- Department of Psychiatry, Affiliated Nanjing Brain Hospital, Nanjing Medical University, Nanjing, Jiangsu, China
Aims: This study aims to explore the gender differences in cognitive improvements after two months of atypical antipsychotic treatment in first episode schizophrenia (FES).
Methods: 82 patients with FES, including 50 male patients and 32 female patients, were enrolled in the present study. Positive and Negative Syndrome Scale (PANSS) and MATRICS Consensus Cognitive Battery (MCCB) were respectively conducted to evaluate the clinical symptoms and cognitive function of patients with FES at baseline and after treatment. Repeated measure ANOVA was performed to compare gender differences in cognitive domains scores between baseline and 2-month follow-up. Stepwise liner regression model was performed to explore the effect factors of cognitive improvements in patients.
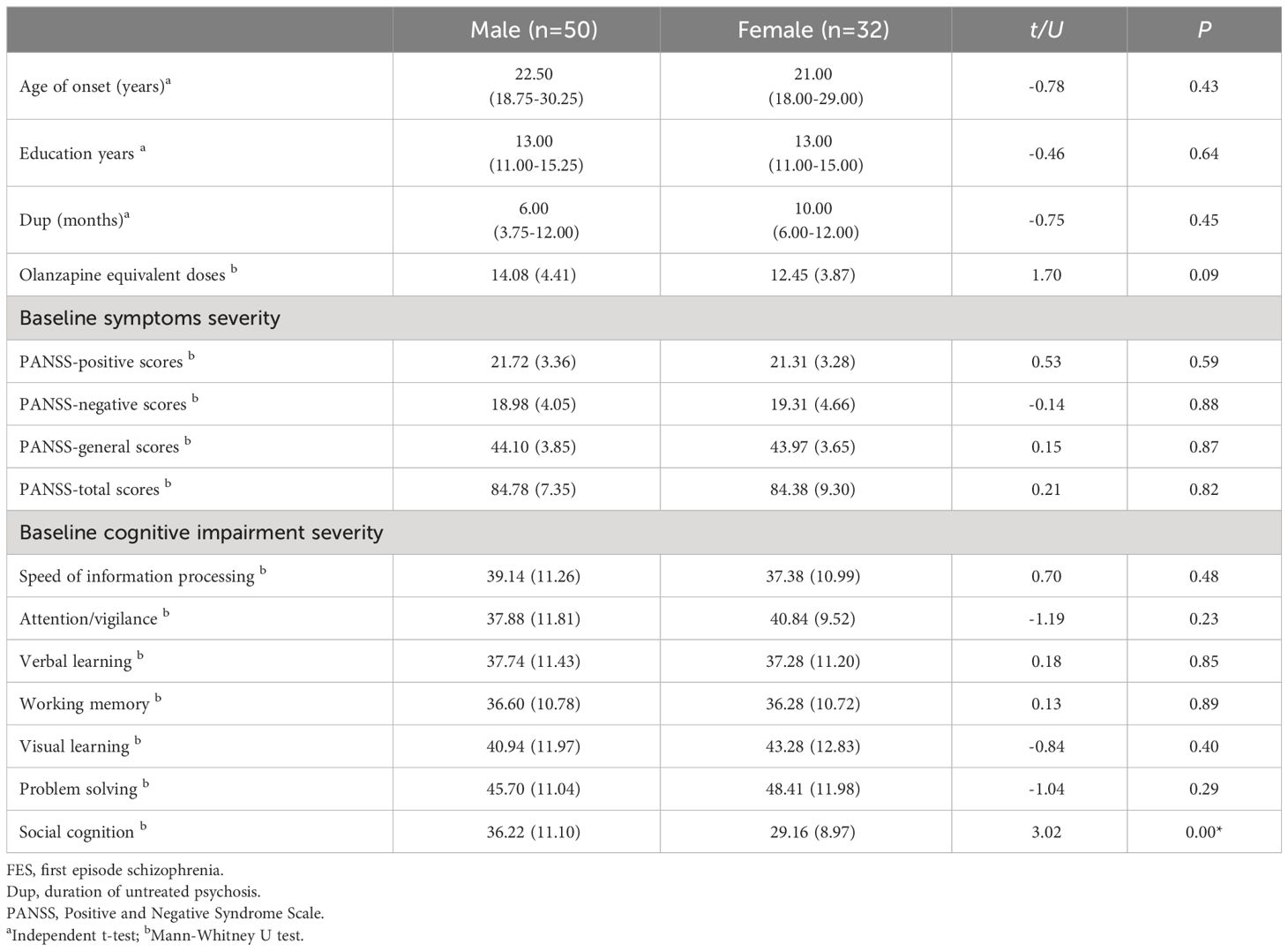
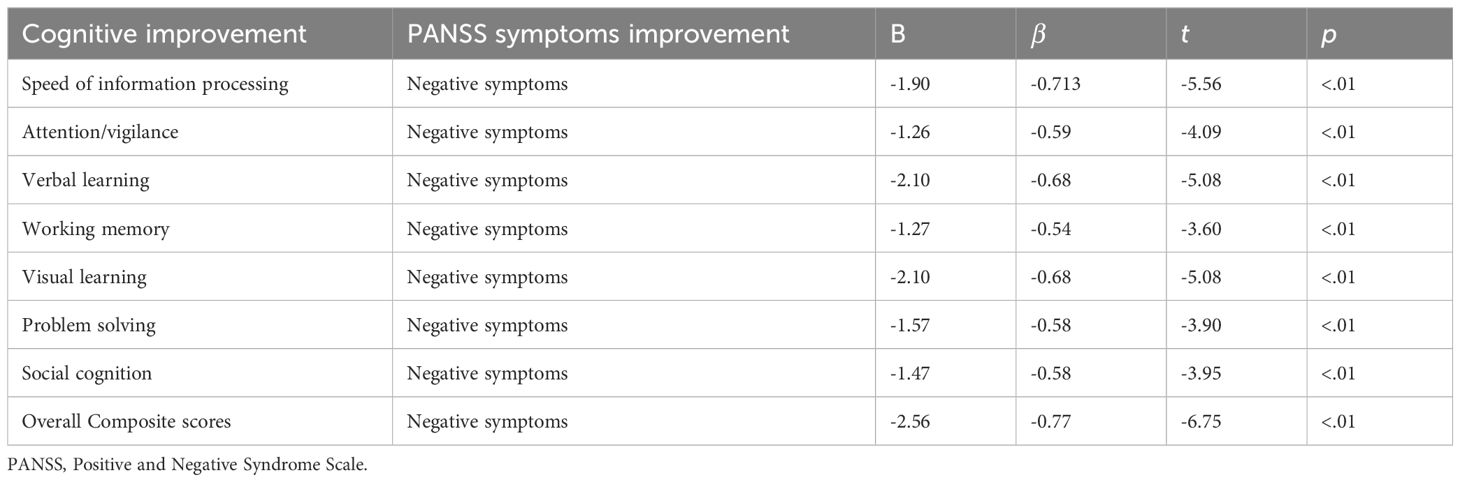
Results: There was no significant difference in age of onset, education years, PANSS scores, duration of untreated psychosis and Olanzapine equivalent doses between male and female patients (all p > 0.05). In the comparisons of cognition function, male patients exhibited better performance in social cognition compared with female patients at baseline (t = 3.20, p < 0.05). After treatment, improvements of attention/vigilance and working memory were both found in male patients and female patients (attention/vigilance, F = 11.867, p < 0.05; working memory, F = 18.265, p < 0.05). In addition, improvement of speed of information processing was only found in female patients (F = 11.65, p < 0.01). Significant interaction between time and gender was found in speed information of processing (F = 4.140, p = 0.045). Stepwise liner regression model revealed that improvements of negative symptoms promote improvements of cognitive function in female patients (all p < 0.05).
Conclusions: Our findings revealed gender differences of cognitive improvements in patients with FES after 2-month treatment. It provides new evidence for gender differences in cognitive symptoms of schizophrenia, and also provides preliminary clues for further individualized cognitive intervention strategies.
Introduction
Gender is one of the most important heterogeneity factors in schizophrenia (1). Over the past few decades, numerous studies have explored difference between male and female patients in several aspects of the disease. For example, male patients with schizophrenia appear to exhibit an earlier age of onset, severer negative symptoms and a higher frequency of alcohol/substance abuse than female patients (2, 3). Female patients display superior occupational, interpersonal and psychosocial function compared with male patients (4). Studies also found gender difference in response to antipsychotic drugs. Male patients tend to respond more poorly to antipsychotic medications, exhibit poorer medication adherence, and therefore have more disabilities (5). Inversely, in a 4-18 years follow-up study, female patients with schizophrenia had both better remission of symptoms and functional outcomes after treatments (6). Gender-related characteristics may play a crucial role in the progression and outcome of schizophrenia. Identifying these differences benefits to specify and implement gender-specific intervention strategies.
As the core features of schizophrenia, cognitive deficits occur early and persist across the course of schizophrenia (7, 8), which may be associated with long-term disability and heavy economic burden on society (9). Several studies have devoted to investigate gender difference of cognitive function in schizophrenia. Evidence indicated that gender differences exhibit in the whole course of schizophrenia, including the prodromal, acute and chronic stages (10, 11). Zhao et al (12) have demonstrated that female patients with FES performed better in speed of processing and verbal learning than male patients. However, Zhang et al (13) also reported no gender difference in the comparisons of cognitive function in first episode schizophrenia. Given the inconsistent results on gender difference in cognitive function, one of the reasons may be the heterogeneity of sample size and methods of analyses. The other may be the different tools of cognitive assessment used in the previous studies. Measurement and Treatment of Schizophrenia Cognition Research (MATRICS) Consensus Cognitive Battery (MCCB) (14, 15), which is considered the gold standard for assessing cognitive function in patients with schizophrenia. Based on these reason, this study adopted appropriate assessment tools to further explore gender differences in cognitive function of schizophrenia.
It’s worth noting that previous studies have shown that gender difference in outcome indicators may affect by the time of evaluation. One of the studies reported that more female patients reached a state of recovery compared to male patients after a 5-year follow-up (16). A 10-year follow-up study indicated that gender differences in outcome were attenuated (17, 18). However, the majority of previous studies have mostly employed long-term longitudinal designs (up to 1 year), and the results of short-term study on gender difference in cognitive improvements remain unclear. Study on gender difference in cognitive function after short-term treatment is beneficial to the formulation of individualized intervention strategies for early schizophrenia. Previous studies have reported about 91.4% schizophrenia with FES had improvements in cognitive function after 2 months of atypical antipsychotic treatment (19). And 2 months is seen as a early time window to observe cognitive improvements (20, 21). Up to now, no study has investigated gender difference in cognitive improvements after 2 months of atypical antipsychotic treatment in Chinese Han population. Whether this cognitive improvement may be influenced by gender remains unknown.
In the present study, the main objective was to investigate gender difference in cognitive improvements of schizophrenia with FES after 2-month treatment. We hypothesize that there is gender difference in cognitive improvement of schizophrenia after 2-month treatment and this difference may be related to the improvement of clinical symptoms.
Materials and methods
Subjects
Patients with FES were recruited from the inpatient and outpatient of Nanjing brain hospital, Jiangsu, China, from January 2017 to September 2023. Patients were diagnosed by two psychiatrists using the DSM-V who were experienced and involved in the usual care process of patients. All patients meets the following criteria: (1) right handed; (2) age between 16 to 45; (3) Intelligence quotient (IQ)>70 the Wechsler Adult Scale of Intelligence (WAIS) was adopted to test the Intelligence Quotient (IQ); (4) Duration of untreated time ≤ 24 months, never treated with antipsychotic medication or physical therapy. Exclusion criteria included: (1) Mental retardation or other serious mental disorders; (2) Serious physical diseases; (3) History of drug or alcohol abuse.
The study was approved by the Medical Research Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University(No. 2021-KY075-01). All participants provided written informed consent.
Nueropsychologic and Clinical measurement
Basic clinical measurements such as age of onset, education years, duration of untreated psychosis were provided by patients and their caregivers. In this study, cognitive assessment was performed using the MCCB, comprised of seven cognitive domains, including speed of information processing, attention and vigilance, verbal learning, working memory, problem solving, visual learning and social cognition (22, 23). Clinical symptoms assessment was performed using the Positive and Negative Syndrome Scale (PANSS), comprising positive symptoms, negative symptoms and general symptoms, and carried out by two experienced psychiatrists (24). All patients were medicated with atypical antipsychotic medication based on routine clinical practice. Seventeen patients were treated with risperidon, thirteen with olanzapine, twenty-one with aripiprazole, fourteen with paliperidone and seventeen with amisulpiride. All drug doses are converted to olanzapine equivalents (25), and cumulative antipsychotic dose was defined as the sum of all daily doses from the start of antipsychotic therapy to the day of retesting MCCB at 2 months follow-up.
Statistical analyses
Statistical analyses were performed using Statistical Package for the Social Sciences version 25.0 (SPSS 25.0). The normality of data distribution was assessed with the Kolmogorov-Smirnov test and Shapiro-Wilk test. Mann-Whitney U test was adopted to compare difference of education years, age of onset and duration of untreated psychosis. Independent t-test was used to compare PANSS scores, MCCB scores at baseline and olanzapine equivalent doses between male and female patients. Repeated measure ANOVA was performed to compare cognitive domain scores between groups. Stepwise liner regression model was performed to explore the effect factors of cognitive improvements in patients.
Results
Demographic and clinical characteristics
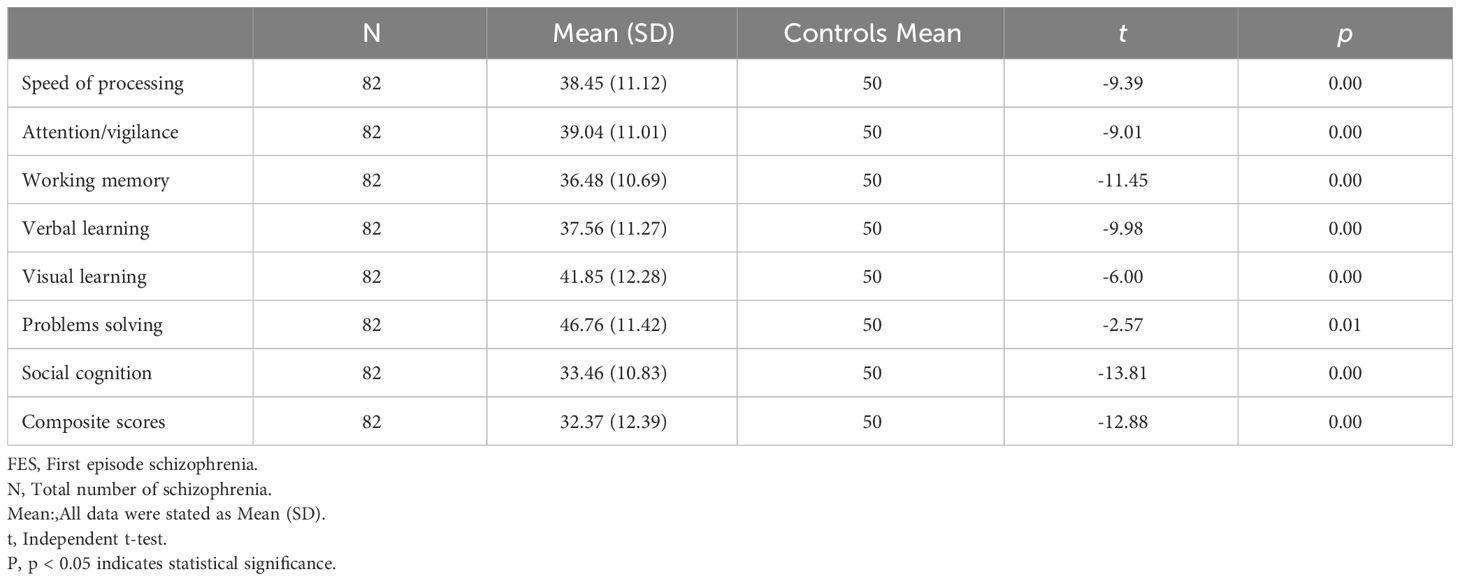
A total of 82 schizophrenia with FES (52 males, 30 females) were enrolled in the present study. Compared to normative data from the Chinese population (26), patients with FES showed deficits in all cognitive domains (all p < 0.05, see Table 1 for details).
There were no significant differences in age of onset, education years, PANSS scores (including positive scores, negative scores and general psychopathology scores) and duration of untreated psychosis between male and female at baseline. There was also no significant differences in Olanzapine equivalent doses between groups (all p > 0.05). In the comparisons of cognition function, male patients exhibited better performance in social cognition compared with female patients at baseline (t = 3.20, p < 0.05, see Table 2 for detalis).
Gender difference in cognitive function at baseline and 2-month follow-up
After 2-month treatment, both male and female patients exhibited improvements in attention/vigilance and working memory (attention/vigilance, F = 11.867, p < 0.05; working memory, F = 18.265, p < 0.05). Furthermore, only female patients showed improvement in speed of information processing (F = 11.65, p < 0.01). All enrolled patients demonstrated significant enhancements in overall cognitive function after 2 months of treatment with atypical antipsychotics (F = 9.708, p < 0.01). Notably, a significant interaction between time and gender was observed in terms of speed of information processing (F = 4.140, p = 0.045). (see Figure 1 for details).
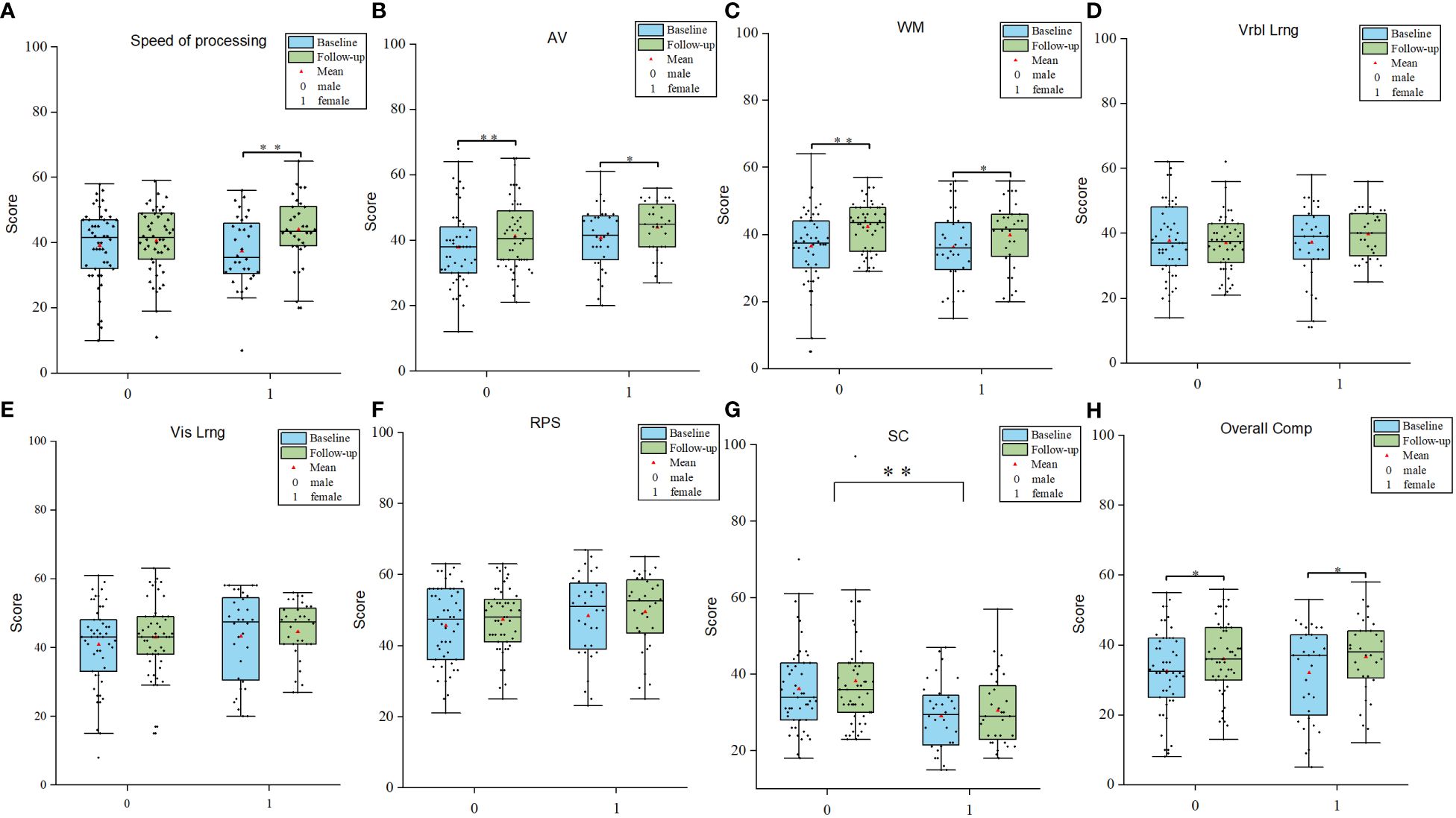
Figure 1 Gender differences in cognitive improvements. (A-H) Comparisions of different cognitive domains between male and female patients. AV Attention/Vigilance; WM Working memory; Vrbl lrng Verbal learning; Vis lrng Visual learning; PRS Problem solving; SC Social cognition. *p < 0.05, **p < 0.01.
Gender difference in effect factors of cognitive improvements
Stepwise liner regression model revealed that improvements of negative symptoms promote improvements of cognitive function in female patients. (all p < 0.05, see details in Table 3). No significant effect factor of cognitive improvements was found in male patients.
Discussion
In the present study, we explored gender differences in cognitive improvements of first episode schizophrenia after 2 months of treatment. The main findings emerged 1) Male patients had better social cognition than female patients at baseline. 2) Improvement of speed of processing was only found in female patients. Significant interaction between time and gender was found in speed information of processing. 3) Negative symptoms improvement may promote cognitive improvements in female patients.
Evidence shows that individuals with schizophrenia exhibit different psychiatric symptoms based on gender. Males tend to experience more severe negative symptoms, while females tend to experience more severe positive symptoms (13, 27–29). However, the present study found no gender differences in psychiatric symptoms of first episode schizophrenia, which was inconsistent with previous research findings. A global study had revealed that gender differences in psychotic symptoms displayed regional heterogeneity, possibly associate with psychosocial and cultural factors in different residential regions (30). Additionally, the heterogeneity in sample size and assessments tools adopted may contribute to potential discrepancies in research outcomes.
Previous studies have reported cognitive impairments in patients with schizophrenia during the early stages of this illness (7, 31–33). In our study, we found that first episode schizophrenia exhibited extensive cognitive deficits compared to normative data from the Chinese population. These findings provide additional evidence to support this viewpoint and further enhance its reliability. When analyzing the data by gender grouping, a noteworthy trend emerged among patients, showing that males scored higher in social cognition, indicating better social function compared to females. However, the conclusions regarding gender differences in social cognition still remain controversial. For instance, previous researches indicated that males exhibited superior social cognition compared to females at the early and chronic stages of schizophrenia (27, 34). It is important to note that some studies suggested there was no significant difference in social cognitive function between gender (13). A potential explanation for the inconsistent of our results could be the complexity of the social cognition functional construct (35). Prior investigations have revealed that social cognition encompasses diverse abilities, including theory of Mind, emotional processing (EP), social perception, and attribution style (36). However, the MCCB used in the present study primarily assessed the EP aspect of individual social cognition. Furthermore, a 10-year follow-up study provided evidence that male patients with schizophrenia exhibited poorer social cognition than female patients (37).Combined with the results of this study, it is possible to speculate that the female advantage in social cognition might become apparent later in the disease course. However, relevant studies need to be further verified.
The present study found that only female patients exhibited improvements in processing speed, which consistent with findings from previous research (34, 38, 39). Notably, a rodent study simulating schizophrenia-like behavior also revealed that female mice demonstrated better processing speed than male mice (40). Nevertheless, the pathophysiological mechanism underlying gender differences in speed of processing improvements in schizophrenia remains inconclusive. Currently, the most widely accepted explanation is that estrogen has a protective impact on cognitive function in patients with schizophrenia (41–43). And previous study also reported that there is an inverse correlation between psychotic symptoms and estrogen levels (44). Some females with psychotic tendencies experience more pronounced symptoms after menopause (45, 46). Estrogen diminished psychotic symptoms by modulating major neurotransmitter systems associated with schizophrenia, such as the dopamine signaling system. However, this protection primarily prevents further cognitive deterioration over time in schizophrenia, rather than preventing damage from the disease itself, as evidenced by the absence of gender differences in processing speed improvements among patients with FES at baseline. Furthermore, the superior response of female patients to antipsychotic drugs may provide an additional explanation. Specifically, estrogen influences the activity of CYP1A2, a major enzyme involved in olanzapine metabolism (47). Consequently, With the same dosage, the drug plasma concentration of olanzapine in female patients with schizophrenia was higher than that in males with schizophrenia and produce better results (48). Numerous prior studies have demonstrated that gender may change brain structure through mediating neurodevelopment, resulting in various psychiatric symptom (49–52). Future magnetic resonance findings should be included to explore gender differences in cognitive structure of processing speed in schizophrenia.
Previous studies have identified a convergence between negative symptoms and cognitive impairments in individuals with schizophrenia (53, 54). Oliver et al. (55) investigated that cognitive changes were associated with negative factors in a one-year follow-up study. Chen et al. also reported that negative symptoms were associated with longitudinal changes of cognitive function (56). Overlapping etiologies may exited between negative symptom subdomains and cognitive function. Cognitive function and clinical symptoms are not independent but affects each other. However, we only found that negative symptoms improvements were correlated with cognitive improvements in female patients, which suggested there may be gender specificity in curative effect. The earlier the intervention for negative symptoms, the greater may be cognitive improvements in female patients.
Cognitive remediation (CR) is an important therapeutic method for cognitive impairments in schizophrenia. CR as a type of behavioral training aims to improve cognitive function with the goal of persistence and generalization in everyday life. CR is effective in promoting the improvement of cognitive function, social and daily living function in patients with schizophrenia (57). Exsting MRI research conducted on individuals in the early stages of schizophrenia has revealed that CR interventions are associated with structural and functional alterations in the brain, particularly in the frontal and limbic regions (58, 59). CR may have a slowing or reversing effect on progressive brain volume deterioration in the early stages of schizophrenia, especially in areas critical to higher cognitive processes (58, 60). Vita et al. also reported that long-term improvement of psychosocial functioning after CR can be observed with significant gender differences, and females exihibited more significant improvements in cognitive function (61). Currently, only a few studies have concentrated on gender differences in cognitive improvements after CR intervention, and the results have been inconsistent. Thus, in the future studies, gender differences in cognitive improvement and brain structure changes after CR interventions need to be further explored at the early stage of the disease by using multimodal techniques. Longitudinal studies are also needed to clarify gender differences in cognitive training and whether its positive effects persist over time.
Limitation
This research has some limitations. First, our study lasted only 2 months, which is much shorter than previous studies. The findings from longitudinal investigations suggested that the duration of 1-3 years may not be sufficient to detect significant changes in cognitive function (62). In our future studies, we intend to conduct ongoing follow-up assessments of the participants who have been enrolled in our study. Second, our research is an observational study, the pathological mechanisms of gender differences in cognitive function in schizophrenia are not yet clear. We need to incorporate magnetic resonance data for further study. Third, the study employed inconsistent drug interventions. Previous research has demonstrated varying degrees of improvement in cognitive function depending on the type of antipsychotic medication utilized (63). Fourth, the sample size after the gender-stratified analysis was relatively small, and our stratified results should be validated in larger studies in the future. Finally, women’s neuropsychological performance has been shown to fluctuate with the menstrual cycle, which was not controlled for in our study (64).
Conclusion
In summary, our results indicated that gender differences of cognitive function exhibited at baseline and 2-month follow-up, which provides some clues for the personalized treatment of schizophrenia patients. Understanding these differences may help develop more precise treatment strategies for individuals with schizophrenia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Research Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WL: Writing – review & editing, Writing – original draft, Methodology, Data curation. XC: Writing – original draft. QL: Writing – review & editing, Data curation. YL: Writing – review & editing, Data curation. CZ: Writing – review & editing, Methodology, Formal Analysis. JD: Writing – review & editing, Supervision, Resources, Funding acquisition. SX: Writing – review & editing, Supervision, Resources, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Medical Science and Technology Development Foundation, Nanjing Municipality Health Bureau (YKK23134), Medical Science and Technology Development Foundation, Nanjing Municipality Health Bureau (ZKX21033).
Acknowledgments
We sincerely appreciate the enthusiastic participation and generous contributions of all research participants. Additionally, we are profoundly grateful to our parents for their unwavering support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1369532/full#supplementary-material
References
1. Fountoulakis KN, Dragioti E, Theofilidis AT, Wiklund T, Atmatzidis X, Nimatoudis I, et al. Gender, age at onset, and duration of being ill as predictors for the long-term course and outcome of schizophrenia: an international multicenter study. CNS Spectr. (2022) 27:716–23. doi: 10.1017/s1092852921000742
2. Riecher-Rössler A, Butler S, Kulkarni J. Sex and gender differences in schizophrenic psychoses-a critical review. Arch Womens Ment Health. (2018) 21:627–48. doi: 10.1007/s00737-018-0847-9
3. Bertani M, Lasalvia A, Bonetto C, Tosato S, Cristofalo D, Bissoli S, et al. The influence of gender on clinical and social characteristics of patients at psychosis onset: A report from the Psychosis Incident Cohort Outcome Study (PICOS). Psychol Med. (2012) 42:769–80. doi: 10.1017/s0033291711001991
4. Häfner H. Gender differences in schizophrenia. Psychoneuroendocrinology. (2003) 28 Suppl 2:17–54. doi: 10.1016/s0306-4530(02)00125-7
5. Lange B, Mueller JK, Leweke FM, Bumb JM. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother. (2017) 18:351–62. doi: 10.1080/14656566.2017.1288722
6. Klærke LR, Baandrup L, Fagerlund B, Ebdrup BH, Pantelis C, Glenthøj BY, et al. Diagnostic stability and long-term symptomatic and functional outcomes in first-episode antipsychotic-naïve patients with schizophrenia. Eur Psychiatry. (2019) 62:130–7. doi: 10.1016/j.eurpsy.2019.07.001
7. Chu AOK, Chang WC, Chan SKW, Lee EHM, Hui CLM, Chen EYH. Comparison of cognitive functions between first-episode schizophrenia patients, their unaffected siblings and individuals at clinical high-risk for psychosis. Psychol Med. (2019) 49:1929–36. doi: 10.1017/s0033291718002726
8. Mucci A, Galderisi S, Green MF, et al. Familial aggregation of MATRICS Consensus Cognitive Battery scores in a large sample of outpatients with schizophrenia and their unaffected relatives. Psychol Med. (2018) 48:1359–66. doi: 10.1017/s0033291717002902
9. Kadakia A, Catillon M, Fan Q, Williams GR, Marden JR, Anderson A, et al. The economic burden of schizophrenia in the United States. J Clin Psychiatry. (2022) 83(6). doi: 10.4088/JCP.22m14458
10. Choi JS, Chon MW, Kang DH, Jung MH, Kwon JS. Gender difference in the prodromal symptoms of first-episode schizophrenia. J Korean Med Sci. (2009) 24:1083–8. doi: 10.3346/jkms.2009.24.6.1083
11. Wei CW, Chen YQ, Ma M, Xiu MH, Zhang XY. Sex differences in the association of body mass index with symptoms and cognitive deficits in Chinese patients with chronic schizophrenia. Transl Psychiatry. (2020) 10:18. doi: 10.1038/s41398-020-0717-x
12. Zhao N, Wang XH, Kang CY, Zheng Y, Yang LY, Guan TF, et al. Sex differences in association between cognitive impairment and clinical correlates in Chinese patients with first-episode drug-naïve schizophrenia. Ann Gen Psychiatry. (2021) 20:26. doi: 10.1186/s12991-021-00347-1
13. Zhang XY, Chen DC, Xiu MH, Yang FD, Haile CN, Kosten TA, et al. Gender differences in never-medicated first-episode schizophrenia and medicated chronic schizophrenia patients. J Clin Psychiatry. (2012) 73:1025–33. doi: 10.4088/JCP.11m07422
14. Mu L, Liang J, Wang H, Chen D, Xiu M, Zhang XY. Sex differences in association between clinical correlates and cognitive impairment in patients with chronic schizophrenia. J Psychiatr Res. (2020) 131:194–202. doi: 10.1016/j.jpsychires.2020.09.003
15. Wang D, Xia L, Zhang Z, Camkurt MA, Issac A, Wu E, et al. Sex difference in association between cognitive and P50 deficits in patients with chronic schizophrenia. Arch Womens Ment Health. (2023) 26:793–801. doi: 10.1007/s00737-023-01367-4
16. Thorup A, Albert N, Bertelsen M, Petersen L, Jeppesen P, Le Quack P, et al. Gender differences in first-episode psychosis at 5-year follow-up–two different courses of disease? Results from the OPUS study at 5-year follow-up. Eur Psychiatry. (2014) 29:44–51. doi: 10.1016/j.eurpsy.2012.11.005
17. Seeman MV. Does gender influence outcome in schizophrenia? Psychiatr Q. (2019) 90:173–84. doi: 10.1007/s11126-018-9619-y
18. Mayston R, Kebede D, Fekadu A, Medhin G, Hanlon C, Alem A, et al. The effect of gender on the long-term course and outcome of schizophrenia in rural Ethiopia: a population-based cohort. Soc Psychiatry Psychiatr Epidemiol. (2020) 55:1581–91. doi: 10.1007/s00127-020-01865-1
19. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. (2006) 163:743–5. doi: 10.1176/ajp.2006.163.4.743
20. Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. (2004) 161:1–56.
21. Keefe RS, Buchanan RW, Marder SR, Schooler NR, Dugar A, Zivkov M, et al. Clinical trials of potential cognitive-enhancing drugs in schizophrenia: what have we learned so far? Schizophr Bull. (2013) 39:417–35. doi: 10.1093/schbul/sbr153
22. Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. (2008) 165:203–13. doi: 10.1176/appi.ajp.2007.07010042
23. Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. (2008) 165:214–20. doi: 10.1176/appi.ajp.2007.07010043
24. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
25. Leucht S, Samara M, Heres S, Patel MX, Furukawa T, Cipriani A, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. (2015) 41:1397–402. doi: 10.1093/schbul/sbv037
26. Shi C, Kang L, Yao S, Ma Y, Li T, Liang Y, et al. The MATRICS consensus cognitive battery (MCCB): co-norming and standardization in China. Schizophr Res. (2015) 169:109–15. doi: 10.1016/j.schres.2015.09.003
27. Pu C, Qiu Y, Zhou T, Yang F, Lu Z, Wang C, et al. Gender differences of neurocognitive functioning in patients with first-episode schizophrenia in China. Compr Psychiatry. (2019) 95:152132. doi: 10.1016/j.comppsych.2019.152132
28. Thorup A, Petersen L, Jeppesen P, Ohlenschlaeger J, Christensen T, Krarup G, et al. Gender differences in young adults with first-episode schizophrenia spectrum disorders at baseline in the Danish OPUS study. J Nerv Ment Dis. (2007) 195:396–405. doi: 10.1097/01.nmd.0000253784.59708.dd
29. Morgan VA, Castle DJ, Jablensky AV. Do women express and experience psychosis differently from men? Epidemiological evidence from the Australian National Study of Low Prevalence (Psychotic) Disorders. Aust N Z J Psychiatry. (2008) 42:74–82. doi: 10.1080/00048670701732699
30. Novick D, Montgomery W, Treuer T, Moneta MV, Haro JM. Sex differences in the course of schizophrenia across diverse regions of the world. Neuropsychiatr Dis Treat. (2016) 12:2927–39. doi: 10.2147/ndt.S101151
31. Zanelli J, Reichenberg A, Sandin S, Morgan C, Dazzan P, Pilecka I, et al. Dynamic and static cognitive deficits in schizophrenia and bipolar disorder after the first episode. Schizophr Bull. (2022) 48:590–8. doi: 10.1093/schbul/sbab150
32. Yang M, Gao S, Zhang X. Cognitive deficits and white matter abnormalities in never-treated first-episode schizophrenia. Transl Psychiatry. (2020) 10:368. doi: 10.1038/s41398-020-01049-0
33. Üçok A, Direk N, Koyuncu A, Keskin-Ergen Y, Yüksel Ç, Güler J, et al. Cognitive deficits in clinical and familial high risk groups for psychosis are common as in first episode schizophrenia. Schizophr Res. (2013) 151:265–9. doi: 10.1016/j.schres.2013.10.030
34. Zhang B, Han M, Tan S, De Yang F, Tan Y, Jiang S, et al. Gender differences measured by the MATRICS consensus cognitive battery in chronic schizophrenia patients. Sci Rep. (2017) 7:11821. doi: 10.1038/s41598-017-12027-w
35. Pinkham AE, Harvey PD, Penn DL. Social cognition psychometric evaluation: results of the final validation study. Schizophr Bull. (2018) 44:737–48. doi: 10.1093/schbul/sbx117
36. Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. (2013) 39:979–92. doi: 10.1093/schbul/sbs080
37. Zhao J, Diao J, Li X, Yang Y, Yao Y, Shi S, et al. Gender differences in psychiatric symptoms and the social functioning of 610 patients with schizophrenia in urban China: A 10-year follow-up study. Neuropsychiatr Dis Treat. (2022) 18:1545–51. doi: 10.2147/ndt.S373923
38. Schaevitz LR, Picker JD, Rana J, Kolodny NH, Shane B, Berger-Sweeney JE, et al. Glutamate carboxypeptidase II and folate deficiencies result in reciprocal protection against cognitive and social deficits in mice: implications for neurodevelopmental disorders. Dev Neurobiol. (2012) 72:891–905. doi: 10.1002/dneu.21000
39. Tsai PC, McDowd J, Tang TC, Su CY. Processing speed mediates gender differences in memory in schizophrenia. Clin Neuropsychol. (2012) 26:626–40. doi: 10.1080/13854046.2012.678887
40. Thomson DM, McVie A, Morris BJ, Pratt JA. Dissociation of acute and chronic intermittent phencyclidine-induced performance deficits in the 5-choice serial reaction time task: influence of clozapine. Psychopharmacol (Berl). (2011) 213:681–95. doi: 10.1007/s00213-010-2020-7
41. Brand BA, de Boer JN, Sommer IEC. Estrogens in schizophrenia: progress, current challenges and opportunities. Curr Opin Psychiatry. (2021) 34:228–37. doi: 10.1097/yco.0000000000000699
42. McGregor C, Riordan A, Thornton J. Estrogens and the cognitive symptoms of schizophrenia: Possible neuroprotective mechanisms. Front Neuroendocrinol. (2017) 47:19–33. doi: 10.1016/j.yfrne.2017.06.003
43. Russell JK, Jones CK, Newhouse PA. The role of estrogen in brain and cognitive aging. Neurotherapeutics. (2019) 16:649–65. doi: 10.1007/s13311-019-00766-9
44. Seeman MV. Psychopathology in women and men: focus on female hormones. Am J Psychiatry. (1997) 154:1641–7. doi: 10.1176/ajp.154.12.1641
45. Gurvich C, Gavrilidis E, Worsley R, Hudaib A, Thomas N, Kulkarni J. Menstrual cycle irregularity and menopause status influence cognition in women with schizophrenia. Psychoneuroendocrinology. (2018) 96:173–8. doi: 10.1016/j.psyneuen.2018.06.022
46. Sommer IE, Brand BA, Gangadin S, Tanskanen A, Tiihonen J, Taipale H. Women with schizophrenia-spectrum disorders after menopause: A vulnerable group for relapse. Schizophr Bull. (2023) 49:136–43. doi: 10.1093/schbul/sbac139
47. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. (2020) 163:107631. doi: 10.1016/j.neuropharm.2019.05.008
48. Eugene AR, Masiak J. A pharmacodynamic modelling and simulation study identifying gender differences of daily olanzapine dose and dopamine D2-receptor occupancy. Nord J Psychiatry. (2017) 71:417–24. doi: 10.1080/08039488.2017.1314011
49. Lang XE, Zhu D, Zhang G, Du X, Jia Q, Yin G, et al. Sex difference in association of symptoms and white matter deficits in first-episode and drug-naive schizophrenia. Transl Psychiatry. (2018) 8:281. doi: 10.1038/s41398-018-0346-9
50. Mendrek A, Mancini-Marïe A. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev. (2016) 67:57–78. doi: 10.1016/j.neubiorev.2015.10.013
51. Yücel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb Cortex. (2001) 11:17–25. doi: 10.1093/cercor/11.1.17
52. Liu X, Lai Y, Wang X, Hao C, Chen L, Zhou Z, et al. Reduced white matter integrity and cognitive deficit in never-medicated chronic schizophrenia: a diffusion tensor study using TBSS. Behav Brain Res. (2013) 252:157–63. doi: 10.1016/j.bbr.2013.05.061
53. Leanza L, Egloff L, Studerus E, Andreou C, Heitz U, Ittig S, et al. The relationship between negative symptoms and cognitive functioning in patients at clinical high risk for psychosis. Psychiatry Res. (2018) 268:21–7. doi: 10.1016/j.psychres.2018.06.047
54. Lim J, Lee SA, Lam M, Rapisarda A, Kraus M, Keefe RS, et al. The relationship between negative symptom subdomains and cognition. Psychol Med. (2016) 46:2169–77. doi: 10.1017/s0033291716000726
55. Olivier MR, Killian S, Chiliza B, Asmal L, Schoeman R, Oosthuizen PP, et al. Cognitive performance during the first year of treatment in first-episode schizophrenia: a case-control study. Psychol Med. (2015) 45:2873–83. doi: 10.1017/s0033291715000860
56. Chan SKW, Liao Y, Hui CLM, Wong TY, Suen Y, Chang WC, et al. Longitudinal changes of cognitive function and its relationship with subdomains of negative symptoms in patients with adult-onset first-episode schizophrenia: A 4-year follow up study. Schizophr Res. (2023) 252:181–8. doi: 10.1016/j.schres.2023.01.004
57. Katsumi A, Hoshino H, Fujimoto S, Yabe H, Ikebuchi E, Nakagome K, et al. Effects of cognitive remediation on cognitive and social functions in individuals with schizophrenia. Neuropsychol Rehabil. (2019) 29:1475–87. doi: 10.1080/09602011.2017.1409639
58. Ramsay IS, Fryer S, Boos A, Roach BJ, Fisher M, Loewy R, et al. Response to targeted cognitive training correlates with change in thalamic volume in a randomized trial for early schizophrenia. Neuropsychopharmacology. (2018) 43:590–7. doi: 10.1038/npp.2017.213
59. Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP, Hogarty SS, et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch Gen Psychiatry. (2010) 67:674–82. doi: 10.1001/archgenpsychiatry.2010.63
60. Eack SM, Newhill CE, Keshavan MS. Cognitive enhancement therapy improves resting-state functional connectivity in early course schizophrenia. J Soc Soc Work Res Summer. (2016) 7:211–30. doi: 10.1086/686538
61. Vita A, Barlati S, Ceraso A, Nibbio G, Durante F, Facchi M, et al. Durability of effects of cognitive remediation on cognition and psychosocial functioning in schizophrenia: A systematic review and meta-analysis of randomized clinical trials. Am J Psychiatry. (2024) 2024:appiajp20230396. doi: 10.1176/appi.ajp.20230396
62. Zanelli J, Mollon J, Sandin S, Morgan C, Dazzan P, Pilecka I, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. (2019) 176:811–9. doi: 10.1176/appi.ajp.2019.18091088
63. Vingerhoets WA, Bloemen OJ, Bakker G, van Amelsvoort TA. Pharmacological interventions for the MATRICS cognitive domains in schizophrenia: what's the evidence? Front Psychiatry. (2013) 4:157. doi: 10.3389/fpsyt.2013.00157
Keywords: first episode schizophrenia, cognitive function, gender difference, follow-up study, MCCB
Citation: Li W, Cao X, Liang Q, Li Y, Zhou C, Du J and Xie S (2024) Gender differences in cognitive improvements after two months of atypical antipsychotic treatment in first episode schizophrenia. Front. Psychiatry 15:1369532. doi: 10.3389/fpsyt.2024.1369532
Received: 12 January 2024; Accepted: 15 April 2024;
Published: 29 April 2024.
Edited by:
Stefano Barlati, University of Brescia, ItalyReviewed by:
Xiao-min Zhu, Suzhou Guangji Hospital, ChinaGabriele Nibbio, University of Brescia, Italy
Copyright © 2024 Li, Cao, Liang, Li, Zhou, Du and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinglun Du, ZHVqaW5nbHVuQDEyNi5jb20=; Shiping Xie, eGllc2hpcGluZ0Buam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Wanyi Li
Wanyi Li Xiang Cao†
Xiang Cao† Chao Zhou
Chao Zhou Shiping Xie
Shiping Xie