- 1Brain and Cognition Clinic, Institute for Cognitive Sciences Studies, Tehran, Iran
- 2School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 3Department of Psychiatry, Imam Hossein Hospital, School of Medicine, Alborz University of Medical Sciences, Karaj, Iran
- 4Unit of Clinical Psychiatry, Department of Neurosciences/DIMSC, Polytechnic University of Marche, Ancona, Italy
- 5Mental Health Research Center, Psychosocial Health Research Institute (PHRI), Department of Psychiatry, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
Background: Obsessive-compulsive disorder (OCD) ranks as the fourth most prevalent psychiatric disorder, with selective serotonin reuptake inhibitors (SSRIs) as its mainstay pharmacological treatment. However, approximately 40 to 60% of patients do not adequately respond to initial treatment, highlighting the need for alternative options. N-acetylcysteine (NAC) is one of the several medications that have been used in augmentation with SSRIs to enhance their efficacy.
Objectives: We aimed to investigate the safety and efficacy of NAC, a glutamate-modulating agent, as an augmentation in the treatment of moderate to severe OCD.
Method: We conducted a thorough search across PubMed, Scopus, Web of science, and ProQuest to identify relevant trials published until December 2023. The primary outcome of interest was the mean difference between the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) scores before and after administrating augmented NAC among patients with moderate to severe OCD. Furthermore, we compared the occurrence of adverse drug events between the experimental and control groups.
Results: We included six randomized controlled trials with 195 patients. The results of our study indicated a positive outcome for the experimental group in terms of the total Y-BOCS score when using the medication for a period of five to eight weeks (p-Value = 0.05). However, no significant difference was observed for durations shorter than five weeks or longer than 12 weeks. Additionally, no significant difference was found between the two groups in terms of the obsession and compulsion Y-BOCS scores. Furthermore, no significant differences were observed in terms of adverse events.
Conclusion: Augmentation of NAC with SSRIs may benefit patients with moderate to severe OCD. However, it is necessary to conduct additional multi-center trials over extended periods to develop a comprehensive strategy for action.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023463683.
Introduction
Obsessive-compulsive disorder (OCD) is a complex and chronic psychiatric disorder affecting millions of individuals worldwide (1). It is characterized by obsessions, compulsions, or both, which not only consume a substantial amount of time but also impair daily functioning, often leading to considerable distress and a diminished quality of life. Obsessions are defined as persistent, intrusive thoughts, urges, or images that the individuals find difficult to dismiss or control. These obsessions often revolve around specific themes, such as fears of contamination, losing control, inappropriate sexual thoughts, religious concerns, or an overwhelming need for symmetry and perfection. In response, individuals with OCD engage in compulsions, repetitive behaviors or mental acts performed with the aim of reducing the anxiety associated with their obsessions. Common compulsions include excessive washing, checking, organizing, or performing mental rituals such as counting or repeating phrases (2).
The underlying pathophysiology of OCD involves a complex interplay of genetic, neurobiological, and environmental factors. Research has long suggested that various neurotransmitters, including serotonin, norepinephrine, acetylcholine, and dopamine, play crucial roles in the development and maintenance of OCD symptoms. This understanding has paved the way for the development of targeted pharmacological treatments (3). First-line treatments for OCD typically include selective serotonin reuptake inhibitors (SSRIs) and clomipramine. Additionally, cognitive behavioral therapy (CBT), particularly exposure and response prevention (ERP), is widely recognized as an effective non-pharmacological treatment for OCD. Despite the availability of these treatments, a significant proportion of patients, ranging from 40 to 60% (4–6), do not respond adequately to first-line therapies or experience intolerable side effects. This situation necessitates the search for efficacious alternative or adjunctive treatment options (7–10).
In recent years, increasing attention has been directed toward the glutamatergic system as a potential therapeutic target in OCD. Glutamatergic medications can act as receptor antagonists, reuptake inhibitors, co-agonists, and ion channel modulators. Altered glutamate levels, may contribute to the development and persistence of OCD symptoms (11). Glutamate has a significant role in the normal development of the cortico-striatal-thalamo-cortical (CSTC) circuitry, neural communication, plasticity, and overall brain function (12). For instance, elevated levels of glutamate and its byproducts are found in the basal ganglia, while reduced levels have been noted in the anterior cingulate cortex in patients with OCD (13). Additionally, disruptions in the balance of glutamate and its interaction with other neurotransmitters, such as GABA and serotonin, which are involved in the flow of glutamate between the cortex, thalamus, and basolateral amygdala, may result in abnormal CSTC pathway activity (14). Over the years, various glutamatergic agents have been investigated, such as lamotrigine, topiramate, memantine, glycine, D-cycloserine, riluzole and N-acetylcysteine (NAC) (15).
NAC, which has emerged as a particularly intriguing candidate, is a derivative of the amino acid cysteine, and has powerful antioxidant properties (16). It is well-known for its role in regulating intracellular levels of glutathione and modulating the glutamatergic system (17). Several clinical trials and studies have evaluated the efficacy of NAC as an adjunctive treatment in OCD, with some suggesting that it may offer significant benefits, particularly in patients who are resistant to conventional therapies. A systematic review and meta-analysis conducted in 2020 reported that NAC is a promising agent for the treatment of patient with OCD (18).
Given the evolving body of evidence regarding the use of NAC in the treatment of moderate to severe OCD, this systematic review and meta-analysis aims to comprehensively evaluate the literature, focusing on the safety and efficacy of NAC as an augmentation in the management of moderate to severe OCD.
Methods
We followed the Preferred Reporting Items For Systematic Reviews And Meta-Analyses (PRISMA) reporting guidelines and the Cochrane Collaboration Handbook (19, 20) (PROSPERO ID: CRD42023463683).
Eligibility criteria
The inclusion criteria for the studies were determined using the PICOT (population, intervention, comparison, outcome, time) framework. Studies were included if they involved individuals over the age of 18 who were diagnosed with OCD, according to either fourth or fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV or DSM-5), and had a total Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) score greater than 16, which is indicative of moderate to severe OCD (21). The intervention of interest was the use of NAC as an augmentation therapy for OCD. The comparison group consisted of patients receiving treatment with SRIs and SSRIs. The primary outcome was the reduction in OCD symptom severity, as measured by the Y-BOCS, a 10-item scale with total scores ranging from 0 to 40, where higher scores indicate more severe symptoms. Included studies were required to have a trial duration of at least 3 weeks to ensure sufficient time for assessing the effects of NAC on OCD symptoms. Quasi-experimental studies, observational studies, case series, and case reports, as well as conference abstracts, protocols, opinion pieces, theses, and review articles were excluded.
Databases and search procedure
We conducted the search across electronic databases of PubMed, Web of Science, Scopus (as databases of journal article) and ProQuest (as a database of grey literature) to identify relevant trials, using the search strategies outlined in Appendix 1 to collect the available evidence from original articles published until December 2023 in English. The search results were imported into an EndNote library (Version 20, Thomson Reuters, USA), and duplicated citations were eliminated using the “Find Duplicates” feature of the software.
Outcome measures
The main measure of interest for our study was the effect size of Y-BOCS scores, which was determined by calculating the mean difference of scores before and after administrating NAC in augmentation with an SSRI. The secondary measure was the effect size of adverse events of NAC throughout the trials.
Study selection
To ensure the accuracy and relevance of the study, two authors (SE and NE) independently screened the titles and abstracts of the selected studies. Following this, the full texts of the remaining articles were obtained and examined, according to the specified inclusion criteria. In the event of any disagreements, the authors resolved them through discussion or sought consultation with a third author (MSH).
In order to identify additional relevant papers, the references cited in the selected articles were also evaluated. The full texts of the studies that were ultimately selected underwent a comprehensive evaluation for quality assessment, data extraction, and analysis.
Data extraction and quality assessment
Detailed data extraction was performed based on the pre-designed data extraction forms, including the study design, first author’s name, country, participants characteristics, interventions and outcomes.
Two authors (SE and NE) independently assessed the risk of bias in studies across six domains: random sequence generation (selection bias for controlled trials), allocation concealment (selection bias for controlled trials), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias) (22).
Evidence synthesis
We adopted systematic methods, including textual descriptions and tabulation, using the Review Manager (RevMan) (Version 5.4. the Cochrane collaboration, 2020), for evidence synthesis. We employed statistical techniques to estimate the missing values using the information provided in the articles. Specifically, we used Wan’s method to calculate the mean and standard deviation based on the sample size, median, range, and interquartile range (23). In cases where data were missing, we considered the participants as non-abstainers. We conducted a meta-analysis using RevMan to determine the weighted average treatment effect across the included studies. To assess heterogeneity among the studies, we utilized the chi-square statistic and calculated I2. Where I2 values greater than 40% or a chi-square statistic with a p-value less than 0.1 indicated significant statistical heterogeneity, a random-effects model was employed (24). To assess the effectiveness of the pharmacological intervention, we calculated the mean difference (MD) of total, compulsion and obsession Y-BOCS scores before and after the intervention. Additionally, we performed a subgroup analysis based on the period of intervention to address any clinical heterogeneity. We used relative risk (RR) with a 95% confidence interval (CI), to assess the occurrence of adverse events (25). To ensure consistency, we utilized data from the study that reported the longest follow-up period for each outcome.
Results
Description of included studies
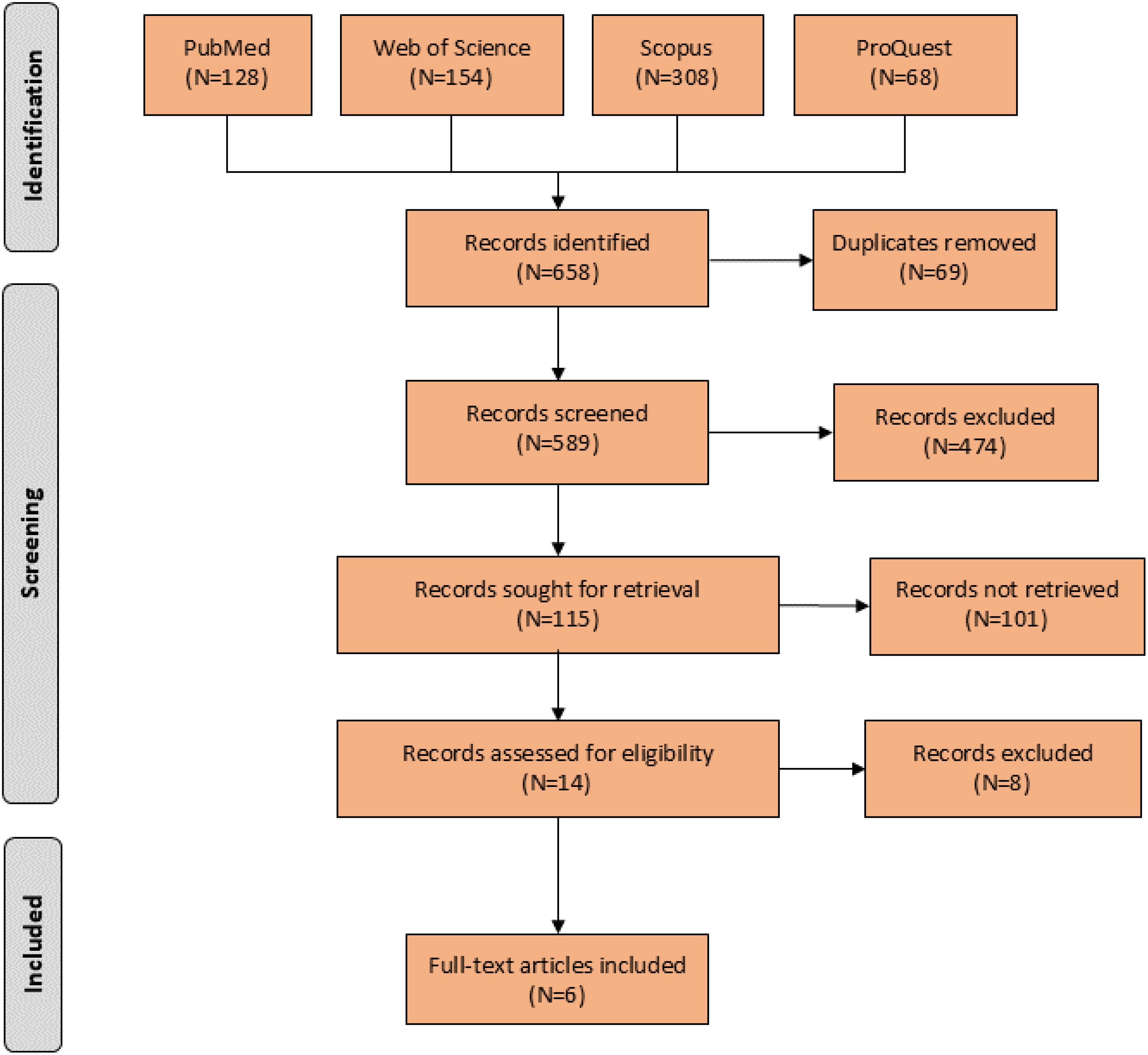
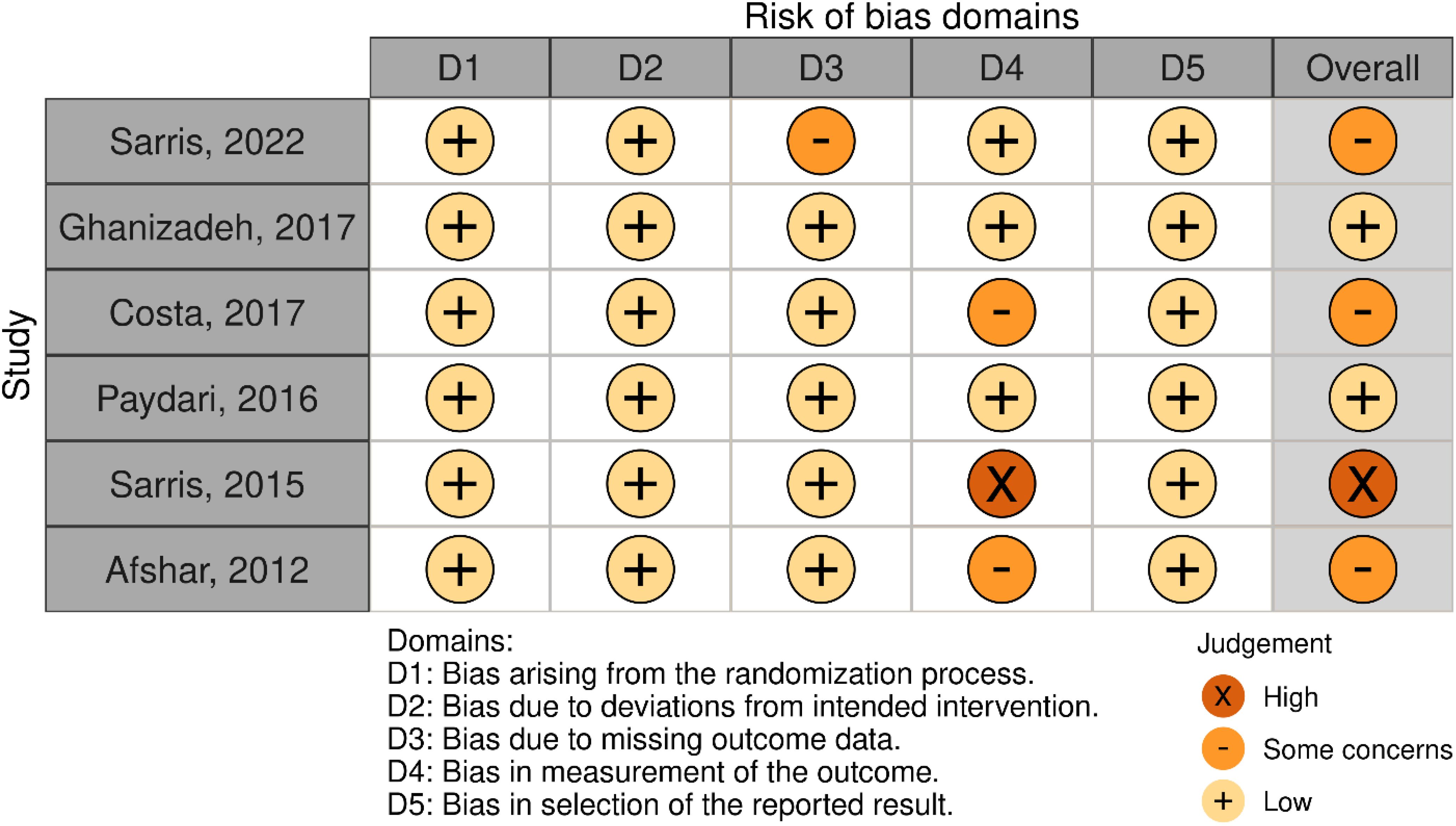
Initial search resulted in 658 records. After assessing the titles and abstracts, a total of 14 studies were selected for full text screening, and six RCTs (26–31) with 195 participants with moderate to severe OCD met the inclusion criteria and were included in the meta-analysis (Figure 1). Their overall quality, as shown in Figure 2, was considered fair. The mean study duration was 15 weeks (ranging from 10 to 20). The dose of NAC ranged from 600 to 3000 mg/day. Table 1 presents the summary of the included studies.
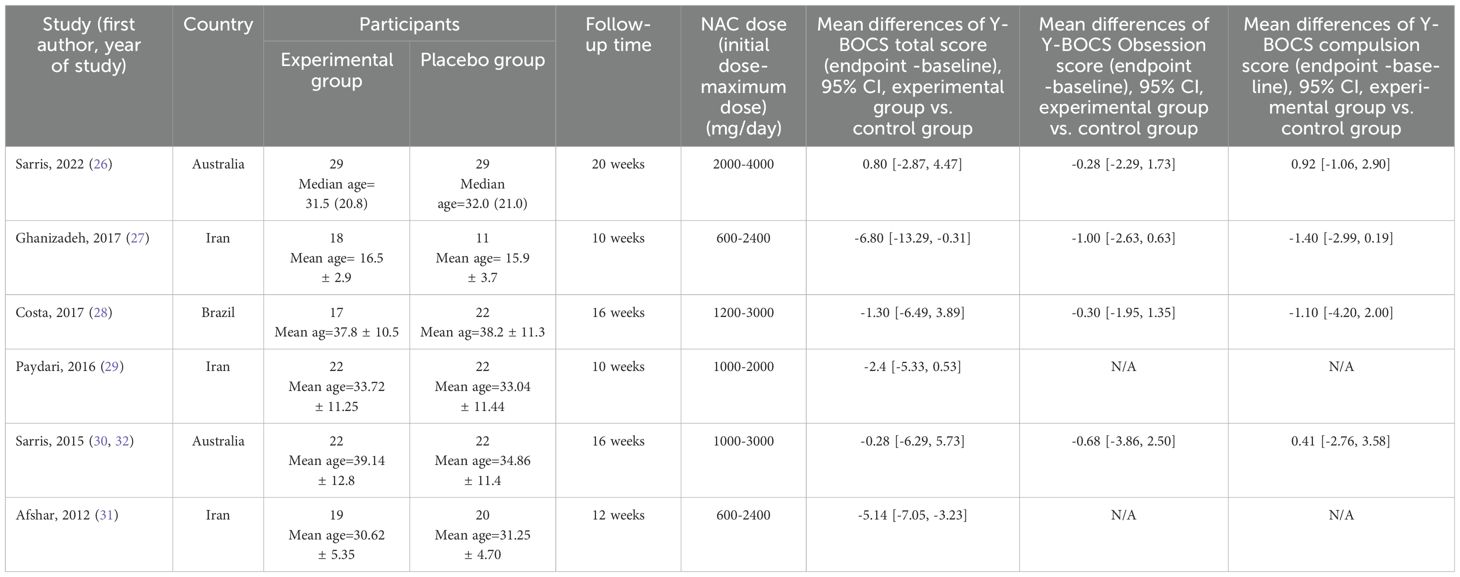
Table 1. Summary of clinical trials investigating the effect of NAC in treating moderate to severe OCD included in meta-analysis.
Total Y-BOCS scores
The results indicated a trend toward the beneficial impact of NAC on total Y-BOCS scores over a duration of 12 weeks or longer (MD = -1.87, 95% CI: [-5.39, -1.66], p-Value = 0.30, I2 = 70%) (Figure 3) (26, 28, 30, 31). Also, the meta-analysis of two studies (27, 31) revealed that using NAC for five to eight weeks is significantly effective (MD = -2.95, 95% CI: [-5.87, -0.04], p-Value = 0.05, I2 = 0%) (Figure 4), while not significantly effective for four weeks or less (MD = 0.74, 95% CI: [-2.27, 3.74], p-Value = 0.63, I2 = 0%) (Figure 5).
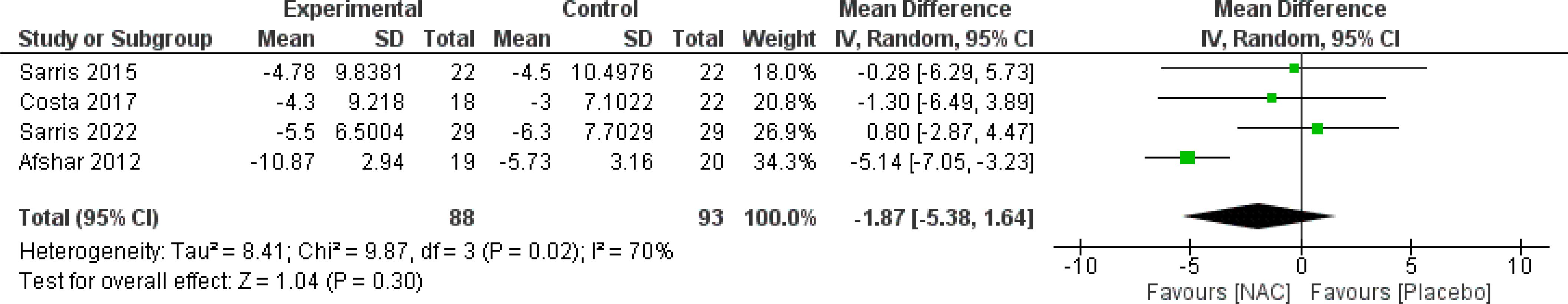
Figure 3. Comparison of total Y-BOCS scores between the NAC and control groups receiving NAC for 12 weeks and more. Also, the meta-analysis of two studies (27, 31) revealed that using NAC for five to eight weeks is significantly effective (MD = -2.95, 95% CI: [-5.87, -0.04], p-Value = 0.05, I2 = 0%) (Figure 4), while not significantly effective for four weeks or less (MD = 0.74, 95% CI: [-2.27, 3.74], p-Value = 0.63, I2 = 0%) (Figure 5).
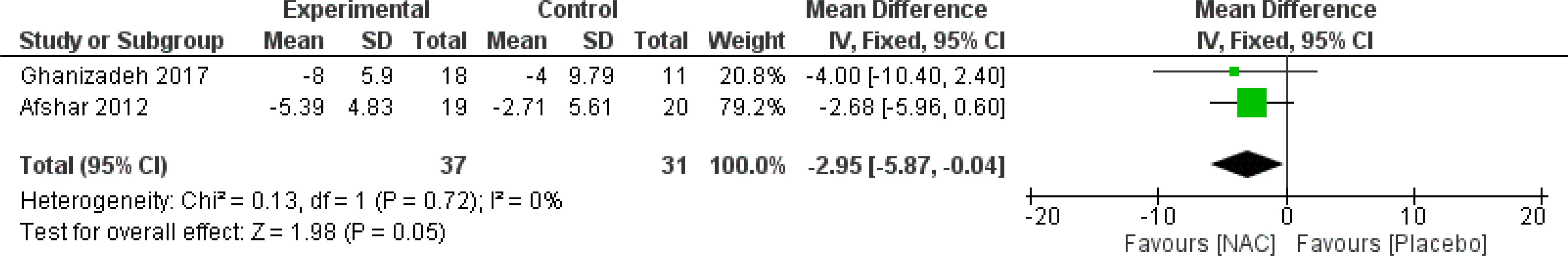
Figure 4. Comparison of total Y-BOCS scores between the NAC and control groups receiving NAC for five to eight weeks.
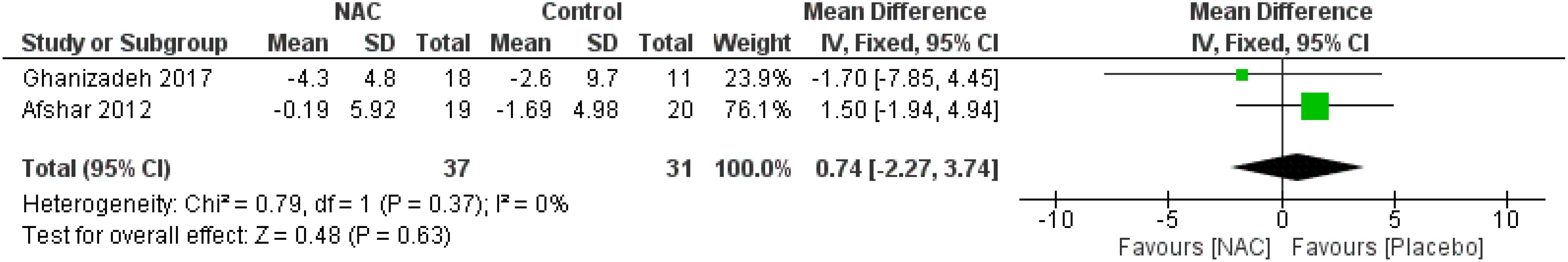
Figure 5. Comparison of total Y-BOCS scores between the NAC and control groups receiving NAC for four weeks and less.
Obsession and compulsion Y-BOCS scores
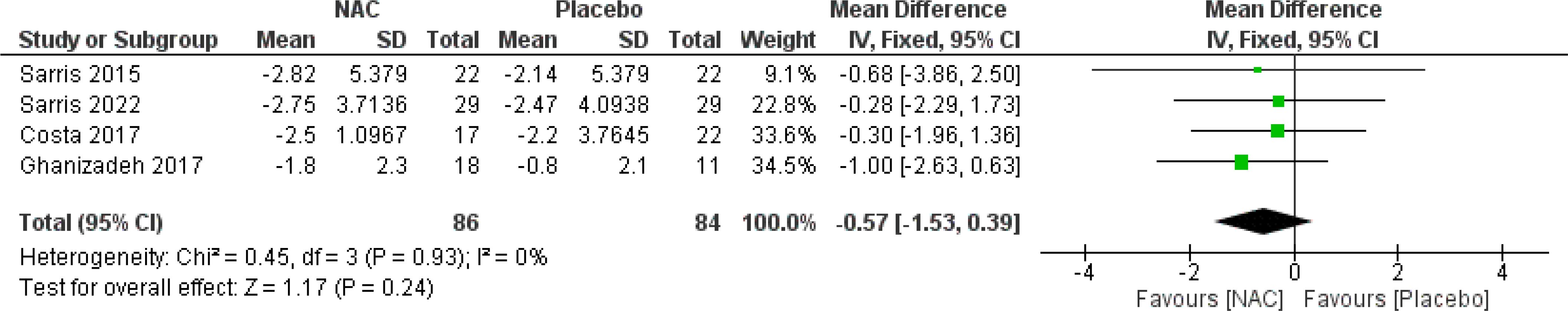
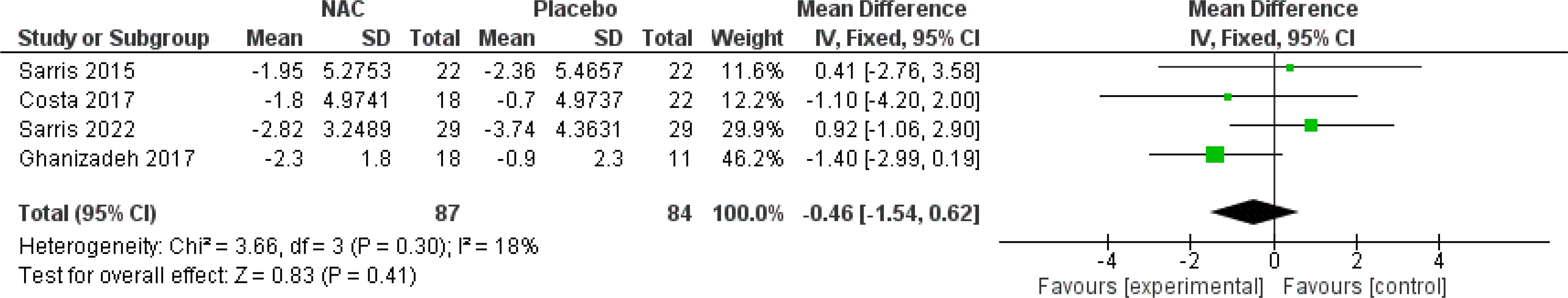
The subgroup analysis of four studies (26–28, 30) revealed that NAC was not significantly effective for obsession (MD = -0.57, 95% CI: [-1.53, 0.39], p-Value = 0.24, I2 = 0%) or compulsion (MD = -0.40, 95% CI: [-1.65, 0.84], p-Value = 0.53, I2 = 18%) (Figures 6, 7).
Adverse events
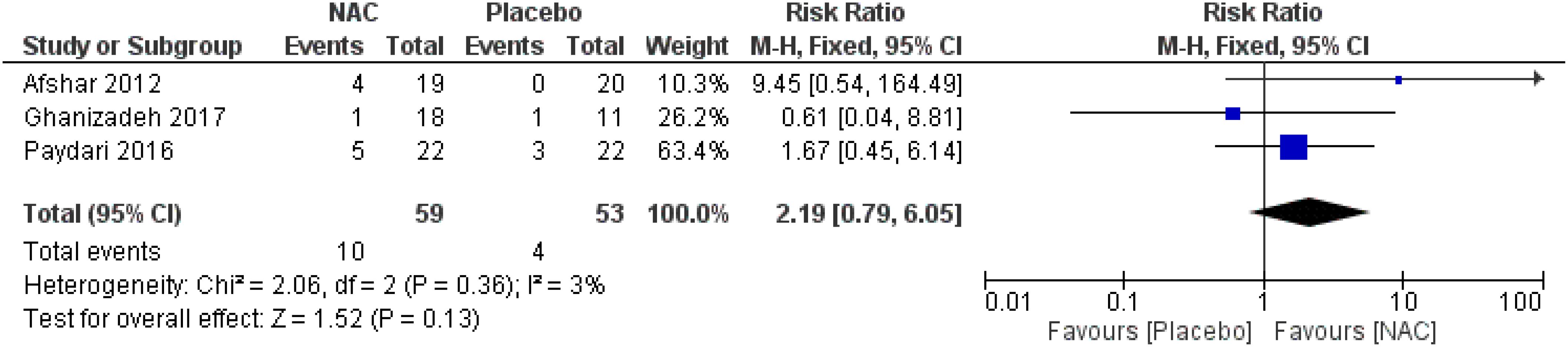
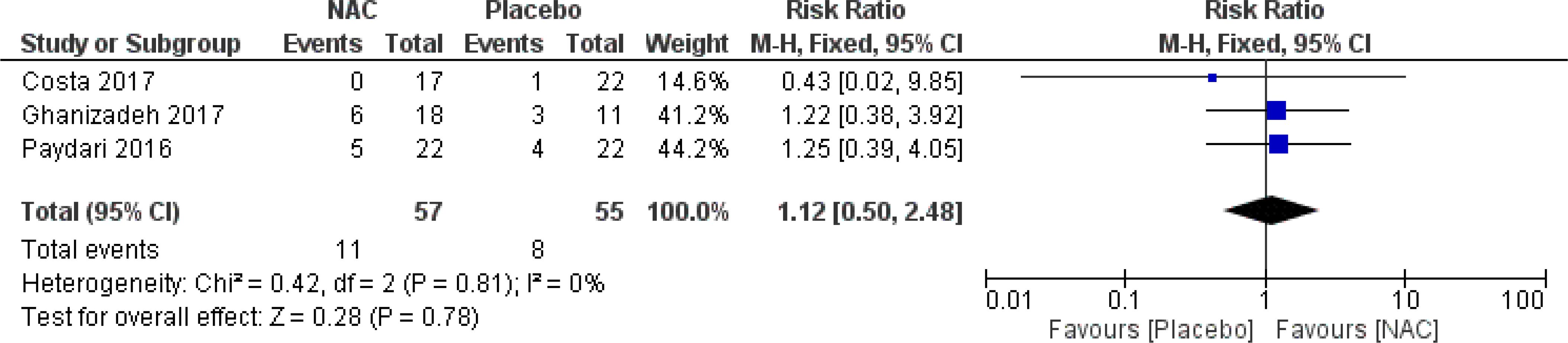
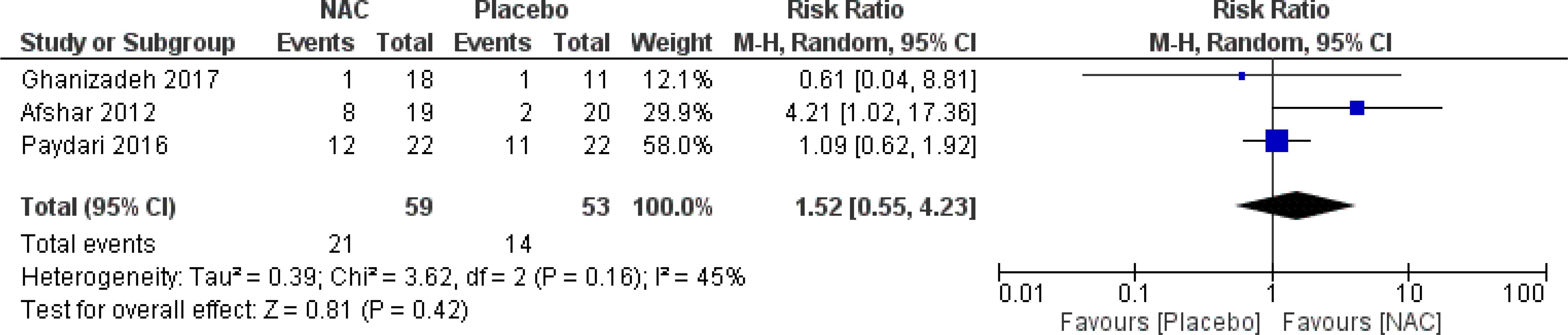
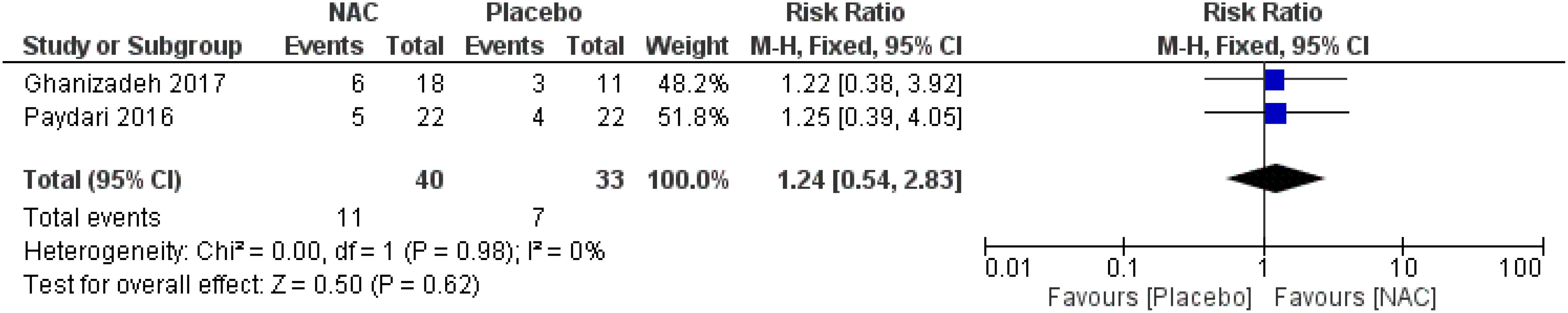
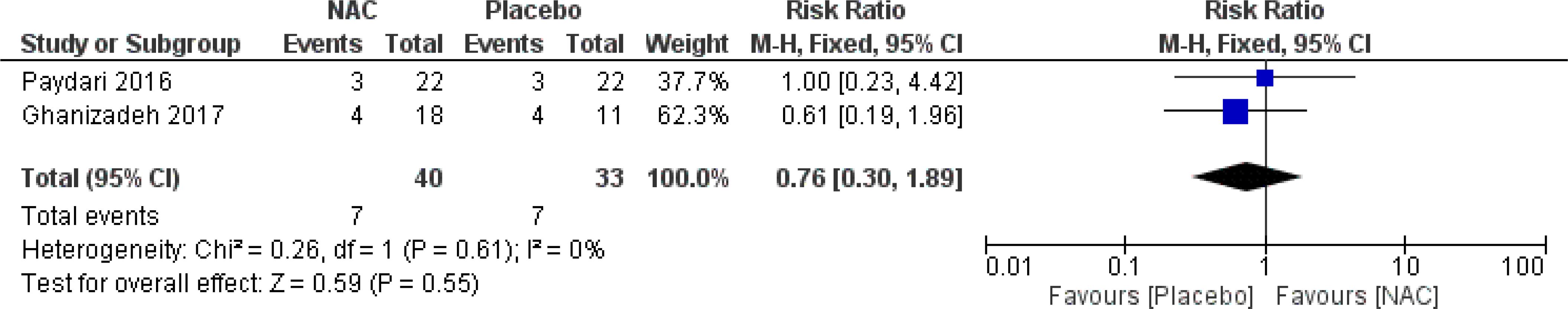
No significant differences were found on the occurrence of diarrhea, nausea/vomiting, dizziness, drowsiness, and dry mouth between the groups (Figures 8–12) (27–29, 31).
Other outcomes
Ghanizadeh et al. (27) have reported that NAC had a positive impact on physical (p-Value = 0.005), emotional (p-Value = 0.001) and social (p-Value = 0.001) function after 10 weeks.
Afshar et al. (31) have reported that the severity of disorder, was significantly different between the NAC and control groups at week 12 (p-Value = 0.01). However, no significant difference was found for clinical improvement.
Costa et al. (28) have reported that there were no significant between-group differences in Dimensional Y-BOCS (DY-BOCS) (aggression/violence, sexual/religious, ordering/symmetry/counting, contamination/cleaning, hoarding, miscellaneous, and total), Beck Anxiety Inventory (BAI), Beck Depression Inventory (BDI), and Brown Assessment of Beliefs Scale (BABS) (insight/delusionality) scores.
Discussion
We conducted a systematic review and meta-analysis on six RCTs to evaluate the safety and efficacy of NAC in the treatment of moderate to severe OCD. Our analysis revealed that NAC has a significant beneficial effect in managing moderate to severe OCD symptoms, between weeks five and eight. Notably, it was well-tolerated and causes only mild side effects, contributing to a favorable safety profile. The effect size, though favorable, was modest, raising questions about whether the observed changes would be perceived as meaningful in a clinical setting. The potential for greater clinical impact might emerge with longer study durations or higher doses of NAC. Indeed, while the studies included in our meta-analysis were limited in scope, there was a trend toward greater efficacy with increased doses of NAC. However, due to the small sample size and variability in study designs, this trend did not reach statistical significance for a period of longer than 12 weeks.
Moreover, our findings align with those of a 2020 systematic review and meta-analysis (18) of five RCTs (20, 21, 23, 26, 27), with a mean duration of 12 weeks (ranged from 10 to 16 weeks), and a maximum dose of NAC ranged from 2000 to 3000 mg/day. They have reported that NAC was statistically superior to placebo in reducing the Y-BOCS scores. Also, it was well-tolerated, with only mild side effects reported. However, our review suggests that the effect might be more nuanced, potentially influenced by factors such as dose and duration of treatment.
In the context of understanding the potential role of NAC in treating moderate to severe OCD, dysregulation of the CSTC circuit, specifically involving disruption of glutamatergic transmission, has been consistently observed in patients with OCD (33, 34). Notably, some studies have reported significantly elevated glutamate levels in the cerebrospinal fluid of patients with OCD, leading to excitotoxicity and oxidative stress, both of which are associated with the severity of OCD symptoms (11). Furthermore, drug-naive adults with OCD have exhibited higher levels of glutamate and glycine in their cerebrospinal fluid (35). Magnetic resonance spectroscopy has further revealed abnormalities in the glutamate-glutamine pathway (36). Moreover, preclinical studies suggest that NAC may influence oxidative stress (37), apoptosis (38), neurogenesis, neuroinflammation, mitochondrial dysfunction (39), and disruptions in the function of dopamine (40) and glutamate, as NAC transforms into cysteine in the CNS, which helps regulate the transportation of glutamate between nerve cells (11), through the glutamate/cystine antiporter (41), mainly found in glial cells (41). These findings collectively suggest that targeting glutamate activity with glutamatergic agents holds promise as a therapeutic strategy for individuals with OCD.
Limitations
It is essential to acknowledge the limitations of this study. First, the inclusion criteria were restricted to English-language publications, potentially introducing language bias. Second, only six RCTs met our inclusion criteria. Third, the diversity in dosing regimens and follow-up periods across studies precluded subgroup analyses based on medication type and dosage. Fourth, the heterogeneity of the included studies in terms of patient populations, comorbidities, and study designs may have influenced the generalizability of the findings.
Future research should aim to conduct larger multi-center trials with longer follow-up periods. Moreover, investigations into the use of NAC in patients with comorbid disorders should be evaluated. Additionally, the impact of NAC on cognitive function (e.g., impairments in set-shifting ability, response inhibition, and nonverbal memory) (42) should be further investigated. Moreover, studies examining the potential benefits of NAC in combination with other emerging treatments, could provide valuable insights into comprehensive treatment strategies for OCD. Finally, a better understanding of patient-specific factors, such as genetic predispositions or comorbidities, may help identify those who are most likely to benefit from NAC therapy.
Conclusions
Our systematic review and meta-analysis suggests that NAC may serve as an effective adjunct treatment for adults with moderate to severe OCD. Additionally, NAC was well-tolerated, with no serious adverse events reported. However, the potential for NAC to maintain or even enhance its therapeutic effects with prolonged use remains unclear, necessitating further high-quality large-scale RCTs for elucidating the optimal dosage and duration of NAC treatment, as well as exploring its mechanisms of action within the glutamatergic system.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The study was approved by the ethics committee of Iran University of Medical Sciences institutional review board (IRB: IR.IUMS.REC.1402.820).
Author contributions
SE: Conceptualization, Data curation, Formal analysis, Writing – original draft. NE: Data curation, Writing – original draft. MHM: Writing – review & editing. MB: Writing – review & editing. LO: Writing – review & editing. MS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Iran University of Medical Sciences to MS (Grant no: 1402-3-99-27232).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1421150/full#supplementary-material
Abbreviations
OCD, Obsessive-Compulsive Disorder; SSRI, selective serotonin reuptake inhibitor; DSM–5, Diagnostic and Statistical Manual of Mental Disorders; Y-BOCS, Yale-Brown obsessive compulsive scale; CBT, cognitive-behavioral therapy; RCTs, randomized-controlled trails; GABA, γ-aminobutyric acid; MD, Mean difference; CI, confidence interval; SD, standard deviation; CSTC, cortico-striatal-thalamo-cortical; NAC, N-acetyl cysteine; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols guidelines; RR, risk ratio.
References
3. Vellucci L, Ciccarelli M, Buonaguro EF, Fornaro M, D’Urso G, De Simone G, et al. The neurobiological underpinnings of obsessive-compulsive symptoms in psychosis, translational issues for treatment-resistant schizophrenia. Biomolecules. (2023) 13. doi: 10.3390/biom13081220
4. Janardhan Reddy YC, Sundar AS, Narayanaswamy JC, Math SB. Clinical practice guidelines for Obsessive-Compulsive Disorder. Indian J Psychiatry. (2017) 59:S74–s90. doi: 10.4103/0019-5545.196976
5. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. (2014) 37:375–91. doi: 10.1016/j.psc.2014.05.006
6. Swierkosz-Lenart K, Dos Santos JFA, Elowe J, Clair AH, Bally JF, Riquier F, et al. Therapies for obsessive-compulsive disorder: Current state of the art and perspectives for approaching treatment-resistant patients. Front Psychiatry. (2023) 14:1065812. doi: 10.3389/fpsyt.2023.1065812
7. Bakizadeh F, Mokhtari S, Saeed F, Mokhtari A, Akbari Koli P, Shalbafan M. Cognitive rehabilitation for adult patients with obsessive-compulsive disorder: A systematic review of randomized controlled trials. BCN. (2024) 15:287–300. doi: 10.32598/bcn.2022.1604.3
8. Shalbafan M, Malekpour F, Tadayon Najafabadi B, Ghamari K, Dastgheib S-A, Mowla A, et al. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: A placebo-controlled, randomized clinical trial. J Psychopharmacology. (2019) 33:1407–14. doi: 10.1177/0269881119878177
9. Askari S, Mokhtari S, Shariat SV, Shariati B, Yarahmadi M, Shalbafan M. Memantine augmentation of sertraline in the treatment of symptoms and executive function among patients with obsessive-compulsive disorder: A double-blind placebo-controlled, randomized clinical trial. BMC Psychiatry. (2022) 22:34. doi: 10.1186/s12888-021-03642-z
10. Eissazade N, Mosavari H, Eghdami S, Boroon M, Ashrafi F, Shalbafan M. Efficacy and safety of 5-hydroxytryptamine-3 (5-HT3) receptor antagonists in augmentation with selective serotonin reuptake inhibitors (SSRIs) in the treatment of moderate to severe obsessive–compulsive disorder: a systematic review and meta-analysis of randomized clinical trials. Sci Rep. (2023) 13:20837. doi: 10.1038/s41598-023-47931-x
11. Ting JT, Feng G. Neurobiology of obsessive–compulsive disorder: insights into neural circuitry dysfunction through mouse genetics. Curr Opin neurobiology. (2011) 21:842–8. doi: 10.1016/j.conb.2011.04.010
12. Hadi F, Kashefinejad S, Kamalzadeh L, Hoobehfekr S, Shalbafan M. Glutamatergic medications as adjunctive therapy for moderate to severe obsessive-compulsive disorder in adults: a systematic review and meta-analysis. BMC Pharmacol Toxicol. (2021) 22:1–11. doi: 10.1186/s40360-021-00534-6
13. Karthik S, Sharma LP, Narayanaswamy JC. Investigating the role of glutamate in obsessive-compulsive disorder: current perspectives. Neuropsychiatr Dis Treat. (2020) 16:1003–13. doi: 10.2147/NDT.S211703
14. Yudkoff M, Daikhin Y, Horyn O, Nissim I, Nissim I. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia. (2008) 49:73–5. doi: 10.1111/j.1528-1167.2008.01841.x
15. Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. (2011) 36:78–86. doi: 10.1503/jpn
16. Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, et al. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radical Res. (2018) 52:751–62. doi: 10.1080/10715762.2018.1468564
17. Morland C, Nordengen K. N-acetyl-aspartyl-glutamate in brain health and disease. Int J Mol Sci. (2022) 23:1268. doi: 10.3390/ijms23031268
18. Gadallah AHA, Ebada MA, Gadallah A, Ahmed H, Rashad W, Eid KA, et al. Efficacy and safety of N-acetylcysteine as add-on therapy in the treatment of obsessive-compulsive disorder: A systematic review and meta-analysis. J Obsessive-Compulsive Related Disord. (2020) 25. doi: 10.1016/j.jocrd.2020.100529
19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Internal Med. (2009) 151:W–65-W-94. doi: 10.1016/j.jclinepi.2009.06.006
20. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). The UK: Cochrane (2023). Available at: www.training.cochrane.org/handbook.
21. Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The yale-brown obsessive compulsive scale: II. Validity. Arch Gen Psychiatry. (1989) 46:1012–6. doi: 10.1001/archpsyc.1989.01810110054008
22. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. (2011) 343. doi: 10.1136/bmj.d5928
23. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res methodology. (2014) 14:1–13. doi: 10.1186/1471-2288-14-135
24. Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical Primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. (2018) 27:317–21. doi: 10.1093/icvts/ivy163
25. Sciences CfIOoM. Evidence synthesis and meta-analysis for drug safety: report of CIOMS working group X. Switzerland: CIOMS (2016).
26. Sarris J, Byrne G, Castle D, Bousman C, Oliver G, Cribb L, et al. N-acetyl cysteine (NAC) augmentation in the treatment of obsessive-compulsive disorder: A phase III, 20-week, double-blind, randomized, placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. (2022) 117:110550. doi: 10.1016/j.pnpbp.2022.110550
27. Ghanizadeh A, Mohammadi MR, Bahraini S, Keshavarzi Z, Firoozabadi A, Alavi Shoshtari A. Efficacy of N-acetylcysteine augmentation on obsessive compulsive disorder: A multicenter randomized double blind placebo controlled clinical trial. Iran J Psychiatry. (2017) 12:134–41.
28. Costa DLC, Diniz JB, Requena G, Joaquim MA, Pittenger C, Bloch MH, et al. Randomized, double-blind, placebo-controlled trial of N-acetylcysteine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. (2017) 78:e766–e73. doi: 10.4088/JCP.16m11101
29. Paydary K, Akamaloo A, Ahmadipour A, Pishgar F, Emamzadehfard S, Akhondzadeh S. N-acetylcysteine augmentation therapy for moderate-to-severe obsessive-compulsive disorder: randomized, double-blind, placebo-controlled trial. J Clin Pharm Ther. (2016) 41:214–9. doi: 10.1111/jcpt.12370
30. Sarris J, Oliver G, Camfield DA, Dean OM, Dowling N, Smith DJ, et al. N-acetyl cysteine (NAC) in the treatment of obsessive-compulsive disorder: A 16-week, double-blind, randomised, placebo-controlled study. CNS Drugs. (2015) 29:801–9. doi: 10.1007/s40263-015-0272-9
31. Afshar H, Roohafza H, Mohammad-Beigi H, Haghighi M, Jahangard L, Shokouh P, et al. N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. (2012) 32:797–803. doi: 10.1097/JCP.0b013e318272677d
32. Sarris J, Oliver G, Camfield DA, Dean OM. Participant characteristics as modifiers of response to N-acetyl cysteine (NAC) in obsessive-compulsive disorder. Clin psychol Sci. (2016) 4:1104–11. doi: 10.1177/2167702616639864
33. Oliver G, Dean O, Camfield D, Blair-West S, Ng C, Berk M, et al. N-acetyl cysteine in the treatment of obsessive compulsive and related disorders: a systematic review. Clin Psychopharmacol Neurosci. (2015) 13:12. doi: 10.9758/cpn.2015.13.1.12
34. Shokrani M, Askari S, Eissazade N, Shariat SV, Shariati B, Yarahmadi M, et al. Agomelatine augmentation of sertraline in the treatment of moderate to severe obsessive-compulsive disorder: a randomized double-blinded placebo-controlled clinical trial. BMC Psychiatry (2023) 23:686. doi: 10.21203/rs.3.rs-2684405/v1
35. Swedo SE, Leonard HL, Kruesi MJ, Rettew DC, Listwak SJ, Berrettini W, et al. Cerebrospinal fluid neurochemistry in children and adolescents with obsessive-compulsive disorder. Arch Gen Psychiatry. (1992) 49:29–36. doi: 10.1001/archpsyc.1992.01820010029004
36. Kellner CH, Jolley RR, Holgate RC, Austin L, Lydiard RB, Laraia M, et al. Brain MRI in obsessive-compulsive disorder. Psychiatry Res. (1991) 36:45–9. doi: 10.1016/0165-1781(91)90116-7
37. Alici D, Bulbul F, Virit O, Unal A, Altindag A, Alpak G, et al. Evaluation of oxidative metabolism and oxidative DNA damage in patients with obsessive–compulsive disorder. Psychiatry Clin neurosciences. (2016) 70:109–15. doi: 10.1111/pcn.12362
38. Gupta R, Mehan S, Sethi P, Prajapati A, Alshammari A, Alharbi M, et al. Smo-Shh agonist Purmorphamine prevents neurobehavioral and neurochemical defects in 8-OH-DPAT-induced experimental model of obsessive-compulsive disorder. Brain Sci. (2022) 12:342. doi: 10.3390/brainsci12030342
39. Lacey CJ, Salzberg MR. Obsessive-compulsive disorder with mitochondrial disease. Psychosomatics. (2008) 49:540–2. doi: 10.1176/appi.psy.49.6.540
40. Denys D, Zohar J, Westenberg H. The role of dopamine in obsessive-compulsive disorder: preclinical and clinical evidence. J Clin Psychiatry. (2004) 65:11–7.
41. Kato S, Ishita S, Sugawara K, Mawatari K. Cystine/glutamate antiporter expression in retinal mu¨ ller glial cells: Implications fordl-alpha-aminoadipate toxicity. Neuroscience. (1993) 57:473–82. doi: 10.1016/0306-4522(93)90080-Y
Keywords: acetylcysteine, obsessive-compulsive disorder, glutamate, systematic review, meta-analysis
Citation: Eghdami S, Eissazade N, Heidari Mokarar M, Boroon M, Orsolini L and Shalbafan M (2024) The safety and efficacy of N-acetylcysteine as an augmentation in the treatment of obsessive-compulsive disorder in adults: a systematic review and meta-analysis of randomized clinical trials. Front. Psychiatry 15:1421150. doi: 10.3389/fpsyt.2024.1421150
Received: 21 April 2024; Accepted: 26 August 2024;
Published: 23 September 2024.
Edited by:
Mirko Manchia, University of Cagliari, ItalyReviewed by:
Donatella Marazziti, University of Pisa, ItalyMaryam Pourshams, Ahvaz Jundishapur University of Medical Sciences, Iran
Irene Martínez-García, University of Castilla La Mancha, Spain
Copyright © 2024 Eghdami, Eissazade, Heidari Mokarar, Boroon, Orsolini and Shalbafan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammadreza Shalbafan, U2hhbGJhZmFuLm1yQGl1bXMuYWMuaXI=
†These authors have contributed equally to this work and share first authorship
 Shayan Eghdami
Shayan Eghdami Negin Eissazade
Negin Eissazade Mohsen Heidari Mokarar3
Mohsen Heidari Mokarar3 Mahsa Boroon
Mahsa Boroon Mohammadreza Shalbafan
Mohammadreza Shalbafan