
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 28 June 2024
Sec. Psychopathology
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1389093
 Daniela V. Pinto Payares1*
Daniela V. Pinto Payares1* Logan Spooner1
Logan Spooner1 Jennifer Vosters1
Jennifer Vosters1 Samantha Dominguez1
Samantha Dominguez1 Lauren Patrick1
Lauren Patrick1 Ann Harris2
Ann Harris2 Shibani Kanungo3,4*
Shibani Kanungo3,4*Introduction: Mitochondrial diseases are known inborn errors affecting energy metabolism and are as common as chronic diseases such as diabetes, affecting approximately 1 in 5,000 people. The role of mitochondrial diseases/dysfunction has been highlighted in neurodevelopmental disorders like ASD, ADHD, intellectual disability, and speech delay, as well as various psychiatric conditions. Neurodevelopmental disorders are increasingly recognized as having behavioral and psychiatric symptoms. Our study aimed to investigate reports of mitochondrial disorders, noting neurodevelopmental disorders and psychiatric/behavioral conditions.
Methods: This was done through a systematic review of literature from PubMed/MEDLINE, Scopus, and Cochrane Library up to November 2022.
Results: We found 277 publications, of which 139 met the inclusion criteria. We mostly found review articles with mention of mitochondrial dysfunction/disorder in relation to ASD with brief mentions of psychiatric/behavioral comorbidities.
Discussion: This suggests a need for broader research efforts beyond ASD to understand the relationship between mitochondrial disorder or dysfunction and various neurodevelopmental and psychiatric/behavioral comorbidities.
Mitochondria are subcellular organelles with numerous functions, including energy production through oxidative phosphorylation (OXPHOS), calcium homeostasis, reactive oxygen species production, scavenging, regulation of apoptosis, and activating caspase family of proteases (1, 2). Defects in mitochondrial gene expression can lead to failure of respiratory chain enzymes and ATP synthase, impairing OXPHOS, which is essential for cellular energy production (3). Mitochondrial disorders are complex multisystemic diseases with heterogenous clinical manifestations and can affect every organ and system (2). Mitochondrial diseases are one of the most prevalent inborn errors in metabolism, affecting about every 1 in 5,000 people (4), up to 1 in 200 infants harbor mtDNA mutations (5). Primary mitochondrial disorders have been traditionally defined as resulting from any mutations in mitochondrial DNA (mtDNA) or nuclear DNA (nDNA). The complex relationship between nDNA and mtDNA genomes and more pathogenic variants (6) has generated debate on the definition based on OXPHOS function or mitochondrial structure and function (2). In primary mitochondrial disorders, high energy demand tissues are affected, resulting in neurologic, vision, hearing, neuromuscular, neurodevelopmental, and behavioral abnormalities.
Mitochondrial disorders are primarily of maternal inheritance but can also have autosomal dominant (AD), autosomal recessive (AR), and X-linked inheritance (6). Additionally, heteroplasmy can have varying effects on different family members (5).
Environmental stimuli producing reactive oxygen species (ROS) can affect mitochondrial function and, with stress, trigger energy deficits of a primary mitochondrial disorder (3). Mitochondrial dysfunction can affect DNA, protein, and lipids and has been implicated in type 2 diabetes, obesity, hypertension, and cardiovascular diseases, severe psychiatric neurological disorders, including schizophrenia, bipolar disorder, Alzheimer’s, and Rett Syndrome (7). Diagnosing mitochondrial disorders comes with many challenges: which tissue will provide the best sample, analyzing hundreds of mtDNA and nDNA gene variations, and epigenetic influences that are not fully understood.
The Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 defines neurodevelopmental disorders (NDDs) as conditions such as intellectual developmental disorder, communication disorders, autism spectrum disorder, attention deficit hyperactivity disorder, specific learning disorder, and motor disorders that affect developmental milestones since infancy, impairing personal, social, academic, motor, or occupational functions (8) as described below in Table 1.
Earlier suggestions of an association between autism and disorders of mitochondrial oxidative phosphorylation (9–11), have moved towards environmental factors affecting mitochondrial dysfunction as a factor in the development and progression of ASD (7, 12–18). The mechanism of how mitochondrial dysfunction leads to an ASD phenotype is not known, but research suggests oxidative defects causing disruption in early brain development is a plausible mechanism (12).
Neurocognitive disorders (NCDs) such as Alzheimer’s, Dementia, and Parkinsonism has acquired decline in cognitive functioning as prominent clinical feature. (8).
Psychiatric disorders are behavioral, emotional, or cognitive dysfunctions in one or more areas, affecting social, occupational, and interpersonal functioning (19, 20). A few disorder related features are as described in Table 2:
Studies have shown that NDD are increasingly recognized as having behavioral and psychiatric symptoms (21). ASD has been associated with higher rates of mood and anxiety disorders, obsessive-compulsive disorder, schizophrenia, and other psychiatric disorders. Suicide attempts are also more common in this population (21). A high comorbidity between autism and ADHD seem to be connected, presenting as social deficits from an etiological point of view (22).
Current literature highlights the role of mitochondrial disorder/dysfunction in numerous neurodevelopmental disorders, such as ASD, ADHD, intellectual disability, and speech delay (2, 4, 7, 11, 23). Mitochondrial abnormalities have been noted in various psychiatric disorders as well. Patients presenting with psychiatric symptoms later were found to have a primary mitochondrial disorder (24). Paranoid psychiatric symptoms and borderline personality disorder have been seen in patients later diagnosed with MELAS (25, 26). Individuals with hereditary sensorimotor neuropathy (HN) and mitochondrial mutations scored higher on depression screening scales and had a higher lifetime prevalence of psychiatric diagnoses and personality disorders (27).
Similarly, environmental factors affecting mitochondria, abnormalities in morphology and density of mitochondria, multiple mtDNA mutations, and SNPs have been implicated in the pathophysiology of many psychiatric disorders (28–30). While a genotype/psychiatric phenotype correlation has not been associated with any specific mitochondrial deletion or copy number variant (28), both animal and human subjects with mitochondrial disease presented with decreased neuronal function and had subsequent behavioral deficits consistent with psychiatric disorders (31). Mitochondrial dysfunction has been implicated in depression and schizophrenia. Specific mitochondrial disorders and dysfunctions have been identified in connection with certain psychiatric and neurodevelopmental disorders (23).
Our study aimed to investigate through a systematic review of literature reports of mitochondrial disorders noting neurodevelopmental disorders and psychiatric/behavioral disorders.
We conducted a (systematic) review of literature in PubMed/MEDLINE, Scopus, and Cochrane Library from inception to November 2022. The PubMed search strategy included keywords with asterisks (*) to capture the variation of the individual keyword as well as terms from the Medical Subject Headings Database (MeSH Database, https://www.ncbi.nlm.nih.gov/mesh/). Specific search tags used included[Mesh]: (“Mitochondrial Diseases”[Mesh] OR mitochondrial disease*) AND (“Neurodevelopmental Disorders”[Mesh] OR neurodevelopmental disorder*) AND (“Behavior”[Mesh] OR behavior*). Literature searches in Scopus and Cochrane Library included keywords (“mitochondrial disease*”) AND (“neurodevelopmental disorder*”) AND (behavior). We also searched the ClinicalTrials.gov database (https://clinicaltrials.gov/) using the keywords “mitochondrial disease,” “neurodevelopmental disorder, and behavior for any relevant clinical trials or studies. No filters or limiters were applied to ensure relevant literature was captured from inception to November 2022. All references identified from the searches were imported into Mendeley Reference Manager citation management software. Software and manual checks were made to remove duplicate citations. All remaining citations were imported and organized into a Microsoft Excel spreadsheet. Full texts of those citations were downloaded and imported into Mendeley.
Inclusion criteria:
● Full text
● Texts with English language availability
● Human subjects
● Translational cell studies with human cell lines
Exclusion criteria:
● No full text available
● Texts without English language available
● Animal studies
Publications were categorized as animal if animals or animal-derived cells were the models used. Publications were classified as human studies when human subjects or cell lines were used. Human studies were further classified by type of publications as below:
● Review
● Metanalysis
● Cohort- Prospective/Retrospective
● Case Reports
● Case Series
● Case Control
● Observation
● Randomized control trial (RCT) including one translational study.
● In Vitro – included cell line studies
● Clinical Trials – all were listed in clinicaltrials.gov and/or Cochrane library
Publications were further noted if the paper discussed a mitochondrial disorder (mtDNA, nDNA, or OXPHOS defects) or a mitochondrial dysfunction and were screened for neurodevelopmental disorders and/or behavioral disorders were mentioned in them.
Data was examined to identify how many publications mentioned any neurodevelopmental disorder and/or psychiatric/behavioral disorder (Intellectual Disability: Mild, Intellectual Disability: Moderate, Intellectual Disability: Severe, Intellectual Disability (Nonspecific), Language Disorder, ASD, ADHD: Hyperactive, Cerebral Palsy, Schizophrenia, PTSD, Major Depressive Disorder: mild (general), Neurocognitive Disorder Possibly Due to Alzheimer, Bipolar disorder (general/nonspecific), Generalized Anxiety Disorder (general), Personality disorders (general/nonspecific), Neurocognitive Disorder Possibly Due to Parkinson’s, OCD, Dementia), in each type of publication (Review, Prospective, Meta, Cohort, Case Reports, Case Series, Case Control, Observation, Retrospective, RCT, In Vitro, Clinical Trials.). If any single publication mentioned more than one neurodevelopmental disorder and/or psychiatric/behavioral disorder, they were counted under each individual disorder category.
Our literature search yielded 277 publications from the above four databases. PubMed returned 168 results, Scopus returned 96 results, ClinicalTrials.gov returned 9 results, and Cochrane Database returned 4 results. Of the 277 total publications, 42 duplicate records were removed. This led to 235 publications being screened with 95 meeting exclusion criteria and 139 meeting inclusion criteria articles. See Figure 1 (flow chart) for more details.
Of the 139 publications included, 72 were identified as reviews where 62 noted mitochondrial dysfunction and 13 included mitochondrial disorders and or dysfunction; 13 were case reports with 5 discussing mitochondrial dysfunction and 7 relating to mitochondrial disorder. 1 case series noting mitochondrial dysfunction; 8 Observational with 5 reported on mitochondrial dysfunction and 2 on mitochondrial disorder; 5 prospective studies contained 3 mitochondrial dysfunction and 2 mitochondrial disorders; 9 retrospective publications were found, 3 of which was mitochondrial dysfunction-related and 5 mitochondrial disorders; 14 case-control studies 11 mentioned dysfunction of the mitochondria while 3 pertained to mitochondrial disorders; 3 randomized clinical trials were found with 3 discussing mitochondrial dysfunction and 0 relating to mitochondrial disorders; 2 in vitro studies both noting mitochondrial dysfunction; 1 meta-analysis mentioning mitochondrial dysfunction; 12 clinical trials where all 12 mentioned mitochondrial dysfunction.
Review of each type of publication with mention of any NDD, neurocognitive disorder and/or psychiatric/behavioral disorder (Intellectual Disability: Mild, Intellectual Disability: Moderate, Intellectual Disability: Severe, Intellectual Disability (Nonspecific), Language Disorder, ASD, ADHD: Hyperactive, Cerebral Palsy, Schizophrenia, PTSD, Major Depressive Disorder: mild (general), Neurocognitive Disorder Possibly Due to Alzheimer, Bipolar disorder (general/nonspecific), Generalized Anxiety Disorder (general), Personality disorders (general/nonspecific), Neurocognitive Disorder Possibly Due to Parkinson’s, OCD, Dementia) is shown in Figure 2.
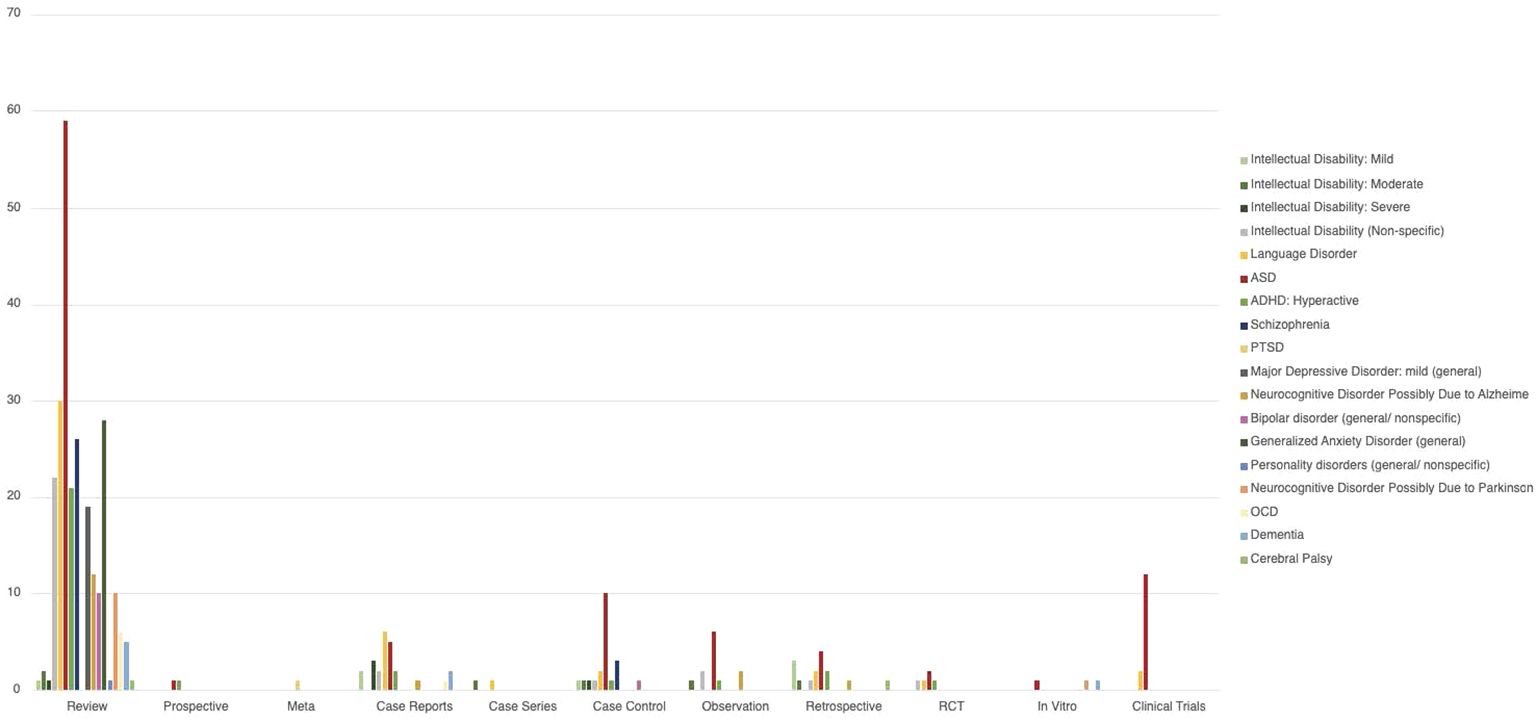
Figure 2 Distribution of the number of number of NDD and behavioral disorders in the different type of papers analyzed. The bars represent the number of paper the condition was mentioned in. Some papers mentioned more than one condition, and in those cases the paper were counted more than once.
Neurodevelopmental disorders mentioned in publications are detailed in Table 3. Neurocognitive disorders mentioned in publications are detailed in Table 4 and Behavioral/psychiatric disorders mentioned in publications are detailed in Table 5.
We examined 139 publications on mitochondrial disease/dysfunction that highlighted the role of mitochondrial disease/dysfunction in NDDs and behavioral/psychiatric disorders. Tables 3 and 5 bring to light the lack of new research studying the relationship between mitochondrial dysfunction and NDDs and psychiatric/behavioral disorders. Additionally, the majority of articles were focused on ASD, leading to the question of whether ASD has a stronger correlation to mitochondrial disease or if it has more research funding.
There is limited research exploring mitochondrial diseases, though more common than is currently understood, affecting 1 in 5000 people (4). Without strong clinical suspicion, mitochondrial diseases can be difficult to diagnose due to their heterogeneous presentation and multiorgan system involvement.
The contents of our systematic review search indicated mitochondrial dysfunction/disorder was mentioned in 100 articles investigating ASD. Of the NDDs and behavioral/psychiatric disorders we searched, the preponderance of the articles focused on elucidating the connection between ASD and mitochondrial function.
In a population-based study, 120 children with ASD were studied for potential etiologies. Of the 69 children tested, 11 likely had a mitochondrial etiology. This connection between mitochondrial function and ASD has led to a shift in terminology, classifying ASD based on pathophysiology—”idiopathic ASD” vs. “mitochondrial ASD” (12). Another study showed that up to 16% of individuals with mtDNA deletions, while only 3.3% of the healthy control had mtDNA deletions. The study concluded that mtDNA mutations were statistically more common in patients with ASD compared to controls (18).
As the relationship between ASD and mitochondrial dysfunction becomes clear, the question of pathophysiology must be accounted for. Individuals with ASD frequently have multiorgan comorbidities. These diverse disorders may be accounted for by concomitant mitochondrial dysfunction. If the mitochondria are affected in ASD and cannot produce enough ATP, it naturally follows that high-energy organ systems would be negatively impacted, resulting in comorbidities (32).
Despite the significant amount of research dedicated to ASD and mitochondrial dysfunction, there are gaps in our knowledge. While ASD is classified as an NDD, there is a behavioral component to its presentation, which is unexplored in these articles. In our review, we found the link between behavioral/psychiatric disorders and mitochondrial dysfunction to be vastly uncharted. There were only 131 mentions of all behavioral/psychiatric disorders, some of which are very brief. Conversely, ASD, which is extensively researched, was the main topic of 100 articles, questioning research bias for such correlation between ASD and mitochondrial disease,.
With an annual budget of over $40 billion, the National Institutes of Health (NIH) is the largest single public funder of biomedical and behavioral research in the world (33). Of the NDDs, neurocognitive disorders, and behavioral/psychiatric disorders we found in our systematic investigation, allocation of NIH research funds (from largest to least) were on: Alzheimer’s disease; intellectual and developmental disabilities; ASD; schizophrenia; Parkinson’s disease; anxiety disorders; MDD; bipolar disorder; ADHD, and Tourette’s.
NIH funding of ASD has increased from $118 million in 2008 to $306 million in 2022, while no funding was provided for mitochondrial disorders (34). This research has resulted in standardized screening tools, such as MCHAT-R/F, that allow pediatricians to identify ASD effectively and systematically. The prevalence of ASD is 1/36 (35) in comparison to 1/5000 for mitochondrial disease. Only with more research and improved standardized screening for mitochondrial disease will we truly know if this difference in prevalence is as substantial as it first appears.
In addition to ASD, our search produced 47 mentions of intellectual disability. One study found a link between genetic variation in mtDNA and ID. In this study, individuals with ID presented with motor coordination deficits, attention deficits, epilepsy, heart conditions, and GI disorders. 79% of the genetic variations were identified as SNPs (36). This study also linked mtDNA mutations to ASD, bipolar disorder, schizophrenia, MELAS, LHON, and Leigh Syndrome, among others. This indicates a common mitochondrial denominator between the NDDs and behavioral/psychiatric disorders we are analyzing in this paper.
Our search generated 44 references to language disorders. One article emphasized the importance of creatine in brain functions. Creatine formation involves mitochondria, and creatine is necessary in the expenditure of ATP. Therefore, in cases of mitochondrial disruption of the creatine pathway, individuals present with intellectual and language disorders (37).
This systematic review search yielded 29 mentions of ADHD. ADHD and ASD were found to have several concomitant biological components, including: increased oxidative stress, increased toxic metal burden, decreased methylation, mitochondrial dysfunction, and cerebral hypoperfusion. These shared features indicate mitochondrial dysfunction plays a key role in the development of both conditions (38). Another article investigated the role of parent-of-origin effects (POE) in the development of ADHD. Specifically, situations in which the unequal passing of genetic information affects mitochondrial function. However, no single mechanism of inheritance for ADHD has been identified, leading us to conclude mitochondrial dysfunction regulating the ADHD phenotype (39).
Similarly, in our analysis of behavioral and psychiatric disorders, the bioenergetic deficits seen in the aforementioned NDDs can contribute to the etiology of several psychiatric disorders, such as schizophrenia, MDD, and bipolar I disorder.
A cross-sectional study done with 12 children with mitochondrial disorders, 33% had depressive symptoms, and 50% of these participants had problems with anxiety (40). In a similar study, 36 adults with confirmed mitochondrial disorders found the lifetime prevalence of major depressive disorder is 54%, 17% for bipolar disorder, 11% for generalized anxiety disorder, and 11% for panic disorder. 17% of subjects were at current risk for suicide, and 8% were considered high risk (41). This raises a question about the correlation between mitochondrial function and prevalent psychiatric disorders.
Twenty-nine articles in our review mention schizophrenia and bring forth compelling evidence of mitochondrial involvement in its pathophysiology, the first of which is mitochondrial morphology. Studies have found mitochondrial swelling and hyperplasia in schizophrenic individuals, as well as inconsistent evidence of altered oxidative phosphorylation due to ETC complex dysfunction. Disease inheritance also implicates mitochondrial involvement, as the rates of schizophrenia are higher in female relatives. Studies indicate that the familial risk of developing schizophrenia was significantly higher in family members who shared mtDNA (42).
Similarly, bipolar disorder was only mentioned 11 times in our systematic review. In addition to several mtDNA SNPs found to correlate with bipolar disorder, previous studies have uncovered significant differences in mitochondrial size and distribution in the CNS of bipolar patients when compared with controls (42). Additionally, as evidenced by increased lipid peroxidation and antioxidant enzyme alterations, it is believed there is an overproduction of ROS in bipolar patients (42). A final, albeit inconsistent, finding points towards a shift from oxidative phosphorylation to glycolysis in bipolar individuals. While intriguing, these findings will remain inconclusive without proper investigation (42).
MDD, referenced 19 times in our literature search on mitochondrial disease, has a lifetime prevalence of 5-17%. Research found in all stress models of depression, there was a bioenergetic impairment such as decreased ATP, increased ROS, and/or decreased antioxidant defense. All of which correspond to an underlying mitochondrial dysfunction, making MDD another example of an exceptionally common condition with evidence of mitochondrial involvement that has not been suitably explored (43).
Delving into neurocognitive disorders, our search noted 16 papers connecting Alzheimer’s disease and mitochondrial dysfunction, even theorizing that deficits in mitochondrial function may be the primary event leading to various neurodegenerative disorders, including schizophrenia and Alzheimer’s disease. The leading postulation of Alzheimer’s pathogenesis involves increased oxidative stress due to mitochondrial impairment. One article suggested an accumulation of deletions and point mutations in mtDNA results in the progressive dementia seen in Alzheimer’s disease (44). Specifically, the most frequent deletion of 4977bp in mtDNA contributes to the reduced functionality of 1+ respiratory chain complexes, effectively blocking the ETC and increasing free radical generation. This increase in free radicals leads to more oxidative damage of mtDNA, continuing the cycle of mitochondrial dysfunction (44).
Our literature search disclosed 11 articles on Parkinson’s disease. One such article investigated the role of monoamine oxidases (MAOs) A and B may play in the pathophysiology of Parkinson’s disease. MAOs are critical to the proper functioning of synaptic neurotransmission, but the byproducts of the reaction have neurotoxic potential. This article suggests in cases where these enzymes are overactive, the accumulation of the byproducts could lead to mitochondrial damage and result in the Parkinsonian phenotype (45).
In conclusion, while these conditions may seem disparate in appearance or clinical presentation, they are all connected by an underlying etiology: mitochondrial dysfunction. ASD, due to its generous research funding, appears to have a stronger correlation with mitochondrial dysfunction. However, with the understanding of research bias, it is more likely that these other conditions have the same connection to mitochondrial impairment but lack the funds to fully investigate.
Diagnosing mitochondrial disorders comes with many challenges: which tissue will provide the best sample, analyzing hundreds of mtDNA and nDNA gene variations, and epigenetic influences that are not fully understood. This makes mitochondrial disease difficult to study, as these difficulties indicate it may frequently go undiagnosed.
In addition to diagnostic complications, treatment and prognostic tools also present challenges. On average, people with mitochondrial disease have 16 major medical issues stemming from dysfunctional energy production (46). Even with research still in its early stages, over 350 pathogenic gene variants have been connected to mitochondrial disease (47). This makes a standardized test to detect mitochondrial disease exceptionally complicated.
With advanced diagnostics, novel therapeutics, and individualized research, we may find mitochondrial defects play a major role in all chronic conditions. That is the future required in order to properly identify and treat mitochondrial disease (48).
Given the aforementioned limitations, there is much that requires improvement in order to enhance care of patients with mitochondrial disease. Specifically, more clinical trials and randomized control trials (RCT) are needed. Of the extensive search we conducted, only 12 were clinical trials and 3 were RCT. 72 of the 139 articles we examined were reviews. We need to cultivate interest, encourage new studies, and advance our understanding of mitochondrial disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DP: Writing – review & editing, Writing – original draft. LS: Writing – review & editing, Writing – original draft. JV: Writing – review & editing, Writing – original draft. SD: Writing – review & editing, Writing – original draft. LP: Writing – review & editing, Writing – original draft. AH: Writing – review & editing, Writing – original draft. SK: Writing – review & editing, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders — A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:1066–77. doi: 10.1016/j.bbadis.2016.11.010
2. Rahman S. Mitochondrial disease in children. J Intern Med. (2020) 287:609–33. doi: 10.1111/JOIM.13054
3. Suomalainen A, Battersby BJ. Mitochondrial diseases: The contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol. (2018) 19(2):77–92. doi: 10.1038/nrm.2017.66
4. Parikh S, Goldstein A, Koenig MK, Scaglia F, Enns GM, Saneto R, et al. Diagnosis and management of mitochondrial disease: A consensus statement from the Mitochondrial Medicine Society. Genet Med. (2015) 17:689–701. doi: 10.1038/gim.2014.177
5. Wallace DC, Chalkia D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb Perspect Biol. (2013) 5:1–47. doi: 10.1101/CSHPERSPECT.A021220
6. Lagier-Tourenne C, Hirano M. Mitochondrial disorders. In: Neurogenetics: Scientific and Clinical Advances. Boca Raton, Florida, USA: CRC Press (2005). p. 261–88.
7. Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol Psychiatry. (2012) 17:290–314. doi: 10.1038/mp.2010.136
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Arlington, Virginia, USA: American Psychiatric Association (2013). doi: 10.1176/appi.books.9780890425596
9. Tsao CY, Mendell JR. Autistic disorder in 2 children with mitochondrial disorders. J Child Neurol. (2007) 22:1121–23. doi: 10.1177/0883073807306266
10. Weissman JR, Kelley RI, Bauman ML, Cohen BH, Murray KF, Mitchell RL, et al. Mitochondrial disease in autism spectrum disorder patients: A cohort analysis. PloS One. (2008) 3:e3815. doi: 10.1371/journal.pone.0003815
11. Haas RH. Autism and mitochondrial disease. Dev Disabil Res Rev. (2010) 16:144–53. doi: 10.1002/ddrr.112
12. Oliveira G, Diogo L, Grazina M, Garcia P, Ataíde A, Marques C, et al. Mitochondrial dysfunction in autism spectrum disorders: A population-based study. Dev Med Child Neurol. (2005) 47:185–9. doi: 10.1017/S0012162205000332
13. Poling JS, Frye RE, Shoffner J, Zimmerman AW. Developmental regression and mitochondrial dysfunction in a child with autism. J Child Neurol. (2006) 21:170–2. doi: 10.1177/08830738060210021401
14. Goh S, Dong Z, Zhang Y, DiMauro S, Peterson BS. Mitochondrial dysfunction as a neurobiological subtype of autism spectrum disorder: Evidence from brain imaging. JAMA Psychiatry. (2014) 71:665–71. doi: 10.1001/jamapsychiatry.2014.179
15. Valenti D, de Bari L, De Filippis B, Henrion-Caude A, Vacca RA. Mitochondrial dysfunction as a central actor in intellectual disability-related diseases: An overview of Down syndrome, autism, Fragile X and Rett syndrome. Neurosci Biobehav Rev. (2014) 46:202–17. doi: 10.1016/j.neubiorev.2014.01.012
16. Rose S, Niyazov DM, Rossignol DA, Goldenthal M, Kahler SG, Frye RE. Clinical and molecular characteristics of mitochondrial dysfunction in autism spectrum disorder. Mol Diagnosis Ther. (2018) 22:571–93. doi: 10.1007/s40291-018-0352-x
17. Valiente-Pallejà A, Torrell H, Muntané G, Cortés MJ, Martínez-Leal R, Abasolo N, et al. Genetic and clinical evidence of mitochondrial dysfunction in autism spectrum disorder and intellectual disability. Hum Mol Genet. (2018) 27:891–900. doi: 10.1093/hmg/ddy009
18. Varga NÁ, Pentelényi K, Balicza P, Gézsi A, Reményi V, Hársfalvi V, et al. Mitochondrial dysfunction and autism: Comprehensive genetic analyses of children with autism and mtDNA deletion. Behav Brain Funct. (2018) 14:1–14. doi: 10.1186/s12993-018-0135-x
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR (4th ed., text rev). Washington, DC: American Psychiatric Publishing Inc (2000).
20. Stein DJ, Phillips KA, Bolton D, Fulford KWM, Sadler JZ, Kendler KS. What is a mental/psychiatric disorder? from DSM-IV to DSM-V. Psychol Med. (2010) 40:1759–65. doi: 10.1017/S0033291709992261
21. King BH. Psychiatric comorbidities in neurodevelopmental disorders. Curr Opin Neurol. (2016) 29:113–7. doi: 10.1097/WCO.0000000000000299
22. Fitzgerald M. The future of psychiatry and neurodevelopmental disorders: A paradigm shift. In: Fitzgerald M (ed.), Neurodevelopment and Neurodevelopmental Disorder. London, UK: IntechOpen (2019). p. 1–13. doi: 10.5772/INTECHOPEN.88540
23. Falk MJ. Neurodevelopmental manifestations of mitochondrial disease. J Dev Behav Pediatr. (2010) 31:610–21. doi: 10.1097/DBP.0b013e3181ef42c1
24. Anglin RE, Tarnopolsky MA, Mazurek MF, Rosebush PI. The psychiatric presentation of mitochondrial disorders in adults. J Neuropsychiatry Clin Neurosci. (2012) 24:394–409. doi: 10.1176/APPI.NEUROPSYCH.11110345
25. Anglin RE, Garside SL, Tarnopolsky MA, Mazurek MF, Rosebush PI. The psychiatric manifestations of mitochondrial disorders: A case and review of the literature. J Clin Psychiatry. (2012) 73:506–12. doi: 10.4088/JCP.11r07237
26. Ge Y-X, Shang B, Chen W-Z, Lu Y, Wang J. Adult-onset of mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome with hypothyroidism and psychiatric disorders. eNeurologicalSci. (2017) 6:16–20. doi: 10.1016/j.ensci.2016.11.005
27. Inczedy-Farkas G, Remenyi V, Gal A, Varga Z, Balla P, Udvardy-Meszaros A, et al. Psychiatric symptoms of patients with primary mitochondrial DNA disorders. Behav Brain Funct. (2012) 8:9. doi: 10.1186/1744-9081-8-9
28. Anglin RES, Mazurek MF, Tarnopolsky MA, Rosebush PI. The mitochondrial genome and psychiatric illness. Am J Med Genet B Neuropsychiatr Genet. (2012) 159B:749–59. doi: 10.1002/AJMG.B.32086
29. Cuperfain AB, Zhang ZL, Kennedy JL, Gonçalves VF. The complex interaction of mitochondrial genetics and mitochondrial pathways in psychiatric disease. Mol Neuropsychiatry. (2018) 4:52–69. doi: 10.1159/000488031
30. Xu H, Yang F. The interplay of dopamine metabolism abnormalities and mitochondrial defects in the pathogenesis of schizophrenia. Transl Psychiatry. (2022) 12:1–13. doi: 10.1038/s41398-022-02233-0
31. Ni P, Ma Y, Chung S. Mitochondrial dysfunction in psychiatric disorders. Schizophr Res. (2022). doi: 10.1016/j.schres.2022.08.027
32. Frye RE, Rossignol DA. Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatr Res. (2011) 69:41–7. doi: 10.1203/PDR.0b013e318212f16b
33. National Institutes of Health. Direct economic contributions. Available at: https://www.nih.gov/about-nih/what-we-do/impact-nih-research/serving-society/direct-economic-contributions (Accessed November 30, 2023).
34. National Institutes of Health. Estimates of funding for various research, condition, and disease categories (RCDC). Available at: https://report.nih.gov/funding/categorical-spending#/ (Accessed November 30, 2023).
35. Centers for Disease Control and Prevention. Data and statistics on autism spectrum disorder. Available at: https://www.cdc.gov/ncbddd/autism/data.html (Accessed November 30, 2023).
36. Cruz ACP, Ferrasa A, Muotri AR, Herai RH. Frequency and association of mitochondrial genetic variants with neurological disorders. Mitochondrion. (2019) 46:345–60. doi: 10.1016/J.MITO.2018.09.005
37. Joncquel-Chevalier Curt M, Voicu PM, Fontaine M, Dessein AF, Porchet N, Mention-Mulliez K, et al. Creatine biosynthesis and transport in health and disease. Biochimie. (2015) 119:146–65. doi: 10.1016/j.biochi.2015.10.022
38. Bradstreet JJ, Smith S, Baral M, Rossignol DA. Biomarker-guided interventions of clinically relevant conditions associated with autism spectrum disorders and attention deficit hyperactivity disorder. Altern Med Rev. (2010) 15:15–32. Available at: https://pubmed.ncbi.nlm.nih.gov/20359266/
39. Zayats T, Johansson S, Haavik J. Expanding the toolbox of ADHD genetics. How can we make sense of parent of origin effects in ADHD and related behavioral phenotypes? Behav Brain Funct. (2015) 11:1–8. doi: 10.1186/s12993-015-0078-4
40. Riquin E, Le Nerzé T, Pasquini N, Barth M, Prouteau C, Colin E, et al. Psychiatric symptoms of children and adolescents with mitochondrial disorders: A descriptive case Series. Front Psychiatry. (2021) 12:685532/PDF. doi: 10.3389/fpsyt.2021.685532
41. Fattal O, Link J, Quinn K, Cohen BH, Franco K. Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectr. (2007) 12:429–38. doi: 10.1017/S1092852900015303
42. Marin SE, Saneto RP. Neuropsychiatric features in primary mitochondrial disease. Neurol Clin. (2016) 34:247–94. doi: 10.1016/j.ncl.2015.08.011
43. Kolar D, Kleteckova L, Brozka H, Vales K. Mini-review: Brain energy metabolism and its role in animal models of depression, bipolar disorder, schizophrenia and autism. Neurosci Lett. (2021) 760:1–13. doi: 10.1016/J.NEULET.2021.136003
44. Marazziti D, Baroni S, Picchetti M, Landi P, Silvestri S, Vatteroni E, et al. Mitochondrial alterations and neuropsychiatric disorders. Curr Med Chem. (2011) 18:4715–21. doi: 10.2174/092986711797379221
45. Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: From pathophysiology to therapeutics. Adv Drug Deliv Rev. (2008) 60:1527–33. doi: 10.1016/j.addr.2008.06.002
46. Cohen B, Balcells C, Hotchkiss B, Aggarwal K, Karaa A. A retrospective analysis of health care utilization for patients with mitochondrial disease in the United States: 2008-2015. Orphanet J Rare Dis. (2018) 13:1–11. doi: 10.1186/s13023-018-0949-5
47. Children’s Hospital of Philadelphia. Mitochondrial medicine. Available at: https://www.research.chop.edu/mitochondrial-medicine (Accessed November 30, 2023).
48. Children’s Hospital of Philadelphia. Mitochondrial medicine program. Available at: https://www.chop.edu/centers-programs/mitochondrial-medicine-program (Accessed November 30, 2023).
Keywords: mitochondrial disorder, mitochondrial dysfunction, neurodevelopmental disorder, psychiatric disorder, ASD, ADHD, depression, anxiety
Citation: Pinto Payares DV, Spooner L, Vosters J, Dominguez S, Patrick L, Harris A and Kanungo S (2024) A systematic review on the role of mitochondrial dysfunction/disorders in neurodevelopmental disorders and psychiatric/behavioral disorders. Front. Psychiatry 15:1389093. doi: 10.3389/fpsyt.2024.1389093
Received: 20 February 2024; Accepted: 16 May 2024;
Published: 28 June 2024.
Edited by:
Joana M. Gaspar, Federal University of Santa Catarina, BrazilReviewed by:
Dejan Budimirovic, Johns Hopkins University United StatesCopyright © 2024 Pinto Payares, Spooner, Vosters, Dominguez, Patrick, Harris and Kanungo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shibani Kanungo, c2hpYmFuaS5rYW51bmdvQHdtZWQuZWR1; Daniela V. Pinto Payares, RGFuaWVsYS5waW50b3BheWFyZXNAd21lZC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.