- 1Department of Internal Medicine, Endocrinology and Diabetology, Czerniakowski Hospital, Warsaw, Poland
- 2Faculty of Medicine, Lazarski University, Warsaw, Poland
- 3Faculty of Medicine, Cardinal Stefan Wyszynski University, Warsaw, Poland
Ghrelin is primarily responsible for regulating energy balance, as it increases appetite. However, in recent years, its new physiological functions have been discovered—it regulates lipogenesis, plays a role in the development of insulin resistance, and even acts protectively on heart muscle. Moreover, ghrelin was associated with many psychiatric disorders, including major depressive disorder (MDD) or schizophrenia. Ghrelin levels were elevated in patients diagnosed with depression and in patients after suicide attempts. Moreover, ghrelin was connected to depression among postmenopausal women and was shown to be a predictive marker of MDD among the elderly. Ghrelin may influence mood disorders in various ways: by regulating stress response or inflammation or altering neurotransmission in the amygdala, dorsal raphe nucleus, or hippocampus, brain regions previously connected to the pathophysiology of MDD. Genetic variants of ghrelin and its receptor have also been associated with depression. Moreover, ghrelin can interfere with the antidepressant’s action and may play a role in treatment resistance. This review highlights ghrelin’s role in depression, summarizes the existing knowledge on the subject, and presents ideas for further research.
1 Introduction
Major depressive disorder (MDD) is among the most common diseases of affluence. In recent studies, ghrelin has been linked to some psychiatric conditions: addictive disorders, obsessive-compulsive disorder, schizophrenia, bipolar disorder, and eating disorders (1). Ghrelin’s antagonist, leptin, has also been associated with bipolar disorder or MDD in previous research.
Ghrelin is an orexigenic hormone that primarily regulates food intake, but its many new functions have been discovered in recent years. Ghrelin regulates glucose homeostasis and might play a role in insulin resistance, another condition linked to MDD (2, 3). The hormone also influences lipogenesis and white adipose tissue utilization. Moreover, it was found to protect cardiac muscle during the recovery phase after myocardial infection by reducing sympathetic tonus (4). Ghrelin indirectly enhances the expression of insulin-like growth factor 1 (IGF-1) via growth hormone. The action of IGF-1 on muscle tissue, combined with increased nutrient availability (due to increased appetite), causes ghrelin to induce muscle mass growth. It was also shown that ghrelin induces the proliferation of osteoblasts and increases bone mineral density (4). Moreover, ghrelin mediates inflammation and is connected to sleep-cycle regulation, reward behavior, and memory (5).
Ghrelin binds to the growth hormone secretagogue receptor (GHSR), which is expressed in various organs of the body, including the brain, pancreas, liver, skeletal muscles, and adipose tissue, which explains its pleiotropic mechanism of action (6). Ghrelin is secreted in an inactive-deacylated form, which is later transformed by ghrelin O-acyltransferase to its acylated, active form (7). As a “hunger hormone”, it acts in the pituitary gland and hypothalamus in which it activates orexigenic neuropeptide Y neurons, which initiate appetite (2).
2 Methods
Articles on ghrelin in relation to depressive symptoms were retrieved from databases Academic Search Ultimate, ERIC, Health Source: Consumer Edition, Health Source: Nursing/Academic Edition, and MEDLINE from their inception to August 2023. The keywords were as follows: “depression” OR “depressive disorder” OR “depressive symptoms” OR “major depressive disorder” AND “ghrelin”.
The following inclusion criteria were applied for studies on humans: 1) ghrelin concentration was measured in peripheral blood, 2) patients were diagnosed with or screened for MDD according to the International Classification of Diseases (ICD) or Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria, and 3) all of the participants were adults. Studies were excluded in the following cases: 1) non-English articles, 2) non-original studies, and 3) co-occurrence of chronic disorders.
3 How can ghrelin affect mood?
3.1 Stress
Stress and anxiety are major components of depression symptoms. They both activate the sympathetic nervous system and hypothalamus–pituitary–adrenal axis (HPA axis) and therefore induce the “fight or flight” response. Studies on animals revealed that ghrelin level rises in stressful settings (8, 9).
In a study conducted by Huang et al., mice were treated with chronic unpredictable mild stress (CUMS) for 8 weeks. CUMS mice had higher acylated ghrelin levels in peripheral blood and significantly higher levels of pre-proghrelin mRNA in their stomachs when compared to untreated controls (p < 0.05). The same trend was observed during the histologic evaluation of their hippocampi—the expression of both GHSR and pre-proghrelin mRNA was elevated (p < 0.01) (8). Another research concluded that ghrelin itself can induce corticosterone release. Intraperitoneal injection of ghrelin into male mice (3 mg/kg) provoked greater release of corticosterone 15 minutes after the procedure when compared to controls injected with saline (160 ± 8.5 and 95.7 ± 9.8 ng/mL, respectively). However, the same trend was not observed among female mice treated with 2 mg/kg ghrelin (10).
Current research provides evidence that excessive ghrelin secretion is mediated by adrenergic signaling, as Gupta et al. have demonstrated. In their experiment, they exposed the mice to chronic social defeat stress (CSDS) for 10 days. The procedure induced depression-like behavior in the subjects. Mice were treated with atenolol [β1-adrenergic receptor (β1AR) antagonist] every day of the experiment. On day 11, the plasma acylated ghrelin was measured, and it was found to be lowered by 36.5% when compared to that of controls not treated with β-blocker. Furthermore, mice that were given atenolol spent significantly less time in the interaction zone than matched controls, indicating exaggeration of their anxiety-like behavior (11).
Other studies have shown that ghrelin signaling protects individuals from excessive stress reactions. Mice that did not secrete ghrelin or had non-functional GHSR could not adapt to stressful situations and exhibited depressive-like behavior. Mahbod et al. assessed if handling would have had an anxiolytic effect on mice secreting no ghrelin. As they observed, ghrelin knock-out mice spent less time in open arms during the elevated plus maze test in comparison to the wild-type, previously handled mice. The outcome of this study highlights the protective and adaptive role of ghrelin in stressful settings. Moreover, mice with non-functional GHSR secreted more corticosterone 30 and 60 minutes after the stressogenic stimuli (12). It suggests that ghrelin/GHSR signaling might limit the excessive HPA activation.
Gathered evidence indicates that ghrelin might link the sympathetic nervous system and HPA axis during stress response. Ghrelin secretion is mediated by β1AR signaling, and functional GHSR is needed to prevent unrestrained corticosterone release. Therefore, ghrelin could mediate the negative feedback loop of stress response. This hypothesis is further supported by the fact that treatment with β1-blocker suppressed ghrelin secretion, which resulted in an increase in anxiety-like behavior in mice.
Adrenergic stimulation and alterations in the HPA axis are known factors engaged in the pathophysiology of depression. Patients suffering from MDD have higher concentrations of circulating norepinephrine and cortisol than healthy subjects (13, 14). Moreover, treatment with antidepressants lowers both catecholamines and ghrelin levels in serum (13, 15, 16). The role of ghrelin in stress response might constitute its link to major depressive disorder.
3.2 Genetics
Ghrelin–receptor gene polymorphism can also contribute to the pathophysiology of depression. Nakashima et al. provided evidence that the Leu72Met variant in the ghrelin gene was more frequent among depressed patients in the Japanese population (17). Moreover, the GHSR DNA methylation profile is also associated with depression among individuals. Cordova-Palomera et al. examined the pattern of DNA methylation in 17 monozygotic twins. The study revealed that epigenetic print in the GHSR gene varied between depressed and non-depressed subjects (18). Both studies provide evidence that genetic variations in the ghrelin signaling system constitute its role in the pathophysiology of depression.
3.3 Monoamines
Ghrelin, as a hormone engaged in food intake, acts on orexigenic neurons in the hypothalamus. It controls the activity of the ventral tegmental area (VTA) that projects to the nucleus accumbens (NAc). These structures consist of dopaminergic neurons and constitute a reward circuit. VTA projects to the prefrontal cortex, amygdala, and hippocampus, which are referred to as brain reward regions (19). In individuals with depression, the VTA–NAc circuit functions abnormally. The activation of NAc in response to rewarding stimuli is reduced (20).
Ghrelin was shown to increase noradrenergic and serotoninergic transmission, which is the main mechanism of action of many antidepressants (1). It would explain the alleviation of depressive symptoms after the administration of ghrelin in studies on animals. Moreover, 5-HT2C receptor antagonists (such as antidepressants, e.g., fluoxetine and agomelatine) decrease serum ghrelin levels in rats (21). This effect could have arisen from the anxiolytic action of the mentioned drugs, proving ghrelin as a hormone engaged in stress response. The same trend was observed in humans with MDD treated with antidepressant or electroconvulsive therapy (ECT) (15).
Intraventricular ghrelin injection increases serotonin metabolism and promotes the expression of several serotonin receptors in both the amygdala and dorsal raphe nucleus (DRN), other brain regions that were previously connected to depression (22). Ghrelin was also found to depolarize serotoninergic neurons in the DRN. Ogaya et al. found out that ghrelin binds to its postsynaptic receptor and directly induces depolarization in DRN neurons (23). Intrahippocampal infusion of ghrelin also induced long-lasting (>120 min) synaptic plasticity. It intensified the presynaptic release of neurotransmitters and changed the amplitude of action potentials (24). Escitalopram [selective serotonin reuptake inhibitor (SSRI)] also induces neuroplasticity, and this effect is now believed to be responsible for its antidepressive mechanism of action (25).
3.4 Glutaminergic signaling
Signaling mediated by N-methyl-d-aspartate receptor (NMDAR) is involved in the development of depression (26, 27). The evidence is further supported by the antidepressive effect of NMDAR antagonists, ketamine and esketamine, used to treat treatment-resistant major depression (28, 29). Studies on animals provided insight into the relationship between glutaminergic signaling and ghrelin.
Bianconi et al. showed that ghrelin administration not only improves depression and spatial memory but also has an impact on NMDAR-mediated transmission in the brain. The study involved intrahippocampal injection of ghrelin to mice that previously underwent olfactory bulbectomy (OB). OB induces depressive-like behavior, causes memory impairment, and downregulates NMDAR. The study showed that ghrelin reversed OB’s effect on both depressive behavior and NMDAR internalization (30).
On the contrary, another research showed that ghrelin administration can antagonize the antidepressant effect of ketamine, an NMDAR antagonist. Landrigan et al. exposed mice to both ketamine and ghrelin. Mice treated with ketamine showed decreased depressive-like behavior in forced swim tests; however, when ghrelin was augmented, the effect did not persist. It was suggested that ketamine’s action is dependent on ghrelin signaling, although the coadministration of GHSR antagonists did not influence the forced swim test outcomes (31). The result of this study could explain why the body mass index (BMI) correlates positively with MDD improvement after intravenous ketamine administration (ghrelin secretion is inversely proportional to BMI) (32).
3.5 Inflammation
Low-grade inflammation is one of the pathophysiological components of depression. Pawar et al. have demonstrated that ghrelin administration has an impact on some of the proinflammatory cytokines. In the experiment, animals were exposed to a high-fat diet and stressors inducing depressive-like behavior. Central ghrelin administration not only improved depression symptoms but also lowered tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and IL-6 concentrations. The reduction of inflammation was also observed during histopathological evaluation as a decrease in cell infiltration and edema in brain tissue (33). The aforementioned cytokines have been associated with depression in previous research (34–36).
The ways in which ghrelin influences mood are illustrated in Figure 1.
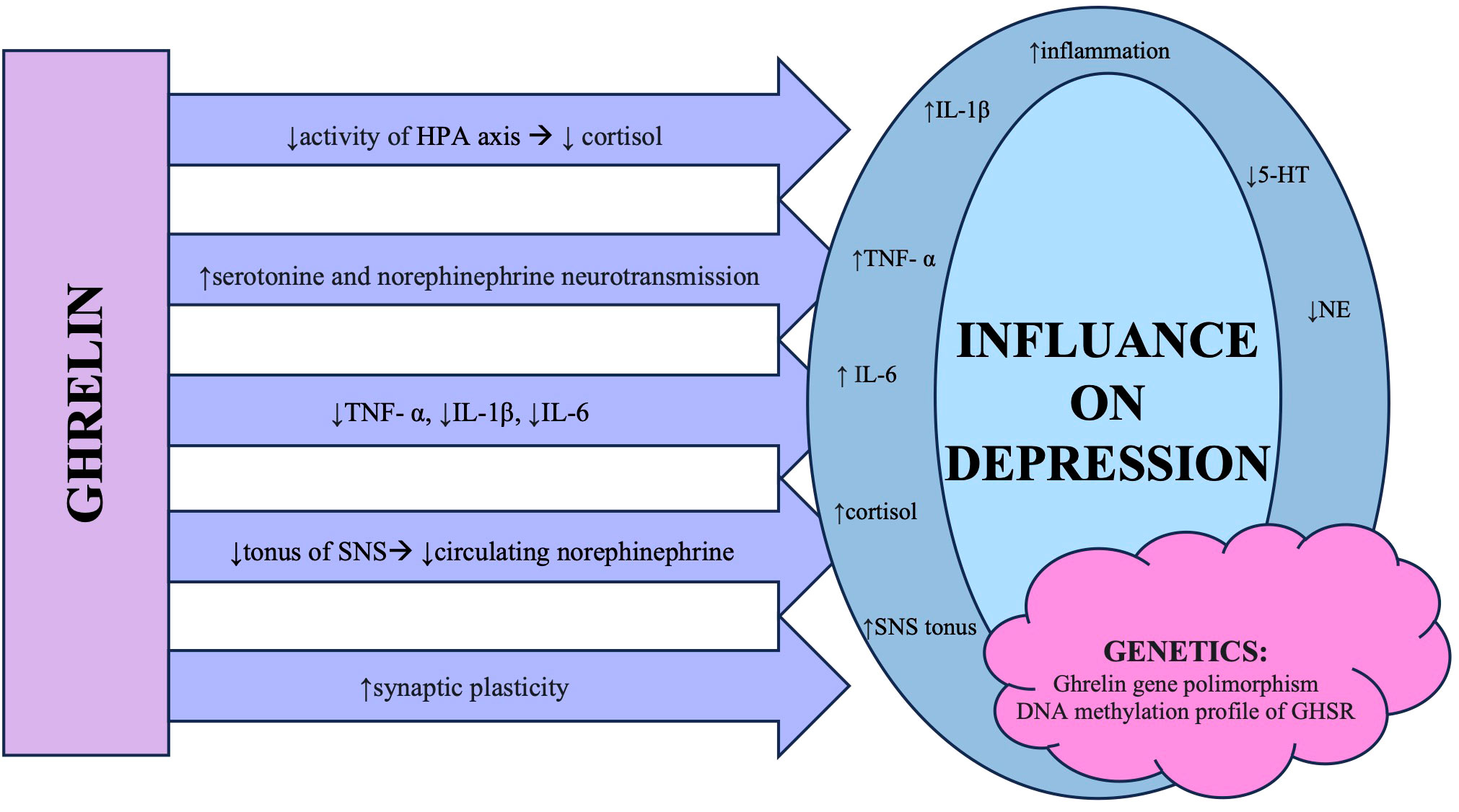
Figure 1 The summary of different mechanisms in which ghrelin influences changes observed in patients diagnosed with major depressive disorder. Abbreviations: HPA axis, hypothalamic–pituitary–adrenal axis; TNF-α, tumor necrosis factor α; IL, interleukin; SNS, sympathetic nervous system; 5-HT, 5-hydroxytryptamine/serotonin; NE, noradrenaline; GHSR, growth hormone secretagogue receptor.
3.6 Ghrelin as an antidepressant
Several studies on animals (rats and mice) showed that ghrelin administration exhibits antidepressant effects (8, 9, 30, 31, 33, 37) expressed in outcomes of the forced swimming test, sucrose preference test, tail suspension test, or elevated plus maze test. On the contrary, some researchers declared that ghrelin induced depression-like behavior in animals (10, 38, 39). Carlini et al. reported that acute ghrelin injection alleviates symptoms of depression and anxiety, while Hansson et al. noted that its chronic administration can provoke the occurrence of depression-like behavior (37, 38). Jackson et al. performed their study on juvenile rats and observed induction of depressive symptoms 1 week after injection of ghrelin (39).
The detailed studies’ characteristics and outcomes are presented in Table 1 and discussed in Section 5 of this review.
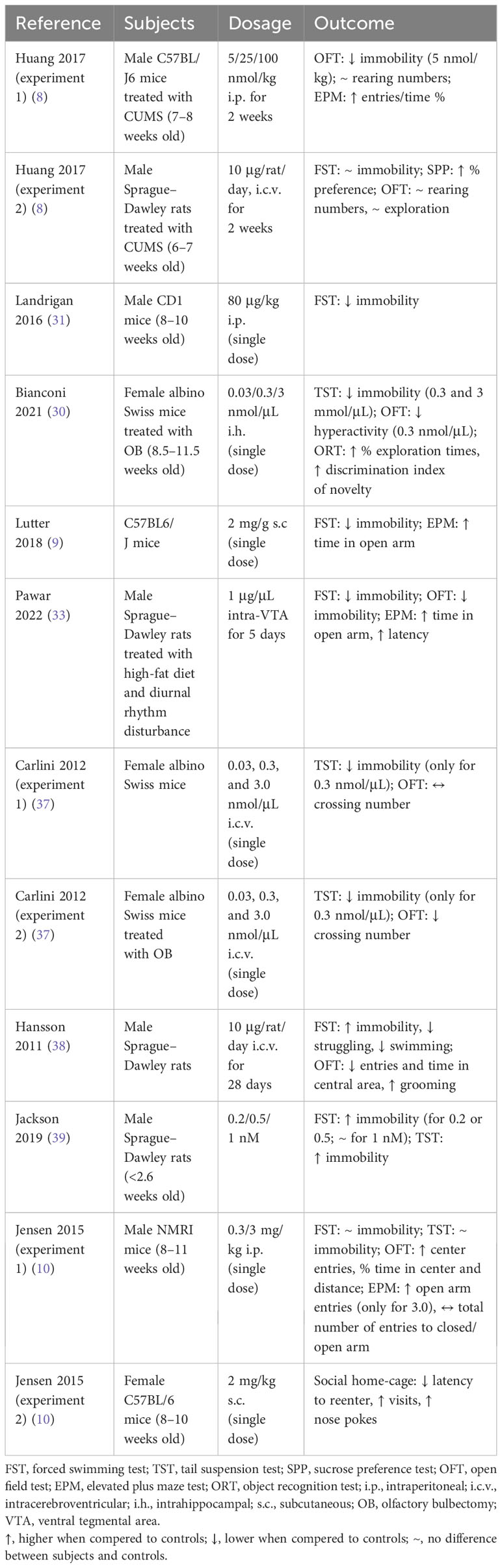
Table 1 The characteristics and outcomes of preclinical experiments regarding antidepressant effect of ghrelin’s administration.
4 Relationship between ghrelin concentration and major depressive disorder in humans
There are several studies on the relationship between ghrelin concentration in blood and depression among adult, unmedicated participants. Some of the authors reported higher ghrelin levels in those diagnosed with depression (15, 40, 41), while others showed decreased (42) or similar ghrelin levels (43–45) in comparison to healthy participants.
Algul et al. noted that ghrelin concentration shows a positive correlation with the severity of depression. Fasting ghrelin concentration was higher in severely depressed patients in comparison to subjects diagnosed with moderate depression or healthy controls (40).
Ozsoy et al. reported that ghrelin levels were elevated in patients with depression and normalized after treatment with antidepressants, ECT, or both. Furthermore, the method of treatment (drugs vs. ECT vs. drugs + ECT) did not have any influence on the decrease in serum ghrelin concentration (15). Kurt et al. reported the same results among depressed patients treated with ECT (46). The outcomes of the mentioned studies stay consistent with the findings of Ischitobi et al. They demonstrated that ghrelin levels are elevated in patients not responding to 8-week SSRI treatment in comparison to responders and healthy controls (16). Furthermore, Ricken et al. observed that ghrelin levels increase in non-responding patients with MDD after lithium augmentation and decrease in responding patients (47). It could suggest that ghrelin signaling mediates patients’ response to antidepressant treatment.
However, Paslakis et al. did not observe any differences in basal ghrelin concentration before and after antidepressant treatment. However, their study showed changes in ghrelin response to a standardized glucose load procedure (patients received 75 g of glucose after an overnight fasting period, and their ghrelin levels were measured at baseline and after 30, 60, 90, and 120 minutes of glucose intake). A significant difference was observed in terms of the time points of the loading procedure as well as between patients and matched healthy controls. The changes in patients’ BMI and the drug they have taken (mirtazapine vs. venlafaxine) showed no statistically significant influence on ghrelin concentration (48).
Active ghrelin has also been associated with postpartum depression. Baker et al. measured ghrelin levels in the 24-hour urinary samples during pregnancy and 6 weeks after labor. Women screened positive for postpartum depression 12 weeks after childbirth had higher acylated ghrelin concentrations during pregnancy. Moreover, ghrelin levels changed differently in women who were depressed during pregnancy in comparison to non-depressed subjects. The mean change of ghrelin concentration decrease from pregnancy to postpartum was less expressed among women who were depressed during pregnancy. However, the results were not statistically significant when ghrelin levels were adjusted to creatinine. It suggests that renal function should be taken into consideration while planning further research in which ghrelin is measured from urine samples (49).
There is also evidence that ghrelin levels are associated with depression severity among post-menopausal women. Naufel et al. observed that both total and acylated ghrelin concentrations were higher among those with severe depression in comparison to mildly depressed individuals. Both active and total ghrelin levels correlated positively with scores on Beck’s Depression Inventory and Patient Health Questionnaire (50).
Wittekind et al. analyzed data from LIFE-Adult-Study and included 1,092 participants whose total ghrelin levels were measured, and Center for Epidemiologic Studies Depression Scale (CES-D) scores were available. They did not observe any significant difference in regard to ghrelin concentration between depressed and non-depressed subjects. Also, there was no association between CES-D scores and total ghrelin levels (51). It should be noted that the diagnosis of depression was not confirmed by a psychiatrist nor any standardized clinical interview. There are also no data on subjects’ history of using psychotropic drugs or psychiatric evaluations. Mentioned factors could have had an influence on study results, as antidepressant treatment tends to lower ghrelin levels (15, 16, 46).
On the contrary, higher ghrelin levels were associated with a higher prevalence of depression among Japanese women. Akter et al. examined 497 participants (287 men and 210 women). They analyzed the subjects using two different cut-off points for depression in the CES-D scale (≥16 points validated for the general population and ≥19 points validated for the Japanese population). In either setting, the results stayed consistent: there was no association between ghrelin concentration in male subjects, and there was a relationship between higher ghrelin levels and higher odds for the development of depression among women (52).
In a population of older Dutch adults (55 years and older), there was no correlation between total ghrelin concentration and depressive symptoms (assessed with the CES-D scale) at the beginning of the study. However, participants with higher ghrelin levels at baseline had higher odds for depression after 3 years at follow-up, expressed specifically in participants younger than 69.7 years and with a waist–hip ratio below 0.96 (53).
Atescelik et al. conducted a study on patients admitted to the emergency department with recent suicide attempts. They reported that both acylated and unacylated ghrelin levels correlated positively with Beck’s Depression Inventory scores. Both active and non-active ghrelin concentrations were significantly higher in patients than in healthy subjects. Some of the patients had a history of psychiatric conditions (major depressive disorder, schizophrenia, schizo-affective disorder, and personality disorder); however, it did not influence the results of hormone assays between patients with different conditions and patients with no psychiatric history (54).
5 Discussion
In recent studies on both animals and humans, ghrelin has been associated with major depressive disorder. This link is expressed in ghrelin’s action involved in stress response and inflammation. The hormone also acts in reward regions of the brain as well as in the amygdala and DRN, regions previously connected to the pathophysiology of MDD. Ghrelin also exhibits some of the antidepressant’s mechanism of action—it influences serotoninergic and glutaminergic transmission and induces synaptic plasticity. Variations in both ghrelin and its receptor genes have also been observed in individuals with depression.
Preclinical studies on animals have revealed that ghrelin influences the results of tests assessing depression-like behavior (Table 2). Researchers have applied many different approaches to evaluate the hypothesis of ghrelin’s antidepressive properties, and therefore, the results are heterogenic and have their limitations. However, it should be noted that in every study in which an animal depression model was induced (by CUMS protocol, disturbed diurnal rhythm, or olfactory bulbectomy), the results stayed consistent, and ghrelin was found as an antidepressive agent independently of the dosage (route of administration, acute vs. chronic). Interestingly, Lutter et al. in the second set of experiments (not included in Table 2) did not use exogenous ghrelin but enhanced its endogenic secretion by calorie restriction, and the antidepressive effect persisted. In terms of future research, it would be crucial to induce an animal model of depression in the treated subjects. The promising results from the mentioned preclinical studies should also endorse research on humans.
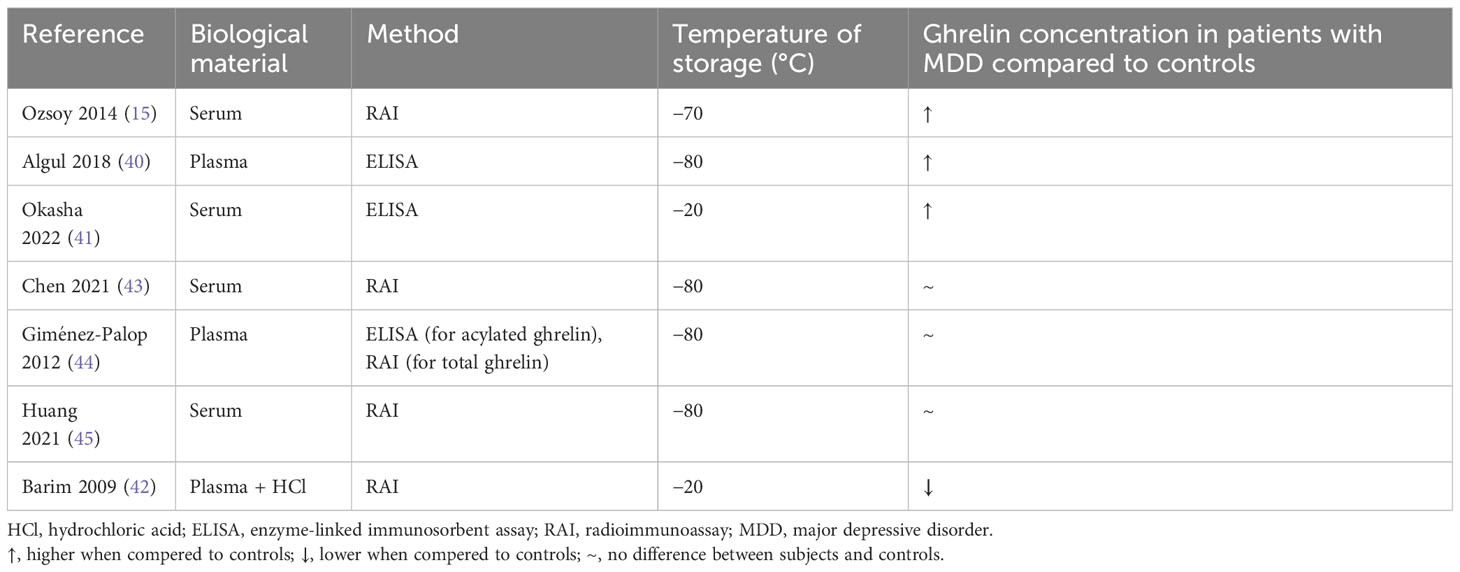
Table 2 The summary of laboratory methods used to measure ghrelin levels in studies on unmedicated humans with healthy control group.
As ghrelin signaling was shown to interfere with the HPA axis, this connection should also be further evaluated. It may be a key to understanding a mechanism in which ghrelin expresses its anxiolytic mechanism of action observed in animals. There is also a need for future research on ghrelin’s influence on inflammation, as immune response is another factor that contributes to the pathophysiology of MDD, and the data available are limited.
Research on human subjects revealed that antidepressant treatment lowers ghrelin secretion. Moreover, higher levels of circulating ghrelin correlated with the development of depression in the future. Levels of these hormones were also elevated in individuals after suicide attempts and correlated positively with MDD severity among post-menopausal women. Ghrelin was also linked to postpartum depression.
The results of current studies on humans are heterogenic, and therefore, it is difficult to determine their final meaning. The problem might arise from different laboratory methods applied to measure ghrelin levels in participants (Table 2). Ghrelin is characterized by its short half-time, and as a relatively small protein circulating in low concentrations, it is vulnerable to pre-laboratory errors. Hence, the observed dispersion of the results may arise from different laboratory approaches: biological material used (plasma vs. serum), temperature of material storage (−20°C, −70°C, or −80°C), and method applied [enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RAI)]. A consistent laboratory approach should be incorporated in future research. Moreover, it would be beneficial if both acylated and deacylated ghrelin were evaluated. This kind of systematic approach would help determine if ghrelin could be a biomarker of depression.
Those results, even if from small sample studies, show that there is a connection between ghrelin and MDD, and it might have clinical consequences. Esketamine is an antidepressant that is recommended for patients with treatment-resistant depression. In studies on animals, ketamine’s action was antagonized by ghrelin administration. Studies on humans revealed that patients with higher BMI, and therefore lower concentration of circulating ghrelin, had a higher chance for remission while being treated with esketamine. On the contrary, among patients treated with escitalopram and venlafaxine, the trend was reversed—those with higher BMI had the lowest remission rate (55). In-depth research on ghrelin signaling and antidepressant treatment could provide insight into treatment resistance mechanism, which still remains a challenge in managing treatment-resistant patients. Moreover, it could determine which antidepressant would be most suitable for a patient in a given metabolic state.
Considering that 20% to 60% of patients do not respond to first-line MDD treatment, broadening the knowledge of biological factors contributing to resistance could shorten the time to recovery. Gathered data suggest that metabolic hormones, including ghrelin, may play a role in treatment response. This possible connection is supported by the fact that ghrelin levels dropped among responders and stayed unchanged among non-responders, as Ischitobi et al. and Ricken et al. have demonstrated. Conducting research that would identify antidepressant medications with a higher response rate in a given metabolic state would benefit both patients and clinicians.
An in-depth understanding of the pathophysiology of depression is highly needed, as it could address some of the problems currently faced by practitioners and patients, which include managing treatment-resistant patients, tolerance to a drug, or weight gain following antidepressant treatment. Future research on the relationship between ghrelin and depression should address the following questions:
● Does ghrelin signaling interfere with antidepressant treatment, and if yes, could it potentially explain some aspects of treatment resistance?
● Could ghrelin concentration determine which antidepressant medication would benefit the patient the most in a given metabolic state?
● Does ghrelin exhibit an antidepressant effect in humans as it was shown in research on animals?
● Does ghrelin concentration differ between depressed and non-depressed patients, and if yes, could ghrelin be a biomarker of MDD?
Author contributions
ML: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing. TM: Investigation, Writing – original draft, Writing – review & editing. MM: Investigation, Writing – review & editing. TZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wittekind DA, Kluge M. Ghrelin in psychiatric disorders - A review. Psychoneuroendocrinology. (2015) 52:176–94. doi: 10.1016/j.psyneuen.2014.11.013
2. Ibrahim Abdalla MM. Ghrelin – physiological functions and regulation. Eur Endocrinol. (2015) 11:90–5. doi: 10.17925/EE.2015.11.02.90
3. Pearson S, Schmidt M, Patton G, Dwyer T, Blizzard L, Otahal P, et al. Depression and insulin resistance. Diabetes Care. (2010) 33:1128–33. doi: 10.2337/dc09-1940
4. Pradhan G, Samson SL, Sun Y. Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care. (2013) 16:619–24. doi: 10.1097/MCO.0b013e328365b9be
5. Morin V, Hozer F, Costemale-Lacoste J-F. The effects of ghrelin on sleep, appetite, and memory, and its possible role in depression: A review of the literature. L’Encephale. (2018) 44:256–63. doi: 10.1016/j.encep.2017.10.012
6. Els S, Beck-Sickinger AG, Chollet C. Chapter Six - Ghrelin Receptor: High Constitutive Activity and Methods for Developing Inverse Agonists. In: Conn PM, editor. Methods in Enzymology. Constitutive Activity in Receptors and Other Proteins, Part B. New York, United States: Academic Press (2010). p. 103–21. doi: 10.1016/B978-0-12-381296-4.00006-3
7. Kojima M, Hamamoto A, Sato T. Ghrelin O-acyltransferase (GOAT), a specific enzyme that modifies ghrelin with a medium-chain fatty acid. J Biochem. (2016) 160:189–94. doi: 10.1093/jb/mvw046
8. Huang H-J, Zhu X-C, Han Q-Q, Wang Y-L, Yue N, Wang J, et al. Ghrelin alleviates anxiety- and depression-like behaviors induced by chronic unpredictable mild stress in rodents. Behav Brain Res. (2017) 326:33–43. doi: 10.1016/j.bbr.2017.02.040
9. Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. (2008) 11:752–3. doi: 10.1038/nn.2139
10. Jensen M, Ratner C, Rudenko O, Christiansen SH, Skov LJ, Hundahl C, et al. Anxiolytic-like effects of increased ghrelin receptor signaling in the amygdala. Int J Neuropsychopharmacol. (2016) 19:1–12. doi: 10.1093/ijnp/pyv123
11. Gupta D, Chuang J-C, Mani BK, Shankar K, Rodriguez JA, Osborne-Lawrence S, et al. β1-adrenergic receptors mediate plasma acyl-ghrelin elevation and depressive-like behavior induced by chronic psychosocial stress. Neuropsychopharmacology. (2019) 44:1319–27. doi: 10.1038/s41386-019-0334-7
12. Mahbod P, Smith EP, Fitzgerald ME, Morano RL, Packard BA, Ghosal S, et al. Desacyl ghrelin decreases anxiety-like behavior in male mice. Endocrinology. (2018) 159:388–99. doi: 10.1210/en.2017-00540
13. Veith RC, Lewis N, Linares OA, Barnes RF, Raskind MA, Villacres EC, et al. Sympathetic nervous system activity in major depression. Basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch Gen Psychiatry. (1994) 51:411–22. doi: 10.1001/archpsyc.1994.03950050071008
14. Dziurkowska E, Wesolowski M. Cortisol as a biomarker of mental disorder severity. J Clin Med. (2021) 10. doi: 10.3390/jcm10215204
15. Ozsoy S, Besirli A, Abdulrezzak U, Basturk M. Serum ghrelin and leptin levels in patients with depression and the effects of treatment. Psychiatry Invest. (2014) 11:167–72. doi: 10.4306/pi.2014.11.2.167
16. Ishitobi Y, Kohno K, Kanehisa M, Inoue A, Imanaga J, Maruyama Y, et al. Serum ghrelin levels and the effects of antidepressants in major depressive disorder and panic disorder. Neuropsychobiology. (2012) 66:185–92. doi: 10.1159/000339948
17. Nakashima K, Akiyoshi J, Hatano K, Hanada H, Tanaka Y, Tsuru J, et al. Ghrelin gene polymorphism is associated with depression, but not panic disorder. Psychiatr Genet. (2008) 18:257. doi: 10.1097/YPG.0b013e328306c979
18. Córdova-Palomera A, Palma-Gudiel H, Forés-Martos J, Tabarés-Seisdedos R, Fañanás L. Epigenetic outlier profiles in depression: A genome-wide DNA methylation analysis of monozygotic twins. PloS One. (2018) 13:e0207754. doi: 10.1371/journal.pone.0207754
19. Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. (2013) 14:609–25. doi: 10.1038/nrn3381
20. Nestler EJ. Role of the brain’s reward circuitry in depression: transcriptional mechanisms. Int Rev Neurobiol. (2015) 124:151–70. doi: 10.1016/bs.irn.2015.07.003
21. Fujitsuka N, Asakawa A, Hayashi M, Sameshima M, Amitani H, Kojima S, et al. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol Psychiatry. (2009) 65:748–59. doi: 10.1016/j.biopsych.2008.10.031
22. Hansson C, Alvarez-Crespo M, Taube M, Skibicka KP, Schmidt L, Karlsson-Lindahl L, et al. Influence of ghrelin on the central serotonergic signaling system in mice. Neuropharmacology. (2014) 79:498–505. doi: 10.1016/j.neuropharm.2013.12.012
23. Ogaya M, Kim J, Sasaki K. Ghrelin postsynaptically depolarizes dorsal raphe neurons in rats. vitro Peptides. (2011) 32:1606–16. doi: 10.1016/j.peptides.2011.07.001
24. Chen L, Xing T, Wang M, Miao Y, Tang M, Chen J, et al. Local infusion of ghrelin enhanced hippocampal synaptic plasticity and spatial memory through activation of phosphoinositide 3-kinase in the dentate gyrus of adult rats. Eur J Neurosci. (2011) 33:266–75. doi: 10.1111/j.1460-9568.2010.07491.x
25. Johansen A, Armand S, Plavén-Sigray P, Nasser A, Ozenne B, Petersen IN, et al. Effects of escitalopram on synaptic density in the healthy human brain: a randomized controlled trial. Mol Psychiatry. (2023) 28:1–8. doi: 10.1038/s41380-023-02285-8
26. Adell A. Brain NMDA receptors in schizophrenia and depression. Biomolecules. (2020) 10:947. doi: 10.3390/biom10060947
27. Amidfar M, Woelfer M, Réus GZ, Quevedo J, Walter M, Kim Y-K. The role of NMDA receptor in neurobiology and treatment of major depressive disorder: Evidence from translational research. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 94:109668. doi: 10.1016/j.pnpbp.2019.109668
28. Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. (2013) 170:1134–42. doi: 10.1176/appi.ajp.2013.13030392
29. Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. (2019) 22:616–30. doi: 10.1093/ijnp/pyz039
30. Bianconi S, Poretti MB, Rodríguez P, Maestri G, Rodríguez PE, de Barioglio SR, et al. Ghrelin restores memory impairment following olfactory bulbectomy in mice by activating hippocampal NMDA1 and MAPK1 gene expression. Behav Brain Res. (2021) 410:113341. N.PAG-N.PAG. doi: 10.1016/j.bbr.2021.113341
31. Landrigan J, Shawaf F, Dwyer Z, Abizaid A, Hayley S. Interactive effects of ghrelin and ketamine on forced swim performance: Implications for novel antidepressant strategies. Neurosci Lett. (2018) 669:55–8. doi: 10.1016/j.neulet.2016.08.015
32. Freeman MP, Hock RS, Papakostas GI, Judge H, Cusin C, Mathew SJ, et al. Body mass index as a moderator of treatment response to ketamine for major depressive disorder. J Clin Psychopharmacol. (2020) 40:287. doi: 10.1097/JCP.0000000000001209
33. Pawar GR, Agrawal YO, Nakhate KT, Patil CR, Sharma C, Ojha S, et al. Ghrelin alleviates depression-like behaviour in rats subjected to high-fat diet and diurnal rhythm disturbance. Am J Trans Res. (2022) 14:7098–108.
34. Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
35. Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, et al. IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep. (2018) 8:12050. doi: 10.1038/s41598-018-30487-6
36. Das R, Emon M, Shahriar M, Nahar Z, Islam SMA, Bhuiyan MA, et al. Higher levels of serum IL-1β and TNF-α are associated with an increased probability of major depressive disorder. Psychiatry Res. (2021) 295:113568. doi: 10.1016/j.psychres.2020.113568
37. Carlini VP, MaChado DG, Buteler F, Ghersi M, Ponzio MF, Martini AC, et al. Acute ghrelin administration reverses depressive-like behavior induced by bilateral olfactory bulbectomy in mice. Peptides. (2012) 35:160–5. doi: 10.1016/j.peptides.2012.03.031
38. Hansson C, Haage D, Taube M, Egecioglu E, Salomé N, Dickson SL. Central administration of ghrelin alters emotional responses in rats: behavioural, electrophysiological and molecular evidence. Neuroscience. (2011) 180:201–11. doi: 10.1016/j.neuroscience.2011.02.002
39. Jackson TM, Ostrowski TD, Middlemas DS. Intracerebroventricular ghrelin administration increases depressive-like behavior in male juvenile rats. Front Behav Neurosci. (2019) 13:77. doi: 10.3389/fnbeh.2019.00077
40. Algul S, Ozcelik O. Evaluating the levels of nesfatin-1 and ghrelin hormones in patients with moderate and severe major depressive disorders. Psychiatry Invest. (2018) 15:214–8. doi: 10.30773/pi.2017.05.24
41. Okasha TA, El-Gabry DA, Ali MH, Gabrielle FF. The role of ghrelin peptide among a sample of Egyptian patients with first episode of major depressive disorder. Middle East Curr Psychiatry. (2022) 29:1–7. doi: 10.1186/s43045-022-00263-4
42. Barim AO, Aydin S, Colak R, Dag E, Deniz O, Sahin I. Ghrelin, paraoxonase and arylesterase levels in depressive patients before and after citalopram treatment. Clin Biochem. (2009) 42:1076–81. doi: 10.1016/j.clinbiochem.2009.02.020
43. Chen M-H, Hsu J-W, Huang K-L, Tsai S-J, Su T-P, Li C-T, et al. Role of appetite hormone dysregulation in the cognitive function among patients with bipolar disorder and major depressive disorder. World J Biol Psychiatry. (2021) 22:428–34. doi: 10.1080/15622975.2020.1819566
44. Giménez-Palop O, Coronas R, Cobo J, Gallart L, Barbero JD, Parra I, et al. Fasting plasma peptide YY concentrations are increased in patients with major depression who associate weight loss. J Endocrinol Invest. (2012) 35:645–8. doi: 10.3275/8180
45. Huang K-L, Chen M-H, Hsu J-W, Tsai S-J, Bai Y-M. Using classification and regression tree modeling to investigate appetite hormones and proinflammatory cytokines as biomarkers to differentiate bipolar I depression from major depressive disorder. CNS spect. (2021) 27: 1–7. doi: 10.1017/S109285292100016X
46. Kurt E, Guler O, Serteser M, Cansel N, Ozbulut O, Altinbaş K, et al. The effects of electroconvulsive therapy on ghrelin, leptin and cholesterol levels in patients with mood disorders. Neurosci Lett. (2007) 426:49–53. doi: 10.1016/j.neulet.2007.08.018
47. Ricken R, Bopp S, Schlattmann P, Himmerich H, Bschor T, Richter C, et al. Ghrelin serum concentrations are associated with treatment response during lithium augmentation of antidepressants. Int J Neuropsychopharmacol. (2017) 20:692–7. doi: 10.1093/ijnp/pyw082
48. Paslakis G, Westphal S, Hamann B, Gilles M, Lederbogen F, Deuschle M. Unstimulated and glucose-stimulated ghrelin in depressed patients and controls. J Psychopharmacol (Oxford England). (2014) 28:582–6. doi: 10.1177/0269881114527655
49. Baker JH, Pedersen C, Leserman J, Brownley KA. Active ghrelin and the postpartum. Arch women’s Ment Health. (2016) 19:515–20. doi: 10.1007/s00737-015-0578-0
50. Naufel MF, Pedroso AP, Oyama LM, Telles MM, Hachul H, Ribeiro EB. Preliminary evidence of acylated ghrelin association with depression severity in postmenopausal women. Sci Rep. (2021) 11:1–9. doi: 10.1038/s41598-021-84431-2
51. Wittekind DA, Kratzsch J, Biemann R, Mergl R, Riedel-Heller S, Witte V, et al. Association between self-rating depression scores and total ghrelin and adipokine serum levels in a large population-based sample. Front Psychiatry. (2022) 13:891325. doi: 10.3389/fpsyt.2022.891325
52. Akter S, Pham NM, Nanri A, Kurotani K, Kuwahara K, Jacka FN, et al. Association of serum leptin and ghrelin with depressive symptoms in a Japanese working population: a cross-sectional study. BMC Psychiatry. (2014) 14:203. doi: 10.1186/1471-244X-14-203
53. van Andel M, van Schoor NM, Korten NC, Heijboer AC, Drent ML. Ghrelin, leptin and high-molecular-weight adiponectin in relation to depressive symptoms in older adults: Results from the Longitudinal Aging Study Amsterdam. J Affect Disord. (2022) 296:103–10. doi: 10.1016/j.jad.2021.09.069
54. Atescelik M, Yilmaz M, Korkmaz S, Goktekin MC, Gurger M, Ilhan N. The relationship between ghrelin and copeptin levels, and anxiety and depression levels in suicide attempts. Clin Psychopharmacol Neurosci. (2017) 15:256–60. doi: 10.9758/cpn.2017.15.3.256
Keywords: ghrelin, metabolic hormone, depression, major depressive disorder (MDD), mood disorder
Citation: Lis M, Miłuch T, Majdowski M and Zawodny T (2024) A link between ghrelin and major depressive disorder: a mini review. Front. Psychiatry 15:1367523. doi: 10.3389/fpsyt.2024.1367523
Received: 08 January 2024; Accepted: 26 February 2024;
Published: 13 March 2024.
Edited by:
Tasuku Hashimoto, International University of Health and Welfare (IUHW), JapanReviewed by:
Gabriel Guillén-Ruiz, Universidad Veracruzana, MexicoTakahiro Sato, Kurume University, Japan
Copyright © 2024 Lis, Miłuch, Majdowski and Zawodny. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tymoteusz Miłuch, dHltb3RldXN6Lm1pbHVjaEBzenBpdGFsY3plcm5pYWtvd3NraS53YXcucGw=
 Michał Lis
Michał Lis Tymoteusz Miłuch
Tymoteusz Miłuch Maciej Majdowski1
Maciej Majdowski1