- 1Molecular Psychiatry Lab, Faculty of Science and Medicine, University of Freiburg, Villars-sur-Glâne, Switzerland
- 2University Psychiatry Research Unit, Freiburg Mental Health Network, Villars-sur-Glâne, Switzerland
- 3Department of Neuropsychology, Lake Lucerne Institute, Vitznau, Switzerland
Social cognitive deficits and social behavior impairments are common in major depressive disorder (MDD) and affect the quality of life and recovery of patients. This review summarizes the impact of standard and novel treatments on social functioning in MDD and highlights the potential of combining different approaches to enhance their effectiveness. Standard treatments, such as antidepressants, psychotherapies, and brain stimulation, have shown mixed results in improving social functioning, with some limitations and side effects. Newer treatments, such as intranasal oxytocin, mindfulness-based cognitive therapy, and psychedelic-assisted psychotherapy, have demonstrated positive effects on social cognition and behavior by modulating self-referential processing, empathy, and emotion regulation and through enhancement of neuroplasticity. Animal models have provided insights into the neurobiological mechanisms underlying these treatments, such as the role of neuroplasticity. Future research should explore the synergistic effects of combining different treatments and investigate the long-term outcomes and individual differences in response to these promising interventions.
1 Introduction
Major depressive disorder (MDD) is a prevalent and disabling psychiatric condition that impairs the social functioning and well-being of millions of people worldwide. In addition to the core symptoms of depressed mood, anhedonia, and altered appetite and sleep, MDD is associated with social cognitive deficits and behavioral impairments that exacerbate the distress and hinder the recovery of affected individuals (1, 2). These social deficits are pervasive and encompass almost every aspect of one’s social capabilities, such as emotion regulation, social cognition, and social behavior. These impairments are more subtle than those observed in other neuropsychiatric disorders, such as autism and schizophrenia, but they may affect a larger proportion of patients and have a greater impact on their quality of life (3–6). For instance, MDD patients show similar attentional biases and difficulties in disengaging from negative emotional stimuli as autistic patients (7). MDD patients are also more vulnerable to interpersonal stressors, such as loss, humiliation, or rejection (8), which can undermine their self-worth and exacerbate their depressive symptoms (9). Additionally, interpersonal stressors have been found to be stronger predictors of future depression than non-interpersonal stressors, such as financial, academic, or health problems (10–12).
Impairments in various domains of social functioning, such as social support, network size, occupational and marital functioning persist even after remission from mood symptoms (13, 14). These residual symptoms not only reduce the quality of life of MDD patients but also increase the risk of relapse and chronicity (15). Social cognition deficits in MDD can emerge early in life and persist across development. Studies have shown that children and adolescents with high levels of depressive symptoms have more conflictual, negative, and less collaborative and mutual social interactions with peers than those with low levels of depressive symptoms (16–19). Thus, social impairment pose significant challenges to successful interpersonal functioning and quality of life for both depressed patients and their families (20, 21) and successful remission from MDD requires not only a decrease in depressive symptoms but also significant improvements in the social domain (22).
Conventional treatments for MDD, such as antidepressants, and some brain stimulation therapies, such as electroconvulsive therapy, have primarily focused on reducing mood symptoms and have shown variable and often limited effects on social functioning (23), with various drawbacks and side effects, including sexual dysfunction (24) or cardiovascular problems (25). In contrast, novel treatments, such as intranasal oxytocin, vagus nerve stimulation (VNS) and mindfulness-based cognitive therapy (MBCT), ketamine and psychedelic-assisted psychotherapy (PAP), have emerged as promising alternatives for enhancing social cognition and behavior in MDD (26–29). These innovative approaches target key aspects of social functioning, such as social bonding, emotion recognition, rumination, emotional regulation, empathy, and connectedness, with rapid and lasting effects. Animal models have complemented human studies by revealing the neurobiological mechanisms underlying the social effects of these treatments (30–32). Therefore, it is crucial to evaluate the effects of both conventional and novel therapeutic interventions on social outcomes in MDD.
In this article, we review the current literature on the impact of standard and novel treatments on social functioning in major depression. We first provide an overview of the social deficits associated with major depression and how they affect the patients, their families, and their friends. We then discuss the effects of conventional treatments, such as antidepressants, psychotherapies, and some brain electro-stimulation therapies, on social functioning. We highlight the limitations and challenges of these treatments and point out the need for alternative approaches. Next, we introduce novel treatments that have emerged as promising options for enhancing social functioning in major depression. These include intranasal oxytocin, vagus nerve stimulation, mindfulness-based cognitive therapy (MBCT), ketamine and psychedelic-assisted psychotherapy (PAP). We describe how these treatments target key aspects of social functioning, such as social bonding, emotion recognition, emotional regulation, empathy, and connectedness. We also present the evidence from animal models that reveal the neurobiological mechanisms underlying the social effects of these treatments. Finally, we identify the gaps and challenges for future research.
2 Methods
In this narrative review, we delve into two critical domains of social dysfunction observed in patients with Major Depressive Disorder (MDD): social cognition and social behavior. These domains play a pivotal role in shaping an individual’s ability to navigate social interactions and maintain healthy relationships. Dysfunction within these domains can significantly impair individuals’ social functioning. Specifically, social cognition encompasses a range of conscious and subconscious psychological processes that influence social behavior, including socio-emotional regulation strategies such as cognitive reappraisal, forgiveness capacity, decentering, empathy, sensitivity to social rejection, cognitive flexibility, emotion recognition deficits, and diminished self-compassion, ultimately impacting social connectedness.
We conducted a targeted literature search specifically focused on depression, deliberately excluding other mental disorders to maintain a narrower scope and reduce the length of the article. This approach allowed us to concentrate on the most relevant studies pertaining to depressive symptoms and treatments without the additional complexity of related conditions.
The search was conducted using several electronic databases, including PubMed and Web of Science. We used a precise set of search terms to locate relevant studies, including ‘depression,’ ‘major depressive disorder,’ ‘depressive symptoms,’ and ‘treatment.’ The inclusion criteria required that studies explicitly address depressive symptoms or treatments. This focused strategy was designed to ensure that our review remained concentrated on depression alone, providing a comprehensive yet specific overview of the literature.
In our review, we have used the term “novel” to refer to treatments that represent a significant departure from traditional therapies commonly used in clinical practice. This includes treatments that are either in the experimental stages or have only recently been introduced into clinical settings. However, our use of “novel” is not strictly limited to the recency of development but also encompasses the extent to which these treatments are integrated into routine clinical practice across different countries. Thus, the perception of novelty may vary based on individual perspectives and regional practices. For example, a treatment method that is considered well-established and widely used in one country may be relatively new or less commonly utilized in another country due to differences in healthcare policies, availability of resources, and cultural attitudes toward mental health treatment. Additionally, regulatory agencies in different countries may have varying standards for approving and endorsing treatment methods, which can influence their adoption and acceptance within the healthcare system. Thus, despite initial testing in research studies or clinical trials, some methods might not yet be widely adopted in clinical practice depending on the country. Novel methods may or may not be recent; they could have been developed recently or may have been around for some time but are only now gaining recognition or undergoing rigorous evaluation.
3 Impairments of social cognition in MDD
3.1 Decreased socio-cognitive reappraisal
The regulation of emotions is essential not only for momentary emotional experiences and behaviors but also for broader and more enduring aspects of psychological functioning (33, 34). Social cognitive reappraisal, a form of emotion regulation, involves changing one’s thoughts about a potentially distressing situation to minimize its emotional impact (35). This type of reappraisal is aimed at reinterpreting negative emotions, thoughts, or situations in a positive light (36). Studies have demonstrated that cognitive reappraisal attenuates negative emotions and promotes mental health (37). It has also been suggested that social difficulties in depression might arise from an ineffective use of socio-cognitive reappraisal (34, 38–40). Moreover, a lower inclination to use positive reappraisal has been consistently linked to subclinical and clinical forms of depression (37) (see Figure 1). Further, children’s use of cognitive reappraisal may also influence their risk of developing depression, with those who have never experienced a depressive episode being 1.3 times more likely to use cognitive reappraisal than those currently in a depressive episode (41). Therefore, encouraging the use of cognitive reappraisal may be a promising strategy for managing and preventing depression.
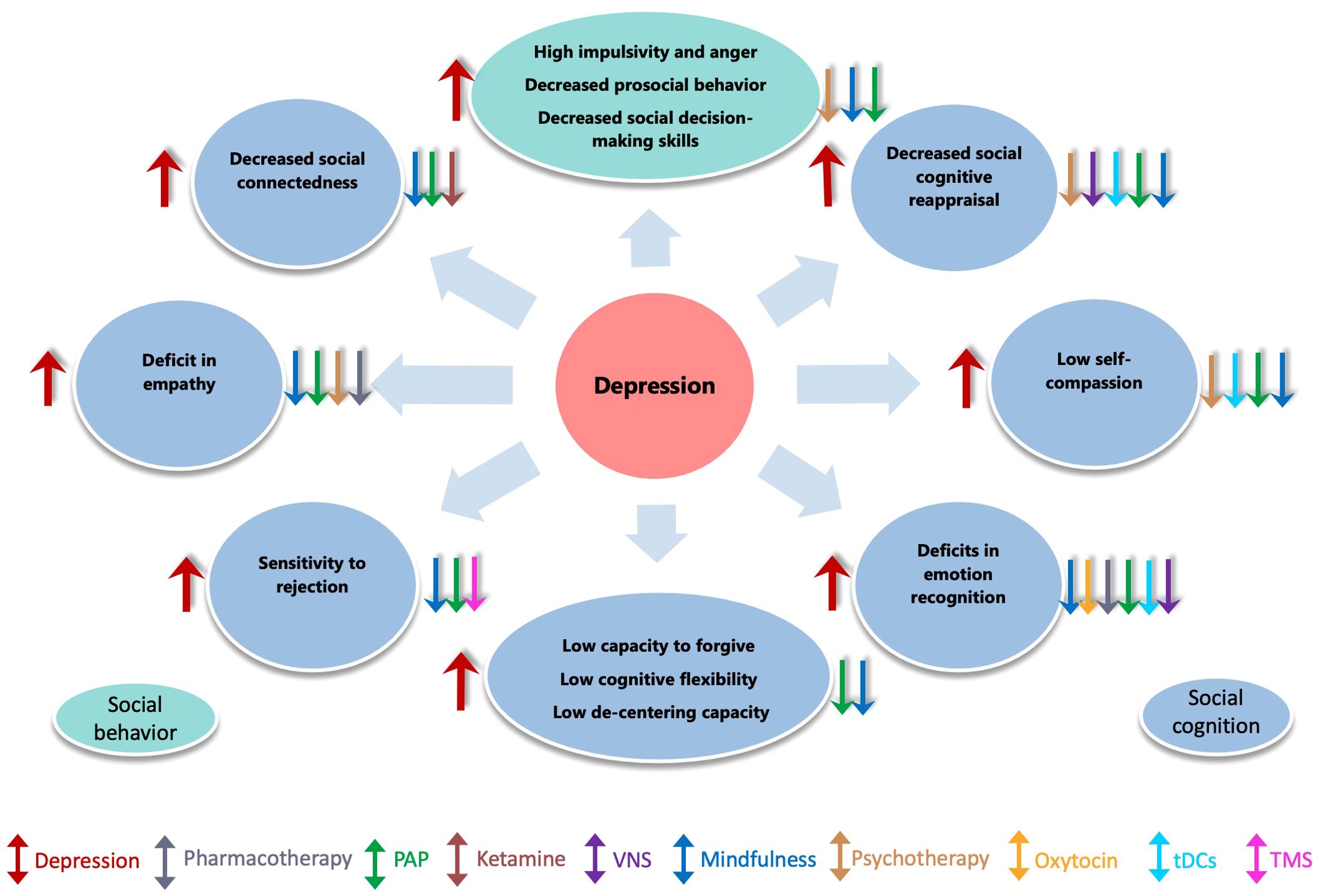
Figure 1. Impact of depression on social behavior, cognition, and the effect of treatment interventions. This figure illustrates how depression impairs empathy, emotion recognition, social connectedness,cognitive flexibility etc. (red arrows). Various interventions, including pharmacotherapy, psychotherapy, mindfulness, ketamine, psychedelic, brain stimulation techniques (TMS, tDCS, VNS) etc., are shown to help alleviate these effects.
These findings suggest that cognitive reappraisal is important for mental health and social functioning because it can minimize emotional impact of social stressors. Therefore, it is important to address the deficits in cognitive reappraisal in the treatment of depression and to improve the social outcomes of depressed individuals.
3.2 Low decentering capability
Decentering is the ability to assume a detached, objective stance toward present-moment awareness, which allows individuals to experience distressing inner events as imperfect models of the real world rather than precise reflections (42). Repeated cognitive reappraisal has been shown to facilitate decentering, attenuate negative emotions and help individuals understand that emotions and thoughts are only temporary mental events that do not necessarily reflect one’s self or external reality (43, 44). Decentering allows individuals to experience distressing inner events as imperfect models of the real-world rather than precise reflections (45). Reduced decentering ability has been linked with several psychiatric disorders, including major depression (46, 47) (see Figure 1).
Individuals with a tendency toward self-rumination have difficulty spontaneously taking a decentered perspective and are consequently prone to depression (44, 48). Thus, decentering is of particular interest in depression since it may reduce levels of depressive rumination by teaching patients more adaptive ways of relating to their thinking (49). Additionally, decentering contributes to preventing the recurrence of depression (42). Notably, the ability to decenter is related to diminishing egocentrism, a state similar to ego-dissolution achieved by the use of psychedelics.
3.3 Low self-compassion and self-acceptance and high self-criticism
Depression is often characterized by a self-critical attitude, which can lead to emotional difficulties such as uncontrolled anger (50) (see Figure 1). Those who are caught up in self-evaluation may also be needy and devote considerable attention to self-aggrandizement in order to compensate for perceived personal deficits. Self-compassion, in contrast to self-criticism, is characterized by a non-judgmental attitude toward one’s inadequacies and failures, feelings of caring and kindness toward oneself, and recognition that one’s experience is part of the common human experience. Higher levels of self-compassion were shown to be related to lower levels of depressive symptoms (51). Thus, individuals with depression, both current and remitted, have significantly lower levels of self-compassion compared to those without depression. For example, higher levels of self-compassion have been associated with lower levels of depressive symptoms (52). Conversely, low levels of self-acceptance are associated with depression (53, 54), and symptom severity in major depressive disorder is negatively associated with being self-soothing when experiencing negative emotions (55) as well as accepting and tolerating negative emotions (56). Therefore, self-compassion may be a protective factor against depression.
These findings suggest that higher levels of self-compassion may protect against depression, and help depressed individuals cope with stress and adversity. Self-compassion may also enhance social functioning and well-being, as it may foster empathy, trust, and cooperation with others. Therefore, it is important to address the deficits in self-compassion in the treatment of depression and to improve the social outcomes of depressed individuals.
3.4 Low socio-cognitive flexibility
Cognitive flexibility is the ability to adjust one’s cognitive strategies or behaviors in response to environmental changes, allowing for greater adaptability and problem-solving skills in social interactions (57, 58). Research has shown that cognitive flexibility is negatively correlated with depressive symptoms and mediates the relationship between coping styles and resilience, highlighting its importance for mental health (59) (see Figure 1). Depressed individuals tend to exhibit rigid and ruminative thinking styles, making it difficult for them to adapt to changing situations (60, 61). Additionally, individuals with depression exhibit compromised ability to shift attention and response from one emotional category to another (62), as well as deficits in the ability to respond appropriately to changing environmental requirements (63). Self-report measures have also revealed that greater levels of depression are associated with lower scores of cognitive flexibility and higher levels of impulsivity (64). Furthermore, experimental studies have shown that MDD patients exhibit higher switch costs in reaction times, indicating decreased cognitive flexibility (65). Therefore, cognitive flexibility may be impaired in depression and affect various aspects of functioning.
These findings show that depression is associated with low cognitive flexibility and high cognitive rigidity which may affect their social functioning and lead to more social problems and conflicts. Therefore, it is important to address these impairments in the treatment of depression and to improve the social outcomes of depressed individuals.
3.5 Low capacity to forgive
Forgiveness is a multifaceted construct that involves considering relationships in a balanced manner, recognizing others’ weaknesses and mistakes, as well as their strengths and talents. The ability to forgive and repair relationships has been linked to a reduction in depression, indicating its effectiveness in regulating negative affect (66, 67). In fact, forgiving others leads to a decrease in depressive and angry emotions, thoughts, and behaviors, and an increase in positive and benevolent ones toward the offending person (68).
Studies across all ages have found that both trait and state forgiveness are significantly associated with lower rates of depression (69). Moreover, forgiving others has been inversely linked to depressive symptoms (70–72), including postpartum depression (73) (see Figure 1). Recent research has highlighted that participants who were more benevolent and forgiving demonstrated greater self-reassurance and reported lower depressive symptomatology (74). Conversely, a lack of forgiveness may be considered a predisposing factor to depression (75), since it perpetuates negative emotions related to perceived offences in relationships ( (76, 77). Thus, the evidence suggests that forgiveness plays a crucial role in regulating negative affect and mitigating the risk of depression across all ages.
These findings suggest that forgiveness can reduce depression and negative affect and increase positive and benevolent emotions toward others. However, a lack of forgiveness may be a risk factor for depression, as it perpetuates negative emotions related to perceived offences in relationships. Therefore, it is important to address the deficits in forgiveness in the treatment of depression and to improve the social outcomes of depressed individuals.
3.6 Deficits in emotion recognition and perception
Social functioning in depression may be impaired by a distorted perception of emotions and mental states. Depressed patients tend to interpret social stimuli in a negative way that matches their mood. For example, they show more attention to sad faces and less to happy faces (78). They also have a higher sensitivity to neutral faces that were previously associated with sad expressions, suggesting a negative memory bias (79). Moreover, depressed patients have difficulty recognizing subtle or complex emotions (80) (see Figure 1). It has been shown that dysphoric individuals tend to perceive ambiguous faces as negative (81). Therefore, the inability to regulate or correct the negative bias may increase the vulnerability to depression (82). These findings indicate that depressed patients have deficits in emotion recognition and a mood-congruent negative bias that affect their social functioning. They tend to perceive social stimuli in a more negative and less positive way than non-depressed individuals and have trouble identifying and interpreting complex or ambiguous emotions. These impairments may lead to reduced social engagement, increased interpersonal conflicts, and lower social support.
These findings suggest that depression impairs the perception of emotions and mental states in social situations leading to impairment of social functioning and leading to more social isolation and conflict. Therefore, it is important to address these deficits in the treatment of depression and to improve the social outcomes of depressed patients.
3.7 Deficits in empathy
Empathy is essential for understanding others by taking their perspective, managing emotions and navigating social situations (83). However, empathy seems to be compromised in depression. Depressed patients show less sensitivity to others’ pain (84), and lower cognitive empathy, as reflected by poor perspective taking, theory of mind, and empathic accuracy (85) (see Figure 1). On the other hand, higher affective empathy may increase the risk of depression, as it may lead to excessive guilt and responsibility for others’ suffering (86).
These findings suggest that empathy may have a dual role in depression: it may facilitate social functioning, but also increase the risk of depression. It is important to explore how empathy can be modulated and optimized in the treatment of depression and to improve the social outcomes of depressed patients.
3.8 Increased sensitivity to social rejection
Depression may make people more sensitive and avoidant of social signals and rejection (87). Depressed individuals may also expect and confirm negative social outcomes by ignoring positive information (88). As a result, they tend to perceive more social rejection than others (1, 89, 90) (see Figure 1). Moreover, they may find rejection more distressing (91) and react more negatively to it (92). Even after recovery, they may remain more vulnerable to interpersonal criticism (93). These impairments may impair social functioning in depression and increase isolation and loneliness. Therefore, it is important to address these impairments in the treatment of depression and to improve the social outcomes of depressed individuals.
3.9 Decreased social connectedness
Social connectedness is the feeling of closeness and belonging to other people and groups . People who have high social connectedness tend to enjoy social interactions, see others as friendly, and relate to them easily (94). However, depression is linked to low social connectedness and integration. Chronically depressed patients may feel less connected and compassionate to their close ones (see Figure 1). They may also experience more depression as a result of low social connectedness, especially in adolescence (95, 96). Thus, depressed individuals may feel less close and compassionate to their close ones and may have difficulty enjoying social interactions and relating to others. A vital part of treating depression is therefore to help them cope with these troubles and to enhance their social quality of life.
4 Impairments of social behavior in MDD
4.1 Increased impulsivity and anger
Depression may be associated with higher impulsivity, according to a recent systematic review of 21 studies (97). Notably, depressed individuals may react more impulsively to both positive and negative emotions, indicating a general over-responsiveness to emotions (98) (see Figure 1). This may impair their ability to cope with stress (35) and negatively affect their social relationships (99–101). This higher impulsivity and emotional over-responsiveness may affect social relationships resulting in more interpersonal problems and conflicts. Therefore, it is important to address these issues in the treatment of depression.
4.2 Decreased prosocial behavior
Depression may diminish the joy and motivation of helping others. Prosocial behavior, or voluntary actions that benefit others, has been found to be inversely related to depressive mood in non-clinical samples (102–104). Further, depressed individuals may be less inclined to help others because they expect less positive outcomes from social situations (104) (see Figure 1). This can lead to reduced social support and happiness. Thus, helping depressed patients to overcome these problems is a key part of treating depression and enhancing their social well-being.
4.3 Deficits in social decision making
Social behavior depends on the ability to evaluate the fairness of social interactions and to cooperate with others. Since depression may affect various aspects of social decision-making, such as fairness, cooperation, trust, and reciprocity, several paradigms have been used to study these abilities, such as the Ultimatum Game (UG), the Prisoner’s Dilemma (PD) and the Trust Game context. The UG involves two players, one of whom proposes how to split a sum of money and the other who accepts or rejects the offer. If the offer is rejected, neither player gets anything. Depressed patients tend to make more fair offers as proposers (105, 106) and to reject more unfair offers as responders than healthy controls (see Figure 1). They also reject more offers when they are paired with emotional facial expressions. Their rejection rate is correlated with their depression severity and does not change after treatment. Depressed individuals may also have a higher preference for fairness and a desire to punish unfair actions, which may be related to decreased forgiveness of others. This behavior of depressed patients may be influenced by several factors, such as fear of rejection , reduced reward sensitivity as well as negative emotions, pessimism, and negative associations (105, 107, 108).
The PD is a game where two players can choose to cooperate or defect, resulting in different payoffs depending on their choices. Cooperation is optimal for both players, but defection is tempting for each individual (109). Depressed patients tend to cooperate less than healthy controls (108, 110) and have more negative emotional reactions to betrayal . Their cooperation may depend on their partner’s behavior: they are more prosocial with cooperative partners but more volatile with unbiased partners (111).
The Trust Game is a game where one player can send some money to another player, who can then return some or none of it. The amount sent by the first player is multiplied by a factor before reaching the second player, creating an incentive for trust and reciprocity (109). Depressed patients show less reciprocity than healthy controls in the Trust Game, except when they are in remission. They may also be more sensitive to the risk of being cheated by their partner.
These findings show that depression affects the ability to evaluate the fairness of social interactions and to cooperate with others. The tendency to make fair offers and reject more unfair offers in the Ultimatum Game may reflect the fear of rejection, reduced reward sensitivity, and higher preference for fairness. They also tend to cooperate less and react more negatively to betrayal in the Prisoner’s Dilemma, which may reflect their pessimism, volatility, and negative emotions. Showing less reciprocity and more risk sensitivity in the Trust Game, which may reflect their distrust and low self-esteem. These impairments may affect their social functioning and lead to more social isolation and conflict. Therefore, it is important to address these impairments in the treatment of depression and to improve the social outcomes of depressed individuals.
5 Diagnosis of social deficits in MDD
Overall, our findings demonstrate that recovery from depression requires significant improvement not only of depressive symptoms but also in social adaptation and functioning. Assessing the severity of MDD only through disease-specific symptoms is therefore insufficient to understand a patient’s needs and the personal burden of disease. We therefore recommend that screening, treatment, and therapeutic approaches should not only target core depressive symptoms but also integrate an assessment of the patient’s social functioning and social network. These assessments could include both subjective measures of social dysfunction, such as the Social Functioning Questionnaire (112), Social Dysfunction Rating Scale (113) or Work and Social Assessment Scale (114), as well as objective measures, such as longitudinal changes in a patient’s social decision-making tendencies in neuroeconomic games (115). Such measures could be used as diagnostic markers for clinical diagnoses and measures of treatment outcomes.
Furthermore, social networks may provide opportunities to screen people for depression, as people are increasingly relying on social media to search for social connections, share emotions, and post about their daily activities. Depressed and non-depressed individuals differ in various measures of social media use, including the number and content of Facebook messages, linguistic variability in tweets, number of followers, and frequency of Instagram use (116). Having fewer Facebook friends or mutual friends, posting frequently, and describing negative emotions, as well as the frequent use of personal pronouns, words related to pain and aggression, or “past focus” words, have all been positively correlated with depression (116). Visual content on social media sites (e.g., photographs posted to Instagram) can also be used as a marker for detecting depression. For example, markers of depression can be observed in the behavior and posts of Instagram users even before the date of the first depression diagnosis (117). Given these social media “signatures” of depression, automated analysis of social media could potentially be used for early detection of depression. If an automated process could detect elevated depression scores in a social media user based on their online content, that individual could be targeted for a more thorough assessment and provided with resources, support, and treatment. Furthermore, automated detection methods that rely on artificial intelligence and machine learning for passive, large-scale monitoring of social media sites may help identify depressed or otherwise at-risk individuals (118–121). For example, the use of certain words (122), as well as other patterns in a user’s language and online activity, could indicate depression (118). Machine learning-based analyses of social media could therefore serve as an additional tool in public mental health screening (Liu et al., 2022), help physicians diagnose mental illnesses, and help psychiatrists analyze patient behavior (123).
6 Well-established treatments of social deficits in MDD
There are a wide variety of biological (i.e., pharmacotherapeutic) and psychological (i.e., psychotherapeutic) therapies available for social deficits in depression; however, these traditional models of treatment delivery have several limitations.
6.1 Pharmacotherapy
Antidepressants are the mainstay of pharmacological treatment for depression, but their effects on social functioning are limited and inconsistent. Antidepressants are primarily aimed at relieving the acute symptoms of depression and restoring the mood, rather than improving social functioning (124). Even when antidepressants reduce depressive symptoms, they often fail to restore normal levels of social functioning in various domains, such as social relationships, occupational and marital functioning, and social support life (125). Moreover, antidepressants may have a negative impact on some aspects of social functioning, such as empathy and emotional reactivity, by reducing the aversive responses to the suffering of others or inducing emotional blunting (126–128).
Among the novel antidepressants, only desvenlafaxine and vortioxetine have consistently improved social functioning, as measured by an improvement in mood and social relationships (129, 130). Some antidepressants, such as citalopram and reboxetine, have also shown some positive effects on social cognition tasks involving facial affect recognition (131, 132) and interpretation of affective pictures (133, 134) (see Figure 1). However, these effects are often small and do not match the much larger changes typically observed for classical symptoms of depression (135, 136). Moreover, these effects are not fully explained by changes in depressive symptom severity and may not translate into real-life social situations (137, 138). Thus, efficacy in relieving the core symptoms of depression does not necessarily guarantee efficacy in relieving impaired social functioning.
Furthermore, antidepressants may have a delayed effect on social functioning compared to mood symptoms. Two Japanese studies showed that antidepressants improved social adaptation after four weeks of treatment, whereas mood symptoms improved after two weeks (139, 140). This suggests that improvement in social functioning tends to lag considerably behind clinical remission from severe depression. Therefore, antidepressants may not be sufficient to address the complex social needs of individuals with depression.
6.2 Psychotherapy
Psychotherapy is another treatment option for depression that can improve social functioning by addressing the interpersonal and emotional aspects of the disorder. It can be combined with antidepressants to achieve better outcomes than medication alone (141).
Psychotherapy approaches can have varying effects on social functioning depending on their specific goals and focus. For instance, a systematic review of five studies aimed at addressing social isolation and depression in aged care clients (142) found that reminiscence therapy was the only approach that significantly reduced both social isolation and depression (143). In contrast, Interpersonal Psychotherapy (IPT) is specifically designed to enhance social functioning. IPT achieves this by helping patients manage new roles within their relationships and resolving interpersonal conflicts (144). IPT has demonstrated its effectiveness in several ways, including bolstering social skills, improving social support, fostering healthier parent-adolescent relationships, and enhancing attachment security (145).
Cognitive behavioral therapy (CBT), another influential psychotherapy, targets mood and cognition improvement. This is achieved by challenging negative thoughts and behaviors while imparting coping skills (146). Although a number of studies indicate that adding to pharmacotherapy produces only moderate improvement in social functioning (146–148), other studies have shown that it can reduce loneliness (149) and improve social functioning (150). Thus, several studies have indicated that CBT can alleviate loneliness and promote better social functioning (149, 150). Moreover, CBT can also be effectively employed to address issues like social media addiction, a problem that can negatively impact both social functioning and overall life satisfaction (151).
A different approach to enhancing social functioning is the Cognitive Behavioral Analysis System of Psychotherapy (CBASP). CBASP seeks to improve social functioning by facilitating corrective interpersonal experiences within the patient-therapist relationship and teaching effective problem-solving techniques (152). Research has shown that CBASP can lead to an increase in empathic behavior (see Figure 1) and a reduction in interpersonal problems among individuals dealing with depression (153).
In conclusion, psychotherapy is a valuable treatment option for depression that can improve some aspects of social functioning by addressing the interpersonal and emotional aspects of the disorder. However, although CBT and IPT might reduce social deficits (145, 154), they do not fully ameliorate impairments in social functioning (23). In addition, many patients do not accept behavioral treatments and they are associated with high drop-out rates and they may not prevent relapses (155). Therefore, it is important to explore additional treatment options that can target the underlying neurobiological and psychological mechanisms of social functioning in depression, such as novel pharmacological agents or psychosocial interventions.
6.3 Electroconvulsive therapy
Between 12% (156) and 30% (157) of individuals with depression do not respond to consecutive courses of antidepressant medication and psychosocial therapy, resulting in a diagnosis of treatment-resistant depression (TRD). For individuals with TRD, various somatic or brain stimulation therapies may be considered. Electroconvulsive therapy (ECT) has proven to be an effective option for TRD (158). However, ECT does not consistently affect negative neurocognitive bias related to social impairments in major depressive disorder (MDD), as measured by the amygdala response to emotional faces (159). Similarly, experimental animal studies utilizing electroconvulsive seizure did not reverse the negative effects of chronic social stress exposure in mice; instead, they provided insights into potential mechanisms contributing to ECT side-effects in humans, such as short and long-term memory loss (160). Nevertheless, a study has suggested that ECT’s effect on memory may have a beneficial effect related to social functioning by reducing negative memory bias (161).
7 Novel treatments of social deficits in MDD
7.1 Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) is a non-invasive technique that modulates neural activity in the brain by generating a magnetic field through a brief discharge of electric current into a stimulated coil. This magnetic field induces depolarization of neural cell membranes in cortical tissue under the coil, affecting related nerve activity loops. It has shown the ability to improve social performance in depressed patients (162) by modifying cognitive control processes related to emotion regulation (163). Specifically, TMS targeting the right ventrolateral prefrontal cortex (PFC) has been shown to mitigate negative emotions experienced during social exclusion situations in depressed patients, suggesting its potential to enhance emotional regulation in response to social pain and social rejection (164).
7.2 Transcranial direct current stimulation
Another noninvasive neuromodulation technique, transcranial direct current stimulation (tDCS), has been employed successfully to induce mood changes in individuals with depression. Activation of the right ventrolateral PFC using tDCS has positively impacted emotional regulation (see Figure 1) while viewing images of social exclusion, particularly when reappraisal techniques are employed (165). Moreover, tDCS over the left dorsolateral PFC has reduced perceived emotional valence for negative images, potentially through enhanced cognitive control over emotional expression (166). Additionally, tDCS has improved interference control in tasks involving sad and neutral faces, as evidenced by faster reaction times (167). Furthermore, tDCS of the PFC has enhanced cognitive reappraisal of negative stimuli in depression (168), while also reducing aggressive responses to social exclusion (169). Moreover, tDCS may have a profound impact on compassion motivation, as revealed in a recent study employing anodal transcranial direct current stimulation (tDCS) on the right insula (170). Compassion motivation, linked to a calm physiological state indicated by heart rate variability (HRV), was significantly influenced by this tDCS technique. It increased HRV during videos evoking empathy, rendering individuals more sensitive to distress signals while concurrently reducing negative feelings and amplifying positive ones during subsequent tasks. These results suggest that tDCS may enhance compassion by modulating the physiological and emotional aspects of compassion motivation, potentially facilitating more empathetic and socially attuned behavior.
In summary, for individuals with treatment-resistant depression, therapies such as ECT, TMS, and tDCS hold promise in improving social functioning and social interactions. These therapies can influence emotional regulation, cognitive control, and memory processing, all of which are critical components of social behavior and social relationships in individuals with depression.
7.3 Vagus nerve stimulation
Vagus nerve stimulation (VNS) offers an alternative approach to treating depression by modulating various brain networks (171) and holds great potential for addressing not only mood-related symptoms but also social deficits commonly observed in depression. The vagus nerve may be an important mediator between depression and social functioning since it connects inner organs such as gut, heart and brain with social expressions, including pitch and tone of the voice as well as facial expression. Given that vagus nerve activity is reduced in MDD (172), therapeutic modulation of its activity has the potential to improve depressive symptoms and social functioning. Historically, VNS required surgical implantation, but a recent development called transcutaneous vagus nerve stimulation (tVNS) eliminates the need for surgical implants. Instead, it employs a bipolar electrode attached to the skin of the left ear conch (173).
In healthy subjects, tVNS has demonstrated promise in enhancing emotional processing and cognitive regulation and the sense of connection. It has improved cognitive reappraisal skills and reduced the perceived intensity of emotion-eliciting pictures (26). Additionally, tVNS has shown the ability to modulate attention to direct gaze (174) and enhance emotion recognition for faces (175). Significantly, transcutaneous electrical stimulation of the auricular branch of the vagus nerve (taVNS) has been found to selectively direct visual attention toward facial features essential for emotional recognition (176). Moreover, this stimulation has been associated with elevated levels of oxytocin in saliva, a neurotransmitter pivotal in modulating social cognition.
Furthermore, tVNS has been observed to enhance emotion recognition, especially for easily discernible emotional cues (177). Recent investigations have revealed that tVNS can reduce emotional biases toward both sad and happy facial expressions (178). In adolescents diagnosed with MDD, tVNS has been found to alter early visual processing of negatively valenced emotional stimuli, potentially reducing responses to sad emotional stimuli (179). These findings suggest that tVNS may reduce emotional reactivity, particularly toward sadness, and enhance the ability to decode social cues, potentially improving social functioning and facilitating more positive social interactions and relationships.
7.4 Intranasal oxytocin
There are also several novel treatments for depression that are less rooted in electrochemical physiology. In addition to using TMS and tVNS to treat social impairment, numerous studies over the last two decades have explored the modulatory role of the neuropeptide oxytocin on social cognition and behavior (180). In healthy participants, intranasal oxytocin administration led to increased eye contact with others, thus improving facial recognition (181) and body language-based emotion recognition (182). In persons at risk for depression, administration of oxytocin facilitated the processing of positive emotional cues (see Figure 1), enhancing the maintenance of attention to the mouth region of happy faces (183). Furthermore, intranasal oxytocin administration improved mood in new mothers who otherwise reported a moderately low mood (184). In mothers with postnatal depression, oxytocin can amplify responses to happy expressions from infants (185). In a related study, mothers with postnatal depression who received intranasal oxytocin were more likely to rate an infant cry as “urgent” (186). Another study has shown that after administration of oxytocin, postnatally depressed mothers were more protective of their baby in the presence of a stranger (187). For these reasons, oxytocin is worth investigating in terms of improving social functioning in MDD.
7.5 Intravenous and intranasal ketamine
Over the past two decades, subanesthetic ketamine treatment has emerged as a groundbreaking approach for addressing treatment-resistant depression (TRD) (188), offering rapid and immediate relief to patients who have previously found little success with other treatments (189). This development has instilled hope among individuals grappling with TRD, representing a promising alternative (158). Ketamine therapy encompasses a racemic mixture comprising two enantiomers, R-ketamine (arketamine) and S-ketamine (esketamine). The Food and Drug Administration (FDA) recognized the efficacy of intranasal esketamine, marketed as ‘Spravato,’ in conjunction with oral antidepressants for TRD treatment, thus offering a novel method of administration (190).
In contrast to traditional antidepressants, which typically require weeks to manifest therapeutic effects, both ketamine and serotonergic psychedelics have shown the capacity to alleviate depressive symptoms and enhance emotion regulation and social functioning (29, 158, 191, 192) within hours following a single administration (193, 194). For example, even a rapid intravenous ketamine injection at a dose of 0.5 mg/kg has proven effective in reducing rumination (195) and inducing sustained improvements in negative self-schema (196).
Ketamine’s positive impact on social functioning extends to its ability to enhance neural responses to positive emotions (see Figure 1), particularly within the right caudate, which appears to correlate with reduced depression severity (197). In the context of MDD, ketamine infusions have led to increased motivation and desire to engage in social interactions (158). Post-treatment, individuals with depression have reported a shift in their perceptions of others, reporting increased feelings of closeness, connectedness, and an enhanced ability to relate (198). These experiences are noteworthy as they suggest that ketamine can induce alterations in self-perception and reduce self-focus, potentially contributing to improved social interactions and emotional well-being. Additionally, research findings from another study indicate that ketamine has the capacity to mitigate pathological self-focus during emotional processing tasks. Specifically, it has been observed that ketamine administration results in increased deactivation of the Default Mode Network (DMN), particularly in response to negative and aversive stimuli, when compared to a placebo in healthy subjects assessed 24 hours after administration (199). This finding implies that ketamine may facilitate a more flexible and adaptive responsiveness of the DMN, reducing excessive self-referential processing during emotionally charged situations.
Recent research has also shed light on ketamine’s role in restoring top-down control of emotion processing. Thus, ketamine’s potential as an antidepressant extends to its ability to normalize brain function during emotionally valenced attentional processing (200). Additionally, by increasing functional connectivity between limbic regions and the central executive network, which plays a crucial role in top-down processing necessary for effective emotion regulation, ketamine may facilitate better emotional control (201). The rapid and positive effects of ketamine on emotional processing not only hold promise for improving social functioning but also have the potential to facilitate the establishment of therapeutic relationships, a cornerstone of various psychotherapies (191).
Ketamine has also been shown to reduce anhedonia, which could lead to improvements in social functioning (202). The research reported significant reductions in anhedonia following repeated ketamine infusions in patients with treatment-resistant depression. Notably, these effects were more pronounced in patients not using benzodiazepines, suggesting that ketamine’s impact on anhedonia might be influenced by concurrent medication use. However, larger placebo-controlled trials are needed to validate these findings conclusively.
The molecular mechanisms underlying the antidepressant actions of ketamine remain incompletely understood (203). At the neuronal level, esketamine holds the potential to bring about significant enhancements in neural plasticity and synaptogenesis, primarily through the upregulation of brain-derived neurotrophic factor (BDNF) production. Stress and depression often manifest as decreased BDNF levels in key brain regions, including the prefrontal cortex and hippocampus (30–32). Notably, studies have indicated that chronic ketamine treatment can effectively counteract the decline in BDNF protein levels in critical regions such as the hippocampus and nucleus accumbens in a rat model of depression (204). These findings suggest that ketamine-induced BDNF release may facilitate the adaptive rewiring of pathological neurocircuitry, ultimately leading to enduring structural brain adaptations that can sustain therapeutic benefits even in the absence of chronic dosing (205). Moreover, it has been postulated that the rapid antidepressant effects of esketamine may be closely linked to synaptic potentiation, a phenomenon that could contribute to a reduction in negative thinking patterns (206). By fostering synaptic potentiation, esketamine might facilitate the formation of new neural connections and the strengthening of existing ones, potentially improving emotional regulation and cognitive processes.
The pharmacodynamics of ketamine are intricately intertwined with multiple neurotransmitter systems, making its mechanisms of action complex. Notably, its rapid alleviation of depressive symptoms and reduction of suicidality have been attributed to its modulation of the NMDA receptor and interaction with the opioid system, as evidenced in a rat model (207). Furthermore, recent research has expanded our understanding of the endogenous mu-opioid system, revealing its involvement in more than just the hedonic aspects of pain. It has been proposed that the mu-opioid system plays a role in reinforcing socially affiliative or protective behavior in response to both positive and negative social experiences, with potential long-term implications for social behavior and health (208).
Furthermore, ketamine’s impact on the serotonin system extends beyond the traditionally recognized 5HT2A/1A receptors, known for their association with antidepressant effects. Ketamine may also activate other serotonin receptors, potentially influencing mood and social behavior. In rodents, ketamine’s antidepressant properties involve both AMPA and 5-HT1B receptors, with low 5-HT1B receptor binding observed in limbic regions in Major Depressive Disorder (MDD). However, human studies have shown that ketamine treatment does not significantly alter 5-HT1B receptor binding overall, although an increase in binding is noted in the hippocampus, suggesting a potential role of 5-HT1B receptors in ketamine’s antidepressant action (209). Additionally, considering the significant role of the glutamatergic system in MDD and social functioning, the surge in glutamatergic activity following ketamine administration is believed to contribute to its rapid antidepressant effects (210).
The observed efficacy of ketamine in depression management underscores its potential utility, particularly in cases where conventional treatments prove ineffective (211). For a substantial subset of depressed individuals facing challenges with traditional therapies, ketamine’s swift antidepressant actions hold promise for enhancing overall well-being and fostering a sense of connectedness and existential fulfillment (212).
7.6 Novel psychotherapeutic interventions
In response to the limitations of conventional treatments for social impairments, several researchers have investigated the effectiveness of novel psychotherapeutic interventions. Two such examples include the “Cognitively Based Compassion Training Programme” for couples (CBCT®-fC), which aims to improve social interaction skills (213), and the Cognitive and Emotional Recovery Training Program for Depression (CERT-D), which aims to improve psychosocial functioning (214). Additionally, various other psychotherapeutic methods hold promise in improving social functioning for individuals experiencing depression. These approaches include social competence training, emotion regulation skills training, and emotional intelligence training. Each of these methods focuses on specific aspects of social functioning such as social competence training (215), emotion regulation skills training (216, 217), or emotional intelligence training (218).
7.7 Mindfulness-based cognitive therapy
Emerging as promising novel treatment approaches deeply rooted in the spiritual traditions of indigenous and religious communities, both mindfulness-based cognitive therapy (MBCT) and psychedelic-assisted psychotherapy (PAP) have garnered significant scientific attention (219, 220). These therapeutic modalities, originating from diverse cultural contexts, have recently demonstrated the potential for complementary effects on enhancing social functioning in individuals grappling with depression (221).
In the realm of MBCT, a structured program integrates mindfulness-based training interventions with elements of cognitive behavioral therapy (222). These interventions are meticulously designed to actively manipulate attention and awareness. Notably, they have proven effective in diminishing self-centered thought patterns (223, 224) and reducing emotional reactivity to the rigors of social stress (225).
One of the core mechanisms through which MBCT contributes to improved social functioning in individuals with depression is the cultivation of self-awareness and self-regulation. By actively altering attention and awareness, MBCT enables individuals to gain insight into their thought processes, emotional reactions, and habitual patterns of behavior. Thus, a recent study has shown that MBCT might effectively reduce negative self-bias and facilitate psychophysiological benefits associated with a more positive self-view (226).
Another mechanism by which mindfulness enhances social functioning in individuals grappling with depression is cognitive reappraisal. Evidence suggests a strong correlation between mindfulness training and the augmentation of cognitive reappraisal as an effective emotion regulation strategy. For instance, one study revealed that individuals who received brief mindfulness training exhibited substantial increases in dispositional nonreactivity, a fundamental aspect of mindfulness (227). Importantly, this heightened dispositional nonreactivity was positively linked with the increased adoption of cognitive reappraisal techniques over time.
Mindful decentering, a core element of mindfulness, plays a pivotal role in facilitating positive reappraisal (228). It involves disengaging from initial appraisals of events and entering a state of metacognitive awareness that reduces semantic evaluations associated with those events. This cognitive shift, inherent to mindfulness, allows individuals to attribute new, more positive meanings to previously distressing situations. By cultivating mindfulness consciousness, individuals can redefine and reframe their circumstances in a way that fosters hope and resilience - a crucial component of meaning-based coping strategies.
In essence, mindfulness, with its capacity for cognitive reappraisal, becomes an integral tool for individuals facing acute or chronic stressors. The natural state of mindfulness, which involves cognitive flexibility, enables individuals to make positive reappraisals even in challenging social situations. Therefore, mindfulness training has the potential to empower individuals to effectively manage their emotions by encouraging them to reevaluate and reinterpret their emotional experiences. This, in turn, contributes significantly to their emotional well-being and improved social functioning.
Moreover, mindfulness has been associated with heightened compassion, extending both to oneself (self-compassion) and others (229). The heightened compassion toward others fosters a profound sense of interconnectedness and facilitates responding to potential conflicts with non-aggressive approaches (230). When individuals develop self-compassion, they are better equipped to manage self-criticism and self-blame, which are often intertwined with social difficulties in depression (231). This, in turn, can lead to more positive and constructive social interactions.
Recent research findings underscore the capacity of MBCT to effectively attenuate negative self-bias, thereby facilitating psychophysiological benefits associated with a more positive self-concept. This shift toward a more positive self-view can directly impact how individuals perceive their social interactions. As they develop a more positive self-image, they may feel more confident and less anxious in social situations, ultimately leading to improved social functioning (226).
Additionally, MBCT demonstrates a positive effect in diminishing self-criticism (232), curtailing ruminative thought processes (233, 234), mitigating self-blame (231), and reducing shame-proneness (235). These changes in cognitive and emotional processes may contribute to more adaptive social behaviors and interactions, as individuals are less burdened by negative self-perceptions and rumination, which can hinder social engagement.
Mindfulness practices offer valuable tools in addressing impulsive behaviors and anger among students. Thus, an older study has shown that mindfulness training can effectively decrease impulsive and aggressive behaviors (236). By cultivating present-moment awareness, mindfulness reduces obsessive rumination and promotes the experience of positive emotions by lowering the likelihood of engaging in impulsive behaviors, which often exacerbate the very emotional issues individuals seek to alleviate or resolve through aggression (237). Mindfulness training further equips individuals with the ability to recognize the initial signs of aggressive impulses, empowering them to inhibit these impulses effectively using the skills honed through mindfulness practice, such as acceptance and equanimity (236). In essence, mindfulness provides essential tools for reducing impulsivity and managing anger, promoting healthier responses to challenging emotions and situations.
Equally compelling is the evidence suggesting that MBCT augments cognitive flexibility (238–240). This can be highly beneficial in social interactions as it allows people to better understand diverse viewpoints and respond more skillfully to differing opinions and behaviors. In conflicts, cognitive flexibility can facilitate compromise and creative problem-solving, leading to healthier relationships.
In addition to enhancing cognitive flexibility, mindfulness practice also heightens empathetic capacities (83, 241). When individuals are more in touch with their own emotions and thoughts, they become more attuned to the feelings and needs of others. This heightened empathy can lead to improved communication, deeper connections, and a greater ability to provide emotional support in relationships.
Additionally, mindfulness training fosters compassion, and facilitates forgiveness toward others (230, 242). When individuals are more compassionate, they tend to be more patient and forgiving, which can lead to more harmonious and fulfilling relationships. Since holding onto grudges or resentment can strain relationships, being capable to let go of past grievances and focus on the present moment might facilitate healing leading to the restoration or improvement of relationships.
Finally, mindfulness training has been shown to increase willingness to cooperate in the ultimatum game (243). Further, two studies have shown that an 8-week mindfulness training program has promoted reactive prosocial behavior while observing suffering or hardship of a confederate (230, 244). Further, mindfulness promoted prosocial responsiveness to a representation of an ostracized stranger (245) (see Figure 1). Another study found that subjects in the mindfulness condition allocated more money to another participant despite not having any information about the financial needs or any other form of distress (246). When individuals are more mindful, they are more likely to respond to the needs of others in a helpful and altruistic manner. This can strengthen bonds, build trust, and contribute to a more positive social environment. The cooperative mindset can lead to better teamwork in various aspects of life, including personal relationships.
Notwithstanding these substantial advancements in social functioning attributed to MBCT, it is important to acknowledge that realizing these benefits often necessitates intensive and prolonged mindfulness practice. This acknowledgment underscores both the potential and the challenges of MBCT as an intervention in ameliorating social deficits among individuals contending with depression. Consequently, it highlights the need for further research to optimize the accessibility and efficiency of MBCT, ultimately ensuring its broad applicability in clinical settings.
7.8 Psychedelic-assisted psychotherapy
Psychedelic-assisted psychotherapy (PAP) has emerged as a therapeutic approach demonstrating analogous outcomes to MBCT, particularly in addressing social impairments associated with depression. PAP encompasses the administration of psychedelic substances, including MDMA, psilocybin, LSD, ayahuasca, ketamine, or ibogaine, within a structured intervention coupled with psychotherapy (247, 248). Within this structured context, psychedelic agents have exhibited the capacity to induce enduring positive alterations in social functioning following a single dosage (249). Thus, a single ingestion of ayahuasca in a social setting resulted in increased cognitive empathy as well as an improved ability to recognize emotions in others when compared to baseline (250) (see Figure 1).
PAP facilitates the emergence of emotions like self-compassion, forgiveness, and self-acceptance, which serve as potent remedies for emotions such as shame, guilt, anger, isolation, disconnection, or other negative feelings that patients may struggle to address in therapy and that traditional antidepressants do not appear to alleviate (251).
One plausible mechanism underlying the improvement of social impairments in depression through psychedelics lies in their ability to modulate self-referential processes. Psychedelics have been found to diminish self-referential awareness (252), and ruminative self-focus (Letheby and Gerrans, 2017), facilitating a shift away from internalized negative thought patterns. This reduction in self-focused rumination can contribute to feelings of unity with others and an augmented sense of social affiliation (253, 254). By disrupting the dominance of depressive self-preoccupation, psychedelics may enable individuals to engage more fully in social interactions and connect with others on a deeper level.
Another mechanism might lie in the increased capability to adopt a decentered stance of mind after the use of psychedelics. It has been shown, that a single dose of ayahuasca led to increases in decentering ratings 24 hours and 7 days following the ingestion (250). These findings align with prior research that has consistently reported enhancements in decentering abilities and related mindfulness capacities at various post-ayahuasca time points, including 24 hours (255, 256), 15 days (257), and even 2 months post-intake (258). Previous research has shown that when individuals are prompted to analyze their feelings about negative autobiographical experiences from a self-distanced, decentered perspective (i.e., by visualizing events from the standpoint of an impartial observer) as opposed to a self-immersed, first-person viewpoint, they exhibit lower levels of acute emotional and physiological reactivity and are less prone to rumination over time (259, 260). This is highly relevant for depressive patients who often experience heightened emotional reactivity to social situations, which can manifest as increased sensitivity to perceived social rejection, criticism, or negative feedback and lead to acute emotional distress and physiological responses such as anxiety or even withdrawal from social interactions. By viewing negative social experiences as an impartial observer, depressed individuals might be able to reduce the intensity of their emotional reactions and decrease physiological arousal in social situations. Consequently, they may become less vulnerable to the negative emotional impact of social interactions and be more willing to initiate them.
Furthermore, psychedelics have been associated with the modulation of emotional processing. They have been found to ameliorate the negative emotional bias observed in depressed individuals during emotion recognition tasks. For instance, multiple studies have indicated that MDMA, commonly known as “ecstasy,” positively skews an individual’s interpretation of emotional facial expressions by augmenting the recognition of positive expressions while potentially impeding the recognition of negative expressions (261–264). This alteration in emotional processing may help individuals with depression perceive social interactions and cues in a more positive and socially attuned manner, ultimately improving their social functioning.
Numerous investigations have corroborated the enhancement of behaviors associated with cognitive flexibility and receptivity to novel experiences following psychedelic use (255, 265–271). This heightened cognitive flexibility may play a pivotal role in improving social impairments by enabling individuals to adapt more effectively to social contexts, read social cues, and engage in more fluid and adaptive social interactions.
Additionally, psychedelics have been associated with heightened emotional empathy (268, 272–275), prosocial behavior (249, 276), increased generosity (277), and self-reported altruism (278). These pro-social effects suggest that psychedelics may enhance individuals’ ability to connect with others on an emotional level, thereby addressing some of the social impairments often seen in depression (279).
Furthermore, psychedelics often induce a state known as ego dissolution, where the boundaries between self and others blur (280). This experience, commonly reported during psychedelic journeys (280, 281), suggests that psychedelics might promote a heightened sense of connection with others and potentially reduce feelings of loneliness. A more recent investigation focusing on male long-term AIDS survivors has suggested that psilocybin-assisted psychotherapy may effectively reduce attachment anxiety and enhance attachment security (282). Consequently, the effects of psychedelics seem to extend beyond individual well-being, offering the potential for therapeutic benefits in terms of improved social connections, better relationships, and more positive social behavior.
Furthermore, individuals consuming psychedelic substances have demonstrated a decreased likelihood of rejecting unfair offers in the ultimatum game (283) and a heightened inclination toward cooperative behavior in the prisoner’s dilemma (284). These findings suggest that psychedelics may promote more cooperative and adaptive social behaviors, potentially mitigating social difficulties commonly associated with depression.
Recent research highlights the potential of psychedelics, such as MDMA and psilocybin, to mitigate the impact of social rejection sensitivity, a phenomenon often exacerbated in individuals with depression. MDMA, for instance, has demonstrated a capacity to diminish the effects of simulated social rejection, as evidenced by improvements in self-reported mood and self-esteem, along with a reduced perception of rejection intensity within the context of virtual social interaction paradigm (Cyberball game) (285). Furthermore, an intriguing observation emerged from this research, indicating that higher doses of MDMA were associated with an increased estimated number of throws received by participants during the rejection condition in the Cyberball game. This suggests that MDMA may not only influence mood but could potentially alter the objectively assessed level of rejection experienced by individuals.
Similarly, psilocybin, administered to healthy volunteers, has exhibited the ability to reduce feelings of social exclusion (286). Intriguingly, during a Cyberball game, psilocybin was found to modulate emotional responses to social exclusion by attenuating activation in brain regions associated with social rejection (287). This dampening of social rejection sensitivity is of particular significance within the realm of psychotherapy. By ameliorating the emotional repercussions of perceived rejection, psychedelics like MDMA may create an environment conducive to open and honest communication. This effect holds significant therapeutic potential, offering individuals with depression a platform to engage more constructively with their emotions and interpersonal challenges during psychotherapeutic interventions.
Finally, the concept of connectedness was emphasized in psychedelic therapy, as revealed in a qualitative research paper for treatment-resistant depression (TRD) clinical trial (254). In 6-month follow-up interviews, participants, who endorsed the treatment’s effectiveness, consistently cited a key factor: a renewed sense of connection. This factor was categorized into three aspects: connection to oneself, others, and the world at large (254).
Psychedelics can potentially improve social functioning through their multifaceted effects on the brain and psychology. Their serotonin (5HT)2A agonist or partial agonist effects, have demonstrated rapid antidepressant properties, and this may contribute to their positive impact on social interactions. The modulation of the serotonin system can influence mood, emotional regulation and reward processing, potentially reducing symptoms of social anhedonia and social anxiety that hinder social interactions. Moreover, psychedelics have been shown to increase neuroplasticity, including effects on spinogenesis, synaptic pruning and brain-derived neurotrophic factor. Likewise, both healthy individuals and individuals with TRD exhibit heightened circulating levels of BDNF following a single administration of ayahuasca (288). Furthermore, psilocybin has been observed to elevate peak BDNF levels, irrespective of whether patients have received pre-treatment with the antidepressant escitalopram or a placebo, indicating that psilocybin exerts a significant influence on BDNF (289). These mechanisms are similar to effect of stimulating the vagus nerve in rats, which quickly increased the levels of neurotransmitters (such as norepinephrine) and the genes that produce neurotrophic factors like BDNF (290). These processes may help improve mood by promoting the growth of new brain cells in areas like the hippocampus, which are linked to mood disorders (291). Further, they could contribute to increased resilience to stressors in social settings (292).
From a neuroscientific perspective, studies using neuroimaging and neurophysiology have suggested that psychedelics may induce changes in brain connectivity. Specifically, they may disrupt the default mode network, consisting of the medial prefrontal cortex, posterior cingulate cortex, precuneus, and angular gyrus, which is typically overactive in conditions like MDD (293). Among individuals with depression, there have been documented decreases in functional connectivity within the DMN following psilocybin treatment (280, 294). More specifically, these reductions were noted in connections between the ventromedial prefrontal cortex (vmPFC) and the right angular gyrus (294). By altering the DMN, psychedelics may reduce self-referential thinking and introspection, potentially leading to more open and empathetic social interactions.
In summary, psychedelics have the potential to improve social functioning by acting on various levels. They may alleviate symptoms of depression and anxiety, enhance resilience to stress, and modify brain connectivity patterns, all of which can contribute to more positive and fulfilling social interactions. However, although clinical trials consistently showcase the efficacy of PAP across diverse mental health conditions, it is imperative to acknowledge that this area of research is still in its formative stages. It is important to note that further research is needed to fully understand the extent and nuances of these effects and to ensure their safe and responsible use. To comprehensively elucidate the potential moderators and mediators of this treatment modality and ascertain the sustainability of its effects over time, larger trials involving more heterogeneous participant samples remain a critical area of future exploration.
8 Role of animal models in treatment of social deficits
Animal studies using genetic, epigenetic, and environmentally-induced models of depression can help researchers test existing MDD treatments and search for novel therapeutics (295). Although human experiences of depression, such as sadness, guilt, or suicidal thoughts, cannot be simulated or assessed in rodents or other animal models, animals can still exhibit depression-related behaviors such as social anhedonia, social defeat, sociability, social anxiety, and social withdrawal (296). Animal models may therefore serve as a powerful tool for the study of social impairments, social behavior, and treatment options in depression (297). For example, raising rodents in constant social isolation immediately after weaning leads to behaviors typical of depression (298), such as despair-like behavior, which is expressed in mice as increasing immobility over time (299). The “social defeat stress” animal model (297) has been used to show that stress-susceptible mice exhibit repeated aggression and develop a long-lasting aversion to social contact. These behavioral changes can be reversed by administering antidepressants (300) or psychedelic drugs such as LSD or ketamine (301–303). For example, low doses of LSD increased sociability in mice by activating serotonin 5-HT2A receptors and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (304). Further, recent study in the rat model of depression has shown that after administration of ketamine, the rapid response entailed topological modifications in cognitive, sensory, emotion, and reward-related circuitry, whereas after 48 h ketamine had mainly mediator effects on cognitive flexibility (305).
Despite their evident imperfections, animal models could also provide invaluable help to understand the cellular and molecular mechanisms underlying the treatment of MDD (295). For example, Gerhard et al. (2020) recently demonstrated that in a mouse model, a specific NMDA receptor subunit on GABAergic interneurons was the initial cellular trigger for ketamine’s rapid antidepressant activity. Further, another study in mice has shown that in the chronic stress model, ketamine selectively reversed stress-induced branch-specific elimination of postsynaptic dendritic spines and led to remission of specific depression-related behaviors such as motivated escape behavior (306). This study has also shown that eliminating newly formed synapses abolished some of the drug’s positive effects, indicating that spinogenesis induced by ketamine might be useful for maintaining remission after ketamine treatment.
The use of animal models in psychiatric research, particularly in studies involving depression, raises significant ethical issues. Inducing depression-like states in animals often involves procedures such as chronic stress exposure, social isolation, and genetic manipulation, which can cause considerable distress and suffering to the animals (307). These methods, while valuable for understanding the mechanisms underlying depression and testing potential treatments, pose ethical dilemmas regarding the humane treatment of research animals.
To address these ethical concerns, several measures are implemented to ensure the welfare of animals used in research. All experimental protocols must undergo rigorous review and approval by Institutional Animal Care and Use Committees (IACUCs) or equivalent ethical review boards. These committees evaluate the scientific merit of the proposed studies and the ethical justification for using animals, ensuring that the research adheres to the principles of the 3Rs: Replacement, Reduction, and Refinement (308).
● Replacement: Whenever possible, alternative methods that do not involve animals, such as in vitro studies or computer simulations, are used to achieve the research objectives.
● Reduction: The number of animals used in experiments is minimized by employing efficient study designs and statistical methods that maximize the data obtained from each animal.
● Refinement: The procedures are refined to minimize pain and distress, including the use of analgesics, anesthetics, and humane endpoints to alleviate suffering.
Despite these measures, the ethical justification for animal research in psychiatry remains a topic of ongoing debate. Increasing community expectations regarding animal rights and welfare necessitate a continuous reassessment of the ethical frameworks guiding such research. Researchers are encouraged to maintain transparency in their methodologies and to engage in open dialogue with the public and ethical bodies to align scientific pursuits with evolving ethical standards (309).
9 Limitations
While this article embraces a narrative review approach, which provides a comprehensive overview of the literature, it is important to acknowledge some inherent limitations. Firstly, the subjectivity in selecting and interpreting studies could introduce bias into the review process. Additionally, the absence of a systematic search strategy might restrict the inclusion of relevant research.
Another limitation of this review is the lack of extensive exploration into the complexities of differentiating social deficits attributable to MDD from those arising from underlying personality traits independent of depressive episodes. Future research endeavors should aim to elucidate these distinctions, as they hold significant implications for accurate diagnosis and tailored treatment approaches. Despite these limitations, our review offers valuable insights into impairments of social functioning in depression and their treatments and serves as a catalyst for generating hypotheses for future research.
10 Summary and conclusion
In this comprehensive exploration of treatments for social deficits in MDD, we have reviewed a range of therapeutic approaches, encompassing both traditional and novel methods. While MDD is commonly associated with emotional distress and cognitive symptoms, its profound impact on social functioning is often overlooked. Traditional pharmacotherapy, represented by antidepressants, while effective in alleviating mood-related symptoms, has shown limited and inconsistent success in improving social deficits. Additionally, some antidepressants may even negatively impact certain aspects of social functioning. The integration of psychotherapy alongside pharmacotherapy has emerged as a promising strategy to enhance social functioning. Cognitive Behavioral Therapy and Interpersonal Therapy are two influential psychotherapeutic approaches that have demonstrated some success in mitigating social deficits associated with MDD. While these therapies can produce moderate improvements, they often fall short of fully ameliorating social impairments that importantly contribute to therapy discontinuation and depressive relapse. Therefore, it becomes imperative to explore additional avenues beyond these traditional approaches.
Brain stimulation therapies, such as electroconvulsive therapy, transcranial magnetic stimulation, and transcranial direct current stimulation, offer alternative paths for addressing social deficits in MDD. These therapies have shown promise in improving social performance, emotion regulation, and cognitive control. The diverse range of brain stimulation techniques suggests that modulating neural circuits holds significant potential for enhancing social functioning in individuals with depression, although further research is required to comprehend the underlying mechanisms fully.
Vagus nerve stimulation, especially through transcutaneous methods, introduces another non-invasive approach to treating depression’s social and emotional impairments. Emerging evidence suggests that transcutaneous vagus nerve stimulation (tVNS) can modulate emotional processing, attention, and social cognition. Its capacity to affect oxytocin release, a critical neuropeptide for social bonding, makes tVNS a compelling avenue for research and potential intervention in social deficits. In line with it, intranasal oxytocin administration offers a more direct pharmacological approach to enhance social cognition and behavior in individuals with MDD. Oxytocin has exhibited promising results in improving emotional processing, enhancing social interactions, and boosting mood in various contexts. Its potential to bridge the gap in social functioning is an area that might benefit from further exploration.
The emergence of ketamine treatment has marked a groundbreaking development in the field of depression therapy. Ketamine, particularly its S-ketamine enantiomer, has demonstrated rapid and substantial antidepressant effects, leading to its approval for the treatment of treatment-resistant depression. Ketamine’s impact extends beyond mood improvement, addressing emotional processing, social anxiety, and social functioning. Its ability to induce rapid and profound changes in emotional and social aspects makes it a promising intervention for social deficits in MDD.
Finally, mindfulness-based cognitive therapy (MBCT) and psychedelic-assisted psychotherapy (PAP) offer novel and complementary approaches to enhance social functioning in depression. MBCT combines mindfulness training with cognitive behavioral therapy, targeting self-focus, emotional reactivity, and self-criticism. In contrast, PAP utilizes psychedelic substances in conjunction with psychotherapy to induce lasting changes in social behavior, emotional processing, and cognitive flexibility. These two approaches, while distinct, share the common goal of improving social deficits, albeit with different mechanisms and timelines.
To gain a deeper understanding of the complex social deficits in MDD and the potential interventions, animal models have proven invaluable. These models allow researchers to explore treatment mechanisms at the cellular and molecular levels. Animal studies have uncovered insights into the action of antidepressants, psychedelics, and other therapeutic modalities, shedding light on the neural underpinnings of social deficits and their amelioration.
In conclusion, MDD poses significant challenges beyond the core emotional and cognitive symptoms, significantly impacting social functioning. Addressing these deficits necessitates a multifaceted approach that combines traditional pharmacotherapy and psychotherapy with innovative treatments like brain stimulation, vagus nerve stimulation, oxytocin administration, ketamine treatment, mindfulness-based cognitive therapy, and psychedelic-assisted psychotherapy. The integration of these diverse approaches offers hope for individuals with depression to regain a fulfilling social life, making it essential to continue researching and refining these interventions. By embracing this holistic perspective and advancing our understanding, we can better support those affected by depression in their journey toward improved social functioning and overall well-being.
Author contributions
AK: Writing – review & editing, Writing – original draft. GH: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kupferberg A, Bicks L, Hasler G. Social functioning in major depressive disorder. Neurosci Biobehav Rev. (2016) 69:313–32. doi: 10.1016/j.neubiorev.2016.07.002
2. Kupferberg A, Hasler G. The social cost of depression: Investigating the impact of impaired social emotion regulation, social cognition, and interpersonal behavior on social functioning. J Affect Disord Rep. (2023) 14:100631. doi: 10.1016/j.jadr.2023.100631
3. Derntl B, Habel U. Deficits in social cognition: a marker for psychiatric disorders? Eur Arch Psychiatry Clin Neurosci. (2011) 261:145–9. doi: 10.1007/s00406-011-0244-0
4. Lee KH, Farrow TFD, Spence SA, Woodruff PWR. Social cognition, brain networks and schizophrenia. Psychol Med. (2004) 34:391–400. doi: 10.1017/S0033291703001284
5. Weniger G, Lange C, Rüther E, Irle E. Differential impairments of facial affect recognition in schizophrenia subtypes and major depression. Psychiatry Res. (2004) 128:135–46. doi: 10.1016/j.psychres.2003.12.027
6. Bazin N, Brunet-Gouet E, Bourdet C, Kayser N, Falissard B, Hardy-Baylé M-C, et al. Quantitative assessment of attribution of intentions to others in schizophrenia using an ecological video-based task: a comparison with manic and depressed patients. Psychiatry Res. (2009) 167:28–35. doi: 10.1016/j.psychres.2007.12.010
7. Unruh KE, Bodfish JW, Gotham KO. Adults with autism and adults with depression show similar attentional biases to social-affective images. J Autism Dev Disord. (2020) 50:2336–47. doi: 10.1007/s10803-018-3627-5
8. Allen NB, Badcock PBT. The social risk hypothesis of depressed mood: evolutionary, psychosocial, and neurobiological perspectives. Psychol Bull. (2003) 129:887–913. doi: 10.1037/0033-2909.129.6.887
9. Macinnes DL. Self-esteem and self-acceptance: an examination into their relationship and their effect on psychological health. J Psychiatr Ment Health Nurs. (2006) 13:483–9. doi: 10.1111/j.1365-2850.2006.00959.x
10. O’Neill SC, Cohen LH, Tolpin LH, Gunthert KC. Affective reactivity to daily interpersonal stressors as a prospective predictor of depressive symptoms. J Soc Clin Psychol. (2004) 23:172–94. doi: 10.1521/jscp.23.2.172.31015
11. Parrish BP, Cohen LH, Laurenceau J-P. Prospective relationship between negative affective reactivity to daily stress and depressive symptoms. J Soc Clin Psychol. (2011) 30:270–96. doi: 10.1521/jscp.2011.30.3.270
12. Sheets ES, Craighead WE. Comparing chronic interpersonal and noninterpersonal stress domains as predictors of depression recurrence in emerging adults. Behav Res Ther. (2014) 63:36–42. doi: 10.1016/j.brat.2014.09.001
13. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. (2004) 80:135–44. doi: 10.1016/S0165-0327(03)00054-5
14. Saris IMJ, Aghajani M, van der Werff SJA, van der Wee NJA, Penninx BWJH. Social functioning in patients with depressive and anxiety disorders. Acta Psychiatr Scand. (2017) 136:352–61. doi: 10.1111/acps.12774
15. Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. (1995) 25:1171–80. doi: 10.1017/s0033291700033146
16. Katz SJ, Conway CC, Hammen CL, Brennan PA, Najman JM. Childhood social withdrawal, interpersonal impairment, and young adult depression: a mediational model. J Abnorm Child Psychol. (2011) 39:1227. doi: 10.1007/s10802-011-9537-z
17. Kyte Z, Goodyer I. “Social cognition in depressed children and adolescents.” In: Social cognition and developmental psychopathology. Oxford University Press, New York, NY, US (2008). p. 201–37. doi: 10.1093/med/9780198569183.003.0008
18. Rudolph KD, Hammen C, Burge D. Interpersonal functioning and depressive symptoms in childhood: addressing the issues of specificity and comorbidity. J Abnorm Child Psychol. (1994) 22:355–71. doi: 10.1007/BF02168079
19. Schwartz-Mette RA, Shankman J, Dueweke AR, Borowski S, Rose AJ. Relations of friendship experiences with depressive symptoms and loneliness in childhood and adolescence: A meta-analytic review. Psychol Bull. (2020) 146:664–700. doi: 10.1037/bul0000239
20. Knight MJ, Baune BT. Social cognitive abilities predict psychosocial dysfunction in major depressive disorder. Depress Anxiety. (2019) 36:54–62. doi: 10.1002/da.22844
21. Weightman MJ, Knight MJ, Baune BT. A systematic review of the impact of social cognitive deficits on psychosocial functioning in major depressive disorder and opportunities for therapeutic intervention. Psychiatry Res. (2019) 274:195–212. doi: 10.1016/j.psychres.2019.02.035
22. Porcelli S, van der Wee N, van der Werff S, Aghajani M, Glennon JC, van Heukelum S, et al. Social brain, social dysfunction and social withdrawal. Neurosci Biobehav Rev. (2019) 97:10–33. doi: 10.1016/j.neubiorev.2018.09.012
23. Byock I. Taking Psychedelics Seriously. J Palliat Med. (2018) 21:417–21. doi: 10.1089/jpm.2017.0684
24. Stimmel GL, Gutierrez MA. Sexual dysfunction and psychotropic medications. CNS Spectr. (2006) 11:24–30. doi: 10.1017/S1092852900026730
25. Yekehtaz H, Farokhnia M, Akhondzadeh S. Cardiovascular considerations in antidepressant therapy: an evidence-based review. J Tehran Univ Heart Cent. (2013) 8:169–76.
26. De Smet S, Baeken C, Seminck N, Tilleman J, Carrette E, Vonck K, et al. Non-invasive vagal nerve stimulation enhances cognitive emotion regulation. Behav Res Ther. (2021) 145:103933. doi: 10.1016/j.brat.2021.103933
27. Heuschkel K, Kuypers KPC. Depression, mindfulness, and psilocybin: possible complementary effects of mindfulness meditation and psilocybin in the treatment of depression. a review. Front Psychiatry. (2020) 11. https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00224.
28. Marazziti D, Diep P-T, Carter S, Carbone MG. Oxytocin: an old hormone, a novel psychotropic drug and its possible use in treating psychiatric disorders. Curr Med Chem. (2022) 29:5615–87. doi: 10.2174/0929867329666220727120646
29. Marchi M, Magarini FM, Galli G, Mordenti F, Travascio A, Uberti D, et al. The effect of ketamine on cognition, anxiety, and social functioning in adults with psychiatric disorders: A systematic review and meta-analysis. Front Neurosci. (2022) 16:1011103. doi: 10.3389/fnins.2022.1011103
30. Viana GSB, do Vale EM, de Araujo ARA, Coelho NC, Andrade SM, da Costa RO, et al. Rapid and long-lasting antidepressant-like effects of ketamine and their relationship with the expression of brain enzymes, BDNF, and astrocytes. Braz J Med Biol Res. (2020) 54:e10107. doi: 10.1590/1414-431X202010107
31. Woelfer M, Li M, Colic L, Liebe T, Di X, Biswal B, et al. Ketamine-induced changes in plasma brain-derived neurotrophic factor (BDNF) levels are associated with the resting-state functional connectivity of the prefrontal cortex. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry. (2020) 21:696–710. doi: 10.1080/15622975.2019.1679391
32. Yang T, Nie Z, Shu H, Kuang Y, Chen X, Cheng J, et al. The role of BDNF on neural plasticity in depression. Front Cell Neurosci. (2020) 14. https://www.frontiersin.org/article/10.3389/fncel.2020.00082.
33. Joormann J, Gotlib IH. Emotion Regulation in Depression: Relation to Cognitive Inhibition. Cognit Emot. (2010) 24:281–98. doi: 10.1080/02699930903407948
34. Joormann J, Stanton CH. Examining emotion regulation in depression: A review and future directions. Behav Res Ther. (2016) 86:35–49. doi: 10.1016/j.brat.2016.07.007
35. Garnefski N, Kraaij V. The Cognitive Emotion Regulation Questionnaire. Eur J Psychol Assess. (2007) 23:141–9. doi: 10.1027/1015-5759.23.3.141
36. Gross JJ. “Emotion regulation: Conceptual and empirical foundations.” In: Handbook of emotion regulation, 2nd ed. The Guilford Press, New York, NY, US (2014). p. 3–20.
37. Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin Psychol Rev. (2010) 30:217–37. doi: 10.1016/j.cpr.2009.11.004
38. Dryman MT, Heimberg RG. Emotion regulation in social anxiety and depression: a systematic review of expressive suppression and cognitive reappraisal. Clin Psychol Rev. (2018) 65:17–42. doi: 10.1016/j.cpr.2018.07.004
39. Ehring T, Tuschen-Caffier B, Schnülle J, Fischer S, Gross JJ. Emotion regulation and vulnerability to depression: spontaneous versus instructed use of emotion suppression and reappraisal. Emot Wash DC. (2010) 10:563–72. doi: 10.1037/a0019010
40. Picó-Pérez M, Radua J, Steward T, Menchón JM, Soriano-Mas C. Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Prog Neuropsychopharmacol Biol Psychiatry. (2017) 79:96–104. doi: 10.1016/j.pnpbp.2017.06.001
41. Yin J. The relationship between cognitive reappraisal and depression in middle childhood. Undergrad J Psychol. (2021). https://journals.uncc.edu/ujop/article/view/1062.
42. Fresco DM, Segal ZV, Buis T, Kennedy S. Relationship of posttreatment decentering and cognitive reactivity to relapse in major depression. J Consult Clin Psychol. (2007) 75:447–55. doi: 10.1037/0022-006X.75.3.447
43. Kobayashi R, Shigematsu J, Miyatani M, Nakao T. Cognitive reappraisal facilitates decentering: a longitudinal cross-lagged analysis study. Front Psychol. (2020) 11. https://www.frontiersin.org/article/10.3389/fpsyg.2020.00103.
44. Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psychol. (2002) 70:275–87. doi: 10.1037//0022-006x.70.2.275
45. Webb TL, Miles E, Sheeran P. Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol Bull. (2012) 138:775–808. doi: 10.1037/a0027600
46. Bennett MP, Knight R, Patel S, So T, Dunning D, Barnhofer T, et al. Decentering as a core component in the psychological treatment and prevention of youth anxiety and depression: a narrative review and insight report. Transl Psychiatry. (2021) 11:1–14. doi: 10.1038/s41398-021-01397-5
47. Soler J, Cebolla A, Feliu-Soler A, Demarzo MMP, Pascual JC, Baños R, et al. Relationship between meditative practice and self-reported mindfulness: the MINDSENS composite index. PLoS One. (2014) 9:e86622. doi: 10.1371/journal.pone.0086622
48. Fresco DM, Moore MT, van Dulmen MHM, Segal ZV, Ma SH, Teasdale JD, et al. Initial psychometric properties of the experiences questionnaire: validation of a self-report measure of decentering. Behav Ther. (2007) 38:234–46. doi: 10.1016/j.beth.2006.08.003
49. Segal Z, Williams M, Teasdale J. Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse: Book review. Cognit Behav Ther. (2002) 31:193–4.
50. Carson SH, Langer EJ. Mindfulness and self-acceptance. J Ration-Emotive Cogn-Behav Ther. (2006) 24:29–43. doi: 10.1007/s10942-006-0022-5
51. Krieger T, Altenstein D, Baettig I, Doerig N, Holtforth MG. Self-compassion in depression: associations with depressive symptoms, rumination, and avoidance in depressed outpatients. Behav Ther. (2013) 44:501–13. doi: 10.1016/j.beth.2013.04.004
52. Bakker AM, Cox DW, Hubley AM, Owens RL. Emotion regulation as a mediator of self-compassion and depressive symptoms in recurrent depression. Mindfulness. (2019) 10:1169–80. doi: 10.1007/s12671-018-1072-3
53. Chamberlain JM, Haaga DAF. Unconditional self-acceptance and responses to negative feedback. J Ration-Emotive Cogn-Behav Ther. (2001) 19:177–89. doi: 10.1023/A:1011141500670
54. Flett GL, Besser A, Davis RA, Hewitt PL. Dimensions of perfectionism, unconditional self-acceptance, and depression. J Ration-Emotive Cogn-Behav Ther. (2003) 21:119–38. doi: 10.1023/A:1025051431957
55. Gilbert P, Baldwin MW, Irons C, Baccus JR, Palmer M. Self-criticism and self-warmth: an imagery study exploring their relation to depression. J Cognit Psychother. (2006) 20:183–200. doi: 10.1891/jcop.20.2.183
56. Campbell-Sills L, Barlow DH, Brown TA, Hofmann SG. Effects of suppression and acceptance on emotional responses of individuals with anxiety and mood disorders. Behav Res Ther. (2006) 44:1251–63. doi: 10.1016/j.brat.2005.10.001
57. Maramis MM, Mahajudin MS, Khotib J. Impaired cognitive flexibility and working memory precedes depression: a rat model to study depression. Neuropsychobiology. (2021) 80:225–33. doi: 10.1159/000508682
58. Schwenke D, Fjaellingsdal TG, Bleichner MG, Grage T, Scherbaum S. An approach to social flexibility: Congruency effects during spontaneous word-by-word interaction. PLoS One. (2020) 15:e0235083. doi: 10.1371/journal.pone.0235083
59. Soltani E, Shareh H, Bahrainian SA, Farmani A. The mediating role of cognitive flexibility in correlation of coping styles and resilience with depression. Pajoohandeh J. (2013) 18:88–96.
60. Schwert C, Aschenbrenner S, Weisbrod M, Schröder A. Cognitive impairments in unipolar depression: the impact of rumination. Psychopathology. (2017) 50:347–54. doi: 10.1159/000478785
61. Wagner CA, Alloy LB, Abramson LY. Trait rumination, depression, and executive functions in early adolescence. J Youth Adolesc. (2015) 44:18–36. doi: 10.1007/s10964-014-0133-8
62. Murphy FC, Michael A, Sahakian BJ. Emotion modulates cognitive flexibility in patients with major depression. Psychol Med. (2012) 42:1373–82. doi: 10.1017/S0033291711002418
63. Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, et al. Control and interference in task switching–a review. Psychol Bull. (2010) 136:849–74. doi: 10.1037/a0019842
64. Yu Y, Yu Y, Lin Y. Anxiety and depression aggravate impulsiveness: the mediating and moderating role of cognitive flexibility. Psychol Health Med. (2020) 25:25–36. doi: 10.1080/13548506.2019.1601748
65. Deuter CE, Wingenfeld K, Otte C, Bustami J, Kaczmarczyk M, Kuehl LK. Noradrenergic system and cognitive flexibility: Disentangling the effects of depression and childhood trauma. J Psychiatr Res. (2020) 125:136–43. doi: 10.1016/j.jpsychires.2020.03.017
66. Akhtar S, Barlow J. Forgiveness therapy for the promotion of mental well-being: a systematic review and meta-analysis. Trauma Violence Abuse. (2018) 19:107–22. doi: 10.1177/1524838016637079
67. Datu JAD. Forgiveness, gratitude and subjective well-being among filipino adolescents. Int J Adv Couns. (2014) 36:262–73. doi: 10.1007/s10447-013-9205-9
68. Wade NG, Hoyt WT, Kidwell JEM, Worthington EL. Efficacy of psychotherapeutic interventions to promote forgiveness: a meta-analysis. J Consult Clin Psychol. (2014) 82:154–70. doi: 10.1037/a0035268
69. Burnette JL, Davis DE, Green JD, Worthington EL, Bradfield E. Insecure attachment and depressive symptoms: The mediating role of rumination, empathy, and forgiveness. Pers Individ Differ. (2009) 46:276–80. doi: 10.1016/j.paid.2008.10.016
70. Derdaele E, Toussaint L, Thauvoye E, Dezutter J. Forgiveness and late life functioning: the mediating role of finding ego-integrity. Aging Ment Health. (2019) 23:238–45. doi: 10.1080/13607863.2017.1399346
71. Dezutter J, Toussaint L, Leijssen M. Forgiveness, ego-integrity, and depressive symptoms in community-dwelling and residential elderly adults. J Gerontol Ser B. (2016) 71:786–97. doi: 10.1093/geronb/gbu146
72. Long KNG, Worthington EL, VanderWeele TJ, Chen Y. Forgiveness of others and subsequent health and well-being in mid-life: a longitudinal study on female nurses. BMC Psychol. (2020) 8:104. doi: 10.1186/s40359-020-00470-w
73. Ripley JS, Worthington EL, Garthe RC, Davis DE, Hook JN, Reid CA, et al. Trait forgiveness and dyadic adjustment predict postnatal depression. J Child Fam Stud. (2018) 27:2185–92. doi: 10.1007/s10826-018-1053-0
74. Barcaccia B, Salvati M, Pallini S, Baiocco R, Curcio G, Mancini F, et al. Interpersonal forgiveness and adolescent depression. the mediational role of self-reassurance and self-criticism. J Child Fam Stud. (2020) 29:462–70. doi: 10.1007/s10826-019-01550-1
75. Ashkani S, Edalati Shateri Z, Birashk B. Lack of forgiveness as a predisposing factor to depression: Comparison of lack of forgiveness in depressed, vulnerable to depression and non-vulnerable to depression subjects. Int J Behav Sci. (2017) 10:162–6.
76. Barcaccia B, Pallini S, Pozza A, Milioni M, Baiocco R, Mancini F, et al. Forgiving adolescents: far from depression, close to well-being. Front Psychol. (2019) 10:1725. doi: 10.3389/fpsyg.2019.01725
77. Chung M-S. Relation between lack of forgiveness and depression: the moderating effect of self-compassion. Psychol Rep. (2016) 119:573–85. doi: 10.1177/0033294116663520
78. Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clin Psychol Rev. (2012) 32:704–23. doi: 10.1016/j.cpr.2012.09.004
79. Zhou L, Liu M, Ye B, Wang X, Liu Q. Sad expressions during encoding enhance facial identity recognition in visual working memory in depression: Behavioural and electrophysiological evidence. J Affect Disord. (2021) 279:630–9. doi: 10.1016/j.jad.2020.10.050
80. Weightman MJ, Air TM, Baune BT. A review of the role of social cognition in major depressive disorder. Front Psychiatry. (2014) 5:179. doi: 10.3389/fpsyt.2014.00179
81. Beevers CG, Wells TT, Ellis AJ, Fischer K. Identification of emotionally ambiguous interpersonal stimuli among dysphoric and nondysphoric individuals. Cognit Ther Res. (2009) 33:283–90. doi: 10.1007/s10608-008-9198-6
82. Neta M, Brock RL. Social connectedness and negative affect uniquely explain individual differences in response to emotional ambiguity. Sci Rep. (2021) 11:3870. doi: 10.1038/s41598-020-80471-2
83. Centeno RPR, Fernandez KTG. Effect of mindfulness on empathy and self-compassion: an adapted mbct program on filipino college students. Behav Sci. (2020) 10:61. doi: 10.3390/bs10030061
84. Fujino J, Yamasaki N, Miyata J, Kawada R, Sasaki H, Matsukawa N, et al. Altered brain response to others׳ pain in major depressive disorder. J Affect Disord. (2014) 165:170–5. doi: 10.1016/j.jad.2014.04.058
85. Schreiter S, Pijnenborg GHM, Aan Het Rot M. Empathy in adults with clinical or subclinical depressive symptoms. J Affect Disord. (2013) 150:1–16. doi: 10.1016/j.jad.2013.03.009
86. Yan Z, Zeng X, Su J, Zhang X. The dark side of empathy: Meta-analysis evidence of the relationship between empathy and depression. Psych J. (2021) 10:794–804. doi: 10.1002/pchj.482
87. Kupferberg A, Hager OM, Fischbacher U, Brändle LS, Haynes M, Hasler G. Testing the social competition hypothesis of depression using a simple economic game. Br J Psychiatry Open. (2016) 2:163–9. doi: 10.1192/bjpo.bp.115.001362
88. Kube T, Schwarting R, Rozenkrantz L, Glombiewski JA, Rief W. Distorted Cognitive Processes in Major Depression: A Predictive Processing Perspective. Biol Psychiatry. (2020) 87:388–98. doi: 10.1016/j.biopsych.2019.07.017
89. Ehnvall A, Mitchell PB, Hadzi-Pavlovic D, Parker G, Frankland A, Loo C, et al. Rejection sensitivity and pain in bipolar versus unipolar depression. Bipolar Disord. (2014) 16:190–8. doi: 10.1111/bdi.12147
90. Platt B, Cohen Kadosh K, Lau JYF. The role of peer rejection in adolescent depression. Depress Anxiety. (2013) 30:809–21. doi: 10.1002/da.22120
91. Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc Cognit Affect Neurosci. (2014) 9:1798–807. doi: 10.1093/scan/nst175
92. Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ, et al. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol Psychiatry. (2015) 20:193–200. doi: 10.1038/mp.2014.185
93. Hooley JM, Gruber SA, Scott LA, Hiller JB, Yurgelun-Todd DA. Activation in dorsolateral prefrontal cortex in response to maternal criticism and praise in recovered depressed and healthy control participants. Biol Psychiatry. (2005) 57:809–12. doi: 10.1016/j.biopsych.2005.01.012
94. Lee RM, Draper M, Lee S. Social connectedness, dysfunctional interpersonal behaviors, and psychological distress: Testing a mediator model. J Couns Psychol. (2001) 48:310–8. doi: 10.1037/0022-0167.48.3.310
95. Armstrong S, Oomen-Early J. Social connectedness, self-esteem, and depression symptomatology among collegiate athletes versus nonathletes. J Am Coll Health J ACH. (2009) 57:521–6. doi: 10.3200/JACH.57.5.521-526
96. Malaquias S, Crespo C, Francisco R. How do Adolescents Benefit from Family Rituals? Links to Social Connectedness, Depression and Anxiety. J Child Fam Stud. (2015) 24:3009–17. doi: 10.1007/s10826-014-0104-4
97. Fields SA, Schueler J, Arthur KM, Harris B. The Role of Impulsivity in Major Depression: A Systematic Review. Curr Behav Neurosci Rep. (2021) 8:38–50. doi: 10.1007/s40473-021-00231-y
98. Carver CS, Johnson SL, Joormann J. Major depressive disorder and impulsive reactivity to emotion: Toward a dual-process view of depression. Br J Clin Psychol. (2013) 52:285–99. doi: 10.1111/bjc.12014
99. Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cognit Affect Neurosci. (2009) 4:143–57. doi: 10.1093/scan/nsp007
100. Riva P. “Emotion regulation following social exclusion: Psychological and behavioral strategies.” In: Social exclusion: Psychological approaches to understanding and reducing its impact. Springer International Publishing, Cham, Switzerland (2016). p. 199–225. doi: 10.1007/978-3-319-33033-4_10
101. Tse WS, Bond AJ. The impact of depression on social skills: a review. J Nerv Ment Dis. (2004) 192:260–8. doi: 10.1097/01.nmd.0000120884.60002.2b
102. Davis AN, Carlo G, Schwartz SJ, Unger JB, Zamboanga BL, Lorenzo-Blanco EI, et al. The longitudinal associations between discrimination, depressive symptoms, and prosocial behaviors in U.S. latino/a recent immigrant adolescents. J Youth Adolesc. (2016) 45:457–70. doi: 10.1007/s10964-015-0394-x
103. Musick MA, Wilson J. Volunteering and depression: the role of psychological and social resources in different age groups. Soc Sci Med. (2003) 56:259–69. doi: 10.1016/S0277-9536(02)00025-4
104. Setterfield M, Walsh M, Frey A-L, McCabe C. Increased social anhedonia and reduced helping behaviour in young people with high depressive symptomatology. J Affect Disord. (2016) 205:372–7. doi: 10.1016/j.jad.2016.08.020
105. Destoop M, Schrijvers D, De Grave C, Sabbe B, De Bruijn ERA. Better to give than to take? Interactive social decision-making in severe major depressive disorder. J Affect Disord. (2012) 137:98–105. doi: 10.1016/j.jad.2011.12.010
106. Scheele D, Mihov Y, Schwederski O, Maier W, Hurlemann R. A negative emotional and economic judgment bias in major depression. Eur Arch Psychiatry Clin Neurosci. (2013) 263, 675–683. doi: 10.1007/s00406-013-0392-5
107. Radke S, Schäfer IC, Müller BW, de Bruijn ERA. Do different fairness contexts and facial emotions motivate ‘irrational’ social decision-making in major depression? An exploratory patient study. Psychiatry Res. (2013) 210:438–43. doi: 10.1016/j.psychres.2013.07.017
108. Pulcu E, Thomas EJ, Trotter PD, McFarquhar M, Juhasz G, Sahakian BJ, et al. Social-economical decision making in current and remitted major depression. Psychol Med. (2015) 45:1301–13. doi: 10.1017/S0033291714002414
109. Kupferberg A, Preuss N, Hasler G. Verwendung ökonomischer Spiele für die Diagnostik psychischer Erkrankungen. Z Für Psychiatr Psychol Psychother. (2017) 65:45–54. doi: 10.1024/1661-4747/a000300
110. Clark C, Thorne CB, Hardy S, Cropsey KL. Cooperation and depressive symptoms. J Affect Disord. (2013) 150:1184–7. doi: 10.1016/j.jad.2013.05.011
111. Sorgi KM, van ’t Wout M. The influence of cooperation and defection on social decision making in depression: A study of the iterated Prisoner’s Dilemma Game. Psychiatry Res. (2016) 246:512–9. doi: 10.1016/j.psychres.2016.10.025
112. Tyrer P, Nur U, Crawford M, Karlsen S, MacLean C, Rao B, et al. The social functioning questionnaire: a rapid and robust measure of perceived functioning. Int J Soc Psychiatry. (2005) 51:265–75. doi: 10.1177/0020764005057391
113. Lozupone M, Panza F, Piccininni M, Copetti M, Sardone R, Imbimbo BP, et al. Social dysfunction in older age and relationships with cognition, depression, and apathy: the greatage study. J Alzheimers Dis JAD. (2018) 65:989–1000. doi: 10.3233/JAD-180466
114. Heissel A, Bollmann J, Kangas M, Abdulla K, Rapp M, Sanchez A. Validation of the German version of the work and social adjustment scale in a sample of depressed patients. BMC Health Serv Res. (2021) 21:593. doi: 10.1186/s12913-021-06622-x
115. Bourke C, Douglas K, Porter R. Processing of facial emotion expression in major depression: a review. Aust N Z J Psychiatry. (2010) 44:681–96. doi: 10.3109/00048674.2010.496359
116. Kim J, Uddin ZA, Lee Y, Nasri F, Gill H, Subramanieapillai M, et al. A Systematic review of the validity of screening depression through Facebook, Twitter, Instagram, and Snapchat. J Affect Disord. (2021) 286:360–9. doi: 10.1016/j.jad.2020.08.091
117. Reece AG, Danforth CM. Instagram photos reveal predictive markers of depression. EPJ Data Sci. (2017) 6:1–12. doi: 10.1140/epjds/s13688-017-0110-z
118. Guntuku SC, Yaden DB, Kern ML, Ungar LH, Eichstaedt JC. Detecting depression and mental illness on social media: an integrative review. Curr Opin Behav Sci. (2017) 18:43–9. doi: 10.1016/j.cobeha.2017.07.005
119. Liu D, Feng XL, Ahmed F, Shahid M, Guo J. Detecting and measuring depression on social media using a machine learning approach: systematic review. JMIR Ment Health. (2022) 9:e27244. doi: 10.2196/27244
120. Owusu PN, Reininghaus U, Koppe G, Dankwa-Mullan I, Bärnighausen T. Artificial intelligence applications in social media for depression screening: A systematic review protocol for content validity processes. PLoS One. (2021) 16:e0259499. doi: 10.1371/journal.pone.0259499
121. Wang L, Liu H, Zhou T. A Sequential emotion approach for diagnosing mental disorder on social media. Appl Sci. (2020) 10:1647. doi: 10.3390/app10051647
122. Mustafa RU, Ashraf N, Ahmed FS, Ferzund J, Shahzad B, Gelbukh A. “A Multiclass Depression Detection in Social Media Based on Sentiment Analysis.” In: Latifi S, editor. 17th International Conference on Information Technology–New Generations (ITNG 2020). Springer International Publishing, Cham (2020). p. 659–62. doi: 10.1007/978-3-030-43020-7_89
123. Hussain J, Satti FA, Afzal M, Khan WA, Bilal HSM, Ansaar MZ, et al. Exploring the dominant features of social media for depression detection. J Inf Sci. (2020) 46:739–59. doi: 10.1177/0165551519860469
124. Silva MJD, Cooper S, Li HL, Lund C, Patel V. Effect of psychosocial interventions on social functioning in depression and schizophrenia: meta-analysis. Br J Psychiatry. (2013) 202:253–60. doi: 10.1192/bjp.bp.112.118018
125. Hengartner MP. How effective are antidepressants for depression over the long term? A critical review of relapse prevention trials and the issue of withdrawal confounding. Ther Adv Psychopharmacol. (2020) 10:2045125320921694. doi: 10.1177/2045125320921694
126. Goodwin GM, Price J, De Bodinat C, Laredo J. Emotional blunting with antidepressant treatments: A survey among depressed patients. J Affect Disord. (2017) 221:31–5. doi: 10.1016/j.jad.2017.05.048
127. Rosenblat JD, Simon GE, Sachs GS, Deetz I, Doederlein A, DePeralta D, et al. Treatment effectiveness and tolerability outcomes that are most important to individuals with bipolar and unipolar depression. J Affect Disord. (2019) 243:116–20. doi: 10.1016/j.jad.2018.09.027
128. Rütgen M, Pletti C, Tik M, Kraus C, Pfabigan DM, Sladky R, et al. Antidepressant treatment, not depression, leads to reductions in behavioral and neural responses to pain empathy. Transl Psychiatry. (2019) 9:1–13. doi: 10.1038/s41398-019-0496-4
129. Ferguson JM, Tourian KA, Rosas GR. High-dose desvenlafaxine in outpatients with major depressive disorder. CNS Spectr. (2012) 17:121–30. doi: 10.1017/S1092852912000508
130. McIntyre RS, Florea I, Pedersen MM, Christensen MC. Head-to-head comparison of vortioxetine versus desvenlafaxine in patients with major depressive disorder with partial response to ssri therapy: results of the vivre study. J Clin Psychiatry. (2023) 84:23m14780. doi: 10.4088/JCP.23m14780
131. Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ. Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. Am J Psychiatry. (2004) 161:166–8. doi: 10.1176/appi.ajp.161.1.166
132. Fu CHY, Williams SCR, Brammer MJ, Suckling J, Kim J, Cleare AJ, et al. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry. (2007) 164:599–607. doi: 10.1176/ajp.2007.164.4.599
133. Fu CHY, Costafreda SG, Sankar A, Adams TM, Rasenick MM, Liu P, et al. Multimodal functional and structural neuroimaging investigation of major depressive disorder following treatment with duloxetine. BMC Psychiatry. (2015) 15:82. doi: 10.1186/s12888-015-0457-2
134. Wang Y, Xu C, Cao X, Gao Q, Li J, Liu Z, et al. Effects of an antidepressant on neural correlates of emotional processing in patients with major depression. Neurosci Lett. (2012) 527:55–9. doi: 10.1016/j.neulet.2012.08.034
135. Hofmann SG, Wu JQ, Boettcher H. Effect of cognitive behavioral therapy for anxiety disorders on quality of life: a meta-analysis. J Consult Clin Psychol. (2014) 82:375–91. doi: 10.1037/a0035491
136. McKnight PE, Kashdan TB. The importance of functional impairment to mental health outcomes: a case for reassessing our goals in depression treatment research. Clin Psychol Rev. (2009) 29:243–59. doi: 10.1016/j.cpr.2009.01.005
137. Briley M, Moret C. Improvement of social adaptation in depression with serotonin and norepinephrine reuptake inhibitors. Neuropsychiatr Dis Treat. (2010) 6:647–55. doi: 10.2147/NDT.S13171
138. Denninger JW, van Nieuwenhuizen AO, Wisniewski SR, Luther JF, Trivedi MH, Rush AJ, et al. Changes in depressive symptoms and social functioning in the sequenced treatment alternatives to relieve depression study. J Nerv Ment Dis. (2011) 199:807–10. doi: 10.1097/NMD.0b013e31822fcbe2
139. Ishida T, Inada Y, Kudo M. Study on the clinical efficacy of milnacipran by using the Social Adaptation Self-evaluation Scale. Jpn J Clin Psychopharmacol. (2010) 13:77–83.
140. Ueda N, Yoshimura R, Houjo T. Effects of milnacipran on social adaptation in patients with depression. Rinshyo Seisin Yakuri. (2008) 11:273–9.
141. Karyotaki E, Smit Y, Holdt Henningsen K, Huibers MJH, Robays J, de Beurs D, et al. Combining pharmacotherapy and psychotherapy or monotherapy for major depression? A meta-analysis on the long-term effects. J Affect Disord. (2016) 194:144–52. doi: 10.1016/j.jad.2016.01.036
142. Franck L, Molyneux N, Parkinson L. Systematic review of interventions addressing social isolation and depression in aged care clients. Qual Life Res. (2016) 25:1395–407. doi: 10.1007/s11136-015-1197-y
143. Chiang K-J, Chu H, Chang H-J, Chung M-H, Chen C-H, Chiou H-Y, et al. The effects of reminiscence therapy on psychological well-being, depression, and loneliness among the institutionalized aged. Int J Geriatr Psychiatry. (2010) 25:380–8. doi: 10.1002/gps.2350
144. Renner F, Cuijpers P, Huibers MJH. The effect of psychotherapy for depression on improvements in social functioning: a meta-analysis. Psychol Med. (2014) 44:2913–26. doi: 10.1017/S0033291713003152
145. Spence SH, O’Shea G, Donovan CL. Improvements in interpersonal functioning following interpersonal psychotherapy (ipt) with adolescents and their association with change in depression. Behav Cognit Psychother. (2016) 44:257–72. doi: 10.1017/S1352465815000442
146. Chiang K-J, Tsai J-C, Liu D, Lin C-H, Chiu H-L, Chou K-R. Efficacy of cognitive-behavioral therapy in patients with bipolar disorder: A meta-analysis of randomized controlled trials. PLoS One. (2017) 12:e0176849. doi: 10.1371/journal.pone.0176849
147. Hirschfeld RMA, Dunner DL, Keitner G, Klein DN, Koran LM, Kornstein SG, et al. Does psychosocial functioning improve independent of depressive symptoms? a comparison of nefazodone, psychotherapy, and their combination. Biol Psychiatry. (2002) 51:123–33. doi: 10.1016/S0006-3223(01)01291-4
148. Scott J. Effects of cognitive therapy on psychological symptoms and social functioning in residual depression. Br J Psychiatry. (2000) 177:440–6. doi: 10.1192/bjp.177.5.440
149. Käll A, Jägholm S, Hesser H, Andersson F, Mathaldi A, Norkvist BT, et al. Internet-Based Cognitive Behavior Therapy for Loneliness: A Pilot Randomized Controlled Trial. Behav Ther. (2020) 51:54–68. doi: 10.1016/j.beth.2019.05.001
150. Matsunaga M, Okamoto Y, Suzuki S, Kinoshita A, Yoshimura S, Yoshino A, et al. Psychosocial functioning in patients with treatment-resistant depression after group cognitive behavioral therapy. BMC Psychiatry. (2010) 10:22. doi: 10.1186/1471-244X-10-22
151. Zhou X, Rau P-LP, Yang C-L, Zhou X. Cognitive behavioral therapy-based short-term abstinence intervention for problematic social media use: improved well-being and underlying mechanisms. Psychiatr Q. (2021) 92:761–79. doi: 10.1007/s11126-020-09852-0
152. McCullough JP, Schramm E, Penberthy JK. CBASP as a distinctive treatment for persistent depressive disorder. New York, NY, US: Routledge/Taylor & Francis Group (2015). xviii, 152 p.
153. Serbanescu I, Backenstrass M, Drost S, Weber B, Walter H, Klein JP, et al. Impact of Baseline Characteristics on the Effectiveness of Disorder-Specific Cognitive Behavioral Analysis System of Psychotherapy (CBASP) and Supportive Psychotherapy in Outpatient Treatment for Persistent Depressive Disorder. Front Psychiatry. (2020) 11. https://www.frontiersin.org/article/10.3389/fpsyt.2020.607300.
154. Ezawa ID, Plate AJ, Strunk DR. What do people really think of me? evaluating bias in interpersonal predictions over the course of cognitive-behavioral therapy of depression. Behav Ther. (2021) 52:1286–95. doi: 10.1016/j.beth.2021.02.007
155. Steinert C, Hofmann M, Kruse J, Leichsenring F. Relapse rates after psychotherapy for depression – stable long-term effects? A meta-analysis. J Affect Disord. (2014) 168:107–18. doi: 10.1016/j.jad.2014.06.043
156. Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. (2007) 68:17–25.
157. Zhdanava M, Pilon D, Ghelerter I, Chow W, Joshi K, Lefebvre P, et al. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. (2021) 82:20m13699. doi: 10.4088/JCP.20m13699
158. Griffiths C, Walker K, Reid I, da Silva KM, O’Neill-Kerr A. A qualitative study of patients’ experience of ketamine treatment for depression: The ‘Ketamine and me’ project. J Affect Disord Rep. (2021) 4:100079. doi: 10.1016/j.jadr.2021.100079
159. Miskowiak KW, Kessing LV, Ott CV, Macoveanu J, Harmer CJ, Jørgensen A, et al. Does a single session of electroconvulsive therapy alter the neural response to emotional faces in depression? A randomised sham-controlled functional magnetic resonance imaging study. J Psychopharmacol (Oxf). (2017) 31:1215–24. doi: 10.1177/0269881117699615
160. van Buel EM, Sigrist H, Seifritz E, Fikse L, Bosker FJ, Schoevers RA, et al. Mouse repeated electroconvulsive seizure (ECS) does not reverse social stress effects but does induce behavioral and hippocampal changes relevant to electroconvulsive therapy (ECT) side-effects in the treatment of depression. PLoS One. (2017) 12:e0184603. doi: 10.1371/journal.pone.0184603
161. Bai T, Xie W, Wei Q, Chen Y, Mu J, Tian Y, et al. Electroconvulsive therapy regulates emotional memory bias of depressed patients. Psychiatry Res. (2017) 257:296–302. doi: 10.1016/j.psychres.2017.07.069
162. Pirmoradi M, Dolatshahi B, Rostami R, Mohammadkhani P, Dadkhah A. The changes of social performance with transcranial magnetic stimulation (TMS) in depressed patients. Eur Psychiatry. (2017) 41:S375–6. doi: 10.1016/j.eurpsy.2017.02.396
163. Lantrip C, Gunning FM, Flashman L, Roth RM, Holtzheimer PE. Effects of Transcranial Magnetic Stimulation on the Cognitive Control of Emotion: Potential Antidepressant Mechanisms. J ECT. (2017) 33:73–80. doi: 10.1097/YCT.0000000000000386
164. Licheng MO, Tianyou GUO, Yueyao Z, Feng XU, Dandan Z. The role of ventrolateral prefrontal cortex on emotional regulation of social pain in depressed patients: A TMS study. Acta Psychol Sin. (2021) 53:494. doi: 10.3724/SP.J.1041.2021.00494
165. He Z, Liu Z, Zhao J, Elliott R, Zhang D. Improving emotion regulation of social exclusion in depression-prone individuals: a tDCS study targeting right VLPFC. Psychol Med. (2020) 50:2768–79. doi: 10.1017/S0033291719002915
166. Peña-Gómez C, Vidal-Piñeiro D, Clemente IC, Pascual-Leone Á, Bartrés-Faz D. Down-Regulation of Negative Emotional Processing by Transcranial Direct Current Stimulation: Effects of Personality Characteristics. PLoS One. (2011) 6:e22812. doi: 10.1371/journal.pone.0022812
167. Nejati V, Majidinezhad M, Nitsche M. The role of the dorsolateral and ventromedial prefrontal cortex in emotion regulation in females with major depressive disorder (MDD): A tDCS study. J Psychiatr Res. (2022) 148:149–58. doi: 10.1016/j.jpsychires.2022.01.030
168. Chrysikou EG, Wing EK, van Dam WO. Transcranial direct current stimulation over the prefrontal cortex in depression modulates cortical excitability in emotion regulation regions as measured by concurrent functional magnetic resonance imaging: an exploratory study. Biol Psychiatry Cognit Neurosci Neuroimaging. (2022) 7:85–94. doi: 10.1016/j.bpsc.2019.12.004
169. Riva P, Romero Lauro LJ, DeWall CN, Chester DS, Bushman BJ. Reducing aggressive responses to social exclusion using transcranial direct current stimulation. Soc Cognit Affect Neurosci. (2015) 10:352–6. doi: 10.1093/scan/nsu053
170. Di Bello M, Giudetti F, Palani S, Petrocchi N, McIntosh R, Ottaviani C. Modulatory effects of transcranial direct current stimulation of right insula on compassion motivation. Int J Clin Health Psychol. (2023) 23:100362. doi: 10.1016/j.ijchp.2022.100362
171. Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front Psychiatry. (2018) 9:44. doi: 10.3389/fpsyt.2018.00044
172. Buchmann A, Ritter C, Müller ST, Haynes M, Ghisleni C, Tuura R, et al. Associations between heart rate variability, peripheral inflammatory markers and major depressive disorder. J Affect Disord. (2022) 304:93–101. doi: 10.1016/j.jad.2022.02.017
173. Hamer HM, Bauer S. Lessons learned from transcutaneous vagus nerve stimulation (tVNS). Epilepsy Res. (2019) 153:83–4. doi: 10.1016/j.eplepsyres.2019.02.015
174. Maraver MJ, Steenbergen L, Hossein R, Actis-Grosso R, Ricciardelli P, Hommel B, et al. Transcutaneous vagus nerve stimulation modulates attentional resource deployment towards social cues. Neuropsychologia. (2020) 143:107465. doi: 10.1016/j.neuropsychologia.2020.107465
175. Sellaro R, de Gelder B, Finisguerra A, Colzato LS. Transcutaneous vagus nerve stimulation (tVNS) enhances recognition of emotions in faces but not bodies. Cortex. (2018) 99:213–23. doi: 10.1016/j.cortex.2017.11.007
176. Zhu S, Zhang X, Qing Y, Zhang Y, Yao S, Kendrick K, et al. Transcutaneous auricular vagus nerve stimulation increases eye-gaze to salient facial features and oxytocin release. Brain Stimul Basic Transl Clin Res Neuromodulation. (2021) 14:1639. doi: 10.1016/j.brs.2021.10.165
177. Colzato LS, Sellaro R, Beste C. Darwin revisited: The vagus nerve is a causal element in controlling recognition of other’s emotions. Cortex. (2017) 92:95–102. doi: 10.1016/j.cortex.2017.03.017
178. Johnson KV-A, Steenbergen L. Gut Feelings: Vagal Stimulation Reduces Emotional Biases. Neuroscience. (2022) 494:119–31. doi: 10.1016/j.neuroscience.2022.04.026
179. Koenig J, Parzer P, Haigis N, Liebemann J, Jung T, Resch F, et al. Effects of acute transcutaneous vagus nerve stimulation on emotion recognition in adolescent depression. Psychol Med. (2021) 51:511–20. doi: 10.1017/S0033291719003490
180. Quintana DS, Lischke A, Grace S, Scheele D, Ma Y, Becker B. Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research. Mol Psychiatry. (2021) 26:80–91. doi: 10.1038/s41380-020-00864-7
181. Wang T, Tang Q, Wu X, Chen X. Attachment anxiety moderates the effect of oxytocin on negative emotion recognition: Evidence from eye-movement data. Pharmacol Biochem Behav. (2020) 198:173015. doi: 10.1016/j.pbb.2020.173015
182. Bernaerts S, Berra E, Wenderoth N, Alaerts K. Influence of oxytocin on emotion recognition from body language: A randomized placebo-controlled trial. Psychoneuroendocrinology. (2016) 72:182–9. doi: 10.1016/j.psyneuen.2016.07.002
183. Boyle A, Johnson A, Ellenbogen M. Intranasal Oxytocin Alters Attention to Emotional Facial Expressions, Particularly for Males and Those with Depressive Symptoms. Psychoneuroendocrinology. (2022) 142, 105796. doi: 10.1016/j.psyneuen.2022.105796
184. Lindley Baron-Cohen K, Feldman R, Fearon P, Fonagy P. Intranasal oxytocin administration improves mood in new mothers with moderate low mood but not in mothers with elevated symptoms of postnatal depression: A randomised controlled trial. J Affect Disord. (2022) 300:358–65. doi: 10.1016/j.jad.2021.11.062
185. Donadon MF, Martin-Santos R L, Osório F. Oxytocin effects on the cognition of women with postpartum depression: A randomized, placebo-controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 111:110098. doi: 10.1016/j.pnpbp.2020.110098
186. Mah BL, Van Ijzendoorn MH, Out D, Smith R, Bakermans-Kranenburg MJ. The Effects of Intranasal Oxytocin Administration on Sensitive Caregiving in Mothers with Postnatal Depression. Child Psychiatry Hum Dev. (2017) 48:308–15. doi: 10.1007/s10578-016-0642-7
187. Mah BL, Bakermans-Kranenburg MJ, Van IJzendoorn MH, Smith R. Oxytocin promotes protective behavior in depressed mothers: a pilot study with the enthusiastic stranger paradigm. Depress Anxiety. (2015) 32:76–81. doi: 10.1002/da.22245
188. McIntyre RS, Rodrigues NB, Lee Y, Lipsitz O, Subramaniapillai M, Gill H, et al. The effectiveness of repeated intravenous ketamine on depressive symptoms, suicidal ideation and functional disability in adults with major depressive disorder and bipolar disorder: Results from the Canadian Rapid Treatment Center of Excellence. J Affect Disord. (2020) 274:903–10. doi: 10.1016/j.jad.2020.05.088
189. Kowalczyk M, Kowalczyk E, Kwiatkowski P, Łopusiewicz Ł, Sienkiewicz M, Talarowska M. Ketamine—New Possibilities in the Treatment of Depression: A Narrative Review. Life. (2021) 11:1186. doi: 10.3390/life11111186
190. Kryst J, Kawalec P, Pilc A. Efficacy and safety of intranasal esketamine for the treatment of major depressive disorder. Expert Opin Pharmacother. (2020) 21:9–20. doi: 10.1080/14656566.2019.1683161
191. Hasler G. Toward specific ways to combine ketamine and psychotherapy in treating depression. CNS Spectr. (2020) 25:445–7. doi: 10.1017/S1092852919001007
192. Jawad MY, Di Vincenzo JD, Badulescu S, Teopiz KM, Tabassum A, Ceban F, et al. The therapeutic role of ketamine and esketamine in treating psychopathological domains of depression. Neuropharmacology. (2023) 223:109299. doi: 10.1016/j.neuropharm.2022.109299
193. Kadriu B, Greenwald M, Henter ID, Gilbert JR, Kraus C, Park LT, et al. Ketamine and Serotonergic Psychedelics: Common Mechanisms Underlying the Effects of Rapid-Acting Antidepressants. Int J Neuropsychopharmacol. (2021) 24:8–21. doi: 10.1093/ijnp/pyaa087
194. Riggs LM, Gould TD. Ketamine and the Future of Rapid-Acting Antidepressants. Annu Rev Clin Psychol. (2021) 17:207–31. doi: 10.1146/annurev-clinpsy-072120-014126
195. Vidal S, Jermann F, Aubry J-M, Richard-Lepouriel H, Kosel M. Effect of Ketamine on Rumination in Treatment-Resistant Depressive Patients. J Clin Psychopharmacol. (2020) 40:607–10. doi: 10.1097/JCP.0000000000001305
196. Hasler G, Suker S, Schoretsanitis G, Mihov Y. Sustained improvement of negative self-schema after a single ketamine infusion: an open-label study. Front Neurosci. (2020) 14. https://www.frontiersin.org/article/10.3389/fnins.2020.00687.
197. Murrough JW, Collins KA, Fields J, DeWilde KE, Phillips ML, Mathew SJ, et al. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry. (2015) 5:e509–9. doi: 10.1038/tp.2015.10
198. Sumner RL, Chacko E, McMillan R, Spriggs MJ, Anderson C, Chen J, et al. A qualitative and quantitative account of patient’s experiences of ketamine and its antidepressant properties. J Psychopharmacol (Oxf). (2021) 35:946–61. doi: 10.1177/0269881121998321
199. Lehmann M, Seifritz E, Henning A, Walter M, Böker H, Scheidegger M, et al. Differential effects of rumination and distraction on ketamine induced modulation of resting state functional connectivity and reactivity of regions within the default-mode network. Soc Cognit Affect Neurosci. (2016) 11:1227–35. doi: 10.1093/scan/nsw034
200. Reed JL, Nugent AC, Furey ML, Szczepanik JE, Evans JW, Zarate CA. Effects of ketamine on brain activity during emotional processing: differential findings in depressed versus healthy control participants. Biol Psychiatry Cognit Neurosci Neuroimaging. (2019) 4:610–8. doi: 10.1016/j.bpsc.2019.01.005
201. Vasavada MM, Loureiro J, Kubicki A, Sahib A, Wade B, Hellemann G, et al. Effects of serial ketamine infusions on corticolimbic functional connectivity in major depression. Biol Psychiatry Cognit Neurosci Neuroimaging. (2021) 6:735–44. doi: 10.1016/j.bpsc.2020.06.015
202. Wilkowska A, Wiglusz MS, Gałuszko-Wegielnik M, Włodarczyk A, Cubała WJ. Antianhedonic effect of repeated ketamine infusions in patients with treatment resistant depression. Front Psychiatry. (2021) 12. https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2021.704330.
203. Wei Y, Chang L, Hashimoto K. Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psychiatry. (2022) 27:559–73. doi: 10.1038/s41380-021-01121-1
204. Réus GZ, Nacif MP, Abelaira HM, Tomaz DB, dos Santos MAB, Carlessi AS, et al. Ketamine treatment partly reverses alterations in brain derived- neurotrophic factor, oxidative stress and energy metabolism parameters induced by an animal model of depression. Curr Neurovasc Res. (2015) 12:73–84. doi: 10.2174/1567202612666150122122924
205. Aleksandrova LR, Phillips AG. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci. (2021) 42:929–42. doi: 10.1016/j.tips.2021.08.003
206. Salahudeen MS, Wright CM, Peterson GM. Esketamine: new hope for the treatment of treatment-resistant depression? A narrative review. Ther Adv Drug Saf. (2020) 11. doi: 10.1177/2042098620937899
207. Klein ME, Chandra J, Sheriff S, Malinow R. Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc Natl Acad Sci. (2020) 117:2656–62. doi: 10.1073/pnas.1916570117
208. Meier IM, van Honk J, Bos PA, Terburg D. A mu-opioid feedback model of human social behavior. Neurosci Biobehav Rev. (2021) 121:250–8. doi: 10.1016/j.neubiorev.2020.12.013
209. Tiger M, Veldman ER, Ekman C-J, Halldin C, Svenningsson P, Lundberg J. A randomized placebo-controlled PET study of ketamine´s effect on serotonin1B receptor binding in patients with SSRI-resistant depression. Transl Psychiatry. (2020) 10:1–8. doi: 10.1038/s41398-020-0844-4
210. Aleksandrova LR, Phillips AG, Wang YT. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J Psychiatry Neurosci JPN. (2017) 42:222–9. doi: 10.1503/jpn.160175
211. Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian network for mood and anxiety treatments (canmat) 2016 clinical guidelines for the management of adults with major depressive disorder. Can J Psychiatry Rev Can Psychiatr. (2016) 61:540–60. doi: 10.1177/0706743716659417
212. Yavi M, Henter ID, Park LT, Zarate C. Key considerations in the pharmacological management of treatment-resistant depression. Expert Opin Pharmacother. (2021) 22:2405–15. doi: 10.1080/14656566.2021.1951225
213. Aguilar-Raab C, Jarczok MN, Warth M, Stoffel M, Winter F, Tieck M, et al. Enhancing Social Interaction in Depression (SIDE study): protocol of a randomised controlled trial on the effects of a Cognitively Based Compassion Training (CBCT) for couples. BMJ Open. (2018) 8:e020448. doi: 10.1136/bmjopen-2017-020448
214. Knight MJ, Baune BT. Psychosocial Dysfunction in Major Depressive Disorder—Rationale, Design, and Characteristics of the Cognitive and Emotional Recovery Training Program for Depression (CERT-D). Front Psychiatry. (2017) 8:280. doi: 10.3389/fpsyt.2017.00280
215. Durães RSS, Khafif TC, Lotufo-Neto F, Serafim A de P. Effectiveness of cognitive behavioral couple therapy on reducing depression and anxiety symptoms and increasing dyadic adjustment and marital social skills: an exploratory study. Fam J. (2020) 28:344–55. doi: 10.1177/1066480720902410
216. Berking M, Ebert D, Cuijpers P, Hofmann SG. Emotion regulation skills training enhances the efficacy of inpatient cognitive behavioral therapy for major depressive disorder: a randomized controlled trial. Psychother Psychosom. (2013) 82:234–45. doi: 10.1159/000348448
217. Berking M, Wupperman P, Reichardt A, Pejic T, Dippel A, Znoj H. Emotion-regulation skills as a treatment target in psychotherapy. Behav Res Ther. (2008) 46:1230–7. doi: 10.1016/j.brat.2008.08.005
218. Jahangard L, Haghighi M, Bajoghli H, Ahmadpanah M, Ghaleiha A, Zarrabian MK, et al. Training emotional intelligence improves both emotional intelligence and depressive symptoms in inpatients with borderline personality disorder and depression. Int J Psychiatry Clin Pract. (2012) 16:197–204. doi: 10.3109/13651501.2012.687454
219. Muttoni S, Ardissino M, John C. Classical psychedelics for the treatment of depression and anxiety: A systematic review. J Affect Disord. (2019) 258:11–24. doi: 10.1016/j.jad.2019.07.076
220. Van Gordon W, Shonin E, Griffiths MD, Singh NN. There is Only One Mindfulness: Why Science and Buddhism Need to Work Together. Mindfulness. (2015) 6:49–56. doi: 10.1007/s12671-014-0379-y
221. Heuschkel K, Kuypers KPC. Depression, mindfulness, and psilocybin: possible complementary effects of mindfulness meditation and psilocybin in the treatment of depression. A Review. Front Psychiatry. (2020) 11:224. doi: 10.3389/fpsyt.2020.00224
222. Segal ZV, Williams M, Teasdale J. Mindfulness-Based Cognitive Therapy for Depression, Second Edition. New York: Guilford Publications (2018). 473 p.
223. Bieling PJ, Hawley LL, Bloch RT, Corcoran KM, Levitan RD, Young LT, et al. Treatment-specific changes in decentering following mindfulness-based cognitive therapy versus antidepressant medication or placebo for prevention of depressive relapse. J Consult Clin Psychol. (2012) 80:365–72. doi: 10.1037/a0027483
224. Hoge EA, Bui E, Goetter E, Robinaugh DJ, Ojserkis RA, Fresco DM, et al. Change in decentering mediates improvement in anxiety in mindfulness-based stress reduction for generalized anxiety disorder. Cognit Ther Res. (2015) 39:228–35. doi: 10.1007/s10608-014-9646-4
225. Britton WB, Shahar B, Szepsenwol O, Jacobs WJ. Mindfulness-based cognitive therapy improves emotional reactivity to social stress: results from a randomized controlled trial. Behav Ther. (2012) 43:365–80. doi: 10.1016/j.beth.2011.08.006
226. Kirschner H, Kuyken W, Karl A. A biobehavioural approach to understand how mindfulness-based cognitive therapy reduces dispositional negative self-bias in recurrent depression. Mindfulness. (2022) 13:928–41. doi: 10.1007/s12671-022-01845-3
227. Garland EL, Hanley A, Farb NA, Froeliger B. State mindfulness during meditation predicts enhanced cognitive reappraisal. Mindfulness. (2015) 6:234–42. doi: 10.1007/s12671-013-0250-6
228. Garland E, Gaylord S, Park J. The role of mindfulness in positive reappraisal. Explore N Y N. (2009) 5:37–44. doi: 10.1016/j.explore.2008.10.001
229. Golden HL, Vosper J, Kingston J, Ellett L. The impact of mindfulness-based programmes on self-compassion in nonclinical populations: a systematic review and meta-analysis. Mindfulness. (2021) 12:29–52. doi: 10.1007/s12671-020-01501-8
230. Condon P, Desbordes G, Miller WB, DeSteno D. Meditation increases compassionate responses to suffering. Psychol Sci. (2013) 24:2125–7. doi: 10.1177/0956797613485603
231. Williams K, Elliott R, Barnhofer T, Zahn R, Anderson IM. Positive shifts in emotion evaluation following mindfulness-based cognitive therapy (mbct) in remitted depressed participants. Mindfulness. (2021) 12:623–35. doi: 10.1007/s12671-020-01521-4
232. Gorgi Y, Tabaeian SM, Shokrolahi M. The effectiveness of mindfulness-based cognitive therapy on self-concept, self-acceptance and self-criticism in women with substance use disorders. Sci Q Res Addict. (2021) 14:73–88. doi: 10.29252/etiadpajohi.14.58.73
233. Foroughi A, Sadeghi K, Parvizifard A, Parsa Moghadam A, Davarinejad O, Farnia V, et al. The effectiveness of mindfulness-based cognitive therapy for reducing rumination and improving mindfulness and self-compassion in patients with treatment-resistant depression. Trends Psychiatry Psychother. (2020) 42:138–46. doi: 10.1590/2237-6089-2019-0016
234. Perestelo-Perez L, Barraca J, Peñate W, Rivero-Santana A, Alvarez-Perez Y. Mindfulness-based interventions for the treatment of depressive rumination: Systematic review and meta-analysis. Int J Clin Health Psychol IJCHP. (2017) 17:282–95. doi: 10.1016/j.ijchp.2017.07.004
235. Proeve M, Anton R, Kenny M. Effects of mindfulness-based cognitive therapy on shame, self-compassion and psychological distress in anxious and depressed patients: A pilot study. Psychol Psychother Theory Res Pract. (2018) 91:434–49. doi: 10.1111/papt.12170
236. Franco C, Amutio A, López-González L, Oriol X, Martínez-Taboada C. Effect of a Mindfulness Training Program on the Impulsivity and Aggression Levels of Adolescents with Behavioral Problems in the Classroom. Front Psychol. (2016) 7. https://www.frontiersin.org/articles/10.3389/fpsyg.2016.01385.
237. Fix RL, Fix ST. The effects of mindfulness-based treatments for aggression: A critical review. Aggress Violent Behav. (2013) 18:219–27. doi: 10.1016/j.avb.2012.11.009
238. Lee JK, Orsillo SM. Investigating cognitive flexibility as a potential mechanism of mindfulness in Generalized Anxiety Disorder. J Behav Ther Exp Psychiatry. (2014) 45:208–16. doi: 10.1016/j.jbtep.2013.10.008
239. Moore A, Malinowski P. Meditation, mindfulness and cognitive flexibility. Conscious Cognit. (2009) 18:176–86. doi: 10.1016/j.concog.2008.12.008
240. Zou Y, Li P, Hofmann SG, Liu X. The Mediating Role of Non-reactivity to Mindfulness Training and Cognitive Flexibility: A Randomized Controlled Trial. Front Psychol. (2020) 11. https://www.frontiersin.org/articles/10.3389/fpsyg.2020.01053.
241. Shapiro SL, Schwartz GE, Bonner G. Effects of mindfulness-based stress reduction on medical and premedical students. J Behav Med. (1998) 21:581–99. doi: 10.1023/a:1018700829825
242. Cheang R, Gillions A, Sparkes E. Do Mindfulness-Based Interventions Increase Empathy and Compassion in Children and Adolescents: A Systematic Review. J Child Fam Stud. (2019) 28:1765–79. doi: 10.1007/s10826-019-01413-9
243. Kirk U, Gu X, Sharp C, Hula A, Fonagy P, Montague PR. Mindfulness training increases cooperative decision making in economic exchanges: Evidence from fMRI. NeuroImage. (2016) 138:274–83. doi: 10.1016/j.neuroimage.2016.05.075
244. Lim D, Condon P, DeSteno D. Mindfulness and compassion: an examination of mechanism and scalability. PLoS One. (2015) 10:e0118221. doi: 10.1371/journal.pone.0118221
245. Berry DR, Cairo AH, Goodman RJ, Quaglia JT, Green JD, Brown KW. Mindfulness increases prosocial responses toward ostracized strangers through empathic concern. J Exp Psychol Gen. (2018) 147:93–112. doi: 10.1037/xge0000392
246. Hafenbrack AC, Cameron LD, Spreitzer GM, Zhang C, Noval LJ, Shaffakat S. Helping people by being in the present: mindfulness increases prosocial behavior. Organ Behav Hum Decis Process. (2020) 159:21–38. doi: 10.1016/j.obhdp.2019.08.005
247. Garcia-Romeu A, Richards WA. Current perspectives on psychedelic therapy: use of serotonergic hallucinogens in clinical interventions. Int Rev Psychiatry Abingdon Engl. (2018) 30:291–316. doi: 10.1080/09540261.2018.1486289
248. Nutt D, Carhart-Harris R. The Current Status of Psychedelics in Psychiatry. JAMA Psychiatry. (2021), 121–122. doi: 10.1001/jamapsychiatry.2020.2171
249. Griffiths RR, Johnson MW, Richards WA, Richards BD, Jesse R, MacLean KA, et al. Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J Psychopharmacol Oxf Engl. (2018) 32:49–69. doi: 10.1177/0269881117731279
250. Kiraga MK, Mason NL, Uthaug MV, van Oorsouw KIM, Toennes SW, Ramaekers JG, et al. Persisting effects of ayahuasca on empathy, creative thinking, decentering, personality, and well-being. Front Pharmacol. (2021) 12. https://www.frontiersin.org/articles/10.3389/fphar.2021.721537.
251. Yehuda R, Lehrner A. Psychedelic therapy-a new paradigm of care for mental health. JAMA. (2023) 330:813–4. doi: 10.1001/jama.2023.12900
252. Nour MM, Evans L, Nutt D, Carhart-Harris RL. Ego-dissolution and psychedelics: validation of the ego-dissolution inventory (EDI). Front Hum Neurosci. (2016) 10:269. doi: 10.3389/fnhum.2016.00269
253. Lyubomirsky S. Toward a new science of psychedelic social psychology: the effects of mdma (ecstasy) on social connection. Perspect Psychol Sci. (2022) 17:1234–57. doi: 10.1177/17456916211055369
254. Watts R, Day C, Krzanowski J, Nutt D, Carhart-Harris R. Patients’ accounts of increased “connectedness” and “acceptance” after psilocybin for treatment-resistant depression. J Humanist Psychol. (2017) 57:520–64. doi: 10.1177/0022167817709585
255. Murphy-Beiner A, Soar K. Ayahuasca’s ‘afterglow’: improved mindfulness and cognitive flexibility in ayahuasca drinkers. Psychopharmacol (Berl). (2020) 237:1161–9. doi: 10.1007/s00213-019-05445-3
256. Soler J, Elices M, Franquesa A, Barker S, Friedlander P, Feilding A, et al. Exploring the therapeutic potential of Ayahuasca: acute intake increases mindfulness-related capacities. Psychopharmacol (Berl). (2016) 233:823–9. doi: 10.1007/s00213-015-4162-0
257. González D, Cantillo J, Pérez I, Farré M, Feilding A, Obiols JE, et al. Therapeutic potential of ayahuasca in grief: a prospective, observational study. Psychopharmacol (Berl). (2020) 237:1171–82. doi: 10.1007/s00213-019-05446-2
258. Sampedro F, de la Fuente Revenga M, Valle M, Roberto N, Domínguez-Clavé E, Elices M, et al. Assessing the psychedelic “after-glow” in ayahuasca users: post-acute neurometabolic and functional connectivity changes are associated with enhanced mindfulness capacities. Int J Neuropsychopharmacol. (2017) 20:698–711. doi: 10.1093/ijnp/pyx036
259. Kross E, Ayduk O, Mischel W. When asking “why” does not hurt. Distinguishing rumination from reflective processing of negative emotions. Psychol Sci. (2005) 16:709–15. doi: 10.1111/j.1467-9280.2005.01600.x
260. Kross E, Ayduk O. Facilitating adaptive emotional analysis: distinguishing distanced-analysis of depressive experiences from immersed-analysis and distraction. Pers Soc Psychol Bull. (2008) 34:924–38. doi: 10.1177/0146167208315938
261. Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacol (Berl). (2009) 207:73–83. doi: 10.1007/s00213-009-1635-z
262. Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacol (Berl). (2012) 222:293–302. doi: 10.1007/s00213-012-2645-9
263. Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H. Effects of MDMA and Intranasal oxytocin on social and emotional processing. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. (2014) 39:1654–63. doi: 10.1038/npp.2014.12
264. Wardle MC, de Wit H. MDMA alters emotional processing and facilitates positive social interaction. Psychopharmacol (Berl). (2014) 231:4219–29. doi: 10.1007/s00213-014-3570-x
265. Close J, Haijen E, Watts R, Roseman L, Carhart-Harris R. Psychedelics and psychological flexibility – Results of a prospective web-survey using the Acceptance in Action Questionnaire II. J Context Behav Sci. (2020) 16, 37–44. doi: 10.1016/j.jcbs.2020.01.005
266. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Context Behav Sci. (2020) 15:39–45. doi: 10.1016/j.jcbs.2019.11.004
267. Doss MK, Považan M, Rosenberg MD, Sepeda ND, Davis AK, Finan PH, et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl Psychiatry. (2021) 11:1–10. doi: 10.1038/s41398-021-01706-y
268. Kuypers KPC. Out of the box: A psychedelic model to study the creative mind. Med Hypotheses. (2018) 115:13–6. doi: 10.1016/j.mehy.2018.03.010
269. Kuypers KPC, Riba J, de la Fuente Revenga M, Barker S, Theunissen EL, Ramaekers JG. Ayahuasca enhances creative divergent thinking while decreasing conventional convergent thinking. Psychopharmacol (Berl). (2016) 233:3395–403. doi: 10.1007/s00213-016-4377-8
270. Mason NL, Mischler E, Uthaug MV, Kuypers KPC. Sub-acute effects of psilocybin on empathy, creative thinking, and subjective well-being. J Psychoactive Drugs. (2019) 51:123–34. doi: 10.1080/02791072.2019.1580804
271. Uthaug MV, van Oorsouw K, Kuypers KPC, van Boxtel M, Broers NJ, Mason NL, et al. Sub-acute and long-term effects of ayahuasca on affect and cognitive thinking style and their association with ego dissolution. Psychopharmacol (Berl). (2018) 235:2979–89. doi: 10.1007/s00213-018-4988-3
272. Dolder PC, Schmid Y, Müller F, Borgwardt S, Liechti ME. LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. (2016) 41:2638–46. doi: 10.1038/npp.2016.82
273. Pokorny T, Preller KH, Kometer M, Dziobek I, Vollenweider FX. Effect of Psilocybin on Empathy and Moral Decision-Making. Int J Neuropsychopharmacol. (2017) 20:747–57. doi: 10.1093/ijnp/pyx047
274. Preller KH, Vollenweider FX. “Phenomenology, Structure, and Dynamic of Psychedelic States.” In: Halberstadt AL, Vollenweider FX, Nichols DE, editors. Behavioral Neurobiology of Psychedelic Drugs. Current Topics in Behavioral Neurosciences. Springer, Berlin, Heidelberg (2018). p. 221–56. doi: 10.1007/7854_2016_459
275. Schmid Y, Hysek CM, Simmler LD, Crockett MJ, Quednow BB, Liechti ME. Differential effects of MDMA and methylphenidate on social cognition. J Psychopharmacol (Oxf). (2014) 28:847–56. doi: 10.1177/0269881114542454
276. Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cognit Affect Neurosci. (2014) 9:1645–52. doi: 10.1093/scan/nst161
277. Kirkpatrick M, Delton AW, Robertson TE, de Wit H. Prosocial effects of MDMA: A measure of generosity. J Psychopharmacol Oxf Engl. (2015) 29:661–8. doi: 10.1177/0269881115573806
278. Schmid Y, Liechti ME. Long-lasting subjective effects of LSD in normal subjects. Psychopharmacol (Berl). (2018) 235:535–45. doi: 10.1007/s00213-017-4733-3
279. Vaid G, Walker B. Psychedelic Psychotherapy: Building Wholeness Through Connection. Glob Adv Health Med. (2022) 11:2164957X221081113. doi: 10.1177/2164957X221081113
280. Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. (2022) 28:844–51. doi: 10.1038/s41591-022-01744-z
281. Carhart-Harris RL, Roseman L, Bolstridge M, Demetriou L, Pannekoek JN, Wall MB, et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep. (2017) 7:13187. doi: 10.1038/s41598-017-13282-7
282. Stauffer CS, Anderson BT, Ortigo KM, Woolley J. Psilocybin-Assisted Group Therapy and Attachment: Observed Reduction in Attachment Anxiety and Influences of Attachment Insecurity on the Psilocybin Experience. ACS Pharmacol Transl Sci. (2021) 4:526–32. doi: 10.1021/acsptsci.0c00169
283. Gabay AS, Carhart-Harris RL, Mazibuko N, Kempton MJ, Morrison PD, Nutt DJ, et al. Psilocybin and MDMA reduce costly punishment in the Ultimatum Game. Sci Rep. (2018) 8:1–9. doi: 10.1038/s41598-018-26656-2
284. Gabay AS, Kempton MJ, Gilleen J, Mehta MA. MDMA increases cooperation and recruitment of social brain areas when playing trustworthy players in an iterated prisoner’s dilemma. J Neurosci. (2019) 39:307–20. doi: 10.1523/JNEUROSCI.1276-18.2018
285. Frye CG, Wardle MC, Norman GJ, de Wit H. MDMA decreases the effects of simulated social rejection. Pharmacol Biochem Behav. (2014) 117:1–6. doi: 10.1016/j.pbb.2013.11.030
286. Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. (2012) 37:630–40. doi: 10.1038/npp.2011.228
287. Preller KH, Pokorny T, Hock A, Kraehenmann R, Stämpfli P, Seifritz E, et al. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U.S.A. (2016) 113:5119–24. doi: 10.1073/pnas.1524187113
288. de Almeida RN, Galvão AC de M, da Silva FS, Silva EA dos S, Palhano-Fontes F, Maia-de-Oliveira JP, et al. Modulation of Serum Brain-Derived Neurotrophic Factor by a Single Dose of Ayahuasca: Observation From a Randomized Controlled Trial. Front Psychol. (2019) 10. https://www.frontiersin.org/articles/10.3389/fpsyg.2019.01234.
289. Becker AM, Holze F, Grandinetti T, Klaiber A, Toedtli VE, Kolaczynska KE, et al. Acute Effects of Psilocybin After Escitalopram or Placebo Pretreatment in a Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Subjects. Clin Pharmacol Ther. (2022) 111:886–95. doi: 10.1002/cpt.2487
290. Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. (2007) 1179:28–34. doi: 10.1016/j.brainres.2007.08.045
291. Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. (2004) 56:140–5. doi: 10.1016/j.biopsych.2004.02.033
292. Salinas J, Beiser A, Himali JJ, Satizabal CL, Aparicio HJ, Weinstein G, et al. Associations between social relationship measures, serum brain-derived neurotrophic factor, and risk of stroke and dementia. Alzheimers Dement Transl Res Clin Interv. (2017) 3:229–37. doi: 10.1016/j.trci.2017.03.001
293. Husain MI, Ledwos N, Fellows E, Baer J, Rosenblat JD, Blumberger DM, et al. Serotonergic psychedelics for depression: What do we know about neurobiological mechanisms of action? Front Psychiatry. (2023) 13. https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1076459.
294. Mertens LJ, Wall MB, Roseman L, Demetriou L, Nutt DJ, Carhart-Harris RL. Therapeutic mechanisms of psilocybin: Changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J Psychopharmacol (Oxf). (2020) 34:167–80. doi: 10.1177/0269881119895520
295. Czéh B, Simon M. Benefits of animal models to understand the pathophysiology of depressive disorders. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 106:110049. doi: 10.1016/j.pnpbp.2020.110049
296. Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, et al. Social approach behaviors in oxytocin knockout mice: Comparison of two independent lines tested in different laboratory environments. Neuropeptides. (2007) 41:145–63. doi: 10.1016/j.npep.2007.02.002
297. Becker M, Pinhasov A, Ornoy A. Animal models of depression: what can they teach us about the human disease? Diagnostics. (2021) 11:123. doi: 10.3390/diagnostics11010123
298. Amiri S, Haj-Mirzaian A, Rahimi-Balaei M, Razmi A, Kordjazy N, Shirzadian A, et al. Co-occurrence of anxiety and depressive-like behaviors following adolescent social isolation in male mice; possible role of nitrergic system. Physiol Behav. (2015) 145:38–44. doi: 10.1016/j.physbeh.2015.03.032
299. Kuramochi M, Nakamura S. Effects of postnatal isolation rearing and antidepressant treatment on the density of serotonergic and noradrenergic axons and depressive behavior in rats. Neuroscience. (2009) 163:448–55. doi: 10.1016/j.neuroscience.2009.06.017
300. Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. (2006) 9:519–25. doi: 10.1038/nn1659
301. Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, et al. Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol Psychiatry. (2017) 81:285–95. doi: 10.1016/j.biopsych.2016.06.012
302. Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA. Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. (2014) 76:550–8. doi: 10.1016/j.biopsych.2013.12.014
303. Newman EL, Covington HE, Suh J, Bicakci MB, Ressler KJ, DeBold JF, et al. Fighting Females: Neural and Behavioral Consequences of Social Defeat Stress in Female Mice. Biol Psychiatry. (2019) 86:657–68. doi: 10.1016/j.biopsych.2019.05.005
304. Gregorio DD, Popic J, Enns JP, Inserra A, Skalecka A, Markopoulos A, et al. Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proc Natl Acad Sci. (2021) 118. doi: 10.1073/pnas.2020705118
305. Gass N, Becker R, Reinwald J, Cosa-Linan A, Sack M, Weber-Fahr W, et al. Differences between ketamine’s short-term and long-term effects on brain circuitry in depression. Transl Psychiatry. (2019) 9:1–11. doi: 10.1038/s41398-019-0506-6
306. Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. (2019) 364:eaat8078. doi: 10.1126/science.aat8078
307. Rollin MDH, Rollin BE. Crazy like a fox. Validity and ethics of animal models of human psychiatric disease. Camb Q Healthc Ethics. (2014) 23:140–51. doi: 10.1017/s0963180113000674
308. MacArthur Clark J. The 3Rs in research: a contemporary approach to replacement, reduction and refinement. Br J Nutr. (2018) 120:S1–7. doi: 10.1017/s0007114517002227
Keywords: major depressive disorder, impairments, psychedelics, vagus, ketamine, antidepressants, treatment
Citation: Kupferberg A and Hasler G (2024) From antidepressants and psychotherapy to oxytocin, vagus nerve stimulation, ketamine and psychedelics: how established and novel treatments can improve social functioning in major depression. Front. Psychiatry 15:1372650. doi: 10.3389/fpsyt.2024.1372650
Received: 18 January 2024; Accepted: 05 August 2024;
Published: 14 October 2024.
Edited by:
Emily R. Stern, New York University, United StatesReviewed by:
Mariusz Stanisław Wiglusz, Medical University of Gdansk, PolandMichael James Weightman, University of Adelaide, Australia
Copyright © 2024 Kupferberg and Hasler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gregor Hasler, Z3JlZ29yLmhhc2xlckB1bmlmci5jaA==
 Aleksandra Kupferberg1
Aleksandra Kupferberg1 Gregor Hasler
Gregor Hasler