- 1Overwaal Centre of Expertise for Anxiety Disorders, Obsessive Compulsive Disorder (OCD) and Posttraumatic Stress-Disorder (PTSD), Institution for Integrated Mental Health Care, Pro Persona, Nijmegen, Netherlands
- 2Behavioural Science Institute, Radboud University, Nijmegen, Netherlands
- 3Altrecht Eating Disorders Rintveld, Zeist, Netherlands
- 4Department of Clinical Psychology, Utrecht University, Utrecht, Netherlands
- 5Rivierduinen Eating Disorders Ursula, Leiden, Netherlands
- 6Bureau Bêta, Nijmegen, Netherlands
- 7Department of Psychiatry, Leiden University Medical Center, Leiden, Netherlands
- 8Radboud University Medical Centre, Department of Psychiatry, Radboud University, Nijmegen, Netherlands
- 9Department of Specialist Training, Institution for Integrated Mental Health Care (GGz) Drenthe, Assen, Netherlands
- 10Department of Psychiatry, University of Groningen & University Medical Center Groningen, Groningen, Netherlands
Objective: Obsessive compulsive disorder (OCD) and anorexia nervosa (AN) are conditions associated with poor cognitive flexibility, a factor considered to interfere with treatment, but research into the relationship between cognitive flexibility and treatment outcome is limited. This study explores whether baseline measures of cognitive flexibility predict outcomes in OCD and AN, evaluates whether changes in these measures contribute to treatment outcome, and evaluates the effectiveness of adjunctive cognitive remediation therapy (CRT) in improving cognitive flexibility.
Methods: This secondary analysis utilized linear mixed model analysis on data from a randomized controlled multicenter clinical trial involving adult participants with OCD (n=71) AND AN (n=61). Participants underwent 10 twice-weekly sessions of either CRT or a non-specific active control intervention (specialized attention therapy; SAT), followed by treatment as usual. Assessments using Yale-Brown Obsessive Compulsive Scale and the Eating Disorder Examination Questionnaire were conducted at baseline, post-CRT/SAT and at 6 and 12 months. Cognitive flexibility was evaluated through the Trail Making Test (TMT), the Color-Word Interference Test (CWIT) and the Detail and Flexibility Questionnaire (DFlex).
Results: Levels of cognitive flexibility at baseline did not predict or moderate treatment outcome, nor did change in cognitive flexibility (baseline post-CRT/SAT) mediate treatment outcome, with CRT providing no greater improvement in measures of cognitive flexibility than SAT.
Conclusions: This study failed to find any relationship between measures of cognitive flexibility and treatment outcome in OCD and AN, and thus questions hypothetical associations between measures of cognitive flexibility and mechanisms of change in patients with OCD and AN.
Introduction
Obsessive-compulsive disorder (OCD) and anorexia nervosa (AN) are severe mental disorders with extensive impact on psychological well-being. Some authors have suggested that OCD and AN belong to the same spectrum of disorders (1, 2), basing their postulations on five similarities. First, these disorders share clinical features, including obsessive worrying, compulsive and ritualistic behavior, and repetitive thinking (3). Second, the presence of OCD has been hypothesized to be a predisposing factor for the development of AN (4); third, OCD frequencies in AN are significantly elevated, ranging between 19% in cross-sectional studies and 44% in longitudinal studies (5). Further, AN is diagnosed in increased frequencies up to 10% in women diagnosed with OCD (6). Fourth, there is a high genetic correlation (rg=0.49 ± 0.13, p<0.01) between AN and OCD (7), and, finally, individuals with AN and OCD have been shown to share inefficiencies in executive functioning (8). There is growing evidence that cognitive inflexibility represents a core feature of the neuropsychological profiles of both OCD and AN (9–11) and entails a candidate neuropsychological endophenotype, i.e. a set of stable behavioral symptoms with a clear genetic connection (12–16). In a recent systematic review of publications on cognitive flexibility in acute AN, Miles et al. (17) found that adult participants with AN perform worse than healthy controls (HCs) on neuropsychological indices and self-report items of cognitive flexibility.
Executive functions refer to the collection of cognitive processes necessary for the cognitive control of goal-directed behavior (18) and include ‘core’ functions such as cognitive flexibility, inhibitory control, working memory as well as the higher-level functions of reasoning, problem-solving, and planning (19). In both OCD and AN, specifically cognitive flexibility has been found to be impaired (17, 20, 21), which function is typically defined as the ability to change perspectives or approaches to a problem and readily adjust to new demands, rules, or priorities (19). When changes take place in the environment, we need to be able to focus our attention on those elements that are changing and, after discovering that a previous approach does no longer apply to the changed environment, we are expected to be able to suppress our earlier response and develop a new strategy. We can thus integrate information and manipulate it in real time to flexibly switch from one response scenario to another (22).
An essential part of cognitive flexibility is set-shifting, the ability to move back and forth between tasks, operations, or mental sets in response to changing goals or environmental experiences (23), which has been suggested to be inhibited in both OCD (24) and AN (25). Snyder et al. (10) found their OCD group to perform worse than the HC group on the Trail Making Test (TMT), with the between-group differences showing medium effect sizes (d=0.54); the Wisconsin Card Sorting Task (WCST) and the Intra-Extra-dimensional Set-shift Task (ID/EDS) yielded both smaller, comparable effect sizes (d=0.44 and d=.50, respectively). A meta-analysis of OCD studies confirmed impaired set-shifting performance on the ID/EDS, with medium-to-large effect sizes, which impairment was also found to extend to the participants’ clinically asymptomatic first-degree relatives (26, 27). Another meta-analysis evaluating 11 studies with participants with the AN restricting subtype reported medium effect sizes (g=-0.51) for inefficient set-shifting (25).
Set-shifting deficits in OCD and AN have been found to be mediated by abnormal activation of fronto-striatal circuitry, areas that are important for executive functions (e.g. dorsolateral/ventrolateral prefrontal and striatal regions) (28–30). Findings revealed that patients with OCD made more errors and lacked activation in the dorsal fronto-striatal regions linked to cognitive flexibility, suggesting that diminished cognitive flexibility contributes to their task deficits. Interestingly, fronto-striatal dysfunction in OCD is amenable to treatment (31). Similarly, in AN, altered activation in fronto-striatal regions and limbic circuits are taken to mediate the development of the disorder (32, 33). In AN, neural mechanisms that define the therapeutic response to CBT are currently being studied but not yet elucidated. Similarly, individuals with AN struggle with flexible behavior adaptation, marked by reduced activity in fronto-striatal circuits associated with behavioral changes (29, 34).
Together, the findings described demonstrate that adults with OCD and those with AN score worse than HCs on neuropsychological as well as subjective measures of cognitive flexibility, including set-shifting. The literature on the association between reduced cognitive flexibility and symptom severity is contradictory. Some studies have found this association, e.g. (35, 36), while others have not, e.g. (37, 38). These mixed findings are likely related to the different methods used to measure cognitive flexibility and the various outcome measures employed (e.g., BMI vs. EDE-Q). Findings revealed that patients with OCD made more errors and lacked activation in the dorsal fronto-striatal regions linked to cognitive flexibility, suggesting that diminished cognitive flexibility contributes to their task deficits. Similarly, individuals with AN struggle with flexible behavior adaptation, marked by reduced activity in fronto-striatal circuits associated with behavioral changes (29, 34).
In addition to the above-mentioned neuropsychological measures, the detail and flexibility questionnaire (DFlex) (39) revealed significantly poorer subjective cognitive flexibility in adolescents and adults with AN when compared to HCs (39–41), while, again compared to HCs, female students with subclinical obsessive-compulsive symptoms likewise showed significantly more self-reported cognitive inflexibility (42). Finally, recently we reported on the baseline neuropsychological and subjective measures of cognitive flexibility using the same patient groups as reported here directly comparing participants with OCD and AN to HCs and found both patient groups to show similar results with inflexibility, where the higher rates of perceived inflexibility did not correlate with the neuropsychological cognitive flexibility scores (43).
Although the identified correlations between cognitive inflexibility and symptom severity do not imply a causal relationship, one hypothesis could be that interventions targeting the enhancement of cognitive flexibility may result in greater symptom reduction and a larger therapeutic effect in both populations.
Cognitive remediation therapy (CRT) is an easy-to-use intervention designed to do just that for people coping with AN (44). AN case series, uncontrolled studies and RCTs had yielded promising results (45–51). For OCD, CRT had not been previously investigated as a treatment enhancer until our study (52). However, there were two studies demonstrating that cognitive training was effective in improving cognitive flexibility, with one study also showing a positive effect on reducing symptom severity (53, 54). On two other studies (55, 56) involving patients with OCD, no significant differences were found between the effects of cognitive training and a non-cognitive training control condition on neuropsychological measures and a symptom-specific outcome measure. Moreover, recent randomized controlled trials (RCTs) and a preliminary systematic review and meta-analysis predominantly reported negative results or non-superiority compared to other control treatments when CRT was used as an enhancer to treatment as usual for eating disorders and OCD (57–62). This suggests that while poor cognitive flexibility may interfere with OCD and AN treatment, CRT has yielded less favorable outcomes than anticipated.
While CRT is assumed to improve cognitive flexibility and central coherence, this premise remains debated. Furthermore, RCTs of CRT with control groups are scarce, particularly those examining neuropsychological outcomes. To address this gap, we conducted an overview of the evidence on the effects of CRT or similar cognitive training interventions on neuropsychological measures in individuals with anorexia nervosa or OCD.
A search of PsycINFO and PubMed, using the terms ((OCD or Obsessive Compulsive Disorder) or (Anore*)) and (cognit* and (remed* or train*)) and (Neuropsychol* or measure), yielded 277 unique results. We excluded studies focused on children or adolescents, as well as those lacking a control condition for comparison. Relevant articles identified through citation tracking were added. This process produced a final selection of 15 studies comparing cognitive training with a control condition or waitlist, summarized in Table 1.
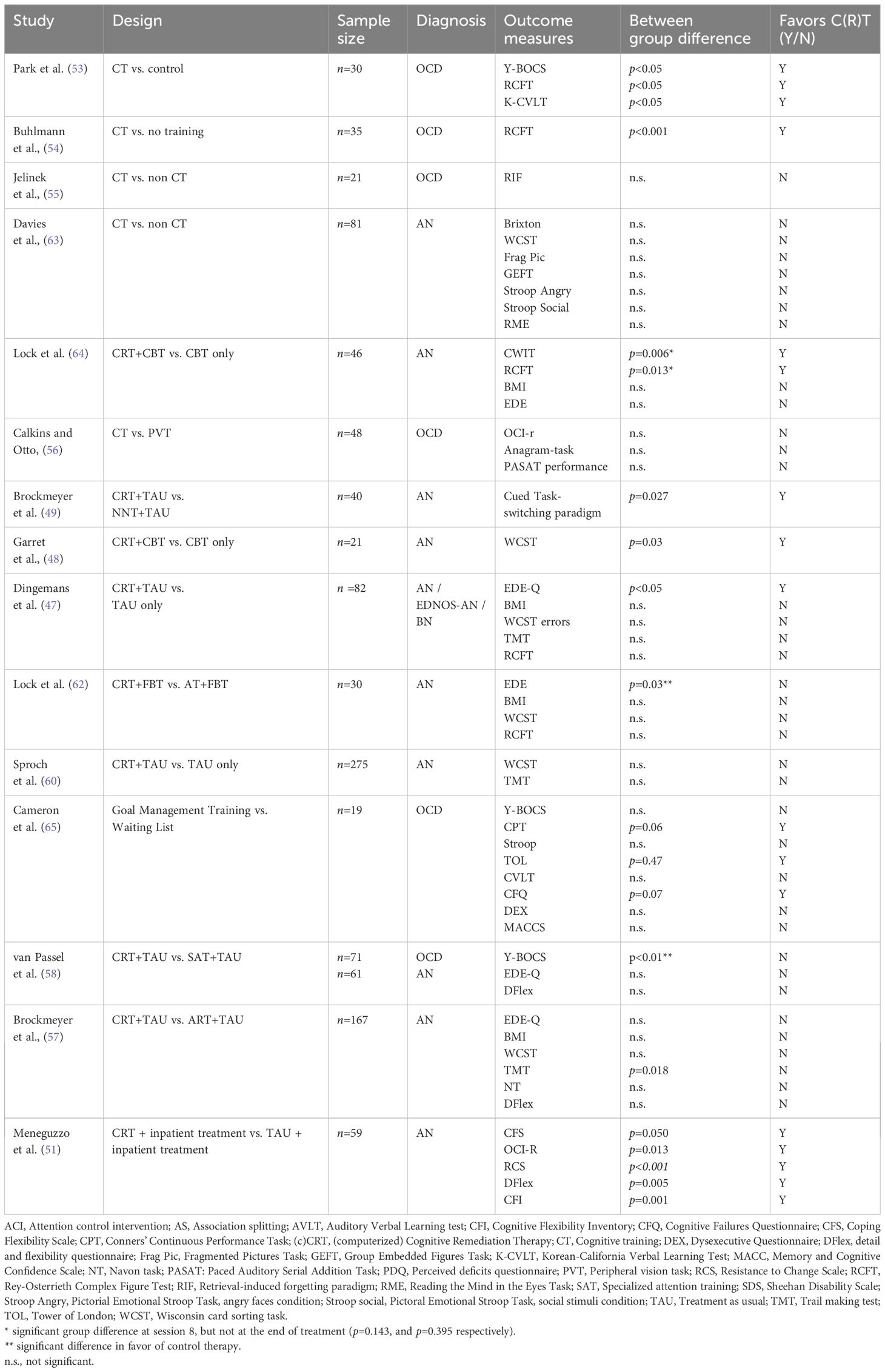
Table 1. Effectiveness of cognitive remediation therapy compared to control interventions on neuropsychologic measures and/or symptom severity.
Table 1 presents studies with CRT, Cognitive Remediation and Emotion Skills training (CREST) or comparable cognitive training focused on cognitive inflexibility in adults with AN or OCD to control treatments. The table shows the effect on symptom reduction and neuropsychological measures. As can be seen, the impact of these cognitive trainings on symptom reduction and neuropsychological measures is mixed. While the evidence summarized in Table 1 highlights the variability in outcomes of cognitive training interventions, it underscores the need for further investigation into their specific effects. To address this, our study aims to explore three key questions: a) to what extend does CRT lead to greater improvements on neuropsychological and subjective measures of cognitive flexibility as compared to Specialized attention therapy (SAT), our custom-designed active control condition, and b) to what extend does cognitive flexibility measured at baseline predict treatment outcomes in OCD and AN, and c) do changes in cognitive flexibility contribute to the treatment outcomes.
Methods
Participants
The participants being evaluated in this study (n=132) were originally enrolled in our RCT evaluating the effectiveness of CRT versus SAT as enhancers of TAU for OCD or AN (52, 58). Participants were between 18 and 66 years old and fulfilled the DSM-IV-TR1 criteria for OCD (n=71) or AN or eating disorder not otherwise specified-AN type (EDNOS-AN, n=61). To verify the DSM-IV-TR diagnoses we used the Structured Clinical Interview for DSM-IV-TR axis-I disorders (66). We included participants with AN and EDNOS-AN because meta-analyses (67, 68) concluded that these conditions fall within the same spectrum in terms of eating pathology, general psychopathology and physical health. Of all participants with AN or EDNOS-AN, (3.3%, n=3) had a BMI ≥ 18.5. AN diagnoses were additionally confirmed using the ED examination (EDE) interview (69) or the self-report questionnaire (EDE-Q) (70). Participants with OCD were included when they scored ≥ 16 on the Yale-Brown obsessive-compulsive scale (Y-BOCS). Comorbid OCD and AN was allowed. Twelve participants fulfilled the diagnostic criteria for both OCD and AN.
Exclusion criteria were severe neurological illness (including a history of seizures, stroke, or Parkinson’s disease), severe comorbid mental disorders (e.g. schizophrenia, clinically significant bipolar disorder, current psychosis, substance dependence/abuse, organic mental disorder), intellectual impairment [defined as an IQ<80 as estimated with the Dutch Adult Reading Test (DART)] (71), and an inability to adequately speak or read Dutch. Antidepressants and antipsychotics were allowed, if dosages were kept constant during the experimental part of the study. Since benzodiazepines can dampen the effect of cognitive treatments (72), only sleep medication was allowed, restricted to a daily dose of up to 20 mg of temazepam (or equivalent dosages of other sleep aids). Participants did not receive renumeration for their participation.
The demographic and clinical characteristics of the study participants are provided in Table 2; detailed descriptions have been published elsewhere (43, 58). Of the 61 participants with AN enrolled, 57 were female (93.4%) and 4 male (6.6%); mean age was 24.90 years (range 18-52, SD 7.28). Of the 72 participants with OCD that were included, 50 were female (69.4%) and 22 male (30.6%) and their mean age was 33.92 years (range 18-66, SD 10.86). Participants were randomized to CRT (n=68) or SAT (n=64). One male participant with OCD withdrew his consent just before randomization and the baseline assessment. The two intervention groups showed no significant differences in self-reported sex/gender, level of education, number of previous treatments, illness duration, or severity of the illness as based on the EDE-Q (AN) and the Y-BOCS scores (OCD). At baseline, there were no differences in the neuropsychological and self-report flexibility indices between the groups receiving CRT and SAT, respectively.

Table 2. Mean, SD and between group differences for demographic, clinical and flexibility variables of all groups.
Procedure
Participants were originally enrolled in our RCT evaluating the effectiveness of CRT versus SAT as enhancers of TAU for OCD or AN (52, 58) of which details have been described elsewhere. In brief, the study was conducted at four highly specialized OCD and AN treatment-centers in The Netherlands. Participants gave informed consent prior to enrollment and were 1:1 randomized to one of two arms (CRT or SAT). CRT, based on the manual of Tchanturia (73) aimed to enhance cognitive flexibility and reduce over-detailed thinking through ten 45-minute biweekly sessions with reflective tasks and homework. The control condition that we designed for this trial, named Specialized Attention Treatment (SAT), was equal to CRT with respect to duration, homework assignments and timing but focused solely on neutral relaxing entertainment and experiences (e.g., board games, listening music, looking at a photo album) without targeting cognitive flexibility or thinking styles (74). After 10 sessions of CRT or SAT, all participants received TAU for OCD or AN, following Dutch and international guidelines (75–78) including CBT with exposure, psychoeducation, cognitive therapy, and pharmacotherapy for OCD, and comprehensive care for AN, such as CBT based protocols, art therapy, social skills training, family therapy, and pharmacotherapy.
Measures
Participants completed all measures described below at baseline (T0), directly after CRT/SAT (T1), and 6 (T2) and 12 (T3) months after baseline.
Symptom severity measures
The Eating Disorder Examination Questionnaire (EDE-Q) (70, 79) is the self-report version of the Eating Disorder Examination (EDE) (80), a semi-structured interview to evaluate ED psychopathology. The EDE-Q assesses attitudinal and behavioral aspects of EDs over a 28-day period using four subscales gauging concerns about shape, weight and eating, and restraint, generating subscale scores and a total scale score. The EDE-Q has excellent internal consistency (Cronbach α 0.78-0.93). The subscales have excellent test-retest reliability over a 2-week period (Pearson’s r ranging from 0.81 to 0.94) (81).
The 10-item Yale-Brown Obsessive-Compulsive Severity Scale (Y-BOCS) (82) is a clinician-rated, semi-structured interview-based scale that is broadly used to assess obsessive-compulsive symptom severity. The scale has two parts, with each 5-item subscale examining five aspects of OCD pathology: 1) time consumed, 2) degree of interference, 3) distress, 4) resistance, and 5) perceived control. The first subscale gives an obsession score (maximum: 20), the second a compulsion score (maximum: 20), together yielding a total score (maximum: 40). The Y-BOCS has a strong internal consistency (Cronbach α.88-.91), inter-rater reliability (r 0.82-0.98), and test-retest reliability in clinical and nonclinical samples was excellent.
Measures of cognitive flexibility
Neuropsychological measures
The neuropsychological measures we used comprised the Trail Making Test (TMT) (83), and the Stroop task (84), including Delis-Kaplan Executive Function System (D-KEFS) card nr. 4 (85).
The TMT (83) was administered to evaluate set-shifting abilities. Originally a pen-and-paper test, we used the computerized version that has recently become available. Patients numerically or alphabetically connect circles on a page in a ‘dot-to-dot’ fashion (trail A), and then alternatively link numbers and letters, i.e. 1–A–2–B–3–C (trail B). The AB ratio score, i.e. the ratio between the time taken to complete trail A and the time needed to complete part B, serves as the index of cognitive flexibility.
The Color-Word Interference Test (CWIT), part of the D-KEFS test battery (85), comprises four components: color naming, word reading, inhibition, and inhibition/switching. In the first part, participants are required to quickly and accurately name color patches. In the second part, participants read out words printed in black ink. The third part involves an inhibition task, where participants identify the ink colors of color words printed in incongruous colors. Lastly, the test assesses the ability to switch between cognitive tasks without explicit cues. Participants are instructed to name the color of the ink when seeing words, but if a word appears within a box, they are instructed to read the word. These boxes are randomly positioned throughout the trial. The time taken to complete the fourth CWIT card is used as the measure of cognitive flexibility. The D-KEFS CWIT has an internal consistency of between 0.72 and.82 and a test-retest reliability of 0.65 for condition 4 (85).
Self-report questionnaire
The DFlex (39) is a self-report scale that measures cognitive rigidity and attention to detail (central coherence). Patients are asked to rate 24 statements on 6-point Likert scales with anchors ‘strongly agree’ and ‘strongly disagree’. The two subscales showed excellent internal consistency (Cronbach α 0.90 and 0.91, respectively). Construct validity (as compared to relevant subscales of the autism-spectrum quotient (AQ) (86) was strong for cognitive rigidity (r = 0.72) but only moderate for attention to detail (r = 0.26) (39).
Statistical analyses
Prior to analysis, variables were evaluated for the presence of outliers and distributional properties were examined. We determined outliers in a univariate way when the measurements exceeded the 5% or 95% threshold from the sample. These measurements were excluded from analyzes. Data analyses were conducted with R version 4.1.1 (87) using R Studio 2021.09.0 + 351. Longitudinal modelling was performed using the R lme4 package (88) and the exceedance probabilities (p values) of the parameters were calculated with the R package lmertest (89). Between-group differences were analyzed using χ2 for categorical variables and independent sample t-tests for continuous variables.
To analyze change in cognitive flexibility from T0 to T1, we specified a linear mixed model for each of the flexibility indices, with the measure of flexibility as the dependent variable and as fixed effects: the actual day of measurement (time), diagnosis (AN/OCD), CRT/SAT, baseline flexibility score, and the interactions between diagnosis and time, between CRT/SAT and time, and between diagnosis, CRT/SAT and time. In the random effects part of the model, random slopes for the time effect were included. Estimated marginal means and within-group differences were calculated for baseline and T1 scores using the emmeans package (90). We included all participants with at least two measurements. From the original sample (n=132; 58 CRT and 47 SAT), 27 patients were excluded (10 from the CRT group and 17 from the SAT group) due to being classified as outliers or having only a single measurement. To specifically address missing data in longitudinal analyzes, we utilized Linear Mixed Models. This approach is more robust in handling missing data as it considers the correlations between measurements from the same participants over time, estimating parameters using all available data and without imputation of missing data.
For the moderation and mediation analyses, linear mixed models were fitted with severity (the z-score from the Y-BOCS for OCD and the z-score from the EDE-Q for AN) as the dependent variable. We specified a linear mixed model with fixed effects: baseline flexibility score as the moderator, or - for the mediation analyses - the baseline-to-T1 difference score for the measures of cognitive flexibility as a mediator, the interaction between moderator (or mediator) and time. We did not include effects for diagnosis or type of adjunctive treatment (CRT or SAT) in this model because there was no significant interaction between time and diagnosis or time and type of adjunctive treatment in the flexibility change models; in our earlier study with the same sample (58), we examined the effects of pharmacotherapy on treatment response and found that differences in psychotropic medication were non-significant and did not confound treatment effects; therefore medication use was not controlled for. To account for the two different phases, we specified a linear B-spline model with a knot at the end of CRT/SAT (knot at T1, degree=1). In the random effects part of the model, random slopes for the time effect were included. To visualize the interaction between the measure of flexibility and flexibility difference score, and severity, we plotted three splines with confidence intervals for three levels of flexibility scores.
Results
Does CRT lead to greater improvements on cognitive flexibility as compared to SAT?
The mixed model analyses revealed no significant change from T0 to T1 for TMT AB-ratio (F(195)=2.07, p=0.152). CWIT-rigidity (F(201)=23.0, p<0.001) and DFlex-rigidity (F(191)=8.38, p<0.01) did show a significant T0-T1 change, which implies that flexibility as measured with the CWIT and DFlex rigidity subscales had improved following CRT/SAT. However, the two-way interactions time*diagnosis and time*type of adjunctive treatment were all non-significant. The three-way interaction time*diagnosis*type of adjunctive treatment was also non-significant, signifying there were no differences in change over time between the CRT and SAT or the OCD and AN groups.
As can be seen in Table 3, the within-group differences for estimated marginal means (EMMs) as based on the model demonstrate significant improvement over time for the CWIT and DFlex but not for TMT AB-ratio in the total study sample. When calculating EMMs for the diagnosis (AN/OCD) and type of adjunctive treatment (CRT/SAT) subgroups, we found that on the CWIT all subgroups showed significant decreases, reflecting an improvement in cognitive flexibility over time. On the DFlex, only the OCD subgroup showed a significant decrease over time. Finally, we saw no significant T0-to-T1 changes in the TMT AB-ratio scores for any of the subgroups.
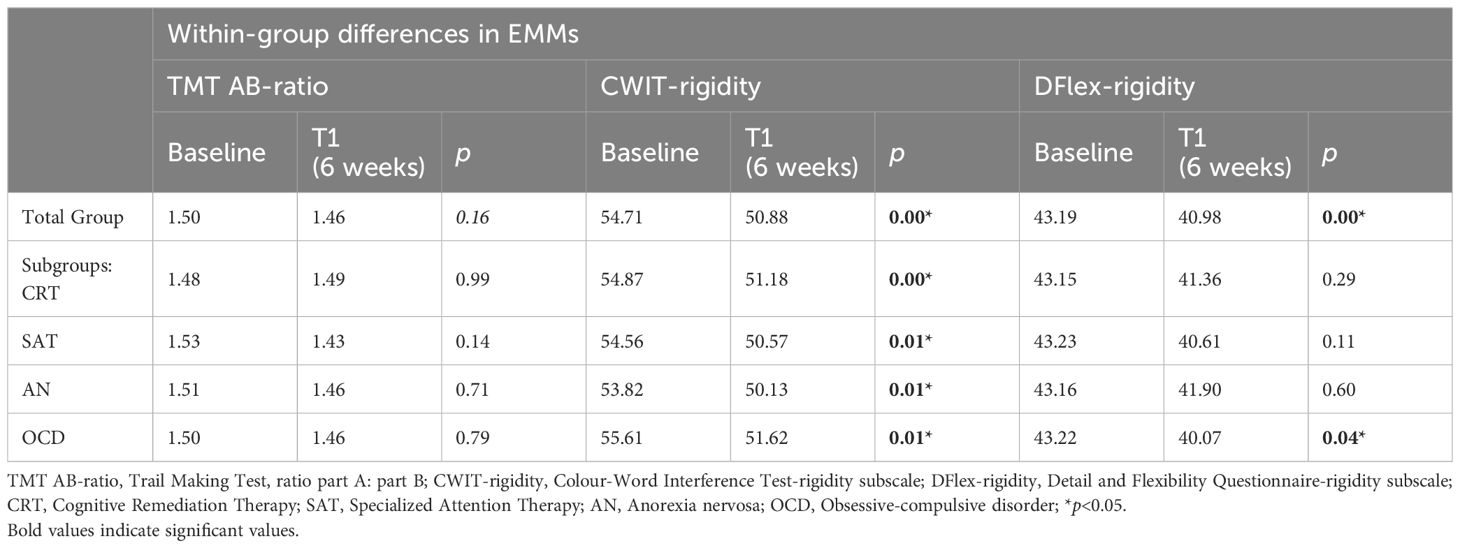
Table 3. Within-group estimated marginal means (EMMs) for the cognitive flexibility measurements at baseline and 6 weeks for the total group and for the four subgroups.
Does cognitive flexibility measured at baseline predict treatment outcomes in OCD and AN?
Presenting the moderator models, Figure 1 (left column) shows there was no significant interaction between the baseline TMT AB-ratio score and time (t(288)=-0.0979, p=0.922) during CRT/SAT or the TAU phase (t(148)=0.540, p=0.590), which tells us that TMT performance did not moderate change in symptom severity during treatment. During CRT/SAT, the interaction between the baseline CWIT-rigidity scores and time was non-significant (t(296)=-0.958, p=0.339), which also applies to TAU (t(163)=-2.54, p=0.012), indicating that CWIT-rigidity did not moderate changes in Y-BOCS and EDE-Q scores during treatment. Moreover, the interaction between baseline DFlex-rigidity scores and time was non-significant both during CRT/SAT (t(289)=-0.464, p=0.643) and TAU (t(91.2)=1.51, p=0.134), implying that DFlex performance did not moderate changes in the Y-BOCS and EDE-Q scores during treatment.
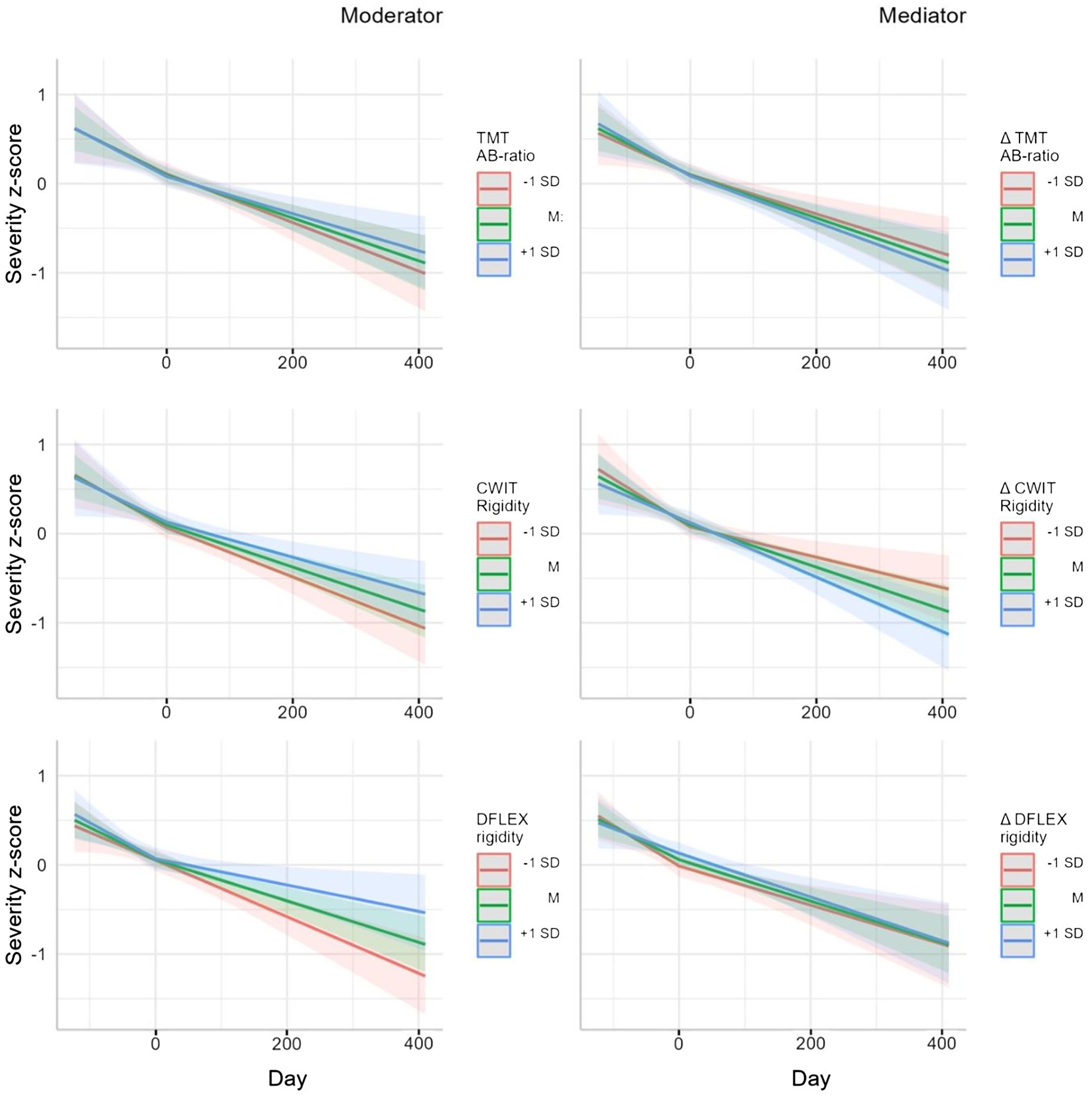
Figure 1. Moderator (left column) and mediator (right column) models: predeicted severity z-score with measurement of flexibility (MF) or MF as covariate.
Do changes in cognitive flexibility mediate the treatment outcomes?
The mediator models depicted in the right column of Figure 1 illustrate that in both treatment phases, i.e. CRT/SAT and TAU, there was no significant interaction between the TMT-AB-ratio T0-T1 difference score and time (t(296)=-0.390, p=0.697 and (t(112)=0.661, p=0.510, respectively). The interaction between the CWIT T0-T1 difference score and time was also not significant for either phase (t(282)=0.641, p=0.522, and t(151)=0.882, p=0.379, respectively), as was the case for the interaction between the DFlex-rigidity T0-T1 difference score and time (t(286)=-0.924, p=0.356; t(92.4)=0.274, p=0.785, respectively). In summary, we found no mediation effect for any of the three measures of flexibility on changes in disease-specific symptom severity.
Discussion
Main findings
This study compared the efficacy of CRT and an active control intervention in improving cognitive flexibility in individuals with OCD or AN. It also explored the connection between baseline flexibility and longer-term outcomes in OCD and AN participants, and whether those showing larger improvements following CRT/SAT achieved better outcomes from TAU.
We observed a time-related effect of both adjunctive treatments on both CWIT-rigidity and DFlex-rigidity but not for TMT AB-ratio in both diagnostic groups. In the absence of any time-related between-group (CRT/SAT or OCD/AN) differences, we conclude that CRT did not enhance cognitive flexibility to a greater degree than SAT in both OCD and AN disproving our first hypothesis that CRT would lead to greater improvements on cognitive flexibility as compared to SAT. We accordingly simplified the moderation and mediation models, excluding diagnosis and type of adjunctive treatment to increase the power of our analyses. Our second hypothesis, suggesting that patients with higher levels of cognitive flexibility would respond more favorably to the treatment, was disproven as the moderator scores failed to predict treatment outcomes. Further, our third hypothesis, proposing that better outcomes during TAU were mediated by improved cognitive flexibility was not supported.
In summary, this study demonstrates that CRT did not improve cognitive flexibility more so than SAT did, nor that the degree of cognitive flexibility had a moderating or mediating effect on the outcomes of OCD and AN treatment.
CRT as an enhancer of cognitive flexibility
Our findings generally align with previous studies that evaluated CRT for AN, which together provide growing evidence that CRT does not enhance cognitive flexibility nor diminish illness severity more so than an active control condition (57–61, 91).
Cognitive flexibility as a moderator of treatment outcomes
Our findings regarding the lack of a moderating effect of cognitive flexibility on the 6- and 12 month outcomes of TAU for AN and OCD are in contrast with one previous OCD study and three AN studies (47, 48, 92, 93) but in line with two other OCD studies and one AN study (94–96).
As to the studies reporting contrasting findings, we can say that the recent study by Schubert et al. (92) including 112 patients with OCD did find higher self-reported levels of flexibility at baseline to predict lower levels of OCD symptoms at the conclusion of a specialized CBT-based group therapy. However, a different concept of cognitive flexibility was employed, one that aligns more closely with the theory of Acceptance and Commitment Therapy (ACT), where cognitive flexibility is defined as the capacity to be in contact with the present and act in accordance with long-term goals rather than short-term urges. Moreover, the participants received an inpatient multimodal treatment program without elements specifically targeting cognitive flexibility, while its assessment solely relied on a short self-report questionnaire. Having 21 women with AN complete the WCST at baseline to assess cognitive flexibility, Garrett et al. (48) documented that the more proficient performers had better outcomes after 16 weeks of CBT, as indicated by higher BMI scores. But, unlike our study, Garrett et al. (48) used functional magnetic resonance imaging to evaluate if regional brain activation associated with cognitive flexibility predicted treatment response. Evaluating a group of 82 patients with AN, Dingemans et al. (47) observed that poor baseline cognitive flexibility, as assessed with the TMT and WCST, were associated with greater long-term improvements in ED-related quality of life for those having received CRT compared to the control (TAU only) group. Consistent with our study, though, was that the authors also found no moderating effect for their flexibility measures on disorder-specific outcomes (EDE-Q and BMI). Finally, the study by Harper et al. (93) involving 46 patients with AN showed that those participants who still met the diagnosis at follow-up had shown a poorer performance on the TMT and WCST at baseline compared to peers with a BMI higher than 19 in the last 12 months. In line with our study, Harper et al. (93) found no significant difference in TMT-AB-ratio, only in the TMT-B subtest. The authors used a different outcome-measure (three groups: remaining ill, recently recovered and sustaining recovery) whilst, for AN, we used the EDE-Q as outcome which allows for the possibility to detect more subtle changes.
The contrast in the findings summarized above and ours may then be due to the variety in outcome measures that were used in the AN studies and the lack of uniformity in the assessment of cognitive flexibility. The AN studies finding a moderation effect of cognitive flexibility used weight or BMI or quality of life as outcome measure, while those employing the EDE or EDE-Q found no such effect.
Looking at the two OCD studies documenting results that are in accordance with our findings, (94, 95), we observe that both studies used neuropsychological tests (including the TMT and Stroop) to assess cognitive flexibility. The Oldershaw-study including 71 women with AN showed that baseline cognitive flexibility predicted 7% of posttreatment weight gain, but there was no predictive effect when the EDE-Q outcomes were considered (96).
In sum, that our findings both agree and contrast with previous findings can thus partly be explained by the differences in the measures of symptom severity and cognitive flexibility, and statistical methods, which differences complicate a sound comparison of the various results.
Cognitive flexibility as a mediator of treatment outcome
We found change in cognitive flexibility during treatment not to predict treatment outcome, which is in line with Schubert et al. (92) who also found no relationship between OCD symptom reductions and an improvement in self-perceived cognitive flexibility, thereby contradicting the earlier findings of Twohig et al. (97) who did note that self-reported cognitive flexibility mediated change in OCD symptoms in their sample. In both studies, a different concept of cognitive flexibility that aligns more closely to ACT was employed.
Consistent with our findings, Oldershaw et al. (96) observed that in their AN sample no measure of change in flexibility correlated with clinical EDE-Q improvement and post-treatment weight. In a recent AN study Duriez et al. (36) did detect that increased cognitive flexibility as measured with the Brixton test (98), mediated the improvement in daily-life functioning as well as ED and depressive symptoms during treatment but not the improvement of BMI. In contrast with our study, Duriez et al. (36) used t-tests to detect differences between baseline and follow-up and did not include an adjunctive treatment targeting cognitive flexibility prior to TAU.
Again, these conflicting findings can be explained by the different concepts and measures of cognitive flexibility, different outcome measures, design, and statistical methods. In the case of AN research, it clearly matters whether the EDE-Q is chosen as the outcome measure or weight/BMI.
Cognitive flexibility - a lack of uniformity in both definition and operationalization
In this study we used two widely recognized neuropsychological tasks and one subjective measure (DFlex) to test cognitive flexibility. We found no correlation between the three baseline flexibility indices in our sample. The concept of cognitive flexibility is a complex one, with a wide variety of tasks and approaches being used to capture its essence (99). Also in the CRT literature, the heterogeneity in the tasks and measures used to operationalize cognitive flexibility is large (61). In their systematic review and meta-analysis, Howlett et al. (100) look at the associations between the two mostly used broad approaches to assess cognitive flexibility: self-report and neuropsychological testing. Self-reporting has the advantage that outcomes are closer to the daily-life experiences of the respondent than those of standardized neuropsychological tasks. However, subjective assessments are susceptible to reporting bias and depend on an individual’s subjective perception of their own abilities, and they, unavoidably, simultaneously gauge other processes of executive functioning. Neuropsychological tests, on the other hand, may not entirely capture all aspects of cognitive flexibility that are important for targeting psychotherapeutic interventions (101). Moreover, both in HCs and patient populations, the relationship between self-report and neuropsychological indices of cognitive flexibility is absent (43, 100), which may be due to task impurities (100, 102) in that any executive task also taps nonexecutive functions that influence the test outcomes just as is the case with self-reported flexibility tools. We hence emphasize that self-report questionnaires cannot be considered valid substitutes of neuropsychological tests of cognitive flexibility and vice versa.
One possibility is that the measures used were insufficiently sensitive to detect meaningful changes over time or failed to capture the complexity of cognitive flexibility and its disorder-specific manifestations. Sample characteristics may also have played a role. Variability in baseline cognitive flexibility, symptom severity, comorbidities, or therapy engagement could obscure potential associations. Additionally, the relatively small sample size may have limited statistical power to detect subtle effects. However, this raises the question of whether neuropsychological measures and self-report questionnaires provide ecologically valid assessments capable of capturing the complexity of cognitive flexibility in real world daily life situations.
Finally, these findings suggest a need to refine our understanding of cognitive flexibility’s role in these disorders. While theoretical models posit that enhanced cognitive flexibility supports treatment responsiveness, the results indicate that this relationship may be more context-dependent, and potentially influenced by factors such as emotional regulation or environmental stressors.
Limitations
With respect to the limitations of our study, we need to mention the dropout at the 12-month timepoint, which, with 49%, was far higher than expected. Relevantly, 36 of the 48 (75%) of the patients dropping out had already ended TAU and their participation to the study before the 52-week assessment. Relevantly, with 19%, the dropout during the first phase of treatment (at 5 weeks) was more acceptable. Since we calculated the moderators and mediators for the total sample over the full 52-week study period, the chance of non-selective dropout cannot be excluded.
Secondly, three participants (3.3%) with a BMI above 18.5 kg/m² were included, which means that according to DSM-IV criteria, they formally do not fulfill an diagnosis. However, according to the diagnostic procedure of the department where they were treated, they fulfilled an diagnosis.
Thirdly, given that TMT AB-ratio scores following CRT/SAT were non-significantly lower than the baseline scores, we cannot fully rule out that enhancing cognitive flexibility needs more time and/or more training for effects to become manifest. Possibly, a more intensive CRT format [as suggested for individuals with schizophrenia (103)] or a dedicated drill-and-practice strategy throughout the OCD or AN treatment or, finally, apps specifically designed to enhance cognitive flexibility might be more effective in training this executive function. We cannot preclude that in the long run significantly lower scores can be achieved that do contribute to a better treatment outcome.
Finally, a limitation of this study is the absence of data on baseline comorbidities and their potential impact on treatment trajectories. Future research should address these factors to clarify their role in outcomes.
Final conclusions and further directions
In view of earlier and recent conflicting findings, we question whether CRT should be applied when the aim is to address cognitive inflexibility. It has already been suggested that CRT may not work by resolving this inflexibility but by having patients reflect on their thinking styles and strategies and by translating new behaviors to everyday life. Furthermore, CRT also addresses the common issue of treatment ambivalence in this population, where low motivation to change behaviors is prevalent. CRT tackles this indirectly by providing patients with EDs opportunities to experience treatment successes (104).
We like to suggest several options for future research. First, colleagues should focus on how to define cognitive flexibility, providing an answer as to whether we should use a broad concept or a more precise definition that aligns either closely with existing or novel neuropsychological or with self-report measures. Furthermore, it would be interesting to look for dedicated interventions that directly train cognitive flexibility in OCD and AN, as various apps are targeting this inefficiency.
In AN research, therapy targeting cognitive flexibility, central coherence, and emotional factors, such as CREST (105, 106), is under investigation. While its application in OCD remains unexplored, further study in both AN and OCD populations may be worthwhile. Moreover, the potential of SAT’s focus on behavioral activation (107) and positive psychology, which taps into elements of positive psychology like addressing pleasure, engagement, meaning making (108, 109) warrants further investigation.
The gain in cognitive flexibility from CRT was not great in our study. Based on this and other results, as an integral treatment for all patients with OCD and AN, CRT appears insufficiently effective in improving this ability. We would like to know whether patients with AN or OCD that have a clearer deficit in cognitive flexibility will benefit more from targeted training on this deficit. Finally, CRT research might investigate other mechanisms of action, such as improving treatment ambivalence, the therapeutic relationship, promoting play, reflecting on thinking styles and meaning making.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the University Medical Centre Utrecht approved the study (METc no. NL43751.041.13 v0.3). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BP: Writing – original draft, Writing – review & editing. UD: Data curation, Supervision, Writing – original draft, Writing – review & editing. AD: Writing – review & editing. TB: Formal analysis, Methodology, Writing – review & editing. LS: Writing – review & editing. EB: Supervision, Writing – review & editing. AE: Writing – review & editing. EF: Writing – review & editing. G-JH: Supervision, Writing – review & editing. DC: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received a ZonMW grant, project number 837001004.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ In the Netherlands, the initial version of the DSM-5 was released in 2014, after participant inclusion for the study had already commenced. The DSM-5 was not fully implemented in the Netherlands until 2017. We chose not to switch DSM versions during the study to maintain consistency.
References
1. Berlin GS, Hollander E. Compulsivity, impulsivity, and the DSM-5 process. CNS Spectrums. (2014) 19:62–8. doi: 10.1017/S1092852913000722
2. Fineberg NA, Apergis-Schoute AM, Vaghi MM, Banca P, Gillan CM, Voon V, et al. Mapping compulsivity in the DSM-5 obsessive compulsive and related disorders: cognitive domains, neural circuitry, and treatment. Int J Neuropsychopharmacol. (2018) 21:42–58. doi: 10.1093/ijnp/pyx088
3. Mahjani B, Bey K, Boberg J, Burton C. Genetics of obsessive-compulsive disorder. psychol Med. (2021) 51:1–13. doi: 10.1017/S0033291721001744
4. Buckner JD, Silgado J, Lewinsohn PM. Delineation of differential temporal relations between specific eating and anxiety disorders. J Psychiatr Res. (2010) 44:781–7. doi: 10.1016/j.jpsychires.2010.01.014
5. Mandelli L, Draghetti S, Albert U, De Ronchi D, Atti AR. Rates of comorbid obsessive-compulsive disorder in eating disorders: A meta-analysis of the literature. J Affect Disord. (2020) 277:927–39. doi: 10.1016/j.jad.2020.09.003
6. Pinto A, Mancebo MC, Eisen JL, Pagano ME, Rasmussen SA. The Brown Longitudinal Obsessive Compulsive Study: clinical features and symptoms of the sample at intake. J Clin Psychiatry. (2006) 67:703–11. doi: 10.4088/jcp.v67n0503
7. Yilmaz Z, Halvorsen M, Bryois J, Yu D, Thornton LM, Zerwas S, et al. Examination of the shared genetic basis of anorexia nervosa and obsessive-compulsive disorder. Mol Psychiatry. (2020) 25:2036–46. doi: 10.1038/s41380-018-0115-4
8. Godier LR, Park RJ. Compulsivity in anorexia nervosa: A transdiagnostic concept [Peer Reviewed. Front Psychol. (2014) 5:778. doi: 10.3389/fpsyg.2014.00778
9. Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin Psychol Rev. (2013) 33:1163–71. doi: 10.1016/j.cpr.2013.09.004
10. Snyder HR, Kaiser RH, Warren SL, Heller W. Obsessive-compulsive disorder is associated with broad impairments in executive function: A meta-analysis. Clin Psychol Sci. (2015) 3:301–30. doi: 10.1177/2167702614534210
11. Sultson H, van Meer F, Sanders N, van Elburg AA, Danner UN, Hoek HW, et al. Associations between neural correlates of visual stimulus processing and set-shifting in ill and recovered women with anorexia nervosa. Psychiatry Res Neuroimaging. (2016) 255:35–42. doi: 10.1016/j.pscychresns.2016.07.004
12. Delorme R, Gousse V, Roy I, Trandafir A, Mathieu F, Mouren-Simeoni MC, et al. Shared executive dysfunctions in unaffected relatives of patients with autism and obsessive-compulsive disorder. Eur Psychiatry. (2007) 22:32–8. doi: 10.1016/j.eurpsy.2006.05.002
13. Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder [Empirical Study; Quantitative Study. Am J Psychiatry. (2007) 164:335–8. doi: 10.1176/ajp.2007.164.2.335
14. Abramovitch A, De Nadai AS, Geller DA. Neurocognitive endophenotypes in pediatric OCD probands, their unaffected parents and siblings. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 110:110283. doi: 10.1016/j.pnpbp.2021.110283
15. Talbot A, Hay P, Buckett G, Touyz S. Cognitive deficits as an endophenotype for anorexia nervosa: an accepted fact or a need for re-examination? Int J Eat Disord. (2015) 48:15–25. doi: 10.1002/eat.22332
16. Tenconi E, Santonastaso P, Degortes D, Bosello R, Titton F, Mapelli D, et al. Set-shifting abilities, central coherence, and handedness in anorexia nervosa patients, their unaffected siblings and healthy controls: Exploring putative endophenotypes. World J Biol Psychiatry. (2010) 11:813–23. doi: 10.3109/15622975.2010.483250
17. Miles S, Gnatt I, Phillipou A, Nedeljkovic M. Cognitive flexibility in acute anorexia nervosa and after recovery: A systematic review. Clin Psychol Rev. (2020) 81:101905. doi: 10.1016/j.cpr.2020.101905
18. Buss AT, Lowery KN. Inhibitory control and executive function. In: Benson JB, editor. Encyclopedia of infant and early childhood development, 2nd ed. Oxford, United Kingdom: Elsevier (2020) 183–93. doi: 10.1016/B978-0-12-809324-5.23669-9
19. Diamond A. Executive functions. Annu Rev Psychol. (2013) 64:135–68. doi: 10.1146/annurev-psych-113011-143750
20. Fenger MM, Gade A, Adams KH, Hansen ES, Bolwig TG, Knudsen GM. Cognitive deficits in obsessive-compulsive disorder on tests of frontal lobe functions. Nord J Psychiatry. (2005) 59:39–44. doi: 10.1080/08039480510018814
21. Dittrich WH, Johansen T. Cognitive deficits of executive functions and decision-making in obsessive-compulsive disorder [Empirical Study; Interview; Quantitative Study. Scandinavian J Psychol. (2013) 54:393–400. doi: 10.1111/sjop.12066
22. Dajani DR, Uddin LQ. Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends Neurosci. (2015) 38:571–8. doi: 10.1016/j.tins.2015.07.003
23. Friederich HC, Herzog W. Cognitive-behavioral flexibility in anorexia nervosa. Curr Top Behav Neurosci. (2011) 6:111–23. doi: 10.1007/7854_2010_83
24. Gruner P, Pittenger C. Cognitive inflexibility in obsessive-compulsive disorder. Neuroscience. (2017) 345:243–55. doi: 10.1016/j.neuroscience.2016.07.030
25. Wu M, Brockmeyer T, Hartmann M, Skunde M, Herzog W, Friederich HC. Set-shifting ability across the spectrum of eating disorders and in overweight and obesity: a systematic review and meta-analysis. Psychol Med. (2014) 44:3365–85. doi: 10.1017/S0033291714000294
26. Chamberlain SR, Solly JE, Hook RW, Vaghi MM, Robbins TW. Cognitive inflexibility in OCD and related disorders. In: Current topics in behavioral neurosciences. Springer, Berlin Heidelberg (2021). p. 1–21. doi: 10.1007/7854_2020_198
27. Isobe M, Vaghi M, Fineberg NA, Apergis-Schoute AM, Bullmore ET, Sahakian BJ, et al. Set-shifting-related basal ganglia deformation as a novel familial marker of obsessive–compulsive disorder. Br J Psychiatry. (2021) 220:1–4. doi: 10.1192/bjp.2021.45
28. Bersani G, Quartini A, Ratti F, Pagliuca G, Gallo A. Olfactory identification deficits and associated response inhibition in obsessive-compulsive disorder: on the scent of the orbitofronto-striatal model. Psychiatry Res. (2013) 210:208–14. doi: 10.1016/j.psychres.2013.05.032
29. Vaghi MM, Vertes PE, Kitzbichler MG, Apergis-Schoute AM, van der Flier FE, Fineberg NA, et al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol Psychiatry. (2017) 81:708–17. doi: 10.1016/j.biopsych.2016.08.009
30. Lao-Kaim NP, Fonville L, Giampietro VP, Williams SC, Simmons A, Tchanturia K. Aberrant function of learning and cognitive control networks underlie inefficient cognitive flexibility in anorexia nervosa: a cross-sectional fMRI study. PloS One. (2015) 10:e0124027. doi: 10.1371/journal.pone.0124027
31. Freyer T, Kloppel S, Tuscher O, Kordon A, Zurowski B, Kuelz AK, et al. Frontostriatal activation in patients with obsessive-compulsive disorder before and after cognitive behavioral therapy. Psychol Med. (2011) 41:207–16. doi: 10.1017/S0033291710000309
32. Fuglset TS, Landrø NI, Reas DL, Rø Ø. Functional brain alterations in anorexia nervosa: a scoping review. J Eat Disord. (2016) 4:32–2. doi: 10.1186/s40337-016-0118-y
33. Su T, Gong J, Tang G, Qiu S, Chen P, Chen G, et al. Structural and functional brain alterations in anorexia nervosa:A multimodal meta-analysis of neuroimaging studies. Hum Brain Mapp. (2021) 42:5154–69. doi: 10.1002/hbm.25602
34. Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, et al. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder [Empirical Study; Quantitative Study. Brain. (2008) 131:155–64. doi: 10.1093/brain/awm277
35. Abramovitch A, Dar R, Schweiger A, Hermesh H. Neuropsychological impairments and their association with obsessive-compulsive symptom severity in obsessive-compulsive disorder. Arch Clin Neuropsychol. (2011) 26:364–76. doi: 10.1093/arclin/acr022
36. Duriez P, Kaya Lefèvre H, Di Lodovico L, Viltart O, Gorwood P. Increased cognitive flexibility mediates the improvement of eating disorders symptoms, depressive symptoms and level of daily life functioning in patients with anorexia nervosa treated in specialised centres. Eur Eating Disord Rev. (2021) 29:600–10. doi: 10.1002/erv.2829
37. Fuglset TS. Is set-shifting and central coherence in anorexia nervosa influenced by body mass index, anxiety or depression? A systematic review. BMC Psychiatry. (2021) 21:137–51. doi: 10.1186/s12888-021-03120-6
38. Bolton D, Raven P, Madronal-Luque R, Marks IM. Neurological and neuropsychological signs in obsessive compulsive disorder: interaction with behavioural treatment. Behav Res Ther. (2000) 38:695–708. doi: 10.1016/S0005-7967(99)00139-4
39. Roberts ME, Barthel FM, Lopez C, Tchanturia K, Treasure JL. Development and validation of the Detail and Flexibility Questionnaire (DFlex) in eating disorders. Eat Behav. (2011) 12:168–74. doi: 10.1016/j.eatbeh.2011.04.001
40. Lang K, Lloyd S, Khondoker M, Simic M, Treasure J, Tchanturia K. Do children and adolescents with anorexia nervosa display an inefficient cognitive processing style? PloS One. (2015) 10:e0131724. doi: 10.1371/journal.pone.0131724
41. Wang SB, Gray EK, Coniglio KA, Murray HB, Stone M, Becker KR, et al. Cognitive rigidity and heightened attention to detail occur transdiagnostically in adolescents with eating disorders. Eat Disord. (2019) 29:1–13. doi: 10.1080/10640266.2019.1656470
42. Sternheim L, van der Burgh M, Berkhout LJ, Dekker MR, Ruiter C. Poor cognitive flexibility, and the experience thereof, in a subclinical sample of female students with obsessive-compulsive symptoms. Scand J Psychol. (2014) 55:573–7. doi: 10.1111/sjop.12163
43. Sternheim L, van Passel B, Dingemans A, Cath D, Danner UN. Cognitive and experienced flexibility in patients with anorexia nervosa and obsessive compulsive disorder. Front Psychiatry. (2022) 13:868921. doi: 10.3389/fpsyt.2022.868921
44. Tchanturia K. Cognitive remediation therapy (CRT) for eating and weight disorders (1st ed). London: Routledge (2015). doi: 10.4324/9781315749266
45. Lindvall Dahlgren C, Rø Ø. A systematic review of cognitive remediation therapy for anorexia nervosa - development, current state and implications for future research and clinical practice. J Eat Disord. (2014) 2:1–12. doi: 10.1186/s40337-014-0026-y
46. Abbate-Daga G, Buzzichelli S, Marzola E, Amianto F, Fassino S. Effectiveness of cognitive remediation therapy (CRT) in anorexia nervosa: a case series. J Clin Exp Neuropsychol. (2012) 34:1009–15. doi: 10.1080/13803395.2012.704900
47. Dingemans AE, Danner UN, Donker JM, Aardoom JJ, van Meer F, Tobias K, et al. The effectiveness of cognitive remediation therapy in patients with a severe or enduring eating disorder: a randomized controlled trial. Psychother Psychosom. (2014) 83:29–36. doi: 10.1159/000355240
48. Garrett AS, Lock J, Datta N, Beenhaker J, Kesler SR, Reiss AL. Predicting clinical outcome using brain activation associated with set-shifting and central coherence skills in Anorexia Nervosa. J Psychiatr Res. (2014) 57:26–33. doi: 10.1016/j.jpsychires.2014.06.013
49. Brockmeyer T, Ingenerf K, Walther S, Wild B, Hartmann M, Herzog W, et al. Training cognitive flexibility in patients with anorexia nervosa: a pilot randomized controlled trial of cognitive remediation therapy. Int J Eat Disord. (2014) 47:24–31. doi: 10.1002/eat.22206
50. Brockmeyer T, Walther S, Ingenerf K, Wild B, Hartmann M, Weisbrod M, et al. Brain effects of computer-assisted cognitive remediation therapy in anorexia nervosa: A pilot fMRI study. Psychiatry Res249. (2016) 249:52–6. doi: 10.1016/j.pscychresns.2016.02.007
51. Meneguzzo P, Tenconi E, Todisco P, Favaro A. Cognitive remediation therapy for anorexia nervosa as a rolling group intervention: Data from a longitudinal study in an eating disorders specialized inpatient unit. Eur Eating Disord Rev. (2021) 29:770–82. doi: 10.1002/erv.2848
52. van Passel B, Danner U, Dingemans A, van Furth E, Sternheim L, van Elburg A, et al. Cognitive remediation therapy (CRT) as a treatment enhancer of eating disorders and obsessive compulsive disorders: study protocol for a randomized controlled trial. BMC Psychiatry. (2016) 16:393. doi: 10.1186/s12888-016-1109-x
53. Park HS, Shin YW, Ha TH, Shin MS, Kim YY, Lee YH, et al. Effect of cognitive training focusing on organizational strategies in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. (2006) 60:718–26. doi: 10.1111/j.1440-1819.2006.01587.x
54. Buhlmann U, Deckersbach T, Engelhard I, Cook LM, Rauch SL, Kathmann N, et al. Cognitive retraining for organizational impairment in obsessive-compulsive disorder. Psychiatry Res. (2006) 144:109–16. doi: 10.1016/j.psychres.2005.10.012
55. Jelinek L, Rietschel L, Kellner M, Muhtz C, Moritz S. The effect of practice on the recall of salient information in obsessive–compulsive disorder. Psychiatry Res. (2012) 198:89–93. doi: 10.1016/j.psychres.2012.03.002
56. Calkins AW, Otto MW. Testing the boundaries of computerized cognitive control training on symptoms of obsessive compulsive disorder [Empirical Study; Quantitative Study. Cogn Ther Res. (2013) 37:587–94. doi: 10.1007/s10608-012-9496-x
57. Brockmeyer T, Schmidt H, Leiteritz-Rausch A, Zimmermann J, Wunsch-Leiteritz W, Leiteritz A, et al. Cognitive remediation therapy in anorexia nervosa-A randomized clinical trial. J Consult Clin Psychol. (2021) 89:805–15. doi: 10.1037/ccp0000675
58. van Passel B, Danner UN, Dingemans AE, Aarts E, Sternheim L, Becker ES, et al. Cognitive remediation therapy does not enhance treatment effect in obsessive-compulsive disorder and anorexia nervosa: A randomized controlled trial. Psychother Psychosom. (2020) 89:228–41. doi: 10.1159/000505733
59. Herbrich L, van Noort B, Pfeiffer E, Lehmkuhl U, Winter S, Kappel V. Follow-up assessment of cognitive remediation therapy in adolescent anorexia nervosa: A pilot study. Eur Eat Disord Rev. (2017) 25:104–13. doi: 10.1002/erv.2501
60. Sproch LE, Anderson KP, Sherman MF, Crawford SF, Brandt HA. A randomized controlled trial of group cognitive remediation therapy for anorexia nervosa: Effects on set-shifting tasks for inpatient adults and adolescents. Int J Eat Disord. (2019) 52:1004–14. doi: 10.1002/eat.23143
61. Hagan KE, Christensen KA, Forbush KT. A preliminary systematic review and meta-analysis of randomized-controlled trials of cognitive remediation therapy for anorexia nervosa. Eat Behav. (2020) 37:101391. doi: 10.1016/j.eatbeh.2020.101391
62. Lock J, Fitzpatrick KK, Agras WS, Weinbach N, Jo B. Feasibility study combining art therapy or cognitive remediation therapy with family-based treatment for adolescent anorexia nervosa. Eur Eat Disord Rev. (2018) 26:62–8. doi: 10.1002/erv.2571
63. Davies H, Fox J, Naumann U, Treasure J, Schmidt U, Tchanturia K. Cognitive remediation and emotion skills training for anorexia nervosa: an observational study using neuropsychological outcomes. Eur Eat Disord Rev. (2012) 20:211–7. doi: 10.1002/erv.2170
64. Lock J, Agras WS, Fitzpatrick KK, Bryson SW, Jo B, Tchanturia K. Is outpatient cognitive remediation therapy feasible to use in randomized clinical trials for anorexia nervosa? Int J Eat Disord. (2013) 46:567–75. doi: 10.1002/eat.22134
65. Cameron DH, McCabe RE, Rowa K, O’Connor C, McKinnon MC. A pilot study examining the use of Goal Management Training in individuals with obsessive-compulsive disorder. Pilot Feasibility Stud. (2020) 6:151–63. doi: 10.1186/s40814-020-00684-0
66. First MB, Spitzer RLG, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, clinician version (SCID-CV). Washington D. C., United States: American Psychiatric Press, Inc (1996).
67. Thomas JJ, Vartanian LR, Brownell KD. The relationship between eating disorder not otherwise specified (EDNOS) and officially recognized eating disorders: meta-analysis and implications for DSM. Psychol Bull. (2009) 135:407–33. doi: 10.1037/a0015326
68. Wilkop M, Wade TD, Keegan E, Cohen-Woods S. Impairments among DSM-5 eating disorders: A systematic review and multilevel meta-analysis. Clin Psychol Rev. (2023) 101:102267. doi: 10.1016/j.cpr.2023.102267
69. Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, assessment and treatment. New York, United States: Guilford Press (1993). p. 317–60.
70. Aardoom JJ, Dingemans AE, Slof Op't Landt MC, Van Furth EF. Norms and discriminative validity of the Eating Disorder Examination Questionnaire (EDE-Q). Eat Behav. (2012) 13:305–9. doi: 10.1016/j.eatbeh.2012.09.002
71. Schmand B, Lindeboom J, Van Harskamp F. De nederlandse leestest voor volwassenen. [the dutch adult reading test]. Lisse, The Netherlands: Swets & Zeitlinger (1992).
72. Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. (2004) 18:37–48. doi: 10.2165/00023210-200418010-00004
73. Tchanturia K, Davies H, Reeder C, Wykes T. Cognitive remediation programme for anorexia nervosa: A manual for practitioners. Institute of Psychiatry (2010). Available at: http://www.katetchanturia.com (Accessed January 11, 2024).
74. van Passel B, Cath DC. Specialized Attention Therapy for patients with anorexia nervosa or obsessive compulsive disorder. Nijmegen, The Netherlands: Radboud University (2013).
75. Wamel A, Wassink M. Landelijk basisprogramma eetstoornissen. Utrecht, The Netherlands: Trimbos-instituut (2006).
76. Balkom A, Vliet IV, Emmelkamp P, Bockting C, Spijker J, Hermens M, et al. Multidisciplinaire richtlijn Angststoornissen. Richtlijn voor de diagnostiek, behandeling en begeleiding van volwassen patiënten met een angststoornis. 3th. Utrecht, The Netherlands: Trimbos-instituut (2013).
77. American Psychiatric Association [APA]. Treatment of patients with eating disorders, third edition. Am J Psychiatry. (2006) 163:4–54. doi: 10.1176/appi.books.9780890423363
78. Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB, American Psychiatric A. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. (2007) 164:5–53.
79. Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? [Peer Reviewed. Int J Eating Disord. (1994) 16:363–70. doi: 10.1002/1098-108X(199412)16:4<363::AID-EAT2260160405>3.0.CO;2-#
80. Cooper Z, Cooper PJ, Fairburn CG. The validity of the eating disorder examination and its subscales. Br J Psychiatry. (1989) 154:807–12. doi: 10.1192/bjp.154.6.807
81. Luce KH, Crowther JH. The reliability of the Eating Disorder Examination-Self-Report Questionnaire Version (EDE-Q). Int J Eat Disord. (1999) 25:349–51. doi: 10.1002/(SICI)1098-108X(199904)25:3<349::AID-EAT15>3.0.CO;2-M
82. Goodman WK, Rasmussen SA, Price LH, Mazure C, Heninger G, Charney D. Yale-brown obsessive compulsive scale (Y-BOCS Delis-Kaplan Executive Function System (D–KEFS) [Database record. [Peer reviewed. Verhaltenstherapie. (1991) 1:226–33. doi: 10.1159/000257973
83. Reitan R. Trail Making test: Manual for administration, scoring, and interpretation. Bloomington: I. University (1956).
84. Stroop JR. Studies of the interference in serial verbal reactions. J Exp Psychol. (1935) 18:643–62. doi: 10.1037/h0054651
85. Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D–KEFS) [Database record]. APA PsycTests. (2001). doi: 10.1037/t15082-000
86. Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. (2001) 31:5–17. doi: 10.1023/a:1005653411471
87. R Core Team. A language and environment for statistical computing (2021). Available online at: https://www.R-project.org (Accessed January 04, 2023).
88. Bates D, Maechler M, Bolker B, Walker S. Lme4: linear mixed-effects models using eigen and S4 (2021). Available online at: https://github.com/lme4/lme4/ (Accessed January 04, 2023).
89. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Software. (2017) 82:1–26. doi: 10.18637/jss.v082.i13
90. Lenth RV. Estimated Marginal Means, aka Least-Squares Means [R package emmeans version 1.8.4-1]. (2022). Available at: https://cran.r-project.org/web/packages/emmeans/.
91. Herbrich-Bowe L, Bentz LK, Correll CU, Kappel V, van Noort BM. Randomized controlled trial of cognitive remediation therapy in adolescent inpatients with anorexia nervosa: Neuropsychological outcomes. Eur Eat Disord Rev. (2022) 30:772–86. doi: 10.1002/erv.2921
92. Schubert C, Hilbert S, Favreau M, Wolstein J, Voderholzer U. Psychological flexibility as a potential change factor in cognitive behavioural therapy of OCD. Behav Cogn Psychother. (2022) 50:381–91. doi: 10.1017/S1352465822000091
93. Harper JA, Brodrick B, Van Enkevort E, McAdams CJ. Neuropsychological and cognitive correlates of recovery in anorexia nervosa. Eur Eating Disord Rev. (2017) 25:491–500. doi: 10.1002/erv.2539
94. Braga DT, Abramovitch A, Fontenelle LF, Ferrao YA, Gomes JB, Vivan AS, et al. Neuropsychological predictors of treatment response to cognitive behavioral group therapy in obsessive-compulsive disorder. Depress Anxiety. (2016) 33:848–61. doi: 10.1002/da.22509
95. Voderholzer U, Schwartz C, Freyer T, Zurowski B, Thiel N, Herbst N, et al. Cognitive functioning in medication-free obsessive-compulsive patients treated with cognitive-behavioural therapy. J Obsessive-Compulsive Related Disord. (2013) 2:241–8. doi: 10.1016/j.jocrd.2013.03.003
96. Oldershaw A, Lavender T, Schmidt U. Are socio-emotional and neurocognitive functioning predictors of therapeutic outcomes for adults with anorexia nervosa? Eur Eating Disord Rev. (2018) 26:346–59. doi: 10.1002/erv.2602
97. Twohig MP, Vilardaga JCP, Levin ME, Hayes SC. Changes in psychological flexibility during acceptance and commitment therapy for obsessive compulsive disorder. J Contextual Behav Sci. (2015) 4:196–202. doi: 10.1016/j.jcbs.2015.07.001
98. Burgess PW, Shallice T. The hayling and brixton tests. Pearson (1997). Available at: https://books.google.nl/books?id=jAs6vwEACAAJ.
99. Wildes JE, Forbes EE, Marcus MD. Advancing research on cognitive flexibility in eating disorders: the importance of distinguishing attentional set-shifting and reversal learning. Int J Eat Disord. (2014) 47:227–30. doi: 10.1002/eat.22243
100. Howlett CA, Wewege MA, Berryman C, Oldach A, Jennings E, Moore E, et al. Same room - different windows? A systematic review and meta-analysis of the relationship between self-report and neuropsychological tests of cognitive flexibility in healthy adults. Clin Psychol Rev. (2021) 88:102061. doi: 10.1016/j.cpr.2021.102061
101. Dennis JP, Vander Wal JS. The cognitive flexibility inventory: instrument development and estimates of reliability and validity. Cogn Ther Res. (2010) 34:241–53. doi: 10.1007/s10608-009-9276-4
102. Miyake A, Emerson MJ, Friedman NP. Assessment of executive functions in clinical settings: problems and recommendations. Semin Speech Lang. (2000) 21:169–83. doi: 10.1055/s-2000-7563
103. Reser MP, Slikboer R, Rossell SL. A systematic review of factors that influence the efficacy of cognitive remediation therapy in schizophrenia. Aust New Z J Psychiatry. (2019) 53:624–41. doi: 10.1177/0004867419853348
104. Danner UN, Dingemans AE, Steinglass JE. Cognitive remediation therapy for eating disorders. Curr Opin Psychiatry. (2015) 28:468–72. doi: 10.1097/YCO.0000000000000192
105. Money C, Genders R, Treasure J, Schmidt U, Tchanturia K. A brief emotion focused intervention for inpatients with anorexia nervosa: a qualitative study. J Health Psychol. (2011) 16:947–58. doi: 10.1177/1359105310396395
106. Meneguzzo P, Bonello E, Tenconi E, Todisco P. Enhancing emotional abilities in anorexia nervosa treatment: A rolling-group cognitive remediation and emotional skills training protocol. Eur Eat Disord Rev. (2024) 32:1026–37. doi: 10.1002/erv.3113
107. Motivala SJ, Arellano M, Greco RL, Aitken D, Hutcheson N, Tadayonnejad R, et al. Relationships between obsessive-compulsive disorder, depression and functioning before and after exposure and response prevention therapy. Int J Psychiatry Clin Pract. (2018) 22:40–6. doi: 10.1080/13651501.2017.1351991
108. Gander F, Proyer RT, Ruch W. Positive psychology interventions addressing pleasure, engagement, meaning, positive relationships, and accomplishment increase well-being and ameliorate depressive symptoms: A randomized, placebo-controlled online study. Front Psychol. (2016) 7:686. doi: 10.3389/fpsyg.2016.00686
Keywords: obsessive compulsive disorder, anorexia nervosa, cognitive flexibility, cognitive remediation therapy, moderation analysis
Citation: van Passel B, Danner UN, Dingemans AE, Broekman TG, Sternheim LC, Becker ES, van Elburg AA, van Furth EF, Hendriks G-J and Cath DC (2025) Remediating cognitive inflexibility in obsessive compulsive disorder and anorexia nervosa neither moderates nor mediates treatment effects: an exploratory study. Front. Psychiatry 15:1456890. doi: 10.3389/fpsyt.2024.1456890
Received: 29 June 2024; Accepted: 09 December 2024;
Published: 13 January 2025.
Edited by:
Karin Meissner, Hochschule Coburg, GermanyReviewed by:
Paolo Meneguzzo, University of Padua, ItalyCristina Segura-Garcia, University of Magna Graecia, Italy
Copyright © 2025 van Passel, Danner, Dingemans, Broekman, Sternheim, Becker, van Elburg, van Furth, Hendriks and Cath. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Boris van Passel, Yi52YW4ucGFzc2VsQHByb3BlcnNvbmEubmw=
 Boris van Passel
Boris van Passel Unna N. Danner
Unna N. Danner Alexandra E. Dingemans
Alexandra E. Dingemans Theo G. Broekman
Theo G. Broekman Lot C. Sternheim
Lot C. Sternheim Eni S. Becker2
Eni S. Becker2 Annemarie A. van Elburg
Annemarie A. van Elburg Eric F. van Furth
Eric F. van Furth Daniëlle C. Cath
Daniëlle C. Cath