
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 28 June 2024
Sec. Autism
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1413961
This article is part of the Research Topic Innovative and Cutting-edge Approaches to the Identification and Management of Autism Spectrum Disorders View all 6 articles
Introduction: Sleep disorders are common in children with autism spectrum disorder (ASD). Transcranial magnetic stimulation (TMS) can influence the excitability of neuronal cells in stimulated areas, leading to improvements in sleep and other autistic symptoms. However, studies on clinical mechanisms of TMS in treating sleep disorders associated with ASD are limited. Therefore, we aimed to explore the effects of TMS on sleep structure and quality in children with ASD.
Methods: Between January 2020 and December 2021, recruitment was advertised through child and adolescent outpatient clinics and online platforms by the Hangzhou Seventh People’s Hospital and Lishui Second People’s Hospital. Sixty children with ASD who met the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, were selected and randomly divided into the active TMS and sham TMS treatment groups. Thirty healthy children of the same age were recruited as controls. The active TMS group received bilateral low-frequency (0.5 Hz) TMS targeting the dorsolateral prefrontal cortex on both sides in children with ASD, whereas the sham TMS group received sham stimulation with the same stimulation time and location as the experimental group. Both groups were treated for 6 weeks, and the participants were assessed using the Sleep Disturbance Scale for Children (SDSC) before treatment, at 3 weeks, and at 6 weeks of intervention. Independent sample t-tests and difference t-tests were used for statistical analysis of the data.
Results: No significant differences were observed in general demographic variables, such as age and sex, between the ASD and control groups (P>0.05). Independent sample t-test analysis showed that the total SDSC score, difficulty falling asleep, sleep maintenance, awakening disorders, sleep-wake transition disorders, excessive daytime sleepiness, and nocturnal hyperhidrosis scores were significantly higher in the ASD group than in the control group (P<0.05). Before treatment, no significant differences were observed in the factor or total SDSC scores between the sham TMS group and the active TMS group (P>0.05). After 15 and 30 treatment sessions, the total SDSC score, difficulty falling asleep, sleep maintenance, sleep-wake transition disorders, and excessive daytime sleepiness scores were significantly higher in the sham TMS group than in the active TMS group (P<0.05). The difference t-test analysis showed that after 30 treatment sessions, the reduction rates of the total SDSC score, difficulty falling asleep, sleep maintenance, awakening disorders, sleep-wake transition disorders, excessive daytime sleepiness, and nocturnal hyperhidrosis dimensions were significantly higher in the active TMS group than in the sham TMS group (P<0.05).
Conclusion: Low-frequency TMS targeting the dorsolateral prefrontal cortex in children with ASD can effectively improve their sleep status, and significant improvement can be achieved after 6 weeks (30 sessions) of treatment.
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition characterized primarily by persistent deficits in social communication and interactions across multiple contexts, along with restricted and repetitive patterns of behavior, interests, and activities (1, 2). According to the 2015 “Report on the Development of Autism Education and Rehabilitation Industry in China,” it is estimated that there may be over 10 million individuals with ASD in China, with > 2 million children aged 0–14 years affected (3). Furthermore, data from the latest National Survey of Children’s Health in the United States in 2016 (4) revealed a prevalence rate of 2.79% among children aged 3–17 years, whereas the prevalence rate of ASD in Chinese children was approximately 0.265% (5).
In addition to core symptoms, children with ASD often exhibit one or more comorbid conditions, including intellectual disability, sleep disorders, epilepsy, and gastrointestinal dysfunction (6, 7). Sleep disorders are particularly common in children with ASD and manifest as prolonged sleep latency, increased sleep arousal, reduced slow-wave sleep, decreased total sleep time, and reduced sleep efficiency. In turn, insufficient sleep exacerbates the core symptoms of ASD (such as repetitive and stereotypical behaviors and social impairments) (8, 9) and other maladaptive behaviors (such as self-injury, aggression, hyperactivity, disobedience, irritability, and emotional issues) (10–15).
Currently, the primary treatment methods for sleep disorders in individuals with ASD include pharmacological therapy, behavioral interventions, and alternative therapies. Although pharmacological therapy and behavioral interventions can improve sleep disorders to varying degrees in children with ASD, there are concerns regarding the potential side effects associated with drug treatment. Additionally, the heterogeneity among children with ASD and the limitations in implementing behavioral interventions, specifically for this population, make it challenging to select the most appropriate intervention measures. Behavioral interventions may not be suitable for younger children. Therefore, there is a pressing need to identify safe, rapid, and tolerable treatment methods to improve sleep quality in this population.
Transcranial magnetic stimulation (TMS) is a neuromodulation technique that uses Faraday’s law of electromagnetic induction to generate time-varying pulsed magnetic fields that penetrate the skull and stimulate the brain. This stimulation alters the membrane potential of neuronal cells in the cerebral cortex, inducing excitatory (at frequencies > 1 Hz, known as high frequency) or inhibitory (at frequencies < 1 Hz, known as low-frequency) effects (16). Research (17–21) has demonstrated that TMS targeting the dorsolateral prefrontal cortex can influence the excitability of neuronal cells in stimulated areas, leading to improvements in anxiety, depression, sleep, repetitive behaviors, and social impairments. However, studies on the clinical mechanisms of TMS in the treatment of sleep disorders associated with ASD are limited.
Therefore, this study aimed to investigate the effects of low-frequency TMS on sleep structure and quality in children with ASD aged 8–15 years who experienced sleep disorders. Specifically, TMS will be used to stimulate the dorsolateral prefrontal cortex bilaterally with the aim of providing evidence for further improving the sleep status of children with ASD.
Between January 2020 and December 2021, recruitment was advertised through child and adolescent outpatient clinics and online platforms at the Seventh People’s Hospital of Hangzhou and the Second People’s Hospital of Lishui. This study recruited 60 children aged 8–15 years with ASD and sleep disorders. Additionally, 30 healthy children in the same age range were recruited as the controls.
The inclusion criteria were as follows: (1) age between 8 and 15 years; (2) right-handedness; (3) no speech, hearing, or vision impairments; (4) no history of severe physical illness or nervous system infection; (5) no use of central nervous system drugs for at least one week before assessment; and (6) fulfillment of the diagnostic criteria for autism in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Exclusion criteria included: (1) inability to complete the intervention tests; (2) history of epilepsy; (3) history of brain injury, brain tumor, encephalitis, or brain metabolic disorders; and (4) presence of metal objects in their bodies. This study was approved by the ethics management committee of the hospital, and patients or their guardians voluntarily agreed to participate. The legal guardians of all the patients included in the study signed informed consent forms.
The Sleep Disturbance Scale for Children (SDSC), which has good reliability and validity (22), was used to assess sleep quality in children aged 6–15 years. Either the child or the main caregiver responded to the questionnaire, and the adults filled out the forms. The SDSC comprises 26 items across six dimensions: difficulties in initiating and maintaining sleep, sleep-disordered breathing, disorders of arousal, sleep-wake transition disorders, daytime excessive sleepiness, and sleep hyperhidrosis (Night Sweats). Higher scores indicate more severe sleep disturbance. Bruni et al. (23) set a total score of 39 as the cutoff value, which corresponds to the upper quartile of the control group. The sensitivity and specificity of this cutoff were 0.89 and 0.74, respectively. Therefore, an SDSC total score > 39 was considered indicative of sleep disturbance.
Before the trial, the researchers underwent training in questionnaire administration and TMS techniques (Transcranial Magnetic Stimulator, S-50, Shenzhen Yingchi Technology Co., Ltd.) to ensure uniform and standardized treatment procedures and minimize measurement bias. During stimulation, the participants wore electrode caps to confirm the stimulation location. Data entry was performed using the double-entry method.
Statistical analysis was conducted using SPSS 25.0. Continuous variables are expressed as mean ± standard deviation (SD). Independent sample t-tests were used to compare the groups. Differences were considered statistically significant at P < 0.05.
Participants with ASD were randomly divided into two groups: the active TMS group, and the sham TMS group, with 30 participants in each group. No statistically significant differences were observed between the groups in terms of various indicators or general characteristics, including age, sex, disease duration, education level, and personality traits. We used the Five Centimeter Rule to identify participants’ DLPFC (24). To ensure data rigor and single-factor variable control, excluding placebo effects, the following intervention methods were adopted:
Normal age-matched control group: No treatment was administered.
The active TMS group: Received real TMS stimulation (100% MT, 0.5 Hz, 300 pulses per side) once daily for 6 weeks (five sessions per week) (25).
The sham TMS group: Received sham TMS stimulation (5% MT, 0.5 Hz, 300 pulses per side) once daily for 6 weeks (five sessions per week).
Assessments were conducted using the SDSC, sleep structure analysis, cortical neural excitability measures, and neural network indicators after 15 and 30 treatment sessions.
Sixty children with ASD were surveyed in this study, with a mean age of 11.20 ± 2.313 years. Among them, 31 were males and 29 were females. Additionally, 30 typically developing children were included, averaging 11.90 ± 2.139 years old, with 12 males and 18 females. Ninety questionnaires were distributed to both the ASD and control groups, with all 90 questionnaires successfully recovered, resulting in 100% questionnaire efficiency. The results indicated no statistically significant differences in age or sex between the ASD and control groups (P>0.05) (Table 1).
Independent sample t-tests were conducted to analyze sleep disorders in children with ASD and typically developing children. The results revealed that the ASD group had significantly higher total SDSC scores, difficulties in falling asleep and maintaining sleep, arousal disorders, sleep-wake transition disorders, excessive somnolence, and nocturnal hyperhidrosis compared to the normal group (P<0.05), as shown in Table 2.
All participants completed 30 sessions of treatment without any dropouts due to adverse effects. Independent sample t-tests were used to compare sleep disorders between the sham TMS group and the active TMS group before treatment. The results showed that there were no significant differences in SDSC total score, difficulties in falling asleep and maintaining sleep, arousal disorders, sleep-wake transition disorders, excessive somnolence, and nocturnal hyperhidrosis between the two groups (P>0.05), as detailed in Table 3.
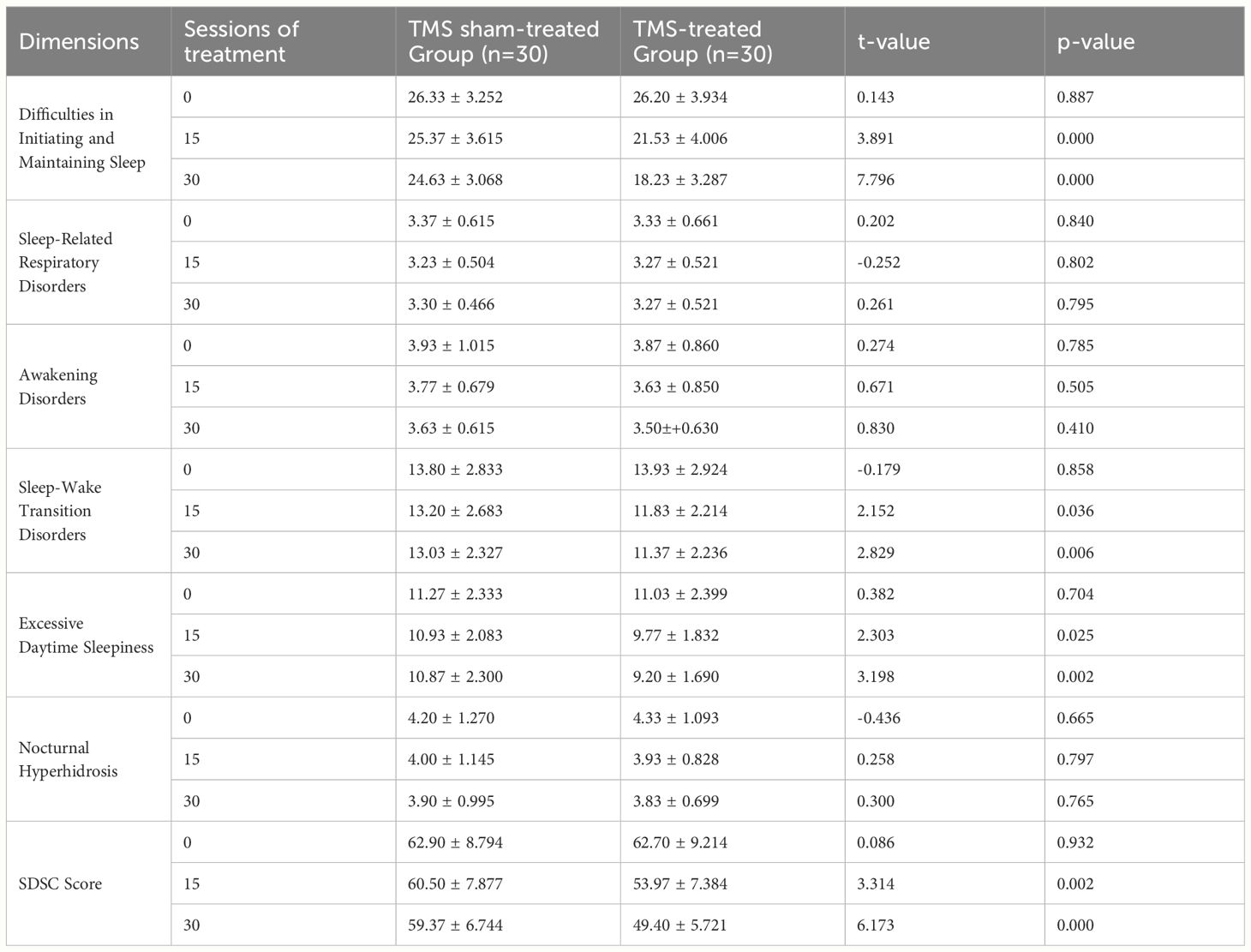
Table 3 Comparison of SDSC scores between the TMS sham-treated and TMS-treated groups after treatment ( ± s).
According to the F and P analysis before and after treatment, the active TMS group showed significant therapeutic effects compared to the sham TMS group, with the effects of Maintaining Sleep, Sleep-Wake Transition Disorders, and Excessive Daytime Sleepiness being particularly significant (P<0.05), as detailed in Tables 4, 5.
Independent sample t-tests were conducted to analyze sleep disorders in the sham TMS group and the active TMS group after 15 and 30 treatment sessions. The findings revealed that the sham TMS group had higher scores in SDSC total score, difficulties in falling asleep and maintaining sleep, sleep-wake transition disorders, and excessive somnolence compared to the active TMS group, with statistically significant differences (P<0.05), as summarized in Table 3.
Difference t-tests were used to assess sleep disorders in the sham and active TMS groups after 30 treatment sessions. The results indicated that the active TMS group had significantly higher reduction rates in SDSC total score, difficulties in falling asleep and maintaining sleep, arousal disorders, sleep-wake transition disorders, excessive somnolence, and nocturnal hyperhidrosis than the sham TMS group (P<0.05) (Table 6)
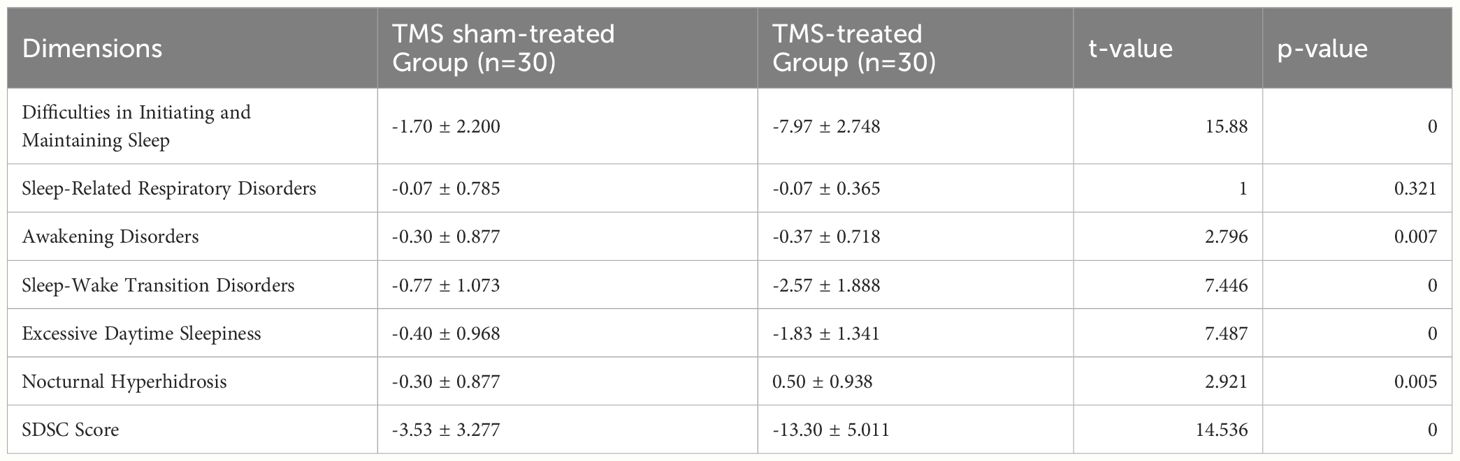
Table 6 Comparison of SDSC score reductions between the TMS sham-treated and TMS-treated groups after 30 sessions of treatment ( ± s).
Adequate sleep is a crucial safeguard for children’s brain development, information processing, cognitive function, memory, and learning capabilities (26). Numerous domestic and international studies have demonstrated that the prevalence of sleep disorders in children with ASD is higher than that in typically developing children, ranging from approximately 44–86% (6, 27–29), while the prevalence in typically developing children is approximately 25–40% (30–32). The prevalence of sleep disorders in children and adolescents with ASD is approximately two to three times higher than that in their typically developing peers. A sleep survey conducted by Li Shiyun et al. in China among 84 children with ASD aged 3–12 years and 91 typically developing children revealed that children with ASD exhibited more difficulties in initiating and maintaining sleep, reduced total sleep time, poorer sleep quality, and night awakenings than typically developing children, which is consistent with the findings of this study.
TMS is a novel neuromodulatory therapy that regulates neural excitability, accelerates synaptogenesis, and modulates neurotransmitter transmission and expression by applying a pulsed magnetic field to the cerebral cortex. In this study, TMS was administered to children with ASD, and the results showed that the total efficacy rate was significantly higher in the active TMS group than in the sham TMS group (P < 0.05). We found that the sleep quality was better after 30 TMS treatment sessions than after 15 sessions, which is consistent with previous studies that reported that 20 TMS treatment sessions showed significant improvement (33). Studies have demonstrated that 6 consecutive weeks (five sessions per week) of low-frequency bilateral TMS stimulation of the dorsolateral prefrontal cortex resulted in significant improvements in sleep structure and functional connectivity strength in different brain regions.
Currently, most studies on insomnia interventions employ low-frequency (1 Hz) TMS to stimulate the right dorsolateral prefrontal cortex (16). In this study, low-frequency (0.5 Hz) TMS was used to stimulate the dorsolateral prefrontal cortex bilaterally in children with ASD. The results revealed improvements in sleep disorders (SDSC factor scores, total scores, and reduction rates) in the active TMS group, whereas no statistically significant changes were observed in the control group that received sham stimulation (P<0.05). This suggests that TMS has a specific effect on patients with ASD and sleep disorders. The therapeutic mechanism may be related to improving the imbalance between excitation and inhibition commonly observed in patients with ASD and enhancing the excitation-inhibition ratio (34–36). Foreign studies have suggested that low-frequency stimulation of the right dorsolateral prefrontal cortex can improve sleep through mechanisms such as increased serum levels of brain-derived neurotrophic factor and gamma-aminobutyric acid and reduced motor-evoked potentials.
This study had some limitations, such as its relatively small sample size and focus only on the dorsolateral prefrontal cortex. Future studies should address these shortcomings. The age range of the participants was set at 8–15 years, without any subgroup analyses regarding the possible effects of puberty. These limitations will be addressed in future studies.
In summary, low-frequency bilateral TMS stimulation of the dorsolateral prefrontal cortex in children with ASD can effectively improve their sleep status, and significant improvements can be achieved after 6 weeks (30 sessions). Behavioral interventions are commonly used to treat sleep disorders in children with ASD. However, given the individual differences and potential side effects of pharmacological treatments, they are not recommended as first-line options unless the sleep disorder symptoms are severe. TMS is a novel, painless, side effect-free, safe, and effective alternative therapy that has been shown to have significant effects on sleep disorders. Therefore, TMS therapy may be considered for the treatment of sleep disorders in children with ASD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of Lishui Second People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
JY: Conceptualization, Data curation, Writing – review & editing. YZ: Conceptualization, Funding acquisition, Writing – original draft. JW: Data curation, Methodology, Writing – original draft, Writing – review & editing. GZ: Data curation, Funding acquisition, Investigation, Writing – review & editing. KF: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. From January 1st, 2020 to December 31st, 2022, YZ and GZ has received sponsorship from LISHUI SCIENCE & TECHNOLOGY BUREAU, and Lishui Second People’s Hospital under Grant 2020RC05.
We thank the participants and families who participated. Special thanks to Haoyu Xing for their help with the formatting.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. The Association AP. Diagnostic and statistical manual of mental disorders, 5th Edition: DSM-5. Int J Offender Ther Comp Criminol. (2013) 57:1546–8.
2. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. (2018) 392:508–20. doi: 10.1016/S0140-6736(18)31129-2
3. WUCAILU Children Behavior Correction Center. Report on the development of autism education and rehabilitation industry in China. Building E2, Huateng Century Headquarters Park, Chaoyang District, Beijing: Beijing Normal University Press (2015).
4. Xu G, Strathearn L, Liu B, O’Brien M, Kopelman TG, Zhu J, et al. Prevalence and treatment patterns of autism spectrum disorder in the United States. JAMA Pediatr. (2019) 173:153–9. doi: 10.1001/jamapediatrics.2018.4208
5. Xian L, Sui Fang L, Wen-xiong C, Fan-fan C, Song-ying S, Xiu Q. Prevalence of autism spectrum disorders among children in China: a systematic review and meta-analysis. Chin J Child Health Care. (2018) 26:402–6.
6. Souders MC, Zavodny S, Eriksen W, Sinko R, Connell J, Kerns C, et al. Sleep in children with autism spectrum disorder. Curr Psychiatry Rep. (2017) 19:34. doi: 10.1007/s11920-017-0782-x
7. Jinming H, Xinghua L, Hongzhu D, Kaiyun C, Xiaobing A, et al. Investigation of sleep quality in children with autism spectrum disorder. J New Med. (2017) 48:99–103.
8. Park S, Cho S-C, Cho IH, Kim B-N, Kim J-W, Shin M-S, et al. Sleep problems and their correlates and comorbid psychopathology of children with autism spectrum disorders. Res Autism Spectr Disord. (2012) 6:1068–72. doi: 10.1016/j.rasd.2012.02.004
9. Tudor ME, Hoffman CD, Sweeney DP. Children with autism: sleep problems and symptom severity. Focus Autism Dev Disabil. (2012) 27:254–62. doi: 10.1177/1088357612457989
10. Henderson JA, Barry TD, Bader SH, Jordan SS. The relation among sleep, routines, and externalizing behavior in children with an autism spectrum disorder. Res Autism Spectr Disord. (2011) 5:758–67. doi: 10.1016/j.rasd.2010.09.003
11. Goldman SE, McGrew S, Johnson KP, Richdale AL, Clemons T, Malow BA. Sleep is associated with problem behaviors in children and adolescents with autism spectrum disorders. Res Autism Spectr Disord. (2011) 5:1223–9. doi: 10.1016/j.rasd.2011.01.010
12. Matson JL, Ancona M, Wilkins J. Sleep disturbances in adults with autism spectrum disorders and severe intellectual impairments. J Ment Health Res Intellect Disabil. (2008) 1:129–39. doi: 10.1080/19315860801988210
13. Malow B, Marzec M, McGrew S, Wang L, Henderson L, Stone W. Characterizing sleep in children with autism spectrum disorders: a multi-dimensional approach. Sleep. (2006) 29:15–63. doi: 10.1093/sleep/29.12.1563
14. Schreck KA, Mulick JA, Smith AF. Sleep problems as possible predictors of intensified symptoms of autism. Res Dev Disabil. (2004) 25:60–6. doi: 10.1016/j.ridd.2003.04.007
15. Williams PG, Sears LL, Allard AM. Sleep problems in children with autism. J Sleep Res. (2004) 13:265–8. doi: 10.1111/j.1365-2869.2004.00405.x
16. Peterman JS, Carper MM, Elkins RM, Comer JS, Pincus DB, Kendall PC, et al. The effects of cognitive-behavioral therapy for youth anxiety on sleep problems. J Anxiety Disord. (2016) 37:78. doi: 10.1016/j.janxdis.2015.11.006
17. Sokhadze EM, El-Baz AS, Sears LL, Opris I, Casanova MF. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front Syst Neurosci. (2014) 8:134. doi: 10.3389/fnsys.2014.00134
18. Scalise A, Pittaro-Cadore I, Serafini A, Simeoni S, Fratticci L, Ecoretti E, et al. Transcranial magnetic stimulation in sleep fragmentation: a model to better understand sleep disorders. Sleep Med. (2014) 15:1386–91. doi: 10.1016/j.sleep.2014.06.007
19. Sánchezescandón O, Aranalechuga Y, Teránpérez G, Ruiz-Chow A, Esqueda-Leon E, González-Robles RO, et al. Transcranial magnetic stimulation improves sleep parameters in patients affected with insomnia associated to electroencephalographic abnormalities. Neurosci Med. (2014) 5:72–7. doi: 10.4236/nm.2014.51010
20. Achterberg M. Repetitive transcranial magnetic stimulation as a possible new treatment strategy to improve social skills in autism spectrum disorder. University of Utrecth, Utrecth (2014). 30 p.
21. Concetta PM, Susanna C, Cristina M, Bignotti S, Gazzoli A, Miniussi C, et al. Dorsolateral prefrontal transcranial magnetic stimulation in patients with major depression locally affects alpha power of REM sleep. Front Hum Neurosci. (2013) 7:433. doi: 10.3389/fnhum.2013.00433
22. Huang MM, Qian Z, Wang J, Vaughn MG, Lee YL, Dong G. Validation of the Sleep Disturbance Scale for Children and prevalence of parent reported sleep disorder symptoms in Chinese children. Sleep Med. (2014) 15:923–8. doi: 10.1016/j.sleep.2014.03.023
23. Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, et al. The sleep disturbance scale for children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. (1996) 5:251–61. doi: 10.1111/j.1365-2869.1996.00251.x
24. George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. (1995) 6:1853–6. doi: 10.1097/00001756-199510020-00008
25. Tang Y, Wu Y, Wang J. Clinical application and operational standards of repetitive transcranial magnetic stimulation: Shanghai Expert Consensus. Shanghai Med J. (2022) 45:65–70. doi: 10.19842/j.cnki.issn.0253-9934.2022.02.01
26. Xiaoming S, Fan J, Shenghui L, Yan C, Tian Y, Wu Z-Q, et al. Study on the effect of sleep on children's growth development and its application. J Shanghai Jiao Tong Univ (Medical Science). (2012) 32:1209–13. doi: CNKI:SUN:SHEY.0.2012-09-016
27. Ravi G, Deepak G, Kandpal DS, Nidhi M, Mohan D, Manish M, et al. Prevalence of sleep disorders among primary school children. Indian J Pediatr. (2016) 83:1232–6. doi: 10.1007/s12098-016-2138-7
28. Van der Heijden KB, Stoffelsen RJ, Popma A, Swaab H. Sleep, chronotype, and sleep hygiene in children with attention-deficit/hyperactivity disorder, autism spectrum disorder, and controls. Eur Child Adolesc Psychiatry. (2018) 27:99–111. doi: 10.1007/s00787-017-1025-8
29. Mazurek MO, Sohl K. Sleep and behavioral problems in children with autism spectrum disorder. J Autism Dev Disord. (2016) 46:1906–15. doi: 10.1007/s10803-016-2723-7
30. Levin A, Scher A. Sleep problems in young children with autism spectrum disorders: A study of parenting stress, mothers' sleep-related cognitions, and bedtime behaviors. CNS Neurosci Ther. (2016) 22:921–7. doi: 10.1111/cns.12651
31. Yunxiao C, Rutayisire E, Xiaoyan W, Huang WP, Yu SH, Chen HY, et al. Emotional and behavioral problems associated with sleep problems in preschool aged children. Chin J Epidemiol. (2017) 38:1191–6.
32. Zhong C, Bin Z, Lingzhi Q, Yaqi Z, Lin Q. A study on the influencing factors of sleep disorders in school-age children. Chin J Pract Pediatrics. (2013) 28:221–3. doi: CNKI:SUN:ZSEK.0.2013-03-025
33. Wenhua W, We F, Yanling C, Qiurong L, Yuying H, Bihua G, et al. Observation of the curative effect of repetitive transcranial magnetic stimulation on sleep disorders in children with autism spectrum disorder. Chin J Rehabil Med. (2022) 37:928–32.
34. Jiang B, He D, Guo Z, Mu Q, Zhang L. Efficacy and placebo response of repetitive transcranial magnetic stimulation for primary insomnia. Sleep Med. (2019) 63:9–13. doi: 10.1016/j.sleep.2019.05.008
35. Port RG, Oberman LM, Roberts TP. Revisiting the excitation/inhibition imbalance hypothesis of ASD through a clinical lens. Br J Radiol Suppl. (2019) 92:20180944. doi: 10.1259/bjr.20180944
Keywords: autism spectrum disorder, transcranial magnetic stimulation, sleep quality, dorsolateral prefrontal cortex, children
Citation: Yan J, Zhang Y, Wang J, Zhu G and Fang K (2024) Effects of transcranial magnetic stimulation on sleep structure and quality in children with autism. Front. Psychiatry 15:1413961. doi: 10.3389/fpsyt.2024.1413961
Received: 19 April 2024; Accepted: 18 June 2024;
Published: 28 June 2024.
Edited by:
Dongchuan Yu, Southeast University, ChinaCopyright © 2024 Yan, Zhang, Wang, Zhu and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhang, ODc2MjIyMDUyQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.