
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 28 May 2024
Sec. Neurostimulation
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1377257
Background: Negative symptoms and cognitive impairments are highly frequent in schizophrenia spectrum disorders (SSD), associated with adverse functional outcomes and quality of life. Repetitive transcranial magnetic stimulation (rTMS) has been considered a promising therapeutic option in SSD. However, placebo effects of rTMS on these symptoms remained unclear.
Objective: To investigate placebo effects of rTMS on alleviating negative symptoms and cognitive impairment in patients with SSD and to explore potential moderators.
Methods: We systematically searched five electronic databases up to 15 July 2023. Randomized, double-blind, sham-controlled trials investigating effects of rTMS on negative symptoms or cognition in patients with SSD were included. The pooled placebo effect sizes, represented by Hedges’ g, were estimated using the random-effects model. Potential moderators were explored through subgroup analysis and meta-regression.
Results: Forty-four randomized controlled trials with 961 patients (mean age 37.53 years; 28.1% female) in the sham group were included. Significant low-to-moderate pooled placebo effect sizes were observed for negative symptoms (g=0.44, p<0.001), memory (g=0.31, p=0.010), executive function (g=0.35, p<0.001), working memory (g=0.26, p=0.004), and processing speed (g=0.36, p=0.004). Subgroup analysis indicated that placebo effects were affected by sham stimulation methods, rTMS targeting approaches, and stimulation frequency.
Conclusions: Placebo effects of rTMS on negative symptoms and cognition in patients with SSD are significant in a small-to-moderate magnitude, which might be mediated by rTMS parameters. Our findings will provide new insights for practitioners to further optimize and establish standardized rTMS protocols for future RCTs tackling cardinal symptoms in SSD.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023390138.
Schizophrenia spectrum disorders (SSD) are devastating neuropsychiatric illnesses with a prevalence of around 3% (1). Cardinal symptoms of SSD include positive symptoms such as delusions and hallucinations, negative symptoms, and cognitive deficits (2). Patients with negative symptoms experience prolonged social withdrawal and decreased interests, which lead to adverse quality of life (3). In addition, cognitive deficits are strongly associated with poor functional outcomes, such as poor ability to live independently or interact with other people (4). It has been reported that 20% to 30% of patients with SSD after pharmacotherapy have residual positive symptoms (5, 6). However, the percentage of patients who still experience residual negative symptoms after long-term antipsychotic treatment is as high as 50% (7, 8). Moreover, a prospective study with a 15-year follow-up showed that cognitive deficits in people with first-episode SSD lasted for more than 15 years (9). These reports reflect necessity of advancing therapy for negative symptoms and cognitive deficits in patients with SSD.
Repetitive transcranial magnetic stimulation (rTMS), a popular non-invasive brain stimulation technique with few side effects such as mild headache (10), is considered promising intervention for ameliorating symptoms in patients with SSD (11). A meta-analysis by Hyde et al. (11) analyzed 59 RCTs and found that active rTMS was significantly more effective than sham rTMS in improving total symptoms, auditory hallucinations, and negative symptoms in individuals with schizophrenia or schizoaffective disorder. rTMS delivers magnetic field pulses to a focused region of the scalp to induce changes in neural activity of the underlying brain areas (12). Because of its promising effectiveness on modulating targeted neural activity and further reducing symptoms (12), increasing research attention has been attracted to examining effects of rTMS on negative symptoms and cognitive deficits in patients with SSD (13, 14). A meta-analysis report published in 2018 (15) has showed that rTMS administrated to the dorsolateral prefrontal cortex induces an overall moderate effect on lessening severity of negative symptoms in patients with SSD. In addition, previous studies have indicated that high-frequency rTMS targeting the dorsolateral prefrontal cortex effectively improves working memory and social cognition in patients with SSD (14, 16). It is noteworthy that even though beneficial effects of rTMS on negative and cognitive symptoms have been indicated, accumulated studies (17, 18) have pointed out a possibility of placebo effects of rTMS.
Placebo effects refer to positive responses in patient’s symptoms or clinical outcomes after receiving a sham treatment (e.g. inert pills, sham neuromodulation or saline injections) as a control in randomized sham-controlled trials (RCT) (19). In research contexts, placebo effects are typically measured as the change in outcome measure compared to baseline after administration of a sham treatment in the sham group (17, 19). Accumulating number of clinical studies and meta-analyses have reported placebo effects existing in the treatment of psychiatric disorders such as depression (17, 20), resistant obsessive-compulsive disorder (21), primary insomnia (22), and auditory hallucinations (23). It has been shown that placebo effects caused by rTMS are attributable to a range of sham stimulation methods (e.g. sham coil or active coil positioned at 45°, 90° or 180° from the skull) used in RCTs, which are associated with patient’s expectations of symptom improvement and memories of former treatment experiences (24). Moreover, rTMS targeting approaches (e.g. 10–20 EEG location system and MRI-neuronavigation system), stimulation frequency, patient characteristics (e.g. age, gender and treatment duration), and study design (e.g. number of research centers) are also possible moderators modifying placebo effects (25). Investigating factors that mediate placebo effects will be beneficial in establishing precise strategies for controlling components that influence placebo effects, optimizing placebo procedures of rTMS, and facilitating the development of novel rTMS protocols in SSD.
Currently, although a growing body of research shows positive effects of rTMS on negative symptoms and cognitive impairments in patients with SSD, no systematic review and meta-analysis has examined the important issue of placebo effects of rTMS. To our best knowledge, only one meta-analysis of 21 RCTs by Dollfus et al. (23) in 2016 has investigated placebo effects of rTMS on auditory hallucinations in patients with SSD, which found that placebo effects on hallucinations were small but evident (Hedges’ g=0.29) and related to sham stimulation methods as well as study design (i.e. parallel-group or crossover). However, it remains unclear how large placebo effects of rTMS are on the remaining cardinal symptoms represented by negative symptoms and cognitive deficits, and which moderators influence the effects.
To sum up, the purpose of this study was to conduct a systematic review and meta-analysis to examine placebo effects of rTMS on negative symptoms and cognitive impairment in patients with SSD. The secondary purpose was to identify possible moderators of the aforementioned placebo effects of rTMS. We believe that elucidating placebo effects of rTMS and associated moderators would help researchers to develop more efficacious rTMS protocols with less placebo effects in treatment of negative and cognitive symptoms. Meanwhile, our results would guide clinicians to improve placebo procedures of rTMS and to apply more effective treatment parameters for core symptoms of SSD in clinical practice.
This report was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (26). The protocol for this study was registered in PROSPERO with the ID CRD42023390138 (https://www.crd.york.ac.uk/prospero/).
Two authors of this study searched papers collected by the Cochrane Library, Medline, Web of Science, CINAHL, and EMBASE until July 15th, 2023 according to a set of key words (Supplementary Table S1). We also manually screened references of included publications for identifying eligible articles.
This study included randomized, double-blind, sham-controlled, parallel-group, and cross-over trials using sham rTMS as a control compared with real rTMS. Participants had to be diagnosed with SSD according to the Diagnostic and Statistical Manual of Mental Disorders (27), the International Classification of Diseases (28) or the Mini International Neuropsychiatric Interview (29). Study outcomes are cognition and/or negative symptoms. Publications were ineligible if they were not written in English, or did not have full-text or raw data available for effect size calculation after we contacted the corresponding authors of the publications by e-mail. Titles and abstracts of publications were independently screened to identify eligibility for full-text evaluation after duplicate publications were removed. Any discrepancies during this process were discussed. The persistent discrepancies were adjudicated by the corresponding author.
We used a standardized spreadsheet to collect relevant data, including first author, publication year, study design, demographic characteristics, sample size of the sham group, rTMS parameters of the sham group, and outcome measures. We defined placebo effects as the mean difference of outcome measures from baseline to the end of treatment in the sham group (30). Therefore, results of outcome measures such as mean and standard deviations at baseline and after intervention in the sham group were collected in detail. Detailed information is shown in Table 1. During the process of data extraction, if a study assumed multiple instruments for the evaluation of a single outcome, the data were collected from the primary outcome as specified by the study authors. In the absence of a clearly defined primary outcome, the data were collected from the instrument that was most commonly investigated. Detailed information about outcome measures employed in each study can be found in Table 1. When an outcome was assessed multiple times, data from the end of treatment were selected for analysis, as in previous studies (73, 74). Moreover, if data were unclear and could not be extracted from graphs, we contacted the corresponding author by e-mail to obtain the data. If there was no response, studies were excluded.
To enable direct cross-comparison between studies assessing different cognitive functioning, cognitive outcomes in this study were specifically divided into five sub-domains, including memory, executive function, working memory, attention, and processing speed based on the Measurement and Treatment Research to Improve Cognition in Schizophrenia (11, 75).
Meta-analyses were performed using the Comprehensive Meta-Analysis (version 3) software. We calculated Hedge’s g, a standardized mean difference, to determine the placebo effect sizes for negative symptoms and cognition. Specifically, it was calculated using the mean and standard deviation of outcome measure at baseline and at the end of intervention in the sham group. Where the above statistics at baseline and at the end of intervention were not reported, we computed the Hedge’s g using the mean difference and its standard deviation of the end of intervention compared to the baseline in the sham group. All calculations of effect sizes were conducted using the Comprehensive Meta-Analysis. The pre- and post-treatment correlations of the sham group for each study were set to be 0.25, which is a modest correlation (30).The magnitude of the Hedges’ g was defined as small (0.2–0.49), medium (0.5–0.79), or large (≥0.8) according to Cohen’s guideline (76).
Study heterogeneity was examined using the I2 statistic, which was classified to low (I2 ≤ 50%), medium (I2: 51–75%), and high (I2>75%) levels (77). Publication bias was assessed using Egger’s tests and funnel plots when 10 or more studies were included (78). The quality (i.e., risk of bias) of the included RCTs was assessed using the Cochrane risk-of-bias tool for randomized trials-version 2 (RoB 2) [29]. This tool classified the risk of bias for each study as high, low, or unclear risk. We assumed random-effects model for data analysis, as stimulation parameters and outcome measures varied between studies (77).
In the sensitivity analysis, we excluded studies with a high risk of bias, as defined according to the Cochrane risk-of-bias tool, to test whether the findings remained unchanged (11). We also examined whether effect size estimates would be stable under the assumption of different levels of correlation (r=0, 0.5 and 0.8) between pretest and posttest data. In addition, four subgroup analyses were run to examine potential moderators of placebo effects, including sham stimulation methods, rTMS targeting approaches, stimulation frequency, and efficacy of active rTMS versus sham rTMS. Subgroup analysis was performed when the number of included RCTs for a particular outcome was two or more (77). Finally, we conducted univariate meta-regressions using a random-effect model to examine whether age, proportion of females, illness duration, sample size, treatment duration, randomization ratio (active vs. sham ratio), and publication time were associated with placebo effects. Meta-regressions were performed when the available studies for a particular outcome was no less than 10 (77). P<0.05 (two-tailed) was considered the threshold for statistical significance.
Our systematic search identified 1927 records from five databases and 2073 records from the reference lists of included studies (see Figure 1). After excluding 2120 duplicates, 1880 records were examined for title and abstract. Of these, 1822 articles were excluded due to non-TMS, non-double-blind RCT and other reasons. Of the 58 retrieved full-text studies, 44 articles were finally included and analyzed in this review.
The characteristics of the included studies are listed in Table 1. They were reported from 14 countries (China=14, India=6, Germany=5, Czech Republic=4, Canada=3, France=2, Israel=2, UK=2, Australia, Finland, Netherlands, Sweden, Turkey, and USA). The included RCTs recruited a total of 2125 patients (26.26% female) and 961 patients (28.10% female) were allocated to the sham rTMS group, with disease duration ranging from 3.10 to 34.50 years and mean age from 22.30 to 57.37 years. Of these, there were 853 patients with schizophrenia, 9 patients with schizoaffective disorder, 7 patients with schizophreniform disorder and 92 patients who had one of the above three diagnoses but did not be clearly classified by study authors. A total of 34 studies (14, 31, 34, 36–46, 48, 50, 52–56, 58, 59, 61–67, 69–72) assessed severity of illness using the PANSS scale, with a mean total score of 75.06, and two studies (35, 47) used the Brief Psychopathological Rating Scale, with a mean score of 40.30; The remaining studies (16, 32, 33, 49, 51, 57, 60, 68) did not report total scores for the PANSS or the BPRS, which only provide scores for negative symptoms using scales such as the PANSS, the SANS or the CAINS. Additionally, as shown in Table 1, sham stimulation methods included sham coils (n=24), coil flipped 45° (n=6), 90° (n=10), or 180° (n=4). rTMS targeting approaches covered anatomical localization (n=18), 10–20 EEG location system (n=11) and MRI-neuronavigation system (n=11). The 8-shaped coil is the most used type, with stimulation frequencies varying between 10Hz, 20 Hz and 50 Hz. The longest duration of rTMS treatment was 60 sessions, while the shortest was a single session. Negative symptoms were reported in 37 studies, memory in 12 studies, executive function in 11 studies, working memory in 9 studies, attention in 7 studies and processing speed in 6 studies.
The quality of the included parallel and cross-over RCTs is shown in Supplementary Figures S2a, S2b, respectively. Two of the 43 included parallel RCTs exhibited a high risk of bias, 28 studies had a moderate risk of bias and the remaining studies had a low risk of bias. Only one of the included RCTs had a crossover design, which has a low risk of bias.
As there were fewer than 10 RCTs for working memory, attention, and processing speed, we assessed publication bias only for negative symptoms (p=0.192, Supplementary Figure S1a), memory (p=0.802, Supplementary Figure S1b), and executive function (p=0.694, Supplementary Figure S1c) using Egger’s tests and funnel plots. No significant publication bias was found for these outcomes.
Thirty-seven RCTs involving 810 (228 female) patients were used to examine placebo effects of rTMS on negative symptoms. Meta-analysis indicated that sham rTMS had a significant placebo effect with low heterogeneity on negative symptoms (g=0.44; 95% CI: 0.32 to 0.56; p<0.001; I2 = 43.12%; Figure 2). When we removed two RCTs with a high risk of bias, the result was still stable (g=0.44; 95% CI: 0.32 to 0.57; p<0.001; I2 = 43.73%; Supplementary Table S3).
Subgroup analysis of sham stimulation methods revealed that sham coil, 45°, and 90° position coil (g=0.50, p<0.001; g=0.46, p=0.002; g=0.35, p=0.001; Supplementary Figure S3a) significantly affected placebo effects, with the lowest effect size found in the 90° position coil. For rTMS targeting approaches, the placebo effect size of the anatomical localization (g=0.53, p<0.001) was higher than that of the 10–20 EEG location system and MRI-neuronavigation system (g=0.47, p=0.002; g=0.41, p=0.001; Supplementary Figure S3b). rTMS at 20-Hz (g=0.82, p<0.001; Supplementary Figure S3c) had a higher effect than other stimulation frequencies; Moreover, a higher placebo effect size was found in studies with no significant efficacy of active rTMS over sham rTMS (g=0.50, p<0.001; Supplementary Figure S3d) (see Table 2).
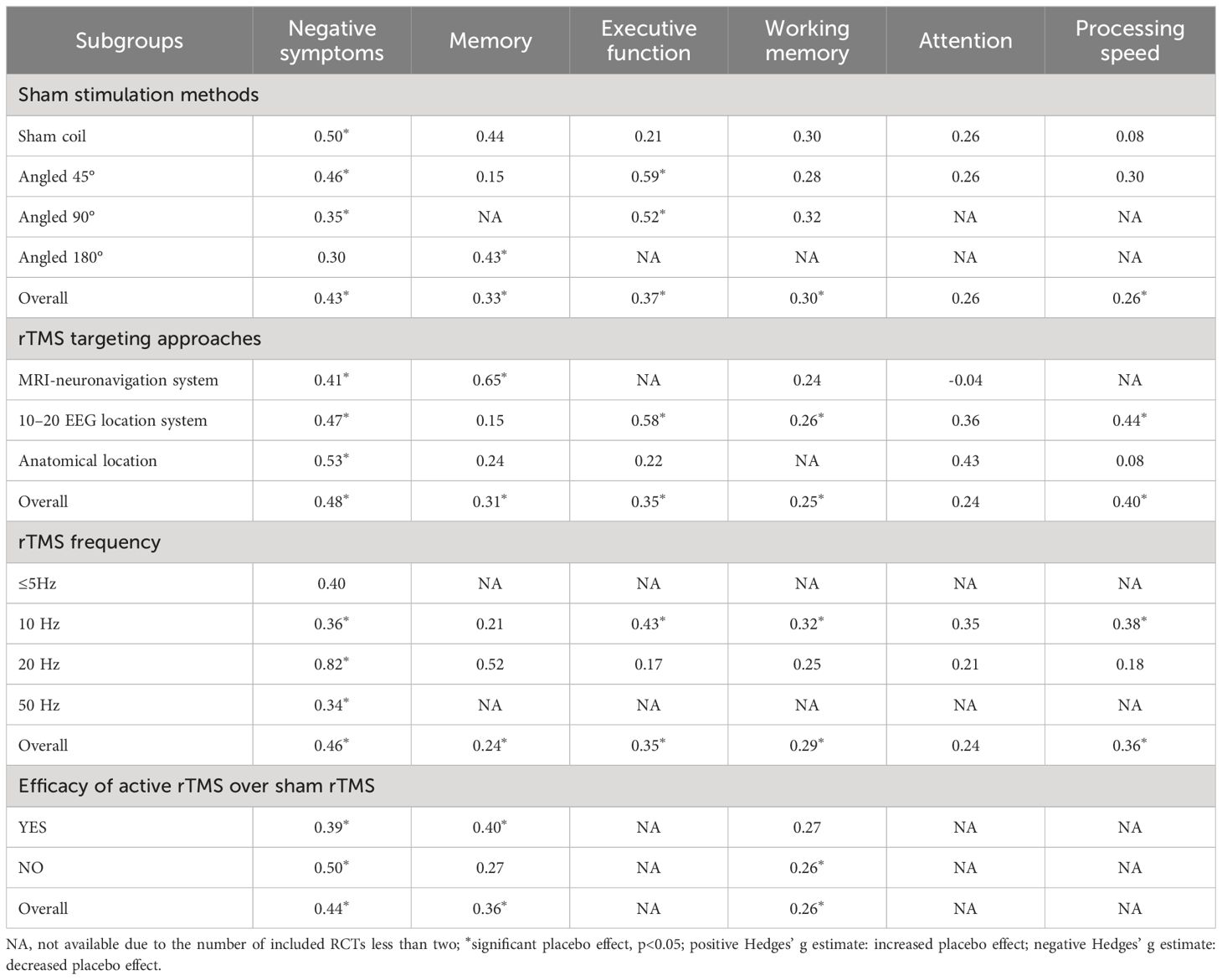
Table 2 Hedges’ g of subgroup analyses for rTMS trials of placebo effects on negative symptoms and cognition.
Meanwhile, meta-regression indicated that placebo effects of rTMS on negative symptoms was not associated with age, proportion of females, illness duration, treatment days, sample size, randomization ratio, or publication time (see Supplementary Table S5).
A total of 12 RCTs with 293 (70 female) patients were included in the analysis. The results showed that there was a significant pooled placebo ES for memory after sham rTMS treatment (g=0.31; 95% CI: 0.08 to 0.55; p=0.01; I2 = 48.80%; Figure 3A). The placebo effect size did not show a substantial difference when an RCT with high risk of bias was excluded (g=0.28; 95% CI: 0.02 to 0.53; p=0.032; I2 = 49.85%; see Supplementary Table S3).
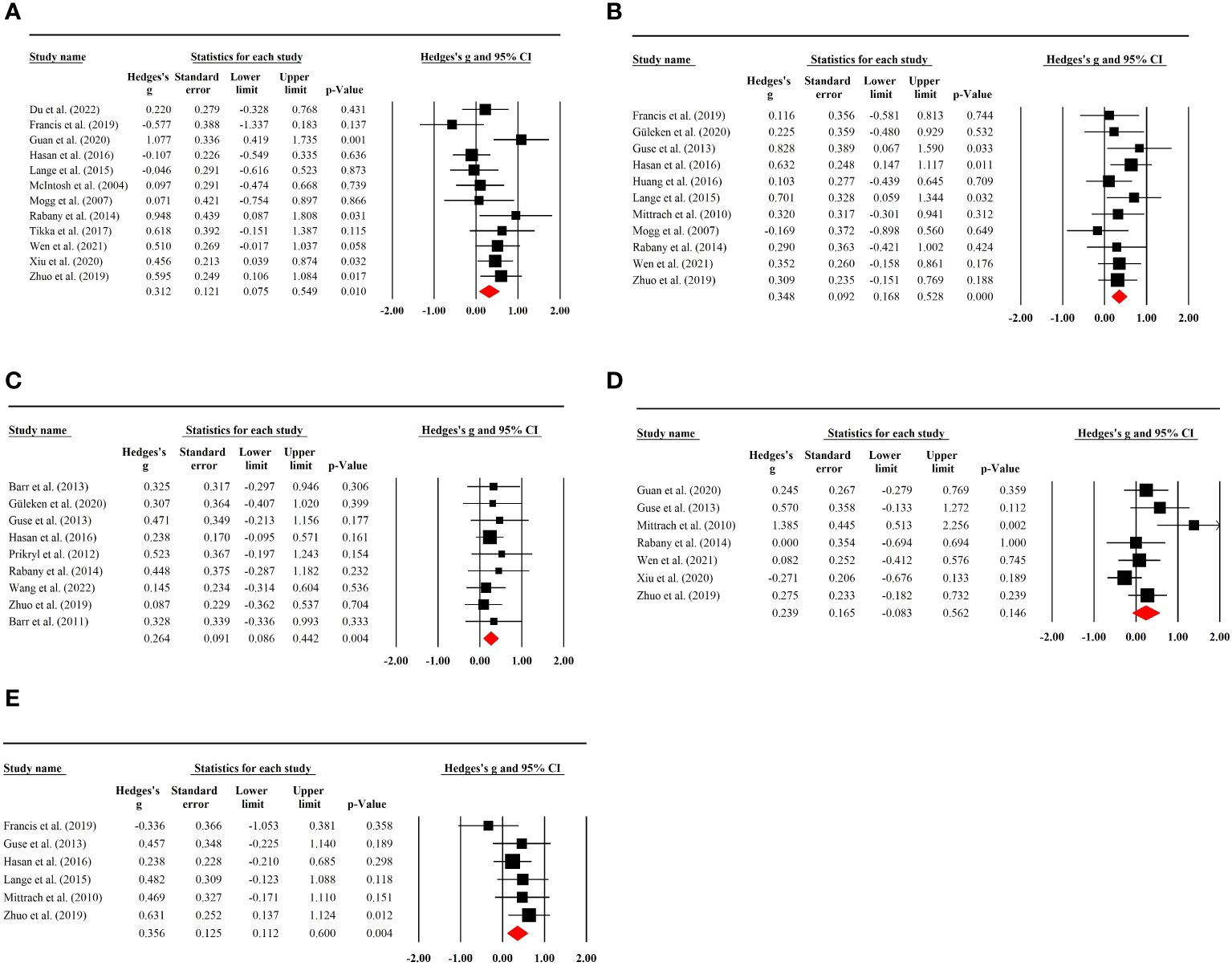
Figure 3 (A) Placebo effects of rTMS on memory in patients with SSD. (B) Placebo effects of rTMS on executive functioning in patients with SSD. (C) Placebo effects of rTMS on working memory in patients with SSD. (D) Placebo effects of rTMS on attention in patients with SSD. (E) Placebo effects of rTMS on processing speed in patients with SSD.
Subgroup analysis of sham stimulation methods indicated that rTMS with 180° position coil (g=0.43, p=0.021; Supplementary Figure S4a), but not with sham coil, 45°, or 90° position coil, had a significant placebo effect. Separate analysis for rTMS targeting approaches revealed that rTMS with MRI-neuronavigation system (g=0.65, p<0.001; Supplementary Figure S4b) showed a significant placebo effect (see Table 2). In addition, a larger placebo effect size was observed in studies of the efficacy of active rTMS over sham rTMS (g=0.40, p=0.002; Supplementary Figure S4d)
No evident association was found between placebo effects of sham rTMS and age, proportion of females, illness duration, treatment days, sample size, randomization ratio, and the publication date (see Supplementary Table S5).
Executive function was reported in 11 RCTs involving 231 (60 female) patients. Sham rTMS had a significant placebo effect on executive function without study heterogeneity (g=0.35; 95% CI: 0.17 to 0.53; p<0.001; I2 = 0.00%; Figure 3B). No significant change in effect size was observed after excluding an RCT with high risk of bias (g=0.36; 95% CI: 0.16 to 0.55; p<0.001; I2 = 0.00%; Supplementary Table S3).
Subgroup analysis showed that sham rTMS with 45° or 90° position coil had an evident placebo effect on executive function (g=0.59, p=0.003; g=0.52, p=0.011; Supplementary Figure S5a), with the highest effect size found for the 45° position coil. The analysis also indicated that placebo effects of sham rTMS with 10–20 EEG location system or frequency of 10 Hz had a significant placebo effect (g=0.58, p<0.001; g=0.43, p<0.001; Supplementary Figures S5b, c) (see Table 2).
Meta-regression analysis revealed that placebo effects of sham rTMS on executive function was not affected by age, proportion of females, illness duration, treatment duration, sample size, randomization ratio, and publication time (see Supplementary Table S5).
Working memory tests were performed in nine rTMS RCTs containing 432 (126 female) patients. A significant placebo effect was found with no study heterogeneity (g=0.26; 95% CI:0.09 to 0.44; p=0.004; I2 = 0.00%; Figure 3C). Sensitivity analysis excluding two studies with high risk of bias showed a stable result (g=0.33; 95% CI: 0.12 to 0.54; p<0.01; I2 = 0.00%; Supplementary Table S3).
Subgroup analysis showed no significant placebo effects in the different sham stimulation methods, but a significant placebo effect of the rTMS protocol with 10–20 EEG location system (g=0.26, p=0.034; Supplementary Figure S6b) or 10-Hz stimulation frequency (g=0.32, p=0.024; Supplementary Figure S6c). Moreover, a significant placebo effect was found in studies where the efficacy of active rTMS was not superior to the sham rTMS (g=0.26, p=0.014; Supplementary Figure S6d)
Seven RCTs with 375 (64 female) patients were used to analyze placebo effects on attention, and no significant placebo effect was observed (g=0.24; 95% CI: -0.08 to 0.56; p=0.146; I2 = 56.86%; Figure 3D). The result was stable when a study with a high risk of bias was excluded (g=0.25; 95% CI: -0.14 to 0.64; p=0.216; I2 = 63.26%; Supplementary Table S3). Additionally, there was no significant placebo effect found in the subgroup analysis (see Table 2).
Six RCTs with 324 (74 female) patients were included in the analysis of placebo effects on processing speed. A significant placebo effect was found with a low degree of heterogeneity (g=0.36; 95% CI: 0.11 to 0.60; p=0.004; I2 = 7.63%; Figure 3E). Sensitivity analysis by removing an RCT with a high risk of bias showed no significant difference in the result (g=0.28; 95% CI: 0.01 to 0.54; p=0.039; I2 = 0.00%; Supplementary Table S3).
Subgroup analysis showed that sham rTMS with 10–20 EEG location system (g=0.44 p=0.001; Supplementary Figure S8b) or 10 Hz stimulation frequency (g=0.38, p=0.010; Supplementary Figure S8c) had a significant placebo effect (also see Table 2).
Apart from excluding studies with a high risk of bias to examine the reliability of the results, as described above for the relevant outcomes, we also estimated the placebo effect sizes assuming different pre-and post- treatment correlations (r=0, 0.5, and 0.8 respectively). As shown in Supplementary Table S4, little variation was observed in the sensitivity estimates. Specifically, the highest and lowest pooled placebo effect sizes were 0.44 and 0.43 for negative symptoms, 0.31 and 0.31 for memory, 0.35 and 0.30 for executive function, 0.26 and 0.26 for working memory, 0.23 and 0.19 for attention, and 0.37 and 0.30 for processing speed.
This is the first systematic review of RCTs comprehensively investigating placebo effects of rTMS on negative symptoms and cognition in SSD. We found significant small-to-moderate placebo effect sizes in negative symptoms (g=0.44), memory (g=0.31), executive function (g=0.35), working memory (g=0.26) and processing speed (g=0.36) with low study heterogeneity and publication bias. However, no significant placebo effects of rTMS on attention were observed. The placebo effect size estimate was robust for each outcome in sensitivity analysis. Moreover, we identified several factors affecting placebo effects, including sham stimulation methods, rTMS targeting approaches, and rTMS frequency.
A meta-analysis by Fraguas et al. (79) was the first to examine placebo effects of pharmacological placebo on alleviating negative symptoms in SSD and found a significant placebo effect size of 2.909 (Cohen’s d). Based on this report, a study in 2022 re-evaluated placebo effects of antipsychotics on negative symptoms and found only a moderate effect size of 0.644 (Cohen’s d) (30). The reason for this difference between the two trials may be related to the different way the placebo effect size was calculated. Specifically, Fraguas et al. (79) calculated the placebo effect size using the mean change in negative symptoms over the follow-up period in the placebo group. Instead, Czobor et al. (30) calculate the placebo effect size by the mean change in negative symptoms between pre- and post-treatment measurements in the sham group. The methodology proposed by Czobor et al. is consistent with that employed in our study. Additionally, Keefe et al. (80) first investigated placebo effects of antipsychotic medications on cognition in SSD and found that the placebo treatment had minimal placebo effects with an effect size of 0.18 (Cohen’s d). Meanwhile, Agid et al. (81) reported a small to medium placebo effect size of about 0.33 for antipsychotics on total symptom severity in patients with SSD. It is matter to note that there is heterogeneity in the inclusion criteria of studies in these studies, and caution should be exercised when making comparisons.
In addition to pharmacological studies, non-invasive stimulation techniques such as rTMS have been considered as a promising method for alleviating symptoms of SSD. However, studies have shown that non-invasive techniques may produce greater placebo effects than pharmacological placebo (82, 83). Brunoni et al. (20) compared placebo effects of sham rTMS and pharmacological placebo in depression and found large placebo effects for both sham rTMS and pharmacological placebo. Dollfus and colleagues (23) found a placebo effect size of 0.29 using Hedges’ g in rTMS trials for the treatment of hallucinations, which is lower than the placebo effect sizes of rTMS in negative symptoms and several neurocognitive domains in this study. The subgroup analysis in their review showed a significant placebo effect in the parallel design RCTs (g=0.44), but not in the crossover trials. The lack of a placebo effect in crossover trials may be related to the fact that patients can differentiate between the active and the sham intervention periods in terms of scalp and auditory sensations, as well as coil placement on the head. Because the crossover design allows patients to compare the scalp and auditory sensations and side effects related to both active and sham stimulation periods, it is relatively easy for patients to guess which type of stimulation has been used. Therefore, awareness of the type of intervention may attenuate placebo effects in crossover trials. Apart from the fact that placebo effects of rTMS are present in SSD, studies have also reported that placebo effects are common in other psychiatric disorders, particularly in depression. A meta-analysis conducted by Xu et al. (84) reported that placebo effects of rTMS for depression were large (Cohen’s d = 1.016), and increasing over the years. Similarly, Razza et al. (17) analyzed 61 RCTs and also found that placebo effects in rTMS depression trials were large (Hedges’s g = 0.8) and positively associated with the year of publication. It should therefore be noted that placebo effects may be a common phenomenon in psychiatric conditions.
In rTMS trials for psychiatric disorders, stimulation parameters are considered as important factors affecting the efficacy of rTMS (85, 86). Meanwhile, the parameters also influence placebo effects of sham rTMS, a view that has been reported in previous studies (20, 23). In our study, we found that sham stimulation methods, coil type, rTMS targeting approaches, and stimulation frequency and intensity influenced placebo effects to varying degrees.
Specifically, our findings indicated that the sham coil produced a significant placebo effect, which higher than that of other three sham stimulation methods in negative symptoms. The ideal sham coil would generally have the same appearance as the active coil. It can generate an identical stimulation sound and scalp sensation, but produces no or only a weak magnetic field (87), which does not induce an active effect on the cortical target. However, it is difficult to guarantee the quality of the sham coils applied in various studies, and therefore the effectiveness of blinding varies. Out of the 44 studies included, only 10 evaluated the effectiveness of blinding using a scale. All 10 studies reported that blinding was effective, as patients were unable to distinguish between the active and sham rTMS treatment. It should be noted that the stimulation sound and scalp sensation generated by sham coils with poor quality are clearly different from the active coils (87). It can therefore be easily distinguished from the active treatment by patients, which would impact the effectiveness of blinding. Moreover, studies have found that poorly designed sham coils can produce active effects, such as biological effects in the brain (88). Therefore, minimizing placebo effects by improving the design of sham coils will facilitate the development of rTMS techniques and provide more reliable evidence.
Moreover, we found that only the 180° or 45° position coil produced significant placebo effects on memory and executive function. Apart from the sham coil, it is also a common method of sham rTMS that an active coil is tilted at specific angles (e.g., 45°, 90°, and 180°) away from the scalp. Although this method is widely used, it has several drawbacks. First, the stimulation sensations in the scalp caused by the active coil tilted at a specific angle are not the same as those generated by the active coil. It can therefore easily lead subjects who have never received rTMS to believe that these feelings are side effects caused by the active treatment. Second, rTMS-induced somatic sensations are useful in increasing placebo effects (89). Meanwhile, the active coil angled away from the scalp has also been shown to produce the active effects in animal studies (24). Therefore, the positive effects of this sham condition may exaggerate the improvement in the clinical outcomes in the sham group. For the remaining cognitive domains, we found no significant placebo effects in any of sham stimulation methods, which may be related to the limited number of included studies. In addition to the aforementioned sham methods, it seems that transcutaneous electrical nerve stimulation (TENS) with a mild current may also be a viable option for simulating active rTMS as a sham control. This method is typically used to simulate the scalp sensation elicited by active rTMS without inducing real neuromodulatory effects. However, TENS was not employed as a sham control in the studies analyzed in our review. In a study by Sheffer et al. (90), it was reported that focal electrical stimulation could serve as an effective sham control for administering high-frequency rTMS at the dorsolateral prefrontal cortex. More RCTs are required to investigate and confirm the mimetic effect of TENS in rTMS studies.
There are currently three main approaches to locating the stimulation target in rTMS studies, including traditional anatomical localization (e.g. 5-cm rule), the 10–20 EEG location system, and the MRI-neuronavigation system (86). In our review, these methods were applied in 18, 11, and 11 studies, respectively. For negative symptoms, the method of anatomical localization was shown to produce the largest placebo effect and the MRI-neuronavigation system the smallest. For memory, a significant placebo effect was only observed in the method of MRI-neuronavigation system. For working memory, attention, and processing speed, only the 10–20 EEG location system shown a significant placebo effect. It has been suggested that when physicians use more advanced technology, particularly the MRI-neuronavigation system, patients are prefer to assume that they are receiving effective treatment and therefore develop good expectations of treatment (23). Meanwhile, other two methods also require careful manipulation by professionals, so their placebo effects may depend heavily on the performance of the professionals. However, it is not yet known whether these effects are real and how large they are. Overall, we need to be problem specific, and that different rTMS targeting approaches may cause the inconsistent size of placebo effects across symptoms.
In general, the duration of an rTMS session increases as the rTMS frequency decreases. Longer stimulation duration may increase the opportunity for patient-doctor interaction and enhance placebo effects. Our results indicate that a stimulation frequency of 10 Hz could induce significant placebo effects in negative symptoms, executive function, working memory, and processing speed. However, the 20 Hz stimulation frequency only produces significant placebo effects for negative symptoms. It is important to note that due to the limited number of studies using 5 Hz and 50 Hz stimulation in our review, placebo effect sizes are missing for all cognitive domains, making it inappropriate to compare them with 10 Hz and 20 Hz for placebo effects. The relationship between stimulation frequency and placebo effects still needs to be investigated due to the limited number of studies analyzed in this review. Moreover, The number of intervention sessions may be positively correlated with the study duration. In our review, the majority of intervention sessions in the included studies ranged from 10 to 40 sessions. Although there is a paucity of evidence demonstrating the relationship between placebo effects and intervention sessions, it is possible that they may play a role in influencing the effects. Thus, further research is necessary to examine the potential relationship.
Publication time and subject demographics have previously been considered as potential factors influencing placebo effects (91). Our results suggest that there were no significant placebo effects of these factors on negative symptoms and cognition in SSD. It is therefore still debated whether factors, such as age, sex, and proportion of females affect placebo effects in psychiatry (92). In a study by Czobor et al. (30), they found that placebo effects were significantly related to study duration. Specifically, it decreased over the 8-week study period. In our included studies, the study duration was mostly between 2 and 4 weeks, with relatively few studies lasting 8 weeks or more. This may be the reason why we did not find a significant association between study duration and placebo effects. In addition, placebo effects may increase over the years of the study (93), but this phenomenon was not found in our study and in the study by Czobor et al. as well. Regarding the relationship between illness duration and placebo effects, although no significant association was found, a negative association has been reported in previous researches (30, 81). Several studies have shown that unbalanced randomization could also increase placebo effects (30, 94), and this association was not significant in our study. Apart from the above factors, several subjective factors also need to be considered. First, previous studies have reported that patients’ expectations and their relationship with the clinician influence placebo effects during treatment (95, 96). Patient expectations are the beliefs that patients hold about the potential benefits of the treatment they receive. Researches indicated that patient expectations are associated with the secretion of dopamine, altered neuronal firing, or changes in brain glucose metabolism (97). The rational promotion of patient expectations in medical practice can help to maximize the efficacy of rTMS, improving the treatment of psychiatric disorders (84). With the increasing recognition of physiotherapy and advancements in psychiatry, rTMS for SSD is gaining wider acceptance among the general public (98). This may result in a sustained increase in placebo effects, and potentially increasing its significance in clinical settings. Second, healthcare professionals’ competence and empathy are also associated with placebo effects (99). It is therefore advisable for researchers to control for the potential effects of those subjective factors to reduce the bias. In medical practice, investigating placebo effects and the moderators may assist clinicians, in comprehending the underlying mechanisms and in developing more effective treatment strategies. It can also facilitate the reduction of placebo effects through proper methods, thereby increasing sensitivity to detect effects of promising rTMS protocols for improving negative symptoms and cognitive impairments in clinical settings.
With regard to the possible neurobiological mechanisms underlying placebo effects, some evidence suggests that it may be related to the release of dopamine in the mesolimbic pathway (100). Dopamine has been shown to activate endogenous reward networks in the brain, including the ventromedial prefrontal cortex, ventral striatum, orbitofrontal cortex, anterior cingulate cortex, and amygdala, leading to clinical benefits. It should also be noted that the mechanisms are complex and not yet well understood, and more research is required.
The findings of the current review should be considered with several limitations. First, although subgroup analysis was performed for each outcome to explore placebo effects under the different conditions, the limited number of studies in some subgroups resulted in missing results. Second, due to the insufficient information in the included RCTs, we are not able to examine all factors that may affect placebo effects in SSD such as study design (parallel and cross-over) and number of research centers. Finally, we did not examine placebo effects of rTMS on social cognition, as the very limited publications.
Overall, our study provides up-to-date evidence of placebo effects of rTMS on negative symptoms and cognitive impairment in SSD. We conclude that placebo effects of rTMS on negative symptoms, memory, executive function, working memory, processing speed, but not on attention, are significant in a small-to-moderate magnitude. Subgroup analysis and meta-regressions showed that placebo effects were significantly associated with sham stimulation methods, rTMS targeting approaches, and stimulation frequency, but not with age, proportion of females, illness or treatment duration, sample size, randomization proportion and publication time. Our findings, therefore, provide novel insights into understanding placebo effects of rTMS on negative symptoms and cognitive deficits, which would accelerate the development of sham-controlled rTMS studies in the treatment of SSD. More well-designed RCTs with larger sample sizes and longer follow-up may be needed to investigate novel rTMS protocols to minimize placebo effects in the treatment of negative and cognitive symptoms in SSD.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
MW: Conceptualization, Data curation, Methodology, Writing – original draft. SL: Data curation, Software, Writing – original draft. LH: Data curation, Writing – review & editing. YX: Software, Writing – review & editing. ZS: Software, Supervision, Writing – review & editing. LS: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1377257/full#supplementary-material
1. Rössler W, Joachim Salize H, van Os J, Riecher-Rössler A. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol. (2005) 15:399–409. doi: 10.1016/j.euroneuro.2005.04.009
2. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. (2016) 388:86–97. doi: 10.1016/s0140–6736(15)01121–6
3. Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. (2018) 5:664–77. doi: 10.1016/s2215–0366(18)30050–6
4. Green MF. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J Clin Psychiatry. (2016) 77:8569. doi: 10.4088/jcp.14074su1c.02
5. Lähteenvuo M, Tiihonen J. Antipsychotic polypharmacy for the management of schizophrenia: Evidence and recommendations. Drugs. (2021) 81:1273–84. doi: 10.1007/s40265–021-01556–4
6. Vasiliu O. Third-generation antipsychotics in patients with schizophrenia and non-responsivity or intolerance to clozapine regimen: What is the evidence? Front Psychiatry. (2022) 13:1069432. doi: 10.3389/fpsyt.2022.1069432
7. Schennach R, Riedel M, Obermeier M, Spellmann I, Musil R, Jäger M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. (2015) 265:107–16. doi: 10.1007/s00406–014-0528–2
8. An der Heiden W, Leber A, Häfner H. Negative symptoms and their association with depressive symptoms in the long-term course of schizophrenia. Eur Arch Psychiatry Clin Neurosci. (2016) 266:387–96. doi: 10.1007/s00406–016-0697–2
9. Albus M, Hubmann W, Mohr F, Tiedemann TV, Pechler S, Drießlein D, et al. Neurocognitive functioning in patients with first-episode schizophrenia: results of a prospective 15-year follow-up study. Eur Arch Psychiatry Clin Neurosci. (2020) 270:689–98. doi: 10.1007/s00406-019-01030-z
10. Lefaucheur J-P, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin Neurophysiol. (2020) 131:474–528. doi: 10.1016/j.clinph.2019.11.002
11. Hyde J, Carr H, Kelley N, Seneviratne R, Reed C, Parlatini V, et al. Efficacy of neurostimulation across mental disorders: systematic review and meta-analysis of 208 randomized controlled trials. Mol Psychiatry. (2022) 27:2709–19. doi: 10.1038/s41380–022-01524–8
12. Ferrarelli F, Phillips ML. Examining and modulating neural circuits in psychiatric disorders with transcranial magnetic stimulation and electroencephalography: Present practices and future developments. AJP. (2021) 178:400–13. doi: 10.1176/appi.ajp.2020.20071050
13. Tseng P-T, Zeng B-S, Hung C-M, Liang C-S, Stubbs B, Carvalho AF, et al. Assessment of noninvasive brain stimulation interventions for negative symptoms of schizophrenia: A systematic review and network meta-analysis. JAMA Psychiatry. (2022) 79:770–9. doi: 10.1001/jamapsychiatry.2022.1513
14. Jin Y, Tong J, Huang Y, Shi D, Zhu N, Zhu M, et al. Effectiveness of accelerated intermittent theta burst stimulation for social cognition and negative symptoms among individuals with schizophrenia: A randomized controlled trial. Psychiatry Res. (2023) 320:115033. doi: 10.1016/j.psychres.2022.115033
15. Aleman A, Enriquez-Geppert S, Knegtering H, Dlabac-de Lange JJ. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: Meta-analysis of controlled trials. Neurosci Biobehav Rev. (2018) 89:111–8. doi: 10.1016/j.neubiorev.2018.02.009
16. Barr MS, Farzan F, Rajji TK, Voineskos AN, Blumberger DM, Arenovich T, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. (2013) 73:510–7. doi: 10.1016/j.biopsych.2012.08.020
17. Razza LB, Moffa AH, Moreno ML, Carvalho AF, Padberg F, Fregni F, et al. A systematic review and meta-analysis on placebo response to repetitive transcranial magnetic stimulation for depression trials. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 81:105–13. doi: 10.1016/j.pnpbp.2017.10.016
18. Burke MJ, Romanella SM, Mencarelli L, Greben R, Fox MD, Kaptchuk TJ, et al. Placebo effects and neuromodulation for depression: a meta-analysis and evaluation of shared mechanisms. Mol Psychiatry. (2022) 27:1658–66. doi: 10.1038/s41380–021-01397–3
19. Evers AWM, Colloca L, Blease C, Annoni M, Atlas LY, Benedetti F, et al. Implications of placebo and nocebo effects for clinical practice: Expert consensus. Psychother Psychosom. (2018) 87:204–10. doi: 10.1159/000490354
20. Brunoni AR, Lopes M, Kaptchuk TJ, Fregni F. Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis. PLoS One. (2009) 4:e4824. doi: 10.1371/journal.pone.0004824
21. Mansur CG, Myczkowki ML, de Barros Cabral S, Sartorelli M do CB, Bellini BB, Dias AM, et al. Placebo effect after prefrontal magnetic stimulation in the treatment of resistant obsessive-compulsive disorder: a randomized controlled trial. Int J Neuropsychopharmacol. (2011) 14:1389–97. doi: 10.1017/s1461145711000575
22. Jiang B, He D, Guo Z, Mu Q, Zhang L. Efficacy and placebo response of repetitive transcranial magnetic stimulation for primary insomnia. Sleep Med. (2019) 63:9–13. doi: 10.1016/j.sleep.2019.05.008
23. Dollfus S, Lecardeur L, Morello R, Etard O. Placebo response in repetitive transcranial magnetic stimulation trials of treatment of auditory hallucinations in schizophrenia: A meta-analysis. Schizophr Bull. (2016) 42:301–8. doi: 10.1093/schbul/sbv076
24. Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiatry. (2001) 49:460–3. doi: 10.1016/s0006–3223(00)01110–0
25. Weimer K, Colloca L, Enck P. Placebo effects in psychiatry: mediators and moderators. Lancet Psychiatry. (2015) 2:246–57. doi: 10.1016/s2215–0366(14)00092–3
26. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
27. Association AP. DSM 5 Diagnostic and statistical manual of mental disorders. Arlington, VA, US: American Psychiatric Publishing, Inc. (2013). p. 947.
28. Tandon R. The nosology of schizophrenia: Toward DSM-5 and ICD-11. Psychiatr Clinics North America. (2012) 35:557–69. doi: 10.1016/j.psc.2012.06.001
29. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59 Suppl 20:22–33.
30. Czobor P, Kakuszi B, Bitter I. Placebo response in trials of negative symptoms in schizophrenia: A critical reassessment of the evidence. Schizophr Bull. (2022) 48:1228–40. doi: 10.1093/schbul/sbac061
31. Barr MS, Farzan F, Arenovich T, Chen R, Fitzgerald PB, Daskalakis ZJ. The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PLoS One. (2011) 6:e22627. doi: 10.1371/journal.pone.0022627
32. Barr MS, Farzan F, Tran LC, Fitzgerald PB, Daskalakis ZJ. A randomized controlled trial of sequentially bilateral prefrontal cortex repetitive transcranial magnetic stimulation in the treatment of negative symptoms in schizophrenia. Brain Stimul. (2012) 5:337–46. doi: 10.1016/j.brs.2011.06.003
33. Basavaraju R, Ithal D, Thanki MV, Ramalingaiah AH, Thirthalli J, Reddy RP, et al. Intermittent theta burst stimulation of cerebellar vermis enhances fronto-cerebellar resting state functional connectivity in schizophrenia with predominant negative symptoms: A randomized controlled trial. Schizophr Res. (2021) 238:108–20. doi: 10.1016/j.schres.2021.10.005
34. Bation R, Magnin C, Poulet E, Mondino M, Brunelin J. Intermittent theta burst stimulation for negative symptoms of schizophrenia-A double-blind, sham-controlled pilot study. NPJ Schizophr. (2021) 7:10. doi: 10.1038/s41537–021-00138–3
35. Bodén R, Bengtsson J, Thörnblom E, Struckmann W, Persson J. Dorsomedial prefrontal theta burst stimulation to treat anhedonia, avolition, and blunted affect in schizophrenia or depression - a randomized controlled trial. J Affect Disord. (2021) 290:308–15. doi: 10.1016/j.jad.2021.04.053
36. Chauhan P, Garg S, Tikka SK, Khattri S. Efficacy of intensive cerebellar intermittent theta burst stimulation (iCiTBS) in treatment-resistant schizophrenia: A randomized placebo-controlled study. Cerebellum. (2021) 20:116–23. doi: 10.1007/s12311–020-01193–9
37. Du X-D, Li Z, Yuan N, Yin M, Zhao X-L, Lv X-L, et al. Delayed improvements in visual memory task performance among chronic schizophrenia patients after high-frequency repetitive transcranial magnetic stimulation. World J Psychiatry. (2022) 12:1169–82. doi: 10.5498/wjp.v12.i9.1169
38. Fitzgerald PB, Herring S, Hoy K, McQueen S, Segrave R, Kulkarni J, et al. A study of the effectiveness of bilateral transcranial magnetic stimulation in the treatment of the negative symptoms of schizophrenia. Brain Stimul. (2008) 1:27–32. doi: 10.1016/j.brs.2007.08.001
39. Francis MM, Hummer TA, Vohs JL, Yung MG, Visco AC, Mehdiyoun NF, et al. Cognitive effects of bilateral high frequency repetitive transcranial magnetic stimulation in early phase psychosis: a pilot study. Brain Imaging Behav. (2019) 13:852–61. doi: 10.1007/s11682–018-9902–4
40. Garg S, Sinha VK, Tikka SK, Mishra P, Goyal N. The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: A randomized rater blind-sham controlled study. Psychiatry Res. (2016) 243:413–20. doi: 10.1016/j.psychres.2016.07.023
41. Guan HY, Zhao JM, Wang KQ, Su XR, Pan YF, Guo JM, et al. High-frequency neuronavigated rTMS effect on clinical symptoms and cognitive dysfunction: a pilot double-blind, randomized controlled study in Veterans with schizophrenia. Transl Psychiatry. (2020) 10:79. doi: 10.1038/s41398–020-0745–6
42. Güleken MD, Akbaş T, Erden SÇ, Akansel V, Al ZC, Özer ÖA. The effect of bilateral high frequency repetitive transcranial magnetic stimulation on cognitive functions in schizophrenia. Schizophr Res Cognit. (2020) 22:100183. doi: 10.1016/j.scog.2020.100183
43. Guse B, Falkai P, Gruber O, Whalley H, Gibson L, Hasan A, et al. The effect of long-term high frequency repetitive transcranial magnetic stimulation on working memory in schizophrenia and healthy controls–a randomized placebo-controlled, double-blind fMRI study. Behav Brain Res. (2013) 237:300–7. doi: 10.1016/j.bbr.2012.09.034
44. Hasan A, Guse B, Cordes J, Wölwer W, Winterer G, Gaebel W, et al. Cognitive effects of high-frequency rTMS in schizophrenia patients with predominant negative symptoms: Results from a multicenter randomized sham-controlled trial. Schizophr Bull. (2016) 42:608–18. doi: 10.1093/schbul/sbv142
45. Holi MM, Eronen M, Toivonen K, Toivonen P, Marttunen M, Naukkarinen H. Left prefrontal repetitive transcranial magnetic stimulation in schizophrenia. Schizophr Bull. (2004) 30:429–34. doi: 10.1093/oxfordjournals.schbul.a007089
46. Huang W, Shen F, Zhang J, Xing B. Effect of repetitive transcranial magnetic stimulation on cigarette smoking in patients with schizophrenia. Shanghai Arch Psychiatry. (2016) 28:309–17. doi: 10.11919/j.issn.1002–0829.216044
47. Klein E, Kolsky Y, Puyerovsky M, Koren D, Chistyakov A, Feinsod M. Right prefrontal slow repetitive transcranial magnetic stimulation in schizophrenia: a double-blind sham-controlled pilot study. Biol Psychiatry. (1999) 46:1451–4. doi: 10.1016/s0006–3223(99)00182–1
48. Kumar N, Vishnubhatla S, Wadhawan AN, Minhas S, Gupta P. A randomized, double blind, sham-controlled trial of repetitive transcranial magnetic stimulation (rTMS) in the treatment of negative symptoms in schizophrenia. Brain Stimul. (2020) 13:840–9. doi: 10.1016/j.brs.2020.02.016
49. Dlabac-de Lange JJ, Bais L, van Es FD, Visser BGJ, Reinink E, Bakker B, et al. Efficacy of bilateral repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: results of a multicenter double-blind randomized controlled trial. Psychol Med. (2015) 45:1263–75. doi: 10.1017/s0033291714002360
50. Li Z, Yin M, Lyu X-L, Zhang L-L, Du X-D, Hung GC-L. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: Findings from a randomized controlled trial. Psychiatry Res. (2016) 240:333–5. doi: 10.1016/j.psychres.2016.04.046
51. McIntosh AM, Semple D, Tasker K, Harrison LK, Owens DGC, Johnstone EC, et al. Transcranial magnetic stimulation for auditory hallucinations in schizophrenia. Psychiatry Res. (2004) 127:9–17. doi: 10.1016/j.psychres.2004.03.005
52. Mittrach M, Thünker J, Winterer G, Agelink MW, Regenbrecht G, Arends M, et al. The tolerability of rTMS treatment in schizophrenia with respect to cognitive function. Pharmacopsychiatry. (2010) 43:110–7. doi: 10.1055/s-0029–1242824
53. Mogg A, Purvis R, Eranti S, Contell F, Taylor JP, Nicholson T, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res. (2007) 93:221–8. doi: 10.1016/j.schres.2007.03.016
54. Pan Z, Xiong D, Xiao H, Li J, Huang Y, Zhou J, et al. The effects of repetitive transcranial magnetic stimulation in patients with chronic schizophrenia: insights from EEG microstates. Psychiatry Res. (2021) 299:113866. doi: 10.1016/j.psychres.2021.113866
55. Prikryl R, Kasparek T, Skotakova S, Ustohal L, Kucerova H, Ceskova E. Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr Res. (2007) 95:151–7. doi: 10.1016/j.schres.2007.06.019
56. Prikryl R, Mikl M, Prikrylova Kucerová H, Ustohal L, Kasparek T, Marecek R, et al. Does repetitive transcranial magnetic stimulation have a positive effect on working memory and neuronal activation in treatment of negative symptoms of schizophrenia? Neuro Endocrinol Lett. (2012) 33:90–7.
57. Prikryl R, Ustohal L, Prikrylova Kucerova H, Kasparek T, Venclikova S, Vrzalova M, et al. A detailed analysis of the effect of repetitive transcranial magnetic stimulation on negative symptoms of schizophrenia: a double-blind trial. Schizophr Res. (2013) 149:167–73. doi: 10.1016/j.schres.2013.06.015
58. Prikryl R, Ustohal L, Kucerova HP, Kasparek T, Jarkovsky J, Hublova V, et al. Repetitive transcranial magnetic stimulation reduces cigarette consumption in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. (2014) 49:30–5. doi: 10.1016/j.pnpbp.2013.10.019
59. Quan WX, Zhu XL, Qiao H, Zhang WF, Tan SP, Zhou DF, et al. The effects of high-frequency repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia and the follow-up study. Neurosci Lett. (2015) 584:197–201. doi: 10.1016/j.neulet.2014.10.029
60. Rabany L, Deutsch L, Levkovitz Y. Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. J Psychopharmacol. (2014) 28:686–90. doi: 10.1177/0269881114533600
61. Saba G, Verdon CM, Kalalou K, Rocamora JF, Dumortier G, Benadhira R, et al. Transcranial magnetic stimulation in the treatment of schizophrenic symptoms: a double blind sham controlled study. J Psychiatr Res. (2006) 40:147–52. doi: 10.1016/j.jpsychires.2005.02.008
62. Singh S, Kumar N, Verma R, Nehra A. The safety and efficacy of adjunctive 20-Hz repetitive transcranial magnetic stimulation for treatment of negative symptoms in patients with schizophrenia: A double-blinded, randomized, sham-controlled study. Indian J Psychiatry. (2020) 62:21–9. doi: 10.4103/psychiatry.Indianjpsychiatry_361_19
63. Su X, Zhao L, Shang Y, Chen Y, Liu X, Wang X, et al. Repetitive transcranial magnetic stimulation for psychiatric symptoms in long-term hospitalized veterans with schizophrenia: A randomized double-blind controlled trial. Front Psychiatry. (2022) 13:873057. doi: 10.3389/fpsyt.2022.873057
64. Tikka SK, Nizamie SH, Venkatesh Babu GM, Aggarwal N, Das AK, Goyal N. Safety and efficacy of adjunctive Θ Burst repetitive transcranial magnetic stimulation to right inferior parietal lobule in schizophrenia patients with first-rank symptoms: A pilot, exploratory study. J ECT. (2017) 33:43–51. doi: 10.1097/yct.0000000000000343
65. Wang L, Li Q, Wu Y, Ji G-J, Wu X, Xiao G, et al. Intermittent theta burst stimulation improved visual-spatial working memory in treatment-resistant schizophrenia: A pilot study. J Psychiatr Res. (2022) 149:44–53. doi: 10.1016/j.jpsychires.2022.02.019
66. Wen N, Chen L, Miao X, Zhang M, Zhang Y, Liu J, et al. Effects of high-frequency rTMS on negative symptoms and cognitive function in hospitalized patients with chronic schizophrenia: A double-blind, sham-controlled pilot trial. Front Psychiatry. (2021) 12:736094. doi: 10.3389/fpsyt.2021.736094
67. Wobrock T, Guse B, Cordes J, Wölwer W, Winterer G, Gaebel W, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. (2015) 77:979–88. doi: 10.1016/j.biopsych.2014.10.009
68. Wölwer W, Lowe A, Brinkmeyer J, Streit M, Habakuck M, Agelink MW, et al. Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain Stimul. (2014) 7:559–63. doi: 10.1016/j.brs.2014.04.011
69. Xiu MH, Guan HY, Zhao JM, Wang KQ, Pan YF, Su XR, et al. Cognitive enhancing effect of high-frequency neuronavigated rTMS in chronic schizophrenia patients with predominant negative symptoms: A double-blind controlled 32-week follow-up study. Schizophr Bull. (2020) 46:1219–30. doi: 10.1093/schbul/sbaa035
70. Zhao S, Kong J, Li S, Tong Z, Yang C, Zhong H. Randomized controlled trial of four protocols of repetitive transcranial magnetic stimulation for treating the negative symptoms of schizophrenia. Shanghai Arch Psychiatry. (2014) 26:15–21. doi: 10.3969/j.issn.1002–0829.2014.01.003
71. Zhu L, Zhang W, Zhu Y, Mu X, Zhang Q, Wang Y, et al. Cerebellar theta burst stimulation for the treatment of negative symptoms of schizophrenia: A multicenter, double-blind, randomized controlled trial. Psychiatry Res. (2021) 305:114204. doi: 10.1016/j.psychres.2021.114204
72. Zhuo K, Tang Y, Song Z, Wang Y, Wang J, Qian Z, et al. Repetitive transcranial magnetic stimulation as an adjunctive treatment for negative symptoms and cognitive impairment in patients with schizophrenia: a randomized, double-blind, sham-controlled trial. Neuropsychiatr Dis Treat. (2019) 15:1141–50. doi: 10.2147/ndt.s196086
73. Chang JR, Fu S-N, Li X, Li SX, Wang X, Zhou Z, et al. The differential effects of sleep deprivation on pain perception in individuals with or without chronic pain: A systematic review and meta-analysis. Sleep Med Rev. (2022) 66:101695. doi: 10.1016/j.smrv.2022.101695
74. Matsusaki A, Kaneko M, Narukawa M. Meta-analysis of placebo response in randomized clinical trials of antipsychotic drugs using PANSS focusing on different approaches to the handling of missing data. Clin Drug Investig. (2018) 38:751–61. doi: 10.1007/s40261–018-0661–1
75. Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. (2004) 56:301–7. doi: 10.1016/j.biopsych.2004.06.023
76. Cohen J. Statistical power analysis for the behavioral sciences. Routledge. (2013) 31:499–500. doi: 10.4324/9780203771587
77. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Chichester (UK): John Wiley & Sons (2019). doi: 10.1002/9781119536604
78. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
79. Fraguas D, Díaz-Caneja CM, Pina-Camacho L, Umbricht D, Arango C. Predictors of placebo response in pharmacological clinical trials of negative symptoms in schizophrenia: A meta-regression analysis. Schizophr Bull. (2019) 45:57. doi: 10.1093/schbul/sbx192
80. Keefe RSE, Davis VG, Harvey PD, Atkins AS, Haig GM, Hagino O, et al. Placebo response and practice effects in schizophrenia cognition trials. JAMA Psychiat. (2017) 74:807–14. doi: 10.1001/jamapsychiatry.2017.1574
81. Agid O, Siu CO, Potkin SG, Kapur S, Watsky E, Vanderburg D, et al. Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am J Psychiatry. (2013) 170:1335–44. doi: 10.1176/appi.ajp.2013.12030315
82. Meissner K, Fässler M, Rücker G, Kleijnen J, Hróbjartsson A, Schneider A, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. (2013) 173:1941–51. doi: 10.1001/jamainternmed.2013.10391
83. Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. (2000) 53:786–92. doi: 10.1016/s0895–4356(00)00206–7
84. Xu Y, Zhang Y, Zhao D, Tian Y, Yuan T-F. Growing placebo response in TMS treatment for depression: a meta-analysis of 27-year randomized sham-controlled trials. Nat Ment Health. (2023) 1:792–809. doi: 10.1038/s44220-023-00118-9
85. Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. (2015) 58:208–13. doi: 10.1016/j.rehab.2015.05.005
86. Rossi S, Antal A, Bestmann S, Bikson M, Brewer C, Brockmöller J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin Neurophysiol. (2021) 132:269–306. doi: 10.1016/j.clinph.2020.10.003
87. Sommer J, Jansen A, Dräger B, Steinsträter O, Breitenstein C, Deppe M, et al. Transcranial magnetic stimulation—a sandwich coil design for a better sham. Clin Neurophysiol. (2006) 117:440–6. doi: 10.1016/j.clinph.2005.09.025
88. Strafella AP, Ko JH, Monchi O. Therapeutic application of transcranial magnetic stimulation in Parkinson’s disease: The contribution of expectation. NeuroImage. (2006) 31:1666–72. doi: 10.1016/j.neuroimage.2006.02.005
89. Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. (2008) 59:565–90. doi: 10.1146/annurev.psych.59.113006.095941
90. Sheffer CE, Mennemeier MS, Landes RD, Dornhoffer J, Kimbrell T, Bickel WK, et al. Focal electrical stimulation as an effective sham control for active rTMS and biofeedback treatments. Appl Psychophysiol Biofeedback. (2013) 38:171–6. doi: 10.1007/s10484-013-9221-x
91. Furukawa TA, Cipriani A, Atkinson LZ, Leucht S, Ogawa Y, Takeshima N, et al. Placebo response rates in antidepressant trials: a systematic review of published and unpublished double-blind randomised controlled studies. Lancet Psychiatry. (2016) 3:1059–66. doi: 10.1016/s2215–0366(16)30307–8
92. Yildiz A, Vieta E, Tohen M, Baldessarini RJ. Factors modifying drug and placebo responses in randomized trials for bipolar mania. Int J Neuropsychopharmacol. (2011) 14:863–75. doi: 10.1017/s1461145710001641
93. Kemp AS, Schooler NR, Kalali AH, Alphs L, Anand R, Awad G, et al. What is causing the reduced drug-placebo difference in recent schizophrenia clinical trials and what can be done about it? Schizophr Bull. (2010) 36:504–9. doi: 10.1093/schbul/sbn110
94. Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. (2009) 19:34–40. doi: 10.1016/j.euroneuro.2008.08.009
95. Beecher HK. The powerful placebo. J Am Med Assoc. (1955) 159:1602–6. doi: 10.1001/jama.1955.02960340022006
96. Bertisch SM, Legedza ART, Phillips RS, Davis RB, Stason WB, Goldman RH, et al. The impact of psychological factors on placebo responses in a randomized controlled trial comparing sham device to dummy pill. J Eval Clin Pract. (2009) 15:14–9. doi: 10.1111/j.1365-2753.2008.00942.x
97. Salvatore MF. Dopamine signaling in substantia nigra and its impact on locomotor function-not a new concept, but neglected reality. Int J Mol Sci. (2024) 25:1131. doi: 10.3390/ijms25021131
98. Jannati A, Oberman LM, Rotenberg A, Pascual-Leone A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacol. (2023) 48:191–208. doi: 10.1038/s41386–022-01453–8
99. Kaptchuk TJ. The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance? Ann Intern Med. (2002) 136:817–25. doi: 10.7326/0003–4819-136–11-200206040–00011
Keywords: schizophrenia spectrum disorders, repetitive transcranial magnetic stimulation, placebo effects, negative symptoms, cognition, randomized controlled trial
Citation: Wang M, Lu S, Hao L, Xia Y, Shi Z and Su L (2024) Placebo effects of repetitive transcranial magnetic stimulation on negative symptoms and cognition in patients with schizophrenia spectrum disorders: a systematic review and meta-analysis. Front. Psychiatry 15:1377257. doi: 10.3389/fpsyt.2024.1377257
Received: 27 January 2024; Accepted: 14 May 2024;
Published: 28 May 2024.
Edited by:
Caroline Emma Wass, University of Gothenburg, SwedenReviewed by:
Wiebke Struckmann, Stanford University, United StatesCopyright © 2024 Wang, Lu, Hao, Xia, Shi and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Su, MTg3MDUzMTcwNzhAMTYzLmNvbQ==; Zhenchun Shi, c2hpemMyMDA4QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.