
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 07 June 2024
Sec. Schizophrenia
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1388442
 Shannon Delaney1,2†‡
Shannon Delaney1,2†‡ Cynthia Robveille3‡
Cynthia Robveille3‡ Ricardo G. Maggi3
Ricardo G. Maggi3 Erin Lashnits4
Erin Lashnits4 Emily Kingston3
Emily Kingston3 Chance Liedig3
Chance Liedig3 Lilly Murray1
Lilly Murray1 Brian A. Fallon1,2
Brian A. Fallon1,2 Edward B. Breitschwerdt3*
Edward B. Breitschwerdt3*Introduction: The potential role of pathogens, particularly vector-transmitted infectious agents, as a cause of psychosis has not been intensively investigated. We have reported a potential link between Bartonella spp. bacteremia and neuropsychiatric symptoms, including pediatric acute onset neuropsychiatric syndrome and schizophrenia. The purpose of this study was to further assess whether Bartonella spp. exposure or infection are associated with psychosis.
Methods: In a blinded manner, we assessed the presence of anti-Bartonella antibodies by indirect immunofluorescence assays (IFA), and infection by amplification of bacterial DNA from blood by quantitative polymerase chain reaction (qPCR), digital PCR (dPCR), and droplet digital PCR (ddPCR) in 116 participants. Participants were categorized into one of five groups: 1) controls unaffected by psychosis (n = 29); 2) prodromal participants (n = 16); 3) children or adolescents with psychosis (n = 7); 4) adults with psychosis (n = 44); and 5) relatives of a participant with psychosis (n = 20).
Results: There was no significant difference in Bartonella spp. IFA seroreactivity between adults with psychosis and adult controls unaffected by psychosis. There was a higher proportion of adults with psychosis who had Bartonella spp. DNA in the bloodstream (43.2%) compared to adult controls unaffected by psychosis (14.3%, p = 0.021). The Bartonella species was determined for 18 of the 31 bacteremic participants, including infection or co-infection with Bartonella henselae (11/18), Bartonella vinsonii subsp. berkhoffii (6/18), Bartonella quintana (2/18), Bartonella alsatica (1/18), and Bartonella rochalimae (1/18).
Discussion: In conjunction with other recent research, the results of this study provide justification for a large national or international multi-center study to determine if Bartonella spp. bacteremia is more prevalent in adults with psychosis compared to adults unaffected by psychosis. Expanding the investigation to include a range of vector-borne and other microbial infections with potential CNS effects would enhance knowledge on the relationship between psychosis and infection.
Psychosis constitutes a severely demoralizing illness for the patient, creates numerous emotional and medical management challenges for family members and physicians, and contributes to a substantial economic burden for society (1, 2). An increasing number of studies supports a role for chronic inflammation in various neurological conditions, including studies that have focused on patients with schizophrenia and psychosis (3–5). In a study involving 638,213 Swedish men, high erythrocyte sedimentation rate, a global indicator of inflammation, was associated with increased risk for schizophrenia and decreased risk for other non-affective psychoses in adulthood (6). Yuan and colleagues performed a systematic meta-analysis of inflammation-related factors in eight major psychiatric disorders, including schizophrenia (SCZ), bipolar disorder, autism spectrum disorder, major depression disorder, post-trauma stress disorder, sleeping disorder, obsessive-compulsive disorder and suicide (7). As their work supported the possibility of differentiating psychiatric disorders by using inflammatory biomarkers, the authors proposed a system-wide longitudinal study using strict analytical procedures to validate sensitive and specific inflammatory biomarkers associated with different types of psychosis. While identifying biomarkers of inflammation helps to clarify a potential mechanism of disease, identifying unrecognized perpetuating agents of inflammation may prove to be a more effective strategy for generating patient specific interventions in the future.
Bartonella spp. are emerging, potentially zoonotic pathogens that are most often transmitted by arthropod vectors or animal bites and scratches (8–12). A substantial number of animal species have co-evolved with a specific Bartonella sp. (now more than 45 named species), for which an animals’ blood serves as a reservoir for blood sucking arthropods. After human beings become incidentally infected, symptoms most often consist of acute onset fever, myalgia, headache and potentially lymphadenopathy (13, 14). Although the acute infection can vary in severity, most people experience a mild to moderate flu-like illness that is most often self-limiting. With the advent of more sensitive diagnostic methods, it is now recognized that some infected individuals develop a longstanding blood borne infection, accompanied by a spectrum of chronic, often non-specific symptoms primarily involving the cardiovascular (endocarditis and myocarditis), neurological (neuropathy, seizures, encephalitis, and other symptoms) and rheumatological (myalgia, fatigue, joint pain) systems (15–19). Pediatric acute onset neuropsychiatric syndrome involves new onset complex psychiatric symptoms emerging in the context of an infectious trigger; a case report has identified Bartonella as a potential contributing factor, with symptom resolution by using antimicrobial treatments (20). Considering the increasingly large number of Bartonella species, the environmental diversity of mammalian reservoir hosts, and the range of competent and suspected vectors for Bartonella spp. transmission, it is increasingly obvious that human exposures to this genus of bacteria are more frequent and ubiquitous than formerly suspected (11, 21–26).
Previously, the authors (Delaney S, Fallon B) investigated the potential role of inflammation in children, adolescents, and young adults with psychosis (27). They found significantly elevated C-reactive protein (CRP) levels and interleukin 6 (IL-6) in the psychosis group compared to the controls unaffected by psychosis (27). In addition, IL-6 levels correlated positively with anti-lipopolysaccharide (LPS) IgA antibodies in the psychosis group, and negatively with vitamin D. At the same time, the corresponding author and his collaborators tested people with schizophrenia or schizoaffective disorder (SCZ/SAD) for Bartonella infection by droplet digital PCR (ddPCR). As the study was halted due to the SARS CoV2 pandemic, the authors elected to unblind and publish the findings as a pilot study that found people with SCZ/SAD were significantly more likely than healthy volunteers to have Bartonella spp. DNA in their blood (28). The goal of the current study was to further assess whether Bartonella spp. exposure or infection are associated with psychosis. Our primary hypothesis was that detection of Bartonella species DNA would be significantly associated with psychotic symptoms.
One hundred and sixteen participants were included in this study. All participants (including children) and parents for those under age 18 signed a consent. The protocol for sample collection (#7029) was approved by the New York State Psychiatric Institute Institutional Review Board. Blood and serum specimens stored at -80°C, including individuals from a previously published cohort (27), were used for molecular and serological testing, respectively. All samples were de-identified and shipped overnight express to the Intracellular Pathogens Research Laboratory, North Carolina State University (NCSU). Investigators and research technicians at NCSU were blinded to all participant categorizations. Results obtained previously using inflammatory markers (CRP, IL-6), anti-LPS antibodies (IgM, IgG, IgA) and vitamins (D, B12, folate) were reanalyzed. Some biomarker values were not available for all participants.
Participants were classified into one of five groups: 1) controls unaffected by psychosis (ages 11–33, n = 29); 2) prodromal participants with psychosis (ages 16–30, n = 16); 3) children or adolescents with psychosis (ages 8–16, n = 7); 4) adults with psychosis (ages 18–37, n = 44); and 5) relatives of a participant with psychosis (ages 21–67, n = 20). Relatives were composed of parents (n = 15), siblings (n = 4), and an aunt (n = 1). Participants with psychosis were recruited from the community and inclusion criteria included those between the ages of 8 and 35 with a psychiatrist verified diagnosis on the MINI Neuropsychiatric structured diagnostic interview, including a positive diagnosis of psychosis (current or lifetime) or a mood disorder with psychotic symptoms. Prodromal participants were recruited solely through the prodromal clinic, the Center for Prevention and Evaluation (COPE), and met criteria for the Attenuated Positive Symptom Syndrome (APSS) (29). Controls unaffected by psychosis were recruited through Columbia’s clinical research website and denied having a history of psychotic symptoms or of autoimmune conditions. The latter were excluded from the original study (27), due to potential increased inflammatory markers. Participants mostly lived in the greater New York City area; their risk of vector exposure was not assessed.
The testing approaches used in this study are depicted in Figure 1.
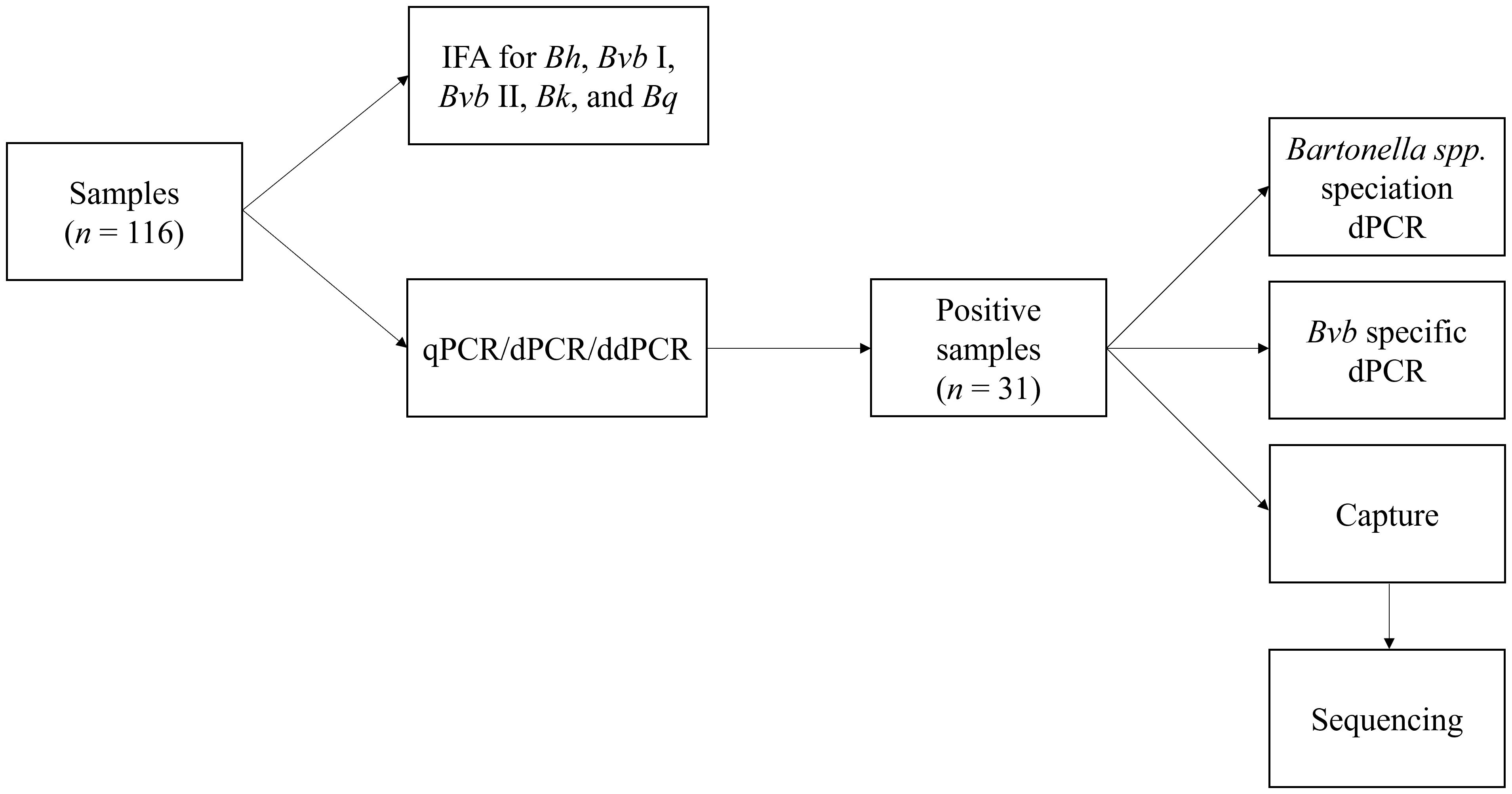
Figure 1 Summary of the Bartonella serological and molecular testing approaches used in this study. IFA, indirect fluorescent antibody; Bh, Bartonella henselae; Bvb I, Bartonella vinsonii subsp. berkhoffii genotype I; Bvb II, Bartonella vinsonii subsp. berkhoffii genotype II; Bk, Bartonella koehlerae; Bq, Bartonella quintana; qPCR, quantitative polymerase chain reaction; dPCR, digital PCR; ddPCR, droplet digital PCR.
As described previously (30, 31), each participant was tested using five indirect fluorescent antibody (IFA) assays, each representing a unique Bartonella species or genotype. Bartonella vinsonii subsp. berkhoffii (genotypes I and II), B. henselae (strain San Antonio 2), B. koehlerae, and B. quintana IgG antibodies were determined using DH82 cell culture-grown bacteria as antigens and following standard IFA techniques with fluorescein conjugated goat anti-human IgG. A sample was considered Bartonella spp. seroreactive if an IFA titer of ≥1:64 was obtained for any one or more antigens.
Following DNA extraction from each whole blood sample, amplification of the human hydroxymethylbilane synthase gene was used as housekeeping reference gene. The Bartonella spp. intergenic spacer 16S-23S rRNA region was targeted by quantitative PCR (qPCR, CFXOpus thermocycler, Bio-Rad, Hercules, CA), digital PCR (dPCR, QIAcuity nanoplate-based digital PCR system, Qiagen, Carlsbad, CA), and droplet digital PCR (ddPCR, QX200 Droplet Digital PCR, Bio-Rad, Hercules, CA) using primers and probes as previously described (28, 32–34). A sample was considered PCR positive if any one or more of the qPCR, dPCR or ddPCR testing modalities generated a positive result.
Attempts to identify Bartonella species using PCR+ samples were performed by qPCR and dPCR using either species specific probes (with minor modifications) as previously described (35), or by biotin-streptavidin DNA amplicon capture with qPCR re-amplification, followed by DNA sequence species comparisons. In addition, DNA amplification specifically targeting the Bartonella vinsoni subsp. berkhoffii intergenic spacer region was also performed using B. vinsoni subsp. berkhoffii specific primers and probes (36).
Summary statistics for demographics and Bartonella test results were calculated for each group. For statistical analysis of the primary outcome, only unrelated adults (18 years of age or older) between the group with psychosis (n = 44) and the control group (group unaffected by psychosis; n = 28) were analyzed. Thus, one child, an 11 year-old female, in the control group was excluded in the statistical analysis. Descriptive results only are presented for prodromal participants, children or adolescents with psychosis, and relatives.
For continuous variables (age and body mass index), the case and control groups were compared using the Wilcoxon rank sum test for nonparametric data. For categorical variables (gender, Bartonella serology result, Bartonella PCR result), adults with psychosis and controls were compared using chi-squared test (or Fisher exact test for small group sizes). To determine agreement between IFA and PCR, the kappa statistic was calculated (37).
Associations between biomarkers (CRP, IL-6, serum anti-LPS IgM/IgG/IgA, vitamin D, folate, and vitamin B12) and seroreactivity or PCR status were determined using Wilcoxon rank sum test for nonparametric data. This test was also used to assess associations between biomarkers and adults with psychosis or controls. Since these biomarker comparisons were being done to generate novel hypotheses, correction for multiple comparisons was not performed to decrease the risk of alpha error in this small sample size.
Statistical significance was set at p ≤ 0.05. All statistical analyses were performed in R v. 4.3.2 (R Core Team 2023) (38).
Demographic information for all groups is summarized in Table 1. There were 44 adults with psychosis and 28 controls included in the statistical analysis. There was no significant difference in age (p = 0.982) or body mass index (p = 0.291) between the two groups. Gender data was available for only 39 participants with psychosis and 19 controls; of those with data available, there was a higher proportion of male participants in the group with psychosis (25/39, 64.1%) compared to the control group (6/19, 31.6%, p = 0.040).
Serological results are summarized by study group in Tables 2, 3. Overall, 72/116 (62.1%) participants were seroreactive to one or more of the five Bartonella spp. antigens by IFA testing. The difference in the proportion of seroreactive adults was not statistically significant between the control group (21/28, 75.0%) and the group with psychosis (25/44, 56.8%) (p = 0.189). Bartonella vinsonii subsp. berkhoffii genotype II and B. henselae were the most frequently seroreactive species among the five antigens tested in the overall population (48.3% and 46.6%, respectively). Patterns of seroreactivity were highly variable among individuals, with some participants reactive to all five antigens (22/116; 19.0%) and others reactive to only one (18/116; 15.5%), two (13/116; 11.2%), three (10/116; 8.6%) or four antigens (9/116; 7.8%). Most seroreactive participants (54/72, 75.0%) were reactive to more than one Bartonella species/genotype. The percentage of seroreactive participants progressively decreased as antibody titers increased from 1:64 to 1:1024, the highest titer recorded to any antigen for any participant. Reciprocal B. henselae and B. vinsonii subsp. berkhoffii genotype I antibody titers ranged from 64 to 1024, whereas reciprocal B. koehlerae, B. quintana and B. vinsonii subsp. berkhoffii genotype II antibody titers ranged from 64 to 512.
Results of qPCR, dPCR and ddPCR testing are summarized by study group in Table 4. DNA from at least one Bartonella species was amplified in 31/116 (26.7%) participants, including 5 controls unaffected by psychosis (5/29; 17.2%), 3 prodromal participants (3/16; 18.8%), 19 adults with psychosis (19/44; 43.2%), and 4 relatives of participants with psychosis (4/20; 1 sibling and 3 parents; 20%). None of the children, except the one in the control group, was PCR+. The difference in the proportion of PCR+ adults was statistically significant between the control group (4/28, 14.3%) and the group with psychosis (19/44, 43.2%) (p = 0.021). The Bartonella species was determined for 18 of the 31 bacteremic participants (Table 5). Bartonella henselae (11/18, 61.1%) and B. vinsonii subsp. berkhoffii (6/18, 33.3%) were the most common species identified. There was co-infection in three adults with psychosis, involving B. quintana (2/18), B. alsatica (1/18), and B. rochalimae (1/18).
Agreement between serological and molecular results was slight or less (kappa = -0.09, 95% CI -0.27–0.09) (Tables 6, 7). This agreement was not significantly more likely than would be expected by chance alone (p = 0.335). There was seroreactivity to at least one antigen in 19 of 31 bacteremic participants (61.3%), and in 53 of 85 non-bacteremic participants (62.4%). There was no significant association between B. henselae PCR positivity and B. henselae seroreactivity (p = 0.549): 11% of participants with B. henselae titers less than 1:64 were PCR+ (7/62), compared to 5% of participants with B. henselae titers 1:64 or 1:128 (2/39) and 13% of participants with B. henselae titers 1:256 or above (2/15, including the child control). Of the six B. vinsonii subsp. berkhoffii PCR+ participants, five were B. vinsonii subsp. berkhoffii genotype I and/or II seroreactive (3 at 1:64 and one each at 1:128 and 1:256). The sole non-seroreactive participant was co-infected with B. alsatica. The participant that was PCR+ for B. quintana and B. rochalimae was seroreactive to all five antigens at titers of 1:256, whereas the participant co-infected with B. quintana and B. henselae was seronegative to all five antigens. One adult with psychosis, diagnosed with anti-NMDA (N-methyl-D-aspartate) receptor antibody encephalitis, was seroreactive to all five antigens at titers of 1:128 (B. quintana) or 1:256 (B. henselae, B. vinsonii subsp. berkhoffii genotype I and II, B. koehlerae); Bartonella spp. DNA was not amplified from the blood specimen.
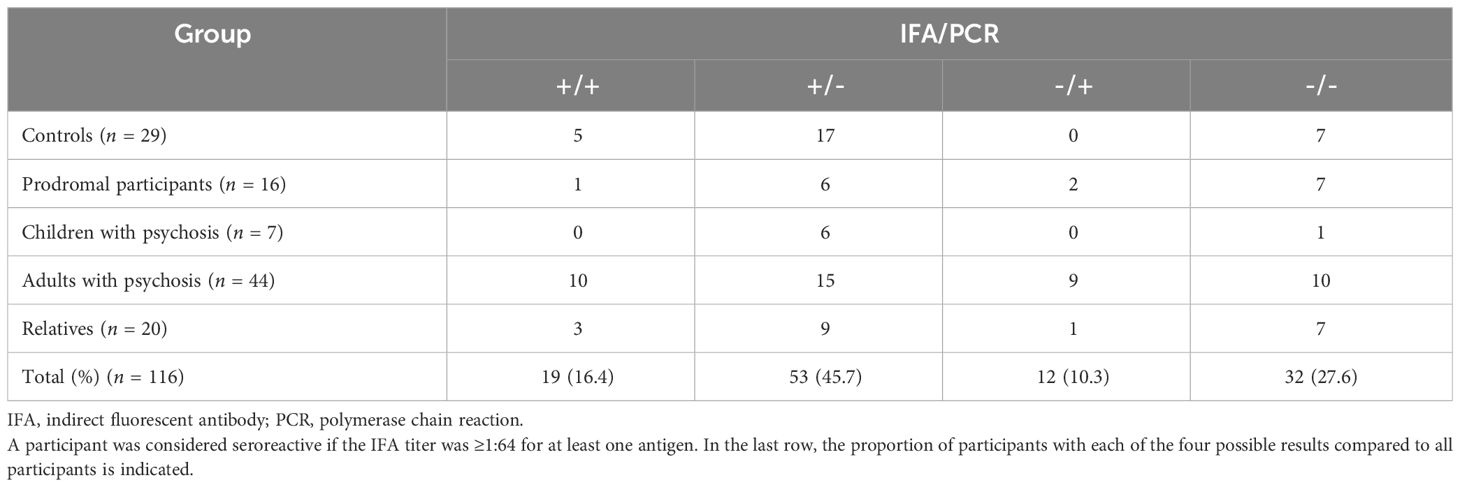
Table 6 Agreement between Bartonella spp. indirect fluorescent antibody results and molecular results for each group.
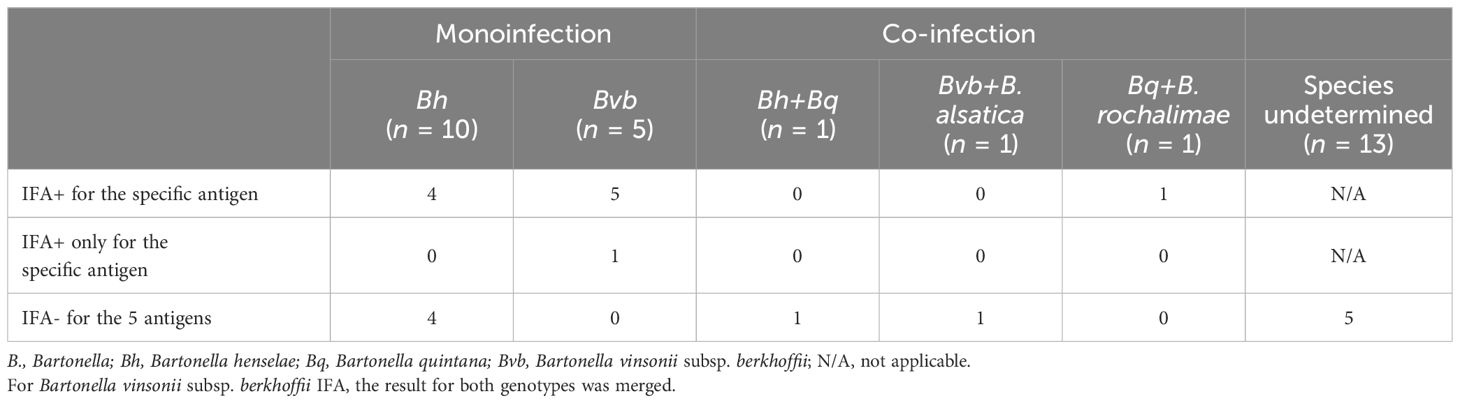
Table 7 Seroreactivity to specific antigens by indirect fluorescent antibody assays in bacteremic participants.
There was no significant association between any individual biomarker (CRP, IL-6, serum anti-LPS IgM, IgG, or IgA, vitamin D, folate or vitamin B12) and seroreactivity in adults (data not shown). For the PCR status, only serum anti-LPS IgG was significantly higher in bacteremic adults (median: 0.1036782, range: 0.0203120–0.3348107, n = 25) compared to non-bacteremic adults (median: 0.0688245, range: 0.0145963–0.4288184, n = 49) (p = 0.028). There were no significant associations between any individual biomarker and participant group (adults with psychosis compared to controls) (data not shown).
In this study, there was a higher proportion of adults with psychosis that had Bartonella spp. DNA in the bloodstream compared to adult controls unaffected by psychosis. This finding is consistent with the results of the pilot study by Lashnits and colleagues, in which a higher proportion of adults with SCZ/SAD had Bartonella spp. DNA amplified from blood (11 of 17 participants) compared to healthy controls (1 of 13 participants) (28). In the current study, a positive qPCR result was obtained for only 9 of 31 bacteremic participants; all of whom were either in the prodromal or the adult psychosis groups. Bartonella spp. DNA was only amplified by dPCR or ddPCR in the remaining 22 PCR+ participants, highlighting the enhanced sensitivity of these two digital PCR techniques when attempting to document (i.e., microbiologically detect) low template bacterial DNA concentrations in participant blood. Sanger sequencing, DNA capture, Taqman® probe-based, and B. vinsonii subsp. berkhoffii-specific PCR assays were used to define the Bartonella species in PCR+ participants.
Despite efforts to determine the Bartonella species, 13 of the 31 bacteremic participants were infected with an undetermined species, possibly due to very low bacterial numbers, or due to novel or known species for which the intergenic spacer primers lack sensitivity. This study confirmed that B. henselae was the most frequent species amplified from participant blood specimens (11/18). In addition, 6 participants, including two controls unaffected by psychosis, were infected with Bartonella vinsonii subsp. berkhoffii, whose primary reservoir host is canids. Infection with B. vinsonii subsp. berkhoffii is considered an occupational risk for veterinary workers and others with extensive animal contact (39, 40). On a comparative medicine/One Health basis, a previous study involving cats examined following necropsy at the Animal Medical Center in New York documented an unexpectedly high prevalence of B. vinsonii subsp. berkhoffii DNA in endomyocarditis-left ventricular endocardial fibrosis cases compared to control cats with cardiomyopathy or histologically normal hearts (41). Findings in cats and humans in the New York City region justify future research efforts to define the mode(s) of transmission, potential reservoir(s), and medical importance of B. vinsonii subsp. berkhoffii in this location.
There was co-infection in three adults with psychosis, involving B. quintana, B. alsatica, and B. rochalimae. Although technically difficult to document with current testing modalities, Bartonella spp. co-infections have been previously reported, most often as a component of rigorous, complex research testing efforts (42–44). To our knowledge, B. alsatica has only been reported in a few human patients from Europe, comprising illnesses targeting the cardiovascular or lymphoid systems (45–48). Infection with B. rochalimae has been described in an American woman with fever, myalgia, and splenomegaly three weeks after multiple insect bites acquired during a trip in Peru (49). Additionally, B. rochalimae has been reported in association with endocarditis in a 22-year-old man who had unrepaired congenital ventricular septal defect, and in dogs in the United States (50, 51).
Similar to the North Carolina SCZ/SAD study, there was no significant difference in Bartonella spp. seroreactivity between the adults with psychosis and the controls. Based upon serology, Bartonella exposure was common among all study groups. As previously reported (28), there was low IFA sensitivity. For example, seven of 11 participants infected with B. henselae did not have detectable antibodies against this species, and five PCR+ participants were seronegative for all 5 antigens. This could be explained by anergy or antigenic variation among Bartonella strains resulting in false-negative IFA results in some participants (52). A significant increase of IgG antibodies against LPS but not against the Bartonella antigens tested in bacteremic adults could also account for anergy. Diminished antigen presentation was found in dogs experimentally infected with B. vinsonii subsp. berkhoffii (53), and IgG subclass deficiency has been reported in two women infected with B. henselae (54). Regardless of mechanism(s), these results suggest that serological tests are not clinically useful when attempting to assess the role of Bartonella spp. infections in participants with chronic psychiatric disorders. In addition to less-than-optimal sensitivity, cross-reactivity occurs across Bartonella spp. antigens, most prominently in endocarditis patients with extremely high IFA titers (55). Participants can also be co-infected with more than one Bartonella species, which further complicates interpretation of species cross-reactivity. In the context of specificity, cross-reactivity to other bacterial genera have been previously reported, but a recent publication failed to identify specific patterns of cross-reactivity across genera in occupationally at risk veterinary workers (10). As previously addressed (39, 40), serological and molecular results often did not agree in this study. None of the 6 participants who had a titer of ≥512 for at least one antigen was bacteremic, suggesting that anti-Bartonella antibodies could decrease the number of circulating bacteria below the level of molecular detection (56).
The frequency and medical importance of Bartonella spp. infections among family members is yet to be clarified. Although presumably an infrequent occurrence, perinatal transmission of B. henselae and B. vinsonii subsp. berkhoffii genotype II to twins in New York facilitated documentation of bacteremic durations that likely spanned a decade (52). In addition to the possibility of in utero infections, which clearly deserve increased research consideration, bacteremic infections with the same or different Bartonella species have been reported in multiple family members (52, 53). In the current study, two out of the four unrelated siblings of participants with psychosis were seroreactive to all five antigens. These two siblings were PCR-, whereas both participants with psychosis were bacteremic with either Bartonella henselae or an undetermined Bartonella species. The parent of one bacteremic participant was also included in this study; similar to her son unaffected by psychosis, she was seroreactive to all five antigens at titers ≥1:256 and PCR-. Three unrelated parents, without history of psychosis and whose offspring with psychosis was a child (1/3) or an adult (2/3), were PCR+. Interestingly, these two adults with psychosis and their parents were bacteremic; however, the Bartonella sp(p). infecting them was not determined using the techniques employed in this study. As long standing Bartonella sp(p). bacteremia is being increasingly confirmed with new, more sensitive diagnostic testing modalities, for example in blood donors and healthy veterinary workers (30, 34, 35, 54–56), documentation of asymptomatic infection in five controls in this study was an expected finding.
There were several limitations in this study. Due to the lack of aseptic technique and the manipulations of blood samples for prior testing purposes, culturing for Bartonella species was not performed. Thus, viable bacterial infection was not confirmed. The prevalence of Bartonella DNA in participants’ blood reported in this study was potentially underestimated because only a single blood specimen was tested, and enrichment blood culture was not performed (40). Bartonella is a highly fastidious bacterium that is difficult to document microbiologically in diagnostic specimens due to slow dividing times (approximately 22 hours), complex nutritional requirements, and intermittent bacteremia. To overcome these limitations in previous studies, our laboratory has required three aseptically collected blood and serum samples during a one-week period from each study participant, to increase the possibility of obtaining a PCR+ result (57). The presence of Bartonella DNA in blood was used to support infection; however, sequential testing would be necessary to confirm long-term bacteremia. In addition, the sample size for several groups was small; therefore statistical comparisons were limited to adults with psychosis compared to adult controls unaffected by psychosis. Finally, this study does not establish whether the presence of Bartonella spp. in the blood of adults with psychosis is a cause, a cofactor, or contributor to disease progression. Also, we cannot exclude the possibility of opportunistic infections, as Bartonella spp. infections have been associated with immune dysfunction (53, 54, 58). Our investigation had a limited infectious disease focus by design; testing for co-infection with other tickborne, vector-borne and non-vector-borne pathogens was not performed. As the Bartonella spp. test results were generated years after the original sample collection, antimicrobial therapy was not considered applicable.
On the basis of the North Carolina pilot study and the results of this study, there is justification for a large multi-center prospective study to determine if Bartonella spp. bacteremia is more prevalent in adults with psychosis compared to adults unaffected by psychosis and adults with other non-psychotic neurological disorders. Participants from different groups should match by age, sex, and socioeconomic status. Age of onset of symptomatology, as well as history of psychiatric hospitalizations (if any) and mental illness in the family, should be recorded. If such a future study supports an association between Bartonella spp. bacteremia and psychosis, Bartonella-targeted antimicrobial therapy trials could be initiated to determine if treatment improves or resolves psychotic behavior. Furthermore, broad infectious disease screening (including Bartonella spp.) should be considered in the setting of new onset neuropsychiatric disease, especially psychosis.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The protocol for human sample collection (#7029) was approved by the New York State Psychiatric Institute Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Stored frozen blood and serum samples were de-identified prior to shipment to North Carolina State University for blinded serological and molecular testing purposes. Written informed consent for participation in this study was provided by the participant and, for those under age 18, by the participant's legal guardians/next of kin. Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
SD: Conceptualization, Resources, Writing – review & editing, Investigation. CR: Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. RM: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing. EL: Writing – review & editing, Formal analysis. EK: Writing – review & editing, Investigation. CL: Investigation, Writing – review & editing. LM: Resources, Writing – review & editing. BF: Conceptualization, Resources, Supervision, Writing – review & editing. EB: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported through donations to the Bartonella/Vector Borne Diseases Research Fund at the North Carolina State University College of Veterinary Medicine, through a grant from the Steven and Alexandra Cohen Foundation, and by the state of North Carolina. SD’s research, which involved collecting samples and data from the study population, was supported by an American Academy of Child and Adolescent Psychiatry (AACAP) Lily Pilot Award and by the Lyme & Tick-borne Diseases Research Center at Columbia University Irving Medical Center established by the Global Lyme Alliance, Inc and the Lyme Disease Association, Inc. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
In conjunction with Dr. S. Sontakke and North Carolina State University, EB holds US Patent No. 7,115,385 Media and Methods for Cultivation of Microorganisms, which was issued on October 3rd, 2006. He is a co-founder, shareholder and Chief Scientific Officer for Galaxy Diagnostics, a company that provides advanced diagnostic testing for the detection of Bartonella spp. infections. RM is a co-founder and the Chief Technical Officer for Galaxy Diagnostics Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
B., Bartonella; CRP, C-reactive protein; dPCR, digital polymerase chain reaction; ddPCR, droplet digital polymerase chain reaction; IFA, indirect fluorescent antibody; IL-6, interleukin 6; LPS, lipopolysaccharide; qPCR, quantitative polymerase chain reaction; SCZ, schizophrenia; SAD, schizoaffective disorder.
1. Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou CF, Chaiyakunapruk N. Global economic burden of schizophrenia: A systematic review. Neuropsychiatr Dis Treat. (2016) 12:357–73. doi: 10.2147/NDT.S96649
2. Chen J, El-Den S, Pham L, O’Reilly CL, Collins JC. Healthcare professionals’ Knowledge, confidence and attitudes in relation to psychosis care: A systematic review. Int J Soc Psychiatry. (2023) 69:1856–68. doi: 10.1177/00207640231194490
3. Rantala MJ, Luoto S, Borraz-Leon JI, Krams I. Schizophrenia: the new etiological synthesis. Neurosci Biobehav Rev. (2022) 142:104894. doi: 10.1016/j.neubiorev.2022.104894
4. Klein HC, Guest PC, Dobrowolny H, Steiner J. Inflammation and viral infection as disease modifiers in schizophrenia. Front Psychiatry. (2023) 14:1231750. doi: 10.3389/fpsyt.2023.1231750
5. Dawidowski B, Gorniak A, Podwalski P, Lebiecka Z, Misiak B, Samochowiec J. The role of cytokines in the pathogenesis of schizophrenia. J Clin Med. (2021) 10. doi: 10.3390/jcm10173849
6. Kappelmann N, Khandaker GM, Dal H, Stochl J, Kosidou K, Jones PB, et al. Systemic inflammation and intelligence in early adulthood and subsequent risk of schizophrenia and other non-affective psychoses: A longitudinal cohort and co-relative study. Psychol Med. (2019) 49:295–302. doi: 10.1017/S0033291718000831
7. Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry. (2019) 9:233. doi: 10.1038/s41398–019-0570-y
8. Cruz T, Goncalves LR, Furquim MEC, Andre MR, Munhoz AD, Carlos RSA, et al. Threat under cats’ Claws: molecular detection and risk factors for zoonotic bartonella species in blood and claw samples from cats in Brazil. Acta Tropica. (2022) 232:106496. doi: 10.1016/j.actatropica.2022.106496
9. Sepulveda-Garcia P, Alabi A, Alvarez K, Rojas L, Mella A, Goncalves LR, et al. Bartonella spp. In households with cats: risk factors for infection in cats and human exposure. One Health. (2023) 16:100545. doi: 10.1016/j.onehlt.2023.100545
10. Thiel N, Baker M, Lipton B, Fuller L, Breitschwerdt EB, Rabinowitz P. Risk factors for bartonella seroreactivity among veterinary workers in the pacific northwest. Vector Borne Zoonotic Dis. (2023) 23:356–63. doi: 10.1089/vbz.2022.0060
11. Billeter SA. A review of bartonella infections in california-implications for public and veterinary health. J Med Entomol. (2022) 59:1154–63. doi: 10.1093/jme/tjac056
12. Kalogeropoulos D, Asproudis I, Stefaniotou M, Moschos MM, Mentis A, Malamos K, et al. Bartonella henselae- and quintana-associated uveitis: A case series and approach of a potentially severe disease with a broad spectrum of ocular manifestations. Int Ophthalmol. (2019) 39:2505–15. doi: 10.1007/s10792–019-01096–7
13. Eyer-Silva WA, Wutke LSC, Paiva ACM, Silva G, Ferry FRA, Signorini D, et al. A case of bartonella neuroretinitis with macular star diagnosed by clinical, epidemiological, serological, and molecular data: resolution after initiation of antimicrobial therapy. Rev da Sociedade Bras Med Trop. (2020) 53:e20190516. doi: 10.1590/0037–8682-0516–2019
14. Murakami K, Tsukahara M, Tsuneoka H, Iino H, Ishida C, Tsujino K, et al. Cat scratch disease: analysis of 130 seropositive cases. J Infect Chemother. (2002) 8:349–52. doi: 10.1007/s10156–002-0194–6
15. Stockmeyer B, Schoerner C, Frangou P, Moriabadi T, Heuss D, Harrer T. Chronic vasculitis and polyneuropathy due to infection with bartonella henselae. Infection. (2007) 35:107–9. doi: 10.1007/s15010–007-6021–3
16. Breitschwerdt EB, Maggi RG, Robert Mozayeni B, Hegarty BC, Bradley JM, Mascarelli PE. Pcr amplification of bartonella koehlerae from human blood and enrichment blood cultures. Parasites Vectors. (2010) 3:76. doi: 10.1186/1756–3305-3–76
17. Breitschwerdt EB, Mascarelli PE, Schweickert LA, Maggi RG, Hegarty BC, Bradley JM, et al. Hallucinations, sensory neuropathy, and peripheral visual deficits in a young woman infected with bartonella koehlerae. J Clin Microbiol. (2011) 49:3415–7. doi: 10.1128/jcm.00833–11
18. Dietmann A, Colin-Benoit E, Tinkhauser G, Meinel TR, Scheidegger O. Pearls & Oy-sters: bilateral mononeuropathic neuralgic amyotrophy triggered by bartonella henselae infection responsive to immunoglobulin. Neurology. (2022) 98:597–600. doi: 10.1212/WNL.0000000000200014
19. Nawrocki CC, Max RJ, Marzec NS, Nelson CA. Atypical manifestations of cat-scratch disease, United States, 2005–2014. Emerging Infect Dis. (2020) 26:1438–46. doi: 10.3201/eid2607.200034
20. Breitschwerdt EB, Greenberg R, Maggi RG, Mozayeni BR, Lewis A, Bradley JM. Bartonella henselae bloodstream infection in a boy with pediatric acute-onset neuropsychiatric syndrome. J Cent Nervous System Dis. (2019) 11:1179573519832014. doi: 10.1177/1179573519832014
21. McCormick DW, Rassoulian-Barrett SL, Hoogestraat DR, Salipante SJ, SenGupta D, Dietrich EA, et al. Bartonella spp. Infections identified by molecular methods, United States. Emerging Infect Dis. (2023) 29:467–76. doi: 10.3201/eid2903.221223
22. Mai BH. Seroprevalence of bartonella quintana infection: A systematic review. J Glob Infect Dis. (2022) 14:50–6. doi: 10.4103/jgid.jgid_220_21
23. Krugel M, Krol N, Kempf VAJ, Pfeffer M, Obiegala A. Emerging rodent-associated bartonella: A threat for human health? Parasites Vectors. (2022) 15:113. doi: 10.1186/s13071–022-05162–5
24. Iannino F, Salucci S, Di Provvido A, Paolini A, Ruggieri E. Bartonella infections in humans dogs and cats. Vet Italiana. (2018) 54:63–72. doi: 10.12834/VetIt.398.1883.2
25. Alvarez-Fernandez A, Breitschwerdt EB, Solano-Gallego L. Bartonella infections in cats and dogs including zoonotic aspects. Parasites Vectors. (2018) 11:624. doi: 10.1186/s13071–018-3152–6
26. Regier Y, OR F, Kempf VA. Bartonella spp. - a chance to establish one health concepts in veterinary and human medicine. Parasites Vectors. (2016) 9:261. doi: 10.1186/s13071–016-1546-x
27. Delaney S, Fallon B, Alaedini A, Yolken R, Indart A, Feng T, et al. Inflammatory biomarkers in psychosis and clinical high risk populations. Schizophr Res. (2019) 206:440–3. doi: 10.1016/j.schres.2018.10.017
28. Lashnits E, Maggi R, Jarskog F, Bradley J, Breitschwerdt E, Frohlich F. Schizophrenia and bartonella spp. Infection: A pilot case-control study. Vector Borne Zoonotic Dis. (2021) 21:413–21. doi: 10.1089/vbz.2020.2729
29. Brucato G, Masucci MD, Arndt LY, Ben-David S, Colibazzi T, Corcoran CM, et al. Baseline demographics, clinical features and predictors of conversion among 200 individuals in a longitudinal prospective psychosis-risk cohort. Psychol Med. (2017) 47:1923–35. doi: 10.1017/S0033291717000319
30. Lantos PM, Maggi RG, Ferguson B, Varkey J, Park LP, Breitschwerdt EB, et al. Detection of bartonella species in the blood of veterinarians and veterinary technicians: A newly recognized occupational hazard? Vector Borne Zoonotic Dis. (2014) 14:563–70. doi: 10.1089/vbz.2013.1512
31. Breitschwerdt EB, Maggi RG, Quach C, Bradley JM. Bartonella spp. Bloodstream infection in a canadian family. Vector Borne Zoonotic Dis. (2019) 19:234–41. doi: 10.1089/vbz.2018.2353
32. Maggi R, Breitschwerdt EB, Qurollo B, Miller JC. Development of a multiplex droplet digital pcr assay for the detection of babesia, bartonella, and borrelia species. Pathogens. (2021) 10. doi: 10.3390/pathogens10111462
33. Maggi RG, Richardson T, Breitschwerdt EB, Miller JC. Development and validation of a droplet digital pcr assay for the detection and quantification of bartonella species within human clinical samples. J Microbiol Methods. (2020) 176:106022. doi: 10.1016/j.mimet.2020.106022
34. Portillo A, Maggi R, Oteo JA, Bradley J, Garcia-Alvarez L, San-Martin M, et al. Bartonella spp. Prevalence (Serology, culture, and pcr) in sanitary workers in la rioja Spain. Pathogens. (2020) 9. doi: 10.3390/pathogens9030189
35. Maggi RG, Harms CA, Hohn AA, Pabst DA, McLellan WA, Walton WJ, et al. Bartonella henselae in porpoise blood. Emerging Infect Dis. (2005) 11:1894–8. doi: 10.3201/eid1112.050969
36. Maggi RG, Chomel B, Hegarty BC, Henn J, Breitschwerdt EB. A bartonella vinsonii berkhoffii typing scheme based upon 16s-23s its and pap31 sequences from dog, coyote, gray fox, and human isolates. Mol Cell Probes. (2006) 20:128–34. doi: 10.1016/j.mcp.2005.11.002
38. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2023).
39. Breitschwerdt EB, Maggi RG, Duncan AW, Nicholson WL, Hegarty BC, Woods CW. Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerging Infect Dis. (2007) 13:938–41. doi: 10.3201/eid1306.061337
40. Maggi RG, Mascarelli PE, Pultorak EL, Hegarty BC, Bradley JM, Mozayeni BR, et al. Bartonella spp. Bacteremia in high-risk immunocompetent patients. Diagn Microbiol Infect Dis. (2011) 71:430–7. doi: 10.1016/j.diagmicrobio.2011.09.001
41. Donovan TA, Balakrishnan N, Carvalho Barbosa I, McCoy T, Breitschwerdt EB, Fox PR. Bartonella spp. As a possible cause or cofactor of feline endomyocarditis-left ventricular endocardial fibrosis complex. J Comp Pathol. (2018) 162:29–42. doi: 10.1016/j.jcpa.2018.05.002
42. Breitschwerdt EB, Maggi RG. Bartonella quintana and bartonella vinsonii subsp. Vinsonii bloodstream co-infection in a girl from North Carolina, USA. Med Microbiol Immunol. (2019) 208:101–7. doi: 10.1007/s00430–018-0563–0
43. Breitschwerdt EB, Maggi RG, Nicholson WL, Cherry NA, Woods CW. Bartonella sp. Bacteremia in patients with neurological and neurocognitive dysfunction. J Clin Microbiol. (2008) 46:2856–61. doi: 10.1128/jcm.00832–08
44. Breitschwerdt EB, Maggi RG, Lantos PM, Woods CW, Hegarty BC, Bradley JM. Bartonella vinsonii subsp. Berkhoffii and bartonella henselae bacteremia in a father and daughter with neurological disease. Parasites Vectors. (2010) 3:29. doi: 10.1186/1756–3305-3–29
45. Puges M, Menard A, Berard X, Genevieve M, Pinaquy JB, Edouard S, et al. An unexpected case of bartonella alsatica prosthetic vascular graft infection. Infection Drug Resistance. (2019) 12:2453–6. doi: 10.2147/IDR.S206805
46. Raoult D, Roblot F, Rolain JM, Besnier JM, Loulergue J, Bastides F, et al. First isolation of bartonella alsatica from a valve of a patient with endocarditis. J Clin Microbiol. (2006) 44:278–9. doi: 10.1128/jcm.44.1.278–279.2006
47. Jeanclaude D, Godmer P, Leveiller D, Pouedras P, Fournier PE, Raoult D, et al. Bartonella alsatica endocarditis in a french patient in close contact with rabbits. Clin Microbiol Infect. (2009) 15 Suppl 2:110–1. doi: 10.1111/j.1469–0691.2008.02187.x
48. Angelakis E, Lepidi H, Canel A, Rispal P, Perraudeau F, Barre I, et al. Human case of bartonella alsatica lymphadenitis. Emerging Infect Dis. (2008) 14:1951–3. doi: 10.3201/eid1412.080757
49. Eremeeva ME, Gerns HL, Lydy SL, Goo JS, Ryan ET, Mathew SS, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. New Engl J Med. (2007) 356:2381–7. doi: 10.1056/NEJMoa065987
50. Ernst E, Qurollo B, Olech C, Breitschwerdt EB. Bartonella rochalimae, a newly recognized pathogen in dogs. J Vet Internal Medicine/American Coll Vet Internal Med. (2020) 34:1447–53. doi: 10.1111/jvim.15793
51. Traver EC, Saharia K, Luethy P, Amoroso A. Severe infective endocarditis caused by bartonella rochalimae. Emerging Infect Dis. (2023) 30:394–6. doi: 10.3201/eid3002.230929
52. Jin X, Gou Y, Xin Y, Li J, Sun J, Li T, et al. Advancements in understanding the molecular and immune mechanisms of bartonella pathogenicity. Front Microbiol. (2023) 14:1196700. doi: 10.3389/fmicb.2023.1196700
53. Pappalardo BL, Brown TT, Tompkins M, Breitschwerdt EB. Immunopathology of bartonella vinsonii (Berkhoffii) in experimentally infected dogs. Vet Immunol Immunopathol. (2001) 83:125–47. doi: 10.1016/s0165-2427(01)00372-5
54. Kaufman DL, Kogelnik AM, Mozayeni RB, Cherry NA, Breitschwerdt EB. Neurological and immunological dysfunction in two patients with bartonella henselae bacteremia. Clin Case Rep. (2017) 5:931–5. doi: 10.1002/ccr3.977
55. Boodman C, Wuerz T, Lagace-Wiens P. Endocarditis due to bartonella quintana, the etiological agent of trench fever. CMAJ: Can Med Assoc J = J l’Association Med Can. (2020) 192:E1723–E6. doi: 10.1503/cmaj.201170
56. Koesling J, Aebischer T, Falch C, Schulein R, Dehio C. Cutting edge: antibody-mediated cessation of hemotropic infection by the intraerythrocytic mouse pathogen bartonella grahamii. J Immunol. (2001) 167:11–4. doi: 10.4049/jimmunol.167.1.11
57. Pultorak EL, Maggi RG, Mascarelli PE, Breitschwerdt EB. Serial testing from a 3-day collection period by use of the bartonella alphaproteobacteria growth medium platform may enhance the sensitivity of bartonella species detection in bacteremic human patients. J Clin Microbiol. (2013) 51:1673–7. doi: 10.1128/JCM.00123–13
Keywords: Bartonella, infection, psychosis, serology, polymerase chain reaction, neurologic diseases
Citation: Delaney S, Robveille C, Maggi RG, Lashnits E, Kingston E, Liedig C, Murray L, Fallon BA and Breitschwerdt EB (2024) Bartonella species bacteremia in association with adult psychosis. Front. Psychiatry 15:1388442. doi: 10.3389/fpsyt.2024.1388442
Received: 19 February 2024; Accepted: 06 May 2024;
Published: 07 June 2024.
Edited by:
Robert Carroll Bransfield, The State University of New Jersey, United StatesReviewed by:
Raphael B. Stricker, Union Square Medical Associates, United StatesCopyright © 2024 Delaney, Robveille, Maggi, Lashnits, Kingston, Liedig, Murray, Fallon and Breitschwerdt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edward B. Breitschwerdt, ZWJicmVpdHNAbmNzdS5lZHU=
†Present address: Shannon Delaney, Private Practice, New York, NY, United States
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.