- 1Department of Psychiatry, Sneka Mind Care Hospital, Tirunelveli, Tamil Nadu, India
- 2Medical Affairs and Clinical Research, Sun Pharmaceutical Industries Limited, Mumbai, India
- 3Medical Affairs and Clinical Research, Sun Pharma Laboratories Limited, Mumbai, India
- 4Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India
Management of negative symptoms is one of the most challenging and important unmet needs of schizophrenia treatment. Negative symptoms together with positive symptoms result in significant psychosocial impairment and poor quality of life. Existing studies on atypical antipsychotics reported limited treatment adherence due to higher prevalence of treatment-emergent adverse events, such as diabetes, weight gain, hyperlipidemia, hyperprolactinemia and hypertension. A compound with greater affinity for dopamine D2/D3 receptors may improve negative symptoms, mood, and cognitive impairment associated with schizophrenia. In 2015, the US FDA has approved cariprazine, a partial D2/D3 agonist for treatment of schizophrenia, mania or mixed episodes. Midlands and Lancashire Commissioning Support Unit, UK (2019) has particularly suggested cariprazine for the treatment of predominant negative symptoms of schizophrenia. India’s Central Drugs Standard Control Organization (CDSCO) has approved cariprazine in 2021 for the treatment of schizophrenia, manic or mixed episodes associated with bipolar I disorder. A ten-fold greater affinity for D3 receptors and partial agonism to serotonin receptors, along with longer half-life make cariprazine distinct when compared with other atypical antipsychotics. Cariprazine is also reported to have fewer incidents of metabolic and hormonal adverse events, and has been shown to provide better relapse prevention. Recent evidence indicates promising effect of cariprazine in ameliorating negative symptoms as well as psychotic symptoms in patients with schizophrenia. In addition, improved adherence to treatment (adjunctive/monotherapy) with cariprazine in patients having inadequate response to an ongoing antipsychotic treatment has also been clinically established. This review presents the evidence-based safety and efficacy of cariprazine for treatment of predominant negative symptoms of schizophrenia.
1 Introduction
Schizophrenia is a complex neuropsychiatric disorder, presenting with positive (hallucinations and delusions), negative (blunted affect, alogia, anhedonia, asociality and avolition), and cognitive (impaired retrieval of information like thinking, learning and memorizing) symptoms (1). In addition to these symptoms, patients with schizophrenia often experience affective symptoms (depression and anxiety) that is associated with increased risk of suicide and poor quality of life (2). Positive symptoms are primarily monitored to diagnose an active state of schizophrenia, but negative symptoms are significant contributors of poor psychosocial functioning and performance, impacting the patient’s quality of life (3). Identification of negative symptoms can be sometimes challenging due to their insidious onset, paucity of psychotic signs and similarity with other clinical features of schizophrenia, resulting in delayed treatment outcomes (1). The fundamental pathophysiological mechanism of negative symptoms is different from positive symptoms (3). Hyperdopaminergic state of dopamine D2 receptor in the mesolimbic area is related to positive symptom prognosis, while hypodopaminergic dysregulation of the prefrontal cortex leads to negative symptoms (1). Only a decade ago, the focus from positive symptoms has shifted to the negative symptoms. Since then, very few pharmaceutical agents have been studied that successfully met the therapeutic target of negative symptoms (4). Typical (first generation) and atypical (second and third generations) antipsychotics are primarily used to modulate the dopaminergic function (5). The mechanism of action of first generation antipsychotics (FGAs) lack preference-based blocking of dopamine pathway, resulting in extrapyramidal symptoms (dyskinesia, akathisia and tremors), hyperprolactinemia-associated sexual dysfunction and aggravation of negative symptoms (6). The second generation antipsychotics (SGAs) are combined D2 and serotonin 5-HT2A receptor antagonists with lower risk for developing extrapyramidal symptoms (EPS) (7). Second generation antipsychotics are effective for negative symptoms, but result in several treatment-emergent side effects including diabetes, ketoacidosis, weight gain, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, hypertension and metabolic syndrome (6, 8, 9).
Negative symptoms of schizophrenia have been consistently related to poor treatment outcome. A well-tolerated and long-term effective treatment option for negative symptoms is one of the most important unmet needs that is to be addressed. Globally approved third generation antipsychotic (TGA), cariprazine is distinct from other antipsychotics and has partial agonist activity at dopamine D3/D2 and serotonin 5-HT1A receptors (10). Because of its dopamine-dependent partial agonism to D2 and D3 receptors, cariprazine is less likely to cause weight gain, metabolic disorder and hyperprolactinemia (6). This review attempts to outline the safety and efficacy of cariprazine for the treatment of predominant negative symptoms of schizophrenia.
2 Epidemiology of schizophrenia
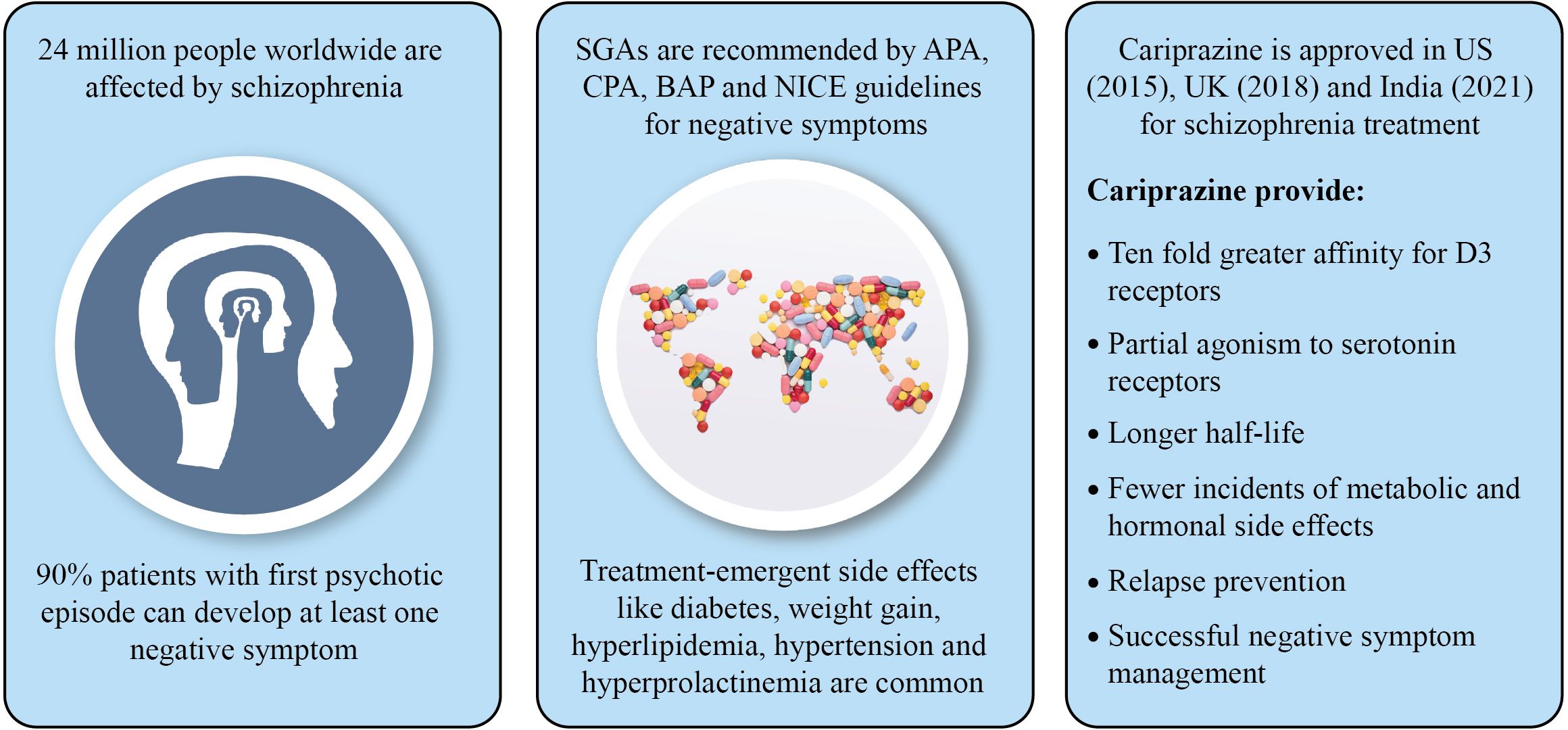
According to World Health Organization (2022), schizophrenia affects about 24 million people worldwide, i.e., 1 in 300 (11). It is one of the top 15 causes of global disability (12). In India, the prevalence of schizophrenia is 1.5-2.5/1000 people, with an annual rate of 0.35-0.38/1000 and 0.44/1000 people from urban and rural area, respectively (13). Many factors including migration, drug abuse, urbanicity (stress, noise and pollution), childhood traumas, psychosocial factors, infections, cannabis use, birth during winter (high chances of respiratory infections and inadequate vitamin D synthesis), and obstetric complications during fetal, childhood, adolescence and early adult life can increase the risk of developing schizophrenia (14). The onset of schizophrenia mostly occurs during late adolescence and early adulthood, and happens earlier in males (average age 18 years) than females (average age 25 years) (1). Late onset of schizophrenia (after the age of 44 years) accounts for 15-20% of all cases (15).
3 Overview of negative symptoms of schizophrenia
Prevalence of negative symptoms is 75% in patients having schizophrenia and 68% in patients with schizoaffective disorder (16). Sometimes negative symptoms appear before the onset of positive symptoms (73% prevalence) or in the same month of developing positive symptoms (20% prevalence) (17). Ninety percent patients with first psychotic episode can develop at least one negative symptom, whereas 35-70% can still suffer from negative symptoms post-treatment (17).
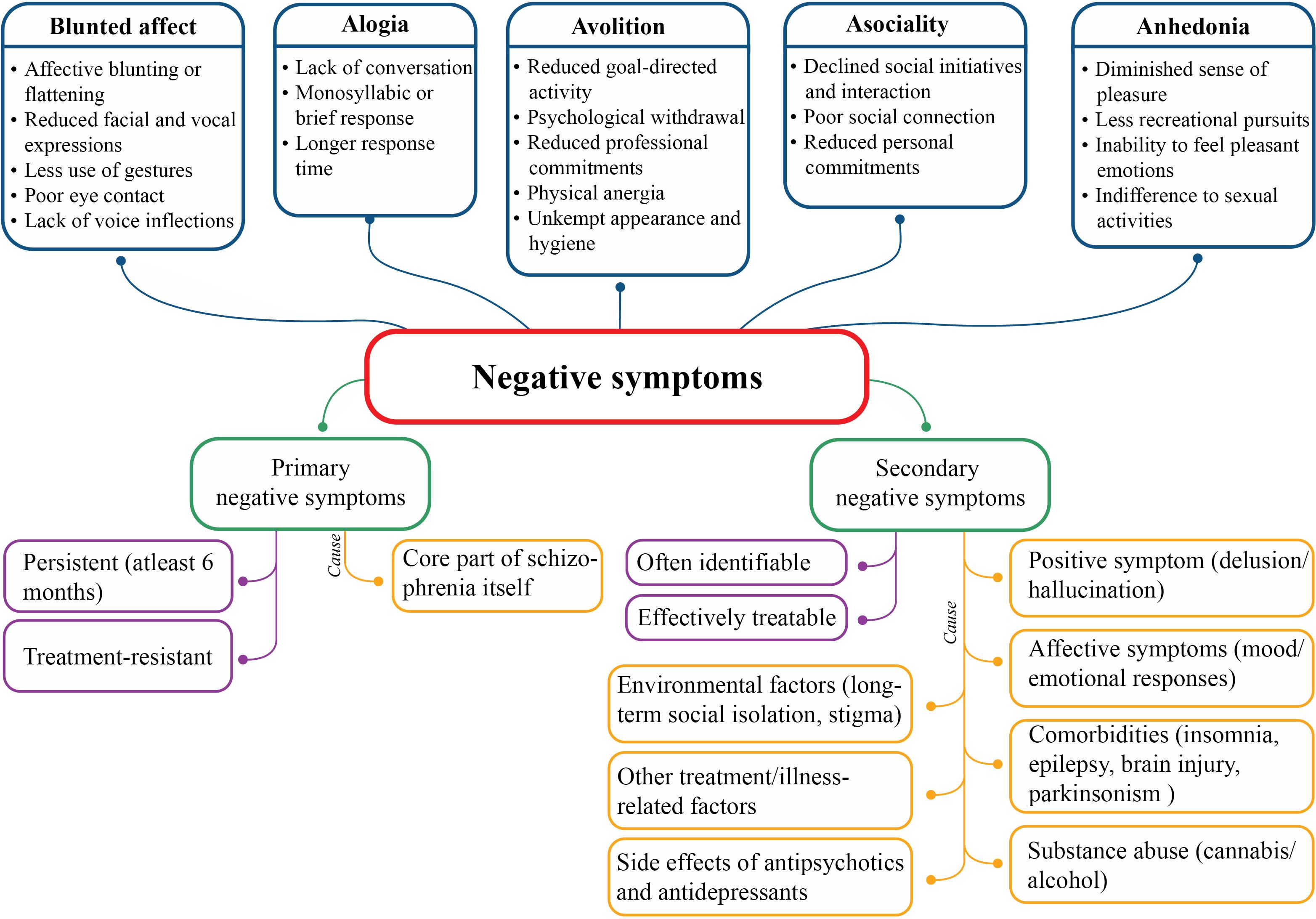
The five constructs of negative symptoms are blunted affect, alogia, anhedonia, asociality and avolition (3) that cluster into two domains: the expressive domain (blunted affect and alogia) and the experiential domain (anhedonia, asociality and avolition); latter has a larger effect on the real-world functioning (18). Moreover, negative symptoms can be primary or secondary depending on their etiology (Figure 1). Primary negative symptoms for more than one year manifest deficit syndrome of schizophrenia and patients without these symptoms are considered to suffer from non-deficit schizophrenia (19). The severity of negative symptoms is also described by persistent (persisting over time, in spite of antipsychotic treatment), predominant (greater severity than co-occurring positive symptoms) and prominent (at least three moderate symptoms or two severe symptoms) negative symptoms (17, 20).
Pathology of negative symptoms includes decreased dopamine transmission in mesocortical pathways, along with decreased serotonergic, glutamatergic and noradrenergic transmission. Of note, hypodopaminergic functioning in the prefrontal lobe and additional mesolimbic structures are responsible for diminished motivation and reward-related processes, leading to negative symptoms (17, 21). Inhibition of glutamate neurotransmission after antagonizing N-methyl-D-aspartate (NMDA) receptors may results in negative symptoms of schizophrenia (17). Brain regions associated with expressive domain are rostral anterior cingulate cortex, amygdala, and ventrolateral prefrontal cortex; and with experiential domain are dorsal and ventral striatum, dorsolateral prefrontal cortex, anterior cingulate cortex and orbitofrontal cortex (21, 22).
Genetic factors, prenatal complications and poor premorbid adjustments prior to development of psychotic illnesses are contributing factors for onset of negative symptoms (17). Males are more prone to develop negative symptoms, especially anhedonia and avolition (23). Negative symptoms greatly affect disease prognosis, physical and psychological health, and personal and social relationships (24–28), as reduced functioning of mental health, health utility and expert-rated quality of life were reported (29). During early phase of the syndrome, negative symptoms increase the risk of self-harm that can persist up to 7 years since first psychiatric visit, and impact different domains of real-life functioning (18). Level of negative symptoms in elderly population is equivalent to that of younger schizophrenia population (30). Anxiety and depression are the central symptoms of population with predominant negative symptoms, hence clinicians must pay attention to these symptoms too, and not only to negative symptoms (31).
4 Assessment of negative symptoms of schizophrenia
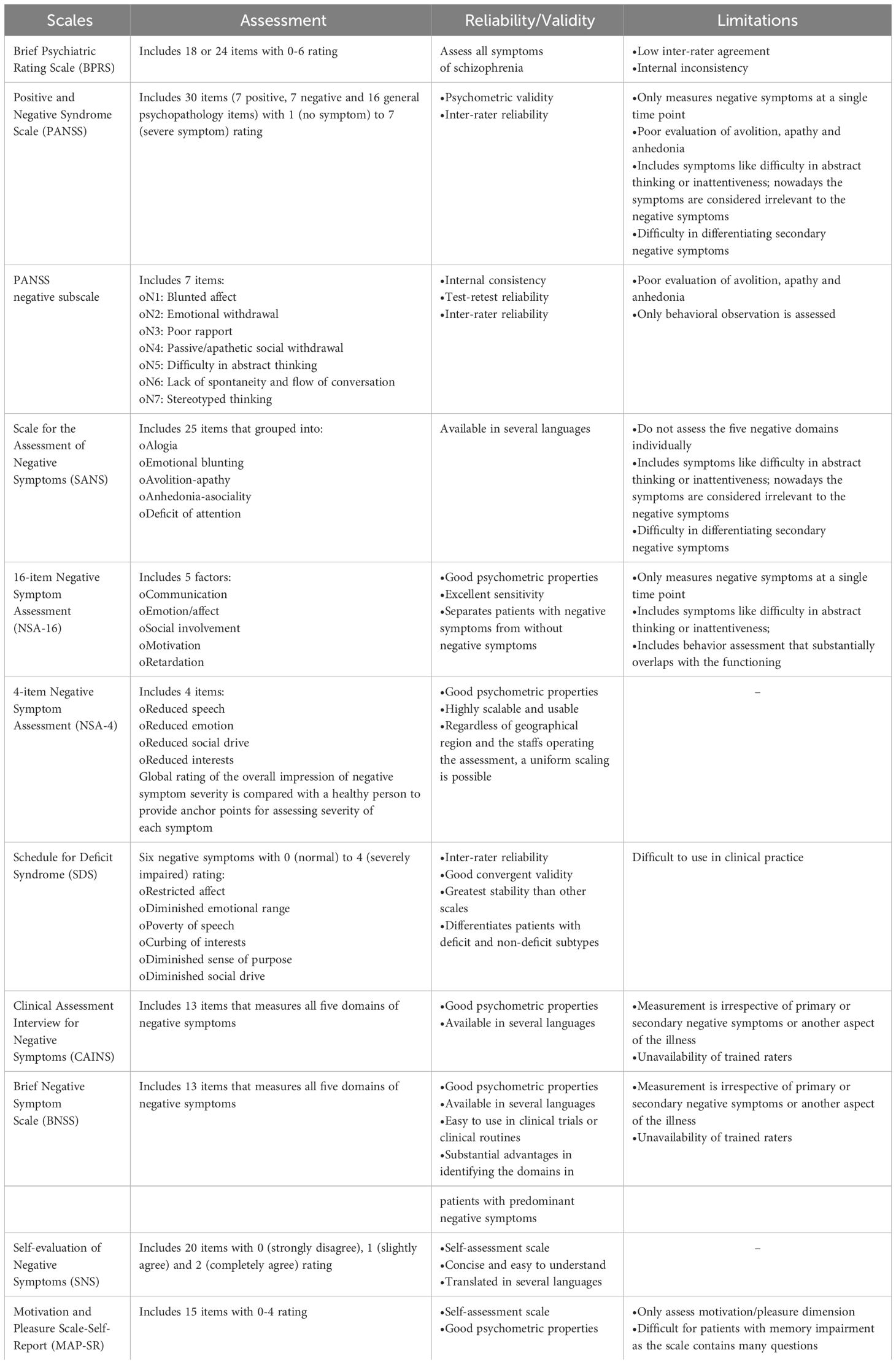
Quantitative (frequency, duration and intensity) and qualitative (difference between anticipatory and consummatory aspects of anhedonia; or difference between behavioral and experiential aspects) aspects of negative symptoms is assessed using validated instruments (18). Different scales for standardized assessments of negative symptoms which are used either by professionals or by the patient (17, 20) is presented in Table 1.
Brief Psychiatric Rating Scale (BPRS), Positive and Negative Syndrome Scale (PANSS) and Scale for the Assessment of Negative Symptoms (SANS) are used for diagnosis of deficit schizophrenia (20). In first episode of schizophrenia, patient’s phenomenological variety of negative symptoms can be evaluated with PANSS based experiential factor (‘poor rapport’, ‘passive/apathetic social withdrawal’, ‘active social avoidance’ and ‘lack of spontaneity’) and expressive factor (‘blunted affect’, ‘emotional withdrawal’ and ‘motor retardation’) (32). Brief Negative Symptom Rating Scale (BNSS) and Clinical Assessment Interview for Negative Symptoms (CAINS) have been developed by National Institute of Mental Health (NIMH) as the ‘next generation’ scale. In the USA, 16-item Negative Symptom Assessment (NSA-16) and SANS are recommended but not PANSS negative symptoms subscale, because of its inadequate coverage; whereas, the European drug authority has endorsed the use of SANS and PANSS negative symptoms subscale in clinical studies (9). Many European countries have approved the use of Self-evaluation of Negative Symptoms (SNS) scale (18).
5 Current treatment options for negative symptoms of schizophrenia and their limitations
Treatment of negative symptoms is challenging as no particular guidelines are available related to treatment algorithms and maintenance of the treatment; patients with treatment-resistant schizophrenia usually develop prominent negative symptoms (33, 34). The European regulatory guidelines and commentary issued by the US regulators have different perspectives with respect to treatment of schizophrenia, as the former recommend splitting negative symptoms from other domain of the disease, while the latter suggested lumping all the aspects of the disease together (9). The World Federation of Societies of Biological Psychiatry guidelines recommended use of FGA for secondary but not for primary negative symptoms of schizophrenia (35). Antipsychotic treatment is recommended by the American Psychiatric Association (APA) and the British Association for Psychopharmacology (BAP) for improvement and remission of both positive and negative symptoms; National Institute for Health and Care Excellence (NICE) and Canadian Psychiatric Association (CPA) suggested this treatment approach for improving functioning and quality of life. Reduced hospitalization and mortality with antipsychotic therapy are demonstrated by APA and CPA (34). United Nations High Commissioner for Refugees (UNHCR) recommended switching from FGA to SGA in case of ineffective treatment of negative symptoms (34). Likewise, the European Psychiatric Association guidelines recommended switching to SGA in patients not responding to FGA, along with social skill training and psychosocial rehabilitation (20).
First generation antipsychotics exhibits narrow efficacy spectrum in managing negative symptoms of schizophrenia (7). Second generation antipsychotics were introduced in the late 80s (24) with a promise to yield higher treatment efficacy, better receptor binding properties and lower side effects compared to FGAs (36). Significant difference in the pharmacological properties and side effect profiles exist between FGAs [fluphenazine, haloperidol, perphenazine and pimozide (D2 antagonists) and chlorpromazine, loxapine, thioridazine and trifluoperazine (D2 and 5-HT2 antagonists)], SGAs [iloperidone, lurasidone, olanzapine and ziprasidone (D2 and 5-HT2 antagonists), asenapine, clozapine, paliperidone and risperidone (5-HT2, D2 and norepinephrine α2 antagonist) and quetiapine (D2 and 5-HT2 antagonist and norepinephrine transporter reuptake inhibitor)], and TGAs [aripiprazole and brexpiprazole (D2 and 5-HT1A partial agonist and 5-HT2A antagonists)] (37, 38). Clozapine is considered as the best evidence-based therapeutic option for treatment-resistant schizophrenia (39). Higher efficacy of clozapine than other SGAs is reported for management of schizophrenia and schizophrenia-like psychoses (40). Despite its efficacy, 40% patients with treatment-resistant schizophrenia were reported to be non-respondent to clozapine treatment (41). Nielsen et al. reported improvement in negative symptoms after treatment with aripiprazole due to its partial D2 receptor agonist effect; however, no improvement in cognitive functions was found (42). Another study on patients with schizophrenia-spectrum disorders reported lower efficacy of aripiprazole in terms of improvement in PANSS negative score and CGI-S score (43). Although brexpiprazole has shown greater efficacy in improving negative symptoms (44), but common adverse effects associated with brexpiprazole are akathisia, headache, somnolence, weight gain and altered triglyceride level. Long-term risk and benefits of brexpiprazole are also not well-established (45).
Treatment-emergent adverse events are frequent with SGAs that commonly include akathisia, EPS, weight gain, sedation, insomnia, hyperprolactinemia and metabolic changes (46). Other adverse events include periorbital edema, parotitis, (inflammation of parotid gland/s) and pseudopheochromocytoma, i.e., severe paroxysmal hypertension (39). Lobos et al. reported higher incidence of akathisia with olanzapine, elevated glucose, triglycerides and prolactin levels with olanzapine and clozapine, hypercholesterolemia and hypersalivation with clozapine and low sexual drive with clozapine and risperidone treatment (40). Clozapine is also associated with other side effects viz. EPS, agranulocytosis, drooling, sedation, headache, dizziness, tremor, tachycardia, lengthening of corrected QT (QTC), weight gain, hypotension, visual abnormality, sweating, dry mouth, constipation, dyslipidemia and flexural intertrigo (39). Increase in prolactin level and EPS with amisulpride treatment and weight gain and elevated serum lipid and prolactin levels with amisulpride, aripiprazole, and olanzapine treatment were reported (43, 47). Additionally, evidence-based international guidelines revealed that SGAs have only moderate effect on negative symptoms; antidepressants and glutamatergic compounds are necessary to use additionally to overcome the disease burden (9). Schizophrenia patient data from 20 placebo-controlled trials reported prominent negative symptoms (8-33.1%), predominant negative symptoms (14.9%) and European Medicines Association (EMA) criteria-based negative symptoms (12.2-45.5%) even after 6 weeks of active treatment with SGA (48).
Poor outcomes with FGAs, and major side effects and inadequate response to SGAs leave a gap regarding the most appropriate treatment of negative symptoms, which is a long-standing challenge for schizophrenia management. Recently, a review on mental health care in central and eastern Europe suggested that many countries across the Europe have incorporated cariprazine as the first-line treatment for negative symptoms (49). Both the EMA and the US Food and Drug Administration (FDA) have approved cariprazine for schizophrenia management (50). The position statement of Polish Psychiatric Association on the use of D2/D3 receptor partial agonists highlighted the benefits of cariprazine in the management of predominant and persistent negative symptoms (51).
6 Cariprazine: a novel third generation antipsychotic
Cariprazine was approved in 2015 by the US FDA and later in 2018 in the UK (5, 7). The antipsychotic is approved in the US for treatment of schizophrenia, mania or mixed episodes, and depressive episodes related to bipolar I disorder and as adjunctive therapy to anti-depressants for the treatment of major depressive disorders, whereas in Europe it is approved for treatment of schizophrenia (26). Midlands and Lancashire Commissioning Support Unit 2019 has also recommended cariprazine for the treatment of predominant negative symptoms of schizophrenia (52). In 2021, India’s national regulatory body for cosmetics, pharmaceuticals and medical devices, Central Drugs Standard Control Organization (CDSCO) approved cariprazine for the treatment of schizophrenia, manic or mixed episodes associated with bipolar I disorder (53).
Cariprazine is available in capsule form with doses of 1.5, 3, 4.5, or 6 mg for schizophrenia treatment (6). At clinically relevant doses, cariprazine appeared to have higher occupancies of D2 and D3 receptors (54). Cariprazine dose of 1.5 mg/day results in 69% occupancy of both D2 and D3 receptors, and 3 mg/day for 14 days leads to 90% occupancy, suggesting adequate efficacy (6). Efficacy, tolerability and safety of cariprazine in patients having acute exacerbation of schizophrenia is established at a daily dose of 3 or 6 mg (55). More rapid onset of action (by 1 to 2 weeks) is achieved at a daily cariprazine dose of ≥3 mg than 1.5 mg; however, efficacy of cariprazine at 6th week remains same with both higher and lower doses (56). Improvement in PANSS total score and CGI-S with cariprazine was reported at a dose of 1.5, 3 and 4.5 mg/day in one study (57), and at 3-6 or 6-9 mg/day in another study (58). Cariprazine dose of 4.5–6 mg/day improves negative symptoms (26); 3-6 or 6-9 mg/day improves PANSS and CAINS negative symptom scores (54) and 3, 6 or 9 mg/day lowers the chances of relapse (59). Cariprazine is also effective for patients with schizophrenia and concomitant substance use disorder, as it appeared to reduce cravings of illicit drugs/alcohol in such patients (60).
6.1 Unique aspect of cariprazine’s pharmacology
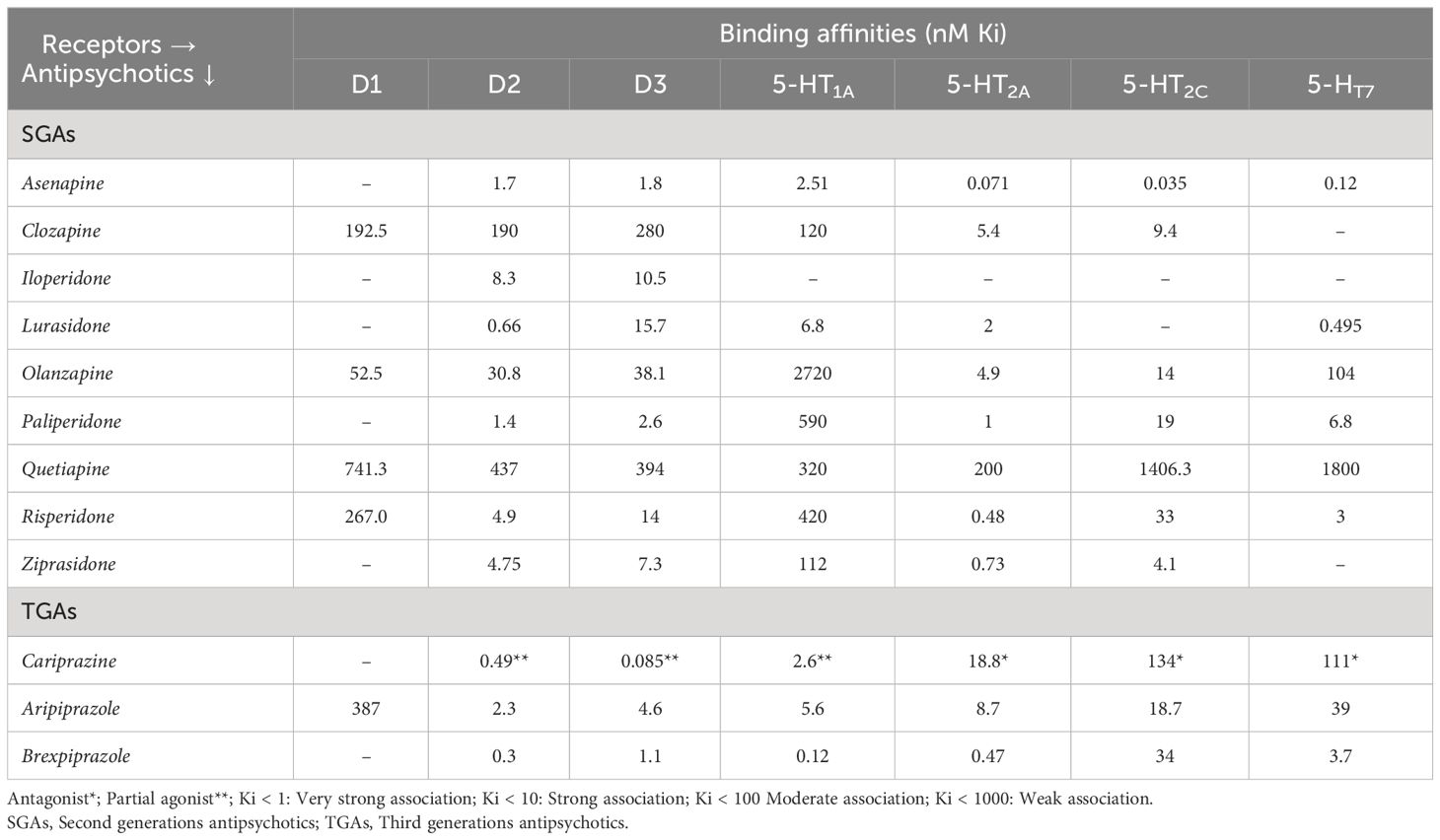
Cariprazine is a potent D2/D3 partial agonist with preferential binding to D3 receptors (61). This differs from two other TGAs like aripiprazole and brexpiprazole, by its distinct receptor-binding characteristics not only at dopamine D2/D3 receptors, but also at serotonin 5HT1A, 5HT2B, 5HT2A, 5HT2C, and histamine H1 receptors (62). Cariprazine acts as an antagonist when dopamine activity is normal and as partial agonist when the activity is low, depending on the available dopamine (63). This feature of cariprazine is proven effective for treatment of predominant primary negative symptoms of schizophrenia (24, 64). It is especially recommended for elder patients, as cariprazine results in procognitive and antidepressant effects due to its partial agonism towards D2/D3 receptors (7). Cariprazine also acts as an antagonist to 5-HT2B and a partial agonist to 5-HT1A. Its strong affinity towards 5-HT1B receptor is the reason for reduced EPS and akathisia; however, the clinical relevance of antagonism to serotonin 5-HT2B receptors is unknown. Partial agonism of cariprazine to 5-HT1A receptors lowers depressant effects of schizophrenia, and weak antagonism to 5-HT2C and H1 receptors reduces risk of weight gain, metabolic abnormalities and sedation than olanzapine and quetiapine (63). Additionally, cariprazine has a lower or negligible affinity for noradrenergic, histaminergic, and cholinergic receptors (65). Because of its lower inhibition of dopaminergic neurotransmission in the striatum, cariprazine has lower risk of developing EPS than other atypical antipsychotics (63). The receptor binding affinities of different anti-psychotics in comparison with cariprazine is shown in Table 2 (5, 36, 66–69).
Greater affinity for D3 receptor together with actions of serotonin receptors makes cariprazine a potential antipsychotic for alleviating the negative symptoms. Moreover, these symptoms are responsible for poor social functioning, impacting patient’s daily functioning and quality of life. Efficacy of cariprazine is well-established in treating negative as well as cognitive and affective symptoms of schizophrenia, thus improving social behavior of the patient. For this reason cariprazine is regarded as a ‘socializing drug’ (70). In addition to ten-fold higher affinity for D3 receptors, cariprazine adds exceptional values to schizophrenia management because of its long half-life and broad-spectrum efficacy and safety (3, 5, 71). A remarkably longer half-life of the active metabolites of cariprazine, desmethyl cariprazine (DCAR) and didesmethyl cariprazine (DDCAR), of 2-4 days and 1-3 weeks (67) respectively, prevents patients from experiencing incidence of relapse even after accidentally missing dose. Early and late efficacy are offered by DCAR and DDCAR, respectively; with both depicting mean concentrations of 400% and 30% respectively even after 12 weeks of cariprazine administration (56). Cariprazine provides a significantly longer time to relapse (defined by occurrence of psychiatric hospitalization, worsening of symptom scores, aggression or violence or suicidal tendency) and lower chance of relapse (59).
It may be noted that, because cariprazine and its active metabolites have long half-lives, the active moiety would take several weeks to reach steady state; this is unlikely to be a problem as efficacy has anyway been demonstrated in clinical trials. However, because of the long half-lives, the active moiety would take long to wash out. This could be positive if patients do not take the drug for one or more days or temporarily discontinue the treatment, as the drug is still in the body. The long half-lives also obviate the risk of a drug discontinuation syndrome. However, the long half-lives could be negative if rapid reduction of blood levels is desired, as when patients experience adverse effects or become pregnant (72).
6.2 Clinical evidences on cariprazine in management of negative symptoms of schizophrenia
6.2.1 Efficacy of cariprazine treatment
The broad-spectrum efficacy of cariprazine in treatment of schizophrenia and predominant negative symptoms in terms of reduction in blunted affect, emotional withdrawal, passive/apathetic social withdrawal, poor rapport and difficulty in abstract thinking according to PANSS score is established (3). Although antipsychotic monotherapy is recommended for schizophrenia treatment, with the evidence of efficacy of polypharmacy in the real world, monotherapy is often challenged (6). On the other hand, adverse effects of using multiple antipsychotics disapproved the idea of polypharmacy (20). Available findings on cariprazine monotherapy or adjunctive therapy for negative symptom treatment are summarized in Table 3. Németh et al. conducted a phase III randomized trial in eleven European countries, and found a significant improvement in predominant negative symptoms with cariprazine than risperidone, starting from ~3 months of treatment, as well as a greater treatment adherence. Moreover, the improvement was independent of EPS, positive and depressive symptoms (71). A recently published study found that a single trajectory best described improvement of negative symptoms with cariprazine: there was steady improvement all through the trial with most improvement occurring during the first 4 weeks (76). Another study demonstrated effectiveness of cariprazine monotherapy in reducing PANSS negative subscale items and PANSS-derived factors by week 26; in comparison to risperidone, the efficacy of cariprazine in negative symptom improvement was an exclusive effect of the antipsychotic only (3). Cariprazine showed to have higher improvement in moderate/severe negative symptoms in patients with acute schizophrenia compared to aripiprazole (24). A lower number needed to treat (NNT) indicates therapeutic effects of a drug compared to the comparator, based on the visible improvements (77). The NNT of cariprazine is lower than risperidone (n=3 vs. 6) and aripiprazole (n=3 vs. 19) in achieving PANSS factor score for negative symptoms, suggesting that cariprazine dose of 1.5–3 mg/day is sufficient to accomplish positive outcomes than risperidone and aripiprazole (26). A small uncontrolled, open label study in patients with early psychosis found that the mean negative PANSS score decreased from 26 (at baseline) to 11 (at 6 months) in patients who tolerated cariprazine (1.5-3.0 mg/day) and responded to it (78). Treatment-resistant or drug-naïve schizophrenia has shown improvement with cariprazine treatment (1, 75). Steady state of paranoid delusions and aggressiveness was achieved with 2 weeks of cariprazine treatment (79). Cariprazine as adjunctive or monotherapy also resulted in remission of negative symptoms (5, 25, 64, 74).
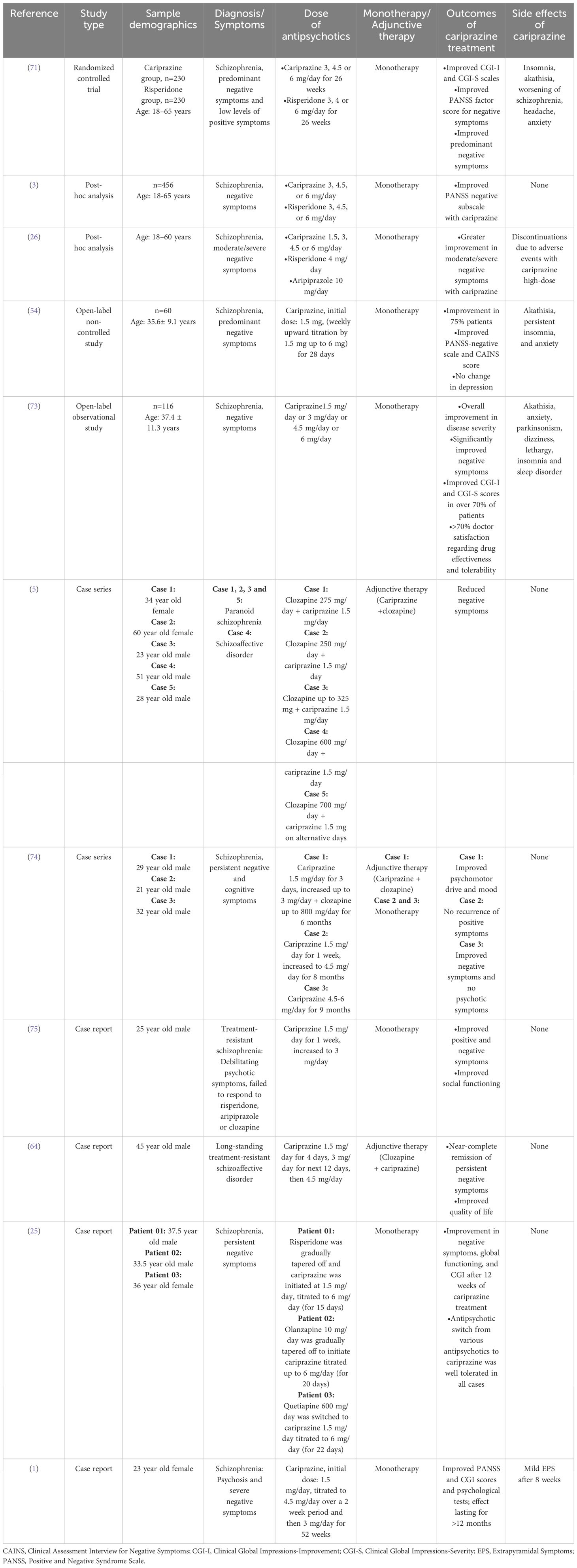
Table 3 Summary of clinical evidence of cariprazine for management of negative symptoms of schizophrenia.
6.2.2 Safety of cariprazine treatment
The most common adverse reactions with cariprazine treatment (incidence rate of ≥ 5%) are EPS and akathisia in patients with schizophrenia; EPS, akathisia, dyspepsia, vomiting, somnolence, and restlessness in bipolar mania; nausea, akathisia, restlessness and EPS in bipolar depression; and akathisia, restlessness, fatigue, constipation, nausea, insomnia, increased appetite, dizziness, and EPS in adjunctive treatment of major depressive disorder (80). Previous studies on safety and tolerability of cariprazine monotherapy demonstrated that treatment with cariprazine is generally well-tolerated and lowers total cholesterol, low-density lipoprotein, high-density lipoprotein and triglyceride levels in patients with schizophrenia (81, 82). Long term safety of cariprazine monotherapy in adults with schizophrenia is established by Cutler et al.; safety and tolerability remained consistent up to one year (83). Normal electrocardiogram (ECG) and occurrence of mild/moderate treatment-emergent adverse events (akathisia, insomnia, headache and weight increased; anxiety and tremor) over the course of 53 weeks of cariprazine treatment was found in patients with acute exacerbation of schizophrenia. Safety and tolerability of cariprazine in terms of vital signs, body weight, clinical laboratory tests and ECG has been recorded by a post hoc analysis including four short (6 weeks) and four long (≥6 months) term studies. The study reported that cariprazine has a good safety profile and is well-tolerated with lower rates of treatment-emergent adverse events, independent of the treatment durations (84). Another post hoc analysis of pooled data from three short term (6 weeks) trials recorded safety of cariprazine in both early (<5 years) and late (>15 years) stage schizophrenia patients. Although insomnia, akathisia, EPS and headache occurred in both groups but discontinuation from the study was not related to the adverse events (85). Insomnia, akathisia, constipation, anxiety, nausea and vomiting are reported to occur with cariprazine treatment in patients with negative symptoms of schizophrenia (Table 3). However, the side effects have lower occurrence rate than other available SGAs (86). Discontinuation of cariprazine due to treatment-emergent adverse events is as low as 9% (62). Despite the recorded side effects, ~70% clinicians rated cariprazine’s effectiveness and tolerability as ‘satisfactory’ or ‘very satisfactory’ (73). If long-term efficacy and tolerability is the chief concern with negative symptom treatment then cariprazine may be used as the first-line treatment for both prominent negative symptoms and severe positive symptoms (25). Observing the clinical changes in negative symptoms with cariprazine, it can be suggested as a good treatment option for predominant negative symptoms of schizophrenia.
7 Summary
This review article provides new insights on the possible use of cariprazine for negative symptom management (Figure 2). In a nutshell, negative symptoms of schizophrenia hinder patient’s quality of life and treatment options are limited. Antipsychotic management of negative symptoms is recommended by various international guidelines. However, FGAs are ineffective for treatment of negative symptoms when they are secondary to positive symptoms, and SGAs have partial benefits on negative symptoms due to frequent incidence of treatment-related side effects. Cariprazine, a recently approved antipsychotic, has high affinity and occupancy for D2/D3 receptors, partial agonism to 5-HT1A and antagonism to 5-HT2B receptors, and longer half-life which is efficacious in management of patients with negative symptoms of schizophrenia. The drug appears to be superior to available SGAs with lower incidence of metabolic disorders and relapse. Therefore, cariprazine can be used as a viable alternative to other antipsychotics for predominant negative symptom treatment. More clinical trials need to be conducted to confirm the beneficial effect of cariprazine for treatment of negative symptoms over other antipsychotics.
Author contributions
PS: Validation, Writing – review & editing. PD: Data curation, Investigation, Writing – original draft. AS: Data curation, Investigation, Writing – original draft. SD: Data curation, Investigation, Writing – original draft. CK: Data curation, Investigation, Writing – original draft. AM: Validation, Writing – review & editing. SM: Validation, Writing – review & editing. CA: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This review article was supported by Sun Pharma Laboratories Limited, Mumbai, India.
Acknowledgments
The authors are grateful to WorkSure® India for providing medical writing assistance for this manuscript.
Conflict of interest
Author PD was employed by the company Sun Pharmaceutical Industries Limited. Authors AS, SD, CK, AM, and SM were employed by the company Sun Pharma Laboratories Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Molnar MJ, Jimoh IJ, Zeke H, Palásti Á, Fedor M. Early-onset schizophrenia with predominantly negative symptoms: A case study of a drug-naive female patient treated with cariprazine. Front Pharmacol. (2020) 11:477. doi: 10.3389/fphar.2020.00477
2. Morrissette DA, Stahl SM. Affective symptoms in schizophrenia. Drug Discovery Today Ther Strateg. (2011) 8:3–9. doi: 10.1016/j.ddstr.2011.10.005
3. Fleischhacker W, Galderisi S, Laszlovszky I, Szatmári B, Barabássy Á, Acsai K, et al. The efficacy of cariprazine in negative symptoms of schizophrenia: Post hoc analyses of PANSS individual items and PANSS-derived factors. Eur Psychiatry. (2019) 58:1–9. doi: 10.1016/j.eurpsy.2019.01.015
4. Fadgyas Stanculete M, Capatina O. The many faces of negative symptoms in schizophrenia. Psychos - Phenomenol Psychopathol Pathophysiol. (2022). doi: 10.5772/intechopen.98412
5. Oloyede E, Clark I, Mace S, Whiskey E, Taylor D. Clozapine augmentation with cariprazine for negative symptoms: a case series and literature review. Ther Adv Vaccines. (2022) 12:1–9. doi: 10.1177/https
6. Edinoff A, Ruoff MT, Ghaffar YT, Rezayev A, Jani D, Kaye AM, et al. Cariprazine to treat schizophrenia and bipolar disorder in adults. Psychopharmacol Bull. (2020) 50:83–117.
7. Reddy Mukku S, Nadella R, Kornapalli S. Cariprazine for late-life psychiatric illness: A review on therapeutic potential and challenges. J Geriatr Ment Heal. (2021) 8:77. doi: 10.4103/jgmh.jgmh_43_21
8. Fabrazzo M, Cipolla S, Camerlengo A, Perris F, Catapano F. Second-generation antipsychotics’ Effectiveness and tolerability: A review of real-world studies in patients with schizophrenia and related disorders. J Clin Med. (2022) 11(15):4530. doi: 10.3390/jcm11154530
9. Möller HJ, Czobor P. Pharmacological treatment of negative symptoms in schizophrenia. Eur Arch Psychiatry Clin Neurosci. (2015) 265:567–78. doi: 10.1007/s00406-015-0596-y
10. McCormack PL. Cariprazine: first global approval. Drugs. (2015) 75:2035–43. doi: 10.1007/s40265-015-0494-7
11. World Health Organization. Schizophrenia (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/schizophrenia.
12. Sabe M, Zhao N, Crippa A, Kaiser S. Antipsychotics for negative and positive symptoms of schizophrenia: dose-response meta-analysis of randomized controlled acute phase trials. NPJ Schizophr. (2021) 7(11):43. doi: 10.1038/s41537-021-00171-2
13. Mathew VK, Sam KG, Samuel B, Das AK. Epidemiology of schizophrenia in an Indian hospital. Res J Pharm Technol. (2020) 13(1):219–23. doi: 10.5958/0974-360x.2020.00044.x
14. Janoutová J, Janáčková P, Šerý O, Zeman T, Ambroz P, Kovalová M, et al. Epidemiology and risk factors of Schizophrenia. Neuroendocrinol Lett. (2016) 37:1–8.
15. Folsom DP, Lebowitz BD, Lindamer LA, Palmer BW, Patterson TL, Jeste DV. Schizophrenia in late life: Emerging issues. Dialogues Clin Neurosci. (2006) 8:45–52. doi: 10.31887/dcns.2006.8.1/dfolsom
16. Mosolov SN, Yaltonskaya PA. Primary and secondary negative symptoms in schizophrenia. Front Psychiatry. (2022) 12:766692. doi: 10.3389/fpsyt.2021.766692
17. Correll CU, Schooler NR. Negative symptoms in schizophrenia: A review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. (2020) 16:519–34. doi: 10.2147/NDT.S225643
18. Giordano GM, Caporusso E, Pezzella P, Galderisi S. Updated perspectives on the clinical significance of negative symptoms in patients with schizophrenia. Expert Rev Neurother. (2022) 22:541–55. doi: 10.1080/14737175.2022.2092402
19. Alabaf S, Kirkpatrick B, Chen S, Cardinal RN, Fernandez-Egea E. Early versus late risk factors for deficit and nondeficit schizophrenia. Rev Psiquiatr Salud Ment. (2022) 15:38–46. doi: 10.1016/j.rpsm.2021.03.002
20. Galderisi S, Mucci A, Dollfus S, Nordentoft M, Falkai P, Kaiser S, et al. EPA guidance on assessment of negative symptoms in schizophrenia. Eur Psychiatry. (2021) 64(1):e23. doi: 10.1192/j.eurpsy.2021.11
21. Wu Q, Wang X, Wang Y, Long YJ, Zhao JP, Wu RR. Developments in biological mechanisms and treatments for negative symptoms and cognitive dysfunction of schizophrenia. Neurosci Bull. (2021) 37:1609–24. doi: 10.1007/s12264-021-00740-6
22. Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Front Psychiatry. (2014) 5:47. doi: 10.3389/fpsyt.2014.00047
23. Barendse MEA, Lara GA, Guyer AE, Swartz JR, Taylor SL, Shirtcliff EA, et al. Sex and pubertal influences on the neurodevelopmental underpinnings of schizophrenia: A case for longitudinal research on adolescents. Schizophr Res. (2023) 252:231–41. doi: 10.1016/j.schres.2022.12.011
24. Căpăţînă O, Micluţia I, Fadgyas−stănculete M. Current perspectives in treating negative symptoms of schizophrenia: A narrative review (Review). Exp Ther Med. (2021) 21(3):276. doi: 10.3892/etm.2021.9707
25. Vasiliu O. Case report: cariprazine efficacy in young patients diagnosed with schizophrenia with predominantly negative symptoms. Front Psychiatry. (2021) 12:786171. doi: 10.3389/fpsyt.2021.786171
26. Earley W, Guo H, Daniel D, Nasrallah H, Durgam S, Zhong Y, et al. Efficacy of cariprazine on negative symptoms in patients with acute schizophrenia: A post hoc analysis of pooled data. Schizophr Res. (2019) 204:282–8. doi: 10.1016/j.schres.2018.08.020
27. Bokhari SQ, Bokhari QM, Mariam A, Majeed R. Correlation between quality of life and positive and negative symptoms of schizophrenia. Pakistan J Med Heal Sci. (2015) 9:367–70.
28. Desalegn D, Girma S, Tessema W, Yeshigeta E, Kebeta T. Quality of Life and Associated Factors among Patients with Schizophrenia Attending Follow-Up Treatment at Jimma Medical Center, Southwest Ethiopia: A Cross-Sectional Study. Psychiatry J. (2020) 2020:1–7. doi: 10.1155/2020/4065082
29. Karow A, Wittmann L, Schöttle D, Schäfer I, Lambert M. The assessment of quality of life in clinical practice in patients with schizophrenia. Dialogues Clin Neurosci. (2014) 16:185–95. doi: 10.31887/dcns.2014.16.2/akarow
30. Cohen CI, Natarajan N, Araujo M, Solanki D. Prevalence of negative symptoms and associated factors in older adults with schizophrenia spectrum disorder. Am J Geriatr Psychiatry. (2013) 21:100–7. doi: 10.1016/j.jagp.2012.10.009
31. Demyttenaere K, Anthonis E, Acsai K, Correll CU. Depressive symptoms and PANSS symptom dimensions in patients with predominant negative symptom schizophrenia: A network analysis. Front Psychiatry. (2022) 13:795866. doi: 10.3389/fpsyt.2022.795866
32. Pelizza L, Leuci E, Maestri D, Quattrone E, Paulillo G, Pellegrini P, et al. Negative symptoms in first episode schizophrenia: Results from the “parma early psychosis” program. Eur Psychiatry. (2021) 64(S1):S168–8. doi: 10.1192/j.eurpsy.2021.446
33. Tsapakis EM, Dimopoulou T, Tarazi FI. Clinical management of negative symptoms of schizophrenia: An update. Pharmacol Ther. (2015) 153:135–47. doi: 10.1016/j.pharmthera.2015.06.008
34. Correll CU, Martin A, Patel C, Benson C, Goulding R, Kern-Sliwa J, et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophrenia. (2022) 8:1–10. doi: 10.1038/s41537-021-00192-x
35. Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthøj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia–a short version for primary care. Int J Psychiatry Clin Pract. (2017) 21:82–90. doi: 10.1080/13651501.2017.1291839
36. Pilla Reddy V, Kozielska M, Suleiman AA, Johnson M, Vermeulen A, Liu J, et al. Pharmacokinetic-pharmacodynamic modelling of antipsychotic drugs in patients with schizophrenia: Part II: The use of subscales of the PANSS score. Schizophr Res. (2013) 146:153–61. doi: 10.1016/j.schres.2013.02.010
37. Ricci V, De Berardis D, Maina G. Third-generation antipsychotics and lurasidone in the treatment of substance-induced psychoses: A narrative review. Healthc. (2024) 12:1–15. doi: 10.3390/healthcare12030339
38. Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, et al. The American psychiatric association practice guideline for the treatment of patients with schizophrenia. The American Journal of Psychiatry. (Washington, DC: American Psychiatric Association Publishing) (2020). pp. 868–872 p. doi: 10.1176/appi.ajp.2020.177901.
39. De Fazio P, Gaetano R, Caroleo M, Cerminara G, Maida F, Bruno A, et al. Rare and very rare adverse effects of clozapine. Neuropsychiatr Dis Treat. (2015) 11:1995–2003. doi: 10.2147/NDT.S83989
40. Lobos C, Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, et al. Clozapine versus other atypical anti psychotics for schizophrenia. Cochrane Database Syst Rev. (2010) 11(11):CD006633. doi: 10.1002/14651858.cd006633.pub2
41. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. (2017) 62:772–7. doi: 10.1177/0706743717718167
42. Nielsen MØ, Kristensen TD, Borup Bojesen K, Glenthøj BY, Lemvigh CK, Ebdrup BH. Differential effects of aripiprazole and amisulpride on negative and cognitive symptoms in patients with first-episode psychoses. Front Psychiatry. (2022) 13:834333. doi: 10.3389/fpsyt.2022.834333
43. Johnsen E, Kroken RA, Løberg EM, Rettenbacher M, Joa I, Larsen TK, et al. Amisulpride, aripiprazole, and olanzapine in patients with schizophrenia-spectrum disorders (BeSt InTro): a pragmatic, rater-blind, semi-randomised trial. Lancet Psychiatry. (2020) 7:945–54. doi: 10.1016/S2215-0366(20)30341-2
44. Ricci V, Paggi A, Cristofori E, Passarello E, Maina G. Efficacy of brexpiprazole for treatment persistent negative symptoms in three schizophrenic patients: A case series. Psychiatry Res Case Rep. (2022) 1:100040. doi: 10.1016/j.psycr.2022.100040
45. Diefenderfer LA, Iuppa C. Brexpiprazole: A review of a new treatment option for schizophrenia and major depressive disorder. Ment Heal Clin. (2017) 7:207–12. doi: 10.9740/mhc.2017.09.207
46. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. (2018) 17:341–56. doi: 10.1002/wps.20567
47. Liang Y, Yu X. Effectiveness of amisulpride in Chinese patients with predominantly negative symptoms of schizophrenia: A subanalysis of the ESCAPE study. Neuropsychiatr Dis Treat. (2017) 13:1703–12. doi: 10.2147/NDT.S140905
48. Rabinowitz J, Werbeloff N, Caers I, Mandel FS, Stauffer V, Menard F, et al. Negative symptoms in schizophrenia - the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr Res. (2013) 150:334–8. doi: 10.1016/j.schres.2013.06.023
49. Bitter I, Mohr P, Raspopova N, Szulc A, Samochowiec J, Micluia IV, et al. Assessment and treatment of negative symptoms in schizophrenia—A regional perspective. Front Psychiatry. (2022) 12:820801. doi: 10.3389/fpsyt.2021.820801
50. Cerveri G, Gesi C, Mencacci C. Pharmacological treatment of negative symptoms in schizophrenia: Update and proposal of a clinical algorithm. Neuropsychiatr Dis Treat. (2019) 15:1525–35. doi: 10.2147/NDT.S201726
51. Wichniak A, Siwek M, Rymaszewska J, Janas-Kozik M, Wolańczyk T, Bieńkowski P, et al. The position statement of the Working Group of the Polish Psychiatric Association on the use of D2/D3 dopamine receptor partial agonists in special populations. Psychiatr Pol. (2021) 55:967–87. doi: 10.12740/PP/140287
52. Midlands and Lancashire Commissioning Support Unit. New Medicine Assessment Cariprazine (Reagila®▼) For the treatment of schizophrenia in adults. In: Lancash South Cumbria Med Manag Gr (2019). Available at: https://www.midlandsandlancashirecsu.nhs.uk/.
53. Central Drugs Standard Control Organization. List of new drugs approved in the year 2021 till date (2021). Available at: https://cdsco.gov.in/opencms/opencms/en/Approval_new/Approved-New-Drugs/.
54. Ivanov SV, Smulevich AB, Voronova EI, Yakhin KK, Beybalaeva TZ, Katok AA. Early clinical effects of novel partial D3/D2 agonist cariprazine in schizophrenia patients with predominantly negative symptoms (Open-label, non-controlled study). Front Psychiatry. (2022) 12:770592. doi: 10.3389/fpsyt.2021.770592
55. Durgam S, Cutler A, Lu K, Migliore R, Ruth A, Laszlovszky I, et al. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo-and active-controlled trial. J Clin Psychiatry. (2015) 76:2310. doi: 10.1111/bdi.12238
56. Campbell RH, Diduch M, Gardner KN, Thomas C. Review of cariprazine in management of psychiatric illness. Ment Heal Clin. (2017) 7:221–9. doi: 10.9740/mhc.2017.09.221
57. Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: A phase II, randomized clinical trial. Schizophr Res. (2014) 152:450–7. doi: 10.1016/j.schres.2013.11.041
58. Kane JM, Zukin S, Wang Y, Lu K, Ruth A, Nagy K, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. (2015) 35:367–73. doi: 10.1097/JCP.0000000000000346
59. Durgam S, Earley W, Li R, Li D, Lu K, Laszlovszky I, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: A randomized, double-blind, placebo-controlled trial. Schizophr Res. (2016) 176:264–71. doi: 10.1016/j.schres.2016.06.030
60. Fagiolini A, Alcalá JÁ, Aubel T, Bienkiewicz W, Bogren MMK, Gago J, et al. Treating schizophrenia with cariprazine: From clinical research to clinical practice. Real world experiences and recommendations from an International Panel. Ann Gen Psychiatry. (2020) 19:1–11. doi: 10.1186/s12991-020-00305-3
61. Stahl SM. Drugs for psychosis and mood: Unique actions at D3, D2, and D1 dopamine receptor subtypes. CNS Spectr. (2017) 22:375–84. doi: 10.1017/S1092852917000608
62. Citrome L. Cariprazine for acute and maintenance treatment of adults with schizophrenia: an evidence-based review and place in therapy. Neuropsychiatr Dis Treat. (2018) 14:2563–77. doi: 10.2147/NDT.S169369
63. Do A, Keramatian K, Schaffer A, Yatham L. Cariprazine in the treatment of bipolar disorder: within and beyond clinical trials. Front Psychiatry. (2021) 12:769897. doi: 10.3389/fpsyt.2021.769897
64. Bogren M, Soltesz M, Hjorth S. Remission of persistent negative symptoms and psychosocial consequences by combined clozapine and cariprazine treatment in a patient with long-standing treatment-resistant schizoaffective disorder. Front Psychiatry. (2022) 13:887547. doi: 10.3389/fpsyt.2022.887547
65. Aubel T. Cariprazine: Patients with treatment-resistant schizophrenia. Neuropsychiatr Dis Treat. (2021) 17:2327–32. doi: 10.2147/NDT.S315653
66. Seneca N, Finnema S, Laszlovszky I, Kiss B, Horváth A, Pásztor G, et al. Occupancy of dopamine D 2 and D 3 and serotonin 5-HT1A receptors by the novel antipsychotic drug candidate, cariprazine (RGH-188), in monkey brain measured using positron emission tomography. Psychopharmacol (Berl). (2011) 218:579–87. doi: 10.1007/s00213-011-2343-z
67. Stahl SM, Laredo S, Morrissette DA. Cariprazine as a treatment across the bipolar I spectrum from depression to mania: mechanism of action and review of clinical data. Ther Adv Psychopharmacol. (2020) 10:1–11. doi: 10.1177/https
68. Kaar SJ, Natesan S, McCutcheon R, Howes OD. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. (2020) 172:107704. doi: 10.1016/j.neuropharm.2019.107704
69. Watanabe Y, Yamada S, Otsubo T, Kikuchi T. Brexpiprazole for the treatment of schizophrenia in adults: An overview of its clinical efficacy and safety and a psychiatrist’s perspective. Drug Des Devel Ther. (2020) 14:5559–74. doi: 10.2147/DDDT.S240859
70. Morozov P, Bekker R, Bykov Y. Cariprazine ‘ s potential in improving social dysfunction in patients with schizophrenia: A perspective. Front Psychiatry. (2022) 13:868751. doi: 10.3389/fpsyt.2022.868751
71. Németh G, Laszlovszky I, Czobor P, Szalai E, Szatmári B, Harsányi J, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. (2017) 389:1103–13. doi: 10.1016/S0140-6736(17)30060-0
72. Andrade C. Psychotropic drugs with long half-lives: implications for drug discontinuation, occasional missed doses, dosing interval, and pregnancy planning. J Clin Psychiatry. (2022) 83(4):22F14593. doi: 10.4088/JCP.22f14593
73. Rancans E, Dombi ZB, Mátrai P, Barabássy Á, Sebe B, Skrivele I, et al. The effectiveness and safety of cariprazine in schizophrenia patients with negative symptoms and insufficient effectiveness of previous antipsychotic therapy: an observational study. Int Clin Psychopharmacol. (2021) 36:154–61. doi: 10.1097/YIC.0000000000000351
74. Viegas F, Ferreira T, Campos C. Using cariprazine to ameliorate negative symptoms and metabolic side effects of clozapine and paliperidone – clinical cases. Neuropsychiatr Dis Treat. (2022) 18:1145–9. doi: 10.2147/NDT.S343747
75. Montgomery A, Rogowska M, Dratcu L. Cariprazine — an alternative treatment for clozapine-resistant schizophrenia? Clin Psychopharmacol Neurosci. (2023) 21:202–6. doi: 10.9758/cpn.2023.21.1.202
76. Leucht S, Dombi ZB, Szabó P, Barabássy Á, Levine SZ. Single trajectory treatment response for predominant negative symptoms: Post-hoc analysis of a clinical trial with cariprazine and risperidone. Schizophr Res. (2023) 261:24–30. doi: 10.1016/j.schres.2023.09.004
77. Mohr P, Masopust J, Kopeˇcek M. Dopamine receptor partial agonists: do they differ in their clinical efficacy? Front Psychiatry. (2022) 12:781946. doi: 10.3389/fpsyt.2021.781946
78. Pappa S, Kalniunas A, Maret J. Cariprazine for negative symptoms in early psychosis: a pilot study with a 6-month follow-up. Front Psychiatry. (2023) 14:1183912. doi: 10.3389/fpsyt.2023.1183912
79. Machetanz L, Lau S, Kirchebner J. Cariprazine in offender patient with acute psychosis and aggressive behavior: Case report. Psychiatry Res Case Rep. (2023) 2:100094. doi: 10.1016/j.psycr.2022.100094
80. VRAYLAR™ (cariprazine) capsules, for oral use. Ref ID 5095981 (2022). Available at: https://shorturl.at/EFHZ4.
81. Earley W, Durgam S, Lu K, Laszlovszky I, Debelle M, Kane JM. Safety and tolerability of cariprazine in patients with acute exacerbation of schizophrenia: A pooled analysis of four phase II/III randomized, double-blind, placebo-controlled studies. Int Clin Psychopharmacol. (2017) 32:319–28. doi: 10.1097/YIC.0000000000000187
82. Nasrallah HA, Earley W, Cutler AJ, Wang Y, Lu K, Laszlovszky I, et al. The safety and tolerability of cariprazine in long-term treatment of schizophrenia: A post hoc pooled analysis. BMC Psychiatry. (2017) 17:1–13. doi: 10.1186/s12888-017-1459-z
83. Cutler AJ, Durgam S, Wang Y, Migliore R, Lu K, Laszlovszky I, et al. Evaluation of the long-term safety and tolerability of cariprazine in patients with schizophrenia: results from a 1-year open-label study. CNS Spectr. (2018) 23:39–50. doi: 10.1017/S1092852917000220
84. Barabássy Á, Sebe B, Acsai K, Laszlovszky I, Szatmári B, Earley WR, et al. Safety and tolerability of cariprazine in patients with schizophrenia: A pooled analysis of eight phase II/III studies. Neuropsychiatr Dis Treat. (2021) 17:957–70. doi: 10.2147/NDT.S301225
85. Falkai P, Dombi ZB, Acsai K, Barabássy Á, Schmitt A, Németh G. The efficacy and safety of cariprazine in the early and late stage of schizophrenia: a post hoc analysis of three randomized, placebo-controlled trials. CNS Spectr. (2023) 28:104–11. doi: 10.1017/S1092852921000997
Keywords: atypical antipsychotics, cariprazine, negative symptoms, schizophrenia, pharmacology, socializing drug, third-generation antipsychotics, D3 receptor
Citation: Selvan P, Devkare P, Shetty A, Dharmadhikari S, Khandhedia C, Mane A, Mehta S and Andrade C (2024) A review on the pharmacology of cariprazine and its role in the treatment of negative symptoms of schizophrenia. Front. Psychiatry 15:1385925. doi: 10.3389/fpsyt.2024.1385925
Received: 14 February 2024; Accepted: 29 March 2024;
Published: 22 April 2024.
Edited by:
Renato de Filippis, University Magna Graecia of Catanzaro, ItalyReviewed by:
Bruce J. Kinon, Karuna Therapeutics, Inc., United StatesEva Ceskova, Masaryk University, Czechia
Copyright © 2024 Selvan, Devkare, Shetty, Dharmadhikari, Khandhedia, Mane, Mehta and Andrade. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prashant Devkare, UHJhc2hhbnRILkRldmthcmVAc3VucGhhcm1hLmNvbQ==
 Panneer Selvan
Panneer Selvan Prashant Devkare
Prashant Devkare Arthik Shetty3
Arthik Shetty3