- 1Department of Psychiatry, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Radiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Clinical Medicine, Chongqing Medical and Pharmaceutical College, Chongqing, China
Introduction: Adverse life events constitute primary risk factors for major depressive disorder (MDD), influencing brain function and structure. Adolescents, with their brains undergoing continuous development, are particularly susceptible to enduring impacts of adverse events.
Methods: We investigated differences and correlations among childhood trauma, negative life events, and alterations of brain function in adolescents with first-episode MDD. The study included 23 patients with MDD and 19 healthy controls, aged 10–19 years. All participants underwent resting-state functional magnetic resonance imaging and were assessed using the beck depression inventory, childhood trauma questionnaire, and adolescent self-rating life events checklist.
Results: Compared with healthy controls, participants with first-episode MDD were more likely to have experienced emotional abuse, physical neglect, interpersonal relationship problems, and learning stress (all p’ < 0.05). These adverse life events were significantly correlated with alterations in brain functions (all p < 0.05).
Discussion: This study contributes novel evidence on the underlying process between adverse life events, brain function, and depression, emphasizing the significant neurophysiological impact of environmental factors.
1 Introduction
Major depressive disorder (MDD) is characterized by an inability to feel joy or continuous emotions of despair (1). In China, MDD is the second most common cause of disability (2). Adolescents with MDD have symptoms that are more intense, last longer, and have higher recurrence rates than adults (3). Moreover, nearly half of all mental disorders begin during adolescence (4), emphasizing the importance of investigating the pathogenesis of first-episode MDD, as well as prevention and early intervention.
Adolescence is crucial for psychological growth and is affected by negative events in families, schools, and physical and psychological aspects (5). These events can directly impact depressive emotions and indirectly influence the emotions by affecting ruminant thinking (6), particularly in individuals with disruptions to default mode network functional connectivity (7). However, the precise neural mechanisms underlying these changes remain unclear.
Studies suggest that adults with MDD may have experienced early trauma and negative life events in their early life (8–10). These individuals display cognitive and emotional impairments associated with dysfunction in the resting-state functional connectivity (rs-FC) of the amygdala, the anterior insula (11), the hippocampus, and the temporal cortex.
The early-childhood stage of brain development renders the brain susceptible to adverse life events, but limited research has explored the effects of these stress on rs-FC among adolescents with first-episode MDD. Therefore, we aimed to investigate the differences in trauma, negative life events, and alterations of brain function between adolescents with first-episode MDD and healthy controls and analyze their correlations.
2 Materials and methods
2.1 Study design and participants
We utilized a case-control design in this study, recruiting 23 patients from the Psychiatry Department of the First Affiliated Hospital of Chongqing Medical Hospital and 19 healthy controls from local schools between May 2018 and August 2019. All participants were right-handed and of native Chinese Han ethnicity. Two licensed psychiatrists diagnosed the patients using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) Patient Edition for MDD. Inclusion criteria encompassed an age range of 10–19 years, a diagnosis of first-episode MDD without significant recovery, and no current use of antidepressants. Exclusion criteria included bipolar disorder, schizophrenia, any other psychotic disorder, severe medical conditions such as cardiac or organic brain disease, and contraindications to magnetic resonance imaging (MRI). Healthy controls adhered to the same exclusion criteria. This study adhered to the Declaration of Helsinki and was approved by the Medical Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Approval No. 2023-058-02). All adolescent participants and their guardians signed a written informed consent form after receiving a complete description of the study.
2.2 Measures
All participants underwent a comprehensive clinical assessment including general information, childhood trauma experience, and adverse life events. We used the beck depression inventory (BDI) (12), the childhood trauma questionnaire (CTQ) (13), and the adolescent self-rating life events checklist (ASLEC) (14) to assess severity of depression, experiences of physical, sexual, and emotional abuse, as well as physical and emotional neglect, and negative life events. The Chinese editions of both the CTQ and ASLEC have demonstrated reliability and validity in adolescents (15, 16).
2.3 Imaging data acquisition and pre-processing
All MRI data were obtained using a Signa HDxt 3.0-T MRI machine (GE Healthcare, Chicago, IL) with an eight-channel phased array head coil at the Department of Radiology of the First Affiliated Hospital of Chongqing Medical Hospital. During the resting-state functional MRI (rs-fMRI) scanning, participants were instructed to close their eyes and avoid thinking. The rs-fMRI data were acquired using a gradient-echo-echo-planar imaging sequence with specific parameters: time repetition/time echo = 2000/40 ms, flip angle = 90°, field of view = 100 × 100 mm, matrix = 64 × 64, slice thickness/slice spacing = 4.0/4.0 mm. High-resolution anatomical T1-weighted destructive gradient recall images were acquired, covering the whole brain, using the following parameters: time repetition/time echo = 8348/3.27 ms, flip angle = 12°, field of view = 75 × 75 mm, matrix = 256 × 256, slice thickness/slice spacing = 1.0/1.0 mm. To ensure the absence of structural abnormalities, two experienced neuroradiologists thoroughly examined all images.
RESTplus 1.25 (17), based on Statistical Parametric Mapping 12 in MATLAB 2013b (MathWorks, Natick, MA), was used for image data preprocessing. The main steps included: (1) removing the first 10 volumes to address scanner instability, (2) slice timing and head motion correction, ensuring displacement of participants < 3 mm/3°, (3) excluding non-neuronal sources, including white matter, cerebrospinal fluid, and 24-parameter head motion profile signals, (4) performing spatially normalized corrected imaging at a resolution of 3 mm × 3 mm × 3 mm, (5) calculating regional homogeneity (ReHo) and using z-standardization to a normal distribution, (6) smoothing the data using a Gaussian kernel of 6-mm full width and half maximum, (7) removing linear trend. We chose different zReHo values between patients with MDD and healthy controls as a region of interest (ROI), used the seed-based functional connectivity (FC) method to assess the abnormalities, and used Fisher’s z transformation to enhance normality.
2.4 Statistical analysis
Demographic data and clinical characteristics were compared using independent-sample t-tests for normal variables, the Mann–Whitney U-test for non-normal variables, and the chi-squared test for sex in SPSS 23.0 (IBM, Armonk, NY). Statistical significance was defined as p < 0.05 for all two-tailed tests. A two-sample t-test was used to compare ReHo between the MDD and healthy control groups, with an uncorrected significance level of p < 0.05. We used a one-sample t-test using false discovery rate (FDR) correction with cluster-level multiple comparison correlation of significance level of p < 0.05 to illustrate the spatial distribution of within-group FC for the MDD and healthy control groups. Following this, we conducted a two-sample t-test to determine whether there were any significant variations in whole-brain FC within the ROI between the two groups. In this part, the Gaussian random field theory was used for cluster-level multiple corrections, with a threshold of Voxel-level p < 0.05 and Cluster-level p-value < 0.05. The mean ReHo and FC values of abnormal brain regions were extracted for examining the correlations between the brain functional values and clinical characteristics, using Spearman’s correlation analysis. The p-values have been adjusted to Benjamini–Hochberg p'-values to control the FDR when necessary. Sex, age, and years of education were considered as nuisance factors.
3 Results
3.1 Demographic and clinical characteristics
In total, 42 participants were selected, of whom 23 were patients with MDD and 19 were healthy controls. The demographic and clinical data of all participants are presented in Table 1. Five patients with MDD were unable to complete the ASLEC and BDI.
3.2 Group comparisons
Age, sex, and years of education did not significantly differ between the MDD and healthy control groups. Adolescents with MDD were much more likely than healthy controls to report experiencing emotional abuse or physical neglect, and their CTQ total score was also significantly higher (all p’ < 0.05). Additionally, compared to the healthy controls, adolescents with MDD exhibited a greater prevalence of interpersonal relationship and learning stress problems (all p’ < 0.001). No differences were observed between the two groups regarding physical and sexual abuse; emotional neglect; punishment; loss of relatives, friends, and property; health and adaptation problems; or others (all p’ > 0.05).
3.3 Comparison of ReHo between groups
The zReHo of the right Rolandic operculum (ROL.R) was significantly higher in the MDD group than in the healthy control group (pFDR < 0.05) (Table 2; Figure 1).
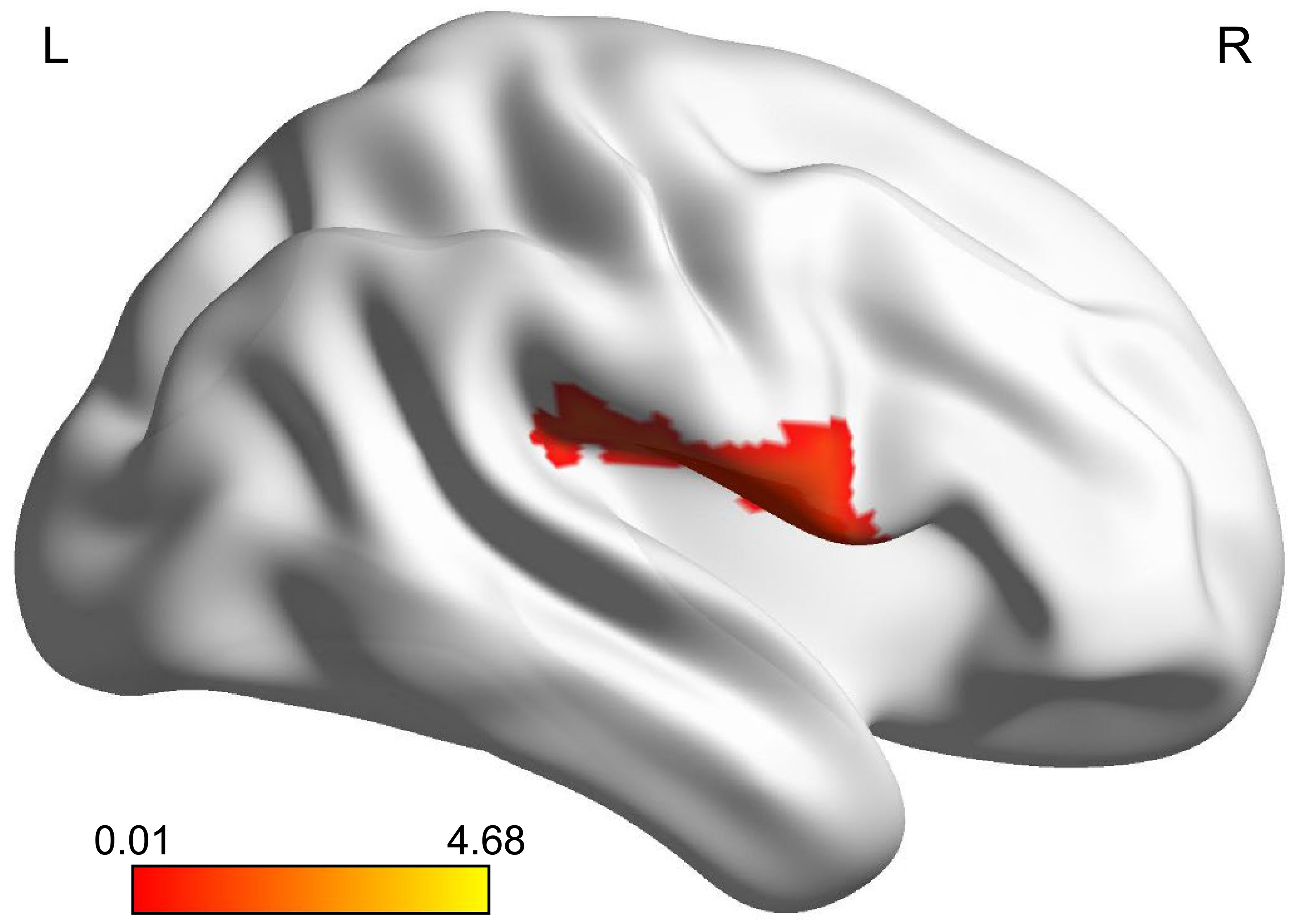
Figure 1 Brain region where adolescents with MDD had significantly more activity than healthy controls. The highlighted region was located on the most significantly different voxel of the right Rolandic operculum (X, Y, Z = 51, -6, 12, T = 4.678, PFDR < 0.05). The color bar represents the T value of zReHo. zReHo, z-standardized regional homogeneity. MDD, major depressive disorder.
3.4 Within-group FC analysis
The results of the one-sample t-test revealed that the ROL.R with high FC values had a strong connection to the cingulate cortex. Furthermore, activities were observed in the temporal cortex, frontal cortex, caudate, angular gyrus, and parahippocampal gyrus (Table 3). The spatial distribution of FC in the MDD group showed similar patterns to those observed among healthy controls (Table 4; Figure 2).
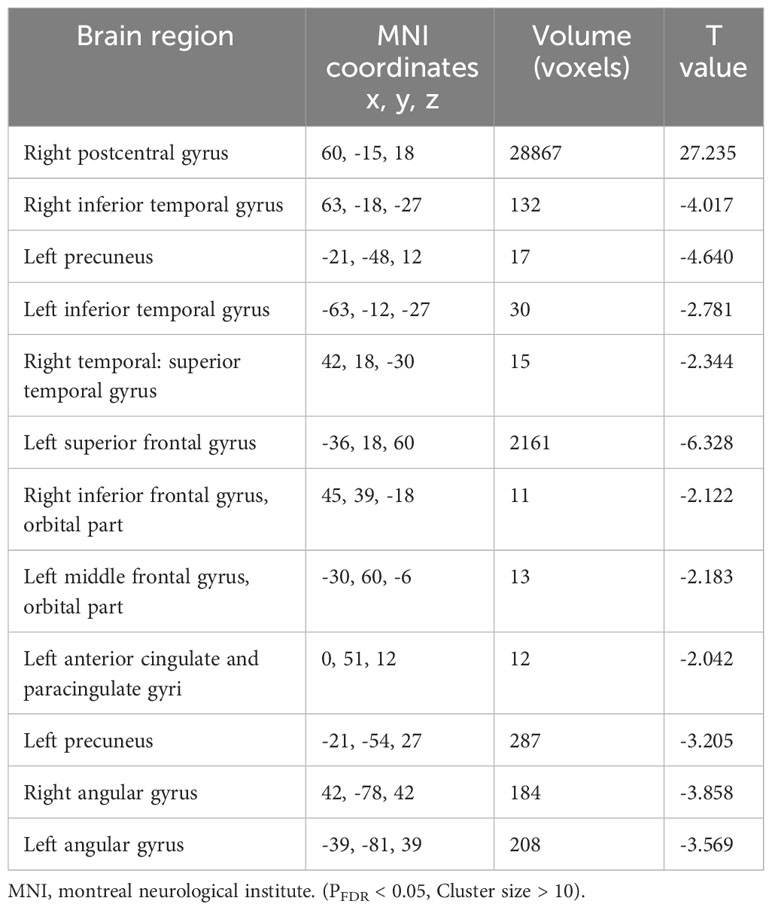
Table 4 Functional connectivity of right Rolandic operculum for patients with major depressive disorder.
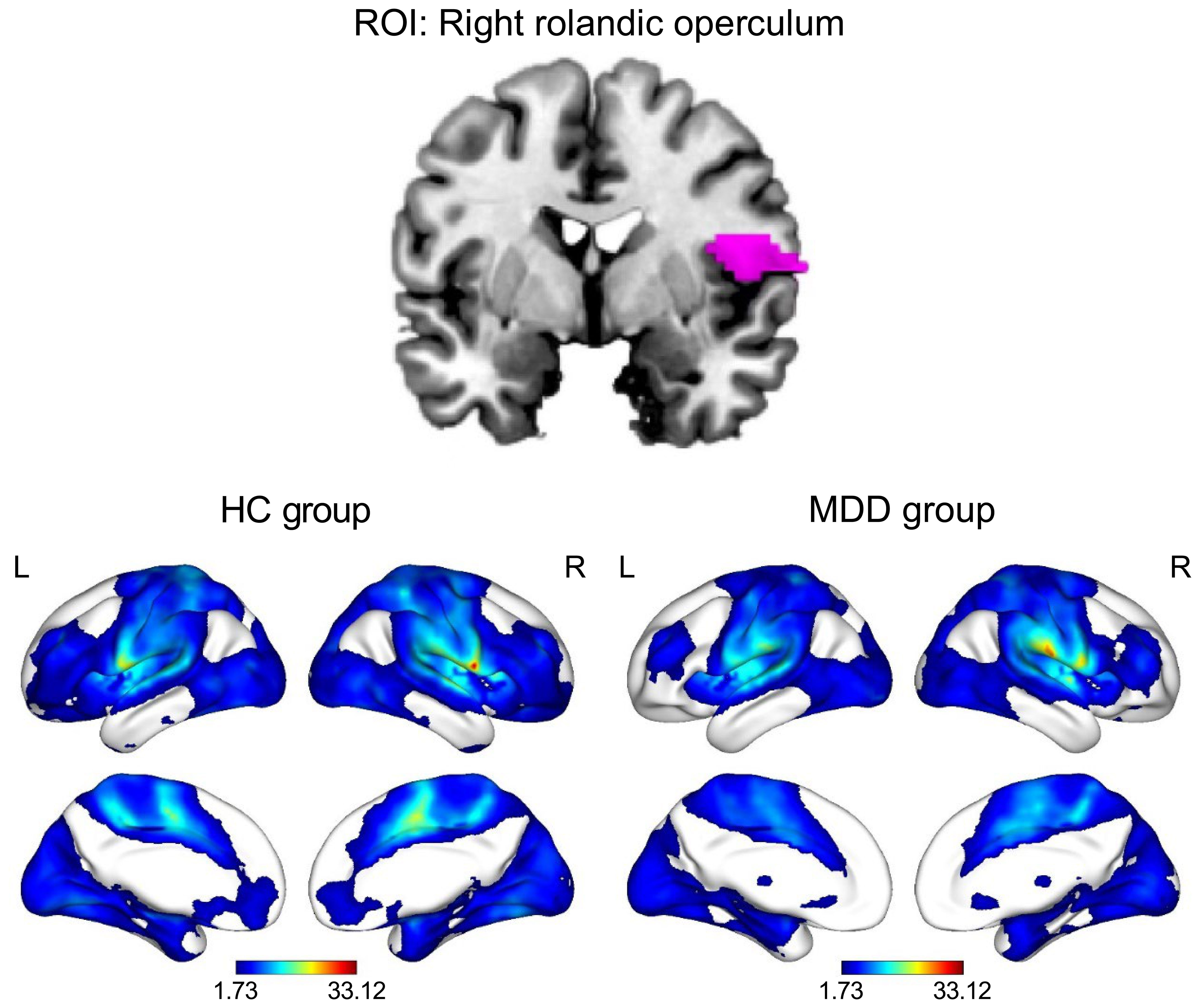
Figure 2 Functional connectivity patterns of the right Rolandic operculum in the HC and MDD groups (PFDR < 0.05, cluster size > 10). The color bar represents the T value of functional connectivity. HC, healthy control; L, left; MDD, major depressive disorder; R, right; ROI, region of interest.
3.5 Between-group FC differences
Decreased FC between the ROL.R, the right orbital part of the middle frontal gyrus (ORBmid.R) and the left postcentral gyrus (PoCG.L) was observed more in the MDD group than in the healthy control group (Table 5; Figure 3).
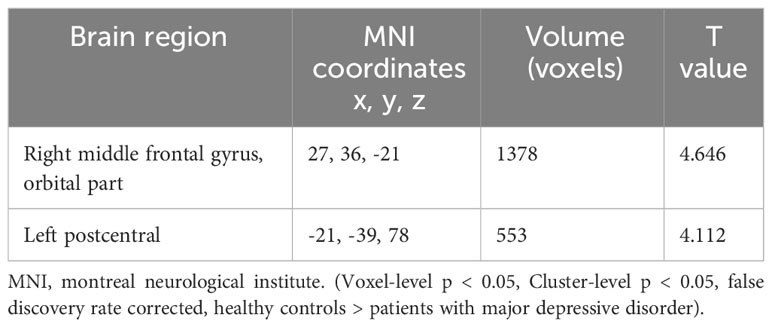
Table 5 Functional connectivity differences between the major depressive disorder and healthy control groups with right Rolandic operculum as region of interest.
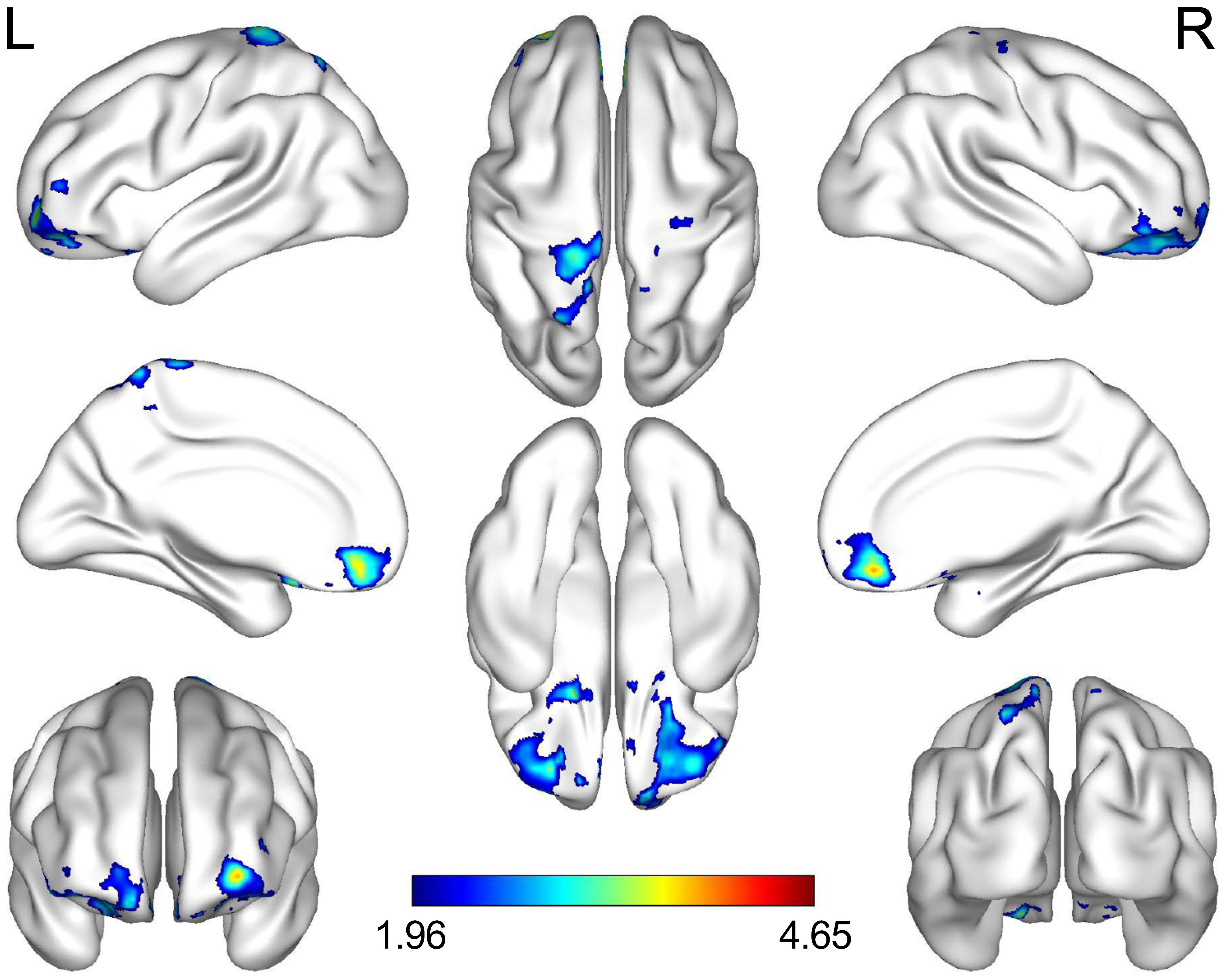
Figure 3 Brain regions where adolescents with MDD had less connectivity than the HCs. The highlighted regions were located on the most significantly different voxels of the right orbital part of the middle frontal and the left postcentral gyrus (X, Y, Z = 27, 36, -21, T = 4.646; X, Y, Z =-21, -39, 78, T = 4.122; voxel p < 0.05, cluster p < 0.05, false discovery rate corrected). The color bar represents the T value of functional connectivity. L, left; R, right; MDD, major depressive disorder; HC, healthy control.
3.6 Correlation analyses
In the MDD group, the BDI score showed a positive correlation with the CTQ total score (p = 0.037, r = 0.524), physical neglect (p = 0.019, r = 0.579), punishment (p = 0.027, r = 0.61), and other (p = 0.003, r = 0.775). The increased ReHo was positively correlated with health and adaption problems (p = 0.004, r = 0.74). The decreased FC between ROL.R and PoCG.L was negatively correlated with sexual abuse (p= 0.039, r = -0.519).
In the HC group, the BDI score showed a positive correlation with emotional abuse (p = 0.004, r = 0.742). Additionally, each factor of the ASLEC showed a positive correlation with the BDI score (interpersonal relationships p = 0.000, r = 0.853; learning stress p = 0.001, r = 0.818; punishment p = 0.012, r = 0.67; loss of relatives, friend, and property p = 0.006, r = 0.715; health and adaption problems p = 0.023, r = 0.623; others p = 0.038, r = 0.579). Furthermore, the decreased ReHo was negatively correlated with emotional neglect (p = 0.028, r = -0.608).
We also calculated the correlations between abnormalities and clinical characteristics in all participants, the BDI score was positively correlated with each factor of the CTQ and ASLEC (all p < 0.05), except health and adaptation problems (p > 0.05) (Table 6). The abnormal ReHo in the ROL.R was positively correlated with the BDI score, CTQ total score, emotional, physical, and sexual abuse, physical neglect, and interpersonal relationships (all p < 0.05). The abnormal FC between the ROL.R and ORBmid.R showed a negative correlation with the BDI score, physical neglect, and interpersonal relationship problems (all p < 0.05). The abnormalFC between the ROL.R and PoCG.L was negatively correlated with the CTQ total score, physical neglect, and learning stress problems (all p < 0.05) (Figures 4, 5).
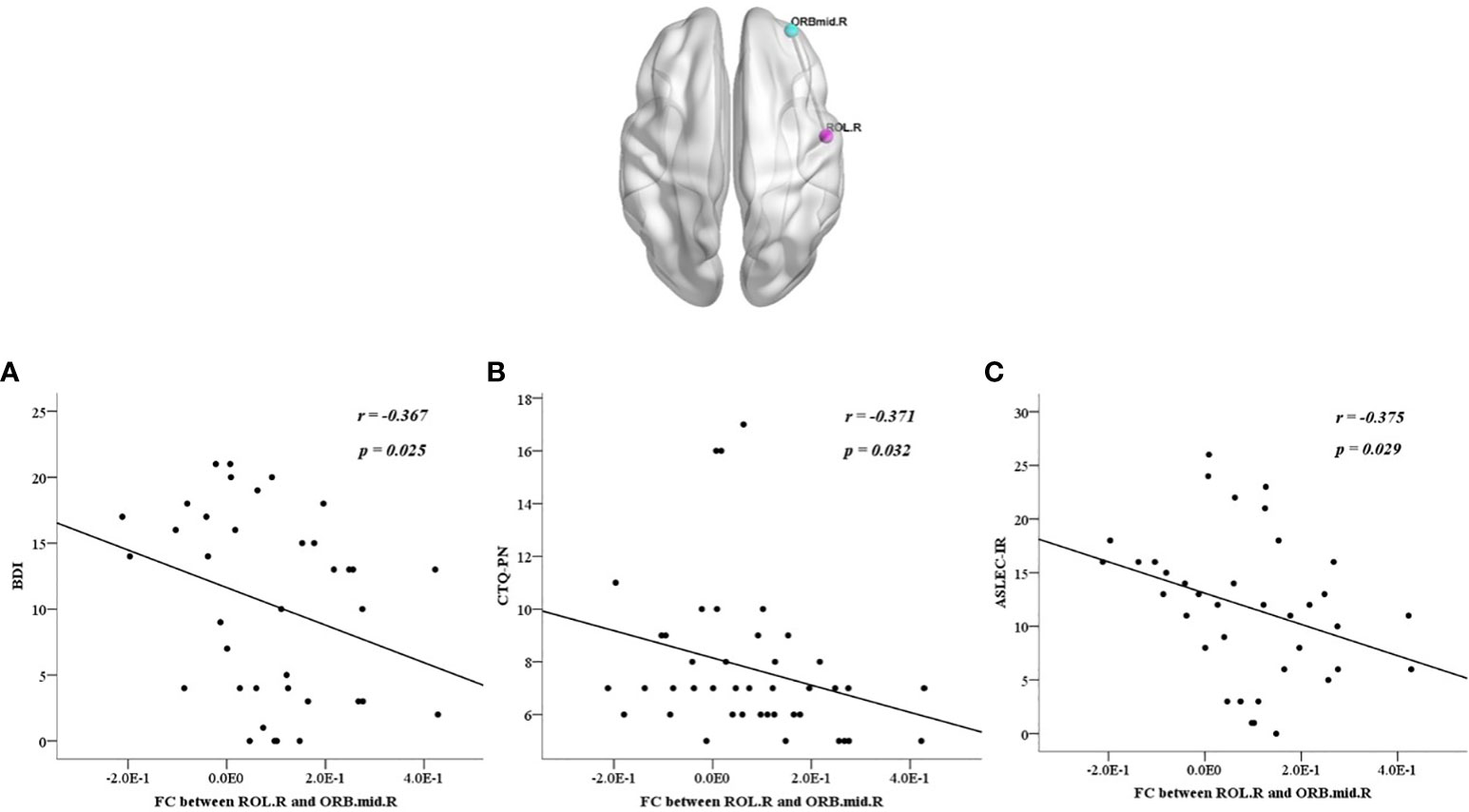
Figure 4 All participants showed negative correlations between the discrepant regions of functional connectivity in the right orbital part of the middle frontal gyrus and the right Rolandic operculum with BDI score (A), CTQ-PN (B), ASLEC, adolescent self-rating life events checklist; BDI, beck depression inventory; CTQ, childhood trauma questionnaire; FC, functional connectivity; ORBmid.R, right orbital part of the middle frontal gyrus; ROL.R, right Rolandic operculum.
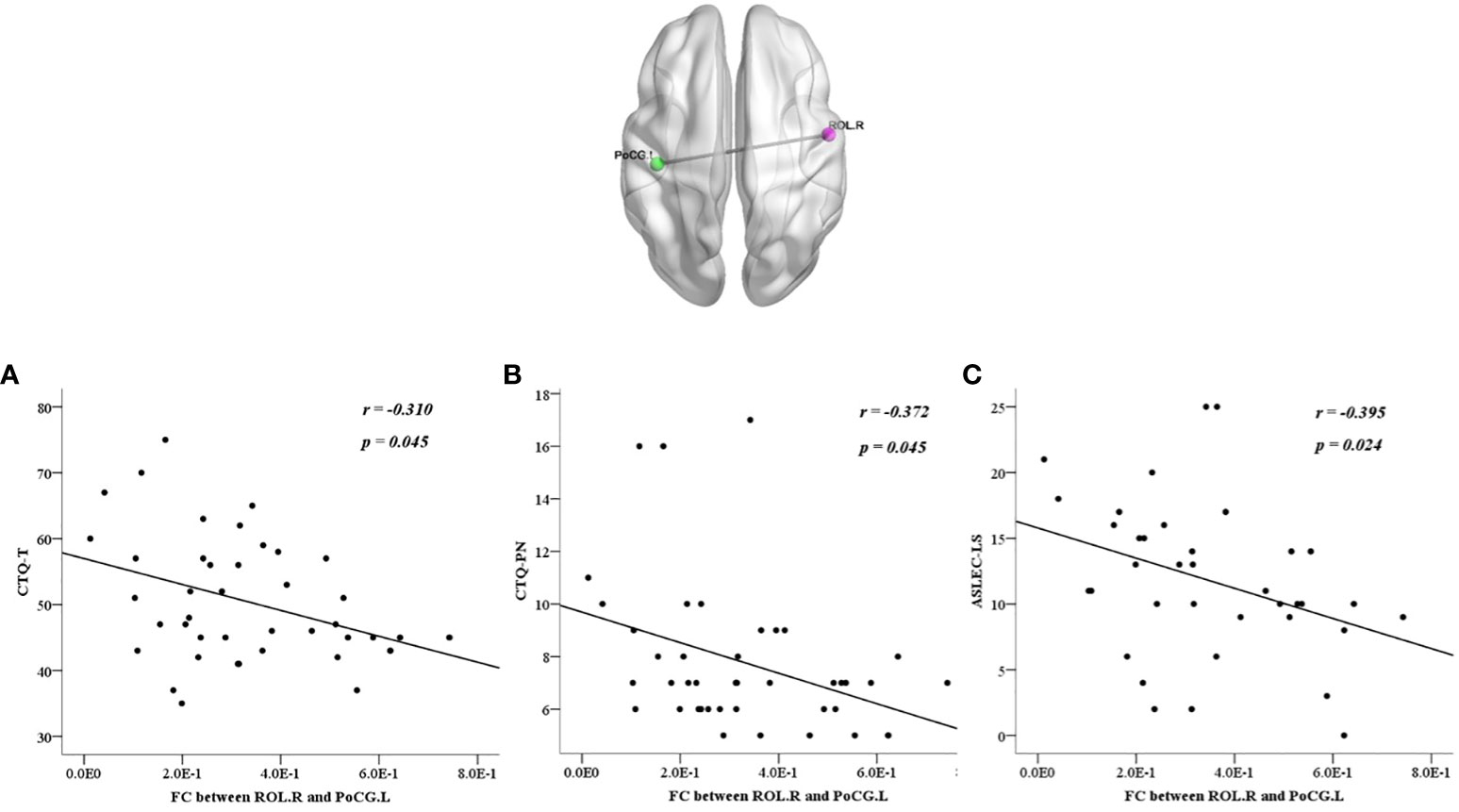
Figure 5 All participants showed negative correlations between the discrepant regions of functional connectivity in the left postcentral gyrus and the right Rolandic operculum with CTQ-T (A), CTQ-PN (B), and ASLEC-LS (C). ASLEC, Adolescent Self-Rating Life Events Checklist; CTQ, Childhood Trauma Questionnaire; FC, functional connectivity; PoCG.L, left postcentral gyrus; ROL.R, right Rolandic operculum.
4 Discussion
We applied the ReHo and rs-FC methods to examine regional activity and distant connectivity, respectively, and found increased ReHo in the ROL.R and decreased FC between the ROL.R, ORBmid.R, and PoCG.L in adolescents with MDD. The abnormal regional ReHo was positively correlated with depression severity and adverse life events, while the abnormal whole-brain FC was negatively correlated with depression severity and adverse experience.
The ROL belongs to the auditory network, which is responsible for integrating and processing auditory stimuli and is associated with human emotions (18). Previous studies have shown that the ROL is involved in interoceptive awareness, which processes signals important for self-awareness (19). In this study, we observed a ReHo anomaly in the ROL.R and as ReHo values increased, the positive relationships between depression severity and adverse life events, although some studies suggested otherwise. Zhang and colleagues discovered that the amplitude of low-frequency fluctuation (ALFF), which quantifies the intensity of local brain activity in a specific frequency bands, was reduced in the ROL.R of patients with MDD (20). Additionally, both ALFF and ReHo are regional functional activity characteristics (21). This discrepancy in results may be attributed to our focus on MDD in adolescents instead of adults. It may provide new possibilities and valuable insights for investigating unique neuroimaging alterations connected to MDD in adolescents.
The middle frontal gyrus, located in the ventral part of the medial prefrontal cortex, is involved in processing and regulation of emotions from top to bottom automatically or implicitly (22). Guo et al. reported that patients with MDD who had not received antidepressant had a decrease in fractional ALFF in the ORBmid.R (23). Furthermore, college students with MDD showed an increased ALFF ratio in the ORBmid.R after exercising (24). Patients with MDD also showed a decrease in the middle frontal and medial orbitofrontal gyri (25). Our findings support previous literature on MDD by identifying abnormalities in the ORBmid.R. The decreased FC between the ROL.R and ORBmid.R related to emotional dysregulation observed in adolescents with MDD could help explain the underlying neurobiological processes contributing to impaired emotion processing in this disorder.
The postcentral gyrus is important in the sensorimotor system (26) and crucial in emotional processing in patients with MDD (27), bipolar disorder (28), and anxiety (29). Luo et al. indicated that PoCG.L activity is linked to childhood trauma and is responsible for the main effect of childhood trauma on adults with MDD. This supports the idea that the postcentral gyrus is involved in the recognition of internal bodily states, as the generation of emotions requires awareness of external and internal bodily states (30). Another study focusing on suicidal behavior of adolescents with depression pointed that higher levels of suicidality were positively associated with functional connectivity between left precuneus and left postcentral gyri (31). This result further supported that the sensorimotor system may underlie the tendency to internal bodily state with negative emotion and suicidal behavior. However, we found decreased FC between the ROL.R and PoCG.L in the adolescents with depression without suicidal behavior in this study. These inconsistent results in the PoCG.L might be due to the differences in the age of participants and the focus of the study. Therefore, we should expand the sample of adolescents with depression and further classify them for analysis according to specific criteria such as whether they have suicidal behavior or whether they have experienced childhood trauma in further studies.
Strong evidence suggests that adverse life events in childhood are the primary risk factors for MDD, as they can affect the ability to recognize and communicate emotions and result in increased psychological distress among adolescents (32, 33). It may be due to an imbalance in the development of subcortical areas (accumbens and amygdala) and prefrontal lobes during the adolescence, therefore, when confronted with negative emotional stimulation, adolescents show less activity in the orbital frontal cortex and more activity in the amygdala than adults (34). The neurological basis for this difference may be a series of prefrontal cortex remodeling that occurs during adolescence, including a decrease in the density of spines on pyramidal cells, a reduction in the volumes and a substantial loss of glutamatergic excitatory synapses (35). Preclinical studies also support the idea that adverse life events have long-lasting effects on neurotransmitters and neurohormonal systems, as well as on a network of brain areas involved in memory and fear, such as the hippocampus, medial prefrontal cortex, and amygdala. These regions interact with neurochemical systems to influence the brain’s response to stress (36). For example, during adolescent development, appropriate thyroid hormone levels could stimulate the differentiation of dopaminergic neurons. However, after adolescence mice experienced early life stress, the thyroid hormone signal transduction development was defective, which further affected the development of ventral tegmental area (37). Other hormones, such as testosterone and estrogen, could also affect the neurotransmission of dopamine, which in turn further impact the maturation of neural circuitry and cognitive processing in adolescence (38). This may be one of the reasons for a large number of female suffering from depression among adolescents, suggesting that the pathological mechanism of hormonal changes related to stress environmental sensitivity in depression should be considered (39).
Despite finding some evidence indicating changes in the neural reactivity of adolescents with MDD who have experienced negative life events and trauma, our study has some limitations. First, as the insufficient sample size restricts the ability to draw precise conclusions, caution is needed when interpreting the findings. Second, the case-control design of this study could only initially explore the correlation between adverse life events and brain function with depression severity in adolescents with depression. In future study, it is necessary to expand the sample size for further cohort studies to explore whether there are changes in brain function and depression symptoms in adolescents with depression after receiving antidepressant medication or cognitive behavioral therapy based on the findings derived from the present study.
In summary, this study expands on the existing literature and emphasizes the critical neurological effects of the cumulative experience of stress and trauma. Additionally, the severity of MDD was found to interact with abnormal activities between the ROL.R and ORBmid.R, and exposure to life stress and trauma was shown to notably influence the PoCG.L and ORBmid.R. These findings provide evidence that there might be distinct developmental patterns of changes in how emotions are processed in the brain, influenced by early adverse life events in adolescents with MDD. Therefore, this study could potentially contribute to research on psychiatric problems during adolescence and necessitate early detection, attention, and individualized intervention to prevent adverse outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Ethical approval number: 2023-058-02). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
XX: Formal Analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. JT: Investigation, Writing – review & editing. YP: Investigation, Writing – review & editing. YL: Investigation, Writing – review & editing. YC: Investigation, Writing – review & editing. MY: Investigation, Writing – review & editing. RY: Data curation, Writing – review & editing. XH: Resources, Writing – review & editing. YF: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Program for Youth Innovation in Future Medicine of Chongqing Medical University (W0107), the Natural Science Foundation Chongqing, China (CSTB2022NSCQ-MSX0121), and Chongqing Municipal Education Commission (KJZD-K202300501)
Acknowledgments
The authors thank all participants in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. (2016) 2:16065. doi: 10.1038/nrdp.2016.65
2. Lu J, Xu X, Huang Y, Li T, Ma C, Xu G, et al. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2021) 8:981–90. doi: 10.1016/S2215-0366(21)00251-0
3. Wang X, Li X, Guo C, Hu Y, Xia L, Geng F, et al. Prevalence and correlates of alexithymia and its relationship with life events in chinese adolescents with depression during the COVID-19 pandemic. Front Psychiatry. (2021) 12:774952. doi: 10.3389/fpsyt.2021.774952
4. Li Y, Jinxiang T, Shu Y, Yadong P, Ying L, Meng Y, et al. Childhood trauma and the plasma levels of IL-6, TNF-α are risk factors for major depressive disorder and schizophrenia in adolescents: A cross-sectional and case-control study. J Affect Disord. (2022) 305:227–32. doi: 10.1016/j.jad.2022.02.020
5. Jiang Z, Wang Z, Diao Q, Chen J, Tian G, Cheng X, et al. The relationship between negative life events and non-suicidal self-injury (NSSI) among Chinese junior high school students: the mediating role of emotions. Ann Gen Psychiatry. (2022) 21:45. doi: 10.1186/s12991-022-00423-0
6. Xia H, Han X, Cheng J, Liu D, Wu Y, Liu Y. Effects of negative life events on depression in middle school students: The chain-mediating roles of rumination and perceived social support. Front Psychol. (2022) 13:781274. doi: 10.3389/fpsyg.2022.781274
7. Son JJ, Schantell M, Picci G, Wang YP, Stephen JM, Calhoun VD, et al. Altered longitudinal trajectory of default mode network connectivity in healthy youth with subclinical depressive and posttraumatic stress symptoms. Dev Cognit Neurosci. (2023) 60:101216. doi: 10.1016/j.dcn.2023.101216
8. Sun T, Zhang L, Liu Y, Wu S, Yang BX, Liu JF, et al. The relationship between childhood trauma and insomnia among college students with major depressive disorder: Mediation by the role of negative life events and dysfunctional attitudes. Compr Psychiatry. (2023) 122:152368. doi: 10.1016/j.comppsych.2023.152368
9. Vinkers CH, Joëls M, Milaneschi Y, Kahn RS, Penninx BW, Boks MP. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety. (2014) 31:737–45. doi: 10.1002/da.2014.31.issue-9
10. Fowler JC, Allen JG, Oldham JM, Frueh BC. Exposure to interpersonal trauma, attachment insecurity, and depression severity. J Affect Disord. (2013) 149:313–8. doi: 10.1016/j.jad.2013.01.045
11. Luo L, Yang T, Zheng X, Zhang X, Gao S, Li Y, et al. Altered centromedial amygdala functional connectivity in adults is associated with childhood emotional abuse and predicts levels of depression and anxiety. J Affect Disord. (2022) 303:148–54. doi: 10.1016/j.jad.2022.02.023
12. Beck AT, Beck RW. Screening depressed patients in family practice. Postgraduate Medicine (1972) 52(6):81–5. doi: 10.1080/00325481.1972.11713319
13. Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M. Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry. (1995) 152:1329–35. doi: 10.1176/ajp.152.9.1329
14. Liu X, Liu L, Yang J, Chai F, Wang A, Sun L, et al. The adolescent self-rating life events checklist and its reliability and validity. Chin J Clin Psychol. (1997) 5:34–6.
15. Fan Q, Liu Q, Liu C, Wang Z. Childhood trauma and suicidal ideation among Chinese adolescents: The mediating effects of character strengths and perceived stress. J Psychiatr Ment Health Nurs. (2023) 30:526–36. doi: 10.1111/jpm.12892
16. Zhang J, Li Y, Li J, Lyu M, Chen Y, Yang S, et al. The effect of life events, resilience, self-esteem, and coping styles on aggressive behavior among left-behind adolescents: Structural equation modeling. Front Psychiatry. (2023) 14:991608. doi: 10.3389/fpsyt.2023.991608
17. Jia XZ, Wang J, Sun HY, Zhang H, Liao W, Wang Z, et al. RESTplus: an improved toolkit for resting-state functional magnetic resonance imaging data processing. Sci Bull (Beijing). (2019) 64:953–4. doi: 10.1016/j.scib.2019.05.008
18. Sun JF, Chen LM, He JK, Wang Z, Guo CL, Ma Y, et al. A comparative study of regional homogeneity of resting-state fMRI between the early-onset and late-onset recurrent depression in adults. Front Psychol. (2022) 13:849847. doi: 10.3389/fpsyg.2022.849847
19. Blefari ML, Martuzzi R, Salomon R, Bello-Ruiz J, Herbelin B, Serino A, et al. Bilateral Rolandic operculum processing underlying heartbeat awareness reflects changes in bodily self-consciousness. Eur J Neurosci. (2017) 45:1300–12. doi: 10.1111/ejn.13567
20. Zhang L, Wei X, Zhao J. Amplitude of low-frequency oscillations in first-episode drug-naive patients with major depressive disorder: A resting state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. (2022) 18:555–61. doi: 10.2147/NDT.S348683
21. Zhang B, Liu S, Liu X, Chen S, Ke Y, Qi S, et al. Discriminating subclinical depression from major depression using multi-scale brain functional features: A radiomics analysis. J Affect Disord. (2022) 297:542–52. doi: 10.1016/j.jad.2021.10.122
22. Zhao P, Yan R, Wang X, Geng J, Chattun MR, Wang Q, et al. Reduced resting state neural activity in the right orbital part of middle frontal gyrus in anxious depression. Front Psychiatry. (2019) 10:994. doi: 10.3389/fpsyt.2019.00994
23. Guo W, Liu F, Yu M, Zhang J, Zhang Z, Liu J, et al. Decreased regional activity and network homogeneity of the fronto-limbic network at rest in drug-naive major depressive disorder. Aust N Z J Psychiatry. (2015) 49:550–6. doi: 10.1177/0004867415577978
24. Zhang J, Gao T, Li Y, Song Z, Cui M, Wei Q, et al. The effect of Bafa Wubu of Tai Chi on college students' anxiety and depression: A randomized, controlled pilot study. Front Physiol. (2023) 14:1036010. doi: 10.3389/fphys.2023.1036010
25. Han KM, Choi S, Jung J, Na KS, Yoon HK, Lee MS, et al. Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J Affect Disord. (2014) 155:42–8. doi: 10.1016/j.jad.2013.10.021
26. Liu P, Tu H, Zhang A, Yang C, Liu Z, Lei L, et al. Brain functional alterations in MDD patients with somatic symptoms: A resting-state fMRI study. J Affect Disord. (2021) 295:788–96. doi: 10.1016/j.jad.2021.08.143
27. Shan X, Cui X, Liu F, Li H, Huang R, Tang Y, et al. Shared and distinct homotopic connectivity changes in melancholic and non-melancholic depression. J Affect Disord. (2021) 287:268–75. doi: 10.1016/j.jad.2021.03.038
28. Yang Y, Cui Q, Pang Y, Chen Y, Tang Q, Guo X, et al. Frequency-specific alteration of functional connectivity density in bipolar disorder depression. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 104:110026. doi: 10.1016/j.pnpbp.2020.110026
29. Kong Z, Zhu X, Chang S, Bao Y, Ma Y, Yu W, et al. Somatic symptoms mediate the association between subclinical anxiety and depressive symptoms and its neuroimaging mechanisms. BMC Psychiatry. (2022) 22:835. doi: 10.1186/s12888-022-04488-9
30. Luo Q, Chen J, Li Y, Wu Z, Lin X, Yao J, et al. Altered regional brain activity and functional connectivity patterns in major depressive disorder: A function of childhood trauma or diagnosis? J Psychiatr Res. (2022) 147:237–47. doi: 10.1016/j.jpsychires.2022.01.038
31. Schreiner MW, Klimes-Dougan B, Cullen KR. Neural correlates of suicidality in adolescents with major depression: resting-state functional connectivity of the precuneus and posterior cingulate cortex. Suicide Life Threat Behav. (2019) 49:899–913. doi: 10.1111/sltb.12471
32. Kercher AJ, Rapee RM, Schniering CA. Neuroticism, life events and negative thoughts in the development of depression in adolescent girls. J Abnorm Child Psychol. (2009) 37:903–15. doi: 10.1007/s10802-009-9325-1
33. Girz L, Driver-Linn E, Miller GA, Deldin PJ. Evaluation of life events in major depression: assessing negative emotional bias. Clin Psychol Psychother. (2017) 24:661–9. doi: 10.1002/cpp.2033
34. Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. (2008) 1124:111–26. doi: 10.1196/annals.1440.010
35. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. (2000) 24:417–63. doi: 10.1016/S0149-7634(00)00014-2
36. Bremner JD, Wittbrodt MT. Stress, the brain, and trauma spectrum disorders. Int Rev Neurobiol. (2020) 152:1–22. doi: 10.1016/bs.irn.2020.01.004
37. Bennett SN, Chang AB, Rogers FD, Jones P, Peña CJ. Thyroid hormones mediate the impact of early-life stress on ventral tegmental area gene expression and behavior. Horm Behav. (2023) 159:105472. doi: 10.1016/j.yhbeh.2023.105472
38. Gupta T, Eckstrand KL, Forbes EE. Annual research review: puberty and the development of anhedonia - considering childhood adversity and inflammation. J Child Psychol Psychiatry. (2024) 65(4):459–80. doi: 10.1111/jcpp.13955
Keywords: major depressive disorder, childhood trauma, negative life events, resting-state functional magnetic resonance imaging, adolescents
Citation: Xia X, Tang J, Peng Y, Liu Y, Chen Y, Yuan M, Yu R, Hou X and Fu Y (2024) Brain alterations in adolescents with first-episode depression who have experienced adverse events: evidence from resting-state functional magnetic resonance imaging. Front. Psychiatry 15:1358770. doi: 10.3389/fpsyt.2024.1358770
Received: 22 December 2023; Accepted: 18 March 2024;
Published: 09 April 2024.
Edited by:
Naseem Akhtar Qureshi, Al-Falah University, IndiaReviewed by:
Xiufeng Xu, The First Affiliated Hospital of Kunming Medical University, ChinaPatrick Köck, Universitäre Psychiatrische Dienste Bern, Switzerland
Copyright © 2024 Xia, Tang, Peng, Liu, Chen, Yuan, Yu, Hou and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renqiang Yu, eXVyZW5xaWFuZ0Bob3NwaXRhbC5jcW11LmVkdS5jbg==; Xiao Hou, aG91eGlhb3hpYW9AZm94bWFpbC5jb20=; Yixiao Fu, ZnV5aXhpYW9yZXNlYXJjaEAxMjYuY29t
 Xiaodi Xia
Xiaodi Xia Jinxiang Tang1
Jinxiang Tang1 Renqiang Yu
Renqiang Yu Yixiao Fu
Yixiao Fu