
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Psychiatry , 23 August 2024
Sec. Digital Mental Health
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1420097
Background: Adverse effects of chronically high levels of stress on physical and mental health are well established. In physicians, the effects of elevated stress levels exceed the individual level and include treatment errors and reduced quality of patient-doctor relationships. Breathing and mindfulness-based exercises have been shown to reduce stress and could serve as an immediate and easy-to-implement anti-stress intervention among physicians. Due to the heterogeneity of their effect on stress, we aim to evaluate the intervention effect of performing a short daily breathwork-based or mindfulness-based intervention on the everyday level of perceived stress in physicians in residence in Germany in a series of N-of-1 trials.
Methods: Study participants will choose between two short interventions, box breathing, and one guided more complex mindfulness-based breathing exercise. Each participant subsequently will be randomly allocated to a sequence of 1-week intervention (A) and control (B, everyday life) phases. Each N-of-1 trial consists of two two-week cycles (AB or BA), resulting in a total trial duration of 4 weeks (ABAB or BABA). Perceived levels of stress will be assessed daily via the StudyU App on the participant’s smartphone. Additionally, participants will be asked to complete a questionnaire at baseline and three months after completion of the study that contains questions about basic participant characteristics, lifestyle factors, individual living situations, and validated psychological questionnaires. Intervention effects will be estimated by Bayesian multi-level random effects models on the individual and population level.
Discussion: This study contributes to the development of short-term solutions to reduce work-related stress for physicians in residence. This is expected to benefit the individual and increase the quality of overall healthcare due to a reduction in treatment errors and an increase in the quality of doctor-patient relationships.
Clinical trial registration: ClinicalTrials.gov, identifier NCT05745545.
Continuously high levels of psychosocial stress are associated with numerous adverse physical and mental health outcomes (1–3). Research has consistently demonstrated that physicians represent a demographic notably susceptible to experiencing elevated stress levels over prolonged durations (4). In a prospective cohort study of 3,588 resident physicians in the USA, 45.2% reported symptoms of burnout (5). Similarly, 48.7% of physicians in surgery in Germany fulfilled the burn-out criteria of the Copenhagen Burnout Inventory (CBI) (6). In a survey of young German clinicians between their first and sixth year of training, 22% of the participants stated that they had taken medication due to work-related stress (7). A high prevalence of burnout (8) and a higher stress level compared to the general population were also reported for physicians in general medical practices in Germany (9). Furthermore, it was repeatedly shown that high levels of stress and burnout in physicians increase the risk for major medical errors (10, 11), and poorer quality of medical care, including poorer doctor-patient relationships and deviation from medical standards (12, 13). A reduction in stress levels among physicians would, therefore, not only lead to individual benefits with respect to physical and mental health in the medical workforce but also to an improvement in patient care (4) with a potentially positive effect on the health of the general population. Furthermore, reductions in stress and burnout rates have been shown to yield substantial economic advantages (14). This improvement is partly attributable to lower rates of physician turnover and increased productivity resulting from decreased stress levels (15, 16). For this reason, this study aims to test stress management interventions that are easy to learn and can be performed quickly without the need for special training or additional tools. Special breathing techniques and mindfulness exercises are two approaches that meet these requirements (4, 17–19). Box breathing, also known as “tactical breathing”, is a relaxation technique that decreases heart rate variability and, among others, is used by the military and law enforcement to decrease stress levels (18, 20–22). Breathwork exercises have an impact on a range of symptoms and can lead to a reduction of anxiety, depression, and feelings of anger but also increase the feeling of comfort and relaxation (23, 24). A recent meta-analysis showed a positive effect of paced breathing on stress perception (25). However, some studies did not find a statistically significant effect of breathing exercises on stress (25–27). Mindfulness interventions vary in type and duration and can positively affect depressive symptoms, anxiety, stress, well-being, and quality of life [meta-analysis in ref (28)]. A meta-analysis of 12 studies reported a positive effect of interventions aimed at reducing stress (including mindfulness-based interventions) (4). Although the majority of studies in the field focus on the structured 8-week mindfulness-based stress reduction (MBSR) program, there are also studies on shorter intervention periods, including app-based mindfulness interventions (17). A study that used 10-minute audio recordings of mindfulness exercises over 8 weeks in patients with anxiety disorders showed positive effects compared to a placebo group (29). A meta-analysis also reported moderate effect sizes of stress-management interventions (30). However, also null results were reported [reviewed in (28)].
Due to interindividual differences and personal preferences, high heterogeneity in intervention effects is expected (31) and has been shown for mindfulness-based interventions before (32). In order to account for this heterogeneity, N-of-1 trials (33) are the gold-standard study design in which every participant applies intervention and non-intervention phases in a cross-over design and continuously assesses the outcome of interest (e.g., daily) over time. After completion of the trial, statistical inference on the intervention effect can be done on the individual level by comparing the outcome in the intervention phases with the outcome in the control phases and on the population level from the same data. In the following, we describe a study protocol for a series of N-of-1 trials to evaluate the effect of breathing and mindfulness exercises on stress levels among physicians in residency in Germany. We hypothesize that the daily performance of the investigated anti-stress intervention reduces the level of perceived stress.
In this study, we aim to assess the causal effect of two anti-stress interventions on the level of perceived stress. To do so, a series of N-of-1 trials will be conducted. Each trial consists of two phases: (1) eligibility and baseline questionnaire as well as the four-week N-of-1 trial; and (2) a three-month follow-up period after which participants will be asked to fill out a follow-up questionnaire. In the N-of-1 trial, every participant is randomly assigned to one of two sequences of one-week intervention (A) and control (B) phases (ABAB or BABA). In the intervention phase (A) participants are asked to perform the anti-stress intervention while in control phases (B) they are instructed to not perform the intervention. Since the data is collected anonymously and the participants will be recruited over a period of 1.5 months, the individual follow-up time between baseline- and follow-up questionnaires can slightly differ between participants. A schematic overview of the study design is shown in Figure 1. Results will be reported following the “CONSORT extension for reporting N-of-1 trials (CENT)” guidelines (34). The study center is the Institute of Public Health at the Charité – Universitätsmedizin Berlin. All participants provided informed consent, and the study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Charité- Universitätsmedizin Berlin— approval number EA4/260/23.
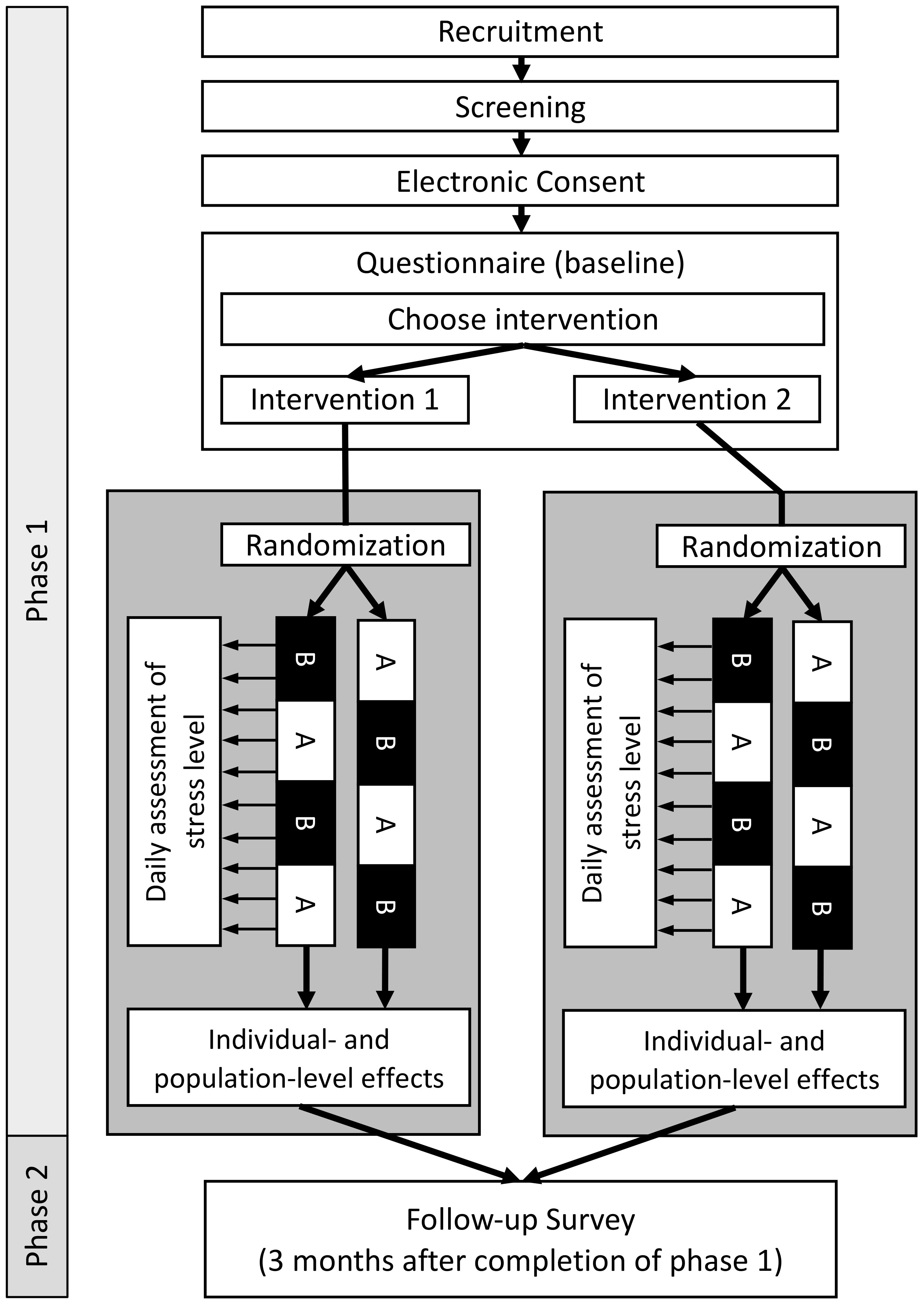
Figure 1. Overview of participant flow and study design. Phase 1: After recruitment and screening for eligibility, participants are asked to give their consent to participate in the study and to fill out the baseline questionnaire. At the end of the questionnaire, participants choose themself which intervention they would like to evaluate. Participants are randomly allocated to one of two possible sequences of intervention (A) and control (B) phases (ABAB or BABA). Phase 2: Three months after completion of Phase 1, participants will be asked to fill out a follow-up questionnaire to assess the sustainability of the intervention.
Participants will be able to choose between two interventions at the beginning of the trial. Both interventions will be provided digitally as video (box breathing) or audio (mindfulness breathing) files. The first intervention, a 6-minute box breathing exercise, is used to reduce stress and is employed, for example, by law enforcement and the military to provide effective stress reduction in dangerous situations (20). Participants are instructed to take some time to relax and sit in a quiet and comforting place. They are then instructed to breathe in for four seconds, hold their breath for four seconds, and breathe out for another four seconds. After holding their breath for four seconds, the next breathing cycle starts. Participants will be provided with a video of a red dot following the outlines of a box at the required speed as a visual aid. The second intervention is a 10-minute guided breathing exercise (audio only) combining mindfulness meditation with simple body movements and breathwork. Participants are instructed to sit in a quiet room and find an upright position. They are then guided through several short exercises that include light upper body stretching and mindful and conscious breathing. Participants are asked to consciously breath in and out and follow the flow of air through their body. As part of the exercise participants should focus on their natural breathing cycle and conscouly experience the calmness they experience as result of the exercise.
The study population is composed of physicians in residence in Germany who work for at least 9 hours per week. Participants will be recruited via email through mailing lists of professional societies and local training centers of the university hospitals in Germany. Interested participants can sign up for a contact list and will subsequently receive the study information as well as a universal access link to the baseline questionnaire per email. Additionally, interested participants can contact the principal investigator via email or phone for further information. Recruitment will be done over the period of 1.5 months, and participants are allowed to start with the study as soon as they consent to study participation.
● Physicians in training in Germany
● Weekly working time in medical activity of at least 9 hours
● Regular access to a smartphone on which the StudyU App can be installed
● Informed consent
● Age <18 years
● Specialist training already completed
● No clinical activity during the study period (e.g. vacation, research activity, etc.)
● Participation in another intervention study during the study period
● Does not speak German
● Does yoga more than 4 times a month
● Meditates or performs breathing exercises on average more than 4 days per month
● Confirmed or suspected pregnancy
● Presence of a psychiatric disorder
● Presence of cardiovascular disease
● Presence of respiratory or pulmonary disease
● Presence of a neurological disease
● Substance abuse (for example, alcohol, drugs, or other)
● Planned surgery within the next 6 months
● Doctor’s recommendation (or self-assessment) not to perform mindfulness or breathing exercises
● Lack of informed consent
● Employee of the Charité - Universitätsmedizin Berlin (due to data protection reasons, employees of the Charité - Universitätsmedizin Berlin will not be included in this study)
The full study is conducted digitally and only anonymized data will be collected. The anti-stress intervention and guidance throughout the study period, as well as the daily assessment of the participant’s reported outcomes (PROs), will be delivered digitally via the StudyU App (35). The StudyU App is a mobile app available for both iOS and Android phones allowing users to participate in N-of-1 trials, get daily reminders of active tasks, record PROs, and receive a summary of their individual results in-app after their trial. It is part of the open-source StudyU platform, which also allows an easy and free design of such trials via the website studyu.health. An overview of the study design and participant flow through the trial is shown in Figure 1, and example screens of the app are shown in Figure 2.
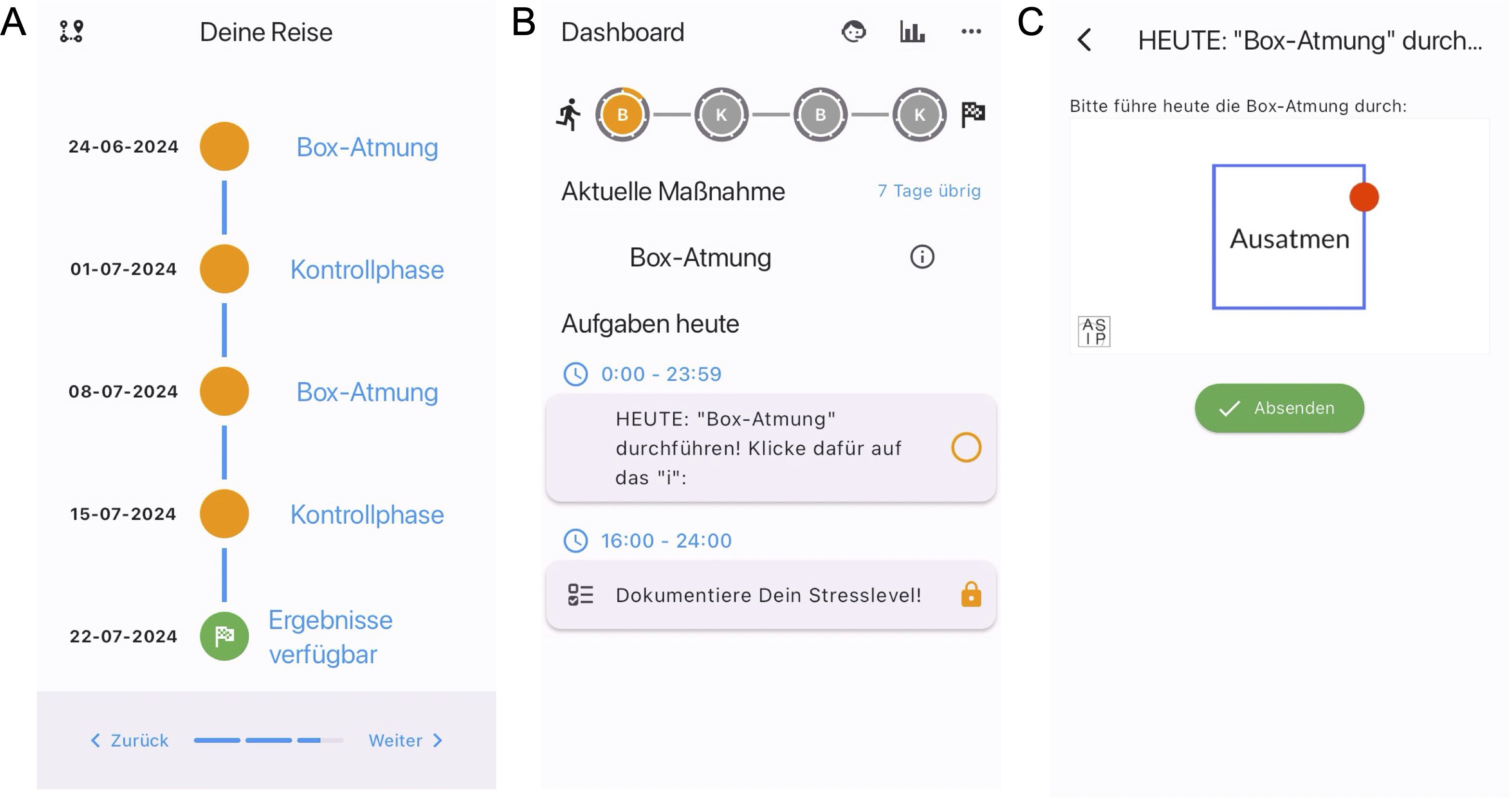
Figure 2. Screenshots of the StudyU-App. In the app, participants are first welcomed to the app, get an overview of its functionality, and have to agree to the terms of use. Then they can select their trial of interest, have to answer and pass eligibility criteria and consent to the study. Next, they are shown an overview of their N-of-1 trial, and after choosing this, they have started their study in which they will see a daily dashboard with tasks. Of the app, the following screens are shown for illustration: (A) Trial overview of N-of-1 trial evaluating box breathing. (B) Participant’s daily tasks in the dashboard. (C) Video intervention (box breathing).
Phase 1: Interested participants will be screened for eligibility and provided with the study information as well as the required consent form via email. If the participants are interested in participating in the study, they can follow a link to the REDCap baseline questionnaire (36) that is provided together with the study documents. After giving consent to participate, the participants are asked to answer the following questions that are used to generate an 8-digit self-generated identification code (SGIC):
● Please enter the first and second letter of your mother’s first name.
● Please enter the first and second letter of your place of birth.
● Please indicate your sex as stated in your birth certificate.
● Please enter the number of your older (not younger!) siblings.
● Please enter the last digit of your parent’s house number.
● Please enter the last digit of your parent’s postal code.
The SGIC is an adapted version of the approach suggested by Schnell and colleagues (37).
After completion of the baseline questionnaire, participants will be provided with an individual linkage code on the final screen of the questionnaire (which is different from the SGIC). This code can be used (1) by the participants to register for the ASIP study in the StudyU App and (2) by the research team to link the data from the baseline questionnaire with data recorded in the StudyU App. The random allocation of participants to intervention sequence ABAB or BABA (where A is intervention and B is control) is displayed in the app to the participants. Daily push notifications will remind participants to conduct the daily anti-stress intervention during the intervention phase. Additional daily reminders will be sent via push notification to remind reporting the outcome. During control phases, the reminders to document the daily stress level will be displayed on the participant’s phone via push notification.
If the minimally required number of PROs was documented, participants will be given access to an automatically generated report with the results of their collected data within the StudyU App after the trial is successfully completed.
Phase 2: Three months after completion of phase 1, participants will be asked to fill out a follow-up questionnaire. At the beginning of this questionnaire, they will be asked to provide their SGIC, which will be used to link it to the baseline questionnaire. The follow-up questionnaire includes the same items as the baseline questionnaire but additionally asks the participants to report on adverse events and if and how the intervention was implemented in everyday life after study completion.
Basic characteristics and additional information about participants will be assessed through digital surveys at baseline and three months after completion of phase 1 using REDCap (Research Electronic Data Capture) (36), a browser-based application designed to manage surveys and databases. Collected information include age, sex, body height and weight, place of residence, work (work hours, speciality, place of work, shift-work), lifestyle variables (alcohol, smoking, sport), and others. Additionally, as part of the N-of-1 trial (Phase 1, Figure 1), daily PROs will be collected with the StudyU App (35).
Primary Outcomes: Participants will be asked to answer the following two questions daily (during both the intervention and control phase) in the StudyU App on a visual analog scale from 1 (“not stressed at all”) to 10 (“extremely stressed out”):
● “Overall, how stressful was your day?”
● “Which level of stress do you expect for the following day?”
Participants will be able to answer these questions after 4 p.m. each day and will be reminded with push notifications if the PRO is not documented. It should be noted that the second question targets the anticipated stress on the following day independently of the intervention effect. This is naturally the case in the control phase, and we assume that participants will also not include the anticipated intervention effect in their anticipated rating of the stress on the following day so that the answers in both phases can be compared. Together with the two questions stated above, participants are asked whether they performed the intervention that day. This item will be used to assess adherence.
Additional secondary outcomes are the level of agreement between expected and actually perceived levels of stress, adherence to study protocol, and successful completion of the study (defined as reporting a minimally required number of PROs (n=16) during phase 1 of the study).
To characterize the study participants and also exploratively investigate potential effect measure modifiers, additional variables are assessed as part of the self-administered surveys at baseline and follow-up. Participants are asked to provide information about age, sex, place of residence, place of work (hospital vs. outpatient/ambulatory setting) regular work hours per week, lifestyle variables (sport, alcohol, smoking), as well as the individual living situation, relationship status and number of children in the household. Additionally, selected questions from the validated German versions of the following standardized and free-to-use questionnaires are used to assess additional psychological variables. These measures will be used for a better description of our study sample and to assess the degree of variation between participants. The instruments used in this study include:
● Copenhagen Psychosocial Questionnaire (COPSOQ, work-life conflicts) (38, 39)
● Copenhagen Burnout Inventory, Personal Burnout (38)
● Effort-reward imbalance questionnaire (ERI) (40, 41)
● Cohen’s Perceived Stress Scale (42, 43)
● Satisfaction with life scale (SWLS) (44)
● System Usability Scale (SUS)
Data will be collected anonymously, i.e., no data is collected that allows to connect the collected data with identifying information at any point in time. Data collected through the REDCap survey at baseline and the data collected in the StudyU App will be linked using a randomly allocated linkage code automatically and digitally provided at the end of the baseline survey. This code is needed to get access to the ASIP study in the StudyU App and will be used to link data from both databases (REDCap and StudyU) after study completion. Data collected with the REDCap survey at baseline is linked with data collected in the follow-up REDCap survey three months after completion of phase 1 by the SGICs that are only known to the participants. Two months after the linkage of the baseline and follow-up questionnaire at the end of the study, the SGICs will be deleted from the data.
Anonymous data from the REDCap surveys will be stored on protected servers of the Charité – Universitätsmedizin Berlin. Anonymous data collected through the StudyU App is saved securely on a backend hosted locally by the Hasso-Plattner-Institute in Potsdam, Germany. Until the SGICs are deleted, all data will be stored password-protected, and only the principal investigator and nominated research staff will have access to the data. After completion of the study and deletion of the SGIC, parts of the data will be published in a public research data repository. Data collected in the StudyU App will be published in the StudyU repository and made openly available.
In this study, the level of stress is assessed between 4 p.m. and the end of the day on each day of the intervention and control phase of the study. If participants are in the intervention phase, they are instructed to only answer the items after they completed the intervention. Participants are instructed to perform the anti-stress intervention via push notification on their mobile phone at 10 a.m. on each day of the intervention phase but are free to perform the intervention whenever they choose. There might not be full adherence to the intervention suggestions. Through StudyU, only information about the availability of the intervention through the pre-specified intervention sequence is available.
Consequently, in this study, we are targeting the causal effect of the availability of each of the two anti-stress interventions among participants who chose the respective intervention prior to the study. In particular, we are interested in the effect of “always performing the intervention” compared to “never performing the intervention.” This constitutes our estimand of interest and under the assumption of full adherence, this estimated causal effect of intervention availability is equal to the effect of the intervention itself. We assume that the treatment effect is constant over time and that there is no effect of time-varying variables throughout the study period. Also, we assume that there are no carry over effects. Participants are specifically instructed to not perform the intervention in the control periods and the audio and video file of the intervention is not available to participants during the control phase. We will calculate individual effects as well as the average effect across participants.
The sample size was calculated for the population effect of the two investigated anti-stress interventions on the daily level of perceived stress assessed on an analog scale between 1 and 10. To calculate the sample size, the approach described by Yang and colleagues (45) was employed, which uses a linear mixed model to estimate sample size in a series of N-of-1 trials. The ShinyApp web application (https://jiabeiyang.shinyapps.io/SampleSizeNof1/) provided by the authors was used, and a fixed intercept and common slope model were assumed, yielding a standard ordinary least squares regression model. We use a fixed intercept for stability reasons of the intervention effect and use a common slope model to provide a robust estimate also for small samples sizes by assuming homogeneous treatment effects for the estimation of population-level effects, see (45) for a detailed description. Calculations were done based on a homogeneous residual standard error of 2.41, which is in line with results published by Lesage and colleagues (46). Based on results published by Naughton and Johnston (47), an autocorrelation between PROs at two adjacent time points (i.e., days) of 0.8 was assumed. For this sample size calculation, an alternating sequence was assumed (ABAB or BABA). Due to the novelty of this study, an estimation of the expected effect size is challenging. Therefore, we conservatively assumed an intervention effect of a standardized mean difference of at least 0.3 points between intervention and control phases, which is 50% of the effect size reported in a meta-analysis of ten studies investigating the effect of mindfulness-based intervention on stress over a longer time period (6 to 12 months) in future health professionals (19). Setting the level of statistical significance as α = 5% and assuming documentation of PROs on at least 4 days per week, a power of 80% will be reached with six participants per sequence (ABAB or BABA), yielding n=12 participants in total for each of the two investigated interventions. Under an assumption of a dropout rate of 30% (so that the full trial data cannot be used), n=17 participants need to be recruited per intervention (n=34 participants in total).
To estimate the estimand of interest described above for each intervention individually, both interventions will be separately analyzed in comparison to the everyday-life control. On the individual level, intervention effects will be estimated using Bayesian repeated-measure models which will include a first-order autoregressive error structure. Autocorrelation of daily stress was observed before (47) and will be considered during the data analysis. For population-level effect estimation, the data from all individual N-of-1 trials will be analyzed together. A Bayesian multilevel repeated-measures model will be used to estimate the posterior distribution of the population-level average intervention effect as well as the within- and between-participant variance. Similarly to the individual-level estimates, the dependency of responses over time will be modeled by a first-order autoregressive error structure. Statistical analyses will be conducted with JAGS (48) and the statistical software package R (49) employing the Markov Chain Monte Carlo (MCMC) method. We will perform an intention-to-treat analysis.
As a secondary analysis, we will perform a joint analysis of both interventions to estimate the effect of the availability of an anti-stress intervention among the participants who chose the respective intervention. Additionally, we plan to use the various measures of psychological variables assessed at baseline for subgroup analyses and exploratory analyses of predictors of intervention effects.
Since the intensity of the breathwork and mindfulness exercises is rather low, we estimate the risk for adverse outcomes to be very low. Nevertheless, in accordance with other studies in the field, a number of exclusion criteria were defined to further minimize the risk of unwanted negative outcomes. As part of the follow-up questionnaire three months after study completion, participants will be asked if and which adverse events were experienced due to the intervention.
The Anti-Stress Intervention Among Physicians (ASIP) study aims to evaluate the effect of two anti-stress interventions on the daily level of perceived stress in physicians in residence in Germany. To assess the efficacy of these interventions, a series of N-of-1 trials will be conducted. Participants can choose between box breathing and a mindfulness-based breathing exercise, thereby studying the effect of their preferred intervention, and will randomly be allocated to a sequence of intervention (A) and control (B) phases (resulting in sequence ABAB or BABA). The study participants will report their perceived stress levels daily through a mobile application. Bayesian analyses will be conducted to estimate the intervention effect and effect of potential effect modifiers such as age, sex, work experience, working hours per week, and others will be investigated.
Strengths of this study include its design (series of N-of-1 trials), which enables the examination of intervention effects on the individual and the population level. The first is of special interest in the case of stress-reducing interventions, as the individual effect is the one that we ultimately want to achieve. Also, for the estimation of effects on the group level, due to repeated assessment of the PROs, a smaller sample size compared to traditional RCTs is sufficient to reach the same statistical power. Both interventions are short and easy to conduct (6 and 10 minutes) and, therefore, easy to implement into everyday life. This potentially increases adherence to the study protocol and long-term use (if proven successful). Additionally, each participant will be given access to their individual N-of-1 trial results. This could potentially motivate participants to use the intervention in their everyday lives even after the completion of the study. Furthermore, by providing two different anti-stress interventions the participants are able to choose an intervention based on individual preferences. This potentially further increases adherence and is also close to a real-life scenario, where interested people are able to choose from a wide variety of anti-stress interventions.
We want to point out several limitations to this study: First, the control phases are general routine in everyday life. Therefore, no placebo control is available, which is a frequent problem in studies evaluating anti-stress interventions (17, 29) and can introduce a bias as the participants might report reduced levels of stress because they expect this as part of the intervention. In this case, a reduction in the daily stress levels would be documented not due to the direct causal effect of the intervention but because of the partcipant’s expectation. However, since we are ultimately interested in the evaluation of the total intervention effect, it could be argued that, although some part of the observed effects might be attributable to the placebo effect, this is acceptable as the intervention itself only introduces a limited burden to the conducting person and adverse outcomes are unlikely. Second, protocol adherence and dropout are expected to be a challenge in this study. Therefore, we aim to limit deviation from the study protocol due to individual guidance through the study procedure as well as the delivery of the intervention and collection of the daily PRO through a mobile application. Furthermore, conservative sample size calculations included a dropout rate of 30%, and the pre-specified power of 80% for the estimation of group effects is reached if PROs are reported on 4 of the 7 days per intervention or control phase. Therefore, a robust study design is in place to deal with incomplete data reporting and high drop-out rates.
High levels of stress are known to be responsible for numerous adverse health outcomes. In the case of physicians, it results in negative outcomes exceeding the individual negative health effects due to increased treatment errors and decreased quality of the patient-doctor relationship. Therefore, if proven effective, the anti-stress interventions investigated in this study could potentially be useful in the sustainable decrease of stress in physicians and thereby improve individual and population-level health.
All participants provided informed consent, and the study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Charité — Universitätsmedizin Berlin, approval number EA4/260/23.
VV: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. TK: Conceptualization, Resources, Supervision, Writing – review & editing. SK: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank Ellen Zitzmann for her valuable input and help in developing the mindfulness-based breathing exercise and recording the intervention audio files. In addition, the authors thank Marco Piccininni for his notes on the design and the statistical analysis plan of this study.
TK reports outside of the submitted work receiving research grants from the Gemeinsamer Bundesausschuss G-BA Federal Joint Committee, Germany and the Bundesministerium für Gesundheit BMG - Federal Ministry of Health, Germany. He has also received personal compensation from Eli Lilly and Company, the BMJ, and Frontiers.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. (2007) 298:1685–7. doi: 10.1001/jama.298.14.1685
2. Kivimäki M, Virtanen M, Elovainio M, Kouvonen A, Väänänen A, Vahtera J. Work stress in the etiology of coronary heart disease—a meta-analysis. Scandinavian J Work Environ Health. (2006) 32:431–42. doi: 10.5271/sjweh.1049
3. Turner AI, Smyth N, Hall SJ, Torres SJ, Hussein M, Jayasinghe SU, et al. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology. (2020) 114:104599. doi: 10.1016/j.psyneuen.2020.104599
4. Regehr C, Glancy D, Pitts A, LeBlanc VR. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J nervous Ment disease. (2014) 202:353–9. doi: 10.1097/NMD.0000000000000130
5. Dyrbye LN, Burke SE, Hardeman RR, Herrin J, Wittlin NM, Yeazel M, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. (2018) 320:1114–30. doi: 10.1001/jama.2018.12615
6. Klein J, Grosse Frie K, Blum K, von dem Knesebeck O. Burnout and perceived quality of care among German clinicians in surgery. Int J Qual Health Care. (2010) 22:525–30. doi: 10.1093/intqhc/mzq056
7. Raspe M, Koch P, Zilezinski M, Schulte K, Bitzinger D, Gaiser U, et al. Arbeitsbedingungen und Gesundheitszustand junger Ärzte und professionell Pflegender in deutschen Krankenhäusern. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2020) 63:113–21. doi: 10.1007/s00103-019-03057-y
8. Dreher A, Theune M, Kersting C, Geiser F, Weltermann B. Prevalence of burnout among German general practitioners: comparison of physicians working in solo and group practices. PloS One. (2019) 14:e0211223. doi: 10.1371/journal.pone.0211223
9. Degen L, Linden K, Seifried-Dübon T, Werners B, Grot M, Rind E, et al. Job satisfaction and chronic stress of general practitioners and their teams: baseline data of a cluster-randomised trial (IMPROVEjob). Int J Environ Res Public Health. (2021) 18:9458. doi: 10.3390/ijerph18189458
10. Tawfik DS, Profit J, Morgenthaler TI, Satele DV, Sinsky CA, Dyrbye LN, et al. Physician burnout, well-being, and work unit safety grades in relationship to reported medical errors. Mayo Clinic Proc. (2018) 93:1571–80. doi: 10.1016/j.mayocp.2018.05.014
11. Owoc J, Mańczak M, Tombarkiewicz M, Olszewski R. Burnout, well being, and self reported medical errors among physicians. Polish Arch Internal Med. (2021) 131:626–32. doi: 10.20452/pamw.16033
12. Firth-Cozens J, Greenhalgh J. Doctors’ perceptions of the links between stress and lowered clinical care. Soc Sci Med. (1997) 44:1017–22. doi: 10.1016/S0277-9536(96)00227-4
13. Yates SW. Physician stress and burnout. Am J Med. (2020) 133:160–4. doi: 10.1016/j.amjmed.2019.08.034
14. Shanafelt T, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. (2017) 177:1826–32. doi: 10.1001/jamainternmed.2017.4340
15. Han S, Shanafelt TD, Sinsky CA, Awad KM, Dyrbye LN, Fiscus LC, et al. Estimating the attributable cost of physician burnout in the United States. Ann Internal Med. (2019) 170:784–90. doi: 10.7326/M18-1422
16. Willard-Grace R, Knox M, Huang B, Hammer H, Kivlahan C, Grumbach K. Burnout and health care workforce turnover. Ann Family Med. (2019) 17:36–41. doi: 10.1370/afm.2338
17. Creswell JD. Mindfulness interventions. Annu Rev Psychol. (2017) 68:491–516. doi: 10.1146/annurev-psych-042716-051139
19. Lu CP, Dijk SW, Pandit A, Kranenburg L, Luik AI, Hunink MM. The effect of mindfulness-based interventions on reducing stress in future health professionals: A systematic review and meta-analysis of randomized controlled trials. Applied Psychology: Health and Well-Being (2023).
20. Röttger S, Theobald DA, Abendroth J, Jacobsen T. The effectiveness of combat tactical breathing as compared with prolonged exhalation. Appl Psychophysiol Biofeedback. (2021) 46:19–28. doi: 10.1007/s10484-020-09485-w
21. Bouchard S, Bernier F, Boivin É, Morin B, Robillard G. Using biofeedback while immersed in a stressful videogame increases the effectiveness of stress management skills in soldiers. PloS One. (2012) 7:e36169. doi: 10.1371/journal.pone.0036169
22. Dillard CC, Martaindale H, Hunter SD, McAllister MJ. Slow breathing reduces biomarkers of stress in response to a virtual reality active shooter training drill. Healthcare. (2023) 11:2351. doi: 10.3390/healthcare11162351
23. Zaccaro A, Piarulli A, Laurino M, Garbella E, Menicucci D, Neri B, et al. How breath-control can change your life: a systematic review on psycho-physiological correlates of slow breathing. Front Hum Neurosci. (2018) 353. doi: 10.3389/fnhum.2018.00353
24. Balban MY, Neri E, Kogon MM, Weed L, Nouriani B, Jo B, et al. Brief structured respiration practices enhance mood and reduce physiological arousal. Cell Rep Med. (2023) 4. doi: 10.1016/j.xcrm.2022.100895
25. Fincham GW, Strauss C, Montero-Marin J, Cavanagh K. Effect of breathwork on stress and mental health: A meta-analysis of randomised-controlled trials. Sci Rep. (2023) 13:432. doi: 10.1038/s41598-022-27247-y
26. Rosenberg A, Hamiel D. Reducing test anxiety and related symptoms using a biofeedback respiratory practice device: A randomized control trial. Appl Psychophysiol Biofeedback. (2021) 46:69–82. doi: 10.1007/s10484-020-09494-9
27. James TA, James D, Larkey LK. Heart-focused breathing and perceptions of burden in Alzheimer’s caregivers: An online randomized controlled pilot study. Geriatric Nursing. (2021) 42:397–404. doi: 10.1016/j.gerinurse.2021.02.006
28. Taylor H, Strauss C, Cavanagh K. Can a little bit of mindfulness do you good? A systematic review and meta-analyses of unguided mindfulness-based self-help interventions. Clin Psychol Review. (2021) 89:102078. doi: 10.1016/j.cpr.2021.102078
29. Boettcher J, Åström V, Påhlsson D, Schenström O, Andersson G, Carlbring P. Internet-based mindfulness treatment for anxiety disorders: a randomized controlled trial. Behav Ther. (2014) 45:241–53. doi: 10.1016/j.beth.2013.11.003
30. Carrington P, Collings GH Jr., Benson H, Robinson H, Wood LW, Lehrer PM, et al. The use of meditation–relaxation techniques for the management of stress in a working population. J Occup Environ Med. (1980) 22:221–31.
31. Goodwin AM, Miller D, D’Angelo S, Perrin A, Wiener R, Greene B, et al. Protocol for randomized personalized trial for stress management compared to standard of care. Front Psychol. (2023) 14. doi: 10.3389/fpsyg.2023.1233884
32. Zainal NH, Newman MG. Mindfulness enhances cognitive functioning: a meta-analysis of 111 randomized controlled trials. Health Psychol Rev. 18(2):369–95. doi: 10.1080/17437199.2023.2248
34. Shamseer L, Sampson M, Bukutu C, Schmid CH, Nikles J, Tate R, et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015: Explanation and elaboration. Bmj. (2015) 350. doi: 10.1136/bmj.h1793
35. Konigorski S, Wernicke S, Slosarek T, Zenner AM, Strelow N, Ruether DF, et al. StudyU: A platform for designing and conducting innovative digital N-of-1 trials. J Med Internet Res. (2022) 24:e35884. doi: 10.2196/35884
36. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed informatics. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
37. Schnell R, Bachteler T, Reiher J. Improving the use of self-generated identification codes. Eval Review. (2010) 34:391–418. doi: 10.1177/0193841X10387576
38. Nübling M, Stößel U, Hasselhorn H, Michaelis M, Hofmann F. Methoden zur Erfassung psychischer Belastungen - Erprobung eines Messinstrumentes (COPSOQ). Bremerhaven, Germany: Wirtschaftsverlag NW (2005).
39. Kristensen TS, Hannerz H, Høgh A, Borg V. The Copenhagen Psychosocial Questionnaire-a tool for the assessment and improvement of the psychosocial work environment. Scandinavian J work Environ Health. (2005) 31(6):438–49. doi: 10.5271/sjweh.948
40. Rödel A, Siegrist J, Hessel A, Brähler E. Fragebogen zur messung beruflicher gratifikationskrisen. Z für Differentielle und Diagnostische Psychologie. (2004) 25:227–38. doi: 10.1024/0170-1789.25.4.227
41. Siegrist J, Wege N, Pühlhofer F, Wahrendorf M. A short generic measure of work stress in the era of globalization: effort–reward imbalance. Int Arch Occup Environ Health. (2009) 82:1005–13. doi: 10.1007/s00420-008-0384-3
43. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. (1983) 24(4):385–96. doi: 10.2307/2136404
44. Janke S, Glöckner-Rist A, Glöckner-Rist A. Deutsche version der satisfaction with life scale (SWLS). Danner D, editor. Mannheim, Germany: Zusammenstellung sozialwissenschaftlicher Items und Skalen Mannheim: GESIS (2014).
45. Yang J, Steingrimsson JA, Schmid CH. Sample size calculations for n-of-1 trials. arXiv preprint arXiv:211008970. (2021). doi: 10.48550/arXiv.2110.08970
46. Lesage F-X, Berjot S, Deschamps F. Clinical stress assessment using a visual analogue scale. Occup Med. (2012) 62:600–5. doi: 10.1093/occmed/kqs140
47. Naughton F, Johnston D. A starter kit for undertaking n-of-1 trials. Eur Health Psychol. (2014) 16:196–205.
48. Depaoli S, Clifton JP, Cobb PR. Just another gibbs sampler (JAGS): flexible software for MCMC implementation. J Educ Behav Statistics. (2016) 41:628–49. doi: 10.3102/1076998616664876
49. Team RDC. R: A language and environment for statistical computing. (2010) Vienna, Austria. Available online at: https://www.R-project.org/.
Keywords: breathwork, mindfulness, box-breathing, stress, N-of-1 trial, physicians, anti-stress intervention, work-related stress
Citation: Vetter VM, Kurth T and Konigorski S (2024) Evaluation of easy-to-implement anti-stress interventions in a series of N-of-1 trials: study protocol of the anti-stress intervention among physicians study. Front. Psychiatry 15:1420097. doi: 10.3389/fpsyt.2024.1420097
Received: 19 April 2024; Accepted: 30 July 2024;
Published: 23 August 2024.
Edited by:
Uffe Kock Wiil, University of Southern Denmark, DenmarkReviewed by:
Frank Vitinius, University Hospital of Cologne, GermanyCopyright © 2024 Vetter, Kurth and Konigorski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentin Max Vetter, dmFsZW50aW4udmV0dGVyQGNoYXJpdGUuZGU=; Stefan Konigorski, c3RlZmFuLmtvbmlnb3Jza2lAaHBpLmRl
†ORCID: Valentin Max Vetter, orcid.org/0000-0001-5003-7766
Tobias Kurth, orcid.org/0000-0001-7169-2620
Stefan Konigorski, orcid.org/0000-0002-9966-6819
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.