- 1School of Medicine, Yunnan University, Kunming, China
- 2Yunnan Technological Innovation Centre of Drug Addiction Medicine, Yunnan University, Kunming, China
- 3Department of General Surgery I, First People’s Hospital of Yunnan Province, Kunming, China
- 4Department of Rehabilitation Education and Corrections, Drug Rehabilitation Administration of Yunnan Province, Kunming, China
- 5Department of Gastrointestinal Surgery, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 6Department of Ultrasound, The First Affiliated Hospital of Kunming Medical University, Kunming, China
Introduction: Methamphetamine is currently one of the most commonly used addictive substances with strong addiction and a high relapse rate. This systematic review aims to examine the effectiveness of physical activity in improving negative emotions, cognitive impairment, and drug craving in people with methamphetamine use disorder (MUD).
Methods: A total of 17 studies out of 133 found from Embase and PubMed were identified, reporting results from 1836 participants from MUD populations. Original research using clearly described physical activity as interventions and reporting quantifiable outcomes of negative mood, cognitive function and drug craving level in people with MUD were eligible for inclusion. We included prospective studies, randomized controlled trials, or intervention studies, focusing on the neurological effects of physical activity on MUD.
Results: Taken together, the available clinical evidence showed that physical activity-based interventions may be effective in managing MUD-related withdrawal symptoms.
Discussion: Physical exercise may improve drug rehabilitation efficiency by improving negative emotions, cognitive behaviors, and drug cravings.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024530359.
1 Introduction
Methamphetamine, the main drug in amphetamine-type stimulants, is highly addictive (1). Methamphetamine’s metabolism in the human body is relatively stable. So it soon became one of the most used drugs in the world (1). The latest World Drug Report from the United Nations Office on Drugs and Crime indicates that an estimated 36 million people used amphetamines in 2021, representing 0.7 percent of the global population (2). Qualitative assessments suggest an increase in the use of amphetamines in 2021 and over the last decade (2). In a cross-sectional study of 195 711 respondents in the United States from 2015 to 2019, it was found that methamphetamine use and overdose deaths due to MUD and psychostimulants were on the rise (3).
MUD can lead to many serious consequences, such as anxiety, depression, cognitive impairment, and anhedonia could occur after withdrawal (1). A large proportion of methamphetamine-dependent patients are repeatedly hospitalized and repeatedly detoxified due to MUD-related psychosis (4).In patients with a positive psychiatric history, drug dependence is more likely to lead to long-term psychiatric consequences (5). Therefore, there is an urgent need for a safe, feasible, and effective way to manage methamphetamine addiction.
Exercise is an important part of a healthy life (6), which is conducive to enhancing cardiopulmonary function, improves the functional status of the blood circulatory system (7), and helps to prevent and treat a variety of metabolic diseases, and enhances cognitive ability (8–10). Even low-intensity exercise such as walking is associated with better health (11). In recent years, many studies have confirmed that physical exercise can effectively suppress drug craving and relapse through multiple modulations of the nervous system, and can improve cognitive impairment (12). Furthermore, exercise intervention can help to recover the brain damage (13), facilitate long-term synaptic potentiation-related pathways (14), and promote recovery from severe mental disorders, which was confirmed by real-world multicenter study (15). Exercise has been found to be an effective intervention in improving social health and mental health in people with MUD (16). Moreover, exercise can promote the release of endorphins in the brain, which are naturally occurring analgesics that help to alleviate stress and depression symptoms (17, 18). Physical exercise can also increase the levels of growth factors in the brain, enhancing cognitive function and brain plasticity (19). Results from multicenter randomized controlled trials demonstrated that 20-min moderate physical activity can improve adherence to pharmacological treatments in patients with severe mental disorders (20).
This study aimed to systematically review and synthesize the literature on the efficacy of physical exercise intervention on negative emotion, cognitive impairment, and drug craving caused by MUD. In this systematic review, we provided an overview of the neurological and behavioral effects of exercise interventions as adjunctive treatments for the prevention and elimination of methamphetamine addiction.
2 Methodology
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) 2020 statement (21), this review was registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42024530359).
To summarize recent findings from the effects of exercise on the management of methamphetamine addiction, we searched the PubMed and Embase databases for articles published in peer-reviewed journals written in English and indexed until October 2023. We used search line such as (((physical exercise) OR (physical activity) OR (exercise)) AND((methamphetamine) OR (methamphetamine use disorder))) AND((depression) OR (anxiety) OR (cognition)). The search strategy was limited to the period from 2014 to 2023. All titles and abstracts found by the search strategy as reported were screened for relevance by GQW, CBL, and XFM. Full searches of titles and abstracts were then conducted to assess eligibility for inclusion in the review by DZH and GQW, independently.
The following eligibility criteria have been considered: published in English; participants met diagnostic criteria for MUD according to the fifth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorder (DSM-5); used clearly described physical activity as interventions; outcomes included negative mood, cognitive function, or drug craving levels with quantifiable indicators. Studies with insufficient evidence or very low quality, studies not focusing on MUD, and studies in animal models or in vitro were excluded. Non-original research, such as conference papers, systematic reviews, meta-analyses, and narrative reviews were excluded.
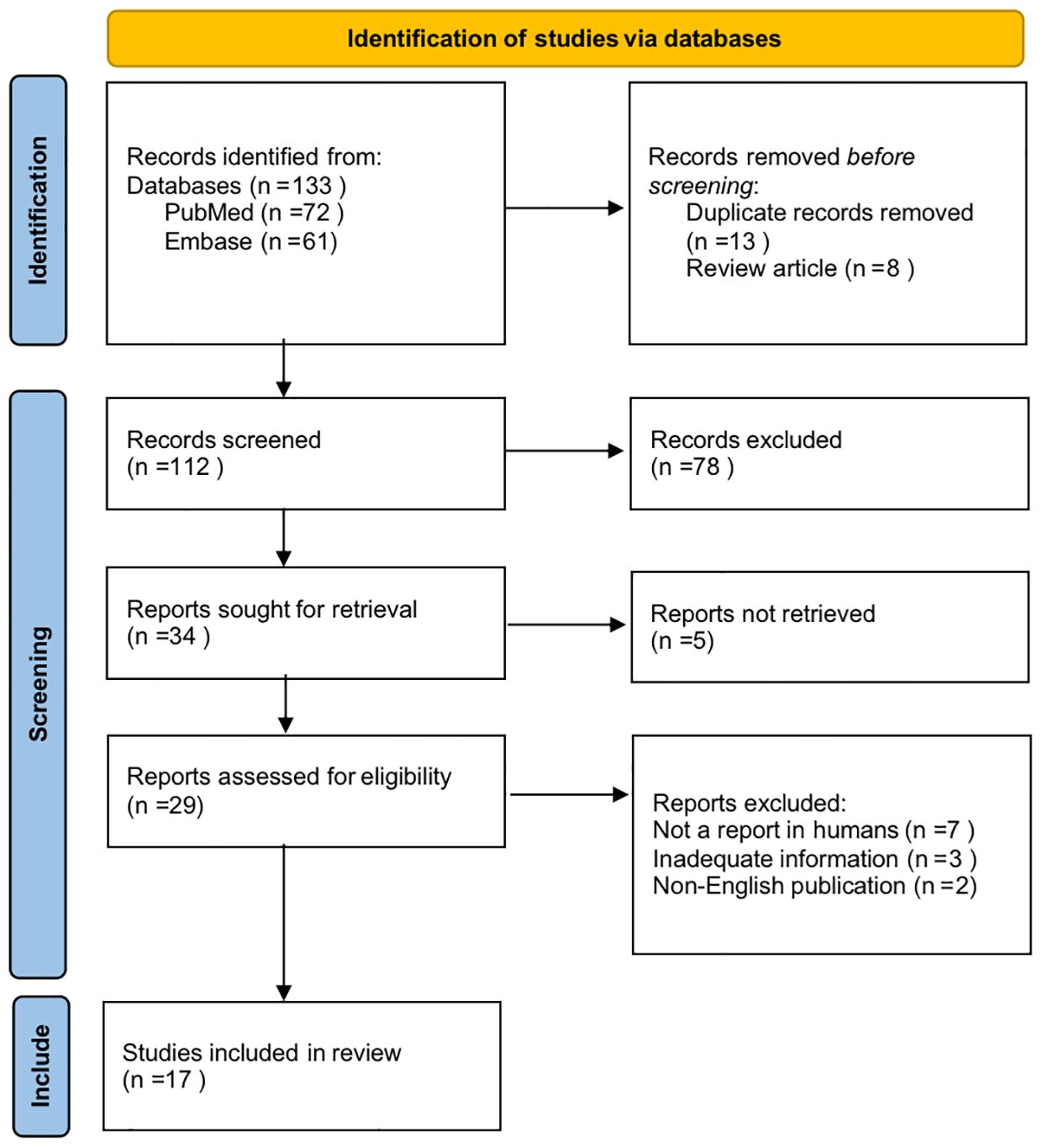
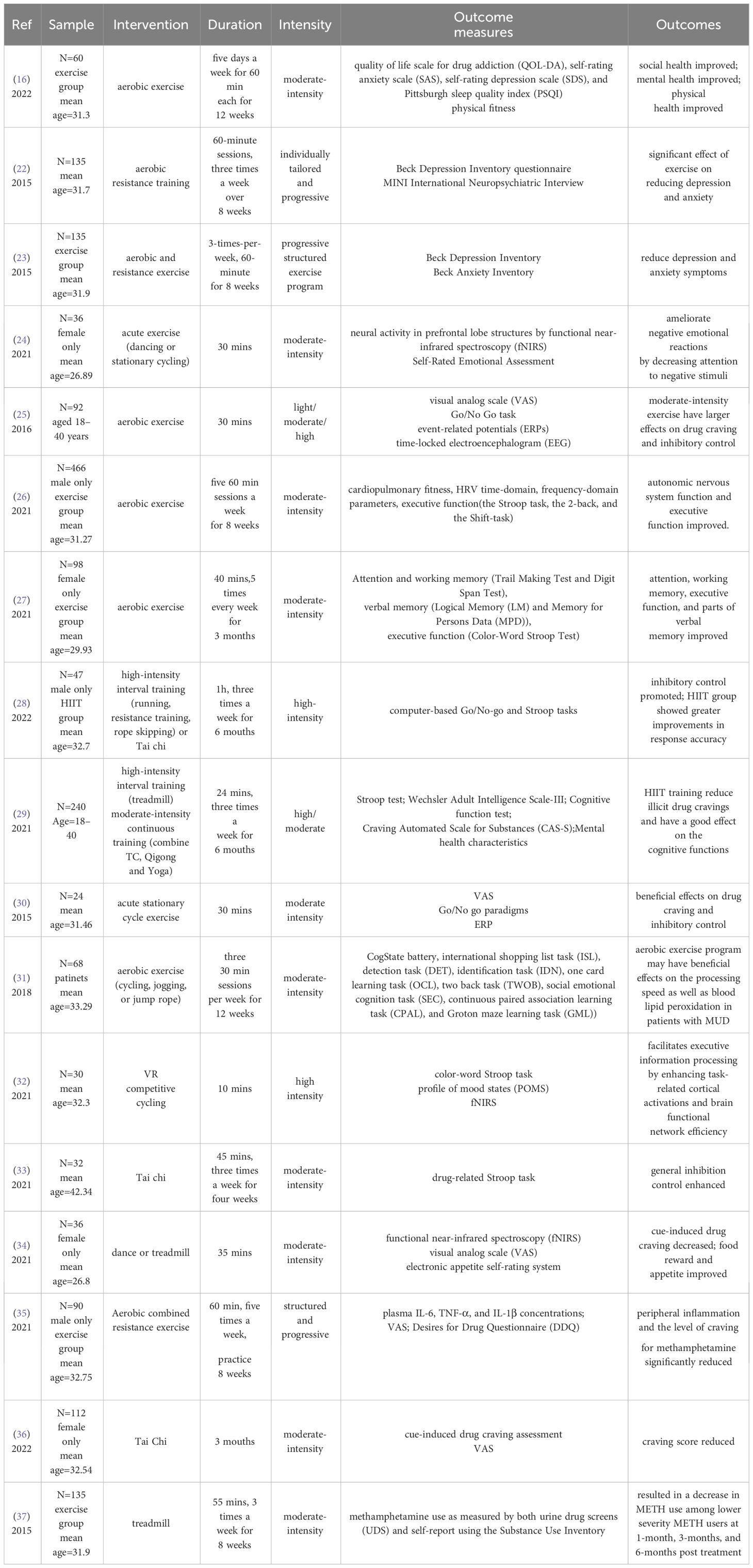
Based on the above criteria, all results were in independently selected for eligibility by CHX, and ZYZ, with disagreements resolved by discussion. CHX and ZYZ independently extracted the data regarding sample characteristics, intervention, duration, intensity, outcome measures, and outcomes for each selected study. HYL, MZ and KHW supervised the entire process. A total of 17 studies out of 133 were included in this review. The detailed process is shown in Figure 1. Characteristics of all studies included in the review were described in Table 1. We included prospective studies, randomized controlled trials, or intervention studies, focusing on the neurological effects of physical activity on MUD. The risk of bias was assessed by two independent reviewers using the AMSTAR2 tool (38).The purpose of this review is to provide an overall assessment discussion of the current state of research. We did not request additional data from authors of published reports, nor did we analyze data not included in the reported articles selected in the systematic review.
3 Results
3.1 Negative emotions
Anxiety and/or depression is the most common mental symptom in the process of methamphetamine use and withdrawal, more than 40% of users have varying degrees of anxiety and/or depression, which directly affects the success rate and relapse rate of withdrawal (39). Effectively improving the negative emotions of patients is one of the key medical problems to be solved urgently in drug treatment (40). Methamphetamine binds to dopamine, norepinephrine, and serotonin transporters in neurons, leading to the rapid accumulation of monoamine neurotransmitters in brain synapses (1). MUD affects the brain’s reward pathway by releasing these neurotransmitters to induce euphoria (41, 42). Long-term abuse of methamphetamine can severely impair the structure and function of the monoamine transmitter system in the brain of methamphetamine users, leading to depletion of the monoamine neurotransmitter (43) and disturbance in the release of neurotransmitters such as dopamine (DA), norepinephrine (NE), and serotonin (5-HT) (1, 44). Besides, long-term abuse of methamphetamine depletes dopamine reserves in the brain and reduces the availability of dopamine receptors (45). Changes in dopamine levels in the brain can regulate emotional states such as depression and anxiety, resulting in reward effects, drug cravings, etc. (1). In addition, 5-HT and NE neurotransmitters can also modulate synaptic plasticity and improve negative mood, which is strongly associated with methamphetamine dependence (46).
Methamphetamine also impairs movement, executive performance, and episodic memory, leading to anxiety and depression (47). Positron emission tomography imaging has found that dopamine transporters in the caudate/putamen and nucleus accumbens of methamphetamine users are significantly reduced, and are significantly correlated with the duration of methamphetamine use and the severity of persistent psychiatric symptoms (48). A positive correlation between methamphetamine dependence severity and incidence of depression and anxiety has also been demonstrated in clinical populations (49). Methamphetamine withdrawal syndrome presents with behavioral psychiatric symptoms such as drug cravings, lack of pleasure, irritability, difficulty concentrating, lethargy, and even suicide (50, 51). Besides, negative emotions like depression and anxiety are also strongly associated with relapse and rehabilitation in methamphetamine-dependent individuals in the early stages of rehabilitation withdrawal (52, 53). Studies have shown that physical activity can improve physiological and neurological damage caused by methamphetamine dependence, increase the release of brain-derived neurotrophic factor (BDNF), and exert antidepressant-like effects (54).
In people with mood disorders, aerobic exercise interventions for 8 to 12 weeks have shown some therapeutic efficacy and significant improvement in negative mood (55). A study of 60 methamphetamine-dependent individuals showed that 12 weeks of moderate-intensity aerobic exercise significantly improved the patients’ social health, mental health, and physical health (16). Some researchers found that after 8 weeks of moderate-intensity exercise, participants who received exercise interventions had significantly lower depression scores than healthy groups, while participants who received the most frequent exercise had the best results. It was further concluded that exercise interventions are more effective in the early stages of addiction withdrawal and can be effective in treating depressive symptoms in individuals (22). Researchers found that an eight-week exercise program significantly improved depression and anxiety symptoms in patients with MUD during the acute withdrawal phase, with significantly lower levels of depression and anxiety in the exercise group than in the healthy control group during the eight-week follow-up period (23). A 30-minute dance session can also improve negativity by reducing the attention of female methamphetamine dependents to negative stimuli (24).
The therapeutic effect of exercise also has been confirmed in animal experiments. For example, wheeled running can reduce central and peripheral inflammation and relieve anxiety-like symptoms in mice with an acute withdrawal model of methamphetamine (56).
3.2 Cognitive performance
Chronic drug abuse is often accompanied by a decrease in an individual’s ability to suppress control, and deficits in inhibitory control in cognitive control are also an important marker of drug dependence (57, 58). In different types of drug users, inhibition control of stimulant users (e.g., cocaine, methamphetamine, etc.) exhibited a higher response error rate, longer response time, and lower inhibition compared with opioid users (59). Studies have found that patients with MUD are often accompanied by abnormal cortical striatal function such as abnormal gray matter and white matter integrity, monoamine neurotransmitter system defects, neuroinflammation, and poor neuronal integrity (1, 60). These neurological abnormalities can seriously affect individual cognitive function (61, 62). Changes in neuroplasticity and the remodeling of specific brain circuits may be the primary neural basis for the effects of drug exposure on learning and memory (63–65).
Hence, patients with methamphetamine addiction often exhibit impaired cognitive function, including executive function, attention, social cognition, flexibility, and working memory (66, 67). Memory is also one of the important aspects of the cognitive abilities. Long-term abuse of methamphetamine may eventually affect learning and memory by altering the neuroplasticity of associated brain regions, such as the dorsal hippocampus (41) and prefrontal cortex (68). Evidence from animal models showed that the synaptic transmission rate in the dorsal hippocampus may be reduced by exposure to methamphetamine during adolescence, ultimately contributing to the development of memory impairment (64, 68, 69).
Physical activity has been shown to improve cognition in cognitively impaired populations, and the therapeutic effect of exercise intensity varies (70–72). In studies of methamphetamine users, experimental results have shown that moderate-intensity aerobic exercise has the most significant effect on inhibition and control (25). A randomized controlled trial of 330 people with MUD has validated the efficacy of 8 weeks of aerobic exercise and showed that aerobic exercise can improve autonomic nervous system function and executive function in individuals with MUD at the same time (26). Randomized controlled trials have also demonstrated that 12 weeks of aerobic exercise can improve attention, working memory, and executive function in women with methamphetamine dependence, and promote cognitive recovery (27). High-intensity intermittent aerobic exercise has also been found to improve the executive ability and response accuracy of methamphetamine-dependent patients (28, 29). Moreover, there are differences in the effects of short-term acute exercise and long-term adaptive exercise on inhibition capacity. Behavioral and electrophysiological measurements were performed by the Go/No Go association task in the absence of pharmacological intervention to examine the effect of acute exercise on craving-related craving and inhibitory control in methamphetamine users, which showed that acute aerobic exercise can increase inhibition (30). The therapeutic effect of chronic aerobic exercise on inhibition has also been reported. For example, MUD patients were given 12 weeks of moderate-intensity aerobic exercise training and found significant improvements in lipid peroxides and cognitive function (31). In addition to this, there have also been studies that have reported the efficacy of various forms of exercise. Acute VR competitive cycling can enhance information processing capacity by enhancing task-related cortical activation and brain functional networking in methamphetamine-dependent individuals (32).
Tai Chi is a traditional Chinese sport that is classified as a moderate-intensity exercise. Attention bias towards medication is an important indicator of MUD, and 4 weeks of tai chi exercise reduced the sensitivity and attention bias of medication-related cues in people with MUD, suggesting that tai chi exercise intervention may promote recovery from MUD through attention control (33).
3.3 Drug cravings
The potentiating effect of the drug depends primarily on dopamine signaling in the nucleus accumbens, and long-term drug exposure triggers glutamate-mediated neuroadaptation in the dopamine striatum-thalamic cortex (prefrontal cortex regions, including the orbitofrontal and anterior cingulate cortex) (60) and marginal pathways (amygdala and hippocampus), which can lead to addiction (73). Drug cravings are a key factor in maintaining drug dependence. methamphetamine users have more severe drug cravings and anxiety than heroin users (74, 75). Long-term drug abuse can induce the release of γ-aminobutyric acid and glutamate and the differential expression of DA neurons in the ventral tegmental region of the midbrain, leading to addiction and perpetuation of drug use (76–78). Some studies have also shown that homeostatic imbalance of midbrain dopamine caused by drug addiction is associated with decreased levels of dopamine D2 receptors in the striatum (42, 79). Numerous studies using positron tomography have demonstrated that the availability of dopamine D2/D3 receptors in the striatum is associated with drug cravings for methamphetamine-addicted (80, 81) and mediates impulsive temperament personality (82).
As a non-pharmacological treatment, exercise can activate the reward system, which in turn affects the neural circuits and neurotransmitter conduction of drug users, so exercise may reduce drug-induced dependent behaviors (17, 18, 83, 84). In the reward pathway of drug addiction, physical activity can enhance the ability of dopamine signaling to reduce drug overuse. In population experiments on methamphetamine addiction, subjects in the exercise group showed significant increases in striatal D2/D3 receptor availability (measured as non-displaceable binding potential (BPND)) (85). Furthermore, study have shown that wheeled running can improve methamphetamine-induced dopamine and serotonin homeostasis imbalances and regulate the normalization of reward pathways (86). Therefore, physical exercise may improve dopamine system function by promoting an increase in dopamine release and increasing the expression of dopamine D2 receptors, thereby improving brain reward pathways, reducing the drug craving (85).
A study reported that acute moderate-intensity dance and aerobic exercise may decrease cue-induced methamphetamine craving and improve food reward and appetite responses in women with MUD (34). 8 weeks of aerobic exercise combined with resistance training reduced levels of inflammatory factors in the peripheral blood and significantly reduced the craving for methamphetamine in people with MUD (35). A study in people dependent on methamphetamine showed that drug craving level decreased significantly during exercise, immediately after exercise, and 50 minutes after exercise, and drug craving scores at these time points after exercise were significantly lower than in the control group. In studies of MUD patients, 20 minutes of moderate-intensity acute aerobic exercise was found to temporarily relieve cravings in those with methamphetamine withdrawal (30). In a follow-up study, the investigators compared the dose-response relationship between different exercise intensities (low, moderate, and high intensity) and methamphetamine cravings. Furthermore, randomized controlled trials have demonstrated that tai chi can help reduce withdrawal symptoms by increasing individual self-awareness and introverted attention, and also have a good therapeutic effect on drug cravings in methamphetamine-dependent patients (36). In terms of intensity, results showed that methamphetamine craving scores decreased more in the moderate- and high-intensity exercise groups than in the low-intensity exercise and control groups during acute exercise, immediately after exercise, and at 50 minutes after exercise (25). Additionally, researchers found a significant reduction in the use of methamphetamine after eight weeks of aerobic exercise intervention (37).
Studies in animal models have shown that six weeks of treadmill exercise upregulate levels of dopamine D2 receptors in the striatum and regulate dopamine homeostasis in the midbrain (87).Furthermore, in methamphetamine-dependent rats, a reduction in methamphetamine craving induced by chronic wheel running is associated with the number of gray matter dopamine neurons around the midbrain aqueduct (88).
4 Discussion
The substance use disorder is a long-standing world problem. The increasing complexity of global drug abuse in the wake of the pandemic, and the inevitable cultivation of illicit drug plants triggered by the economic downturn, have given rise to flexible drug delivery models (89). Drug rehabilitation is a difficult and necessary task. Together, the results from this systematic review suggest there is consistent evidence that exercise therapy may play a positive role in drug rehabilitation through a variety of neurobiological mechanisms to regulate the physiological function and psychological state of people with MUD.
We included 17 studies within the last decade. The majority of included studies adopted randomized controlled design. The sample size ranged from 24 to 466 with a total of 1836 participants. The age of these participants ranged from 18 to 45 years old, with the average age mostly around 31 years old. They did not receive any specific treatment other than exercise interventions. The exercise group and controls were matched for age, education. Of these studies, exercise interventions include treadmill, tai chi, resistance exercises, cycling, and dance, with varying intensity and duration. The control group was given health education by watching relevant materials or videos.
In terms of intervention intensity, moderate-intensity exercise (16, 24–27, 30, 31, 34, 37)and high-intensity interval training (28, 29, 32) have been shown to have greater benefits. However, due to experimental constraints, these studies have been inconsistent on the duration of exercise, ranging from short-term acute aerobic exercise (24, 25, 30, 32, 34) to sustained exercise lasting weeks or months (16, 22, 23, 26–29, 31, 33, 35–37). Analyzing the effects of different intervention duration and intensity on people with MUD is conducive to the subsequent formulation of a unified exercise intervention treatment plan.
These studies confirmed that exercise interventions can reduce anxiety and depression symptoms (16, 22–24), enhance inhibitory control (25, 28–30, 33) and executive capacity (26, 27), and reduce the level of craving for methamphetamine (25, 30, 34–37) in people with MUD. Although further research on the efficacy of exercise interventions is required, based on the available evidence, we assume that exercise interventions have some advantages in the treatment of MUD.
The role of exercise in MUD is well reported. However, significant problems and challenges still remain, hindering the large-scale adoption of exercise interventions, that future research should address to better understand its clinical implications. Firstly, differences in the role of different exercise patterns, exercise frequency and duration in suppressing addictive behaviors are not exactly clear. Secondly, no efficient, sensitive and objective biomarkers have been found, the possibility of BDNF and proBNDF as biomarkers to predicate disease stage, synaptic plasticity, and therapeutic efficacy could be considered in the future (90, 91). Finally, patients with different states need a normative guideline for exercise interventions. To address these issues, further analyses of the neurobiological mechanisms of physical activity interventions are necessary.
In particular, more specific neural network mechanisms, and more in vivo evidence of the addiction model are needed to provide more theoretical support. Besides, preclinical studies and clinical trials could combine physical activity with some promising treatments or medicaments, such as repetitive transcranial magnetic stimulation (92) or bupropion, mirtazapine, and methylphenidate (93).Psychosocial and psychological interventions have also been proved to be effective in patients with mood disorders (94). Physical activity may improve the efficacy of these promising therapy. Moreover, there are many types of drugs except methamphetamine. The role of physical exercise in patients who use different types of drugs, as well as the development of personalized exercise prescriptions, are also the directions of further research and analysis.
Additionally, many forms of exercise can be used as effective intervention methods in drug treatment, such as mindfulness meditation (95, 96), traditional Chinese health exercises (28, 33, 36, 97)(e.g. Health Qigong, Tai Chi), and other forms of exercise that take into account both physical and psychological aspects. The practice of those therapies requires the elimination of distractions, concentration, and a calm mind. This is actually a process of psychological adjustment, allowing people in forced rehabilitation to regain their concentration, strengthen their inner self-awareness, and then get rid of negative emotions. Furthermore, traditional health-preserving exercises are mostly group activities and group communication activities, which can promote people’s socialization and interpersonal relationship development, thereby regulating negative emotions and enhancing happiness. More evidence-based medical evidence is needed on these forms of exercise.
5 Conclusion
In summary, exercise could be used as a safe, accessible, and effective intervention to alleviate the mental health problems associated with MUD. It is anticipated that continued research on the therapeutic role of physical exercise interventions will aid in the development of effective drug rehabilitation programs for the treatment of drug-dependent individuals. However, there is a need to collect more high-quality evidences all over the world, and the efficacy and sustainability of physical activity interventions should be evaluated in more longer-term studies.
6 Limitations of the study
This systematic review presents several limitations: The number of studies included in this review was limited. Moreover, due to study constraints, the gender distribution of study participants, the modalities of exercise interventions, and the interventions in the control group were also inconsistent. Most of these studies came from China due to its compulsory drug rehabilitation institutions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Author contributions
CX: Writing – original draft, Writing – review & editing. ZZ: Writing – review & editing. DH: Writing – review & editing. GW: Writing – review & editing. CL: Writing – review & editing. XM: Writing – review & editing. KW: Funding acquisition, Writing – original draft, Writing – review & editing. HL: Funding acquisition, Writing – original draft, Writing – review & editing. MZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Yunnan Technological Innovation Centre of Drug Addiction Medicine (Grant Number: 202305AK340001), Yunnan Province Expert Workstation of Cangming Huang (NO. 202105AF150040), Yunnan Province Technology Industry Leading Talents Support Program, Yunnan Fundamental Research projects(Grant Number: 202202AS070077, 202201AY070001-079, 202201AU070227), and Yunnan Provincial Department of Education (2023J0011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MUD, methamphetamine use disorder; AMTSASR2, A Measurement Tool to Assess Systematic Review, version 2; DA, dopamine; NE, norepinephrine; 5-HT, 5-Hydroxytryptamine; BDNF, brain-derived neurotrophic factor; HPA, hypothalamic-pituitary-adrenal axis; BPND, nondisplaceable binding potential.
References
1. Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: A review. JAMA Psychiatry. (2020) 77:959. doi: 10.1001/jamapsychiatry.2020.0246
2. United Nations Office on Drugs and Crime. World Drug Report 2023. United Nations publication: The United Nations Office on Drugs and Crime (UNODC (2023).
3. Han B, Compton WM, Jones CM, Einstein EB, Volkow ND. Methamphetamine use, methamphetamine use disorder, and associated overdose deaths among US adults. JAMA Psychiatry. (2021) 78:1329–42. doi: 10.1001/jamapsychiatry.2021.2588
4. Jones CM, Compton WM, Mustaquim D. Patterns and characteristics of methamphetamine use among adults - United States, 2015–2018. MMWR. Morbidity mortality weekly Rep. (2020) 69:317–23. doi: 10.15585/mmwr.mm6912a1
5. Martinotti G, Merino Del Villar C, Garcia Cordoba A, Andrés Tubau L, Castro Sánchez I, Di Carlo F, et al. Club drugs and psychiatric sequelae: an issue of vulnerability and previous psychiatric history. Int J Environ Res Public Health. (2021) 18:6944. doi: 10.3390/ijerph18136944
6. Powell KE, Paluch AE, Blair SN. Physical activity for health: What kind? How much? How intense? On top of what? Annu Rev Public Health. (2011) 32:349–65. doi: 10.1146/annurev-publhealth-031210-101151
7. Voelcker-Rehage C, Niemann C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci Biobehav Rev. (2013) 37:2268–95. doi: 10.1016/j.neubiorev.2013.01.028
8. Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. (2018) 52:154–60. doi: 10.1136/bjsports-2016-096587
9. Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. (2008) 9:58–65. doi: 10.1038/nrn2298
10. Chen C, Nakagawa S. Physical activity for cognitive health promotion: An overview of the underlying neurobiological mechanisms. Ageing Res Rev. (2023) 86:101868. doi: 10.1016/j.arr.2023.101868
11. Lb A. Physical activity and health. BMJ (Clinical Res ed.). (2007) 334(7605):1173. doi: 10.1136/bmj.39225.414537.80
12. Liu Xx, Wang S. Effects of aerobic exercise combined with attentional bias training on cognitive function and psychiatric symptoms of individuals with methamphetamine dependency: a randomized controlled trial. Int J Ment Health Addict. (2021) 29(4):e164. doi: 10.1007/s11469-021-00686-w
13. Cheng T, Huang XD, Hu XF, Wang SQ, Chen K, Wei JA, et al. Physical exercise rescues cocaine-evoked synaptic deficits in motor cortex. Mol Psychiatry. (2021) 26:6187–97. doi: 10.1038/s41380-021-01336-2
14. Vints WAJ, Levin O, Fujiyama H, Verbunt J, Masiulis N. Exerkines and long-term synaptic potentiation: Mechanisms of exercise-induced neuroplasticity. Front Neuroendocrinol. (2022) 66:100993. doi: 10.1016/j.yfrne.2022.100993
15. Sampogna G, Luciano M, Di Vincenzo M, Andriola I, D’Ambrosio E, Amore M, et al. The complex interplay between physical activity and recovery styles in patients with severe mental disorders in a real-world multicentric study. Front Psychiatry. (2022) 13:945650. doi: 10.3389/fpsyt.2022.945650
16. Xu J, Zhu Z, Liang X, Huang Q, Zheng T, Li X. Effects of moderate-intensity exercise on social health and physical and mental health of methamphetamine-dependent individuals: A randomized controlled trial. Front Psychiatry. (2022) 13:997960. doi: 10.3389/fpsyt.2022.997960
17. Schoenfeld TJ, Swanson C. A runner’s high for new neurons? Potential role for endorphins in exercise effects on adult neurogenesis. Biomolecules. (2021) 11:1077. doi: 10.3390/biom11081077
18. Kang GM, Min SH, Lee CH, Kim JY, Lim HS, Choi MJ, et al. Mitohormesis in hypothalamic POMC neurons mediates regular exercise-induced high-turnover metabolism. Cell Metab. (2021) 33:334–349.e6. doi: 10.1016/j.cmet.2021.01.003
19. Cassilhas RC, Tufik S, de Mello MT. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life sciences: CMLS. (2016) 73:975–83. doi: 10.1007/s00018-015-2102-0
20. Sampogna G, Luciano M, Di Vincenzo M, Toni C, D’Ambrosio E, Rampino A, et al. Physical activity influences adherence to pharmacological treatments in patients with severe mental disorders: results from the multicentric, randomized controlled LIFESTYLE trial. Front Pharmacol. (2023) 14:1285383. doi: 10.3389/fphar.2023.1285383
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed.). (2021) 372:n71. doi: 10.1136/bmj.n71
22. Haglund M, Ang A, Mooney L, Gonzales R, Chudzynski J, Cooper CB, et al. Predictors of depression outcomes among abstinent methamphetamine-dependent individuals exposed to an exercise intervention. Am J Addict. (2015) 24(3):246–51. doi: 10.1111/j.1521-0391.2014.12175.x
23. Rawson RA, Chudzynski J, Gonzales R, Mooney L, Dickerson D, Ang A, et al. The impact of exercise on depression and anxiety symptoms among abstinent methamphetamine-dependent individuals in A residential treatment setting. J Subst Abuse Treat. (2015) 57:36–40. doi: 10.1016/j.jsat.2015.04.007
24. Tao Q, Zhang C, Li X. Dancing improves emotional regulation in women with methamphetamine use disorder but use of a cycle ergometer does not. Front Neurosci. (2021) 15. doi: 10.3389/fnins.2021.629061
25. Wang D, Zhou C, Zhao M, Wu X, Chang YK. Dose-response relationships between exercise intensity, cravings, and inhibitory control in methamphetamine dependence: An ERPs study. Drug Alcohol Depend. (2016) 161:331–9. doi: 10.1016/j.drugalcdep.2016.02.023
26. Liu Xx, Wang S. Effect of aerobic exercise on executive function in individuals with methamphetamine use disorder: Modulation by the autonomic nervous system. Psychiatry Res. (2021) 306:114241. doi: 10.1016/j.psychres.2021.114241
27. Liu J, Chen C, Liu M, Zhuang S. Effects of aerobic exercise on cognitive function in women with methamphetamine dependence in a detoxification program in Tianjin, China: A randomized controlled trial. J Nurs research: JNR. (2021) 29(4):e164. doi: 10.1097/JNR.0000000000000440
28. Yin Y, Yang S, Xiao K, Wang T, Wang J, Schöllhorn WI, et al. Comparison of the acute effects of Tai chi versus high-intensity interval training on inhibitory control in individuals with substance use disorder. Front Psychol. (2022) 13:941719. doi: 10.3389/fpsyg.2022.941719
29. Menglu S, Suyong Y, Xiaoyan W, Schöllhorn WI, Dong Z. Cognitive effectiveness of high-intensity interval training for individuals with methamphetamine dependence: a study protocol for randomised controlled trial. Trials. (2021) 22:650. doi: 10.1186/s13063-021-05615-9
30. Wang D, Zhou C, Chang YK. Acute exercise ameliorates craving and inhibitory deficits in methamphetamine: An ERP study. Physiol Behav. (2015) 147:38–46. doi: 10.1016/j.physbeh.2015.04.008
31. Zhang K, Zhang Q, Jiang H, Du J, Zhou C, Yu S, et al. Impact of aerobic exercise on cognitive impairment and oxidative stress markers in methamphetamine-dependent patients. Psychiatry Res. (2018) 266:328–33. doi: 10.1016/j.psychres.2018.03.032
32. Qi L, Yin Y, Bu L, Tang Z, Tang L, Dong G. Acute VR competitive cycling exercise enhanced cortical activations and brain functional network efficiency in MA-dependent individuals. Neurosci Lett. (2021) 757:135969. doi: 10.1016/j.neulet.2021.135969
33. He M, Yang S, Miao Y, Zhang W, Zhu D, Xu D. Four-week Tai Chi intervention decreases attention bias to drug cues in individuals with methamphetamine use disorder. Am J Drug Alcohol Abuse. (2021) 47:638–48. doi: 10.1080/00952990.2021.1950745
34. Zhou YU, Finlayson G, Liu X, Zhou Q, Liu T, Zhou C. Effects of acute dance and aerobic exercise on drug craving and food reward in women with methamphetamine dependence. Med Sci Sports Exercise. (2021) 53:2245–53. doi: 10.1249/MSS.0000000000002723
35. Wang J, Lu C, Zheng L, Zhang J. Peripheral inflammatory biomarkers of methamphetamine withdrawal patients based on the neuro-inflammation hypothesis: the possible improvement effect of exercise. Front Psychiatry. (2021) 12:795073. doi: 10.3389/fpsyt.2021.795073
36. Wang M, Chen Y, Xu Y, Zhang X, Sun T, Li H, et al. A randomized controlled trial evaluating the effect of Tai Chi on the drug craving in women. Int J Ment Health Addict. (2022), 1–13. doi: 10.1007/s11469-022-00917-8
37. Rawson RA, Chudzynski J, Mooney L, Gonzales R, Ang A, Dickerson D, et al. Impact of an exercise intervention on methamphetamine use outcomes post-residential treatment care. Drug Alcohol Depend. (2015) 156:21–8. doi: 10.1016/j.drugalcdep.2015.08.029
38. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical Res ed.). (2017) 358:j4008. doi: 10.1136/bmj.j4008
39. Glasner- Edwards S, Lj M. Methamphetamine psychosis: epidemiology and management. CNS Drugs. (2014) 28(12):1115–26. doi: 10.1007/s40263-014-0209-8
40. Stellern J, Xiao KB, Grennell E, Sanches M, Gowin JL, Sloan ME. Emotion regulation in substance use disorders: a systematic review and meta-analysis. Addict (Abingdon England). (2023) 118:30–47. doi: 10.1111/add.16001
41. Shukla M, Vincent B. Methamphetamine abuse disturbs the dopaminergic system to impair hippocampal-based learning and memory: An overview of animal and human investigations. Neurosci Biobehav Rev. (2021) 131:541–59. doi: 10.1016/j.neubiorev.2021.09.016
42. Proebstl L, Kamp F, Manz K, Krause D, Adorjan K, Pogarell O, et al. Effects of stimulant drug use on the dopaminergic system: A systematic review and meta-analysis of in vivo neuroimaging studies. Eur Psychiatry: J Assoc Eur Psychiatrists. (2019) 59:15–24. doi: 10.1016/j.eurpsy.2019.03.003
43. Miller DR, Bu M, Gopinath A, Martinez LR, Khoshbouei H. Methamphetamine dysregulation of the central nervous system and peripheral immunity. J Pharmacol Exp Ther. (2021) 379:372–85. doi: 10.1124/jpet.121.000767
44. Inserra A, De Gregorio D, Gobbi G. Psychedelics in psychiatry: neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacol Rev. (2021) 73:202–77. doi: 10.1124/pharmrev.120.000056
45. Wise RA, Robble MA. Dopamine and addiction. Annu Rev Psychol. (2020) 71:79–106. doi: 10.1146/annurev-psych-010418-103337
46. Ferrucci M, Giorgi FS, Bartalucci A, Busceti CL, Fornai F. The effects of locus coeruleus and norepinephrine in methamphetamine toxicity. Curr Neuropharmacology. (2013) 11:80–94. doi: 10.2174/157015913804999522
47. Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clinics North America. (2013) 36:261–75. doi: 10.1016/j.psc.2013.02.005
48. Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. (2001) 158:1206–14. doi: 10.1176/appi.ajp.158.8.1206
49. Prakash MD, Tangalakis K, Antonipillai J, Stojanovska L, Nurgali K, Apostolopoulos V, et al. Methamphetamine: Effects on the brain, gut and immune system. Pharmacol Res. (2017) 120:60–7. doi: 10.1016/j.phrs.2017.03.009
50. Harro J. Neuropsychiatric adverse effects of amphetamine and methamphetamine. Int Rev Neurobiol. (2015) 120:179–204. doi: 10.1016/bs.irn.2015.02.004
51. O’Malley KY, Hart CL, Casey S, Downey LA. Methamphetamine, amphetamine, and aggression in humans: A systematic review of drug administration studies. Neurosci Biobehav Rev. (2022) 141:104805. doi: 10.1016/j.neubiorev.2022.104805
52. Hellem TL. A review of methamphetamine dependence and withdrawal treatment: A focus on anxiety outcomes. J Subst Abuse Treat. (2016) 71:16–22. doi: 10.1016/j.jsat.2016.08.011
53. Chang X, Sun Y, Zhang Y, Muhai J, Lu L, Shi J. A review of risk factors for methamphetamine-related psychiatric symptoms. Front Psychiatry. (2018) 9:603. doi: 10.3389/fpsyt.2018.00603
54. Malhi GS, Mann J. Depression. Lancet (London England). (2018) 392:2299–312. doi: 10.1016/S0140-6736(18)31948-2
55. Smith PJ, Merwin RM. The role of exercise in management of mental health disorders: an integrative review. Annu Rev Med. (2021) 72:45–62. doi: 10.1146/annurev-med-060619-022943
56. Re GF, Li H, Yang JQ, Li Y, Zhang Z, Wu X, et al. Exercise modulates central and peripheral inflammatory responses and ameliorates methamphetamine-induced anxiety-like symptoms in mice. Front Mol Neurosci. (2022) 15:955799. doi: 10.3389/fnmol.2022.955799
57. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. (2016) 67:23–50. doi: 10.1146/annurev-psych-122414-033457
58. Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. (2018) 21:1520–9. doi: 10.1038/s41593-018-0246-6
59. Smith JL, Mattick RP, Jamadar SD, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend. (2014) 145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009
60. Tian W, Zhao D, Ding J, Zhan S, Zhang Y, Etkin A, et al. An electroencephalographic signature predicts craving for methamphetamine. Cell Rep Med. (2024) 5:101347. doi: 10.1016/j.xcrm.2023.101347
61. London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. (2015) 1628:174–85. doi: 10.1016/j.brainres.2014.10.044
62. Jia X, Wang J, Jiang W, Kong Z, Deng H, Lai W, et al. Common gray matter loss in the frontal cortex in patients with methamphetamine-associated psychosis and schizophrenia. NeuroImage. Clin. (2022) 36:103259. doi: 10.1016/j.nicl.2022.103259
63. Ding J, Huang J, Tang X, Shen L, Hu S, He J, et al. Low and high dose methamphetamine differentially regulate synaptic structural plasticity in cortex and hippocampus. Front Cell Neurosci. (2022) 16:1003617. doi: 10.3389/fncel.2022.1003617
64. Liang M, Zhu L, Wang R, Su H, Ma D, Wang H, et al. Methamphetamine exposure in adolescent impairs memory of mice in adulthood accompanied by changes in neuroplasticity in the dorsal hippocampus. Front Cell Neurosci. (2022) 16:892757. doi: 10.3389/fncel.2022.892757
65. Iino Y, Sawada T, Yamaguchi K, Tajiri M, Ishii S, Kasai H, et al. Dopamine D2 receptors in discrimination learning and spine enlargement. Nature. (2020) 579:555–60. doi: 10.1038/s41586-020-2115-1
66. Mizoguchi H, Yamada K. Methamphetamine use causes cognitive impairment and altered decision-making. Neurochemistry Int. (2019) 124:106–13. doi: 10.1016/j.neuint.2018.12.019
67. Fitzpatrick RE, Rubenis AJ, Lubman DI, Verdejo-Garcia A. Cognitive deficits in methamphetamine addiction: Independent contributions of dependence and intelligence. Drug Alcohol Depend. (2020) 209:107891. doi: 10.1016/j.drugalcdep.2020.107891
68. Bernheim A, See RE, Reichel CM. Chronic methamphetamine self-administration disrupts cortical control of cognition. Neurosci Biobehav Rev. (2016) 69:36–48. doi: 10.1016/j.neubiorev.2016.07.020
69. Chen X, Wu X, Wu H, Zhang M. Phase separation at the synapse. Nat Neurosci. (2020) 23:301–10. doi: 10.1038/s41593-019-0579-9
70. Sanders LMJ, Hortobágyi T, Karssemeijer EGA, Van der Zee EA, Scherder EJA, van Heuvelen MJG. Effects of low- and high-intensity physical exercise on physical and cognitive function in older persons with dementia: a randomized controlled trial. Alzheimer’s Res Ther. (2020) 12:28. doi: 10.1186/s13195-020-00597-3
71. Yu F, Vock DM, Zhang L, Salisbury D, Nelson NW, Chow LS, et al. Cognitive effects of aerobic exercise in alzheimer’s disease: A pilot randomized controlled trial. J Alzheimer’s disease: JAD. (2021) 80:233–44. doi: 10.3233/JAD-201100
72. Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ (Clinical Res ed.). (2018) 361:k1675. doi: 10.1136/bmj.k1675
73. Volkow ND, Michaelides M, Baler R. The neuroscience of drug reward and addiction. Physiol Rev. (2019) 99:2115–40. doi: 10.1152/physrev.00014.2018
74. Yuan J, Liu XD, Han M, Lv RB, Wang YK, Zhang GM, et al. Comparison of striatal dopamine transporter levels in chronic heroin-dependent and methamphetamine-dependent subjects. Addict Biol. (2017) 22:229–34. doi: 10.1111/adb.12271
75. Luo D, Tan L, Shen D, Gao Z, Yu L, Lai M, et al. Characteristics of depression, anxiety, impulsivity, and aggression among various types of drug users and factors for developing severe depression: a cross-sectional study. BMC Psychiatry. (2022) 22:274. doi: 10.1186/s12888-022-03933-z
76. Yang J, Chen J, Liu Y, Chen KH, Baraban JM, Qiu Z. Ventral tegmental area astrocytes modulate cocaine reward by tonically releasing GABA. Neuron. (2023) 111:1104–1117.e6. doi: 10.1016/j.neuron.2022.12.033
77. Buck SA, Torregrossa M, Logan RW, Freyberg Z. Roles of dopamine and glutamate co-release in the nucleus accumbens in mediating the actions of drugs of abuse. FEBS J. (2021) 288:1462–74. doi: 10.1111/febs.15496
78. Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, et al. Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron. (2012) 73:978–89. doi: 10.1016/j.neuron.2011.12.031
79. Ashok AH, Mizuno Y, Volkow ND, Howes OD. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: A systematic review and meta-analysis. JAMA Psychiatry. (2017) 74:511–9. doi: 10.1001/jamapsychiatry.2017.0135
80. Morales AM, Kohno M, Robertson CL, Dean AC, Mandelkern MA, London ED. Gray-matter volume, midbrain dopamine D2/D3 receptors and drug craving in methamphetamine users. Mol Psychiatry. (2015) 20:764–71. doi: 10.1038/mp.2015.47
81. Moeller SJ, Okita K, Robertson CL, Ballard ME, Konova AB, Goldstein RZ, et al. Low striatal dopamine D2-type receptor availability is linked to simulated drug choice in methamphetamine users. Neuropsychopharmacology: Off Publ Am Coll Neuropsychopharmacol. (2018) 43:751–60. doi: 10.1038/npp.2017.138
82. Kohno M, Okita K, Morales AM, Robertson CL, Dean AC, Ghahremani DG, et al. Midbrain functional connectivity and ventral striatal dopamine D2-type receptors: link to impulsivity in methamphetamine users. Mol Psychiatry. (2016) 21:1554–60. doi: 10.1038/mp.2015.223
83. Landolfi E. Exercise addiction. Sports Med (Auckland N.Z.). (2013) 43:111–9. doi: 10.1007/s40279-012-0013-x
84. Dohnalova L, Lundren P, Jre C, Goldstein N, Wenski SL, Nanudorn P, et al. A microbiome-dependent gut-brain pathway regulates motivation for exercise. Nature. (2022) 612(7941):739–47. doi: 10.1038/s41586-022-05525-z
85. Robertson CL, Ishibashi K, Chudzynski J, Mooney LJ, Rawson RA, Dolezal BA, et al. Effect of exercise training on striatal dopamine D2/D3 receptors in methamphetamine users during behavioral treatment. Neuropsychopharmacology: Off Publ Am Coll Neuropsychopharmacol. (2016) 41:1629–36. doi: 10.1038/npp.2015.331
86. O’Dell SJ, Galvez BA, Ball AJ, Marshall JF. Running wheel exercise ameliorates methamphetamine-induced damage to dopamine and serotonin terminals. Synapse (New York N.Y.). (2012) 66:71–80. doi: 10.1002/syn.20989
87. Robison LS, Swenson S, Hamilton J, Thanos PK. Exercise reduces dopamine D1R and increases D2R in rats: implications for addiction. Med Sci Sports Exercise. (2018) 50:1596–602. doi: 10.1249/MSS.0000000000001627
88. Sobieraj JC, Kim A, Fannon MJ, Mandyam CD. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Structure Funct. (2016) 221:261–76. doi: 10.1007/s00429-014-0905-7
89. United Nations Office on Drugs and Crime. World Drug Report 2022. United Nations publication: The United Nations Office on Drugs and Crime (UNODC (2022).
90. Pettorruso M, Miuli A, Clemente K, Mancusi G, Migliara G, Di Carlo F, et al. Enhanced peripheral levels of BDNF and proBDNF: elucidating neurotrophin dynamics in cocaine use disorder. Mol Psychiatry. (2024). doi: 10.1038/s41380-023-02367-7
91. Miuli A, d’Andrea G, Pettorruso M, Mancusi G, Mosca A, Di Carlo F, et al. From a cycle to a period: the potential role of BDNF as plasticity and phase-specific biomarker in cocaine use disorder. Curr Neuropharmacology. (2022) 20:2024–8. doi: 10.2174/1570159X20666220114152052
92. Martinotti G, Pettorruso M, Montemitro C, Spagnolo PA, Acuti Martellucci C, Di Carlo F, et al. Repetitive transcranial magnetic stimulation in treatment-seeking subjects with cocaine use disorder: A randomized, double-blind, sham-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. (2022) 116:110513. doi: 10.1016/j.pnpbp.2022.110513
93. Siefried KJ, Acheson LS, Lintzeris N, Ezard N. Pharmacological treatment of methamphetamine/amphetamine dependence: A systematic review. CNS Drugs. (2020) 34(4):337–65. doi: 10.1007/s40263-020-00711-x
94. Chiappini S, Di Carlo F, Mosca A, d’Andrea G, Di Paolo M, Lorenzini C, et al. Efficacy of psychosocial and psychological interventions in addition to drug therapy to improve global functioning of inpatients with schizophrenia spectrum and mood disorders: A real-world observational study. Neuropsychiatr Dis Treat. (2023) 19:1887–97. doi: 10.2147/NDT.S418627
95. Jarrett O, London ED, Mahmoudie T, Suh J, Ghahremani D, Dean AC. Mindfulness and clinical correlates in methamphetamine use disorder. Drug Alcohol Depend. (2023) 253:111029. doi: 10.1016/j.drugalcdep.2023.111029
96. Maneesang W, Hengpraprom S, Kalayasiri R. Effectiveness of Mindfulness - Based Therapy and Counseling programs (MBTC) on relapses to methamphetamine dependence at a substance dependency treatment center. Psychiatry Res. (2022) 317:114886. doi: 10.1016/j.psychres.2022.114886
Keywords: methamphetamine use disorder, physical exercise, depression, anxiety, cognition, drug craving
Citation: Xu C, Zhang Z, Hou D, Wang G, Li C, Ma X, Wang K, Luo H and Zhu M (2024) Effects of exercise interventions on negative emotions, cognitive performance and drug craving in methamphetamine addiction. Front. Psychiatry 15:1402533. doi: 10.3389/fpsyt.2024.1402533
Received: 19 March 2024; Accepted: 30 April 2024;
Published: 17 May 2024.
Edited by:
Giovanni Martinotti, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Stefania Chiappini, Saint Camillus International University of Health and Medical Sciences, ItalyFrancesco Di Carlo, University of Studies G. d’Annunzio Chieti and Pescara, Italy
Copyright © 2024 Xu, Zhang, Hou, Wang, Li, Ma, Wang, Luo and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kunhua Wang, d2FuZ2t1bmh1YTE5NjRAMTI2LmNvbQ==; Huayou Luo, MTY3NzU0NjI5NkBxcS5jb20=; Mei Zhu, emh1bWVpc0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Conghui Xu
Conghui Xu Zunyue Zhang
Zunyue Zhang Dezhi Hou1,3
Dezhi Hou1,3