
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 07 May 2024
Sec. ADHD
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1365159
 Zarah van der Pal1*
Zarah van der Pal1* Kristine B. Walhovd2,3
Kristine B. Walhovd2,3 Inge K. Amlien2,3
Inge K. Amlien2,3 Carlijn Jamila Guichelaar2
Carlijn Jamila Guichelaar2 Antonia Kaiser1,4
Antonia Kaiser1,4 Marco A. Bottelier5
Marco A. Bottelier5 Hilde M. Geurts6
Hilde M. Geurts6 Liesbeth Reneman1
Liesbeth Reneman1 Anouk Schrantee1
Anouk Schrantee1Background: Stimulant medication is commonly prescribed as treatment for attention-deficit/hyperactivity disorder (ADHD). While we previously found that short-term stimulant-treatment influences apparent cortical thickness development in an age-dependent manner, it remains unknown whether these effects persist throughout development into adulthood.
Purpose: Investigate the long-term age-dependent effects of stimulant medication use on apparent cortical thickness development in adolescents and adults previously diagnosed with ADHD.
Methods: This prospective study included the baseline and 4-year follow-up assessment of the “effects of Psychotropic drugs On the Developing brain-MPH” (“ePOD-MPH”) project, conducted between June-1-2011 and December-28-2019. The analyses were pre-registered (https://doi.org/10.17605/OSF.IO/32BHF). T1-weighted MR scans were obtained from male adolescents and adults, and cortical thickness was estimated for predefined regions of interest (ROIs) using Freesurfer. We determined medication use and assessed symptoms of ADHD, anxiety, and depression at both time points. Linear mixed models were constructed to assess main effects and interactions of stimulant medication use, time, and age group on regional apparent cortical thickness.
Results: A total of 32 male adolescents (aged mean ± SD, 11.2 ± 0.9 years at baseline) and 24 men (aged mean ± SD, 29.9 ± 5.0 years at baseline) were included that previously participated in the ePOD-MPH project. We found no evidence for long-term effects of stimulant medication use on ROI apparent cortical thickness. As expected, we did find age-by-time interaction effects in all ROIs (left prefrontal ROI: P=.002, right medial and posterior ROIs: P<.001), reflecting reductions in apparent cortical thickness in adolescents. Additionally, ADHD symptom severity (adolescents: P<.001, adults: P=.001) and anxiety symptoms (adolescents: P=0.03) were reduced, and more improvement of ADHD symptoms was associated with higher medication use in adults (P=0.001).
Conclusion: We found no evidence for long-term effects of stimulant-treatment for ADHD on apparent cortical thickness development in adolescents and adults. The identified age-dependent differences in apparent cortical thickness development are consistent with existing literature on typical cortical development.
Stimulant medication, such as methylphenidate- (MPH) and dexamphetamine-based formulations, is commonly prescribed as treatment for ADHD, a prevalent neurodevelopmental disorder characterized by age-inappropriate levels of inattentive, hyperactive and impulsive behavior (1, 2). Stimulant medication has been shown to be highly effective in alleviating core ADHD symptoms of hyperactivity and inattention, as well as ancillary symptoms such as emotional dysregulation (3, 4). Although children and adolescents often receive stimulant-treatment for extended periods of time, possible long-lasting effects of extended stimulant-treatment on cortical development of the brain remain unclear.
Cortical morphology undergoes continuous development throughout the lifetime, with magnetic resonance imaging (MRI) studies reporting rapid reductions in apparent cortical thickness (i.e., cortical thinning) during adolescence and continued cortical thinning at a slower rate throughout adulthood (5, 6). In contrast, changes in cortical surface area predominantly occur during childhood and early adolescence (7, 8). Previous studies investigating cortical maturation in individuals with ADHD using MRI suggest that children and adolescents with ADHD ‘lag behind’ typically developing peers in development of grey matter volume and cortical thickness, particularly in prefrontal regions (9). Moreover, alterations in cortical thickness, surface area and grey matter volume have been negatively associated with clinical outcomes such as ADHD symptom severity and depressive symptoms (10, 11). Of note, apparent changes in cortical thickness during development may in part result from other factors such as increased myelination, which impacts MR contrast and the grey-white matter boundaries used for cortical thickness estimation (12).
However, studies investigating the effect of stimulant medication on brain morphology are less clear and yielded inconsistent results. For instance, a longitudinal study reported more rapid cortical thinning in ADHD participants off stimulant medication, compared with ADHD participants on stimulant medication and typically developing peers (13). Furthermore, a voxel-based morphometry meta-analysis found that stimulant medication use was associated with higher (i.e., more “normalized”) grey matter volume in the right caudate (14). In contrast, two large-scale studies using cross-sectional data in adolescents and adults with ADHD identified no associations between various stimulant-treatment parameters and cortical thickness (15, 16). Notably, prior research mostly examined children, adolescents, or adults separately, without considering potential age-related effects of stimulant-treatment on cortical development. Such age-dependent effects of stimulant medication are supported by animal studies, suggesting that stimulant medication use during early adolescence has lasting effects on brain development (“the neurochemical imprinting hypothesis”) (17, 18). Moreover, we previously found that 4-month MPH-treatment resulted in less rapid cortical thinning in children with ADHD, but not in adults or placebo groups (19). Thus, (preclinical) findings so far suggest that the short-term effects of stimulant-treatment on apparent cortical thickness development are modulated by age. However, it remains unclear whether these effects last throughout development into adulthood.
We hypothesized that stimulant medication use would induce persistent (long-term) age-dependent effects on regional cortical thickness. Specifically, we hypothesized that higher stimulant exposure would be associated with less rapid regional cortical thinning in adolescents, but not in adults with ADHD. Therefore, the present study aimed to investigate whether stimulant medication modulates regional cortical thickness development in a 4-year naturalistic follow-up of the children and adults previously diagnosed with ADHD.
This study is part of the prospective “effects of Psychotropic drugs On the Developing brain - MPH” (ePOD-MPH) project. The initial ePOD-MPH randomized controlled trial (RCT) was a 16-week double-blind, randomized, placebo-controlled, multicenter trial of MPH-treatment conducted between June 1 2011 and June 15 2015, with a blinded endpoint evaluation in stimulant treatment-naive participants with ADHD (20). The present study constitutes the naturalistic 4-year follow-up assessment of the ePOD-MPH RCT, conducted between March 1 2016 and December 28 2019. The ePOD-MPH RCT protocol applied the code of medical ethics and was registered by the Central Committee on Research Involving Human Subjects (an independent registry) on March 24, 2011 (identifier NL34509.000.10) and subsequently at The Netherlands National Trial Register (identifier NTR3103), with enrolment of the first patient on October 13, 2011. The 4-year follow-up assessment was approved by the local medical ethical committee of the Academic Medical Center (NL54972.018.15). All participants and parents or legal representatives of the children provided written informed consent.
This study’s design and analysis plan were pre-registered at the Open Science Foundation registry (https://doi.org/10.17605/OSF.IO/32BHF). For deviations from the pre-registered analysis plan, please see Supplementary Materials.
For the initial ePOD-MPH RCT, we included 50 children (aged 10-12 years) and 49 adult (aged 23-30 years) male outpatients diagnosed with ADHD (all subtypes). ADHD diagnosis, as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV (1)), was determined by an experienced psychiatrist using a structured interview (Diagnostic Interview Schedule for Children fourth edition, DISC-IV (21)) in children or in parents and the Diagnostic Interview for Adult ADHD (22) in adults (for details on recruitment and exclusion criteria, see Supplementary Materials). All participants were eligible for pharmacological treatment (with stimulant medication). For the current study, the 4-year follow-up assessment, participants were contacted by phone and/or email to ask if they wanted to participate in the follow-up assessment. Exclusion criteria were contraindications to MRI.
Stimulant medication use per participant was calculated based on medication received during the initial ePOD-MPH RCT and medication use information between the ePOD-MPH RCT and 4-year follow-up assessment obtained from pharmacies. The following medication use variables were calculated: cumulative dose (mg), exposure duration (months), mean daily dose (mg/day), and age at start of medication use (years). Moreover, we determined stimulant medication use at follow-up (yes/no) and stimulant treatment-naivety at follow-up (yes/no) (for details, see Supplementary Materials).
During the ePOD-MPH RCT, the treating physician prescribed short-acting MPH under double-blind clinical guidance (reduction of ADHD symptoms) following Dutch treatment guidelines. Cumulative dose of MPH was calculated from the prescribed medication and the treatment compliance rate. Exposure duration was 4 months for participants who received MPH, and 0 months for participants who received placebo.
Stimulant medication use between ePOD-MPH RCT end and 4-year follow-up assessment was calculated based on medication history information obtained from participants’ pharmacies. MPH- and dexamphetamine-based formulations were considered as stimulant medication as treatment for ADHD, and cumulative dose was converted to MPH-equivalents (23). Exposure duration to stimulant medication was determined by calculating the time between the start date and end date of stimulant medication use, with a 30-day permissible gap to allow for commonly occurring “medication holidays”, and rounding to months. Next, mean daily dose between baseline and 4-year follow-up assessment was calculated by dividing cumulative dose (in mg) by exposure duration (in days). The age at start of medication use was determined based on the date of the baseline assessment for participants who received MPH during the ePOD-MPH RCT, and date of first stimulant medication prescription in the pharmacy overview for participants who received placebo during the ePOD-MPH RCT.
Participants with no stimulant medication use between baseline and 4-year follow-up assessment were considered stimulant treatment-naive, and cumulative dose, exposure duration, and mean daily dose were set to zero.
ADHD symptoms were assessed at baseline and follow-up using the Disruptive Behavior Disorder Rating-Scale (DBD-RS, inattention and hyperactivity/impulsivity subscales (24)) in adolescents, and the ADHD-Rating Scale (ADHD-RS (22)) in adults. Symptoms of depression and anxiety were assessed at baseline and follow-up using the Child Depression Inventory (CDI (25)) and the child version of the Screen for Child Anxiety Related Disorders (SCARED (26)) in adolescents, and the Beck Depression Inventory (BDI-II (27)) and the Beck Anxiety Inventory (BAI (28)) in adults. While these scales alone are insufficient for clinical diagnosis and primarily serve as continuous measures of symptom severity, we include the cut-off values to contextualize the outcomes more broadly. For adolescents, thresholds indicating clinically significant symptoms were >15 for the DBD-RS inattention and hyperactive/impulsive subscales (29), >14 for the CDI (indicating mild depression (25)), and >24 for the SCARED (30). For adults, clinically significant symptoms were defined by values exceeding >10 for the ADHD-RS (22), >13 for the BDI (indicating mild depression (31)), and >7 for the BAI (indicating mild anxiety (32)). These cut-off values provide valuable context for interpreting the results within a broader framework.
At baseline, MR imaging was performed using a 3T Intera or Achieva MR scanner, and at 4-year follow-up using a 3T Ingenia MR scanner (Philips Medical Systems, Best, The Netherlands). 3D T1-weighted fast-field echo sequences (TR/TE/FA=9.8/4.6ms/8°, voxel size=0.875x0.875x1.2 mm, slices=120, reconstruction matrix=256) were acquired at baseline and 4-year follow-up using an 8-channel receive-only head coil. We used a one-week washout period prior to the follow-up assessment to exclude possible acute effects of medication use (half-life: 2-10 hours).
Vertex-wise cortical thickness was estimated across the brain surface using the FreeSurfer longitudinal processing stream (version 7.1 (33)). Predefined ROIs in the left prefrontal, right medial and right posterior parietal cortices, were selected based on prior findings of psychostimulant effects on apparent cortical thickness by a 4-year longitudinal observational study (13) as well as the initial 16-week ePOD-MPH RCT (19). The Montreal Neurological Institute (MNI) coordinates for previously (13) reported peak group differences were as follows: left frontal: −26, 61, 6; right medial frontal: 6, −23, 40; right posterior: 30, −93, 12. MNI coordinates of the vertices corresponding to these peak group differences were converted to the Talairach coordinate system, and dilated 15 times to create hexagonal ROIs of 550 mm2 when transformed to subjects’ brain surfaces. Surface measures were extracted from the individual participants. In line with the initial ePOD-MPH RCT, scans were visually inspected and rated for the presence of motion and were excluded unless rated 1 (no sign of motion) or 2 (minor signs of motion, but no major distortion and acceptable reconstruction). Reconstructed datasets were not edited, to avoid manual interference.
Analyses were performed using R version 4.0.3 (R Development Core Team, 2011). To identify potential covariates to include in the statistical analysis, we assessed correlations between medication use variables and other (clinical) outcome measures (Supplementary Materials).
For the confirmatory ROI analysis, linear mixed models (LMMs) were constructed to assess main effects and interactions of stimulant medication use, time (baseline, follow-up) and age group (adolescents, adults) on regional cortical thickness. Separate models were used for each ROI and medication use variable (cumulative dose, exposure duration). Medication use variables were included as 1) continuous, and 2) grouped (none/low vs. high; median split) variables. In addition, to assess whether the effects of the initial ePOD-MPH RCT were still present at 4-year follow-up, we constructed LMMs to assess main effects and interactions of ePOD-MPH RCT treatment group (MPH, placebo), time (baseline, follow-up) and age group (adolescents, adults) on regional cortical thickness. Two covariates were included in all models: demeaned age at baseline (per age group; to correct for the larger age range at baseline among adults compared with adolescents) and standardized scan interval (offset from 48 months, corresponding to 4-year follow-up). MR scanner at baseline was included as a covariate, and removed if it did not significantly improve the model. The significance level was set at P<0.05. Additionally, Bayes Factors were calculated comparing the models with medication use to the models without medication use, to determine the strength of the reported evidence (for details, see Supplementary Materials).
We performed exploratory analyses evaluating relations between regional cortical thickness and clinical outcomes. LMMs were constructed separately per age group to assess main effects and interactions of clinical outcome measures and time (baseline, follow-up) on regional apparent cortical thickness. Separate models were used for each ROI and clinical outcome measure (ADHD symptom severity, anxiety and depressive symptoms). In line with the ROI cortical thickness analysis, demeaned age at baseline and standardized scan interval were included in the models as covariates. Benjamini-Hochberg multiple comparison correction (FDR=5%) was applied to adjust for the three clinical scales evaluated.
In addition to the predefined ROIs evaluated in the main analysis, additional brain regions may also be impacted by stimulant medication use. Therefore, we performed an exploratory whole-brain analysis in Freesurfer to assess associations between medication use and vertex-wise cortical thickness and surface area, separately for adolescents and adults. Again, demeaned age at baseline and standardized scan interval were included in the models as covariates. Separate models were used for each surface measure (cortical thickness, surface area), and medication use variable (mean daily dose, medication use at time of 4-year follow-up). First, each individual’s surface was sampled to the fsaverage surface and the difference between the two time points (baseline, follow-up) was computed. Next, these differences were concatenated into one file and smoothing was performed (full-width-at-half-maximum (FWHM 10)). Subsequently, paired analysis was performed using mri_glmfit, and cluster correction was performed using mri_glmfit-sim (cluster threshold: P<.001, FDR=5%) to account for the amount of smoothing and the number of contiguous significant vertices.
Of the 50 children and 49 adults who participated in the initial ePOD-MPH RCT, 33 children (66% return rate) and 25 adults (50% return rate) were included in the 4-year follow-up assessment (Table 1; Figure 1). Baseline characteristics were comparable for participants that did and did not return for follow-up (see Supplementary Table 2). Of those that returned at follow-up, six adolescents were scanned using MR scanner 1 (Intera) and the remaining participants were scanned using MR scanner 2 (Achieva) at the baseline assessment. One adolescent and one adult were excluded from analysis due to missing MRI data and undisclosed prior exposure to stimulant medication, respectively. The final sample consisted of 32 adolescents (aged 11.2 ± 0.9 years at baseline) and 24 men (aged 29.9 ± 5.0 years at baseline). For two adolescents, MRI data from one time point was excluded (one baseline scan, one follow-up scan), due to incorrect/failed segmentation resulting from motion. In contrast to the ePOD-MPH RCT, two participants with structural brain abnormalities (one adolescent with a posterior/cerebellar cyst, one adolescent with a benign tumor in the right frontal lobe) were not excluded from analysis. This decision was made, since these structural abnormalities did not change between baseline and follow-up, and sample size at follow-up was limited. Results were robust when these participants were excluded in a sensitivity analysis (Supplementary Materials).
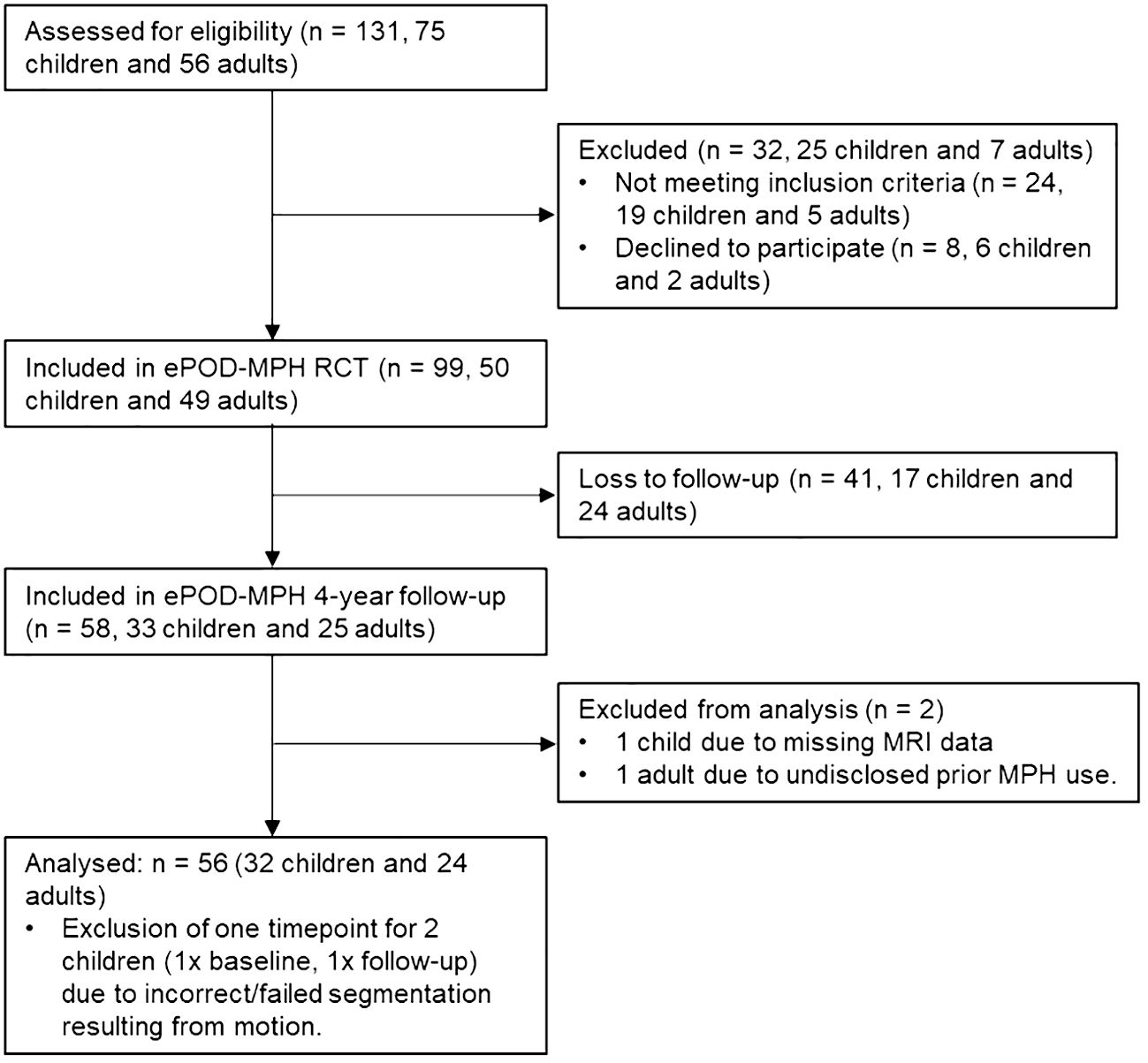
Figure 1 Flow diagram showing participant inclusion process. For consistency, child/adolescent participants are referred to as children throughout the flow diagram. MPH, methylphenidate; RCT, randomized controlled trial.
Exposure duration was higher in adolescents than adults (W=509, P=0.04), whereas cumulative and mean daily dose of stimulant medication did not differ between age groups (Table 1; Figure 2). Two adult participants were prescribed the non-stimulant medication atomoxetine as treatment for ADHD, in addition to stimulant medication.
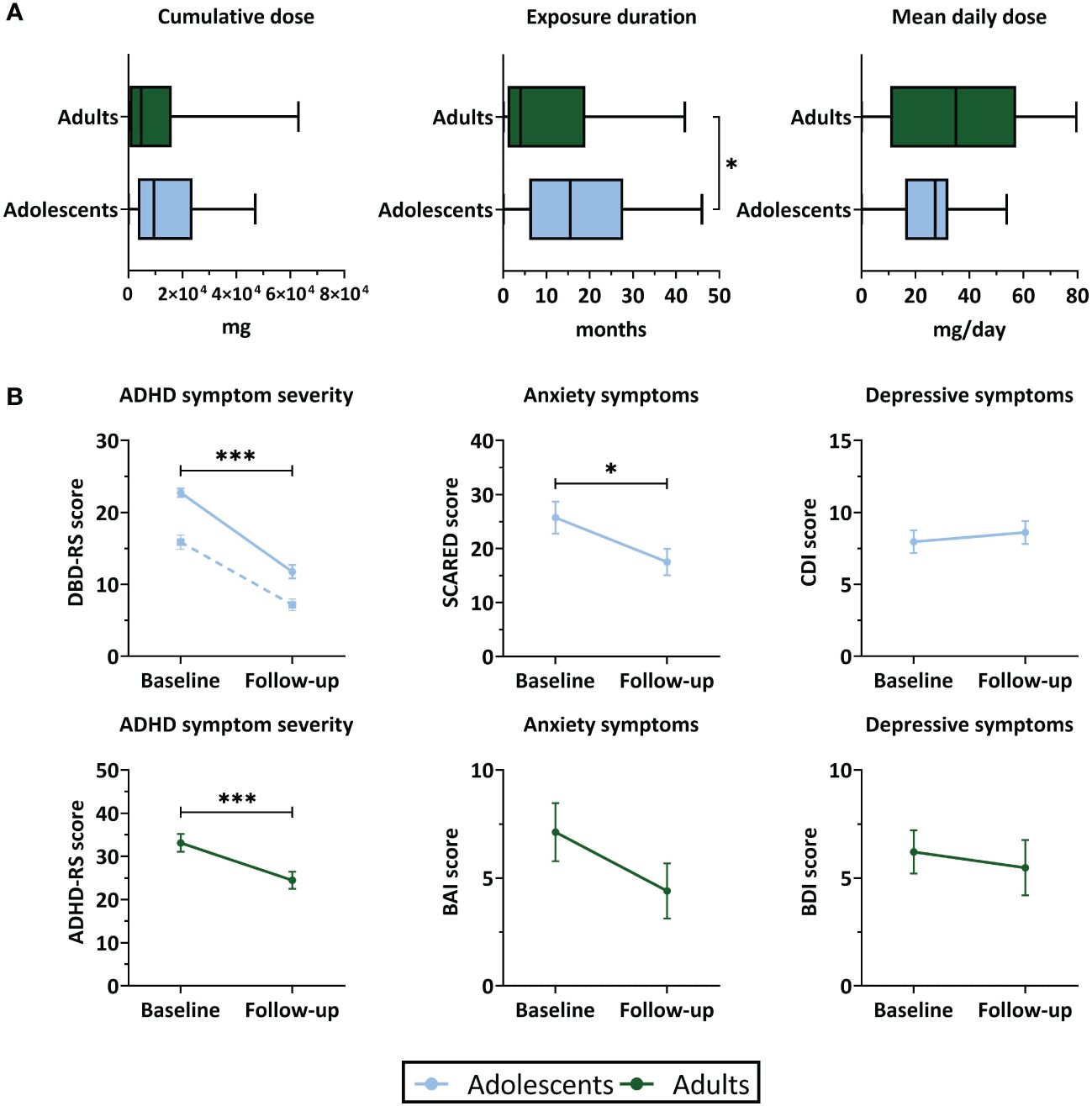
Figure 2 Stimulant medication use and clinical outcomes. (A) Boxplots representing stimulant medication use (median and interquartile range) between baseline and 4-year follow-up assessment. (B) ADHD symptom severity, anxiety and depressive symptom scores (mean ± SEM) at baseline and follow-up. For ADHD symptom severity in adolescents, the solid line represents inattentive symptoms and the dotted line represents hyperactive/impulsive symptoms. ADHD, attention-deficit/hyperactivity disorder; ADHD-RS, ADHD-Rating Scale; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CDI, Child Depression Inventory; DBD-RS, Disruptive Behavioral Disorder Rating Scale (inattentive and hyperactive/impulsive subscales); SCARED, Screen for Child Anxiety Related Disorders. ***P<.001, *P<.05.
In both age groups, we found a reduction in ADHD symptom severity at 4-year follow-up compared to baseline. Symptoms of anxiety were reduced at follow-up in adolescents (t(28)=2.40, P=0.03), but not in adults (t(19)=1.51, P=0.15). Symptoms of depression did not significantly change between baseline and follow-up in either age group (adolescent: t(29)=-0.50, P=0.62; adults: t(20)=0.28, P=0.79) (Table 1; Figure 2). In adolescents, the reductions in ADHD symptom severity and symptoms of anxiety were accompanied by a substantial reduction in the number of participants with clinically significant symptom scores. In adults, most participants still showed clinically significant ADHD symptoms at follow-up, despite reductions in ADHD symptom severity compared to baseline. In both age groups, for most participants the depressive symptom scores did not exceed the clinical cut-off values at baseline or follow-up (Table 1).
In adults, we found an association between medication use and change in ADHD symptom scores (cumulative dose: r=-0.07, P=0.001; exposure duration: r=-0.73, P=0.001), such that higher medication use was associated with more improvement of ADHD symptoms. No further associations were identified between medication use variables and baseline ADHD symptom severity, age at follow-up assessment, age at start of medication use, or change in weight between baseline and follow-up (Supplementary Table 3).
LMM analysis revealed no evidence for effects of cumulative dose and exposure duration on apparent cortical thickness development between baseline and follow-up of all ROIs, both for the models with continuous and grouped medication use variables, as well as when running the analysis split by age group (Table 2). All Bayes Factors were <1/100, providing strong evidence for the null hypothesis that cumulative dose and exposure duration have no effect on regional cortical thickness (Table 3). Furthermore, in adults, demeaned age at baseline had an effect on apparent cortical thickness of the right medial ROI (cumulative dose: t(24)=-2.36, P=0.03; exposure duration: t(24)=-2.39, P=0.03), indicating that higher age at baseline was associated with lower apparent cortical thickness of the right medial ROI. This is in line with literature on typical development reporting continued slow cortical thinning throughout adulthood (6), although the age at baseline cannot be considered entirely separate from the clinical manifestation of ADHD. At 4-year follow-up, we found no evidence for effects of ePOD-MPH RCT treatment groups (MPH, placebo) on apparent cortical thickness of all ROIs (Table 2).
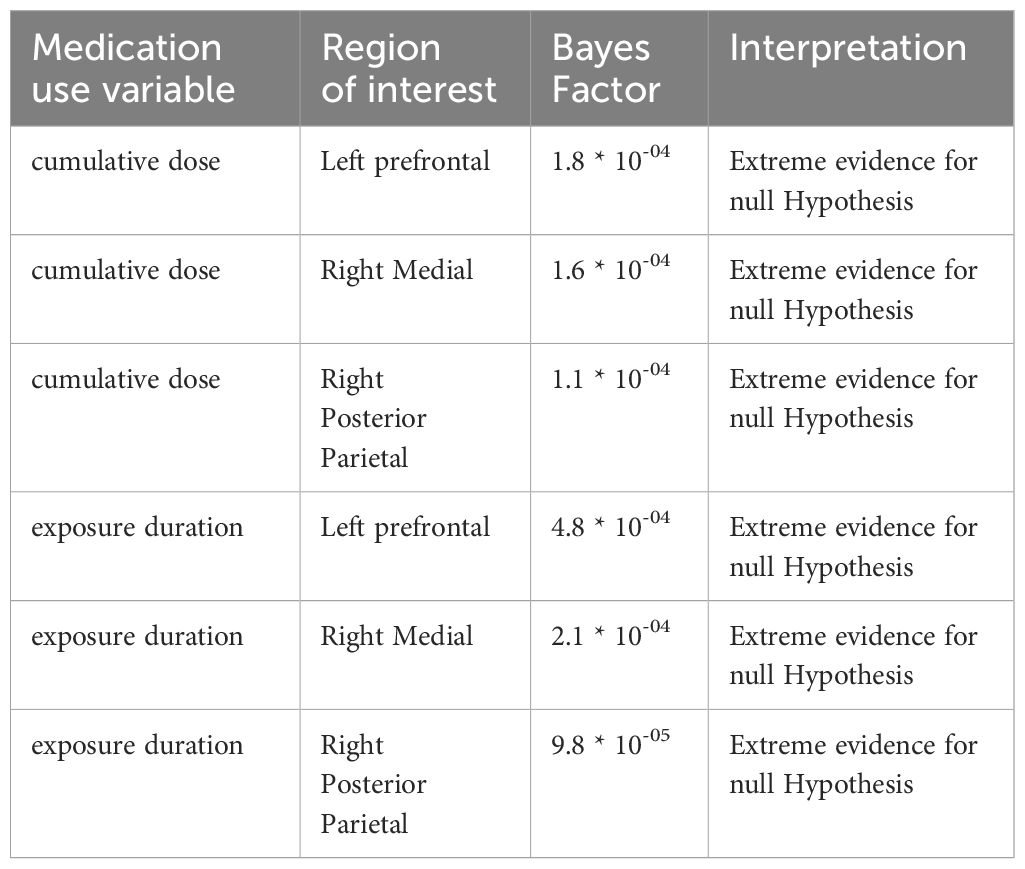
Table 3 Bayes Factors comparing the models with (continuous) medication use to the models without medication use.
In all ROIs, we identified an age-by-time interaction effect on apparent cortical thickness (left prefrontal ROI: t(55)=3.26, P=0.002; right medial ROI: t(54)=4.75, P<0.001; right posterior parietal ROI: t(54)=4.68, P<0.001) (Figure 3B). Post hoc analyses revealed a decrease in cortical thickness of all ROIs between baseline and 4-year follow-up in adolescents (left prefrontal ROI: t(31)=-4.53, P<0.001; right medial ROI: t(30)=-6.74, P<0.001; right posterior parietal ROI: t(30)=-5.75, P<0.001), but not in adults (left prefrontal ROI: t(24)=0.14, P=0.89; right medial ROI: t(24)=-0.97, P=0.34; right posterior parietal ROI: t(24)=0.68, P=0.50). Figure 3C presents the rate of change (mm/year) for each ROI (Table 4).
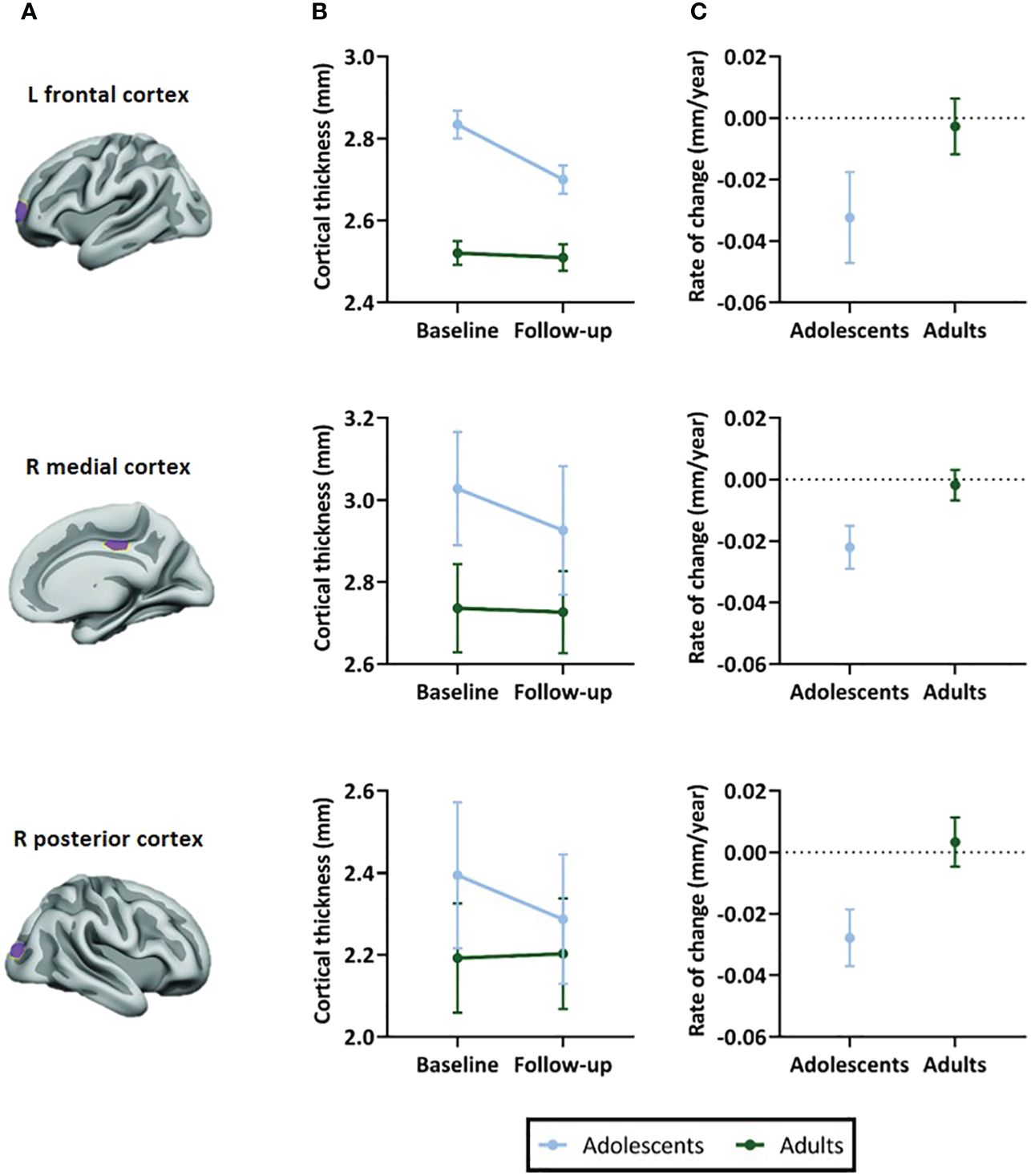
Figure 3 Selected regions of interest (ROIs) and apparent cortical thickness (mm) per ROI. (A) Brain templates showing the selected ROIs in purple. (B) Apparent cortical thickness (mean ± SEM) at baseline and 4-year follow-up assessment. Linear mixed models revealed an age-by-time interaction effect in all ROIs (P=.002 in left frontal ROI, P<.001 in right medial and posterior ROIs), reflecting apparent cortical thinning in adolescents but not adults. (C) Rate of change in apparent cortical thickness (mean ± SEM) between baseline and 4-year follow-up assessment. The plotted values are the raw cortical thickness trajectories, without taking into account the covariates demeaned age at baseline and demeaned scan interval. ROI figures adapted from Walhovd et al. (2020); with permission from American Society of Neuroradiology (19).
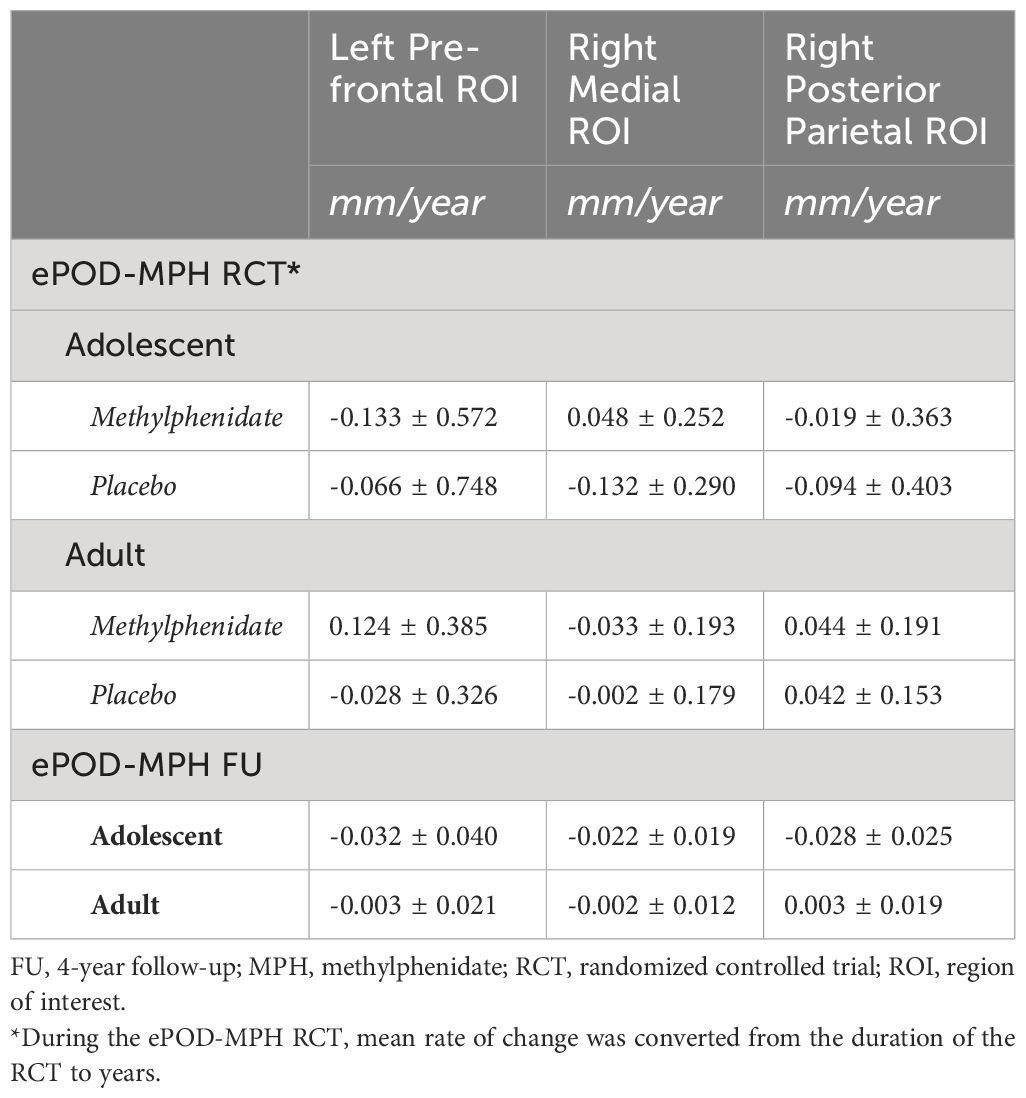
Table 4 Mean rate of change in apparent cortical thickness (mm/year) during the ePOD-MPH RCT and 4-year follow-up.
For clinical outcomes, in adolescents, after correction for multiple comparisons we identified a depression score-by-time interaction effect on cortical thickness of the right posterior parietal ROI (t(32) = -2.99, adjusted P = .008). In adults, we identified ADHD symptom score-by-time (t(20) = 2.36, adjusted P = .04) and anxiety score-by-time (t(21) = 3.00, adjusted P = .005) interaction effects on cortical thickness of the left prefrontal ROI. Post hoc analysis revealed opposite correlations between apparent cortical thickness and clinical scores at baseline compared with 4-year follow-up, although none of these associations were significant (Table 5). We would like to note that, in adults at baseline, the association between ADHD symptom severity and cortical thickness of the left prefrontal ROI was near-significant (r = -0.40, P = .056). We found no other main or interaction effects of clinical outcomes on apparent cortical thickness in adolescents or adults.
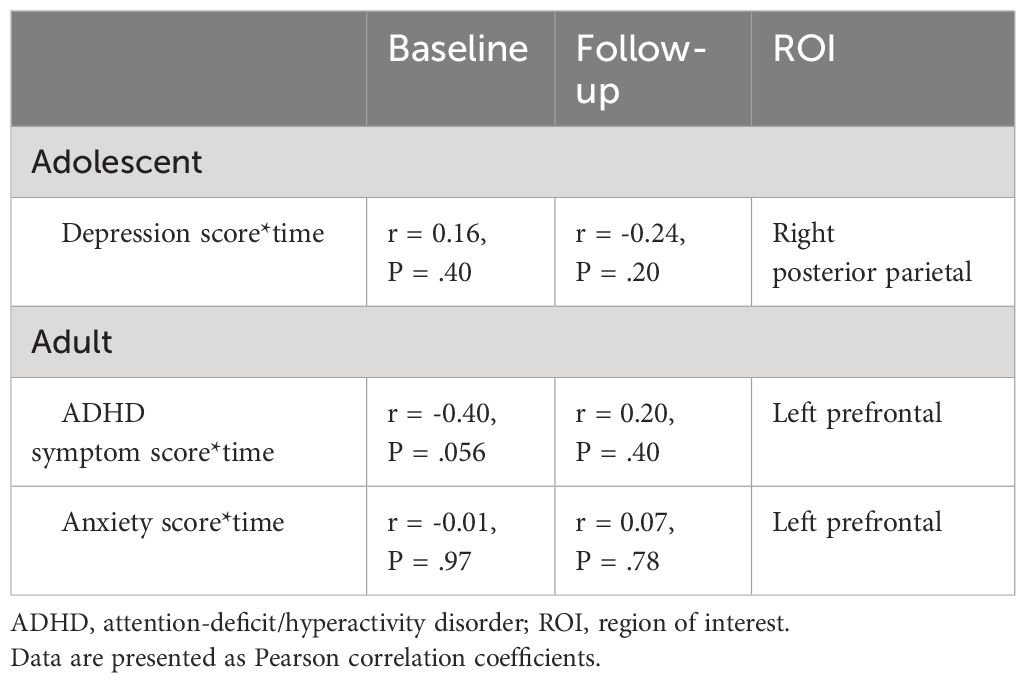
Table 5 Associations between clinical outcomes and regional apparent cortical thickness at baseline and 4-year follow-up assessment.
In order to evaluate whether additional brain regions were impacted by stimulant treatment, in addition to the three ROIs assessed in the main analysis, we performed an exploratory whole-brain analysis of apparent cortical thickness and surface area. In both age groups, we identified no additional associations between medication use and apparent cortical thickness or surface area.
This study investigated the age-dependency of long-term effects of stimulant-treatment on regional apparent cortical thickness in adolescents and adults previously diagnosed with ADHD. In contrast to the 4-month ePOD-MPH RCT, in this 4-year naturalistic follow-up we observed no evidence for long-term effects of stimulant medication use on apparent cortical thickness in any of the 3 ROIs investigated (left prefrontal, right medial and right posterior parietal ROIs). Moreover, as hypothesized, the treatment conditions from the ePOD-MPH RCT could no longer be distinguished. We did, however, identify differences in apparent cortical thickness development between adolescents and adults, which were consistent with existing literature on typical cortical development (7, 34). In addition, we observed improvements in clinical outcomes, as well as an association between higher medication use and more improvement of ADHD symptoms in adults. A possible explanation for this association may be that stimulant medication is effective in alleviating ADHD symptom severity, leading participants to continue using this medication. Finally, we found clinical scores-by-time interaction effects on apparent cortical thickness for depression scores in adolescents, and for ADHD symptom severity and anxiety scores in adults.
In contrast to our hypothesis and prior short-term findings (7), we observed no evidence for long-term effects of stimulant-treatment on (regional) cortical thickness development, either in adolescents or adults. This finding is in contrast with the neurochemical imprinting hypothesis, which states that stimulant administration during development may have lasting effects (17, 18). We propose several explanations for our findings. First, the short-term effects of stimulant medication use identified in the initial ePOD-MPH RCT may be transient, supporting the neural plasticity hypothesis that the (human) brain is able to adapt in response to internal and external stimuli (35). Alternatively, the current study may have been unable to detect subtle stimulant-treatment effects due to limited sample size related to loss-to-follow-up of participants, as well as heterogeneous medication use and prescription adherence among participants (36, 37). Nonetheless, the Bayes Factors calculated here provide strong evidence for the null hypothesis, supporting our findings of lack of evidence for stimulant medication effects on apparent cortical thickness development. Moreover, previous large-scale multicenter projects with heterogeneous study populations also reported no evidence for stimulant-treatment effects on cortical thickness (15, 16).
Discrepant findings in literature regarding stimulant-treatment effects on apparent cortical thickness in ADHD can be attributed to various reasons. Firstly, differences in age of the study participants may influence findings, as the cerebral cortex continues to develop throughout childhood and adolescence into adulthood. As a result, assessment of stimulant medication effects in different developmental stages may yield different findings. Furthermore, previous studies used differing approaches to calculate stimulant medication use or treatment profiles (13, 15, 16). Findings may also be impacted by methodological decisions, such as use of an ROI or whole-brain approach, MR field strength and scanning parameters, or cortical thickness estimation technique (12, 19).
A previous study observed that the mean rate of apparent cortical thinning in stimulant-treated ADHD participants was comparable to typically developing peers, while ADHD participants off stimulant medication showed more rapid apparent cortical thinning (13). Similarly, in our study, we observed comparable changes in apparent cortical thickness development during the naturalistic follow-up. However, we need to be cautious with speculations about associations of medication use with ‘normalization’ of cortical thickness development, since only few participants in our sample (3 adolescents, 5 adults) remained stimulant treatment-naïve and no typically developing control group was included. To gain further insights, it is essential to conduct large-scale longitudinal studies that include stimulant-treated and untreated individuals with ADHD, as well as typically developing peers.
Our findings that apparent cortical thickness was related to clinical outcomes are partially in line with previous studies. For instance, a cross-sectional study reported lower cortical thickness of frontal brain regions in children, adolescents and adults with ADHD, compared with typically developing peers (10). In these regions, lower cortical thickness was associated with higher ADHD symptom severity. Another study evaluated differences in gray matter morphology associated with symptoms of depression, anxiety, and impulsivity in typically developing children and adolescents, and found that higher impulsivity and depressive symptoms were associated with lower cortical thickness of frontal regions and lower hippocampal and pallidal volume (11). However, we note that in our study the clinical scores-by-time interaction effects reflected opposite, but weak and non-significant, associations between apparent cortical thickness and clinical symptoms scores at baseline versus follow-up assessment, making interpretation challenging. Importantly, more generally, the relationship between (individual differences in) brain structure, brain function, and behavioral outcomes remains largely unknown, and may be dynamic and change e.g. with age. Therefore, the relations between gray matter morphology and symptomatology, and the potential influence of stimulant medication thereon, should be interpreted with caution.
A critical strength of this study is its longitudinal design with stimulant treatment-naive participants at baseline, ruling out the influence of a history of medication use on cortical development. Moreover, we replicated previous findings of age-dependent apparent cortical thickness development (6, 34) and rate of regional cortical thinning in stimulant-treated adolescents with ADHD (13). Some limitations should also be considered. First, analogous to previous naturalistic studies, we assumed similar prescription adherence (complete adherence) for all participants. Nevertheless, medication adherence rates have been found to vary substantially (36, 37), therefore future studies should consider using reliable treatment adherence measures (38). Another limitation is that different MR scanners were used at baseline and follow-up assessment, although we were still able to identify general neurodevelopmental patterns of relatively faster reductions in apparent cortical thickness in adolescents than adults, in line with literature (6, 35). Furthermore, we could not include stimulant treatment-naive participants as a control group, since most participants in our sample used stimulant medication during the 4-year naturalistic follow-up, and we included no typically developing control group. Finally, sample size at follow-up was limited and we included only male participants within a specific age range, limiting the generalizability of this study’s results. The choice for male participants was based on the knowledge that patterns of brain development differ considerably between males and females (39), and that the prevalence of ADHD is higher in males than in females (2).
In conclusion, this study found no evidence for long-term effects of stimulant-treatment on apparent cortical thickness development in adolescents and adults previously diagnosed with ADHD. Future research should include prescription adherence measures and employ standardized/homogeneous approaches for acquisition and analysis. Moreover, there is a need for longitudinal studies including stimulant-treated and untreated individuals with ADHD, as well as typically developing peers, to improve our understanding of (age-dependent) effects of stimulant-treatment on the developing brain.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Central Committee on Research Involving Human Subjects & the local Medical ethical committee of the Academic Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by all participants and parents or legal representatives of the children.
Zv: Writing – review & editing, Writing – original draft. KW: Writing – review & editing. IA: Writing – review & editing. CG: Writing – review & editing. AK: Writing – review & editing. MB: Writing – review & editing. HG: Writing – review & editing. LR: Writing – review & editing. AS: Writing – review & editing, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Dutch non-profit organizations Kiddy GoodPills and Suffigium. The ePOD-MPH RCT (baseline data) was funded by a personal research grant awarded to LR by the Academic Medical Center, University of Amsterdam, and 11.32050.26ERA-NET PRIOMEDCHILD FP 6(EU).
We would like to thank all participants and their parents for their contribution to this study and all students that helped with collection and analysis of the data. A preprint version of this manuscript is available at medRxiv (40).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1365159/full#supplementary-material
ADHD, attention-deficit/hyperactivity disorder; ePOD-MPH, effects of Psychotropic drugs On the Developing brain – methylphenidate; MPH, methylphenidate; RCT, randomized controlled trial; ROI, region of interest.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV Vol. 4. . Washington, DC: American psychiatric association (1994).
2. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. (2007) 164:942–8. doi: 10.1176/ajp.2007.164.6.942
3. Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. (2018) 5:727–38. doi: 10.1016/S2215-0366(18)30269-4
4. Jaeschke RR, Sujkowska E, Sowa-Kućma M. Methylphenidate for attention-deficit/hyperactivity disorder in adults: a narrative review. Psychopharmacol (Berl). (2021) 238:2667–91. doi: 10.1007/s00213-021-05946-0
5. Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. (2008) 28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008
6. Vidal-Pineiro D, Parker N, Shin J, French L, Grydeland H, Jackowski AP, et al. Cellular correlates of cortical thinning throughout the lifespan. Sci Rep. (2020) 10:21803. doi: 10.1038/s41598-020-78471-3
7. Walhovd KB, Krogsrud SK, Amlien IK, Bartsch H, Bjørnerud A, Due-Tønnessen P, et al. Neurodevelopmental origins of lifespan changes in brain and cognition. Proc Natl Acad Sci U S A. (2016) 113:9357–62. doi: 10.1073/pnas.1524259113
8. Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, et al. Dynamic development of regional cortical thickness and surface area in early childhood. Cereb Cortex. (2015) 25:2204–12. doi: 10.1093/cercor/bhu027
9. Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. (2007) 104:19649–54. doi: 10.1073/pnas.0707741104
10. Almeida LG, Ricardo-Garcell J, Prado H, Barajas L, Fernández-Bouzas A, Avila D, et al. Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: a cross-sectional study. J Psychiatr Res. (2010) 44:1214–23. doi: 10.1016/j.jpsychires.2010.04.026
11. Merz EC, He X, Noble KG. Pediatric Imaging, Neurocognition, and Genetics Study. Anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage Clin. (2018) 20:243–51. doi: 10.1016/j.nicl.2018.07.020
12. Walhovd KB, Fjell AM, Giedd J, Dale AM, Brown TT. Through thick and thin: a need to reconcile contradictory results on trajectories in human cortical development. Cereb Cortex. (2017) 27:1472–81. doi: 10.1093/cercor/bhv301
13. Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, et al. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. (2009) 166:58–63. doi: 10.1176/appi.ajp.2008.08050781
14. Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. (2011) 168:1154–63. doi: 10.1176/appi.ajp.2011.11020281
15. Schweren LJ, Hartman CA, Heslenfeld DJ, van der Meer D, Franke B, Oosterlaan J, et al. Thinner medial temporal cortex in adolescents with attention-deficit/hyperactivity disorder and the effects of stimulants. J Am Acad Child Adolesc Psychiatry. (2015) 54:660–7. doi: 10.1016/j.jaac.2015.05.014
16. Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, et al. Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am J Psychiatry. (2019) 176:531–42. doi: 10.1176/appi.ajp.2019.18091033
17. Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. (2003) 27:3–18. doi: 10.1016/s0149-7634(03)00005-8
18. Urban KR, Waterhouse BD, Gao WJ. Distinct age-dependent effects of methylphenidate on developing and adult prefrontal neurons. Biol Psychiatry. (2012) 72:880–8. doi: 10.1016/j.biopsych.2012.04.018
19. Walhovd KB, Amlien I, Schrantee A, Rohani DA, Groote I, Bjørnerud A, et al. Methylphenidate effects on cortical thickness in children and adults with attention-deficit/hyperactivity disorder: a randomized clinical trial. AJNR Am J Neuroradiol. (2020) 41:758–65. doi: 10.3174/ajnr.A6560
20. Bottelier MA, Schouw ML, Klomp A, Tamminga HG, Schrantee AG, Bouziane C, et al. The effects of Psychotropic drugs On Developing brain (ePOD) study: methods and design. BMC Psychiatry. (2014) 14:48. doi: 10.1186/1471-244X-14-48
21. Ferdinand RF, van der Ende J. DISC-IV Diagnostic Interview Schedule for Children [Dutch translation NIMH-DISC-IV]. Sophia Kinderziekenhuis/Academisch Ziekenhuis Rotterdam: Afdeling Kinder- en Jeugdpsychiatrie (1998).
22. Kooij JJS. Diagnostic instruments. In: Adult ADHD. Springer, London (2013). doi: 10.1007/978-1-4471-4138-9_3
23. Nasky KM. Stimulant dose conversion calculator(2018). Available online at: https://psychopharmacopeia.com/stimulant_conversion.php.
24. Pelham WE Jr, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. (1992) 31:210–8. doi: 10.1097/00004583-199203000-00006
26. Muris PEHM, Bodden D, Hale WW, Birmaher B, Mayer B. SCARED-NL. In: Vragenlijst over angst en bang-zijn bij kinderen en adolescenten. Amsterdam, Netherlands: Boom Uitgevers (2007).
27. Beck AT, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. (1961) 4:561–71. doi: 10.1001/archpsyc.1961.01710120031004
28. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. (1988) 56:893–7. doi: 10.1037//0022-006x.56.6.893
29. Oosterlaan J, Baeyens D, Scheres A, Antrop I, Roeyers H, Sergeant JA. VvGK6-16: Vragenlijst voor Gedragsproblemen bij Kinderen. Amsterdam, Netherlands: Pearson (2008).
30. Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. (1999) 38:1230–6. doi: 10.1097/00004583-199910000-00011
31. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation (1996). doi: 10.1037/t00742-000
32. Leach C, Lucock M, Iveson S, Noble R. Evaluating psychological therapies services: A review of outcome measures and their utility. Ment Health Learn Disabil Res Practice. (2004) 1:53–6. doi: 10.5920/mhldrp.2004.1153
33. Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. (2012) 61:1402–18. doi: 10.1016/j.neuroimage.2012.02.084
34. Fjell AM, Grydeland H, Krogsrud SK, Amlien I, Rohani DA, Ferschmann L, et al. Development and aging of cortical thickness correspond to genetic organization patterns. Proc Natl Acad Sci U S A. (2015) 112:15462–7. doi: 10.1073/pnas.1508831112
35. Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. (2000) 23:649–711. doi: 10.1146/annurev.neuro.23.1.649
36. Ahmed R, Aslani P. Attention-deficit/hyperactivity disorder: an update on medication adherence and persistence in children, adolescents and adults. Expert Rev Pharmacoecon Outcomes Res. (2013) 13:791–815. doi: 10.1586/14737167.2013.841544
37. Charach A, Gajaria A, Skyba A, Chen S. Documenting adherence to psychostimulants in children with ADHD. J Can Acad Child Adolesc Psychiatry. (2008) 17:131–6.
38. Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophr Res. (2000) 42:241–7. doi: 10.1016/s0920-9964(99)00130-9
39. Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. (1999) 2:861–3. doi: 10.1038/13158
Keywords: attention-deficit/hyperactivity disorder (ADHD), stimulant medication, cortical thickness, gray matter, FreeSurfer, cortical development
Citation: van der Pal Z, Walhovd KB, Amlien IK, Guichelaar CJ, Kaiser A, Bottelier MA, Geurts HM, Reneman L and Schrantee A (2024) Stimulant medication use and apparent cortical thickness development in attention-deficit/hyperactivity disorder: a prospective longitudinal study. Front. Psychiatry 15:1365159. doi: 10.3389/fpsyt.2024.1365159
Received: 03 January 2024; Accepted: 09 April 2024;
Published: 07 May 2024.
Edited by:
Roberto Canitano, Siena University Hospital, ItalyCopyright © 2024 van der Pal, Walhovd, Amlien, Guichelaar, Kaiser, Bottelier, Geurts, Reneman and Schrantee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zarah van der Pal, ei52YW5kZXJwYWxAYW1zdGVyZGFtdW1jLm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.