Introduction
The presence of agitation in dementia patients with Alzheimer’s Disease (AD) is a complex behavioral phenomenon that arises as a result of the progression of the disease. According to reports, the prevalence of agitation in mild cognitive impairment and AD is 60% and 76%, respectively (1). The exact cause of agitation in AD remains unclear; however, various pathological cellular events have been linked to it. These include the upregulation of neuroinflammation in specific regions of the brain (2), the involvement of inflammasomes -3 (3), the reduction of CB1 receptor (CB1r) functionality, and imbalances in brain neurotransmitters (4). The development of therapeutic approaches that target these hallmarks suggests the potential effect of reducing agitated behavior in clinical settings. This opinion will explore agitation in AD by describing the molecular basis of agitation and how therapeutic agents THC and melatonin reduce agitation by targeting neuroinflammation, CB1r imbalance, and neurotransmitter imbalances.
Agitation arising from specific brain regions
Recent positron emission tomography (PET) studies in AD patients have demonstrated that the upregulation of inflammation in the brain’s frontal and medial temporal regions is associated with agitation behavior in AD patients (2). Research has explored the mechanism underlying the onset of agitation, revealing associations with structural and functional deficits in key brain regions, including the frontal cortex (FC), the anterior cingulate cortex (ACC), the posterior cingulate cortex (PCC), the insula, and the hippocampus (5). These structures are associated with cognitive processes, decision-making, motivation, emotion processing, memory, and spatial navigation (6). CB1r is essential in regulating emotional behaviors, such as anxiety and fear, and cognitive functions, such as memory (7). The brain regions associated with agitation and other behaviors within the spectrum of hyperactivity syndrome (8) are rich in the CB1 receptors gene and protein expression (9), suggesting the role of endocannabinoids in regulating these behaviors. Equally important, the damage in these same regions is associated with hyperarousal in insomnia patients (10). In AD, aggressive behaviors have been also linked to circadian disturbance and the melatonin system (11).
Neuroinflammation regulation by cannabinoids
The endocannabinoid system is a crucial player in neuroinflammation regulation. This system is composed of two G-proteins coupled cannabinoid receptors, CB1r and CB2r. Endogenous cannabinoids, such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG), are produced from lipid precursors on demand and act as retrograde transmitters in neuron-to-neuron communication as well as mediate neuron-glia interaction. They are also involved in intracellular signaling that regulates mitochondrial activity (12). CB1r, the most prevalent receptor in the brain, and is primarily found in neurons. In contrast, CB2r expression is minimal, and it is primarily found in glial cells. The local CB1r activation in the hippocampus is crucial for sustaining neurogenesis, mitigating neuroinflammation, and averting cognitive impairment (12). The loss of CB1r in the brain may lead to elevated neuroinflammation due to the disruption of CB1r’s communication with CB2r (12).
Neurotransmitter imbalance stability by cannabinoids
The endocannabinoid system plays a significant role in regulating neurotransmitter release and their receptor function. Studies have revealed that deficits in the GABAergic and serotonergic functions are associated with agitation in AD (4). Specifically, reduced GABA levels/transmission and altered serotonergic system functions, which are linked to the loss of serotonergic neurons in the raphe nuclei and their projections to the cortex (mainly PFC) and the amygdala may be implicated in associated agitation behavior in AD (4, 13, 14). In normal conditions, the GABA/Glutamate ratios are inversely correlated in the salience network (SN) and the default mode network (DMN) of the brain, suggesting that the levels of GABA in the SN play a role in the resting state functional connectivity of the SN and its interactions with the DMN (15). The SN contributes to various brain functions such as communication, social behavior, and self-awareness, which is possible through the integration of sensory, emotional, and cognitive information, and the DMN refers to areas in the brain activated when people’s mind wander at rest (16). Research suggests that in pathological conditions, the SN excitatory/inhibitory interactions get dysregulated, as reflected by the reduction of the GABAergic inhibitory signaling (17) and the dysregulation of serotonin function (18). Studies have shown that cannabinoid agonists stimulate [3H]GABA release by activating the CB1r in rats (19). Additionally, they have also been shown to impair the functionality of 5-HT1A and 5-HT2A/C receptors CB1r knockout mice (18). These findings suggest that the activation of CB1r using CB1r agonist will regulate GABA release and stabilize the serotonergic receptor functionality without affecting the 5-HT levels. CB1r partial agonist, THC, may provide a therapeutic effect against agitation.
Melatonin role in aggressive behavior
Melatonin is a neurohormone synthesized in the pineal gland from its precursor serotonin. Several animal studies have supported the idea that melatonin or melatonin receptor agonists reduce aggressive behaviors in various animal paradigms (11). Elderly persons have decreased levels of melatonin in CSF, blood, saliva, and urine, and patients with AD may even have more declined levels of melatonin than control subjects (20). This may be due to the reduction in functional pineal gland volume and suprachiasmatic nucleus cells (20). The clinical studies have shown mixed results on the efficacy of melatonin on agitation due to variability in dosing (11).
Role of inflammasomes-3 in aggressive behavior and inhibition by THC and melatonin
Chronic neuroinflammation has been shown to be a major factor in the pathophysiology of AD by several studies (21). One of the most important molecular connections in the AD neuroinflammatory pathway is the nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 3 (NLRP3) inflammasome (21). The NLRP3 inflammasome-driven inflammatory response has been implicated in aggressive behavior in animals (3). An animal study demonstrated that the NLRP3 inflammasome-driven inflammatory response contributed to resident intruder paradigm-induced aggressive behavior in mice (3). Both CB1r partial agonist THC (22) and melatonin (23) inhibit inflammasome -3 in-vitro models. These studies imply that both THC and melatonin attenuate inflammasome activity and bring beneficial effects on aggressive behavior, suggesting a potential path for therapeutic intervention.
Protection against aggressive behavior by CB1r agonist
CB1r knockout mice display aggressive behaviors, but the administration of the CB1r agonist Arachidonyl-2’-chloroethylamide (ACEA; 2 mg/kg) to these mice has been shown to decrease their aggression behavior significantly (24). These results suggest the involvement of CB1r in aggressive behavior and social interaction (24). The loss of CB1r has been linked to aggressive behavior in CB1r knockout mice, but research suggests that this behavior was corrected with acute administration of CB1r agonists (24). It is also worth noting that reduced hippocampal CB1r activity was reported in AD patients (25).
Decrease in agitation behavior in AD by THC and melatonin
Several off-label studies on synthetic THC and medicinal cannabis oil containing THC (26–28) have demonstrated a significant reduction in agitation behavior in AD. A study comprising 17 melatonin trials that ranged in dosage from 3 to 10 mg over 10 days to 35 months showed improvement in agitation behaviors in AD (29). It concluded that longer trials on melatonin duration had the most improvement in agitation behaviors, further reinforcing confidence in the potential of these therapeutic agents. Several off-label antipsychotic drugs and the recent FDA-approved brexiprazole drug have been used to treat agitation in AD, however, the complete mode of action is not clear (30). The effects of brexpiprazole on serotonin and dopamine receptors are considered to be the mechanism of action (31). Brexpiprazole exhibits partial agonism on some dopamine and serotonin receptors while antagonistically acting on other serotonin receptors, suggesting that its receptor selectivity is advantageous (31). Combinational therapy of THC and melatonin for agitation behavior provides a synergetic effect on neurobiological pathways and may bring out early therapeutic effects.
Discussion
The proposed action of THC and melatonin displays a synergistic effect on mitigating various neuroinflammatory pathways, neurotransmitter imbalances, and CB1r imbalances, all hallmarks implicated in the development of agitated behavior in the Alzheimer’s brain (Figure 1). The potential of this combination is expected to stabilize mood, as seen in its effect on GABA levels by both THC and melatonin (19, 32). In support of this notion, a case study with Gabapentin showed positive results that suggested the reduction of agitation behavior in AD (33). However, these assertions are constrained by certain limitations, such as a non-randomized trial design, a limited sample size, and the lack of placebo-controlled studies. Accumulating evidence supports the role of GABA as an anti-inflammatory agent (34), further indicating a more indirect effect on the reduction of agitation as promoted by the combination of THC and melatonin. As reported in previous experiments with animal models, the administration of CB1r agonists has also reported decreased levels of aggression (24), suggesting a positive effect for treating agitation in AD. The combination with melatonin will likely display longer-term reductions of agitation, opening new possibilities for therapeutic interventions. In line with this notion, there are seven cannabis-based trials related to dementia that are currently listed on clinicaltrials.gov. One is on general neuropsychiatric symptoms, while the other six are on agitation. The compounds used in the experiments include synthetic THC, CBD, THC-CBD, and a THC+CBD+CBC combination (35). As research in this field continues to evolve, the synergistic effects observed between THC and melatonin may present them as potential candidates for developing treatments that could significantly contribute to the management of mood-related disorders. Different pathways may be involved in bringing out the beneficial effects that THC and melatonin target, and more extensive placebo-controlled studies are warranted to determine the efficacy of combinational therapy of THC and melatonin, which will be critical when including the effect of metabolism. The cytochrome 2C9 enzyme is polymorphic and is responsible for the most significant metabolism of THC (36). Since cytochrome 2C9 is polymorphic, it impacts the effectiveness of THC and should be carefully titrated when administering THC dosages. In a recent pilot study on the safety and tolerability of multiple ascending doses of THC and melatonin in patients with AD, no serious adverse events or deaths were reported. However, a larger sample study is needed to fully understand the long-term effects of THC and melatonin on potential adverse events on these groups (37). The development of therapeutic agents containing CB1r agonist and melatonin may provide an efficient therapeutic effect in treating agitation in AD patients. Further trials are warranted to examine their effect on these unmet AD behavioral conditions.
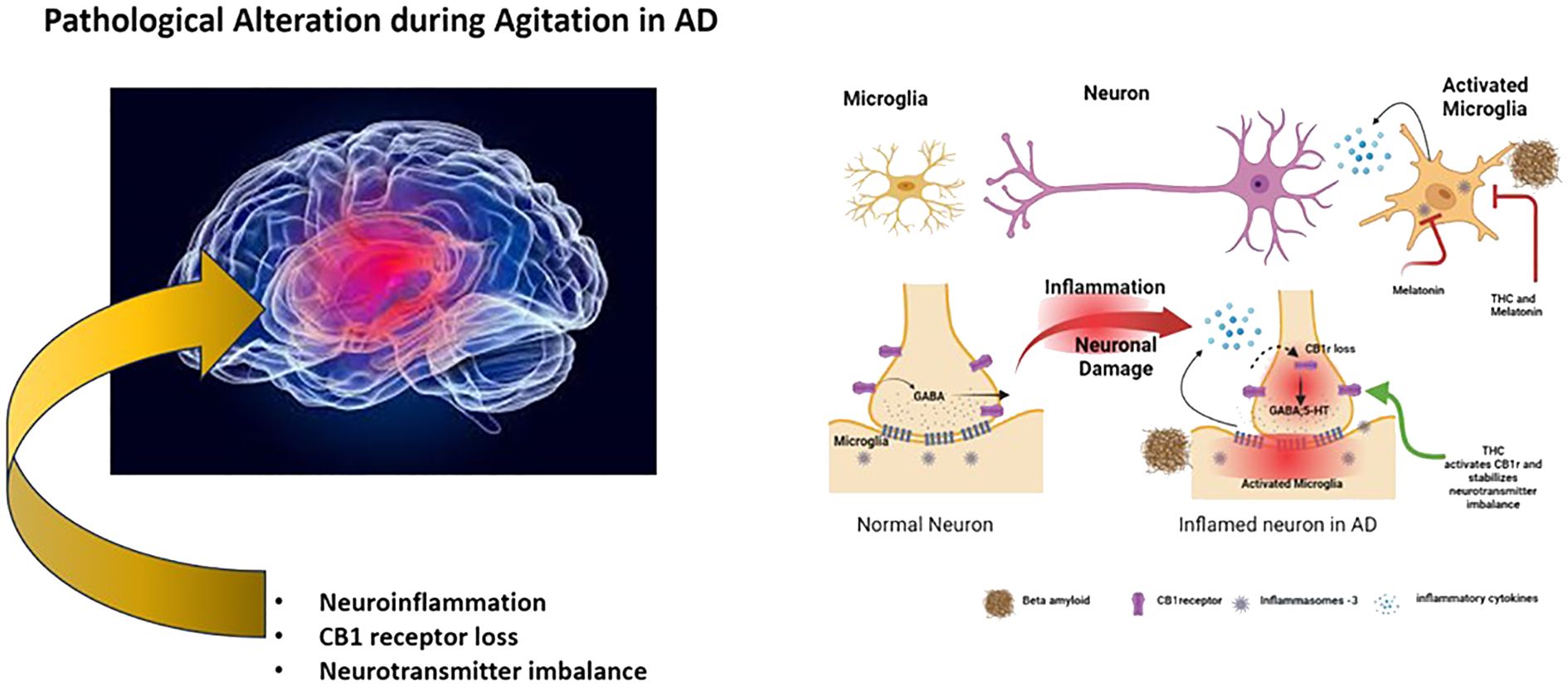
Figure 1 Schematic representation of pathological alterations in agitation of AD and its intervention by THC and melatonin.
Author contributions
JR: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing. MT: Writing – review & editing. RM: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by IGC Pharma LLC, MD, USA.
Conflict of interest
Authors JR, MT and RM are employed by the company IGC Pharma LLC and declare that this study received funding from IGC Pharma LLC. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Van der Mussele S, Le Bastard N, Saerens J, Somers N, Marien P, Goeman J, et al. Agitation-associated behavioral symptoms in mild cognitive impairment and Alzheimer’s dementia. Aging Ment Health. (2015) 19:247–57. doi: 10.1080/13607863.2014.924900
2. Yasuno F, Kimura Y, Ogata A, Ikenuma H, Abe J, Minami H, et al. Involvement of inflammation in the medial temporal region in the development of agitation in Alzheimer’s disease: an in vivo positron emission tomography study. Psychogeriatrics. (2023) 23:126–35. doi: 10.1111/psyg.12915
3. Yu Q, Liu M, Dai W, Xiong Y, Mu X, Xia M, et al. The NLRP3 inflammasome is involved in resident intruder paradigm-induced aggressive behaviors in mice. Front Pharmacol. (2023) 14:974905. doi: 10.3389/fphar.2023.974905
5. Rosenberg PB, Nowrangi MA, Lyketsos CG. Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol Aspects Med. (2015) 43-44:25–37. doi: 10.1016/j.mam.2015.05.005
6. Rolls ET. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct. (2019) 224:3001–18. doi: 10.1007/s00429-019-01945-2
7. Rolls ET. The cingulate cortex and limbic systems for action, emotion, and memory. Handb Clin Neurol. (2019) 166:23–37. doi: 10.1016/B978-0-444-64196-0.00002-9
8. Balthazar ML, Pereira FR, Lopes TM, da Silva EL, Coan AC, Campos BM, et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp. (2014) 35:1237–46. doi: 10.1002/hbm.22248
9. Pak K, Kantonen T, Pekkarinen L, Nuutila P, Nummenmaa L. Association of CNR1 gene and cannabinoid 1 receptor protein in the human brain. J Neurosci Res. (2023) 101:327–37. doi: 10.1002/jnr.25149
10. Chen Y, Dang M, Zhang Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: a systematic review of symptom-general and -specific lesion patterns. Mol Neurodegener. (2021) 16:38. doi: 10.1186/s13024-021-00456-1
11. Paribello P, Manchia M, Bosia M, Pinna F, Carpiniello B, Comai S. Melatonin and aggressive behavior: A systematic review of the literature on preclinical and clinical evidence. J Pineal Res. (2022) 72:e12794. doi: 10.1111/jpi.12794
12. Palmisano M, Gargano A, Olabiyi BF, Lutz B, Bilkei-Gorzo A. Hippocampal deletion of CB1 receptor impairs social memory and leads to age-related changes in the hippocampus of adult mice. Int J Mol Sci. (2022) 24:2. doi: 10.3390/ijms24010026
13. Chakraborty S, Lennon JC, Malkaram SA, Zeng Y, Fisher DW, Dong H. Serotonergic system, cognition, and BPSD in Alzheimer’s disease. Neurosci Lett. (2019) 704:36–44. doi: 10.1016/j.neulet.2019.03.050
14. Rodriguez JJ, Noristani HN, Verkhratsky A. The serotonergic system in ageing and Alzheimer’s disease. Prog Neurobiol. (2012) 99:15–41. doi: 10.1016/j.pneurobio.2012.06.010
15. Levar N, Van Doesum TJ, Denys D, Van Wingen GA. Anterior cingulate GABA and glutamate concentrations are associated with resting-state network connectivity. Sci Rep. (2019) 9:2116. doi: 10.1038/s41598-018-38078-1
16. Buckner RL. The brain’s default network: origins and implications for the study of psychosis. Dialogues Clin Neurosci. (2013) 15:351–8. doi: 10.31887/DCNS.2013.15.3/rbuckner
17. Xu Y, Zhao M, Han Y, Zhang H. GABAergic inhibitory interneuron deficits in alzheimer’s disease: implications for treatment. Front Neurosci. (2020) 14:660. doi: 10.3389/fnins.2020.00660
18. Mato S, Aso E, Castro E, Martin M, Valverde O, Maldonado R, et al. CB1 knockout mice display impaired functionality of 5-HT1A and 5-HT2A/C receptors. J Neurochem. (2007) 103:2111–20. doi: 10.1111/j.1471-4159.2007.04961.x
19. Gonzalez B, Paz F, Floran L, Aceves J, Erlij D, Floran B. Cannabinoid agonists stimulate [3H]GABA release in the globus pallidus of the rat when G(i) protein-receptor coupling is restricted: role of dopamine D2 receptors. J Pharmacol Exp Ther. (2009) 328:822–8. doi: 10.1124/jpet.108.145425
20. Nous A, Engelborghs S, Smolders I. Melatonin levels in the Alzheimer’s disease continuum: a systematic review. Alzheimers Res Ther. (2021) 13:52. doi: 10.1186/s13195-021-00788-6
21. Liang T, Zhang Y, Wu S, Chen Q, Wang L. The role of NLRP3 inflammasome in alzheimer’s disease and potential therapeutic targets. Front Pharmacol. (2022) 13:845185. doi: 10.3389/fphar.2022.845185
22. Suryavanshi SV, Zaiachuk M, Pryimak N, Kovalchuk I, Kovalchuk O. Cannabinoids alleviate the LPS-induced cytokine storm via attenuating NLRP3 inflammasome signaling and TYK2-mediated STAT3 signaling pathways in vitro. Cells. (2022) 11:6–7. doi: 10.3390/cells11091391
23. Arioz BI, Tarakcioglu E, Olcum M, Genc S. The role of melatonin on NLRP3 inflammasome activation in diseases. Antioxidants (Basel). (2021) 10. doi: 10.3390/antiox10071020
24. Rodriguez-Arias M, Navarrete F, Daza-Losada M, Navarro D, Aguilar MA, Berbel P, et al. CB1 cannabinoid receptor-mediated aggressive behavior. Neuropharmacology. (2013) 75:172–80. doi: 10.1016/j.neuropharm.2013.07.013
25. Manuel I, Gonzalez de San Roman E, Giralt MT, Ferrer I, Rodriguez-Puertas R. Type-1 cannabinoid receptor activity during Alzheimer’s disease progression. J Alzheimers Dis. (2014) 42:761–6. doi: 10.3233/JAD-140492
26. Walther S, Schupbach B, Seifritz E, Homan P, Strik W. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia. J Clin Psychopharmacol. (2011) 31:256–8. doi: 10.1097/JCP.0b013e31820e861c
27. Woodward MR, Harper DG, Stolyar A, Forester BP, Ellison JM. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. (2014) 22:415–9. doi: 10.1016/j.jagp.2012.11.022
28. Shelef A, Barak Y, Berger U, Paleacu D, Tadger S, Plopsky I, et al. Safety and efficacy of medical cannabis oil for behavioral and psychological symptoms of dementia: an-open label, add-on, pilot study. J Alzheimers Dis. (2016) 51:15–9. doi: 10.3233/JAD-150915
29. Joseph BlaisMonica ZZ, Sadowski C. Treatment options for sundowning in patients with dementia. Ment Health Clinician. (2014) 4:189–95. doi: 10.9740/mhc.n204525
30. Carrarini C, Russo M, Dono F, Barbone F, Rispoli MG, Ferri L, et al. Agitation and dementia: prevention and treatment strategies in acute and chronic conditions. Front Neurol. (2021) 12:644317. doi: 10.3389/fneur.2021.644317
31. Eaves S, Rey JA. Brexpiprazole (Rexulti): A new monotherapy for schizophrenia and adjunctive therapy for major depressive disorder. P T. (2016) 41:418–22.
32. Rosenstein RE, Cardinali DP. Melatonin increases in vivo GABA accumulation in rat hypothalamus, cerebellum, cerebral cortex and pineal gland. Brain Res. (1986) 398:403–6. doi: 10.1016/0006-8993(86)91505-2
33. Roane DM, Feinberg TE, Meckler L, Miner CR, Scicutella A, Rosenthal RN. Treatment of dementia-associated agitation with gabapentin. J Neuropsychiatry Clin Neurosci. (2000) 12:40–3. doi: 10.1176/jnp.12.1.40
34. Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. (2010) 107:2580–5. doi: 10.1073/pnas.0915139107
35. Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer’s disease drug development pipeline: 2023. Alzheimers Dement (N Y). (2023) 9:e12385. doi: 10.1002/trc2.12385
36. Theken KN, Lee CR, Gong L, Caudle KE, Formea CM, Gaedigk A, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2C9 and nonsteroidal anti-inflammatory drugs. Clin Pharmacol Ther. (2020) 108:191–200. doi: 10.1002/cpt.1830
37. Tangarife MA, Venegas M, Arbelaez MJ, Gutiérrez E, Sanchez LT, Naranjo MP, et al. Reduction of neuropsychiatric symptoms and associated caregiver distress using a tetrahydrocannabinol and melatonin combination in dementia due to Alzheimer’s disease. (2023) 19(21):e080322. doi: 10.1002/alz.080322
Keywords: agitation, Alzheimer’s disease, neuroinflammation, GABA, CBR-1, THC, melatonin
Citation: Rao JS, Tangarife MA and Mukunda R (2024) Neurobiological alteration in agitation in Alzheimer’s disease and possible interventions. Front. Psychiatry 15:1412901. doi: 10.3389/fpsyt.2024.1412901
Received: 05 April 2024; Accepted: 21 June 2024;
Published: 09 July 2024.
Edited by:
Xi Chen, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, ChinaReviewed by:
Kathy C. Richards, The University of Texas at Austin, United StatesCopyright © 2024 Rao, Tangarife and Mukunda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jagadeesh S. Rao, anJhb0BpZ2NwaGFybWEuY29t
 Jagadeesh S. Rao
Jagadeesh S. Rao María Alejandra Tangarife
María Alejandra Tangarife Ram Mukunda
Ram Mukunda