- 1Department of Cardiology, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 3Department of Integrative Cardiology, China-Japan Friendship Hospital, Beijing, China
Background: Emerging studies reveal a shared pathophysiological underpinning for metabolic problems and mental illnesses. The present study aimed to determine the association between atherogenic index of plasma (AIP) and the incidence of major depressive disorder (MDD).
Methods: 7,951 subjects of US adults were collected from the National Health and Nutrition Examination Survey (NHANES) 2005-2018. MDD was evaluated through the Patient Health Questionnaire (PHQ-9). Multivariate logistic regression, sensitivity analysis, and spline smoothing plot method were used to identify the relationship between AIP and MDD. The cut-off point was calculated using recursive partitioning analysis when segmenting effects emerged. The area under the receiver operating characteristic (ROC) curve (AUC) and Hosmer-Lemeshow test were conducted to evaluate the performance of AIP in identifying MDD. Subgroup analyses and interaction tests were used to explore whether the association was stable in different populations.
Results: A positive correlation between AIP and PHQ-9 score and MDD was both observed in 7,951 subjects included in the study, with a significant threshold of -0.42 determined using recursive partitioning analysis. In the fully adjusted model, a positive association between AIP and PHQ-9 score and MDD was observed (β=0.46, 95% CI 0.14~0.78; OR=1.42, 95% CI 1.04~1.93). Individuals in the highest AIP quartile had a 0.39-unit higher PHQ-9 score (β=0.39, 95% CI 0.12~0.66) and a significantly 33% greater risk of MDD than those in the lowest AIP quartile (OR=1.33, 95% CI 1.02~1.73). Spline smoothing plot analysis further confirmed the positive and non-linear association between AIP and PHQ-9 and MDD. ROC analysis (AUC=0.771) and the Hosmer-Lemeshow test (χ2 = 14.239, P=0.076) suggested an excellent performance and goodness-of-fit of the relatively optimal model. DCA and CIC analysis also revealed a favorable overall net benefit and clinical impact of the model. Subgroup analyses and interaction tests revealed that the association between AIP and PHQ-9 score and MDD remained consistent across different subgroups and was not modified by other covariates, and this positive correlation was more pronounced in those with diabetes or hypertension.
Conclusion: An elevated AIP is linked to a higher chance of MDD, especially in those with diabetes or hypertension. Resolving dyslipidemia and managing comorbidities may help reduce the likelihood of developing MDD.
1 Introduction
Depressive disorder is a widespread, severe, and possibly lethal mental condition that also harms physical health and imposes a significant cost on public health (1). Around 300 million individuals worldwide live in the shadow of depressive disorder, and the Global cases of major depressive disorder (MDD) increased by 27.6% in the wake of the novel coronavirus pneumonia pandemic, posing a significant challenge to mental health systems and services (2). Depressive disorders are expected to surpass cardiovascular diseases and tumors as the first global burden of disease in 2030 (3). Currently, the management of depressive disorder comprises a variety of ways such as pharmacotherapy, psychotherapy, electroconvulsive treatment, and integrative therapy (4). Although antidepressants are widely available and relatively effective, they often produce adverse reactions and take weeks before exhibiting effectiveness, thus limiting their use in certain populations, including those with acute illnesses, adolescents, and pregnant women (5, 6). Accordingly, predicting and preventing depression is crucial, and an easily accessible and reliable predictor to assess the occurrence and development of depressive disorders is of paramount importance in clinical settings.
The pathogenesis of depressive disorders is complicated and can be influenced by numerous factors, including genetic, psychological, environmental, and biological factors. The links between depression and oxidative stress, pro-inflammatory response, as well as the widely studied relationships between depression and glucose tolerance disorders were well developed (7, 8). Recently, lipid metabolism has been shown to play an important role in the progression of psychiatric disorders such as depressive disorder (9). According to earlier studies, individuals who suffered from depressive disorder frequently had certain alterations in their lipid profiles, including raised levels of total cholesterol (TC), elevated levels of low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG), and lower levels of high-density lipoprotein cholesterol (HDL-C) (10, 11). The atherogenic index of plasma (AIP), an important biomarker for predicting cardiovascular diseases and depressive symptoms, is mathematically defined as the logarithmic ratio of TG to HDL-C (12). The relationship between cardiovascular events and mental health has been well established (13–15). As studies increasingly suggests that AIP is associated with the incident cardiovascular events, it becomes imperative to identify whether AIP may also reveal complex interactions between cardiovascular diseases and depression. Previous studies have confirmed the relationship between AIP and cardiovascular diseases as well as the association of cardiovascular diseases with depression, respectively (16, 17). Nevertheless, there is still a lack of evidence on the potential direct association between AIP and MDD. Most importantly, determining new and dependable biomarkers that may help predict MDD would contribute to early detection, risk stratification and timely intervention, ultimately improving patient outcomes.
Therefore, this study aims to examine the relationship between AIP and depressive disorder using the continuous cross-sectional data from the NHANES database with national representation and complex multi-stage sampling. Our findings may provide convincing evidence on the involvement of lipid metabolism in MDD and serve as an informative guide for revealing the potential mechanism and correlation between AIP and MDD.
2 Materials and methods
2.1 Study population
This study was conducted with data sourced from the National Health and Nutrition Examination Survey (NHANES) carried out from 2005 to 2018. The NHANES database is a national nutrition and health survey of the U.S. population conducted annually since 1999 by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The survey utilized household questionnaires, telephone interviews, and examinations conducted by medical professionals and trained personnel to collect data and to create a representative sample of the U.S. population through complex multistage, stratified, clustered probability sample. The survey protocol was formally approved by the CDC Institutional Review Board, and all participants gave voluntary informed consent.
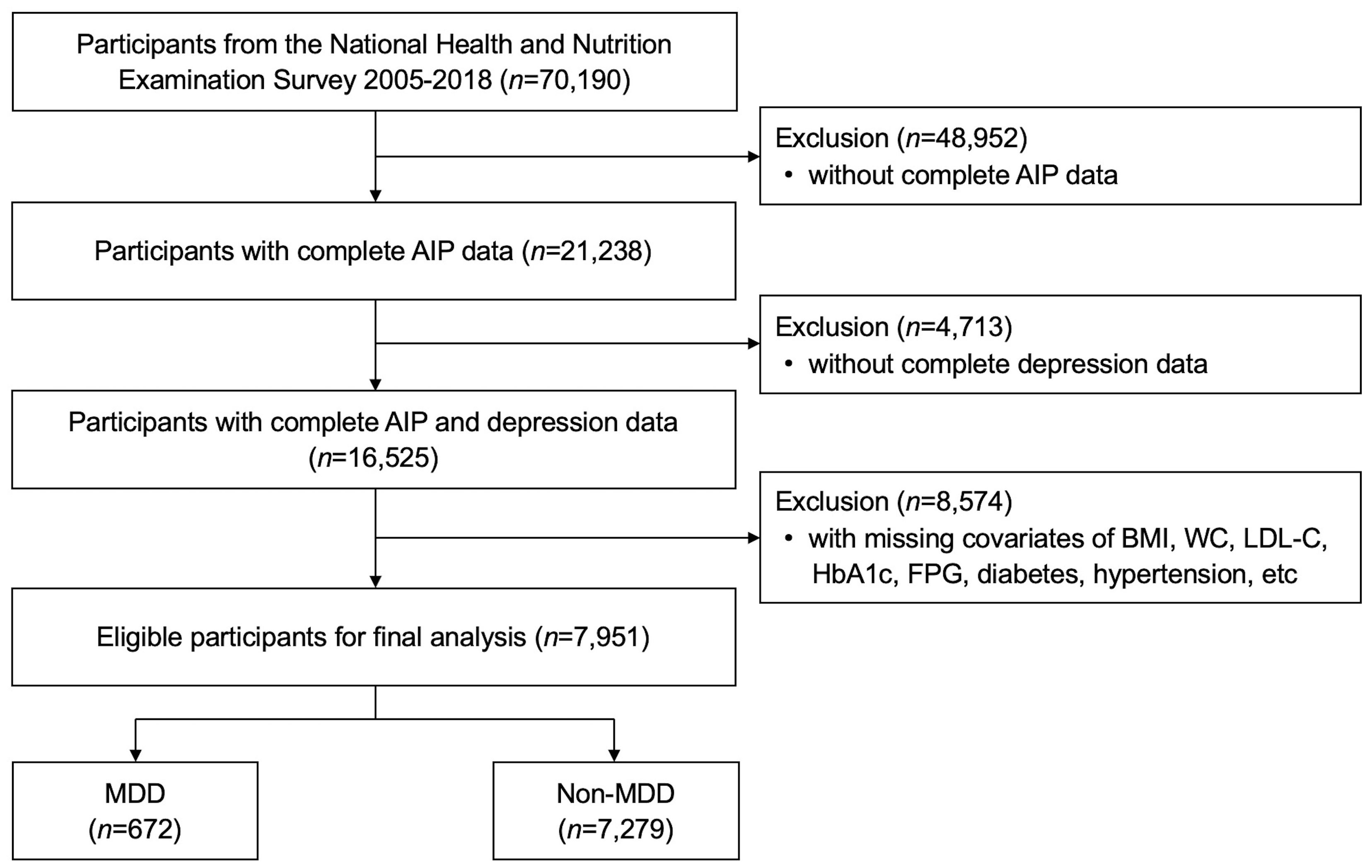
As shown in Figure 1, seven cycles of dataset with full information were used for analysis 2005-2018. Among the 70,190 subjects in the NHANES over the period 2005-2018, a total of 7,951 US adults were enrolled in the present study finally after exclusion of patients with no complete AIP data (n=48,952) and MDD information (n=4,713), and with missing covariates (n=8,574).
2.2 Major depressive disorder
Depressive disorder was assessed using the Patient Health Questionnaire (PHQ-9), a self-report depression screening instrument from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (18). It is a dependable and reliable tool of depression severity based on nine items reflecting depressive disorder that capture the signs and symptoms of depression experienced over the past two weeks. For each symptom question, points ranging from 0 to 3, are associated with the response categories “not at all”, “several days”, “more than half the days”, and “nearly every day”. Total scores range from 0 to 27, with a score greater than or equal to 10 representing MDD (19).
2.3 Atherogenic index of plasma
AIP is derived from two vital lipid indicators, including TG and HDL-C. Rigorous laboratory tests for relevant indicators were conducted in accordance with standardized sampling protocols to assure the validity and comparability of data. Blood samples are typically collected on survey vehicles or at designated sampling sites, followed by processing and testing in the laboratory. The computational formula for AIP was presented as follows: AIP=Lg [TG (mmol/L)/HDL-C (mmol/L)] (12).
2.4 Covariates
The covariates were selected based on previous studies and clinical practice that demonstrated a potential association with AIP levels and MDD prevalence. Standardized questionnaires were utilized to gather information on various factors, including demographic, medical history, and laboratory data. Demographic data comprised age, gender, race, education level, marital status, family income-to-poverty ratio (FIPR), body mass index (BMI), and waist circumference (WC). Medical history information included PHQ-9 score, cigarette use, diabetes, hypertension, hyperlipidemia, cardiovascular disease (CVD), and chronic kidney disease (CKD). Laboratory data encompassed, white blood cell (WBC), segmented neutrophil (Neu), lymphocyte (Lym), platelet (PLT), hemoglobin (Hb), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), serum creatinine (Scr), uric acid (UA), TC, TG, LDL-C, HDL-C, fasting plasma glucose (FPG), glycated hemoglobin A1c (HbA1c), iron, phosphorus, potassium, sodium, and calcium. The demographic data and medical history information were collected through interviews. Race was categorized into four groups: Mexican American, Non-Hispanic White, Non-Hispanic Black, and other races. Education was stratified into three classes: less than high school, high school or equivalent, and college graduate or above. Marital status was organized into three groups: never married, married or living with partner, and others (widowed, divorced, and separated). Smoking at least 100 cigarettes in entire life was defined as a smoker. Detailed information on the specimen collection, processing, quality assurance, and monitoring are described in the section of biospecimen program in NHANES.
2.5 Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median [interquartile range (IQR)], and the t-test or the Mann-Whitney U test was selected for hypothesis testing for those with normal and skewed distributions, respectively. Categorical variables were summarized as percentage-based figures and compared by the Chi-Square test. Multivariate linear and logistic regression model was used to explore the relationship between AIP and PHQ-9 score and MDD, respectively. AIP was treated as a continuous variable in the present analysis, and subjects were grouped based on the AIP quartiles for further analysis. Moreover, we established three regression models by adjusting different indicators to control for confounding biases. In addition to the Model 1 without any adjustments for confounders, two other models were fitted. In Model 2, gender, age, race, education level, marital status, and FIPR were modified. Model 3 was a fully adjusted model that took gender, age, race, education level, marital status, FIPR, BMI, diabetes, hypertension, hyperlipidemia, CVD, CKD, and FPG into account. Indicators that were found to be associated with MDD in previous studies or were statistically different at baseline characteristics were also considered to construct the model. The performance of AIP in identifying MDD was assessed using the area under the receiver operating characteristic (ROC) curve (AUC). Meanwhile, we assessed the goodness-of-fit and reliability of the relatively optimal models using calibration curve and Hosmer-Lemeshow test, with P>0.05 regarded as an acceptable model. The decision curve analysis (DCA) and clinical impact curve (CIC) were employed to assess the predictive accuracy and clinical value of the model. Subgroup analyses and interaction tests were used to confirm the stability of the association between AIP and PHQ-9 score and MDD across different subgroups.
Statistical analyses were performed using IBM-SPSS (version 26.0, Chicago, IL, USA) and R (version 4.1.2, Vienna, Austria). A two-sided P<0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
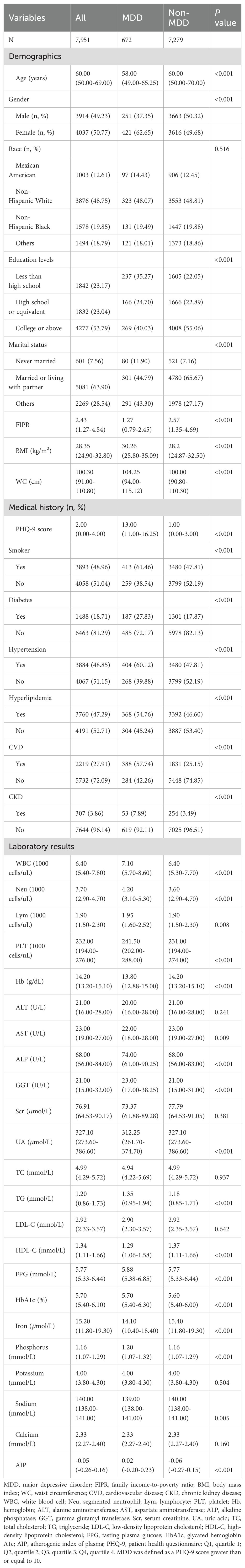
A total of 7,951 subjects with an average age of 60 years were enrolled in the present study, of whom 49.23% were male. The mean AIP levels was -0.05 (-0.26-0.16) for all participants, with the prevalence of MDD was 8.45% overall.
The baseline characteristics of the study subjects according to the PHQ-9 score as a column stratification variable are shown in Table 1. MDD was defined as a PHQ-9 score greater than or equal to 10. Individuals with higher PHQ-9 scores were more likely to be female, younger, less than high school/high school or equivalent, never married, and low income. Depressive disorder was also significantly different in smoking, diabetes, hypertension, hyperlipidemia, CVD, and CKD (P<0.001). Meanwhile, subjects in the MDD group tended to have elevated AIP, BMI, WC, WBC, Neu, Lym, PLT, ALP, GGT, TG, FPG, HbA1c, serum phosphorus, and lower Hb, AST, UA, HDL-C, serum iron, and sodium levels (P<0.05). No statistically significant differences were recorded between the two group in race, ALT, Scr, TC, LDL-C, serum potassium, and calcium (P>0.05).
3.2 AIP cut-off value for MDD
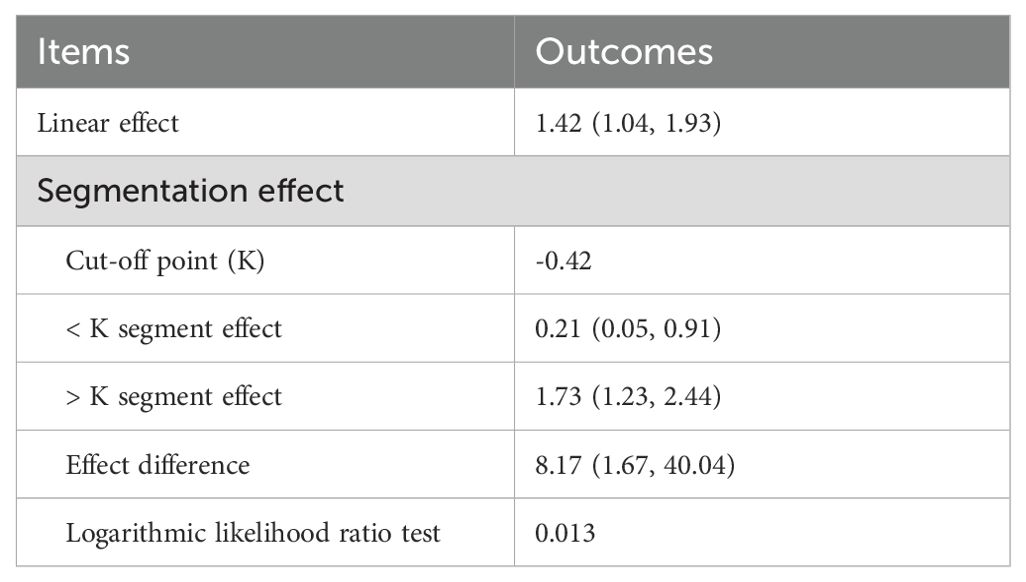
As shown in Table 2, a statistically significant cut-off point (cut-off point=-0.42, P=0.013) in the relationship between AIP and MDD was identified using recursive partitioning analysis. An AIP of -0.42 is the threshold for the risk differentiation of MDD, and the OR value of AIP is close to 1. When the AIP is less than -0.42, it was negatively associated with the risk of MDD (OR=0.21, 95% CI 0.05~0.91). Conversely, when the AIP was greater than -0.42, it was positively related to the risk of MDD (OR=1.73, 95% CI 1.23~2.44).
3.3 The relationship between AIP and depression
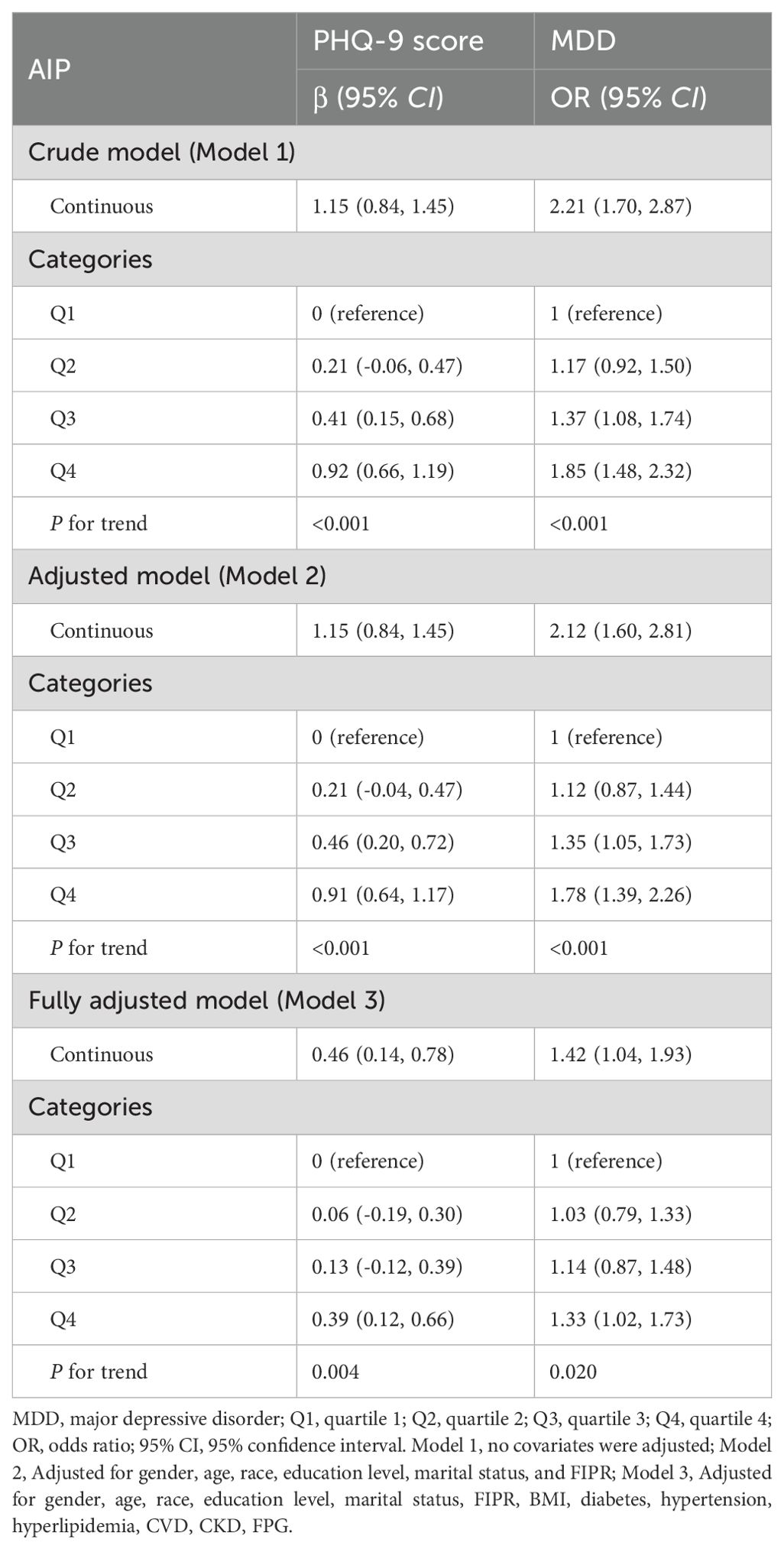
Furthermore, multivariate linear and logistic regression model was performed to explore the correlation between AIP and PHQ-9 score and MDD, respectively (Table 3). In more detail, the results initially demonstrated a significant positive relationship between AIP and PHQ-9 scores in the crude model (β=1.15, 95% CI 0.84~1.45). In the fully adjusted model, a positive association between the AIP and PHQ-9 score was also observed (β=0.46, 95% CI 0.14~0.78), suggesting that each unit of increased AIP score was associated with 0.46 increased units of PHQ-9 score. We further converted the AIP from a continuous variable to a categorical variable to conduct the sensitivity analysis. Compared with the lowest AIP quartile, the PHQ-9 score increased with the higher AIP groups. The mean PHQ-9 score of the highest AIP quartile was 0.39 units higher than that of the lowest quartile (β=0.39, 95% CI 0.12~0.66, P for trend=0.004).
For MDD, we also found a positive association between AIP and the increased likelihood of depressive disorder with statistical significance. After full adjustment in Model 3, subjects with a unit higher AIP had a 42% increased risk of MDD (OR=1.42, 95% CI 1.04~1.93). The association remained statistically significant after AIP was treated as quartiles (P for trend=0.020). Participants in the highest AIP quartile had a significantly 33% higher risk than those in the lowest AIP quartile (OR=1.33, 95% CI 1.02~1.73). Overall, the present findings implied that a higher AIP was associated with a higher PHQ-9 score and an increased risk of MDD. Smoothing plot analysis further validated the positive and non-linear association between AIP and PHQ-9 score and MDD (Figure 2).
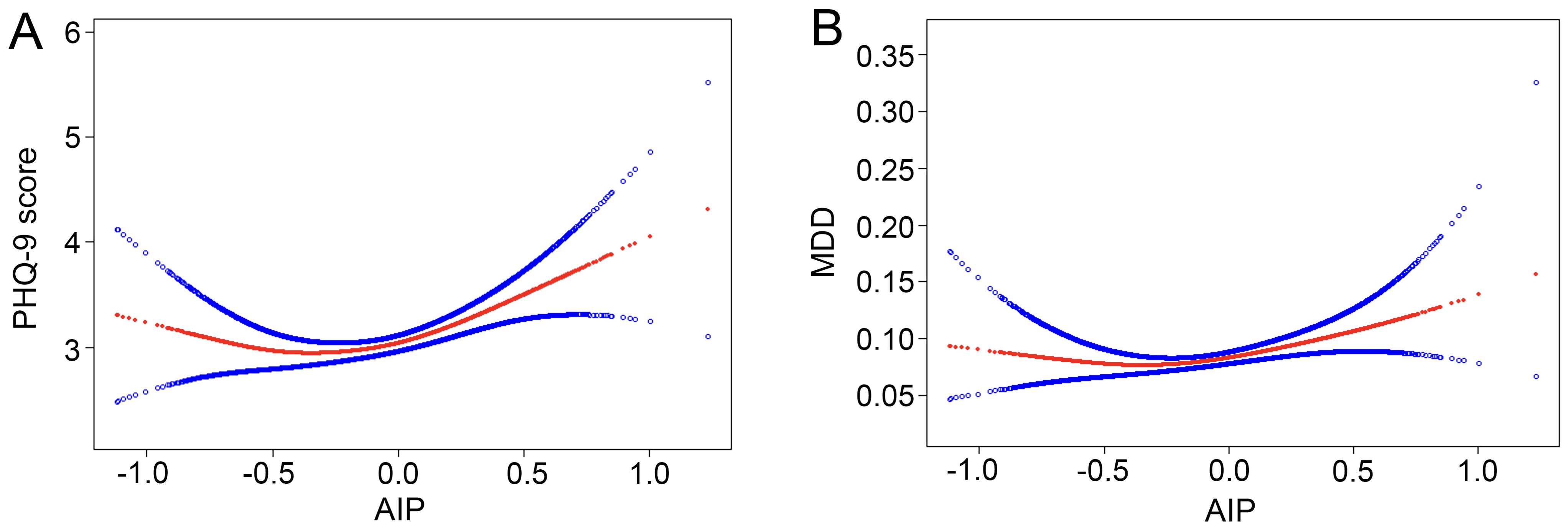
Figure 2. Nonlinear relationship between AIP and depression. The red line represents the smooth curve fit between variables, and the blue bands represent the 95% of confidence interval from the fit. (A) AIP and PHQ-9 score; (B) AIP and MDD. MDD, major depressive disorder.
3.4 Predictive power of AIP for MDD
To synthetically assess the performance of AIP in identifying MDD, the ROC curve, calibration curve, Hosmer-Lemeshow test, as well as the DCA and CIC were employed to process the analysis (Figure 3). After adjustment for confounding factors, ROC analysis showed that the AUC of AIP (AUC=0.771) was larger than that of TG (AUC=0.561) and HDL-C (AUC=0.614) alone, respectively. Moreover, the Hosmer-Lemeshow test (χ2 = 14.239, P=0.076) and calibration curve all suggested an excellent performance and goodness-of-fit of the multivariate model. DCA and CIC analysis were used to examine the clinical utility of the model, indicating a favorable overall net benefit and clinical impact within most reasonable threshold probability of the model.
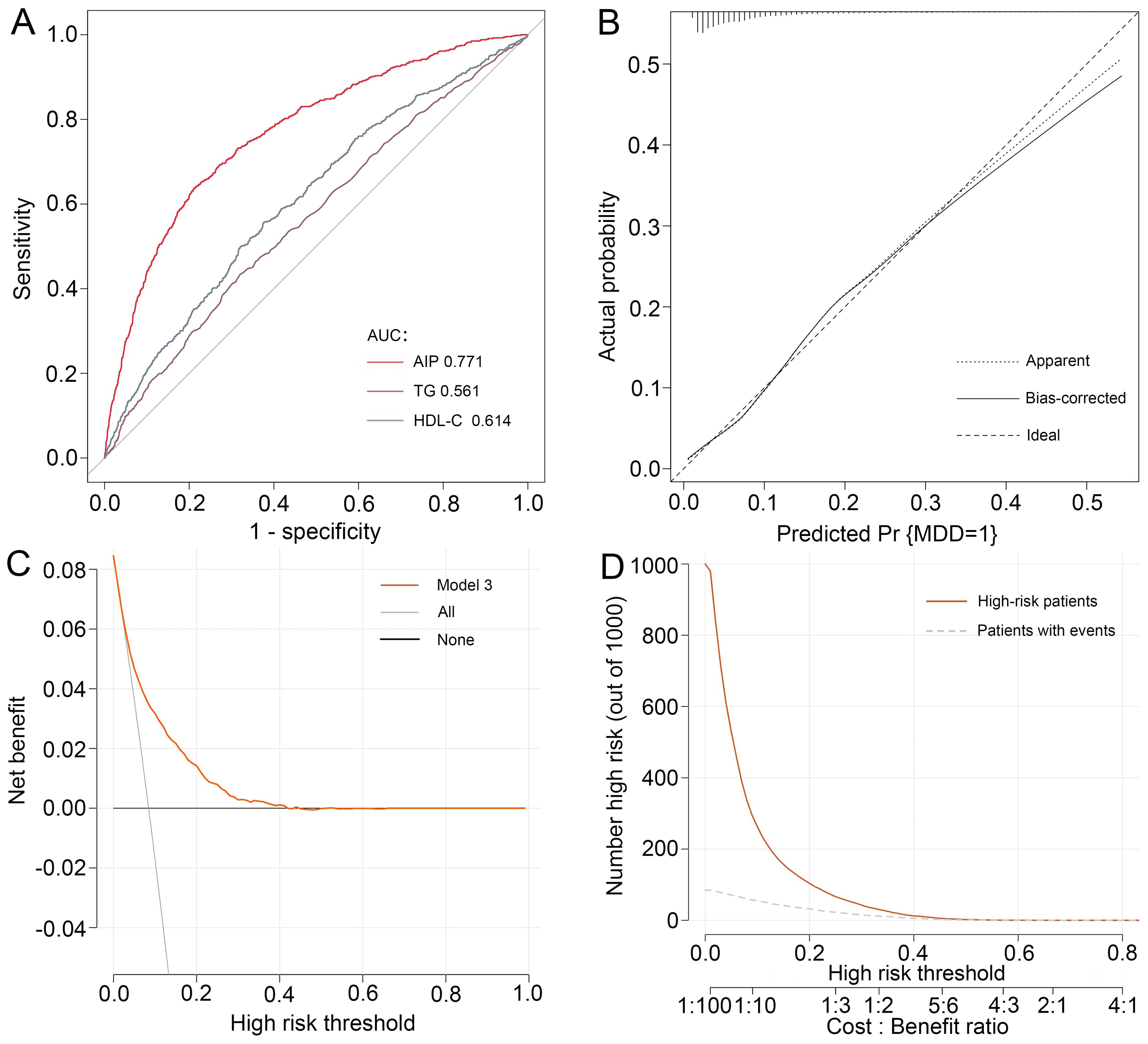
Figure 3. Performance evaluation of AIP for predicting MDD. After adjustment for confounding factors, predictive power of AIP for MDD was examined using (A) the area under the receiver operating characteristic curve (AUC), (B) calibration curve, (C) decision curve analysis (DCA), and (D) clinical impact curve (CIC) analysis. MDD, major depressive disorder.
3.5 Subgroup analysis
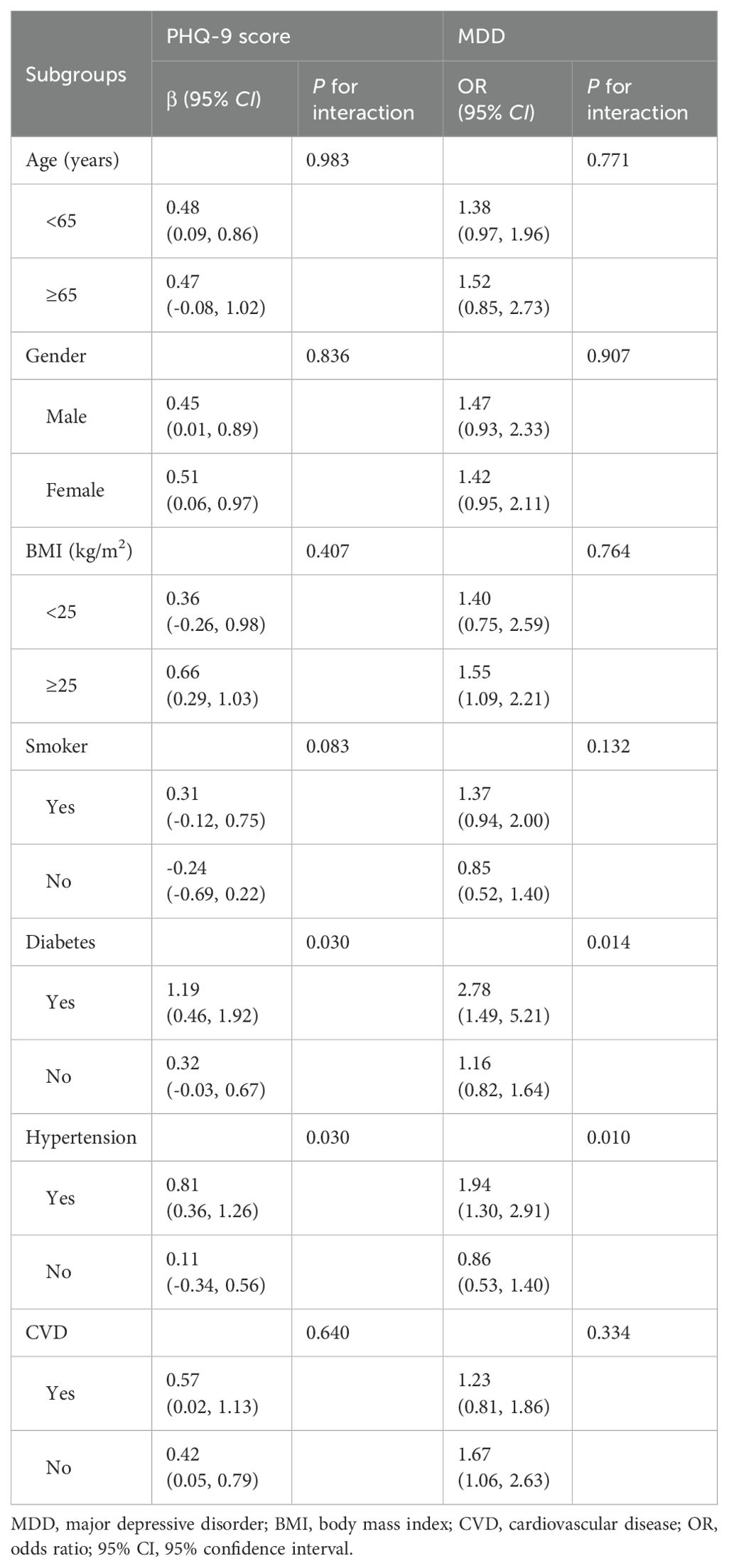
We conducted subgroup analyses and interaction tests stratified by age, gender, BMI, smoker, diabetes, hypertension, and CVD, and to evaluate whether the correlation between AIP and MDD was consistent in the general population and to determine any potentially different population settings (Table 4). The results of our study revealed that the association between AIP and PHQ-9 score and MDD was significantly different in the individuals with diabetes and hypertension (P for interaction<0.05). In the population with diabetes, the risk of MDD was 2.78-fold higher for every unit increase in AIP (OR=2.78, 95% CI 1.49~5.21). In hypertensive patients, individuals with a unit higher AIP had a 94% increased risk of MDD (OR=1.94, 95% CI 1.30~2.91).
4 Discussion
This population-based study of 7,951 US adults form NHANES 2005-2018 explored the relationship between AIP and the incidence of depressive disorder. Notably, the results confirmed that the elevated AIP levels were linked to a higher prevalence of MDD, and this relationship also remained significant even after accounting for all confounding factors, with the highest AIP quartile raising the risk by 33% in the population. Meanwhile, predictive power test indicated an excellent performance and a favorable overall net benefit of the multivariate model. In subgroup analyses and interaction tests, our findings implied that AIP was positively correlated with MDD and a significant dependence of diabetes and hypertension on this correlation was further observed, indicating that elevated levels of AIP may lead to higher PHQ-9 scores and increased the risk of developing MDD, particularly in diabetic and hypertensive populations.
Recently, the metabolic profile leading to depression has attracted much attention (20). AIP serves as a reliable indicator of the atherogenic potential of lipoproteins, particularly the small, dense, and cholesterol-rich particles that play a significant role in the pathogenesis of atherosclerosis (21). Atherosclerosis, as a primary pathological mechanism, significantly accelerates the initiation and progression of multiple cardiovascular diseases (22). Of note, a clear correlation between the presence of CVD and the incident depressive disorder has been well established (5, 23, 24). Moreover, previous evidence (25, 26) implicated that chronic inflammatory states act as a bridge linking depression and atherosclerosis. Studies (27–29) have shown that depression is often accompanied by elevated levels of pro-inflammatory markers such as C-reactive protein and interleukin-6, which not only lead to the onset and progression of depression but also significantly increase the risk of CVD. AIP, which is considered to be involved in both atherosclerosis and inflammation, is expected to be a key link between the two health problems (30, 31). Thus, further study of AIP as a predictor of the presence of depressive disorder may markedly contribute to enhancing our understanding of the intricate biological processes linking mental disorders with cardiovascular health.
Consistent with previous study (32), our current study demonstrated a significant association between elevated AIP levels and a greater prevalence of MDD, even after adjusting for potential confounding factors. This revealed that a more severe disturbance in metabolic profile is related to a higher incidence of MDD, and this correlation between AIP levels and MDD prevalence remained consistent across most subgroups. Notably, individuals with diabetes or hypertension showed a robust association between AIP levels and MDD prevalence, unlike their non-diabetic or non-hypertensive counterparts. Diabetes has been shown to be an important risk factor for MDD (33). Recent research (34) shows that depressive disorder is associated with a higher risk of diabetes, and comorbidity of the two worsens long-term survival. Consequently, blood glucose management and prevention of diabetes should be emphasized in depressed patients, and diabetic patients should also be routinely screened for depression risk considering the impact of comorbid diabetes and depressive disorder. Similarly, hypertension also plays an important role in the development of depressive disorder (35). Results from a cohort (36) comprising 8,677 participants suggested depressive disorder is associated with the risk of cardiovascular death in hypertensive patients, and the more severe the depressive symptoms, the higher the risk of cardiovascular death. Furthermore, a significant bidirectional phenotypic association between depression and hypertension was further recorded in an observational analysis of more than 400,000 participants (37). Most importantly, a multi-center, parallel, cluster-randomized, controlled, implementation trial (38) confirmed that the addition of depression care to chronic medical care exerted a more positive effect on improving mental health outcomes for individuals with hypertension and diabetes. Surprisingly, AIP levels were positively correlated with MDD in individuals younger than 65 years of age or with CVD, but the risk of MDD in the subgroups was almost consistent from the perspective of the two general populations. The difference may derive from the multifaceted physiological and psychological effects of age and CVD history, thus masking a clear link between depressive disorder and the two. Elderly people and those with CVD history may be more aware of risk factors for depressive disorder and tend to adopt healthier lifestyle habits that positively alter lipid metabolism and cardiovascular health, partially offsetting the impact of age and comorbidities on the association of depression. However, residual physiological effects of advanced age and CVD on depression may still remain, and these potential effects can indirectly influence the association of AIP with depression (39, 40). Our findings highlight the importance of having to consider numerous factors when addressing depressive disorder, including multiple lipid factors and comorbidities.
Several reasons may explain the strong association between elevated AIP levels and increased incident depressive disorder. First, previous study (41) suggested that increased lipids are often accompanied by an increase in chronic inflammation, which may affect the immune system and emotional regulation, thereby leading to depressive disorders. Dysregulated neuroinflammatory status might underlie the antidepressive-like behaviors (42). Substantial evidences have demonstrated that monophosphoryl lipid A, through immune stimulation, may prevent stress-induced depression-like behaviors in mice by preventing neuroinflammatory responses (43, 44). Second, metabolic disorders often cause neuroendocrine disorders, such as insulin resistance, increased glucocorticoid secretion, and disturbed glucocorticoid sensitivity associated with systemic cortisol activity, which may interfere with neurogenesis and neuroplasticity (45–47). Moreover, oxidative stress response and endothelial dysfunction, widely recognized as central to the development of CVD, may also occupy a key position in the pathophysiology of depression (48, 49). Given the strong link between AIP and MDD, it is critical to reduce the likelihood of MDD in clinical settings by thoroughly evaluating individuals with elevated AIP levels and implementing effective interventions to mitigate risk factors.
This study has several strengths. Firstly, the data used in this study came from NHANES, a nationwide population-based sample database that followed a standard protocol. All analyses were conducted with consideration of appropriate NHANES sampling weights to make the study samples more representative. Secondly, after adjusting for confounder factors, the present study simultaneously evaluated nonlinear associations between AIP and PHQ-9 score and MDD, calculated the cut-off point, and determined segmented effects between variables to provide more robust evidence. However, several limitations of the present study must be acknowledged. Firstly, due to the cross-sectional study design, we cannot obtain a clear causal relationship between AIP and the incidence of depression. Besides, it is worth noting that the AIP measurement was assessed in a timely manner in this study, and the results may not reflect the patients’ long-term situation. the diagnosis of MDD in this study was obtained through self-reporting by participants. Secondly, although certain measures were taken during data collection to avoid systematic errors, there may still remain information bias. Thirdly, we converted AIP into a categorical variable for further analysis, which may lead to a lack of confidence in the evidence due to the reduced sample size in each group. Therefore, future studies can be conducted by expanding the sample size or focusing on a specific population to explore the potential mechanisms of lipid management and depressive disorder.
5 Conclusion
The current investigation showed a positive and non-linear correlation between elevated AIP levels and higher PHQ-9 scores and a greater risk of MDD. Our findings emphasized the necessity of lipid management in identifying patients at risk of depressive disorder and suggested that AIP may serve as a valuable indicator for evaluating MDD risk in individuals, particularly those with diabetes and hypertension. Most importantly, the present study suggested a potential method of evaluating lipid levels and optimizing the risk stratification of depression.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
ST: Writing – review & editing, Writing – original draft. LY: Writing – review & editing. JL: Writing – review & editing, Writing – original draft. XH: Writing – review & editing. TX: Writing – review & editing. DY: Writing – review & editing. YT: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the High Level Chinese Medical Hospital Promotion Project (No. HLCMHPP2023065) and the National Key Research and Development Program of China (No. 2022YFC3500102).
Acknowledgments
In addition to the general gratitude expressed to the participants of the National Health and Nutrition Examination Survey (NHANES) for their invaluable contributions to scientific inquiry, we would also like to express our appreciation to the Centers for Disease Control and Prevention (CDC) for making the NHANES data publicly available, which was of vital importance for helping to carry out this study. Moreover, we also appreciate EmpowerStats (http://www.empowerstats.net) for the assistance in processing the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Collaborators GBDMD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/S2215-0366(21)00395-3
2. Collaborators C-MD. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. (2021) 398:1700–12. doi: 10.1016/S0140-6736(21)02143-7
3. Murray CJL. The Global Burden of Disease Study at 30 years. Nat Med. (2022) 28:2019–26. doi: 10.1038/s41591-022-01990-1
4. Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. (2014) 35:1365–72. doi: 10.1093/eurheartj/eht462
5. Jha MK, Mathew SJ. Pharmacotherapies for treatment-resistant depression: how antipsychotics fit in the rapidly evolving therapeutic landscape. Am J Psychiatry. (2023) 180:190–9. doi: 10.1176/appi.ajp.20230025
6. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. (2018) 391:1357–66. doi: 10.1016/S0140-6736(17)32802-7
7. Krupa AJ, Dudek D, Siwek M. Consolidating evidence on the role of insulin resistance in major depressive disorder. Curr Opin Psychiatry. (2024) 37:23–8. doi: 10.1097/YCO.0000000000000905
8. Wei YY, Gao HQ, Luo YH, Feng JH, Li GG, Wang TT, et al. Systemic inflammation and oxidative stress markers in patients with unipolar and bipolar depression: A large-scale study. J Affect Disord. (2024) 346:154–66. doi: 10.1016/j.jad.2023.10.156
9. Enko D, Brandmayr W, Halwachs-Baumann G, Schnedl WJ, Meinitzer A, Kriegshauser G. Prospective plasma lipid profiling in individuals with and without depression. Lipids Health Dis. (2018) 17:149. doi: 10.1186/s12944-018-0796-3
10. Kim JA, Choi S, Choi D, Park SM. Pre-existing depression among newly diagnosed dyslipidemia patients and cardiovascular disease risk. Diabetes Metab J. (2020) 44:307–15. doi: 10.4093/dmj.2019.0002
11. Khalfan AF, Campisi SC, Lo RF, McCrindle BW, Korczak DJ. The association between adolescent depression and dyslipidemia. J Affect Disord. (2023) 338:239–45. doi: 10.1016/j.jad.2023.06.017
12. Dobiasova M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: theoretical and practical implications. Clin Chem. (2004) 50:1113–5. doi: 10.1373/clinchem.2004.033175
13. Beekman AT. Promoting mental health and promoting cardiovascular health: two sides of the same coin. Am J Geriatr Psychiatry. (2024) 32:550–1. doi: 10.1016/j.jagp.2023.11.007
14. Charlson FJ, Moran AE, Freedman G, Norman RE, Stapelberg NJ, Baxter AJ, et al. The contribution of major depression to the global burden of ischemic heart disease: a comparative risk assessment. BMC Med. (2013) 11:250. doi: 10.1186/1741-7015-11-250
15. Tang BW, Yuan S, Xiong Y, He QQ, Larsson SC. Major depressive disorder and cardiometabolic diseases: a bidirectional Mendelian randomisation study. Diabetologia. (2020) 63:1305–11. doi: 10.1007/s00125-020-05131-6
16. Sadeghi M, Heshmat-Ghahdarijani K, Talaei M, Safaei A, Sarrafzadegan N, Roohafza H. The predictive value of atherogenic index of plasma in the prediction of cardiovascular events; a fifteen-year cohort study. Adv Med Sci. (2021) 66:418–23. doi: 10.1016/j.advms.2021.09.003
17. Huang X, Zhang J, Liang J, Duan Y, Xie W, Zheng F. Association of cardiovascular health with risk of incident depression and anxiety. Am J Geriatr Psychiatry. (2024) 32:539–49. doi: 10.1016/j.jagp.2023.10.017
18. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. (1999) 282:1737–44. doi: 10.1001/jama.282.18.1737
19. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
20. Pinto B, Conde T, Domingues I, Domingues MR. Adaptation of lipid profiling in depression disease and treatment: A critical review. Int J Mol Sci. (2022) 23:2032. doi: 10.3390/ijms23042032
21. Fernandez-Macias JC, Ochoa-Martinez AC, Varela-Silva JA, Perez-Maldonado IN. Atherogenic index of plasma: novel predictive biomarker for cardiovascular illnesses. Arch Med Res. (2019) 50:285–94. doi: 10.1016/j.arcmed.2019.08.009
22. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44:3720–826. doi: 10.1093/eurheartj/ehad191
23. Nakada S, Ho FK, Celis-Morales C, Jackson CA, Pell JP. Individual and joint associations of anxiety disorder and depression with cardiovascular disease: A UK Biobank prospective cohort study. Eur Psychiatry. (2023) 66:e54. doi: 10.1192/j.eurpsy.2023.2425
24. El-Battrawy I, Behnes M, Akin I. Depression and incident cardiovascular disease. JAMA. (2021) 325:1679–80. doi: 10.1001/jama.2021.2029
25. Alcocer-Gomez E, Cordero MD. NLRP3 inflammasome: common nexus between depression and cardiovascular diseases. Nat Rev Cardiol. (2017) 14:124. doi: 10.1038/nrcardio.2016.214
26. Caruso G, Fresta CG, Grasso M, Santangelo R, Lazzarino G, Lunte SM, et al. Inflammation as the common biological link between depression and cardiovascular diseases: can carnosine exert a protective role? Curr Med Chem. (2020) 27:1782–800. doi: 10.2174/0929867326666190712091515
27. Brydges CR, Bhattacharyya S, Dehkordi SM, Milaneschi Y, Penninx B, Jansen R, et al. Metabolomic and inflammatory signatures of symptom dimensions in major depression. Brain Behav Immun. (2022) 102:42–52. doi: 10.1016/j.bbi.2022.02.003
28. Burger PM, Koudstaal S, Mosterd A, Fiolet ATL, Teraa M, van der Meer MG, et al. C-reactive protein and risk of incident heart failure in patients with cardiovascular disease. J Am Coll Cardiol. (2023) 82:414–26. doi: 10.1016/j.jacc.2023.05.035
29. Jia X, Buckley L, Sun C, Al Rifai M, Yu B, Nambi V, et al. Association of interleukin-6 and interleukin-18 with cardiovascular disease in older adults: Atherosclerosis Risk in Communities study. Eur J Prev Cardiol. (2023) 30:1731–40. doi: 10.1093/eurjpc/zwad197
30. Lan Y, Chen G, Wu D, Ding X, Huang Z, Wang X, et al. Temporal relationship between atherogenic dyslipidemia and inflammation and their joint cumulative effect on type 2 diabetes onset: a longitudinal cohort study. BMC Med. (2023) 21:31. doi: 10.1186/s12916-023-02729-6
31. Nunes SO, Piccoli de Melo LG, Pizzo de Castro MR, Barbosa DS, Vargas HO, Berk M, et al. Atherogenic index of plasma and atherogenic coefficient are increased in major depression and bipolar disorder, especially when comorbid with tobacco use disorder. J Affect Disord. (2015) 172:55–62. doi: 10.1016/j.jad.2014.09.038
32. Mehdi SMA, Costa AP, Svob C, Pan L, Dartora WJ, Talati A, et al. Depression and cognition are associated with lipid dysregulation in both a multigenerational study of depression and the National Health and Nutrition Examination Survey. Transl Psychiatry. (2024) 14:142. doi: 10.1038/s41398-024-02847-6
33. Okasha T, Mostafa BM, Ibrahim I, Abdelgawad AA, Lloyd CE, Sartorius N, et al. Comorbidity of depression and type 2 diabetes in Egypt results from the International Prevalence and Treatment of Diabetes and Depression (INTERPRET-DD) study. Int J Soc Psychiatry. (2024) 70:730–8. doi: 10.1177/00207640241228431
34. Cai J, Zhang S, Wu R, Huang J. Association between depression and diabetes mellitus and the impact of their comorbidity on mortality: Evidence from a nationally representative study. J Affect Disord. (2024) 354:11–8. doi: 10.1016/j.jad.2024.03.003
35. Nemcsik-Bencze Z, Korosi B, Gyongyosi H, Batta D, Laszlo A, Torzsa P, et al. Depression and anxiety in different hypertension phenotypes: a cross-sectional study. Ann Gen Psychiatry. (2022) 21:23. doi: 10.1186/s12991-022-00400-7
36. Shen W, Su Y, Guo T, Ding N, Chai X. The relationship between depression based on patient health questionaire-9 and cardiovascular mortality in patients with hypertension. J Affect Disord. (2024) 345:78–84. doi: 10.1016/j.jad.2023.10.059
37. Xu Z, Wu X, Xiao C, Zhang W, Yan P, Yang C, et al. Observational and genetic analyses of the bidirectional relationship between depression and hypertension. J Affect Disord. (2024) 348:62–9. doi: 10.1016/j.jad.2023.12.028
38. Pence BW, Gaynes BN, Udedi M, Kulisewa K, Zimba CC, Akiba CF, et al. Two implementation strategies to support the integration of depression screening and treatment into hypertension and diabetes care in Malawi (SHARP): parallel, cluster-randomised, controlled, implementation trial. Lancet Glob Health. (2024) 12:e652–e61. doi: 10.1016/S2214-109X(23)00592-2
39. Wardhani YF, Nantabah ZK, Machfutra ED, Lestyoningrum SD, Oktarina, Nurjana MA. The prevalence and distribution of risk factors for depression and emotional mental disorders in the elderly in Indonesia. Int J Soc Psychiatry. (2024) 70:763–71. doi: 10.1177/00207640241227381
40. Krittanawong C, Maitra NS, Qadeer YK, Wang Z, Fogg S, Storch EA, et al. Association of depression and cardiovascular disease. Am J Med. (2023) 136:881–95. doi: 10.1016/j.amjmed.2023.04.036
41. Kang L, Wang W, Nie Z, Gong Q, Yao L, Xiang D, et al. Dysregulated cerebral blood flow, rather than gray matter Volume, exhibits stronger correlations with blood inflammatory and lipid markers in depression. NeuroImage Clin. (2024) 41:103581. doi: 10.1016/j.nicl.2024.103581
42. Li W, Ali T, Zheng C, He K, Liu Z, Shah FA, et al. Anti-depressive-like behaviors of APN KO mice involve Trkb/BDNF signaling related neuroinflammatory changes. Mol Psychiatry. (2022) 27:1047–58. doi: 10.1038/s41380-021-01327-3
43. Li F, Lu X, Ma Y, Gu Y, Ye T, Huang C. Monophosphoryl lipid A tolerance against chronic stress-induced depression-like behaviors in mice. Int J Neuropsychopharmacol. (2022) 25:399–411. doi: 10.1093/ijnp/pyab097
44. Li F, Xiang H, Gu Y, Ye T, Lu X, Huang C. Innate immune stimulation by monophosphoryl lipid A prevents chronic social defeat stress-induced anxiety-like behaviors in mice. J Neuroinflamm. (2022) 19:12. doi: 10.1186/s12974-021-02377-8
45. Kivimaki M, Bartolomucci A, Kawachi I. The multiple roles of life stress in metabolic disorders. Nat Rev Endocrinol. (2023) 19:10–27. doi: 10.1038/s41574-022-00746-8
46. Steptoe A, Frank P. Obesity and psychological distress. Philos Trans R Soc Lond B Biol Sci. (2023) 378:20220225. doi: 10.1098/rstb.2022.0225
47. Borsini A, Nicolaou A, Camacho-Munoz D, Kendall AC, Di Benedetto MG, Giacobbe J, et al. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: relevance for major depression and for human hippocampal neurogenesis. Mol Psychiatry. (2021) 26:6773–88. doi: 10.1038/s41380-021-01160-8
48. Correia AS, Cardoso A, Vale N. Oxidative stress in depression: the link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants (Basel). (2023) 12:470. doi: 10.3390/antiox12020470
Keywords: atherogenic index of plasma, major depressive disorder, dyslipidemia, PHQ-9, NHANES
Citation: Tao S, Yu L, Li J, Huang X, Xue T, Yang D and Tan Y (2024) Higher atherogenic index of plasma is associated with increased major depressive disorder: insights from a nationally representative study. Front. Psychiatry 15:1441119. doi: 10.3389/fpsyt.2024.1441119
Received: 30 May 2024; Accepted: 27 September 2024;
Published: 10 October 2024.
Edited by:
Dominik Strzelecki, Medical University of Lodz, PolandReviewed by:
Kamyar Moradi, Johns Hopkins University, United StatesJun Ma, Wuhan University, China
Marcin Siwek, Medical College, Krakow, Poland
Luca Steardo, University Magna Graecia of Catanzaro, Italy
Copyright © 2024 Tao, Yu, Li, Huang, Xue, Yang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, Z2FteXlsakAxNjMuY29t
 Shiyi Tao
Shiyi Tao Lintong Yu2
Lintong Yu2