
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry, 23 April 2024
Sec. Public Mental Health
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1363875
This article is part of the Research TopicPsychiatric Illness Across the Menstrual CycleView all 10 articles
Premenstrual syndrome (PMS) is a common disorder affecting women of reproductive age, with an estimated global prevalence of 47.8%, with severe symptoms occurring in 3-8%, significantly affecting daily functioning. GABA conductance and changes in neurosteroid levels, particularly allopregnanolone, are suspected to play a substantial role in the disorder’s etiology. In this paper, we provide an overview of recent reports on the etiology and recognized therapeutic approaches, encompassing both pharmacological and non-pharmacological interventions. Our examination includes studies on SSRIs, hormonal agents, neurosteroids, supplementation, and therapeutic roles. We aim to determine the most favorable treatment regimen by comparing medication effects and alternative methods. The treatment of PMS is crucial for enhancing the quality of life for affected women. Medications used in PMS treatment should be individually selected to achieve the best therapeutic effect, considering the clinical situation of the patients.
Many women of reproductive age experience dysphoria and physical symptoms approximately two weeks before menstruation (1). The mentioned discomfort, both physical and psychological, associated with the luteal phase of the menstrual cycle and typically resolving when menstruation ends, is defined as premenstrual syndrome (PMS) (2). The global prevalence of premenstrual syndrome is estimated at 47.8% (3), while the most severe form of PMS - premenstrual dysphoric disorder (PMDD) affects 3-8% of women of reproductive age (4). What is more, the PMDD is classified as a gynecological diagnosis in the ICD-11 classification and as a psychiatric diagnosis in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (5). That indicates the complexity of the disorder and is a reminder of the widespread spectrum of symptoms. The most common mental symptoms of PMS include irritability, tearfulness, anxiety, and depressed mood. Physical ones, on the other hand, mainly involve abdominal bloating, breast tenderness, and headaches (6). Hormonal changes, stress, diet, and alterations in neurotransmission are considered the most significant risk factors (7). It is also suspected that the severity of PMS is higher in unmarried women compared to married women, those with lower economic status, and those with a family history of similar cases (8). Behavioral and social factors also play a role, including medication use (including contraceptives), smoking, alcohol and caffeine consumption, and even education. Age, past pregnancies, and previous menstrual history have also been evaluated, but there is still no complete consensus on how they impact the development of the disorder (9). Diagnosing premenstrual syndrome is possible only after ruling out other conditions that could better explain the experienced discomforts (10). The treatment primarily focuses on alleviating symptoms, and we will delve into this aspect further in our discussion.
The pathogenesis of PMS is intricate and not fully understood. Several theories attempt to explain the causes of its symptoms.
Classically, PMS has been linked to hormonal fluctuations during the monthly cycle, with mood deterioration and increased anxiety primarily associated with decreases in estrogen and progesterone (Figure 1).
Recently, particular attention has been given to the progesterone metabolite allopregnanolone (11). Allopregnanolone is an allosteric modulator of the GABA receptor in the CNS, which binds to the alpha and beta subunits at residues m1-m3 (12), which explains its broad effects on multiple CNS pathways (13). Moreover, allopregnanolone synthesis can occur de novo not only in the brain but also in the ovaries and adrenal glands due to the presence of necessary enzymes in these organs needed for its production (14). Understanding the significance of allopregnanolone in alleviating PMS symptoms may provide crucial information about the cause of the disorder itself. Significantly, when utilized as a novel drug (brexanolone) for postpartum depression (PPD) treatment, it not only mitigates affective disorders (15) but also suppresses the inflammatory response. This dual action could potentially alleviate the severity of peripheral symptoms, including pain (16). The steroidal structure of progesterone and its metabolites enables them to penetrate the blood-brain barrier when formed peripherally, as observed in the ovaries (14). It is important to note that the presence of PMS is a risk factor for PPD (17). Both conditions are believed to be caused by hormonal changes, specifically the increase and subsequent withdrawal of sex hormones (18, 19), and the existence of subgroups of susceptible individuals (20, 21). Due to these associations and the increased interest in neurosteroids, allopregnanolone has become one of the most commonly linked substances to the etiology of PMS in recent years (20).
Women experiencing premenstrual symptoms demonstrate an impaired stress response (22). This may be precisely linked to the action of steroid hormones, which, through various mechanisms, inhibit the activity of the HPA axis, starting at the level of the PVN (23). Progesterone, or more specifically, its metabolite — allopregnanolone, enhances GABA conductance and suppresses CRH formation in hypothalamic cells. In contrast, estrogen inhibits the generation of free radicals, resulting in a reduction of oxidative stress in the body (24). What is more, Granda et al. suggest that abnormal oxidative and inflammatory activity may occur in PMS (25). It is possible that in PMS, there is an abnormal response to estradiol and an increase in oxidative stress, given that antioxidants in high concentrations have a pro-inflammatory effect and estradiol has a second peak concentration in the early luteal phase (26). The significance of estrogen metabolites producing oxygen radicals (27) is noteworthy. However, the current research does not allow for a clear assessment of the role of oxidative stress (28–30). Interestingly, there are no discernible differences in hormone levels during the monthly cycle between healthy women and those suffering from PMS (31). However, concentrations of allopregnanolone and its conversion from progesterone are higher in women with the PMDD (32). This suggests a disturbance in the metabolic pathway of progesterone in women who are affected and implies the existence of a subgroup of women sensitive to hormone concentrations. This sensitivity is supported by the findings of Schmidt et al., who demonstrated that re-administration of progesterone to women suffering from PMS while taking a leuprolide resulted in a recurrence of symptoms (33).
Furthermore, women with PMS, after blocking 5-alpha-reductase, a crucial enzyme for allopregnanolone production, experienced significantly reduced premenstrual symptoms (34). In contrast, during the follicular phase, women with PMDD who took allopregnanolone as part of another study showed reduced GABA-A receptor sensitivity (35). These data underscore the crucial role of this metabolite in the described disorder: High allopregnanolone levels may explain why the stress response in women with premenstrual disorders is blunted (36), given the mentioned above impact of GABA conductance on CRH.
However, as explained above, blocking its synthesis provides relief to patients. The explanation for this situation may lie in the reaction to substances in the CNS itself. Due to their structure, steroid hormones can interact not only trans-membrane to the cell but also through the G-protein-bound receptor, leading to changes in the cell genome (37). Theoretically, with an increase in progesterone, there is an unimodal increase in allopregnanolone, and an adaptation - a down-regulation of the receptor to maintain constant inhibition of GABA (14). With a decrease in the concentration of the substance in the later luteal phase, the physiological GABA-glutamate balance could be disturbed: Adaptive changes do not keep up with the contraction in allopregnanolone, which was higher at baseline in affected women, and GABA receptors are not restored in time, leading to impaired GABA conductance. This could explain, among other things, the increased activity of the prefrontal cortex (38), as observed in imaging studies.
According to this assumption, it would not be the neurosteroid concentration itself that causes the onset of symptoms, but rather the decrease in concentration. The GABA-A receptor appears to adapt to neurosteroid concentrations through changes in its conformation (39). Women with PMDD have lower sensitivity to benzodiazepines, as well as pregnanolone, which may be related to receptor adaptation involving increased expression of the delta subunit. This subunit is insensitive to benzodiazepines but highly sensitive to allopregnanolone (40). Its increased expression, along with the other subunits, may reflect an attempt to adapt to falling allopregnanolone concentrations at the end of the luteal phase, especially since a study by Timby et al. indicates that women with PMDD have altered sensitivity to allopregnanolone (35). A similar theory regarding PPD was presented by Maguire et al. (41). In the case of the monthly cycle, it appears that the distinction lies not so much in the concept of abnormal adaptations taking place but in the severity of their proportions. The long-drawn modulation in PPD throughout the 3rd and 4th trimesters of pregnancy may be linked to both the effects of prolonged exposure of GABA cells to allopregnanolone, leading to adaptations at the level of the receptor and cell genome, and significantly higher progesterone concentrations than in the luteal phase (42). In contrast, changes in PMS may occur only through a pathway of rapid adaptation to allopregnanolone involving structural changes in the GABA A receptor, which would explain the lower severity of symptoms. Additionally, the relationship between the two pathologies is indicated by the fact that PMS predisposes to PPD (17), and similar to PPD, in PMS, we observe a subgroup of women sensitive to hormonal fluctuations (21) (Figure 2).
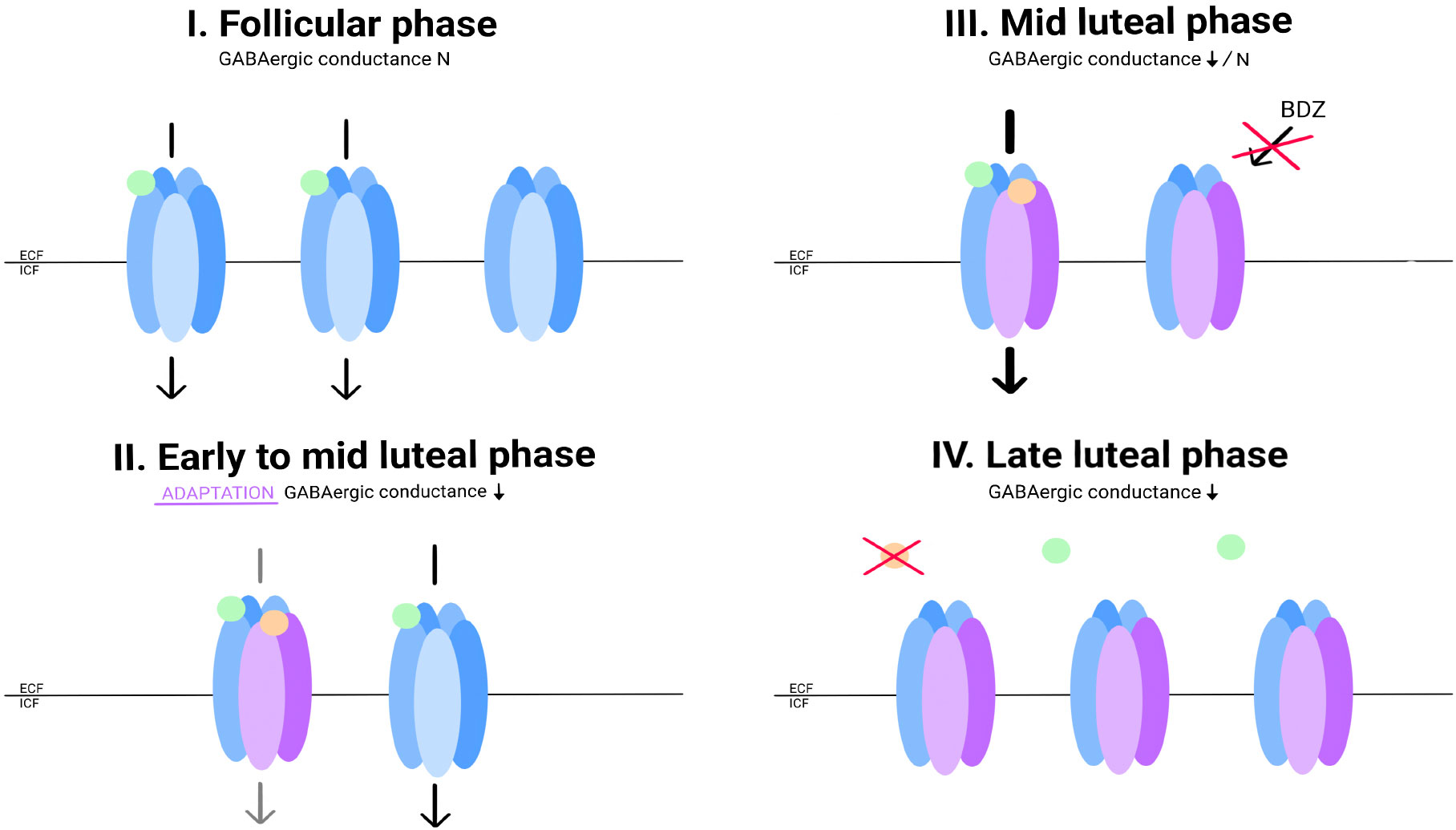
Figure 2 Allopregnanolone concentrations and hypothetical changes in GABA conductance in PMS patients. During the follicular phase, progesterone and allopregnanolone concentrations are low. Expression of selected GABA receptor subunits is not increased. GABA conductance functions properly (I). With increasing concentrations of allopregnanolone, the conformation of the GABA-A receptor is affected: the expression of the alpha4 subunit, and probably delta, is increased. In a group of women with PMS, there is a paradoxical decrease in GABA conductance under the influence of allopregnanolone (II). This condition explains the paradoxical effect of flumenazil in women with PPMD. The GABA-A receptor in this conformation is insensitive to BZDs. At the highest concentration of allopregnanolone in the cycle, GABA-A conductance is mainly regulated by it (III). Allopregnanolone does not reach high enough concentrations in the cycle to induce the expected allosteric modulator effect. Its concentration begins to fall, forcing readaptations within the GABA-A receptor. Until the conformation of the molecules returns to ''physiological'', inhibition may be impaired (IV). - Gamma-aminobutyric acid (GABA); - Allopregnanolone; - GABA receptor; - GABA receptor with altered subunit expression in response to allopregnanolone.
However, it is important to remain skeptical when discussing the connection between the onset of PMS and fluctuations in progesterone derivative levels. The studies by Schmidt et al. (33, 43) showed that eliminating hormone fluctuations during the luteal phase is not enough to prevent the onset of PMS symptoms. What is interesting, the researchers found that there was a subgroup that was sensitive to hormonal fluctuations: only patients with a history of PMS responded to hormonal interventions compared to a group of healthy women. Based on these findings and considering the abnormal response to BDZ in PMS patients, it can be concluded that abnormal adaptive responses of the GABA A receptor are one of the main, but not the only, problems faced by women with PMS. Furthermore, Schmid et al. also indicated in their more recent study that it is not high progesterone levels sustained over a long period, but changes in progesterone concentration that are key in triggering symptoms. This study provides a more complete understanding of the role of sex hormones in the disorder - the findings indicate that it is not re-administration but changes in sex hormone concentration that may be crucial. In both cases, this may indicate abnormal adaptations of the GABA A receptor (44).
The additional importance of hormones is underscored by estrogen’s ability to promote growth factors, such as BDNF (22, 45). SSRIs, used in the treatment of PMS, also stimulate its formation, and their effectiveness in treatment serves as indirect evidence of the importance of disturbances in serotonergic conduction in the etiopathogenesis of this pathology (46). Imaging studies further provide evidence of altered GABA and serotonergic conduction in the amygdaloid nucleus and prefrontal cortex in patients affected by PMDD (47).
Due to the rapid response to treatment with SSRI drugs, a different mechanism should be considered compared to the classical model found in affective disorders (48). In the classical model, the drugs take effect after about 3 weeks, while in the case of PMS, no such time gap is observed. A strong argument for the importance of serotonergic conduction is the lower peripheral blood serotonin levels during the luteal phase in women with PMS (49, 50). Use of drugs from SSRI group, leads to an increase in serotonin concentration in the synaptic cleft. An increase in serotonergic neurotransmission is the result (51). Furthermore, recent studies have shown that during the monthly cycle in women suffering from PMDD, there is an increase in serotonin uptake during the premenstrual period. Furthermore, increased serotonin transporter correlated with increased depressive symptoms. This indicates that the key may be the change in extracellular serotonin levels itself (52).
Another theory proposes the thesis that SSRIs promote an enzyme necessary for the production of allopregnanolone, and this enzyme is responsible for the immediate effect (53), which would explain the achievement of rapid clinical effects after brexanolone administration (54).
Within the allopregnanolone pathway, the enzyme 5α-reductase initiates the transformation of progesterone into 5α-dihydroprogesterone (5α-DHP). Subsequently, another enzyme, 3α-hydroxysteroid dehydrogenase (3α-HSD), facilitates the conversion of 5α-DHP to allopregnanolone (55, 56).
Progesterone can also be transformed into 5β-DHP with the enzyme 5β-reductase. Subsequently, 3α-HSD acts on 5β-DHP to produce pregnanolone (57).
Allopregnanolone and pregnanolone are positive allosteric modulators of GABAA, enhancing its function. Conversely, their isomers, isoallopregnanolone and epipregnanolone, are negative allosteric modulators, thereby inhibiting GABAergic neurotransmission. Dehydroepiandrosterone (DHEA) is another pregnanolone derivative and negative allosteric modulator, which potentially may compete with allopregnanolone for the substrate. Furthermore, a potential mechanism for PMS/PMDD could involve higher levels of negative allosteric modulators compared to positive allosteric modulators (57, 58).
Griffin et al. suggest that SSRIs (fluoxetine, paroxetine and sertraline were included in the study) may modulate the activity of neurosteroidogenic enzymes by enhancing their substrate affinity. For instance, they propose that SSRIs could increase the affinity of 3α-HSD for 5α-DHP, potentially augmenting its function. The specific mechanism of SSRIs influence on enzyme is currently unknown (51).
The mechanism of action of SSRIs in managing PMS/PMDD is convoluted, encompassing the modulation of GABA via neuroactive steroids. The SSRI’s impact on neuroactive steroid levels involves processes such as the redirection of biosynthetic pathways from progesterone towards neuroactive metabolites. Additionally, substrates are directed towards enhancing GABAA function positively, while competitive inhibition of enzyme substrates also plays a role. These mechanisms may contribute to the modulation of neuroactive steroid levels, suggesting the impact of SSRIs in addressing PMS/PMDD symptoms (58, 59).
One clue to the development of the disorder is immune dysregulation in women experiencing PMS. A study by Gold et al. revealed elevated levels of hs-CRP in women with PMS, indicating an immune component to the disorder (60). This study confirms the theory about the role of inflammation in its development, but there is insufficient evidence indicating a central effect of these substances in PMS.
The strong correlation of hsCRP with abdominal pain may suggest a local inflammatory process. Still, the central levels of cytokines are unknown, even though hsCRP was associated with mood disorders in the study. Unfortunately, the study had several limitations that could impact the CRP result: the patients’ status, prevalence, and BMI at the time the samples were taken were not considered (61). A relevant study by Puder et al. demonstrated that regardless of BMI, hsCRP levels are similar in women with high BMI and those within normal limits, and the course of low-grade inflammation is independent of BMI (62). It’s important to note that the study sample included only 15 women. Furthermore, hsCRP concentrations correlated once again with women’s mood, and hsCRP level itself was highest during the early follicular phase, associated with physiological processes. Another study, which excluded conditions such as smoking and a history of mood disorders, provided more robust evidence by demonstrating elevated levels of inflammatory cytokines in affected women (including IFN-gamma, IL-2, IL-10, IL-12, IL-4) (63). However, the study did not clarify the important time criterion for the appearance of these markers in the blood. The markers themselves, such as IFN-gamma, indirectly indicate T-lymphocyte activity, with correspondingly elevated IL-1 levels, highlighting the interconnectedness of anti- and pro-inflammatory factors. An additional argument supporting the importance of inflammation is the results of treatment of selected PMS using anti-inflammatory drugs (64).
Meta-analysis by Klusmann et al., showed that the HPA axis exhibits stronger reactivity during the luteal phase compared to the follicular phase (65). This is also linked to elevated cortisol levels during the luteal phase. Additionally, Hou et al. found that there is a blunted morning cortisol response in PMS (66). The dysregulation of the HPA axis may be caused by cyclical stressors experienced over time. In addition, PMS has been found to result in an impaired cortisol response to stress (67). Affective disorders are also linked to altered HPA axis function (68). It is important to note that the cortisol response and sympathetic nervous system response are impaired in PMS, but only during the luteal phase (69). However, the study by Schmidt et al. mentioned earlier does not provide enough evidence to determine whether it is progesterone alone via allopregnanolone, or both progesterone and estrogen, that contribute to this dysregulation. However, the available data suggest that estradiol-containing drugs may be effective in improving HPA function, as demonstrated by the improvement in function following estrogen administration (70). In addition, regulation of progesterone levels may prevent abnormal adaptations of GABA-A receptors and thus prevent changes in the HPA axis.
In the context of the etiology of PMS, the role of prolactin was also considered. Studies with bromocriptine provide indirect evidence for the effect of prolactin on PMS (71, 72). Additionally, higher prolactin concentrations are observed during the luteal phase, which is associated with PMS symptoms (73). Elevated levels of prolactin have been linked to mastalgia, and decreasing these levels has been shown to result in clinical improvement in patients (74). Based on the concentrations of estrogen and progesterone, high levels of prolactin may exacerbate PMS symptoms, in line with the theory proposed by Carroll and Steiner (72).
Genetic studies have not provided clear conclusions regarding the specific genes that are reliably involved in the development of PMS. However, family studies suggest a discernible genetic component and align with the theory of the existence of a subgroup of susceptible patients (75). Research conducted by Widholm et Kantero found that children of mothers with PMS have a higher likelihood of developing the disorder (76). Additionally, a study on monozygotic and dizygotic twins highlighted a greater than 40% probability of developing the disorder if one of the twins suffers from PMS (77).
Although etiopathogenesis remains incompletely understood, studies on PMS markers and advancements in imaging techniques provide a rationale for the use of many drugs in the treatment of PMS (Figure 3).
Hormone treatment aims to eliminate fluctuations in sex hormones during the menstrual cycle. This can prevent adaptive changes in the Central Nervous System that occur under the influence of progesterone and estrogen derivatives. Theoretically, this could eliminate a group of women particularly sensitive to hormonal fluctuations. The attempted maladaptation of GABA-A receptors to allopregnanolone could be prevented by the absence of a progesterone peak (34, 78). Eliminating this phenomenon could potentially increase serotonin levels in women who suffer from PMS (50). Based on these interactions, monophasic COC preparations seem to be a better treatment option than multiphasic preparations. Biphasic and triphasic formulations gradually increase the amount of gestagens in the second half of the cycle, corresponding to the physiological fluctuations of sex hormones. This removes the progesterone peak, making abnormal adaptation of the body impossible. Monophasic preparations are recommended for controlling mood disorders during PMS, according to guidelines (10). A more detailed description of endocrine disruption is described above.
The most effective drug in the oral contraceptive (OC) group seems to be formulations containing ethinylestradiol and drospirenone. This preparation is FDA-approved for the treatment of PMDD (79). These drugs are intended to improve the patient’s condition through several mechanisms, including the suppression of ovulation, which results from the stabilization of hormone levels by both components of the pill. Theoretically, this is also expected to lead to an improvement in mood. The preparation is also intended to have an anti-androgenic effect, which would reduce symptoms such as irritability and aggression. However, the role of androgen hormones in PMS is not yet fully understood. Eriksson et al. found higher serum testosterone concentrations in women with premenstrual symptoms regardless of cycle (80), while another study (81) found no differences in testosterone concentrations between sick and healthy women. However, it should be noted that the latter study was limited by a small sample size. In addition, the heightened levels of DHEA during the periovulatory period in women with PMS highlight the significance of neurosteroids in the disorder. DHEA is a precursor for the synthesis of neurosteroids and has a protective effect on the CNS (82). However, the concentration of DHEA is higher for a short period during the cycle, indicating a different DHEA processing pathway in affected women.
Additionally, drospirenone is responsible for the anti-androgenic effect in the cited preparation (83). This substance is a progesterone derivative with up to 10 times the anti-androgenic effect. Drospirenone has been found to have a beneficial effect in reducing PMS and PMDD symptoms due to its antagonism to the mineralocorticoid receptor (84). This substance is an analog of spironolactone, a diuretic, which has been shown to nullify symptoms related to water retention and also has mood-enhancing effects (85). It is important to note that in the natural cycle, progesterone competes with aldosterone for access to the mineralocorticoid receptor, thereby antagonizing its action. While most progesterone analogs do not mimic this action, drospirenone is an exception. Spironolactone inhibits the action that results from the earlier dominance of estrogens in the cycle, which leads to the promotion of angiotensin formation (86). This is particularly relevant because angiotensin is responsible for various changes in the body, including its influence on the Central Nervous System. For instance, it regulates acetylcholinergic conductance (68). The earlier-mentioned improvement in mood after spironolactone may be correlated with its ability to lower and normalize progesterone concentrations (87). This suggests that spironolactone may block the body’s abnormal adaptation to progesterone and allopregnanolone. Drospirenone is an analog of spironolactone that performs the important function of progesterone in the periphery more effectively, with anti-androgenic and anti-mineral corticosteroid actions. Additionally, drospirenone lowers the concentration of progesterone in the body, which prevents abnormal adaptation reactions in the CNS. The initial studies on drospirenone were inconclusive. The relatively long period of placebo intake (21/7 days) may have been related to these observations: improvements in aspects of acne, appetite and hunger, and breast pain, but no significant improvement in mood was achieved (88, 89). Only studies using a shorter duration of placebo intake (24/4 days) demonstrated significant improvement in physical symptoms such as breast tenderness, swelling, bloating, headaches, and muscle pain, as well as mood. However, the authors highlighted that previous studies on the use of contraception in PMDD indicate its superiority in treating physical symptoms over mood, where SSRIs are still more potent (79). Lopez et al. (2008) demonstrated significant improvements in productivity and social relationships following a three-month treatment with ethyl estradiol and drospirenone (90). Additionally, this drug can reduce the risk of PMDD recurrence (79). To achieve maximum treatment efficacy, it is recommended to administer the specified preparation for 24 days with a 4-day interval. The preparation contains 20ug of ethinylestradiol and 3mg of drospirenone. If treatment is ineffective, increase the dose of ethinylestradiol to 30ug and take the preparation in a cycle of 21 days with a 7-day interval, along with 3mg of drospirenone. According to the cited data, a shorter medication interval improves the mood of sufferers (10, 79). Therapy with this contraceptive, like other drugs, may cause side effects (Table 1). Patients may experience nausea, breast pain, and intermenstrual bleeding (95).
The use of oral contraceptives that contain only progesterone is not recommended for the treatment of PMS and PMDD symptoms. This is because such therapy may exacerbate mood fluctuations and other PMS-related symptoms (91, 96). Evidence supporting this position comes from a study that found that patients with mood disorders have higher levels of progesterone in their blood compared to the control group (97). It is known that the development of progesterone-induced mood disorders is strongly dependent on the individual’s sensitivity to the hormone, its concentration in the blood, and the timing of exposure. It is worth noting that it is progesterone administered in doses that mimic the luteal phase, and therefore in lower concentrations comparatively to pregnancy, that may be associated with mood side effects in OC users (91, 98–100). In contrast, during pregnancy, high concentrations of the substance exhibit anti-anxiety and sedative effects (101–104). Consequently, the effectiveness of progesterone in alleviating premenstrual symptoms strongly depends on its blood concentration. In conclusion, the use of progesterone alone in the treatment of PMS and PMDD does not show the same efficacy as therapy with oral contraceptives containing drospirenone with ethinylestradiol. Furthermore, it appears that premenstrual symptoms may be induced by the use of progestogen as part of hormone replacement therapy (105). In terms of targeted treatment, dutasteride may be a more suitable option as it inhibits the conversion of progesterone to allopregnanolone (34). However, there is limited data on the efficacy of this substance, and as an androgen, it may have negative effects on male fetal development in women who are planning pregnancies.
The use of oral contraceptives containing only estrogens is not recommended for the treatment of PMS and PMDD symptoms. Studies suggest that such preparations may be ineffective in alleviating premenstrual symptoms or may even worsen them (106). In addition, it has been shown that estrogens are significantly associated with an increased risk of endometrial cancer. However, this risk can be effectively reduced by concomitant use of progesterone (106). Therefore, a more effective and safer approach would be the use of combination preparations, such as the OC and COC preparations cited earlier (107).
Researchers have also considered the issue of the placebo-drug interval. Although OC treatment is effective, it does not fully eliminate hormonal fluctuations. This may be related to a treatment regimen involving a placebo (92). The use of COC - continued contraception - could eliminate LH, FSH, oestradiol, and progesterone fluctuations, thus improving patient comfort (108). Halbreich et al. (2011) studied the effectiveness of levonorgestrel (LNG) 90 mcg/EE 20 mcg for 4 cycles of 28 days. The study found that over half of the patients experienced a significant improvement, defined as a 50% reduction in symptom intensity (92). Furthermore, as the therapy duration increased, more patients responded positively to the treatment. In the initial cycle, during the late luteal phase, typically associated with the onset of symptoms, there was a decrease in symptom intensity according to the DRSP scale. However, according to Freeman et al.’s analysis of studies, the efficacy of COC treatment is similar to that of SSRIs. It should be noted that the effect of COC treatment is not as well demonstrated for low symptom severity (109). One possible reason for the PMS trials showing less clear outcomes than the PMDD trials is that the PMS trials had lower criteria for symptom severity at the start of the study. This could have made it harder to see the differences in how much the LNG/EE and placebo groups improved, compared to the PMDD trials where the participants had more severe symptoms and more room for improvement. It is important to note that these studies are limited by high responses in the placebo group, ranging from 27-53% for PMDD. Additionally, COC treatment offers better control of bleeding days and reduces pain associated with the 5 most severe days of the cycle (110). Therefore, these drugs appear to be particularly effective in more severe cases of PMS - PMDD, especially when physical symptoms are inadequately controlled. COCs have been shown to improve patients’ mood and physical symptoms.
It is noteworthy that the use of a levonorgestrel-releasing IUD may increase stress sensitivity. Women using this type of IUD exhibited significantly higher blood cortisol levels than those who took oral levonorgestrel in combination with estrogen. This phenomenon may be due to the potential effect of this type of contraception on increasing autonomic system reactivity to stimuli such as stress (111). It is worth noting that several studies have suggested that the use of levonorgestrel-releasing IUDs may worsen mood disorders (111–115). In conclusion, it is important to note that the effectiveness of levonorgestrel in alleviating PMS symptoms appears to depend on its method of administration. Oral formulations containing levonorgestrel demonstrate greater efficacy than IUDs, which may even exacerbate symptoms associated with the disorder.
Alongside oral contraceptives, gonadotropin-releasing hormone (GnRH) agonists also play a significant role in the treatment of PMS and PMDD. The mechanism of action involves inhibiting the central hypothalamic-pituitary-ovarian system, which leads to the inhibition of ovulation. This has been confirmed in studies (116). Inhibiting ovulation is expected to reduce hormonal fluctuations in the menstrual cycle. However, it is important to note that these drugs induce a menopausal state, which can cause symptoms such as bone mass loss and hot flashes. To minimize the side effects of therapy, progestogens or tibolone are often added. Another option is to use a progesterone receptor blocker, which, if given early enough in the cycle, also prevents ovulation. Ulipristal acetate is a progesterone receptor blocker used to treat uterine myoma. Receptors for progesterone are present in the hippocampus and frontal cortex (117). This highlights the significance of this steroid in the disorder. Blocking its receptor would prevent interactions between progesterone and the genome, inhibiting potential negative changes (118). Comasco et al. (2020) conducted a study that found that taking ulipristal significantly improved psychiatric/mental symptoms in PMDD sufferers compared to placebo. The study lasted for three months and outcomes were measured using the Daily Record of Severity of Problems (DRSP) scale (93).
The group of GnRH agonists match the efficacy of first-line drugs - SSRIs. Due to their induction of the perimenopausal state/suppression of estrogen and progesterone synthesis (mentioned above), they cause several side effects characteristic of the menopausal period (119) These side effects limit the duration of therapy to a maximum of 6 months, with the main limitation being the loss of bone mass (120). To address the problems of therapy with GnRH analogs, attempts are being made to use add-back hormone therapy to reduce the incidence of side effects (121).
However, this is a controversial approach due to the etiology of PMS in which hormonal fluctuations seem to be the predominant problem. In theory, this could lead to counteracting the therapeutic effect of GnRH. Progestogens themselves can trigger a worsening of mood in women, presumably through their effect on the GABAa receptor (82). According to Schmidt et al.’s theory, not only progesterone but also estradiol administered alone can induce a relapse of PMS symptoms (33). Similar conclusions were reached by Leathear et al. in whose study of GnRH with add-back hormone therapy as many as nine out of 20 subjects discontinued therapy, when in the case of the GnRH analog alone it was three out of 20, including only one for medical indications. The entire study lasted six months and showed that people on add-back hormone therapy did not achieve clinically significant improvements compared to placebo (121). Given that progestogen was given only for one week into a cycle in this study, it is debatable to use add-back therapy alone with estrogen. This would eliminate the effect of progestogen, which, when administered during the luteal phase, can mimic premenstrual symptoms (122). On top of this, a study by Erkkola et al. indicates that progestogen supply every 3 months for 14 days was sufficient in menopausal women to prevent endometrial hyperplasia (123). Furthermore, in a study by Mezrow et al, it was shown that add-back estrogen was also effective, however, each time Medroxyprogesterone acetate (MPA) was administered for 10 days every 4 cycles, this was accompanied by a worsening of mood (124). Further studies, on a group of PMS and PMDD patients, are needed to confirm these reports and effectively apply this type of therapy in selected patients. In agreement with the data presented here correlates with the study by Segebladh et al. who showed that the addition of HRT in women with PMDD specifically worsened the control of mood-related symptoms, however, the addition of 1.5mg of oestradiol alone (gel, daily) least interfered with the outcome of leuprolide acetate treatment (125). Furthermore, the higher the concentration of estradiol relative to progesterone in the other groups of the study, the more pronounced the premenstrual symptoms were. This evidence indirectly suggests that the co-occurrence of hormones in the cycle potentiates their interaction with mood, lowering it even more strongly in predisposed women. This also challenges the approach that progesterone metabolites alone are crucial for the development of the premenstrual disorders (126).
There are only a limited number of studies that have examined the effect of GnRH agonists along with add-back hormone treatment, which makes it difficult to draw clear conclusions. A meta-analysis conducted by Wyatt et al. indicated that add-back therapy does not reduce the effectiveness of GnRH agonists based on several studies (127). However, more recent studies have raised doubts about these findings. Ultimately, a high placebo effect, typical of PMS studies, reduces the quality of the results. The mere administration of a placebo may suggest the reappearance of hormone fluctuations and subsequent symptoms in female patients (128). Additionally, it should be noted that the effectiveness of GnRH therapy decreases in patients with a co-existing psychiatric diagnosis, which is more frequent in the PMS population than in the general population. There is no doubt that the problem of adverse effects of GnRH analogs requires replacement therapy, and studies suggest that the best combination would be oestradiol alone with progesterone administered approximately every 3–4 cycles. However, it should be noted that progesterone administration may be accompanied by an increase in symptoms, and this should be brought to the patient’s attention when attempting such therapy. On top of this, the small amount of evidence limits such an approach.
When discussing GnRH agonists, GnRH antagonists should also be considered. GnRH antagonists rapidly inhibit pituitary gonadotropin secretion through competition for GnRH receptors, eliminating the initial stimulatory phase typical of agonists. They have indications, among others, in the treatment of endometriosis (129).
The reason why they can be considered for use is their rapid onset of action and rapid return of pituitary function after cessation of therapy (130). GnRH agonists must be administered for a longer period and on a relatively continuous basis to maintain their effect. While the therapeutic regimen would not differ in terms of continuity of therapy in the case of GnRH antagonists for PMS and PMDD, these drugs are more predictable in their use. However, they can be expensive and may require hormone replacement therapy (131). While there are no studies that discuss the use of these drugs for PMS and PMDD, they may become more convenient for clinicians to use in the future.
As blocking the synthesis of progesterone metabolites, including allopregnanolone, has been found to provide relief to patients with PMDD, it is important to attempt to normalize the concentration of this substance (132). Low concentrations of allopregnanolone can worsen mood in certain cases (133). Conversely, when its concentration peaks, its activity has been associated with a decrease in amygdala impulsivity (134).
The appearance of allopregnanolone appears to affect GABA receptor modulation, with only high concentrations being beneficial in a therapeutic context. This is observed in the group where a paradoxical anxiety mechanism is described at low concentrations (135). However, a potential issue in this scenario is determining the appropriate timing for terminating treatment with the drug. The decrease in allopregnanolone concentration appears to trigger the re-conformation of the GABA receptor (39). Our current knowledge is insufficient to use the substance that is blamed for mood fluctuations. The abnormal receptor response appears to underlie the pathogenesis of the disorder, given the somewhat common paradoxical mechanism of action of GABA-A modulators, which also involves the action of benzodiazepines (136) and ethanol (135). Another issue is blocking progesterone metabolism to inhibit allopregnanolone synthesis. SSRI drugs appear to normalize allopregnanolone concentrations, which may explain their rapid effect in women with premenstrual symptoms (137). In contrast, isoallopregnanolone is a negative modulator of GABA, unlike allopregnanolone, but its effect on GABA is small (138, 139). In studies conducted on rats, isoallopregnanolone was found to reverse the effects of allopregnanolone (140). Additionally, even a half dose of isoallopregnanolone was able to reverse the sedative effects of allopregnanolone as well as the SEV test, which measures the intensity of anesthesia (141). To ensure the drug’s effectiveness, it should be administered based on the predicted concentrations of allopregnanolone during the cycle. This means that its concentration should be highest in the later luteal phase to counteract the fall of allopregnanolone. Bäckström et al.’s study showed the greatest improvement in patients with the highest concentrations of the drug in the late luteal phase. Despite methodological errors, such as administering the drug outside of the late luteal phase, it still demonstrated efficacy (142). A recent study found that women who took isoallopregnanolone had a lower incidence of PMDD symptoms than those who took a placebo (94). However, both studies have methodological problems. In the first study, inaccurate adjustment of the drug’s administration timing to the luteal phase and an unselective inclusion criterion were noted. Women with symptoms outside the luteal phase were also admitted. Furthermore, the initial analysis in the second study only considered the 5 days of the cycle with the most severe symptoms. It was not until the extension to 9 days that a significant benefit from the drug was observed. Additional research is required to establish definitive conclusions. Currently, it is understood that isoallopregnanolone is particularly effective in improving mood, reducing tension, and alleviating anxiety. The medication appears to be beneficial for patients with mental disorders during their menstrual cycle.
In conclusion, for PMS therapy, oral contraceptives containing drospirenone and ethinylestradiol (at a dose of 3 mg drospirenone and 20 mcg ethinylestradiol) are the most effective. If bleeding and abdominal pain are not controlled, an alternative solution is to use COCs with levonorgestrel and EE. Transdermal patches may be used as an alternative to oral contraceptive pills for patients who have difficulty taking them regularly. However, the effectiveness of transdermal patches is still a matter of debate (106). It is not recommended to use formulations that contain only progesterone or only estrogen. The available research on the effectiveness of new treatments that selectively target progesterone and its metabolites is insufficient to draw firm conclusions.
It is important to note that many PMS and PMDD symptoms, including breast tenderness, depression, and headaches, can occur as side effects of taking contraception, which limits the effectiveness of this approach (143). Although side effects were rare in most of the studies cited, it is still necessary to consider alternative therapeutic approaches for premenstrual disorders. It is worth noting that the preparations discussed above mainly affected physical symptoms, and only some had a clear effect on improving mood.
The treatment of PMS and PMDD uses drugs from the SSRI group, which block serotonin reuptake in the presynaptic area. This leads to an increase in serotonin concentration in the synaptic cleft, increasing serotonergic neurotransmission (46).
According to the latest guidelines from the Royal College of Obstetricians and Gynaecologists, SSRIs should be used as first-line drugs in the pharmacotherapy of severe PMS (144). Primarily because they are considered most effective in alleviating the anxiety and irritability symptoms characteristic of the disorder (145). Studies on the use of SSRIs to treat PMDD have shown a beneficial effect of the therapy in 60% to 90% of patients, with a range of 30% to 40% to placebo (22).
The exact percentages depended on the criteria the patients met. Among the most important were the severity, type, and number of symptoms reported (50). Such criteria limit the determination of the percentage beneficial therapeutic effect for the entire group of women suffering from PMS.
An advantage of the use of SSRIs in the course of PMS, as opposed to their use in the treatment of depression, is their rapid effect, achieved even within days of starting medication. This indicates a different mechanism of activity than that observed in depression therapy, where measurable improvement can be observed after a few weeks of taking the drugs (46, 146, 147).
The rapid effects of SSRIs in women with PMS or PMDD are likely due to their simultaneous effects on serotonin receptors and allopregnanolone levels in the brain, thereby indirectly modulating GABAA receptor function. Increasing the efficiency of DHP conversion to allopregnanolone SSRI group drugs also alters the levels of this neurosteroid (22, 148).
The swift effects of SSRIs in treating PMS and PMDD allow them to be used not only continuously, but also intermittently (only during the luteal phase) (149). Currently, there are no studies that show a clear difference in the efficacy of SSRIs in relieving PMS, comparing administration either continuously or only during the luteal phase. However, it should be noted that at this point the number of studies is insufficient to draw confident conclusions (46). Taking SSRIs only in the luteal phase avoids the withdrawal syndrome associated with long-term antidepressant use (150).
The choice of route of administration, i.e.: continuously or only in the luteal phase in women with severe PMS or PMDD without comorbidities, may be based on patient or physician preference and individual experience of side effects occurring in a given patient (151). It is worth mentioning that it is necessary to gradually discontinue the intake of SSRI drugs when they are administered continuously (144). Otherwise, there is a risk of adverse effects, the most common of which are nausea and weakness. Marjoribanks et al. showed that there is a correlation between the dose of an SSRI and the appearance of side effects. It seems that higher doses of the drug are associated with an increased likelihood of experiencing its side effects (46).
The entire group of SSRI drugs can be used to treat premenstrual symptoms. According to the Marjoribanks et al. (46), too few studies have been conducted using a specific drug from the SSRI group to indicate significant differences in the effectiveness of PMS treatment. The choice of drug should be based on the individual clinical situation of the patient, this is to minimize the severity and frequency of adverse effects.
In the literature, it is possible to distinguish SSRI drugs for the treatment of PMS such as fluoxetine, sertraline, paroxetine, citalopram, and escitalopram, the first 3 of which are approved by the FDA (50).
The criteria for selecting patients for the study were most often similar. They included aspects such as an age range of 18 to 45 years, regular menstrual cycles of 22 to 35 days, and evidence of probable ovulation. They also included meeting the criteria for a diagnosis of PMS/PMDD, and the absence of psychiatric comorbidities. Side effects that can occur with specific SSRI drugs are typically common to the entire group. Such adverse effects as decreased libido, nausea, weakness, drowsiness, fatigue, and sweating can be mentioned (46, 50).
Preclinical studies suggest that low doses of fluoxetine may increase allopregnanolone concentrations in the brain (152).
A pilot study on the use of fluoxetine to treat PMS conducted on 40 women showed the potential to alleviate the emotional symptoms of PMS. The administration of a dose of 10 mg/day during the luteal phase of the menstrual cycle, 7 days before the probable date of menstruation, was found to yield the most favorable outcomes. This led to a reduction of emotional PMS symptoms by more than 40% in 70% of the study participants, in comparison to placebo. The study was a randomized, double-blind, placebo-controlled trial (152).
Another double-blind pilot study of 39 women reported the efficacy of fluoxetine compared to placebo and calcium. Fluoxetine and calcium carbonate were administered for a period of 4 menstrual cycles. The dose taken by the patients was 10 mg of fluoxetine twice daily. Calcium carbonate was administered at 600 mg twice a day. The study shows noticeable benefits in treating PMS with fluoxetine. Efficacy with calcium was significantly lower, although higher than with placebo. Limitations of this study were the significance achieved in only 2 of the 5 symptom assessment instruments and the small study sample. According to the authors, there is no need to further compare the efficacy of fluoxetine with calcium in the treatment of PMS (153).
A study by Hedayat et al. conducted on 100 women compared the efficacy of fluoxetine and buspirone in treating PMS. The study was single-blind. The doses the patients were given were 20 mg/d of fluoxetine in one group and 10 mg/d of buspirone in the other. In both cases, the administration period was 2 months. Both drugs showed significant efficacy in treating PMS, with no significant differences between them. The authors believe that buspirone may be a better choice for treatment, due to fewer side effects. However, a limitation related to the lack of a placebo group should be taken into account here (154).
Hunter et al. demonstrated that fluoxetine, used in the treatment of PMDD, had a faster and more effective impact on alleviating anxiety-related symptoms compared to CBT therapy. However, after six months, the effectiveness of CBT therapy and fluoxetine use yielded similar results. The combination of both treatments showed no additional benefits. Such findings may contribute to better-tailoring therapy to the unique requirements of each patient. The female participants in the study were administered a daily dose of 20 mg for six months, and the study included forty-five women (155).
A study comparing the efficacy of fluoxetine with placebo in the treatment of PMDD showed that of the side effects, only decreased libido was observed with a statistically significant higher frequency among patients taking fluoxetine. Efficacy in alleviating physical symptoms was observed only among those administered a daily dose of 20 mg of fluoxetine. The reduction in the severity of problems was estimated at 38% for the 20 mg/d group, administering the drug daily only during the luteal phase (156).
According to a study from 2003, the difference in efficacy between a dose of 20 mg/d and 60 mg/d of fluoxetine was not statistically significant. In both cases, compared to placebo, efficacy was higher. At the 60 mg/d dose, adverse effects were more common (157).
Another clinical trial also proves there are no statistical differences between the efficacy of a 20 mg/d dose and a 60 mg/d dose in treating the physical symptoms of PMDD. Statistically significant differences were observed when tolerance to fluoxetine developed, favoring the 20 mg/d dose. At the 60 mg/d dose, patients were significantly more likely to discontinue treatment due to side effects (158).
Another study involving 405 women also reported that a 60 mg/d dose of fluoxetine resulted in a higher incidence of side effects compared to a 20 mg/d dose (159).
Fluoxetine, due to its high efficacy and the relatively high number of studies compared to other SSRI drugs in the treatment of PMS/PMDD, appears to be an appropriate form of medication as a first-line drug. It is worth noting that using the lowest effective dose is advisable, considering that, in selected studies, doses as low as 10 mg effectively controlled symptoms and carried a lower risk of side effects (Table 2).
A 1997 study observed significant improvement in PMDD symptom relief with sertraline administered continuously. The overall evaluation showed a great or very great improvement in 62% of those given sertraline and 34% of those in the placebo group (160).
A study by Freeman et al. on the use of sertraline to treat PMS found improvements in mood and relief of physical symptoms in women using sertraline. Doses ranged from 50 mg/d to 100 mg/d. Improvements occurred as early as the first month of treatment. The study was randomized, double-blind, and placebo-controlled (151).
In a 3-month, placebo-controlled comparison of sertraline and desipramine, the study revealed a significant advantage of the SSRI drug over the noradrenergic affinity drug. The degree of improvement was measured using the Penn Daily Symptom Report (DSR), indicating that symptoms decreased by more than 50% in 65% of the women studied (161).
According to a 2015 study, which investigated the efficacy of sertraline, including a placebo, on 188 women, treatment with this SSRI drug is not universally effective when administered ad hoc. The study utilized doses of 50 mg/d and 100 mg/d of sertraline (162).
A 2006 randomized clinical trial involving 314 women suggests the effectiveness of sertraline in alleviating moderate to severe PMS symptoms. Patients received sertraline throughout the luteal phase for the first two cycles, followed by continuous administration for one cycle and initiation of treatment at the onset of symptoms for one cycle. The doses used were 25 mg/d and 50 mg/d. Each mode of administration exhibited comparable efficacy, with the lower dose of 25 mg/d showing a favorable outcome (163).
According to the study by Freeman et al., the recurrence rate of PMS symptoms was significantly higher after short-term treatment compared to long-term treatment with sertraline. However, it should be noted that prolonged treatment also exhibited a high rate of symptom recurrence. Patients experiencing severe symptoms at the beginning of medication indicated the highest risk of relapse, regardless of the treatment duration. This study suggests that the high severity of complaints (before the start of treatment) is a marker for a worse prognosis in patients. The dosage used in this study ranged from 50 mg/d to 100 mg/d of sertraline (164).
On the other hand, the Yonkers et al. study shows no evidence of sertraline withdrawal symptoms after sudden discontinuation after 2 weeks of treatment for 2 cycles. This correlates with the theory cited above that short-term administration of sertraline is less likely to be fraught with side effects. The dose used ranged from 50 mg/d to 150 mg/d of sertraline, but the researchers did not consider the severity of initial symptoms (165).
According to a 2004 study, a dosing regimen (either continuous or luteal phase only) using 50 mg/d to 100 mg/d of sertraline does not show differences in efficacy for treating PMS/PMDD (151).
Due to the risk of relapse documented in the studies discussed above, this drug appears to be slightly inferior to fluoxetine. It should be noted that sertraline, in most studies, demonstrated efficacy in selected patients even at doses as low as 25 mg, emphasizing the necessity of individualizing therapy. Initiating therapy with a lower dose could potentially reduce symptoms and the risk of withdrawal syndrome, consequently lowering the risk of relapse, assuming its efficacy (Table 3). Moreover, sertraline has a shorter half-life than fluoxetine, which means it could be more convenient to use during luteal phase (166).
A multicenter study using a placebo and paroxetine yielded a result indicating that paroxetine was effective in relieving PMDD symptoms. The study involved 327 women. Paroxetine was administered at doses of 12.5 mg/d or 25 mg/d or placebo once daily for three treatment cycles. The method for evaluating efficacy was the VAS-Mood score (focusing on symptoms such as irritability, tension, affective lability, and depressed mood) during the luteal phase. Both doses of paroxetine were found to be effective according to the VAS-Mood scale (167).
A clinical trial conducted by Landen et al. in 2006 demonstrated that continuous treatment of PMDD with paroxetine effectively reduced symptoms such as irritability, achieving a response rate of 85% compared to the placebo. Luteal-phase-only treatment showed comparable effectiveness to continuous administration for symptoms like irritability, affect lability, and mood swings. A modest effect on reducing the severity of symptoms was observed for depressed mood and somatic symptoms. Dosages ranging from 10 mg/d to 20 mg/d were utilized (168).
A 2008 study demonstrated the effectiveness of treating PMDD with paroxetine at a dose of 20 mg/d. The continuous treatment group achieved a response rate ranging from 50% to 78.6%, while the intermittent treatment group achieved a response rate ranging from 37.5% to 93.8%. The study was subject to limitations, including a small sample size of 36 participants and the absence of a placebo group (169).
In the Steiner et al., 2005 study, within the group using paroxetine during the luteal phase at a dose of 25 mg, at least one side effect was observed in 76.7% of patients. At a dose of 12.5 mg, it was observed in 67.7% of subjects. In the placebo trial, this percentage was 56.7%. The most commonly observed side effects associated with taking paroxetine were nausea, asthenia, headaches, and decreased libido (170).
According to the Landén et al., 2008 study, paroxetine demonstrates a rapid reduction in symptoms, which is uncommon for a serotonin-dependent antidepressant. Such a swift response to treatment has not been observed previously (146).
Paroxetine has shown high efficacy in treating premenstrual symptoms, although its use is associated with the frequent occurrence of at least one side effect (Table 4). It may emerge as an alternative to classical treatments for some patients due to its notably rapid action and very high efficacy.
A study by Eriksson on the efficacy of escitalopram suggests a higher effectiveness of this drug than a placebo. The study involved 151 women, and the drug was administered intermittently for 3 months, only during the luteal phase. The doses used were 10 mg/d and 20 mg/d. The use of the 20 mg/d dose showed a symptom-reducing effect of up to 90%. The primary measurements focused on the sum of symptoms such as irritability, depressed mood, tension, and affective lability. Irritability alone, considered the main symptom of PMDD in this study, was reduced by 80% compared to the placebo group — a reduction of 30%. Side effects, such as nausea and reduced libido, were not observed more frequently in patients receiving escitalopram at 20 mg/d than in those receiving a lower dose (46, 171).
The Freeman et al., 2005 study also demonstrated the efficacy of treating PMDD with escitalopram at doses ranging from 10 mg/d to 20 mg/d. However, the study was constrained by limiting factors, such as a low number of participants and the absence of a placebo trial (172).
Escitalopram, due to the limited number of available studies, cannot be conclusively evaluated as an effective drug for the treatment of PMS (Table 5).
In a 1998 study by Wikander et al., examining how the use of citalopram affects the treatment of premenstrual dysphoria with severe irritability, pharmacological medication was shown to be more effective than placebo. The drugs were administered either continuously or during the luteal phase only. The study revealed that the administration of the 20 mg drug during the luteal phase alone led to better control of irritability and improvement in well-being compared to continuous use of the drug (173).
Another study, in turn, demonstrated that citalopram administered as needed, in doses ranging from 10 mg/d to 20 mg/d, also showed efficacy in relieving PMDD symptoms (174).
On the other hand, Freeman et al. suggest that the treatment of PMS with citalopram is effective for patients in whom prior treatment with SSRIs has failed, whether used throughout the entire menstrual cycle or only during the luteal phase (175).
Studies on the treatment of PMS or PMDD with citalopram are riddled with limitations, including small subject numbers and insufficient independent research, hindering a comprehensive evaluation of citalopram’s efficacy in PMS treatment (Table 6). Most studies underscore the effectiveness of citalopram when used intermittently—specifically, during the luteal phase of the monthly cycle.
A randomized controlled double-blind clinical trial evaluating the efficacy of venlafaxine as a representative of the SNRI group demonstrated its significant superiority over placebo in reducing PMDD symptoms. The study included 143 women who were administered venlafaxine for four menstrual cycles at doses ranging from 50 to 200 mg/d. In the group receiving the drug, 60% of patients experienced symptom relief, compared to 35% in the placebo group (176).
A study by Cohen et al. suggests that venlafaxine is effective and well-tolerated in the treatment of PMDD at doses ranging from 75 mg/d to 112.5 mg/d. However, this open-label study is significantly limited by a small sample size (177).
The study by Hsiao et al. also indicates the efficacy of venlafaxine in the treatment of PMDD. Patients reported relief from symptoms such as anxiety and depression. Doses ranging from 18.25 mg/d to 150 mg/d were used. However, the study was limited by a small number of participants and excessive variability in the doses administered, which were modified by the patients themselves (178).
Venlafaxine appears to be effective in treating premenstrual symptoms such as anxiety and depression, but the limited number of studies is a significant drawback, preventing a comprehensive evaluation of the drug’s effectiveness. It should be noted that the effect of venlafaxine at the doses used in the studies primarily corresponds to an enhancement of serotonergic rather than noradrenergic conduction. It is plausible to consider the use of this drug in cases of high intolerance to SSRI drugs as an alternative in the treatment of PMS/PMDD.
Duloxetine is a medication that is not only used in the treatment of psychiatric disorders but is also indicated for alleviating painful physical symptoms that may accompany depression (179). This suggests, in combination with its serotonergic component, that the drug could be effective for PMS associated with increased pain.
Ramos et al. present two female patients suffering from PMDD. In the case of one patient who had an isolated premenstrual disorder, there was an improvement of up to 94% in the premenstrual DRSP score with a daily dose of 60 mg. On the other hand, the second patient, being treated for Major Depressive Disorder (MDD), continued to experience severe mental symptoms despite previous psychiatric treatment, including venlafaxine 375 mg/d and clomipramine 150 mg/d. After the administration of 120 mg/d duloxetine, not only was there a satisfactory control of depression observed but, notably, the patient did not manifest premenstrual symptoms for the first time (180).
In contrast, a study by Mazza et al. indicated that duloxetine at a dose of 60 mg caused a significant improvement in symptoms (50% improvement) in almost 80% of patients. However, this study did not include a placebo group. Significantly notable was the elevated rate of improvement within a brief timeframe—following the initial two cycles during which the patients underwent drug administration (181). Another single-blind study demonstrated a swift clinical response in female patients, manifesting as early as the first cycle when duloxetine was administered at a dosage of 60 mg (182).
Duloxetine is a drug characterized by a dual mechanism of action that is particularly beneficial in the context of heightened physical pain. It is feasible to administer a relatively low dose of 60 mg/d. Adverse effects were observed in a small percentage of subjects, encompassing symptoms such as nausea, insomnia, decreased libido, and reduced appetite. However, it is crucial to acknowledge that studies evaluating this substance are hampered by significant limitations, including the absence of double-blinding, a limited cohort of female patients, or the complete absence of a placebo group. Further investigations employing double-blind, placebo-controlled trials are imperative to advance our understanding.
Buspirone affects serotonergic conduction through the 5HT1A receptor and also has properties that affect dopaminergic pathways (183). Both of these actions suggest a potential use of the drug in the treatment of PMS and PMDD.
In a single-blind study, Nazari et al. compared buspirone 10 mg/d with fluoxetine 20 mg/d, demonstrating that both formulations were effective with no advantage for either of them. However, it is conceivable that buspirone, due to its lower rate of side effects, may be the preferred drug to fluoxetine (154). Nevertheless, the study lacked a control group and had a short duration of 2 months. Conversely, another study comparing buspirone and nefazodone found that buspirone showed a better treatment effect than placebo, in contrast to nefazodone (184).
The limited number of studies and the absence of double-blind trials constrain the robustness of utilizing this substance. However, it appears to be effective and may be considered an option for certain patients.
In conclusion, SSRI drugs are highly effective in treating PMS and PMDD, particularly the irritability associated with the syndrome. Their use is linked to relatively mild side effects, which can be mitigated by employing the drugs intermittently. These characteristics justify their use as first-line drugs in the treatment of PMS. Fluoxetine demonstrates significant therapeutic efficacy and induces relatively few side effects at therapeutic doses in a luteal-phase-only regimen. Moreover, fluoxetine has undergone extensive study for PMS treatment, making it the most suitable drug for managing the disorder. Paroxetine also exhibits high efficacy in treating PMS, though its elevated rate of side effects renders it less preferable compared to fluoxetine.
Sertraline, due to its high rate of symptom recurrence, does not appear to be the best drug for treating premenstrual symptoms. Assessing the actual effectiveness of escitalopram, citalopram, and venlafaxine in the treatment of PMS is challenging due to the small number of studies and the limited number of participants. However, the selection of a particular SSRI drug should be based on individual patient preference and adjusted for efficacy and tolerability, as mentioned by the authors of the 2013 Marjoribanks et al. review (46). The treatment regimen should also be based on the patient’s needs. While it can be assumed that other SSRI drugs are also effective, they may differ in the occurrence of side effects. It is presumed that all doses used in the cited studies are effective in treating PMS. However, there is a correlation indicating that the incidence of SSRI side effects increases with dosage, making high doses potentially intolerable for patients. It is noteworthy that a significant number of patients with PMS were administered doses that were akin to those utilized in the treatment of affective disorders. Doses developed theoretically for a more severe disorder and on groups, typically not including women, can often be too high. This is worth bearing in mind, as individual studies using lower doses in selected cases have proven effective and reduced the risk of side effects.
Most of the herbal research is fraught with significant limitations, constraining the ability to arrive at conclusive evaluations (Table 7). The predominant focus in existing studies centers around VAC, saffron, and curcumin.
VAC is a herbal preparation, and its mechanism of action primarily involves enhancing dopaminergic conduction (197). An empirical argument supporting the use of a drug with this affinity is the frequent occurrence of hyperprolactinemia in women experiencing some symptoms of PMS (198).
In his study, Schellenberg demonstrated that, over time, the number of patients responding to VAC treatment increased. By the study’s conclusion, which encompassed three cycles, more than half of the women taking VAC experienced a symptom reduction of over 50%. In contrast, the placebo group exhibited a reduction of only 24%. Another study by Schellenberg et al. revealed that the most optimal therapeutic effects were achieved with a 20 mg dose taken once daily, with no additional benefits observed when increasing the dose to 30 mg (199).
Furthermore, according to Cerqueira et al., VAC demonstrated improvements in both physical and psychological symptoms of PMS and PMDD (200). Another study also underscored the efficacy of VAC extract, indicating enhancements in all PMS symptom domains as measured by PMSD, except for abdominal cramping (201). It is worth noting, however, that abdominal cramping may be inherent to the nature of PMS.
In contrast, Van Die et al. suggested the potential use of VAC in combination with St. John’s wort for premenopausal women with PMS. The results showed promise, especially in addressing mood swings. Despite the observation of improvements in anxiety and hyperhydration levels, the degree of enchantment was not significantly different from that observed with a placebo. It is essential to note that this study was conducted with a small group (14 people) (202).
Moreover, Ma et al. highlighted the efficacy of the substance in controlling symptoms related to water retention in a study with a larger participant pool (203). A noteworthy concurrence with this study is the observed progressive improvements over time, which align with findings in the earlier mentioned Schellenberg study. Interestingly, the study also reported a relatively high percentage of placebo results, potentially attributed to the subjective measurement methods employed. This methodology may explain the results in the He et al. study, where VAC demonstrated improvement but placebo results were as high as 50% (204).
Ambrosini et al. supported the effectiveness of VAC in controlling PMS-related headaches (205). Presumably, the herb’s impact on headaches is linked to its high affinity for μ and κ subtype opioid receptors (197). However, it is essential to note that the study lacked a control sample. Additionally, the Bergel et al. study observed that persistent headaches associated with VAC led one patient to discontinue treatment. Interestingly, in this study, VAC did not demonstrate an impact on prolactin levels (206).
This raises questions about the credibility of the previously mentioned study, particularly since this study, revealing headaches as a side effect despite high limitations, provides more qualitative data than the research discussed earlier. On the other hand, He et al. whose study also identified headaches after VAC administration, suggested that many reported side effects might be attributed to the inherent nature of PMS (204).
Most of the cited studies face significant limitations, a point emphasized by Verkaik et al. In their meta-analysis on VAC studies in PMS, they highlighted the high risk of bias, heterogeneity, subjective methods, and underpowered inclusion criteria, collectively diminishing the quality of evidence regarding the effectiveness of VAC (207). More discerning and selective studies are imperative. Furthermore, many herbal studies exhibit notable methodological flaws, such as the absence of a placebo group.
In conclusion, while VAC appears to be an effective formulation, further research is essential to conclusively demonstrate its benefits. It is important to keep in mind that herbal therapies are frequently associated with various unpredictable interactions, potentially contributing to the high number of side effects, as seen in the Berg et al. study where the group was allowed to take other drugs in addition to VAC (206).
The argument in favor of using saffron finds support in studies on the substance conducted in affective disorders (208). In rat studies, saffron has been shown to increase BDNF expression (209) in the hippocampus. The suspected mechanism of action involves the safranal and crocin compounds, which impact serotonergic conduction (210). Additionally, this preparation contains flavonoids and carotenoids, which exhibit antioxidant effects and prevent the formation of prostaglandins, potentially explaining its analgesic effect (211).
Conversely, a study by Fukui et al. discovered that a 20-minute exposure to the scent of the preparation lowered cortisol levels and elevated estrogen levels, irrespective of the menstrual cycle timing. This exposure was associated with symptom relief, as measured by the STAI (State-Trait Anxiety Inventory). It is worth noting that the rapid decrease in cortisol levels may indicate a beneficial short-term effect of saffron exposure in situations of heightened tension and stress (212).
In the study by Beiranvand et al., saffron was administered once a day for two menstrual cycles at a dose of 30 mg, revealing a significant decrease in PMS severity in the saffron group compared to the placebo group (213). This finding aligns with an earlier study by Agha-Hosseini et al., where saffron, at a total dose of 30 mg divided into two doses of 15 mg each, demonstrated a notable reduction in PMS symptoms for up to 76% of women after two monthly cycles of administration (214).
Rajabi et al. explored the impact of saffron on PMDD symptoms by comparing it with an antidepressant and a placebo, similar to previous studies on depression. Both preparations were administered twice a day. The dosage was limited to the luteal phase based on the mechanism of action. The results indicated comparable efficacy between fluoxetine and saffron (215). Notably, side effects were less frequently observed with saffron, potentially favoring the herbal preparation.
Saffron appears to offer an alternative to SSRI drug treatment for PMS. However, further studies are warranted to both indicate and confirm its effectiveness.
Curcumin, a member of the ginger family, is a curcuminoid derived from turmeric. Its suspected mechanism of action in addressing PMS is linked to the modulation of neurotransmitter levels, including serotonin (216). Additionally, a study by Fanaei et al. demonstrated an increase in BDNF levels in women with PMS following curcumin supplementation, which correlated with clinical improvement in patients (217). Another pivotal aspect of curcumin’s action involves the inhibition of prostaglandin synthesis by suppressing COX-2 (218, 219).
Khayat et al. demonstrated that administering curcumin for 10 days starting 7 days before menstruation is an effective method for relieving PMS symptoms compared to a placebo (220). They utilized a dose of 100 mg twice daily. On the contrary, Bahrami et al. used a higher dose of 500 mg once a day with the same dosing schedule but found no clear advantage of curcumin over placebo (221). However, both studies are limited by the relatively small number of subjects and the short study duration of 3 cycles.
Another aspect of curcumin’s impact on women with PMS was explored by Arabnezhad et al., who studied vitamin D levels using a 500 mg dose of curcuminoid and 5 mg of piperine following the same schedule. They reported a slight improvement in vitamin D levels relative to the placebo. On the contrary, the markers of liver and kidney function measured in this study did not exhibit differences between the study group and the placebo (222). Another study analyzed the effects of curcuminoid and piperine on inflammatory markers and iron metabolism in women with PMS. However, it failed to show changes indicative of a benefit from curcumin, except for a reduction in hsCRP. It is noteworthy that the baseline hsCRP value was already low (223).
In conclusion, despite the theoretically beneficial effects of curcumin, its conclusive efficacy for PMS symptoms has not been established. While a recent study by Bahrami et al. suggests improvements in cognitive function for women with PMS (224), isolated reports on its effectiveness are insufficient to draw concrete conclusions.
The most common treatment for PMS is pharmacotherapy. However, non-pharmacological methods, such as cognitive-behavioral therapy (CBT), regular aerobic exercise, yoga, vitamin supplementation, and leading a healthy lifestyle, are increasingly recommended as additional options (225).
Cognitive-behavioral therapy (CBT) is a psychotherapy that aims to identify negative, disturbing, or destructive thought patterns and develop coping strategies (10) to alleviate associated symptoms, such as depression, stress, and anxiety (226).
Ussher and Perz suggest that couples may benefit more from CBT than individual therapy. Women may feel symptoms such as depression, anger, and irritability during the premenstrual phase (226, 227), which can lead to increased conflict in the relationship with their partner (228, 229). Conversely, women may experience feelings of guilt or other negative thoughts during quiescence. The study involved four 90-minute therapy sessions with a clinical psychologist over 5 months. The focus of the meetings was to challenge these negative thoughts and develop coping strategies. Women were encouraged to engage in self-care and make lifestyle changes, such as exercise and diet. Regardless of the treatment modality, both treatment groups showed improvements in women’s well-being compared to the control group. The study found a sustained reduction in depression, anxiety, and stress over the following three months (226). The results emphasize the significance of receiving understanding and support from loved ones during PMS treatment, as well as the importance of informed education about PMS.
The authors of a separate study demonstrated that CBT can enhance the quality of life for young women with PMS. They highlighted that the knowledge gained during therapy sessions on problem-solving, stress management, and education about a healthy diet can significantly impact positive treatment outcomes (230).
Zinc (Zn) supplementation can provide antioxidant, anti-inflammatory, and antidepressant benefits as a micronutrient (231, 232). Several studies have found that women with PMS have lower levels of zinc (Zn) (233–236). Jafari et al. confirmed that zinc supplementation for 12 weeks reduced the severity of both physical and psychological symptoms of PMS. The study reported an increase in BDNF and TAC levels, which may have contributed to the positive effects of the treatment (232).
Vitamin D plays a crucial role in maintaining calcium homeostasis, sex hormone concentrations, and the normal functioning of neurotransmitters (237–240). In addition, it reduces the production of prostaglandins (241). Furthermore, it has significant effects on the female reproductive system (242). Bahrami et al. demonstrated that high doses of vitamin D (50,000 IU cholecalciferol/week for nine weeks) alleviate PMS symptoms and painful menstruation in adolescent females. The treatment also reduces the incidence of back pain, tendency to cry, and possibly nausea, as well as loss of concentration or lack of energy (243). However, the results of another study contradict this, as it found no significant effect of vitamin D supplementation on PMS symptoms despite administering a dose of 2000 IU every other day for 12 weeks (244).
One study found that calcium supplementation can reduce affective and behavioral symptoms of PMS, such as depression, changes in appetite, and early fatigue (245). Another research confirmed that taking 500mg of calcium daily for two months can reduce mood disturbances related to PMS (246). Earlier studies have also noted a reduction in PMS symptoms (247, 248).
B vitamins play a crucial role in the metabolic processes of many of our body’s systems (19). In a comparative study by Chocano-Bedoya PO et al., it was demonstrated that vitamins B1 and B2 significantly reduce the risk of PMS (249). Abdollahifard et al. found that taking vitamin B1 during the luteal phase reduces both physical and psychological symptoms of PMS (250). Some studies have shown that vitamin B6 can alleviate and reduce the occurrence of PMS symptoms (251–253). B vitamins affect neurotransmitter metabolism through various mechanisms (249). Vitamin B1 is important in the metabolism of GABA precursors, which regulate conductance thought to be crucial in the pathogenesis of PMS (254, 255). Vitamin B2 is essential for the activation of vitamin B6. Vitamin B6 plays a crucial role as a cofactor in the production of serotonin. In contrast, several other studies have not found clear evidence of vitamin B6 effectively modifying PMS symptoms (256–258). However, despite the conflicting results, it is recommended that B vitamins be supplemented as they may help alleviate mild PMS symptoms through the mechanisms described above (259).
Magnesium (Mg) is a cofactor for many enzymes and influences many biochemical reactions. It is responsible for protein synthesis, proper muscle and nerve function, and maintaining blood osmoticity (260). According to Moslehi et al.’s meta-analysis, there is currently no significant relationship between serum or erythrocyte magnesium concentrations.
Additionally, there was no observed vitamin A deficiency in either the luteal or follicular phases. However, it is important to note that the study had a small sample size, consisting of only 10 PMS patients in the study group and 10 women in the control group (261).
In addition to the non-pharmacological treatments mentioned above, such as supplementation and physical activity, other factors can influence a healthy lifestyle and potentially improve quality of life.
Research has shown that diet can affect the likelihood of experiencing PMS symptoms. Two studies have confirmed that consuming high amounts of unhealthy foods, such as fast food, soft drinks, processed meat, salt, sugar, sweets, hydrogenated fats, mayonnaise, high-fat dairy products and red meat, can increase the risk of PMS (262, 263). In contrast, consuming meals that are rich in fruits, vegetables, dried fruits, nuts, legumes, garlic, and fish may decrease the likelihood of experiencing PMS (263).
Some studies have indicated that the consumption of caffeine and caffeinated beverages is linked to PMS symptoms, particularly increased breast pain. Therefore, it is recommended to avoid consuming caffeine (264–267). However, several studies have failed to find any correlation (268–270). Therefore, further research is required to determine conclusively whether caffeine has any impact on PMS.
In addition to consuming high-calorie, fatty, sugary, and salty foods, smoking (268, 271–273) and alcohol consumption (274) are also significant risk factors for PMS.
Physical activity may alleviate some symptoms, such as depression or increased pain tolerance, by increasing the secretion of endorphins and reducing cortisol concentrations (275). Aerobic exercise can also reduce feelings of fatigue, improve concentration, and reduce the intensity of other PMS symptoms. Regular physical activity has been shown to have a regulating effect on prolactin, oestradiol, and progesterone concentrations, as well as an increase in hemoglobin, erythrocytes, and thrombocytes (276).
It is important to note that regular exercise can alleviate PMS symptoms, while occasional physical activity may exacerbate them (277).
Mohebbi Dehnavi et al. confirmed that regular aerobic exercise has a positive effect on PMS symptoms. In their study, 35 female students performed high-intensity aerobic activity for 30 minutes, 3 times a week, for a total of 8 weeks. The completion of it resulted in a reduction in the severity of some physical symptoms of PMS (278).
However, the results of studies on this topic are contradictory. Several of them indicate that aerobic exercises, such as walking, running, or swimming, have a positive impact on PMS symptoms (279–281). Others have found no significant association between physical activity and physical symptoms of PMS (282, 283).
Yoga has been found to have a positive effect on reducing both physical and psychological symptoms of PMS, as evidenced by several studies (284–286). Yoga comprises three key elements: breath control (pranayama), postures (asana), and meditation (dhyana) (287). The breathing techniques employed during yoga have a significant impact on various bodily systems, including the nervous system. They regulate the function of the autonomic nervous system (ANS) and inhibit its sympathetic part. They also reduce heart rate and blood pressure (288, 289). Presumably, this helps relax the mind and body and can relieve feelings of tension, anxiety, or depression (284). Yoga poses involve limb positioning and muscle contraction, which stimulates pressure receptors under the skin. By increasing vagus nerve activity, cortisol production is reduced (290). Furthermore, yoga increases BDNF levels (291), a factor whose increase is one of the resultant effects of SSRI drugs. This effect can be linked both to the activation of the autonomic system during exercise and to the stimulation of prefrontal cortex activity during meditation (292) (293). This can have positive effects on pain, depression, and immune function (284). Meditation, also known as relaxation training or relaxation exercises, involves achieving calmness and inner tranquility by maintaining a continuous state of mindfulness. Furthermore, they stimulate the secretion of melatonin, which enhances the quality of sleep and aids in falling asleep (291).
According to a study conducted by Chang et al., yoga alleviates both physical and psychological symptoms associated with PMS. In a research conducted by Vaghel et al., 65 women performed yoga exercises at home using a 30-minute DVD program, at least three times a week for three menstrual cycles (284).
Both yoga and aerobic exercise were found to have a positive effect on reducing the intensity of pain and other somatic symptoms of PMS. However, the group of women who regularly practiced yoga had better results compared to the group who regularly did aerobic exercise. Yoga may alleviate psychological symptoms of PMS, such as stress and anxiety, due to its focus on breath control and meditation in addition to physical activity, unlike aerobic exercise (294). Yoga can support a focus on feelings, which correlates with some of the components of behavioral-cognitive therapy.
Non-pharmacological treatments can significantly alleviate physical symptoms of PMS. These include supplementation with vitamin B6, vitamin D, zinc, calcium. CBT therapy can positively reduce the severity of PMS symptoms related to the psyche. Regular aerobic exercise and yoga can relieve physical complaints such as headaches, fatigue, cramps, swelling, and breast pain. By using breathing techniques and meditation during yoga, it is possible to reduce feelings of tension, depression, or anxiety. Limiting the intake of unhealthy food, alcohol, and smoking can also reduce the risk of symptoms.
It is worth noting that the treatment methods listed are interrelated and, when used together, can produce positive results. CBT therapy is designed to provide patients with appropriate psychoeducation. Women experiencing PMS can acquire a fundamental understanding of the condition, its causes, and coping mechanisms. Therapy sessions often promote a healthier lifestyle, including regular exercise, and dietary changes, which can alleviate most physical symptoms. Overall, this may improve women’s well-being and quality of life. However, there is a lack of research that considers most non-pharmacological treatments simultaneously.
It is believed that hysterectomy combined with bilateral removal of the ovaries and fallopian tubes (TAH/BSO) can be considered an effective treatment for severe PMS or PMDD. However, it is important to be aware of the consequences it carries - patients are at risk of premature menopause with this form of treatment (295). Ovarian failure after hysterectomy alone is estimated to occur in 15-50% of cases. Due to the risk of the consequences of premature menopause: osteoporosis, premature death, mood disorders, infertility, neurological and cardiovascular diseases, patients are recommended hormone therapy (HRT) (296).
A study by Cronje et al. showed the efficacy of (TAH/BSO) in 47 patients with severe PMS, who continued hormone therapy after surgery. Interestingly, 96% of the women were ‘satisfied’ or ‘very satisfied’ with TAH/BSO, and 93.6% reported complete resolution of cyclic symptoms. The study concluded that TAH/BSO, in combination with HRT, was an extremely effective treatment for PMS (297).
Another invasive treatment for PMS is thermal ablation of the endometrium. A study that tested the efficacy of this form of therapy involved 36 patients reporting heavy periods and PMS symptoms. The average age of the women undergoing treatment was 41.4 years and the average body mass index was 26.7. Most of them, as many as 75 percent, had not undergone effective hormone therapy. Virtually all the women, 97%, reported improvement in PMS after endometrial ablation, both in terms of the severity of symptoms and in terms of the symptoms themselves (298). At this point, it should be noted that symptom relief may be related to the cessation of bleeding itself. In particular, a study involving 73 women showed that initially after treatment when patients did not experience monthly bleeding, they did not report PMS symptoms at the same time, whereas when bleeding recurred, PMS-related complaints also appeared (299).
Surgical methods of treating severe PMS or PMDD, are very effective, however, the complications they may entail should not be overlooked. TAH/BSO appears to be the riskiest, and the potential health risks associated with organ removal require the implementation of hormone replacement. It should also be borne in mind that the studies referred to included a small group and that ablation is not a recognized form of treatment for PMS and PMDD and the impression of a reduction in the severity of complaints may have been correlated with the non-occurrence of bleeding.
Non-pharmacological treatments should be considered before introducing medications. However, in cases of severe symptoms, consider them complementary support for pharmacological treatment. Psychoeducation plays a pivotal role at every stage, helping patients prepare for the most challenging days of the cycle. It should cover elements such as diet and physical activity levels, while fostering awareness of the disorder’s complexity. CBT remains a viable option at any stage of the treatment process.
We recommend that pharmacological treatment should be tailored to the individual clinical needs of the patient, taking into account the entire clinical picture. In the case of the predominance of psychological symptoms, SSRI drugs are preferred, while physical symptoms are treated with OC drugs. It is important to personalize the treatment not only based on the patient’s predominant symptoms but also taking into consideration their preferences and individual circumstances. For instance, for patients who often forget to take their medication, SSRIs might be a better option as they are fast-acting in PMS. Similarly, it is crucial to consider the significant side effects of the drug. For example, paroxetine has a strong anticholinergic effect and can cause weight gain, making it necessary to use caution when considering its inclusion in cases of coexisting diabetes or obesity. On the other hand, escitalopram may cause QT prolongation at higher doses, making it better to choose another drug such as sertraline for patients who are in the habit of self-medication and increasing doses. In case a patient chooses venlafaxine, it is important to keep in mind that it may raise blood pressure.
The most effective approach to treatment with SSRIs appears to be their administration during the luteal phase. Continuous administration is feasible, but it should be reserved for severe cases of PMS (e.g., in patients burdened with other psychiatric disorders) due to the higher risk of side effects. Notably, comparable therapeutic effects can be achieved by administering the drug solely during the luteal phase, making non-continuous administration the preferred treatment method. A continuous treatment regimen may also be considered if there is no response to the intermittent method (only in the luteal phase).
Based on the information presented in our study, the optimal selection among SSRIs would be fluoxetine, which can be used at a maximum dose of 20 mg. Scientific reports suggest no significant differences in side effects between 20 mg and 10 mg doses due to the interval in administration. However, we recommend restricting the administration of the maximum dose to the most severe cases. For others, the preferred starting dose is 10 mg, as studies indicate noticeable improvements in patients’ conditions even at low doses. A follow-up visit is recommended after 3 cycles, as this is when response rates are typically high.
In cases where physical symptoms and irregular cycles predominate, OC drugs appear to be a preferable choice. Among the EE drugs, drospirenone has been extensively studied, with no demonstrated advantage of other preparations in the same group.
We recommend using a dosing schedule of 24/4 instead of 21/7, as supported by the studies we cited. These studies indicate that limiting placebo days has a beneficial effect on treatment. If the placebo regimen proves ineffective in symptom reduction, especially during the placebo phase, transitioning to a COC may be considered (Figure 4).
Considering the disorder’s etiology, pain medications may offer a viable option. Additionally, research on neurosteroids suggests that they can be employed to alleviate PMS symptoms by inhibiting the action or synthesis of allopregnanolone. The use of isoallopregnanolone appears particularly promising, although further research is needed to conclusively demonstrate its therapeutic effectiveness.
Herbal treatment serves as an alternative to pharmacological drugs; however, due to significant limitations in the methodology of research on herbs, they should not be considered a therapeutic option in more severe cases. Moreover, numerous interactions restrict the possibility of their use, and while VAC or saffron seem to be the most promising, evidence of their efficacy is limited. Due to the widespread popularity of herbal supplements (300), it is advisable to ensure that patients do not concurrently use such preparations, as monotherapy is preferred to avoid potential interactions. When combining herbs with pharmacological drugs, there is a specific concern regarding their impact on hepatic drug metabolism. For example, St. John’s wort (Hypericum perforatum) acts as an inducer of CYP3A4, CYP1A2, and CYP2C9, which may lead to decreased efficacy of several antidepressants, such as paroxetine, sertraline, and fluoxetine, due to increased liver metabolism. On the other hand, the use of Ginkgo biloba, by inhibiting cytochromes, may elevate plasma concentrations of drugs, potentially increasing side effects and exacerbating the antiplatelet effects of SSRI and SNRI drugs (301). Additionally, it is essential to note that patients using saffron are at a higher risk of developing serotonin syndrome when concurrently taking SSRI drugs.
It is essential to note that our review is not systematic, and despite attempts to cover all studies, one should keep in mind significant limitations.
SM: Conceptualization, Data curation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. AO: Data curation, Investigation, Project administration, Resources, Writing – review & editing. XZ: Data curation, Methodology, Resources, Writing – original draft. KI: Data curation, Investigation, Resources, Visualization, Writing – original draft. ZS: Conceptualization, Visualization, Writing – original draft. NW: Funding acquisition, Project administration, Supervision, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Medical University of Bialystok, grant number B.SUB.24.445. The financial sponsor played no role in the design, execution, analysis, and interpretation of data.
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Farahmand M, Ramezani Tehrani F, Khalili D, Amin G, Negarandeh R. Factors associated with the severity of premenstrual syndrome among Iranian college students. J Obstet Gynaecol Res. (2017) 43:1726–31. doi: 10.1111/jog.13439
2. Freeman EW. Premenstrual syndrome and premenstrual dysphoric disorder: definitions and diagnosis. Psychoneuroendocrinology. (2003) 28 Suppl 3:25–37. doi: 10.1016/S0306-4530(03)00099-4
3. Direkvand-Moghadam A, Sayehmiri K, Delpisheh A, K S. Epidemiology of premenstrual syndrome (PMS)-A systematic review and meta-analysis study. J Clin Diagn Res. (2014) 8:106–9. doi: 10.7860/JCDR/2014/8024.4021
4. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. (2012) 18:48–51. doi: 10.1258/mi.2012.012013
5. Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. (2012) 169:465–75. doi: 10.1176/appi.ajp.2012.11081302
6. Tkaczuk-Włach J, Sobstyl M, Syty K, Łepecka-Klusek C, Jakiel G. Zespół napięcia przedmiesiączkowego. Menopause Review/Przegląd Menopauzalny. (2009) 8:339–43.
7. Grady-Weliky TA. Clinical practice. Premenstrual dysphoric disorder N Engl J Med. (2003) 348:433–8. doi: 10.1056/NEJMcp012067
8. Sadler C, Smith H, Hammond J, Bayly R, Borland S, Panay N, et al. Lifestyle factors, hormonal contraception, and premenstrual symptoms: the United Kingdom Southampton Women's Survey. J Womens Health (Larchmt). (2010) 19:391–6. doi: 10.1089/jwh.2008.1210
9. Deuster PA, Adera T, South-Paul J. Biological, social, and behavioral factors associated with premenstrual syndrome. Arch Fam Med. (1999) 8:122–8. doi: 10.1001/archfami.8.2.122
10. Dilbaz B, Aksan A. Premenstrual syndrome, a common but underrated entity: review of the clinical literature. J Turk Ger Gynecol Assoc. (2021) 22:139–48. doi: 10.4274/jtgga.
11. Oracz A, Modzelewski S, Iłendo K, Sokół A. Brexanolone and current methods of treating postpartum and perinatal depression. Pharmacotherapy Psychiatry Neurology/Farmakoterapia w Psychiatrii i Neurologii. (2023) 39:53–64. doi: 10.5114/fpn.2023.127424
12. Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA(A) receptors. Pharmacol Ther. (2007) 116:7–19. doi: 10.1016/j.pharmthera.2007.03.011
13. Chua HC, Chebib M. GABA(A) receptors and the diversity in their structure and pharmacology. Adv Pharmacol. (2017) 79:1–34. doi: 10.1016/bs.apha.2017.03.003
14. Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology. (2009) 34 Suppl 1:S48–58. doi: 10.1016/j.psyneuen.2009.08.009
15. Tang GY, Parekh J. Brexanolone injection for post-partum depression treatment. Lancet. (2019) 394:379. doi: 10.1016/S0140-6736(19)30714-7
16. Balan I, Patterson R, Boero G, Krohn H, O'Buckley TK, Meltzer-Brody S, et al. Brexanolone therapeutics in post-partum depression involves inhibition of systemic inflammatory pathways. EBioMedicine. (2023) 89:104473. doi: 10.1016/j.ebiom.2023.104473
17. Cao S, Jones M, Tooth L, Mishra GD. History of premenstrual syndrome and development of postpartum depression: A systematic review and meta-analysis. J Psychiatr Res. (2020) 121:82–90. doi: 10.1016/j.jpsychires.2019.11.010
18. O'Brien S, Rapkin A, Dennerstein L, Nevatte T. Diagnosis and management of premenstrual disorders. BMJ. (2011) 342:d2994. doi: 10.1136/bmj.d2994
19. Modzelewski S, Oracz A, Iłendo K, Sokół A, Waszkiewicz N. Biomarkers of postpartum depression: A narrative review. J Clin Med. (2023) 12. doi: 10.20944/preprints202309.1485.v1
20. Bannister E. There is increasing evidence to suggest that brain inflammation could play a key role in the aetiology of psychiatric illness. Could inflammation be a cause of the premenstrual syndromes PMS and PMDD? Post Reprod Health. (2019) 25:157–61. doi: 10.1177/2053369119875386
21. Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. (2000) 157:924–30. doi: 10.1176/appi.ajp.157.6.924
22. Lanza di Scalea T, Pearlstein T. Premenstrual dysphoric disorder. Med Clin North Am. (2019) 103:613–28. doi: 10.1016/j.mcna.2019.02.007
23. Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. (1994) 62:265–71. doi: 10.1016/0306-4522(94)90330-1
24. Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. (2005) 68:959–65. doi: 10.1124/mol.105.014662
25. Granda D, Szmidt MK, Kaluza J. Is premenstrual syndrome associated with inflammation, oxidative stress and antioxidant status? A systematic review of case-control and cross-sectional studies. Antioxidants (Basel). (2021) 10. doi: 10.3390/antiox10040604
26. Draper CF, Duisters K, Weger B, Chakrabarti A, Harms AC, Brennan L, et al. Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci Rep. (2018) 8:14568. doi: 10.1038/s41598-018-32647-0
27. Reed SC, Levin FR, Evans SM. Changes in mood, cognitive performance and appetite in the late luteal and follicular phases of the menstrual cycle in women with and without PMDD (premenstrual dysphoric disorder). Horm Behav. (2008) 54:185–93. doi: 10.1016/j.yhbeh.2008.02.018
28. Balat O, Dikensoy E, Ugur MG, Atmaca R, Cekmen M, Yurekli M. Malon dialdehyde, nitrite and adrenomedullin levels in patients with premenstrual syndrome. Arch Gynecol Obstet. (2007) 275:361–5. doi: 10.1007/s00404-006-0269-1
29. Duvan CI, Cumaoglu A, Turhan NO, Karasu C, Kafali H. Oxidant/antioxidant status in premenstrual syndrome. Arch Gynecol Obstet. (2011) 283:299–304. doi: 10.1007/s00404-009-1347-y
30. Incebiyik A, Camuzcuoglu A, Hilali NG, Ulas T, Vural M, Camuzcuoglu H, et al. Serum oxidative stress, visfatin and apelin in healthy women and those with premenstrual syndrome. J Obstet Gynaecol. (2015) 35:188–92. doi: 10.3109/01443615.2014.948399
31. Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, et al. Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. Am J Obstetrics Gynecology. (1988) 158:5–11. doi: 10.1016/0002-9378(88)90765-X
32. Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. (2001) 49:788–97. doi: 10.1016/S0006-3223(00)01044-1
33. Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. (1998) 338:209–16. doi: 10.1056/NEJM199801223380401
34. Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. (2016) 41:1093–102. doi: 10.1038/npp.2015.246
35. Timby E, Bäckström T, Nyberg S, Stenlund H, Wihlbäck AN, Bixo M. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls-a pilot study. Psychopharmacol (Berl). (2016) 233:2109–17. doi: 10.1007/s00213-016-4258-1
36. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacol (Berl). (2014) 231:3619–34. doi: 10.1007/s00213-014-3572-8
37. Rønnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. (2005) 26:65–84. doi: 10.1016/j.yfrne.2005.05.001
38. Comasco E, Sundström-Poromaa I. Neuroimaging the menstrual cycle and premenstrual dysphoric disorder. Curr Psychiatry Rep. (2015) 17:77. doi: 10.1007/s11920-015-0619-4
39. Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, et al. Allopregnanolone and mood disorders. Prog Neurobiol. (2014) 113:88–94. doi: 10.1016/j.pneurobio.2013.07.005
40. Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. (2002) 2:795–816. doi: 10.2174/1568026023393507
41. Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci. (2009) 29:9592–601. doi: 10.1523/JNEUROSCI.2162-09.2009
42. Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I Normal pregnancy Am J Obstet Gynecol. (1972) 112:1095–100. doi: 10.1016/0002-9378(72)90185-8
43. Schmidt PJ, Nieman LK, Grover GN, Muller KL, Merriam GR, Rubinow DR. Lack of effect of induced menses on symptoms in women with premenstrual syndrome. N Engl J Med. (1991) 324:1174–9. doi: 10.1056/NEJM199104253241705
44. Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. (2017) 174:980–9. doi: 10.1176/appi.ajp.2017.16101113
45. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. (2015) 17:87. doi: 10.1007/s11920-015-0628-3
46. Marjoribanks J, Brown J, O'Brien PM, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. (2013) 2013:Cd001396. doi: 10.1002/14651858
47. Dubol M. Neuroimaging premenstrual dysphoric disorder: A systematic and critical review. Front Neuroendocrinol. (2020) 57:100838. CNEaRLaIS-PaEC. doi: 10.1016/j.yfrne.2020.100838
48. Perez-Caballero L, Torres-Sanchez S, Romero-López-Alberca C, González-Saiz F, Mico JA, Berrocoso E. Monoaminergic system and depression. Cell Tissue Res. (2019) 377:107–13. doi: 10.1007/s00441-018-2978-8
49. Rapkin AJ. The role of serotonin in premenstrual syndrome. Clin Obstet Gynecol. (1992) 35:629–36. doi: 10.1097/00003081-199209000-00022
50. Yonkers KA, O'Brien PM, Eriksson E. Premenstrual syndrome. Lancet. (2008) 371:1200–10. doi: 10.1016/S0140-6736(08)60527-9
51. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. (1999) 96:13512–7. doi: 10.1073/pnas.96.23.13512
52. Sacher J, Zsido RG, Barth C, Zientek F, Rullmann M, Luthardt J, et al. Increase in serotonin transporter binding in patients with premenstrual dysphoric disorder across the menstrual cycle: A case-control longitudinal neuroreceptor ligand positron emission tomography imaging study. Biol Psychiatry. (2023) 93:1081–8. doi: 10.1016/j.biopsych.2022.12.023
53. Guidotti A, Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3 alpha, 5 alpha-tetrahydroprogesterone (allopregnanolone) availability? Biol Psychiatry. (1998) 44:865–73. doi: 10.1016/S0006-3223(98)00070-5
54. Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. (2018) 392:1058–70. doi: 10.1016/S0140-6736(18)31551-4
55. Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. (2009) 9:24–30. doi: 10.1016/j.coph.2008.12.006
56. Matsumoto K, Puia G, Dong E, Pinna G. GABA(A) receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress. (2007) 10:3–12. doi: 10.1080/10253890701200997
57. Wenzel ES, Pinna G, Eisenlohr-Moul T, Bernabe BP, Tallon RR, Nagelli U, et al. Neuroactive steroids and depression in early pregnancy. Psychoneuroendocrinology. (2021) 134:105424. doi: 10.1016/j.psyneuen.2021.105424
58. Miller KN, Standeven L, Morrow AL, Payne JL, Epperson CN, Hantsoo L. GABAergic neuroactive steroid response to sertraline in premenstrual dysphoric disorder. Psychoneuroendocrinology. (2024) 160:106684. doi: 10.1016/j.psyneuen.2023.106684
59. Trauger JW, Jiang A, Stearns BA, LoGrasso PV. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. (2002) 41:13451–9. doi: 10.1021/bi026109w
60. Gold EB, Wells C, Rasor MO. The association of inflammation with premenstrual symptoms. J Womens Health (Larchmt). (2016) 25:865–74. doi: 10.1089/jwh.2015.5529
61. Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. (2013) 14:232–44. doi: 10.1111/obr.12003
62. Puder JJ, Blum CA, Mueller B, De Geyter C, Dye L, Keller U. Menstrual cycle symptoms are associated with changes in low-grade inflammation. Eur J Clin Invest. (2006) 36:58–64. doi: 10.1111/j.1365-2362.2006.01591.x
63. Bertone-Johnson ER, Ronnenberg AG, Houghton SC, Nobles C, Zagarins SE, Takashima-Uebelhoer BB, et al. Association of inflammation markers with menstrual symptom severity and premenstrual syndrome in young women. Hum Reprod. (2014) 29:1987–94. doi: 10.1093/humrep/deu170
64. Martin VT, Ballard J, Diamond MP, Mannix LK, Derosier FJ, Lener SE, et al. Relief of menstrual symptoms and migraine with a single-tablet formulation of sumatriptan and naproxen sodium. J Womens Health (Larchmt). (2014) 23:389–96. doi: 10.1089/jwh.2013.4577
65. Klusmann H, Luecking N, Engel S, Blecker MK, Knaevelsrud C, Schumacher S. Menstrual cycle-related changes in HPA axis reactivity to acute psychosocial and physiological stressors - A systematic review and meta-analysis of longitudinal studies. Neurosci Biobehav Rev. (2023) 150:105212. doi: 10.1016/j.neubiorev.2023.105212
66. Hou L, Huang Y, Zhou R. Premenstrual syndrome is associated with altered cortisol awakening response. Stress. (2019) 22:640–6. doi: 10.1080/10253890.2019.1608943
67. Huang Y, Zhou R, Wu M, Wang Q, Zhao Y. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. (2015) 18:160–8. doi: 10.3109/10253890.2014.999234
68. Nobis A, Zalewski D, Waszkiewicz N. Peripheral markers of depression. J Clin Med. (2020) 9. doi: 10.3390/jcm9123793
69. Klatzkin RR, Bunevicius A, Forneris CA, Girdler S. Menstrual mood disorders are associated with blunted sympathetic reactivity to stress. J Psychosom Res. (2014) 76:46–55. doi: 10.1016/j.jpsychores.2013.11.002
70. Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. (1991) 129:2503–11. doi: 10.1210/endo-129-5-2503
71. Mansel RE, Dogliotti L. European multicentre trial of bromocriptine in cyclical mastalgia. Lancet. (1990) 335:190–3. doi: 10.1016/0140-6736(90)90278-D
72. Carroll BJ, Steiner M. The psychobiology of premenstrual dysphoria: the role of prolactin. Psychoneuroendocrinology. (1978) 3:171–80. doi: 10.1016/0306-4530(78)90005-7
73. Wuttke W, Jarry H, Christoffel V, Spengler B, Seidlová-Wuttke D. Chaste tree (Vitex agnus-castus)–pharmacology and clinical indications. Phytomedicine. (2003) 10:348–57. doi: 10.1078/094471103322004866
74. Schwibbe M, Becker D, Wuttke W. EEG and psychological effects of lisuride in women with premenstrual tension. In: Lisuride and Other Dopamine Agonists Calne. Raven Press, New York (1983). p. 345–55.
75. Hantsoo L, Payne JL. Towards understanding the biology of premenstrual dysphoric disorder: From genes to GABA. Neurosci Biobehav Rev. (2023) 149:105168. doi: 10.1016/j.neubiorev.2023.105168
76. Widholm O, Kantero RL. A statistical analysis of the menstrual patterns of 8,000 Finnish girls and their mothers. Acta Obstet Gynecol Scand Suppl. (1971) 14:Suppl 14:1–36.
77. Jahanfar S, Lye MS, Krishnarajah IS. The heritability of premenstrual syndrome. Twin Res Hum Genet. (2011) 14:433–6. doi: 10.1375/twin.14.5.433
78. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Fac Rev. (2022) 11:11. doi: 10.12703/r
79. Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. (2005) 72:414–21. doi: 10.1016/j.contraception.2005.08.021
80. Eriksson E, Sundblad C, Lisjö P, Modigh K, Andersch B. Serum levels of androgens are higher in women with premenstrual irritability and dysphoria than in controls. Psychoneuroendocrinology. (1992) 17:195–204. doi: 10.1016/0306-4530(92)90058-F
81. Dougherty DM, Bjork JM, Moeller FG, Swann AC. The influence of menstrual-cycle phase on the relationship between testosterone and aggression. Physiol Behav. (1997) 62:431–5. doi: 10.1016/S0031-9384(97)88991-3
82. Marecki R, Kałuska J, Kolanek A, Hakało D, Waszkiewicz N. Zuranolone - synthetic neurosteroid in treatment of mental disorders: narrative review. Front Psychiatry. (2023) 14:1298359. doi: 10.3389/fpsyt.2023.1298359
83. Muhn P, Fuhrmann U, Fritzemeier KH, Krattenmacher R, Schillinger E. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci. (1995) 761:311–35. doi: 10.1111/j.1749-6632.1995.tb31386.x
84. Fuhrmann U, Krattenmacher R, Slater EP, Fritzemeier KH. The novel progestin drospirenone and its natural counterpart progesterone: biochemical profile and antiandrogenic potential. Contraception. (1996) 54:243–51. doi: 10.1016/S0010-7824(96)00195-3
85. Vellacott ID, O'Brien PM. Effect of spironolactone on premenstrual syndrome symptoms. J Reprod Med. (1987) 32:429–34.
86. Hellberg D, Claesson B, Nilsson S. Premenstrual tension: a placebo-controlled efficacy study with spironolactone and medroxyprogesterone acetate. Int J Gynaecol Obstet. (1991) 34:243–8. doi: 10.1016/0020-7292(91)90357-B
87. Shapiro G, Evron S. A novel use of spironolactone: treatment of hirsutism. J Clin Endocrinol Metab. (1980) 51:429–32. doi: 10.1210/jcem-51-3-429
88. Freeman EW, Kroll R, Rapkin A, Pearlstein T, Brown C, Parsey K, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med. (2001) 10:561–9. doi: 10.1089/15246090152543148
89. Graham CA, Sherwin BB. A prospective treatment study of premenstrual symptoms using a triphasic oral contraceptive. J Psychosom Res. (1992) 36:257–66. doi: 10.1016/0022-3999(92)90090-O
90. Lopez LM, Kaptein A, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. (2008) 1:Cd006586. doi: 10.1002/14651858.CD006586.pub2
91. Lundin C, Danielsson KG, Marie B, Moby L, Bengtsdotter H, Jawad I, et al. Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle—A double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology. (2017) 76:135–43. doi: 10.1016/j.psyneuen.2016.11.033
92. Halbreich U, Freeman EW, Rapkin AJ, Cohen LS, Grubb GS, Bergeron R, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. (2012) 85:19–27. doi: 10.1016/j.contraception.2011.05.008
93. Comasco E, Kopp Kallner H, Bixo M, Hirschberg AL, Nyback S, de Grauw H, et al. Ulipristal acetate for treatment of premenstrual dysphoric disorder: A proof-of-concept randomized controlled trial. Am J Psychiatry. (2021) 178:256–65. doi: 10.1176/appi.ajp.2020.20030286
94. Bäckström T, Ekberg K, Hirschberg AL, Bixo M, Epperson CN, Briggs P, et al. A randomized, double-blind study on efficacy and safety of sepranolone in premenstrual dysphoric disorder. Psychoneuroendocrinology. (2021) 133:105426. doi: 10.1016/j.psyneuen.2021.105426
95. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. (2009) 2:Cd006586. doi: 10.1002/14651858.CD006586.pub3
96. Inger Sundström-Poromaa and Erika Comasco and Rachael Sumner and Eileen L. Progesterone – friend or foe? Front Neuroendocrinol. (2020) 59:100856. doi: 10.1016/j.yfrne.2020.100856
97. Hardoy MC, Serra M, Carta MG, Contu P, Pisu MG, Biggio G. Increased neuroactive steroid concentrations in women with bipolar disorder or major depressive disorder. J Clin Psychopharmacol. (2006) 26:379–84. doi: 10.1097/01.jcp.0000229483.52955.ec
98. Sundström-Poromaa I, Comasco E, Sumner R, Luders E. Progesterone - friend or foe? Front Neuroendocrinol. (2020) 59:100856.
99. Gingnell M, Engman J, Frick A, Moby L, Wikström J, Fredrikson M, et al. Oral contraceptive use changes brain activity and mood in women with previous negative affect on the pill–a double-blinded, placebo-controlled randomized trial of a levonorgestrel-containing combined oral contraceptive. Psychoneuroendocrinology. (2013) 38:1133–44. doi: 10.1016/j.psyneuen.2012.11.006
100. Spinelli MG. Depression and hormone therapy. Clin Obstet Gynecol. (2004) 47:428–36. doi: 10.1097/00003081-200406000-00019
101. Taylor MA, Abrams R. Gender differences in bipolar affective disorder. J Affect Disord. (1981) 3:261–71. doi: 10.1016/0165-0327(81)90027-6
102. Tondo L, Baldessarini RJ. Rapid cycling in women and men with bipolar manic-depressive disorders. Am J Psychiatry. (1998) 155:1434–6. doi: 10.1176/ajp.155.10.1434
103. Paul SM, Purdy RH. Neuroactive steroids. FASEB J. (1992) 6:2311–22. doi: 10.1096/fasebj.6.6.1347506
104. Backström T. Neuroendocrinology of premenstrual syndrome. Clin Obstet Gynecol. (1992) 35:612–28. doi: 10.1097/00003081-199209000-00021
105. Henshaw C, Foreman D, Belcher J, Cox J, O'Brien S. Can one induce premenstrual symptomatology in women with prior hysterectomy and bilateral oophorectomy? J Psychosom Obstet Gynaecol. (1996) 17:21–8.
106. Naheed B, Kuiper JH, Uthman OA, O'Mahony F, O'Brien PM. Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome. Cochrane Database Syst Rev. (2017) 3:Cd010503. doi: 10.1002/14651858.CD010503.pub2
107. Carlini SV, Lanza di Scalea T, McNally ST, Lester J, Deligiannidis KM. Management of premenstrual dysphoric disorder: A scoping review. Int J Womens Health. (2022) 14:1783–801. doi: 10.2147/IJWH.S297062
108. Archer DF, Kovalevsky G, Ballagh SA, Grubb GS. Ovarian activity and safety of a novel levonorgestrel/ethinyl estradiol continuous oral contraceptive regimen. Contraception. (2009) 80:245–53. doi: 10.1016/j.contraception.2009.03.006
109. Freeman EW, Halbreich U, Grubb GS, Rapkin AJ, Skouby SO, Smith L, et al. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. (2012) 85:437–45. doi: 10.1016/j.contraception.2011.09.010
110. Kwiecien M, Edelman A, Nichols MD, Jensen JT. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. (2003) 67:9–13. doi: 10.1016/S0010-7824(02)00445-6
111. Aleknaviciute J, Tulen JHM, De Rijke YB, Bouwkamp CG, van der Kroeg M, Timmermans M, et al. The levonorgestrel-releasing intrauterine device potentiates stress reactivity. Psychoneuroendocrinology. (2017) 80:39–45. doi: 10.1016/j.psyneuen.2017.02.025
112. Merki-Feld GS, Apter D, Bartfai G, Grandi G, Haldre K, Lech M, et al. ESC expert statement on the effects on mood of the natural cycle and progestin-only contraceptives. Eur J Contracept Reprod Health Care. (2017) 22:247–9. doi: 10.1080/13625187.2017.1353075
113. Tewari R, Kay VJ. Compliance and user satisfaction with the intra-uterine contraceptive device in Family Planning Service: the results of a survey in Fife, Scotland, August 2004. Eur J Contracept Reprod Health Care. (2006) 11:28–37. doi: 10.1080/13625180500431422
114. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. (1994) 49:56–72. doi: 10.1016/0010-7824(94)90109-0
115. Sivin I, el Mahgoub S, McCarthy T, Mishell DR Jr., Shoupe D, Alvarez F, et al. Long-term contraception with the levonorgestrel 20 mcg/day (LNg 20) and the copper T 380Ag intrauterine devices: a five-year randomized study. Contraception. (1990) 42:361–78. doi: 10.1016/0010-7824(90)90046-X
116. Baker LJ, O'Brien PM. Premenstrual syndrome (PMS): a peri-menopausal perspective. Maturitas. (2012) 72:121–5. doi: 10.1016/j.maturitas.2012.03.007
117. Guerra-Araiza C, Cerbón MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sci. (2000) 66:1743–52. doi: 10.1016/S0024-3205(00)00497-5
118. Rabe T, Saenger N, Ebert AD, Roemer T, Tinneberg HR, De Wilde RL, et al. Selective progesterone receptor modulators for the medical treatment of uterine fibroids with a focus on ulipristal acetate. BioMed Res Int. (2018) 2018:1374821. doi: 10.1155/2018/1374821
119. Nworie KM. Premenstrual syndrome: etiology, diagnosis and treatment. A mini literature review. J Obstetrics Gynecological Investigations. (2018) 1:41–6. doi: 10.5114/jogi.2018.78010
120. Ismaili E, Walsh S, O'Brien PMS, Bäckström T, Brown C, Dennerstein L, et al. Fourth consensus of the International Society for Premenstrual Disorders (ISPMD): auditable standards for diagnosis and management of premenstrual disorder. Arch Womens Ment Health. (2016) 19:953–8. doi: 10.1007/s00737-016-0631-7
121. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with "add-back" estrogen therapy. Obstet Gynecol. (1993) 81:104–7.
122. Jungheim ES, Kenerson JJ, Foyouzi-Yousefi N, Allsworth JE, Marquard KL. Discussion: 'Add-back regimens in patients using a GnRH agonist for premenstrual dysphoric disorder' by Segebladh et al. Am J Obstet Gynecol. (2009) 201:e1–5. doi: 10.1016/j.ajog.2009.06.001
123. Erkkola R, Kumento U, Lehmuskoski S, Mattila L, Mustonen M. No increased risk of endometrial hyperplasia with fixed long-cycle oestrogen-progestogen therapy after five years. J Br Menopause Soc. (2004) 10:9–13. doi: 10.1258/136218004322986717
124. Mezrow G, Shoupe D, Spicer D, Lobo R, Leung B, Pike M. Depot leuprolide acetate with estrogen and progestin add-back for long-term treatment of premenstrual syndrome. Fertil Steril. (1994) 62:932–7. doi: 10.1016/S0015-0282(16)57053-8
125. Segebladh B, Borgström A, Nyberg S, Bixo M, Sundström-Poromaa I. Evaluation of different add-back estradiol and progesterone treatments to gonadotropin-releasing hormone agonist treatment in patients with premenstrual dysphoric disorder. Am J Obstet Gynecol. (2009) 201:139.e1–8. doi: 10.1016/j.ajog.2009.03.016
126. Wang M, Seippel L, Purdy RH, Bãckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J Clin Endocrinol Metab. (1996) 81:1076–82. doi: 10.1210/jcem.81.3.8772579
127. Wyatt KM, Dimmock PW, Ismail KM, Jones PW, O'Brien PM. The effectiveness of GnRHa with and without 'add-back' therapy in treating premenstrual syndrome: a meta analysis. BJOG. (2004) 111:585–93. doi: 10.1111/j.1471-0528.2004.00135.x
128. Mortola JF, Girton L, Fischer U. Successful treatment of severe premenstrual syndrome by combined use of gonadotropin-releasing hormone agonist and estrogen/progestin. J Clin Endocrinol Metab. (1991) 72:252A–F. doi: 10.1210/jcem-72-2-252
129. Rzewuska AM, Żybowska M, Sajkiewicz I, Spiechowicz I, Żak K, Abramiuk M, et al. Gonadotropin-releasing hormone antagonists-A new hope in endometriosis treatment? J Clin Med. (2023) 12. doi: 10.3390/jcm12031008
130. Copperman AB, Benadiva C. Optimal usage of the GnRH antagonists: a review of the literature. Reprod Biol Endocrinol. (2013) 11:20. doi: 10.1186/1477-7827-11-20
131. Hung SW, Zhang R, Tan Z, Chung JPW, Zhang T, Wang CC. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: A review. Med Res Rev. (2021) 41:2489–564. doi: 10.1002/med.21802
132. Sikes-Keilp C, Rubinow DR. GABA-ergic modulators: new therapeutic approaches to premenstrual dysphoric disorder. CNS Drugs. (2023) 37:679–93. doi: 10.1007/s40263-023-01030-7
133. van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar JK, et al. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. (2008) 13:325–33. doi: 10.1038/sj.mp.4002030
134. van Wingen G, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar J, et al. How progesterone impairs memory for biologically salient stimuli in healthy young women. J Neurosci. (2007) 27:11416–23. doi: 10.1523/JNEUROSCI.1715-07.2007
135. Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. (2003) 44:242–57. doi: 10.1016/j.yhbeh.2003.04.002
136. Gourley SL, Debold JF, Yin W, Cook J, Miczek KA. Benzodiazepines and heightened aggressive behavior in rats: reduction by GABA(A)/alpha(1) receptor antagonists. Psychopharmacol (Berl). (2005) 178:232–40. doi: 10.1007/s00213-004-1987-3
137. Gracia CR, Freeman EW, Sammel MD, Lin H, Sheng L, Frye C. Allopregnanolone levels before and after selective serotonin reuptake inhibitor treatment of premenstrual symptoms. J Clin Psychopharmacol. (2009) 29:403–5. doi: 10.1097/JCP.0b013e3181ad8825
138. Belelli D, Lambert JJ, Peters JA, Gee KW, Lan NC. Modulation of human recombinant GABAA receptors by pregnanediols. Neuropharmacology. (1996) 35:1223–31. doi: 10.1016/S0028-3908(96)00066-4
139. Bäckström T, Das R, Bixo M. Positive GABA(A) receptor modulating steroids and their antagonists: Implications for clinical treatments. J Neuroendocrinol. (2022) 34:e13013. doi: 10.1111/jne.13013
140. Bäckström T, Wahlström G, Wahlström K, Zhu D, Wang MD. Isoallopregnanolone; an antagonist to the anaesthetic effect of allopregnanolone in male rats. Eur J Pharmacol. (2005) 512:15–21. doi: 10.1016/j.ejphar.2005.01.049
141. Bengtsson SK, Nyberg S, Hedström H, Zingmark E, Jonsson B, Bäckström T, et al. Isoallopregnanolone antagonize allopregnanolone-induced effects on saccadic eye velocity and self-reported sedation in humans. Psychoneuroendocrinology. (2015) 52:22–31. doi: 10.1016/j.psyneuen.2014.10.025
142. Bixo M, Ekberg K, Poromaa IS, Hirschberg AL, Jonasson AF, Andréen L, et al. Treatment of premenstrual dysphoric disorder with the GABA(A) receptor modulating steroid antagonist Sepranolone (UC1010)-A randomized controlled trial. Psychoneuroendocrinology. (2017) 80:46–55. doi: 10.1016/j.psyneuen.2017.02.031
143. Rapkin A. A review of treatment of premenstrual syndrome and premenstrual dysphoric disorder. Psychoneuroendocrinology. (2003) 28 Suppl 3:39–53. doi: 10.1016/S0306-4530(03)00096-9
144. Green LJ, O’Brien PMS, Panay N, Craig M, on behalf of the Royal College of Obstetricians and Gynaecologists. Management of premenstrual syndrome: green-top guideline no. 48. Bjog. (2017) 124:e73–e105. doi: 10.1111/1471-0528.14260
145. Halbreich U, O'Brien PM, Eriksson E, Bäckström T, Yonkers KA, Freeman EW. Are there differential symptom profiles that improve in response to different pharmacological treatments of premenstrual syndrome/premenstrual dysphoric disorder? CNS Drugs. (2006) 20:523–47. doi: 10.2165/00023210-200620070-00001
146. Landén M, Erlandsson H, Bengtsson F, Andersch B, Eriksson E. Short onset of action of a serotonin reuptake inhibitor when used to reduce premenstrual irritability. Neuropsychopharmacology. (2009) 34:585–92. doi: 10.1038/npp.2008.86
147. Harmer CJ, Cowen PJ. 'It's the way that you look at it'–a cognitive neuropsychological account of SSRI action in depression. Philos Trans R Soc Lond B Biol Sci. (2013) 368:20120407. doi: 10.1098/rstb.2012.0407
148. Sundström Poromaa I, Comasco E, Bäckström T, Bixo M, Jensen P, Frokjaer VG. Negative association between allopregnanolone and cerebral serotonin transporter binding in healthy women of fertile age. Front Psychol. (2018) 9:2767. doi: 10.3389/fpsyg.2018.02767
149. Sundström-Poromaa I, Comasco E. New pharmacological approaches to the management of premenstrual dysphoric disorder. CNS Drugs. (2023) 37:371–9. doi: 10.1007/s40263-023-01004-9
150. Reilly TJ, Wallman P, Clark I, Knox CL, Craig MC, Taylor D. Intermittent selective serotonin reuptake inhibitors for premenstrual syndromes: A systematic review and meta-analysis of randomised trials. J Psychopharmacol. (2023) 37:261–7. doi: 10.1177/02698811221099645
151. Freeman EW, Rickels K, Sondheimer SJ, Polansky M, Xiao S. Continuous or intermittent dosing with sertraline for patients with severe premenstrual syndrome or premenstrual dysphoric disorder. Am J Psychiatry. (2004) 161:343–51. doi: 10.1176/appi.ajp.161.2.343
152. Maranho M, Guapo VG, de Rezende MG, Vieira CS, Brandão ML, Graeff FG, et al. Low doses of fluoxetine for the treatment of emotional premenstrual syndrome: a randomized double-blind, placebo-controlled, pilot study. Psychoneuroendocrinology. (2023) 157:106360. doi: 10.1016/j.psyneuen.2023.106360
153. Yonkers KA, Pearlstein TB, Gotman N. A pilot study to compare fluoxetine, calcium, and placebo in the treatment of premenstrual syndrome. J Clin Psychopharmacol. (2013) 33:614–20. doi: 10.1097/JCP.0b013e31829c7697
154. Nazari H, Yari F, Jariani M, Marzban A, Birgandy M. Premenstrual syndrome: a single-blind study of treatment with buspirone versus fluoxetine. Arch Gynecol Obstet. (2013) 287:469–72. doi: 10.1007/s00404-012-2594-x
155. Hunter MS, Ussher JM, Cariss M, Browne S, Jelley R, Katz M. Medical (fluoxetine) and psychological (cognitive-behavioural therapy) treatment for premenstrual dysphoric disorder: a study of treatment processes. J Psychosom Res. (2002) 53:811–7. doi: 10.1016/S0022-3999(02)00338-0
156. Cohen LS, Miner C, Brown EW, Freeman E, Halbreich U, Sundell K, et al. Premenstrual daily fluoxetine for premenstrual dysphoric disorder: a placebo-controlled, clinical trial using computerized diaries. Obstet Gynecol. (2002) 100:435–44. doi: 10.1097/00006250-200209000-00008
157. Steiner M, Brown E, Trzepacz P, Dillon J, Berger C, Carter D, et al. Fluoxetine improves functional work capacity in women with premenstrual dysphoric disorder. Arch Womens Ment Health. (2003) 6:71–7. doi: 10.1007/s00737-002-0162-2
158. Steiner M, Romano SJ, Babcock S, Dillon J, Shuler C, Berger C, et al. The efficacy of fluoxetine in improving physical symptoms associated with premenstrual dysphoric disorder. Bjog. (2001) 108:462–8. doi: 10.1111/j.1471-0528.2001.00120.x
159. Steiner M, Steinberg S, Stewart D, Carter D, Berger C, Reid R, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. (1995) 332:1529–34. doi: 10.1056/NEJM199506083322301
160. Yonkers KA, Halbreich U, Freeman E, Brown C, Endicott J, Frank E, et al. Symptomatic improvement of premenstrual dysphoric disorder with sertraline treatment. A randomized controlled trial. Sertraline Premenstrual Dysphoric Collaborative Study Group. Jama. (1997) 278:983–8. doi: 10.1001/jama.278.12.983
161. Freeman EW, Rickels K, Sondheimer SJ, Polansky M. Differential response to antidepressants in women with premenstrual syndrome/premenstrual dysphoric disorder: a randomized controlled trial. Arch Gen Psychiatry. (1999) 56:932–9. doi: 10.1001/archpsyc.56.10.932
162. Yonkers KA, Kornstein SG, Gueorguieva R, Merry B, Van Steenburgh K, Altemus M. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: A randomized clinical trial. JAMA Psychiatry. (2015) 72:1037–44. doi: 10.1001/jamapsychiatry.2015.1472
163. Kornstein SG, Pearlstein TB, Fayyad R, Farfel GM, Gillespie JA. Low-dose sertraline in the treatment of moderate-to-severe premenstrual syndrome: efficacy of 3 dosing strategies. J Clin Psychiatry. (2006) 67:1624–32. doi: 10.4088/JCP.v67n1020
164. Freeman EW, Rickels K, Sammel MD, Lin H, Sondheimer SJ. Time to relapse after short- or long-term treatment of severe premenstrual syndrome with sertraline. Arch Gen Psychiatry. (2009) 66:537–44. doi: 10.1001/archgenpsychiatry.2008.547
165. Yonkers KA, Pearlstein T, Fayyad R, Gillespie JA. Luteal phase treatment of premenstrual dysphoric disorder improves symptoms that continue into the postmenstrual phase. J Affect Disord. (2005) 85:317–21. doi: 10.1016/j.jad.2004.10.006
166. Altamura AC, Moro AR, Percudani M. Clinical pharmacokinetics of fluoxetine. Clin Pharmacokinet. (1994) 26:201–14. doi: 10.2165/00003088-199426030-00004
167. Cohen LS, Soares CN, Yonkers KA, Bellew KM, Bridges IM, Steiner M. Paroxetine controlled release for premenstrual dysphoric disorder: a double-blind, placebo-controlled trial. Psychosom Med. (2004) 66:707–13. doi: 10.1097/01.psy.0000140005.94790.9c
168. Landén M, Nissbrandt H, Allgulander C, Sörvik K, Ysander C, Eriksson E. Placebo-controlled trial comparing intermittent and continuous paroxetine in premenstrual dysphoric disorder. Neuropsychopharmacology. (2007) 32:153–61. doi: 10.1038/sj.npp.1301216
169. Wu KY, Liu CY, Hsiao MC. Six-month paroxetine treatment of premenstrual dysphoric disorder: continuous versus intermittent treatment protocols. Psychiatry Clin Neurosci. (2008) 62:109–14. doi: 10.1111/j.1440-1819.2007.01785.x
170. Steiner M, Hirschberg AL, Bergeron R, Holland F, Gee MD, Van Erp E. Luteal phase dosing with paroxetine controlled release (CR) in the treatment of premenstrual dysphoric disorder. Am J Obstet Gynecol. (2005) 193:352–60. doi: 10.1016/j.ajog.2005.01.021
171. Eriksson E, Ekman A, Sinclair S, Sörvik K, Ysander C, Mattson UB, et al. Escitalopram administered in the luteal phase exerts a marked and dose-dependent effect in premenstrual dysphoric disorder. J Clin Psychopharmacol. (2008) 28:195–202. doi: 10.1097/JCP.0b013e3181678a28
172. Freeman EW, Sondheimer SJ, Sammel MD, Ferdousi T, Lin H. A preliminary study of luteal phase versus symptom-onset dosing with escitalopram for premenstrual dysphoric disorder. J Clin Psychiatry. (2005) 66:769–73. doi: 10.4088/JCP.v66n0616
173. Wikander I, Sundblad C, Andersch B, Dagnell I, Zylberstein D, Bengtsson F, et al. Citalopram in premenstrual dysphoria: is intermittent treatment during luteal phases more effective than continuous medication throughout the menstrual cycle? J Clin Psychopharmacol. (1998) 18:390–8. doi: 10.1097/00004714-199810000-00007
174. Ravindran LN, Woods SA, Steiner M, Ravindran AV. Symptom-onset dosing with citalopram in the treatment of premenstrual dysphoric disorder (PMDD): a case series. Arch Womens Ment Health. (2007) 10:125–7. doi: 10.1007/s00737-007-0181-0
175. Freeman EW, Jabara S, Sondheimer SJ, Auletto R. Citalopram in PMS patients with prior SSRI treatment failure: a preliminary study. J Womens Health Gend Based Med. (2002) 11:459–64. doi: 10.1089/15246090260137635
176. Freeman EW, Rickels K, Yonkers KA, Kunz NR, McPherson M, Upton GV. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. (2001) 98:737–44. doi: 10.1097/00006250-200111000-00006
177. Cohen LS, Soares CN, Lyster A, Cassano P, Brandes M, Leblanc GA. Efficacy and tolerability of premenstrual use of venlafaxine (flexible dose) in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol. (2004) 24:540–3. doi: 10.1097/01.jcp.0000138767.53976.10
178. Hsiao MC, Liu CY. Effective open-label treatment of premenstrual dysphoric disorder with venlafaxine. Psychiatry Clin Neurosci. (2003) 57:317–21. doi: 10.1046/j.1440-1819.2003.01123.x
179. Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, Schatzberg AF. Duloxetine 60 mg once-daily in the treatment of painful physical symptoms in patients with major depressive disorder. J Psychiatr Res. (2005) 39:43–53. doi: 10.1016/j.jpsychires.2004.04.011
180. Ramos MG, Hara C, Rocha FL. Duloxetine treatment of Premenstrual Dysphoric Disorder: case reports. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:579–80. doi: 10.1016/j.pnpbp.2007.09.006
181. Mazza M, Harnic D, Catalano V, Janiri L, Bria P. Duloxetine for premenstrual dysphoric disorder: a pilot study. Expert Opin Pharmacother. (2008) 9:517–21. doi: 10.1517/14656566.9.4.517
182. Ramos MG, Hara C, Rocha FL. Duloxetine treatment for women with premenstrual dysphoric disorder: a single-blind trial. Int J Neuropsychopharmacol. (2009) 12:1081–8. doi: 10.1017/S1461145709000066
183. Anderson IM. Serotonin receptors, buspirone, and premenstrual syndrome. Lancet. (1989) 2:615. doi: 10.1016/S0140-6736(89)90730-7
184. Landén M, Eriksson O, Sundblad C, Andersch B, Naessén T, Eriksson E. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacol (Berl). (2001) 155:292–8. doi: 10.1007/s002130100718
185. Ozgoli G, Selselei EA, Mojab F, Majd HA. A randomized, placebo-controlled trial of Ginkgo biloba L. in treatment of premenstrual syndrome. J Altern Complement Med. (2009) 15(8):845–51.
186. Sharifi F, Simbar M, Mojab F, Majd HA. Comparison of the effects of Matricaria chamomila (Chamomile) extract and mefenamic acid on the intensity of premenstrual syndrome. Complement Ther Clin Pract. (2014) 20(1):81–8.
187. Yamada K, Kanba S. Effectiveness of kamishoyosan for premenstrual dysphoric disorder: open-labeled pilot study. Psychiatry Clin Neurosci. (2007) 61(3):323–5.
188. Jung-Gum R, Sae-Il C, Young-Jin L, Ho-Suk S. The effects of St. John's Wort on premenstrual syndrome in single women: a randomized double-blind, placebo-controlled study. Clin Psychopharmacol Neurosci. (2010) 8(1):30–7.
189. Canning S, Waterman M, Orsi N, Ayres J, Simpson N, Dye L. The efficacy of Hypericum perforatum (St John's wort) for the treatment of premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. CNS Drugs. (2010) 24(3):207–25.
190. Stevinson C, Ernst E. A pilot study of Hypericum perforatum for the treatment of premenstrual syndrome. BJOG. (2000) 107(7):870–6.
191. Farahmand M, Khalili D, Ramezani Tehrani F, Amin G, Negarandeh R. Could Anise decrease the intensity of premenstrual syndrome symptoms in comparison to placebo? A double-blind randomized clinical trial. J Complement Integr Med. (2020).
192. Farahmand M, Khalili D, Ramezani Tehrani F, Amin G, Negarandeh R. Effectiveness of Echium amoenum on premenstrual syndrome: a randomized, double-blind, controlled trial. BMC Complement Med Ther. (2020) 20(1):295.
193. Khayat S, Kheirkhah M, Behboodi Moghadam Z, Fanaei H, Kasaeian A, Javadimehr M. Effect of treatment with ginger on the severity of premenstrual syndrome symptoms. ISRN Obstet Gynecol. (2014) 2014:792708.
194. Akbarzadeh M, Dehghani M, Moshfeghy Z, Emamghoreishi M, Tavakoli P, Zare N. Effect of melissa officinalis capsule on the intensity of premenstrual syndrome symptoms in high school girl students. Nurs Midwifery Stud. (2015) 4(2):e27001.
195. Tjandrawinata RR, Nofiarny D, Susanto LW, Hendri P, Clarissa A. Symptomatic treatment of premenstrual syndrome and/or primary dysmenorrhea with DLBS1442, a bioactive extract of Phaleria macrocarpa. Int J Gen Med. (2011) 4:456–76.
196. Sodouri M, Masoudi Alavi N, Fathizadeh N, Taghizadeh M, Azarbad Z, Memarzadeh M. Effects of Zataria Multi-Flora, Shirazi thyme, on the Severity of Premenstrual Syndrome. Nurs Midwifery Stud. (2013) 2(4):57–63.
197. Meier B, Berger D, Hoberg E, Sticher O, Schaffner W. Pharmacological activities of Vitex agnus-castus extracts in vitro. Phytomedicine. (2000) 7:373–81. doi: 10.1016/S0944-7113(00)80058-6
198. Jarry H, Leonhardt S, Gorkow C, Wuttke W. In vitro prolactin but not LH and FSH release is inhibited by compounds in extracts of Agnus castus: direct evidence for a dopaminergic principle by the dopamine receptor assay. Exp Clin Endocrinol. (1994) 102:448–54. doi: 10.1055/s-0029-1211317
199. Schellenberg R, Zimmermann C, Drewe J, Hoexter G, Zahner C. Dose-dependent efficacy of the Vitex agnus castus extract Ze 440 in patients suffering from premenstrual syndrome. Phytomedicine. (2012) 19:1325–31. doi: 10.1016/j.phymed.2012.08.006
200. Cerqueira RO, Frey BN, Leclerc E, Brietzke E. Vitex agnus castus for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. (2017) 20:713–9. doi: 10.1007/s00737-017-0791-0
201. Ma L, Lin S, Chen R, Zhang Y, Chen F, Wang X. Evaluating therapeutic effect in symptoms of moderate-to-severe premenstrual syndrome with Vitex agnus castus (BNO 1095) in Chinese women. Aust N Z J Obstet Gynaecol. (2010) 50:189–93. doi: 10.1111/j.1479-828X.2010.01137.x
202. van Die MD, Bone KM, Burger HG, Reece JE, Teede HJ. Effects of a combination of Hypericum perforatum and Vitex agnus-castus on PMS-like symptoms in late-perimenopausal women: findings from a subpopulation analysis. J Altern Complement Med. (2009) 15:1045–8. doi: 10.1089/acm.2008.0539
203. Ma L, Lin S, Chen R, Wang X. Treatment of moderate to severe premenstrual syndrome with Vitex agnus castus (BNO 1095) in Chinese women. Gynecol Endocrinol. (2010) 26:612–6. doi: 10.3109/09513591003632126
204. He Z, Chen R, Zhou Y, Geng L, Zhang Z, Chen S, et al. Treatment for premenstrual syndrome with Vitex agnus castus: A prospective, randomized, multi-center placebo controlled study in China. Maturitas. (2009) 63:99–103. doi: 10.1016/j.maturitas.2009.01.006
205. Ambrosini A, Di Lorenzo C, Coppola G, Pierelli F. Use of Vitex agnus-castus in migrainous women with premenstrual syndrome: an open-label clinical observation. Acta Neurol Belg. (2013) 113:25–9. doi: 10.1007/s13760-012-0111-4
206. Berger D, Schaffner W, Schrader E, Meier B, Brattström A. Efficacy of Vitex agnus castus L. extract Ze 440 in patients with pre-menstrual syndrome (PMS). Arch Gynecol Obstet. (2000) 264:150–3. doi: 10.1007/s004040000123
207. Verkaik S, Kamperman AM, van Westrhenen R, Schulte PFJ. The treatment of premenstrual syndrome with preparations of Vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. (2017) 217:150–66. doi: 10.1016/j.ajog.2017.02.028
208. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. (2005) 97:281–4. doi: 10.1016/j.jep.2004.11.004
209. Ghasemi T, Abnous K, Vahdati F, Mehri S, Razavi BM, Hosseinzadeh H. Antidepressant Effect of Crocus sativus Aqueous Extract and its Effect on CREB, BDNF, and VGF Transcript and Protein Levels in Rat Hippocampus. Drug Res (Stuttg). (2015) 65:337–43. doi: 10.1055/s-00023610
210. Kleinstäuber M, Witthöft M, Hiller W. Cognitive-behavioral and pharmacological interventions for premenstrual syndrome or premenstrual dysphoric disorder: a meta-analysis. J Clin Psychol Med Settings. (2012) 19:308–19. doi: 10.1007/s10880-012-9299-y
211. Ait Tastift M, Makbal R, Bourhim T, Omari Z, Isoda H, Gadhi C. Safety assessment and pain relief properties of saffron from taliouine region (Morocco). Molecules. (2022) 27. doi: 10.3390/molecules27103339
212. Fukui H, Toyoshima K, Komaki R. Psychological and neuroendocrinological effects of odor of saffron (Crocus sativus). Phytomedicine. (2011) 18:726–30. doi: 10.1016/j.phymed.2010.11.013
213. Beiranvand SP, Beiranvand NS, Moghadam ZB, Birjandi M, Azhari S, Rezaei E, et al. The effect of Crocus sativus (saffron) on the severity of premenstrual syndrome. Eur J Integr Med. (2016) 8:55–61. doi: 10.1016/j.eujim.2015.06.003
214. Agha-Hosseini M, Kashani L, Aleyaseen A, Ghoreishi A, Rahmanpour H, Zarrinara AR, et al. (saffron) in the treatment of premenstrual syndrome: a double-blind, randomised and placebo-controlled trial. Bjog. (2008) 115:515–9. doi: 10.1111/j.1471-0528.2007.01652.x
215. Rajabi F, Rahimi M, Sharbafchizadeh MR, Tarrahi MJ. Saffron for the management of premenstrual dysphoric disorder: A randomized controlled trial. Adv BioMed Res. (2020) 9:60. doi: 10.4103/abr.abr_49_20
216. Kulkarni S, Dhir A, Akula KK. Potentials of curcumin as an antidepressant. ScientificWorldJournal. (2009) 9:1233–41. doi: 10.1100/tsw.2009.137
217. Fanaei H, Khayat S, Kasaeian A, Javadimehr M. Effect of curcumin on serum brain-derived neurotrophic factor levels in women with premenstrual syndrome: A randomized, double-blind, placebo-controlled trial. Neuropeptides. (2016) 56:25–31. doi: 10.1016/j.npep.2015.11.003
218. Lopresti AL, Hood SD, Drummond PD. Multiple antidepressant potential modes of action of curcumin: a review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J Psychopharmacol. (2012) 26:1512–24. doi: 10.1177/0269881112458732
219. Moriyuki K, Sekiguchi F, Matsubara K, Nishikawa H, Kawabata A. Curcumin Inhibits the proteinase-activated receptor-2-triggered prostaglandin E2 production by suppressing cyclooxygenase-2 upregulation and Akt-dependent activation of nuclear factor-κB in human lung epithelial cells. J Pharmacol Sci. (2010) 114:225–9. doi: 10.1254/jphs.10126SC
220. Khayat S, Fanaei H, Kheirkhah M, Moghadam ZB, Kasaeian A, Javadimehr M. Curcumin attenuates severity of premenstrual syndrome symptoms: A randomized, double-blind, placebo-controlled trial. Complement Ther Med. (2015) 23:318–24. doi: 10.1016/j.ctim.2015.04.001
221. Bahrami A, Zarban A, Rezapour H, Agha Amini Fashami A, Ferns GA. Effects of curcumin on menstrual pattern, premenstrual syndrome, and dysmenorrhea: A triple-blind, placebo-controlled clinical trial. Phytother Res. (2021) 35:6954–62. doi: 10.1002/ptr.7314
222. Arabnezhad L, Mohammadifard M, Rahmani L, Majidi Z, Ferns GA, Bahrami A. Effects of curcumin supplementation on vitamin D levels in women with premenstrual syndrome and dysmenorrhea: a randomized controlled study. BMC Complement Med Ther. (2022) 22:19. doi: 10.1186/s12906-022-03515-2
223. Talebpour A, Mohammadifard M, Zare Feyzabadi R, Mahmoudzadeh S, Rezapour H, Saharkhiz M, et al. Effect of curcumin on inflammatory biomarkers and iron profile in patients with premenstrual syndrome and dysmenorrhea: A randomized controlled trial. Physiol Rep. (2023) 11:e15763. doi: 10.14814/phy2.15763
224. Bahrami A, Jafari-Nozad AM, Karbasi S, Ayadilord M, Ferns GA. Efficacy of curcumin on cognitive function scores in women with premenstrual syndrome and dysmenorrhea: A triple-blind, placebo-controlled clinical trial. Chin J Integr Med. (2023) 29:387–93. doi: 10.1007/s11655-023-3732-3
225. Tolossa FW, Bekele ML. Prevalence, impacts and medical managements of premenstrual syndrome among female students: cross-sectional study in College of Health Sciences, Mekelle University, Mekelle, northern Ethiopia. BMC Womens Health. (2014) 14:52. doi: 10.1186/1472-6874-14-52
226. Ussher JM, Perz J. Evaluation of the relative efficacy of a couple cognitive-behaviour therapy (CBT) for Premenstrual Disorders (PMDs), in comparison to one-to-one CBT and a wait list control: A randomized controlled trial. PloS One. (2017) 12:e0175068. doi: 10.1371/journal.pone.0175068
227. Brown MA, Zimmer PA. Personal and family impact of premenstrual symptoms. J Obstet Gynecol Neonatal Nurs. (1986) 15:31–8. doi: 10.1111/j.1552-6909.1986.tb01364.x
228. Stout AL, Steege JF. Psychological assessment of women seeking treatment for premenstrual syndrome. J Psychosom Res. (1985) 29:621–9. doi: 10.1016/0022-3999(85)90071-6
229. Rebecca Ryser and Leslie LF. Premenstrual syndrome and the marital relationship. Am J Family Ther. (1992) 20:179–90. doi: 10.1080/01926189208250887
230. Izadi-Mazidi M, Davoudi I, Mehrabizadeh M. Effect of group cognitive-behavioral therapy on health-related quality of life in females with premenstrual syndrome. Iran J Psychiatry Behav Sci. (2016) 10:e4961. doi: 10.17795/ijpbs
231. Siminiuc R, Ţurcanu D. Impact of nutritional diet therapy on premenstrual syndrome. Front Nutr. (2023) 10:1079417. doi: 10.3389/fnut.2023.1079417
232. Jafari F, Amani R, Tarrahi MJ. Effect of zinc supplementation on physical and psychological symptoms, biomarkers of inflammation, oxidative stress, and brain-derived neurotrophic factor in young women with premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. (2020) 194:89–95. doi: 10.1007/s12011-019-01757-9
233. Fathizadeh S, Amani R, Haghighizadeh MH, Hormozi R. Comparison of serum zinc concentrations and body antioxidant status between young women with premenstrual syndrome and normal controls: A case-control study. Int J Reprod Biomed. (2016) 14:699–704. doi: 10.18502/ijrm.v14i11.699
234. Mitsuya H, Omata N, Kiyono Y, Mizuno T, Murata T, Mita K, et al. The co-occurrence of zinc deficiency and social isolation has the opposite effects on mood compared with either condition alone due to changes in the central norepinephrine system. Behav Brain Res. (2015) 284:125–30. doi: 10.1016/j.bbr.2015.02.005
235. Chuong CJ, Dawson EB. Zinc and copper levels in premenstrual syndrome. Fertil Steril. (1994) 62:313–20. doi: 10.1016/S0015-0282(16)56884-8
236. Posaci C, Erten O, Uren A, Acar B. Plasma copper, zinc and magnesium levels in patients with premenstrual tension syndrome. Acta Obstet Gynecol Scand. (1994) 73:452–5. doi: 10.3109/00016349409013429
237. Chen Y, Zhi X. Roles of vitamin D in reproductive systems and assisted reproductive technology. Endocrinology. (2020) 161. doi: 10.1210/endocr/bqaa023
238. Moridi I, Chen A, Tal O, Tal R. The association between vitamin D and anti-müllerian hormone: A systematic review and meta-analysis. Nutrients. (2020) 12. doi: 10.37766/inplasy2020.4.0204
239. Pilz S, Zittermann A, Obeid R, Hahn A, Pludowski P, Trummer C, et al. The role of vitamin D in fertility and during pregnancy and lactation: A review of clinical data. Int J Environ Res Public Health. (2018) 15. doi: 10.3390/ijerph15102241
240. Grzesiak M. Vitamin D3 action within the ovary - an updated review. Physiol Res. (2020) 69:371–8. doi: 10.33549/physiolres
241. Lasco A, Catalano A, Benvenga S. Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: results of a randomized, double-blind, placebo-controlled study. Arch Intern Med. (2012) 172:366–7. doi: 10.1001/archinternmed.2011.715
242. Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: a narrative review. Int J Clin Pract. (2013) 67:225–35. doi: 10.1111/ijcp.12031
243. Bahrami A, Avan A, Sadeghnia HR, Esmaeili H, Tayefi M, Ghasemi F, et al. High dose vitamin D supplementation can improve menstrual problems, dysmenorrhea, and premenstrual syndrome in adolescents. Gynecol Endocrinol. (2018) 34:659–63. doi: 10.1080/09513590.2017.1423466
244. Abdollahi R, Abiri B, Sarbakhsh P, Kashanian M, Vafa M. The effect of vitamin D supplement consumption on premenstrual syndrome in vitamin D-deficient young girls: A randomized, double-blind, placebo-controlled clinical trial. Complement Med Res. (2019) 26:336–42. doi: 10.1159/000500016
245. Ghanbari Z, Haghollahi F, Shariat M, Foroshani AR, Ashrafi M. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. (2009) 48:124–9. doi: 10.1016/S1028-4559(09)60271-0
246. Shobeiri F, Araste FE, Ebrahimi R, Jenabi E, Nazari M. Effect of calcium on premenstrual syndrome: A double-blind randomized clinical trial. Obstet Gynecol Sci. (2017) 60:100–5. doi: 10.5468/ogs.2017.60.1.100
247. Penland JG, Johnson PE. Dietary calcium and manganese effects on menstrual cycle symptoms. Am J Obstet Gynecol. (1993) 168:1417–23. doi: 10.1016/S0002-9378(11)90775-3
248. Thys-Jacobs S. Micronutrients and the premenstrual syndrome: the case for calcium. J Am Coll Nutr. (2000) 19:220–7. doi: 10.1080/07315724.2000.10718920
249. Chocano-Bedoya PO, Manson JE, Hankinson SE, Willett WC, Johnson SR, Chasan-Taber L, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. (2011) 93:1080–6. doi: 10.3945/ajcn.110.009530
250. Abdollahifard S, Rahmanian Koshkaki A, Moazamiyanfar R. The effects of vitamin B1 on ameliorating the premenstrual syndrome symptoms. Glob J Health Sci. (2014) 6:144–53. doi: 10.5539/gjhs.v6n6p144
251. Kashanian M, Mazinani R, Jalalmanesh S, Babayanzad Ahari S. Pyridoxine (vitamin B6) therapy for premenstrual syndrome. Int J Gynaecol Obstet. (2007) 96:43–4. doi: 10.1016/j.ijgo.2006.09.014
252. Sharma P, Kulshreshtha S, Singh GM, Bhagoliwal A. Role of bromocriptine and pyridoxine in premenstrual tension syndrome. Indian J Physiol Pharmacol. (2007) 51:368–74.
253. Williams MJ, Harris RI, Dean BC. Controlled trial of pyridoxine in the premenstrual syndrome. J Int Med Res. (1985) 13:174–9. doi: 10.1177/030006058501300305
254. Frankenburg FR. The role of one-carbon metabolism in schizophrenia and depression. Harv Rev Psychiatry. (2007) 15:146–60. doi: 10.1080/10673220701551136
255. Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. (2008) 13:216–26.
256. Retallick-Brown H, Blampied N, Rucklidge JJ. A pilot randomized treatment-controlled trial comparing vitamin B6 with broad-spectrum micronutrients for premenstrual syndrome. J Altern Complement Med. (2020) 26:88–97. doi: 10.1089/acm.2019.0305
257. Hagen I, Nesheim BI, Tuntland T. No effect of vitamin B-6 against premenstrual tension. A controlled clinical study. Acta Obstet Gynecol Scand. (1985) 64:667–70. doi: 10.3109/00016348509158211
258. De Souza MC, Walker AF, Robinson PA, Bolland K. A synergistic effect of a daily supplement for 1 month of 200 mg magnesium plus 50 mg vitamin B6 for the relief of anxiety-related premenstrual symptoms: a randomized, double-blind, crossover study. J Womens Health Gend Based Med. (2000) 9:131–9. doi: 10.1089/152460900318623
259. Johnson SR. Premenstrual syndrome, premenstrual dysphoric disorder, and beyond: a clinical primer for practitioners. Obstet Gynecol. (2004) 104:845–59. doi: 10.1097/01.AOG.0000140686.66212.1e
260. Grubbs RD, Maguire ME. Magnesium as a regulatory cation: criteria and evaluation. Magnesium. (1987) 6:113–27.
261. Chuong CJ, Dawson EB, Smith ER. Vitamin A levels in premenstrual syndrome. Fertil Steril. (1990) 54:643–7. doi: 10.1016/S0015-0282(16)53822-9
262. Farasati N, Siassi F, Koohdani F, Qorbani M, Abashzadeh K, Sotoudeh G. Western dietary pattern is related to premenstrual syndrome: a case-control study. Br J Nutr. (2015) 114:2016–21. doi: 10.1017/S0007114515003943
263. MoradiFili B, Ghiasvand R, Pourmasoumi M, Feizi A, Shahdadian F, Shahshahan Z. Dietary patterns are associated with premenstrual syndrome: evidence from a case-control study. Public Health Nutr. (2020) 23:833–42. doi: 10.1017/S1368980019002192
264. Rossignol AM. Caffeine-containing beverages and premenstrual syndrome in young women. Am J Public Health. (1985) 75:1335–7. doi: 10.2105/AJPH.75.11.1335
265. Rossignol AM, Bonnlander H. Caffeine-containing beverages, total fluid consumption, and premenstrual syndrome. Am J Public Health. (1990) 80:1106–10. doi: 10.2105/AJPH.80.9.1106
266. Rossignol AM, Bonnlander H, Song L, Phillis JW. Do women with premenstrual symptoms self-medicate with caffeine? Epidemiology. (1991) 2:403–8. doi: 10.1097/00001648-199111000-00003
267. Pinar G, Colak M, Oksuz E. Premenstrual Syndrome in Turkish college students and its effects on life quality. Sex Reprod Healthc. (2011) 2:21–7. doi: 10.1016/j.srhc.2010.10.001
268. Gold EB, Bair Y, Block G, Greendale GA, Harlow SD, Johnson S, et al. Diet and lifestyle factors associated with premenstrual symptoms in a racially diverse community sample: Study of Women's Health Across the Nation (SWAN). J Womens Health (Larchmt). (2007) 16:641–56. doi: 10.1089/jwh.2006.0202
269. Purdue-Smithe AC, Manson JE, Hankinson SE, Bertone-Johnson ER. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. (2016) 104:499–507. doi: 10.3945/ajcn.115.127027
270. Caan B, Duncan D, Hiatt R, Lewis J, Chapman J, Armstrong MA. Association between alcoholic and caffeinated beverages and premenstrual syndrome. J Reprod Med. (1993) 38:630–6.
271. Bertone-Johnson ER, Hankinson SE, Johnson SR, Manson JE. Cigarette smoking and the development of premenstrual syndrome. Am J Epidemiol. (2008) 168:938–45. doi: 10.1093/aje/kwn194
272. Dorn LD, Negriff S, Huang B, Pabst S, Hillman J, Braverman P, et al. Menstrual symptoms in adolescent girls: association with smoking, depressive symptoms, and anxiety. J Adolesc Health. (2009) 44:237–43. doi: 10.1016/j.jadohealth.2008.07.018
273. Hashim MS, Obaideen AA, Jahrami HA, Radwan H, Hamad HJ, Owais AA, et al. Premenstrual syndrome is associated with dietary and lifestyle behaviors among university students: A cross-sectional study from sharjah, UAE. Nutrients. (2019) 11. doi: 10.3390/nu11081939
274. Fernández MDM, Saulyte J, Inskip HM, Takkouche B. Premenstrual syndrome and alcohol consumption: a systematic review and meta-analysis. BMJ Open. (2018) 8:e019490. doi: 10.1136/bmjopen-2017-019490
275. Omidali fa. The effect of Pilates exercise and consuming Fennel on pre-menstrual syndrome symptoms in non-athletic girls. Complementary Med J. (2015) 5:1203–13.
276. El-Lithy A, El-Mazny A, Sabbour A, El-Deeb A. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. (2015) 35:389–92. doi: 10.3109/01443615.2014.960823
277. Farahani A. Relationship between pre menstrual syndrome with body mass index among university students. J Hayat. (2012) 17:85–95.
278. Mohebbi Dehnavi Z, Jafarnejad F, Sadeghi Goghary S. The effect of 8 weeks aerobic exercise on severity of physical symptoms of premenstrual syndrome: a clinical trial study. BMC Womens Health. (2018) 18:80. doi: 10.1186/s12905-018-0565-5
279. Yilmaz Akyuz E. The effect of diet and aerobic exercise on Premenstrual Syndrome: Randomized controlled trial. Rev Nutrição. (2019) 32. doi: 10.1590/1678-9865201932e180246
280. Ravichandran H, Janakiraman B. Effect of aerobic exercises in improving premenstrual symptoms among healthy women: A systematic review of randomized controlled trials. Int J Womens Health. (2022) 14:1105–14. doi: 10.2147/IJWH.S371193
281. Maged AM, Abbassy AH, Sakr HRS, Elsawah H, Wagih H, Ogila AI, et al. Effect of swimming exercise on premenstrual syndrome. Arch Gynecol Obstet. (2018) 297:951–9. doi: 10.1007/s00404-018-4664-1
282. Silva CM, Gigante DP, Carret ML, Fassa AG. [Population study of premenstrual syndrome]. Rev Saude Publica. (2006) 40:47–56. doi: 10.1590/S0034-89102006000100009
283. Beckvid Henriksson G, Schnell C, Lindén Hirschberg A. Women endurance runners with menstrual dysfunction have prolonged interruption of training due to injury. Gynecol Obstet Invest. (2000) 49:41–6. doi: 10.1159/000010211
284. Chang HC, Cheng YC, Yang CH, Tzeng YL, Chen CH. Effects of yoga for coping with premenstrual symptoms in Taiwan-A cluster randomized study. Healthcare (Basel). (2023) 11. doi: 10.3390/healthcare11081193
285. Zhang H, Zhu M, Song Y, Kong M. Baduanjin exercise improved premenstrual syndrome symptoms in Macau women. J Tradit Chin Med. (2014) 34:460–4. doi: 10.1016/S0254-6272(15)30047-9
286. Dutta A, Aruchunan M, Mukherjee A, Metri KG, Ghosh K, Basu-Ray I. A comprehensive review of yoga research in 2020. J Integr Complement Med. (2022) 28:114–23. doi: 10.1089/jicm.2021.0420
287. Ciezar-Andersen SD, Hayden KA, King-Shier KM. A systematic review of yoga interventions for helping health professionals and students. Complement Ther Med. (2021) 58:102704. doi: 10.1016/j.ctim.2021.102704
288. Ma X, Yue ZQ, Gong ZQ, Zhang H, Duan NY, Shi YT, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front Psychol. (2017) 8:874. doi: 10.3389/fpsyg.2017.00874
289. Cramer H, Lauche R, Dobos G. Characteristics of randomized controlled trials of yoga: a bibliometric analysis. BMC Complement Altern Med. (2014) 14:328. doi: 10.1186/1472-6882-14-328
290. Field T. Yoga clinical research review. Complement Ther Clin Pract. (2011) 17:1–8. doi: 10.1016/j.ctcp.2010.09.007
291. Cahn BR, Goodman MS, Peterson CT, Maturi R, Mills PJ. Yoga, meditation and mind-body health: increased BDNF, cortisol awakening response, and altered inflammatory marker expression after a 3-month yoga and meditation retreat. Front Hum Neurosci. (2017) 11:315. doi: 10.3389/fnhum.2017.00315
292. Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. (2015) 16:213–25. doi: 10.1038/nrn3916
293. Aarse J, Herlitze S, Manahan-Vaughan D. The requirement of BDNF for hippocampal synaptic plasticity is experience-dependent. Hippocampus. (2016) 26:739–51. doi: 10.1002/hipo.22555
294. Vaghela N, Mishra D, Sheth M, Dani VB. To compare the effects of aerobic exercise and yoga on Premenstrual syndrome. J Educ Health Promot. (2019) 8:199. doi: 10.4103/jehp.jehp_50_19
295. Dimmock PW, Wyatt KM, Jones PW, O'Brien PM. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. (2000) 356:1131–6. doi: 10.1016/S0140-6736(00)02754-9
296. Okeke T, Anyaehie U, Ezenyeaku C. Premature menopause. Ann Med Health Sci Res. (2013) 3:90–5. doi: 10.4103/2141-9248.109458
297. Cronje WH, Vashisht A, Studd JW. Hysterectomy and bilateral oophorectomy for severe premenstrual syndrome. Hum Reprod. (2004) 19:2152–5. doi: 10.1093/humrep/deh354
298. Lukes AS, McBride RJ, Herring AH, Fried M, Sherwani A, Dell D. Improved premenstrual syndrome symptoms after NovaSure endometrial ablation. J Minim Invasive Gynecol. (2011) 18:607–11. doi: 10.1016/j.jmig.2011.06.001
299. Lefler HT, Lefler CF. Origin of premenstrual syndrome: assessment by endometrial ablation. J Am Assoc Gynecol Laparosc. (1994) 1:207–12. doi: 10.1016/S1074-3804(05)81011-4
300. Singh BB, Berman BM, Simpson RL, Annechild A. Incidence of premenstrual syndrome and remedy usage: a national probability sample study. Altern Ther Health Med. (1998) 4:75–9.
Keywords: PMS, PMDD, SSRI, allopregnanolone, treatment
Citation: Modzelewski S, Oracz A, Żukow X, Iłendo K, Śledzikowka Z and Waszkiewicz N (2024) Premenstrual syndrome: new insights into etiology and review of treatment methods. Front. Psychiatry 15:1363875. doi: 10.3389/fpsyt.2024.1363875
Received: 22 January 2024; Accepted: 03 April 2024;
Published: 23 April 2024.
Edited by:
Liisa Hantsoo, Johns Hopkins University, United StatesReviewed by:
Melissa Wagner, University of Cincinnati, United StatesCopyright © 2024 Modzelewski, Oracz, Żukow, Iłendo, Śledzikowka and Waszkiewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Modzelewski, Mzg3MzhAc3R1ZGVudC51bWIuZWR1LnBs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.