- 1Department of Psychology and Neuroscience, University of North Carolina-Chapel Hill, Chapel Hill, NC, United States
- 2Department of Psychiatry, University of North Carolina-Chapel Hill, Chapel Hill, NC, United States
- 3Carolina Institute for Developmental Disabilities , University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, United States
Premenstrual dysphoric disorder (PMDD), a more severe manifestation of premenstrual syndrome (PMS), is characterized by emotional, behavioral, and physical symptoms that begin in the mid-to-late luteal phase of the menstrual cycle, when estradiol and progesterone levels precipitously decline, and remit after the onset of menses. Remotely monitoring physiologic variables associated with PMDD depression symptoms, such as heart rate variability (HRV), sleep, and physical activity, holds promise for developing an affective state prediction model. Switching into and out of depressive states is associated with an increased risk of suicide, and therefore, monitoring periods of affective switching may help mitigate risk. Management of other chronic health conditions, including cardiovascular disease and diabetes, has benefited from remote digital monitoring paradigms that enable patients and physicians to monitor symptoms in real-time and make behavioral and medication adjustments. PMDD is a chronic condition that may benefit from real-time, remote monitoring. However, clinical practice has not advanced to monitoring affective states in real-time. Identifying remote monitoring paradigms that can detect within-person affective state change may help facilitate later research on timely and efficacious interventions for individuals with PMDD. This narrative review synthesizes the current literature on behavioral and physiological correlates of PMDD suitable for remote monitoring during the menstrual cycle. The reliable measurement of heart rate variability (HRV), sleep, and physical activity, with existing wearable technology, suggests the potential of a remote monitoring paradigm in PMDD and other depressive disorders.
1 Introduction
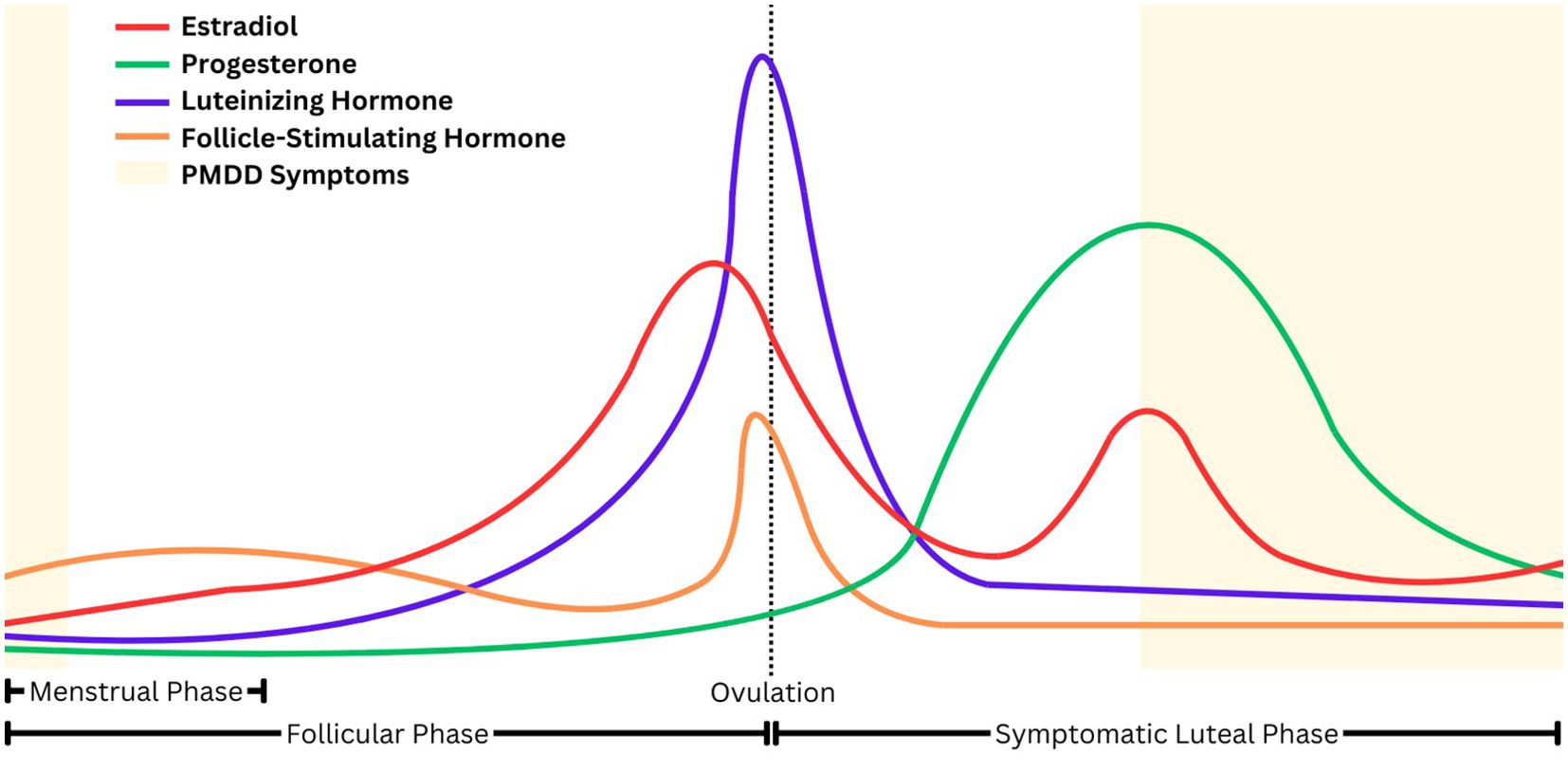
Premenstrual dysphoric disorder (PMDD) is characterized by emotional, behavioral, and physical symptoms that begin in the mid-to-late luteal phase of the menstrual cycle, when estradiol and progesterone levels precipitously decline, and remit after the onset of menses (Figure 1) (1–3). Research suggests that the withdrawal of the neuroactive steroid allopregnanolone (ALLO), a metabolite of progesterone, during the luteal phase may diminish the effect of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) among those with PMDD, leading to a heightened stress response and reduced parasympathetic nervous system activity (3, 4).
PMDD may be thought of as a more impairing and severe manifestation of premenstrual syndrome (PMS), which is also characterized by a combination of physical and emotional symptoms that commonly include anger, depression, irritability, mood swings, breast tenderness, headache, and bloating (5). A recent meta-analysis found a 3.2% global prevalence estimate of confirmed PMDD, whereas PMS has global prevalence estimates of nearly 50% (6, 7). Like other mood disorders, the precipitous onset and remission of symptoms, which can be severe and impairing, often leaves individuals with PMDD uncertain about when symptoms will begin and the degree of impact they will have each menstrual cycle.
Management of other chronic health conditions, including cardiovascular disease and diabetes, has benefited from remote digital monitoring paradigms that enable patients and physicians to monitor symptoms in real-time and make behavioral and medication adjustments (8–12). Although digital health has not been widely adopted in mental healthcare, preliminary evidence suggests it may help individuals with depression and their healthcare providers better identify personalized patterns of risk and enable just-in-time interventions (13). PMDD is an ideal mood disorder to begin building within-person algorithms to detect mood changes, given the frequent cadence of affective switching (i.e., switching from euthymia to depression and back again each month) and the clear benefit of detecting an impending affective switch early enough to prevent or reduce its severity.
In mood disorders generally, the transition into and out of depression (i.e., “affective switching”) is characterized by increased rates of suicide (14–16). Thus, PMDD presents a unique risk due to the frequency of affective switching. Indeed, those with PMDD are seven times more likely to attempt suicide than individuals without PMDD (Prasad et al., 2021).
Despite the high mortality rate in PMDD and the associated importance of monitoring risk, PMDD is difficult to diagnose correctly and monitor over time. Specifically, both the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and the International Classification of Diseases, Eleventh Revision (ICD-11) require prospective daily assessment during two menstrual cycles (17–19). To meet the criteria for PMDD, symptoms must be present for the week before menstruation (i.e., the luteal phase), and symptoms must clear out within the first couple of days of menstruation. Per the DSM-5, at least five symptoms must be endorsed, including at least one symptom from criterion B (marked irritability or anger or increased interpersonal conflicts; markedly depressed mood, feelings of hopelessness, or self-deprecating thoughts; marked anxiety, tension, and/or feelings of being keyed up or on edge) and at least one symptoms from criterion C (decreased interested in usual activity; subjective difficulty concentrating; lethargy, easy fatigability, or marked lack of energy; marked change in appetite, overeating or specific cravings; hypersomnia or insomnia; a sense of being overwhelmed or out of control; physical symptoms such as breast tenderness or swelling, joint or muscle pain, a sensation of “bloating” or weight gain) (18).
At present, there are no commercially available apps or trackers to assist with PMDD diagnosis. The gold standard method of diagnosing PMDD calls for clinicians to use paper and pencil to hand-score prospective symptom ratings (17). Moreover, for individuals with PMDD, the timing of affective state transitions into and out of depression is contingent on the menstrual cycle. However, not all individuals have a reliably consistent cycle length, making it difficult to predict the highest-risk period (20). Ideally, clinicians would monitor those with PMDD during the highest risk period and introduce just-in-time interventions to mitigate impairment and prevent suicidality. Yet, current practice generally precludes prediction of symptom onset and timely intervention.
Other chronic conditions with heightened mortality rates have benefited from real-time remote monitoring. For example, the use of continuous glucose monitoring devices for diabetes mellitus reduces HbA1c by an additional 17-43% compared to usual care (8, 9). Similar impacts have been observed in cardiovascular disease. Compared with usual care, the use of combined remote monitoring and consultation decreases cardiovascular-related mortality and hospitalization by 17% and 28%, respectively (10). Encouragingly, 83% of adults with cardiovascular disease are willing to share wearable device data with their clinicians to improve their care (21).
Like diabetes mellitus and cardiovascular disease, PMDD is a chronic condition that may benefit from real-time, remote monitoring. However, clinical practice has not advanced to monitoring affective states in real-time (22, 23). Given that 29% of Americans already use fitness tracking devices, remote monitoring may be a feasible and affordable way to monitor affective switching (21). Consequently, using wearable devices for remote monitoring of mood disorder symptoms holds the potential for advancing population health in depressive disorders, as it has with cardiovascular disease and diabetes. However, since there is a lack of studies aimed at detecting affective switching through remote monitoring, reviewing the potential physiological biomarkers that could serve as endpoints for affective switching is warranted. PMDD, a relatively homogenous depression subtype with a known, frequent, and regularly occurring trigger of affective switching, holds promise for developing an affective state prediction model (24).
This review aims to synthesize the current literature on behavioral and physiological biomarkers of affective switching in PMDD and depressive disorders, with a focus on their suitability for remote monitoring. In particular, biomarkers were selected for review that are 1) able to be passively monitored with modern wearable technology 2) have an established association with mood and 3) have some literature supporting a relationship with the menstrual-cycle related changes. As a result, heart rate variability (HRV), sleep, and physical activity were selected for review. The review will also explore the predictive utility of passively monitoring smartphone behavior and social smartphone behavior. Finally, gaps in the existing literature will be identified and potential next steps toward applying remote digital monitoring to PMDD and other depressive disorders will be described.
2 Heart rate variability (HRV)
Heart rate variability (HRV), the variation in time between successive heartbeats, is a noninvasive measure of autonomic nervous system (ANS) activity, with higher HRV thought to reflect greater physiologic flexibility and ability to regulate emotional responses (25).
2.1 HRV measurement
HRV is measured using both time-domain measurements or frequency-domain measurements (26, 27), computed in several different ways: time-domain measurements look at the time between successive heartbeats; RR intervals refer to the time between all successive heartbeats; and NN intervals refer to the time between intervals from which artifacts, or abnormal beats, have been removed. Time-domain measures include the standard deviation of NN intervals (SDNN), the square root of the mean squared differences of NN intervals (rMSSD), and the standard deviation of the average NN intervals over a short time period (SDANN). SDNN provides an overall estimate of HRV, while rMSSD provides an overall estimate of short-term components of HRV, and SDANN provides an overall estimate of long-term components of HRV (26).
Frequency-domain measurements look at the relative power of a frequency band. Frequency domain measurements of HRV consist of very low frequency (VLF) power, low frequency (LF) power, high frequency (HF) power, and very high frequency (VHF) power. HF power represents parasympathetic nervous system (PNS) activity, while LF power can be produced by both sympathetic nervous system (SNS) and PNS activity. The LF/HF ratio is thought to represent the balance between the PNS and SNS (26, 27).
The gold standard for measuring HRV is electrocardiography (EKG), which involves measuring electric signals from the heart to measure heart activity (26, 28). HRV can also be measured with photoplethysmography (PPG) (29, 30). PPG uses LED light and a photodetector to measure the amount of light reflected by tissue, representing blood volume changes (31, 32). Thus, PPG measures pulse rate variability (PRV) as a proxy for RR intervals (29, 30). PPG devices are commonly worn on the wrist; however, newer PPG devices with increased accuracy can be worn on the finger (33).
2.2 Remote monitoring of HRV
Wearable devices with EKG or PPG capabilities enable remote monitoring of HRV. For example, the second-generation Oura ring, which uses PPG, demonstrated high agreement with EKG for nocturnal rMSSD (r2 = 0.980) (34). A systematic review of 18 studies compared HRV derived from classic EKGs with HRV derived from commercially available wearable devices (30). Results indicated correlation ranges of r=0.98-0.99 and r=0.85-0.94 for time-domain and frequency-domain indices of HRV respectively, when measured in a resting state. However, the correlations decreased when HRV was not measured in a resting state (30), indicating that wearable devices may be less accurate during activities. A separate review investigating the accuracy of PRV concluded a strong agreement between PRV and EKG when HRV is measured at rest. At the same time, the review noted that physical activity and mental stress may impair agreement. However, quantitative conclusions were precluded by heterogeneity across reviewed studies (29). Thus, currently available wearable devices are as accurate as EKG for measuring HRV and PRV during rest, though they may be less accurate during physical activity.
2.3 HRV and psychopathology
HRV is related to stress, including perceived stress, response to stressful life events, and adaptability to stress (35, 36). HRV has been shown to be significantly reduced in patients with major depressive disorder (MDD) and other psychiatric conditions, including schizophrenia, posttraumatic stress disorder, and bipolar disorder (37, 38). For example, two meta-analyses have demonstrated that individuals with depression have lower HRV, and lower HRV is associated with more severe depression symptoms (36, 39). However, emerging literature suggests that this association may not hold across all populations. Specifically, one study demonstrated that higher resting HRV was associated with more severe depression among Black Americans, especially among Black Americans who endorse the use of culturally compelled coping (40, 41). Thus, additional studies that investigate HRV functioning among diverse populations are needed.
2.4 HRV in PMDD and during the menstrual cycle
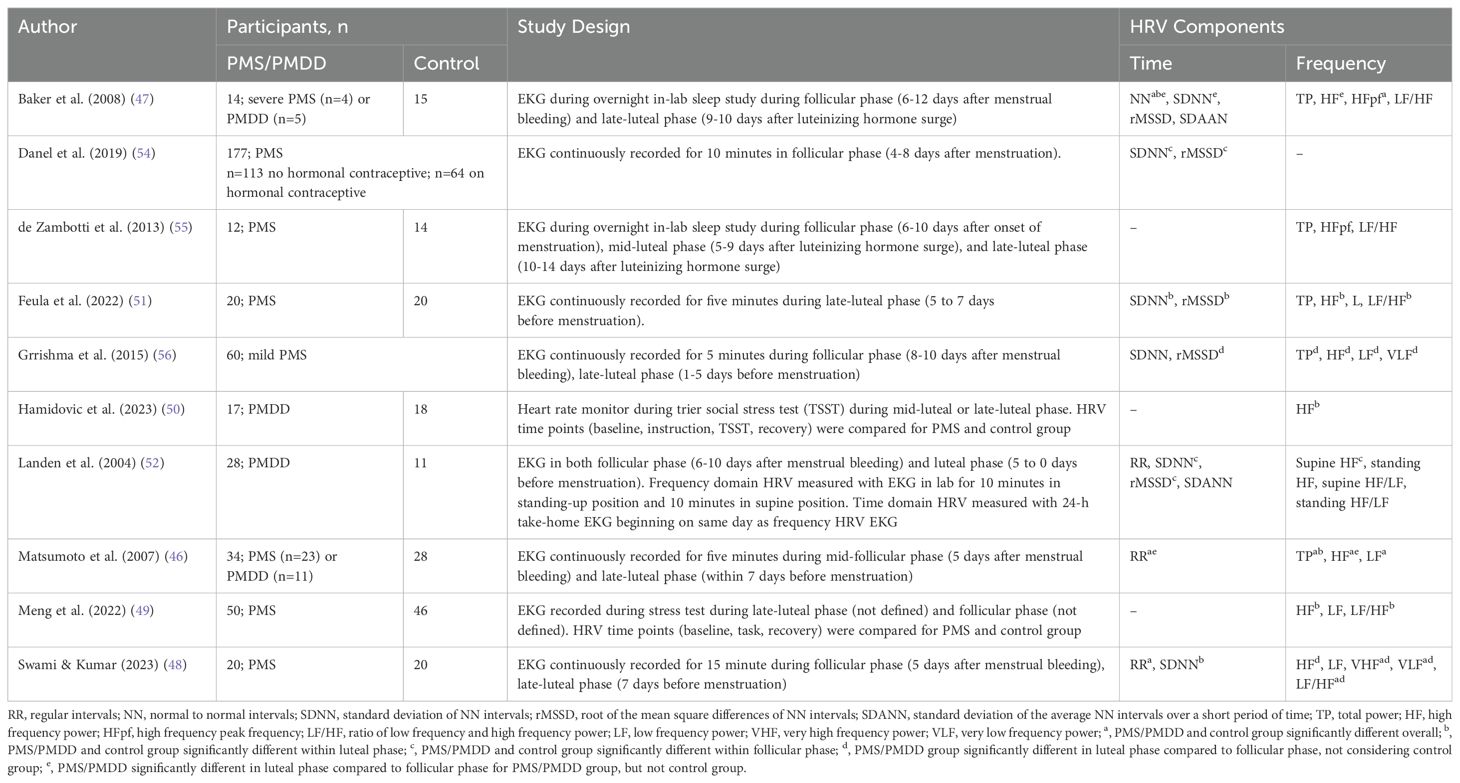
Evidence suggests that HRV changes throughout the menstrual cycle (42–45). However, this relationship may be particularly apparent for those with PMS/PMDD (46, 47). Obtaining a clear understanding of the interaction between PMDD, menstrual phase, and HRV presents challenges because 1) there are two domains of measuring HRV (i.e., frequency and time); 2) there are multiple components of HRV that can be analyzed within each domain (i.e., HF, LF, SSDN, RR intervals, SDAAN, etc.); and 3) there are multiple time points that can be compared (i.e., comparing HRV in a particular cycle phase versus comparing changes in HRV between cycle phases). Several studies have examined HRV in PMS and PMDD, both of which will be discussed to identify themes to guide future studies. Details regarding study samples, design, measurement devices, and findings of studies investigating HRV in PMS and PMDD can be found in Table 1.
2.4.1 Differences in HRV between PMDD, PMS, and asymptomatic control groups during the menstrual cycle
Individuals with PMS/PMDD have been shown to differ from those without PMS/PMDD in certain HRV metrics, regardless of cycle phase. While some studies indicate that those with PMS/PMDD experience lower HRV, on average, results are mixed across studies and thus may be driven by specific HRV components. For instance, while Matsumoto et al. (2007) found that individuals with PMDD have lower HF power throughout the menstrual cycle compared with individuals with PMS or no premenstrual symptoms, Baker et al. (2008) and Swami and Kumar (2023) found that overall HF power was not significantly different between groups (46–48). In contrast, Swami and Kumar found lower VHF power in those with PMS compared to controls throughout the menstrual cycle, and Baker et al. found smaller mean NN intervals in those with PMS compared with controls throughout the menstrual cycle (47, 48).
Results are similarly mixed with regard to LF power. Matsumoto et al. found that LF power was lower in those with PMDD compared with those with PMS and those without PMS/PMDD; Swami and Kumar did not find any group differences; and Baker et al. did not report LF power (46–48). A similar pattern emerged with the LF/HF ratio, where Swami and Kumar found an increased ratio in those with PMS overall; Baker et al. did not find any group differences; and Matsumoto et al. did not report the LF/HF ratio (46–48). Finally, Matsumoto et al. found decreased total power in the PMDD group compared with the PMS and control groups; while Baker et al. did not find any group differences; and Swami and Kumar did not report total power (46–48). Thus, the literature is mixed with regard to differences between PMDD, PMS, and control groups in HRV and its components when measured during the menstrual cycle rather than examining specific cycle phases.
2.4.2 Differences in HRV between PMS and asymptomatic control groups within specific cycle phases
Some studies have examined components of HRV between those with PMS and asymptomatic controls during the follicular phase, luteal phase, or both. For instance, two studies that implemented a social stress test in the luteal phase indicated a delay in HF power recovery after the stress task for the PMS group, compared with controls (49, 50). An additional study indicated lower SDNN and rMSSD in the luteal phase in those with PMS compared with controls (51). However, one study found a lower SDNN, rMSSD, and HF power in the follicular phase for the PMS group compared with controls and did not indicate any within or between-group differences within the luteal phase (52). Similarly, a study that investigated the relationship between PMS symptoms and HRV during the follicular phase found a positive association between PMS symptoms and SDNN and rMSSD for those not on hormonal contraceptives, which is the opposite direction of the effect one would expect based on Landén et al., 2004 (52). This effect did not remain among those on hormonal contraceptives, which could be because hormonal contraceptives have been shown to stabilize the hormonal shifts that occur during the menstrual cycle and reduce PMS symptoms (53, 54). Taken together, these studies suggest HRV and its components are lower in the symptomatic luteal phase in those with PMS compared with those without PMS.
2.4.3 Within-group changes in HRV during the menstrual cycle in PMDD
A considerable number of studies have indicated that the symptomatic luteal phase is characterized by reduced HRV in those with PMS/PMDD. Specifically, women with PMS/PMDD show decreased HF power in the luteal phase compared with the follicular phase (46–48, 55, 56). Additionally, individuals without PMS/PMDD do not show cycle phase differences in HF power, suggesting the luteal phase reduction in HRV is unique to those with PMS/PMDD (46, 47, 55). Time-domain measures such as SDNN and rMSSD are also lower in the luteal phase compared to the follicular phase for those with PMS/PMDD (47, 48, 56). Thus, studies have consistently shown certain aspects of HRV are lower in the luteal phase compared with the follicular phase in those with PMS/PMDD, but not in asymptomatic controls.
2.4.4 Methodological challenges and HRV summary
Across the three sets of studies reviewed, methodologic variation may account for important differences in findings. Of the studies reviewed, two measured HRV during sleep (47, 55), two measured HRV during a stress test (49, 50), and the remaining six measured HRV with a supine or standing EKG sample of varying lengths of time (46, 48, 51, 52, 54, 56). Additionally, as indicated in Table 1, different components of HRV are reported in each study, precluding a full comparison of study results. Finally, sample sizes are consistently small or moderate, and small sample sizes may obscure true findings and may also contribute to false discoveries. Adequately powered studies are needed to determine the extent to which HRV may be associated with the onset of mood symptoms, physiologic symptoms, or their combination during the menstrual cycle in those with PMDD, PMS, and asymptomatic controls.
Despite these methodologic differences, one clear and consistent pattern of results emerged. Across studies, some components of HRV were lower in individuals with PMS/PMDD during the luteal phase compared with the follicular phase, and this difference was unique to individuals with PMS/PMDD. This suggests that HRV variation may be a valid physiologic marker of within-person symptom variation in those with menstrually-related mood disorders (i.e., PMS or PMDD). In contrast, while some studies indicate PMDD is defined by lower HRV across the menstrual cycle compared with those with PMS and asymptomatic controls, these results have not been consistently replicated across studies and should be interpreted with caution. As such, HRV may not be a good diagnostic marker for PMDD.
3 Sleep
3.1 Sleep measurement
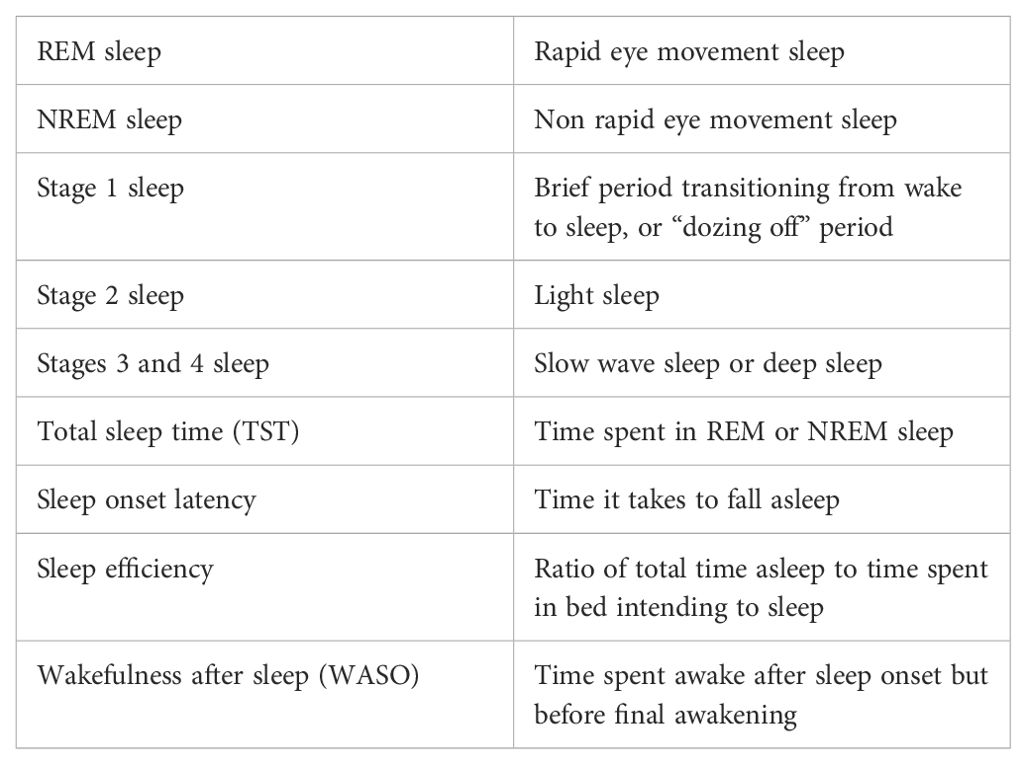
In the literature, sleep is typically assessed by examining the duration of sleep, sleep staging, or both using multi-modal physiologic assessment (Table 2). Polysomnography (PSG) uses a combination of electroencephalogram (EEG), electrooculogram, electromyogram, EKG, pulse oximetry, and airflow and respiratory effort to determine wakefulness and sleep as well as staging (57). PSG offers a comprehensive look at the structural organization of sleep, or sleep architecture, and is considered the gold standard for measuring sleep and diagnosing sleep disorders (58, 59).
Alternative ways to measure sleep physiology include actigraphy and photoplethysmography (PPG). Actigraphs are wearable devices, typically worn on the wrist, that measure sleep by detecting physical movements. Most modern actigraphs include accelerometers for movement detection (60). Additionally, PPG measures heart rate, HRV, blood oxygen saturation, and respiratory rate, which can be used to indicate sleep (32, 33, 61).
Subjective sleep measures, such as sleep diaries or questionnaires, prompt an individual to retrospectively report on sleep components (e.g., time in bed, sleep onset latency). While the most common subjective sleep measures demonstrate strong internal consistency and test-retest reliability, subjective sleep measures are not strongly correlated with objective sleep measures (62–64). In particular, the accuracy of self-reported sleep quality is vulnerable to being impacted by memory processes, personality, mood states, and subjective well-being (64–66). However, subjective sleep measures are low-cost and highly feasible while offering some insight into sleep habits and may help place physiological sleep assessments into context (e.g., knowing that an individual woke up several times in one night because of a thunderstorm can help with the interpretation of physiologic measures).
3.2 Remote monitoring of sleep
PSG typically involves an individual spending at least one night sleeping in a sleep laboratory setting and consists of a specialist observing and interpreting the gathered PSG sleep data. Despite PSG being the gold standard of sleep measurement, wearable devices therefore offer a more unobtrusive, affordable, and feasible way to monitor sleep on an ongoing basis. In determining the validity of remote sleep monitoring devices, attention is paid to sensitivity (i.e., ability to detect sleep), specificity (i.e., ability to detect wake), and staging (i.e., ability to detect sleep stage) (67, 68).
Actigraphy can assess sleep-wake patterns in individuals with average or good sleep with reasonable reliability and validity compared to PSG (60, 69, 70). Additionally, the accuracy of consumer actigraphy devices is comparable to that of research-grade actigraphy devices (71–73). Actigraphy has strong sensitivity (ability to detect sleep) but tends to overestimate total sleep time. However, specificity (ability to detect wake) is consistently low (32, 74–77). Moreover, accuracy may diminish among people with lower sleep quality depending on the device being used (74, 78–80). Another disadvantage of actigraphy is its lack of validation for identifying sleep stages (68, 81). Taken together, these studies suggest that both actigraphy and commercial grade wearable devices can validly measure sleep initiation and duration.
Newer consumer devices include a combination of PPG, accelerometry, and body temperature to achieve increased sleep/wake scoring accuracy compared to actigraphy alone. Moreover, PPG can predict sleep staging with moderate accuracy compared to PSG (32, 68, 71, 82–84). With few exceptions, PPG-based devices that classify sleep into three or four stages have 65-75% staging accuracy (68). Despite this potential for remotely monitoring sleep staging, consumer wearable devices have distinct disadvantages in the research context: 1) the scoring algorithms used by the consumer devices are often proprietary and 2) ongoing improvements to these algorithms may impact within-person reliability during ongoing sleep studies (32).
Newer studies have begun to look at wearable and portable EEG devices, such as in-ear or headband EEG devices. Some wearable EEG devices, such as the Dreem headband or an in-ear EEG, may be more accurate than accelerometers and PPG and are capable of identifying all five sleep stages when used properly (32, 68, 82, 85, 86). Thus, portable and wearable EEG technologies hold promise for studying sleep/wake and sleep staging.
3.3 Sleep and psychopathology
Sleep disturbances are transdiagnostic precipitants and symptoms of MDD and other psychiatric disorders, including bipolar disorder and schizophrenia (87–89). Sleep disturbances are thought to indicate an underlying circadian dysfunction in MDD and mood disorders more generally (90), though circadian dysfunction has not been well studied in PMDD.
3.4 Sleep and the menstrual cycle
Sleep varies by menstrual phase among menstruating individuals, irrespective of PMS/PMDD status. The exact nature of this relationship, however, is not fully understood. Women generally report decreases in perceived sleep quality in the luteal phase compared with the follicular phase (91, 92). One study of 163 women used actigraphy to measure sleep and found that sleep efficiency declined gradually across the menstrual cycle, with a more apparent decline in the luteal phase. However, participants were not all regularly menstruating and were also in different stages of the reproductive life cycle (pre-, early-, and late-perimenopause) (92). Thus, the relationship between sleep efficiency and the menstrual cycle may be somewhat obscured by the inclusion of those who were not regularly menstruating.
Additional studies have directly examined the relationship between sleep and ovarian hormone changes during the menstrual cycle. Rising progesterone levels have been associated with objective sleep measures, including decreased sleep HRV (55) and increased PSG-measured sleep disturbances (93). A recent review found that endogenous progesterone has a sleep-promoting effect and that hormone-related sleep problems were more associated with the rate of change in reproductive hormones than the absolute levels of hormones (94). Taken together, progesterone may regulate sleep during the menstrual cycle in regularly menstruating individuals and may be responsible for cycle phase effects.
3.5 Sleep and PMDD
The menstrual cycle may have a greater impact on sleep among those with PMS or PMDD who report higher levels of insomnia and fatigue and perceive lower sleep quality throughout the entire menstrual cycle compared to those without PMS/PMDD, with the greatest differences occurring during the luteal phase (47, 92, 95–98). However, few studies have examined objective measures of sleep in this population (99).
Baker et al. (2012) compared objective and subjective sleep measures in 18 women with severe PMS and 18 women with minimal menstrual symptoms. The PMS group exhibited poorer subjective sleep quality in the luteal phase and increased levels of slow-wave sleep, as measured by PSG, throughout the menstrual cycle, compared with controls (100). Similar results were found by Shechter et al. (2012), where women with PMDD and luteal-phase insomnia (n=7) experienced more slow-wave sleep during the luteal phase compared with a control group (n=5). However, the sample size was small and the control selection criteria were not well defined (101). In a study done by de Zambotti et al. (2013) the PMS group (n=12) appeared to spend more time in slow-wave sleep in the luteal phase compared with controls (n=14) (17.4 vs 14.3% TST in mid-luteal; 16.2 vs 11.3% TST late-luteal), yet, these results did not reach significance (55).
In contrast, earlier studies indicated that individuals with PMS/PMDD display decreased slow-wave sleep compared to a control group during both the follicular and luteal phases although small sample sizes limit these findings (84, 95). A larger study (n=23 PMDD; n=18 controls) found no difference in slow-wave sleep during the mid-follicular phase or the late-luteal phase, however, results should be interpreted within the context of a clinical trial looking at sleep deprivation therapy (102).
The relevance of sleep in PMDD is further indicated by a series of studies that demonstrated a delayed reduction in endogenous melatonin levels in mornings during the luteal phase compared with the follicular phase in those with PMDD (102–106).
Overall, future studies should focus on delineating the relationship between PMDD and sleep at the within-person level to determine if remote sleep monitoring devices can be used to predict or detect affective switching.
4 Physical activity
4.1 Physical activity measurement
Physical activity can be measured via self-report, accelerometers, pedometers, heart rate monitors, and sensors that combine different measurement modalities (108, 109). Aspects of physical activity that can be measured may include energy expenditure, step count, distance traveled, and time spent in different postures. The gold-standard method for measuring physical activity involves quantifying energy expenditure using the doubly labeled water method, which entails measuring elimination rates of specific isotypes following the ingestion of deuterium and heavy oxygen-labeled water (110). This method is expensive, burdensome, and time-intensive and is therefore not feasible for remote monitoring of physical activity (for a review, see Sylvia et al. (108).
4.2 Remote monitoring of physical activity
Wearable devices are overall an accurate and feasible way to track physical activity, although validity varies between brand. Fuller et al. (2020) conducted a systematic review of commercially available wearable devices for measuring steps, energy expenditure, and heart rate. The review indicated that criterion validity depends on the device, study type (controlled or naturalistic), and type of measurement. Validity for step count was best for Apple Watch and Garmin, while Fitbit, Samsung, and Withings were within +/-3 mean percentage error on average. Heart rate was also accurately measured; all brands fell within +/-3 mean percentage error on average, with a small tendency for underestimation. Wearable devices were found to be unreliable for measuring energy expenditure (111). However, this review did not include devices designed to be worn on the finger (e.g., Oura ring), which emerging studies demonstrate to be highly correlated with gold-standard measures of step counts, heart rate, and energy expenditure (112, 113).
4.3 Physical activity and psychopathology
Depressive symptoms and physical activity have a well-established link. A meta-analysis of 42 studies reported a significant inverse relationship between physical activity (i.e. actigraphy or pedometer) and rates of depression. However, these findings were based on cross-sectional studies, so the directionality of the effects cannot be inferred (114). Decreased physical activity has been consistently linked to risk for depression, although findings regarding the impact of depression on subsequent physical activity are mixed (115–119) Nevertheless, objective measures support a strong negative relationship between depressive symptom severity and daily step count (118, 119).
A wealth of research has been conducted on the effectiveness of physical activity as an intervention for depression. Hu et al. (2020) conducted a systematic review of eight meta-analyses across 134 studies concerning exercise as an intervention for depression symptoms. They concluded that exercise interventions have a moderate effect on reducing depressive symptoms (120). A separate systematic review of 13 studies reported that 10 studies showed a statistically significant reduction in depression symptoms following a randomized-controlled exercise intervention. The review concluded that any physical activity for 30-45 minutes at least three times a week, preferably performed under supervision, is recommended to treat MDD (121). Given both the naturalistic and experimental results linking depression and physical activity, physical activity may be a reliable physiologic indicator of depressed mood.
4.4 Physical activity, the menstrual cycle, and PMDD
To date, there is a lack of research on the relationship between physical activity, the menstrual cycle, and PMDD. A recent meta-analysis on the effects of the menstrual cycle phase on exercise indicated that there may be a trivial reduction in exercise during the early follicular phase (122). A separate study indicated no reduction in step count as a result of menstrual phase (123). Another review looked at the performance of athletes throughout the menstrual cycle and concluded mixed findings regarding levels of physical activity or athletic performance and the menstrual phase (124).
Studies investigating changes in physical activity throughout the menstrual cycle among individuals with PMS/PMDD are lacking. However, one study indicated that women with severe PMS walked 1,411 fewer steps during the luteal phase and menses compared with asymptomatic control women (125). Additionally, observational studies support a negative relationship between looking at PMS/PMDD symptoms and general exercise (126–128). Additionally, there is growing evidence supporting physical activity as an effective intervention for PMS (128, 129). A systematic review of five RCTs with 492 participants concluded that aerobic exercises effectively improve premenstrual symptoms (130).
Physical activity and depression symptom severity are likely bidirectionally related. As depression is a common feature of PMDD, more research is warranted to determine the extent to which objective measures of physical activity can be used to predict PMDD symptoms.
5 Social behaviors and smartphone use
5.1 Remote monitoring of social behavior with smartphones
Aspects of social behavior can be gleaned by tracking smartphone use. For instance, smartphone use for interpersonal connection is positively associated with a higher likelihood of participating in social activities (131), greater belonging support, and greater tangible social support over time. Problematic smartphone use, involving an excessive psychological attachment to one’s smartphone, is associated with less tangible social support over time (132). Thus, smartphone activity, and specific types of smartphone activity, may be a feasible proxy for social behavior.
5.2 Remote monitoring of mood with smartphones
Smartphone data may be associated with mood. Objectively monitored speech patterns from smartphone voice data can predict mood states with up to 97.4% accuracy (133, 134). Applying machine learning models to passively collected smartphone data has been shown to accurately detect fluctuations in mood states, including in those with MDD (135–138). Given the established predictive utility of passively collected smartphone-use data on mood fluctuations, applying these findings to affective switching among people with PMDD is a promising area for future investigation.
5.3 Social media use and mood
Research strongly supports a relationship between social impairments and depressive symptoms (139–141). Moreover, social interaction and support are known to influence clinical outcomes in depression (142–144). The emergence of smartphones and social media introduces new considerations when studying social impairment. While some social media interactions improve mood, most studies show that increased time spent engaging with cell phones and social media apps is associated with greater depression severity (145–149).
Studies of social media use and mood have produced mixed findings. A systematic review of 13 studies investigating adolescent social media use demonstrated a positive association between psychological distress and social media use across multiple measures (150). However, a separate review noted that research on social media use and adolescents has been mostly cross-sectional and has generated conflicting results and small effect sizes (151). Another review indicated a positive association between social media use and mood (152). Social media findings are thus hard to interpret. More detailed studies measuring how an individual uses social media will likely provide better information about the impact of social media on mood. So far, studies of the type of social medial interactions (i.e., active vs passive, private vs public) have yielded similarly mixed findings (153–155).
5.4 Social impairment, PMDD, and the menstrual cycle
PMDD is associated with social impairment during the luteal phase, including interference in relationships with friends, classmates, and coworkers (156–160). Rubinow and colleagues (2017) administered a Facial Discrimination Task in the luteal and follicular phases of women with PMDD and asymptomatic controls. They found that women with PMDD exhibited increased negative judgments and impaired specificity of judgments during the luteal phase compared with the follicular phase, while controls did not experience any menstrual effects (157). These findings suggest that facial recognition is impaired during the luteal phase in PMDD, which could have downstream effects on social behavior.
Women with PMDD also report higher levels of hostility regardless of menstrual phase (158) and more aggressive tactics to solve conflict during the luteal phase (161). Kaiser and colleagues found that among women with PMDD, pain and somatic dysphoria in the luteal phase is correlated with impairment in social activities, while premenstrual irritability in the luteal phase is correlated with impairment in relationships (159). These findings support previous research suggesting that those with PMDD suffer from increased irritability in the luteal phase, which could negatively impact social engagement (158, 162).
Overall, considering the feasibility of smartphone data collection, future research regarding the predictive utility of smartphone data both generally and as a proxy for social behavior among those with PMDD is warranted. However, current research methods of social media use are crude proxies for more nuanced social interactions that could be collected by monitoring social media and smartphone use.
6 Discussion
This review synthesized the current literature on behavioral and physiological correlates of PMDD suitable for remote monitoring during the menstrual cycle. PMDD is marked by the onset and offset of a depressive state provoked by hormonal fluctuations during the menstrual cycle. Switching into and out of depressive states is associated with an increased risk of suicide, therefore, periods of affective switching may be important to monitor to enable just-in-time interventions. Given the cyclical and chronic nature of affective switching in PMDD and attendant suicide risk, identifying remote monitoring paradigms that can detect within-person affective state change may help facilitate later research on timely and efficacious interventions. The reliable measurement of key physiologic variables associated with depression symptoms, HRV, sleep, and physical activity, with existing wearable technology, suggests the potential of a remote monitoring paradigm in PMDD.
6.1 HRV
HRV is an indicator of ANS activity that can be effectively monitored with remote wearable devices, particularly during rest period (29, 30, 34). HRV has been found to relate to stress and depression severity, and is significantly reduced in patients with mental illness (35–39). Although few studies have examined the relationship between PMDD and HRV, recent evidence suggests reduced HRV during the symptomatic luteal phase in those with PMS/PMDD (46–48, 55, 56). Findings consistently demonstrate decreased HF power during the luteal phase, which can be interpreted as a reduction in overall PNS activity (26). These findings align with MDD literature that indicates lower HRV in individuals with symptomatic MDD, compared with controls (36, 39). Although existing studies demonstrated group-level reductions in HRV between the follicular and luteal phases in those with PMDD/PMS, additional research to establish within-person changes in HRV or HF power will be needed to establish the use of this variable as a correlate or predictor of symptom onset to guide clinical practice.
6.2 Sleep
Sleep disturbances are an established precipitant and symptom of psychiatric disorders that can be tracked easily and accurately with remote monitoring (68, 87, 88, 90). In particular, remote monitoring is an effective tool for capturing total sleep-wake time, and newer technology has begun to track sleep staging reliably (32, 68, 71, 82–86). Individuals with PMS/PMDD have more of a negative perception of sleep quality, particularly heightened during the luteal phase, compared to those without PMS/PMDD (47, 92, 95–98). Evidence suggests that the circadian rhythm may be disturbed in the luteal phase among those with PMS/PMDD, with some indications of altered melatonin secretion and slow-wave sleep (55, 100–107). However, because some studies indicate that sleep abnormalities persist throughout the menstrual cycle without showing phasic differences, sleep may be a less useful metric of affective switching in PMDD. Despite this, the prominent finding that perceived sleep quality diminishes in the luteal phase should not be disregarded. It is plausible that sleep quality perception is influenced by psychological state rather than actual sleep quality. However, it is also plausible that changes in perceived sleep quality can be attributed to changes in sleep architecture that are not detectable with between-person study designs. Future studies should focus on delineating the relationship between PMDD and sleep at the within-person level to determine if remote sleep monitoring devices can predict affective switching and help inform the implementation of effective sleep interventions.
6.3 Physical activity
Physical activity can be easily and accurately tracked with remote monitoring methods (111–113). Despite a well-established relationship between depression and physical activity, the bidirectional nature of this relationship has not been well articulated (114–119). In PMS/PMDD, there is not enough evidence that physical activity and exercise (both performance and amount) vary by menstrual phase. Research regarding physical activity/exercise as an intervention is more well-established. Evidence suggests that exercise can meaningfully reduce depression symptoms among those with a depressive disorder (120, 121). Further, growing evidence supports physical activity as an effective intervention for PMS/PMDD (128–130). Additional research is needed to determine whether objective measures of physical activity can be used to predict PMDD symptom onset.
6.4 Social behaviors and smartphone use
Machine learning models applied to passively collected smartphone data to predict mood and social behavior (133–138). Thus, the justification for studying PMDD and smartphone data is two-fold. First, PMDD is marked by social impairment during the symptomatic luteal phase (156, 157, 159, 160, 162). Thus, passively collected smartphone data may be a feasible and unobtrusive proxy for social behavior that can identify affective switching and inform effective social interventions. Second, smartphone data has been demonstrated to predict affective switching with reliable accuracy among individuals with psychiatric illnesses (133–138). Thus, studying the application of machine learning capabilities to model smartphone use in individuals with PMDD is a logical next step. Overall, smartphone data has the potential to reliably predict affective switching among those with PMDD and be used as a marker of social behavior. However, more granular data regarding social communication with smartphones and social media seem necessary, compared with rough metrics of smartphone and social media use.
6.5 Theoretical model of affective switching in PMDD
Based on prior research, withdrawal of the neuroactive steroid allopregnanolone (ALLO) during the luteal phase may diminish the inhibitory effect of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) among those with PMDD, leading to a heightened stress response and reduced parasympathetic nervous system activity (3, 4). Although not fully understood, in mammals, GABA activity may modulate the activity of neurons in the suprachiasmatic nucleus, which regulates melatonin secretion (163, 164). Thus, alterations in GABA functioning might have downstream effects on melatonin secretion and circadian rhythms, contributing to sleep disturbances. As a result, emotional regulation may become compromised by reduced GABA function and fatigue. Although the exact mechanisms of HRV are unclear, both depression symptoms and GABAergic activity may lead to decreased HRV, further impairing stress and emotion regulation abilities. The subsequent cycle of stress, fatigue, and depressive symptoms may yield social withdrawal and inactivity, creating a compounding effect on overall well-being. Importantly, the endpoints of sleep disturbances, HRV, physical activity, and social engagement can be unobtrusively monitored with widely used wearable devices and smartphones.
6.6 Limitations
Given that PMDD was not added to the DSM until 2013, there is less research on PMDD as a diagnostic entity, and earlier studies included those with PMDD in studies of PMS. Thus, the extent to which PMDD and PMS are distinct or overlapping entities concerning physiologic markers remains somewhat unclear. The research conducted since 2013 seems to indicate that PMDD is distinct from asymptomatic controls with regard to certain physiologic markers, while those with PMS appear similar to those with PMDD in some studies and more similar to controls in others. Due to the relative lack of research focusing specifically on PMDD, PMS studies were included. However, PMS findings should be considered preliminary as they pertain to individuals with PMDD.
Moreover, existing studies on PMS or PMDD often included small sample sizes and had certain methodological issues. For example, PMDD diagnoses in the reviewed studies were not always based on the gold standard prospective reporting method. Additionally, existing studies did not always control for factors that may affect mood states and the menstrual cycle. For example, study results can be impacted by hormonal contraceptive use, comorbid diagnoses, cycle regularity, pregnancy status, and demographic factors such as age, race, or ethnicity (165). Although assessing and controlling for these factors can be challenging, future studies should measure and report such variables, and where appropriate, control for these confounding variables in statistical analyses.
Additionally, existing studies have predominately been conducted on cis-gendered women and neglect to consider the impact of alternate gender identities (transgender, non-binary, gender non-conforming, etc.). As such, those who do not identify as a cis-gendered woman are underrepresented in this area of research. Because people of alternate gender identities are at heightened risk for adverse mental health outcomes, excluding this population may perpetuate systemic barriers to accessing care (166). Considering gender identity in analyses, oversampling non-cisgender individuals, or not excluding people of non-cisgender identities is imperative.
7 Conclusion
PMDD is marked by frequent affective switching, with depressive symptoms beginning during the luteal phase and ending shortly after the onset of menses. Affective switching is a period of increased risk of suicide. Given the frequency of affective switching and the chronicity of PMDD, identifying an unobtrusive strategy for identifying periods of heightened risk could enable the delivery of just-in-time interventions. Additionally, given the frequency of affective switching, PMDD may serve as an ideal model for prospectively identifying physiologic markers of affective switching that could be applied to identifying depressive episodes in other depressive disorders that are more difficult to predict.
Remote monitoring is a promising, non-invasive, and passive mechanism for predicting affective switching and providing real-time intervention, as exemplified in chronic conditions such as diabetes and heart conditions. Prospectively identifying within-person physiological and behavioral correlates and predictors of affective switching suitable for remote monitoring is the first step in implementing such a strategy for PMDD. Whether phase-dependent variations in HRV, sleep, physical activity, social variations, and smartphone data that can be monitored remotely will be able to predict affective switching at the individual level will require additional research. If these physiologic variables predict within-person affective switching in those with PMDD, remote monitoring would hold tremendous promise for advancing population health by identifying personalized, scalable intervention strategies for those with PMDD.
Author contributions
RB: Writing – original draft, Writing – review & editing. EB: Writing – original draft, Writing – review & editing. GD: Writing – original draft, Writing – review & editing. JP: Writing – original draft, Writing – review & editing. CS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by funding from the Foundation of Hope.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. (2012) 169:465–75. doi: 10.1176/appi.ajp.2012.11081302
2. Epperson CN. Premenstrual Dysphoric disorder and the brain. AJP. (2013) 170:248–52. doi: 10.1176/appi.ajp.2012.12121555
3. Hantsoo L, Epperson CN. Allopregnanolone in premenstrual dysphoric disorder (PMDD): Evidence for dysregulated sensitivity to GABA-A receptor modulating neuroactive steroids across the menstrual cycle. Neurobiol Stress. (2020) 12:100213. doi: 10.1016/j.ynstr.2020.100213
4. Gao Q, Sun W, Wang YR, Li ZF, Zhao F, Geng XW, et al. Role of allopregnanolone-mediated γ-aminobutyric acid A receptor sensitivity in the pathogenesis of premenstrual dysphoric disorder: Toward precise targets for translational medicine and drug development. Front Psychiatry. (2023) 14. doi: 10.3389/fpsyt.2023.1140796
5. Nappi RE, Cucinella L, Bosoni D, Righi A, Battista F, Molinaro P, et al. Premenstrual syndrome and premenstrual dysphoric disorder as centrally based disorders. Endocrines. (2022) 3:127–38. doi: 10.3390/endocrines3010012
6. Direkvand-Moghadam A, Sayehmiri K, Delpisheh A, Kaikhavandi S. Epidemiology of premenstrual syndrome (PMS)-A systematic review and meta-analysis study. J Clin Diagn Res. (2014) 8:106–9. doi: 10.7860/JCDR/2014/8024.4021
7. Reilly TJ, Patel S, Unachukwu IC, Knox CL, Wilson CA, Craig MC, et al. The prevalence of premenstrual dysphoric disorder: Systematic review and meta-analysis. J Affect Disord. (2024) 349:534–40. doi: 10.1016/j.jad.2024.01.066
8. Maiorino MI, Signoriello S, Maio A, Chiodini P, Bellastella G, Scappaticcio L, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: A systematic review with meta-analysis of randomized controlled trials. Diabetes Care. (2020) 43:1146–56. doi: 10.2337/dc19-1459
9. Kieu A, King J, Govender RD, Östlundh L. The benefits of utilizing continuous glucose monitoring of diabetes mellitus in primary care: A systematic review. J Diabetes Sci Technol. (2023) 17:762–74. doi: 10.1177/19322968211070855
10. Kuan PX, Chan WK, Ying DKF, Rahman MAA, Peariasamy KM, Lai NM, et al. Efficacy of telemedicine for the management of cardiovascular disease: a systematic review and meta-analysis. Lancet Digital Health. (2022) 4:e676–91. doi: 10.1016/S2589-7500(22)00124-8
11. Kario K. Management of hypertension in the digital era. Hypertension. (2020) 76:640–50. doi: 10.1161/HYPERTENSIONAHA.120.14742
12. Joubert M, Benhamou PY, Schaepelynck P, Hanaire H, Catargi B, Farret A, et al. Remote monitoring of diabetes: A cloud-connected digital system for individuals with diabetes and their health care providers. J Diabetes Sci Technol. (2019) 13:1161–8. doi: 10.1177/1932296819834054
13. Walsh AEL, Naughton G, Sharpe T, Zajkowska Z, Malys M, van Heerden A, et al. A collaborative realist review of remote measurement technologies for depression in young people. Nat Hum Behav. (2024) 8:480–92. doi: 10.1038/s41562-023-01793-5
14. Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv. (2001) 52:828–33. doi: 10.1176/appi.ps.52.6.828
15. Inagaki M, Kawashima Y, Kawanishi C, Yonemoto N, Sugimoto T, Furuno T, et al. Interventions to prevent repeat suicidal behavior in patients admitted to an emergency department for a suicide attempt: A meta-analysis. J Affect Disord. (2015) 175:66–78. doi: 10.1016/j.jad.2014.12.048
16. Brodsky BS, Spruch-Feiner A, Stanley B. The zero suicide model: applying evidence-based suicide prevention practices to clinical care. Front Psychiatry. (2018) 9. doi: 10.3389/fpsyt.2018.00033
17. Eisenlohr-Moul TA, Girdler SS, Schmalenberger KM, Dawson DN, Surana P, Johnson JL, et al. Toward the reliable diagnosis of DSM-5 premenstrual dysphoric disorder: the carolina premenstrual assessment scoring system (C-PASS). Am J Psychiatry. (2017) 174:51–9. doi: 10.1176/appi.ajp.2016.15121510
18. APA. Mood Disorders in the DSM-5. 5th ed. Arlington, VA: American Psychiatric Publishing (2013).
19. World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). Geneva: World Health Organization (2019).
20. Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstetric Gynecologic Neonatal Nursing. (2006) 35:376–84. doi: 10.1111/j.1552-6909.2006.00051.x
21. Dhingra LS, Aminorroaya A, Oikonomou EK, Nargesi AA, Wilson FP, Krumholz HM, et al. Use of wearable devices in individuals with or at risk for cardiovascular disease in the US, 2019 to 2020. JAMA Network Open. (2023) 6:e2316634. doi: 10.1001/jamanetworkopen.2023.16634
22. Matcham F, Leightley D, Siddi S, Lamers F, White KM, Annas P, et al. Remote Assessment of Disease and Relapse in Major Depressive Disorder (RADAR-MDD): recruitment, retention, and data availability in a longitudinal remote measurement study. BMC Psychiatry. (2022) 22:136. doi: 10.1186/s12888-022-03753-1
23. Pedrelli P, Fedor S, Ghandeharioun A, Howe E, Ionescu DF, Bhathena D, et al. Monitoring changes in depression severity using wearable and mobile sensors. Front Psychiatry. (2020) 11. doi: 10.3389/fpsyt.2020.584711
24. Rubinow DR, One Small Step for PMDD. One large step for affective disorders. AJP. (2021) 178:215–7. doi: 10.1176/appi.ajp.2020.20121793
25. Porges SW. The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic J Med. (2009) 76:S86. doi: 10.3949/ccjm.76.s2.17
26. Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability. Circulation. (1996) 93:1043–65. doi: 10.1161/01.CIR.93.5.104
27. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:258. doi: 10.3389/fpubh.2017.00258
28. Institute for Quality and Efficiency in Health Care (IQWiG). In brief: What is an electrocardiogram (ECG)? NCBI Bookshelf. (2023). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK536878/.
29. Schäfer A, Vagedes J. How accurate is pulse rate variability as an estimate of heart rate variability?: A review on studies comparing photoplethysmographic technology with an electrocardiogram. Int J Cardiol. (2013) 166:15–29. doi: 10.1016/j.ijcard.2012.03.119
30. Georgiou K, Larentzakis AV, Khamis NN, Alsuhaibani GI, Alaska YA, Giallafos EJ. Can wearable devices accurately measure heart rate variability? A systematic review. Folia Med (Plovdiv). (2018) 60:7–20. doi: 10.2478/folmed-2018-0012
31. Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. (2007) 28:R1. doi: 10.1088/0967-3334/28/3/R01
32. Lujan MR, Perez-Pozuelo I, Grandner MA. Past, present, and future of multisensory wearable technology to monitor sleep and circadian rhythms. Front Digit Health. (2021) 3. doi: 10.3389/fdgth.2021.721919
33. Longmore SK, Lui GY, Naik G, Breen PP, Jalaludin B, Gargiulo GD. A comparison of reflective photoplethysmography for detection of heart rate, blood oxygen saturation, and respiration rate at various anatomical locations. Sensors. (2019) 19:1874. doi: 10.3390/s19081874
34. Kinnunen H, Rantanen A, Kenttä T, Koskimäki H. Feasible assessment of recovery and cardiovascular health: accuracy of nocturnal HR and HRV assessed via ring PPG in comparison to medical grade ECG. Physiol Meas. (2020) 41:04NT01. doi: 10.1088/1361-6579/ab840a
35. Jarczok MN, Jarczok M, Mauss D, Koenig J, Li J, Herr RM, et al. Autonomic nervous system activity and workplace stressors—A systematic review. Neurosci Biobehav Rev. (2013) 37:1810–23. doi: 10.1016/j.neubiorev.2013.07.004
36. Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol Psychiatry. (2010) 67:1067–74. doi: 10.1016/j.biopsych.2009.12.012
37. Jung W, Jang KI, Lee SH. Heart and brain interaction of psychiatric illness: A review focused on heart rate variability, cognitive function, and quantitative electroencephalography. Clin Psychopharmacol Neurosci. (2019) 17:459–74. doi: 10.9758/cpn.2019.17.4.459
38. Moon E, Lee SH, Kim DH, Hwang B. Comparative study of heart rate variability in patients with schizophrenia, bipolar disorder, post-traumatic stress disorder, or major depressive disorder. Clin Psychopharmacol Neurosci. (2013) 11:137–43. doi: 10.9758/cpn.2013.11.3.137
39. Koch C, Wilhelm M, Salzmann S, Rief W, Euteneuer F. A meta-analysis of heart rate variability in major depression. psychol Med. (2019) 49:1948–57. doi: 10.1017/S0033291719001351
40. Keen L, Tan AY, Abbate A. Inverse associations between parasympathetic activity and cognitive flexibility in African Americans: Preliminary findings. Int J Psychophysiology. (2020), 155:204–9. doi: 10.1016/j.ijpsycho.2020.06.013
41. Brownlow BN, Cheavens JS, Vasey MW, Thayer JF, Hill LK. Culturally compelled coping and depressive symptoms in Black Americans: Examining the role of psychophysiological regulatory capacity. Emotion. (2023). No Pagination Specified-No Pagination Specified. doi: 10.1037/emo0001323
42. Sato N, Miyake S, Akatsu J, Kumashiro M. Power spectral analysis of heart rate variability in healthy young women during the normal menstrual cycle. Psychosomatic Med. (1995) 57:331. doi: 10.1097/00006842-199507000-00004
43. Vallejo M, Márquez MF, Borja-Aburto VH, Cárdenas M, Hermosillo AG. Age, body mass index, and menstrual cycle influence young women’s heart rate variability. Clin Auton Res. (2005) 15:292–8. doi: 10.1007/s10286-005-0272-9
44. Brar TK, Singh KD, Kumar A. Effect of different phases of menstrual cycle on heart rate variability (HRV). J Clin Diagn Res. (2015) 9:CC01–4. doi: 10.7860/JCDR/2015/13795.6592
45. Yazar Ş, Yazıcı M. Impact of menstrual cycle on cardiac autonomic function assessed by heart rate variability and heart rate recovery. Med Principles Practice. (2016) 25:374–7. doi: 10.1159/000444322
46. Matsumoto T, Ushiroyama T, Kimura T, Hayashi T, Moritani T. Altered autonomic nervous system activity as a potential etiological factor of premenstrual syndrome and premenstrual dysphoric disorder. BioPsychoSocial Med. (2007) 1:24. doi: 10.1186/1751-0759-1-24
47. Baker FC, Colrain IM, Trinder J. Reduced parasympathetic activity during sleep in the symptomatic phase of severe premenstrual syndrome. J Psychosomatic Res. (2008) 65:13–22. doi: 10.1016/j.jpsychores.2008.04.008
48. Swami G, Kumar P. Heart rate variability & Behavioral changes in normal & Premenstural syndrome cases in follicular and late leuteal phase in medical students. Int J Med Pharm Res. (2023) 4:328–33.
49. Meng Y, Chang L, Hou L, Zhou R. Menstrual attitude and social cognitive stress influence autonomic nervous system in women with premenstrual syndrome. Stress. (2022) 25:87–96. doi: 10.1080/10253890.2021.2024163
50. Hamidovic A, Davis J, Soumare F, Naveed A, Ghani Y, Semiz S, et al. Allopregnanolone is associated with a stress-induced reduction of heart rate variability in premenstrual dysphoric disorder. J Clin Med. (2023) 12:1553. doi: 10.3390/jcm12041553
51. Feula JM, Yerrabelli D, Pal GK, Subhashri S. Decreased cognition is associated with altered cardiovascular autonomic functions and decreased baroreflex sensitivity in women with premenstrual syndrome. J Mind Med Sci. (2022) 9:181–6. doi: 10.22543/7674.91.P181186
52. Landén M, Wennerblom B, Tygesen H, Modigh K, Sörvik K, Ysander C, et al. Heart rate variability in premenstrual dysphoric disorder. Psychoneuroendocrinology. (2004) 29:733–40. doi: 10.1016/S0306-4530(03)00117-3
53. Bäckström T, Andreen L, Birzniece V, Björn I, Johansson IM, Nordenstam-Haghjo M, et al. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs. (2003) 17:325–42. doi: 10.2165/00023210-200317050-00003
54. Danel DP, Kozak K, Szala A, Kunert-Keil C, Dziedzic-Danel A, Siennicka A. The relationship between the premenstrual syndrome and resting cardiac vagal tone in young healthy females: role of hormonal contraception. Neurophysiology. (2019) 51:447–54. doi: 10.1007/s11062-020-09841-w
55. de Zambotti M, Nicholas CL, Colrain IM, Trinder JA, Baker FC. Autonomic regulation across phases of the menstrual cycle and sleep stages in women with premenstrual syndrome and healthy controls. Psychoneuroendocrinology. (2013) 38:2618–27. doi: 10.1016/j.psyneuen.2013.06.005
56. Grrishma B, Gaur GS, Chaturvedula L, Velkumary S, Subramanian SK, Gurunandan U. Assessment of cardiovascular autonomic functions and baroreceptor reactivity in women with premenstrual syndrome. Indian J Physiol Pharmacol. (2015) 59:148–54.
57. Rundo JV, Downey R. Chapter 25 - polysomnography. In: Levin KH, Chauvel P, editors. Handbook of Clinical Neurology. Amsterdam, Netherlands: Elsevier (2019) 160, 381–92. Available at: https://www.sciencedirect.com/science/article/pii/B9780444640321000254.
58. Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J Jr, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. (2005) 28:499–523. doi: 10.1093/sleep/28.4.499
59. Colten HR, Altevogt BM. Research I of M (US) C on SM and. Sleep Physiology. In: Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, D.C., United States: National Academies Press (US) (2006). Available at: https://www.ncbi.nlm.nih.gov/books/NBK19956/.
60. Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep: J Sleep Sleep Disord Res. (2003) 26:342–92. doi: 10.1093/sleep/26.3.342
61. Fukushima H, Kawanaka H, Bhuiyan MS, Oguri K. Estimating heart rate using wrist-type Photoplethysmography and acceleration sensor while running. Annu Int Conf IEEE Eng Med Biol Soc. (2012) 2012:2901–4. doi: 10.1109/EMBC.2012.6346570
62. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. (2008) 19:838. doi: 10.1097/EDE.0b013e318187a7b0
63. Landry GJ, Best JR, Liu-Ambrose T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci. (2015) 7:7. doi: 10.3389/fnagi.2015.00166
64. Fabbri M, Beracci A, Martoni M, Meneo D, Tonetti L, Natale V. Measuring subjective sleep quality: A review. Int J Environ Res Public Health. (2021) 18:1082. doi: 10.3390/ijerph18031082
65. Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. (2008) 9:S10–7. doi: 10.1016/S1389-9457(08)70011-X
66. Lemola S, Ledermann T, Friedman EM. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLoS One. (2013) 8:e71292. doi: 10.1371/journal.pone.0071292
67. Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. (2013) 36:1747–55. doi: 10.5665/sleep.3142
68. Imtiaz SA. A systematic review of sensing technologies for wearable sleep staging. Sensors. (2021) 21:1562. doi: 10.3390/s21051562
69. Sadeh A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med Rev. (2011) 15:259–67. doi: 10.1016/j.smrv.2010.10.001
70. Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. (2018) 14:1209–30. doi: 10.5664/jcsm.7228
71. Cheung J, Zeitzer JM, Lu H, Mignot E. Validation of minute-to-minute scoring for sleep and wake periods in a consumer wearable device compared to an actigraphy device. Sleep Sci Practice. (2018) 2:11. doi: 10.1186/s41606-018-0029-8
72. Hamill K, Jumabhoy R, Kahawage P, de Zambotti M, Walters EM, Drummond SPA. Validity, potential clinical utility and comparison of a consumer activity tracker and a research-grade activity tracker in insomnia disorder II: Outside the laboratory. J Sleep Res. (2020) 29:e12944. doi: 10.1111/jsr.12944
73. Kahawage P, Jumabhoy R, Hamill K, de Zambotti M, Drummond SPA. Validity, potential clinical utility, and comparison of consumer and research-grade activity trackers in Insomnia Disorder I: In-lab validation against polysomnography. J Sleep Res. (2020) 29:e12931. doi: 10.1111/jsr.12931
74. Van De Water ATM, Holmes A, Hurley DA. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography – a systematic review. J Sleep Res. (2011) 20:183–200. doi: 10.1111/j.1365-2869.2009.00814.x
75. Evenson KR, Goto MM, Furberg R. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Activity. (2015) 12:159–9. doi: 10.1186/s12966-015-0314-1
76. Mantua J, Gravel N, Spencer RMC. Reliability of sleep measures from four personal health monitoring devices compared to research-based actigraphy and polysomnography. Sensors (Basel). (2016) 16:646. doi: 10.3390/s16050646
77. Baron KG, Duffecy J, Berendsen MA, Mason IC, Lattie EG, Manalo NC. Feeling validated yet? A scoping review of the use of consumer-targeted wearable and mobile technology to measure and improve sleep. Sleep Med Rev. (2018) 40:151–9. doi: 10.1016/j.smrv.2017.12.002
78. Lichstein KL, Stone KC, Donaldson J, Nau SD, Soeffing JP, Murray D, et al. Actigraphy validation with insomnia. Sleep. (2006) 29:232–9. doi: 10.1093/sleep/29.2.232
79. Baandrup L, Jennum PJ. A validation of wrist actigraphy against polysomnography in patients with schizophrenia or bipolar disorder. Neuropsychiatr Dis Treat. (2015) 11:2271–7. doi: 10.2147/NDT.S88236
80. Kang SG, Kang JM, Ko KP, Park SC, Mariani S, Weng J. Validity of a commercial wearable sleep tracker in adult insomnia disorder patients and good sleepers. J Psychosomatic Res. (2017) 97:38–44. doi: 10.1016/j.jpsychores.2017.03.009
81. Pollak CP, Tryon WW, Nagaraja H, Dzwonczyk R. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep. (2001) 24:957–65. doi: 10.1093/sleep/24.8.957
82. Chinoy ED, Cuellar JA, Jameson JT, Markwald RR. Performance of four commercial wearable sleep-tracking devices tested under unrestricted conditions at home in healthy young adults. Nat Sci Sleep. (2022) 14:493–516. doi: 10.2147/NSS.S348795
83. Fonseca P, Weysen T, Goelema MS, Møst EIS, Radha M, Lunsingh Scheurleer C, et al. Validation of photoplethysmography-based sleep staging compared with polysomnography in healthy middle-aged adults. Sleep. (2017) 40:zsx097. doi: 10.1093/sleep/zsx097
84. Lee KA, Shaver JF, Giblin EC, Woods NF. Sleep patterns related to menstrual cycle phase and premenstrual affective symptoms. Sleep: J Sleep Res Sleep Med. (1990) 13:403–9. doi: 10.1093/sleep/13.5.403
85. Arnal PJ, Thorey V, Debellemaniere E, Ballard ME, Bou Hernandez A, Guillot A, et al. The Dreem Headband compared to polysomnography for electroencephalographic signal acquisition and sleep staging. Sleep. (2020) 43:zsaa097. doi: 10.1093/sleep/zsaa097
86. Nakamura T, Alqurashi YD, Morrell MJ, Mandic DP. Hearables: automatic overnight sleep monitoring with standardized in-ear EEG sensor. IEEE Trans Biomed Engineering. (2020) 67:203–12. doi: 10.1109/TBME.10
87. Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Arch Gen Psychiatry. (1992) 49:651–68. doi: 10.1001/archpsyc.1992.01820080059010
88. Bartlett D, Jackson ML. The bidirectional nature of sleep problems and psychopathology. Med Today: peer reviewed J Clin practice. (2016) 17:23–8.
89. Freeman D, Sheaves B, Waite F, Harvey AG, Harrison PJ. Sleep disturbance and psychiatric disorders. Lancet Psychiatry. (2020) 7:628–37. doi: 10.1016/S2215-0366(20)30136-X
90. Harvey AG. Sleep and circadian functioning: critical mechanisms in the mood disorders? Annu Rev Clin Psychol. (2011) 7:297–319. doi: 10.1146/annurev-clinpsy-032210-104550
91. Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosomatic Res. (2004) 56:239–43. doi: 10.1016/S0022-3999(03)00067-9
92. Zheng H, Harlow SD, Kravitz HM, Bromberger J, Buysse DJ, Matthews KA, et al. Actigraphy-defined measures of sleep and movement across the menstrual cycle in midlife menstruating women: SWAN sleep study. Menopause. (2015) 22:66–74. doi: 10.1097/GME.0000000000000249
93. Sharkey KM, Crawford SL, Kim S, Joffe H. Objective sleep interruption and reproductive hormone dynamics in the menstrual cycle. Sleep Med. (2014) 15:688–93. doi: 10.1016/j.sleep.2014.02.003
94. Haufe A, Leeners B. Sleep disturbances across a woman’s lifespan: what is the role of reproductive hormones? J Endocrine Soc. (2023) 7:bvad036. doi: 10.1210/jendso/bvad036
95. Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. (2007) 30:1283–91. doi: 10.1093/sleep/30.10.1283
96. Gupta R, Lahan V, Bansal S. Subjective sleep problems in young women suffering from premenstrual dysphoric disorder. N Am J Med Sci. (2012) 4:593–5. doi: 10.4103/1947-2714.103326
97. Lin PC, Ko CH, Lin YJ, Yen JY. Insomnia, inattention and fatigue symptoms of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. (2021) 18:6192. doi: 10.3390/ijerph18126192
98. Jeon B, Baek J. Menstrual disturbances and its association with sleep disturbances: a systematic review. BMC Women’s Health. (2023) 23:470. doi: 10.1186/s12905-023-02629-0
99. Meers JM, Nowakowski S. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. (2020) 34:43–9. doi: 10.1016/j.copsyc.2019.09.003
100. Baker FC, Sassoon SA, Kahan T, Palaniappan L, Nicholas CL, Trinder J, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. (2012) 21:535–45. doi: 10.1111/j.1365-2869.2012.01007.x
101. Shechter A, Lespérance P, Ng Ying Kin NMK, Boivin DB. Nocturnal polysomnographic sleep across the menstrual cycle in premenstrual dysphoric disorder. Sleep Med. (2012) 13:1071–8. doi: 10.1016/j.sleep.2012.05.012
102. Parry BL, Mostofi N, LeVeau B, Nahum HC, Golshan S, Laughlin GA, et al. Sleep EEG studies during early and late partial sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Psychiatry Res. (1999) 85:127–43. doi: 10.1016/S0165-1781(98)00128-0
103. Parry BL, Berga SL, Kripke DF, Klauber MR, Laughlin GA, Yen SSC, et al. Altered waveform of plasma nocturnal melatonin secretion in premenstrual depression. Arch Gen Psychiatry. (1990) 47:1139–46. doi: 10.1001/archpsyc.1990.01810240059010
104. Parry BL, Meliska CJ, Martínez LF, López AM, Sorenson DL, Hauger RL, et al. Late, but not early, wake therapy reduces morning plasma melatonin: Relationship to mood in Premenstrual Dysphoric Disorder. Psychiatry Res. (2008) 161:76–86. doi: 10.1016/j.psychres.2007.11.017
105. Parry BL, Meliska CJ, Martinez LF, Lopez AM, Sorenson DL, Dawes SE, et al. A 1-week sleep and light intervention improves mood in premenstrual dysphoric disorder in association with shifting melatonin offset time earlier. Arch Womens Ment Health. (2023) 26:29–37. doi: 10.1007/s00737-022-01283-z
106. Shechter A, Boivin DB. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. (2010) 2010:e259345. doi: 10.1155/2010/259345
107. Shechter A, Lespérance P, Kin NMKNY, Boivin DB. Pilot investigation of the circadian plasma melatonin rhythm across the menstrual cycle in a small group of women with premenstrual dysphoric disorder. PloS One. (2012) 7:e51929. doi: 10.1371/journal.pone.0051929
108. Sylvia LG, Bernstein EE, Hubbard JL, Keating L, Anderson EJ. A practical guide to measuring physical activity. J Acad Nutr Diet. (2014) 114:199–208. doi: 10.1016/j.jand.2013.09.018
109. Dowd KP, Szeklicki R, Minetto MA, Murphy MH, Polito A, Ghigo E, et al. A systematic literature review of reviews on techniques for physical activity measurement in adults: a DEDIPAC study. Int J Behav Nutr Phys Act. (2018) 15:15. doi: 10.1186/s12966-017-0636-2
110. Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiology-Regulatory Integr Comp Physiol. (1986) 250:R823–30. doi: 10.1152/ajpregu.1986.250.5.R823
111. Fuller D, Colwell E, Low J, Orychock K, Tobin MA, Simango B, et al. Reliability and validity of commercially available wearable devices for measuring steps, energy expenditure, and heart rate: systematic review. JMIR mHealth uHealth. (2020) 8:e18694. doi: 10.2196/18694
112. Henriksen A, Svartdal F, Grimsgaard S, Hartvigsen G, Hopstock LA. Polar vantage and oura physical activity and sleep trackers: validation and comparison study. JMIR Formative Res. (2022) 6:e27248. doi: 10.2196/27248
113. Kristiansson E, Fridolfsson J, Arvidsson D, Holmäng A, Börjesson M, Andersson-Hall U. Validation of Oura ring energy expenditure and steps in laboratory and free-living. BMC Med Res Methodol. (2023) 23:50. doi: 10.1186/s12874-023-01868-x
114. Gianfredi V, Blandi L, Cacitti S, Minelli M, Signorelli C, Amerio A, et al. Depression and objectively measured physical activity: A systematic review and meta-analysis. Int J Environ Res Public Health. (2020) 17:3738. doi: 10.3390/ijerph17103738
115. Azevedo Da Silva M, Singh-Manoux A, Brunner EJ, Kaffashian S, Shipley MJ, Kivimäki M, et al. Bidirectional association between physical activity and symptoms of anxiety and depression: the Whitehall II study. Eur J Epidemiol. (2012) 27:537–46. doi: 10.1007/s10654-012-9692-8
116. Stavrakakis N, de Jonge P, Ormel J, Oldehinkel AJ. Bidirectional prospective associations between physical activity and depressive symptoms. TRAILS Study J Adolesc Health. (2012) 50:503–8. doi: 10.1016/j.jadohealth.2011.09.004
117. Pinto Pereira SM, Geoffroy MC, Power C. Depressive symptoms and physical activity during 3 decades in adult life: bidirectional associations in a prospective cohort study. JAMA Psychiatry. (2014) 71:1373–80. doi: 10.1001/jamapsychiatry.2014.1240
118. Hsueh MC, Stubbs B, Lai YJ, Sun CK, Chen LJ, Ku PW. A dose response relationship between accelerometer assessed daily steps and depressive symptoms in older adults: a two-year cohort study. Age Ageing. (2021) 50:519–26. doi: 10.1093/ageing/afaa162
119. Ramsey CM, Lynch KG, Gehrman PR, Vairavan S, Narayan VA, Li QS, et al. Daily steps and depressive symptoms: A longitudinal evaluation of patients with major depressive disorder in the precision medicine in mental health care study. J Affect Disord. (2022) 300:334–40. doi: 10.1016/j.jad.2021.12.116
120. Hu MX, Turner D, Generaal E, Bos D, Ikram MK, Ikram MA, et al. Exercise interventions for the prevention of depression: a systematic review of meta-analyses. BMC Public Health. (2020) 20:1255. doi: 10.1186/s12889-020-09323-y
121. Nyström MBT, Neely G, Hassmén P, Carlbring P. Treating major depression with physical activity: A systematic overview with recommendations. Cogn Behav Ther. (2015) 44:341–52. doi: 10.1080/16506073.2015.1015440
122. McNulty KL, Elliott-Sale KJ, Dolan E, Swinton PA, Ansdell P, Goodall S, et al. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: A systematic review and meta-analysis. Sports Med. (2020) 50:1813–27. doi: 10.1007/s40279-020-01319-3
123. Lin G, Siddiqui R, Lin Z, Blodgett JM, Patel SN, Truong KN, et al. Blood glucose variance measured by continuous glucose monitors across the menstrual cycle. NPJ Digit Med. (2023) 6:1–8. doi: 10.1038/s41746-023-00884-x
124. Carmichael MA, Thomson RL, Moran LJ, Wycherley TP. The impact of menstrual cycle phase on athletes’ Performance: A narrative review. Int J Environ Res Public Health. (2021) 18:1667. doi: 10.3390/ijerph18041667
125. Lang AL, Bruhn RL, Fehling M, Heidenreich A, Reisdorf J, Khanyaree I, et al. Feasibility study on menstrual cycles with fitbit device (FEMFIT): prospective observational cohort study. JMIR mHealth uHealth. (2024) 12:e50135. doi: 10.2196/50135
126. Aganoff JA, Boyle GJ. Aerobic exercise, mood states and menstrual cycle symptoms. J Psychosomatic Res. (1994) 38:183–92. doi: 10.1016/0022-3999(94)90114-7
127. Witkoś J, Hartman-Petrycka M. The influence of running and dancing on the occurrence and progression of premenstrual disorders. Int J Environ Res Public Health. (2021) 18:7946. doi: 10.3390/ijerph18157946
128. Sanchez BN, Kraemer WJ, Maresh CM. Premenstrual syndrome and exercise: A narrative review. Women. (2023) 3:348–64. doi: 10.3390/women3020026
129. Pearce E, Jolly K, Jones LL, Matthewman G, Zanganeh M, Daley A. Exercise for premenstrual syndrome: a systematic review and meta-analysis of randomised controlled trials. BJGP Open. (2020) 4. doi: 10.3399/bjgpopen20X101032
130. Ravichandran H, Janakiraman B. Effect of aerobic exercises in improving premenstrual symptoms among healthy women: A systematic review of randomized controlled trials. Int J Women’s Health. (2022) 14:1105–14. doi: 10.2147/IJWH.S371193
131. Kim Y, Wang Y, Oh J. Digital media use and social engagement: how social media and smartphone use influence social activities of college students. Cyberpsychology Behavior Soc Networking. (2016) 19:264–9. doi: 10.1089/cyber.2015.0408
132. Lapierre MA, Zhao P. Smartphones and social support: longitudinal associations between smartphone use and types of support. Soc Sci Comput Review. (2022) 40:831–43. doi: 10.1177/0894439320988762
133. Osmani V. Smartphones in mental health: detecting depressive and manic episodes. IEEE Pervasive Computing. (2015) 14:10–3. doi: 10.1109/MPRV.2015.54
134. Flanagan O, Chan A, Roop P, Sundram F. Using acoustic speech patterns from smartphones to investigate mood disorders: scoping review. JMIR mHealth uHealth. (2021) 9:e24352. doi: 10.2196/24352
135. Antosik-Wójcińska AZ, Dominiak M, Chojnacka M, Kaczmarek-Majer K, Opara KR, Radziszewska W, et al. Smartphone as a monitoring tool for bipolar disorder: a systematic review including data analysis, machine learning algorithms and predictive modelling. Int J Med Informatics. (2020) 138:104131. doi: 10.1016/j.ijmedinf.2020.104131
136. Jacobson NC, Chung YJ. Passive sensing of prediction of moment-to-moment depressed mood among undergraduates with clinical levels of depression sample using smartphones. Sensors. (2020) 20:3572. doi: 10.3390/s20123572
137. Bai R, Xiao L, Guo Y, Zhu X, Li N, Wang Y, et al. Tracking and monitoring mood stability of patients with major depressive disorder by machine learning models using passive digital data: prospective naturalistic multicenter study. JMIR mHealth uHealth. (2021) 9:e24365. doi: 10.2196/24365
138. Ren B, Balkind EG, Pastro B, Israel ES, Pizzagalli DA, Rahimi-Eichi H, et al. Predicting states of elevated negative affect in adolescents from smartphone sensors: a novel personalized machine learning approach. psychol Med. (2023) 53:5146–54. doi: 10.1017/S0033291722002161
139. Hirschfeld RMA, Montgomery SA, Keller MB, Kasper S, Schatzberg AF, Möller HJ, et al. Social functioning in depression: A review. J Clin Psychiatry. (2000) 61:268–75. doi: 10.4088/JCP.v61n0405
140. Saeri AK, Cruwys T, Barlow FK, Stronge S, Sibley CG. Social connectedness improves public mental health: Investigating bidirectional relationships in the New Zealand attitudes and values survey. Aust N Z J Psychiatry. (2018) 52:365–74. doi: 10.1177/0004867417723990
141. Santini ZI, Jose PE, Cornwell EY, Koyanagi A, Nielsen L, Hinrichsen C, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. (2020) 5:e62–70. doi: 10.1016/S2468-2667(19)30230-0
142. Nezlek JB, Hampton CP, Shean GD. Clinical depression and day-to-day social interaction in a community sample. J Abnormal Psychol. (2000) 109:11–9. doi: 10.1037/0021-843X.109.1.11
143. Owen R, Gooding P, Dempsey R, Jones S. The reciprocal relationship between bipolar disorder and social interaction: A qualitative investigation. Clin Psychol Psychotherapy. (2017) 24:911–8. doi: 10.1002/cpp.v24.4
144. Alsubaie MM, Stain HJ, Webster LAD, Wadman R. The role of sources of social support on depression and quality of life for university students. Int J Adolescence Youth. (2019) 24:484–96. doi: 10.1080/02673843.2019.1568887
145. Demirci K, Akgönül M, Akpinar A. Relationship of smartphone use severity with sleep quality, depression, and anxiety in university students. J Behav Addictions. (2015) 4:85–92. doi: 10.1556/2006.4.2015.010
146. Alhassan AA, Alqadhib EM, Taha NW, Alahmari RA, Salam M, Almutairi AF. The relationship between addiction to smartphone usage and depression among adults: a cross sectional study. BMC Psychiatry. (2018) 18:148. doi: 10.1186/s12888-018-1745-4
147. Hunt MG, Marx R, Lipson C, Young J. No more FOMO: limiting social media decreases loneliness and depression. J Soc Clin Psychol. (2018) 37:751–68. doi: 10.1521/jscp.2018.37.10.751
148. Masud MT, Mamun MA, Thapa K, Lee DH, Griffiths MD, Yang SH. Unobtrusive monitoring of behavior and movement patterns to detect clinical depression severity level via smartphone. J Biomed Informatics. (2020) 103:103371. doi: 10.1016/j.jbi.2019.103371
149. Pfefferbaum B, North CS. Mental health and the covid-19 pandemic. N Engl J Med. (2020) 383:510–2. doi: 10.1056/NEJMp2008017
150. Keles B, McCrae N, Grealish A. A systematic review: the influence of social media on depression, anxiety and psychological distress in adolescents. Int J Adolescence Youth. (2020) 25:79–93. doi: 10.1080/02673843.2019.1590851
151. Odgers CL, Jensen MR. Annual Research Review: Adolescent mental health in the digital age: facts, fears, and future directions. J Child Psychol Psychiatry. (2020) 61:336–48. doi: 10.1111/jcpp.13190
152. Boulianne S. Social media use and participation: a meta-analysis of current research. Information Communication Society. (2015) 18:524–38. doi: 10.1080/1369118X.2015.1008542
153. Thorisdottir IE, Sigurvinsdottir R, Asgeirsdottir BB, Allegrante JP, Sigfusdottir ID. Active and passive social media use and symptoms of anxiety and depressed mood among Icelandic adolescents. Cyberpsychology Behavior Soc Networking. (2019) 22:535–42. doi: 10.1089/cyber.2019.0079
154. Beyens I, Pouwels JL, van Driel II, Keijsers L, Valkenburg PM. Social media use and adolescents’ Well-being: developing a typology of person-specific effect patterns. Communication Res. (2021) 51(6):00936502211038196. doi: 10.1177/00936502211038
155. Valkenburg PM, van Driel II, Beyens I. The associations of active and passive social media use with well-being: A critical scoping review. New Media Society. (2022) 24:530–49. doi: 10.1177/14614448211065425
156. Robinson RL, Swindle RW. Premenstrual symptom severity: impact on social functioning and treatment-seeking behaviors. J Women’s Health Gender-Based Med. (2000) 9:757–68. doi: 10.1089/15246090050147736
157. Rubinow DR, Smith MJ, Schenkel LA, Schmidt PJ, Dancer K. Facial emotion discrimination across the menstrual cycle in women with Premenstrual Dysphoric Disorder (PMDD) and controls. J Affect Disord. (2007) 104:37–44. doi: 10.1016/j.jad.2007.01.031
158. Yen JY, Chen CC, Chang SJ, Ko CH, Chen CS, Yen CF. Hostility, impulsivity, and behavior inhibition among women with PMDD. CNS Spectrums. (2011) 16:205–13. doi: 10.1017/S1092852912000387
159. Kaiser G, Janda C, Kleinstäuber M, Weise C. Clusters of premenstrual symptoms in women with PMDD: Appearance, stability and association with impairment. J Psychosomatic Res. (2018) 115:38–43. doi: 10.1016/j.jpsychores.2018.10.004
160. KV LG, Chellamma V. Prevalence, pattern, and predictors OF premenstrual syndrome and premenstrual dysphoric disorder among College girls. Asian J Pharm Clin Res. (2023) 16(2):124–6. doi: 10.22159/ajpcr.2023v16i2.46373
161. Bond AJ, Critchlow DG, Wingrove J. Conflict resolution in women is related to trait aggression and menstrual cycle phase. Aggressive Behavior. (2003) 29:228–38. doi: 10.1002/ab.10025
162. Yen JY, Chang SJ, Long CY, Tang TC, Chen CC, Yen CF. Working memory deficit in premenstrual dysphoric disorder and its associations with difficulty in concentrating and irritability. Compr Psychiatry. (2012) 53:540–5. doi: 10.1016/j.comppsych.2011.05.016
163. Kalsbeek A, Garidou ML, Palm IF, van der Vliet J, Simonneaux V, Pévet P, et al. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur J Neurosci. (2000) 12:3146–54. doi: 10.1046/j.1460-9568.2000.00202.x
164. Ono D, Honma Ki, Yanagawa Y, Yamanaka A, Honma S. Role of GABA in the regulation of the central circadian clock of the suprachiasmatic nucleus. J Physiol Sci. (2018) 68:333–43. doi: 10.1007/s12576-018-0604-x
165. Schmalenberger KM, Tauseef HA, Barone JC, Owens SA, Lieberman L, Jarczok MN, et al. How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology. (2021) 123:104895. doi: 10.1016/j.psyneuen.2020.104895
166. Mayer KH, Bradford JB, Makadon HJ, Stall R, Goldhammer H, Landers S. Sexual and gender minority health: what we know and what needs to be done. Am J Public Health. (2008) 98:989–95. doi: 10.2105/AJPH.2007.127811
167. Reed DL, Sacco WP. Measuring sleep efficiency: what should the denominator be? J Clin Sleep Med. (2016) 12:263–6. doi: 10.5664/jcsm.5498
168. Patel AK, Reddy V, Shumway KR, Araujo JF. Physiology, sleep stages. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2024). Available at: http://www.ncbi.nlm.nih.gov/books/NBK526132/.
Keywords: PMDD, PMS, remote monitoring, sleep, HRV, physical activity
Citation: Brown RD, Bondy E, Prim J, Dichter G and Schiller CE (2024) The behavioral and physiological correlates of affective mood switching in premenstrual dysphoric disorder. Front. Psychiatry 15:1448914. doi: 10.3389/fpsyt.2024.1448914
Received: 14 June 2024; Accepted: 01 October 2024;
Published: 04 November 2024.
Edited by:
Lauren M. Osborne, Cornell University, United StatesReviewed by:
Elena M. D. Schönthaler, University Hospital Graz, AustriaLiisa Hantsoo, Johns Hopkins University, United States
Copyright © 2024 Brown, Bondy, Prim, Dichter and Schiller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robin Dara Brown, Um9iaW5kYkB1bmMuZWR1
 Robin Dara Brown
Robin Dara Brown Erin Bondy
Erin Bondy Julianna Prim2
Julianna Prim2 Gabriel Dichter
Gabriel Dichter Crystal Edler Schiller
Crystal Edler Schiller