
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Psychiatry , 13 June 2024
Sec. Digital Mental Health
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1409173
This article is part of the Research Topic Usable and Effective Digital Health for Autism Care and Treatment View all 5 articles
 Helen L. Coulter1,2
Helen L. Coulter1,2 Mark P. Donnelly3*
Mark P. Donnelly3* Anita Yakkundi4,5
Anita Yakkundi4,5 Helen McAneney5,6
Helen McAneney5,6 Owen G. Barr2,7
Owen G. Barr2,7 W. George Kernohan2,7*
W. George Kernohan2,7*There is a reported high prevalence of anxiety in people with autism spectrum disorder. This mini review appraises existing research investigating heart rate variability biofeedback to help manage symptoms of anxiety in people with autism spectrum disorder. A thorough search of electronic databases was conducted to find relevant literature. Consultation with experts and a librarian helped develop search terms following the PICO framework. Five databases were searched, and screening was undertaken using Covidence software, with the process outlined in a PRISMA flowchart. The latest review showed positive short-term effects but there is a need for long-term follow-up. Future investigations should consider device type, training settings, and control interventions. Accurate heart rate variability assessment independent of biofeedback devices is crucial. Additional measures like cortisol assessment and user feedback are recommended for comprehensive evaluation. The findings highlight progress in the evidence base and offer insight to future directions.
Heart rate variability (HRV) is a term used to describe the natural variability in heart rhythm which reflects the activity of both the sympathetic and parasympathetic actions of the autonomic nervous system (1). HRV is a complex variable which constantly changes according to the individual’s responses to their environment, and which also declines with age (2, 3). A detailed review of HRV metrics has been produced (4) and normative values have been reported (5, 6). A number of theoretical models have been proposed describing the links between HRV and health, mediated via connections between the heart and the brain (7–9). HRV is now frequently used as a physiological marker and is considered a sensitive indicator of the stress response (10) and an index of an individual’s ability to self-regulate behaviour (11).
Biofeedback involves monitoring physiology by actively involving the user, enabling them to learn to change their unique physiological responses to improve health (12). Several systematic reviews have been conducted highlighting the potential of biofeedback as a cost-effective digital health intervention to help people manage anxiety (13, 14). A review of the types of biofeedback modalities and devices being trialled for stress management has been carried out by Yu et al. (15). HRV measurement has been used in conjunction with sensor technology to develop a form of biofeedback now referred to as heart rate variability biofeedback or HRVB (16). HRVB involves breathing training to develop a phenomenon called Respiratory Sinus Arrhythmia (RSA), where heart rate acceleration and deceleration synchronizes with respiration and typically occurs when breathing is slowed to a rate between 4.5–7 breaths per minute (17). A guide to the process of HRVB training to develop what has been termed ‘Resonance Frequency’ breathing has been outlined (18) and the possible mechanisms of effect underlying HRVB have been described (19, 20). Several meta-analytic reviews have now demonstrated efficacy for HRVB to reduce anxiety in a range of populations (14, 21).
People with autism spectrum disorder (ASD) frequently experience high levels of anxiety (22, 23) and reviews have indicated higher prevalence rates of anxiety in young people with ASD, in comparison with typically developing peers (24). A range of interventions have been employed to treat anxiety in people with ASD (25). Despite widespread use of medication, the evidence for its effectiveness is limited and side effects and adverse events can occur (26). There is evidence for effectiveness of interventions such as cognitive behavioural therapy adapted for people with ASD (27), however the availability of interventions for anxiety is limited by difficulties with adoption of interventions (28) and lack of support and training for those working with people with ASD (29).
As a non-invasive digital health solution, HRVB may represent a useful method of engaging people with ASD. HRVB removes the complex social and communication demands of traditional cognitive and behavioural therapies (30), bypasses the risks of medication and through often intuitive digital displays, leverages the characteristic visual strengths and interests of people with ASD. People with ASD do, however, present with a wide range of differences in physiological reactions compared to neurotypical peers (31–33) and further investigation into interventions to help improve autonomic system regulation may be particularly important.
This paper presents a review conducted to assess and summarise literature that currently exists on the use of HRVB in people with Autistic Spectrum Disorder (ASD).
A comprehensive search of electronic databases followed by the screening of the articles was undertaken to identify relevant literature. In consultation with experts in the areas of ASD and HRV, and then further refinement with advice from a subject librarian, the search terms were developed and followed the PICO framework (Population, Intervention, Comparator and Outcome) (34). These search terms are listed in Table 1 and were combined using Boolean logic. Five databases, CINAHL ultimate, Embase (via OVID), Medline (via OVID), PsycINFO (via OVID) and Scopus were searched to capture relevant literature across the domains represented by these databases. An initial search was carried out on 19th July 2017, with no date restrictions applied, and further updated with the final search performed on 16th November 2023, in which relevant publications between 2017 and 2023 were then added to form the full list of included studies in this mini review. Inclusion and exclusion criteria applied during the screening process are also outlined in Table 1. That is, the inclusion of peer reviewed research articles, with no restriction on date published or language. Papers were excluded if not ASD/autism, not HRV/biofeedback, no English abstract, or if it was a review article or of a single case study design. Only peer reviewed articles were included in the review. Single case studies were also excluded as they were viewed to lack generalisability due to their focus on a singular instance, limiting the applicability and reliability of findings in broader contexts.
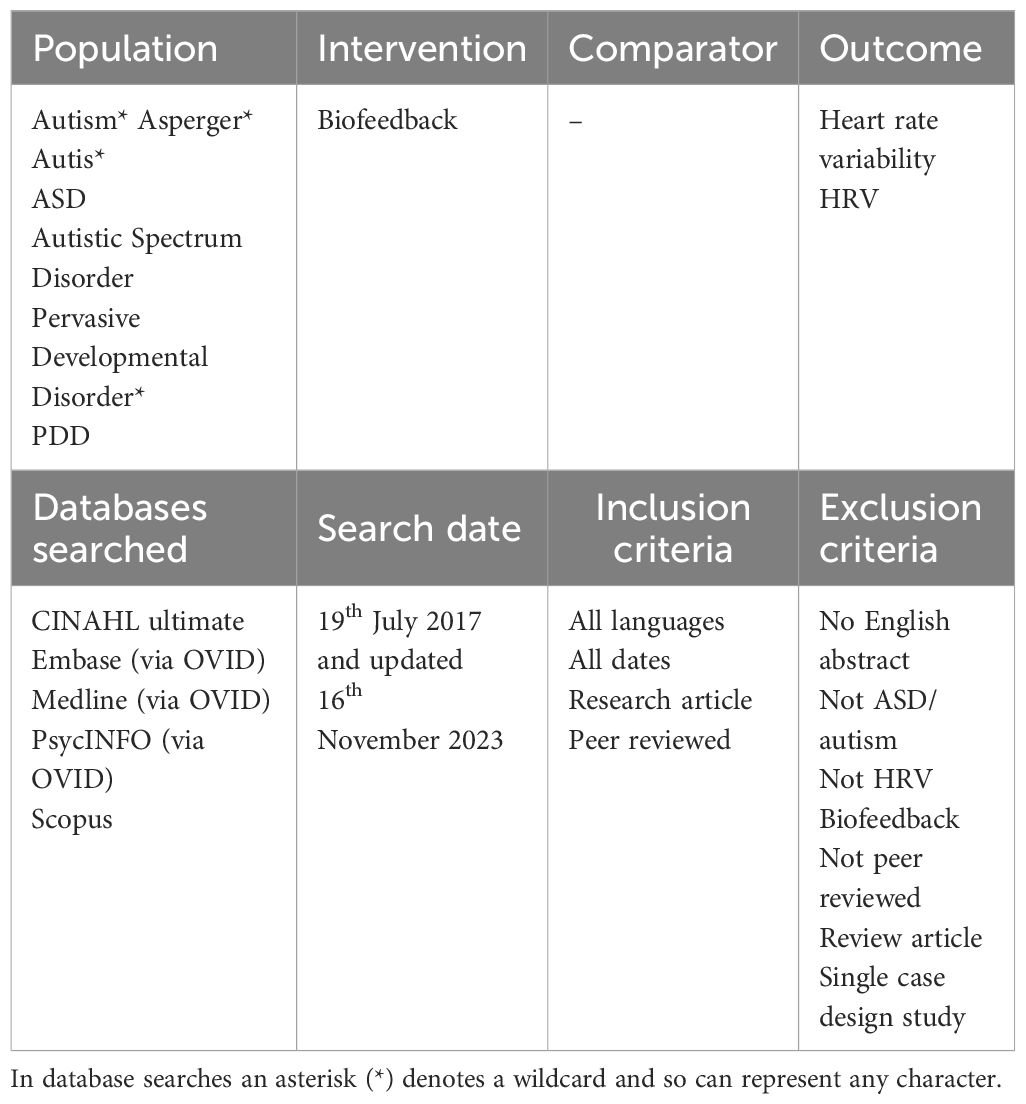
Table 1 Search terms used, and inclusion and exclusion criteria applied during the screening process.
The screening process was facilitated using the software Covidence (35), which automatically removes duplicates. Articles were initially screened by title and abstract, carried out by HC & AY, followed by full text screening. A PRISMA flowchart provides an overview of this screening process (36).
Summary details of included articles were then extracted, including details of population, intervention, comparator, and outcome aspects, followed by an assessment of the study, using the CASP framework (37) as a guide, which was carried out in duplicate.
An overview of the screening process is provided in the PRISMA flowchart (36) presented in Figure 1. In total 38 articles were returned, 30 from the searches of the five databases (CINAHL ultimate n = 0, Embase (via OVID) n = 12, Medline (via OVID) n = 4, PsycINFO (via OVID) n = 5, Scopus n = 9) and 8 additional articles from manual searches. After duplicates were removed, 24 articles were screened for inclusion with 20 articles excluded for the reasons as listed in Figure 1. Four remaining articles are included in this review (38–41).
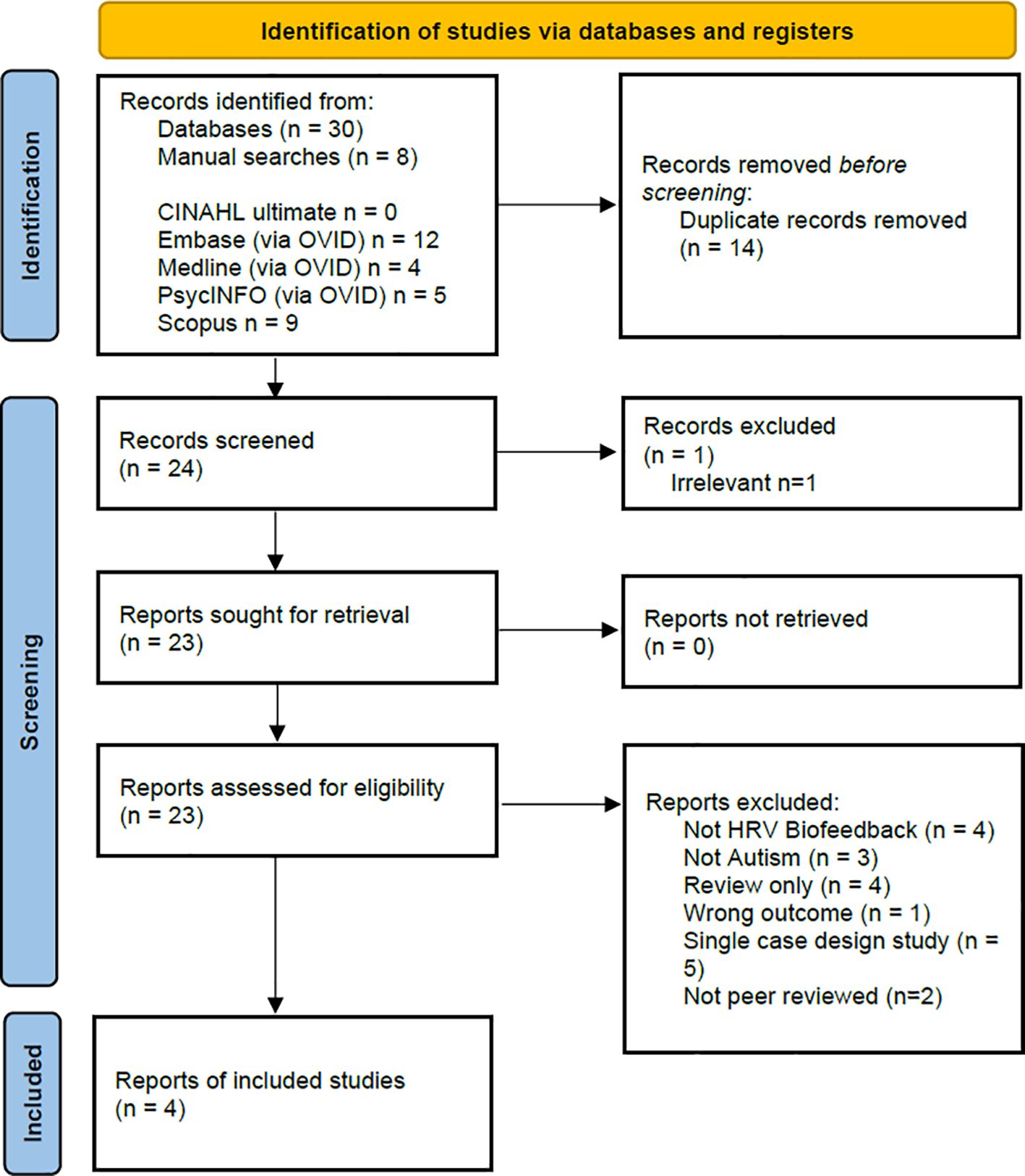
Figure 1 PRISMA Flowchart (36) showing the overview of the identification of and screening of studies for inclusion in this review.
Table 2 summarises the extracted data and critical assessment of the four included articles. Two of the four articles are published within the last two years (40, 41), with two studies conducted in the USA (38, 39), one in the UK (40) and one in Belgium (41). The design of the interventions in these studies varies but all comprise exploratory or pilot studies, with appropriately small sample sizes. Across the papers, there is variation in the measures used and demographics reported.
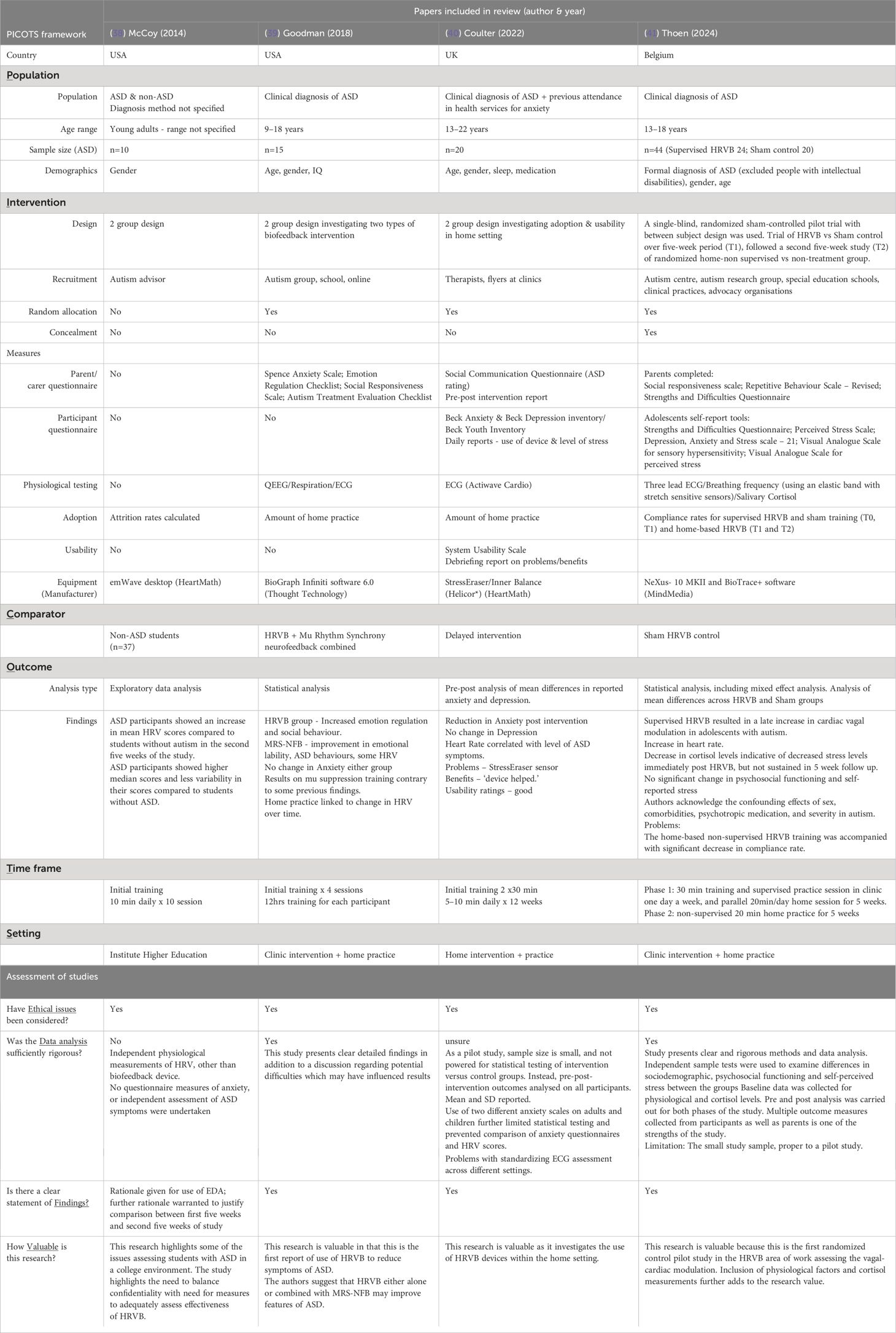
Table 2 Summary of four included studies on HRVB interventions in people with ASD showing key features in design, measures, and intervention.
Based on a review of existing literature on the use of HRVB in people with ASD, several themes emerge regarding current studies and several recommendations are made for the development of future research in this area.
Analysis of the studies reviewed here highlight wide variations in study design and the methodologies employed, which have employed different devices, training protocols and diverse outcome measurements. The current studies all provide important information on specific areas of focus and are typical of early-stage research under development however the heterogeneity of design makes direct comparisons difficult. This problem has been highlighted in several biofeedback reviews (15, 42) and there is a clear need for larger randomised control studies particularly with this new population. The most recent study reviewed in this paper, Thoen et al. (41), did employ this type of design and showed some positive effects for HRVB but also highlighted the need for more follow up to assess the longer-term effects of HRVB for ASD populations.
The type of biofeedback device used, and the type and location of training employed may also be important to consider in future investigations. Two studies reviewed here employed more real-world training in either school or home environments (38, 40) and did not adopt protocols using multi sensor devices in a clinical environment. Whilst this type of study remains problematic in terms of standardising environment conditions the need for follow up testing of devices in real world situations with clinical populations has been emphasized (43).
Resonance frequency breathing rates within individuals may not remain stable over time (44, 45) and assessing any HRVB intervention in conjunction with repeated HRV measures and age-related HRV norms is vital. The type of control intervention used may also be important. As noted by Goodman et al. (39), it is possible that simply teaching diaphragmatic breathing alone may be enough to create changes in symptoms such as anxiety. This type of slow breathing intervention has been shown to have positive effects (46, 47) and may be useful as an active control intervention in future studies to assess its effects on HRV in people with ASD.
A further issue highlighted by this review is the need to accurately assess and record HRV independently from biofeedback devices. We argue that studies should include measures of HRV measured via 12-lead ECG which are independent from the biofeedback device itself. Heart rate variability is now a commonly collected data variable which can be measured via apps on iPhones and activity trackers. However, this belies the complexity underlying the multiple influences which contribute to HRV on a moment-to-moment basis (3). The increasing number of studies now assessing HRV and the variability of data collection, analysis, and reporting, highlight the need to adhere to standardize data collection according to agreed guidelines (48). Separate independent measurement of HRV pre and post intervention is needed to elucidate whether HRVB practice does change HRV responses.
The importance of using additional measures of participants reactions is recommended to help elucidate the links between biofeedback and anxiety in ASD. The use of physiological assessments such as cortisol assessment (41) and the use of tests of EEG functioning (39) represent valuable methods of gathering vital information on physiological and neurological reactions.
The involvement of both users and carers in providing direct reports on symptoms and perceived stress in this population is also seen as a vital area which is needed. The use of participant reports on levels of stress and sources of stress (40, 41) and usability reports on device function highlight how user input can help to develop future work and highlight risks and benefits of an intervention.
Assessing the longer-term effectiveness of any intervention is important, and the use of remote monitoring to assess home practice and stress levels will be important in future work to assess adoption and usage of any intervention in this area. In addition, capturing and recording data from the biofeedback devices or from home practice reports as used by Goodman et al. (39) may help assess whether there is a dose response relationship for this type of intervention.
Several key issues are apparent regarding the specific vulnerabilities of people with ASD. Foremost in this area is the need for larger studies to establish normative values for HRV in people with ASD under a range of conditions. Several previous studies assessing the physiological responses of people with ASD have indicated that this population may have different responses to stress compared to neurotypical peers (33) and increased levels of anxiety and depression (23, 24). Assessment of autonomic nervous system functioning, and physical and mental health conditions is particularly important for people with ASD considering the increased level of these co-morbidities in this population (49–51).
In addition, the use of the ‘stress test’ paradigm may not be appropriate or indeed ethical to use for people with ASD. One feasibility study reviewed here noted that the level of ASD symptoms was correlated with increased heart rate during a stress test and noted several previously undetected mental health and cardiac health difficulties in participants during assessments (40). Recent systematic reviews have highlighted links between heart disease and ASD (52, 53) and the implications of these issues should be considered in future studies. Further work in this area should consider not employing a stress test paradigm for people with ASD and instead should consider longer term HRV recording (54) or repeated daily recordings under rest conditions and follow standardised recording and assessment guidelines (48). In addition, recording severity of symptoms in individuals with a diagnosis of ASD, as well as levels of mental and physical health symptoms pre and post intervention are important to provide more accurate information on the links between ASD and HRV.
ASD is now a common condition (55) with significant economic health care costs (56, 57). Providing interventions for symptoms that affect the day to day lives of people with ASD such as anxiety have been emphasized as a vital area for research (58, 59). Despite concerns regarding overuse, there has also been a recognition of the potential for digital technology to manage the growing levels of mental health in the general population to help reduce the increasing burden of mental illness (60, 61). HRVB has been found to be an important adjunct to existing interventions for neurotypical populations with mental health conditions (62, 63) and people with ASD should not be excluded from any future research developments in this area (64, 65).
We argue that there exists evidence from recent studies to suggest the potential for this intervention to help people with ASD manage anxiety. Future studies should aim to address some of the issues outlined in this review to determine both the type and level of intervention appropriate in this vulnerable population and to further assess the mechanism of effect of HRVB.
HC: Writing – original draft, Writing – review & editing. MD: Writing – original draft, Writing – review & editing. AY: Writing – original draft, Writing – review & editing. HM: Writing – review & editing. OB: Writing – review & editing. WK: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. HC was supported by a Health & Social Care Doctoral Fellowship (2013-2018) from the Research and Development Division of the Public Health Agency, Northern Ireland, United Kingdom [2013 Doctoral Fellowship: Heart Rate Variability Biofeedback in Young People with Autism - a Feasibility grant # EAT/4730/12]. The NIPHRN is an HSC Public Health Agency Research and Development Division funded initiative to support Public Health Research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. (1996) 17:354–81. doi: 10.1093/oxfordjournals.eurheartj.a014868
2. Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol. (2014) 5:1040. doi: 10.3389/fpsyg.2014.01040
3. Lombardi F, Huikuri H, Schmidt G, Malik M. e-Rhythm Study Group of European Heart Rhythm Association. Short-term heart rate variability: Easy to measure, difficult to interpret. Heart Rhythm. (2018) 15:1559–60. doi: 10.1016/j.hrthm.2018.05.023
4. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:258. doi: 10.3389/fpubh.2017.00258
5. Nunan D, Sandercock GR, Brodie DA. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin electrophysiology: PACE. (2010) 33:1407–17. doi: 10.1111/pace.2010.33.issue-11
6. Dantas EM, Kemp AH, Andreão RV, da Silva VJD, Brunoni AR, Hoshi RA, et al. Reference values for short-term resting-state heart rate variability in healthy adults: Results from the Brazilian Longitudinal Study of Adult Health-ELSA-Brasil study. Psychophysiology. (2018) 55:e13052. doi: 10.1111/psyp.13052
7. Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. (2001) 42:123–46. doi: 10.1016/s0167–8760(01)00162–3
8. Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. (2000) 61:201–16. doi: 10.1016/s0165–0327(00)00338–4
9. Kemp AH, Koenig J, Thayer JF. From psychological moments to mortality: A multidisciplinary synthesis on heart rate variability spanning the continuum of time. Neurosci Biobehavioural Rev. (2017) 83:547–67. doi: 10.1016/j.neubiorev.2017.09.006
10. Kim HG, Cheon EJ, Bai DS, Lee YH, Koo BH. Stress and heart rate variability: A meta-analysis and review of literature. Psychiatry Invest. (2018) 15:235–45. doi: 10.30773/pi.2017.08.17
11. Holzman JB, Bridgett DJ. Heart rate variability indices as biomarkers of top-down self-regulatory mechanisms: A meta-analytic review. Neurosci Biobehavioural Rev. (2017) 74:233–55. doi: 10.1016/j.neubiorev.2016.12.032
12. Tan G, Shaffer F, Lyle R, Teo I. Evidence-based Practice in Biofeedback and Neurofeedback. 3rd edition. Wheat Ridge, CO: AAPB Press (2016). Available at: https://www.amazon.com/Evidence-based-Practice-Biofeedback-Neurofeedback-3rd/dp/0984297960.
13. Schoenberg PL, David AS. Biofeedback for psychiatric disorders: a systematic review. Appl Psychophysiol Biofeedback. (2014) 39:109–35. doi: 10.1007/s10484–014-9246–9
14. Goessl VC, Curtiss JE, Hofmann SG. The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychol Med. (2017) 47:2578–86. doi: 10.1017/S0033291717001003
15. Yu B, Funk M, Hu J, Wang Q, Feijs L. Biofeedback for everyday stress management: A systematic review. Front ICT. (2018) 5:23. doi: 10.3389/fict.2018.00023
16. Lehrer PM, Vaschillo E, Vaschillo B. Resonant frequency biofeedback training to increase cardiac variability: rationale and manual for training. Appl Psychophysiol Biofeedback. (2000) 25:177–91. doi: 10.1023/a:1009554825745
17. Lehrer P, Vaschillo B, Zucker T, Graves J, Katsamanis M, Aviles M, et al. Protocol for heart rate variability biofeedback training. Biofeedback. (2013) 41:98–109. doi: 10.5298/1081–5937-41.3.08
18. Shaffer F, Meehan ZM. A practical guide to resonance frequency assessment for heart rate variability biofeedback. Front Neurosci. (2020) 14:570400. doi: 10.3389/fnins.2020.570400
19. Lehrer PM, Gevirtz R. Heart rate variability biofeedback: how and why does it work? Front Psychol. (2014) 5:756. doi: 10.3389/fpsyg.2014.00756
20. Sevoz-Couche C, Laborde S. Heart rate variability and slow-paced breathing: when coherence meets resonance. Neurosci Biobehavioural Rev. (2022) 135:104576. doi: 10.1016/j.neubiorev.2022.104576
21. Lehrer P, Kaur K, Sharma A, Shah K, Huseby R, Bhavsar J, et al. Heart rate variability biofeedback improves emotional and physical health and performance: A systematic review and meta-analysis. Appl Psychophysiol Biofeedback. (2020) 45:109–29. doi: 10.1007/s10484-020-09466-z
22. Mattila ML, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Kielinen M, et al. Comorbid psychiatric disorders associated with Asperger syndrome/high-functioning autism: a community- and clinic-based study. J Autism Dev Disord. (2010) 40:1080–93. doi: 10.1007/s10803–010-0958–2
23. Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. (2019) 6:819–29. doi: 10.1016/s2215–0366(19)30289–5
24. van Steensel FJA, Heeman EJ. Anxiety levels in children with autism spectrum disorder: A meta-analysis. J Child Family Stud. (2017) 26:1753–67. doi: 10.1007/s10826–017-0687–7
25. Kerns K, Renno P, Storch E, Kendall P, Wood J. Anxiety in Children and Adolescents with Autism Spectrum Disorder: Evidence-Based Assessment and Treatment. London: Academic Press (2017). Available at: https://shop.elsevier.com/books/anxiety-in-children-and-adolescents-with-autism-spectrum-disorder/kerns/978–0-12–805122–1.
26. Vasa RA, Mazurek MO, Mahajan R, Bennett AE, Bernal MP, Nozzolillo AA, et al. Assessment and treatment of anxiety in youth with Autism Spectrum Disorders. Paediatrics. (2016) 137 Suppl 2:S115–23. doi: 10.1542/peds.2015-2851J
27. Perihan C, Burke M, Bowman-Perrott L, Bicer A, Gallup J, Thompson J, et al. Effects of Cognitive Behavioural Therapy for reducing anxiety in children with high functioning ASD: A systematic review and meta-analysis. J Autism Dev Disord. (2020) 50:1958–72. doi: 10.1007/s10803–019-03949–7
28. Dingfelder HE, Mandell DS. Bridging the research-to-practice gap in autism intervention: an application of diffusion of innovation theory. J Autism Dev Disord. (2011) 41:597–609. doi: 10.1007/s10803–010-1081–0
29. Cooper K, Loades ME, Russell A. Adapting psychological therapies for autism. Res Autism Spectr Disord. (2018) 45:43–50. doi: 10.1016/j.rasd.2017.11.002
30. Mottron L. Should we change targets and methods of early intervention in autism, in favour of a strengths-based education? Eur Child Adolesc Psychiatry. (2017) 26:815–25. doi: 10.1007/s00787–017-0955–5
31. DuBois D, Ameis SH, Lai MC, Casanova MF, Desarkar P. Interoception in autism spectrum disorder: A review. Int J Dev Neurosci. (2016) 52:104–11. doi: 10.1016/j.ijdevneu.2016.05.001
32. Arora I, Bellato A, Ropar D, Hollis C, Groom MJ. Is autonomic function during resting-state atypical in Autism: A systematic review of evidence. Neurosci Biobehavioural Rev. (2021) 125:417–41. doi: 10.1016/j.neubiorev.2021.02.041
33. Makris G, Agorastos A, Chrousos GP, Pervanidou P. Stress system activation in children and adolescents with autism spectrum disorder. Front Neurosci. (2022) 15:756628. doi: 10.3389/fnins.2021.756628
34. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inf Decision Making. (2007) 7:16. doi: 10.1186/1472–6947-7–16
35. Covidence systematic review software. Veritas Health Innovation . Melbourne Australia. Available online at: www.Covidence.org (Accessed March 28, 2024).
36. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
37. The Critical Skills Appraisal Programme. The critical skills appraisal programme: making sense of evidence (2006). England: Public Health Resource Unit. Available online at: http://www.casp-uk.net/ (Accessed March 28, 2024).
38. McCoy KM, Westlake G, Zucker SH, DiGangi SA. Evaluation of biofeedback intervention in college students diagnosed with an autism spectrum disorder. J Division Autism Dev Disabil Council Exceptional Children. (2014) 1:121–35. Available at: https://exceptionalchildren.org/sites/default/files/2023-04/may28_dadd_online_journal_v1_2014.pdf?_gl=1*1ee1fnl*_ga*MTg4NDE4NTM1OS4xNzExNjY0MzU3*_ga_L4ZFTNESGT*MTcxNzQzMjI1Mi4zLjEuMTcxNzQzMjI3MC40Mi4wLjA (Accessed March 28, 2024).
39. Goodman M, Castro N, Sloan M, Sharma R, Widdowson M, Herrera E, et al. A neurovisceral approach to autism: Targeting self-regulation and core symptoms using neurofeedback and biofeedback. NeuroRegulation. (2018) 5:9–29. doi: 10.15540/nr.5.1.9
40. Coulter H, Donnelly M, Mallett J, Kernohan WG. Heart rate variability biofeedback to treat anxiety in young people with Autism Spectrum Disorder: Findings from a home-based pilot study. JMIR Form Res. (2022) 6:e37994. doi: 10.2196/37994
41. Thoen A, Alaerts K, Prinsen J, Steyaert J, Van Damme T. The physiological and clinical-behavioural effects of heart rate variability biofeedback in adolescents with Autism: A pilot randomized controlled trial. Appl Psychophysiol Biofeedback. (2024). doi: 10.1007/s10484–024-09638–1
42. Wheat AL, Larkin KT. Biofeedback of heart rate variability and related physiology: a critical review. Appl Psychophysiol Biofeedback. (2010) 35:229–42. doi: 10.1007/s10484-010-9133-y
43. Lin B, Prickett C, Woltering S. Feasibility of using a biofeedback device in mindfulness training - a pilot randomized controlled trial. Pilot Feasibility Study. (2021) 7:84. doi: 10.1186/s40814–021-00807–1
44. Lehrer PM, Vaschillo EG, Vidali V. Heart rate and breathing are not always in phase during resonance frequency breathing. Appl Psychophysiol Biofeedback. (2020) 45:145–52. doi: 10.1007/s10484-020-09459-y
45. Capdevila L, Parrado E, Ramos-Castro J, Zapata-Lamana R, Lalanza JF. Resonance frequency is not always stable over time and could be related to the inter-beat interval. Sci Rep. (2021) 11:8400. doi: 10.1038/s41598–021-87867–8
46. Laborde S, Allen MS, Borges U, Dosseville F, Hosang TJ, Iskra M, et al. Effects of voluntary slow breathing on heart rate and heart rate variability: A systematic review and a meta-analysis. Neurosci Biobehavioural Rev. (2022) 138:104711. doi: 10.1016/j.neubiorev.2022.104711
47. Fincham GW, Strauss C, Montero-Marin J, Cavanagh K. Effect of breathwork on stress and mental health: A meta-analysis of randomised-controlled trials. Sci Rep. (2023) 13:432. doi: 10.1038/s41598-022-27247-y
48. Quintana D, Alvares G, Heathers J. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): recommendations to advance research communication. Transl Psychiatry. (2016) 6:e803. doi: 10.1038/tp.2016.73
49. Hirvikoski T, Mittendorfer-Rutz E, Boman M, Larsson H, Lichtenstein P, Bölte S. Premature mortality in autism spectrum disorder. Br J Psychiatry. (2016) 208:232–8. doi: 10.1192/bjp.bp.114.160192
50. Davignon MN, Qian Y, Massolo M, Croen LA. Psychiatric and medical conditions in transition-aged individuals with ASD. Paediatrics. (2018) 141:S335–45. doi: 10.1542/peds.2016-4300K
51. Weir E, Allison C, Warrier V, Baron-Cohen S. Increased prevalence of non-communicable physical health conditions among autistic adults. Autism. (2021) 25:681–94. doi: 10.1177/1362361320953652
52. Gu S, Katyal A, Zhang Q, Chung W, Franciosi S, Sanatani S. The association between congenital heart disease and autism spectrum disorder: A systematic review and meta-analysis. Pediatr Cardiol. (2023) 44:1092–107. doi: 10.1007/s00246–023-03146–5
53. Dhanasekara CS, Ancona D, Cortes L, Hu A, Rimu AH, Robohm-Leavitt C, et al. Association between autism spectrum disorders and cardiometabolic diseases: A systematic review and meta-analysis. JAMA Paediatrics. (2023) 177:248–57. doi: 10.1001/jamapediatrics.2022.5629
54. Brown SBRE, Brosschot JF, Versluis A, Thayer JF, Verkuil B. New methods to optimally detect episodes of non-metabolic heart rate variability reduction as an indicator of psychological stress in everyday life. Int J Psychophysiol. (2018) 131:30–6. doi: 10.1016/j.ijpsycho.2017.10.007
55. Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. (2011) 68:459–65. doi: 10.1001/archgenpsychiatry.2011.38
56. Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Paediatrics. (2014) 168:721–8. doi: 10.1001/jamapediatrics.2014.210
57. Rogge N, Janssen J. The economic costs of autism spectrum disorder: A literature review. J Autism Dev Disord. (2019) 49:2873–900. doi: 10.1007/s10803-019-04014-z
58. Pellicano E, Dinsmore A, Charman T. What should autism research focus upon? Community views and priorities from the United Kingdom. Autism. (2014) 18:756–70. doi: 10.1177/1362361314529627
59. Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, et al. Autism spectrum disorder. Nat Rev Dis Primers. (2020) 6:5. doi: 10.1038/s41572–019-0138–4
60. Kazdin AE, Blase SL. Rebooting psychotherapy research and practice to reduce the burden of mental illness. Perspect Psychol Sci. (2011) 6:21–37. doi: 10.1177/1745691610393527
61. Naslund JA, Aschbrenner KA. . Digital technology for health promotion: opportunities to address excess mortality in persons living with severe mental disorders. Evidence-Based Ment Health. (2019) 22:17–22. doi: 10.1136/ebmental-2018–300034
62. Caldwell YT, Steffen PR. Adding HRV biofeedback to psychotherapy increases heart rate variability and improves the treatment of major depressive disorder. Int J Psychophysiol. (2018) 131:96–101. doi: 10.1016/j.ijpsycho.2018.01.001
63. De Witte NAJ, Buyck I, Van Daele T. Combining biofeedback with stress management interventions: A systematic review of physiological and psychological effects. Appl Psychophysiol Biofeedback. (2019) 44:71–82. doi: 10.1007/s10484–018-09427–7
64. Fletcher-Watson S, Adams J, Brook K, Charman T, Crane L, Cusack J, et al. Making the future together: Shaping autism research through meaningful participation. Autism. (2019) 23:943–53. doi: 10.1177/1362361318786721
65. Milton DEM. Beyond Tokenism: Autistic people in autism research. Psychol. (2019). Available at: https://thepsychologist.bps.org.uk/volume-32/october-2019/beyond-tokenism-autistic-people-autism-research (Accessed March 28, 2024).
Keywords: heart rate variability biofeedback, anxiety, autism spectrum disorder, digital health, intervention
Citation: Coulter HL, Donnelly MP, Yakkundi A, McAneney H, Barr OG and Kernohan WG (2024) Heart rate variability biofeedback to reduce anxiety in autism spectrum disorder – a mini review. Front. Psychiatry 15:1409173. doi: 10.3389/fpsyt.2024.1409173
Received: 29 March 2024; Accepted: 29 May 2024;
Published: 13 June 2024.
Edited by:
Wenbing Zhao, Cleveland State University, United StatesReviewed by:
Shinya Sakai, Hokkaido University, JapanCopyright © 2024 Coulter, Donnelly, Yakkundi, McAneney, Barr and Kernohan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark P. Donnelly, bXAuZG9ubmVsbHlAdWxzdGVyLmFjLnVr; W. George Kernohan, d2cua2Vybm9oYW5AdWxzdGVyLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.