- 1Department of Psychiatry, Beijing Children’s Hospital, Capital Medical University, National Center for Children Healthy, Beijing, China
- 2Peking University Sixth Hospital, Peking University Institute of Mental Health, National Health Commission (NHC) Key Laboratory of Mental Health (Peking University), Beijing, China
- 3Big Data Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- 4Department of Psychosomatic Medicine, Beijing Children’s Hospital, Capital Medical University, National Center for Children Healthy, Beijing, China
Background: Sensory symptoms linked to tic disorder (TD) are challenging to quantify via self- or parent-reported measures. The current study aimed to develop a novel observer-rated semi-structured interview, namely, the Sensory Phenomenon Assessment Scale (SPAS), to aid clinical evaluation on symptoms of TD among children.
Methods: To test its psychometric properties, tic, premonitory urge (PU), and obsessive–compulsive symptoms (OCS) were also assessed in 223 children via the Yale Global Tic Severity Scale (YGTSS), the Premonitory Urge for Tic Scale (PUTS), and the Children’s Yale–Brown Obsessive–Compulsive Scale (CY-BOCS). Factor analysis and internal consistency test were carried out using data from TD-diagnosed individuals.
Results: Good internal consistency and test–retest reliability were observed. Criterion validity was established by significant correlations between the PUTS, the YGTSS, the CY-BOCS, and scores of the SPAS. Factor analyses supported a single-factor model of the SPAS, in which the five items each showed a factor loading above 0.6.
Conclusion: This study demonstrated that the SPAS is reliable and valid and, thus, can serve as a good and concise measure of clinical symptoms among children and adolescents with TD.
Introduction
Tic disorder (TD) is a neurodevelopmental disorder commonly found in children and adolescents (1). Premonitory urge (PU) can occur before, during, or after the onset of tic symptoms. Typical PU symptoms include itchiness, pressure, or a sense of incompleteness (2, 3). Several studies (2, 4) have shown that PU is prevalent in patients with TD, especially those with Tourette syndrome (TS). The sensations are also very salient, as many patients with TS describe the PU as more distressing than the tics themselves (5). Recent behavioral models suggest that PU is the cognition-behavioral basis for tic symptoms. After the onset of the tic symptoms, the individual is relieved from the pain of PU, even if sometimes temporarily (5, 6). Based on cross-sectional data (7, 8), a close positive correlation between severity of PU and tic symptoms in TD individuals should be noticed, which also implies that a higher degree of PU predicts more severe tics. These high correlations of theirs may derive from similar neural mechanisms; for example, both motor tic and PU production are correlated with right insula (9) and cingulate cortex volume (10). PU is related to the production, duration, and inhibitory control of tics (11, 12). Fostering awareness and understanding of PU stands as the foundational and pivotal stage in a well-established behavioral intervention for treating TD, Habit Reversal Training (HRT) (13, 14), for example.
Assessing PU objectively holds significance in the examination of TD. Methods devised for evaluating PU encompass both neuropsychological paradigms (11) and assessment scales (15–20). Neuropsychological paradigms were discouraged in clinical evaluation due to their complexity and high demand for equipment. Validated scales for measuring PU include the Premonitory Urges for Tic Disorders Scale (PUTS) (15), its adapted version Premonitory Urges for Tic Disorders Scale-Revised (PUTS-R) (16), Individualized Premonitory Urge for Tics Scale (I-PUTS) (17), Sensory Processing and Self-Regulation Checklist (SPSRC) (18), Rumination and Awareness Scale for Tic-Associated Sensations (RASTS) (19), and University of Sao Paulo Sensory Phenomena Scale (USP-SPS) (20). Among these tools, the PUTS (15) stands out as the most widely used, with translations and validations available in numerous languages (21–23). The majority of clinical studies (4, 24) employed the PUTS to gauge the intensity of PU. The PUTS was developed by Professor Woods (15) and first published in 2005. This self-rating scale has a total of nine items and mainly focuses on the number and frequency of PU. In an earlier study, the reliability and validity of the PUTS (23) have been verified in a Chinese setting by our research team.
However, issues that have not been addressed exist within the current scales. Symptom-related tensity and functional impairment are not fully assessed, while reliability and validity of self-report scales are less solid for children under the age of 10 (15, 25). Therefore, a new type of observer-rated scale is in need to provide more accurate and comprehensive assessment.
The current study aimed to develop and validate a new observer-rated semi-structured interview, namely, the Sensory Phenomenon Assessment Scale (SPAS). Drawing on the latest research on children’s mental health and behavior, we strived to build a tool that accurately reflects the complexity of TD children’s PU symptoms and is suitable for clinical practice. We hypothesized that the new instrument would have good reliability and validity and would be suitable for assessing tic-related sensory symptoms.
Methods
Participants
Participants were recruited from the outpatient clinic of Beijing Children’s Hospital from 1 May 2022 to 30 April 2023. Inclusion criteria were as follows (1): patients aged 6–17 years (2); patients who had TD diagnosed by a child psychiatrist according to the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5); and (3) patients experiencing PUs (with a total PUTS score exceeding 12). Exclusion criteria were as follows: (1) patients with traumatic brain injury, epilepsy, or intracranial tumors; (2) patients who had comorbid mental disorders; and (3) patients with difficulty communicating.
Informed consent was obtained from each patient and their main caregiver. The procedure of the current study was approved by the Ethics Committee of Beijing Children’s Hospital (approval number: [2023]-E-105-R).
Measures
The Sensory Phenomenon Assessment Scale (SPAS) was developed by the Delphi method. The final version of the SPAS consisted of two parts with 13 items. The first part was the observer-described “symptom list”, which contained eight items (itch, sense of suffocation, pressure, sense of energy release, sense of energy tension, sense of uncompletion, indescribable discomfort, and other). The second part is “severity” and contains five items, namely, number, frequency, tensity, degree of transformation, and functional impairment. Each item was scored on a six-point scale from 0 to 5, with higher scores indicating severer symptoms. For more details, see Appendix 1 (Development of SPAS), Appendix 2 (Final version of SPAS), and Appendix 3 (SPAS User Manual).
The Yale Global Tic Severity Scale (YGTSS) mainly consists of three parts. The first part is a tic inquiry item. In the second part, the number, frequency, intensity, complexity, and interference of tics were scored. Each aspect was scored from 0 to 5, and the maximum total score was 50. The final section is the overall impairment score, with a maximum score of 50. Higher YGTSS score indicates more serious tic symptoms. The reliability and validity of the scale have been verified in the Chinese-Taiwanese population (26). In addition, we had revisited the structure of this scale in a sample of Chinese children with TDs (27).
The PUTS consists of nine items, each item has five scales with a 0 to 4 score scale (a score of 0 = “none”, 1 = ‘‘not at all true,’’ 2 = ‘‘a little true,’’ 3 = ‘‘pretty much true,’’ and 4 = ‘‘very much true”). Score ranges from 9 to 36. Nine items were used to measure the frequency of sensory symptoms of a different nature, the frequency of sensory transformation into tics, and whether sensory phenomena persisted after tics. The reliability and validity of this scale had been validated in a Chinese population (23).
The Children’s Yale–Brown Obsessive–Compulsive Scale (CY-BOCS) (28) is a 10-item, clinician-rated, semi-structured scale designed to assess the symptom severity of OCD during a subject’s previous week. Each item is rated by a five-point Likert scale (0–4). The total score ranges from 0 to 40. The CY-BOCS consists of two dimensions: obsessive thoughts and compulsive behaviors. Our team has verified the reliability and validity of this scale in the Chinese population (29).
All patients’ severity of tics was evaluated using the SPAS, YGTSS, and CY-BOCS by two experienced child psychiatrists (Yanlin Li and Xianbin Wang). The Pearson correlation coefficient of consistency was 0.91. All the patients and their parents were then asked to fill in the PUTS.
Statistical analysis
To verify the reliability of the SPAS, we calculated the internal consistency (Cronbach’s α) on all five items of part 2 (“severity of sensory phenomena”), because part 1 (“symptom list”) was not scoring. A split-half reliability analysis was performed on odd and even items and the Spearman–Brown coefficient was computed. Dozens of participants were selected from mild tic patients, who required only clinical observation (no medication or other intervention), and repeated the test 1 month later to calculate the test–retest reliability.
Validity test included criterion-related validity based on the PUTS, YGTSS, and CY-BOCS and exploratory factor analysis (EFA). Correlation coefficients between the SPAS and the PUTS, YGTSS, and CY-BOCS were calculated, respectively. Factor loadings of each item were estimated by EFA.
All the above calculations were performed separately on a sample younger than 10 years of age (group 1), and a sample of age greater than or equal to 10 (group 2) used IBM SPSS Statistics 19.
Results
Demographic characteristics and clinical profiles
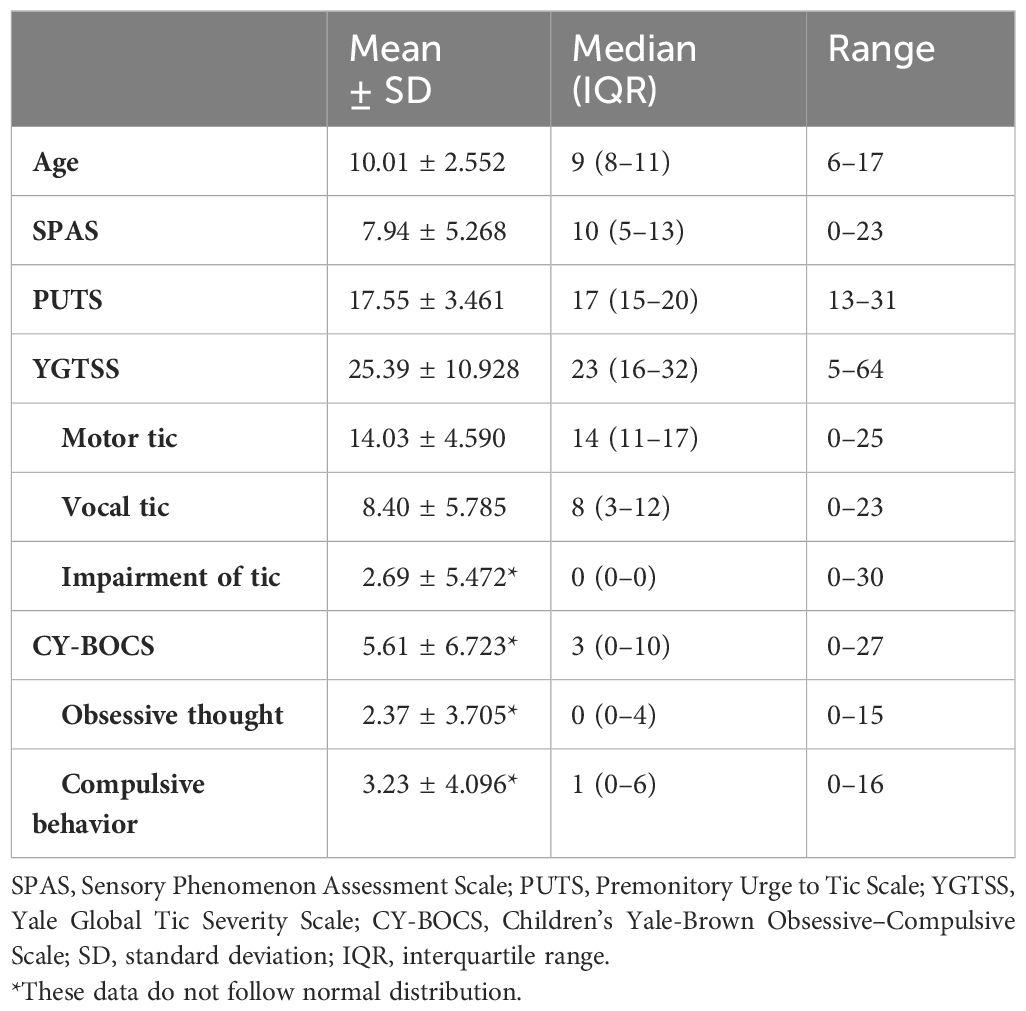
A total of 223 children and adolescents with TD (187 male and 36 female participants) were enrolled in the testing sample according to inclusion and exclusion criteria. All continuous variables, except for the CY-BOCS and its two subscales, exhibited a normal distribution. The mean age was 10.01 years with a standard deviation of 2.552. The mean SPAS score was 7.94 with a standard deviation of 5.268, while the mean PUTS score was 17.55 with a standard deviation of 3.461. Additionally, the mean YGTSS total score (total score of motor tic, vocal tic, and impairment of tic) was 25.39 with a standard deviation of 10.928, and the CY-BOCS had a median of 2, with an interquartile range of 0–8. See Table 1 for details.
Reliability of SPAS
Internal consistency
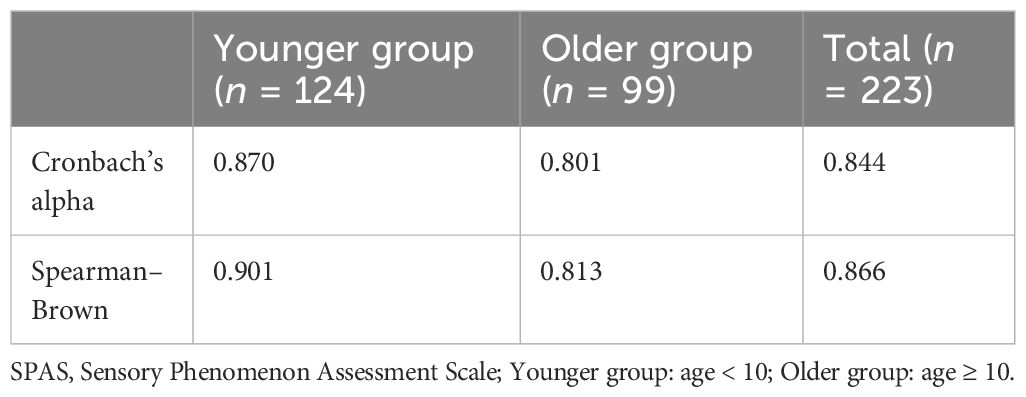
The Cronbach’s α coefficients for the SPAS were 0.844 for the total sample (n = 223), 0.870 for group 1 (aged less than 10 years), and 0.801 for group 2 (aged more than or equal to 10 years). Furthermore, the Spearman–Brown coefficients for these samples were 0.866, 0.901, and 0.813, respectively, indicating strong split-half reliability. For more details, see Table 2.
Test–retest reliability
Fifty-three patients were selected and refilled the SPAS 1 month later. The correlation coefficient between the two measurements was 0.987 (p < 0.01).
Validity of SPAS
Criterion-related validity
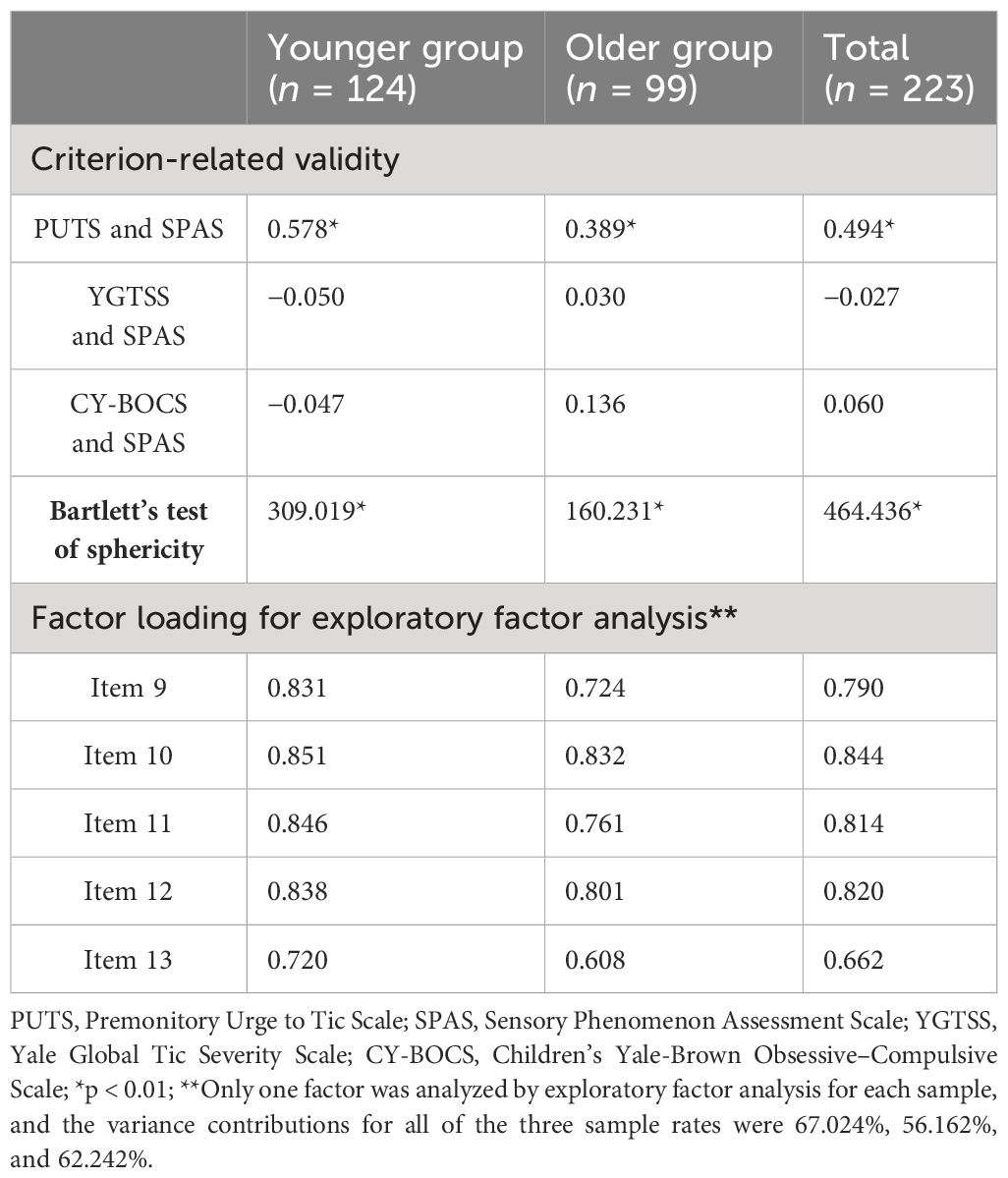
The total SPAS scores of the total, younger, and older groups were significantly positively correlated with scores of the PUTS (p < 0.01), but not with the YGTSS (p > 0.05) or the CY-BOCS (p > 0.05). Each correlation coefficient is shown in Table 3. The correlations among each item of the SPAS and the respective scales and subscales are presented in Supplementary Table S3. Supplementary Table S4 displays the correlations among each item of the SPAS.
Validity of construct
The score of each item of the SPAS was significantly correlated with the total score. In addition, the EFA with the total sample identified one dimension that explained 62.242% of the variance. Furthermore, each item from both total sample and younger or older sample had a factor loading greater than 0.5. For more details, see Table 3.
Discussion
The present study reports the development and validation of the SPAS, a new tool to quantitatively measure individual differences in tic-related sensory phenomena. Concurring with our hypotheses, the SPAS demonstrated good psychometric properties.
The final version of the SPAS was developed through literature search and Delphi expert consultation. The overall framework of the SPAS appeared to be reasonable for assessing sensory symptoms associated with TD in children and adolescents. Some reasons supporting the reasonableness of the framework were as follows.
On the one hand, the SPAS has been designed to be an observer-rated scale. Previous scales (15, 18, 19) used to assess PU or sensory symptoms were all self-reported, with the exception of I-PUTS (17). The SPAS and I-PUTS were clinician-rated scales, which might avoid the interference of subjectivity from patients. A meta-analysis (30) indicated that clinician-rated instruments commonly enjoy significantly higher effect sizes than their self-reported counterparts, though another study (31) stated that both self-report scales and clinician-rated scales were irreplaceable and complement each other.
On the other hand, the two-part structure of SPAS, with a symptom list and a severity assessment, was a well-thought-out design. The symptom list captured the variety of sensory phenomena associated with TDs, while the severity assessment quantified the intensity and impact of these symptoms on the child’s daily life. The severity assessment part of the SPAS included items such as number, frequency, tensity, degree of transformation, and functional impairment. Given that the I-PUTS evaluated PU only by three dimensions (number, frequency, and intensity), the SPAS may serve as a more comprehensive tool for assessing PU in TD.
The SPAS demonstrated satisfactory performance in terms of these reliability and validity. The 1-month test–retest reliability assessment of SPAS revealed highly significant correlations (p < 0.01), with a correlation coefficient of 0.987. Our findings affirm the stability and reliability of the measurement instrument, meeting the prescribed criteria for reliability. There is strong reliability for both the younger population, under 10 years of age, and the older group comprising children and adolescents aged 10 years and above. Furthermore, this observation compensates for the less than satisfactory reliability exhibited by the previous tool PUTS when applied to patients with TS under the age of 10 (32). Evidence suggested that the incidence of PU in patients with TD increases with age (33). The reason for this may involve a growing physiological awareness (or body awareness) with age. This also made older children more aware of the presence of PU. Other studies have suggested that this body awareness was negatively related to inhibitory function (34), and PU seemed to be related to inhibitory function (12); thus, we hypothesized that the incidence of PU increases with age, possibly because of increased body awareness. This also made it difficult for younger children (especially under the age of 10) to understand the meaning of the items when they complete the self-rating questionnaire (because they may never be aware of the intuitive feeling).
Subsequently, the validity analysis had factor loadings greater than 0.5 after EFA, indicating that these items together contribute to one dimension—the severity of PU. The unidimensional scale has demonstrated benefits in clinical settings due to its simplicity for clinical assessors, straightforward administration, and the ease with which results can be shared for clinical reference.
We then selected the PUTS, YGTSS, and CY-BOCS as criteria for calculating criterion validity. The total score of the SPAS exhibited a significant positive correlation with the total score of the PUTS (p < 0.001). However, there was no observed correlation between the total score of the SPAS and the total score of the YGTSS and CY-BOCS. These findings suggest that the SPAS demonstrates robust criterion validity, primarily assessing the severity of PU (as indicated by its lack of correlation with the YGTSS and CY-BOCS total score), rather than other measures such as tic symptoms and obsessive–compulsive symptoms. However, in most prior investigations of the PUTS (15), PUTS-R (16), and I-PUTS (17), a least moderate significant correlation with the YGTSS was presented. The reason for our inconsistency with previous results may be that our sample consisted of only patients with TD aged 6–16 years with PU. A larger sample size and more age-stratified patients with TD (such as young adults) may be needed in the future to explore the correlation between the results of the SPAS and other scales.
Our study has several limitations. Firstly, for convenient access, our sample was composed of children and adolescents exclusively. Future investigations should include adults with TD for gaining comprehensive insight. Secondly, while we employed the YGTSS, the PUTS, and the CY-BOCS as concurrent validity measures, alternative scales such as the USP-SPS (20), specifically designed for PU assessment, might offer alternative results. Lastly, we acknowledge that the lack of direct measurement of participants’ intelligence quotient (IQ) is a limitation of our study. While clinicians excluded participants who demonstrated difficulties in communication, which often correlates with lower IQ, we did not administer formal IQ tests as part of our protocol. In future studies, we will exclude the effect of IQ on the results.
Conclusion
Following psychometric testing, the newly developed scale SPAS demonstrated robust reliability and validity, fulfilling the prerequisites for clinical scales. Subsequent clinical applications confirmed the utility of this scale, indicating its capacity to provide a comprehensive evaluation of PU severity in children and adolescents with TD aged 6–17 years.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
We obtained the informed consent of all patients and obtained the ethical approval issued by the Ethics Committee of Beijing Children’s Hospital.
Author contributions
XW: Writing – review & editing, Writing – original draft, Software, Resources, Project administration, Methodology, Data curation. YLL: Writing – original draft, Software, Resources, Project administration, Investigation, Data curation. LY: Writing – review & editing, Supervision, Methodology. HX: Writing – review & editing, Methodology, Investigation. AZ: Writing – review & editing, Supervision, Methodology, Investigation. WZ: Writing – review & editing, Supervision, Methodology, Investigation. ZJ: Writing – review & editing, Supervision, Methodology, Investigation. YC: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. YL: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NSFC) under Grant Nos. 82171538 and 82001445; the Natural Science Foundation of Beijing Municipality under Grant No. 7212035; Beijing Hospitals Authority Youth Programme Grant No. QML202112031; and the Beijing High level Public Health Technology Talent Construction Project No. 2022-2-007.
Acknowledgments
We thank all the participants for their support in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1387417/full#supplementary-material
References
1. Plessen KJ. Tic disorders and Tourette's syndrome. Eur Child Adolesc Psychiatry. (2013) 22 Suppl 1:S55–60. doi: 10.1007/s00787-012-0362-x
2. Essing J, Jakubovski E, Psathakis N, Cevirme SN, Leckman JF, Muller-Vahl KR. Premonitory urges reconsidered: urge location corresponds to tic location in patients with primary tic disorders. J Mov Disord. (2022) 15:43–52. doi: 10.14802/jmd.21045
3. Steinberg T, Shmuel Baruch S, Harush A, Dar R, Woods D, Piacentini J, et al. Tic disorders and the premonitory urge. J Neural Transm (Vienna). (2010) 117:277–84. doi: 10.1007/s00702-009-0353-3
4. Edwards KR, Raines JM, Winnick JB, Sherman MF, Higginson CI, Navin K, et al. Sex and psychiatric comorbidity correlates of the premonitory urge for tic scale in youth with persistent tic disorders. J Neural Transm (Vienna). (2020) 127:977–85. doi: 10.1007/s00702-020-02151-9
5. Reese HE, Scahill L, Peterson AL, Crowe K, Woods DW, Piacentini J, et al. The premonitory urge to tic: measurement, characteristics, and correlates in older adolescents and adults. Behav Ther. (2014) 45:177–86. doi: 10.1016/j.beth.2013.09.002
6. Woods DW, Piacentini JC, Chang S, Deckersbach T, Ginsburg GS, Peterson AL, et al. Managing Tourette syndrome: A behavioral intervention for children and adults. New York: Oxford University Press (2008). doi: 10.1093/med:psych/9780195341287.001.0001
7. Gu Y, Li Y, Cui Y. Correlation between premonitory urges and tic symptoms in a Chinese population with tic disorders. Pediatr Investig. (2020) 4:86–90. doi: 10.1002/ped4.12189
8. Li Y, Wang F, Liu J, Wen F, Yan C, Zhang J, et al. The correlation between the severity of premonitory urges and tic symptoms: A meta-analysis. J Child Adolesc Psychopharmacol. (2019) 29:652–8. doi: 10.1089/cap.2019.0048
9. Jackson SR, Loayza J, Crighton M, Sigurdsson HP, Dyke K, Jackson GM. The role of the insula in the generation of motor tics and the experience of the premonitory urge-to-tic in Tourette syndrome. Cortex. (2020) 126:119–33. doi: 10.1016/j.cortex.2019.12.021
10. Jackson SR, Sigurdsson HP, Dyke K, Condon M, Jackson GM. The role of the cingulate cortex in the generation of motor tics and the experience of the premonitory urge-to-tic in Tourette syndrome. J Neuropsychol. (2021) 15:340–62. doi: 10.1111/jnp.12242
11. Langelage J, Verrel J, Friedrich J, Siekmann A, Schappert R, Bluschke A, et al. Urge-tic associations in children and adolescents with Tourette syndrome. Sci Rep. (2022) 12:16008. doi: 10.1038/s41598-022-19685-5
12. Sturm A, Ricketts EJ, McGuire JF, Lerner J, Lee S, Loo SK, et al. Inhibitory control in youth with Tourette's Disorder, attention-deficit/hyperactivity disorder and their combination and predictors of objective tic suppressibility. Psychiatry Res. (2021) 304:114163. doi: 10.1016/j.psychres.2021.114163
13. Liu S, Li Y, Cui Y. Review of habit reversal training for tic disorders. Pediatr Investig. (2020) 4:127–32. doi: 10.1002/ped4.12190
14. Singer HS. Treatment of tics and tourette syndrome. Curr Treat Options Neurol. (2010) 12:539–61. doi: 10.1007/s11940-010-0095-4
15. Woods DW, Piacentini J, Himle MB, Chang S. Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. J Dev Behav Pediatr JDBP. (2005) 26:397–403. doi: 10.1097/00004703-200512000-00001
16. Baumung L, Muller-Vahl K, Dyke K, Jackson G, Jackson S, Golm D, et al. Developing the premonitory urges for tic disorders scale-revised (PUTS-R). J Neuropsychol. (2021) 15:129–42. doi: 10.1111/jnp.12216
17. McGuire JF, McBride N, Piacentini J, Johnco C, Lewin AB, Murphy TK, et al. The premonitory urge revisited: an individualized premonitory urge for tics scale. J Psychiatr Res. (2016) 83:176–83. doi: 10.1016/j.jpsychires.2016.09.007
18. Lai CYY, Yung TWK, Gomez INB, Siu AMH. Psychometric properties of sensory processing and self-regulation checklist (SPSRC). Occup Ther Int. (2019) 2019:8796042. doi: 10.1155/2019/8796042
19. Matsuda N, Nonaka M, Kono T, Fujio M, Nobuyoshi M, Kano Y. Premonitory awareness facilitates tic suppression: subscales of the premonitory urge for tics scale and a new self-report questionnaire for tic-associated sensations. Front Psychiatry. (2020) 11:592. doi: 10.3389/fpsyt.2020.00592
20. Rosario MC, Prado HS, Borcato S, Diniz JB, Shavitt RG, Hounie AG, et al. Validation of the University of Sao Paulo Sensory Phenomena Scale: initial psychometric properties. CNS Spectr. (2009) 14:315–23. doi: 10.1017/s1092852900020319
21. Forcadell E, Garcia-Delgar B, Nicolau R, Perez-Vigil A, Cordovilla C, Lazaro L, et al. Tic disorders and premonitory urges: validation of the Spanish-language version of the Premonitory Urge for Tics Scale in children and adolescents. Trastornos de tics e impulso premonitorio: validación de la versión española de la «Escala para el Impulso Premonitorio al Tic» en niños y adolescentes. Neurologia. (2020) S0213-4853(20)30427-8. doi: 10.1016/j.nrl.2020.09.006
22. Kim M, Chung SK, Yang JC, Park JI, Nam SH, Park TW. Development of the korean form of the premonitory urge for tics scale: a reliability and validity study. Soa Chongsonyon Chongsin Uihak. (2020) 31:146–53. doi: 10.5765/jkacap.200013
23. Li Y, Woods DW, Gu Y, Yu L, Yan J, Wen F, et al. Psychometric properties of the chinese version of the premonitory urge for tics scale: a preliminary report. Front Psychol. (2021) 12:573803. doi: 10.3389/fpsyg.2021.573803
24. Kyriazi M, Kalyva E, Vargiami E, Krikonis K, Zafeiriou D. Premonitory urges and their link with tic severity in children and adolescents with tic disorders. Front Psychiatry. (2019) 10:569. doi: 10.3389/fpsyt.2019.00569
25. Raines JM, Edwards KR, Sherman MF, Higginson CI, Winnick JB, Navin K, et al. Premonitory Urge for Tics Scale (PUTS): replication and extension of psychometric properties in youth with chronic tic disorders (CTDs). J Neural Transmission. (2017) 125:727–34. doi: 10.1007/s00702-017-1818-4
26. Ho CS, Huang JY, Yang CH, Lin YJ, Huang MY, Su YC. Is the Yale Global Tic Severity Scale a valid tool for parent-reported assessment in the paediatric population? A prospective observational study in Taiwan. BMJ Open. (2020) 10:e034634. doi: 10.1136/bmjopen-2019-034634
27. Wen F, Gu Y, Yan J, Liu J, Wang F, Yu L, et al. Revisiting the structure of the Yale Global Tic Severity Scale (YGTSS) in a sample of Chinese children with tic disorders. BMC Psychiatry. (2021) 21:394. doi: 10.1186/s12888-021-03399-5
28. Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, et al. Children's yale-brown obsessive compulsive scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. (1997) 36(6):844–52. doi: 10.1097/00004583-199706000-00023
29. Yan J, Gu Y, Wang M, Cui Y, Li Y. The obsessive-compulsive symptoms in tic disorders and the psychometric properties of children's yale-brown obsessive-compulsive scale: an evidence-based survey in a chinese sample. Front Pediatr. (2022) 10:794188. doi: 10.3389/fped.2022.794188
30. Cuijpers P, Li J, Hofmann SG, Andersson G. Self-reported versus clinician-rated symptoms of depression as outcome measures in psychotherapy research on depression: a meta-analysis. Clin Psychol Rev. (2010) 30:768–78. doi: 10.1016/j.cpr.2010.06.001
31. Uher R, Perlis RH, Placentino A, Dernovšek MZ, Henigsberg N, Mors O, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depression Anxiety. (2012) 29:1043–9. doi: 10.1002/da.21993
32. Raines JM, Edwards KR, Sherman MF, Higginson CI, Winnick JB, Navin K, et al. Premonitory Urge for Tics Scale (PUTS): replication and extension of psychometric properties in youth with chronic tic disorders (CTDs). J Neural Transm (Vienna). (2018) 125:727–34. doi: 10.1007/s00702-017-1818-4
33. Brandt V, Essing J, Jakubovski E, Muller-Vahl K. Premonitory urge and tic severity, comorbidities, and quality of life in chronic tic disorders. Mov Disord Clin Pract. (2023) 10:922–32. doi: 10.1002/mdc3.13742
Keywords: tic disorder, premonitory urge, Sensory Phenomenon Assessment Scale, reliability, validity
Citation: Wang X, Li Y, Yu L, Xu H, Zhang A, Zhang W, Jiang Z, Cui Y and Li Y (2024) Sensory Phenomenon Assessment Scale: a new tool for assessment of tic-associated sensations. Front. Psychiatry 15:1387417. doi: 10.3389/fpsyt.2024.1387417
Received: 17 February 2024; Accepted: 31 May 2024;
Published: 24 June 2024.
Edited by:
Tjhin Wiguna, University of Indonesia, IndonesiaCopyright © 2024 Wang, Li, Yu, Xu, Zhang, Zhang, Jiang, Cui and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghua Cui, Y3VpeW9uZ2h1YUBiY2guY29tLmNu; Ying Li, bGl5aW5nQGJjaC5jb20uY24=
†These authors have contributed equally to this work
 Xianbin Wang
Xianbin Wang Yanlin Li1,2†
Yanlin Li1,2† Hui Xu
Hui Xu Anyi Zhang
Anyi Zhang Zhongliang Jiang
Zhongliang Jiang Yonghua Cui
Yonghua Cui Ying Li
Ying Li