- 1Department of Psychiatry, Taipei Veteran General Hospital, Taipei, Taiwan
- 2Division of Psychiatry, School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 3Department of Nursing, Mackay Medical College, Taipei, Taiwan
- 4Department of Psychiatry, Tri-Service General Hospital, National Defense Medical Centre, Taipei, Taiwan
- 5Department of Psychiatry, Beitou Branch, Tri-Service General Hospital, Taipei, Taiwan
- 6Institute of Biomedical Sciences, National Sun Yat-sen University, Kaohsiung, Taiwan
- 7Department of Psychology, College of Medical and Health Science, Asia University, Taichung, Taiwan
- 8Prospect Clinic for Otorhinolaryngology & Neurology, Kaohsiung, Taiwan
- 9Institute of Precision Medicine, National Sun Yat-sen University, Kaohsiung, Taiwan
- 10Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 11Department of Pharmacy, Chang Gung Memorial Hospital Linkou, Taipei, Taiwan
- 12Department of Neurology, Tri-Service General Hospital, National Defense Medical Centre, Taipei, Taiwan
- 13Centre for Chronic Illness and Ageing, University of Greenwich, London, United Kingdom
- 14Department of Psychiatry, E-DA Dachang Hospital, I-Shou University, Kaohsiung, Taiwan
- 15Department of Psychiatry, E-DA Hospital, I-Shou University, Kaohsiung, Taiwan
- 16Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
Objective: Psilocybin-assisted therapy has shown promising efficacy on clinical depressive symptoms. However, diverse psychological support or psychotherapy was performed with psilocybin treatment. This study aimed to explore the association of psychological protocols with the efficacy of psilocybin-assisted therapy for depressive symptoms.
Method: Five major databases were systemic searched for clinical trials addressing psilocybin-assisted therapy for patients with clinical depressive symptoms. A Bayesian random-effects meta-analysis and meta-regression were performed. The effect size was mean difference (with 95% credible interval) measured by 17-Item Hamilton Depression Rating Scale.
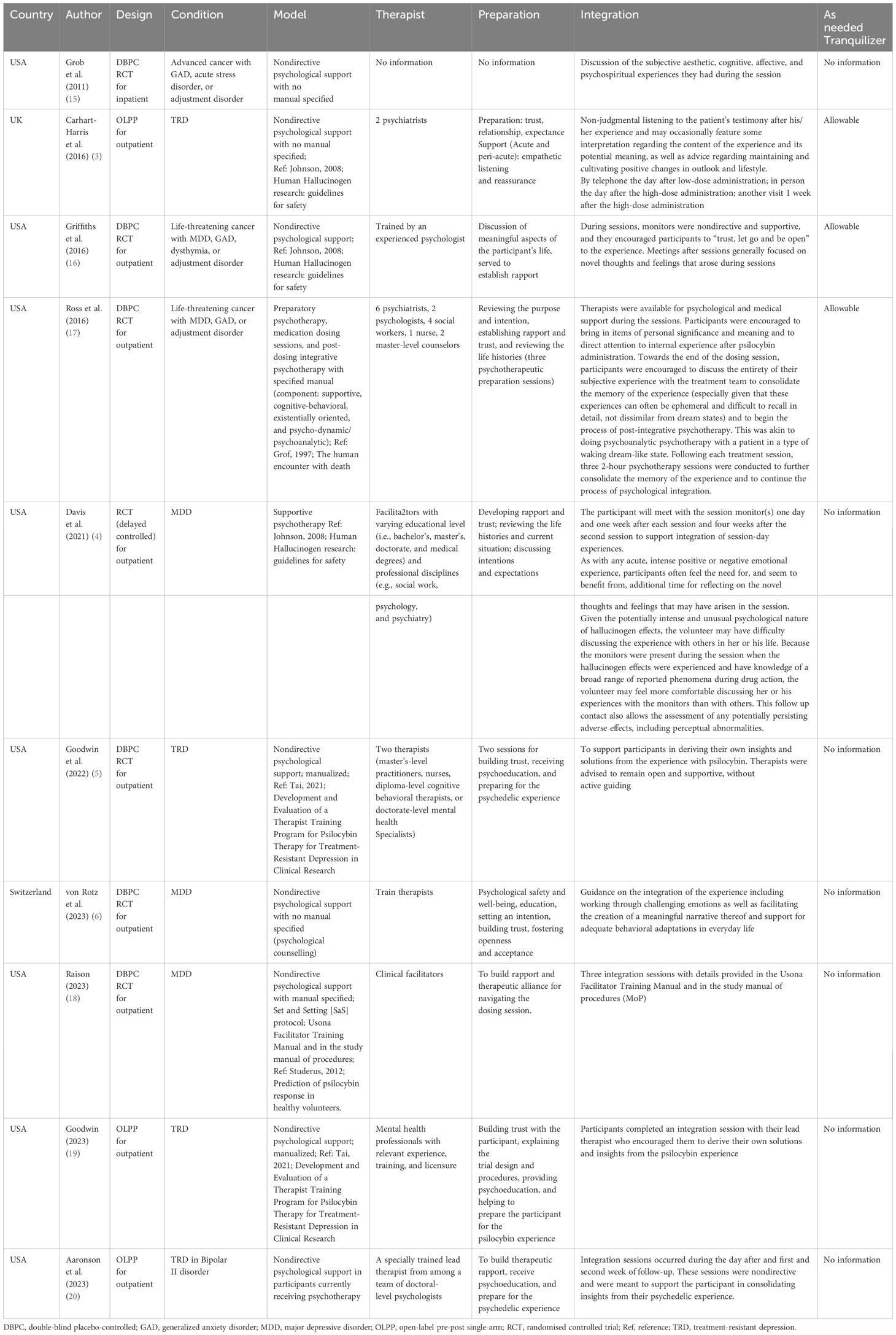
Results: There were 10 eligible studies including 515 adult patients with clinically diagnosed depression. The psychological protocols could be categorized into four types: (i) manualized directive psychotherapy(k=1); (ii) manualized nondirective psychological support(k=3), (iii) non-manualized nondirective psychological support(k=5); and (iv) non-manualized supportive psychotherapy(k=1). The pooled standard mean difference of psilocybin-assisted therapy was 10.08 (5.03-14.70).
Conclusion: Compared with manualized nondirective psychological support, the other three psychological approaches did not differ significantly. The improvement of depressive symptoms was not associated with the psychological protocols in adult patients receiving psilocybin-assisted therapy.
Systemic review registration: Open Science Framework: identifier (osf.io/3YUDV).
Introduction
Major Depressive Disorder (MDD), a complex and deliberating mental disorder, differs significantly from typical transient sadness or the misery experienced in adverse circumstances (1). Substantial evidence indicates that MDD has recurrent or prolonged impacts on various domains, including activities of daily living, quality of life, cognitive function, and employment status. According to the report of World Health Organization, depression is a leading cause of disability worldwide and a major contributor to the overall global burden of disease (2). However, the treatment of MDD is suboptimal, and a third to half of people with MDD do not achieve adequate treatment response with multiple antidepressant trials (1). This underscores the demand for innovative therapeutic strategies with rapid and sustained antidepressant effects.
In recent decades, psychedelic medicine has been advancing, revealing promising evidence for the treatment of various psychiatric disorders. Psilocybin, a classic psychedelic, when paired with psychological support or psychotherapy, has demonstrated rapid and enduring antidepressant effects in several clinical trials (3–6). In 2018, the US Food and Drug Administration (FDA) awarded two breakthrough therapy designations for psilocybin in the treatment of treatment-resistant depression (TRD), followed by a designation for MDD in 2019 (7). On July 1, 2023, Australia has approved psilocybin for the treatment of depression (8). The first country classifies psychedelics as medicines at a national level.
The resurgent exploration of psilocybin for MDD management has sparked enthusiasm. In standard clinical trials, psilocybin is administered with accompanying psychological support or psychotherapy. The efficacy of psilocybin-assisted therapy in MDD is conceptualized as a synergistic interplay between psychotherapeutic techniques and psilocybin. However, the psychotherapeutic approaches employed in previous clinical trials vary widely (9). For example, the study by Carhart-Harris et al. conducted nondirective (or client-led) psychological support without specified manual (3), while another study performed directive psychotherapy with selecting Acceptance and Commitment Therapy (10). The absence of a standardized psychological support or psychotherapy poses challenges in training therapists in the best way to conduct psilocybin-assisted therapy, thereby limiting the application of RCT findings in clinical practice.
Pharmaceutical companies have significantly invested in clinical research and filed patents on the production of psilocybin as well as the therapeutic processes (e.g., training of therapists). In psilocybin clinical trials, the inclusion of psychological support or psychotherapy is expected to influence future labeling. The aim of the current study was to explore the association between psychological approaches and antidepressant efficacy in adult patients receiving psilocybin-assisted therapy. Our paper will provide important information for further studies to select the optimal psychological approaches is psilocybin-assisted therapy for adult patients with clinically diagnosed depression.
Methods
The study protocol was registered with PROSPERO (521995). We followed the Preferred Reporting Items for Systematic Reviews and Meta analyses (PRISMA) (11), which can be found in Appendix 1.
Data sources and search
Two reviewers independently searched MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, ClinicalTrial.gov, and PubMed without language restrictions from database inception to January 15, 2024. We also searched grey literature and reviewed reference lists of included studies and related systematic reviews (12). Two authors independently screened and selected the studies, and discrepancies were resolved by consulting a third author. Appendix 2 demonstrates the complete search strategies, and Appendix 3 shows the reasons for exclusion.
Eligibility criteria
Eligible studies were clinical trials in adult patients (≥18 years) with clinical depression (i.e., diagnosed depression), such as distress related to life-threatening diagnoses and terminal illness, MDD, or other psychiatric disorders with comorbid clinical depression. We considered clinical trials with the following study designs: blinded randomized controlled trials (RCTs), open-label RCTs, and single-arm pre-post studies. We excluded microdosing studies, follow-up studies (follow-up after trial completion), and studies with healthy volunteers. We also excluded conference abstracts, editorials, reviews, meta-analyses, case reports, and case series, as well as publications reporting duplicate data.
Definitions and data extraction
The primary outcome was change in depressive symptoms from baseline (continuous outcome), as measured by a validated rating scale such as the Hamilton Rating Scale for Depression. When multiple measurement tools were used, they were selected in the following order: the Hamilton Rating Scale for Depression, Montgomery-Åsberg Depression Rating Scale, and Beck Depression Inventory (second edition). Regarding the classifications of psychotherapy, they are primarily based on the descriptions in the methods or e-methods sections of the clinical trial studies we included, as well as on whether the psychotherapy is manualized or directive. Outcome data were extracted from the original intention-to-treat analysis. Two authors independently extracted and reviewed the data, which was further verified by another author. WebPlot Digitizer (https://automeris.io/WebPlotDigitizer/) was used to extract numerical data from the figures. Two reviewers independently extracted data and discrepancies were resolved by consensus and, when necessary, by consulting corresponding authors. We extracted the methodology of the psychological support or psychotherapy of each trial. The extracted study-level variables include author, year, condition, psychotherapeutic model, therapist, preparation session, and integration session. We also extracted sample size, study design, dose of psilocybin, age, sex, comorbidities, and baseline and post-treatment severity of depressive symptoms.
Risk of bias assessment
Two authors independently used the Cochrane randomized trial Risk of Bias tool (version 2.0) to assess the risk of bias in the included trials (13). Conflicts resolved through discussion with a third reviewer.
Statistical analysis
All statistical analyses were performed using R version 4.3.1 (The R Foundation for Statistical Computing; Vienna, Austria). The meta-analysis within a Bayesian framework was fitted using the Bayesian statistical software Stan called multinma (14). The posterior distributions were obtained from tour Markov chains. There were 50000 iterations per chain and the first 20000 iterations for each chain were discarded as a warm-up. Convergence was assessed by visual inspection of the trace plots of the key parameters for each analysis. The prior settings and convergence results are shown in Appendix 4. The funnel plots and the test for funnel plot asymmetry were conducted using the R package metafor.
Results
A total of 821 papers were identified in the search. After removing duplicates and excluding papers by title and abstract, 24 papers were considered for full-text review. Then,10 were considered eligible (Supplementary Figure 1).
Demographic characteristics
There were ten eligible studies (n=515) that examined the antidepressant effects of psilocybin on adult patients with clinically diagnosed depression. The mean age was 44.0 (6.7) years with 62.1% of female patients. Figure 1 illustrates the demographic and clinical characteristics of the included studies. These ten trials were conducted between 2011 and 2023. Seven studies recruited adult patients with MDD, and three included mixed conditions (e.g., life-threatening cancer with MDD, generalized anxiety disorder, or persistent depressive disorders). There were seven RCTs, and three pre-post single-arm open-label trials. Six studies used non-manualized psychological approaches and four used manualized ones. Finally, the psychological protocols could be categorized into four types: (i) manualized directive psychotherapy (k=1); (ii) manualized nondirective psychological support (k=3), (iii) non-manualized nondirective psychological support (k=5); and (iv) non-manualized supportive psychotherapy (k=1).
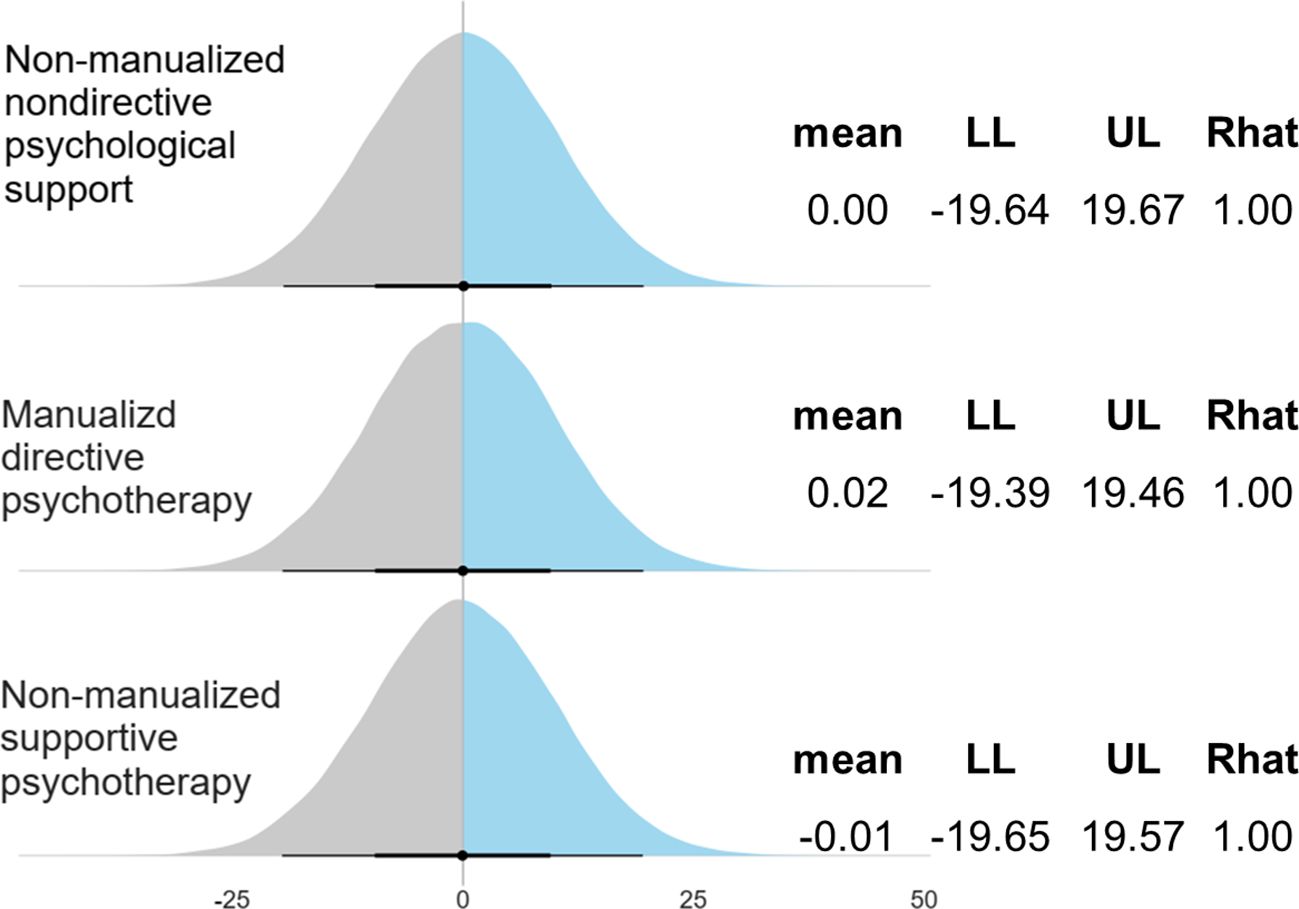
Figure 1. Study characteristics and models of psychological approaches. RCT, randomised controlled trial; Pre-Post, pre-post single-arm design; PS, psychological support; PT, psychotherapy; MDPT, manualized directive psychotherapy; MNDPS, manualized nondirective psychological support; NMNDPS, non-manualized nondirective psychological support; NMSPT, non-manualized supportive psychotherapy. Note: Dose: total dose (mg).
Table 1 shows the details of the psychological approaches among the ten studies. The components of the manualized directive psychotherapy included supportive, cognitive-behavioral, existentially oriented, and psychoanalytic dimension (17). The manual was based on the guide by Grof (1977): The human encounter with death (21). For the manualized nondirective psychological support, the manuals were based on the following guides (22, 23): (i). Studerus 2012: Prediction of psilocybin response in healthy volunteers. (ii). Tai 2021: Development and evaluation of a therapist training program for psilocybin therapy for treatment-resistant depression in clinical research. Only one study employed supportive psychotherapy (4); however, the content of the supportive psychotherapy resembled that of the other two studies with nondirective psychological support (3, 16). All of these three studies were based on the guide (24): (i). Johnson 2008: Human Hallucinogen research: guidelines for safety.
Risk of bias of the included studies
Three studies (3/10) was rated as having a high overall ROB (Supplementary Figure 2). The percentages of studies with high, some concerns, and low ROB for the individual items of psychedelic trials were as follows: 0%, 14.3%, and 85.7% for randomization; 0%, 14.3%, and 85.7% for deviations from intended interventions; 0%, 30.0%, and 70.0% for missing outcome data; 30.0%, 30.0%, and 40.0% for measurements of outcomes; 0%, 0%, and 100% for selection of reported results.
Primary outcome: depressive symptoms
Figure 2 shows the pooled effect size of psilocybin-assisted therapy with a mean difference of 10.08 (5.03-14.70).
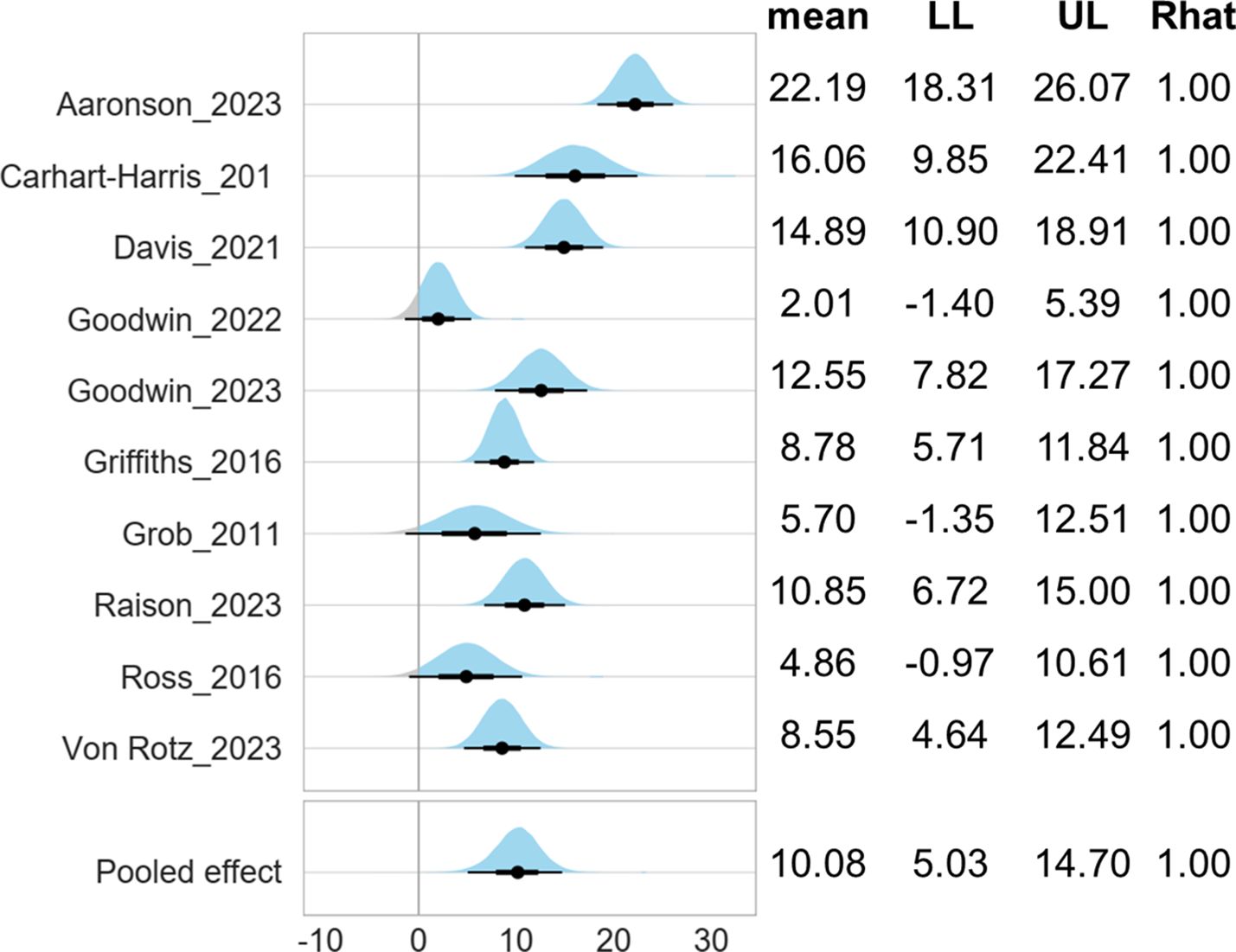
Figure 2. Forest plot of psilocybin-assisted therapy for patients with depressive symptoms. LL, lower limit of 95% credible interval; UL, upper limit of 95% credible interval. Note 1: The mean score was measured by 17-Item Hamilton Depression Rating Scale. Note 2: The Gelman and Rubin convergence diagnostic, or Rhat, approaching 1 indicates that multiple independent Markov Chain Monte Carlo (MCMC) chains have converged to similar distributions, suggesting that the simulation has reached a stable and reliable result.
Subgroup analysis
Figure 3 shows the comparable effectiveness of different psychological approaches. Compared with manualized nondirective psychological support, the other three psychological approaches did not differ significantly, with adjustment of baseline severity, age, female proportion, dose of psilocybin.
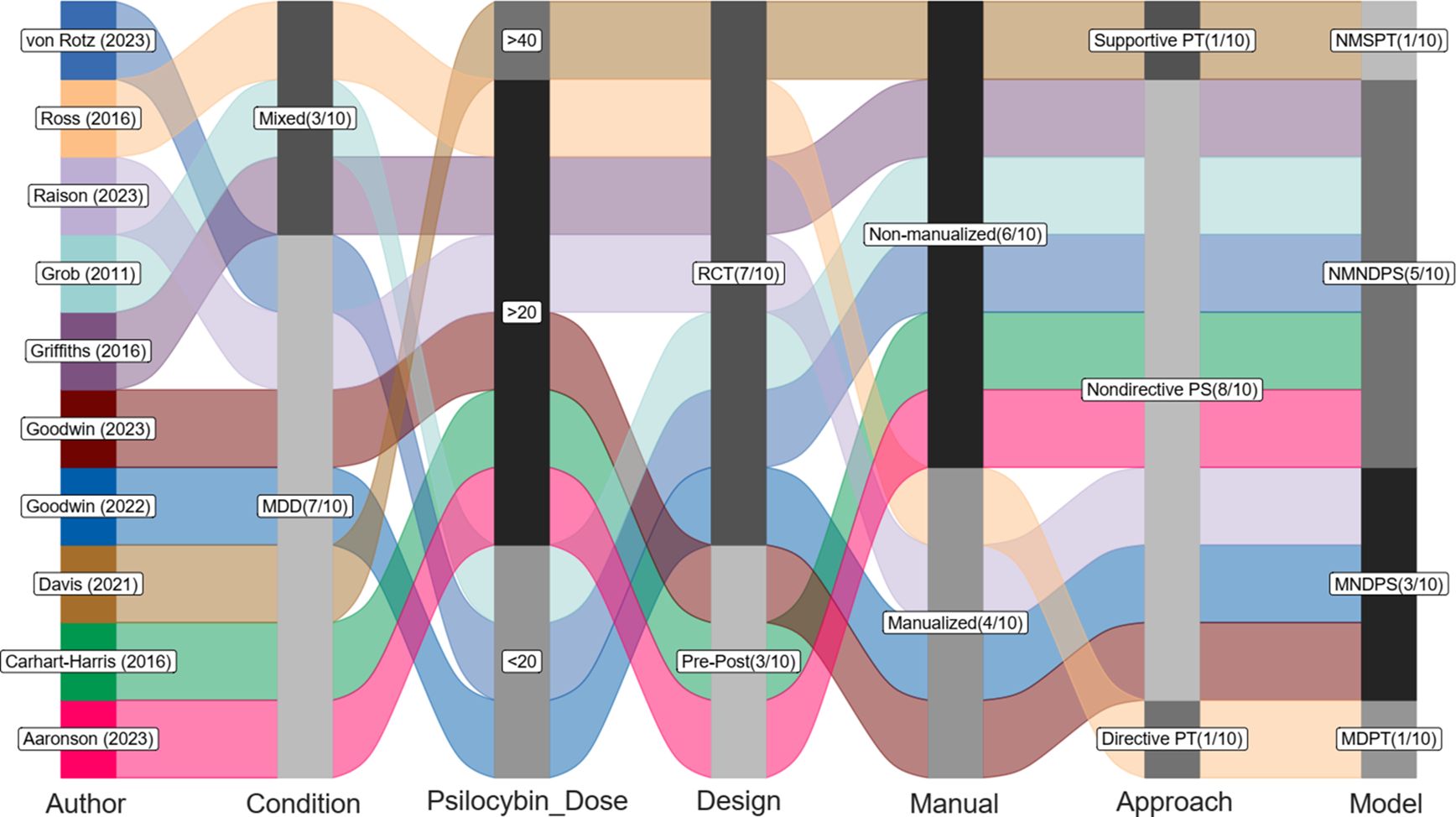
Figure 3. Comparisons between different psychological approaches (reference: manualized nondirective psychological support). LL, lower limit of 95% credible interval; UL, upper limit of 95% credible interval. Note 1: The mean score was measured by 17-Item Hamilton Depression Rating Scale. Note 2: The Gelman and Rubin convergence diagnostic, or Rhat, approaching 1 indicates that multiple independent Markov Chain Monte Carlo (MCMC) chains have converged to similar distributions, suggesting that the simulation has reached a stable and reliable result. Note 3: Compared with manualized nondirective psychological support with adjustment of baseline severity, age, female proportion, dose of psilocybin.
Publication bias
Visual inspection of the funnel plot did not show apparent publication bias, and the Egger’s test was not statistically significant (p=0.51, Figure 4).
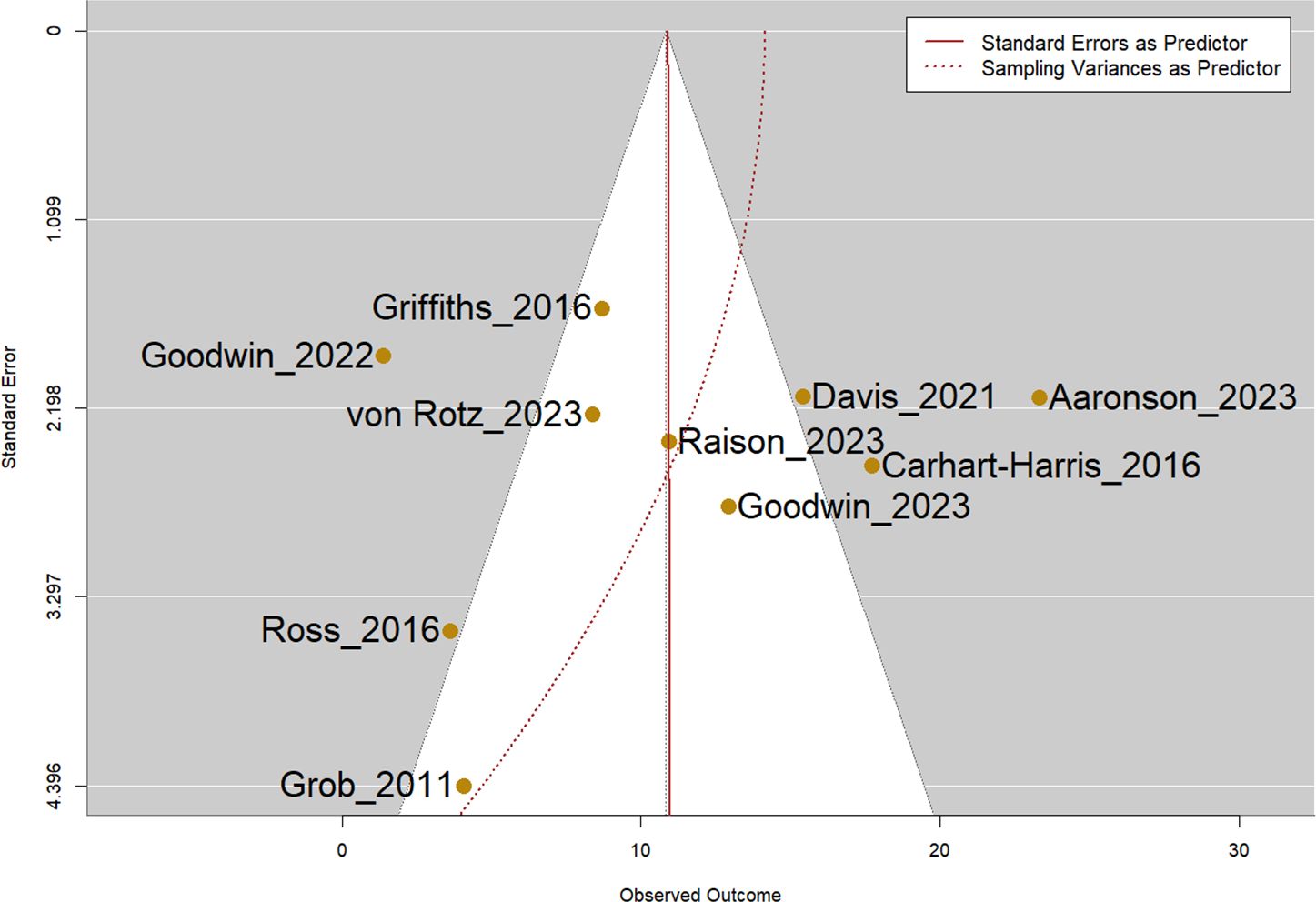
Figure 4. Funnel plot of all included studies. Note: Egger’s test was not statistically significant (p=0.51).
Discussion
Psilocybin-assisted therapy is an emerging therapeutic approach that combines psilocybin, a classic psychedelic drug, with psychological support or psychotherapy to address MDD or TRD. The integration of psychedelics and psychotherapy may leverage distinctive interplay between pharmacological, neural, and psychological effects, providing new promising avenues in the challenging landscape of MDD (12). However, the psychological approaches in previous psilocybin trials were not applied in a standardized way (9). The complex psychotherapeutic components complicate the protocols of psilocybin-assisted therapy. As expected, it is difficult to establish a rigid set of standardized psychological support or psychotherapy in psilocybin-assisted therapy even though psilocybin has been approved for TRD in clinical practice in Australia (8). Based on our systematic review, we found that most of the included psilocybin trials were combined with nondirective psychological support without specific manuals, and three used manualized nondirective psychological support. There was one study using non-manualized supportive psychotherapy (4), but the content was similar to those of nondirective psychological support. Only one study using manualized directive psychotherapy (17). However, with adjustment of baseline depression severity, age, female proportion, dose of psilocybin, there were not significant difference between these psychological protocols in association with the efficacy of psilocybin-assisted therapy.
The therapeutic mechanisms of psilocybin for MDD remains incompletely understood. It is believed that pharmacological, neural, and psychological underpinning contributed to its efficacy on MDD (25). At the pharmacological level, psilocybin primarily acts on the receptor with indirect effects on the dopaminergic and glutamatergic systems, leading to gene expression, increased neuroplasticity, and anti-inflammation. At the pharmacological level, psilocybin primarily activates the 5-HT2A (5-hydroxytryptamine) receptor and indirectly modulates the dopaminergic and glutamatergic systems. These interactions results in gene expression, heightened neuroplasticity, and anti-inflammatory responses (25). At the neural level, the response to psilocybin correlated with decreases in large-scale network modularity, suggesting that psilocybin’s antidepressant action may depend on a global increase in how different brain networks are integrated (26). At the psychological level, psilocybin is administered with psychological support or psychotherapy. When delivered in a secure and therapeutic environment, along with psychological assistance, participants often describe transformative experiences marked by profound shifts in values, beliefs, and perspectives. These changes can result in enhanced subjective well-being, increased openness, and improved cognitive flexibility (25, 27).
For the potential clinical application, psilocybin-assisted psychotherapy has sparked debate among psychiatrists, psychologists, neuroscientists, and advocates of psychedelics. This has led to the emergence of two factions: psilocybin therapy as a pure biological intervention and psilocybin as a catalyst for psychotherapy (28). Clearly, both sides are supported by equally valid arguments. On one hand, proponents of psilocybin-assisted therapy as a form of psychotherapy emphasize the following points. First, the effects of psychedelics have been observed to be highly dependent on contextual factors (ie, set and setting) (29). The psychopathology may exacerbate if the psychedelic experiences develop in adverse environments. Second, the nondirective psychological support is still a process-directive psychotherapeutic procedure (23). The authors of the therapist training program acknowledge that their psychological support framework incorporates elements from cognitive-behavioral therapy, mindfulness, Acceptance and Commitment Therapy, and Gestalt therapy model of focusing (23).
On the other hand, the effects of psilocybin have been argued to result from the drug itself and not from psychological interventions (30). In a RCT involving 233 patients with TRD, a dose-response relationship was observed between psilocybin dosage and depression improvement (5). Specifically, a separation of 25 mg, 10 mg, and 1mg psilocybin doses was identified. The psychological support was consistently administered across all three groups, indicating that the efficacy of psilocybin was largely contingent on mechanisms (5). Besides, a recent study found that psychedelics can function as positive allosteric modulators, fostering neuroplasticity through direct binding to the BDNF (brain-derived neurotrophic factor) receptor TrkB (tropomyosin receptor kinase) (31). This means psychedelics may exert antidepressant effect without inducing hallucinogenic effects, further supporting that psilocybin may produce therapeutic effects without experiencing psychedelic experiences. The United States FDA has published a new draft guidance to provide general considerations to sponsors developing psychedelic drugs for treatment of medical conditions (https://www.fda.gov/news-events/press-announcements/fda-issues-first-draft-guidance-clinical-trials-psychedelic-drugs, last access on Feb. 4 2024). In this guidance, it is recommended to employ a factorial design in future RCTs to distinguish and assess the individual contributions of drug and psychotherapy to any observed treatment response. Although our findings indicate that different psychotherapies do not show significant differences in antidepressant efficacy with psilocybin, these psychotherapies may still have some therapeutic effects. Through the factorial design RCTs, clinicians might build a standardized treatment protocol and a standardized tutor training program to ensure the effectiveness and safety of the psilocybin-assisted treatment.
Our study still has several limitations. First, some studies did not specify which guidance they followed, and we only could provide the information from the published papers (15, 20). Second, we did not include psilocybin studies addressing other psychiatric conditions such as substance use disorder. We also did not include other psychedelic-assisted therapy such as 3,4-methylenedioxymethamphetamine. However, focusing on a single psychedelic (psilocybin) and a single condition (clinically diagnosed depression) allows for a better understanding of the inconsistencies and diversity in protocols used simultaneously.
Conclusion
Over the past decades, accumulative evidence from clinical trials reveals promising results of psychedelic drugs in the treatment of various psychiatric disorders. Our study clearly showed diverse protocols of psychological support or psychotherapy with psilocybin treatment. Nondirective client-led therapy was the most commonly employed, and most of these studies used non-manualized protocols. The establishment of a consistent and standardized protocol for psychological strategies might be crucial for the development of a training program among clinical practitioners. However, we did not find significant difference in the association with the antidepressant efficacy among different psychological protocols. In the future, standardized psychological protocols and standardized tutor training programs should be established to ensure the quality of treatment. Furthermore, longitudinal studies or follow-up designs of RCTs are needed to assess the long-term effects of psilocybin-assisted therapy. These suggestions would provide a clearer roadmap for advancing the field.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
M-HC: Writing – review & editing, Validation, Conceptualization. S-LC: Writing – review & editing, Validation, Funding acquisition. Y-CK: Writing – review & editing, Validation. P-TT: Writing – review & editing, Validation. C-WH: Writing – review & editing, Validation. C-LY: Writing – original draft, Formal analysis, Data curation. F-CY: Writing – review & editing, Validation. TT: Writing – review & editing, Validation, Supervision. T-WH: Writing – original draft, Data curation, Conceptualization. C-SL: Writing – review & editing, Methodology, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. MacKay Medical College in Taiwan (MMC-RD-111-1B-P003, for S-LC) and the National Science and Technology Council in Taiwan (NSTC 112-2314-B-715-003, for S-LC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1439347/full#supplementary-material
References
1. Marwaha S, Palmer E, Suppes T, Cons E, Young AH, Upthegrove R. Novel and emerging treatments for major depression. Lancet. (2023) 401:141–53. doi: 10.1016/S0140-6736(22)02080-3
2. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
3. Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. (2016) 3:619–27. doi: 10.1016/S2215-0366(16)30065-7
4. Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, et al. Effects of psilocybin-assisted therapy on major depressive disorder: A randomized clinical trial. JAMA Psychiatry. (2021) 78:481–9. doi: 10.1001/jamapsychiatry.2020.3285
5. Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med. (2022) 387:1637–48. doi: 10.1056/NEJMoa2206443
6. von Rotz R, Schindowski EM, Jungwirth J, Schuldt A, Rieser NM, Zahoranszky K, et al. Single-dose psilocybin-assisted therapy in major depressive disorder: a placebo-controlled, double-blind, randomized clinical trial. eClinicalMedicine. (2023) 56:101809. doi: 10.1016/j.eclinm.2022.101809
7. Heal DJ, Smith SL, Belouin SJ, Henningfield JE. Psychedelics: threshold of a therapeutic revolution. Neuropharmacology. (2023) 236:109610. doi: 10.1016/j.neuropharm.2023.109610
8. Nogrady B. Australia’s approval of MDMA and psilocybin for PTSD and depression is premature, say critics. BMJ. (2023) 382:p1599. doi: 10.1136/bmj.p1599
9. Butlen-Ducuing F, McCulloch DEW, Haberkamp M, Mattila T, Bałkowiec-Iskra E, Aislaitner G, et al. The therapeutic potential of psychedelics: the European regulatory perspective. Lancet. (2023) 401:714–6. doi: 10.1016/S0140-6736(23)00264-7
10. Sloshower J, Skosnik PD, Safi-Aghdam H, Pathania S, Syed S, Pittman B, et al. Psilocybin-assisted therapy for major depressive disorder: An exploratory placebo-controlled, fixed-order trial. J Psychopharmacol. (2023) 37:698–706. doi: 10.1177/02698811231154852
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
12. Ko K, Kopra EI, Cleare AJ, Rucker JJ. Psychedelic therapy for depressive symptoms: A systematic review and meta-analysis. J Affect Disord. (2023) 322:194–204. doi: 10.1016/j.jad.2022.09.168
13. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
14. Phillippo DM. multinma: Bayesian network meta-analysis of individual and aggregate data. Bristol, England: University of Bristol (2020). doi: 10.32614/CRAN.packages
15. Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. (2011) 68:71–8. doi: 10.1001/archgenpsychiatry.2010.116
16. Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol. (2016) 30:1181–97. doi: 10.1177/0269881116675513
17. Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. (2016) 30:1165–80. doi: 10.1177/0269881116675512
18. Raison CL, Sanacora G, Woolley J, Heinzerling K, Dunlop BW, Brown RT, et al. Single-dose psilocybin treatment for major depressive disorder: a randomized clinical trial. JAMA. (2023) 330(9):843–853. doi: 10.1001/jama.2023.14530
19. Goodwin GM, Croal M, Feifel D, Kelly JR, Marwood SM, Mistry S, et al. Psilocybin for treatment resistant depression in patients taking a concomitant SSRI medication. Neuropsychopharmacology. (2023) 48(10):1492–99. doi: 10.1038/s41386-023-01648-7
20. Aaronson ST, van der Vaart A, Miller T, LaPratt J, Swartz K, Shoultz A, et al. Single-dose synthetic psilocybin with psychotherapy for treatment-resistant bipolar type II major depressive episodes: A nonrandomized controlled trial. JAMA Psychiatry. (2023) 81:552–62. doi: 10.1001/jamapsychiatry.2023.4685
22. Studerus E, Gamma A, Kometer M, Vollenweider FX. Prediction of psilocybin response in healthy volunteers. PloS One. (2012) 7:e30800. doi: 10.1371/journal.pone.0030800
23. Tai SJ, Nielson EM, Lennard-Jones M, Johanna Ajantaival RL, Winzer R, Richards WA, et al. Development and evaluation of a therapist training program for psilocybin therapy for treatment-resistant depression in clinical research. Front Psychiatry. (2021) 12:586682. doi: 10.3389/fpsyt.2021.586682
24. Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. (2008) 22:603–20. doi: 10.1177/0269881108093587
25. van Elk M, Yaden DB. Pharmacological, neural, and psychological mechanisms underlying psychedelics: A critical review. Neurosci Biobehav Rev. (2022) 140:104793. doi: 10.1016/j.neubiorev.2022.104793
26. Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. (2022) 28:844–51. doi: 10.1038/s41591-022-01744-z
27. Doss MK, Považan M, Rosenberg MD, Sepeda ND, Davis AK, Finan PH, et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl Psychiatry. (2021) 11:574. doi: 10.1038/s41398-021-01706-y
28. Gründer G, Brand M, Mertens LJ, Jungaberle H, Kärtner L, Scharf DJ, et al. Treatment with psychedelics is psychotherapy: beyond reductionism. Lancet Psychiatry. (2024) 11:231–6. doi: 10.1016/S2215-0366(23)00363-2
29. Carhart-Harris RL, Roseman L, Haijen E, Erritzoe D, Watts R, Branchi I, et al. Psychedelics and the essential importance of context. J Psychopharmacol. (2018) 32:725–31. doi: 10.1177/0269881118754710
30. Goodwin GM, Malievskaia E, Fonzo GA, Nemeroff CB. Must psilocybin always “Assist psychotherapy”? Am J Psychiatry. (2024) 181:20–5. doi: 10.1176/appi.ajp.20221043
Keywords: psilocybin, psilocybin-assisted therapy, Bayesian meta-analysis, depressive symptoms, psychotherapy outcome research
Citation: Chen M-H, Cheng S-L, Kao Y-C, Tseng P-T, Hsu C-W, Yu C-L, Yang F-C, Thompson T, Hsu T-W and Liang C-S (2024) The association between diverse psychological protocols and the efficacy of psilocybin-assisted therapy for clinical depressive symptoms: a Bayesian meta-analysis. Front. Psychiatry 15:1439347. doi: 10.3389/fpsyt.2024.1439347
Received: 27 May 2024; Accepted: 22 July 2024;
Published: 13 August 2024.
Edited by:
Alina Wilkowska, Medical University of Gdansk, PolandReviewed by:
João Carlos Alchieri, Federal University of Rio Grande do Norte, BrazilMariusz Stanisław Wiglusz, Medical University of Gdansk, Poland
Gniewko Więckiewicz, Medical University of Silesia, Poland
Copyright © 2024 Chen, Cheng, Kao, Tseng, Hsu, Yu, Yang, Thompson, Hsu and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trevor Thompson, VC5UaG9tcHNvbkBncmVlbndpY2guYWMudWs=; Tien-Wei Hsu, czk4MDExMDFAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
‡ORCID: Trevor Thompson, orcid.org/0000-0001-9880-782X
Tien-Wei Hsu, orcid.org/0000-0003-4136-1251
 Mu-Hong Chen
Mu-Hong Chen Shu-Li Cheng3†
Shu-Li Cheng3† Ping-Tao Tseng
Ping-Tao Tseng Chih-Wei Hsu
Chih-Wei Hsu Tien-Wei Hsu
Tien-Wei Hsu Chih-Sung Liang
Chih-Sung Liang