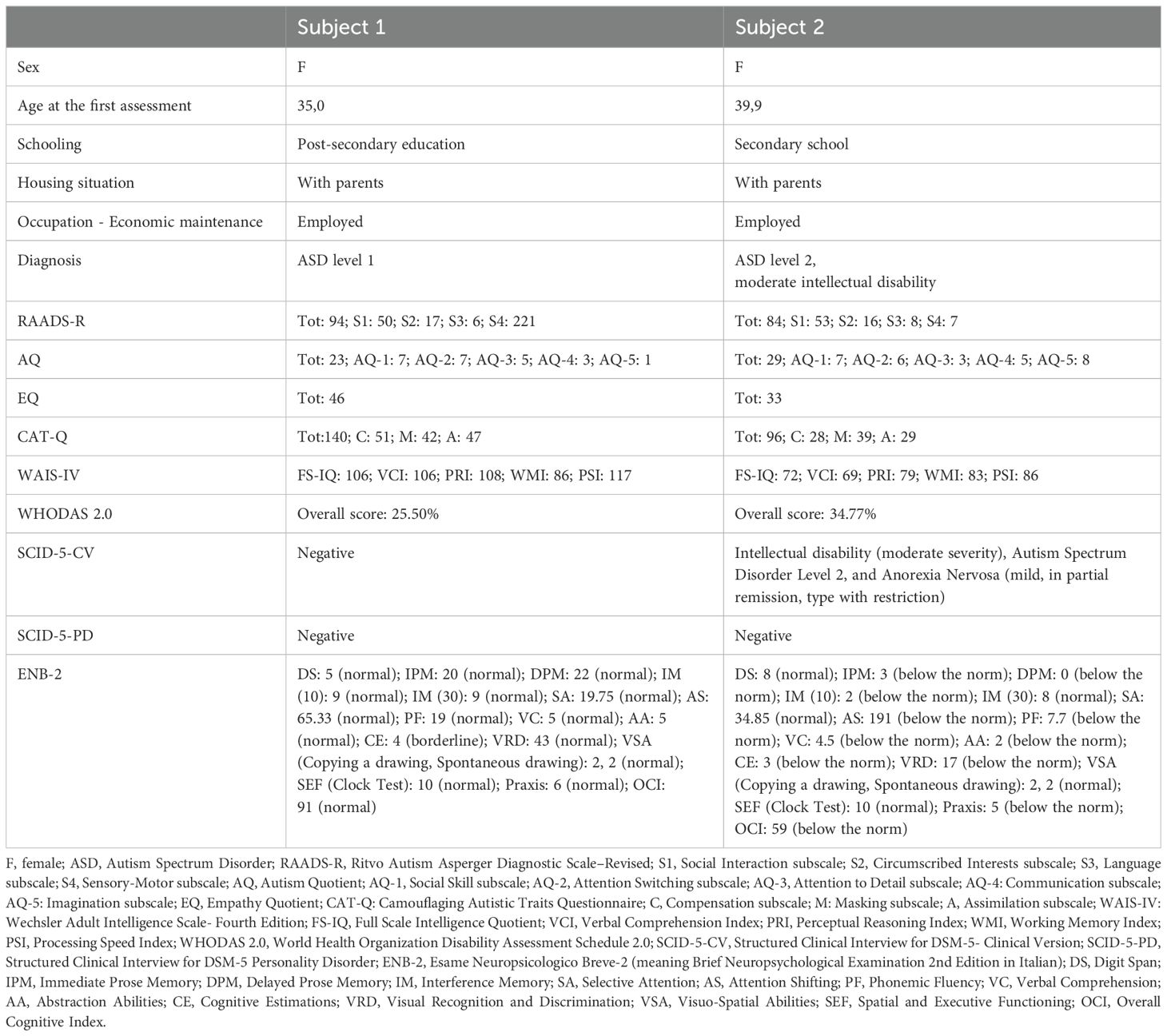
2 Case reports
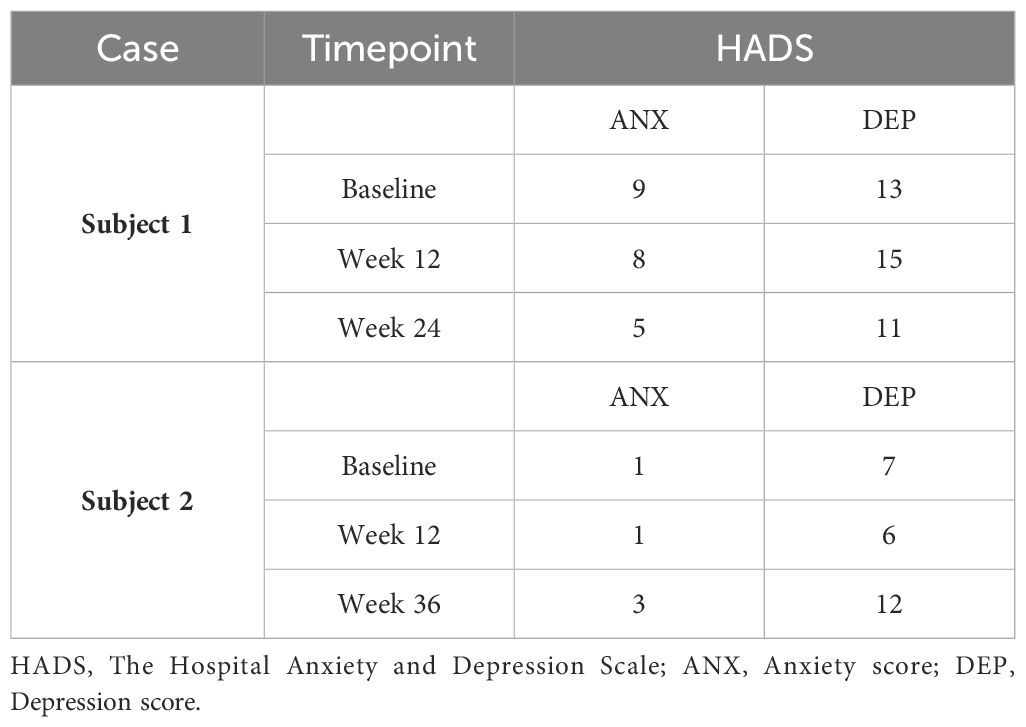
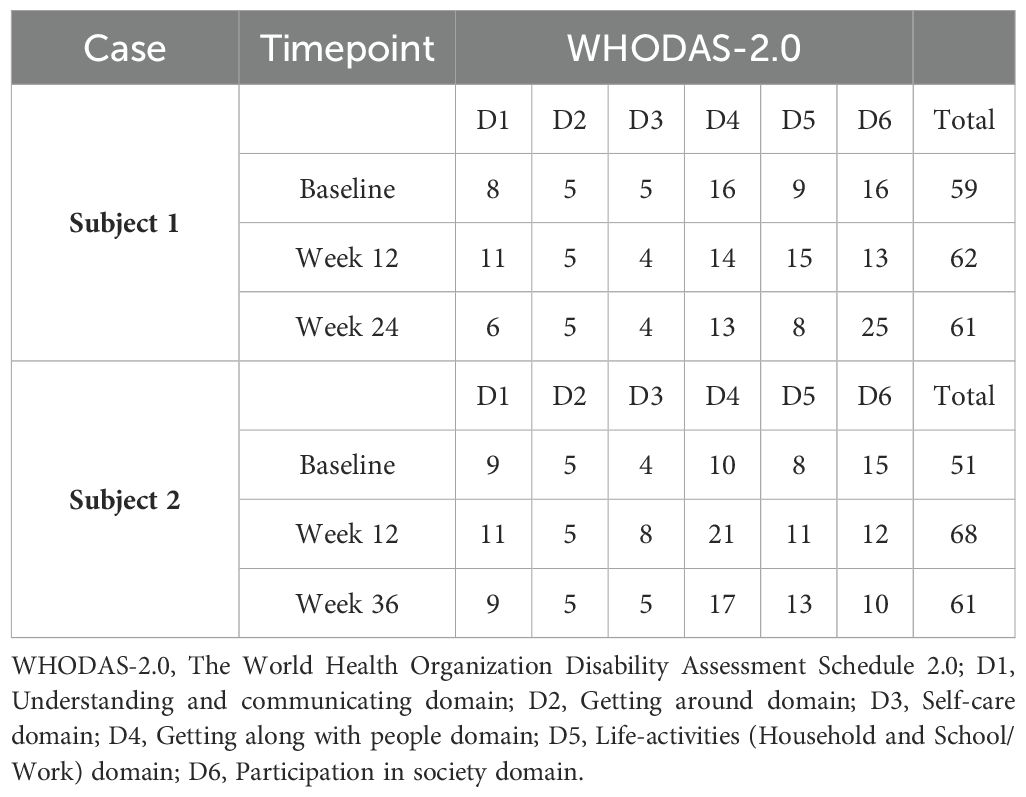
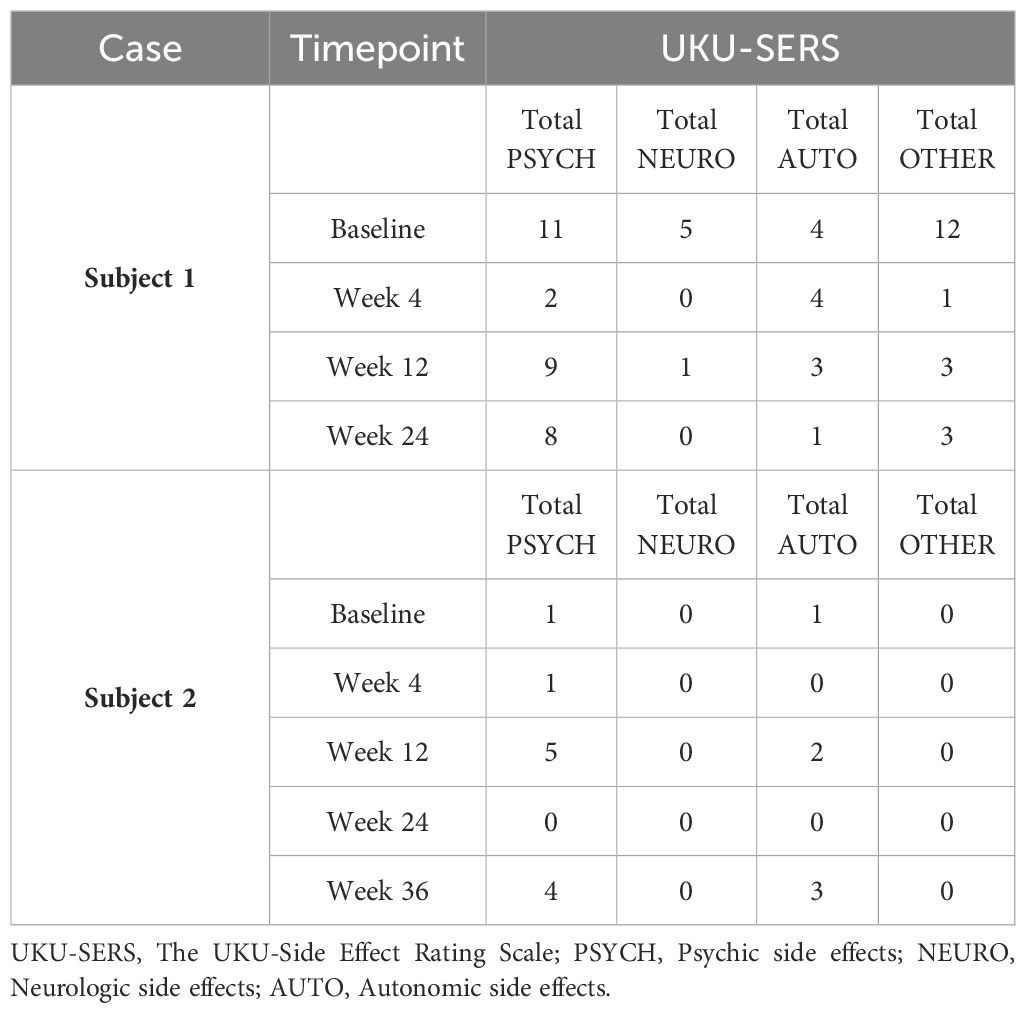
Both patients were help-seeking women presenting with symptoms of psychic distress to the Unit of Psychiatry at the University Hospital of Udine, where they were diagnosed with ASD in adulthood according to the Diagnostic and Statistical Manual of Mental Disorders—Fifth Edition (DSM-5) (1). Baseline evaluations were carried out to define neurodevelopmental characteristics [the Ritvo Autism Asperger Diagnostic Scale-Revised (RAADS-R) (43), the Autism Quotient (AQ) (44), the Empathy Quotient (EQ) (45), the Camouflaging Autistic Traits Questionnaire (CAT-Q) (46), the Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV) (47), the World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0; 36 items, interviewer-administered) (48)] and to assess possible neuropsychiatric comorbidities [the Structured Clinical Interview for DSM-5 Disorders, Clinician Version (SCID-CV) (49), the Structured Clinical Interview for DSM-5 Disorders, Personality Disorders Version (SCID-PD) (50), the Esame Neuropsicologico Breve-2 (ENB-2, meaning Brief Neuropsychological Examination 2nd Edition in Italian) (51)] (Table 1). The patients were approached by trained investigators to initiate um-PEA monotherapy, as the first two consecutive autistic individuals with heterogeneous ASD severity levels consenting to an internal pilot trial approved by the Department of Medicine (DMED) at the University of Udine (Institutional Review Board: 146/2024) in May 2024. They were proposed to undergo sustained daily treatment with oral um-PEA (600 mg/day with breakfast, tablet form, Normast®), to assess the feasibility of completing a 12-week follow-up. The medication was dispensed by qualified physicians in units of 60 tablets at each timepoint. Clinical progress was evaluated at baseline, 4 weeks, and 12 weeks using (i) the Symptom Checklist-90-Revised (SCL-90-R) (52) to assess um-PEA effect on levels of psychic distress (Table 2A), (ii) the Hospital Anxiety and Depression Scale (HADS) (53) to assess um-PEA effect on anxiety and depressive symptoms (Table 2B), and (iii) the WHODAS 2.0 (48) to assess um-PEA effect on adaptive functioning (Table 2C). The occurrence of adverse effects was quantified using the UKU Side Effect Rating Scale (54) (Table 2D). After completing the initial 12-week phase, the patients were invited to enter an extension phase follow-up to assess the clinical stability of the treatment for a maximum observation period of 36 weeks. At each timepoint, patients underwent blood analyses, including hematological, biochemical, inflammatory, and neuroaxonal impairment markers (Table 3). Specifically, a large panel of cytokines and chemokines was analyzed on available serum samples using customized multiplex immunoenzymatic assays (Ella instrument, Bio-Techne, USA). Reference ranges suggested by the manufacturer were validated in our population by indirect methods. Adjunctive serum samples were collected to measure the biochemical reaction pathways resulting from um-PEA supplementation using Surface-Enhanced Raman Scattering (SERS) technique (55) (Figure 1; Supplementary Material) and to measure endocannabinoidome (eCBome) mediators using liquid chromatography-mass spectrometry (LC-MS) (56, 57) (Table 3). Blood samples collection was performed at 8 in the morning, after fasting for approximately 12 hours, before um-PEA daily intake.
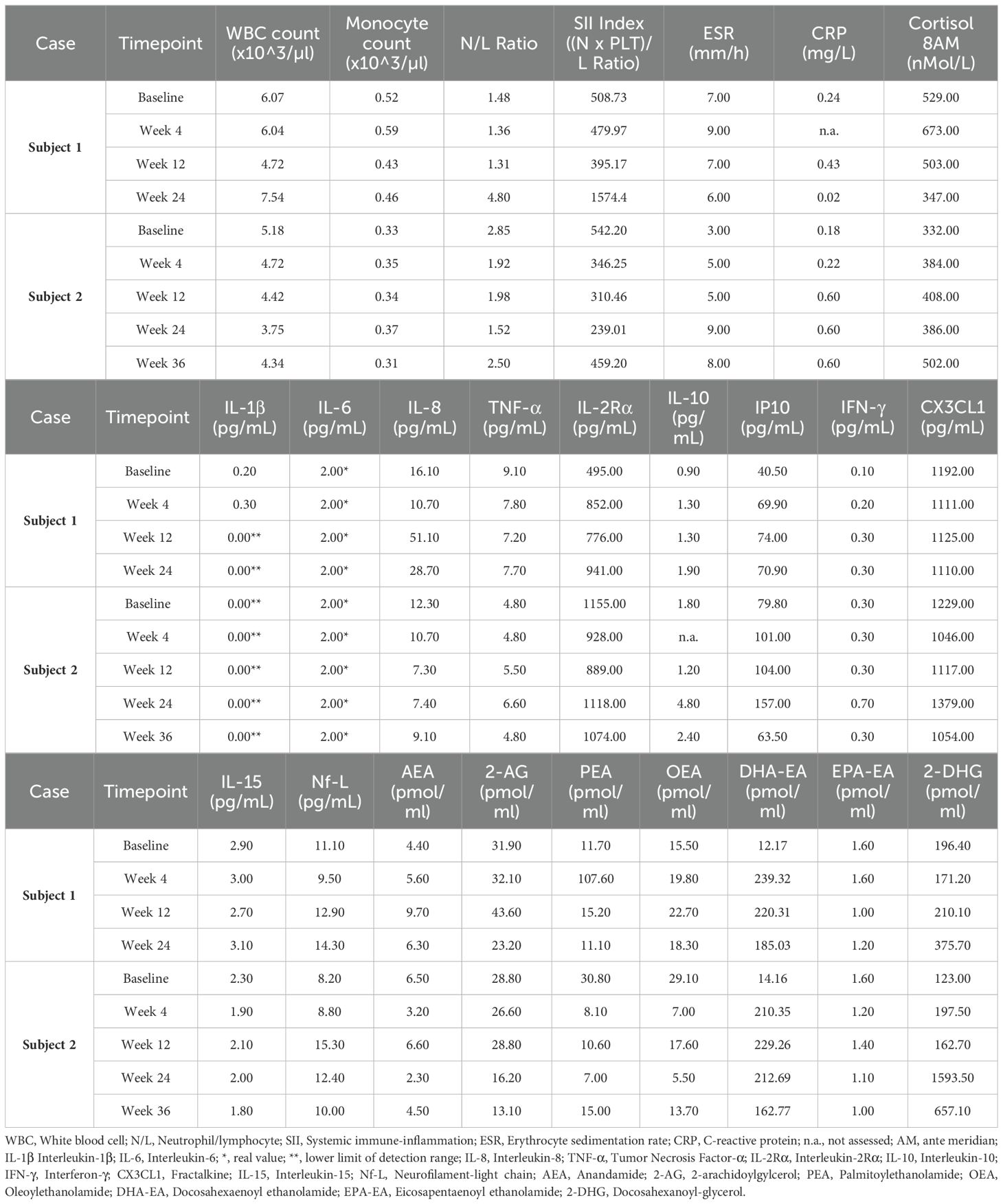
Table 3. Main hematological, biochemical, inflammatory, neuroaxonal impairment, and endocannabinoidome (eCBome) markers.
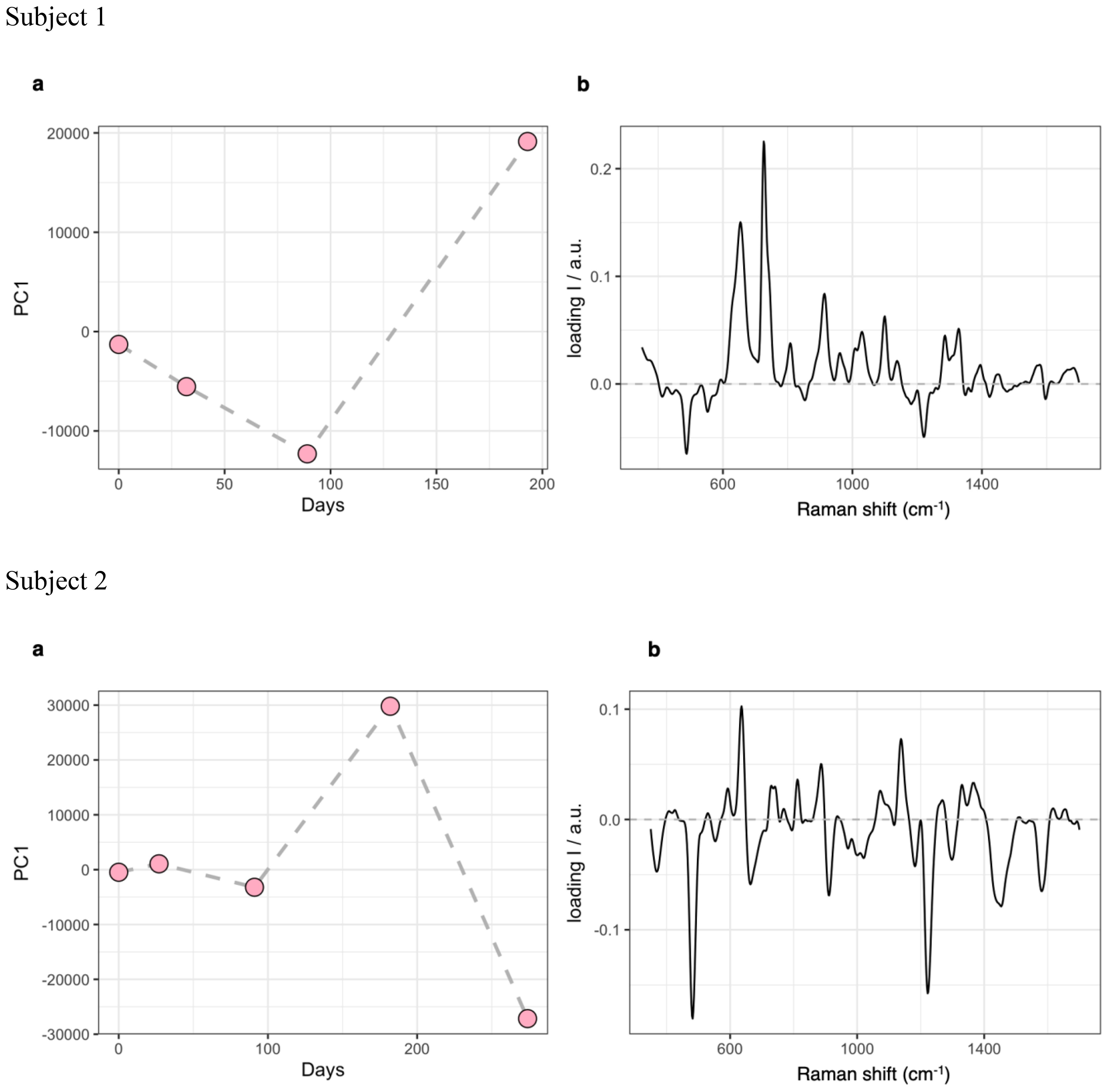
Figure 1. PCA results for the analysis SERS dataset. (A), evolution of the PC1 score profile over time. (B), spectral loadings for PC1. PCA, Principal Components Analysis; SERS, Surface-Enhanced Raman Scattering; PC1, Principal Component 1.
A synthesis of the two cases is presented below and summarized in Tables 1–3, and Figure 1.
2.1 Subject 1
The patient is a 35-year-old woman, who was diagnosed in adulthood with ASD. She lives with her parents. Allegedly, her family history is negative for any neuropsychiatric conditions. Her developmental milestones were reached on time. She experienced slight difficulties in math during schoolyears, although no failures were reported. She finally obtained a degree in Cultural Heritage Conservation and currently works in a library. Premorbid social adjustment was described as subnormal, with tendency to rigidity, avoidance of social contacts, and difficulties to develop age-appropriate relationships. Signs of tactile, gustative, and auditory hypersensitivity were referred.
A clinical diagnosis of level 1 ASD was based on the DSM-5 criteria (1, 49) and supported by the RAADS-R (43) and the CAT-Q (46). The EQ test (45) displayed good empathic abilities. The AQ test (44) displayed subthreshold results. Her intelligence quotient (IQ) was within normal limits, as measured using the WAIS-IV (47). Further evaluation with the WHODAS 2.0 showed a level of daily functioning with mild impairment. Although by the time of her presentation to the Unit of Psychiatry outpatient service she exhibited long-standing depressive symptoms, irritability, low concentration, restrictive eating behaviors, and sleep disturbances, their severity was not sufficient to diagnose comorbid major psychiatric or personality disorders, as measured with the SCID-5-CV (49) and SCID-5-PD (50), respectively (Table 1). She was not on any psychotropic medications. In the past, the patient was prescribed sertraline 50 mg/day, perphenazine 2 mg/day, and gabapentin 900 mg/day to treat the aforementioned symptoms of psychic distress. However, these medications provided little benefit and resulted in the occurrence of unpleasant side effects, so that the patient was reluctant to initiate further conventional psychopharmacological treatments.
She then agreed to start supplementation monotherapy with um-PEA 600 mg/day for 12 weeks, resulting in improvement on the Global Severity Index (GSI) and on the somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, phobia, paranoia, psychoticism, and sleep subscales of the SCL-90-R (52) (Table 2A). Despite discontinuing um-PEA at 4 months, most beneficial effects persisted and were accompanied by improvements in the total anxiety and depression scores of the HADS (53) (Table 2B) when the patient was reassessed at Week 24. Conversely, the depression subscale of the SCL-90-R (52) (Table 2A) at Week 24 partially regressed compared to Week 12, along with the interpersonal sensitivity, phobia, psychoticism, and sleep subscales. Most domains of the WHODAS 2.0 (48) improved at Week 24 compared to the treatment initiation, although the total score of the scale remained nearly unchanged, possibly due to a sharp decline in the Participation in society domain from Week 12 to Week 24 (Table 2C). No serious side effects were reported throughout the observation period (54) (Table 2D).
At Week 12, several changes in blood and serum immune response biomarkers were observed compared to baseline, including decreased total white blood cell (WBC) count, neutrophil/lymphocyte ratio, monocyte count, systemic immune-inflammation (SII) index, cortisol at 8 ante meridiem (AM), Interleukin-1β (IL-1β, normal range <0.16 pg/mL), Tumor Necrosis Factor-α (TNF-α, normal range 7.80-12.20 pg/mL), Interleukin-15 (IL-15, normal range 1.90-2.60 pg/mL), and fractalkine (CX3CL1, normal range 892.00-1502.00 pg/mL), with some of these markers reversing to higher levels by Week 24 after um-PEA discontinuation. Conversely, increasing patterns in Interleukin-8 (IL-8, normal range 6.70-16.20 pg/mL), Interleukin-2Rα (IL-2Rα, normal range 440.00-1435.00 pg/mL), Interleukin-10 (IL-10, normal range 1.80-3.80 pg/mL), Interferon-γ (IFN-γ, normal range <0.99 pg/mL), Interferon-inducible Protein 10 (IP10, normal range 37.20-222.00 pg/mL), and C-reactive protein (CRP) were detected at Week 12 compared to baseline, mostly maintaining stable levels at Week 24 after um-PEA discontinuation. Erythrocyte sedimentation rate (ESR) and Interleukin-6 (IL-6, normal range <7.00 pg/mL) levels remained stable and within normal range for the entire observation period. Neurofilament-light chain (Nf-L, normal range 9.00-22.00 pg/mL) showed a tendency toward increase, although maintaining within normal limits (Table 3).
The Principal Components Analysis (PCA) of samples used for SERS spectroscopy indicated that the main molecular fingerprint was related to spectral bans commonly linked to serum levels of purine degradation products (at 654 and 726 cm−1) (58). These bands showed a significant decrease from baseline to Week 12, partially reflecting changes related to reduced inflammation, followed by a sharp increase by Week 24 (Figure 1).
Markers of eCBome modulation included elevated levels of serum AEA, 2-AG, OEA, PEA, docosahexaenoyl ethanolamide (DHA-EA), and 2-docosahexanoyl-glycerol (2-DHG) by Week 12 compared to baseline, with most levels subsiding after the discontinuation of um-PEA. Besides, levels of eicosapentaenoyl ethanolamide (EPA-EA) decreased at Week 12 but increased again by Week 24 (Table 3).
2.2 Subject 2
The patient is a 40-year-old woman, who was diagnosed in adulthood with ASD and comorbid moderate intellectual disability. She lives with her parents. The patient’s family history is negative for any neuropsychiatric conditions. She was born preterm (after eight months of gestation) out of her parents’ second pregnancy. At the time of her presentation to the Unit, no anamnestic data were available regarding postnatal electroencephalography (EEG) recordings or brain magnetic resonance imaging (MRI). She presented delayed expressive language development with need for one-year speech therapy when she was 3 years old. Allegedly, all other developmental milestones were reached on time. The patient has presented serious impairment in relationships with peers since early childhood, showing a tendency to self-isolation and difficulties to integrate herself within social groups. Her premorbid role functioning was marked by poor performance in mainstream school courses, ongoing challenges in generating and organizing homemaking tasks, and inability to maintain an independent job after high school. The latter difficulty was mainly due to struggles with manual activities and relating to customers. According to her mother, the patient faced all novelties with deep anxiety, driven by her extreme rigidity, which also affected her limited interests. Following poor performance evaluations at her first work placements when she was nineteen years old, the patient displayed initial signs of psychic distress, including avoidant and restrictive eating behaviors, increased tension with everyday stressors, tearful episodes, emotional dullness, and occasionally slowed spontaneous movements. She received a tentative diagnosis of psychosis with prevalent negative symptoms and aberrant eating behaviors. As a result, when she was 25 years old, she started working as an employee in a supported job environment. The patient presented several physical comorbidities, including Hashimoto’s thyroiditis (treated with levothyroxine), recurrent episodes of anemia, bone density loss, and amenorrhea secondary to malnutrition. A revised diagnosis of level 2 ASD was determined based on the patient’s medical and neurodevelopmental history, the SCID-5-CV (1, 49), and the RAADS-R (43). Despite a borderline full scale-intelligence quotient (FS-IQ) of 72 as measured with the WAIS-IV (47), comorbid moderate intellectual disability was diagnosed due to prominent impairments in adaptive functioning based on the WHODAS 2.0 (48). Further neuropsychological assessment conducted as part of the routine diagnostic process corroborated the diagnosis, showing an Overall Cognitive Index below the norm, as well as deficits in immediate and delayed prose memory, interference memory, attention shifting, phonemic fluency, verbal comprehension, abstraction abilities, cognitive estimations, visual recognition and discrimination, and praxis (Table 1) (51). By the time of her presentation to the Unit of Psychiatry outpatient service, she was not on any psychotropic medications. She exhibited mild obsessive-compulsive symptoms, irritability, anxiety, and depressive thoughts, although not sufficient to diagnose a comorbid major mental health disturbance. Past episodes of acute psychic distress were treated with short-term psychotropic medications, including amisulpride, aripiprazole, cariprazine, and quetiapine, but these attempts never achieved complete symptom remission and often led to the occurrence of adverse effects.
The patient was then prescribed um-PEA 600 mg/day. Hematology and biochemistry tests (i.e., full blood count, electrolytes, renal profile, hepatic profile, bone profile, lipids, thyroid profile), alongside physical examination, were performed and resulted in norm both before initiating the treatment and throughout the whole observation. Substantial clinical stability emerged through the GSI and most subscales of the SCL-90-R (52), as well as in the total anxiety and depression scores of the HADS (53) during the initial 12 weeks of treatment. However, the hostility and sleep subscales of the SCL-90-R (52) slightly worsened (Tables 2A, B).
At Week 12, better cognitive readiness and work performance were reported according to the patient’s employer, although the total score on the WHODAS 2.0 (48) indicated a global worsening of adaptive functioning, with the Getting along with people domain declining the most (Table 2C). She maintained um-PEA for 4 months, with no reported side effects (Table 2D). Subsequently, the clinical picture deteriorated due to the occurrence of racing intrusive thoughts and worries, accelerated speech, increased irritability, and disturbed sleeping pattern. This led the patient to seek emergency care, resulting in the initiation of olanzapine at a dose of up to 7.5 mg/day. Concurrently um-PEA supplementation was discontinued.
Despite resulting in good control over observed symptoms, perceived psychic distress got worse by Week 36, as measured using the GSI of the SCL-90-R (52). Consistently, the somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, phobia, and psychoticism subscales of the SCL-90-R (52) worsened, as well as anxiety and depression scores of the HADS (53) (Tables 2A, B). Adaptive functioning globally improved from Week 12 to Week 24 according to the WHODAS 2.0 (48) (Table 2C). Peripheral immune response biomarkers fluctuated throughout the observation period, with decreases in total WBC count, neutrophil/lymphocyte ratio, SII index, IL-8, IL-2Rα, and IL-10 at either Week 12 or Week 24 compared to baseline, most of which rebounded to higher levels by Week 36. Levels of TNF-α, IP10, IFN-γ, ESR, CRP, and monocyte count increased either progressively or abruptly until Week 24 compared to baseline, before generally decreasing by Week 36. IL-1β and IL-6 were stable within normal limits throughout the observation period. Nf-L exhibited a trend toward an increase but remained within normal limits (Table 3). The score profile of the first component at the PCA-SERS revealed an almost flat trend from baseline to Week 12, followed by a sharp rise by Week 24, and then a decline. The spectral loadings for the main principal component – accounting for nearly 92% of the total variance – suggest that the significant change observed after the third month was linked to a decrease in the antioxidant response (negative ergothioneine and glutathione bands at 482, 1224 cm−1 and 664, 912 cm−1, respectively) and an increase in inflammatory response (uric acid bands at 636, 812, 886, and 1135 cm−1) (58, 59). This trend was reversed by Week 36 (Figure 1). Markers of eCBome modulation included a decrease in serum levels of AEA, 2-AG, OEA, PEA, and EPA-EA from baseline to Week 36. Notably, PEA serum levels sharply dropped during um-PEA treatment and remained persistently lower at Weeks 24 and 36 compared to baseline. Conversely, DHA-EA and 2-DHG serum levels increased from baseline to Week 36 (Table 3).
3 Discussion
Both autistic patients presented above underwent um-PEA 600 mg/day monotherapy to treat symptoms of psychic distress for the intended 12-week period, which was extended by an additional month, without experiencing any adverse effects necessitating treatment discontinuation. Overall, um-PEA supplementation was associated with either improvement or at least stability of symptoms for the initial 12 weeks of treatment. Interestingly, the first subject experienced partially relapsed symptoms of depression and interpersonal sensitivity after treatment discontinuation, which may have also impacted the patient’s participation in social activities. The second subject exhibited a sudden decline in her clinical condition after four months of treatment, resulting in the discontinuation of um-PEA and the initiation of antipsychotic medication. Changes in both clinical presentations were accompanied by fluctuations in peripheral immune and eCBome biomarkers throughout the entire observation period. Some considerations from the illustrated reports deserve to be highlighted.
Um-PEA 600 mg/day monotherapy in a level 1 autistic subject appeared to alleviate symptoms of psychic distress and their impact on daily living, with partial regression observed after treatment discontinuation. Notably, some improvements persisted a few months after um-PEA suspension, underscoring the need for further studies to fully understand the symptom-specific properties of um-PEA monotherapy across several psychopathological constructs and adaptive functioning domains in level 1 autistic adults. Contrastingly, um-PEA effects were perceived as more nuanced in a level 2 autistic subject with comorbid intellectual disability, being limited to anecdotal improvements in cognitive performance. Additionally, um-PEA was apparently insufficient to prevent the worsening of psychic distress symptoms in the latter subject, such as thought perseverance, increased irritability, and sleep/wake rhythm disturbances. These issues necessitated psychopharmacological intervention, suggesting caution when considering um-PEA monotherapy for level 2/level 3 autistic individuals. This finding seems noteworthy, because prior research has shown that PEA add-on therapy in risperidone-treated autistic children with moderate to high levels of irritability has led to improved behavior, albeit over shorter durations and with higher dosages of the dietary supplement (40). As add-on therapy for autistic adults with substantial or very substantial support needs, um-PEA could serve for enhancing the effectiveness of conventional psychotropic medications, potentially reducing the necessary dosage of treatments that often come with adverse effects (e.g., second-generation antipsychotics, mood stabilizers) (60, 61). Besides, um-PEA add-on may help treat either somatic and autonomic symptoms often associated with ASD (e.g., dental/periodontal issues, bowel disturbances) (62–66), or commonly comorbid immune disorders (e.g., atopic dermatitis) (67, 68).
Individual fluctuations in the peripheral levels of immune biomarkers and PCA-SERS analysis would indicate diverse inflammatory tendencies between the two patients in parallel to um-PEA intake, with the first subject showing a trend toward reduced inflammation, while the second subject maintained a basically stable response. Intriguingly, peripheral levels of proinflammatory cytokines most implicated in ASD pathophysiology (i.e., IL-1β, TNF-α, and IL-6) showed a decreasing or at least unvarying trend in the level 1 autistic patient, while they increased or remained stable in the level 2 autistic patient throughout the treatment (15–18). Nevertheless, only few cytokines exhibited significant peripheral tone variations following um-PEA supplementation, particularly indicating diametral effects on T-cell homeostasis (i.e., IL-2Rα) and anti-inflammatory response (i.e., IL-10), as well as overlapping effects on interferon activation (i.e., IP10). Further studies will be needed to better explain whether and how um-PEA exerts its immunomodulatory functions in autism with different severity degrees.
No remarkable changes in Nf-L levels were detected in either patient, aligning with early sparse evidence on the role of this specific neuroaxonal injury biomarker in ASD (69, 70), possibly due to its high inter-individual variability and still unclear release pattern across neuropsychiatric conditions (71).
Levels of eCBs and NAEs largely varied over the observation period, potentially following um-PEA intake, though yielding contrasting findings between the two individuals. Consistent with um-PEA mechanism of action (31, 32), the first subject exhibited increased eCBs and NAEs levels during its supplementation. Particularly, AEA signaling paralleled to changes in depressive symptoms and social engagement during the entire observation period, coherent with previous clinical and preclinical evidence accounting for increased AEA levels as a plausible mechanism underlying um-PEA effects on these manifestations (36, 72–74). On the other hand, the second subject exhibited mostly stable eCBs and NAEs levels during treatment, except for a keen decrease in initially elevated PEA tone from baseline to Week 12, only partially halting after um-PEA discontinuation.
This reducing pattern observed during um-PEA supplementation could have been caused by either a negative feedback mechanism, or by gut malabsorption related to her malnutrition state, or other individual factors, such as genetics, long-term effects of preterm birth, or autism severity degree, and could underpin the patient’s unchanged or even worsened clinical condition. Given the second patient’s medical history, it is possible to speculate that at the time of her presentation she was exhibiting early symptoms of psychosis recrudescence, which have previously been linked to elevated PEA serum levels (37) and may have precipitated despite um-PEA treatment.
Although the use of robust psychometric tools (48, 52–54) and biological assessments (55–58) would indicate diametrical effects of um-PEA in autistic adults with different severity levels, the exploratory nature and inherent biases of case reports, including self-assessments with limited insight and physiological variability in inflammatory biomarkers or eCBs/NAEs levels independent of um-PEA intake, and the scarcity of existing literature on the subject (40–42), should not be overlooked. Therefore, it is essential to interpret these findings with caution.
Future research involving patient cohorts and in the context of more rigorous clinical trials should systematically investigate the biological underpinnings of um-PEA monotherapy for the treatment of psychic distress among autistic adults. This would help clarify potential correlations between symptom improvement and biomarker fluctuations, thereby parsing um-PEA potential as a putative disease-modifying drug (75). Finally, whether the biobehavioral effects of um-PEA, either as monotherapy or add-on therapy with a safe and tolerable profile, are to be considered age- and level-specific in autism will require further robust investigations involving patients across the entire spectrum.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board, Department of Medicine (DMED), University of Udine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RB: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing, Resources, Visualization. FP: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing, Resources, Visualization. MBas: Data curation, Investigation, Methodology, Writing – review & editing, Resources, Validation, Visualization. CS: Data curation, Investigation, Methodology, Writing – review & editing, Resources, Validation, Visualization. CC: Data curation, Investigation, Methodology, Writing – review & editing, Resources, Validation, Visualization. RF: Data curation, Investigation, Methodology, Writing – review & editing, Resources, Validation, Visualization. SF: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing. PB: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing. DP: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing. OS: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing. MF: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing. FC: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing. MBal: Data curation, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. MC: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge Epitech Group for supporting their research activity through the generous supply of the study medication.
Conflict of interest
MC has been a consultant and advisor to GW Pharma Limited, F. Hoffmann-La Roche Limited, and GW Pharma Italy SRL outside of this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
FP and MC declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1463849/full#supplementary-material
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: American Psychiatric Association (APA) (2013) p. 50–9.
2. Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. (2004) 113:e472–865. doi: 10.1542/peds.113.5.e472
3. Grabrucker AM. Environmental factors in autism. Front Psychiatry. (2013) 3:118. doi: 10.3389/fpsyt.2012.00118
4. Schneider M, VanOrmer J, Zlomke K. Adverse childhood experiences and family resilience among children with autism spectrum disorder and attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. (2019) 40:573–805. doi: 10.1097/DBP.0000000000000703
5. Berg KL, Shiu CS, Feinstein RT, Msall ME, Acharya K. Adverse childhood experiences are associated with unmet healthcare needs among children with autism spectrum disorder. J Pediatr. (2018) 202:258–64.e1. doi: 10.1016/j.jpeds.2018.07.021
6. Bortoletto R, Bassani L, Garzitto M, Lamberti M, Simonati A, Darra F, et al. Risk of psychosis in autism spectrum disorder individuals exposed to psychosocial stressors: A 9-year chart review study. Autism Res. (2023) 16:2139–495. doi: 10.1002/aur.3042
7. Howlin P, Magiati I. Autism spectrum disorder: Outcomes in adulthood. Curr Opin Psychiatry. (2017) 30:69–765. doi: 10.1097/YCO.0000000000000308
8. Hollocks MJ, Leno VC, Chandler S, White P, Yorke I, Charman T, et al. Psychiatric conditions in autistic adolescents: Longitudinal stability from childhood and associated risk factors. Eur Child Adolesc Psychiatry. (2022) 32(11):2197–208. doi: 10.1007/s00787-022-02065-9
9. Thiel T, Riedelbauch S, Gaigg S, Roessner V, Ring M. The impact of depressive and anxious symptoms on quality of life in adults on the autism spectrum. Autism Res. (2024) 17(6):1161–74. doi: 10.1002/aur.3144
10. Lugo-Marin J, Magan-Maganto M, Rivero-Santana A, Cuellar-Pompa L, Alviani M, Jenaro-Rio C, et al. Prevalence of psychiatric disorders in adults with autism spectrum disorder: A systematic review and meta-analysis. Res Autism Spectr Disord. (2019) 59:22–335. doi: 10.1016/j.rasd.2018.12.004
11. Lever AG, Geurts HM. Psychiatric co-occurring symptoms and disorders in young, middle-aged, and older adults with autism spectrum disorder. J Autism Dev Disord. (2016) 46:1916–305. doi: 10.1007/s10803-016-2722-8
12. Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (ssris) for autism spectrum disorders (asd). Cochrane Database Syst Rev. (2013), CD004677. doi: 10.1002/14651858.CD004677.pub3
13. Hurwitz R, Blackmore R, Hazell P, Williams K, Woolfenden S. Tricyclic antidepressants for autism spectrum disorders (asd) in children and adolescents. Cochrane Database Syst Rev. (2012), CD008372. doi: 10.1002/14651858.CD008372.pub2
14. Rutter M. Aetiology of autism: Findings and questions*. J Intellectual Disability Res. (2005) 49:231–385. doi: 10.1111/j.1365-2788.2005.00676.x
15. Arteaga-Henriquez G, Lugo-Marin J, Gisbert L, Setien-Ramos I, Martinez-Gallo M, Pujol-Borrell R, et al. Activation of the monocyte/macrophage system and abnormal blood levels of lymphocyte subpopulations in individuals with autism spectrum disorder: A systematic review and meta-analysis. Int J Mol Sci. (2022) 23:105.3390/ijms232214329. doi: 10.3390/ijms232214329
16. Ferencova N, Visnovcova Z, Ondrejka I, Hrtanek I, Bujnakova I, Kovacova V, et al. Peripheral inflammatory markers in autism spectrum disorder and attention deficit/hyperactivity disorder at adolescent age. Int J Mol Sci. (2023) 24:105.3390/ijms241411710. doi: 10.3390/ijms241411710
17. Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol Psychiatry. (2015) 20:440–65. doi: 10.1038/mp.2014.59
18. Saghazadeh A, Ataeinia B, Keynejad K, Abdolalizadeh A, Hirbod-Mobarakeh A, Rezaei N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: Effects of age, gender, and latitude. J Psychiatr Res. (2019) 115:90–1025. doi: 10.1016/j.jpsychires.2019.05.019
19. Cristiano C, Lama A, Lembo F, Mollica MP, Calignano A, Mattace Raso G. Interplay between peripheral and central inflammation in autism spectrum disorders: Possible nutritional and therapeutic strategies. Front Physiol. (2018) 9:184. doi: 10.3389/fphys.2018.00184
20. Matta SM, Hill-Yardin EL, Crack PJ. The influence of neuroinflammation in autism spectrum disorder. Brain Behavior Immun. (2019) 79:75–905. doi: 10.1016/j.bbi.2019.04.037
21. El-Ansary A, Al-Ayadhi L. Gabaergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J Neuroinflamm. (2014) 11:1895. doi: 10.1186/s12974-014-0189-0
22. Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, et al. Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. (2006) 30:1472–75. doi: 10.1016/j.pnpbp.2006.06.013
23. Blaylock RL, Strunecka A. Immune-glutamatergic dysfunction as a central mechanism of the autism spectrum disorders. Curr Med Chem. (2009) 16:157–705. doi: 10.2174/092986709787002745
24. Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S. Effect of cannabis on glutamate signalling in the brain: A systematic review of human and animal evidence. Neurosci Biobehav Rev. (2016) 64:359–815. doi: 10.1016/j.neubiorev.2016.03.010
25. Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. (2004) 141:775–855. doi: 10.1038/sj.bjp.0705667
26. Colizzi M, Ruggeri M, Bhattacharyya S. Unraveling the intoxicating and therapeutic effects of cannabis ingredients on psychosis and cognition. Front Psychol. (2020) 11:833. doi: 10.3389/fpsyg.2020.00833
27. Aran A, Harel M, Cassuto H, Polyansky L, Schnapp A, Wattad N, et al. Cannabinoid treatment for autism: A proof-of-concept randomized trial. Mol Autism. (2021) 12:65. doi: 10.1186/s13229-021-00420-2
28. Raz N, Heller I, Lombardi T, Marino G, Davidson EM, Eyal AM. Terpene-enriched cbd oil for treating autism-derived symptoms unresponsive to pure cbd: Case report. Front Pharmacol. (2022) 13:979403. doi: 10.3389/fphar.2022.979403
29. Shrader SH, Mellen N, Cai J, Barnes GN, Song ZH. Cannabidiol is a behavioral modulator in btbr mouse model of idiopathic autism. Front Neurosci. (2024) 18:1359810. doi: 10.3389/fnins.2024.1359810
30. Arturo IF, Fabiana P. Endocannabinoidome. Els. (2018), 1–10. doi: 10.1002/9780470015902.a0028301
31. Petrosino S, Schiano Moriello A, Cerrato S, Fusco M, Puigdemont A, De Petrocellis L, et al. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at trpv1 cation channels. Br J Pharmacol. (2016) 173:1154–625. doi: 10.1111/bph.13084
32. Petrosino S, Schiano Moriello A. Palmitoylethanolamide: A nutritional approach to keep neuroinflammation within physiological boundaries-a systematic review. Int J Mol Sci. (2020) 21:105.3390/ijms21249526. doi: 10.3390/ijms21249526
33. Bortoletto R, Balestrieri M, Bhattacharyya S, Colizzi M. Is it time to test the antiseizure potential of palmitoylethanolamide in human studies? A systematic review of preclinical evidence. Brain Sci. (2022) 12:101. doi: 10.3390/brainsci12010101
34. Orefice NS, Alhouayek M, Carotenuto A, Montella S, Barbato F, Comelli A, et al. Oral palmitoylethanolamide treatment is associated with reduced cutaneous adverse effects of interferon-beta1a and circulating proinflammatory cytokines in relapsing-remitting multiple sclerosis. Neurotherapeutics. (2016) 13:428–385. doi: 10.1007/s13311-016-0420-z
35. Colizzi M, Bortoletto R, Colli C, Bonomo E, Pagliaro D, Maso E, et al. Therapeutic effect of palmitoylethanolamide in cognitive decline: A systematic review and preliminary meta-analysis of preclinical and clinical evidence. Front Psychiatry. (2022) 13:1038122. doi: 10.3389/fpsyt.2022.1038122
36. Ghazizadeh-Hashemi M, Ghajar A, Shalbafan MR, Ghazizadeh-Hashemi F, Afarideh M, Malekpour F, et al. Palmitoylethanolamide as adjunctive therapy in major depressive disorder: A double-blind, randomized and placebo-controlled trial. J Affect Disord. (2018) 232:127–335. doi: 10.1016/j.jad.2018.02.057
37. Bortoletto R, Piscitelli F, Candolo A, Bhattacharyya S, Balestrieri M, Colizzi M. Questioning the role of palmitoylethanolamide in psychosis: A systematic review of clinical and preclinical evidence. Front Psychiatry. (2023) 14:1231710. doi: 10.3389/fpsyt.2023.1231710
38. Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol. (2017) 174:1349–655. doi: 10.1111/bph.13580
39. Colizzi M, Bortoletto R, Costa R, Zoccante L. Palmitoylethanolamide and its biobehavioral correlates in autism spectrum disorder: A systematic review of human and animal evidence. Nutrients. (2021) 13:1346. doi: 10.3390/nu13041346
40. Khalaj M, Saghazadeh A, Shirazi E, Shalbafan MR, Alavi K, Shooshtari MH, et al. Palmitoylethanolamide as adjunctive therapy for autism: Efficacy and safety results from a randomized controlled trial. J Psychiatr Res. (2018) 103:104–15. doi: 10.1016/j.jpsychires.2018.04.022
41. Antonucci N, Cirillo A, Siniscalco D. Beneficial effects of palmitoylethanolamide on expressive language, cognition, and behaviors in autism: A report of two cases. Case Rep Psychiatry. (2015) 2015):325061. doi: 10.1155/2015/325061
42. Bertolino B, Crupi R, Impellizzeri D, Bruschetta G, Cordaro M, Siracusa R, et al. Beneficial effects of co-ultramicronized palmitoylethanolamide/luteolin in a mouse model of autism and in a case report of autism. CNS Neurosci Ther. (2017) 23:87–985. doi: 10.1111/cns.12648
43. Sturm A, Huang S, Bal V, Schwartzman B. Psychometric exploration of the raads-r with autistic adults: Implications for research and clinical practice. Autism. (2024) 28:13623613241228329. doi: 10.1177/13623613241228329
44. Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (aq): Evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. (2001) 31:5–175. doi: 10.1023/a:1005653411471
45. Baron-Cohen S, Wheelwright S. The empathy quotient: An investigation of adults with asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. (2004) 34:163–755. doi: 10.1023/b:jadd.0000022607.19833.00
46. Hull L, Mandy W, Lai MC, Baron-Cohen S, Allison C, Smith P, et al. Development and validation of the camouflaging autistic traits questionnaire (cat-q). J Autism Dev Disord. (2019) 49:819–335. doi: 10.1007/s10803-018-3792-6
47. Benson N, Hulac DM, Kranzler JH. Independent examination of the wechsler adult intelligence scale—fourth edition (wais-iv): What does the wais-iv measure? psychol Assess. (2010) 22:1215. doi: 10.1037/a0017767
48. Yen CF, Hwang AW, Liou TH, Chiu TY, Hsu HY, Chi WC, et al. Validity and reliability of the functioning disability evaluation scale-adult version based on the whodas 2.0–36 items. J Formos Med Assoc. (2014) 113:839–495. doi: 10.1016/j.jfma.2014.08.008
49. Shabani A, Masoumian S, Zamirinejad S, Hejri M, Pirmorad T, Yaghmaeezadeh H. Psychometric properties of structured clinical interview for dsm-5 disorders-clinician version (scid-5-cv). Brain Behav. (2021) 11:e018945. doi: 10.1002/brb3.1894
50. First MB. Structured clinical interview for the dsm (scid). In: The encyclopedia of clinical psychology (2014). p. 1–6. Arlington, VA: American Psychiatric Association (APA).
51. Mondini S, Mapelli D, Vestri A, Arcara G, Bisiacchi PS. Esame neuropsicologico breve 2. R. C. Editore (2011). Available online at: https://multimedia.raffaellocortina.it/strumenti/cortina/multimedia/testenb/pdfjs/web/pdf/testenb_protocollo.pdf (Accessed September 25, 2024).
52. Derogatis LR, Cleary PA. Factorial invariance across gender for the primary symptom dimensions of the scl-90. Br J Soc Clin Psychol. (1977) 16:347–565. doi: 10.1111/j.2044-8260.1977.tb00241.x
53. Snaith RP, Zigmond AS. The hospital anxiety and depression scale. Br Med J (Clin Res Ed). (1986) 292:3445. doi: 10.1136/bmj.292.6516.344
54. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The uku side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. (1987) 334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x
55. Lu Y, Lin L, Ye J. Human metabolite detection by surface-enhanced raman spectroscopy. Mater Today Bio. (2022) 13:1002055. doi: 10.1016/j.mtbio.2022.100205
56. Ferrara AL, Piscitelli F, Petraroli A, Parente R, Galdiero MR, Varricchi G, et al. Altered metabolism of phospholipases, diacylglycerols, endocannabinoids, and n-acylethanolamines in patients with mastocytosis. J Immunol Res. (2019) 2019):5836476. doi: 10.1155/2019/5836476
57. Maia J, Iannotti FA, Piscitelli F, Fonseca BM, Braga A, Braga J, et al. The endocannabinoidome in human placenta: Possible contribution to the pathogenesis of preeclampsia. Biofactors. (2023) 49:887–995. doi: 10.1002/biof.1952
58. Premasiri WR, Lee JC, Ziegler LD. Surface-enhanced raman scattering of whole human blood, blood plasma, and red blood cells: Cellular processes and bioanalytical sensing. J Phys Chem B. (2012) 116:9376–865. doi: 10.1021/jp304932g
59. Fornasaro S, Sergo V, Bonifacio A. The key role of ergothioneine in label-free surface-enhanced raman scattering spectra of biofluids: A retrospective re-assessment of the literature. FEBS Lett. (2022) 596:1348–555. doi: 10.1002/1873-3468.14312
60. Marquis S, Marquis NE, Lunsky Y, McGrail KM, Baumbusch J. Prescriptions for antipsychotics: Youth with intellectual/developmental disabilities compared to youth without intellectual/developmental disabilities. J Autism Dev Disord. (2024). doi: 10.1007/s10803-024-06344-z
61. Odalovic M, Gorman A, Paul A, McCallion P, Burke E, MacLachlan M, et al. Psychotropic medicines’ prevalence, patterns and effects on cognitive and physical function in older adults with intellectual disability in Ireland: Longitudinal cohort study, 2009-2020. BJPsych Open. (2024) 10:e395. doi: 10.1192/bjo.2023.607
62. Di Nardo G, Bernardo L, Cremon C, Barbara G, Felici E, Evangelisti M, et al. Palmitoylethanolamide and polydatin in pediatric irritable bowel syndrome: A multicentric randomized controlled trial. Nutrition. (2024) 122:1123975. doi: 10.1016/j.nut.2024.112397
63. Cremon C, Stanghellini V, Barbaro MR, Cogliandro RF, Bellacosa L, Santos J, et al. Randomised clinical trial: The analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment Pharmacol Ther. (2017) 45:909–225. doi: 10.1111/apt.13958
64. Jaber MA. Dental caries experience, oral health status and treatment needs of dental patients with autism. J Appl Oral Sci. (2011) 19:212–75. doi: 10.1590/s1678-77572011000300006
65. Zhu J, Meng H, Zhang L, Li Y. Exploring the molecular mechanism of comorbidity of autism spectrum disorder and inflammatory bowel disease by combining multiple data sets. J Transl Med. (2023) 21:3725. doi: 10.1186/s12967-023-04218-z
66. Isola G, Polizzi A, Iorio-Siciliano V, Alibrandi A, Ramaglia L, Leonardi R. Effectiveness of a nutraceutical agent in the non-surgical periodontal therapy: A randomized, controlled clinical trial. Clin Oral Investig. (2021) 25:1035–455. doi: 10.1007/s00784-020-03397-z
67. Bakkaloglu B, Anlar B, Anlar FY, Oktem F, Pehlivanturk B, Unal F, et al. Atopic features in early childhood autism. Eur J Paediatr Neurol. (2008) 12:476–95. doi: 10.1016/j.ejpn.2007.12.008
68. Rao A, Moussa AA, Erickson J, Briskey D. Efficacy of topical palmitoylethanolamide (levagen+) for the management of eczema symptoms: A double-blind, comparator-controlled, randomized clinical trial. Skin Pharmacol Physiol. (2023) 36:288–955. doi: 10.1159/000536670
69. Paketci C, Ermis C, Sisman AR, Hiz S, Baykara B, Yis U. Blood neurofilament light chain and thrombospondin-1 levels of patients with autism spectrum disorder. Turk J Med Sci. (2022) 52:1041–495. doi: 10.55730/1300-0144.5406
70. Simone M, De Giacomo A, Palumbi R, Palazzo C, Lucisano G, Pompamea F, et al. Serum neurofilament light chain and glial fibrillary acidic protein as potential diagnostic biomarkers in autism spectrum disorders: A preliminary study. Int J Mol Sci. (2023) 24:105.3390/ijms24033057. doi: 10.3390/ijms24033057
71. Bavato F, Barro C, Schnider LK, Simren J, Zetterberg H, Seifritz E, et al. Introducing neurofilament light chain measure in psychiatry: Current evidence, opportunities, and pitfalls. Mol Psychiatry. (2024) 29(8):2543–59. doi: 10.1038/s41380-024-02524-6
72. Patel S, Hillard CJ. Role of endocannabinoid signaling in anxiety and depression. Curr Top Behav Neurosci. (2009) 1:347–715. doi: 10.1007/978-3-540-88955-7_14
73. Doenni VM, Gray JM, Song CM, Patel S, Hill MN, Pittman QJ. Deficient adolescent social behavior following early-life inflammation is ameliorated by augmentation of anandamide signaling. Brain Behav Immun. (2016) 58:237–475. doi: 10.1016/j.bbi.2016.07.152
74. Minichino A, Jackson MA, Francesconi M, Steves CJ, Menni C, Burnet PWJ, et al. Endocannabinoid system mediates the association between gut-microbial diversity and anhedonia/amotivation in a general population cohort. Mol Psychiatry. (2021) 26:6269–765. doi: 10.1038/s41380-021-01147-5
75. Bortoletto R, Basaldella M, Candolo A, Garzitto M, Comacchio C, Curcio F, et al. The Supplementation Therapy in Autism and Response to Treatment (START) study: an open-label feasibility trial of ultramicronized palmitoylethanolamide potential to alleviate psychic distress among autistic adults. Clin Trans Neurosci. (2024) 8:20. doi: 10.3390/ctn8020020
Keywords: neurodevelopmental disorders, peroxisome proliferator activated receptor alpha, glutamate signaling, nutraceutical, supplementary food, cannabinoids, entourage effect
Citation: Bortoletto R, Piscitelli F, Basaldella M, Scipioni C, Comacchio C, Fiorino R, Fornasaro S, Barbieri P, Pagliaro D, Sepulcri O, Fabris M, Curcio F, Balestrieri M and Colizzi M (2024) Assessing the biobehavioral effects of ultramicronized-palmitoylethanolamide monotherapy in autistic adults with different severity levels: a report of two cases. Front. Psychiatry 15:1463849. doi: 10.3389/fpsyt.2024.1463849
Received: 12 July 2024; Accepted: 13 September 2024;
Published: 22 October 2024.
Edited by:
Antonio Narzisi, Stella Maris Foundation (IRCCS), ItalyCopyright © 2024 Bortoletto, Piscitelli, Basaldella, Scipioni, Comacchio, Fiorino, Fornasaro, Barbieri, Pagliaro, Sepulcri, Fabris, Curcio, Balestrieri and Colizzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riccardo Bortoletto, Ym9ydG9sZXR0by5yaWNjYXJkb0BzcGVzLnVuaXVkLml0
†These authors have contributed equally to this work
 Riccardo Bortoletto
Riccardo Bortoletto Fabiana Piscitelli
Fabiana Piscitelli Marta Basaldella1
Marta Basaldella1 Carla Comacchio
Carla Comacchio Stefano Fornasaro
Stefano Fornasaro Pierluigi Barbieri
Pierluigi Barbieri Martina Fabris
Martina Fabris Francesco Curcio
Francesco Curcio Matteo Balestrieri
Matteo Balestrieri Marco Colizzi
Marco Colizzi