- 1Department of Psychiatry and Neurosciences, Charité Campus Mitte (CCM), Charité Universitätsmedizin Berlin, Berlin, Germany
- 2Department of Psychiatry and Psychotherapy, Psychiatric University Hospital Charité at St. Hedwig Hospital, Berlin, Germany
- 3Department of Psychiatry, Depression Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 4Department of Psychiatry, Hallym University Sacred Heart Hospital, Anyang, Republic of Korea
- 5Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Suwon, Republic of Korea
- 6Department of Statistics and Actuarial Science, Soongsil University, Seoul, Republic of Korea
- 7Department of Health Sciences and Technology, Department of Medical Device Management and Research, and Department of Clinical Research Design and Evaluation, Samsung Advanced Institute for Health Sciences and Technology (SAIHST), Sungkyunkwan University, Seoul, Republic of Korea
Introduction: Early age at menopause has been linked to various adverse health outcomes, but its association with suicide risk remains underexplored. This study aims to assess the relationship between age at menopause and suicide risk among postmenopausal women.
Methods: This retrospective cohort study analyzed data from the Korean National Health Insurance System (NHIS), covering 1,315,795 postmenopausal women aged 30 years and above, from 2009 to 2021. Menopausal age was classified as primary ovarian insufficiency (under 40 years), early menopause (40-44 years), average menopause (45-49 and 50-54 years), and late menopause (55 years and older). Suicide incidence was identified using ICD-10 codes for primary cause of death. Multivariable Cox proportional hazards models were utilized to estimate hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: Across the 12-year follow-up, there were 2,986 suicides. Women with primary ovarian insufficiency exhibited the highest suicide risk (HR, 1.43; 95% CI, 1.14–1.78, p < 0.001), followed by those with early menopause (HR, 1.31; 95% CI, 1.15–1.50, p < 0.001), and those with menopause between 45 and 49 (HR, 1.13; 95% CI, 1.04–1.23, p < 0.001) compared to the reference group undergoing menopause at age of 50-54.
Discussion: Early onset of menopause, particularly primary ovarian insufficiency, is associated with a significantly elevated risk of suicide. These findings underscore the need for targeted interventions and support for women experiencing early menopause. This study highlights the importance of monitoring mental health in postmenopausal women and suggests further research to explore the underlying mechanisms linking early menopause to increased suicide risk.
1 Introduction
Menopause, defined as the cessation of menstruation for 12 consecutive months, marks a pivotal transition in a woman’s life (1). Occurring at a median age of 51.4 years (2), this period is characterized by hormonal changes, including declining estrogen levels and rising follicle-stimulating hormone (FSH) concentrations, signaling the end of reproductive capacity (3). Beyond physical symptoms such as hot flashes and vaginal dryness, menopause impacts mental health, with hormonal fluctuations linked to an array of psychiatric comorbidities, including depression, cognitive decline, and insomnia (4–6). The perimenopausal period marked by estrogen fluctuations is associated with an increased risk of depression (7). The decline in estrogen disrupts stress response and emotional regulation, potentially exacerbating depressive symptoms (8). Moreover, estrogen acts as a neuroprotective factor, influencing brain function and protecting against cognitive decline. Although the precise mechanisms are still under investigation, the decline in estrogen during menopause may disrupt these protective systems, potentially leading to an increased vulnerability to mental health problems (9).
The timing of menopause has been shown to affect mental health: Primary ovarian insufficiency (POI), defined as the depletion or dysfunction of ovarian follicles with cessation of menses before the age of 40, affects about 1% of women globally and 2.4% in South Korean (10) (hereafter referred to as Korea). Characterized by premature ovarian function decline, earlier cessation of menstruation is associated with several health risks (11). The etiology of POI is complex and varied, including genetic factors, autoimmune disorders, environmental exposures, and impact of medical treatments such as chemotherapy and radiation. However, the main mechanism remains unknown in most cases (12). Women with POI face increased risks of cardiovascular disease (13), osteoporosis (14), mental health conditions including depression and anxiety (15, 16), and cognitive decline (17). Psychosocial effects of POI are significant, often resulting in grief, anxiety, and depression. They are amplified by societal stigma and uncertainties surrounding the disorder (18). Notably, a previous study conducted in Korea has linked POI to an increased tendency towards suicidal thoughts (19). These findings indicate a connection between early menopause and elevated mental health risks, including suicide.
Despite the recognized impact of menopause on mental health, the potential connection between timing of menopause and suicide risk remains underexplored. Suicide remains a significant global health concern, accounting for over 700,000 deaths annually, highlighting the urgency of addressing all potential risk factors (20). Its gravity is particularly pronounced in Korea, which has been reported to have the highest suicide rate among OECD countries from 2003 to 2020, with 24.1 suicides per 100,000 persons (21). While various biological, psychological, clinical, and socio-environmental determinants have been investigated, it is crucial to continue exploring under-recognized or emerging risk factors that may contribute to mental health vulnerability in women (22).
Our study aimed to explore the association between menopausal timing and suicide risk among postmenopausal women, leveraging the comprehensive dataset from the Korean National Health Insurance System (NHIS). In addition to established risk factors such as depression and chronic physical conditions, we included detailed reproductive data, such as parity and breastfeeding duration, due to their potential impact on hormonal stability and mental health. Previous studies suggest that reproductive factors, including parity (23) and breastfeeding (24), may influence estrogen and progesterone levels, thereby affecting neuroendocrine pathways involved in mood regulation and mental health (25, 26). By investigating a comprehensive set of reproductive and health-related covariates, we aim to provide a nuanced understanding of how menopausal timing may influence suicide risk, contributing to a broader understanding of menopause’s impact on women’s mental health. We hypothesize that earlier onset of menopause is associated with an increased risk of suicide, thus contributing to a broader understanding of menopause’s impact on women’s health.
2 Methods and analysis
2.1 Study design and data source
This study was a retrospective cohort analysis utilizing a database provided by the NHIS. The NHIS is a single-payer, mandatory, comprehensive health insurance program covering 97% of Korea’s population, while the remaining 3% is covered by the Medical Aid Program for low-income individuals (27). The NHIS database includes a broad range of population-based health data, such as demographic information, health examination records, disease diagnoses, and treatment data coded according to the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) (28). Since 2002, the NHIS has continuously compiled this information, enabling reliable population-level research for nearly all residents in Korea (29). The NHIS provides biennial health screenings for all insured individuals, including laboratory tests and self-reported questionnaires on health behaviors (30). This extensive dataset allows for representative analyses of health outcomes across the Korean population.
2.2 Study population
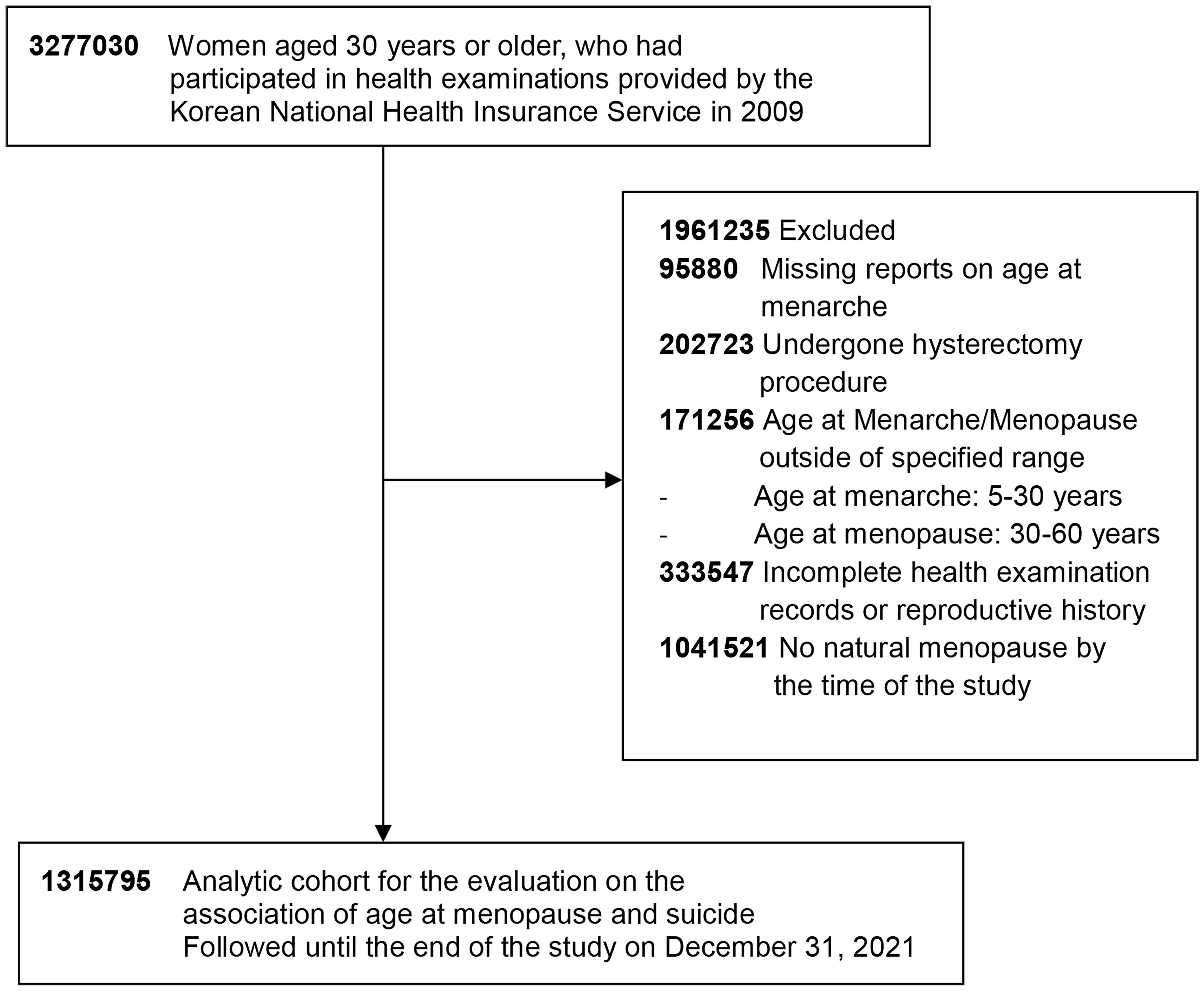
The initial cohort included 3,277,030 women aged 30 years and older who had participated in health examinations provided in 2009. We excluded those without a reported age at menarche (n = 95,880) and those who had undergone hysterectomy procedures (n = 202,723). Women with reported ages at menarche and menopause outside the specified range of 5 to under 30 years for menarche and 30 to under 60 years for menopause were also excluded (n = 171,256). Additional exclusions were made for individuals with incomplete health examination records or reproductive history (n = 333,547) and for those who had not experienced natural menopause by the time of the study (n = 1,041,521). After these criteria were applied, our final cohort for this study consisted of 1,315,795 postmenopausal women (Figure 1).
2.3 Study endpoint and follow-up
The primary outcome, death by suicide, was ascertained through linkage with data from Statistics Korea, the National Statistical Office of Korea. Causes of death were determined using ICD-10 codes (X60-X84) for suicide recorded as the primary cause of death in the database. Selected individuals were followed up until the end of the study on December 31, 2021. The mean follow-up duration was 12 years, starting from the baseline health check-up in 2009 to the end of the study period or the occurrence of suicide.
2.4 Menopausal status
In this study, menopause was defined as permanent cessation of menstrual periods, which is determined retrospectively following a span of 12 consecutive months of amenorrhea. Menopause typically occurs between ages of 45 and 55 years. We categorized postmenopausal women into four groups based on their age at the onset of menopause: under 40 years (POI), 40-44 years (early menopause), 45-49 years and 50-54 years (both falling in the normal range), and those aged ≥ 55 years.
2.5 Covariates
Our study was adjusted for multiple covariates, including detailed reproductive factors using standardized self-reporting questionnaires. Age at menarche was categorized into four groups: 5−12, 13−14, 15−16, and ≥ 17 years. Parity (0, 1, ≥ 2 children), duration of oral contraceptive use (none, < 1 year, ≥ 1 year), breastfeeding duration (none, < 6 months, 6 months up to < 1 year, ≥ 1 year), and use of HRT (none, < 2 years, 2 to 5 years, ≥ 5 years) were also recorded. The duration of fertility was calculated as the interval between menarche and menopause. It was categorized as < 30, 30-34, 35-39, and ≥ 40 years.
Demographic information, such as age and sex, was taken into consideration during the analysis. Economic status was determined based on health insurance premiums, which are indicative of income level in Korea. Participants were categorized as having a low income if they received medical aid or fell within the bottom 25% income bracket versus those outside of this category. Lifestyle factors were obtained from self-reported questionnaires completed by participants during health screenings. Smoking behavior was divided into current smokers and non-smokers. Alcohol consumption was assessed by categorizing individuals as drinkers or non-drinkers based on their self-reported intake frequency. Physical activity was classified into two categories: 1) those engaging in regular moderate-intensity exercise for at least 30 minutes on 5 or more days per week or vigorous activity for at least 20 minutes on 3 or more days per week, and 2) those not engaging such exercise. Obesity was defined by a body mass index (BMI) ≥ 25 kg/m2 (31). Other clinical measures included waist circumference, blood pressure, and serum levels of glucose and cholesterol.
Baseline comorbidities in our study were determined through a combination of ICD-10, pharmacy records, and physical examination findings, supplemented by medical service claims data. Hypertension was determined by either a claim for antihypertensive medication under codes I10−I13 and I15 or recorded blood pressure readings ≥ 140/90 mmHg. Type 2 diabetes was confirmed by at least one annual claim for antidiabetic medication under codes E11−E14 or fasting glucose level ≥ 126 mg/dL. Dyslipidemia was defined as having at least one yearly claim for lipid-lowering medication under code E78 or total cholesterol level ≥ 240 mg/dL. Chronic kidney disease was classified based on an estimated glomerular filtration rate below 60 mL/min/1.73 m2, calculated according to the Modification of Diet in Renal Disease study equation (32) under the code N18 or N19. Depression was identified using codes F32 and F33, anxiety under codes F40, and F41 and schizophrenia under F20. The selected covariates encompass reproductive, lifestyle, and mental health-related factors relevant to menopausal timing and suicide risk.
2.6 Statistical analysis
Baseline characteristics are described using means ± standard deviations for continuous variables and frequencies with percentages for categorical variables, stratified by age at menopause. Comparison of continuous variables across groups was performed using analysis of variance, while the χ2 test was employed for categorical variables. Incidence rates of suicide were calculated as the number of events per 1,000 person-years of follow-up. Kaplan-Meier curves were generated to depict cumulative event rates of suicide by age at menopause.
The association between age at menopause and the risk of suicide were analyzed using multivariable Cox proportional hazards regression, yielding hazard ratios (HRs), and 95% confidence intervals (CIs). Prior to performing the Cox proportional hazards regression, we confirmed that the proportional hazards assumption was met. Model 1 was unadjusted. Model 2 was adjusted for age. Model 3 was further adjusted for socioeconomic and lifestyle factors such as income, smoking status, alcohol consumption, and physical activity. Model 4 was further adjusted for clinical factors, including obesity, type 2 diabetes, hypertension, dyslipidemia, chronic kidney disease, and depression. Model 5 extended adjustments to include reproductive factors, including age at menarche, parity, breastfeeding history, use of oral contraceptives, and hormone replacement therapy (HRT). We included an additional model, Model 6, that extends Model 5 by adjusting for schizophrenia and anxiety to account for a broader range of mental health factors.
All statistical tests were two-sided, and a P-value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc.).
This study was conducted following the principles of the Declaration of Helsinki. It was approved by the Institutional Review Board (IRB) of Soongsil University (No. SSU-202007-HR-236-01). Given the use of de-identified data, informed consent was waived by the IRB. The study design and analysis adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline (33).
3 Results
3.1 Baseline characteristics of the study population
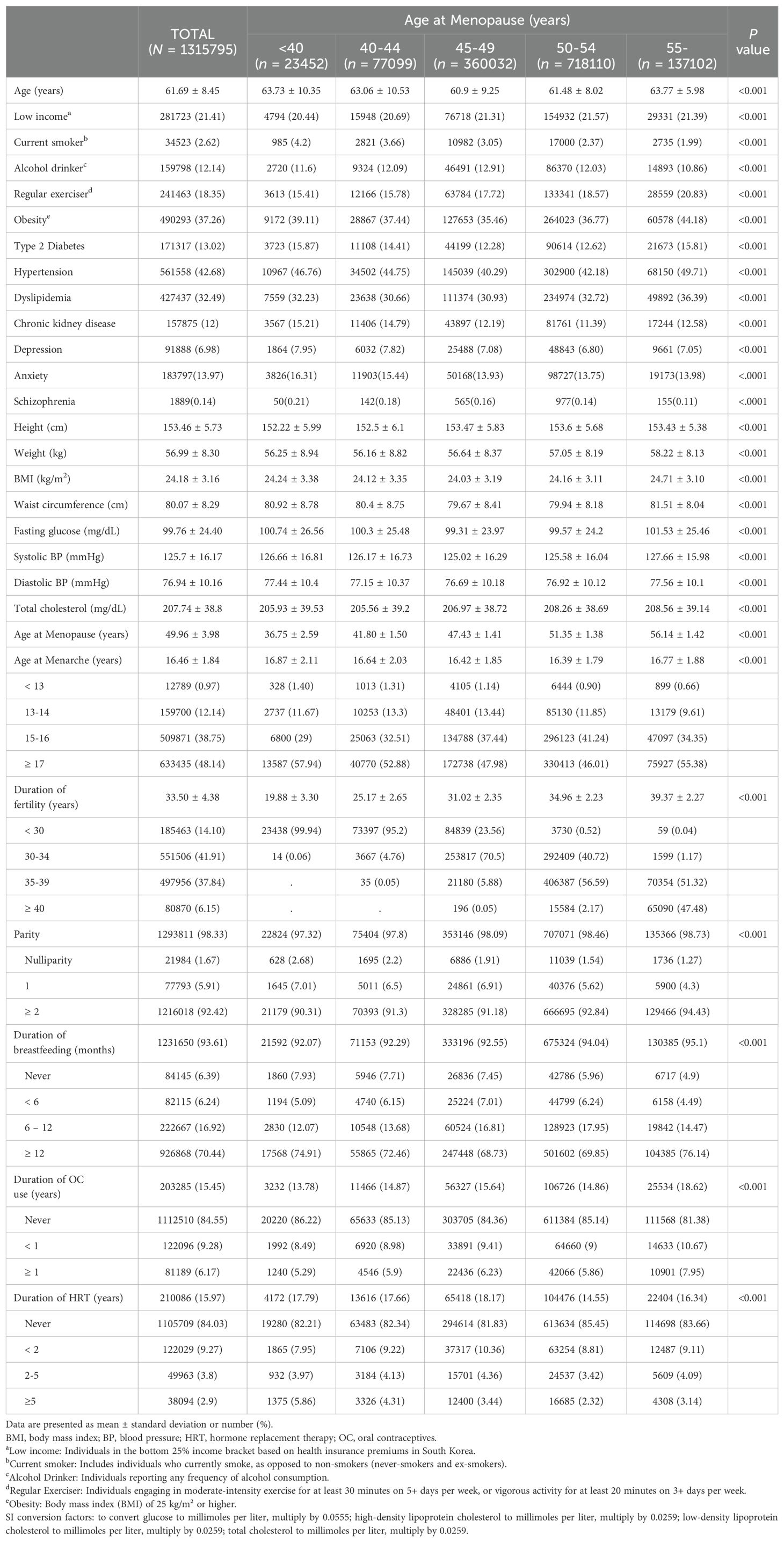
The study population’s baseline characteristics are summarized in Table 1. The study cohort comprised 1,315,795 postmenopausal women with a mean age of 61.7 years. Mean menarche occurred at 16.46 years and mean menopause at 49.96 years. Of the study cohort, 1.8% had POI with an average onset at 36.75 years old. Lifestyle factors varied by age at menopause. Younger menopausal age was associated with a higher rate of smoking (4.2%) but a lower rate of regular exercise (15.4%). Comorbidities such as chronic kidney disease (15.2%) and depression (7.95%) were more prevalent in those with POI. Differences across menopause age groups were statistically significant (P < 0.001) for all comparisons.
3.2 Mortality distribution across menopausal age groups
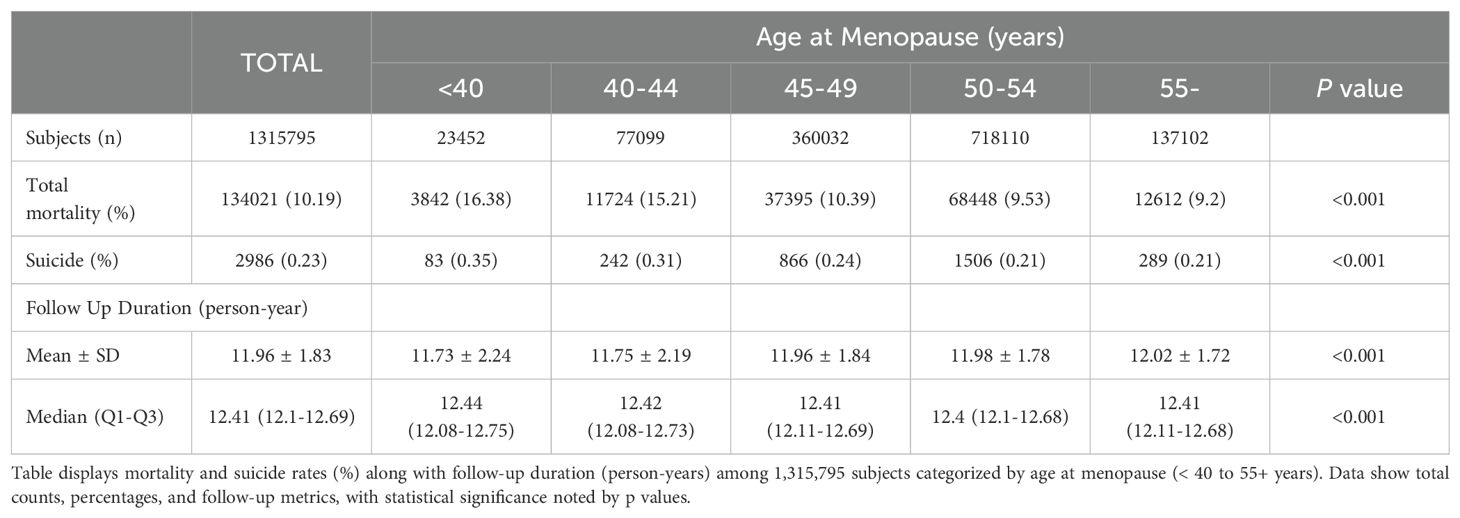
Table 2 outlines data on total mortality and follow-up duration categorized by menopausal age. Among the cohort, a total of 134,021 (10.19%) deaths were recorded, with the highest occurrence observed in women with POI (16.38%). Suicide accounted for 2,986 (0.23%) of all deaths. Again, the highest rate of suicide was observed in the under 40 group (0.35%). The average follow-up period for the entire cohort was approximately 11.96 ± 1.83 years. A slightly longer duration was seen in the group who experienced menopause after age of 55 years (12.02 ± 1.72 years). Median durations remained consistent at around 12.41 years across all age categories examined. Statistically significant variations in mortality rates were evident across different ages at menopause (P < 0.001).
3.3 Incidence and risk of suicide according to age at menopause
Figure 2 illustrates cumulative event rates of suicide, stratified by menopausal age group, with rates increasing inversely with age at menopause. This trend was statistically significant across all groups (p < 0.001). Incidence rates and HRs for suicide, as detailed in Table 3, varied with age at menopause. Women with POI exhibited the highest incidence rate of 0.30 per 1000 person-years and faced a 42.8% increased risk of suicide (HR, 1.428; 95% CI, 1.144-1.782) compared to the reference group (50-54 years) even after adjusting for covariates including demographic, lifestyle, clinical and reproductive factors in Model 5. Relative to the reference group, those in the 40-44 age range had a 31.2% increased risk of suicide (HR, 1.312; 95% CI, 1.145-1.503), while those in the age range of 45-49 had a 13.4% increased risk (HR, 1.134; 95% CI, 1.043-1.233). For women aged 55 and older, the risk was marginally reduced (HR, 0.931; 95% CI, 0.821-1.057). The trend across age groups was significant (P < 0.001). It remained consistent even when multiple variables were adjusted for in successive models.
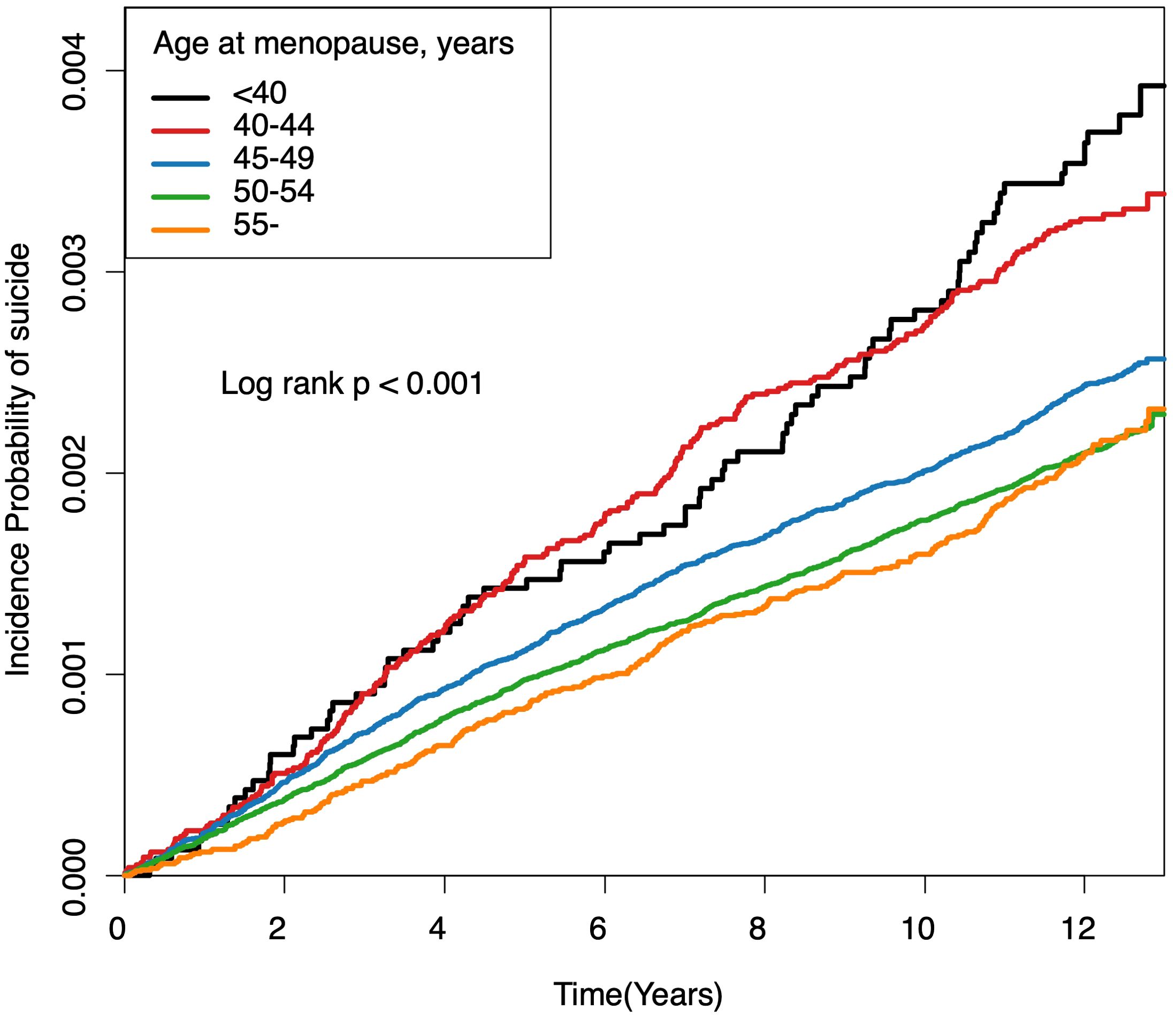
Figure 2. Cumulative event rates of suicide associated with age at menopause. Kaplan-Meier estimates for cumulative incidence of suicide over a 12-year period, stratified by age at menopause. Each line represents an age category: under 40, 40-44, 45-49, 50-54, and 55 years and older. Log-rank tests indicate significant differences among groups (all log-rank P-Values < 0.001).
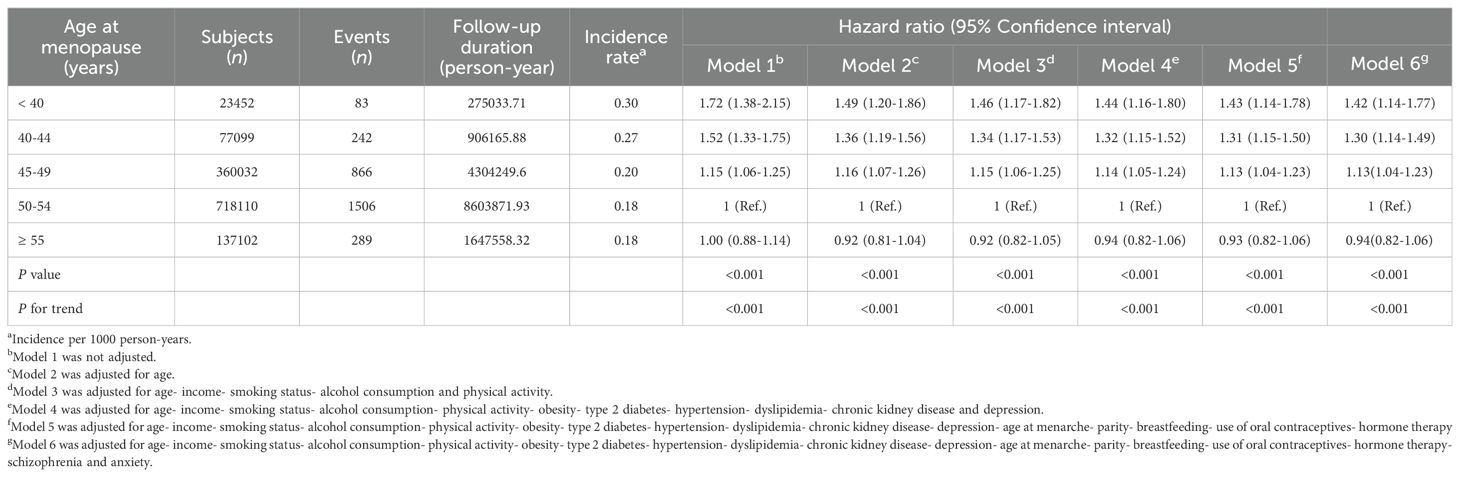
Table 3. Hazard ratios and 95% confidence intervals for association of age at menopause and risk of suicide.
3.4 Subgroup analysis
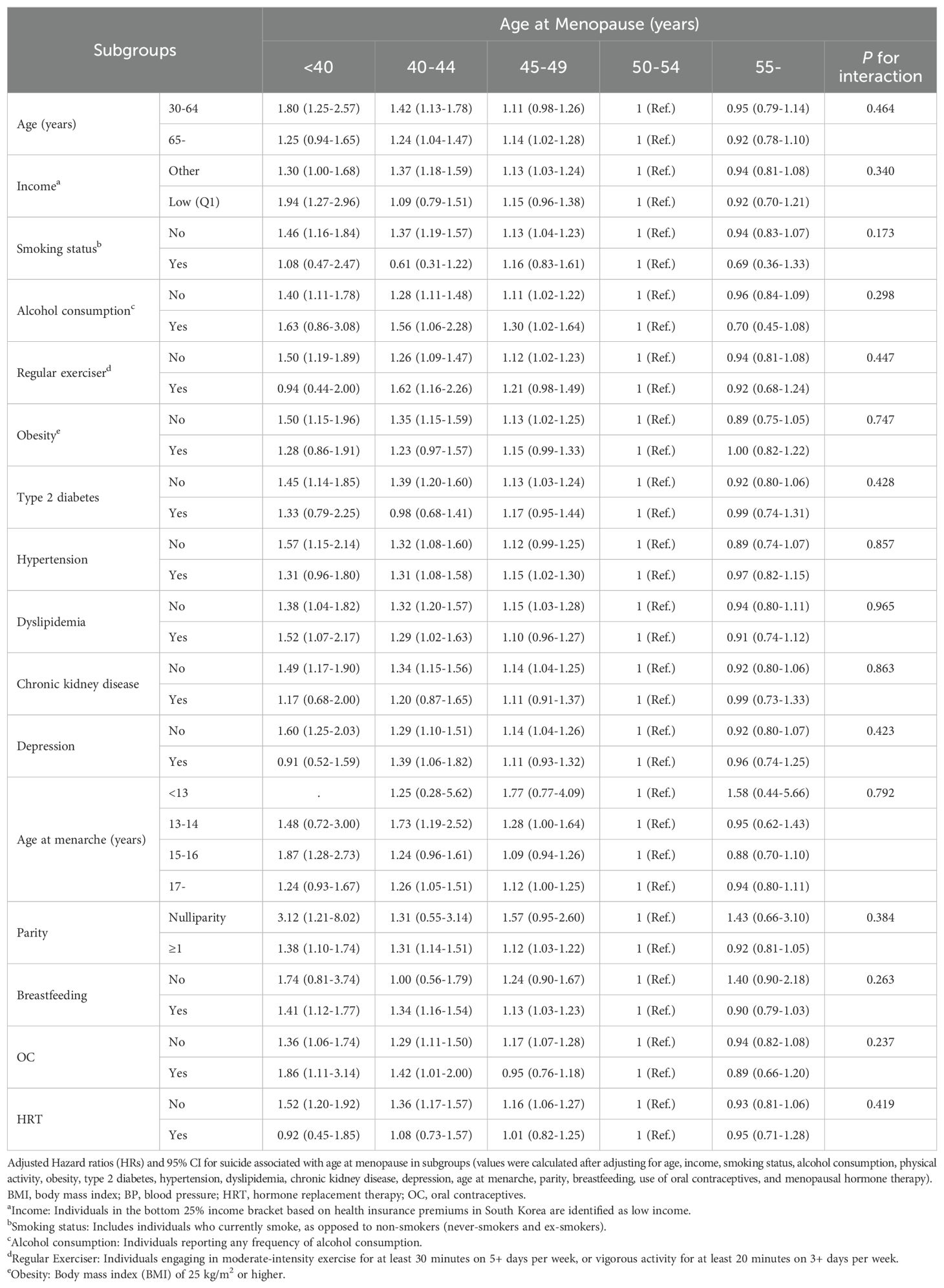
In the subgroup analysis shown in Table 4, HRs for suicide risk associated with age at menopause were calculated after adjusting for demographic, lifestyle, and health-related variables. The analysis revealed that across subgroups (categorized by age, income level, smoking status, alcohol consumption, physical activity, obesity, type 2 diabetes, hypertension, dyslipidemia, chronic kidney disease, depression, age at menarche, parity, breastfeeding, and use of oral contraceptives or hormone therapy), the relationship between earlier age at menopause and increased suicide risk remained consistent. Results showed no significant differences in this association among different subgroups based on these factors (P for interaction > 0.05 for all subgroups).
4 Discussion
This study examined the association between age at menopause and risk of suicide. Our analysis yielded two primary findings (1): women who experienced menopause before the age of 40 exhibited a significantly increased suicide risk, and (2) this risk progressively decreased with increasing age at menopause, showing a potential dose-response relationship. These findings remained robust after adjusting for demographic, lifestyle, clinical, and reproductive factors, underscoring the impact of menopausal timing on women’s mental health. Subgroup analyses supported these associations across different demographic and health-related variables including depression status.
Our results align with previous studies indicating increased mortality and psychiatric disorders in women experiencing POI or early menopause (15). However, they diverge from a Japanese study that found no link between age at menopause and suicide risk, indicating potential demographic or regional differences (34). These mixed findings highlight the importance of considering population-specific factors in understanding menopausal mental health outcomes.
This study provides a novel perspective by examining completed suicide risk rather than suicidal ideation alone, which has been the primary focus of previous research (19). For instance, a cross-sectional study has typically linked early menopause and POI to an increased risk of suicidal ideation but lacked longitudinal follow-up to assess completed suicide as an outcome (19). Our longitudinal approach contributes essential evidence on the cumulative impact of early menopause on mental health, complementing findings from studies focused on ideation.
This study further contributes to understanding menopause-related mental health beyond depression, as early menopause has traditionally been associated with elevated depression risk (16, 35, 36). While depression is a widely recognized risk factor for suicide (22), our findings indicate a direct link between early menopause and elevated suicide risk independent of depression, consistent with a previous study showing genetic correlations with suicide attempts (37). Additionally, a study on Korean women with POI reports a heightened risk of suicidal ideation regardless of major depressive disorder diagnosis (19). This indicates that the mental health implications of early menopause extend beyond depression and may involve other mechanisms.
The increased suicide risk associated with early menopause may be partially attributable to hormonal changes. The transition into menopause brings physiological changes, particularly alterations in neurosteroids such as estrogen and progesterone, which are vital to neuroendocrine regulation, mood stability (38), and control of various behavioral processes (39). Estrogen impacts mood and cognitive functions through its interactions with neurotransmitters neuroprotective mechanisms (40, 41). Estrogen has been associated with a decreased risk of neurodegenerative diseases in postmenopausal women. Its neuroprotective effect is attributed to its activation of estrogen receptors, modulation of gene transcription, and antioxidant effects (42–44). These neurobiological processes are critical for understanding its comprehensive influence on mitigating mood disorders and cognitive decline post-menopause (45, 46). Progesterone plays a crucial role in the central nervous system during menopause. It impacts cognitive processes, mood regulation, inflammation, neurogenesis, and recovery (47), linking hormonal changes to reproductive mood disorders (48). Additionally, progesterone contributes to neural homeostasis, neuroprotection (49), and the development of oligodendrocytes, which are essential for myelination and cognitive function (50). Abrupt hormonal changes during early menopause could disrupt established neuroendocrine balance and neural functions, potentially aggravating mental health conditions during this critical transition period (51). In our study, we included various clinical variables and reproductive factors such as parity, breastfeeding duration, as well as the use of HRT or OC, as these can affect cumulative estrogen and progesterone exposure (23, 24, 26). Our findings remained robust after accounting for these variables, suggesting that menopausal timing itself plays a distinct role in suicide risk beyond the effects of reproductive history and mental health status.
Furthermore, menopause entails profound psychosocial effects. Women may experience a sense of loss related to declining fertility, changes in physical health, and societal attitudes toward aging (52). The stigma surrounding menopause and the perceived loss of youth and fertility can lead to decreased self-worth and identity crises, which are risk factors for mental health issues (18, 53). For those with POI, these experiences can be more intense due to a premature onset, leading to a potentially greater psychosocial impact and a risk of developing mental health conditions predisposing to suicidal ideation and behavior (15). These psychosocial factors likely interact with the biological changes occurring during early menopause, compounding mental health vulnerabilities. This underscores the complex interaction between hormonal changes, reproductive history, and mental health in influencing suicide risk, reinforcing the need for a comprehensive approach to managing mental health in women experiencing early menopause.
Our study utilized the NHIS database of over 52 million individuals with national coverage and long follow-up (27). This database offers robust statistical power and a wide array of data, enhancing the validity and reliability of our findings (29). The incorporation of a wide range of reproductive and health-related factors in our analysis provides a nuanced understanding of the relationship between menopausal timing and suicide risk. However, our study has limitations, including its retrospective nature that precludes the establishment of the causality between menopausal timing and suicide risk. It has potential for recall bias and misclassification due to reliance on self-reported data. Additionally, the findings may be subject to selection bias and are primarily generalizable to Korean women, limiting their applicability to other populations. Furthermore, the identification based on ICD-10 codes in medical records might not fully capture nuances of mental health conditions as this approach does not include systematic assessments of mental health. Cultural factors specific to Korea, such as stigma or reluctance to seek psychiatric help (54–56), might have led to underreporting or underdiagnosis of mental health conditions. Certain confounders, such as gravidity and psychological factors influencing early menopause (e.g., cognitive status, perceived stress), were not available, which may contribute to residual confounding. Also our study did not account for genetic predispositions or autoimmune conditions, which are known to influence both menopausal timing (12) and mental health outcomes. Future studies could benefit from incorporating a broader range of psychological, genetic, autoimmune, and reproductive factors to provide a more comprehensive view of the relationship between menopausal timing and suicide risk. To expand our understanding of menopause’s impact on mental health, longitudinal studies with diverse populations and data including precise menopause onset dates and extensive clinical histories are essential to validate our findings and enhance their relevance across different demographic groups. Exploring psychosocial aspects such as societal perceptions and individual coping strategies will provide further insight into postmenopausal mental health challenges.
Our research highlights the necessity of a proactive, integrated clinical approach to address the suicide risk associated with early menopause and POI. Healthcare providers should implement targeted risk assessments and regular screenings. Timely interventions are crucial for identifying and managing mental health issues effectively. While HRT is a recommended treatment (57), its application requires caution due to potential risks such as increased depression, especially after initiation (35, 58). A comprehensive approach combining cautious use of HRT, psychoeducation, and psychological support is vital for preventing suicide and enhancing the overall well-being of women undergoing early menopause or POI (18, 59).
In conclusion, findings of this study suggest that postmenopausal women who experience menopause at an earlier age, particularly those with POI, may face an increased risk of suicide. Such insight into the relationship between menopausal age and suicide risk emphasizes the need for incorporating reproductive health factors into mental health assessments and suicide prevention strategies for postmenopausal women.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://nhiss.nhis.or.kr/.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Soongsil University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it involved retrospective analyses of de-identified data.
Author contributions
DM: Conceptualization, Methodology, Visualization, Writing – original draft, Writing – review & editing. HK: Writing – review & editing. JJ: Formal analysis, Writing – review & editing. KH: Conceptualization, Formal analysis, Methodology, Writing – review & editing. HJ: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant (No. 2021M3A9E4080784) of the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT and by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), sponsored by the Ministry of Health & Welfare, Republic of Korea (No. HR21C0885).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. (2001) 153:865–74. doi: 10.1093/aje/153.9.865
2. Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. (2013) 178:70–83. doi: 10.1093/aje/kws421
3. Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women's Midlife Health Project. Hum Reprod Update. (2007) 13:559–65. doi: 10.1093/humupd/dmm020
4. Santoro N, Roeca C, Peters BA, Neal-Perry G. The menopause transition: signs, symptoms, and management options. J Clin Endocrinol Metab. (2021) 106:1–15. doi: 10.1210/clinem/dgaa764
5. Lobo RA, Davis SR, De Villiers TJ, Gompel A, Henderson VW, Hodis HN, et al. Prevention of diseases after menopause. Climacteric. (2014) 17:540–56. doi: 10.3109/13697137.2014.933411
6. Baker FC, de Zambotti M, Colrain IM, Bei B. Sleep problems during the menopausal transition: prevalence, impact, and management challenges. Nat Sci Sleep. (2018) 10:73–95. doi: 10.2147/NSS.S125807
7. Newhouse P, Albert K. Estrogen, stress, and depression: A neurocognitive model. JAMA Psychiatry. (2015) 72:727–9. doi: 10.1001/jamapsychiatry.2015.0487
8. Albert KM, Newhouse PA. Estrogen, stress, and depression: cognitive and biological interactions. Annu Rev Clin Psychol. (2019) 15:399–423. doi: 10.1146/annurev-clinpsy-050718-095557
9. Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. (2015) 16:17–29. doi: 10.1038/nrn3856
10. Lim YM, Jeong K, Lee SR, Chung HW, Lee W. Association between premature ovarian insufficiency, early menopause, socioeconomic status in a nationally representative sample from Korea. Maturitas. (2019) 121:22–7. doi: 10.1016/j.maturitas.2018.12.004
11. Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. (2009) 360:606–14. doi: 10.1056/NEJMcp0808697
12. De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. (2010) 376:911–21. doi: 10.1016/S0140-6736(10)60355-8
13. Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. (2019) 4:e553–e64. doi: 10.1016/S2468-2667(19)30155-0
14. Okeke T, Anyaehie U, Ezenyeaku C. Premature menopause. Ann Med Health Sci Res. (2013) 3:90–5. doi: 10.4103/2141-9248.109458
15. Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. (2010) 65:161–6. doi: 10.1016/j.maturitas.2009.08.003
16. Georgakis MK, Thomopoulos TP, Diamantaras AA, Kalogirou EI, Skalkidou A, Daskalopoulou SS, et al. Association of age at menopause and duration of reproductive period with depression after menopause: A systematic review and meta-analysis. JAMA Psychiatry. (2016) 73:139–49. doi: 10.1001/jamapsychiatry.2015.2653
17. Ryan J, Scali J, Carriere I, Amieva H, Rouaud O, Berr C, et al. Impact of a premature menopause on cognitive function in later life. BJOG. (2014) 121:1729–39. doi: 10.1111/1471-0528.12828
18. Groff AA, Covington SN, Halverson LR, Fitzgerald OR, Vanderhoof V, Calis K, et al. Assessing the emotional needs of women with spontaneous premature ovarian failure. Fertil Steril. (2005) 83:1734–41. doi: 10.1016/j.fertnstert.2004.11.067
19. Ryu KJ, Park H, Jeong Y, Nam S, Jeong HG, Kim T. Age at menopause and suicidal ideation in menopausal women: A study of korea national health and nutrition examination survey data. J Korean Med Sci. (2022) 37:e330. doi: 10.3346/jkms.2022.37.e330
20. World Health Organization. Suicide worldwide in 2019: global health estimates. Available online at: https://www.who.int/publications/i/item/9789240026643 (accessed January 6, 2024).
22. Turecki G, Brent DA, Gunnell D, O'Connor RC, Oquendo MA, Pirkis J, et al. Suicide and suicide risk. Nat Rev Dis Primers. (2019) 5:74. doi: 10.1038/s41572-019-0121-0
23. Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. (2017) 20:287–96. doi: 10.1038/nn.4458
24. Kendall-Tackett K. A new paradigm for depression in new mothers: the central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. Int Breastfeed J. (2007) 2:6. doi: 10.1186/1746-4358-2-6
25. Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel Schetter C. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol. (2015) 11:99–137. doi: 10.1146/annurev-clinpsy-101414-020426
26. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. (2015) 9:37. doi: 10.3389/fnins.2015.00037
27. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. (2014) 38:395–403. doi: 10.4093/dmj.2014.38.5.395
28. World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. (1992). Available online at: https://iris.who.int/handle/10665/37958
29. Kim HK, Song SO, Noh J, Jeong IK, Lee BW. Data configuration and publication trends for the korean national health insurance and health insurance review & Assessment database. Diabetes Metab J. (2020) 44:671–8. doi: 10.4093/dmj.2020.0207
30. Shin DW, Cho J, Park JH, Cho B. National General Health Screening Program in Korea: history, current status, and future direction. Precis Future Med. (2022) 6:9–31. doi: 10.23838/pfm.2021.00135
31. World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment . Available online at: https://iris.who.int/bitstream/handle/10665/206936/0957708211_eng.pdf (accessed January 6, 2024).
32. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. (2007) 53:766–72. doi: 10.1373/clinchem.2006.077180
33. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bmj. (2007) 335:806–8. doi: 10.1136/bmj.39335.541782.AD
34. Tanaka S, Abe SK, Sawada N, Yamaji T, Shimazu T, Goto A, et al. Female reproductive factors and risk of external causes of death among women: The Japan Public Health Center-based Prospective Study (JPHC Study). Sci Rep. (2019) 9:14329. doi: 10.1038/s41598-019-50890-x
35. Kim H, Jung JH, Han K, Lee DY, Fava M, Mischoulon D, et al. Ages at menarche and menopause, hormone therapy, and the risk of depression. Gen Hosp Psychiatry. (2023) 83:35–42. doi: 10.1016/j.genhosppsych.2023.04.001
36. Jung M, Koo H, Noh JH. Association between depression and early menopause in South Korean women. Internet J Gynecology Obstetrics. (2019) 23. doi: 10.5580/IJGO.54305
37. Fujikane D, Ohi K, Kuramitsu A, Takai K, Muto Y, Sugiyama S, et al. Genetic correlations between suicide attempts and psychiatric and intermediate phenotypes adjusting for mental disorders. Psychol Med. (2024) 54:488–94. doi: 10.1017/S0033291723002015
38. Graham BM, Denson TF, Barnett J, Calderwood C, Grisham JR. Sex hormones are associated with rumination and interact with emotion regulation strategy choice to predict negative affect in women following a sad mood induction. Front Psychol. (2018) 9:937. doi: 10.3389/fpsyg.2018.00937
39. Do Rego JL, Seong JY, Burel D, Leprince J, Vaudry D, Luu-The V, et al. Regulation of neurosteroid biosynthesis by neurotransmitters and neuropeptides. Front Endocrinol (Lausanne). (2012) 3:4. doi: 10.3389/fendo.2012.00004
40. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cognit Neurosci Rev. (2005) 4:43–58. doi: 10.1177/1534582305277152
41. Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Hormones Behavior. (2015) 74:125–38. doi: 10.1016/j.yhbeh.2015.06.010
42. Green PS, Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci. (2000) 18:347–58. doi: 10.1016/s0736-5748(00)00017-4
43. Dhandapani KM, Brann DW. Protective effects of estrogen and selective estrogen receptor modulators in the brain. Biol Reprod. (2002) 67:1379–85. doi: 10.1095/biolreprod.102.003848
44. Simpkins JW, Singh M, Brock C, Etgen AM. Neuroprotection and estrogen receptors. Neuroendocrinology. (2012) 96:119–30. doi: 10.1159/000338409
45. Wharton W, Gleason CE, Olson SR, Carlsson CM, Asthana S. Neurobiological underpinnings of the estrogen - mood relationship. Curr Psychiatry Rev. (2012) 8:247–56. doi: 10.2174/157340012800792957
46. Zarate S, Stevnsner T, Gredilla R. Role of estrogen and other sex hormones in brain aging. Neuroprotection and DNA repair. Front Aging Neurosci. (2017) 9:430. doi: 10.3389/fnagi.2017.00430
47. Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, et al. Progesterone receptors: form and function in brain. Front Neuroendocrinol. (2008) 29:313–39. doi: 10.1016/j.yfrne.2008.02.001
48. Schiller CE, Schmidt PJ, Rubinow DR. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacol (Berl). (2014) 231:3557–67. doi: 10.1007/s00213-014-3599-x
49. Melcangi RC, Giatti S, Calabrese D, Pesaresi M, Cermenati G, Mitro N, et al. Levels and actions of progesterone and its metabolites in the nervous system during physiological and pathological conditions. Prog Neurobiol. (2014) 113:56–69. doi: 10.1016/j.pneurobio.2013.07.006
50. Ghoumari AM, Baulieu EE, Schumacher M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience. (2005) 135:47–58. doi: 10.1016/j.neuroscience.2005.05.023
51. Riecher-iecher A. Menopause and mental health. In: Chandra PS, Herrman H, Fisher J, Riecher-Rössler A, editors. Mental health and illness of women. Springer Nature, Singapore (2020). p. 147–73.
52. Ishizuka B. Current understanding of the etiology, symptomatology, and treatment options in premature ovarian insufficiency (POI). Front Endocrinol (Lausanne). (2021) 12:626924. doi: 10.3389/fendo.2021.626924
53. Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. (2005) 3:47. doi: 10.1186/1477-7525-3-47
54. Seo HY, Song GY, Ku JW, Park HY, Myung W, Kim HJ, et al. Perceived barriers to psychiatric help-seeking in South Korea by age groups: text mining analyses of social media big data. BMC Psychiatry. (2022) 22:332. doi: 10.1186/s12888-022-03969-1
55. Henderson C, Evans-Lacko S, Thornicroft G. Mental illness stigma, help seeking, and public health programs. Am J Public Health. (2013) 103:777–80. doi: 10.2105/AJPH.2012.301056
56. Zhang Z, Sun K, Jatchavala C, Koh J, Chia Y, Bose J, et al. Overview of stigma against psychiatric illnesses and advancements of anti-stigma activities in six asian societies. Int J Environ Res Public Health. (2019) 17:280. doi: 10.3390/ijerph17010280
57. Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. (2016) 106:1588–99. doi: 10.1016/j.fertnstert.2016.09.046
58. Wium-Andersen MK, Jørgensen TSH, Halvorsen AH, Hartsteen BH, Jørgensen MB, Osler M. Association of hormone therapy with depression during menopause in a cohort of danish women. JAMA Network Open. (2022) 5:e2239491. doi: 10.1001/jamanetworkopen.2022.39491
Keywords: age at menopause, suicide, primary ovarian insufficiency, early menopause, postmenopausal women, menopause
Citation: Moon DU, Kim H, Jung J-H, Han K and Jeon HJ (2024) Association of age at menopause and suicide risk in postmenopausal women: a nationwide cohort study. Front. Psychiatry 15:1442991. doi: 10.3389/fpsyt.2024.1442991
Received: 03 June 2024; Accepted: 25 November 2024;
Published: 17 December 2024.
Edited by:
Wulf Rössler, Charité Department of Psychiatry and Psychotherapy, Charité University Medicine Berlin, GermanyReviewed by:
Gabriel Guillén-Ruiz, Universidad Veracruzana, MexicoQiang Yao, Sichuan University, China
Copyright © 2024 Moon, Kim, Jung, Han and Jeon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Jin Jeon, amVvbmhqQHNra3UuZWR1; amhqMDAxMDAxQGdtYWlsLmNvbQ==; Kyungdo Han, aGtkOTE3QG5hdmVyLmNvbQ==
†These authors have contributed equally to this work
 Daa Un Moon
Daa Un Moon Hyewon Kim
Hyewon Kim Jin-Hyung Jung
Jin-Hyung Jung Kyungdo Han
Kyungdo Han Hong Jin Jeon
Hong Jin Jeon