- 1School of Pharmacy, Zunyi Medical University, Zunyi, Guizhou, China
- 2Department of Pharmacy, Zhuhai People’s Hospital (Zhuhai Clinical Medical College of Jinan University), Zhuhai, Guangdong, China
Purpose: The rising prevalence of postpartum depression (PPD) is harmful to women and families. While there is a growing body of evidence suggesting an association between PPD and autoimmune diseases (ADs), the direction of causality remains uncertain. Therefore, Mendelian randomization (MR) study was employed to investigate the potential causal relationship between the two.
Methods: This study utilized large-scale genome-wide association study genetic pooled data from two major databases: the IEU OpenGWAS project and the FinnGen databases. The causal analysis methods used inverse variance weighting (IVW). The weighted median, MR-Egger method, MR-PRESSO test, and the leave-one-out sensitivity test have been used to examine the results’ robustness, heterogeneity, and horizontal pleiotropy.
Results: A total of 23 ADs were investigated in this study. In the IVW model, the MR study showed that PPD increased the risk of type 1 diabetes (OR , = 1.15 (1.05–1.26),p<0.01),Hashimoto’s thyroiditis((OR) = 1.21 (1.09–1.34),p<0.0001),encephalitis((OR) = 1.66 (1.06–2.60),p<0.05). Reverse analysis showed that ADs could not genetically PPD. There was no significant heterogeneity or horizontal pleiotropy bias in this result.
Conclusion: Our study suggests that PPD is a risk factor for type 1 diabetes, Hashimoto’s thyroiditis, and encephalitis from a gene perspective, while ADs are not a risk factor for PPD. This finding may provide new insights into prevention and intervention strategies for ADs according to PPD patients.
1 Introduction
Postpartum depression (PPD), a prevalent major depressive following childbirth, affects 17.22% women worldwide with a higher prevalence in developing countries compared to developed countries (1). PPD is characterized by symptoms such as depression, emotional instability, feelings of guilt, loss of appetite, low self-esteem, and sleep disturbances, along with a 20% increase in suicidal ideation (2–4). Not only does PPD affect the women themselves, but may also increase the risk of depression in partners and mental retardation in children, increasing the economic burden on families (5–7). Life circumstances (8), social and psychological stress (9), postpartum grief (10), prenatal depression (11), lifestyle (12), vaginal delivery (13), hormonal changes (14), and marital or partner dissatisfaction (11) have been shown to be the common risk factors for PPD. Notably, the correlation between autoimmune diseases (ADs) and PPD has been underexplored in existing studies.
Recent research has highlighted the complex interplay between ADs and PPD. ADs are disease states caused by an immune response of the body’s immune system against its own components due to the fact that it is impossible to distinguish between self and non-self (15). A recent nationwide sibling comparison study demonstrated a bidirectional association between PPD and ADs (16). Although several observational studies have shown that women with ADs are at higher risk for postpartum depression, studies on MS are conflicting (17–21). Notably, preliminary evidence suggests a potential risk of subsequent ADs development in individuals with PPD (22, 23). Despite these insights, the genetic underpinnings of the relationship between PPD and ADs remain poorly understood, with a gap in research elucidating this aspect.
The objective of this study was to examine the causal relationship between PPD and autoimmune diseases (ADs) using bi-directional Mendelian randomization (MR) analysis. MR is a robust method that utilizes genetic variation as instrumental variables to establish causal relationships between risk factors and diseases (24). This method can play a critical role in addressing issues related to confounding factors and reverse causality in observational studies (25). Bi-directional MR analysis can effectively investigate the impact of ADs on the risk of postpartum depression (PPD), as well as the risk of ADs following PPD.
2 Methods
2.1 Study overview
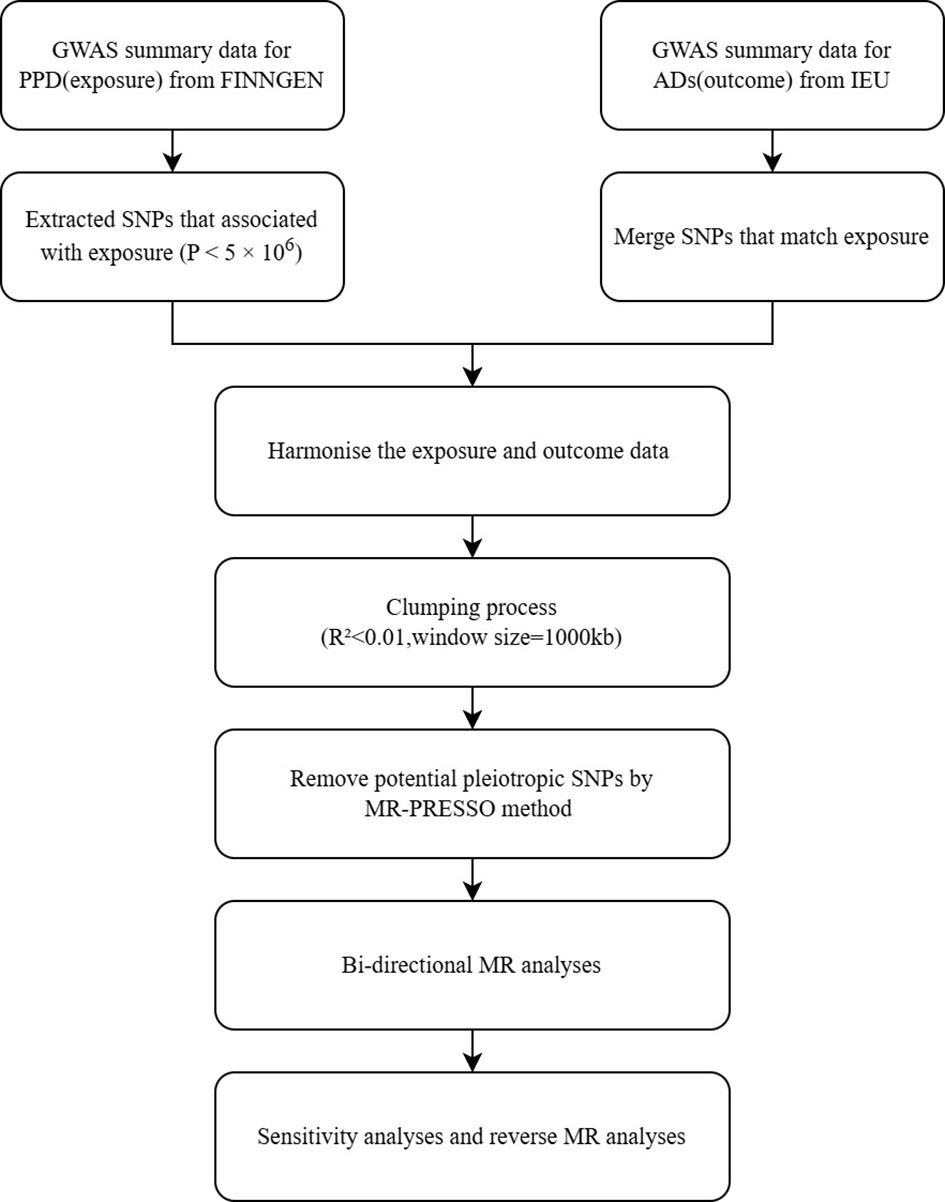
In this study, we investigated the causal relationship between ADs and PPD using a bi-directional Mendelian randomization (MR) approach. We selected 23 subtypes of autoimmune disease diagnoses and obtained their Genome-Wide Association Study (GWAS) summary statistics data from publicly accessible databases. The initial research received ethical approval and informed consent. The study’s flow chart is depicted in Figure 1.
2.2 Data sources
Genome-wide association study (GWASs) summary statistics data for PPD from FinnGen R8, survey of 14,116 European women (prevalence 7.11%, mean age 41.03 years) (26). The diagnostic criteria for PPD are delivery status and International Classification of Diseases, Tenth Edition (ICD-10) codes F32, F33 and F53.0.
GWAS data for ADS were obtained from the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/), which includes data from the UK Biobank (27), FINNGEN (26), and the International Multiple Sclerosis Genetics Consortium (28). The study includes the following ADs: Type 1 diabetes (29), Graves’ disease (30), Hashimoto thyroiditis (30), Rheumatoid arthritis (30), Ankylosing spondylitis (26), Giant cell arteritis with polymyalgia rheumatica (26), Polyarteritis nodosa and related conditions (26), Allergic purpura (26), Behcet’s disease (30), and Systemic lupus erythematosus (30), Psoriasis vulgaris (30), vitiligo (26), alopecia areata (26), idiopathic thrombocytopenic purpura (30), multiple sclerosis (28), myasthenia gravis (31), encephalitis (26), Guillain-Barre syndrome (26), ulcerative colitis (26), Crohn’s disease (32), coeliac disease (26), IgA nephropathy (30), and sarcoidosis (30). Detailed information on the ADs data is in Supplementary Table 1.
2.3 Instrumental variable selection
In magnetic resonance analyses of PPDs with ADs, the following three conditions were employed in order to select the optimal instrumental variable (IV) in order to ensure that the results were true and accurate: (I) the IV was closely related to PPDs, (II) the IV was not related to confounders and (III) the IV was not related to ADs (33). We selected SNPs that were significantly associated with PPD as IVs, choosing only those that were smaller than the genome-wide statistical significance threshold (5 × 10-6). To ensure independence between IVs, SNPs with linkage disequilibrium were filtered using a clump window of 10,000 kb and r2 > 0.001. F-statistics were calculated for all independent variables (IV) to ensure that the F-statistics for the SNPs used in the analyses were all greater than 10. SNPs significantly associated with the results (p < 10-8) were also excluded. To prevent any distortion of strand orientation or allele coding, we removed palindromic SNPs (e.g. A/T or G/C alleles).
2.4 MR analysis
The inverse-variance weighted (IVW) MR method was applied as the primary method to identify potential associations between ADs and PPD (24). To evaluate the stability of the IVW results, we also used MR-PRESSO weighted median, heterogeneity test, MR-Egger regression heterogeneity test, Cochrane’s Q test, and weighted median (34, 35). The Cochrane Q test was used to assess the heterogeneity of the SNPs, and heterogeneity was present if P < 0.05 (36). Directed pleiotropy of genetic tools was tested using MR-Egger regression (37). To exclude SNPs whose abnormalities would affect our results, we also performed a leave-one-out sensitivity test (36). By analyzing the same trends in IVW and weighted median and MR-Egger analyses, the relationship between exposure and outcome was confirmed.
Analysis was performed using R 4.3.2 and the TwoSampleMR package.
3 Results
3.1 IVs selection
After rigorous screening, a total of 28 SNPs strongly associated with PPD (p<10-6) were used in this study. Detailed snps information can be found in Supplementary Table 2.
3.2 MR analysis of PPD for ADs
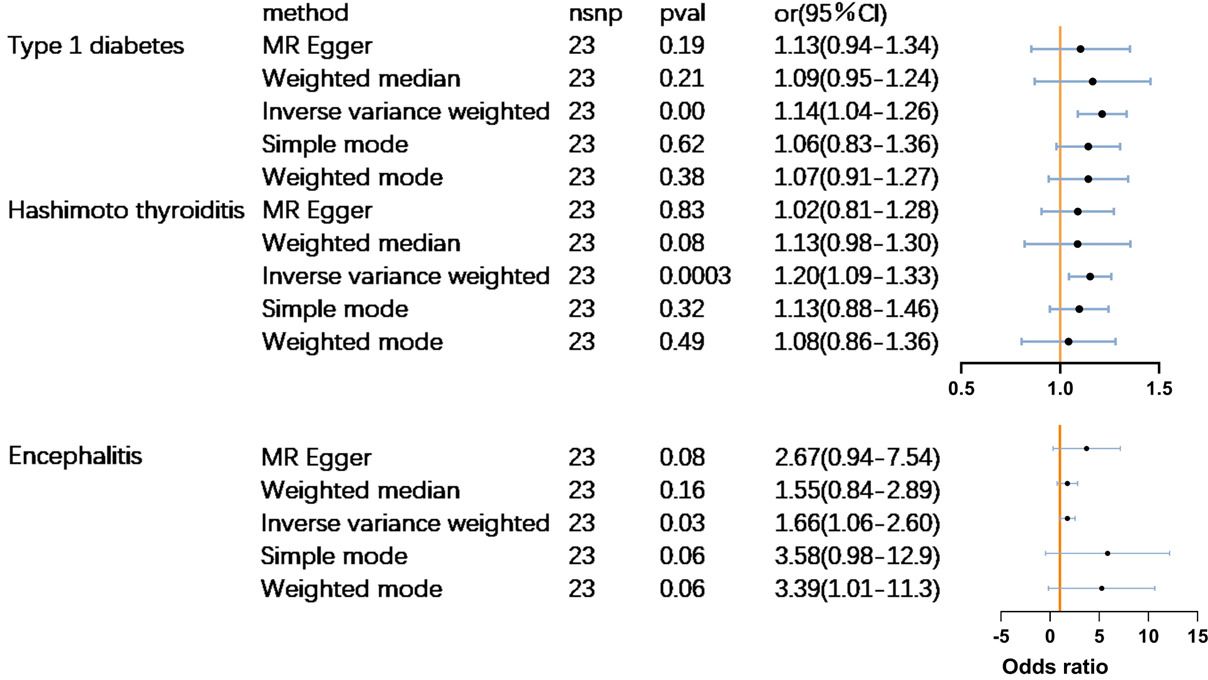
MR analysis revealed that PPD had a significant causal relationship(p<0.05) with three out of the 23 autoimmune diseases. Women who suffer from PPD are at a higher risk of developing type 1 diabetes (odds ratio (OR) = 1.15 (1.05–1.26),p<0.01), Hashimoto’s thyroiditis [(OR) = 1.21 (1.09–1.34],p<0.0001), and encephalitis [(OR) = 1.66 (1.06–2.60),p<0.05] (Figure 2). No significant causal association was found between PPD and the other 20 subtypes of ADs. Complete results are available in Supplementary Table 3.
3.3 Sensitivity analysis of MR
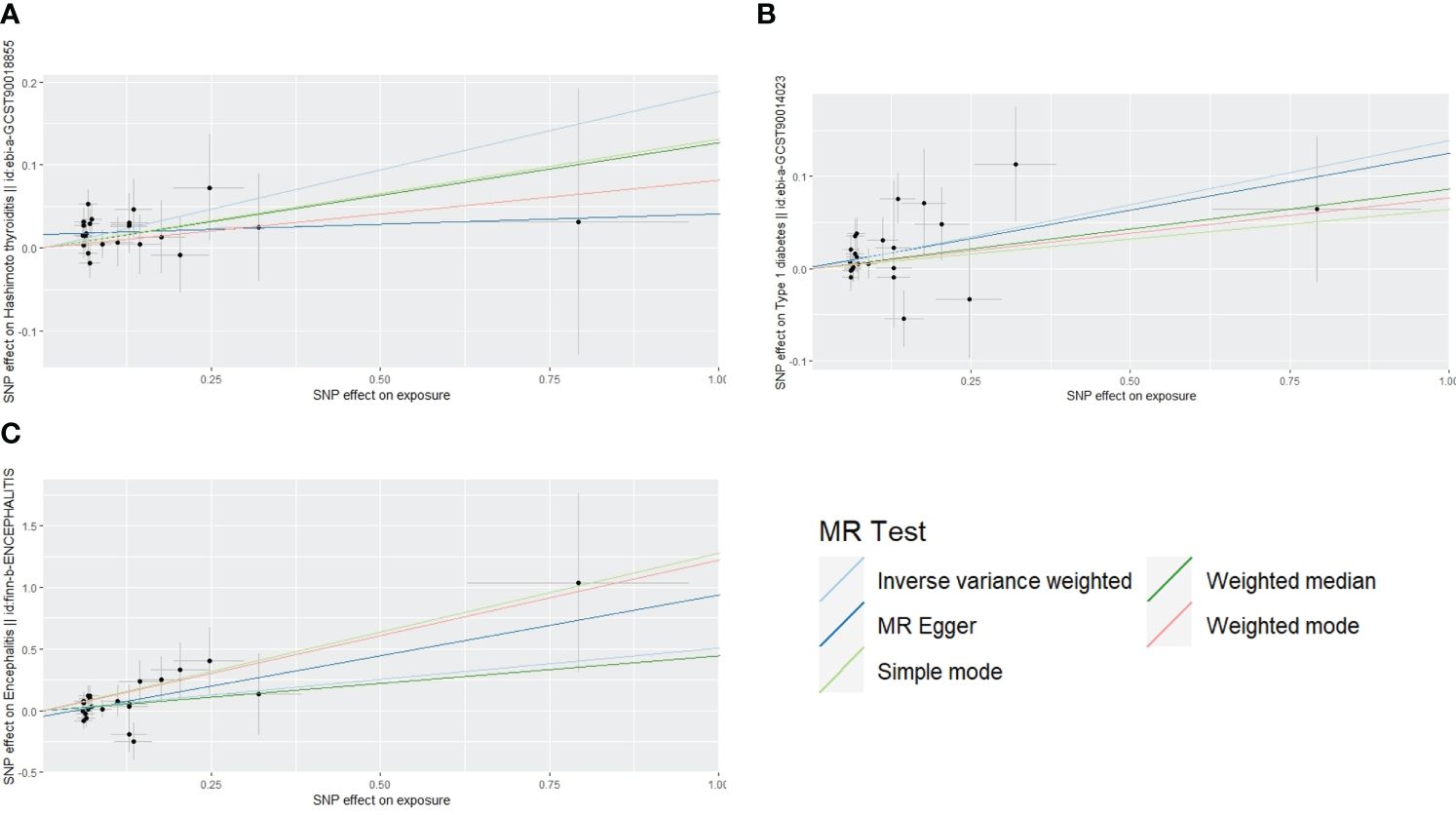
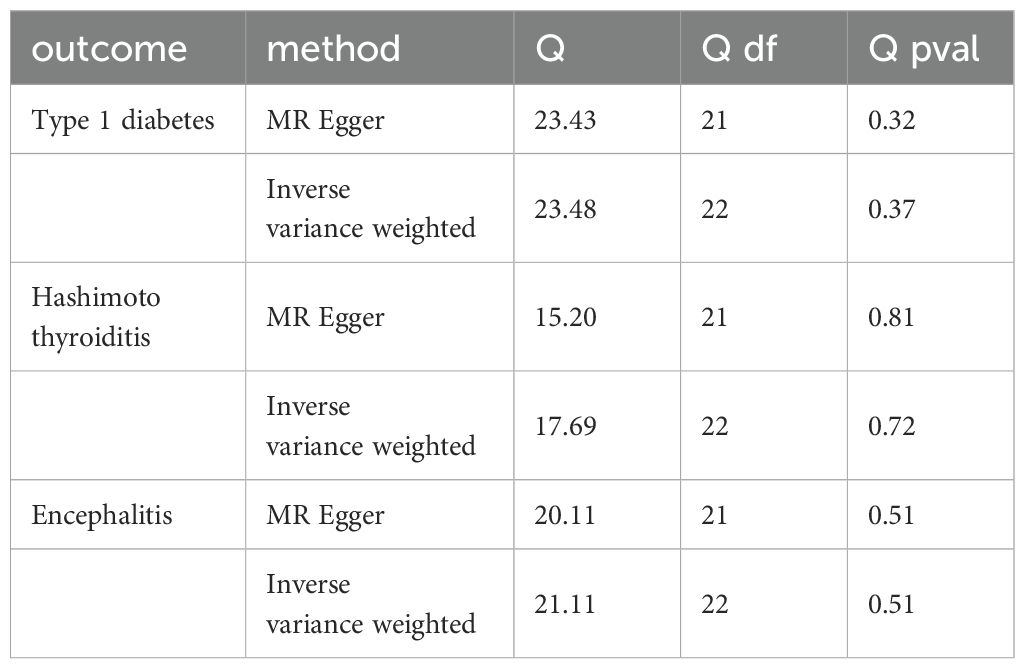
The scatterplot shows that MR-Egger, weighted median, weighted mode, and simple mode results all follow the same trend as IVW:A for Hashimoto’s thyroiditis, B for type 1 diabetes, C for encephalitis (Figure 3). The test for heterogeneity was conducted using Cochrane’s Q-statistics, and no heterogeneity (p>0.05) was found for any of the three outcomes (Table 1). Tests of pleiotropy indicated no horizontal pleiotropy for type 1 diabetes (p=0.84), Hashimoto’s thyroiditis (p=0.13), and encephalitis (p=0.33).
4 Discussion
In this study we further validated the causal relationship between PPD and ADs using MR in a European population. Our findings provide robust support for the involvement of PPD in the risk of Type 1 diabetes, Hashimoto’s thyroiditis, and Encephalitis. Sensitivity analyses also confirmed the reliability of our results, indicating that MR analyses of PPD are trustworthy. However, no association was observed between PPD and the remaining 20 ADs (p>0.05).
The association between PPDs and ADs is a topic of complexity and controversy. An observational study in Sweden found a bidirectional association between certain ADs (such as autoimmune thyroid disease, psoriasis, multiple sclerosis, ulcerative colitis, and celiac disease) and perinatal depression among unaffected sisters, independent of psychiatric comorbidity (16). However, previous studies have produced conflicting results (21, 38–41). Additionally, various studies have shown that inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and psoriasis can increase the risk of PPD (18–20, 22, 40). However, the focus of most previous studies has been on investigating the risk of PPD following ADs, with limited research on the risk of subsequent ADs associated with PPD. A survey from Canada indicated that women with perinatal psychiatric disorders were at a higher risk of developing ADs, though not significantly different from women with non-perinatal psychiatric disorders (17). These findings were mainly based on observational studies that were unable to consider all potential mediators influencing the results, leading to controversies. Factors such as small sample sizes, confounding variables, reverse causation, and differences in study designs may contribute to the inconsistencies in the literature.
In this study, evidence was found suggesting a potential association between Postpartum Depression (PPD) and Type 1 diabetes, Hashimoto’s thyroiditis, and Encephalitis. Type 1 diabetes is a chronic autoimmune disease typically occurring in childhood, although the American Diabetes Association (ADA) has classified latent autoimmune diabetes in adults as T1DM as well (42, 43). This form of diabetes is thought to stem from a combination of genetic and environmental factors, presenting as heterogeneous at different stages (44). The progression of the disease may be exacerbated by psychological factors and obesity, leading to pancreatic beta cell exhaustion and autoimmune destruction (45). Moreover, latent autoimmune diabetes in adults and PPD are commonly linked to obesity, physical inactivity, and lifestyle factors (46, 47). Physiologically, PPD may be associated with type 1 diabetes through thalamic damage and HbA1c levels, indicating a potential pathway connecting the two conditions (48–50).
Hashimoto’s thyroiditis, an autoimmune thyroid disorder, is predominantly observed in women and results from a blend of genetic and environmental factors (51). Hormonal fluctuations during the perinatal phase and heightened stress in the postpartum period are believed to heighten the vulnerability of women with postpartum depression (PPD) to developing Hashimoto’s thyroiditis (52, 53). The occurrence of pregnancy leads to notable modifications in thyroid function, with variations in hormone levels such as human chorionic gonadotropin, estrogen, and progesterone being associated with the initiation and progression of Hashimoto’s thyroiditis (22, 52). It is crucial to closely monitor thyroxine levels in women with PPD to forestall autoimmune thyroid disease. Research findings suggest that individuals with chronic mental disorders have a low likelihood of acquiring autoimmune encephalitis (54). There is a proposition that individuals enduring postpartum psychosis may exhibit higher susceptibility to encephalitis (55). Our study’s outcomes reveal that postpartum depression is linked to an elevated risk of encephalitis, as indicated by a higher odds ratio (OR=1.6). Our results suggest that patients with PPD have an increased risk of type 1 diabetes, Hashimoto’s thyroiditis, and encephalitis associated with their genetic susceptibility.
The use of genetic variation consistent with Mendel’s law of random assignment as an instrumental variable allows for the exclusion of confounding factors in MR analysis (24). This method addresses the issue of reduced confidence in previous observational studies on the relationship between PPD and ADs, which was attributed to the presence of confounding factors and reverse causality that were difficult to avoid. Furthermore, since these single nucleotide polymorphisms (SNPs) are strongly associated with disease and exist before the onset of disease, reverse causation is no longer a concern (56). The GWAS summary data selected for this study were derived from research with large sample sizes, enhancing the reliability of the results.
Several limitations need to be considered in our study. Firstly, the analysis was confined to GWAS studies conducted in Europe, thus it would be advantageous to incorporate data from other regions. Moreover, the study population consisted solely of females; however, it is worth mentioning that most studies utilizing GWAS data for autoimmune diseases did not differentiate between genders.
This is the first study to employ MR to investigate the potential causal relationship between ADs and PPD. The findings indicate that PPD is associated with an increased risk of developing Type 1 diabetes, Hashimoto’s thyroiditis, and Encephalitis. Further experimental and mechanistic studies are required to validate the results obtained.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
WY: Writing – original draft, Writing – review & editing, Data curation, Methodology, Project administration. BS: Data curation, Formal analysis, Project administration, Writing – original draft, Writing – review & editing. CW: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. QX: Investigation, Software, Supervision, Writing – review & editing. YS: Data curation, Investigation, Project administration, Software, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1425623/full#supplementary-material
References
1. Wang Z, Liu J, Shuai H, Cai Z, Fu X, Liu Y, et al. Mapping global prevalence of depression among postpartum women. Trans Psychiatry. (2021) 11:543. doi: 10.1038/s41398-021-01663-6
2. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. (2000) 182:1080–2. doi: 10.1067/mob.2000.105409
3. Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. (2004) 26:289–95. doi: 10.1016/j.genhosppsych.2004.02.006
4. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch women's Ment Health. (2005) 8:77–87. doi: 10.1007/s00737-005-0080-1
5. Le J, Alhusen J, Dreisbach C. Screening for partner postpartum depression: A systematic review. MCN. Am J Maternal Child Nurs. (2023) 48:142–50. doi: 10.1097/NMC.0000000000000907
6. Zhu Y, Li X, Chen J, Gong W. Perinatal depression trajectories and child development at one year: a study in China. BMC pregnancy childbirth. (2024) 24:176. doi: 10.1186/s12884-024-06330-4
7. Bauer A, Knapp M, Parsonage M. Lifetime costs of perinatal anxiety and depression. J Affect Disord. (2016) 192:83–90. doi: 10.1016/j.jad.2015.12.005
8. Cadman T, Strandberg-Larsen K, Calas L, Christiansen M, Culpin I, Dadvand P, et al. Urban environment in pregnancy and postpartum depression: An individual participant data meta-analysis of 12 European birth cohorts. Environ Int. (2024) 185:108453. doi: 10.1016/j.envint.2024.108453
9. Schalla MA, Stengel A. The role of stress in perinatal depression and anxiety - A systematic review. Front Neuroendocrinol. (2024) 72:101117. doi: 10.1016/j.yfrne.2023.101117
10. Liu W, Li W, Wang Y, Yin C, Xiao C, Hu J, et al. Comparison of the EPDS and PHQ-9 in the assessment of depression among pregnant women: Similarities and differences. J Affect Disord. (2024) 351:774–81. doi: 10.1016/j.jad.2024.01.219
11. Hutchens BF, Kearney J. Risk factors for postpartum depression: an umbrella review. J midwifery women's Health. (2020) 65:96–108. doi: 10.1111/jmwh.13067
12. Hu N, Luo J, Xiang W, Yang G, Huang T, Guan L, et al. The relationship between postpartum negative life events and postpartum depression: a moderated mediation model of neuroticism and psychological flexibility. BMC Psychiatry. (2024) 24:147. doi: 10.1186/s12888-024-05594-6
13. Froeliger A, Deneux-Tharaux C, Loussert L, Bouchghoul H, Madar H, Sentilhes L. Prevalence and risk factors for postpartum depression 2 months after a vaginal delivery: a prospective multicenter study. Am J Obstet Gynecol. (2024) 230:S1128–s1137.6. doi: 10.1016/j.ajog.2023.08.026
14. Björväng RD, Walldén Y, Fransson E, Comasco E, Sundström-Poromaa I, Skalkidou A. Mid-pregnancy allopregnanolone levels and trajectories of perinatal depressive symptoms. Psychoneuroendocrinology. (2024) 164:107009. doi: 10.1016/j.psyneuen.2024.107009
15. Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Internal Med. (2015) 278:369–95. doi: 10.1111/joim.12395
16. Bränn E, Chen Y, Song H, László KD, D'Onofrio BM, Hysaj E, et al. Bidirectional association between autoimmune disease and perinatal depression: a nationwide study with sibling comparison. Mol Psychiatry. (2024) 29:602–10. doi: 10.1038/s41380-023-02351-1
17. Brown HK, Wilton A, Liu N, Ray JG, Dennis CL, Vigod SN. Perinatal mental illness and risk of incident autoimmune disease: A population-based propensity-score matched cohort study. Clin Epidemiol. (2021) 13:1119–28. doi: 10.2147/CLEP.S344567
18. Shridharmurthy D, Lapane KL, Nunes AP, Baek J, Weisman MH, Kay J, et al. Postpartum depression in reproductive-age women with and without rheumatic disease: A population-based matched cohort study. J Rheumatol. (2023) 50:1287–95. doi: 10.3899/jrheum.2023-0105
19. Luan M, Yang F, Miao M, Yuan W, Gissler M, Arkema EV, et al. Rheumatoid arthritis and the risk of postpartum psychiatric disorders: a Nordic population-based cohort study. BMC Med. (2023) 21:126. doi: 10.1186/s12916-023-02837-3
20. Eid K, Torkildsen Ø F, Aarseth J, Flemmen H, Holmøy T, Lorentzen Å R, et al. Perinatal depression and anxiety in women with multiple sclerosis: A population-based cohort study. Neurology. (2021) 96:e2789–800. doi: 10.1212/WNL.0000000000012062
21. Krysko KM, Anderson A, Singh J, McPolin K, Rutatangwa A, Rowles W, et al. Risk factors for peripartum depression in women with multiple sclerosis. Multiple sclerosis (Houndmills Basingstoke England). (2022) 28:970–9. doi: 10.1177/13524585211041108
22. Bergink V, Pop VJM, Nielsen PR, Agerbo E, Munk-Olsen T, Liu X. Comorbidity of autoimmune thyroid disorders and psychiatric disorders during the postpartum period: a Danish nationwide register-based cohort study. psychol Med. (2018) 48:1291–8. doi: 10.1017/S0033291717002732
23. Lin CY, Li CK, Liu JM, Hsu RJ, Chuang HC, Chang FW. Postpartum depression and subsequent autoimmune diseases in Taiwan. Int J Environ Res Public Health. (2018) 15:1783. doi: 10.3390/ijerph15081783
24. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
25. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
26. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
27. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. (2015) 12:e1001779. doi: 10.1371/journal.pmed.1001779
28. International Multiple Sclerosis Genetics Consortium, MultipleMS Consortium. Locus for severity implicates CNS resilience in progression of multiple sclerosis. Nature. (2023) 619:323–31. doi: 10.1038/s41586-023-06250-x
29. Chiou J, Geusz RJ, Okino ML, Han JY, Miller M, Melton R, et al. Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature. (2021) 594:398–402. doi: 10.1038/s41586-021-03552-w
30. Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. (2021) 53:1415–24. doi: 10.1038/s41588-021-00931-x
31. Chia R, Saez-Atienzar S, Murphy N, Chiò A, Blauwendraat C, Roda RH, et al. Identification of genetic risk loci and prioritization of genes and pathways for myasthenia gravis: a genome-wide association study. Proc Natl Acad Sci USA. (2022) 119:e2108672119. doi: 10.1073/pnas.2108672119
32. de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. (2017) 49:256–61. doi: 10.1038/ng.3760
33. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (Clinical Res ed.). (2018) 362:k601. doi: 10.1136/bmj.k601
34. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
35. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
36. Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. (2019) 48:728–42. doi: 10.1093/ije/dyy258
37. Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34:2926–40. doi: 10.1002/sim.6522
38. Chalitsios CV, Meena D, Manou M, Papagiannopoulos C, Markozannes G, Gill D, et al. Multiple long-term conditions in people with psoriasis: a latent class and bidirectional Mendelian randomization analysis. Br J Dermatol. (2024) 190:364–73. doi: 10.1093/bjd/ljad410
39. Li W, Kan H, Zhang W, Zhong Y, Liao W, Huang G, et al. Mendelian randomization study on the causal effects of systemic lupus erythematosus on major depressive disorder. J Hum Genet. (2023) 68:11–6. doi: 10.1038/s10038-022-01080-7
40. Vigod SN, Kurdyak P, Brown HK, Nguyen GC, Targownik LE, Seow CH, et al. Inflammatory bowel disease and new-onset psychiatric disorders in pregnancy and post partum: a population-based cohort study. Gut. (2019) 68:1597–605. doi: 10.1136/gutjnl-2018-317610
41. Luo J, Xu Z, Noordam R, van Heemst D, Li-Gao R. Depression and inflammatory bowel disease: A bidirectional two-sample Mendelian randomization study. J Crohn's colitis. (2022) 16:633–42. doi: 10.1093/ecco-jcc/jjab191
42. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S19–s40. doi: 10.2337/dc23-S002
43. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet (London England). (2018) 391:2449–62. doi: 10.1016/S0140-6736(18)31320-5
44. Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. (2019) 15:635–50. doi: 10.1038/s41574-019-0254-y
45. Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet (London England). (2016) 387:2340–8. doi: 10.1016/S0140-6736(16)30507-4
46. Buzzetti R, Maddaloni E, Gaglia J, Leslie RD, Wong FS, Boehm BO. Adult-onset autoimmune diabetes. Nat Rev Dis Primers. (2022) 8:63. doi: 10.1038/s41572-022-00390-6
47. Pavlik LB, Rosculet K. Maternal obesity and perinatal depression: an updated literature review. Cureus. (2020) 12:e10736. doi: 10.7759/cureus.10736
48. Korczak DJ, Pereira S, Koulajian K, Matejcek A, Giacca A. Type 1 diabetes mellitus and major depressive disorder: evidence for a biological link. Diabetologia. (2011) 54:2483–93. doi: 10.1007/s00125-011-2240-3
49. Bächle C, Lange K, Stahl-Pehe A, Castillo K, Holl RW, Giani G, et al. Associations between HbA1c and depressive symptoms in young adults with early-onset type 1 diabetes. Psychoneuroendocrinology. (2015) 55:48–58. doi: 10.1016/j.psyneuen.2015.01.026
50. Moulton CD, Costafreda SG, Horton P, Ismail K, Fu CH. Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav. (2015) 9:651–62. doi: 10.1007/s11682-014-9348-2
51. Petranović Ovčariček P, Görges R, Giovanella L. Autoimmune thyroid diseases. Semin Nucl Med. (2024) 54:219–36. doi: 10.1053/j.semnuclmed.2023.11.002
52. Bogović Crnčić T, Girotto N, Ilić Tomaš M, Krištofić I, Klobučar S, Batičić L, et al. Innate immunity in autoimmune thyroid disease during pregnancy. Int J Mol Sci. (2023) 24:15442. doi: 10.3390/ijms242015442
53. Barić A, Brčić L, Gračan S, Škrabić V, Brekalo M, Šimunac M, et al. Thyroglobulin antibodies are associated with symptom burden in patients with Hashimoto's thyroiditis: A cross-sectional study. Immunol Invest. (2019) 48:198–209. doi: 10.1080/08820139.2018.1529040
54. Pollak TA, McCormack R, Peakman M, Nicholson TR, David AS. Prevalence of anti-N-methyl-D-aspartate (NMDA) receptor [corrected] antibodies in patients with schizophrenia and related psychoses: a systematic review and meta-analysis. psychol Med. (2014) 44:2475–87. doi: 10.1017/S003329171300295X
55. Bergink V, Armangue T, Titulaer MJ, Markx S, Dalmau J, Kushner SA. Autoimmune encephalitis in postpartum psychosis. Am J Psychiatry. (2015) 172:901–8. doi: 10.1176/appi.ajp.2015.14101332
Keywords: postpartum depression, autoimmune disease, Mendelian randomization, genetic cause, etiology
Citation: Yu W, Su B, Wang C, Xia Q and Sun Y (2024) Postpartum depression and autoimmune disease: a bidirectional Mendelian randomization study. Front. Psychiatry 15:1425623. doi: 10.3389/fpsyt.2024.1425623
Received: 30 April 2024; Accepted: 19 July 2024;
Published: 29 August 2024.
Edited by:
Yari Gvion, Bar-Ilan University, IsraelCopyright © 2024 Yu, Su, Wang, Xia and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinxiang Sun, c3VueWlueGlhbmdfM0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Wenlong Yu
Wenlong Yu Bingxue Su1,2†
Bingxue Su1,2†